Lymphogranuloma venereum
Lymphogranuloma venereum is an ulcerative disease of the genital area that is caused by the gram-negative bacteria Chlamydia trachomatis, especially serovars L1, L2, and L3 1. Lymphogranuloma venereum is an uncommon, sexually transmitted disease (STD). Lymphogranuloma venereum is transmittable by vaginal, oral or anal sex. There are increasing numbers of reports in men who have sex with men 2.
Characteristically, lymphogranuloma venereum has three stages of infection 3:
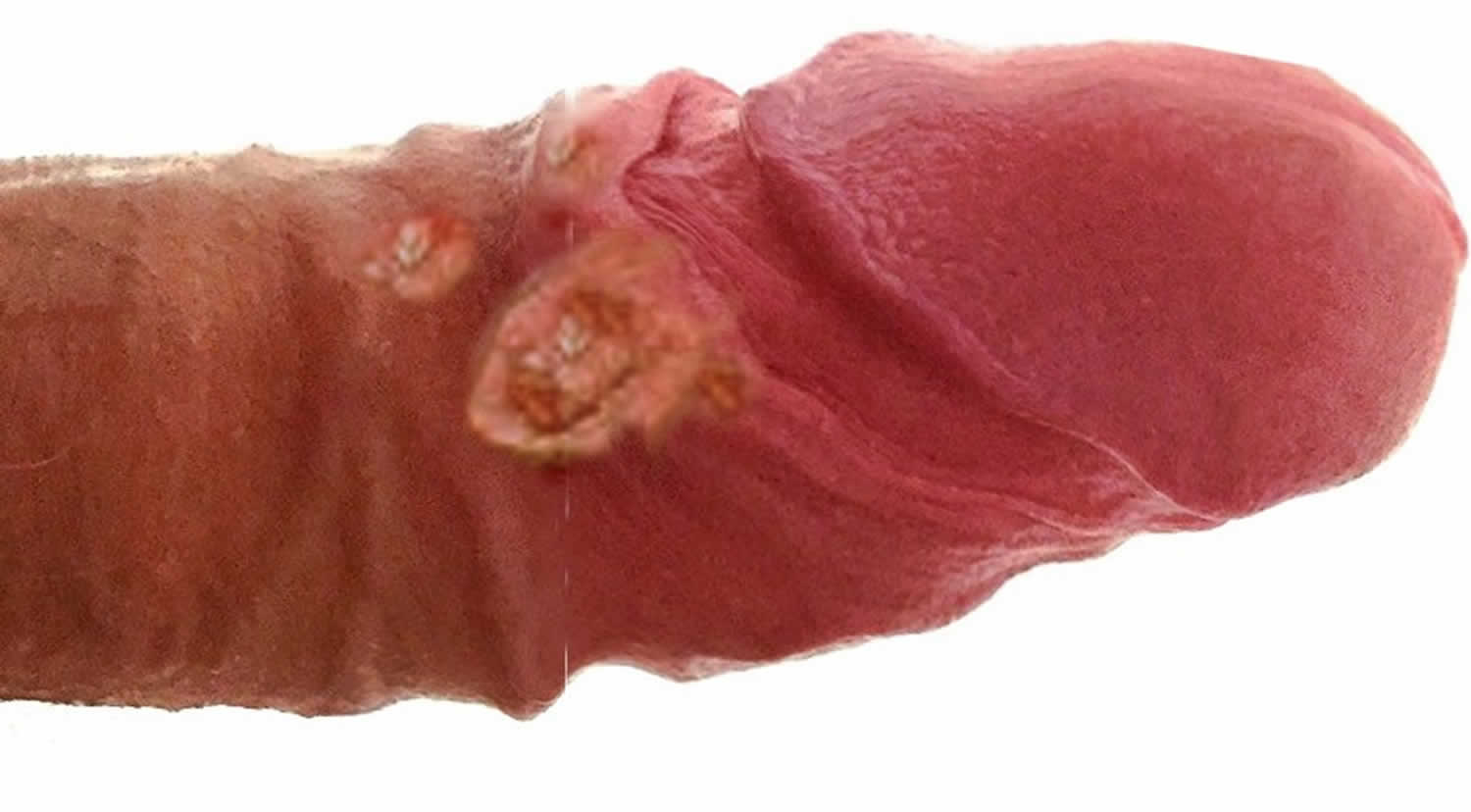
- Primary stage characterized by the development of painless genital ulcer or papules.
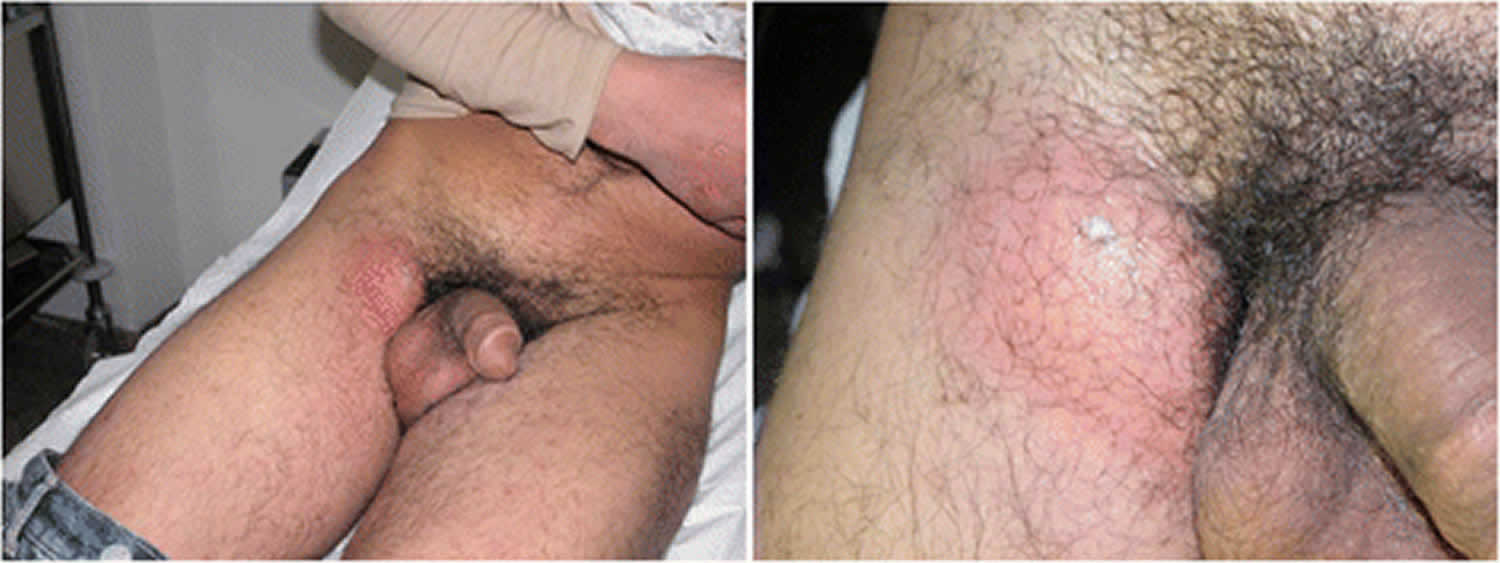
- Secondary stage with the development of unilateral or bilateral tender inguinal and/or femoral lymphadenopathy (also called buboes).
- Late stage with strictures, fibrosis, and fistulae of the anogenital area.
Lymphogranuloma venereum is common in the tropical and subtropical regions around the world (e.g. Africa, Asia, the Caribbean, Central and South America). Lymphogranuloma venereum is rare in the United States 4. Lymphogranuloma venereum may occur at any age; however, the highest incidence of lymphogranuloma venereum is in the sexually active population between 15 and 40 years. Lymphogranuloma venereum probably affects both sexes equally although it is more commonly reported in men because early manifestations of lymphogranuloma venereum are more apparent in men 5. Lymphogranuloma venereum is endemic to men who have sex with men. There is a significant association between HIV-infected patients and lymphogranuloma venereum 6. Men typically present with the acute form of the lymphogranuloma venereum disease whereas women often present when they develop complications from later stages of the lymphogranuloma venereum disease 5.
Figure 1. Lymphogranuloma venereum ulcer
Lymphogranuloma venereum causes
Lymphogranuloma venereum is a long-term (chronic) infection of the lymphatic system caused by Chlamydia trachomatis, serovars L1, L2, L3 7. The bacteria are spread by sexual contact. The infection is not caused by the same bacteria that cause genital chlamydia. Chlamydia trachomatis serovars extend from the primary infection site to the regional lymph nodes and cause a lymphoproliferative reaction, facilitated by binding of Chlamydia trachomatis to the epithelial cells. Binding occurs via heparin sulfate receptors 8.
Chlamydia is a ubiquitous ovoid or obligate intracellular bacteria with a cell wall and ribosomes similar to those of gram-negative organisms 9. The Chlamydia trachomatis cell wall is unique in that it contains an outer lipopolysaccharide membrane, but it lacks peptidoglycan; within the cell wall, cysteine-rich proteins act as the functional peptidoglycan equivalent. The absence of peptidoglycan explains why the organism is not seen with standard Gram’s staining and why beta-lactam antimicrobials are not effective for treatment. Chlamydia trachomatis is a member of the Chlamydiaceae family. The genus Chlamydia includes three species that infect humans: Chlamydia trachomatis, Chlamydia pneumoniae, and Chlamydia psittaci. The species Chlamydia trachomatis, which exclusively infects humans, can cause (1) trachoma in persons of all ages, (2) anogenital infections, lymphogranuloma venereum and conjunctivitis in adults, and (3) conjunctivitis and pneumonia in neonates.
Sexually-acquired Chlamydia trachomatis is highly transmissible, with chlamydial infection rates between sexual partners reported of approximately 55%, with a per-act transmission risk of about 10% 10. Sexual transmission rates per sex act are thought to be slightly higher from men-to-women than from women-to-men, but given the number of asymptomatic carriers in the general population, estimates for the rate of transmission remain imprecise. Transmission of Chlamydia trachomatis can also occur from mother-to-infant via the genital tract during birth.
Lymphogranuloma venereum prevention
Not having any sexual activity is the only way to prevent a sexually transmitted infection. Safer sex behaviors may reduce the risk.
The proper use of condoms, either the male or female type, greatly decreases the risk of catching a sexually transmitted infection. You need to wear the condom from the beginning to the end of each sexual activity.
Lymphogranuloma venereum symptoms
Symptoms of lymphogranuloma venereum can begin a few days to a month after coming in contact with the bacteria. Symptoms include:
- Drainage through the skin from lymph nodes in the groin
- Painful bowel movements (tenesmus)
- Small painless sore on the male genitals or in the female genital tract
- Swelling and redness of the skin in the groin area
- Swelling of the labia (in women)
- Swollen groin lymph nodes on one or both sides; it may also affect lymph nodes around the rectum in people who have anal intercourse
- Blood or pus from the rectum (blood in the stools)
Characteristically lymphogranuloma venereum has three stages 11:
Primary stage:
- Begins in 3 to 12 days after exposure or sometimes it may be longer up to 30 days.
- The patient characteristically develops a painless genital ulcer or papules which are about 1 to 6 mm in size. Sores can also be present in the mouth or throat. An inflammatory reaction can occur at the site of inoculation.
- This stage often goes unnoticed due to the location of the lesions and as the lesions are usually small and there are no associated symptoms.
- The lesions resolve or heal spontaneously after few days.
Secondary stage:
- The secondary stage presents with the development of unilateral or bilateral tender inguinal and/or femoral lymphadenopathy (also called buboes), which occurs two to six weeks after the primary stage; this is called the inguinal syndrome.
- An anorectal syndrome also presents which is characterized by proctitis or proctocolitis-like symptoms. Pain during urination, rectal bleeding, pain during passing stools, abdominal pain, anal pain, tenesmus. Generalized symptoms like body aches, headache, and fever can occur during this stage. This syndrome usually occurs when the transmission is via the anal route 12.
- An oral syndrome can occur in people get lymphogranuloma venereum through the oral route. Cervical lymphadenopathy can occur.
- There are also reports of systemic complications like pneumonia and hepatitis 13.
Late stage:
Usually, occur when the disease is left untreated.
- Necrosis and rupture of the lymph nodes
- Anogenital fibrosis, and strictures
- Anal fistulae
- Elephantiasis of the genital organs can also occur in some cases
Lymphogranuloma venereum complications
Complications can occur many years after you are first infected. Complications usually occur when lymphogranuloma venereum is left untreated include— necrosis and rupture of the lymph nodes, anogenital fibrosis, and strictures, anal fistulae. Elephantiasis of the genital organs can also occur in some cases. Systemic complications like pneumonia and hepatitis also have been reported 14.
Health problems that may result from lymphogranuloma venereum infection include:
- Abnormal connections between the rectum and vagina (fistula)
- Brain inflammation (encephalitis – very rare)
- Infections in the joints, eyes, heart, or liver
- Long-term inflammation and swelling of the genitals
- Scarring and narrowing of the rectum
Lymphogranuloma venereum diagnosis
Lymphogranuloma venereum diagnosis is by clinical suspicion. Other causes of genital ulceration and inguinal adenopathy should be excluded 15.
The basis for a definitive diagnosis of lymphogranuloma venereum is on serology tests (complement fixation or micro-immunofluorescence) or identification of Chlamydia trachomatis in genital, rectal and lymph node specimens (by culture, nucleic acid amplification test or direct immunofluorescence) 16.
Men who have sex with men who have signs and symptoms of proctocolitis should receive testing for lymphogranuloma venereum. In these patients testing of rectal specimens with nucleic acid amplification test is the preferred approach 17.
HIV testing should be a consideration in patients with a sexually transmitted disease.
Lymphogranuloma venereum testing
The selection of a laboratory test to detect the presence of Chlamydia trachomatis is a critical component of disease management and prevention 18. The testing technology has shifted from culture-based methods to molecular-based techniques and this represents a substantial improvement in test sensitivity and ease of specimen collection.
Nucleic acid amplification tests (NAATs)
Nucleic acid amplification tests (NAATs) amplify nucleic acid sequences (either DNA or RNA) that are specific for the organism being detected. Similar to other nonculture tests, NAATs can detect live or non-viable organisms. Multiple commercially-available NAATs are FDA-cleared as diagnostic tests for Chlamydia trachomatis on (2) urine specimens from men and women, urethral swabs in men, and endocervical swabs in women; some tests are cleared for vaginal swabs 19. In addition, in May 2019 the FDA cleared two NAATs for diagnostic testing of chlamydia at extragenital sites (pharynx and rectum); the two tests are the Aptima Combo 2 Assay and the Xpert CT/NG 20. For chlamydia testing in men, NAATs are highly sensitive for detecting Chlamydia trachomatis on either a urethral swab or first-catch urine specimen, but most most experts prefer using urine samples 19. For women, vaginal swabs are preferred over urine samples and several studies have shown that self-collected vaginal swabs are preferred by women and perform equal to or better than clinician-collected vaginal swabs 21. In addition, in men and women, self-collected rectal swabs for NAAT have also performed well 22.
Non-amplification molecular tests
Molecular tests that do not use nucleic acid amplification encompass a variety of antigen detection and nucleic acid hybridization methods. These include enzyme-immunoassays (EIA), direct fluorescent antibody tests (DFA), and nucleic acid hybridization tests, a distinct non-NAAT methodology that detects Chlamydia trachomatis-specific DNA or RNA sequences in rRNA, genomic DNA, or plasmid DNA. All have significantly lower sensitivity (range 50% to 75%) than nucleic acid amplification tests (NAATs) 23. These non-amplification tests are rarely used in clinical practice and they are classified as “not recommended” by the Centers for Disease Control and Prevention 19.
Culture
Historically, cell culture to detect Chlamydia trachomatis was the most sensitive and specific method available to detect chlamydial infection. Cell culture, however, is technically complex, expensive, difficult to standardize, and has a lower sensitivity than amplification tests (50% versus 80%). In addition, performing Chlamydia trachomatis cell culture requires collection of columnar cells from relevant anatomical site(s) and use of stringent transport requirements. The excellent sensitivity and specificity of the nucleic acid amplification test (NAAT) has led to its use in place of culture for most clinical situations; the use of culture for Chlamydia trachomatis is limited to evaluation of suspected cases of sexual assault in children.
Serology
Serologic testing is rarely used to diagnose uncomplicated genital infections caused by Chlamydia trachomatis because chlamydia serologic tests do not reliably distinguish current from prior infection. Two main types of serologic tests are used for diagnosis: (1) chlamydia complement fixation test (CFT), which measures antibody against group specific lipopolysaccharide antigen, and (2) micro-immunofluorescence (MIF) 18. Serology may be of value in the diagnosis of lymphogranuloma venereum because many clinicians do not have access to OmpA serotyping or genotyping. Complement fixation titers of 1:64 or greater can support the diagnosis of lymphogranuloma venereum in the appropriate clinical context 24. The more sensitive and species-specific micro-immunofluorescence (MIF) has replaced the chlamydia complement fixation test (CFT). High background prevalence and infrequent rises and falls in IgG and IgM make serology less practical to use as a diagnostic test for uncomplicated genital chlamydial infection. Serology may be useful in evaluation of inguinal lymphogranuloma venereum and selected chlamydia complications (e.g., perihepatitis and infertility).
Lymphogranuloma venereum treatment
The recommended treatment regimen for lymphogranuloma venereum is doxycycline 100 mg orally twice a day given for 21 days 25.
An alternate regimen is erythromycin 500 mg orally four times a day given for 21 days.
Azithromycin 1 gm orally once weekly for 3 weeks is also an effective alternative regimen 26.
All patients who are suspected to have lymphogranuloma venereum (either genito-ulcerative disease with lymphadenopathy or proctocolitis) should be empirically treated for lymphogranuloma venereum before an official diagnosis is certain.
Pregnant patients can have treatment with erythromycin. Doxycycline and other tetracyclines should be avoided in pregnancy due to the risk of disruption of bone and teeth development.
Patients who have fluctuant or pus-filled buboes can benefit from aspiration of the node, which provides symptomatic relief, although incision and drainage of the nodes are not recommended as it can delay the healing process 27.
Management of sexual partners of lymphogranuloma venereum patients
All exposed partners of lymphogranuloma venereum probable or confirmed patients in the last 60 days should receive testing and empiric treatment with a chlamydial regimen (doxycycline 100mg oral twice daily for 7 days or 1gm azithromycin one-time dose). Appropriate testing should be done based on the route of transmission and can include either cervical, urethral or rectal specimens. If testing for chlamydia and/or lymphogranuloma venereum comes back positive, then treatment should be continued to complete a 21-day course. If testing is negative for chlamydia or lymphogranuloma venereum, then treatment should cease after 7 days.
A diagnosis of lymphogranuloma venereum should be taken into consideration by gastroenterologists and histologists in patients presenting with proctitis or inguinal lymphadenopathy, particularly in men who have sex with men 28.
Lymphogranuloma venereum prognosis
With prompt and appropriate antibiotic therapy, lymphogranuloma venereum prognosis is excellent and patients typically make a full recovery.
Patients must be informed that reinfection and relapses may occur.
References- Rawla P, Limaiem F. Lymphogranuloma Venereum. [Updated 2019 Jun 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537362
- Blank S, Schillinger JA, Harbatkin D. Lymphogranuloma venereum in the industrialised world. Lancet. 2005 May 7-13;365(9471):1607-8.
- Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect. 2002 Apr;78(2):90-2.
- Chen JC, Stephens RS. Trachoma and LGV biovars of Chlamydia trachomatis share the same glycosaminoglycan-dependent mechanism for infection of eukaryotic cells. Mol. Microbiol. 1994 Feb;11(3):501-7.
- Ceovic R, Gulin SJ. Lymphogranuloma venereum: diagnostic and treatment challenges. Infect Drug Resist. 2015;8:39-47.
- Rönn MM, Ward H. The association between lymphogranuloma venereum and HIV among men who have sex with men: systematic review and meta-analysis. BMC Infect. Dis. 2011 Mar 18;11:70.
- Spaargaren J, Fennema HS, Morré SA, de Vries HJ, Coutinho RA. New lymphogranuloma venereum Chlamydia trachomatis variant, Amsterdam. Emerging Infect. Dis. 2005 Jul;11(7):1090-2.
- Su H, Raymond L, Rockey DD, Fischer E, Hackstadt T, Caldwell HD. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1996 Oct 01;93(20):11143-8.
- Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis. 2010;201 Suppl 2:S114-25.
- Althaus CL, Turner KM, Mercer CH, et al. Effectiveness and cost-effectiveness of traditional and new partner notification technologies for curable sexually transmitted infections: observational study, systematic reviews and mathematical modelling. Health Technol Assess. 2014;18:1-100, vii-viii.
- Dal Conte I, Mistrangelo M, Cariti C, Chiriotto M, Lucchini A, Vigna M, Morino M, Di Perri G. Lymphogranuloma venereum: an old, forgotten re-emerging systemic disease. Panminerva Med. 2014 Mar;56(1):73-83.
- Harrison T, Som M, Stroup J. Lymphogranuloma venereum proctitis. Proc (Bayl Univ Med Cent). 2016 Oct;29(4):418-419.
- Schachter J. Chlamydial infections. West. J. Med. 1990 Nov;153(5):523-34.
- Stoner BP, Cohen SE. Lymphogranuloma Venereum 2015: Clinical Presentation, Diagnosis, and Treatment. Clin. Infect. Dis. 2015 Dec 15;61 Suppl 8:S865-73.
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae–2014. MMWR Recomm Rep. 2014 Mar 14;63(RR-02):1-19.
- Bachmann LH, Johnson RE, Cheng H, Markowitz L, Papp JR, Palella FJ, Hook EW. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J. Clin. Microbiol. 2010 May;48(5):1827-32.
- Meyer T. Diagnostic Procedures to Detect Chlamydia trachomatis Infections. Microorganisms. 2016 Aug 05;4(3).
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae–2014. MMWR Recomm Rep. 2014;63:1-19.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. Chlamydial infections. MMWR Recomm Rep. 2015;64(No. RR-3):1-137.
- U.S. Food and Drug Administration. FDA clears first diagnostic tests for extragenital testing for chlamydia and gonorrhea. FDA news release. May 23, 2019.
- Lunny C, Taylor D, Hoang L, et al. Self-Collected versus Clinician-Collected Sampling for Chlamydia and Gonorrhea Screening: A Systemic Review and Meta-Analysis. PLoS One. 2015;10:e0132776.
- Van der Helm JJ, Hoebe CJ, van Rooijen MS, et al. High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women. Sex Transm Dis. 2009;36:493-7.
- Jensen IP, Fogh H, Prag J. Diagnosis of Chlamydia trachomatis infections in a sexually transmitted disease clinic: evaluation of a urine sample tested by enzyme immunoassay and polymerase chain reaction in comparison with a cervical and/or a urethral swab tested by culture and polymerase chain reaction. Clin Microbiol Infect. 2003;9:194-201.
- Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect. 2002;78:90-2.
- Handsfield HH. Lymphogranuloma Venereum Treatment and Terminology. Sex Transm Dis. 2018 Jun;45(6):409-411.
- Workowski KA, Bolan GA., Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015 Jun 05;64(RR-03):1-137.
- White J, O’Farrell N, Daniels D., British Association for Sexual Health and HIV. 2013 UK National Guideline for the management of lymphogranuloma venereum: Clinical Effectiveness Group of the British Association for Sexual Health and HIV (CEG/BASHH) Guideline development group. Int J STD AIDS. 2013 Aug;24(8):593-601.
- Martin IM, Alexander SA, Ison CA, Macdonald N, McCarthy K, Ward H. Diagnosis of lymphogranuloma venereum from biopsy samples. Gut. 2006 Oct;55(10):1522-3.