What is ganglioglioma
Ganglioglioma is a rare, usually low-grade and slow growing brain tumor that accounts for approximately 1-2% of all primary brain tumors in adults 1 and up to 10% of primary brain tumors in children 2. Most gangliogliomas grow slowly and are considered benign. However, up to 10% of gangliogliomas may grow more rapidly and become malignant, meaning the tumor affects the surrounding brain tissue 3. Ganglioglioma tumor contains nerve cells and glial (supportive) cells and typically produces seizures beginning in childhood. In fact, ganglioglioma is the most common epilepsy-associated brain tumor and the seizures may be difficult to control with medicine. Gangliogliomas predominantly affected young (87.5%), male patients, with a peak incidence in the second decade of life 4 and most frequently presented with seizures (64%) 5. Surgical removal is often feasible and is associated with good long-term tumor and seizure control for most patients. Gangliogliomas are most commonly seen in children and young adults and there is no gender preponderance 6.
Gangliogliomas often arise in the temporal lobe (up to 58%) of children and young adults often in association with seizures. Gangliogliomas are the most common tumor related to temporal lobe epilepsy 7. Other common locations of ganglioglioma are the frontal, parietal, and occipital lobes. However, gangliogliomas can occur at any age and may be found at virtually any location of the central nervous system (CNS), such as the spinal cord, cerebellum, thalamus, hypothalamus, lateral ventricle, brainstem and optic nerve 6. A recent study reported that the frontal and parietal lobes are the second most frequent site of gangliogliomas 8.
Ganglioglioma is a well-differentiated and typically slow-growing glioneuronal neoplasm composed of dysplastic ganglion cells in combination with neoplastic glial cells 9. Ganglioglioma is thought to be a tumor of low malignancy potential with a benign clinical course 10. Most gangliogliomas correspond histologically to World Health Organization (WHO) grade 1 and do not recur after complete resection. However, gangliogliomas are both histologically and clinically variable, and tumor recurrence or anaplastic progression occurs in a subset of cases.
Histologically, gangliogliomas are mixed tumors composed of relatively mature neoplastic glial and ganglion cells in varying proportions 11. Both components usually exhibit low grades of malignancy, but it is the grade of the gliomatous element that predicts the biological behavior of the tumor. The cell of origin of each component has long been a clinicopathologic mystery. Many mechanisms could account for the presence of neoplastic glial and neuronal cells in gangliogliomas. These two components could arise independently, with either independent expansions of glioblasts and apolar neuroblasts within the same tumor, or through interaction between these precursors in a paracrine fashion. Alternatively, the two components could be derived from a single abnormal progenitor cell, such as a neuroepithelial cell, which then differentiates into both glial and neuronal elements during pathogenetic development 12. Some findings support the hypothesis that gangliogliomas represent developmental lesions. These include histopathologic evidence of associated disordered neuronal migration and a long clinical history in many patients 6. Histologic diagnosis may be complicated because of difficulty in determining whether the ganglion cells are truly neoplastic, or simply “trapped” neurons in an infiltrating glioma 10. Ganglioglioma was named for its histologic components of differentiated nerve cells, in the form of ganglion cells, with a glial background that is typically astrocytic 10. Malignant transformation is unusual and, when it occurs, most often involves glial cells (glioblastoma) and, less frequently, ganglion cells (neuroblastoma). Leptomeningeal extension is rare 13. The favorable prognosis associated with ganglioglioma makes early recognition important for treatment and patient counseling.
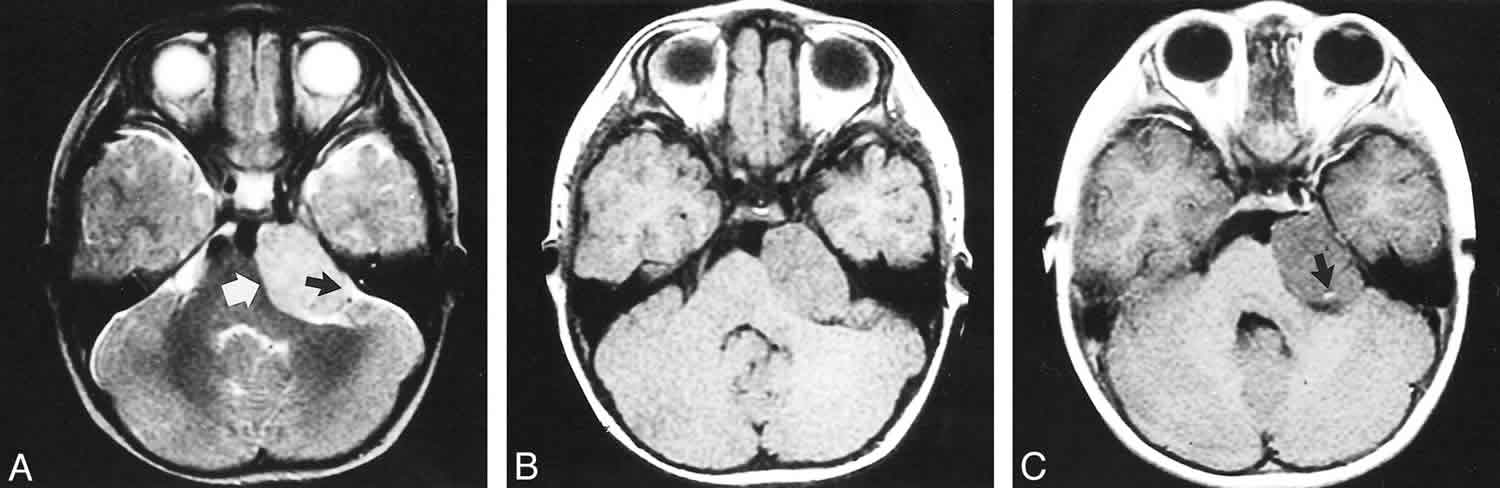
Ganglioglioma neuroimaging appearance is variable, but they often display a mix of solid and cystic components.
The main treatment for ganglioglioma is removal of the entire tumor during surgery and it determines prognosis. In the brain, where a reasonable resection margin can be achieved, the prognosis is good, with recurrence-free survival reported to be 97% at 7.5-year follow-up 14.
In contrast, in the spinal cord where complete resection is often not possible without devastating deficits, local recurrence is very common.
If the entire tumor is not removed, it has the potential to recur and may require additional surgery or treatments, such as radiation therapy or chemotherapy. Unfortunately, because gangliogliomas are quite rare, there is limited information to show that radiation therapy or chemotherapy are effective treatments for ganglioglioma 3.
Ganglioglioma causes
The cause of ganglioglioma is unknown. Gangliogliomas occur when a single cell in the brain starts to divide into more cells, forming a tumor 15. This can occur when the cell randomly acquires changes (mutations) in genes that regulate how a cell divides. The activating p.V600E hotspot mutation in the BRAF oncogene has been identified in a subset of gangliogliomas, ranging from approximately 10–60% depending on the study and anatomic site, with highest frequencies reported in cortical tumors and lower frequency reported in spinal cord tumors 2. However, BRAF p.V600E mutation is not specific to ganglioglioma and has been described in a wide spectrum of neuroepithelial tumors including pilocytic astrocytoma, dysembryoplastic neuroepithelial tumor (DNET), pediatric IDH-wildtype diffuse astrocytoma, polymorphous low-grade neuroepithelial tumor of the young (PLNTY), pleomorphic xanthoastrocytoma, and epithelioid glioblastoma 16. Additionally, the genetic alterations responsible for BRAF p.V600 wildtype gangliogliomas are largely unknown, as is the spectrum of any additional cooperating gene mutations or copy number alterations.
Ganglioglioma pathology
Gangliogliomas are benign, typically slow-growing and well-differentiated neuroepithelial tumors composed of variable proportions of dysplastic neuronal elements (ganglion cells) and neoplastic glial elements 9. Gangliogliomas are World Health Organization (WHO) grade 1 tumors, most commonly found in the temporal lobe (up to 85%) 17, but do occur anywhere in the central nervous system. In a minority of cases (5%), gangliogliomas show aggressive behavior and histopathologic features and are then called anaplastic gangliogliomas (WHO grade 3 tumors) 5. Anaplastic gangliogliomas are even rarer and can occur de novo or as a result of malignant transformation of a pre-existing lesion 18. At this stage, no criteria for WHO grade 2 gangliogliomas have been established 14.
Microscopic appearance
Gangliogliomas, as their name suggests, are composed of two cell populations:
- Ganglion cells (large mature neuronal elements): ganglio-
- Neoplastic glial element: -glioma
- primarily astrocytic, although oligodendroglial or pilocytic astrocytoma components are also encountered 14
The proportion of each component varies widely, and it is the grade of the glial component that determines biological behavior.
Dedifferentiation into high-grade tumors does occasionally occur, and it is usually the glial component (into a glioblastoma multiforme). Only rarely is it the neuronal component (into neuroblastoma).
They are closely related to both gangliocytomas (which contain only the mature neural ganglion cellular component) and ganglioneurocytoma (which also have small mature neoplastic neurons).
Immunophenotype
Neuronal origin is demonstrated by positivity to neuronal markers 14:
- synaptophysin: positive
- neurofilament protein: positive
- MAP2: positive
- chromogranin-A: positive (usually negative in normal neurons) 14
- CD34: positive in 70-80%
The glial component may also show cytoplasmic positivity for GFAP.
Genetics
- BRAF V600E mutations are encountered in 20-60% of cases 14
- IDH: negative (if positive then the tumor is most likely a diffuse glioma) 14
Ganglioglioma symptoms
Most patients with gangliogliomas present with long histories of seizures, whereas focal neurologic deficits or increased intracranial pressure are unusual. The most common symptom is with temporal lobe epilepsy, with an incidence rate of 75-100%, presumably due to the temporal lobes being a favored location 19. Children and young patients are usually affected, and no gender predominance is recognized.
Ganglioglioma diagnosis
If it’s suspected that you have a ganglioglioma, your doctor may recommend a number of tests and procedures, including:
- A neurological exam. A neurological exam may include, among other things, checking your vision, hearing, balance, coordination, strength and reflexes. Difficulty in one or more areas may provide clues about the part of your brain that could be affected by a brain tumor.
- Imaging tests. Magnetic resonance imaging (MRI) is commonly used to help diagnose brain tumors. In some cases a dye may be injected through a vein in your arm during your MRI study. A number of specialized MRI scan components — including functional MRI, perfusion MRI and magnetic resonance spectroscopy — may help your doctor evaluate the ganglioglioma tumor and plan treatment. Sometimes other imaging tests are recommended, including computerized tomography (CT). Positron emission tomography (PET) may be used for brain imaging, but is generally not as useful for creating images of brain cancer as it is for other types of cancer.
- Tests to find cancer in other parts of your body. If it’s suspected that your brain tumor may be a result of cancer that has spread from another area of your body, your doctor may recommend tests and procedures to determine where the cancer originated. One example might be a CT or PET scan to look for signs of lung cancer.
- Collecting and testing a sample of abnormal tissue (biopsy). A biopsy can be performed as part of an operation to remove the brain tumor, or a biopsy can be performed using a needle. A stereotactic needle biopsy may be done for brain tumors in hard to reach areas or very sensitive areas within your brain that might be damaged by a more extensive operation. Your neurosurgeon drills a small hole into your skull. A thin needle is then inserted through the hole. Tissue is removed using the needle, which is frequently guided by CT or MRI scanning. The biopsy sample is then viewed under a microscope to determine if it is cancerous or benign. Sophisticated laboratory tests can give your doctor clues about your prognosis and your treatment options.
Similar to other brain tumors, imaging techniques define the location of gangliogliomas and their relationship to adjacent structures. Because of the relative rarity and nonspecific appearance of these lesions, gangliogliomas are only infrequently considered a presurgical diagnosis 20.
Magnetic resonance imaging (MRI) with contrast enhancement is more sensitive and specific than other imaging techniques in determining the presence and location of gangliogliomas and in characterizing the lesions’ cystic and/or solid components 21. Partially cystic mass with an enhancing mural nodule is seen in ~45% of cases. They may also simply present as a solid mass expanding the overlying gyrus. An infiltrating mass is uncommon and may reflect higher grade. However, the imaging appearances of these tumors are nonspecific, and the diagnosis is usually established by histology and immunohistochemistry.
Ganglioglioma treatment
Microsurgery is the gold standard in the treatment of gangliogliomas, and complete tumor removal, when possible, is the only curative option 22. Complete resection, however, is not always feasible because of tumor location at deep and critical sites adjacent to vital brain areas. In particular, brainstem gangliogliomas often require alternative or additional treatments such as radiation therapy or chemotherapy because of the limited role of microsurgery in the treatment of these lesions.
Patients who underwent gross total resection showed the lowest recurrence rate at 12.5% (1/8), in contrast to a rate of 50% (3/6) in patients who underwent incomplete resection (subtotal resection or partial resection). Among the patients with incompletely resected tumors, those who received adjuvant treatments (gamma knife radiosurgery and conventional radiotherapy) had a better long-term control rate than those who did not (67% vs. 33%) 5. Consequently, gross total resection resulted in the best local control, as reported in previous studies 22 and adjuvant radiation therapy reduced the risk of recurrence. Our result is consistent with those of previous small sized studies that reported favorable results of adjuvant radiation therapy after incomplete resection of nonmalignant gangliogliomas, although gamma knife radiosurgery was not included in these studies 23.
Recurrence occurred in 29% of surgically resected gangliogliomas (12.5% of completely resected tumors and 50% of incompletely resected tumors). Luyken et al. 22 analyzed 184 patients with supratentorial gangliogliomas and reported recurrence rates of 1% among cases with no residual tumor and 8% among cases showing residual tumor. The discrepancy in the recurrence rate between our series and Luyken’s study could be explained by differences in the sample size and distribution of tumor location. For salvage purposes, both gamma knife radiosurgery and repeat surgery were effective, accounting for the lack of further recurrence after salvage treatment 5.
Radiation therapy
Low-grade gangliogliomas do not require post-operative radiotherapy as long as they are completely removed. In cases of incomplete resection, as described above, adjuvant radiation therapy has yielded favorable results in terms of tumor control 23. However, the acceptable long-term tumor control rates obtained with observation alone following subtotal resection suggest that the routine use of adjuvant radiation therapy after subtotal resection may be overtreatment 22. Meanwhile, radiotherapy for the management of recurrence or high-grade gangliogliomas has not produced satisfactory results 23. Furthermore, potential radiation-related malignant changes in low-grade tumors are an ongoing concern 24, despite data suggesting that radiotherapy may not be the major causative factor in malignant transformation 25. Accordingly, there is no consensus regarding the need for radiotherapy in patients with residual or recurrent ganglioglioma. Several authors have suggested that adjuvant radiotherapy should be restricted to cases where recurrence would result in neurologic compromise or patients with anaplastic ganglioglioma in whom radical resection is not feasible because of uncertainty regarding its benefit on tumor control or the detrimental effects of radiation on the CNS 26.
Epilepsy
Epilepsy associated with ganglioglioma, even if it is medically refractory, can be controlled by tumor resection 27. Seizure relief was reported to be accomplished in 76% to 88% of epileptic patients 28 and 59% of seizure-free patients were off antiepileptic medication 29.
Ganglioglioma prognosis
The prognosis of ganglioglioma is favorable, with a 10-year survival rate of 84-93% 30. One series reported a 5-year survival rate of 93% 31 and another study showed a 7.5-year survival rate of 98% 22.
More than half of the residual tumors after subtotal resection remain silent for a long time 29, underscoring the need to identify prognostic factors for the detection of residual tumors at risk of recurrence. Although prognostic factors are difficult to reliably define because of the low incidence of ganglioglioma, certain features such as complete resection, temporal location, epilepsy presentation 32, and absence of cellular atypia 30 are associated with favorable prognosis.
The degree of resection shows a strong correlation with recurrence 32, and the best tumor control and progression-free survival rates are achieved with complete tumor resection 22.
Another important prognostic factor is histologic grade. Although the criteria for WHO grade 2 gangliogliomas have not been established, WHO grade 2 tumors have atypical histological features, such as increased cellularity and increased mitotic activity, and lack unequivocal anaplastic features 32. Several studies based on a 3-tiered histopathologic grading system reported that WHO grade 2 tumors accounted for approximately 10% and WHO grade 3 tumors accounted for 2.5-5% of all gangliogliomas 32. Majores et al. 32 investigated the survival and recurrence rates according to tumor grade. The 5-year survival rates of patients with WHO grade 1, 2, and 3 tumors were 99%, 79%, 53%, and the recurrence rates were 2%, 33%, 60%, respectively. DeMarchi et al. 33 investigated the survival time of anaplastic gangliogliomas based on previous publications and found that nine (39%) of 23 patients with anaplastic gangliogliomas were dead at an average of 13 months after diagnosis. Although some authors reported that histological grade is not a predictor of poor outcome 31, it is likely to play a role in determining the clinical course and hence should be taken into consideration in choosing the treatment modalities.
The extent of resection is thought to be the main prognostic factor in the treatment of gangliogliomas 6. Low-grade gangliogliomas can be cured surgically, and complete tumor resection is the most effective treatment. Radiotherapy is reserved for progressive or malignant tumors after surgical treatment 34. Long-term epilepsy 35 and temporal tumor location 22 are among the favorable prognostic factors. Tumors in the temporal lobe commonly present with epilepsy, and in most cases, a temporal lobe location allows complete resection of the ganglioglioma tumors. As a result, epilepsy and temporal lobe location are associated with favorable outcomes. On the contrary, infratentorial ganglioglioma tumors, which usually cause cerebellar dysfunction or cranial nerve deficits rather than epilepsy, are frequently difficult to completely remove and consequently lead to less favorable outcomes.
References- Zhang D, Henning TD, Zou LG, et al. Intracranial ganglioglioma: clinicopathological and MRI findings in 16 patients. Clin Radiol. 2008;63:80–91
- Pekmezci M, Villanueva-Meyer JE, Goode B, et al. The genetic landscape of ganglioglioma. Acta Neuropathol Commun. 2018;6(1):47. Published 2018 Jun 7. doi:10.1186/s40478-018-0551-z https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5992851
- Selvanathan SK, Hammouche S, Salminen HJ, Jenkinson MD. Outcome and prognostic features in anaplastic ganglioglioma: analysis of cases from the SEER database. Journal of Neuro-oncology. 2011; Epub ahead of print:http://www.ncbi.nlm.nih.gov/pubmed/21626070
- Isimbaldi G, Sironi M, Tonnarelli GP, Roncoroni M, Declich P, Galli C. Ganglioglioma: a clinical and pathological study of 12 cases. Clin Neuropathol. 1996;15:192–199.
- Song JY, Kim JH, Cho YH, Kim CJ, Lee EJ. Treatment and outcomes for gangliogliomas: a single-center review of 16 patients. Brain Tumor Res Treat. 2014;2(2):49–55. doi:10.14791/btrt.2014.2.2.49 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4231627
- Zentner J, Wolf HK, Ostertun B, et al. Gangliogliomas: clinical, radiological, and histopathological findings in 51 patients. J Neurol Neurosurg Psychiatry 1994;57:1497-1502
- Karamitopoulou E, Perentes E, Probst A, Wegmann W. Ganglioglioma of the brain stem: neurological dysfunction of 16-year duration. Clin Neuropathol. 1995;14:162–168.
- Safavi-Abbasi S, Di Rocco F, Chantra K, et al. Posterior cranial fossa gangliogliomas. Skull Base. 2007;17:253–264.
- Becker AJ, Wiestler OD, Figarella-Branger D, Blumcke I, Capper D (2016) Ganglioglioma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) WHO Classification of Tumours of the Central Nervous System, revised 4th edn, International Agency for Research on Cancer, Lyon, pp 138–141
- Blatt GL, Ahuja A, Miller LL, Ostrow PT, Soloniuk DS. Cerebellomedullary ganglioglioma: CT and MR findings. AJNR Am J Neuroradiol 1995;16:790-792
- Cerebellopontine Angle Ganglioglioma: MR Findings. American Journal of Neuroradiology August 2001, 22 (7) 1377-1379 http://www.ajnr.org/content/ajnr/22/7/1377.full.pdf
- Zhu JJ, Leon SP, Folkerth RD, Guo SZ, Wu JK, Black PM. Evidence for clonal origin of neoplastic neuronal and glial cells in gangliogliomas. Am J Pathol 1997;151:565-571
- Berenguer J, Bargallo N, Bravo E, Sanchez M, Cardenal C, Mercader JM. An unusual frontal ganglioglioma: CT and MRI. Neuroradiology 1994;36:311-312
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK “WHO Classification of Tumours of the Central Nervous System. 4th Edition Revised” ISBN: 9789283244929
- Majores, M. , von Lehe, M. , Fassunke, J. , Schramm, J. , Becker, A. J. and Simon, M. (2008), Tumor recurrence and malignant progression of gangliogliomas. Cancer, 113: 3355-3363. doi:10.1002/cncr.23965
- Chappe C, Padovani L, Scavarda D, et al. Dysembryoplastic neuroepithelial tumors share with pleomorphic xanthoastrocytomas and gangliogliomas BRAF(V600E) mutation and expression. Brain Pathol. 2013;23:574–583. doi: 10.1111/bpa.12048
- Rumboldt Z, Castillo M, Huang B et-al. Brain Imaging with MRI and CT. Cambridge University Press. (2012) ISBN:1139576399
- Schittenhelm J, Reifenberger G, Ritz R, et al. Primary anaplastic ganglioglioma with a small-cell glioblastoma component. Clin Neuropathol. 2008;27:91–95.
- Silver JM, Rawlings CE, 3rd, Rossitch E, Jr, Zeidman SM, Friedman AH. Ganglioglioma: a clinical study with long-term follow-up. Surg Neurol. 1991;35:261–266.
- Zhang S, Ai L, Chen XZ, Wang K. Radiological Evaluation of Infratentorial Gangliogliomas in Various Anatomic Locations of the Cerebellum and Brainstem. Clin Neuroradiol. 2016 Jan 7.
- Balaji R, Ramachandran K. Imaging of desmoplastic infantile ganglioglioma: a spectroscopic viewpoint. Childs Nerv Syst. 2009 Jan 13
- Luyken C, Blümcke I, Fimmers R, Urbach H, Wiestler OD, Schramm J. Supratentorial gangliogliomas: histopathologic grading and tumor recurrence in 184 patients with a median follow-up of 8 years. Cancer. 2004;101:146–155
- Liauw SL, Byer JE, Yachnis AT, Amdur RJ, Mendenhall WM. Radiotherapy after subtotally resected or recurrent ganglioglioma. Int J Radiat Oncol Biol Phys. 2007;67:244–247
- Tarnaris A, O’Brien C, Redfern RM. Ganglioglioma with anaplastic recurrence of the neuronal element following radiotherapy. Clin Neurol Neurosurg. 2006;108:761–767
- Mittelbronn M, Schittenhelm J, Lemke D, et al. Low grade ganglioglioma rapidly progressing to a WHO grade IV tumor showing malignant transformation in both astroglial and neuronal cell components. Neuropathology. 2007;27:463–467
- Rumana CS, Valadka AB. Radiation therapy and malignant degeneration of benign supratentorial gangliogliomas. Neurosurgery. 1998;42:1038–1043
- Park YS, Kim DS, Shim KW, Kim JH, Choi JU. Factors contributing to resectability and seizure outcomes in 44 patients with ganglioglioma. Clin Neurol Neurosurg. 2008;110:667–673
- Im SH, Chung CK, Cho BK, Lee SK. Supratentorial ganglioglioma and epilepsy: postoperative seizure outcome. J Neurooncol. 2002;57:59–66
- Im SH, Chung CK, Cho BK, et al. Intracranial ganglioglioma: preoperative characteristics and oncologic outcome after surgery. J Neurooncol. 2002;59:173–183
- Hakim R, Loeffler JS, Anthony DC, Black PM. Gangliogliomas in adults. Cancer. 1997;79:127–131
- Lang FF, Epstein FJ, Ransohoff J, et al. Central nervous system gangliogliomas. Part 2: clinical outcome. J Neurosurg. 1993;79:867–873
- Majores M, von Lehe M, Fassunke J, Schramm J, Becker AJ, Simon M. Tumor recurrence and malignant progression of gangliogliomas. Cancer. 2008;113:3355–3363
- DeMarchi R, Abu-Abed S, Munoz D, Loch Macdonald R. Malignant ganglioglioma: case report and review of literature. J Neurooncol. 2011;101:311–318
- Mickle JP. Ganglioglioma in children. A review of 32 cases at the University of Florida. Pediatr Neurosurg. 1992;18:310–314.
- DeMarchi R, Abu-Abed S, Munoz D, Loch Macdonald R. Malignant ganglioglioma: case report and review of literature. J Neurooncol. 2011;101:311–318.