Gonorrhea
Gonorrhea also known as ‘the clap’, is a sexually transmitted disease (sexually transmitted infection) caused by the Gram-negative diplococci Neisseria gonorrhoeae (gonorrhea) bacteria (Figure 4). Gonorrhea is most common in young adults. Reported cases of gonorrhea have increased significantly in recent years, particularly among sexually active persons who are 15 to 24 years of age 1. The Neisseria gonorrhoeae bacteria or gonorrhea bacteria that cause gonorrhea can infect the genital tract, including the cervix (the opening of the uterus at the top of the vagina), uterus and fallopian tubes in women, and the urethra (the tube for urine) in women and men. Neisseria gonorrhoeae bacteria can also infect the mucous membranes of your mouth, throat, eyes, and rectum 2. You can get gonorrhea during vaginal, oral, or anal sex with an infected partner. A pregnant woman can pass gonorrhea to her baby during childbirth. In babies, gonorrhea most commonly affects the eyes.
In the United States, an estimated 1,568,000 new Neisseria gonorrhoeae infections occur each year 3, 4 and gonorrhea is the second most common notifiable sexually transmitted infection (STD) in the United States and is a major public health problem 5. Infections due to gonorrhea (Neisseria gonorrhoeae), like those resulting from Chlamydia trachomatis, are a major cause of pelvic inflammatory disease (PID) in the United States. Pelvic inflammatory disease (PID) can lead to serious outcomes in women, such as tubal infertility, ectopic pregnancy, and chronic pelvic pain. In addition, epidemiologic and biologic studies provide evidence that gonococcal infections facilitate the transmission of HIV infection 6. For 2017, the rate of reported gonorrhea cases among men (202.5 cases per 100,000 males) was significantly higher than among women (141.8 cases per 100,000 females) 7. Over the 1-year period of 2016–2017, the rates of reported gonorrhea increased 19.3% among men and 17.8% among women 7. In 2017, the highest rates of gonorrhea among women were observed among those aged 20-24 years (648.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females). Among men, the rate was highest among those aged 20-24 years (705.2 cases per 100,000 males) and 25–29 years (645.9 cases per 100,000 males) 7.
Gonorrhea often has no symptoms 8, 9:
- In men, gonorrhea can cause pain when urinating and thick, yellow discharge from the penis. The opening of the penis may be sore. If untreated, it can cause problems with the prostate and testicles, causing permanent damage and infertility in men.
- In women, the early symptoms of gonorrhea often are mild. Later, it can cause bleeding between periods, heavy bleeding during a period, fever, pain when urinating, and increased white, green, yellow, or bloody discharge from the vagina. If untreated, it can lead to pelvic inflammatory disease (PID), which causes problems with pregnancy and infertility.
- Both women and men can get sore throats if they’ve had oral contact with an infected person.
Gonorrhea can cause serious complications if it’s not treated.
If you think you have gonorrhea it is important to see a doctor as soon as possible. Your doctor can confirm the diagnosis with testing and start treatment. Treatment is with antibiotics. Treating gonorrhea is becoming more difficult because drug-resistant strains are increasing. Correct usage of latex condoms greatly reduces, but does not eliminate, the risk of catching or spreading gonorrhea. The most reliable way to avoid gonorrhea infection is to not have anal, vaginal, or oral sex.
Gonorrhea can be effectively treated with antibiotics. Sometimes you may need to be re-tested after your treatment to make sure the treatment has worked.
It is important to avoid having sex, even with a condom, until treatment is finished and tests show you are cured.
It is also very important to tell all your sexual partners from the past three months that you have been diagnosed with gonorrhea. They will need to be tested for gonorrhea and treated if infected.
Your doctor will help you decide who you need to tell and how you can tell them.
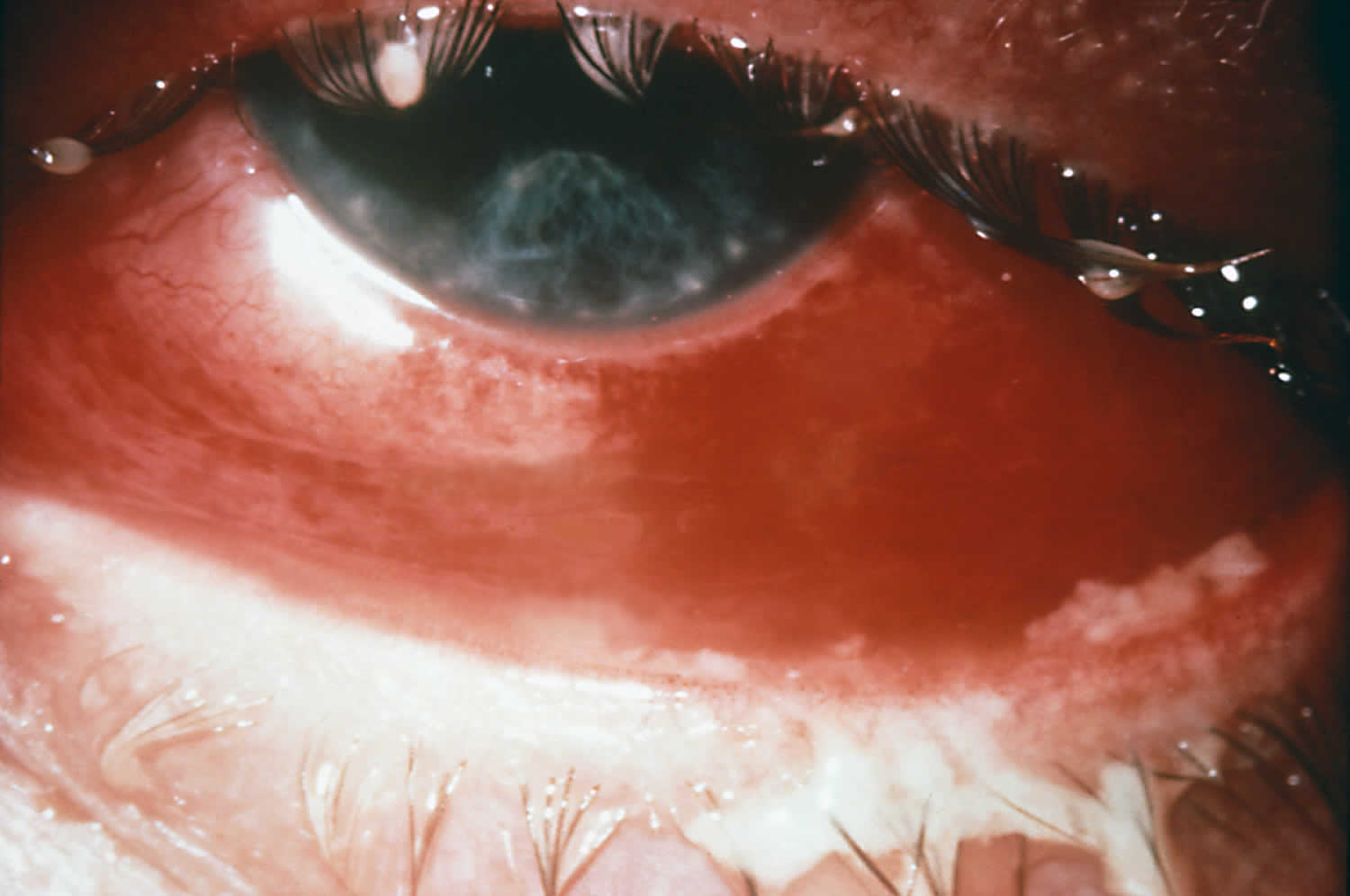
Figure 1. Gonorrhea eye infection
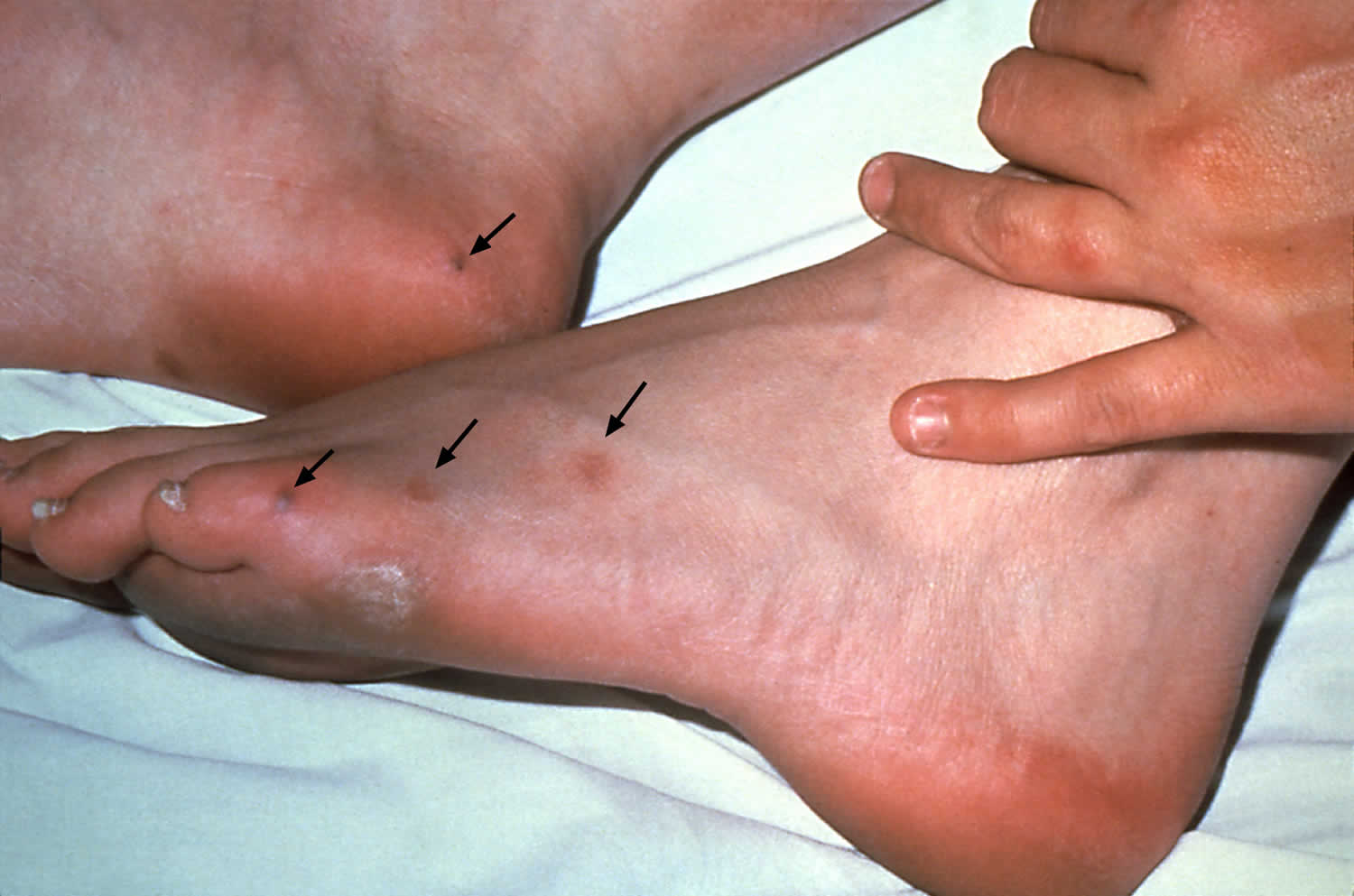
[Source 10 ]Figure 2. Gonorrhea skin rash
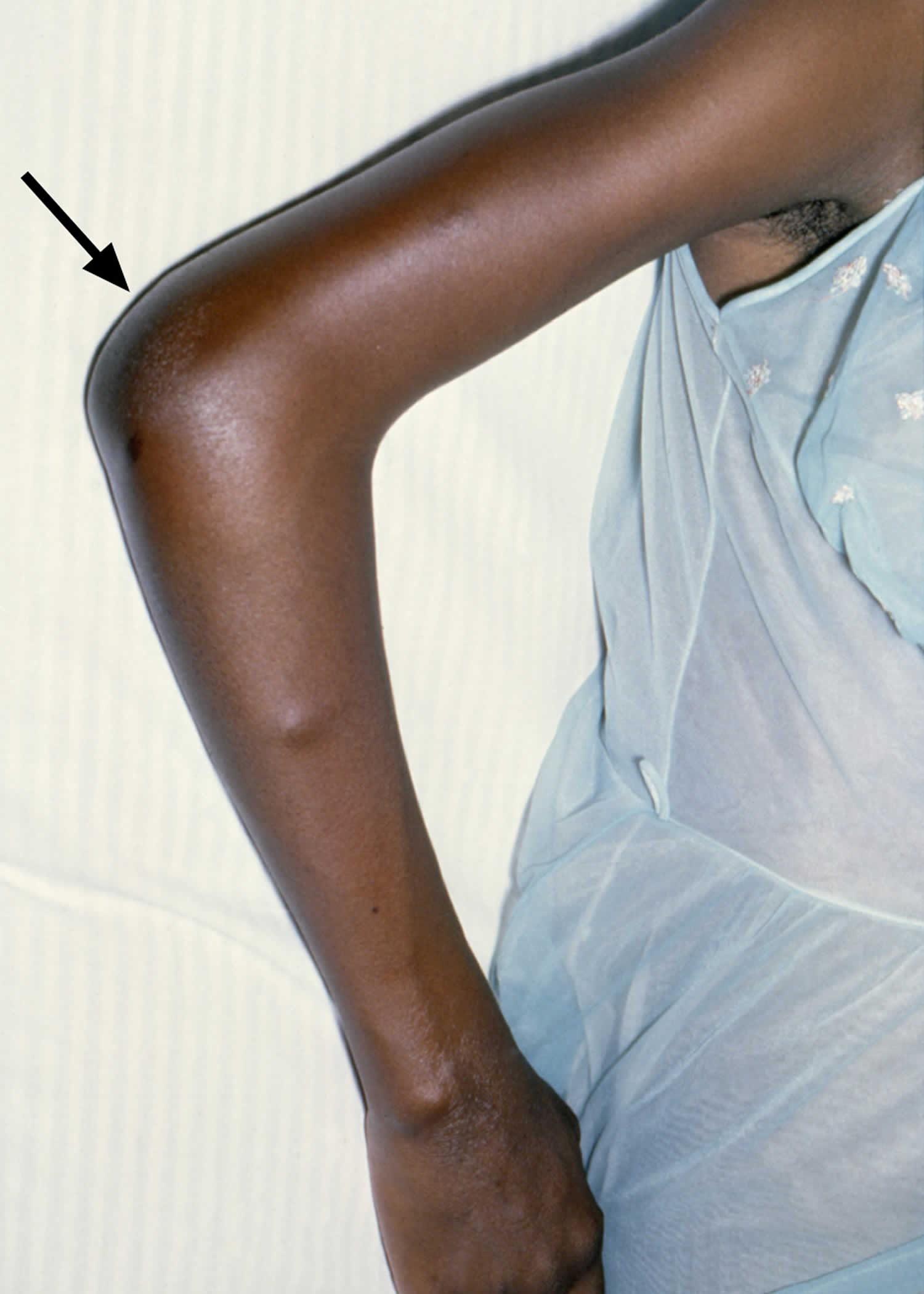
[Source 10 ]Figure 3. Disseminated gonococcal infection with arthritis
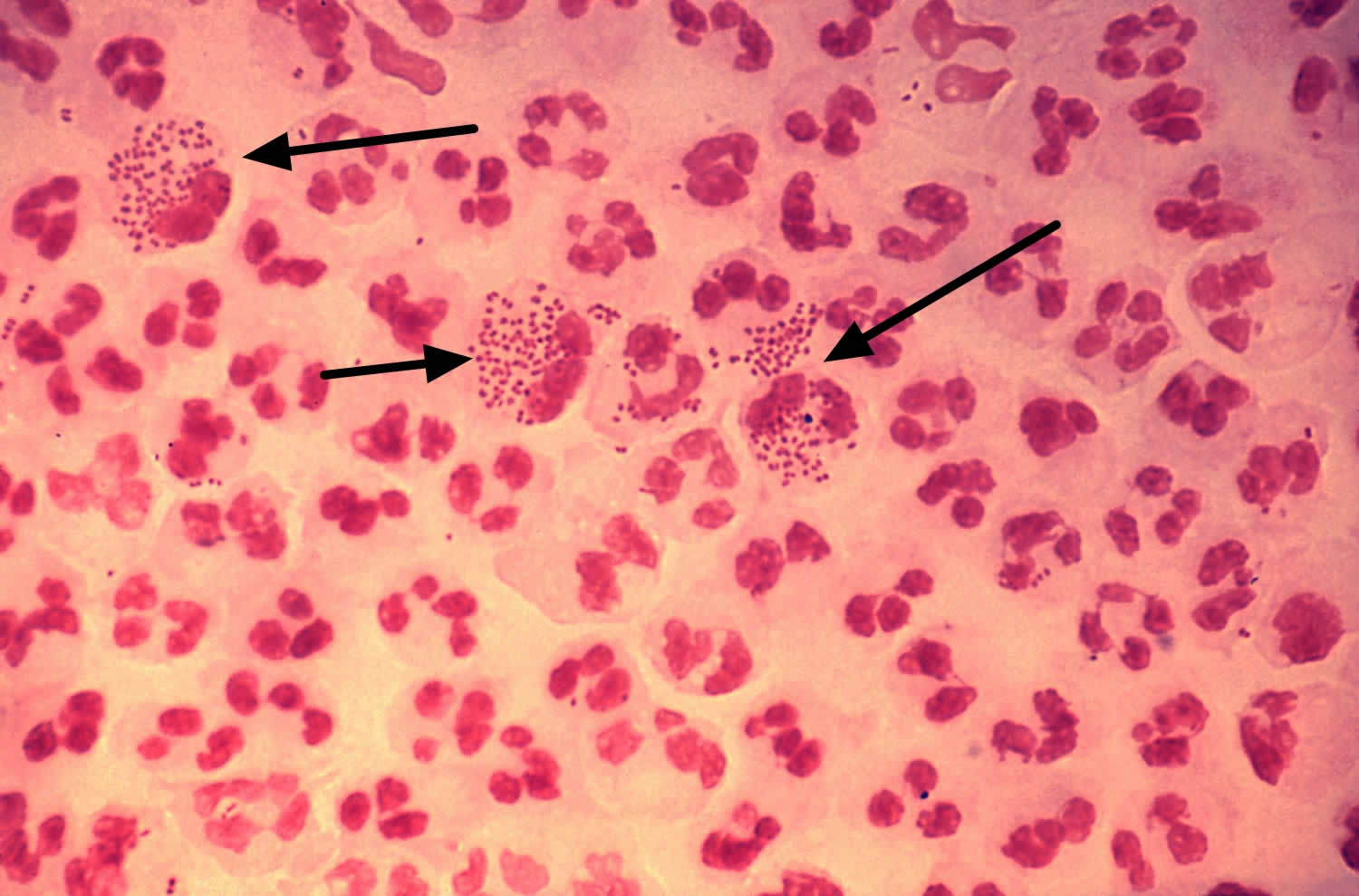
[Source 10 ]Figure 4. Neisseria gonorrhoeae bacteria
Footnotes: This low-resolution photomicrograph reveals the histopathology in an acute case of gonococcal urethritis using Gram-stain technique, demonstrated the presence of Gram-negative diplococci (black arrows). This slide is used to demonstrate the non-random distribution of Neisseria gonorrhoeae bacteria among polymorphonuclear neutrophils (a type of white blood cell). Note that there are both intracellular (inside the cell) and extracellular (outside the cell) gonorrhea bacteria in the field of view. For a presumptive diagnosis of gonorrhea to be made, additional tests would need to still be conducted.
[Source 11 ]Make an appointment with your doctor if you notice any troubling signs or symptoms, such as a burning sensation when you urinate or a pus-like discharge from your penis, vagina or rectum.
Also make an appointment with your doctor if your partner has been diagnosed with gonorrhea. You may not experience signs or symptoms that prompt you to seek medical attention. But without treatment, you can reinfect your partner even after he or she has been treated for gonorrhea.
What causes gonorrhea?
Gonorrhea is caused by infection with the bacterium Neisseria gonorrhea (Figure 4) and is spread by having unprotected vaginal, anal or oral sex with an infected person with gonorrhea. It can be passed by fingers or hands from the genitals to the eyes.
Gonorrhea can also be passed from an infected mother to her baby during birth, which can cause eye infection (neonatal conjunctivitis) and even blindness.
How does gonorrhea spread?
You can get gonorrhea by having vaginal, anal, or oral sex with someone who has gonorrhea. A pregnant person with gonorrhea can give the infection to their baby during childbirth.
How can I reduce my risk of getting gonorrhea?
The only way to completely avoid sexually transmitted diseases (STDs) is to not have vaginal, anal, or oral sex.
If you are sexually active, the following things can lower your chances of getting gonorrhea:
- Being in a long-term mutually monogamous relationship with a partner who has been tested and does not have gonorrhea.
- Using condoms the right way every time you have sex.
Am I at risk for gonorrhea?
Sexually active people can get gonorrhea through vaginal, anal, or oral sex without a condom with a partner who has gonorrhea.
If you are sexually active, have an honest and open talk with your healthcare provider. Ask them if you should get tested for gonorrhea or other STDs. If you are a sexually active gay or bisexual man, you should get tested for gonorrhea every year. If you are a sexually active woman, you should get tested for gonorrhea every year if you are:
- Younger than 25 years.
- 25 years and older with risk factors, such as new or multiple sex partners, or a sex partner who has a sexually transmitted infection.
I’m pregnant. How does gonorrhea affect my baby?
If you are pregnant and have gonorrhea, you can give the infection to your baby as your baby passes through your birth canal during delivery. This can cause cause blindness, joint infection, or a life-threatening blood infection in your baby 12. If you are pregnant, talk to your healthcare provider about getting the correct examination, testing, and treatment. Treating gonorrhea as soon as possible will make health problems for your baby less likely.
Gonorrhea infection in children
Perinatal infections most often occur during childbirth when the neonatal conjunctiva, pharynx, respiratory tract, or anal canal may become infected. Conjunctivitis (ophthalmia neonatorum) is preventable by ocular antimicrobial prophylaxis in the newborn. All cases of gonorrhea beyond the newborn period should be considered possible evidence of sexual abuse. Vulvovaginitis (not cervicitis) is the most common manifestation in prepubescent girls. Signs and symptoms may include vaginal discharge (often purulent or crusting), dysuria, odor, irritation, and pruritus. The anorectum and the pharynx are the most frequently infected sites in abused boys; urethritis is less frequently seen. If specimens are to be collected, proper guidelines for collecting forensic evidence must be followed. When evaluating a child who has potentially suffered sexual abuse, the clinician should consult individual state laws concerning reporting and counseling.
How do I know if I have gonorrhea?
Gonorrhea often has no symptoms, but it can cause serious health problems, even without symptoms.
Most women with gonorrhea do not have any symptoms. Even when a woman has symptoms, they are often mild and can be mistaken for a bladder or vaginal infection. Symptoms in women can include:
- Painful or burning sensation when peeing;
- Increased vaginal discharge; and
- Vaginal bleeding between periods.
Men who do have symptoms may have:
- A burning sensation when peeing;
- A white, yellow, or green discharge from the penis; and
- Painful or swollen testicles (although this is less common).
Rectal infections may either cause no symptoms or cause symptoms in both men and women that may include:
- Discharge;
- Anal itching;
- Soreness;
- Bleeding; and
- Painful bowel movements.
See your healthcare provider if you notice any of these symptoms. You should also see a provider if your partner has an STD or symptoms of one. Symptoms can include an unusual sore, a smelly discharge, burning when peeing, or bleeding between periods.
Is gonorrhea curable?
Yes. Gonorrhea can be cured with the right treatment. The Centers for Disease Control and Prevention (CDC) recommends either a single therapy or using two drugs, to treat gonorrhea – Ceftriaxone 250 mg IM in a single dose (for persons weighing <150 kg) with or without oral doxycycline 100 mg twice daily for 7 days depending on whether chlamydia infection has been excluded 13. If chlamydial infection has not been excluded then add doxycycline 100 mg orally 2 times/day for 7 days. For pregnant women, if chlamydia has not been excluded, oral azithromycin 1 gram should be used in place of doxycycline 13. If ceftriaxone is not available, the two options are (1) intramuscular gentamicin 240 mg plus oral azithromycin 2 grams or (2) oral cefixime 800 mg 14. It is important to take all of the medication prescribed to cure gonorrhea. Medication for gonorrhea should not be shared with anyone. Although medication will stop the infection, it will not repair any permanent damage done by the disease. Antimicrobial resistance in gonorrhea is of increasing concern, and successful treatment of gonorrhea is becoming more difficult. If a person’s symptoms continue for more than a few days after receiving treatment, he or she should return to a health care provider to be reevaluated.
Neisseria gonorrhoeae
Neisseria gonorrhoeae also known as gonococcus (singular), or gonococci (plural), is a species of Gram-negative diplococci bacteria isolated by Albert Neisser in 1879 15. Neisseria gonorrhoeae causes the sexually transmitted genitourinary infection gonorrhea as well as other forms of gonococcal disease including disseminated gonococcemia, septic arthritis, and gonococcal ophthalmia neonatorum.
Neisseria gonorrhoeae sexual transmission is through vaginal, anal, or oral sex 2. Sexual transmission may be prevented through the use of latex condoms 16. Perinatal transmission may occur during childbirth, and may be prevented by antibiotic treatment of the mother before birth and the application of antibiotic eye gel on the eyes of the newborn. After an episode of gonococcal infection, infected persons do not develop immunity to future infections. Reinfection is possible due to Neisseria gonorrhoeae’s ability to evade the immune system by varying its surface proteins 17.
Neisseria gonorrhoeae can cause infection of the genitals, throat, and eyes. Asymptomatic infection is common in males and females 18. Untreated gonococcal infection may spread to the rest of the body (disseminated gonorrhea infection), especially the joints (septic arthritis). Untreated infection in women may cause pelvic inflammatory disease (PID) and possible infertility due to the resulting scarring. Diagnosis is through gonorrhea culture or using nucleic acid amplification testing (NAAT), of a urine sample, urethral swab (for men), or endocervical or vaginal swab (for women) 13, 5. Chlamydia co-testing and testing for other STDs is recommended due to high rates of co-infection 13. FDA-cleared rectal and oral diagnostic tests for gonorrhea (as well as chlamydia) have been validated for clinical use 13.
Gonorrhea can be cured with the right treatment. The Centers for Disease Control and Prevention (CDC) now recommends a single 500 mg intramuscular (IM) dose of ceftriaxone for the treatment of gonorrhea. Alternative regimens are available when ceftriaxone cannot be used to treat urogenital or rectal gonorrhea 2.
Antibiotic resistance in Neisseria gonorrhoeae is a growing public health concern, especially given its propensity to develop resistance easily 19, 20.
How do you get gonorrhea?
Anyone who is sexually active can get gonorrhea. Gonorrhea is spread by having unprotected vaginal, anal or oral sex with an infected person.
Gonorrhea can also be passed from an infected mother to her baby during birth, which can cause eye infection (neonatal conjunctivitis) and even blindness.
The transmission of gonorrhea can occur in several ways:
- Male-to-female transmission of gonorrhea via semen occurs at a rate of approximately 50% to 70% per episode of vaginal intercourse with ejaculation; male-to-female transmission of gonorrhea can occur without ejaculation 21.
- An infected woman can transmit gonorrhea to the urethra of a male sex partner; the rate of transmission is approximately 20% per episode from vaginal intercourse, and it increases to approximately 60% to 80% after four or more intercourse exposures 22.
- Pharyngeal gonorrhea is readily acquired by fellatio; it is less efficiently acquired by cunnilingus. Gonorrhea can also be transmitted from the pharynx to the urethra during fellatio (and presumably to vagina with cunnilingus).
- Perinatal transmission (mother-to-infant) can occur during vaginal delivery, when the infected mother has not been treated during the perinatal period.
Rectal intercourse transmission rates have not been quantified, but rectal intercourse appears to be an efficient mode of transmission. - Gonorrhea is associated with increased susceptibility to HIV acquisition. It is also associated with an increase in HIV transmission, because gonococcal urethritis increases HIV shedding in men 23.
Risk factors for getting gonorrhea
Risk factors and risk markers for acquiring gonorrhea include:
- Younger age
- Being adolescent (especially female)
- Sexually active women younger than 25 and men who have sex with men
- A new sex partner
- Having more than one sex partner
- Having a sex partner who has other partners
- Inconsistent or incorrect condom use
- Living in an urban area where gonorrhea prevalence is high
- Having a lower socio-economic status
- Using drugs including alcohol (in association with higher risk sex)
- Exchanging sex for drugs or money
- African American race
- Previous gonorrhea diagnosis
- Having other sexually transmitted infections
Gonorrhea prevention
The surest way to avoid transmission of gonorrhea or other STDs is to abstain from vaginal, anal, and oral sex, or to be in a long-term mutually monogamous relationship with a partner who has been tested and is known to be uninfected. Latex condoms, when used consistently and correctly, can reduce the risk of transmission of gonorrhea 16.
Take steps to reduce your risk of gonorrhea:
- Always use condoms with a water-based lubricant if you choose to have sex. Abstaining from sex is the surest way to prevent gonorrhea. But if you choose to have sex, use a condom during any type of sexual contact, including anal sex, oral sex or vaginal sex. Use a male latex condom or a female polyurethane condom during each sexual contact. Condoms used properly during every sexual encounter reduce but don’t eliminate the risk of infection. Latex condoms greatly reduces, but does not completely eliminate, the risk of catching or spreading STDs. If your or your partner is allergic to latex, you can use polyurethane condoms.
- Always use dental dams for oral sex (a dental dam is a thin square of latex placed over the vulva or anus during oral sex).
- Limit your sex partners or have a long-term monogamous relationship where neither of you is already infected. Having multiple sex partners puts you at a high risk of contracting chlamydia and other sexually transmitted infections.
- Ask your partner to be tested for sexually transmitted infections. Find out whether your partner has been tested for sexually transmitted infections, including gonorrhea. If not, ask whether he or she would be willing to be tested.
- Don’t have sex with someone who has any unusual symptoms. If your partner has signs or symptoms of a sexually transmitted infection, such as burning during urination or a genital rash or sore, don’t have sex with that person.
- Avoid sex with someone infected with gonorrhea until after they have finished treatment and are cured.
- Have regular sexually transmitted infection (STI) check-ups. Annual screening is recommended for all sexually active women less than 25 years of age and for older women at increased risk of infection, such as those who have a new sex partner, more than one sex partner, a sex partner with concurrent partners, or a sex partner who has a sexually transmitted infection. Regular screening is also recommended for men who have sex with men, as well as their partners.
- Avoid douching. Douching decreases the number of good bacteria in the vagina, which can increase the risk of infection.
To avoid reinfection with gonorrhea, abstain from unprotected sex for seven days after you and your sex partner have completed treatment and after resolution of symptoms, if present.
How to use male condoms
- Put the condom on before any contact is made.
- Unroll the condom over an erect penis to the base of the penis. (Uncircumcised men should pull back their foreskin before unrolling.) The unrolled ring should be on the outside. Leave about 1/2 inch of space in the tip so semen can collect there. Squeeze the tip to get the air out.
- Pull out after ejaculating and before the penis gets soft. To pull out, hold the rim of the condom at the base of the penis to make sure it doesn’t slip off.
- Don’t reuse condoms.
How to use female condoms
- Follow the directions on the condom package for correct placement. Be sure the inner ring goes as far into the vagina as it can. The outer ring stays outside the vagina.
- Guide the penis into the condom.
- After sex, remove the condom before standing up by gently pulling it out.
- Don’t reuse condoms.
Screening for gonococcal infection
Routine screening for gonorrhea infection in women is recommended in order to decrease morbidity as well as to reduce the burden of disease in the community 24. Urethral infections caused by gonorrhea among men usually produce symptoms that cause them to seek curative treatment soon enough to prevent sequelae, but transmission to others may occur in this interim. Among women, gonococcal infections are commonly asymptomatic until complications (such as pelvic inflammatory disease with resultant risk for infertility and ectopic pregnancy) have occurred. The following summarizes gonorrhea screening recommendations issued by the CDC and the U.S. Preventive Services Task Force (USPSTF) for different patient populations 24, 25:
- Sexually Active Women Who Have Sex with Men: The CDC 24 and the U.S. Preventive Services Task Force 25 recommend (1) annual screening for gonorrhea in all sexually active women younger than 25 years of age, and (2) annual screening for gonorrhea in sexually active women age 25 years and older if they are considered to have increased risk for gonococcal infection. The most important identified risk factors for gonococcal infection include a new sex partner, multiple sex partners, a sex partner with concurrent partners, or a sex partner with a sexually transmitted infection; additional factors that indicate risk of gonococcal infection include inconsistent condom use in persons not in a mutually monogamous relationship, exchange of sex for money or drugs, one or more previous sexually transmitted infections, or a coexistent sexually transmitted infection. Women diagnosed with gonorrhea infection should have repeat testing approximately 3 months after completing treatment.
- Women Who Have Sex with Women: The CDC recommends gonococcal screening for women who have sex with women should occur according to the current screening guidelines for sexually active women who have sex with men 26.
- Women Who are Pregnant: The CDC recommends screening for gonorrhea should be performed at the first prenatal visit for (1) women younger than age 25 and (2) women age 25 years and older who are at increased risk for gonorrhea (e.g. women with a new sex partner, a sex partner who has a sexually transmitted infection, more than one sex partner, or a sex partner with concurrent partners 26. Additional factors associated with increased risk of gonococcal infection include inconsistent condom use in persons not in a mutually monogamous relationship, exchange of sex for money or drugs, and previous or coexisting sexually transmitted infections. A repeat test for gonococcal infection should be performed during the third trimester for those at continued risk. Pregnant women diagnosed with gonorrhea infection should have repeat testing approximately 3 months after completing treatment 26.
- Men Who Have Sex Only with Women: Routine screening for gonococcal infection is not recommended by either the CDC or the USPSTF for men who have sex only with women.[10,27]
- Men Who Have Sex with Men: The CDC 26 recommends screening for gonococcal infection in men who have sex with men at least annually, regardless of a history of condom use during sexual contact; the sites tested should correspond with sites involved in sexual activity with other men during the prior year (e.g. urethral testing if insertive intercourse, rectal testing if receptive anal intercourse, and pharyngeal testing with receptive oral intercourse). The U.S. Preventive Services Task Force 25 does not recommend routine screening for gonorrhea in men, including men who have sex with men.
- Transgender Men and Women: The CDC 26 recommends screening for gonorrhea in transgender men (“trans-men”) and transgender women (“trans-women”) should be based on age, current anatomy, and sexual practices.
- Persons with HIV Infection: The CDC 27 recommends performing routine screening for gonorrhea for persons with HIV infection who are sexually active; testing for gonorrhea should be performed at the initial evaluation and at least annually thereafter (more frequent screening may be indicated based on risk). The testing should consist of obtaining samples from the anatomic sites of sexual exposure.
- Persons in Correctional Facilities: The CDC 26 recommends performing routine gonococcal screening at the initial intake in a correctional facility for women 35 years of age and younger and men younger than age 30.
Gonorrhea signs and symptoms
Gonorrhea often has no symptoms 8, 9.
Most women with gonorrhea have no symptoms at all.
Some men, especially those with throat or anus infection, also have no symptoms.
Occasionally gonorrhea can involve the eyes, joints, heart or brain, causing permanent damage.
In women, if symptoms do occur, they usually develop within 10 days of infection. In women, symptoms may include:
- unusual vaginal discharge
- pain, discomfort or burning sensation when passing urine
- pelvic pain, especially during sex
- irregular bleeding, especially between periods or after sex
- anal discharge and discomfort
- sore, dry throat
In men, if symptoms do occur, they usually develop within 1 to 3 days. In men, symptoms may include:
- thick, yellow or white discharge from the penis
- pain, discomfort or burning sensation when passing urine
- pain in the testes (balls)
- redness around the opening of the penis
- anal discharge and discomfort
- sore, dry throat
Gonorrhea symptoms in men
In men, when symptoms do occur, they usually develop within one to three days. In men, gonorrhea symptoms may include:
- thick, yellow or white discharge from the penis
- pain, discomfort or burning sensation when passing urine
- pain or swelling in one testis (balls)
- redness around the opening of the penis
- anal discharge and discomfort
- sore, dry throat.
Urethritis
Urethritis is a common manifestation of gonorrhea in men. Most men develop overt, symptomatic urethritis, but a small percentage will develop asymptomatic (unrecognized) infection. Asymptomatic gonorrhea may act as a reservoir that perpetuates transmission in the community 28. The typical symptoms of gonococcal urethritis, when present, include a purulent or mucopurulent urethral discharge, often accompanied by dysuria. The discharge may also be clear or cloudy. The incubation period ranges from 1 to 14 days, with most men becoming symptomatic within 2 to 5 days after exposure 29.
Anorectal infections
Anorectal infection most often occurs in men who have sex with men, with acquisition of rectal gonorrhea occurring through receptive anal intercourse, but it also has been reported in women with gonococcal cervicitis who do not acknowledge rectal sexual contact. These infections may result from perineal contamination with infected cervical secretions. Most patients with anorectal infection are asymptomatic, although proctitis can occur. Symptoms of proctitis include anal irritation, painful defecation, constipation, scant rectal bleeding, painless mucopurulent discharge, anal pruritus, and tenesmus 30. When proctitis is suspected, an anoscopic examination is recommended to assess for inflammation and mucosal injury. The anorectal mucosa may appear normal, but purulent discharge, erythema, or easily induced bleeding may be observable under anoscopy.
Complications of genital infection in men
Men with untreated gonococcal genital infection can develop epididymitis, with typical symptoms of unilateral testicular pain and swelling, and epididymal tenderness. Epididymitis is infrequent following gonococcal infection, but it is the most common local complication of gonorrhea infection in men. When it does occur, epididymitis is often associated with overt or subclinical urethritis. Urethral discharge may or may not be present. Notably, up to 70% of epididymitis caused by a sexually transmitted pathogen are due to Chlamydia trachomatis. Other less common complications associated with gonococcal infection in men include inguinal lymphadenitis, penile edema, periurethral abscess or fistula, accessory gland infection (Tyson’s glands), balanitis, urethral stricture, and prostatitis, and rarely perirectal abscess.
Gonorrhea symptoms in women
In women, when symptoms do occur, they usually develop within 10 days of infection. In women, gonorrhea symptoms may include:
- unusual vaginal discharge
- pain, discomfort or burning sensation when passing urine
- pelvic pain, especially during sex
- irregular vaginal bleeding, especially between periods or after sex
- abdominal or pelvic pain
- anal discharge and discomfort
- sore, dry throat.
Cervicitis
Symptomatic gonococcal infection in women most often manifests as cervicitis and/or urethritis, but at least 50% of women with genital gonococcal infection are asymptomatic. Symptoms of cervicitis vary and may include a nonspecific vaginal discharge, intermenstrual bleeding, dysuria, lower abdominal pain, and dyspareunia. Clinically, examination of the cervix may show mucopurulent or purulent cervical discharge and easily bleed with minimal contact. The incubation period in women is variable, but symptoms, when they do occur, usually develop within 10 days of the exposure 31. Seventy to ninety percent of women with genital gonococcal infection have laboratory evidence of urethral infection (urethritis); dysuria may be present, but these women frequently do not have specific urethral symptoms.
Anorectal infections
Anorectal gonococcal infection is uncommon in women, but can occur via anal intercourse. Anorectal infection has been reported in women with gonococcal cervicitis who do not acknowledge rectal sexual contact, presumably these infections result from perineal contamination with infected cervical secretions.
Complications in genital infection in women
There are several complications associated with gonorrhea in women:
- Accessory gland (Bartholin’s glands or Skene’s glands) infections: Infection of female sex accessory glands (Bartholin’s glands or Skene’s glands) is often a unilateral infection. Occlusion of the ducts of these glands due to inflammation may result in the formation of an abscess.
- Pelvic inflammatory disease (PID): If cervical gonococcal infection ascends to the endometrium and/or fallopian tubes, PID may develop, typically causing symptoms that include lower abdominal pain, vaginal discharge, dyspareunia, intermenstrual bleeding, and fever 32. In some women, PID may also be asymptomatic. Presumptive treatment for PID should be considered if one or more of the following minimum criteria are present on pelvic examination—uterine or adnexal tenderness or cervical motion tenderness. The long-term sequelae of untreated PID can include chronic pelvic pain, tubal infertility, and increased risk for ectopic pregnancy.
- Perihepatitis (Fitz-Hugh-Curtis Syndrome): In situations where gonococcal infection ascends from the cervix, infection may produce inflammation of the liver capsule and the adjacent peritoneum. Most women with perihepatitis have associated PID, but perihepatitis can occur independently. Historically, perihepatitis was attributed only to gonococcal infection, but now it is often associated with chlamydial infection. Gonococcal perihepatitis is characterized by right upper quadrant pain, and may be accompanied by abnormal liver function tests.
Gonorrhea at other sites in the body
Gonorrhea can also affect these parts of the body:
- Rectum. Signs and symptoms include anal itching, pus-like discharge from the rectum, spots of bright red blood on toilet tissue and having to strain during bowel movements.
- Eyes. Gonorrhea that affects your eyes may cause eye pain, sensitivity to light, and pus-like discharge from one or both eyes.
- Throat. Signs and symptoms of a throat infection may include a sore throat and swollen lymph nodes in the neck.
- Joints. If one or more joints become infected by bacteria (septic arthritis), the affected joints may be warm, red, swollen and extremely painful, especially when you move an affected joint.
Gonorrhea throat infection
Gonorrhea throat infection also called gonococcal pharyngeal infection is most often asymptomatic. The pharynx may be the sole site of infection if the only exposure was receptive orogenital intercourse. Exudative pharyngitis is rare. Symptoms of pharyngeal infection may include pharyngitis, tonsillitis, fever, and cervical adenitis.
Gonorrhea eye infection
Gonococcal infection of the eye, when it does occur, typically presents as conjunctivitis. Gonococcal conjunctivitis in adults most often results from autoinoculation in persons with genital gonococcal infection. Patients may initially develop a mild non-purulent conjunctivitis, that, if untreated, typically progress to marked conjunctival redness, copious purulent discharge, and conjunctival edema 33. Less often, the manifestations include an ulcerative keratitis. Untreated gonococcal conjunctivitis can cause complications that may include corneal perforation, endophthalmitis, and blindness.
Disseminated gonococcal infection
If left untreated, gonorrhea can also spread to the blood and cause disseminated gonococcal infection (DGI) 34. Disseminated gonococcal infection, a systemic gonococcal infection, occurs infrequently and is more common in women than in men. Disseminated gonococcal infection is associated with some gonococcal strains that have a propensity to produce bacteremia without associated urogenital symptoms. In addition, patients with complement deficiency have greater risk of developing disseminated gonococcal infection. Disseminated gonococcal infection is usually characterized by arthralgia, arthritis, tenosynovitis, hepatitis, myocarditis, endocarditis, meningitis and/or dermatitis 34. This condition can be life threatening. Rates of disseminated gonococcal infection have decreased due to the declining proportion of gonococcal strains prone to disseminate 35.
Gonorrhea complications
Untreated gonorrhea can cause serious and permanent health problems in both women and men, such as:
- Infertility in women. Untreated gonorrhea can spread into the uterus and fallopian tubes, causing pelvic inflammatory disease (PID), which may result in scarring of the tubes, greater risk of pregnancy complications and infertility. Pelvic inflammatory disease (PID) is a serious infection that requires immediate treatment. Pelvic inflammatory disease (PID) symptoms may be quite mild or can be very severe and can include abdominal pain and fever 36. PID can lead to internal abscesses and chronic pelvic pain. PID can also damage the fallopian tubes enough to cause infertility or increase the risk of ectopic pregnancy.
- Infertility in men. Men with untreated gonorrhea can experience epididymitis — inflammation of a small, coiled tube in the rear portion of the testicles where the sperm ducts are located (epididymis). Epididymitis is treatable, but if left untreated, it may lead to infertility 37.
- Infection that spreads to the joints and other areas of your body. The bacterium that causes gonorrhea can spread through the bloodstream and infect other parts of your body, including your joints. Fever, rash, skin sores, joint pain, swelling and stiffness are possible results.
- Increased risk of HIV/AIDS. Having gonorrhea makes you more susceptible to infection with human immunodeficiency virus (HIV), the virus that leads to AIDS. People who have both gonorrhea and HIV are able to pass both diseases more readily to their partners.
- Complications in babies. Babies who contract gonorrhea from their mothers during birth can develop blindness, sores on the scalp and infections.
- Disseminated gonococcal infection (DGI). If left untreated, gonorrhea can also spread to the blood and cause disseminated gonococcal infection (DGI). Disseminated gonococcal infection is usually characterized by arthralgia, arthritis, tenosynovitis, hepatitis, myocarditis, endocarditis, and meningitis and/or dermatitis 34. This condition can be life threatening.
Gonorrhea diagnosis
Testing for gonorrhea involves taking a swab (sample) from the urethra in men and the cervix in women. It can also be tested by taking a urine sample.
Sometimes swabs are also be taken from the throat, urethra, vagina or anus.
It is also important to get tested for other sexually transmitted infections such as syphilis, chlamydia and HIV.
To determine whether the gonorrhea bacterium is present in your body, your doctor will analyze a sample of cells. Samples can be collected by:
- Urine test. This may help identify bacteria in your urethra.
- Swab of affected area. A swab of your throat, urethra, vagina or rectum may collect bacteria that can be identified in a laboratory.
For women, home test kits are available for gonorrhea. Home test kits include vaginal swabs for self-testing that are sent to a specified lab for testing. If you prefer, you can choose to be notified by email or text message when your results are ready. You may then view your results online or receive them by calling a toll-free hotline.
Testing for other sexually transmitted infections
Your doctor may recommend tests for other sexually transmitted infections. Gonorrhea increases your risk of these infections, particularly chlamydia, which often accompanies gonorrhea. Testing for HIV also is recommended for anyone diagnosed with a sexually transmitted infection. Depending on your risk factors, tests for additional sexually transmitted infections could be beneficial as well.
Gonorrhea test
The approach to diagnostic testing for gonorrhea has evolved from traditional cultivation to widespread use of nucleic acid amplification tests (NAAT) 38. Gram’s stain, another non-culture test, is used for the diagnosis of urethral gonorrhea in symptomatic males. Culture is still recommended if antimicrobial resistance is a concern, especially in cases of treatment failure.
Nucleic Acid Detection Tests
There are two types of nucleic acid detection tests: non-amplified tests and amplified tests:
- Amplified Tests: The nucleic acid amplification tests (NAATs) include polymerase chain reaction (PCR) (Roche Amplicor; Cepheid GeneXpert CT/NG), transcription-mediated amplification (TMA) (Gen-Probe Aptima), and strand displacement amplification (SDA) (Becton-Dickinson BDProbeTec ET) 39. Amplified tests are FDA-cleared for endocervical specimens from women, urethral specimens from men, and urine specimens from men and women. Some NAATs are also cleared for vaginal swabs. For many of the commercially available tests, the same specimen can be used to test for Chlamydia trachomatis infection. NAATs are the most sensitive test to detect gonorrhea infections. In May 2019, the FDA cleared two NAATs (Aptima Combo 2 Assay and the Xpert CT/NG) for extragenital diagnostic testing of Neisseria gonorrhoeae and Chlamydia trachomatis in rectal and pharyngeal samples 40. Multiple studies have shown NAATs are the most sensitive test to detect Neisseria gonorrhoeae infections. At present, antimicrobial susceptibility cannot be determined with NAATs, but research in this area is ongoing. In addition, the major limitation of NAATs is the potential for false-positive results due to remnant nucleic acids, either from contamination or dead organisms; this property limits the utility of NAATs for immediate post-treatment testing.
- Non-Amplified Tests: Non-amplified tests used for gonorrhea include the DNA probe (e.g. Gen-Probe PACE 2 and Digene Hybrid Capture II). A non-amplified test is less likely to be affected by transport conditions than culture, and has the potential for more timely results. These tests are FDA-cleared for endocervical specimens from women and urethral specimens from men. They are not FDA-cleared for pharyngeal, rectal, or urine specimens. The same specimen can be evaluated for Chlamydia trachomatis infection 38. Antimicrobial susceptibility cannot currently be determined with non-amplified tests.
Point-of-Care NAAT Testing
In March 2021, the FDA approved the first point-of-care nucleic acid amplification test (NAAT) (Binx Health IO CT/NG Assay) for the diagnosis of urogenital chlamydia and gonorrhea 41. This point-of-care test can be run on vaginal swabs obtained from women or on urine samples collected from men 41. This assay can provide a result in approximately 30 minutes 41. In a cross-sectional study, investigators evaluated this point-of-care NAAT for the diagnosis of chlamydia and gonorrhea using vaginal swabs obtained from 1,523 women and urine samples collected from 922 men 42. For gonorrhea, the sensitivity estimates were 100.0% in women and 97.3% in men; the specificity estimates were 99.9% for women and 100.% for men 42. In addition, the investigators found that self-obtained vaginal swabs in women performed equivalent to clinician-collected vaginal swabs 42.
Gram’s Stain
The use of Gram’s stain is a non-culture test that can make a presumptive diagnosis of gonorrhea. In the clinical setting, a Gram’s stain to detect gonorrhea is most often performed on a male with purulent urethral discharge. A Gram’s stain on a specimen positive for gonorrhea shows polymorphonuclear leukocytes (PMNs) with intracellular gram-negative diplococci. A Gram’s stain, with proper laboratory technique, has greater than 95% sensitivity and greater than 99% specificity for diagnosing symptomatic male gonococcal urethritis 38. Thus, the Gram’s stain is considered reliable both to diagnose and to exclude gonococcal urethritis in symptomatic men 24. The sensitivity of a Gram’s stain is lower for mane with asymptomatic urethral infection and thus not considered adequate to rule out infection in asymptomatic men 24. Performing a Gram’s stain is not recommended on endocervical, pharyngeal, or rectal specimens due to poor sensitivity 24.
Bacterial culture
Obtaining a bacterial culture is the historic standard for detection of gonorrhea. It has several advantages over non-culture tests, including low cost, use for a variety of specimen sites, and antimicrobial susceptibility testing can be performed if gonorrhea is isolated from the specimen. Despite having some advantages, culture is not as sensitive as NAAT and is more laboratory intensive, which has led to infrequent use in modern practice. At present, culture is primarily used for antimicrobial resistance surveillance by collecting specimens from either symptomatic urethral infections or from screen-positive sites of infection prior to treatment 13.
Disseminated Gonococcal Infection special diagnostic considerations
When evaluating persons with suspected disseminated gonococcal infection, diagnostic testing should consist of (1) obtaining and ordering NAAT and culture for specimens from all applicable urogenital and extragenital mucosal sites, (2) ordering culture for all specimens obtained from disseminated sites of infection (e.g. skin, synovial fluid, blood, or cerebrospinal fluid), and (3) performing antimicrobial susceptibility testing on all Neisseria gonorrhoeae isolates obtained in culture specimens.
Gonorrhea treatment
Adults with gonorrhea are treated with antibiotics. Due to emerging strains of drug-resistant Neisseria gonorrhoeae, the Centers for Disease Control and Prevention (CDC) recommends that uncomplicated gonorrhea be treated only with the antibiotic ceftriaxone — given as an injection — in combination with either azithromycin (Zithromax, Zmax) or doxycycline (Monodox, Vibramycin, others) — two antibiotics that are taken orally.
Some research indicates that oral gemifloxacin (Factive) or injectable gentamicin, combined with oral azithromycin, is highly successful in treating gonorrhea. This treatment may be helpful in treating people who are allergic to cephalosporin antibiotics, such as ceftriaxone.
The following summarizes several key new recommendations from the 2021 Sexually Transmitted Infections Treatment Guidelines 13, 14:
- For the treatment of uncomplicated gonococcal infection of the cervix, urethra, or rectum in persons who weigh less than 150 kg, the single intramuscular ceftriaxone dose has been increased from 250 mg to 500 mg; for persons who weigh 150 kg or greater, the dose should be increased to 1 gram. The increased dose of ceftriaxone is recommended based on pharmacokinetic and pharmacodynamic data that show ceftriaxone concentrations at 24 hours with a 500 mg dose provides more reliable eradication of Neisseria gonorrhoeae than with a 250 mg dose 43, 44.. Furthermore, because ceftriaxone produces variable levels in the pharynx, the 500 mg ceftriaxone dose should provide a more reliable treatment for pharyngeal gonorrhea.
- For persons with uncomplicated gonococcal infection of the cervix, urethra, or rectum in whom chlamydia infection has been ruled out, ceftriaxone monotherapy is recommended, which is in contrast to the prior recommendation to add azithromycin as dual therapy for all gonococcal infections 45. The rationale for eliminating the routine use of azithromycin as dual therapy for the treatment of gonorrhea is twofold: (1) the concern for azithromycin causing antimicrobial resistance in commensal organisms and concurrent pathogens, and (2) the trend of increasing Neisseria gonorrhoeae resistance to azithromycin 46, 47.
- For persons with gonococcal infection in whom chlamydia infection has not been excluded, oral doxycycline is added to ceftriaxone for the purpose of treating chlamydia (for nonpregnant persons). This new recommendation is based on emerging data suggesting better chlamydia treatment efficacy with doxycycline than with azithromycin, especially with rectal chlamydia 48, 49, 50.
- For persons with pharyngeal gonococcal infection, a test-of-cure is recommended 7 to 14 days after treatment, regardless of the treatment regimen used. The prior recommendation was to perform a test-of-cure after treatment of pharyngeal gonococcal infection only if an alternative regimen was used.
- The recommended expedited partner therapy (EPT) with a single oral dose of cefixime has increased from 400 mg to 800 mg and routine dual therapy with azithromycin is no longer recommended; if concurrent chlamydia was not excluded in the source individual who was diagnosed with gonorrhea, then the expedited partner therapy should include oral doxycycline 100 mg for 7 days (for nonpregnant persons).
Antibiotics for gonorrhea
The CDC treatment guidelines recommend using dual therapy for the treatment of gonococcal infections in adults and adolescents. Ceftriaxone is the most effective cephalosporin for treatment of gonorrhea and should be used in combination with azithromycin. The recommendation for dual therapy is based on the premise that using two antimicrobials with different mechanisms of action (e.g. a cephalosporin plus azithromycin) may improve treatment efficacy and potentially slow the emergence and spread of resistance. In addition, azithromycin and doxycycline will effectively treat concomitant C. trachomatis infection, if present. Azithromycin is preferred over doxycycline as a second agent due to convenience (single-dose therapy versus 7-day therapy) and the substantially lower prevalence of gonococcal resistance to azithromycin than with doxycycline, particularly for gonococcal strains that have an elevated cefixime MIC. In the case of azithromycin allergy or severe intolerance, doxycycline (100 mg orally twice a day for 7 days) can be used as a substitute for azithromycin, but doxycycline should only be used as an alternative, primarily because of the high prevalence of gonococcal tetracycline resistance. For details regarding these alternative regimens, refer to the section on gonococcal infections in the 2015 STD Treatment Guidelines 51. The following recommendations for treatment are based on the 2015 STD Treatment Guidelines.
Uncomplicated Gonococcal Infections of the Cervix, Urethra, and Rectum
Recommended Regimen
- Ceftriaxone 250 mg IM in a single dose (for persons weighing <150 kg)
- PLUS
- Doxycycline 100 mg orally 2 times/day for 7 days (if chlamydial infection has not been excluded)
For pregnant women, if chlamydia has not been excluded, oral azithromycin 1 gram should be used in place of doxycycline 13. If ceftriaxone is not available, the two options are (1) intramuscular gentamicin 240 mg plus oral azithromycin 2 grams or (2) oral cefixime 800 mg 14
Alternative Regimens
If cephalosporin allergy:
- Gentamicin 240 mg IM in a single dose
- PLUS
- Azithromycin 2 g orally in a single dose
If ceftriaxone is not available:
- Cefixime 800 mg* orally in a single dose
- PLUS
- * If chlamydial infection has not been excluded, treat for chlamydia with doxycycline 100 mg orally 2 times/day for 7 days.
Uncomplicated Gonococcal Infections of the throat (pharynx)
Most gonococcal infections of the pharynx are asymptomatic and can be relatively common in some populations 52. Gonococcal infections of the pharynx are more difficult to eradicate than are infections at urogenital and anorectal sites 53. Few antimicrobial regimens, including those involving oral cephalosporins, can reliably cure >90% of gonococcal pharyngeal infections 54. Doctors should ask their patients with urogenital or rectal gonorrhea about oral sexual exposure; if reported, patients should be treated with a regimen with acceptable efficacy against pharyngeal gonorrhea infection.
The recommended treatment of pharyngeal gonorrhea:
- Ceftriaxone 500 mg IM in a single dose (for persons weighing <150 kg)
- PLUS
- Doxycycline 100 mg orally 2 times/day for 7 days (if chlamydial infection has not been excluded)
Concomitant treatment for pharyngeal chlamydial infection is not indicated if testing for pharyngeal chlamydia was not performed or if the testing for chlamydia was negative 13. If treatment for chlamydia pharyngeal infection is indicated and the person is pregnant, oral azithromycin 1 gram should be used in place of doxycycline. Note there are no reliable alternative regimens for the treatment of pharyngeal gonorrhea 13. For persons with an anaphylactic or other severe reaction (e.g., Stevens Johnson syndrome) to ceftriaxone, consult an infectious disease specialist for an alternative treatment recommendation.
Gonococcal conjunctivitis
In the only published study of the treatment of gonococcal conjunctivitis among adults, all 12 study participants responded to a single 1 g intramuscular injection of ceftriaxone 55. Based on this study, the recommended treatment for gonococcal conjunctivitis is a single ceftriaxone 1 gram intramuscular dose 13. In addition, a one-time lavage of the infected eye with saline should be considered 13. Because gonococcal conjunctivitis is uncommon and data regarding treatment of gonococcal conjunctivitis among adults are limited, consultation with an infectious disease specialist should be considered.
Recommended Regimen
- Ceftriaxone 1 g IM in a single dose.
Gonococcal Infections in Pregnancy
As with other patients, pregnant women infected with gonorrhea should be treated ceftriaxone 500 mg in a single IM dose plus azithromycin if chlamydia infection was not ruled out 14. When cephalosporin allergy or other considerations preclude treatment with this regimen, consultation with an infectious disease specialist or an STD clinical expert is recommended. Gentamicin use is cautioned during pregnancy because of risk for neonatal birth defects, nephrotoxicity, or ototoxicity 56. Pregnant persons should not be treated with any fluoroquinolone or tetracycline drugs.
Gonorrhea treatment for babies
Babies born to mothers with gonorrhea receive a medication in their eyes soon after birth to prevent infection. If an eye infection develops, babies can be treated with antibiotics.
Disseminated Gonococcal Infection
Disseminated gonococcal infection frequently results in petechial or pustular acral skin lesions, asymmetric polyarthralgia, tenosynovitis, or oligoarticular septic arthritis. The infection is complicated occasionally by perihepatitis and rarely by endocarditis or meningitis. Because of the possibility of potentially severe sequelae associated with these complications, the 2021 STD Treatment Guidelines recommend hospitalization and consultation with an infectious diseases specialist for patients suspected of having disseminated gonococcal infection. Examination for clinical evidence of endocarditis and meningitis should be performed. If disseminated gonococcal infection is suspected, NAAT and culture specimens from all exposed urogenital and extragenital sites should be collected and processed, in addition to NAAT and culture specimens from disseminated sites of infection (e.g., skin, synovial fluid, blood, or CNS). All N. gonorrhoeae isolates should be tested for antimicrobial susceptibility. Risk factors for dissemination have included female sex, menstruation, pregnancy, and terminal complement deficiency 57; however, reports are increasing among men 58, 59. People receiving eculizumab, a monoclonal antibody that inhibits terminal complement activation, also might be at higher risk for disseminated gonococcal infection 60.
The recommended initial antimicrobial therapy is ceftriaxone 1 gram intramuscularly or intravenously every 24 hours; if chlamydia has not been excluded, then treatment of chlamydia with doxycycline 100 mg twice daily should be given. The first dose of ceftriaxone should be given after promptly obtaining appropriate diagnostic samples. The duration of therapy for disseminated gonococcal infection with arthritis-dermatitis syndrome is at least 7 days, and the ceftriaxone can transition to oral therapy if antimicrobial sensitivity testing shows an effective oral choice 13. For patients diagnosed with disseminated gonococcal infection and meningitis, parenteral therapy should continue for 10 to 14 days; with endocarditis, parenteral therapy should be given for at least 4 weeks 13.
Treatment of Arthritis and Arthritis-Dermatitis Syndrome
Recommended Regimen
- Ceftriaxone 1 g IM or IV every 24 hours
- PLUS
- If chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally 2 times/day for 7 days.
Alternative Regimens
- Cefotaxime 1 g IV every 8 hours
- OR
- Ceftizoxime 1 g IV every 8 hours
- PLUS
- If chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally 2 times/day for 7 days.
When treating for the arthritis-dermatitis syndrome, your doctor can switch to an oral agent guided by antimicrobial susceptibility testing 24–48 hours after substantial clinical improvement, for a total treatment course of at least 7 days.
Treatment of Gonococcal Meningitis and Endocarditis
Recommended Regimen
- Ceftriaxone 1–2 g IV every 12–24 hours
- PLUS
- If chlamydial infection has not been excluded, providers should treat for chlamydia with doxycycline 100 mg orally 2 times/day for 7 days.
No recent studies have been published regarding treatment of disseminated gonococcal infection involving the the brain and spinal cord (central nervous system or CNS) or cardiovascular system. The duration of treatment for disseminated gonococcal infection in these situations has not been systematically studied and should be determined in consultation with an infectious disease specialist. Treatment for disseminated gonococcal infection should be guided by the results of antimicrobial susceptibility testing. Length of treatment should be determined based on clinical presentation. Therapy for meningitis should be continued with recommended parenteral therapy for 10–14 days. Parenteral antimicrobial therapy for endocarditis should be administered for >4 weeks. Treatment of gonococcal perihepatitis should be managed in accordance with the recommendations for PID in these guidelines 13.
Management of Antibiotic-Resistant Gonorrhea
Although there are no confirmed cases of treatment failure due to cephalosporin-resistant gonorrhea in the United States, the gradual upwards trend of MICs documented by the United States Gonococcal Isolate Surveillance Project remains worrisome 61. Criteria for resistance to cefixime and ceftriaxone have not been defined by the Clinical and Laboratory Standards Institute, but isolates with cefixime or ceftriaxone MICs equal to or greater than 0.5 μg/mL are considered to have decreased susceptibility. Only five isolates with ceftriaxone MIC equal to or greater than 0.5 μg/mL have been reported during the history of the United States Gonococcal Isolate Surveillance Project. In 2019, one isolate from Nevada was reported with a ceftriaxone MIC of 1.0 μg/mL 62. Notably, isolates with high-level cefixime and ceftriaxone MICs (cefixime MICs 1.5 to 8 μg/mL and ceftriaxone MICs 1.5 to 4 μg/mL) have been identified in Japan, France, and Spain 63, 64, 65.
Allergy to Penicillins or Cephalosporin
Allergic reactions to first-generation cephalosporins occur in less than 2.5% of persons with a history of penicillin allergy and are less common with third-generation cephalosporins such as ceftriaxone and cefixime 66. Ceftriaxone is contraindicated in patients with a history of IgE-mediated anaphylaxis to penicillin. Given these considerations, expert consultation with an infectious diseases specialist (and possibly also an allergy specialist), is recommended for treating gonorrhea among persons who have documented severe cephalosporin allergy. Cephalosporin desensitization is preferred but impractical in many settings. Potential therapeutic options in this situation for adults and adolescents include (1) dual treatment with single doses of oral gemifloxacin 320 mg plus a single dose of oral azithromycin 2 g, or (2) dual treatment with single doses of intramuscular gentamicin 240 mg plus a single dose of oral azithromycin 2 g 24. Note that since May 2015, gemifloxacin has not available for use in the United States because of a legal dispute regarding the license to manufacture and distribute this drug. For patients with documented severe cephalosporin allergy, recent evidence supports superior effectiveness of dual therapy when compared with azithromycin monotherapy. In this setting, spectinomycin monotherapy has been effective in clinical trials, curing 98.2% of uncomplicated urogenital and anorectal gonococcal infections, but it has poor efficacy against pharyngeal infection and is not currently available in the United States. Although true allergic reactions to third-generation cephalosporins are uncommon among persons who report a history of penicillin allergy, use of ceftriaxone is contraindicated in persons with a history of IgE-mediated penicillin allergy.
Management of Suspected Gonococcal Treatment Failure
Clinicians who diagnose gonorrhea infection in a person with suspected cephalosporin treatment failure should (1) perform culture and susceptibility testing of all relevant clinical specimens; (2) obtain expert opinion for guidance in clinical management (through the STD Clinical Consultation Network [https://stdccn.org], a local STD/HIV Prevention Training Center clinical expert, the CDC, or an infectious diseases specialist); and (3) report the case to the CDC through state and local public health authorities 24. Isolates that grow gonorrhea should be saved and sent to the CDC through state public health laboratory mechanisms. Health departments should prioritize notification and culture evaluation for sex partner(s) of persons with gonorrhea infection suspected for cephalosporin treatment failure or persons whose isolates demonstrate decreased susceptibility to cephalosporins. In this setting, a test-of-cure at relevant clinical sites should be obtained 7 to 14 days after retreatment; culture is the recommended test, preferably with simultaneous NAAT and susceptibility testing of gonorrhea if isolated.
- Initial Approach to Suspected Treatment Failure: Persons with suspected cephalosporin treatment failure should first receive retreatment and receive a single dose of intramuscular ceftriaxone 500 mg, with doxycycline if chlamydial infection exists. This initial approach is recommended in the United States because most suspected treatment failures actually result from reinfection.
- Antimicrobial Options for Likely Treatment Failure: For individuals considered to have high likelihood of true treatment failure, especially those with a documented elevated cephalosporin MIC for N. gonorrhoeae, the suggested option to consider is single-dose oral therapy with azithromycin 2 grams plus a single intramuscular injection of a 240 mg dose of gentamicin.
- Investigational Therapy for N. gonorrhoeae: Several antimicrobials under investigation have shown promise in the treatment of gonorrhea in phase 2 trials, including single-dose oral gepotidacin and single-dose oral zoliflodacin 67, 68. These two agents are now under study in phase 3 trials, and both may have a future role in treating drug-resistant gonorrhea and gonorrhea in persons with serious penicillin or cephalosporin allergy. Two oral agents—solithromycin and delafloxacin—showed promising early results, but phase 3 studies have been disappointing, and these agents are not likely to have a clinical role in the treatment of gonorrhea 69, 70.
Follow-Up
Due to the significant risk of reinfection in persons diagnosed with gonorrhea, all persons diagnosed with gonorrhea should have repeat testing in 3 months at the anatomic site of exposure, regardless of whether they have symptoms; this is considered a test for reinfection, not a test-of-cure 13. For persons with uncomplicated gonococcal infections of the cervix, urethra, or rectum, a routine test-of-cure at 7 to 14 days post-treatment is not recommended 13. For persons with pharyngeal gonorrhea, however, a routine test-of-cure (using either culture or NAAT) is recommended 7 to 14 days after completing treatment, regardless of the treatment regimen 13. For these individuals, if the test-of-cure NAAT is positive, an effort should be made to perform a confirmatory culture before retreatment; all positive cultures for test-of-cure should undergo antimicrobial susceptibility testing 45. For persons with suspected cephalosporin-resistant gonorrhea who receive retreatment, a test-of-cure at relevant clinical sites should be obtained 7 to 14 days after retreatment; culture is the recommended test, preferably with simultaneous NAAT and susceptibility testing of N. gonorrhoeae if isolated 13.
Management of Sex Partners
Your partner also should undergo testing and treatment for gonorrhea, even if he or she has no signs or symptoms. Your partner receives the same treatment you do. Even if you’ve been treated for gonorrhea, you can be reinfected if your partner isn’t treated.
All recent sex partners (within the 60 days preceding the onset of symptoms or gonorrhea diagnosis) should be referred for evaluation, testing, and presumptive treatment of gonorrhea. If the most recent contact with a sex partner occurred more than 60 days preceding onset of symptoms or gonorrhea diagnosis, that partner should be referred for evaluation and treatment. Sex partners who are treated should also be instructed to abstain from sexual activity for 7 days after they have completed antimicrobial treatment.
Expedited partner therapy
In settings where prompt referral and treatment are unavailable or impractical, providers should consider expedited partner therapy 71. This entails provision of appropriate antibiotics as well as educational and pharmacy information for the partner. The documentation should include notification that partner(s) have been exposed, information about the importance of treatment, signs and symptoms of potential complications, as well as possible therapy-related potential allergic reactions and adverse effects 24.
The expedited partner therapy regimen for sex partners of patients with gonorrhea infection is oral cefixime 800 mg with delivery of the prescription to the partner by either the person diagnosed with gonorrhea, a disease investigation specialist, or a collaborating pharmacy as permitted by law; if concurrent chlamydia was not excluded in the source individual who was diagnosed with gonorrhea, then the expedited partner therapy should include oral doxycycline 100 mg for 7 days (for nonpregnant persons); if there is concern regarding the partner taking multidose therapy for chlamydia, then azithromycin 1 gram orally as a single dose can be given 13.
It is essential to check with one’s state health department to clarify the policies, as the use of expedited partner therapy is not legal in all states. The CDC maintains an updated information page Legal Status of Expedited Partner Therapy that identifies the legal status of expedited partner therapy in each state in the United States, as well as providing links to each state for more detailed state policies. Notably, provision of expedited partner therapy alone is not sufficient and each partner should ideally be seen in follow-up for repeat testing to confirm resolution of infection and check for reinfection. Although offering expedited partner therapy to female partners is acceptable, this approach may result in undertreatment of pelvic inflammatory disease. The use of expedited partner therapy for gonorrhea is contraindicated in a female partner who have current signs or symptoms that are suggestive of PID. Female partners who have current signs and symptoms suggestive of PID should undergo prompt evaluation by a health care provider. In addition, the use of expedited partner therapy should not be considered a routine partner management strategy in men who have sex with men with gonorrhea for several reasons, including the high risk for coexisting infections (especially HIV and syphilis infection), inadequate data regarding the efficacy of expedited partner therapy in this patient population, and concerns regarding the increased proportion of gonococcal isolates among men who have sex with men with reduced susceptibility to cefixime.
References- Gonorrhea – CDC Basic Fact Sheet. https://www.cdc.gov/std/gonorrhea/stdfact-gonorrhea.htm
- Gonorrhea – CDC Detailed Fact Sheet. https://www.cdc.gov/std/gonorrhea/stdfact-gonorrhea-detailed.htm
- Kreisel KM, Spicknall IH, Gargano JW, Lewis FMT, Lewis RM, Markowitz LE, Roberts H, Johnson AS, Song R, St Cyr SB, Weston EJ, Torrone EA, Weinstock HS. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2018. Sex Transm Dis. 2021 Apr 1;48(4):208-214. doi: 10.1097/OLQ.0000000000001355
- Sexually Transmitted Disease Surveillance 2021. https://www.cdc.gov/std/statistics/2021
- Gonococcal Infections Among Adolescents and Adults. https://www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
- Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999; 75(1):3–17.
- Sexually Transmitted Disease Surveillance 2017. https://www.cdc.gov/std/stats17/gonorrhea.htm
- Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N Engl J Med. 1974 Jan 17;290(3):117-23. doi: 10.1056/NEJM197401172900301
- Peterman TA, Tian LH, Metcalf CA, Satterwhite CL, Malotte CK, DeAugustine N, Paul SM, Cross H, Rietmeijer CA, Douglas JM Jr; RESPECT-2 Study Group. High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: a case for rescreening. Ann Intern Med. 2006 Oct 17;145(8):564-72. doi: 10.7326/0003-4819-145-8-200610170-00005
- Gonococcal Infections. https://www.std.uw.edu/go/comprehensive-study/gonococcal-infections/core-concept/all
- https://phil.cdc.gov/details.aspx?pid=4085
- Thadepalli H, Rambhatla K, Maidman JE, Arce JJ, Davidson EC Jr. Gonococcal sepsis secondary to fetal monitoring. Am J Obstet Gynecol. 1976 Oct 15;126(4):510-2. doi: 10.1016/0002-9378(76)90650-5
- Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021 Jul 23;70(4):1-187. doi: 10.15585/mmwr.rr7004a1
- St Cyr S, Barbee L, Workowski KA, Bachmann LH, Pham C, Schlanger K, Torrone E, Weinstock H, Kersh EN, Thorpe P. Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep. 2020 Dec 18;69(50):1911-1916. doi: 10.15585/mmwr.mm6950a6
- O’Donnell, Judith A.; Gelone, Steven P. (2009). Pelvic Inflammatory Disease. Infobase Publishing. ISBN 9781438101590.
- Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bull World Health Organ. 2004 Jun;82(6):454-61. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2622864
- Hill SA, Masters TL, Wachter J. Gonorrhea – an evolving disease of the new millennium. Microb Cell. 2016 Sep 5;3(9):371-389. doi: 10.15698/mic2016.09.524
- Chlamydia and Gonorrhea: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
- Hook EW 3rd, Kirkcaldy RD. A brief history of evolving diagnostics and therapy for gonorrhea: lessons learned. Clin Infect Dis 2018;67:1294–9. 10.1093/cid/ciy271
- Sánchez-Busó L, Golparian D, Corander J, et al. The impact of antimicrobials on gonococcal evolution. Nat Microbiol 2019;4:1941–50. 10.1038/s41564-019-0501-y
- Hooper RR, Reynolds GH, Jones OG, et al. Cohort study of venereal disease. I: the risk of gonorrhea transmission from infected women to men. Am J Epidemiol. 1978;108:136-44.
- Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis. 1998;178:1707-12.
- Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868-73.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. Gonococcal infections. MMWR Recomm Rep. 2015;64(No. RR-3):1-137.
- LeFevre ML; U.S. Preventive Services Task Force. Screening for Chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:902-10.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. Special populations. MMWR Recomm Rep. 2015;64(No. RR-3):1-137.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. HIV infection: detection, counseling, and referral. MMWR Recomm Rep. 2015;64(No. RR-3):1-137.
- Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N Engl J Med. 1974;290:117-23.
- Harrison WO, Hooper RR, Wiesner PJ, et al. A trial of minocycline given after exposure to prevent gonorrhea. N Engl J Med. 1979;300:1074-8.
- Jeffrey D. Klausner, Robert Kohn, Charlotte Kent, Etiology of Clinical Proctitis among Men Who Have Sex with Men, Clinical Infectious Diseases, Volume 38, Issue 2, 15 January 2004, Pages 300–302, https://doi.org/10.1086/380838
- McCormack WM, Stumacher RJ, Johnson K, Donner A. Clinical spectrum of gonococcal infection in women. Lancet. 1977 Jun 4;1(8023):1182-5. doi: 10.1016/s0140-6736(77)92720-9
- Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015 May 21;372(21):2039-48. doi: 10.1056/NEJMra1411426
- Wan WL, Farkas GC, May WN, Robin JB. The clinical characteristics and course of adult gonococcal conjunctivitis. Am J Ophthalmol. 1986;102:575-83.
- Holmes KK, Counts GW, Beaty HN. Disseminated gonococcal infection. Ann Intern Med. 1971 Jun;74(6):979-93. doi: 10.7326/0003-4819-74-6-979
- Bleich AT, Sheffield JS, Wendel GD Jr, Sigman A, Cunningham FG. Disseminated gonococcal infection in women. Obstet Gynecol. 2012;119:597-602.
- Svensson L, Weström L, Ripa KT, Mårdh PA. Differences in some clinical and laboratory parameters in acute salpingitis related to culture and serologic findings. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):1017-21. doi: 10.1016/0002-9378(80)91099-6
- Berger RE, Alexander ER, Harnisch JP, Paulsen CA, Monda GD, Ansell J, Holmes KK. Etiology, manifestations and therapy of acute epididymitis: prospective study of 50 cases. J Urol. 1979 Jun;121(6):750-4. doi: 10.1016/s0022-5347(17)56978-5
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae–2014. MMWR Recomm Rep. 2014;63:1-19.
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae–2014. MMWR Recomm Rep. 2014;63:1-19. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4047970
- FDA clears first diagnostic tests for extragenital testing for chlamydia and gonorrhea. https://www.fda.gov/news-events/press-announcements/fda-clears-first-diagnostic-tests-extragenital-testing-chlamydia-and-gonorrhea
- FDA Allows for First Point-of-Care Chlamydia and Gonorrhea Test to be Used in More Near-Patient Care Settings. https://www.fda.gov/news-events/press-announcements/fda-allows-first-point-care-chlamydia-and-gonorrhea-test-be-used-more-near-patient-care-settings
- Van Der Pol B, Taylor SN, Mena L, Lebed J, McNeil CJ, Crane L, Ermel A, Sukhija-Cohen A, Gaydos CA. Evaluation of the Performance of a Point-of-Care Test for Chlamydia and Gonorrhea. JAMA Netw Open. 2020 May 1;3(5):e204819. doi: 10.1001/jamanetworkopen.2020.4819
- Connolly KL, Eakin AE, Gomez C, Osborn BL, Unemo M, Jerse AE. Pharmacokinetic Data Are Predictive of In Vivo Efficacy for Cefixime and Ceftriaxone against Susceptible and Resistant Neisseria gonorrhoeae Strains in the Gonorrhea Mouse Model. Antimicrob Agents Chemother. 2019 Feb 26;63(3):e01644-18. doi: 10.1128/AAC.01644-18
- Stephanie A. Chisholm, Johan W. Mouton, David A. Lewis, Tom Nichols, Catherine A. Ison, David M. Livermore, Cephalosporin MIC creep among gonococci: time for a pharmacodynamic rethink?, Journal of Antimicrobial Chemotherapy, Volume 65, Issue 10, October 2010, Pages 2141–2148, https://doi.org/10.1093/jac/dkq289
- Screening Recommendations and Considerations Referenced in Treatment Guidelines and Original Sources. https://www.cdc.gov/std/treatment-guidelines/screening-recommendations.htm
- Sexually Transmitted Disease Surveillance 2020. https://www.cdc.gov/std/statistics/2020/overview.htm#Gonorrhea
- Gernert KM, Seby S, Schmerer MW, Thomas JC 4th, Pham CD, Cyr SS, Schlanger K, Weinstock H, Shafer WM, Raphael BH, Kersh EN; Antimicrobial-Resistant Neisseria gonorrhoeae Working Group *. Azithromycin susceptibility of Neisseria gonorrhoeae in the USA in 2017: a genomic analysis of surveillance data. Lancet Microbe. 2020 Aug;1(4):e154-e164. doi: 10.1016/S2666-5247(20)30059-8
- Khosropour CM, Dombrowski JC, Barbee LA, Manhart LE, Golden MR. Comparing azithromycin and doxycycline for the treatment of rectal chlamydial infection: a retrospective cohort study. Sex Transm Dis. 2014 Feb;41(2):79-85. doi: 10.1097/OLQ.0000000000000088
- Fabian Yuh Shiong Kong, Sepehr N. Tabrizi, Christopher Kincaid Fairley, Lenka A. Vodstrcil, Wilhelmina M. Huston, Marcus Chen, Catriona Bradshaw, Jane S. Hocking, The efficacy of azithromycin and doxycycline for the treatment of rectal chlamydia infection: a systematic review and meta-analysis, Journal of Antimicrobial Chemotherapy, Volume 70, Issue 5, May 2015, Pages 1290–1297, https://doi.org/10.1093/jac/dku574
- F. Y. S. Kong, S. N. Tabrizi, M. Law, L. A. Vodstrcil, M. Chen, C. K. Fairley, R. Guy, C. Bradshaw, J. S. Hocking, Azithromycin Versus Doxycycline for the Treatment of Genital Chlamydia Infection: A Meta-analysis of Randomized Controlled Trials, Clinical Infectious Diseases, Volume 59, Issue 2, 15 July 2014, Pages 193–205, https://doi.org/10.1093/cid/ciu220
- 2015 Sexually Transmitted Diseases Treatment Guidelines. Gonococcal Infections. https://www.cdc.gov/std/tg2015/gonorrhea.htm
- Schachter J, Moncada J, Liska S, et al. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008;35:637–42.
- Ota KV, Fisman DN, Tamari IE, et al. Incidence and treatment outcomes of pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections in men who have sex with men: a 13-year retrospective cohort study. Clin Infect Dis 2009;48:1237–43.
- Newman LM, Moran JS, Workowski KA. Update on the management of gonorrhea in adults in the United States. Clin Infect Dis 2007;44(Suppl 3):S84–101.
- Haimovici R, Roussel TJ. Treatment of gonococcal conjunctivitis with single-dose intramuscular ceftriaxone. Am J Ophthalmol. 1989 May 15;107(5):511-4. doi: 10.1016/0002-9394(89)90495-9
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee opinion No. 717: sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol 2017;130:e150–2. 10.1097/AOG.0000000000002300
- Bleich AT, Sheffield JS, Wendel GD Jr, Sigman A, Cunningham FG. Disseminated gonococcal infection in women. Obstet Gynecol 2012;119:597–602. 10.1097/AOG.0b013e318244eda9
- Belkacem A, Caumes E, Ouanich J, et al.; Working Group FRA-DGI. Changing patterns of disseminated gonococcal infection in France: cross-sectional data 2009–2011. Sex Transm Infect 2013;89:613–5. 10.1136/sextrans-2013-051119
- Birrell JM, Gunathilake M, Singleton S, Williams S, Krause V. Characteristics and impact of disseminated gonococcal infection in the “Top End” of Australia. Am J Trop Med Hyg 2019;101:753–60. 10.4269/ajtmh.19-0288
- Crew PE, Abara WE, McCulley L, et al. Disseminated gonococcal infections in patients receiving eculizumab: a case series. Clin Infect Dis 2019;69:596–600. 10.1093/cid/ciy958
- Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-6.
- Picker MA, Knoblock RJ, Hansen H, Bautista I, Griego R, Barber L, Bendik W, Lam K, Adelman E, Qiu-Shultz Z, Raphael BH, Pham CD, Kersh EN, Weinstock H, St Cyr SB. Notes from the Field: First Case in the United States of Neisseria gonorrhoeae Harboring Emerging Mosaic penA60 Allele, Conferring Reduced Susceptibility to Cefixime and Ceftriaxone. MMWR Morb Mortal Wkly Rep. 2020 Dec 11;69(49):1876-1877. doi: 10.15585/mmwr.mm6949a5
- Deguchi T, Yasuda M, Hatazaki K, et al. New Clinical Strain of Neisseria gonorrhoeae with Decreased Susceptibility to Ceftriaxone, Japan. Emerg Infect Dis. 2016;22:142-4.
- Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother. 2011 Jul;55(7):3538-45. doi: 10.1128/AAC.00325-11
- Jordi Cámara, Judit Serra, Josefina Ayats, Teresa Bastida, Dolors Carnicer-Pont, Antònia Andreu, Carmen Ardanuy, Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain, Journal of Antimicrobial Chemotherapy, Volume 67, Issue 8, August 2012, Pages 1858–1860, https://doi.org/10.1093/jac/dks162
- Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross-reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy. 2001;31:438-43.
- Taylor SN, Marrazzo J, Batteiger BE, Hook EW 3rd, Seña AC, Long J, Wierzbicki MR, Kwak H, Johnson SM, Lawrence K, Mueller J. Single-Dose Zoliflodacin (ETX0914) for Treatment of Urogenital Gonorrhea. N Engl J Med. 2018 Nov 8;379(19):1835-1845. doi: 10.1056/NEJMoa1706988
- Taylor SN, Morris DH, Avery AK, Workowski KA, Batteiger BE, Tiffany CA, Perry CR, Raychaudhuri A, Scangarella-Oman NE, Hossain M, Dumont EF. Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose-Ranging, Single-Oral Dose Evaluation. Clin Infect Dis. 2018 Aug 1;67(4):504-512. doi: 10.1093/cid/ciy145
- Chen MY, McNulty A, Avery A, Whiley D, Tabrizi SN, Hardy D, Das AF, Nenninger A, Fairley CK, Hocking JS, Bradshaw CS, Donovan B, Howden BP, Oldach D; Solitaire-U Team. Solithromycin versus ceftriaxone plus azithromycin for the treatment of uncomplicated genital gonorrhoea (SOLITAIRE-U): a randomised phase 3 non-inferiority trial. Lancet Infect Dis. 2019 Aug;19(8):833-842. doi: 10.1016/S1473-3099(19)30116-1
- Hook EW 3rd, Golden MR, Taylor SN, Henry E, Tseng C, Workowski KA, Swerdlow J, Nenninger A, Cammarata S. Efficacy and Safety of Single-Dose Oral Delafloxacin Compared With Intramuscular Ceftriaxone for Uncomplicated Gonorrhea Treatment: An Open-Label, Noninferiority, Phase 3, Multicenter, Randomized Study. Sex Transm Dis. 2019 May;46(5):279-286. doi: 10.1097/OLQ.0000000000000971
- Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-85.