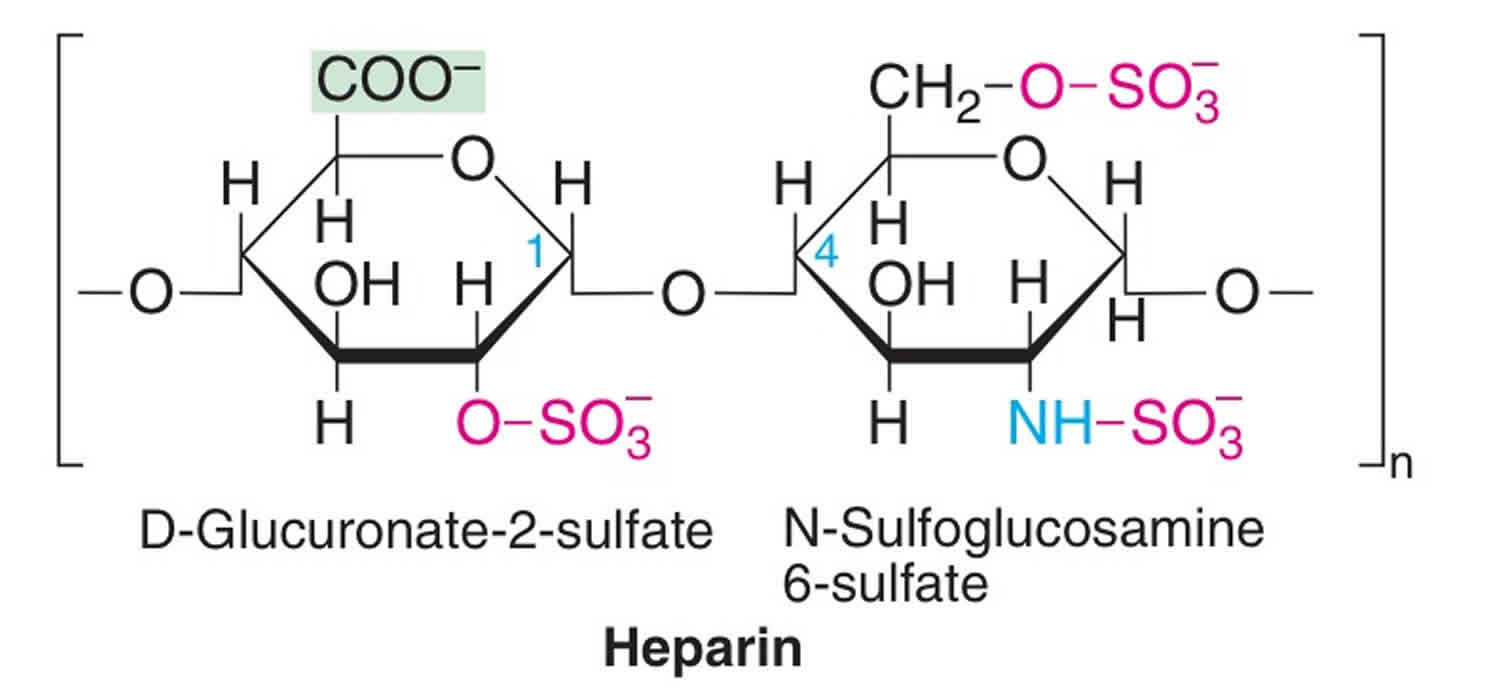
What is heparin
Heparin injection is a complex mixture of naturally occurring glycosaminoglycans that have potent anticoagulant activity. Heparin is used to prevent blood clots from forming in people who have certain medical conditions or who are undergoing certain medical procedures that increase the chance that clots will form. Heparin is also used to stop the growth of clots that have already formed in the blood vessels, but it cannot be used to decrease the size of clots that have already formed. Heparin is also used in small amounts to prevent blood clots from forming in catheters (small plastic tubes through which medication can be administered or blood drawn) that are left in veins over a period of time. Heparin is in a class of medications called anticoagulants (‘blood thinners’). It works by decreasing the clotting ability of the blood. Heparin has been used to treat or prevent venous thromboses for more than 50 years.
Heparin is sometimes called a blood thinner, although it does not actually thin the blood. Heparin will not dissolve blood clots that have already formed, but it may prevent the clots from becoming larger and causing more serious problems.
Heparin is used as the initial treatment of venous thrombosis and pulmonary embolism because of its rapid onset of action, while awaiting the slower onset of activity of oral anticoagulants (such as warfarin). Heparin is also used in the setting of acute myocardial infarction (heart attack) and unstable angina and in prophylaxis of venous thrombosis during and/or after surgery. In addition, heparin is used to maintain patency of intravenous indwelling catheters (“heparin lock”), usually in low doses (10 to 100 units), and is not meant for therapeutic purposes.
Heparin therapeutic indications:
- For the treatment of thrombo-embolic disorders such as deep vein thrombosis, acute arterial embolism or thrombosis, thrombophlebitis, pulmonary embolism and fat embolism.
- For prophylaxis against deep vein thrombosis and thrombo-embolic events in susceptible patients.
- For the prevention of clotting in the extracorporeal circuit during hemodialysis.
Heparin is used to prevent or treat certain blood vessel, heart, and lung conditions. Heparin is also used to prevent blood clotting during open-heart surgery, bypass surgery, kidney dialysis, and blood transfusions. It is used in low doses to prevent the formation of blood clots in certain patients, especially those who must have certain types of surgery or who must remain in bed for a long time. Heparin may also be used to diagnose and treat a serious blood condition called disseminated intravascular coagulation.
Heparin is available only with your doctor’s prescription.
Heparin is available in the following dosage forms:
- Solution
Standard or unfractionated heparin is a complex mixture of naturally occurring glycosaminoglycans and is used as an anticoagulant to treat venous thrombosis or to prevent thrombosis in high risk patients. Multiple generic forms of heparin are available, usually in ampoules or vials of 1000 to 40,000 units per mL. Heparin is typically given initially as 5000 to 10,000 units intravenously, followed by intravenous or subcutaneous boluses every 4 to 12 hours to keep the activated partial thrombin time in the range of 1.5 to 2 times the control value. Common side effects of standard heparin include dizziness, fatigue, headache, indigestion, nausea, excess bleeding, ecchymoses, rash and urticaria.
Low molecular weight heparins (LMWHs) (1000 to 10,000 daltons) are isolated from standard heparin preparations, which are then partially depolymerized and purified by gel chromatography and alcohol precipitation. Commercial preparations of low molecular weight heparins are standardized in a bioassay based upon inhibition of coagulation factor Xa. Heparin is a large glycosaminoglycan and is not absorbed through the gastrointestinal mucosa and must be given intravenously or by subcutaneous injection. Recently, low molecular weight heparins (LMWHs) have replaced standard unfractionated heparin heparin in many situations, their advantage being a more predictable pharmacokinetics which allows for subcutaneous administration and outpatient management.
Heparin comes as a solution (liquid) to be injected intravenously (into a vein) or deeply under the skin and as a dilute (less concentrated) solution to be injected into intravenous catheters. Heparin should not be injected into a muscle. Heparin is sometimes injected one to six times a day and sometimes given as a slow, continuous injection into the vein. When heparin is used to prevent blood clots from forming in intravenous catheters, it is usually used when the catheter is first put in place, and every time that blood is drawn out of the catheter or medication is given through the catheter.
Heparin may be given to you by a nurse or other healthcare provider, or you may be told to inject the medication by yourself at home. If you will be injecting heparin yourself, a healthcare provider will show you how to inject the medication. Ask your doctor, nurse, or pharmacist if you do not understand these directions or have any questions about where on your body you should inject heparin, how to give the injection, or how to dispose of used needles and syringes after you inject the medication.
If you will be injecting heparin yourself, follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Use heparin exactly as directed. Do not use more or less of it or use it more often than prescribed by your doctor.
Heparin solution comes in different strengths, and using the wrong strength may cause serious problems. Before giving an injection of heparin, check the package label to make sure it is the strength of heparin solution that your doctor prescribed for you. If the strength of heparin is not correct do not use the heparin and call your doctor or pharmacist right away.
Your doctor may increase or decrease your dose during your heparin treatment. If you will be injecting heparin yourself, be sure you know how much medication you should use.
Other uses for heparin
Heparin is also sometimes used alone or in combination with aspirin to prevent pregnancy loss and other problems in pregnant women who have certain medical conditions and who have experienced these problems in their earlier pregnancies. Talk to your doctor or pharmacist about the risks of using this medication to treat your condition.
Heparin may be prescribed for other uses; ask your doctor or pharmacist for more information.
Low molecular weight heparin
Low-molecular-weight heparins (LMWHs), for example, dalteparin, enoxaparin, are anticoagulants 1. Low-molecular-weight heparins are used in the prophylaxis of venous thromboembolic disease on acute or elective admission to hospital, and they are used in the treatment of deep vein thromboses (DVT) and pulmonary embolism (PE) 2. The British National Formulary (BNF) and National Institute for Health and Care Excellence (NICE) have stated the use of low-molecular-weight heparins are licensed for:
- DVT prophylaxis in medium and high-risk groups (surgical, orthopedic and medical patients)
- Treatment of venous thromboembolism in pregnancy
- Treatment of DVT and pulmonary embolism (PE) in nonpregnant women (those with both high and low risk of recurrence)
- Treatment of ST-Elevation Myocardial Infarction (STEMI) (in both those undergoing percutaneous coronary intervention and those not)
- Unstable angina
- Prevention of clotting in extracorporeal circuits
Low molecular weight heparins are anticoagulants, acting by inhibition of the final common pathway of the coagulation cascade 3. The coagulation cascade’s goal is to form blood clot, thus preventing bleeding. The final common pathway is the conversion of fibrinogen into fibrin by the activity of thrombin. Low molecular weight heparin inhibits coagulation by activating antithrombin III. Antithrombin III binds to and inhibits factor Xa. In doing so it prevents activation of the final common path; Xa inactivation means that prothrombin is not activated to thrombin, thereby not converting fibrinogen into fibrin for the formation of a clot. LMHW is a small fragment of a larger mucopolysaccharide, heparin 3. Heparin works similarly, by binding antithrombin III and activating it. Heparin also has a binding site for thrombin, so thrombin can interact with antithrombin III and heparin, thus inhibiting coagulation. Heparin this has a faster onset of anticoagulant action as it will inhibit not only Xa but also thrombin, while low molecular weight heparin acts only on Xa inhibition 3.
Heparin induced thrombocytopenia
Heparin-induced thrombocytopenia is a severe complication that can occur in patients exposed to any form or amount of heparin products 4. A fall in platelet counts and a hypercoagulable state characterize heparin-induced thrombocytopenia 4. Patients who experience heparin-induced thrombocytopenia may also develop thromboembolic complications that are associated with morbidity and mortality. This is a significant burden since heparin is widely used for treatment and prophylaxis of thromboembolism, for line flushes, and in heparin-coated catheters.
A heparin-induced thrombocytopenia can occur in up to 5% of patients exposed to heparin products. Heparin-induced thrombocytopenia causes an extremely hypercoagulable state, where up to 50% of patients develop thromboembolic complications, associated with a mortality rate up to 30% 4.
There are several medication-related as well as patient-related factors that can increase the risk of a heparin-induced thrombocytopenia. Because of the difference in structure and function, heparin-induced thrombocytopenia is more likely to occur with unfractionated heparin (UFH) than with low molecular weight heparin (LMWH). Fondaparinux is a heparin-like drug that does not cause heparin-induced thrombocytopenia. Unfractionated heparin is a heterogeneous product that consists of long saccharide chains of varying lengths and molecular weights; the average unfractionated heparin molecule is 45 saccharide units long. Low molecular weight heparin is also a heterogeneous product; however, low molecular weight heparin is, on average, 15 saccharide units long. Fondaparinux is a synthetic pentasaccharide, consisting of only the 5 sugars. The shorter the saccharide chain and the smaller the molecular weight, the less likely the drug is to bind to plasma proteins and cells. Therefore, there is a reduced risk of a heparin-induced thrombocytopenia with low molecular weight heparin compared to unfractionated heparin, whereas fondaparinux does not cause heparin-induced thrombocytopenia, and can be safely utilized in patients with a history of heparin-induced thrombocytopenia and potentially in the treatment of acute heparin-induced thrombocytopenia.
Although no amount of heparin is too small to cause this reaction, heparin-induced thrombocytopenia is more likely to occur in patients exposed to higher doses of the drug; and the longer the duration of therapy, the higher the risk. Furthermore, females and elderly patients appear to be at an increased risk. The incidence of heparin-induced thrombocytopenia is also higher among surgical patients, and this may be due to increased platelet activation and PF4 activity due to mechanical intervention and injury.
Types of heparin-induced thrombocytopenia (occur secondary to heparin use)
- Type 1 heparin-induced thrombocytopenia, also known as Heparin-associated thrombocytopenia (HAT), is a non-immune mediated reaction. Type 1 heparin-induced thrombocytopenia is much more common than type 2 and can occur as early as day 1 of therapy. This is a mild reaction, it is not associated with any complications, and platelet counts will spontaneously normalize even if heparin is continued.
- Type 2 heparin-induced thrombocytopenia is an immune, antibody-mediated reaction. Because it takes time for the antibodies to form, this reaction usually occurs after 5 to 14 days of receiving heparin. However, if a patient has been exposed to heparin within the last 100 days, antibodies may remain in the system, causing this reaction to manifest as soon as day one of heparin therapy. This is a very serious reaction that causes a hypercoagulable state and can lead to life-threatening complications. See the pathophysiology, diagnosis and treatment of type 2 heparin-induced thrombocytopenia below.
Pathophysiology of heparin-induced thrombocytopenia
Under normal physiological conditions, PF4 is stored in alpha-granules of the platelets and is released upon platelet activation. PF4 is positively charged and can, therefore, bind to the negatively charged heparan (a heparin-like substance normally present on the endothelial cell surface); PF4 can also bind to exogenous heparin with much higher affinity than heparin.
PF4 binding to heparin may trigger the formation of IgG, IgA, or IgM antibodies specific to the heparin-PF4 complex. A heparin-induced thrombocytopenia can only occur if IgG, while attached to the heparin-PF4 complex, binds to the FC receptor on the platelet surface and leads to platelet activation. Activated platelets then release pro-thrombotic substances (such as thrombin) and PF4. As IgG activates more platelets, more PF4 is released forming more complexes with heparin, thus activating more platelets. This creates a severely hypercoagulable state and a continuous cycle that can only be broken when heparin is discontinued, and appropriate treatment is initiated.
The most characteristic clinical feature of heparin-induced thrombocytopenia is the thrombocytopenia. Platelet counts fall because macrophages consume the IgG-coated platelets and the removed by the reticuloendothelial system removes them. Simultaneously, as platelets become activated, they aggregate, and the platelet count drops as thrombus forms.
Because heparin-induced thrombocytopenia causes a hypercoagulable state, venous and/or arterial thrombosis can occur. The most common complications are DVT, pulmonary embolism or skin necrosis. The latter is particularly a risk if warfarin is administered in the acute phase. The risk of these complications is highest within the first 10 days, but the pro-thrombotic state persists up to 30 days after stopping heparin.
Heparin-induced thrombocytopenia diagnosis
heparin-induced thrombocytopenia should be suspected when there is an unexplained drop in platelet counts in a patient currently on heparin or recently exposed to heparin products. heparin-induced thrombocytopenia typically presents as a steady drop in platelet counts (no fluctuations), while hemoglobin and hematocrit counts remain relatively stable.
The first step in the diagnosis of heparin-induced thrombocytopenia is the calculation of the 4T score. This is a scoring system used to determine the likelihood of a patient having heparin-induced thrombocytopenia based on the presence or absence of certain parameters. The score may be calculated using the following table.
A 4T score of 0 to 3 points means a heparin-induced thrombocytopenia is unlikely, and heparin therapy may continue while the clinician looks for other causes of thrombocytopenia. A score of 4 to 5 corresponds to intermediate probability and a score of 6 to 8 means high probability. All forms of heparin, including line flushes, should be immediately discontinued and treatment with an alternative anticoagulant should be pursued in any patient who scores 4 or more. In addition, the clinical diagnosis with the 4T score should be confirmed with the PF4 ELISA and the Serotonin Release Assay (SRA).
The PF4 ELISA detects the presence of antibodies. This test is highly sensitive and has a high negative predictive value; heparin-induced thrombocytopenia can be ruled out if this test is negative. However, if the PF4 ELISA is positive, the result should be confirmed with the SRA, a more specific test that is also more costly and takes several days for the result to be reported. The PF4 ELISA detects not only IgG but also IgA and/or IgM, leading to false positives.
The SRA is the gold standard test for confirming heparin-induced thrombocytopenia with high sensitivity and specificity. Unlike ELISA, which detects the presence of antibodies, the SRA is a functional test, which detects the activation of platelets in the presence of antibodies. A donor platelet that becomes activated in the presence of heparin and a sample of the patient’s blood (containing IgG) will release serotonin. A positive SRA confirms the diagnosis of heparin-induced thrombocytopenia, and a negative SRA rules out heparin-induced thrombocytopenia, even in the setting of a positive ELISA.
Heparin-induced thrombocytopenia treatment
The treatment of heparin-induced thrombocytopenia should start as soon as a 4T score of 4 or more is calculated. The first step in the treatment is the discontinuation of all forms of heparin. Next, an alternative anticoagulant must be initiated to prevent or treat any heparin-induced thrombocytopenia-induced thrombosis. In patients recently started on warfarin, warfarin should be held, and phytonadione (vitamin K) should be administered to replete protein C and S stores. The PF4 ELISA an SRA should be sent to confirm the diagnosis.
The anticoagulant of choice in an acute heparin-induced thrombocytopenia is argatroban. Argatroban is a direct thrombin inhibitor that does not interact with PF4 or heparin-induced antibodies. Argatroban has a short half-life of about 50 minutes; it is given as a continuous infusion and requires aPTT monitoring, similar to heparin. This drug is hepatically metabolized and requires adjustment to the starting rate in patients with hepatic dysfunction, heart failure and/or multi-organ failure. Argatroban can profoundly increase INR; however, no therapeutic range for INR has been established for patients on argatroban. Argatroban’s effect on the INR must be considered when bridging a patient to warfarin.
Bivalirudin is another direct thrombin inhibitor that may safely be used in this patient population; however, this agent is usually reserved for use during cardiac catheterization procedures as an alternative to heparin. It is FDA approved for patients undergoing percutaneous coronary intervention with or without a heparin-induced thrombocytopenia. It is more expensive than argatroban.
Another anticoagulant that may be used in a heparin-induced thrombocytopenia is fondaparinux, although not FDA-approved for this indication. Fondaparinux is given as a once-daily subcutaneous injection. Unlike unfractionated heparin and low molecular weight heparin, fondaparinux does not interact with PF4 or heparin-induced antibodies.
Heparin special precautions
Before using heparin:
- tell your doctor and pharmacist if you are allergic to heparin, any other medications, beef products,pork products, or any of the ingredients in heparin injection. Ask your doctor or pharmacist for a list of the ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention any of the following: other anticoagulants such as warfarin (Coumadin); antihistamines (in many cough and cold products); antithrombin III (Thrombate III); aspirin or aspirin-containing products and other nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen (Advil, Motrin) and naproxen (Aleve, Naprosyn); dextran; digoxin (Digitek, Lanoxin); dipyridamole (Persantine, in Aggrenox); hydroxychloroquine (Plaquenil); indomethacin (Indocin); phenylbutazone (Azolid) (not available in the US); quinine; and tetracycline antibiotics such as demeclocycline (Declomycin), doxycycline (Monodox, Vibramycin), minocycline (Dynacin, Minocin) and tetracycline (Bristacycline, Sumycin). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if you have a low level of platelets (type of blood cells needed for normal clotting) in your blood and if you have heavy bleeding that cannot be stopped anywhere in your body. Your doctor may tell you not to use heparin.
- tell your doctor if you are currently experiencing your menstrual period; if you have a fever or an infection; and if you have recently had a spinal tap (removal of a small amount of the fluid that bathes the spinal cord to test for infection or other problems), spinal anesthesia (administration of pain medication in the area around the spine), surgery, especially involving the brain, spinal cord or eye, or a heart attack. Also tell your doctor if you have or have ever had a bleeding disorder such as hemophilia (condition in which the blood does not clot normally), antithrombin III deficiency (condition that causes blood clots to form), blood clots in the legs, lungs, or anywhere in the body, unusual bruising or purple spots under the skin, cancer, ulcers in the stomach or intestine, a tube that drains the stomach or intestine, high blood pressure, or liver disease.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while using heparin, call your doctor.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are using heparin.
- tell your doctor if you smoke or use tobacco products and if you stop smoking at any time during your treatment with heparin. Smoking may decrease the effectiveness of this medication.
It is very important that your doctor check you at regular visits after you leave the hospital for any problems or unwanted effects that may be caused by heparin. If you are using heparin at home, blood tests will be needed to check for unwanted effects. Be sure to keep all appointments.
Do not take aspirin, ibuprofen, or other anti-inflammatory medicines (e.g., NSAIDs) while you are using heparin. Many nonprescription (over-the-counter [OTC]) medicines and some prescription medicines contain these ingredients. Check the labels of all medicines you take. There are many other medicines that may change the way heparin works or increase the chance of bleeding if they are used together with heparin. It is best to check with your doctor before taking any other medicine while you are using heparin.
You may bleed and bruise more easily while you are using heparin. Stay away from rough sports or other situations where you could be bruised, cut, or injured. Tell your doctor about any falls, blows to the body or head, or other injuries, since serious bleeding may occur inside the body with heparin. Be careful when using sharp objects, including razors and fingernail clippers. Avoid picking your nose. If you need to blow your nose, blow it gently. Check with your doctor right away if you notice any unusual bleeding or bruising; black, tarry stools; blood in the urine or stools; or pinpoint red spots on your skin.
Be careful when using a regular toothbrush, dental floss, or toothpick. Your medical doctor, dentist, or nurse may recommend other ways to clean your teeth and gums. Check with your medical doctor before having any dental work done.
Heparin may cause a serious type of allergic reaction called anaphylaxis. Anaphylaxis can be life-threatening and requires immediate medical attention. Tell your doctor right away if you have a rash; itching; swelling of the face, tongue, and throat; trouble breathing; or chest pain after you receive heparin.
Heparin may cause new blood clots to form in some people while they are receiving the medicine or after it is stopped. Stop using heparin and check with your doctor right away if you have pain in the chest, groin, or legs, especially the calves; difficulty with breathing; a sudden, severe headache; slurred speech; a sudden, unexplained shortness of breath; a sudden loss of coordination; or vision changes while using this medicine.
Make sure any doctor or dentist who treats you knows that you are using heparin. You may need to stop using heparin several days before having surgery or medical tests.
Allergies
- Tell your doctor if you have ever had any unusual or allergic reaction to this medicine or any other medicines. Also tell your health care professional if you have any other types of allergies, such as to foods, dyes, preservatives, or animals. For non-prescription products, read the label or package ingredients carefully.
Pediatric
- Appropriate studies performed to date have not demonstrated pediatric-specific problems that would limit the usefulness of heparin injection in children. However, because heparin contains benzyl alcohol, use in newborn babies is not recommended.
Geriatric
- Appropriate studies performed to date have not demonstrated geriatric-specific problems that would limit the usefulness of heparin injection in the elderly. However, elderly patients are more likely to develop bleeding problems, which may require an adjustment in the dose for patients receiving heparin injection.
Pregnancy
- Pregnancy Category C: Animal studies have shown an adverse effect and there are no adequate studies in pregnant women OR no animal studies have been conducted and there are no adequate studies in pregnant women.
Breastfeeding
- Studies in women suggest that this medication poses minimal risk to the infant when used during breastfeeding.
Drug Interactions
Although certain medicines should not be used together at all, in other cases two different medicines may be used together even if an interaction might occur. In these cases, your doctor may want to change the dose, or other precautions may be necessary. When you are receiving heparin, it is especially important that your healthcare professional know if you are taking any of the medicines listed below. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using heparin with any of the following medicines is not recommended. Your doctor may decide not to treat you with this medication or change some of the other medicines you take.
- Defibrotide
- Oritavancin
- Telavancin
Using heparin with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Abciximab
- Aceclofenac
- Acemetacin
- Alipogene Tiparvovec
- Alprostadil
- Alteplase, Recombinant
- Amtolmetin Guacil
- Anagrelide
- Anistreplase
- Antithrombin, Recombinant
- Apixaban
- Argatroban
- Aspirin
- Bemiparin
- Betrixaban
- Bivalirudin
- Bromfenac
- Bufexamac
- Cangrelor
- Cefamandole
- Cefoperazone
- Celecoxib
- Chamomile
- Choline Salicylate
- Cilostazol
- Citalopram
- Clonixin
- Clopidogrel
- Collagenase, Clostridium histolyticum
- Dabigatran Etexilate
- Dalteparin
- Danaparoid
- Desvenlafaxine
- Dexibuprofen
- Dexketoprofen
- Dextran
- Diclofenac
- Diflunisal
- Dipyridamole
- Dipyrone
- Drotrecogin Alfa
- Droxicam
- Edoxaban
- Enoxaparin
- Epoprostenol
- Eptifibatide
- Escitalopram
- Etodolac
- Etofenamate
- Etoricoxib
- Felbinac
- Fenofibrate
- Fenofibric Acid
- Fenoprofen
- Fepradinol
- Feprazone
- Floctafenine
- Flufenamic Acid
- Fluoxetine
- Flurbiprofen
- Fluvoxamine
- Garlic
- Ginkgo
- Ibrutinib
- Ibuprofen
- Iloprost
- Indomethacin
- Inotersen
- Ketoprofen
- Ketorolac
- Levomilnacipran
- Lornoxicam
- Loxoprofen
- Lumiracoxib
- Meclofenamate
- Mefenamic Acid
- Meloxicam
- Milnacipran
- Morniflumate
- Moxalactam
- Nabumetone
- Nadroparin
- Naproxen
- Nepafenac
- Niflumic Acid
- Nimesulide
- Nimesulide Beta Cyclodextrin
- Nintedanib
- Nitroglycerin
- Omadacycline
- Orlistat
- Oxaprozin
- Oxyphenbutazone
- Papaya
- Parecoxib
- Paroxetine
- Pentosan Polysulfate Sodium
- Phenylbutazone
- Piketoprofen
- Piracetam
- Piroxicam
- Pranoprofen
- Prasugrel
- Proglumetacin
- Propyphenazone
- Proquazone
- Reteplase, Recombinant
- Rivaroxaban
- Rofecoxib
- Sarecycline
- Selexipag
- Sertraline
- St John’s Wort
- Streptokinase
- Sulfinpyrazone
- Sulindac
- Tan-Shen
- Tenecteplase
- Tenoxicam
- Tiaprofenic Acid
- Ticlopidine
- Tirofiban
- Tolfenamic Acid
- Tolmetin
- Treprostinil
- Urokinase
- Valdecoxib
- Venlafaxine
- Vilazodone
- Vorapaxar
- Vortioxetine
Using heparin with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Chondroitin
- Coenzyme Q10
- Curcumin
- Dong Quai
- Ginger
- Palifermin
- Vitamin A
- Warfarin
Other Interactions
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using heparin with any of the following may cause an increased risk of certain side effects but may be unavoidable in some cases. If used together, your doctor may change the dose or how often you use this medicine, or give you special instructions about the use of food, alcohol, or tobacco.
- Avocado
Other Medical Problems
The presence of other medical problems may affect the use of heparin. Make sure you tell your doctor if you have any other medical problems, especially:
- Bacterial endocarditis (heart infection) or
- Bleeding problems (eg, hemophilia) or
- Hypertension (high blood pressure), severe or
- Liver disease or
- Major surgery (eg, eye, brain, or spine) or
- Menstrual bleeding (periods), heavy or unusual or
- Spinal anesthesia (numbing medicine placed in the back) or
- Stomach or intestinal ulcer—Use with caution. The risk of bleeding may be increased.
- Bleeding, active or
- Thrombocytopenia (low platelets in the blood) caused by heparin, history of or
- Thrombocytopenia (low platelets in the blood), severe—Should not be used in patients with these conditions.
Heparin contraindications
National Institute for Health and Care Excellence (NICE) and the British National Formulary suggest that contraindications to all heparins include trauma, epidural half-life, hemorrhagic disorders, peptic ulcer disease, recent cerebral hemorrhage, severe hypertension, and recent surgery to the eye or nervous system 1. In these cases, risk-benefit the risks of anticoagulation and bleeding outweighs the potential benefit from heparin acting as a venous thromboembolic disease prophylaxis or at treatment doses. As heparin are self-administered, it is important to consider dosing in cases of chronic kidney disease, where there is a risk of accumulation and thus, higher chances of problematic bleeding.
Heparin mechanism of action
Heparin inhibits coagulation by activating antithrombin III. Heparin binds to antithrombin III to form a heparin-antithrombin III complex. The complex binds to and irreversibly inactivates thrombin and other activated clotting factors, such as factors IX, X, XI, and XII, thereby preventing the polymerization of fibrinogen to fibrin and the subsequent formation of clots.
Heparin dosage
A nurse or other trained health professional will give you heparin in a hospital. Heparin is given through a needle placed in one of your veins or as a shot under your skin.
If you are using heparin at home, your doctor will explain how this medicine is to be given. Your doctor will prescribe your exact dose and tell you how often it should be given.
Use heparin exactly as directed by your doctor. Do not use more of it, do not use it more often, and do not use it for a longer time than your doctor ordered.
You will be shown the body areas where the heparin injection can be given. Use a different body area each time you give yourself a shot. Keep track of where you give each shot to make sure you rotate body areas. This will help prevent skin problems from the shots.
It is recommended that you carry an identification card stating that you are using heparin. If you have any questions about what kind of identification to carry, check with your doctor.
Giving the heparin injection
Wash your hands with soap and water. Dry them well.
Choose where to give the shot. Keep a chart of places you have used, so you do not put the heparin in the same place all the time. Ask your doctor for a chart.
- Keep your shots 1 inch (2.5 centimeters) away from scars and 2 inches (5 centimeters) away from your navel.
- Do not put a shot in a spot that is bruised, swollen, or tender.
The site you choose for the heparin injection should be clean and dry. If your skin is visibly dirty, clean it with soap and water. Or use an alcohol wipe. Allow the skin to dry before giving the shot.
The heparin needs to go into the fat layer under the skin.
- Pinch the skin and put the needle in at a 45º angle.
- Push the needle all the way into the skin. Let go of the pinched skin. Inject the heparin slowly and steadily until it is all in.
After all the medicine is in, leave the needle in for 5 seconds. Pull the needle out at the same angle it went in. Put the syringe down and press the shot site with a piece of gauze for a few seconds. Do not rub. If it bleeds or oozes, hold it longer.
Throw away the needle and syringe in a safe hard container (sharps container). Close the container, and keep it safely away from children and animals. Never reuse needles or syringes.
Write down the date, time, and place on the body where you put the heparin injection.
Adult Dose for Deep Vein Thrombosis
Uses:
- Prophylaxis and treatment of venous thrombosis and pulmonary embolism.
- Prophylaxis and treatment of thromboembolic complications associated with atrial fibrillation.
- Treatment of acute and chronic consumption coagulopathies (disseminated intravascular coagulation).
- Prophylaxis and treatment of peripheral arterial embolism.
The manufacturer provides the following dosing guidelines based on clinical experience:
- Continuous IV infusion:
- Initial dose: 5000 units by IV injection
- Maintenance dose: 20,000 to 40,000 units per 24 hours by continuous IV infusion
- Intermittent IV injection:
- Initial dose: 10,000 units IV
- Maintenance dose: 5000 to 10,000 units IV every 4 to 6 hours
- Deep subcutaneous (intrafat) injection:
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
- 5000 units by IV injection followed by 10,000 to 20,000 units subcutaneously, and then 8000 to 10,000 units subcutaneously every 8 hours or 15,000 to 20,000 units subcutaneously every 12 hours.
- 5000 units subcutaneously 2 hours before surgery and 5000 units subcutaneously every 8 to 12 hours thereafter for 7 days or until the patient is ambulatory, whichever is longer.
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
Comments:
- Recommended doses are based on a 68 kg patient.
- Consult the latest guidelines regarding duration and intensity of anticoagulation for the indicated conditions.
- Dosage and administration must be individualized according to the results of suitable laboratory tests.
- Administer by deep subcutaneous (intrafat, e.g., above the iliac crest or abdominal fat layer, arm, or thigh) injection with a fine (25 to 26 gauge) needle to minimize tissue trauma.
Adult Dose for Deep Vein Thrombosis – Prophylaxis
Uses:
- Prophylaxis and treatment of venous thrombosis and pulmonary embolism.
- Prophylaxis and treatment of thromboembolic complications associated with atrial fibrillation.
- Treatment of acute and chronic consumption coagulopathies (disseminated intravascular coagulation).
- Prophylaxis and treatment of peripheral arterial embolism.
The manufacturer provides the following dosing guidelines based on clinical experience:
- Continuous IV infusion:
- Initial dose: 5000 units by IV injection
- Maintenance dose: 20,000 to 40,000 units per 24 hours by continuous IV infusion
- Intermittent IV injection:
- Initial dose: 10,000 units IV
- Maintenance dose: 5000 to 10,000 units IV every 4 to 6 hours
- Deep subcutaneous (intrafat) injection:
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
- 5000 units by IV injection followed by 10,000 to 20,000 units subcutaneously, and then 8000 to 10,000 units subcutaneously every 8 hours or 15,000 to 20,000 units subcutaneously every 12 hours.
- 5000 units subcutaneously 2 hours before surgery and 5000 units subcutaneously every 8 to 12 hours thereafter for 7 days or until the patient is ambulatory, whichever is longer.
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
Comments:
- Recommended doses are based on a 68 kg patient.
- Consult the latest guidelines regarding duration and intensity of anticoagulation for the indicated conditions.
- Dosage and administration must be individualized according to the results of suitable laboratory tests.
- Administer by deep subcutaneous (intrafat, e.g., above the iliac crest or abdominal fat layer, arm, or thigh) injection with a fine (25 to 26 gauge) needle to minimize tissue trauma.
Adult Dose for Prevention of Thromboembolism in Atrial Fibrillation
Uses:
- Prophylaxis and treatment of venous thrombosis and pulmonary embolism.
- Prophylaxis and treatment of thromboembolic complications associated with atrial fibrillation.
- Treatment of acute and chronic consumption coagulopathies (disseminated intravascular coagulation).
- Prophylaxis and treatment of peripheral arterial embolism.
The manufacturer provides the following dosing guidelines based on clinical experience:
- Continuous IV infusion:
- Initial dose: 5000 units by IV injection
- Maintenance dose: 20,000 to 40,000 units per 24 hours by continuous IV infusion
- Intermittent IV injection:
- Initial dose: 10,000 units IV
- Maintenance dose: 5000 to 10,000 units IV every 4 to 6 hours
- Deep subcutaneous (intrafat) injection:
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
- 5000 units by IV injection followed by 10,000 to 20,000 units subcutaneously, and then 8000 to 10,000 units subcutaneously every 8 hours or 15,000 to 20,000 units subcutaneously every 12 hours.
- 5000 units subcutaneously 2 hours before surgery and 5000 units subcutaneously every 8 to 12 hours thereafter for 7 days or until the patient is ambulatory, whichever is longer.
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
Comments:
- Recommended doses are based on a 68 kg patient.
- Consult the latest guidelines regarding duration and intensity of anticoagulation for the indicated conditions.
- Dosage and administration must be individualized according to the results of suitable laboratory tests.
- Administer by deep subcutaneous (intrafat, e.g., above the iliac crest or abdominal fat layer, arm, or thigh) injection with a fine (25 to 26 gauge) needle to minimize tissue trauma.
Adult Dose for Pulmonary Embolism
Uses:
- Prophylaxis and treatment of venous thrombosis and pulmonary embolism.
- Prophylaxis and treatment of thromboembolic complications associated with atrial fibrillation.
- Treatment of acute and chronic consumption coagulopathies (disseminated intravascular coagulation).
- Prophylaxis and treatment of peripheral arterial embolism.
The manufacturer provides the following dosing guidelines based on clinical experience:
- Continuous IV infusion:
- Initial dose: 5000 units by IV injection
- Maintenance dose: 20,000 to 40,000 units per 24 hours by continuous IV infusion
- Intermittent IV injection:
- Initial dose: 10,000 units IV
- Maintenance dose: 5000 to 10,000 units IV every 4 to 6 hours
- Deep subcutaneous (intrafat) injection:
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
- 5000 units by IV injection followed by 10,000 to 20,000 units subcutaneously, and then 8000 to 10,000 units subcutaneously every 8 hours or 15,000 to 20,000 units subcutaneously every 12 hours.
- 5000 units subcutaneously 2 hours before surgery and 5000 units subcutaneously every 8 to 12 hours thereafter for 7 days or until the patient is ambulatory, whichever is longer.
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
Comments:
- Recommended doses are based on a 68 kg patient.
- Consult the latest guidelines regarding duration and intensity of anticoagulation for the indicated conditions.
- Dosage and administration must be individualized according to the results of suitable laboratory tests.
- Administer by deep subcutaneous (intrafat, e.g., above the iliac crest or abdominal fat layer, arm, or thigh) injection with a fine (25 to 26 gauge) needle to minimize tissue trauma.
Adult Dose for Thrombotic and Thromboembolic Disorder
Uses:
- Prophylaxis and treatment of venous thrombosis and pulmonary embolism.
- Prophylaxis and treatment of thromboembolic complications associated with atrial fibrillation.
- Treatment of acute and chronic consumption coagulopathies (disseminated intravascular coagulation).
- Prophylaxis and treatment of peripheral arterial embolism.
The manufacturer provides the following dosing guidelines based on clinical experience:
- Continuous IV infusion:
- Initial dose: 5000 units by IV injection
- Maintenance dose: 20,000 to 40,000 units per 24 hours by continuous IV infusion
- Intermittent IV injection:
- Initial dose: 10,000 units IV
- Maintenance dose: 5000 to 10,000 units IV every 4 to 6 hours
- Deep subcutaneous (intrafat) injection:
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
- 5000 units by IV injection followed by 10,000 to 20,000 units subcutaneously, and then 8000 to 10,000 units subcutaneously every 8 hours or 15,000 to 20,000 units subcutaneously every 12 hours.
- 5000 units subcutaneously 2 hours before surgery and 5000 units subcutaneously every 8 to 12 hours thereafter for 7 days or until the patient is ambulatory, whichever is longer.
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
Comments:
- Recommended doses are based on a 68 kg patient.
- Consult the latest guidelines regarding duration and intensity of anticoagulation for the indicated conditions.
- Dosage and administration must be individualized according to the results of suitable laboratory tests.
- Administer by deep subcutaneous (intrafat, e.g., above the iliac crest or abdominal fat layer, arm, or thigh) injection with a fine (25 to 26 gauge) needle to minimize tissue trauma.
Adult Dose for Disseminated Intravascular Coagulation
Uses:
- Prophylaxis and treatment of venous thrombosis and pulmonary embolism.
- Prophylaxis and treatment of thromboembolic complications associated with atrial fibrillation.
- Treatment of acute and chronic consumption coagulopathies (disseminated intravascular coagulation).
- Prophylaxis and treatment of peripheral arterial embolism.
The manufacturer provides the following dosing guidelines based on clinical experience:
- Continuous IV infusion:
- Initial dose: 5000 units by IV injection
- Maintenance dose: 20,000 to 40,000 units per 24 hours by continuous IV infusion
- Intermittent IV injection:
- Initial dose: 10,000 units IV
- Maintenance dose: 5000 to 10,000 units IV every 4 to 6 hours
- Deep subcutaneous (intrafat) injection:
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
- 5000 units by IV injection followed by 10,000 to 20,000 units subcutaneously, and then 8000 to 10,000 units subcutaneously every 8 hours or 15,000 to 20,000 units subcutaneously every 12 hours.
- 5000 units subcutaneously 2 hours before surgery and 5000 units subcutaneously every 8 to 12 hours thereafter for 7 days or until the patient is ambulatory, whichever is longer.
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
Comments:
- Recommended doses are based on a 68 kg patient.
- Consult the latest guidelines regarding duration and intensity of anticoagulation for the indicated conditions.
- Dosage and administration must be individualized according to the results of suitable laboratory tests.
- Administer by deep subcutaneous (intrafat, e.g., above the iliac crest or abdominal fat layer, arm, or thigh) injection with a fine (25 to 26 gauge) needle to minimize tissue trauma.
Adult Dose for Venous Thromboembolism
Uses:
- Prophylaxis and treatment of venous thrombosis and pulmonary embolism.
- Prophylaxis and treatment of thromboembolic complications associated with atrial fibrillation.
- Treatment of acute and chronic consumption coagulopathies (disseminated intravascular coagulation).
- Prophylaxis and treatment of peripheral arterial embolism.
The manufacturer provides the following dosing guidelines based on clinical experience:
- Continuous IV infusion:
- Initial dose: 5000 units by IV injection
- Maintenance dose: 20,000 to 40,000 units per 24 hours by continuous IV infusion
- Intermittent IV injection:
- Initial dose: 10,000 units IV
- Maintenance dose: 5000 to 10,000 units IV every 4 to 6 hours
- Deep subcutaneous (intrafat) injection:
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
- 5000 units by IV injection followed by 10,000 to 20,000 units subcutaneously, and then 8000 to 10,000 units subcutaneously every 8 hours or 15,000 to 20,000 units subcutaneously every 12 hours.
- 5000 units subcutaneously 2 hours before surgery and 5000 units subcutaneously every 8 to 12 hours thereafter for 7 days or until the patient is ambulatory, whichever is longer.
- 333 units/kg subcutaneously followed by 250 units/kg subcutaneously every 12 hours; the following dosage regimen has also been recommended:
Comments:
- Recommended doses are based on a 68 kg patient.
- Consult the latest guidelines regarding duration and intensity of anticoagulation for the indicated conditions.
- Dosage and administration must be individualized according to the results of suitable laboratory tests.
- Administer by deep subcutaneous (intrafat, e.g., above the iliac crest or abdominal fat layer, arm, or thigh) injection with a fine (25 to 26 gauge) needle to minimize tissue trauma.
Adult Dose for Cardiothoracic Surgery
Uses:
- Prevention of clotting in arterial and cardiac surgery.
- Total body perfusion for open-heart surgery.
Initial dose: At least 150 units/kg; frequently, 300 units/kg is used for procedures estimated to last less than 60 minutes or 400 units/kg for those estimated to last longer than 60 minutes.
Comments:
- Consult the latest guidelines regarding duration and intensity of anticoagulation for the indicated conditions.
- Dosage and administration must be individualized according to the results of suitable laboratory tests.
Adult Dose for Vascular Surgery
Uses:
- Prevention of clotting in arterial and cardiac surgery.
- Total body perfusion for open-heart surgery.
Initial dose: At least 150 units/kg; frequently, 300 units/kg is used for procedures estimated to last less than 60 minutes or 400 units/kg for those estimated to last longer than 60 minutes.
Comments:
- Consult the latest guidelines regarding duration and intensity of anticoagulation for the indicated conditions.
- Dosage and administration must be individualized according to the results of suitable laboratory tests.
Adult Dose for Blood Transfusion
Use:
- To aid in the maintenance of catheter patency.
- Anticoagulant use in blood transfusions.
Addition of 400 to 600 USP units per 100 mL of whole blood is usually employed to prevent coagulation
Usual Adult Dose for Patency Maintenance of Indwelling Intravenous Devices
- 6 units/hr (using 2 units/mL formulation) has been found to be satisfactory
Comments: Rate of infusion depends upon age, weight, clinical condition, and procedure being employed.
Pediatric Dose for Thrombotic/Thromboembolic Disorder
Neonates:
- Systemic to pulmonary artery shunt thrombosis: 50 to 100 units/kg IV bolus; consideration should be given to ongoing infusion.
- Central venous access device patency: 0.5 units/kg/hr IV continuous infusion
Systemic heparinization:
- Initial dose: 75 to 100 units/kg IV bolus over 10 minutes
- Maintenance dose: 25 to 30 units/kg/hr IV continuous infusion
Infants and Children:
- Systemic to pulmonary artery shunt thrombosis: 50 to 100 units/kg IV bolus; consideration should be given to ongoing infusion.
- Systemic to pulmonary artery shunt thromboprophylaxis: 10 to 15 units/kg/hr IV continuous infusion
- Central venous line thromboprophylaxis in high-risk congenital heart disease (CHD) patients: 10 to 15 units/kg/hr IV continuous infusion
Systemic heparinization:
- Initial dose: 75 to 100 units/kg IV bolus over 10 minutes
- Maintenance dose: Infants: 25 to 30 units/kg/hr IV continuous infusion; Children: 18 to 20 units/kg/hr IV continuous infusion.
Comments:
- Use preservative-free formulations of this drug in neonates and infants.
- Adjust dose to maintain activated partial thromboplastin time (aPTT) of 60 to 85 seconds, assuming this reflects an anti-Factor Xa level of 0.35 to 0.7.
- Infants less than 2 months have the highest requirements (average dose of 28 units/kg/hr).
- Older children may require less of this drug, similar to weight-adjusted adult dosage.
Pediatric Dose for Patency Maintenance of Indwelling Intravenous Devices
Neonates:
- Systemic to pulmonary artery shunt thrombosis: 50 to 100 units/kg IV bolus; consideration should be given to ongoing infusion.
- Central venous access device patency: 0.5 units/kg/hr IV continuous infusion
Systemic heparinization:
- Initial dose: 75 to 100 units/kg IV bolus over 10 minutes
- Maintenance dose: 25 to 30 units/kg/hr IV continuous infusion
Infants and Children:
- Systemic to pulmonary artery shunt thrombosis: 50 to 100 units/kg IV bolus; consideration should be given to ongoing infusion.
- Systemic to pulmonary artery shunt thromboprophylaxis: 10 to 15 units/kg/hr IV continuous infusion
- Central venous line thromboprophylaxis in high-risk congenital heart disease (CHD) patients: 10 to 15 units/kg/hr IV continuous infusion
Systemic heparinization:
- Initial dose: 75 to 100 units/kg IV bolus over 10 minutes
- Maintenance dose: Infants: 25 to 30 units/kg/hr IV continuous infusion; Children: 18 to 20 units/kg/hr IV continuous infusion.
Comments:
- Use preservative-free formulations of this drug in neonates and infants.
- Adjust dose to maintain activated partial thromboplastin time (aPTT) of 60 to 85 seconds, assuming this reflects an anti-Factor Xa level of 0.35 to 0.7.
- Infants less than 2 months have the highest requirements (average dose of 28 units/kg/hr).
- Older children may require less of this drug, similar to weight-adjusted adult dosage.
Renal Dose Adjustments
- Mild to moderate renal dysfunction: Data not available
- Severe renal dysfunction: Use with caution
Dialysis
Extracorporeal dialysis: If equipment manufacturers’ recommendations are not available, the following doses are suggested based on pharmacodynamic data:
- Initial dose: 25 to 30 units/kg
- Maintenance dose: 1500 to 2000 units/hour
Peritoneal dialysis: Data not available
Liver Dose Adjustments
- Use with caution
Dose Adjustments
Converting to warfarin: Continue full heparin therapy for several days until INR has reached a stable therapeutic range. Heparin may then be discontinued without tapering.
Converting to oral anticoagulants other than warfarin:
- Patients receiving heparin continuous IV infusion: Stop heparin immediately after administering the first dose of oral anticoagulant.
- Patients receiving heparin intermittent IV injection: Start oral anticoagulant 0 to 2 hours before the time the next dose of heparin was to have been administered.
Concomitant use of antithrombin III (human): In patients with antithrombin III deficiency, consider a lower dose of this drug when coadministered with antithrombin III (human).
What should I do if I forget a dose?
If you miss a dose of this medicine, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule. Do not double doses.
Heparin side effects
Heparin may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- redness, pain, bruising, or sores at the spot where heparin was injected
- hair loss
Some side effects can be serious. If you experience any of these symptoms, call your doctor immediately:
- unusual bruising or bleeding
- vomit that is bloody or looks like coffee grounds
- stool that contains bright red blood or is black and tarry
- blood in urine
- excessive tiredness
- nausea
- vomiting
- chest pain, pressure, or squeezing discomfort
- discomfort in the arms, shoulder, jaw, neck, or back
- coughing up blood
- excessive sweating
- sudden severe headache
- lightheadedness or fainting
- sudden loss of balance or coordination
- sudden trouble walking
- sudden numbness or weakness of the face, arm or leg, especially on one side of the body
- sudden confusion, or difficulty speaking or understanding speech
- difficulty seeing in one or both eyes
- purple or black skin discoloration
- pain and blue or dark discoloration in the arms or legs
- itching and burning, especially on the bottoms of the feet
- chills
- fever
- hives
- rash
- wheezing
- shortness of breath
- difficulty breathing or swallowing
- hoarseness
- painful erection that lasts for hours
Less common side effects of heparin:
- abdominal or stomach pain or swelling
- back pain or backaches
- bleeding from the gums when brushing teeth
- blood in the urine
- constipation
- coughing up blood
- dizziness
- headaches, severe or continuing
- heavy bleeding or oozing from cuts or wounds
- joint pain, stiffness, or swelling
- menstrual bleeding, unexpected or unusually heavy
- unexplained bruising or purplish areas on the skin
- unexplained nosebleeds
- vomiting of blood or material that looks like coffee grounds
Rare side effects of heparin:
- blood under the skin (blood blister) at the place of injection
- chest pain
- chills or fever
- fast or irregular breathing
- irritation, pain, redness, or ulcers at the place of injection
- itching and burning feeling, especially on the bottom of the feet
- nausea or vomiting
- numbness or tingling in the hands or feet
- pain, coldness, or blue color of the skin on the arms or legs
- peeling of the skin
- puffiness or swelling of the eyelids or around the eyes
- shortness of breath
- skin color change, especially near the place of injection or in the fingers, toes, arms, or legs
- skin rash, hives, or itching
- tearing of the eyes
- tightness in the chest
- trouble with breathing
- wheezing
Heparin may cause osteoporosis (condition in which the bones become weak and may break easily) and spontaneous fractures 5, especially in people who use the medication for a long time. Other, less common, adverse effects include heparin-induced thrombocytopaenia 6, hypoaldosteronism 7 and hypersensitivity reactions 8. Talk to your doctor about the risks of using this medication.
After you stop using heparin, it may still produce some side effects that need attention. During this period of time, check with your doctor immediately if you notice the following side effects:
- Other side effects not listed may also occur in some patients. If you notice any other effects, check with your doctor.
Heparin may cause other side effects. Call your doctor if you have any unusual problems while using heparin.
Heparin overdose
In case of heparin overdose, call the poison control helpline at 1-800-222-1222. Information is also available online at https://www.poisonhelp.org/help. If the victim has collapsed, had a seizure, has trouble breathing, or can’t be awakened, immediately call your local emergency services number.
Symptoms of heparin overdose may include:
- nosebleed
- blood in urine
- black, tarry stools
- easy bruising
- unusual bleeding
- red blood in stools
- vomit that is bloody or looks like coffee grounds.
Treatment of bleeding associated with heparin overdose is stopping the drug and administering protamine sulfate, a strong half-life protein forming a strong bond with the heparin producing an inactive complex 9.
References- Solari F, Bhimji SS. Low Molecular Weight Heparin (LMWH) [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525957
- Dong K, Song Y, Li X, Ding J, Gao Z, Lu D, Zhu Y. Pentasaccharides for the prevention of venous thromboembolism. Cochrane Database Syst Rev. 2016 Oct 31;10:CD005134.
- Mulloy B, Hogwood J, Gray E, Lever R, Page CP. Pharmacology of Heparin and Related Drugs. Pharmacol. Rev. 2016 Jan;68(1):76-141. http://pharmrev.aspetjournals.org/content/68/1/76.long
- Nicolas D, Reed M. Heparin Induced Thrombocytopenia (HIT) [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482330
- Gajic-Veljanoski O, Phua CW, Shah PS, Cheung AM. Effects of Long-Term Low-Molecular-Weight Heparin on Fractures and Bone Density in Non-Pregnant Adults: A Systematic Review With Meta-Analysis. J Gen Intern Med. 2016 Aug;31(8):947-57.
- Prince M, Wenham T. Heparin-induced thrombocytopaenia. Postgrad Med J. 2018 Aug;94(1114):453-457.
- Levesque H, Verdier S, Cailleux N, Elie-Legrand MC, Gancel A, Basuyau JP, Borg JY, Moore N, Courtois H. Low molecular weight heparins and hypoaldosteronism. BMJ. 1990 Jun 02;300(6737):1437-8.
- Cesana P, Scherer K, Bircher AJ. Immediate Type Hypersensitivity to Heparins: Two Case Reports and a Review of the Literature. Int. Arch. Allergy Immunol. 2016;171(3-4):285-289.
- Mulloy B, Hogwood J, Gray E, Lever R, Page CP. Pharmacology of Heparin and Related Drugs. Pharmacol. Rev. 2016 Jan;68(1):76-141.