What is IGF
IGFs (insulin-like growth factors) were previously known as somatomedins. IGF-2 (insulin-like growth factor-2) is believed to be the major fetal growth factor while IGF-1 (insulin-like growth factor-1) is responsible for post natal growth 1. IGF-2 (insulin-like growth factor-2) stimulates placental and fetal growth, and is less growth hormone (GH) dependent than IGF-1 2. However, results of in vivo and in vitro studies, describing growth hormone as a physiological regulator of IGF-2 gene expression in humans in a promoter-specific way are also present 3.
Insulin-like growth factor 1 (IGF-1) and insulin-like growth factor 2 (IGF-2) were named insulin-like growth factors because of their structural homology with insulin (~50% identical sequence) and similar metabolic actions 4. They are functionally related to insulin but have a much higher growth-promoting activity 5. Quantitatively, IGF-2 is the predominant circulating IGF, present in adults at a concentration up to ~700 ng/mL of blood, three times that of IGF-1 2.
IGF-1 plays a central role in pre- and postnatal growth in humans and other mammals, as a key mediator of the growth hormone actions, while also being involved in the control of intermediary metabolism, tissue repair and disease pathogenesis throughout life 6. Both IGFs are central hormones involved in metabolic signaling, affecting glucose uptake, lipogenesis, glycogen storage, and suppression of protein degradation 7. IGF-1 is necessary for normal insulin sensitivity, and impairment of IGF-1 synthesis results in a worsening state of insulin resistance 8.
IGF-1 is a hormone along with growth hormone (GH), helps promote normal bone and tissue growth and development. Growth hormone (GH) stimulates production of IGF-1, which binds to its receptors resulting in increased cell size by increasing protein synthesis 1. Growth hormone acts both directly through the growth hormone receptor, via the activation of tyrosine kinases, and via IGF-1 to increase linear growth in children. Growth hormone is the main regulator for IGF-1 levels in plasma. Nutritional status and thyroid hormone also affect IGF-1 levels. In children with growth hormone insensitivity and growth hormone receptor mutations, IGF-1 injections can be effective.
It is thought that placental growth hormone (GH) may influence fetal growth via IGF-1, as strong correlations of maternal serum concentrations of placentally derived growth hormone and IGF-1 have been shown throughout gestation 9. A prospective longitudinal study of normal pregnant women showed that placental growth hormone (GH) levels rise from 5 weeks gestation to a peak at around 37 weeks, from which time they decreased until birth. Between 24.5 and 37.5 weeks gestation, fetal growth, weight, birth weight and IGF-1 levels were associated with the change in placental growth hormone levels 10.
The main action of growth hormone (GH) via IGF-1 in children is increase in linear growth. The site of action is the epiphyseal plates of long bones, also known as the growth plates. IGF-1 secreted by the liver works alongside locally secreted IGF-1 by the chondrocytes at the growth plate. This stimulates chondrocyte cell division resulting in bone growth and increase in linear growth in children.
Excess of growth hormone in the prepubertal children where the epiphyses are not fused results in gigantism with uncontrolled linear growth. Once the epiphyses are fused, the result of excess growth hormone (GH) is acromegaly. Similarly, a deficiency of growth hormone (GH) or an inability of growth hormone (GH) to exert its actions, i.e. growth hormone resistance, results in short stature or in severe cases dwarfism.
Insulin-like growth factor 1 (IGF-1) is a 70-amino acid polypeptide (molecular weight 7.6 kDa). IGF-1 is a member of a family of closely related growth factors with high structural homology with insulin (~50% identical sequence) to insulin that signal through a corresponding group of highly homologous tyrosine kinase receptors. IGF-1 is produced by many tissues, but the liver is the main source of circulating IGF-1. IGF-1 is the major mediator of the anabolic and growth-promoting effects of growth hormone (GH). IGF-1 is transported by IGF-binding proteins, in particular insulin-like growth factor-binding protein 3 (IGFBP3), which also controls its bioavailability and half-life.
The liver is the major organ for IGF 1 synthesis (see Figure 1) and with smaller production from skeletal muscle as well as many other tissues in response to growth hormone stimulation. IGF-1 mediates many of the actions of growth hormone, stimulating the growth of bones and other tissues and promoting the production of lean muscle mass. Since growth hormone is released into the blood in pulses throughout the day, it is difficult to interpret the results from a single growth hormone test. IGF-1 mirrors growth hormone excesses and deficiencies, but unlike growth hormone, its level is stable throughout the day. This makes IGF-1 a useful indicator of average growth hormone levels. The IGF-1 test is therefore often used to help evaluate for growth hormone deficiency or growth hormone excess.
IGF-1 levels, like growth hormone (GH), are normally low in early childhood, increase gradually during childhood, peak during puberty, and then decline in adult life. Deficiencies in growth hormone and IGF-1 may be caused by a dysfunctional pituitary gland with decreased pituitary hormones (hypopituitarism) or by the presence of a non-growth hormone-producing pituitary tumor that damages hormone-producing cells. Deficiencies in IGF-1 also occur where there is a lack of responsiveness to growth hormone. This insensitivity may be primary (genetic) or secondary to conditions such as malnutrition, hypothyroidism, sex hormone deficiency, and chronic diseases. Genetic growth hormone insensitivity (growth hormone resistance) is very rare.
IGF-1 deficiencies early in life, usually the result of growth hormone deficiency, can inhibit bone growth and overall development and can result in a child with a shorter than normal stature. In adults, decreased production can lead to low bone density, less muscle mass, and altered lipid levels. However, testing for IGF-1 deficiency, or growth hormone deficiency, is not routine in adults who have decreased bone density and/or muscle strength or increased lipids. growth hormone deficiency and consequent IGF-1 deficiency is a very rare cause of these disorders.
Excess growth hormone and IGF-1 can cause abnormal growth of the skeleton and other signs and symptoms characteristic of two rare conditions, gigantism and acromegaly. In children, gigantism causes bones to grow longer, resulting in a very tall person with large feet and hands. In adults, acromegaly causes bones to thicken and soft tissues, such as the nose, to swell. Both conditions can lead to enlarged organs, such as the heart, and to other complications such as type 2 diabetes, increased risk of cardiovascular disease, high blood pressure, arthritis, and a decreased lifespan.
The most common reason for the pituitary to secrete excessive amounts of growth hormone is a growth hormone-producing pituitary tumor (usually benign). Frequently, the tumor can be surgically removed and/or treated with drugs or radiation. In most cases, this will cause growth hormone and IGF-1 levels to return to normal or near normal levels.
Insulin-like growth factors (IGFs) in biological fluids are associated with IGF binding proteins (IGFBPs), which are the principal regulators of IGFs’ bioactivity and activity in metabolic signaling pathways 4. Moreover, these proteins extend the half-life of IGFs in the bloodstream, store IGFs in specific tissue compartments, inhibit activity of IGFs by lowering accessibility of their receptors, and protect them from proteolytic degradation 11.
In blood ~90% of IGF-1 is associated with IGFBP-3 (insulin-like growth factor binding protein 3), circulating as a 150 kDa complex that consists of IGF-1, IGFBP-3 (insulin-like growth factor binding protein 3), and an acid-labile subunit 12. Free IGF1 has a half-life of ~8 min in serum. This can be increased to ~30 min if bound to IGFBP3 and up to ~15h in the ternary complex with IGFBP3 and acid-labile subunit 13.
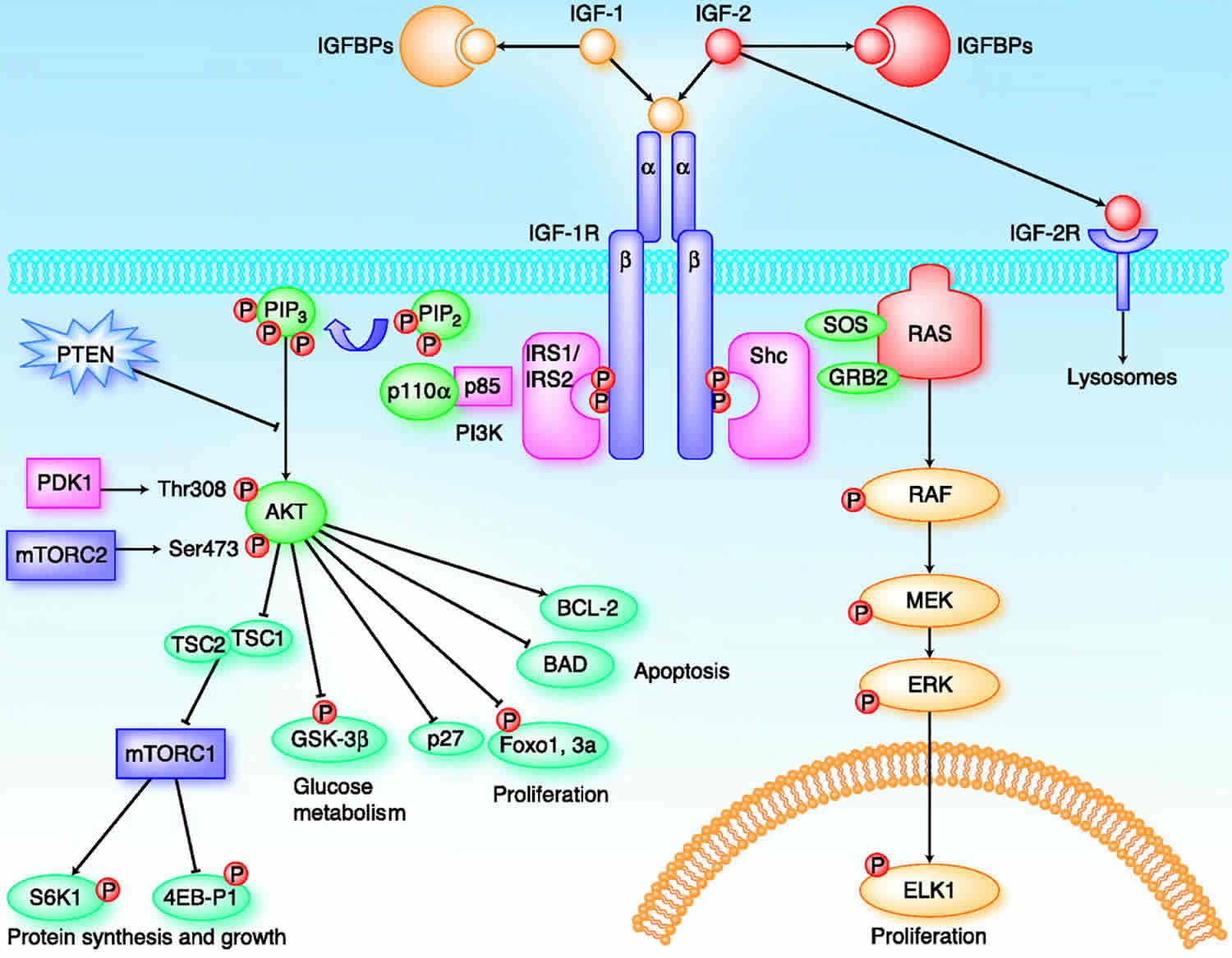
IGFs affect cells through specifically binding three various surface receptors, type-I IGF receptor (IGF1R), type-II IGF receptor (IGF2R), insulin receptor (IR) and hybrid receptors (IGF1R/IR) 14. Most activities of both IGFs are mediated by a type I receptor, with these interactions regulated by high-affinity insulin-like growth factor binding protein (IGFBPs), which can inhibit or enhance the action of IGFs and IGF low-affinity IGFBP-related proteins (IGFBPrP1-10). In addition, insulin-like growth factor binding proteins’ activity is also regulated by IGFBP proteases 15.
Figure 1. Regulation of growth by IGF 1 and growth hormone
 Footnote: 1. Appetite centers in brain affecting calorie intake. #2. Signal transduction in hepatocyte. #3. Release of IGF-1 from binding proteins. #4. IGF-1 expression in growth plate. #5. Proliferation of chondrocytes at growth plate
[Source 1 ]
Footnote: 1. Appetite centers in brain affecting calorie intake. #2. Signal transduction in hepatocyte. #3. Release of IGF-1 from binding proteins. #4. IGF-1 expression in growth plate. #5. Proliferation of chondrocytes at growth plate
[Source 1 ]Figure 2. Summary of actions of growth hormone and IGF-1
IGF test
IGF-1 test for may be used to help:
- Identify growth hormone (GH) deficiency; it is not diagnostic of a GH deficiency but may be ordered along with GH stimulation tests to offer additional information.
- As follow-up to abnormal results on other hormone tests
- Evaluate pituitary function
Less commonly, IGF-1 tests may be used to detect excess growth hormone and to help diagnose and monitor treatment of two rare conditions, acromegaly and gigantism.
IGF-1 is a hormone that, along with growth hormone (GH), helps promote normal bone and tissue growth and development. An IGF-1 test is often ordered along with growth hormone (GH). IGF-1 mirrors growth hormone (GH) excesses and deficiencies, but the level in the blood is stable throughout the day, making it a useful indicator of average growth hormone (GH) levels.
IGF-1 may be ordered with other pituitary hormone tests, such as prolactin or follicle-stimulating hormone (FSH) and luteinizing hormone (LH), to help diagnose pituitary gland dysfunction and decreased pituitary hormones (hypopituitarism).
IGF-1 testing and a growth hormone (GH) suppression test can be used to detect and monitor treatment of a growth hormone-producing pituitary tumor. An anterior pituitary tumor is typically confirmed with imaging scans that help identify and locate the tumor. If surgery is necessary, growth hormone and IGF-1 levels are measured after the tumor’s removal to determine whether the entire tumor was successfully removed. Drug and/or radiation therapy may be used in addition to, or sometimes instead of, surgery to try to decrease growth hormone production and return IGF-1 to a normal or near normal concentration. IGF-1 may be ordered to monitor the effectiveness of this therapy at regular intervals for years afterward to monitor growth hormone production and to detect tumor recurrence.
IGF-1 levels and the measurement of growth hormone can also provide information related to growth hormone insensitivity. Prior to performing definitive growth hormone testing, if the IGF-1 level is found to be normal for age and sex, growth hormone deficiency is excluded and definitive testing is not necessary.
When is IGF-1 test ordered?
IGF-1 testing may be ordered, along with a growth hormone stimulation test, when:
- A child has symptoms of growth hormone deficiency, such as a slowed growth rate and short stature
- Adults have symptoms that a health practitioner suspects may be due to a growth hormone deficiency, such as decreased bone density, fatigue, adverse changes to lipid levels, and reduced exercise tolerance. However, testing for IGF-1 deficiency is not routine in adults who have these symptoms; growth hormone and IGF-1 deficiency are only very rare causes of these disorders.
An IGF-1 also may be ordered when a health practitioner suspects that someone has an underactive pituitary gland and at intervals to monitor those on growth hormone therapy.
Less commonly, IGF-1 testing may be ordered, along with a growth hormone suppression test, when a child has symptoms of gigantism or when an adult shows signs of acromegaly.
When a growth hormone-producing pituitary tumor is found, growth hormone and IGF-1 are ordered after the tumor is surgically removed to determine whether all of the tumor has been extracted. IGF-1 also is ordered at regular intervals when someone is undergoing the drug and/or radiation therapy that frequently follow tumor surgery.
IGF-1 levels may be ordered at regular intervals for many years to monitor a person’s growth hormone production and to watch for pituitary tumor recurrence.
Normal IGF-1 levels by age
Insulin-like growth factor 1 (IGF-1) reference ranges are highly age dependent and results must always be interpreted within the context of the patient’s age.
IGF-1 assays also exhibit significant variability among platforms and manufacturers. Direct comparison of results obtained by different assays is problematic. If IGF-1 is being used for serial monitoring, rebaselining of patients is preferred if assays are changed.
Several amino acid polymorphisms within IGF-1 have been discovered. At least 4 of these are known to result in IGF-1 isoforms with diminished biological activity. IGF-1 immunoassays vary in their ability to detect these reduced-function mutants. If they do detect the mutants, then this will result in an overestimation of functionally active IGF-1 in an affected patient. By contrast, a mass spectrometry-based IGF-1 assay can usually selectively detect the active IGF-1 isoforms. However, there might be as yet unknown functionally different variants of IGF-1, which even mass spectrometry cannot distinguish from wild-type (normal) IGF-1.
Males:
- Age 0-11 months: 18-156 ng/mL
- Age 1 year: 14-203 ng/mL
- Age 2 years: 16-222 ng/mL
- Age 3 years: 22-229 ng/mL
- Age 4 years: 30-236 ng/mL
- Age 5 years: 39-250 ng/mL
- Age 6 years: 47-275 ng/mL
- Age 7 years: 54-312 ng/mL
- Age 8 years: 61-356 ng/mL
- Age 9 years: 67-405 ng/mL
- Age 10 years: 73-456 ng/mL
- Age 11 years: 79-506 ng/mL
- Age 12 years: 84-551 ng/mL
- Age 13 years: 90-589 ng/mL
- Age 14 years: 95-618 ng/mL
- Age 15 years: 99-633 ng/mL
- Age 16 years: 104-633 ng/mL
- Age 17 years: 107-615 ng/mL
- Age 18-22 years: 91-442 ng/mL
- Age 23-25 years: 66-346 ng/mL
- Age 26-30 years: 60-329 ng/mL
- Age 31-35 years: 54-310 ng/mL
- Age 36-40 years: 48-292 ng/mL
- Age 41-45 years: 44-275 ng/mL
- Age 46-50 years: 40-259 ng/mL
- Age 51-55 years: 37-245 ng/mL
- Age 56-60 years: 34-232 ng/mL
- Age 61-65 years: 33-220 ng/mL
- Age 66-70 years: 32-209 ng/mL
- Age 71-75 years: 32-200 ng/mL
- Age 76-80 years: 33-192 ng/mL
- Age 81-85 years: 33-185 ng/mL
- Age 86-90 years: 33-179 ng/mL
- Age >91 years: 32-173 ng/mL
Females:
- Age 0-11 months: 14-192 ng/mL
- Age 1 year: 23-243 ng/mL
- Age 2 years: 28-256 ng/mL
- Age 3 years: 31-249 ng/mL
- Age 4 years: 33-237 ng/mL
- Age 5 years: 36-234 ng/mL
- Age 6 years: 39-246 ng/mL
- Age 7 years: 44-279 ng/mL
- Age 8 years: 51-334 ng/mL
- Age 9 years: 61-408 ng/mL
- Age 10 years: 73-495 ng/mL
- Age 11 years: 88-585 ng/mL
- Age 12 years: 104-665 ng/mL
- Age 13 years: 120-719 ng/mL
- Age 14 years: 136-729 ng/mL
- Age 15 years: 147-691 ng/mL
- Age 16 years: 153-611 ng/mL
- Age 17 years: 149-509 ng/mL
- Age 18-22 years: 85-370 ng/mL
- Age 23-25 years: 73-320 ng/mL
- Age 26-30 years: 66-303 ng/mL
- Age 31-35 years: 59-279 ng/mL
- Age 36-40 years: 54-258 ng/mL
- Age 41-45 years: 49-240 ng/mL
- Age 46-50 years: 44-227 ng/mL
- Age 51-55 years: 40-217 ng/mL
- Age 56-60 years: 37-208 ng/mL
- Age 61-65 years: 35-201 ng/mL
- Age 66-70 years: 34-194 ng/mL
- Age 71-75 years: 34-187 ng/mL
- Age 76-80 years: 34-182 ng/mL
- Age 81-85 years: 34-177 ng/mL
- Age 86-90 years: 33-175 ng/mL
- Age > or =91 years: 25-179 ng/mL
Tanner Stage reference ranges:
Males
- Stage I: 81-255 ng/mL
- Stage II: 106-432 ng/mL
- Stage III: 245-511 ng/mL
- Stage IV: 223-578 ng/mL
- Stage V: 227-518 ng/mL
Females
- Stage I: 86-323 ng/mL
- Stage II: 118-451 ng/mL
- Stage III: 258-529 ng/mL
- Stage IV: 224-586 ng/mL
- Stage V: 188-512 ng/mL
Note: Puberty onset (transition from Tanner stage I to Tanner stage II) occurs for boys at a median age of 11.5 (+/-2) years and for girls at a median age of 10.5 (+/-2) years. There is evidence that it may occur up to 1 year earlier in obese girls and in African American girls. For boys, there is no definite proven relationship between puberty onset and body weight or ethnic origin. Progression through Tanner stages is variable. Tanner stage V (young adult) should be reached by age 18.
What are Tanner Stages?
Tanner Staging, also known as Sexual Maturity Rating, is an objective classification system that healthcare providers use to document and track the development and sequence of secondary sex characteristics of children during puberty 16. Tanner Staging was developed by Marshall and Tanner while conducting a longitudinal study during the 1940s-1960s in England. Based on observational data, they developed separate scales for the development of external genitalia: phallus, scrotum, and testes volume in males; breasts in females; and pubic hair in both males and females.
Below are the Tanner Stages described in detail for clinical reference. For all three sites of development, Tanner Stage 1 corresponds to the pre-pubertal form with progression to Tanner Stage 5, the final adult form. Breast and genital staging, as well as other physical markers of puberty such as height velocity, should be relied on more than pubic hair staging to assess pubertal development because of the independent maturation of adrenal axis.
Pubic Hair Scale (both males and females)
- Stage 1: No pubic hair at all (prepubertal) (typically age 10 and younger)
- Stage 2: Downy hair with slight pigmentation at the base of the penis and scrotum (males) or on the labia majora (females) (age 10–11.5)
- Stage 3: Scant terminal hair – hair becomes more coarse and curly, and begins to extend laterally (age 11.5–13)
- Stage 4: Terminal hair that fills the entire triangle overlying the pubic region but sparing medial thighs (age 13–15)
- Stage 5: Terminal hair that extends beyond the inguinal crease onto the thigh (age 15+)
Female Breast Development Scale
- Stage 1: No glandular breast tissue palpable – areola follows the skin contours of the chest (prepubertal) (typically age 10 and younger)
- Stage 2: Breast bud palpable under areola (1st pubertal sign in females) – areola begins to widen (age 10–11.5)
- Stage 3: Breast begins to become more elevated, and extends beyond the borders of the areola, which continues to widen but remains in contour with surrounding breast (age 11.5–13)
- Stage 4: Areola elevated above contour of the breast, forming “double scoop” appearance (age 13–15)
- Stage 5: Areolar mound recedes back into single breast contour with areolar hyperpigmentation, papillae development and nipple protrusion (age 15+)
Figure 3. Tanner Stage Female
Male External Genitalia Scale
- Stage 1: Testicular volume < 4 ml or long axis < 2.5 cm
- Stage 2: 4 ml-8 ml (or 2.5-3.3 cm long), 1st pubertal sign in males
- Stage 3: 9 ml-12 ml (or 3.4-4.0 cm long)
- Stage 4: 15-20 ml (or 4.1-4.5 cm long)
- Stage 5: > 20 ml (or > 4.5 cm long)
Figure 4. Tanner Stage Male
What does abnormal IGF-1 test result mean?
A normal level of IGF-1 must be considered in context. Some people can have a growth hormone deficiency and still have a normal IGF-1 level.
If an IGF-1 level is normal and a healthcare practitioner still strongly suspects a growth hormone deficiency, then the healthcare provider may order another test, an IGFBP-3 (insulin-like growth factor binding protein 3), to help confirm the growth hormone deficiency. Almost all IGF-1 in the blood is bound to binding proteins, with IGFBP-3 being the most prevalent form, and IGFBP-3 production is also stimulated by growth hormone.
IGFBP3 (insulin-like growth factor binding protein 3) is a 264-amino acid peptide (MW 29kD) produced by the liver. Insulin-like growth factor binding protein 3 (IGFBP3) is the most abundant of a group of insulin-like growth factor binding proteins that transport, and control bioavailability and half-life of IGFs, in particular IGF-1, the major mediator of the anabolic- and growth-promoting effects of growth hormone. In addition to its IGF binding-function, insulin-like growth factor binding protein 3 (IGFBP3) also exhibits intrinsic growth-regulating effects that are not yet fully understood, but have evoked interest with regards to a possible role of IGFBP3 as a prognostic tumor marker. Noncomplexed IGF-1 and IGFBP3 have short half-lives (T1/2) of 10 and 30 to 90 minutes, respectively, while the IGFBP3/IGF-1 complex is cleared with a much slower T1/2 of 12 hours.
The secretion patterns of IGF-1 and insulin-like growth factor binding protein 3 (IGFBP3) mimic each other, their respective syntheses being controlled by growth hormone (GH). Unlike growth hormone secretion, which is pulsatile and demonstrates significant diurnal variation, IGF-1 and IGFBP3 levels show only minor fluctuations. IGF-1 and IGFBP3 serum levels, therefore, represent a stable and integrated measurement of growth hormone production and tissue effect.
Low IGF-1 and IGFBP3 levels are observed in growth hormone deficiency or growth hormone resistance. If acquired in childhood, these conditions result in short stature. Serum IGF-1 and IGFBP3 concentrations below the 2.5th percentile (Standard deviation score, Z-score of <-2) for age are consistent with growth hormone deficiency or severe growth hormone resistance, but patients with incomplete growth hormone deficiency or mild-to-moderate growth hormone resistance may have levels within the reference range. In growth hormone deficiency, growth hormone levels may also be low and can show suboptimal responses in stimulation tests (eg exercise, clonidine, arginine, ghrelin, growth hormone-releasing hormone, insulin-induced hypoglycemia), while in severe growth hormone resistance, growth hormone levels might be substantially elevated. However, dynamic growth hormone testing is not always necessary for diagnosis. If it is undertaken, it should be performed and interpreted in endocrine testing centers under the supervision of a pediatric or adult endocrinologist.
Childhood growth hormone deficiency can be an isolated abnormality or associated with deficiencies of other pituitary hormones. Some of the latter cases may be due to pituitary or hypothalamic tumors, or result from cranial radiation or intrathecal chemotherapy for childhood malignancies.
Most growth hormone resistance in childhood is mild to moderate, with causes ranging from poor nutrition to severe systemic illness (eg, renal failure). These individuals may have IGF-1 and IGFBP3 levels within the reference range. Severe childhood growth hormone resistance is rare and usually due to defects of the growth hormone-receptor, its downstream signaling cascades, or deleterious mutations in IGF-1, its binding proteins, or its receptor signaling cascades.
Both growth hormone deficiency and mild-to-moderate growth hormone resistance can be treated with recombinant human growth hormone (rh-GH) injections, while severe resistance will usually not respond to growth hormone. However, such patients might respond to recombinant IGF-1 therapy, unless the underlying defect is in the IGF-1 receptor or its downstream signaling systems.
The exact prevalence and causes of adult growth hormone resistance are uncertain, but adult growth hormone deficiency is seen mainly in pituitary tumor patients. It is associated with decreased muscle bulk and increased cardiovascular morbidity and mortality, but replacement therapy remains controversial.
Elevated serum IGF-1 and IGFBP3 levels often indicate a sustained overproduction of growth hormone, or excessive growth hormone therapy. Endogenous growth hormone excess is caused mostly by growth hormone-secreting pituitary adenomas, resulting in gigantism, if acquired before epiphyseal closure, and in acromegaly thereafter. Both conditions are associated with generalized organomegaly, hypertension, diabetes, cardiomyopathy, osteoarthritis, compression neuropathies, a mild increase in cancer risk (breast, colon, prostate, lung), and diminished longevity. It is plausible, but unproven, that long-term recombinant human growth hormone (rh-GH) overtreatment may result in similar adverse outcomes. In successfully treated patients, both IGF-1 and IGFBP3 levels should be within the normal range, ideally within the lower third. In both gigantism and acromegaly cases, follow-up IGF1 levels correlate better with clinical disease activity than IGFBP3 levels. After transsphenoidal removal of pituitary tumors in patients with acromegaly, IGF-I concentration starts to decrease and returns to normal levels in most patients postoperatively by the fourth day. If IGF-1 is still elevated after the surgical removal of a pituitary tumor, then the surgery may not have been fully effective. Decreasing IGF-1 levels during subsequent drug and/or radiation therapies indicate that the treatment is lowering growth hormone production.
How long do I have to be monitored?
As long as you are considered to have abnormal (low or high) growth hormone production or are receiving growth hormone replacement therapy, your IGF-1 will need to be monitored. However, IGF-1 is not routinely measured to monitor growth hormone treatment of growth hormone-deficient children. The best index of the effectiveness of growth hormone treatment of children who are growth hormone-deficient is observation of an increase in their growth rate and their absolute height.
Low IGF-1
If the IGF-1 level is decreased, then it is likely that there is a growth hormone deficiency or an insensitivity to growth hormone. If this is in a child, the growth hormone deficiency may have already caused short stature and delayed development and may be treated with growth hormone supplementation. Adults will have an age-related decrease in production, but lower than expected levels may reflect a growth hormone deficiency or insensitivity.
If a decrease in IGF-1 is suspected to be due to a more general decrease in pituitary function (hypopituitarism), then several other endocrine glands and their pituitary regulating hormones will need to be evaluated to decide on appropriate treatment. Reduced pituitary function may be due to inherited defects or can develop as a result of pituitary damage following conditions such as trauma, infections, and inflammation.
Decreased levels of IGF-1 also may be seen with nutritional deficiencies (including anorexia nervosa), chronic kidney or liver disease, inactive/ineffective forms of growth hormone, and with high doses of estrogen. Malnutrition results in low serum IGF-1 concentrations, which recover with restoration of adequate nutrition. Persons with anorexia or malnutrition have low values of IGF-1. IGF-1 is a more sensitive indicator than prealbumin, retinol-binding protein, or transferrin for monitoring nutritional repletion.
The aim of both pediatric and adult growth hormone replacement therapy is to achieve IGF-1 and insulin-like growth factor binding protein 3 (IGFBP3) levels within the reference range, ideally within the middle-to-upper third. Higher levels are rarely associated with any further therapeutic gains, but could potentially lead to long-term problems of growth hormone excess.
What signs and symptoms are seen with deficient growth hormone and IGF-1?
In children, the following may indicate growth hormone and/or IGF-1 deficiency:
- Slowed growth rate in early childhood relative to group norms
- Shorter stature than others of the same chronological age
- Delayed puberty
- X-rays showing delayed bone development
In adults, abnormally low levels of growth hormone and/or IGF-1 may cause subtle, nonspecific symptoms such as:
- Decreased bone density
- Fatigue
- Adverse lipid changes
- Reduced exercise tolerance
High IGF-1
Elevated levels of IGF-1 usually indicate an increased production of growth hormone. Since growth hormone levels vary throughout the day, IGF-1 levels are a reflection of average growth hormone production, not of the actual amount of growth hormone in the blood at the time that the sample for the IGF-1 measurement was taken. This is accurate up to the point at which the liver’s capacity to produce IGF-1 is reached. With severely increased growth hormone production, the IGF-1 level will stabilize at an elevated maximum level.
Increased levels of growth hormone and IGF-1 are normal during puberty and pregnancy but otherwise are most frequently due to pituitary tumors (usually benign).
If IGF-1 is still elevated after the surgical removal of a pituitary tumor, then the surgery may not have been fully effective. Decreasing IGF-1 levels during subsequent drug and/or radiation therapies indicate that the treatment is lowering growth hormone production. If levels of IGF-1 become “normalized,” then the person is no longer producing excess amounts of growth hormone. When someone is undergoing long-term monitoring, an increase in IGF-1 levels may indicate a recurrence of the pituitary tumor.
What signs and symptoms are seen with excess growth hormone and IGF-1 production?
In a child, it is unusual tallness. With an adult, it may be more subtle: a larger nose, thicker lips, a more prominent jaw, or rings and shoes that no longer fit. Other signs and symptoms may include:
- Deepened, husky voice
- Enlarged organs (liver, heart, kidneys, and spleen)
- Enlarged tongue
- Erectile dysfunction
- Fatigue
- Headaches and visual disturbances
- Joint pain and swelling
- Menstrual cycle irregularities
- Muscle weakness
- Snoring
- Sweating and body odor
- Thickening of the skin, skin tags
- Trapped nerves (Carpal tunnel syndrome)
- Rozario KS, Lloyd C, Ryan F. GH and IGF-1 Physiology In Childhood. [Updated 2015 Nov 20]. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK343487
- Livingstone C. IGF2 and cancer. Endocr. Relat. Cancer. 2013;20:R321–R339. doi: 10.1530/ERC-13-0231
- GH is a regulator of IGF2 promoter-specific transcription in human liver. von Horn H, Ekström C, Ellis E, Olivecrona H, Einarsson C, Tally M, Ekström TJ J Endocrinol. 2002 Mar; 172(3):457-65.
- Adamek A, Kasprzak A. Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int J Mol Sci. 2018;19(5):1308. Published 2018 Apr 27. doi:10.3390/ijms19051308 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5983723/
- Weroha S.J., Haluska P. The insulin-like growth factor system in cancer. Endocrinol. Metab. Clin. N. Am. 2012;41:335–350. doi: 10.1016/j.ecl.2012.04.014
- Kasprzak A., Kwasniewski W., Adamek A., Gozdzicka-Jozefiak A. Insulin-like growth factor (IGF) axis in cancerogenesis. Mutat. Res. Rev. Mutat. Res. 2017;772:78–104. doi: 10.1016/j.mrrev.2016.08.007
- Clemmons D.R. Role of insulin-like growth factor in maintaining normal glucose homeostasis. Horm. Res. 2004;62(Suppl. S1):77–82. doi: 10.1159/000080763
- Clemmons D.R. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J. Clin. Investig. 2004;113:25–27. doi: 10.1172/JCI20660
- Caufriez A, Frankenne F, Hennen G, Copinschi G. (1993) Regulation of maternal IGF-I by placental GH in normal and abnormal human pregnancies. Am J Physiol. 265:E572–E577.
- Chellakooty M, Vangsgaard K, Larsen T, Scheike T, Falck-Larsen J, Legarth J, Andersson AM, Main KM, Skakkebaek NE, Juul A. (2004) A longitudinal study of intrauterine growth and the placental growth hormone (GH)-insulin-like growth factor I axis in maternal circulation: association between placental GH and fetal growth. J Clin Endocrinol Metab.89:384–91
- Shin M., Kang H.S., Park J.H., Bae J.H., Song D.K., Im S.S. Recent Insights into Insulin-Like Growth Factor Binding Protein 2 Transcriptional Regulation. Endocrinol. Metab. 2017;32:11–17. doi: 10.3803/EnM.2017.32.1.11
- Murphy L.J. Insulin-like growth factor-binding proteins: Functional diversity or redundancy? J. Mol. Endocrinol. 1998;21:97–107. doi: 10.1677/jme.0.0210097
- Rechler M.M., Clemmonds D.R. Regulatory actions of insulin-like growth factor-binding proteins. Trends Endocrinol. Metab. 1998;9:176–183. doi: 10.1016/S1043-2760(98)00047-2
- LeRoith D., Bondy C., Yakar S., Liu J.L., Butler A. The somatomedin hypothesis: 2001. Endocr. Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419
- Durzyńska J. IGF axis and other factors in HPV-related and HPV-unrelated carcinogenesis (review) Oncol. Rep. 2014;32:2295–2306. doi: 10.3892/or.2014.3505
- Emmanuel M, Bokor BR. Tanner Stages. [Updated 2017 Dec 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470280