Intestine transplant
An intestine transplant may involve the whole intestine or an intestine segment. Most of the intestine transplants are whole organ transplants and are performed in conjunction with a liver transplant 1. Intestine transplants usually involve a cadaveric donor though it is possible for a living donor to donate an intestine segment. Intestinal transplantation has evolved in the past few decades from an experimental procedure to what is currently considered the only long-term option for patients with intestinal failure who have developed irreversible complications associated with the long-term use of parenteral nutrition. The number of intestinal transplants performed has increased sharply, from five in 1990 to 146 in 2016 in the United States alone, according to the Organ Procurement and Transplantation Network 2.
In addition to intestine-only and intestine-liver transplants, multivisceral transplants represent a third type of intestinal transplant (see Figures 1 to 3 below). The United Network for Organ Sharing (UNOS) defines a multivisceral transplant as one that includes the intestine and liver and either the pancreas or kidney; however, several combinations may be used, depending on the extent of disease.
The Organ Procurement and Transplant Network and the Scientific Registry for Transplant Recipients 2011 annual report reveals a change in the demographics of the candidates waiting for transplantation with nearly 60% of candidates in 2011 over the age of 18 compared to only 29% of recipients in 1998 3. At the same time, the medical urgency of the candidates (status 1) declined from 84% to 69%, while time on the waitlist increased and rate of transplantation per 100 waitlist years declined from 92 to 49 (from 2005 compared to 2011). Overall, a decrease in mortality on the waitlist has been observed, likely multi‐factorially related to the shift noted above in the age of candidates listed for intestine only (i.e. without liver) transplantation, improved medical therapies (as noted above) along with changes in organ allocation policy.
Short gut syndrome (68% of cases) and functional bowel problems (15%) are the major sources of intestinal failure leading to intestinal transplantation 4. Rare indications include vascular abdominal catastrophes and selected low-grade neoplastic tumors (eg, neuroendocrine pancreatic tumors and desmoids involving the mesenteric root) 5.
Overall, with the exception of 2012, the number of adult intestine transplants has remained relatively steady in the United States in the past decade, ranging from 77 to 92 adult intestine transplant cases per calendar year (median, 85 cases) 6. This has differed substantially with the pediatric intestinal transplant experience, which has observed a precipitous decrease in the number of intestinal transplants since 2007 7. Much of the decrease in the number of pediatric intestinal transplants can be attributed to specialized intestinal care centers that capitalize on the increased potential that the infant pediatric gut has for adaptation, and thus rehabilitation. This condition differs from the adult patients with a short gut, who have generally maintained and sustained decades of established intestinal function before the loss of gut and, thus, harbors less potential for growth and adaptation of the remnant intestine 8. Prolonged parenteral nutrition exposure in the adult population, however, does account for the need for a proportion of liver-containing grafts in the adult population, and this is an area for potential improvement in decreasing the high pre-transplant mortality in the adult liver–intestine transplant population 8. Early referral to a specialized adult intestinal transplant center and early recognition of adult recipients with intestinal failure could serve to provide the preferred option of isolated intestine transplant and subsequent withdrawal of parenteral nutrition before the development of irreversible parenteral nutrition-associated liver disease, thus, obviating the requirement for a liver-inclusive graft 8. Recognizing the extremely high mortality that adult patients have on the combined liver–intestine waiting list, the United Network for Organ Sharing revised allocation policy for this specific subset in 2013, allowing for a greater, nationwide pool of potential adult multivisceral donors to help mitigate the high mortality risk 9. Current policy for adults also allocates an additional increase in their Model for End-Stage Liver Disease score equivalent to a 10% increase in risk of 3-month mortality.
In 2012, there were 44 transplants per 100 wait-list years for adults and pediatric transplant candidates had 32 transplants per 100 patient-years. 15% were removed from the waiting list because their condition improved and 11.6% were removed from the waiting list because they had died.
In 2012, the 90-day graft failure rate was 15.7%. Ninety-day graft failure rates have remained relatively unchanged since the year 2003.
Reasons for intestine transplant
Intestinal failure is characterized by the inability to maintain protein energy, fluid, electrolyte, or micronutrient balance due to gastrointestinal disease when on a normal diet. Intestinal failure ultimately leads to malnutrition and even death if the patient does not receive parenteral nutrition or become a recipient of an intestinal transplant. Worldwide, the leading cause of intestinal failure is short bowel syndrome caused by surgical removal. Short gut syndrome (68%) and functional bowel problems (15%) are two major indications for intestinal transplantation 4.
The Intestine Transplant Association convened experts in the field and summarized indications for intestine transplantation to treat patients with life‐threatening complications of parenteral nutrition 10. The indications identified by these experts include loss of venous access (thrombosis of two or more central venous access sites), recurrent life‐threatening catheter‐associated bloodstream infections and the development of Intestinal failure–associated liver disease (IFALD) 10. Although the Centers for Medicare & Medicaid Services in April 2001 approved coverage of intestine or multi‐visceral transplantation for patients with these complications of intestine failure 11, recurrent life‐threatening sepsis has recently been challenged as an indication 12. Subsequently, others have suggested additional indications including patients with ultra‐short remnant bowel lengths (<10 cm in children and <20 cm in adults), porto‐mesenteric venous thrombosis, slow growing central abdominal tumors involving the mesenteric root (such as desmoids tumors or neuroendocrine tumors), pseudoobstruction and frozen abdomen 13.
Intestinal failure–associated liver disease (IFALD) is partly caused by omega-6 fatty acids in parenteral nutrition formulas, which can be synthesized into inflammatory molecules. intestinal failure–associated liver disease can range from steatohepatitis, cholestasis, or hepatic fibrosis to end-stage liver disease 14. Children are more likely to have cholestatic liver disease than steatohepatitis 15. Severe liver injury has been reported in as many as 50% of patients with intestinal failure who receive parenteral nutrition for longer than 5 years; this is typically fatal. If patients have life-threatening infections, intestinal failure–associated liver disease, or lose their venous access, 1 year mortality is 70% without intestinal transplantation 16.
As an early alternative to transplantation or total parenteral nutrition (TPN) for patients with short bowel syndrome, surgical bowel lengthening without transplant may be attempted. This requires the serial transverse enteroplasty (STEP) or longitudinal intestinal lengthening and tailoring (LILT) procedures.
Serial transverse enteroplasty (STEP) and longitudinal intestinal lengthening and tailoring (LILT) are particularly successful in patients with decreased transit times and dilated bowel. These procedures lengthen the small bowel while keeping the total surface area the same. Bowel is either split lengthwise or cut obliquely at multiple points. This will lengthen the bowel and shrink the luminal diameter 17. If successful, this may reduce the amount of Tparenteral nutrition required, or negate its use altogether. In one study, 27 children underwent the longitudinal intestinal lengthening and tailoring (LILT) procedure. Overall survival was 92%, and more than 90% of survivors no longer required parenteral nutrition 18.
If patients are not acceptable candidates for STEP or LILT, sometimes a reversal of small bowel direction may effectively increase transit times. If none of these operations are successful, the standard of care is TPN. Intestinal transplantation should be recommended in lieu of TPN in patients with failure of the parenteral nutrition, as indicated by the following:
- Impending or overt liver failure secondary to intestinal failure–associated liver disease
- Thrombosis of two or more central veins
- Two or more episodes per year of systemic sepsis secondary to line infections, or a single episode of fungal sepsis 19
- Frequent episodes of severe dehydration
Reasons for intestine transplant:
- Short Gut Syndrome: Intestinal Atresia
- Short Gut Syndrome: Necrotizing Enterocolitis
- Short Gut Syndrome: Intestinal Volvulus Secondary to Malrotation
- Short Gut Syndrome: Intestinal Volvulus Secondary to Adhesions
- Short Gut Syndrome: Intestinal Volvulus Secondary to Persistent Omphalomesenteric Duct
- Short Gut Syndrome: Gastroschisis
- Short Gut Syndrome: Massive Resection Secondary to Inflammatory Bowel Disease (Crohn’s Disease)
- Short Gut Syndrome: Massive Resection Secondary to Tumor
- Short Gut Syndrome: Massive Resection Secondary to Mesenteric Arterial Thrombosis or Embolus
- Short Gut Syndrome: Massive Resection Secondary to Mesenteric Venous Thrombosis
- Short Gut Syndrome: Specify
- Short Gut Syndrome: Unspecified
- Functional Bowel Problem: Hirschsprung’s Disease
- Functional Bowel Problem: Neuronal Intestinal Dysplasia
- Functional Bowel Problem: Pseudo-obstruction, Neuropathic
- Functional Bowel Problem: Pseudo-obstruction, Myopathic
- Functional Bowel Problem: Protein-losing Enteropathy
- Functional Bowel Problem: Microvillous Inclusion Disease
- Functional Bowel Problem: Specify
- Functional Bowel Problem: Unspecified
- Graft Failure
- Retransplant
- Other Intestinal Disease
The leading causes of intestinal failure differ between adult and pediatric populations.
In children, the following are the leading causes of intestinal failure:
- Intestinal atresia
- Gastroschisis
- Crohn disease
- Microvillus involution disease
- Necrotizing enterocolitis
- Midgut volvulus
- Chronic intestinal pseudo-obstruction
- Massive resection secondary to tumor
- Hirschsprung disease
The following are the leading causes of intestinal failure in adults:
- Crohn disease
- Superior mesenteric artery thrombosis
- Superior mesenteric vein thrombosis
- Trauma
- Desmoid tumor
- Volvulus
- Pseudo-obstruction
- Massive resection secondary to tumor
- Radiation enteritis
Additional indications for intestinal transplantation include the following:
- High risk of death
- Severe short bowel syndrome (gastrostomy, duodenostomy, residual small bowel [< 10 cm in infants, < 20 cm in adults])
- Intestinal failure with frequent hospitalizations, narcotic dependency, or pseudoobstruction
- Patient unwillingness to accept long-term parenteral nutrition
Intestine transplant contraindications
The contraindications of intestinal transplantation are essentially the same as is seen in other types of transplants. Examples include the following:
- Significant coexistent medical conditions that have no potential for improvement following transplantation
- An active uncontrolled infection or malignancy that would not be eliminated by the transplant process
- Psychosocial factors (eg, the lack of capability to assume the responsibilities of the day-to-day management following the transplant or the absence of family support)
Pretransplant workup
The evaluation of a potential recipient needs to be done by a multidisciplinary team including transplant surgery, gastroenterology, nutritional services, psychiatry, social work, anesthesia, and financial services. Further consultation with other specialties may be required.
Laboratory studies should include the following:
- Complete blood count (CBC)
- Coagulation profile
- Complete metabolic panel
- ABO blood group determination
- Human leukocyte antigen (HLA) status
- Panel reactive antibody status
- Screening for HIV and hepatitis B and C virus infection
- Serologies for cytomegalovirus (CMV) and Epstein-Barr virus (EBV)
The gastrointestinal tract should be assessed both radiologically and endoscopically. If liver disease is suspected, a liver biopsy should be performed. Since 2007, 23 points are added to patients’ Pediatric End Stage Liver Disease (PELD) score if their liver disease is due to intestinal failure 20. This is because patients with intestinal failure–associated liver disease (IFALD) have higher mortality rates on the transplant wait list.
A newer scoring system, the Pediatric Hepatology Score (PHD), has been shown to be more specific for the detection of wait list mortality than the Pediatric End Stage Liver Disease 20. Developed in the United Kingdom, this scoring system has yet to become prevalent in the United States 20.
Doppler ultrasonography or magnetic resonance venography should be performed to assess vascular access. Many patients will have at least one central venous stenosis or obstruction. Matsusaki et al reported no difference in recipient outcome between standard vascular access (percutaneous line via the upper body veins) and alternative vascular access (percutaneous line via the lower body veins; vascular access secured surgically, with interventional radiology, or using nonvenous sites) 21.
Patients with dysmotility disorders may require manometry of the stomach, esophagus, and rectum. Children with necrotizing enterocolitis require a full neurologic and pulmonary workup to exclude the possibility of associated intraventricular hemorrhage and bronchopulmonary dysplasia.
Living related donor transplantation can be discussed as an option if a potential living related donor is available. Most often, the terminal ileum is used 22. It is possible to remove the graft laparoscopically to minimize cosmetic concerns 23. The ethics of living donation are important. The risks and benefits of the procedure should be discussed, including the risk of complications from graft removal 24.
It is also important to consider that there have been mixed results when patients are surveyed about their quality of life following intestinal transplantation 25. When parents were questioned regarding their child’s quality of life, scores were lower than when children were directly surveyed. Children’s responses did not reach statistical significance when compared with a general pediatric population. Furthermore, when compared with prior quality of life assessments, patients tended to score more highly once they were transplanted.
While on the waiting list, the stable patient should be frequently reassessed, with specific attention given to any change in medical status, deterioration in liver function, or further loss of vascular access. These patients also need ongoing maintenance of their central lines to minimize line-related complications, such as infections and thrombosis.
Other tests
Plasma Citrulline
Plasma citrulline levels have emerged as a measure for overall for intestinal health. Citrulline is made almost exclusively by enterocytes. Thus, clinicians can measure citrulline trends to assess whether a patient is indeed in intestinal failure and not recovering bowel function 26. This would support a more urgent need for total parenteral nutrition (TPN), and possibly transplantation. A study by Lopez et al 27 noted that citrulline values greater than 15 micromoles/liter could predict successful withdrawal of TPN. It is important to note that citrulline is excreted from the kidneys; hence renal damage can obscure interpretation of results.
Workup for cadaveric donors
Although ABO-compatible donors can be used, ABO-identical donors are preferred in most circumstances because of the risk of graft versus host disease (GVHD). Research indicates that intestinal transplantation virtual crossmatch is comparable in terms of 1 year survival with in vitro crossmatching 28. Flow cytometry was used, survival, freedom from rejection, and graft survival were comparable in patients with a high donor-specific antibody level and controls.
If a suitable match cannot be found, patients can successfully reduce their hazardous antibody load via a protocol involving intravenous immune globulin (IVIg), and possibly plasmapheresis and rituximab prior to transplant 29. The size of the donor must be 50-75% of the size of the recipient. In certain circumstances, segments of the intestine from a larger donor may be considered.
The donor should have no previous history of significant intestinal pathology. As with all transplants, the donor should have no significant hemodynamic instability, sepsis, history of malignancy or chronic infection, severe hypoxia, or severe acidosis, and negative serology for human immunodeficiency virus (HIV) and hepatitis B and C is preferable.
Cytomegalovirus (CMV) and Epstein Barr Virus (EBV) serologic status of the donors and recipients should be taken in consideration. Transplantation from a serologically positive donor into a serologically negative recipient for either of these viruses can have serious consequences. In addition to the risk of a systemic CMV infection, CMV enteritis can occur, which can lead to graft loss. A new EBV infection combined with posttransplant immunosuppression puts the patient at high risk for developing a posttransplant lymphoproliferative disease.
Workup for living donors
A potential living donor also needs to be evaluated by a multidisciplinary team. As with any living donor procedure, possible complications including bleeding and death should be explained in great detail. The living donor should have a complete workup, including CBC, electrolytes, liver function tests, electrocardiogram, and chest radiography. The gastrointestinal tract should be endoscopically evaluated, and, if any concerns are noted, gastrointestinal contrast studies should be performed. The mesenteric vasculature should be studied to ensure that the terminal superior mesenteric artery and vein are adequate.
Complications after intestine transplantation
Rejection
The Organ Procurement and Transplant Network/Scientific Registry for Transplant Recipients annual report suggests that nearly 50% of intestine transplant recipients develop at least one episode of rejection in the first year after intestine transplantation 3. After the 2005 International Intestinal Transplant Registry report demonstrated improved patient survival in patients who received induction therapy (combined with tacrolimus immunosuppression) 30, use of induction therapy has become standard practice. In the Organ Procurement and Transplant Network/Scientific Registry for Transplant Recipients annual report (2011) over 75% of intestine transplant recipients received induction therapy 3. Although the frequent use of induction therapy has markedly decreased the rate of early severe acute rejection, the consequences of severe rejection are considerably higher than other solid organs, that is, a 50% mortality rate 31. While traditionally treatment for acute rejection with bolus steroids or anti‐lymphocyte therapy has been aimed to control the T cell–mediated response to the allograft, antibody‐mediated mechanisms in intestine rejection have achieved increasing attention 32. Although donor‐specific antibody formation in the serum of the recipient associated with graft injury is suggestive of antibody‐mediated intestine rejection (similar to other solid organ transplants), the histologic findings of antibody‐mediated rejection in the intestine are not yet well‐defined due to nonspecific C4d staining in mucosal biopsies and the absence of graft mesenteric arterial structures in the typical intestine biopsy 33. Nonetheless, graft and patient survival have been shown to correlate with factors that clearly are associated with antibody‐mediated rejection including panel reactive antibody (panel reactive antibody), donor‐specific antibody, use of induction therapy and crossmatch results 34. However, further studies are required to identify indications for use and optimal strategies for newer immunosuppressive medications targeting cytokines (e.g. infliximab), B cells (e.g. Rituximab), plasma cells (e.g. Bortezomib) and complement (e.g. Eculizimab), which are not yet well defined in intestine transplantation 35.
Infection
Sepsis is the most frequent cause of death after intestine transplantation and along with rejection, diarrhea and dehydration one of the most frequent causes for readmission to the hospital 30. Bacterial bloodstream infections occur in more than two‐thirds of intestine transplant recipients and are associated with 15% lower patient survival at 1 year than intestine recipients without bacterial infections. The source of infection is the central venous catheter in 50% and intra‐abdominal sources in 33% 36. The most common viral infection after intestine transplantation is cytomegalovirus (cytomegalovirus), historically reported to occur in 24% of recipients 37. More recently, cytomegalovirus viremia has been reported in 11% and cytomegalovirus disease in 7% of pediatric intestine transplant recipients. In those developing cytomegalovirus disease there is a high rate of relapse and an 11‐fold increased risk of posttransplant death 38.
Renal dysfunction
In 2003, Ojo et al 39 demonstrated that renal failure after nonrenal transplantation was common and that intestine transplant recipients appeared to be at the highest risk (21.3% at 5 years posttransplant) compared to all other organ transplant recipients. A recent single center study suggests improvement with only 16% of 62 intestine transplant recipients developing renal failure 40. Predictors for the development of renal failure described include pretransplant GFR below 75% of normal, preoperative intensive care and high dose of tacrolimus immunotherapy 40. No comprehensive multi‐center evaluation of renal failure after intestine transplantation has been accomplished since Ojo et al’s manuscript 39, but the increased use of induction immunotherapy has been associated with decreasing target levels for tacrolimus in recent years, which is anticipated to translate to better preservation of renal function in the future.
De novo malignancy
The most common malignancy to occur after intestine transplantation has been posttransplant lymphoproliferative disorder, occurring in 13% of recipients 41. Seventy‐one percent of posttransplant lymphoproliferative disorder episodes occur in the first year and 95% are related to Epstein‐Barr virus infection, which is especially common in children. Nonlymphoid malignancies have also been reported in 3.2% of recipients and primarily in adults 41.
Nutrition, growth and development
Most patients are weaned from parenteral nutrition and remain parenteral nutrition‐free after intestinal transplantation 42. In a study of 31 parenteral nutrition‐free pediatric patients in France 2 years posttransplant, 84% remained parenteral nutrition‐free at most recent follow‐up (average follow‐up = 7 years) 43. Oral aversion however remains problematic, with 45% of children requiring tube feedings 2 years after intestine transplantation and 30% demonstrating moderate to severe anorexia at most recent follow‐up.
Balance studies in 15 children demonstrated normal protein and carbohydrate absorption (>96–98%), but fat malabsorption was associated with significant steatorrhea (10 g/24 h in 14 samples and 5 with >20 g/24 h) and abnormal serum cholesterol levels in nearly half of recipients 43. Serum triglyceride levels were normal in all patients. Similar studies have not been reported in adult intestine transplant recipients. Despite growth failure in 23% of pediatric recipients at the time of transplant, linear growth velocity was normal in 25 of 31 recipients, but remained delayed in 5 patients posttransplant 43.
Quality of life
Quality‐of‐life has been demonstrated to be better after intestine transplantation compared to patients remaining on parenteral nutrition 44. In two studies of quality‐of‐life after intestine transplantation, the presence of G‐tubes or ostomies and repeat hospitalization were factors associated with decreased scores 44. The Pittsburgh group 45 compared quality‐of‐life before and after intestine transplantation in 76 surviving adult recipients with mixed results. Significant improvements were identified in the domains of anxiety, emotional/cognitive ability, coping skills, sleep patterns, impulse control and social support. Unfortunately, substantial worsening was identified in the domains of depression and financial obligations as well as requirement for sleep medication, forgetfulness and some decreased physical functioning. The latter three symptoms the authors attribute in part to the neurotoxicity of tacrolimus 45.
Biomarkers for allograft monitoring
In light of the need for invasive endoscopy and biopsy for routine monitoring (which frequently requires temporary creation of an ostomy with associated patient and family dissatisfaction and poor quality‐of‐life) investigations into potential biomarkers is of critical importance to further advances in the field. Early results of stool testing for calprotectin, an S‐100 protein released from infiltrating lymphocytes, has shown promise for surveillance of the intestine allograft with elevations noted in some prior to the onset of histologic changes of acute rejection and normal levels consistently associated with normal allograft histology 46.. Citrulline, a protein released from healthy intestine has likewise shown promise with levels demonstrating inverse correlation with the function of the small bowel allograft 47. Unfortunately, rapid turnaround of results is not available for either of these innovative markers and at this time routine monitoring has not been established 48. Ashokkumar et al 49 reported an alternative marker with >90% sensitivity and specificity for acute intestine allograft rejection. This apparently highly reliable marker utilizes an assessment of donor‐induced response of recipient cytotoxic T memory cells in a mixed lymphocyte reaction 49. These results have yet to be confirmed in other centers, but appear quite promising if timely results can be achieved. Farmer et al 50, reported that panel reactive antibody >20%, presence of preformed donor‐specific antibody and positive crossmatch all had adverse effects on outcome. Large scale studies of tissue typing and testing for preformed or de novo anti‐HLA antibodies are lacking in the intestine transplant population. In light of the small numbers of intestine transplants performed nationally (and specifically at any individual center), multi‐center studies will clearly be required to achieve adequate volumes to make meaningful and timely assessments of these or other potential biomarkers 49.
Tissue engineering
Although still in experimental stages, recent developments in identification and characterization of intestine stem cells and advances in tissue engineering suggest that in the future we may have realistic alternatives to cadaveric allograft transplantation. Grikscheit et al 51 has demonstrated the development of short segments of small bowel by seeding intestine stem cell “organoids” onto collagen scaffolds. In this animal model, they were able to demonstrate surgical placement of these tissue engineered intestine pieces in continuity with remnant intestine in animals that had previously undergone extensive resection demonstrating improved growth compared to control animals without the tissue engineered bowel 52. Although clearly the field is still in early stages of development, these and other important contributions characterizing the stem cells in the crypts and the factors that influence their behavior and decellularization/recellularization methods for human small intestine are likely to dramatically change the field of intestine transplantation 53. In contrast to xenografts, which have been fraught with numerous unforeseen immunologic hurdles, these modalities suggest that future efforts may be aimed more toward repair of injured intestine or “growth” of new intestine segments from autologous tissue which could obviate the need for immunosuppression.
Emerging issues
Access to care
There are two areas of concern regarding access to care that disproportionately affect intestine transplant candidates compared to recipients of other solid organ allografts. First, the number of programs actively performing intestine transplantation is dramatically fewer (18 centers in the United States in 2012 and rare centers outside the United States) compared to other solid organ transplants 3. Due to the paucity of programs, patients must often travel to other states at substantial distances from their home and due to the complexity, they must stay near the transplant center for prolonged periods of time. The rates of intestine transplantation vary considerably across the country and, for the average patient, the financial burden is difficult if not impossible to manage 54. Further, the proportion of pediatric candidates is dramatically higher for intestine transplantation relative to other solid organ grafts and the predominant payor in this population is Medicaid. As some states’ Medicaid reimbursement levels are so low, they do not cover the cost of the intestine transplant services and not all Medicaid programs will negotiate a case rate, some programs are unable to provide care to these patients further limiting the potential sites for intestine transplantation. For adult candidates, Medicare has limited coverage to programs that transplant more than 10 intestine allografts per year, further restricting access for potential adult recipients (on average fewer than five of the centers nationally each year). Finally, although organ allocation changes in (2003) have improved the availability of intestine allografts and decreased pretransplant mortality for children, adult mortality rates have remained more than double those seen in liver alone transplant candidates. Changes to the adult intestine allocation policy were implemented in June 2013 to address this disparity, however, the time has been too short to assess the impact. In addition to the frequent lack of awareness by the medical and lay community noted above, these geographical and financial barriers likely contribute to further limit access to care.
Future advances/scientific investigation
Clearly with the small number of intestine transplants performed around the world, scientific investigations are difficult to perform due to inadequate case volume at single centers. In order to gather enough patients to make meaningful and generalizable observations, it will be crucial to form multi‐institutional consortium focused on intestinal transplantation. Topics of high priority for multi‐institutional studies will include biomarkers for identification of acute rejection, delineation of antibody‐mediated rejection in intestine transplant recipients and identification of causes and contributing factors to chronic allograft loss.
Intestine transplant techniques
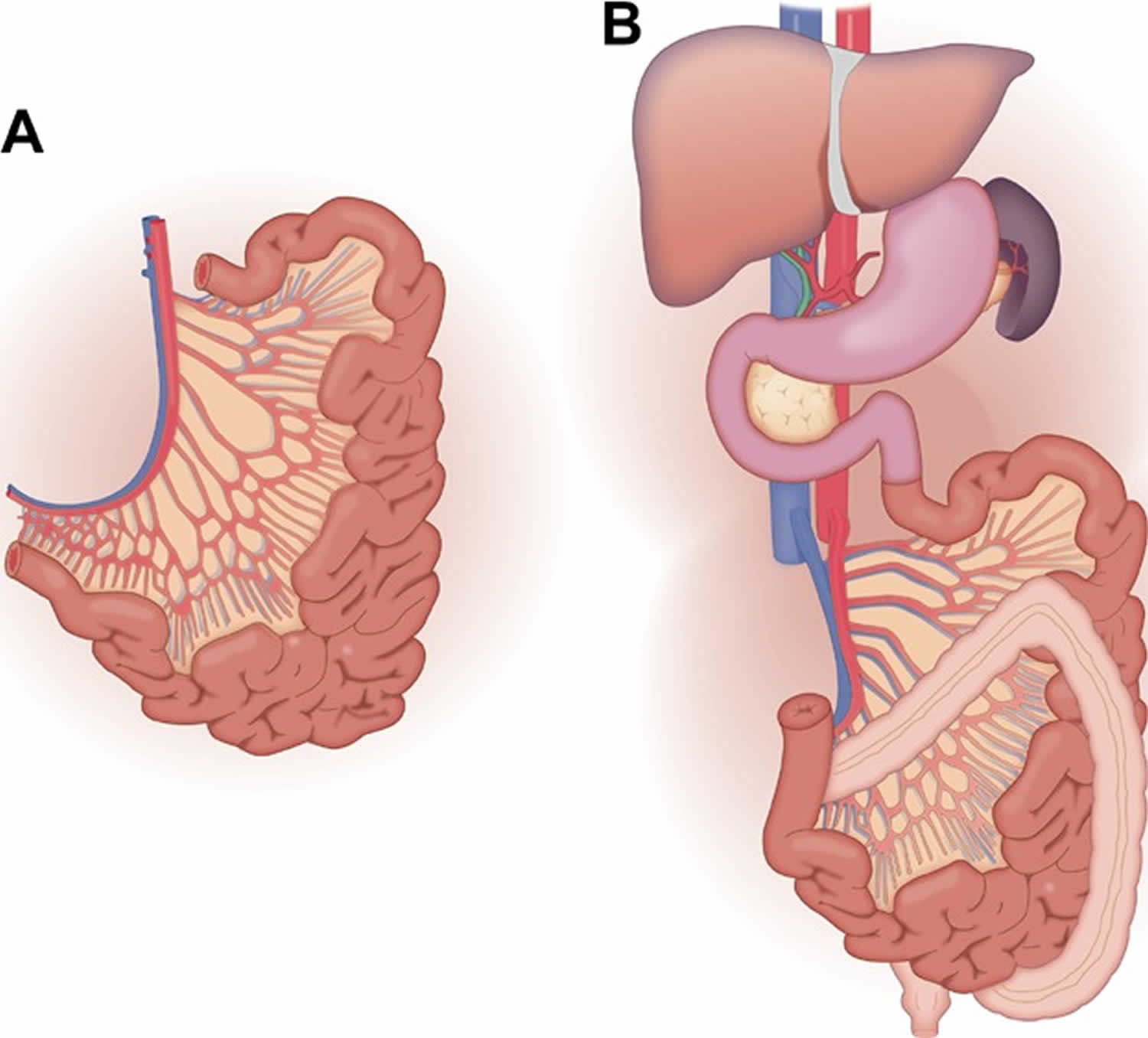
The first and most universally agreed upon type of intestine allograft is the isolated intestine, which typically includes the entire jejunum and ileum as depicted in Figure 1A. Transplantation of the intestine allograft alone (demonstrated in Figure 1B) is increasing in frequency and for the first time (in 2009) exceeded the number of combined liver and intestine transplants 3.
Figure 1. Intestine transplant
Footnote: (A) The isolated small bowel graft consists of the entire jejunum and ileum. The arterial inflow is supplied by the superior mesenteric artery and the superior mesenteric vein drains the isolated small bowel graft. (B) In the small bowel allograft recipient, gastrointestinal continuity is re‐established by anastomosis of the proximal native and donor jejunum and the distal bowel is brought out as a Bishop‐Koop (shown) or loop ileostomy with (shown) or without anastomosis to the remnant native colon.
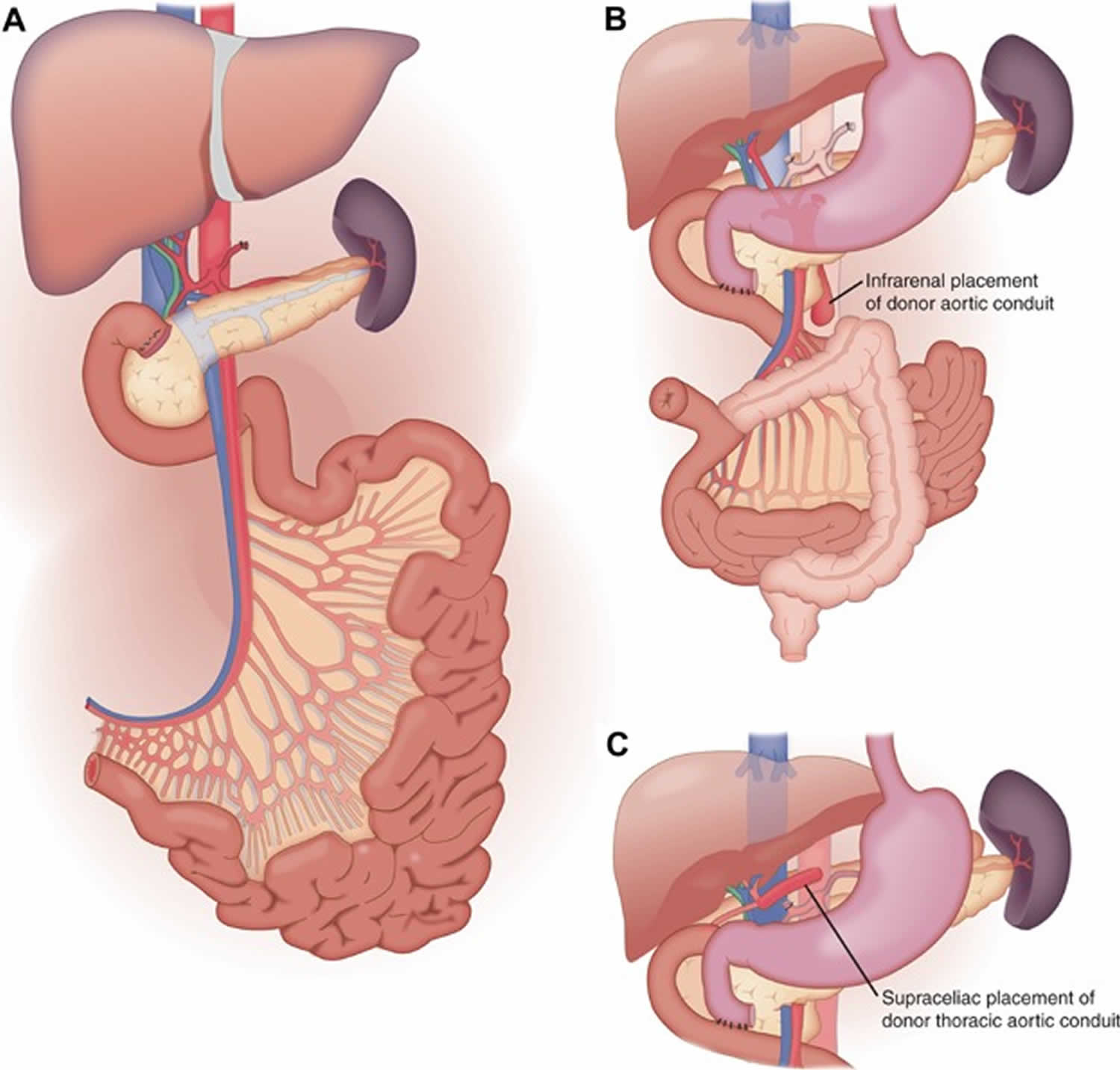
[Source 55 ]The most common combination of organs included with the intestine allograft historically was the liver and pancreas; however, the frequency has declined from 68% in 2007 to 39% of all intestine transplants in 2011 3. A widely used technique for combined liver, small bowel and pancreas allograft (referred to by various centers as a multi‐visceral graft, composite graft or Omaha technique) are to procure all these organs simultaneously from the donor and transplant them en bloc as shown in Figure 2A and B 56. The advantage of the combined liver, small bowel and pancreas allograft is that no hilar dissection of the graft is needed and therefore, there are fewer risks for donor‐related vascular or biliary complications 56. Alternatively, the liver and intestine can be transplanted as individual organs, without the pancreas graft, which has the advantage that if the intestine allograft should develop severe rejection, it could potentially be removed without requiring retransplantation of the liver 57. The disadvantage is that it requires multiple vascular anastomoses and biliary reconstruction with the attendant risk for complications.
Figure 2. Intestine transplant technique
Footnote: (A) The combined liver, small bowel and pancreas allograft is usually procured en bloc without disruption of the hilar structures. The inflow is provided from the donor aorta in continuity to the celiac axis and superior mesenteric artery. The venous outflow is from the hepatic veins/donor vena cava. (B) In the liver, small bowel and pancreas recipient operation, the thoracic aortic conduit is attached to the infrarenal (shown) or suprarenal (see C) aorta and the caval anastomosis is done either in standard caval replacement or piggyback fashion. The bowel continuity is established between native and transplanted jejunum proximally and between donor ileum and native remnant colon distally.
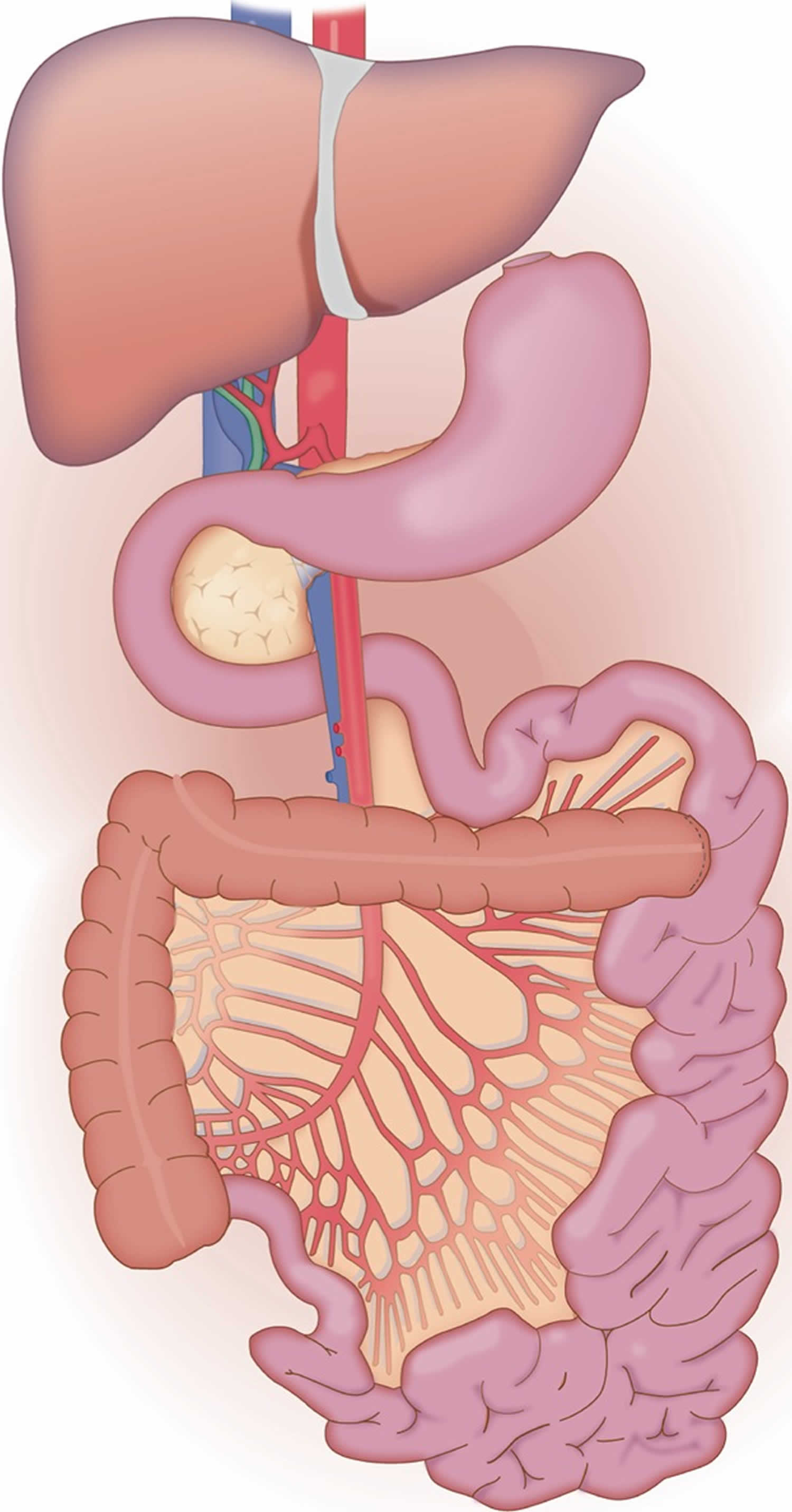
[Source 55 ]In some centers, additional gastrointestinal organs including the stomach, duodenum and/or colon may be procured and transplanted in continuity with the intestine allograft (with or without donor liver and/or pancreas) (Figure 3). The nomenclature and frequency of these technical variants may also be referred to as a multi‐visceral or modified multi‐visceral graft based on the presence or absence of the liver as part of the graft. Although some authors restrict the term multi‐visceral to grafts containing the stomach 30, others include any combination of abdominal organs. In addition, there is substantial inconsistency as to whether or not native organs are simultaneously removed and whether or not this contributes to the decision as to whether or not the term multi‐visceral is used 13. Early series had suggested that inclusion of the colon might increase the risk for infection, several recent single center series and a recent review challenge this long held assumption. In fact, inclusion of the colon has been reported in 20% of small bowel grafts over the past decade 42. Not only has inclusion of the colon been reported to be safe, but it has been associated with a decrease in the water content in the stool leading to fewer episodes of dehydration and fewer hospital readmissions 58. The inclusion of stomach with the allograft for patients with or without upper abdominal exenteration remains a center‐specific decision with some centers applying it universally while others rarely or never including the stomach. Objective data regarding either benefit or harm with inclusion of the stomach has not been demonstrated.
Figure 3. Multivisceral transplant
Footnote: The multi‐visceral allograft is similar to the liver, small bowel and pancreas graft with the addition of the stomach and/or the colon in continuity.
[Source 55 ]Intestine transplant prognosis
The field of intestine transplantation has maintained a registry, the International Intestinal Transplant Registry, reporting outcomes every 2 years and although voluntary boasts 100% participation of intestine transplant centers worldwide. In 2005, the report of the International Intestinal Transplant Registry was published summarizing the results of nearly 1000 intestinal transplant procedures (from 61 centers in 19 countries) 30. This was updated by presentation in 2013 at the meeting of the Intestinal Transplantation Association in Oxford, England to describe the outcomes of 2887 intestine transplant recipients from 87 centers 42. When examined over time, the results for overall graft survival have continued to improve in 5‐year increments for pediatric recipients, although the graft survival in adult recipients has been stable over the last 10 years 42. Historically the best 1‐year patient/graft survival was notably in recipient’s of an isolated intestine transplant (77%/65%, respectively, which compares favorably to 60%/59% in liver/small bowel recipients) 30. Experienced single center reports demonstrate improved 1‐year patient survival of 86–93% for isolated intestine transplantation 59. Unfortunately, the early graft and patient survival advantage does not appear to be sustained long term. Instead, liver‐containing small bowel allograft recipients have demonstrated better graft survival more than 5 years after transplantation than isolated bowel grafts 50. For pediatric recipients of isolated small bowel grafts, the advantage is less clear. In the most recent era (from 2008 to 2012), although the survival gap appears to narrow over time, better 3‐year patient survival is maintained for isolated intestine recipients compared to recipients of other graft types 42. The 2005 IITR further demonstrated a positive correlation between center volume (>10 intestine transplant procedures) and outcomes (improved recipient survival) as has been demonstrated in other fields of surgery 30. Other factors that impact patient and graft survival are unclear. Single center studies, for example, have reported conflicting outcomes from recipient splenectomy at the time of intestine transplantation including lower risk for posttransplant lymphoproliferative disorder (PTLD) and allograft rejection versus worsened patient survival 50. Large and multi‐centered studies to evaluate the factors that impact patient and graft survival (particularly long term) are necessary.
References- Intestine. https://optn.transplant.hrsa.gov/data/organ-datasource/intestine/
- Organ Procurement and Transplantation Network. National Data. OPTN. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data
- Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2011 annual data report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, 2012.
- Cai J, Wu G, Qing A, Everly M, Cheng E, Terasaki P. Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients 2014 Data Report: Intestine. Clin Transpl. 2014. 33-47.
- Varkey J, Simrén M, Jalanko H, Oltean M, Saalman R, Gudjonsdottir A, et al. Fifteen years’ experience of intestinal and multivisceral transplantation in the Nordic countries. Scand J Gastroenterol. 2015 Mar. 50 (3):278-90.
- Organ Procurement and Transplantation Network(OPTN). https://optn.transplant.hrsa.gov/data/organ-datasource/intestine
- Khan K, Desai C, Mete M, et al. Developing trends in the intestinal transplant waitlist. Am J Transpl 2014;14(12):2830.
- Matsumoto CS, Subramanian S, Fishbein TM. Adult Intestinal Transplantation. Gastroenterol Clin North Am. 2018;47(2):341–354. doi:10.1016/j.gtc.2018.01.011 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6433128
- Organ Procurement and Transplantation Network (OPTN). Policy 9.1.F liver and intestine candidates. Effective Date September 12, 2017. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf
- Kaufmann SS, Atkinson JB, Bianchi A, et al. Indications for pediatric intestinal transplantation: A position paper of the American Society of Transplantation. Pediatr Transplant 2001; 5: 80–87.
- Department of Health and Human Services. Centers for Medicare and Medicaid Services. Program memorandum intermediaries/carriers. Intestinal and multi‐visceral transplantation. AB‐02‐040. 2002.
- Zamvar V, Lazonby G, Puntis JW. Recurrent life‐threatening sepsis in intestinal failure: Transplantation or foster care? Arch Dis Child 2013; 98: 556–557.
- Mangus RS, Tector AJ, Kubal CA, Fridell JA, Vianna RM. Multivisceral transplantation: Expanding indications and improving outcomes. J Gastrointest Surg 2013; 17: 179–186; discussion 186–187.
- Intestinal Transplantation. https://emedicine.medscape.com/article/1013245-overview
- Kumpf VJ. Parenteral nutrition-associated liver disease in adult and pediatric patients. Nutr Clin Pract. 2006 Jun. 21(3):279-90.
- Reyes JD. Intestinal transplantation: an unexpected journey. J Pediatr Surg. 2014 Jan. 49(1):13-8.
- Iyer KR. Surgical management of short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014 May. 38(1 Suppl):53S-59S.
- Pitha J, Teplan V, Kalousova M, Racek J, Stollova M, Marecková O. Gender-Related Determinants of Advanced Subclinical Atherosclerosis in Patients Undergoing Kidney Transplantation. Kidney Blood Press Res. 2010 Jun 24. 33(3):227-234.
- Primeggia J, Matsumoto CS, Fishbein TM, Karacki PS, Fredette TM, Timpone JG. Infection among adult small bowel and multivisceral transplant recipients in the 30-day postoperative period. Transpl Infect Dis. 2013 Oct. 15(5):441-8.
- Beath SV, Davies P, Mukherjee A, Lloyd C, Sharif K, McKiernan PJ. A comparison of two validated scores for estimating risk of mortality of children with intestinal failure associated liver disease and those with liver disease awaiting transplantation. Clin Res Hepatol Gastroenterol. 2014 Feb. 38(1):32-9.
- Matsusaki T, Sakai T, Boucek CD, Abu-Elmagd K, Martin LM, Amesur N, et al. Central venous thrombosis and perioperative vascular access in adult intestinal transplantation. Br J Anaesth. 2012 Feb 23.
- Li M, Ji G, Feng F, Song W, Ling R, Chen D. Living-related small bowel transplantation for three patients with short gut syndrome. Transplant Proc. 2008 Dec. 40(10):3629-33.
- Kim WW, Gagner M, Fukuyama S, Hung TI, Biertho L, Jacob BP, et al. Laparoscopic harvesting of small bowel graft for small bowel transplantation. Surg Endosc. 2002 Dec. 16(12):1786-9.
- Panocchia N, Bossola M, Silvestri P, Midolo E, Teleman AA, Tazza L. Ethical evaluation of risks related to living donor transplantation programs. Transplant Proc. 2013 Sep. 45(7):2601-3.
- Abu-Elmagd KM, Kosmach-Park B, Costa G, Zenati M, Martin L, Koritsky DA. Long-term survival, nutritional autonomy, and quality of life after intestinal and multivisceral transplantation. Ann Surg. 2012 Sep. 256(3):494-508.
- Diamanti A, Panetta F, Gandullia P, Morini F, Noto C, Torre G. Plasma citrulline as marker of bowel adaptation in children with short bowel syndrome. Langenbecks Arch Surg. 2011 Oct. 396(7):1041-6.
- Vecino López R, Andrés Moreno AM, Ramos Boluda E, Martinez-Ojinaga Nodal E, Hernanz Macías A, Prieto Bozano G, et al. [Plasma citrulline concentration as a biomarker of intestinal function in short bowel syndrome and in intestinal transplant]. An Pediatr (Barc). 2013 Oct. 79(4):218-23.
- Hawksworth JS, Rosen-Bronson S, Island E, Girlanda R, Guerra JF, Valdiconza C. Successful isolated intestinal transplantation in sensitized recipients with the use of virtual crossmatching. Am J Transplant. 2012 Dec. 12 Suppl 4:S33-42.
- Gondolesi G, Blondeau B, Maurette R, Hoppenhauer L, Rodriguez-Laiz G, Schiano T. Pretransplant immunomodulation of highly sensitized small bowel transplant candidates with intravenous immune globulin. Transplantation. 2006 Jun 27. 81(12):1743-6.
- Grant D, Abu‐Elmagd K, Reves J, et al. 2003 report of the intestine transplant registry: A new era has dawned. Ann Surg 2005; 241: 604–613.
- Lauro A, Bagni C, Zanfi S, et al. Mortality after steroid‐resistant acute cellular rejection and chronic rejection episodes in adult intestinal transplants: Report from a single center in induction/preconditioning era. Transplant Proc 2013; 45: 2032–2033.
- Abu‐Elmaqd KM, Wu G, Costa G, et al. Preformed and de novo donor specific antibodies in visceral transplantation: Long‐term outcome with special reference to the liver. Am J Transplant 2012; 12: 3047–3060.
- Gerlach UA, Lachmann N, Sawitzki B, et al. Clinical relevance of the de novo production of anti‐HLA antibodies following intestinal and multivisceral transplantation. Transpl Int 2014; 27: 280–289.
- Kubal CA, Mangus RS, Vianna RM, et al. Impact of positive flow cytometry crossmatch on outcomes of intestinal/multivisceral transplantation: Role anti‐IL‐2 receptor antibody. Transplantation 2013; 95: 1160–1166.
- De Greef E, Avitzur Y, Grant D, et al. Infliximab as salvage therapy in paediatric intestinal transplant with steroid‐and thymoglobulin‐resistant late acute rejection. J Pediatr Gastroenterol Nutr 2012; 54: 565–567.
- Florescu DF, Qui F, Langnas AN, et al. Bloodstream infections during the first year after pediatric small bowel transplantation. Pediatr Infect Dis J 2001; 31: 700–704.
- Bueno J, Green M, Kocoshis S, et al. Cytomegalovirus infection after intestinal transplantation in children. Clin Infect Dis 1997; 25: 1078–1083.
- Florescu DF, Langnas AN, Sandkovksy U. Opportunistic viral infections in intestinal transplantation. Expert Rev Anti Infect Ther 2014; 11: 367–381.
- Ojo AO, Held PJ, Wolfe RA, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349: 931–940.
- Watson MJ, Venick RS, Kaldas F, et al. Renal function impacts outcomes after intestinal transplantation. Transplantation 2008; 86: 117–122.
- Abu‐Elmagd KM, Mazariegos G, Costa G, et al. Lymphoproliferative disorders and de novo malignancies in intestinal and multivisceral recipients: Improved outcomes with new outlooks. Transplantation 2009; 88: 926–934.
- ITR. 2013 bi‐annual report. In: Grant D, eds. Intestinal Transplant Registry. Toronto, ON: Intestinal Transplant Association, 2014.
- Lacaille F, Vass N, Sauvat F, et al. Long‐term outcome, growth and digestive function in children 2 to 18 years after intestinal transplantation. Gut 2008; 57: 455–461.
- Pironi L, Baxter JP, Lauro A, et al. Assessment of quality of life on home parenteral nutrition and after intestinal transplantation using treatment‐specific questionnaires. Am J Transplant 2012; 12 (Suppl 4): S60–S66.
- Abu‐Elmagd KM, Kosmach‐Park B, Costa G, et al. Long‐term survival, nutritional autonomy, and quality of life after intestinal and multivisceral transplantation. Ann Surg 2012; 256: 494–508.
- Akpinar E, Vargas J, Kato T, et al. Fecal calprotectin level measurements in small bowel allograft monitoring: A pilot study. Transplantation 2008; 85: 1281–1286.
- Andersen DA, Horslen S. An analysis of the long‐term complications of intestine transplant recipients. Prog Transplant 2004; 14: 277–282.
- Hibi T, Nishida S, Garcia J, et al. Citrulline level is a potent indicator of acute rejection in the long term following pediatric intestinal/multivisceral transplantation. Am J Transplant 2013; 12 (Suppl 4): S27–S32.
- Ashokkhumar C, Gupta A, Sun Q, et al. Allospecific CD154+ T cells identify rejection‐prone recipients after pediatric small‐bowel transplantation. Surgery 2009; 146: 166–173.
- Farmer DG, Venick RS, Colangelo J, et al. Pretransplant predictors of survival after intestinal transplantation: Analysis of a single‐center experience of more than 100 transplants. Transplantation 2010; 90: 1574–1580.
- Grikscheit TC, Siddique A, Ochoa ER, et al. Tissue‐engineered small intestine improves recovery after massive small bowel resection. Ann Surg 2004; 240: 748–754.
- Sala FG, Kunisaki SM, Ochoa ER, et al. Tissue‐engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J Surg Res 2009; 156: 205–212.
- Patil PB, Chougule PB, Kumar VK, et al. Recellularization of acellular human small intestine using bone marrow stem cells. Stem Cells Transl Med 2013; 2: 307–315.
- Mazariegos GV, Steffick DE, Horslen S, et al. Intestine transplantation in the United States, 1999–2008. Am J Transplant 2010; 10: 1020–1034.
- Sudan, D. (2014), The Current State of Intestine Transplantation: Indications, Techniques, Outcomes and Challenges. American Journal of Transplantation, 14: 1976-1984. doi:10.1111/ajt.12812
- Sudan DL, Iver KR, Deroover A, et al. A new technique for combined liver/small intestinal transplantation. Transplantation 2001; 72: 1846–1848.
- Fishbein T, Florman S, Gondolesi G, Decker R. Noncomposite simultaneous liver and intestinal transplantation. Transplantation 2003; 75: 564–565.
- Matsumoto CA, Kaufman SS, Fishbein TM. Inclusion of the colon in intestinal transplantation. Curr Opin Organ Transplant 2011; 16: 312–315.
- Kato T, Mittal N, Nishida S, et al. The role of intestinal transplantation in the management of babies with extensive gut resections. J Pediatr Surg 2003; 38: 145–149.