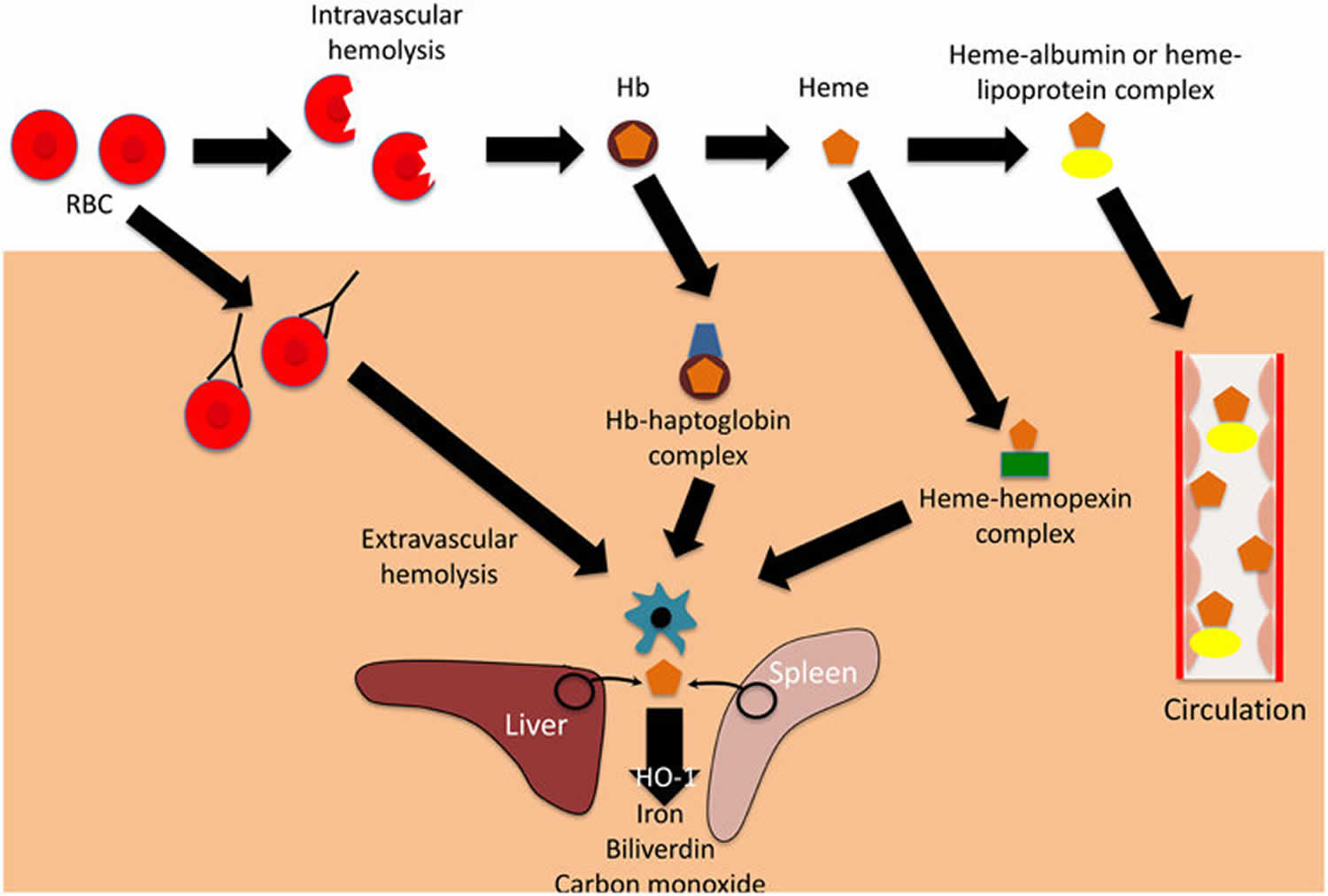
Intravascular hemolysis
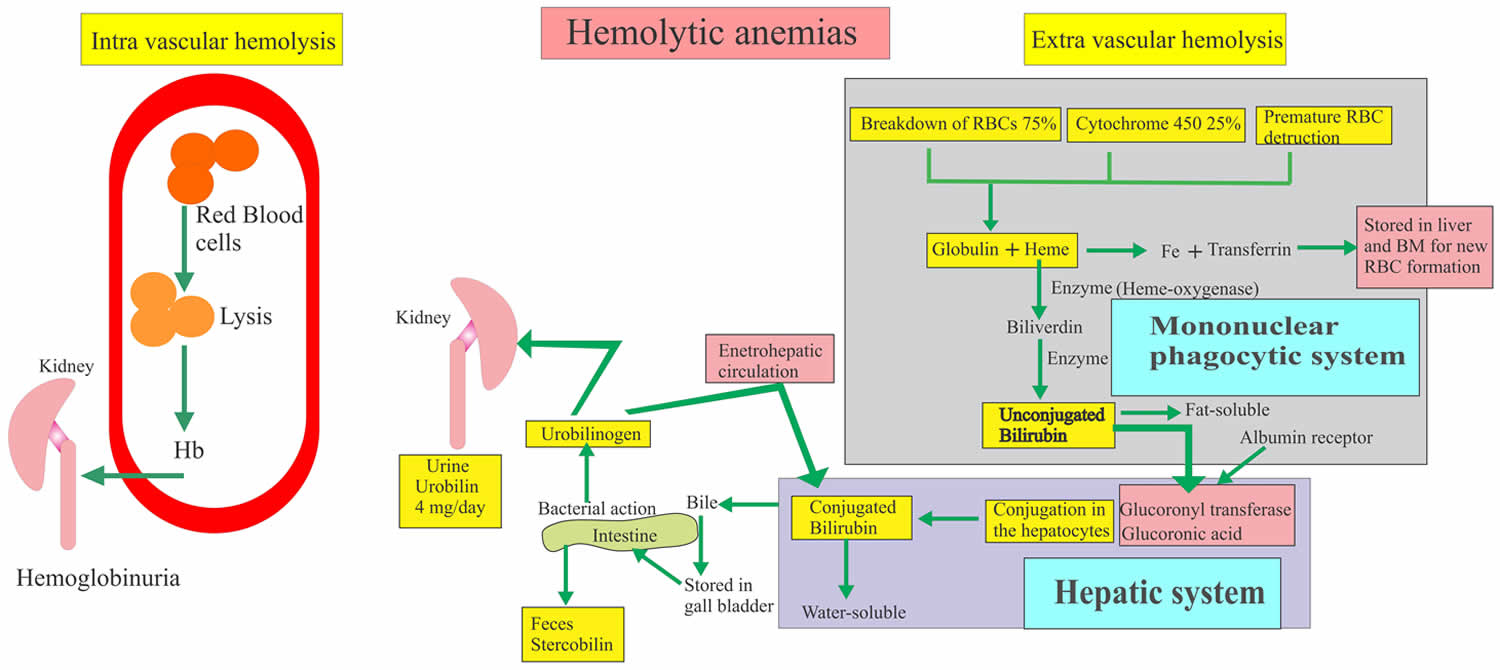
Hemolysis is the premature destruction of red blood cells (RBCs) before the end of their normal life span, and hemolytic anemia occurs when the production of new red blood cells from bone marrow fails to compensate for this loss of red blood cells 1. The causes of hemolysis can be broadly divided into disorders intrinsic or extrinsic to the red blood cell and the location of hemolysis can be subdivided into intravascular (within blood vessels) or extravascular (outside of the blood vessels) (Figure 1). Intravascular hemolysis when the destruction of the red blood cells takes place in the blood vessels and hemoglobin is released. Hemoglobin in the blood gives rise to hemoglobinuria. Intravascular hemolysis are caused by the following: prosthetic cardiac valves, glucose-6-phosphate dehydrogenase (G6PD) deficiency,sickle cell disease, thrombotic thrombocytopenic purpura, disseminated intravascular coagulation, transfusion of ABO incompatible blood and paroxysmal nocturnal hemoglobinuria (PNH) 2. Extravascular hemolysis when the destruction of the red blood cells takes place in the spleen and other reticuloendothelial tissues (also known as mononuclear phagocytic system). Autoimmune hemolytic anemia and hereditary spherocytosis are examples of extravascular hemolysis 3.
Red blood cells normally live for 110 to 120 days. After that, they naturally break down and are most often removed from the circulation by the spleen. Some diseases and processes cause red blood cells to break down too soon. This requires the bone marrow to make more red blood cells than normal. The balance between red blood cell breakdown and production determines how low the red blood cell count becomes. If red blood cells destruction rate is high enough to determine a decrease in hemoglobin values below the normal range, hemolytic anemia occurs.
Most causes of pathological hemolys is occur in the extravascular compartment, primarily in the spleen. Macrophages and other specialized phagocytic cells of the reticuloendothelial system remove defective red blood cells from the circulation. Intravascular hemolysis follows substantial damage to the red blood cell membrane. An important distinction between these processes is the fate of the red blood cell contents, particularly the heme moiety of hemoglobin (Hb) and its iron. Iron is an essential nutrient for pathogen and host, and access to iron within the body is the focus of an intense evolutionary battle 4. In extravascular hemolysis red blood cell contents become localized within reticuloendothelial cells, whereas in intravascular hemolysis hemoglobin (Hb) enters the circulation and can interact with all molecules and cells in contact with the blood 5.
Figure 1. Intravascular hemolysis and extravascular hemolysis
Footnote: Mechanisms and consequences of hemolysis. The fate of the contents of red blood cells (RBCs) depends on whether hemolysisis is extravascular or intravascular. Following intravascular hemolysis, hemoglobion (Hb) is bound by haptoglobin and taken up by monocytes and macrophages. When haptoglobin is depleted, heme is released from hemoglobion (Hb) and is bound by hemopexin.The heme-hemopexin complex is primarily cleared by macrophages and hepatocytes. If hemolysis overwhelms the capacity of both haptoglobin and hemopexin, heme remains within the circulation, weakly binding to albumin and lipoproteins, and can interact with other cell types. In extravascular hemolysis, red blood cells are removed by phagocytic cells, primarily in the spleen and liver. Heme released from both intra-and extra-vascular hemolysis induces the expression of hemeoxygenase-1 (HO-1), which degrades heme to iron, biliverdin and carbon monoxide.
[Source 6 ]Causes of intravascular hemolysis
Intravascular hemolysis can be caused by following:
- Prosthetic cardiac valves
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency
- Thrombotic thrombocytopenic purpura
- Disseminated intravascular coagulation
- Transfusion of ABO incompatible blood
- Paroxysmal nocturnal hemoglobinuria (PNH)
- Sickle cell disease 7
Intravascular hemolysis symptoms
Signs and symptoms of hemolytic anemia are diverse and are due to anemia, the extent of compensation, previous treatment, and the underlying disorder. Patients with minimal or long-standing hemolytic anemia may be asymptomatic, and hemolysis is often found incidentally during routine laboratory testing. Depending on the causes of your anemia, you might have no symptoms. Signs and symptoms, if they do occur, might include:
- In intravascular hemolysis, iron deficiency due to chronic hemoglobinuria can exacerbate anemia and weakness.
- Fatigue
- Weakness
- Pale or yellowish skin
- Irregular heartbeats
- Tachycardia, dyspnea, angina, and weakness occur in patients with severe anemia, as cardiac function is sensitive to anoxia.
- Shortness of breath
- Dizziness or lightheadedness
- Chest pain
- Cold hands and feet
- Headaches
- Dark urine may be due to hemoglobinuria.
- Persistent hemolysis may result in the development of bilirubin gallstones; these patients may present with abdominal pain.
- In patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency, hemolysis can be triggered by oxidant drugs and stress from infections. Fava beans can induce hemolysis in susceptible individuals with the Mediterranean variant of G6PD deficiency.
- Leg ulcers may develop in patients with sickle cell anemia and other hemolytic disorders, as a result of decreased red blood cell (RBC) deformability and endothelial changes.
- In addition to hemolysis, patients with thrombotic thrombocytopenic purpura (TTP) may experience fever, neurologic signs, renal failure, and thrombocytopenia.
- Symptoms of disseminated intravascular coagulation (DIC) may include any of the following:
- Bleeding, from many sites in the body
- Blood clots
- Bruising
- Drop in blood pressure
- Shortness of breath
- Confusion, memory loss or change of behavior
- Fever
At first, anemia can be so mild that you don’t notice it. But symptoms worsen as anemia worsens.
Make an appointment with your doctor if you’re feeling fatigued and you don’t know why.
Fatigue has many causes besides anemia, so don’t assume that if you’re tired you must be anemic. Some people learn that their hemoglobin is low, which indicates anemia, when they donate blood. If you’re told that you can’t donate because of low hemoglobin, make an appointment with your doctor.
Intravascular hemolysis complications
Left untreated, anemia can cause many health problems, such as:
- Severe fatigue. Severe anemia can make you so tired that you can’t complete everyday tasks.
- Pregnancy complications. Pregnant women with folate deficiency anemia may be more likely to have complications, such as premature birth.
- Heart problems. Anemia can lead to a rapid or irregular heartbeat (arrhythmia). When you’re anemic your heart must pump more blood to make up for the lack of oxygen in the blood. This can lead to an enlarged heart or heart failure.
- Death. Some inherited anemias, such as sickle cell anemia, can lead to life-threatening complications. Losing a lot of blood quickly results in acute, severe anemia and can be fatal.
Intravascular hemolysis diagnosis
To diagnose anemia, your doctor is likely to ask you about your medical and family history, perform a physical exam, and run the following tests. Standard blood studies for the workup of suspected hemolytic anemia include the following:
- Complete blood cell count (CBC). A complete blood cell count is used to count the number of blood cells in a sample of your blood. For anemia, your doctor will be interested in the levels of the red blood cells contained in your blood (hematocrit) and the hemoglobin in your blood. Normal adult hematocrit values vary among medical practices but are generally between 40% and 52% for men and 35% and 47% for women. Normal adult hemoglobin values are generally 14 to 18 grams per deciliter for men and 12 to 16 grams per deciliter for women.
- Peripheral blood smear
- Serum lactate dehydrogenase (LDH)
- Serum haptoglobin
- Indirect bilirubin
Hemolysis of collected blood is more likely to occur in standard large vacuum tubes than in small tubes. Thus, it might better to collect blood for these tests in small vacuum tubes, since hemolysis of collected blood can skew the results of these tests 8.
Changes in the lactate dehydrogenase (LDH) and serum haptoglobin levels are the most sensitive general tests because the indirect bilirubin is not always increased.
Hemolytic anemia shows the following lab changes:
- Hemoglobin (Hb) is reduced and may be mild to moderate decreased from 6 to 10 G/dl.
- Reticulocytes are increased from 5 to 20 % (Reticulocytosis).
- MCV is normal or slightly increased,
- MCHC is increased.
- Bone marrow shows erythroid hyperplasia.
- Normal myeloid: erythroid ratio of 2:1 to 12:1 is reduced to 1:1 or much more reduced.
- Coombs test direct is negative which differentiate it from autoimmune hemolytic anemia.
- Peripheral blood smear shows poikilocytosis and polychromatophilia due to reticulocytes.
- There are elliptocytes and fragmented red blood cells.
- There is normochromic and normocytic anemia picture.
- RDW is increased due to anisocytosis and poikilocytosis. Unlike other normochromic and normocytic anemias.
- Increased reticulocytes lead to increased MCV but not like megaloblastic anemias.
- Serum bilirubin is raised.
- Urine urobilinogen is positive.
- Stercobilinogen is increased
- Serum haptoglobin is absent.
Other laboratory studies may be directed by history, physical examination, peripheral smear, and other laboratory findings. Ultrasonography is used to estimate the spleen size, since the physical examination occasionally does not detect significant splenomegaly. Chest radiography, electrocardiography (ECG), and other studies are used to evaluate cardiopulmonary status.
Intravascular hemolysis treatment
Intravascular hemolytic anemia treatment depends on the cause.
- Sickle cell anemia. Treatment might include oxygen, pain relievers, and oral and intravenous fluids to reduce pain and prevent complications. Doctors might also recommend blood transfusions, folic acid supplements and antibiotics. A cancer drug called hydroxyurea (Droxia, Hydrea, Siklos) also is used to treat sickle cell anemia.
- Disseminated intravascular coagulation (DIC). There is no specific treatment for DIC. The goal is to determine and treat the underlying cause of disseminated intravascular coagulation (DIC). Supportive treatments may include:
- Plasma transfusions to replace blood clotting factors if a large amount of bleeding is occurring.
- Blood thinner medicine (heparin) to prevent blood clotting if a large amount of clotting is occurring.
- Thrombotic thrombocytopenic purpura (TTP) can be fatal or cause lasting damage, such as brain damage or a stroke, if it’s not treated right away. In most cases, thrombotic thrombocytopenic purpura occurs suddenly and lasts for days or weeks, but it can go on for months. Relapses (flareups) can occur in up to 60 percent of people who have acquired thrombotic thrombocytopenic purpura. Flareups also occur in most people who have inherited thrombotic thrombocytopenic purpura. Plasma exchange treatments are the most common way to treat thrombotic thrombocytopenic purpura. Other treatments include medicines and surgery. Treatments are done in a hospital.
- Plasma exchange treatment removes your abnormal plasma and replaces it with normal plasma from a healthy donor. Plasma is the liquid part of blood that contains blood cells and platelets. Plasma exchange also replaces the missing enzyme. The procedure is done as follows:
- First, you have your blood drawn as if donating blood. As the blood is passed through a machine that separates blood into its different parts, the abnormal plasma is removed and your blood cells are saved.
- Your blood cells are then combined with normal plasma from a donor, and then given back to you.
- This treatment is repeated daily until blood tests show improvement.
- People who do not respond to this treatment or whose condition often returns may need to:
- Have surgery to remove their spleen
- Get medicines that suppress the immune system, such as steroids or rituximab
- Plasma exchange treatment removes your abnormal plasma and replaces it with normal plasma from a healthy donor. Plasma is the liquid part of blood that contains blood cells and platelets. Plasma exchange also replaces the missing enzyme. The procedure is done as follows:
- Paroxysmal nocturnal hemoglobinuria (PNH): Paroxysmal nocturnal hemoglobinuria is treated with steroids or other medicines that suppress the immune system may help slow the breakdown of red blood cells. Blood transfusions may be needed. Supplemental iron and folic acid are provided. Blood thinners may also be needed to prevent clots from forming. Soliris (eculizumab) is a drug used to treat paroxysmal nocturnal hemoglobinuria. It blocks the breakdown of red blood cells. Bone marrow transplantation can cure paroxysmal nocturnal hemoglobinuria. It may also stop the risk of developing paroxysmal nocturnal hemoglobinuria in people with aplastic anemia. All people with paroxysmal nocturnal hemoglobinuria should receive vaccinations against certain types of bacteria to prevent infection. Ask your health care provider which ones are right for you.
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency treatment may involve:
- Medicines to treat an infection, if present
- Stopping any drugs that are causing red blood cell destruction
- Transfusions, in some cases.
- Guillaud,C.,Loustau,V.,andMichel,M.(2012).Hemolyticanemiain adults: maincausesanddiagnosticprocedures. Exp.Rev.Hem. 5,229–241.doi: 10.1586/ehm.12.3
- Hemolytic Anemia. https://emedicine.medscape.com/article/201066-overview
- Coetzer TI. Erythrocyte Membrane Disorders. Kaushansky K, Lichtman MA, Prchal JT, Levi MM, Press OW, Burns LJ, Caligiuri MA, eds. Williams Hematology. 9th ed. New York, NY: McGraw-Hill Education; 2016. 661-88.
- Barber,M.F.,and Elde,N.C.(2014).Nutritional immunity. Escape from bacterial iron piracy through rapid evolution of transferrin. Science 346,1362–1366.doi: 10.1126/science.1259329
- Schaer,D.J.,Buehler,P.W.,Alayash,A.I.,Belcher,J.D.,and Vercellotti,G. M.(2013). Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121, 1276–1284.doi:10.1182/blood-2012-11-451229
- Orf, Katharine & Cunnington, Aubrey. (2015). Infection-related hemolysis and susceptibility to Gram-negative bacterial co-infection. Frontiers in microbiology. 6. 666. 10.3389/fmicb.2015.00666
- Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127(3):750–760. doi:10.1172/JCI89741 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5330745
- Phelan MP, Reineks EZ, Berriochoa JP, Schold JD, Hustey FM, Chamberlin J, et al. Impact of Use of Smaller Volume, Smaller Vacuum Blood Collection Tubes on Hemolysis in Emergency Department Blood Samples. Am J Clin Pathol. 2017 Oct 1. 148 (4):330-335.