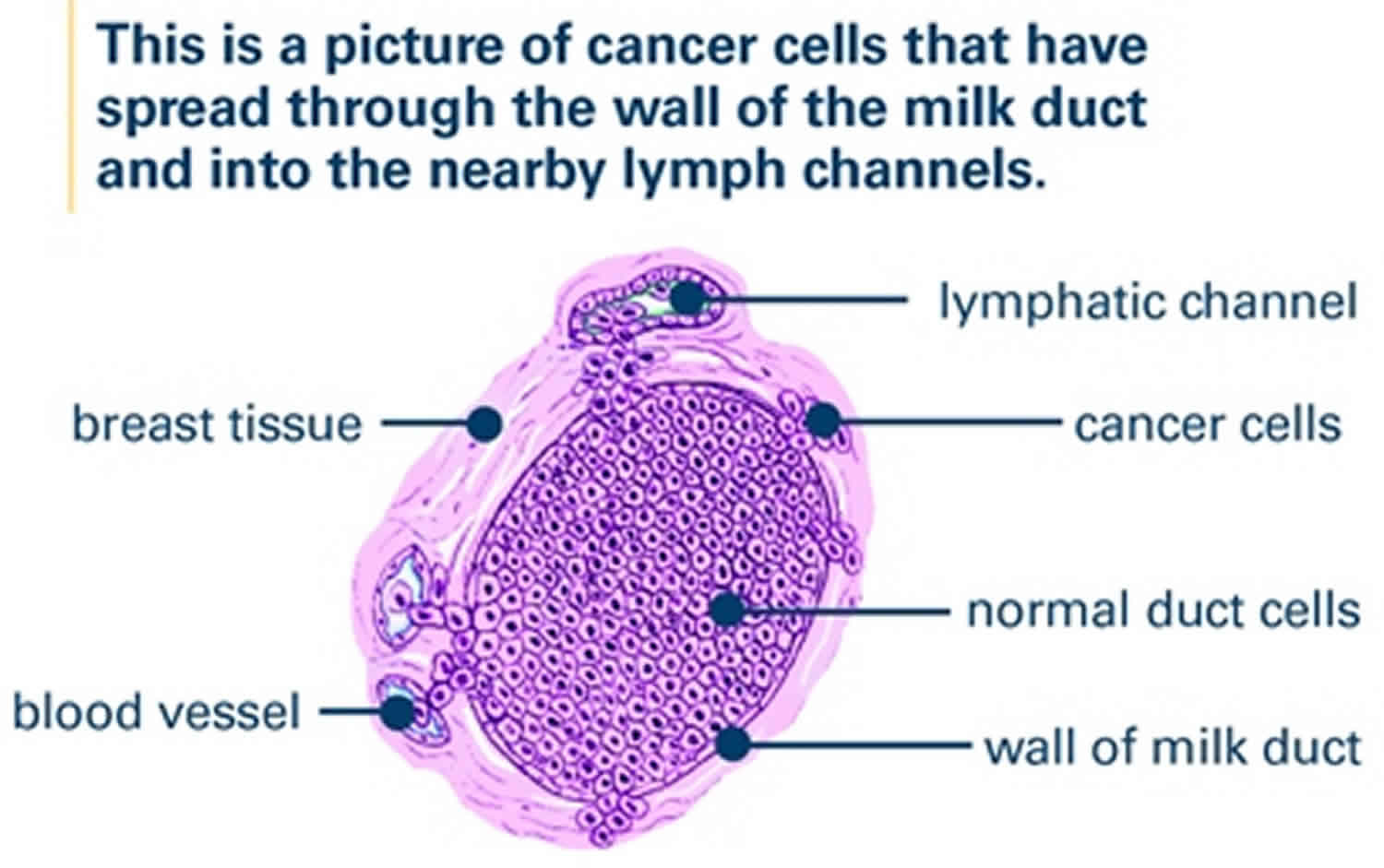
Lymphovascular invasion
Lymphovascular invasion (LVI) is the presence of malignant tumor cells within lymphatic spaces, blood vessels, or both (lymphovascular channels), is a crucial step in the invasion-metastasis cascade. Lymphovascular invasion is defined as the presence of tumor cells within a definite endothelial-lined space (lymphatics or blood vessels) in the breast surrounding invasive carcinoma 1. Lymphovascular invasion, when identified morphologically in the peritumoral area by microscopic examination, is regarded as a marker of metastatic potential and is strongly associated with a poor prognosis in many solid tumors, including breast cancer 2. Although the mechanism of lymphovascular invasion has not been clearly proven, lymphovascular invasion could reflect a surrounding tumor microenvironment that predicts underlying aggressive tumor and worse prognosis. Tumors that don’t have lymphovascular invasion have a better prognosis than tumors that have lymphovascular invasion. Despite molecular mechanisms associated with the development of lymphovascular invasion have been extensively studied, details of driver genes, and molecular pathways and mechanisms involved in its development in breast cancer, remain poorly defined. Although invasive breast cancer cells have the ability to invade surrounding stroma, only those that can interact with endothelial cells, penetrate the vascular wall and withstand the intravascular stress will develop lymphovascular invasion and complete metastatic dissemination. Identification of additional molecular events associated with lymphovascular invasion in the primary tumor and characterization of the contribution of the tumor micro-environment to modulating biological processes leading to lymphovascular invasion in breast cancer remain a challenging task. This stems not only from the complexity of the molecular alterations in the primary tumor and the interactions with different components of its micro-environment but also from the subjective nature of lymphovascular invasion assessment in human breast cancer.
Breast cancer (breast cancer) is recognized as a heterogeneous disease with varied presentation, morphology, behavior and response to therapy. Although a minority of breast cancer patients (<10%) present with metastatic disease, 20-30% of patients presenting with early-stage disease will develop distant metastases over time 3. Distant metastases, which are considered as a prognostically poor event in cancer, develop through coordinated and highly selective biological processes involving several related mechanisms, including cell proliferation, cell-matrix interactions, stromal invasion, cellular migration, lymphovascular invasion and evasion of host immune responses 4.
The prognostic value of lymphovascular invasion has been demonstrated by several independent studies 5, 6, 7, 8. In a previous study of 3,812 cases of breast cancer 8, it has been demonstrated that lymphovascular invasion is not only an independent prognostic variable in the whole series but also in the various prognostic subgroups, including the lymph node-negative cohort. In this subgroup, lymphovascular invasion could be used as a high-risk criterion conferring survival disadvantage equivalent to that provided by involvement of one or two lymph nodes and to that provided by one higher size category 8.
At the molecular level, there are several biochemical and biophysical interactions that cancer cells utilize to facilitate their metastatic progression through vascular and lymphatic channels 5. Exploration of these signalling pathways would be crucial to find novel molecules controlling critical steps of lymphovascular invasion and could be relevant therapeutic candidates. The hallmarks of cancer have been extensively elaborated, and they all pointed to the dominant intrinsic and extrinsic qualities of the tumor cells that govern their genetic regulation 9. Elevated activity of protein expression, resistance to apoptotic signals, increased proliferation rate, adhesion, invasion and cellular motility leading ultimately to cellular migration are some characteristics of malignant cells 10. Although these criteria are not directly related to the occurrence of lymphovascular invasion and can be identified in lymphovascular invasion-negative and lymphovascular invasion-positive cancers, they are critically required traits for its development, and the molecular mechanisms controlling these processes must remain active, at least in some cellular clones, throughout the carcinogenic pathway from initiation to dissemination through vascular channels.
Lymphovascular invasion breast cancer
Lymphovascular invasion has been a predictor of worse survival outcomes in breast cancer. The presence of lymphovascular invasion is associated with an increased risk of axillary lymph node and distant metastases 11. The presence of carcinoma cells in either lymphatic vessels (lymphatic invasion), blood vessels (vascular invasion) or both (lymphovascular invasion) is a significant prognostic factor in invasive breast cancer, with respect to local and distance recurrence 12 and poorer survival 13. One study reported that lymphovascular invasion is not an independent predictor of locoregional control or survival in early-stage breast cancer 14. However, lymphovascular invasion is an independent prognostic factor in lymph node negative breast cancer treated with adjuvant chemotherapy and in operable breast cancer with positive axillary lymph nodes 15. Uematsu et al 16 classified the degrees of lymphovascular invasion as no, minimal, moderate, and marked. They suggested that the degree of lymphovascular invasion is an important factor for prediction of neoadjuvant chemotherapy efficacy in breast cancer. Liu et al 17 reported that patients with no lymphovascular invasion with tumors positive for hormone receptor or HER2 overexpression had the most favorable recurrence-free survival and overall survival when stratified by molecular subtype. Additionally, the authors showed that the patients with lymphovascular invasion and triple negative subtype had the worst recurrence-free survival and overall survival. The population-based study showed that the presence of lymphovascular invasion in patients with operable breast cancer was associated with shorter recurrence-free survival and overall survival in high-risk patients (positive lymph nodes, tumor size >2 cm, high histologic grade, negative hormone receptor status, or age younger than 35 years) 11.
Although the mechanism of lymphovascular invasion has not been completely elucidated, lymphovascular invasion is considered as a prognostic factor in patients with operable breast cancer with or without metastatic axillary lymph nodes who are undergoing adjuvant treatment 15. Post-neoadjuvant chemotherapy lymphovascular invasion was associated with impaired disease-free survival and the magnitude of this effect depended on breast cancer subtype 18. Post-neoadjuvant chemotherapy lymphovascular invasion was an independent predictor of local relapse, distant metastasis, and overall survival. Patients with hormone receptor-positive cancer did not have a survival benefit compared to those with hormone receptor-negative cancer 1. However, when lymphovascular invasion was stratified according to presence or absence of hormone receptor, the patients with hormone receptor-negative status and lymphovascular invasion had unfavorable rates of recurrence and cancer-related death 1.
One study reported that the grade of lymphovascular invasion in surgical specimens obtained after neoadjuvant chemotherapy was significantly associated with increasing hazard ratios for tumor recurrence and tumor-related death 19. Another study showed that absence of lymphovascular invasion in surgical specimens following neoadjuvant chemotherapy correlated with pathologic response 20. Therefore, assessment of lymphovascular invasion in surgical specimens acquired after neoadjuvant chemotherapy is important for prediction of survival outcomes.
In this study 1, lymphovascular invasion was observed in 65 (34.8%) patients. Patients with lymphovascular invasion had high rates of mastectomy and axillary lymph node dissection because these patients had more advanced tumor characteristics. Therefore, the patients with lymphovascular invasion had greater severity of tumor burden and lymph node involvement after neoadjuvant chemotherapy 1. Among the patients with lymphovascular invasion, there were none who achieved pathologic complete response, as we had expected. However, this study did not show that pathologic complete response indicated better survival outcomes 1. To the contrary, lymphovascular invasion showed a significant association with recurrence and death. When lymphovascular invasion was stratified by hormone receptor status, the patients with hormone receptor-positive cancer and no lymphovascular invasion had the most favorable survival outcomes. Hormone receptor-negative status with lymphovascular invasion was associated with higher risk of recurrence and death 1.
References- Ryu YJ, Kang SJ, Cho JS, Yoon JH, Park MH. Lymphovascular invasion can be better than pathologic complete response to predict prognosis in breast cancer treated with neoadjuvant chemotherapy. Medicine (Baltimore). 2018;97(30):e11647. doi:10.1097/MD.0000000000011647 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6078671
- Aleskandarany M, A, Sonbul S, N, Mukherjee A, Rakha E, A: Molecular Mechanisms Underlying Lymphovascular Invasion in Invasive Breast Cancer. Pathobiology 2015;82:113-123. https://doi.org/10.1159/000433583
- Kennecke H, Yerushalmi R, Woods R, et al: Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271-3277.
- Sun Y, Wang X, Zhou Q, et al: Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol Rep 2015;33:338-346.
- Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG: Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014;14:159-172.
- Ugras S, Stempel M, Patil S, Morrow M: Estrogen receptor, progesterone receptor, and HER2 status predict lymphovascular invasion and lymph node involvement. Ann Surg Oncol 2014;21:3780-3786.
- Song YJ, Shin SH, Cho JS, Park MH, Yoon JH, Jegal YJ: The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J Breast Cancer 2011;14:198-203.
- Rakha EA, Martin S, Lee AH, et al: The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 2012;118:3670-3680.
- Hanahan D, Weinberg RA: The hallmarks of cancer. Cell 2000;100:57-70.
- Bray K, Gillette M, Young J, et al: Cdc42 overexpression induces hyperbranching in the developing mammary gland by enhancing cell migration. Breast Cancer Res 2013;15:R91.
- Ejlertsen B, Jensen MB, Rank F, et al. Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J Natl Cancer Inst 2009;101:729–35.
- Rakha EA, Martin S, Lee AH, et al.: The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 2012, 118:3670–3680.
- Mohammed RA, Martin SG, Mahmmod AM, et al.: Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: findings from a large case series with long-term follow-up. J Pathol 2011, 223:358–365.
- Freedman GM, Li T, Polli LV, et al. Lymphatic space invasion is not an independent predictor of outcomes in early stage breast cancer treated by breast-conserving surgery and radiation. Breast J 2012;18:415–9.
- Song YJ, Shin SH, Cho JS, et al. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J Breast Cancer 2011;14:198–203.
- Uematsu T, Kasami M, Watanabe J, et al. Is lymphovascular invasion degree one of the important factors to predict neoadjuvant chemotherapy efficacy in breast cancer? Breast Cancer 2011;18:309–13.
- Liu YL, Saraf A, Lee SM, et al. Lymphovascular invasion is an independent predictor of survival in breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 2016;157:555–64.
- Hamy AS, Lam GT, Laas E, et al. Lymphovascular invasion after neoadjuvant chemotherapy is strongly associated with poor prognosis in breast carcinoma. Breast Cancer Res Treat. 2018;169(2):295-304. doi:10.1007/s10549-017-4610-0
- Tamura N, Hasebe T, Okada N, et al. Tumor histology in lymph vessels and lymph nodes for the accurate prediction of outcome among breast cancer patients treated with neoadjuvant chemotherapy. Cancer Sci 2009;100:1823–33.
- Sullivan PS, Apple SK. Should histologic type be taken into account when considering neoadjuvant chemotherapy in breast carcinoma? Breast J 2009;15:146–54.