Non-Hodgkin’s Lymphoma
Non-Hodgkin lymphoma also known as NHL or just lymphoma, is a cancer that starts when a type of white blood cell, called lymphocyte (T cell or B cell), becomes abnormal. About 85-90 percent of NHL cases start in the B cells 1. In non-Hodgkin’s lymphoma, lymphocytes undergo a malignant (cancerous) change and then multiplies, eventually crowding out healthy cells and creating tumors. Non-Hodgkin lymphoma generally develop in the lymph nodes or in lymphatic tissue found in organs such as the stomach, intestines or skin. Furthermore, these abnormal cells can spread to almost any other part of the body. In some cases, non-Hodgkin’s lymphoma involves blood and the bone marrow (the spongy tissue in the hollow, central cavity of bones, where blood cell formation occurs). Lymphoma cells may develop in just one place or in many sites in the body. Most of the time, doctors don’t know why a person gets non-Hodgkin lymphoma. You are at increased risk if you have a weakened immune system or have certain types of infections.
More than 60 specific non-Hodgkin lymphoma (NHL) subtypes have been identified and assigned names, so classifying it can be quite confusing (even for doctors). Several different systems have been used, but the most recent system is the World Health Organization (WHO) classification 2. The Revised European American Lymphoma and World Health Organization (REAL/WHO) classification of non-Hodgkin’s lymphoma categorizes non-Hodgkin’s lymphoma subtypes by the characteristics of the lymphoma cells, including their appearance, the presence of proteins on the surface of the cells and their genetic features. One way that non-Hodgkin’s lymphoma subtypes are classified is by cell type. Some non-Hodgkin’s lymphoma subtypes, such as diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL), involve lymphocytes called “B cells.” Other subtypes, such as peripheral T-cell lymphoma (PTCL) and cutaneous T-cell lymphoma (CTCL), involve lymphocytes called “T cells” or “natural killer (NK) cells.”
Specialists further classify the non-Hodgkin’s lymphoma subtypes according to the rate of disease progression, either fast growing (aggressive) or slow growing (indolent). Aggressive lymphoma subtypes also called “high-grade non-Hodgkin’s lymphoma”, account for about 60 percent of all non-Hodgkin’s lymphoma cases. Diffuse large B-cell lymphoma is the most common aggressive non-Hodgkin’s lymphoma subtype. Slow-growing (indolent) subtypes account for about 40 percent of all non-Hodgkin’s lymphoma cases. Follicular lymphoma (FL) is the most common subtype of indolent non-Hodgkin’s lymphomas. When indolent lymphomas are first diagnosed, patients have fewer signs and symptoms than patients with aggressive lymphoma subtypes. Whether the diagnosed subtype is aggressive or indolent determines the appropriate treatment, so getting an accurate diagnosis is very important. In some cases, indolent forms of non-Hodgkin’s lymphoma later transform into an aggressive form of the disease.
Non-Hodgkin lymphoma (NHL) is one of the most common cancers in the United States, accounting for about 4.2% of all cancers. The American Cancer Society’s most recent estimates for non-Hodgkin’s lymphoma are for 2022 3:
- New cases: About 80,470 people (44,120 males and 36,350 females) will be diagnosed with non-Hodgkin lymphoma. This includes both adults and children.
- Deaths: About 20,250 people will die from non-Hodgkin lymphoma (11,700males and 8,550 females).
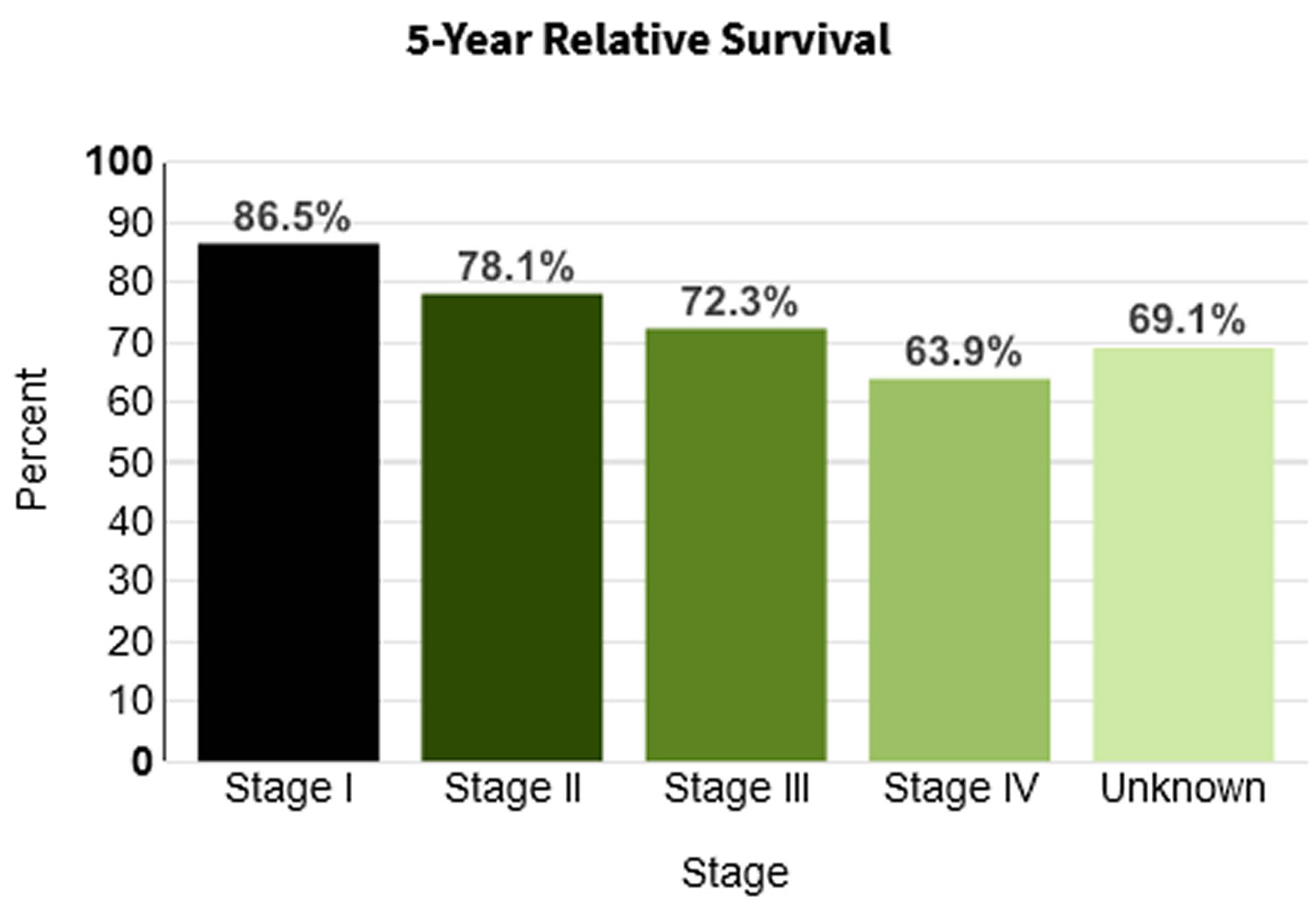
- 5-Year Relative Survival: 73.8%. Relative survival is an estimate of the percentage of patients who would be expected to survive the effects of their cancer. It excludes the risk of dying from other causes. Because survival statistics are based on large groups of people, they cannot be used to predict exactly what will happen to an individual patient. No two patients are entirely alike, and treatment and responses to treatment can vary greatly.
- Percentage of All Cancer Deaths: 3.3%.
- Rate of New Cases and Deaths per 100,000: The rate of new cases of non-Hodgkin lymphoma was 19.0 per 100,000 men and women per year. The death rate was 5.3 per 100,000 men and women per year. These rates are age-adjusted and based on 2015–2019 cases and deaths.
- Lifetime Risk of Developing Cancer: Approximately 2.1 percent of men and women will be diagnosed with non-Hodgkin lymphoma at some point during their lifetime, based on 2017–2019 data.
- In 2019, there were an estimated 763,400 people living with non-Hodgkin lymphoma in the United States.
The average American’s risk of developing non-Hodgkin’s lymphoma during a man’s lifetime is about 1 in 42; for a woman, the risk is about 1 in 52 4. But each person’s risk can be affected by a number of risk factors.
Non-Hodgkin’s lymphoma can occur at any age. In fact, it is one of the more common cancers among children, teens, and young adults. Still, the risk of developing non-Hodgkin’s lymphoma increases throughout life, and more than half of patients are 65 or older at the time of diagnosis. The aging of the American population is likely to lead to an increase in non-Hodgkin’s lymphoma cases during the coming years.
Treatment for non-Hodgkin lymphoma depends on which type it is, so it’s important for doctors to find out the exact type of lymphoma you have. The type of lymphoma depends on what type of lymphocyte is affected (B cells or T cells), how mature the cells are when they become cancerous, and other factors.
The prognosis and treatment approach for different non-Hodgkin’s lymphoma subtypes are influenced by findings from studying the diseased cells and tissues under a microscope, so biopsy samples should be examined by a hematopathologist (a doctor who specializes in the diagnosis of blood disorders and blood cancers).
The Lymphatic System
To understand what lymphoma is, it helps to know about the lymph system (also known as the lymphatic system). The lymph system is part of the immune system, which helps fight infections and some other diseases. The lymphatic system plays a role in:
- fighting bacteria and other infections
- destroying old or abnormal cells, such as cancer cells
The lymphatic system also helps the flow of fluids in the body.
The lymph system is made up mainly of cells called lymphocytes, a type of white blood cell. There are 3 main types of lymphocytes:
- B lymphocytes (B cells): B cells make proteins called antibodies to help protect the body from germs (bacteria and viruses).
- T lymphocytes (T cells): There are several types of T cells. Some T cells destroy germs or abnormal cells in the body. Other T cells help boost or slow the activity of other immune system cells.
- Natural killer (NK) cells, which attack virus-infected cells or tumor cells
The lymphatic system is a vast collection of cells and biochemicals that travel in lymphatic vessels, and the organs and glands that produce them. The lymphatic system includes a network of vessels (like the arteries and veins that carry blood) that assist in circulating body fluids (a colorless liquid called lymph), so it is closely associated with your cardiovascular system. Lymphatic vessels transport excess fluid away from interstitial spaces in most tissues and return it to the bloodstream (Figure 3). This fluid carries food to the cells and bathes the body tissues to form tissue fluid. The fluid then collects waste products, bacteria, and damaged cells. It also collects any cancer cells if these are present. This fluid then drains into the lymph vessels. Without the lymphatic system, this fluid would accumulate in tissue spaces. Special lymphatic capillaries, called lacteals, are located in the lining of the small intestine. They absorb digested fats and transport them to the venous circulation.
The lymphatic system is a system of thin tubes and lymph nodes that run throughout the body. Lymph nodes are bean shaped glands. The thin tubes are called lymph vessels or lymphatic vessels. Tissue fluid called lymph circulates around the body in these vessels and flows through the lymph nodes.
The lymph system is an important part of your immune system. It plays a role in fighting bacteria and other infections and destroying old or abnormal cells, such as cancer cells.
The major sites of lymphoid tissue are:
- Lymph nodes: Lymph nodes are bean-sized collections of lymphocytes and other immune system cells throughout the body, including inside the chest, abdomen, and pelvis. They are connected to each other by a system of lymphatic vessels.
- Spleen: The spleen is an organ under the lower ribs on your left side. The spleen makes lymphocytes and other immune system cells. It also stores healthy blood cells and filters out damaged blood cells, bacteria, and cell waste.
- Bone marrow: The bone marrow is the spongy tissue inside certain bones. New blood cells (including some lymphocytes) are made there.
- Thymus: The thymus is a small organ behind the upper part of the breastbone and in front of the heart. Thymus is an organ in which T lymphocytes mature and multiply.
- Adenoids and tonsils: These are collections of lymphoid tissue in the back of your throat. They help make antibodies against germs that are breathed in or swallowed.
- Digestive tract: The stomach, intestines, and many other organs also have lymph tissue.
The lymphatic system has a second major function— it enables you to live in a world with different types of organisms. Some of them live in or on the human body and in some circumstances may cause infectious diseases. Cells and biochemicals of the lymphatic system launch both generalized and targeted attacks against “foreign” particles, enabling the body to destroy infectious agents. This immunity against disease also protects against toxins and cancer cells. When the immune response is abnormal, persistent infection, cancer, allergies, and autoimmune disorders may result.
The larger lymphatic vessels lead to specialized organs called lymph nodes. After leaving the lymph nodes, the vessels merge to form still larger lymphatic trunks.
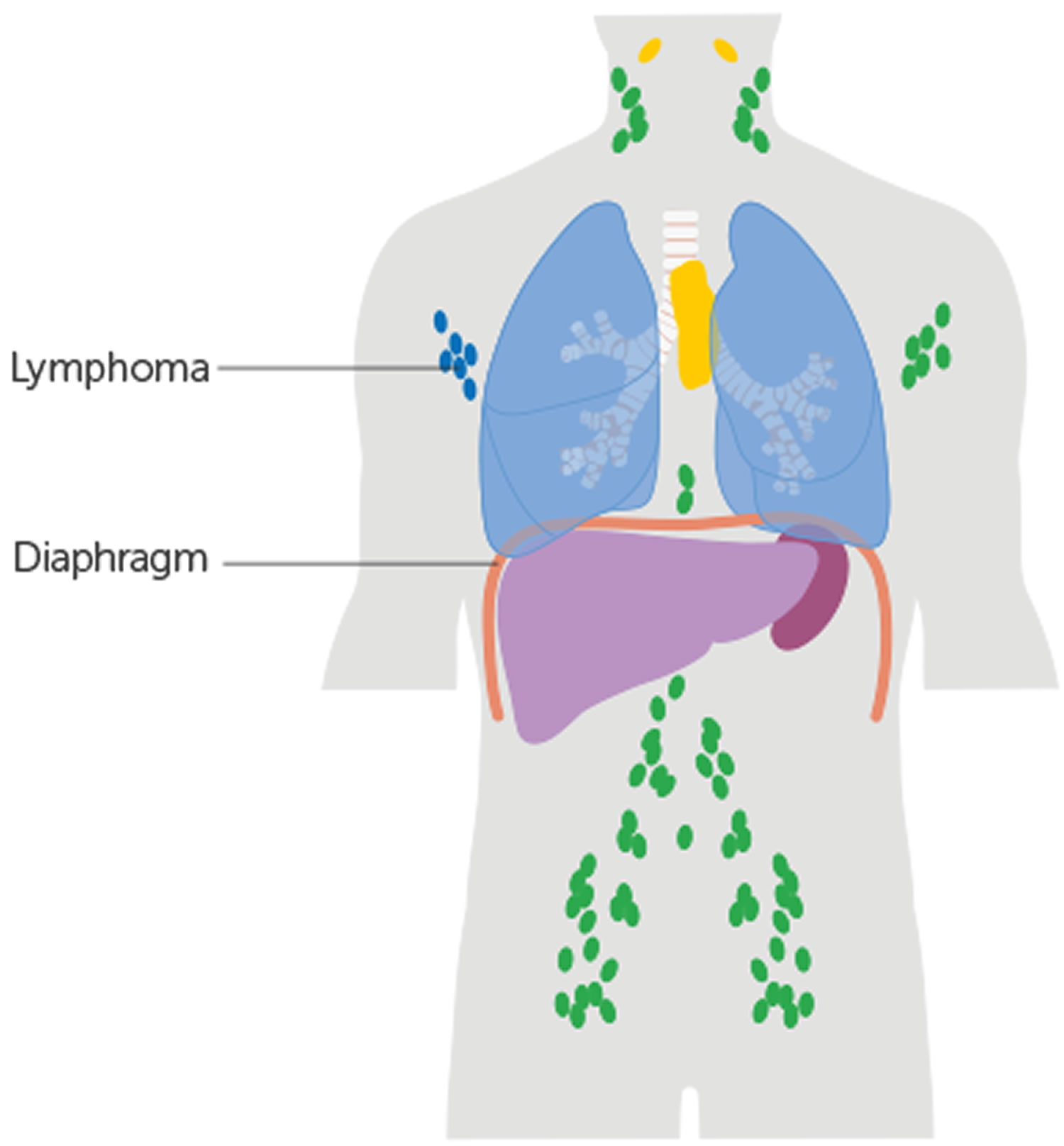
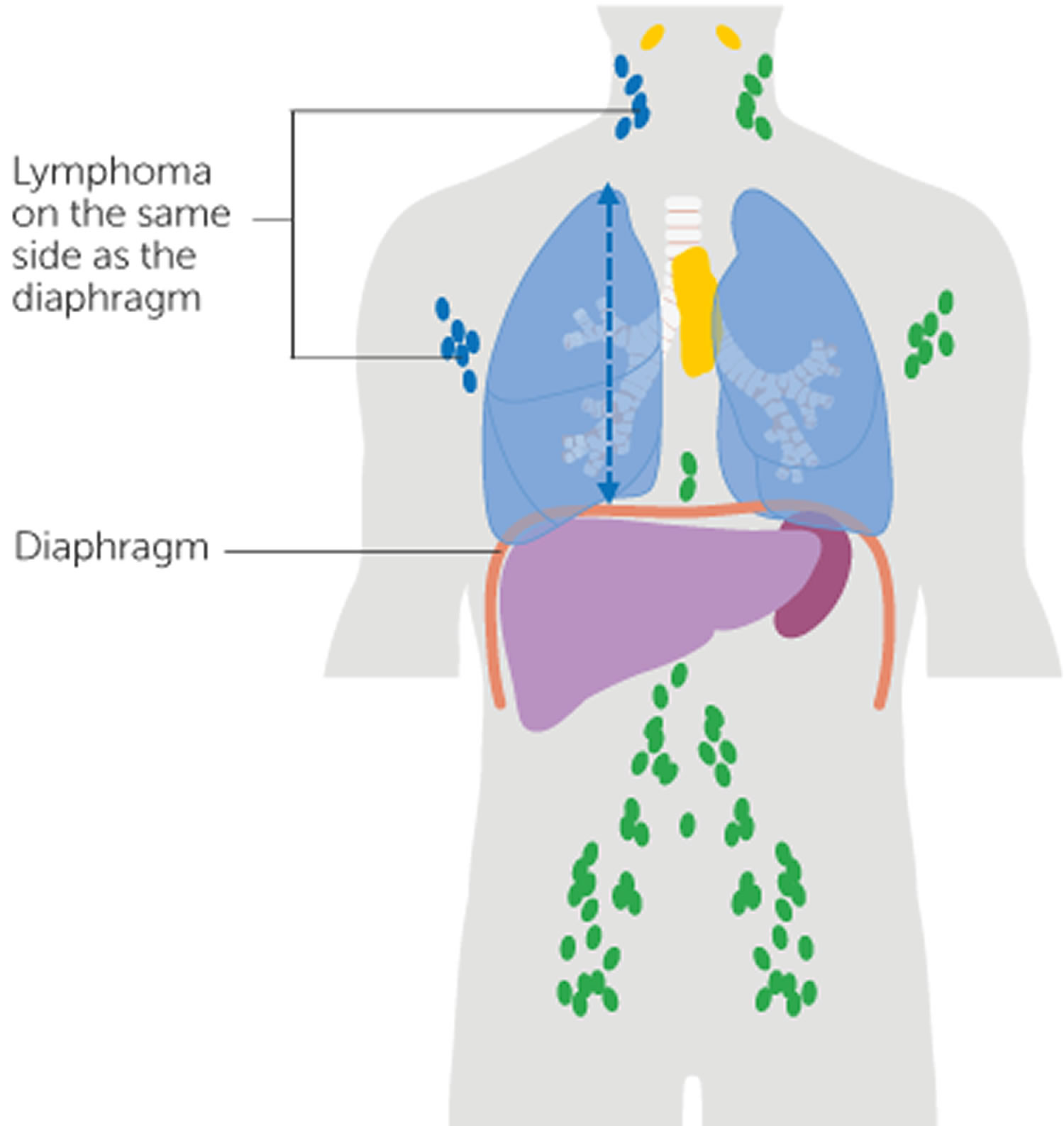
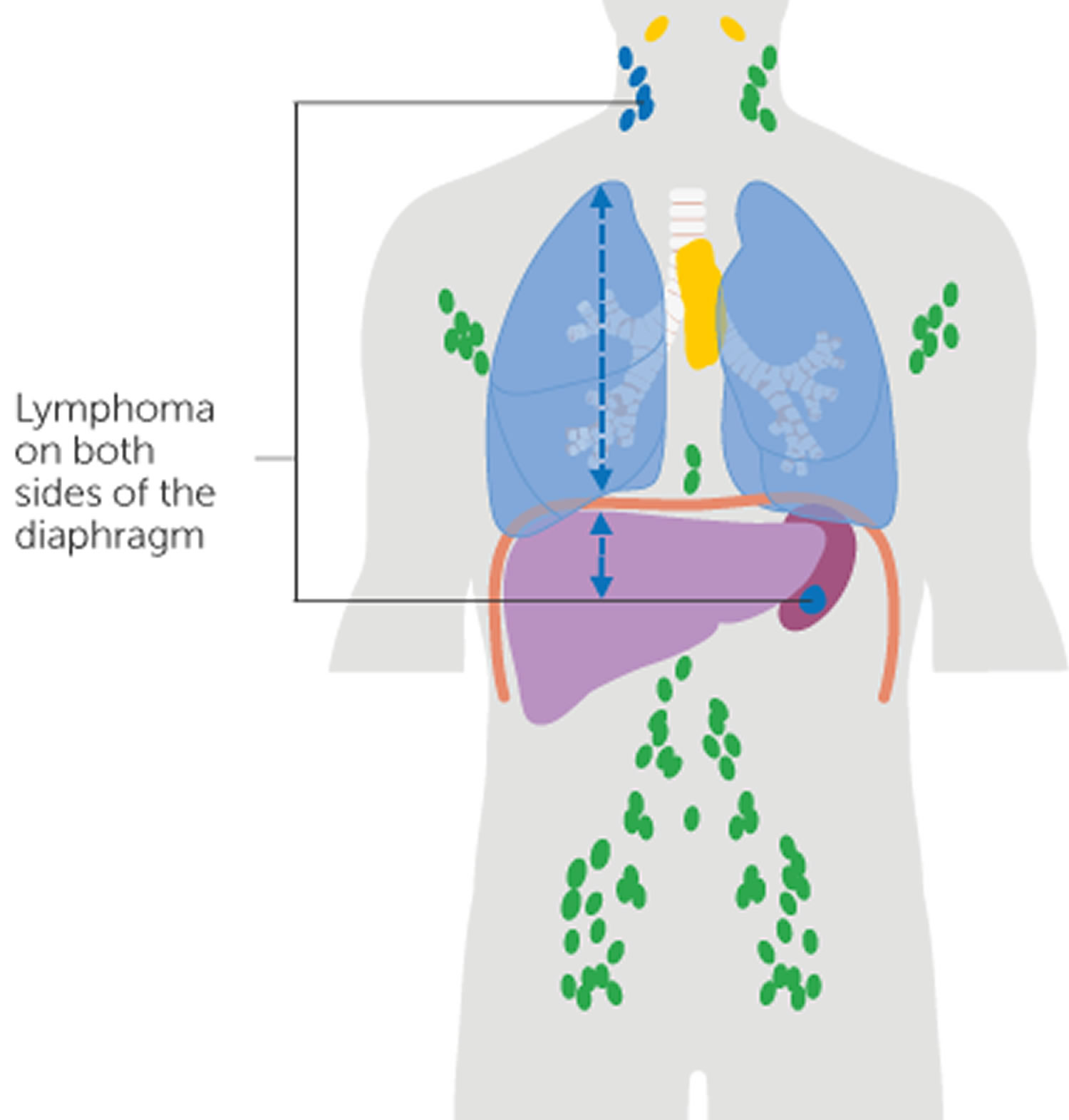
Figure 1. Locations of major lymph nodes
Figure 2. Functions of lymph nodes in the lymphatic system
Figure 3. Schematic representation of lymphatic vessels transporting fluid from interstitial spaces to the bloodstream. Depending on its origin, lymph enters the right or left subclavian vein.
Types of Non-Hodgkin’s Lymphoma
More than 60 specific non-Hodgkin lymphoma (NHL) subtypes have been identified and assigned names, so classifying it can be quite confusing (even for doctors). Several different systems have been used, but the most recent system is the World Health Organization (WHO) classification.
The WHO system groups lymphomas based on:
- The type of lymphocyte the lymphoma starts in
- How the lymphoma looks under a microscope
- The chromosome features of the lymphoma cells
- The presence of certain proteins on the surface of the cells
The more common types of lymphoma are listed below according to whether they start in B lymphocytes (B cells) or T lymphocytes (T cells) with B-cell lymphomas being the most common. Some rarer forms of non-Hodgkin lymphoma are not listed here.
Specialists further classify the non-Hodgkin lymphoma subtypes according to the rate of disease progression, either fast growing (aggressive) or slow growing (indolent).
- Indolent non-Hodgkin lymphomas also called “low-grade non-Hodgkin lymphoma”, are slow-moving and tend to grow more slowly and have fewer signs and symptoms when first diagnosed. Some indolent lymphomas might not need to be treated right away, but can be watched closely instead. Slow-growing (indolent) subtypes account for about 40 percent of all non-Hodgkin lymphoma cases. The most common type of indolent lymphoma in the United States is follicular lymphoma (FL).
- Aggressive non-Hodgkin lymphomas also called “high-grade non-Hodgkin lymphoma”, grow and spread quickly, and usually need to be treated right away. Aggressive lymphoma subtypes account for about 60 percent of all non-Hodgkin lymphoma cases. The most common type of aggressive lymphoma in the United States is diffuse large B cell lymphoma (DLBCL).
- Some types of lymphoma, like mantle cell lymphoma, don’t fit neatly into either of these categories.
When indolent lymphomas are first diagnosed, patients have fewer signs and symptoms than patients with aggressive lymphoma subtypes. Whether the diagnosed subtype is aggressive or indolent lymphoma determines the appropriate treatment, so getting an accurate diagnosis is very important. In some cases, indolent forms of non-Hodgkin lymphoma later transform into an aggressive form of the disease 5.
Non-Hodgkin’s lymphoma Subtypes
Mature B-cell lymphomas (about 85%-90% of non-Hodgkin’s lymphoma cases)
- Aggressive lymphomas (high-grade non-Hodgkin lymphomas)
- Diffuse large B-cell lymphoma (DLBCL) (31%)
- Mantle cell lymphoma (MCL) (can present as aggressive or indolent) (6%)
- Lymphoblastic lymphoma (2%)
- Burkitt lymphoma (BL) (2%)
- Primary mediastinal (thymic) large B-cell lymphoma (PMBCL) (2%)
- Transformed follicular and transformed mucosa-associated lymphoid tissue (MALT) lymphomas
- High-grade B-cell lymphoma with double or triple hits (HBL)
- Primary cutaneous DLBCL, leg type
- Primary DLBCL of the central nervous system
- Primary central nervous system (CNS) lymphoma
- Acquired immunodeficiency syndrome (AIDS)-associated lymphoma
- Indolent lymphomas
- Follicular lymphoma (FL) (22%)
- Marginal zone lymphoma (MZL) (8%)
- Chronic lymphocytic leukemia/small-cell lymphocytic lymphoma (CLL/SLL) (6%)
- Gastric mucosa-associated lymphoid tissue (MALT) lymphoma (5%)
- Lymphoplasmacytic lymphoma (1%)
- Waldenström macroglobulinemia (WM)
- Nodal marginal zone lymphoma (NMZL) (1%)
- Splenic marginal zone lymphoma (SMZL)
Mature T-cell and natural killer (NK)-cell lymphomas (about 10%-15% of non-Hodgkin’s lymphoma cases)
- Aggressive lymphomas (high-grade non-Hodgkin lymphomas)
- Peripheral T-cell lymphoma (PTCL), not otherwise specified (6%)
- Systemic anaplastic large-cell lymphoma (ALCL) (2%)
- Lymphoblastic lymphoma (2%)
- Hepatosplenic gamma/delta T-cell lymphoma
- Subcutaneous panniculitis-like T-cell lymphoma (SPTCL)
- Enteropathy-type intestinal T-cell lymphoma
- Primary cutaneous anaplastic large-cell lymphoma
- Indolent lymphomas
- Cutaneous T-cell lymphoma (CTCL) (4%)
- Mycosis fungoides (MF)
- Sézary syndrome (SS)
- Angioimmunoblastic T-cell lymphoma (AITL)
- Adult T-cell leukemia/lymphoma
- Extranodal NK/T-cell lymphoma (ENK/TCL), nasal type
Non-Hodgkin lymphoma Grades
Doctors sometimes put non-Hodgkin lymphoma into 2 groups, depending on how quickly they are likely to grow and spread. The 2 groups are low grade and high grade. The different grades of non-Hodgkin lymphoma are treated in slightly different ways.
- Low grade non-Hodgkin lymphoma also called indolent non-Hodgkin lymphoma tends to grow very slowly. Follicular lymphoma is the most common type of low grade lymphoma. Other types of non Hodgkin’s lymphomas include:
- Mantle cell lymphoma
- Marginal zone lymphoma
- Small lymphocytic lymphoma
- Lymphoplasmacytic lymphoma
- Skin lymphoma
- Over time, low grade lymphomas may change into a high grade type lymphoma.
- High grade non-Hodgkin lymphoma also called aggressive non-Hodgkin lymphoma tends to grow more quickly than low grade lymphomas. Because they grow quickly your doctor might call your lymphoma an aggressive type. Diffuse large B cell lymphoma (DLBCL) is the most common type of high grade non-Hodgkin lymphoma. Other types of high grade non-Hodgkin’s lymphoma are:
- Burkitt lymphoma
- Peripheral T cell lymphoma
- Lymphoblastic lymphoma
- Blastic NK cell lymphoma
- Enteropathy associated T cell lymphoma (EATL)
- Hepatosplenic gamma delta T cell lymphoma
- Treatment related T cell lymphoma
- Angioimmunoblastic T-cell lymphoma (AITL)
B-cell lymphomas
B-cell lymphomas make up most (about 85%) of non-Hodgkin lymphoma in the United States. These are types of lymphoma that affect B lymphocytes. The most common types of B-cell lymphomas are listed below.
Diffuse large B-cell lymphoma (DLBCL)
This is the most common type of non-Hodgkin lymphoma in the United States, accounting for about 1 out of every 3 lymphomas. The lymphoma cells look fairly large when seen with a microscope.
Diffuse large B-cell lymphoma (DLBCL) can affect people of any age, but it occurs mostly in older people. The average age at the time of diagnosis is mid-60s. It usually starts as a quickly growing mass in a lymph node deep inside the body, such as in the chest or abdomen, or in a lymph node you can feel, such as in the neck or armpit. It can also start in other areas such as the intestines, bones, or even the brain or spinal cord.
Diffuse large B-cell lymphoma (DLBCL) tends to be a fast-growing (aggressive) lymphoma, but it often responds well to treatment. Overall, about 3 out of 4 people will have no signs of disease after the initial treatment, and many are cured.
A common subtype of diffuse large B-cell lymphoma is primary mediastinal B-cell lymphoma. This type of lymphoma occurs mostly in young women. It starts in the mediastinum (the area in the middle of the chest behind the breastbone). It can grow quite large and can cause trouble breathing because it often presses on the windpipe (trachea) leading into the lungs. It can also block the superior vena cava (the large vein that returns blood to the heart from the arms and head), which can make the arms and face swell. This is a fast-growing lymphoma, but it usually responds well to treatment.
There are several other subtypes of diffuse large B-cell lymphoma, but these are rare.
Follicular lymphoma
About 1 out of 5 lymphomas in the United States is a follicular lymphoma. This is usually a slow-growing (indolent) lymphoma, although some follicular lymphomas can grow quickly.
The average age for people with follicular lymphoma is about 60. It’s rare in very young people. Usually, follicular lymphoma occurs in many lymph node sites throughout the body, as well as in the bone marrow.
Follicular lymphomas often respond well to treatment, but they are hard to cure. These lymphomas may not need to be treated when they are first diagnosed. Instead, treatment may be delayed until the lymphoma starts causing problems. Over time, some follicular lymphomas turn into a fast-growing diffuse large B-cell lymphoma.
Chronic lymphocytic leukemia (CLL) /small lymphocytic lymphoma (SLL)
Chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) are closely related diseases. In fact, many doctors consider them different versions of the same disease. The same type of cancer cell (known as a small lymphocyte) is seen in both chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). The only difference is where the cancer cells are found. In chronic lymphocytic leukemia, most of the cancer cells are in the blood and bone marrow. In small lymphocytic lymphoma, the cancer cells are mainly in the lymph nodes and spleen.
Both chronic lymphocytic leukemia and small lymphocytic lymphoma are usually slow-growing (indolent) diseases, although chronic lymphocytic leukemia, which is much more common, tends to grow more slowly. Treatment is the same for chronic lymphocytic leukemia and small lymphocytic lymphoma. They are usually not curable with standard treatments, but many people can live a long time (even decades) with them. Sometimes, these can turn into a more aggressive (fast-growing) type of lymphoma over time.
Mantle cell lymphoma (MCL)
About 5% of lymphomas are mantle cell lymphomas (MCL). Mantle cell lymphoma is much more common in men than in women, and it most often appears in people older than 60. When mantle cell lymphoma is diagnosed, it is usually widespread in the lymph nodes, bone marrow, and often the spleen.
Mantle cell lymphoma (MCL) can be challenging to treat. It tends to grow faster than indolent (slow-growing) lymphomas, but it doesn’t usually respond to treatment as well as aggressive (fast-growing) lymphomas. But newer treatments might offer a better chance for long-term survival for patients now being diagnosed.
Marginal zone lymphomas
Marginal zone lymphomas account for about 5% to 10% of lymphomas. They tend to be slow-growing (indolent). The cells in these lymphomas look small under the microscope. There are 3 main types of marginal zone lymphomas:
- Extranodal marginal zone B-cell lymphoma, also known as mucosa-associated lymphoid tissue (MALT) lymphoma: This is the most common type of marginal zone lymphoma. It starts in places other than the lymph nodes (extranodal). Most MALT lymphomas start in the stomach and are linked to infection by Helicobacter pylori (the bacteria that causes many stomach ulcers). MALT lymphoma might also start in the lung, skin, thyroid, salivary glands, or tissues surrounding the eye. Usually the lymphoma stays in the area where it begins and is not widespread. Many of these other MALT lymphomas have also been linked to infections with bacteria or viruses. The average age of people with MALT lymphoma at the time of diagnosis is about 60. This lymphoma tends to grow slowly and is often curable if found early. Doctors often use antibiotics as the first treatment for MALT lymphoma of the stomach, because treating the Helicobacter pylori infection often cures the lymphoma.
- Nodal marginal zone B-cell lymphoma: This is a rare disease, found mainly in older women. It starts and usually stays in the lymph nodes, although lymphoma cells can also sometimes be found in the bone marrow. Nodal marginal zone B-cell lymphoma tends to be slow-growing (although not usually as slow as MALT lymphoma), and is often curable if found early.
- Splenic marginal zone B-cell lymphoma: This is a rare lymphoma. Most often the lymphoma is found only in the spleen and bone marrow. Splenic marginal zone B-cell lymphoma is most common in older men, and often causes fatigue and discomfort due to an enlarged spleen. Because the disease is slow-growing, it might not need to be treated unless the symptoms become troublesome. This type of lymphoma has been linked to infection with the hepatitis C virus. Sometimes treating the hepatitis C virus can also treat this lymphoma.
Burkitt lymphoma
Burkitt lymphoma is a fast-growing lymphoma is named after the doctor who first described this disease in African children and young adults. Burkitt lymphoma makes up about 1% to 2% of all lymphomas. It is rare in adults, but is more common in children. It’s also much more common in males than in females.
The cells in Burkitt lymphoma are medium-sized. A similar kind of lymphoma, Burkitt-like lymphoma, has slightly larger cells. Because it is hard to tell these lymphomas apart, the WHO classification combines them.
Different varieties of Burkitt lymphoma are seen in different parts of the world:
- In the African (or endemic) variety, Burkitt lymphoma often starts as a tumor of the jaw or other facial bones. It is often linked to infection with the Epstein-Barr virus (EBV, which can also cause infectious mononucleosis or “mono”). This type of Burkitt lymphoma is rare in the United States.
- In the type seen more often in the United States, the lymphoma usually starts in the abdomen (belly), where it forms a large tumor. It can also start in the ovaries, testicles, or other organs, and can spread to the brain and spinal fluid. It is not usually linked to EBV infection.
- Another type (immunodeficiency-associated) of Burkitt lymphoma is associated with immune system problems, such as in people with HIV or AIDS or who have had an organ transplant.
Burkitt lymphoma grows very quickly, so it needs to be treated right away. But more than half of patients can be cured by intensive chemotherapy.
Lymphoplasmacytic Lymphoma and Waldenström Macroglobulinemia
Lymphoplasmacytic lymphoma and Waldenström macroglobulinemia are both slow-growing types of lymphoma that originate in a B-lymphocyte precursor. Waldenström macroglobulinemia is a type of lymphoplasmacytic lymphoma. It is when a monoclonal immunoglobulin M (IgM) protein is identified in the blood, along with lymphoplasmacytic lymphoma in the bone marrow, that the disease is referred to as Waldenström macroglobulinemia. The immunoglobulin M (IgM) protein makes the blood thicker.
Lymphoplasmacytic lymphoma and Waldenström macroglobulinemia is a slow-growing lymphoma is not common, accounting for only 1% to 2% of lymphomas. Lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia) is rare, with an incidence rate of about 3 cases per million people per year in the United States. About 1,000 to 1,500 people are diagnosed with lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia) each year in the United States. Lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia) is more common in men than it is in women and it is much more common among Whites than African Americans.
There are few cases of lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia) in younger people, but the chance of developing this disease goes up as people get older. The average age of people when they are diagnosed with lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia) is 70.
Lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia) cells make large amounts of a certain type of antibody (immunoglobulin M, or IgM), which is known as a macroglobulin. Each antibody (protein) made by the lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia) cells is the same, so it is called a monoclonal protein or just an M protein. The buildup of this M protein in the body can lead to many of the symptoms of Waldenstrom macroglobulinemia, including excess bleeding, problems with vision, and nervous system problems.
The lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia) cells grow mainly in the bone marrow, where they can crowd out the normal cells that make the different types of blood cells. This can lead to low levels of red blood cells (called anemia), which can make people feel tired and weak. It can also cause low numbers of white blood cells, which makes it hard for the body to fight infection. The numbers of platelets in the blood can also drop, leading to increased bleeding and bruising.
Hairy cell leukemia
Despite the name, hairy cell leukemia (HCL) is sometimes considered to be a type of lymphoma. Hairy cell leukemia (HCL) is rare – about 700 people in the United States are diagnosed with it each year. Men are much more likely to get hairy cell leukemia (HCL) than women, and the average age at diagnosis is around 50.
Hairy cell leukemia (HCL) cells are small B lymphocytes with projections coming off them that give them a “hairy” appearance. They are typically found in the bone marrow and spleen and in the blood.
Hairy cell leukemia is slow-growing, and some people may never need treatment. An enlarging spleen or low blood cell counts (due to cancer cells invading the bone marrow) are the usual reasons to begin treatment. If treatment is needed, it’s usually very effective.
Primary central nervous system (CNS) lymphoma
This lymphoma involves the brain or spinal cord (the central nervous system, or CNS). The lymphoma is also sometimes found in tissues around the spinal cord or the eye. Over time, it tends to become widespread in the central nervous system.
Primary CNS lymphoma is rare overall, but it’s more common in older people and in people with immune system problems, such as those who have had an organ transplant or who have AIDS. Most people develop headaches and confusion. They can also have vision problems; weakness or altered sensation in the face, arms, or legs; and in some cases, seizures.
Historically, the outlook for patients with primary CNS lymphoma has not been as good as for many other lymphomas, but this is at least partly because people with CNS lymphoma tend to be older or have other serious health problems. However, the outlook for patients with primary CNS lymphoma has improved over the years mainly due to advances in treatment.
Primary intraocular lymphoma (lymphoma of the eye)
This is a rare type of lymphoma that starts in the eyeball and is often seen along with primary CNS lymphoma. It is the second most common cancer of the eye in adults, with ocular melanoma (eye melanoma) being the first. Most people with primary intraocular lymphoma are elderly or have immune system problems which may be due to AIDS or anti-rejection drugs after an organ or tissue transplant.
People may notice bulging of the eyeball without pain, vision loss, or a blurry vision. Many of the tests done to diagnose ocular melanoma are the same used to diagnose lymphoma of the eye.
The main treatment for lymphoma of the eye is external radiation therapy if the cancer is limited to the eye. Chemotherapy (chemo) or chemotherapy in combination with radiation may be used depending on the type of lymphoma and how far it has spread outside of the eye.
T-cell lymphomas
T-cell lymphomas make up less than 15% of non-Hodgkin lymphomas in the United States. These are types of lymphoma that affect T lymphocytes. There are many types of T-cell lymphoma, but they are all fairly rare.
T-lymphoblastic lymphoma/leukemia
T-lymphoblastic lymphoma accounts for about 1% of all lymphomas. It can be considered either a lymphoma or a type of acute lymphoblastic leukemia (ALL), depending on how much of the bone marrow is involved (leukemias have more bone marrow involvement). The cancer cells are very early forms of T cells.
T-lymphoblastic lymphoma often starts in the thymus (a small organ behind the breastbone and in front of the heart, which is where many T cells are made), and can grow into a large tumor in the mediastinum (the area between the lungs). This can cause trouble breathing and swelling in the arms and face.
T-lymphoblastic lymphoma is most common in teens or young adults, with males being affected more often than females.
T-lymphoblastic lymphoma is fast-growing, but if it hasn’t spread to the bone marrow when it is first diagnosed, the chance of cure with chemotherapy is quite good.
Often, the lymphoma form of this disease is treated in the same way as the leukemia form. For more information, see Acute Lymphocytic Leukemia.
Peripheral T-cell lymphomas
Peripheral T-cell lymphomas are uncommon types of lymphomas that develop from more mature forms of T cells.
Cutaneous T-cell lymphomas (mycosis fungoides, Sezary syndrome, and others)
Cutaneous T-cell lymphomas start in the skin. Skin lymphomas account for about 5% of all lymphomas.
- Mycosis fungoides (MF): Nearly half of all skin lymphomas are mycosis fungoides. Mycosis fungoides can occur in people of any age, but most who get it are in their 50s or 60s. Men are almost twice as likely as women to develop this lymphoma. The first sign of mycosis fungoides is one or more patchy, scaly, red lesions (abnormal areas) on the skin. Mycosis fungoides lesions can be very itchy. Often these lesions are the only symptom of mycosis fungoides. But in some people the disease can progress to more solid, raised tumors on the skin (called plaques). Because mycosis fungoides can be confused with other skin problems, it can be hard to diagnose at first. Several biopsies of the lesions might be needed before the diagnosis is confirmed. Over time, mycosis fungoides can spread across the skin or invade lymph nodes and organs like the liver. In many people this disease grows slowly, but it can sometimes grow more quickly, especially in older people. Some people with mycosis fungoides go on to develop Sezary syndrome. Rare variants of mycosis fungoides include folliculotropic mycosis fungoides, pagetoid reticulosis, and granulomatous slack skin.
- Sezary syndrome (SS): This is often thought of as an advanced form of mycosis fungoides, but these are actually different diseases. In Sezary syndrome, most or all of the skin is affected, instead of just patches of skin. People with Sezary syndrome typically have a very itchy, scaly red rash that can look like a severe sunburn. This is called generalized erythroderma. The skin is often thickened. Lymphoma cells, called Sezary cells, can be found in the blood (as well as in the lymph nodes). Whereas mycosis fungoides. is usually slow growing, Sezary syndrome tends to grow and spread faster, and is harder to treat. People with Sezary syndrome also often have further weakened immune systems, which increases their risk of serious infections.
- Adult T cell leukemia-lymphoma (ATLL): This rare type of T-cell lymphoma is more likely to start in other parts of the body, but it can sometimes be confined to the skin. It is linked to infection with the HTLV-1 virus (although most people infected with this virus do not get lymphoma). It is much more common in Japan and the Caribbean islands than other parts of the world. Adult T cell leukemia-lymphoma often grows quickly, but in some cases it advances slowly, or even shrinks on its own for a time.
- Primary cutaneous anaplastic large cell lymphoma (C-ALCL): This lymphoma usually starts as one or a few tumors on the skin, which can vary in size. Some of these may break open (ulcerate). Most people with this disease are in their 50s or 60s, but it can also occur in children. It is found at least twice as often in men as in women. In most cases it does not spread beyond the skin, and the prognosis (outlook) is very good.
- Lymphomatoid papulosis: This is a benign, slow-growing disease that often comes and goes on its own, even without treatment. In fact, some doctors think of it not as a lymphoma, but rather as an inflammatory disease that might progress to a lymphoma. But under a microscope, it has features that look like primary cutaneous anaplastic large cell lymphoma (C-ALCL). Lymphomatoid papulosis often begins as several large pimple-like lesions that may break open in the middle. Lymphomatoid papulosis is seen in younger people more often than other T-cell skin lymphomas, with an average age of around 45. Men get this disease more often than women. Lymphomatoid papulosis often goes away without treatment, but it can take anywhere from a few months to many years to go away completely. Lymphomatoid papulosis doesn’t spread to internal organs and is not fatal. Rarely, some people with this skin disorder develop another, more serious type of lymphoma.
- Subcutaneous panniculitis-like T-cell lymphoma: Subcutaneous panniculitis-like T-cell lymphoma is a rare lymphoma that invades the deepest layers of the skin, where it causes nodules (lumps) to form. Most often these are on the legs, but they can occur anywhere on the body. Subcutaneous panniculitis-like T-cell lymphoma affects all ages and both sexes equally. It usually grows slowly and tends to have a good outlook.
- Extranodal NK/T-cell lymphoma, nasal type: Extranodal NK/T-cell lymphoma, nasal type is a rare type of lymphoma can start in T-cells or in other lymphocytes known as natural killer (NK) cells. It typically starts in the nose or sinuses, but sometimes it can start in the skin. Extranodal NK/T-cell lymphoma, nasal type has been linked to infection with the Epstein-Barr virus (EBV), and is more common in Asia and Central and South America. It tends to grow quickly.
- Primary cutaneous peripheral T-cell lymphoma (CTCL), rare subtypes: This is a group of rare skin lymphomas that don’t fit into any of the above categories. There are several types.
- Primary cutaneous gamma/delta T-cell lymphoma develops as thickened plaques (raised lesions) or actual tumors, mainly on skin of the arms and legs, but sometimes in the intestines or lining of the nose. This type of lymphoma tends to grow and spread quickly.
- Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma develops as widespread patches, nodules and tumors that often break open in the middle. This type of lymphoma can sometimes look like mycosis fungoides, but a biopsy can tell them apart. This lymphoma tends to grow and spread quickly.
- Primary cutaneous acral CD8+ T-cell lymphoma is very rare, and typically starts as a nodule on the ear, although it can also start on other parts of the body, such as the nose, hand, or foot. It tends to grow slowly and can often be cured with treatment.
- Primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder often starts as a single area of thickening of the skin or a tumor on the head, neck, or upper body. This disease tends to grow slowly and can often be cured with treatment.
Adult T-cell leukemia/lymphoma
Adult T-cell leukemia/lymphoma is caused by infection with a virus called HTLV-1. It is rare in the United States, and much more common in Japan, the Caribbean, and parts of Africa – where infection with HTLV-1 is more common. It can affect the bone marrow (where new blood cells are made), lymph nodes, spleen, liver, skin, and other organs. There are 4 subtypes:
- The smoldering subtype tends to grow slowly and has a good prognosis.
- The chronic subtype also grows slowly and has a good prognosis.
- The acute subtype is the most common. It grows quickly like acute leukemia, so it needs to be treated right away.
- The lymphoma subtype grows more quickly than the chronic and smoldering types, but not as fast as the acute type.
Angioimmunoblastic T-cell lymphoma
Angioimmunoblastic T-cell lymphoma accounts for about 4% of all lymphomas. It is more common in older adults. It tends to involve the lymph nodes as well as the spleen or liver, which can become enlarged. People with this lymphoma usually have fever, weight loss, and skin rashes and often develop infections. Angioimmunoblastic T-cell lymphoma often progresses quickly. Treatment is often effective at first, but the lymphoma tends to come back (recur).
Extranodal natural killer/T-cell lymphoma, nasal type
Extranodal natural killer/T-cell lymphoma, nasal type is a rare type often involving the upper airway passages, such as the nose and upper throat, but it can also invade the skin, digestive tract, and other organs. Extranodal natural killer/T-cell lymphoma, nasal type is much more common in parts of Asia and South America. Cells of this lymphoma are similar in some ways to natural killer (NK) cells, another type of lymphocyte.
Enteropathy-associated intestinal T-cell lymphoma (EATL)
Enteropathy-associated intestinal T-cell lymphoma (EATL) is a lymphoma that occurs in the lining of the intestine. This lymphoma is most common in the small intestine, but can also occur in the colon. Symptoms can include severe abdominal (belly) pain, nausea, and vomiting. There are 2 subtypes of enteropathy-associated intestinal T-cell lymphoma (EATL):
- Type I enteropathy-associated intestinal T-cell lymphoma (EATL) occurs in some people with celiac disease also called gluten-sensitive enteropathy. Celiac disease is an autoimmune disease in which eating gluten, a protein found mainly in wheat and barley, causes the immune system to attack the lining of the intestine and other parts of the body. Type I EATL is rare among people who have had celiac disease since childhood, and is more common in people diagnosed as older adults. This lymphoma is more common in men than women.
- Type II enteropathy-associated intestinal T-cell lymphoma (EATL) is not linked to celiac disease and is less common than type I.
Anaplastic large cell lymphoma (ALCL)
About 2% of lymphomas are of anaplastic large cell lymphoma (ALCL). It is more common in young people (including children), but it can also affect older adults. Anaplastic large cell lymphoma (ALCL) tends to be fast-growing, but many people with this lymphoma can be cured.
There are different forms of anaplastic large cell lymphoma (ALCL):
- Primary cutaneous anaplastic large cell lymphoma (ALCL) only affects the skin. This is discussed in more detail in Lymphoma of the Skin.
- Systemic anaplastic large cell lymphoma (ALCL) can affect the lymph nodes and other organs, including the skin. Systemic ALCL is divided into 2 types based on whether the lymphoma cells have a change in the ALK gene. ALK-positive ALCL is more common in younger people and tends to have a better prognosis (outlook) than the ALK-negative type.
- Breast implant-associated anaplastic large cell lymphoma (ALCL) is a rare type of ALCL that can develop in the breasts of women who have had implants. It seems to be more likely to occur if the implant surfaces are textured (as opposed to smooth).
Peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS)
This name is given to T-cell lymphomas that don’t readily fit into any of the groups above. Most people diagnosed with these lymphomas are in their 60s. These lymphomas often involve the lymph nodes, but they can affect the skin, bone marrow, spleen, liver, and digestive tract, as well. As a group, these lymphomas tend to be widespread and grow quickly. Some patients respond well to chemotherapy, but over time these lymphomas tend to become harder to treat.
Non hodgkin’s lymphoma signs and symptoms
The most common symptom of non-Hodgkin lymphoma is a painless swelling in a lymph node (also known as lymphadenopathy), usually in the neck, armpit or groin.
Some people have no symptoms and the disease may only be discovered during a routine medical examination or while the patient is under care for an unrelated condition.
B Symptoms. Fever, drenching night sweats and loss of more than 10 percent of body weight over six months are sometimes termed “B symptoms” and are significant to the prognosis and staging of the disease. Other non-Hodgkin’s lymphoma symptoms, such as itching and fatigue, do not have the same prognostic importance as the symptoms designated as B symptoms. Furthermore, they are not considered to be B symptoms.
Non-Hodgkin lymphoma can cause many symptoms, such as:
- Painless swelling in one or more lymph node(s)
- Unexplained weight loss
- Fever
- Soaking night sweats
- Coughing, trouble breathing or chest pain
- Weakness and tiredness that don’t go away
- Loss of appetite
- Pain, swelling or a feeling of fullness in the abdomen (due to an enlarged spleen)
- Itchy skin
- Enlargement of the spleen or liver
- Rashes or skin lumps
The signs and symptoms of non-Hodgkin lymphoma are also associated with a number of other, less serious diseases.
A person who has signs or symptoms that suggest the possibility of non-Hodgkin lymphoma (NHL) is usually referred to a blood cancer specialist called a hematologist-oncologist. The doctor will order additional tests and a tissue biopsy to make a diagnosis.
A few people with lymphoma have abnormal cells in their bone marrow when they’re diagnosed.
This may lead to:
- persistent tiredness or fatigue
- an increased risk of infections
- excessive bleeding, such as nosebleeds, heavy periods and spots of blood under the skin.
Non-Hodgkin’s lymphoma complications
Life-threatening emergent complications of NHL should be considered during the initial workup and evaluation. Early recognition and prompt therapy are critical for these situations, which may interfere with and delay treatment of the underlying non-Hodgkin’s lymphoma. These can include:
- Febrile neutropenia
- Hyperuricemia and tumor lysis syndrome – Presents with fatigue, nausea, vomiting, decreased urination, numbness, and tingling of legs and joint pain. Laboratory findings include an increase in uric acid, potassium, creatinine, phosphate, and a decrease in calcium level. This can be prevented with vigorous hydration and allopurinol.
- Spinal cord or brain compression
- Focal compression depending on the location and type of non-Hodgkin’s lymphoma – airway obstruction (mediastinal lymphoma), intestinal obstruction and intussusception, ureteral obstruction
- Superior or inferior vena cava obstruction
- Hyperleukocytosis
- Adult T-cell leukemia-lymphoma can cause hypercalcemia.
- Pericardial tamponade
- Lymphoplasmacytic lymphoma with Waldenstrom macroglobulinemia can cause hyperviscosity syndrome.
- Hepatic dysfunction 6
- Venous thromboembolic disease 7
- Autoimmune hemolytic anemia and thrombocytopenia – can be observed with small lymphocytic lymphoma
Non-Hodgkin’s lymphoma causes
The exact cause of non-Hodgkin’s lymphoma is not known but there are risk factors that may increase a person’s likelihood of developing the disease. Non-Hodgkin lymphoma is caused by a change (mutation) in the DNA of a type of white blood cell called lymphocytes, although the exact reason why this happens isn’t known. Non-Hodgkin lymphomas may be associated with various factors, including infections, environmental factors, immunodeficiency states, and chronic inflammation 8.
Various viruses have been attributed to different types of non-Hodgkin lymphoma 8:
- Epstein-Barr virus (EBV), a DNA virus, is associated with the causation of certain types of non-Hodgkin lymphoma, including an endemic variant of Burkitt lymphoma.
- Human T-cell leukemia virus type 1 (HTLV-1) causes adult T-cell lymphoma. It induces chronic antigenic stimulation and cytokine dysregulation, resulting in uncontrolled B- or T-cell stimulation and proliferation.
- Hepatitis C virus (HCV) results in clonal B-cell expansions. Splenic marginal zone lymphoma and diffuse large B cell lymphoma are some subtypes of NHL due to the Hepatitis C virus.
- Helicobacter pylori infection is associated with an increased risk of gastric mucosa-associated lymphoid tissue (MALT) lymphomas, a primary gastrointestinal lymphoma.
Drugs like phenytoin, digoxin, TNF antagonist are also associated with non-Hodgkin lymphoma 8. Moreover, organic chemicals, pesticides, phenoxy-herbicides, wood preservatives, dust, hair dye, solvents, chemotherapy, and radiation exposure are also associated with the development of non-Hodgkin lymphoma 9, 10.
Congenital immunodeficiency states associated with increased risk of NHL are Wiskott-Aldrich syndrome, severe combined immunodeficiency disease (SCID), and induced immunodeficiency states like immunosuppressant medications. Patients with AIDS (Acquired immunodeficiency syndrome) can have primary central nervous system (brain and spinal cord) lymphoma.
The autoimmune disorders like Sjögren syndrome, rheumatoid arthritis, and Hashimoto thyroiditis are associated with an increased risk of NHL. Hashimoto’s thyroiditis is associate with primary thyroid lymphomas 11. Celiac disease is also associated with an increased risk of non-Hodgkin lymphoma.
Non-Hodgkin lymphoma can occur at any age, but a third cases are diagnosed in people 60 or over. Overall, non-Hodgkin lymphoma is common in age 65 to 74, the median age being 67 years. And the condition is slightly more common in men than women.
Non-Hodgkin’s lymphoma usually starts with an abnormal change in a white cell in a lymph node or lymphoid tissue called a lymphocyte. It can start in one of three major types of lymphocytes:
- B lymphocytes (B cells): B cells normally help protect the body against germs (bacteria or viruses) by making proteins called antibodies. The antibodies attach to the germs, marking them for destruction by other parts of the immune system.
- T lymphocytes (T cells): There are several types of T cells. Some T cells destroy germs or abnormal cells in the body. Other T cells help boost or slow the activity of other immune system cells.
- Natural killer (NK) cells, which attack virus-infected cells or tumor cells
About 85 percent of non-Hodgkin’s lymphoma cases start in the B cells. Your doctor plans your treatment according to the type of cell your non-Hodgkin’s lymphoma developed in.
The abnormal lymphocyte grows out of control and produces more abnormal cells like it.
- These abnormal lymphocytes (lymphoma cells) accumulate and form masses (tumors). If non-Hodgkin’s lymphoma isn’t treated, the cancerous cells crowd out normal white cells, and the immune system can’t guard against infection effectively.
- Non-Hodgkin’s lymphoma that develops in or spreads to other areas of the body where lymphoid tissue is found, such as the spleen, digestive tract and bone marrow, is called primary extranodal lymphoma.
- Non-Hodgkin’s lymphoma is classified into more than 60 different subtypes. Doctors classify the non-Hodgkin’s lymphoma subtypes into categories that describe how rapidly or slowly the disease is progressing:
- Aggressive non-Hodgkin’s lymphoma
- Indolent (slow-growing) non-Hodgkin’s lymphoma
Risk Factors non-Hodgkin’s lymphoma
A risk factor is anything that increases your chance of getting a disease like cancer. However, most people diagnosed with non-Hodgkin’s lymphoma don’t have any obvious risk factors. And many people who have risk factors for the disease never develop it.
Researchers have found several factors that can affect a person’s chance of getting non-Hodgkin lymphoma. There are many types of lymphoma, and some of these factors have been linked only to certain types.
Immune suppression or having a weakened immune system is one of the most clearly established risk factors for non-Hodgkin’s lymphoma. People with autoimmune disease, acquired immunodeficiencies including human immunodeficiency virus infection (HIV) and acquired immune deficiency syndrome (AIDS), and organ transplant recipients have an elevated risk for non-Hodgkin’s lymphoma. In some genetic (inherited) syndromes, such as ataxia-telangiectasia and Wiskott-Aldrich syndrome, children are born with a deficient immune system. Along with an increased risk of serious infections, these children also have a higher risk of developing non-Hodgkin lymphoma. In addition, factors that suppress the immune system, such as chemical exposures or treatments for autoimmune diseases, may contribute to the development of non-Hodgkin’s lymphoma.
Autoimmune diseases. Some autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE or lupus), Sjogren (Sjögren) disease, celiac disease (gluten-sensitive enteropathy), and others have been linked with an increased risk of NHL. In autoimmune diseases, the immune system mistakenly sees the body’s own tissues as foreign and attacks them, as it would a germ. Lymphocytes (the cells from which lymphomas start) are part of the body’s immune system. The overactive immune system in autoimmune diseases may make lymphocytes grow and divide more often than normal. This might increase the risk of them developing into lymphoma cells.
Exposure to certain chemicals and drugs. Some studies have suggested that chemicals such as benzene and certain herbicides and insecticides (weed- and insect-killing substances) may be linked to an increased risk of non-Hodgkin lymphoma. Research to clarify these possible links is still in progress.
Farming communities have a higher incidence of non-Hodgkin’s lymphoma. Some studies suggest that specific ingredients in herbicides and pesticides such as organochlorine, organophosphate and phenoxy acid compounds, are linked to lymphoma. The number of lymphoma cases caused by such exposures has not been determined. More studies are needed to understand these associations.
Some chemotherapy drugs used to treat other cancers may increase the risk of developing non-Hodgkin lymphoma many years later. For example, patients who have been treated for Hodgkin lymphoma have an increased risk of later developing NHL. But it’s not totally clear if this is related to the disease itself or if it is an effect of the treatment.
Some studies have suggested that certain drugs used to treat rheumatoid arthritis, such as methotrexate and the tumor necrosis factor (TNF) inhibitors, might increase the risk of non-Hodgkin lymphoma. But other studies have not found an increased risk. Determining if these drugs increase risk is complicated by the fact that people with rheumatoid arthritis, which is an autoimmune disease, already have a higher risk of NHL.
Exposure to certain viruses and bacteria is associated with non-Hodgkin’s lymphoma. It is thought that infection with either a virus or a bacterium can lead to intense lymphoid cell proliferation, increasing the probability of a cancer-causing event in a cell. Here are some examples:
- Epstein-Barr virus (EBV) infection. Infection with the Epstein-Barr virus (EBV) is an important risk factor for Burkitt lymphoma in some parts of Africa. In developed countries such as the United States, EBV is more often linked with lymphomas in people also infected with HIV, the virus that causes AIDS. EBV has also been linked with some less common types of lymphoma.
- Human T-cell lymphotropic virus-1 (HTLV-1). Human T-cell lymphotropic virus (HTLV-1) is most common in some parts of Japan and in the Caribbean region, but it’s found throughout the world. In the United States, it causes less than 1% of lymphomas. HTLV-1 spreads through sex and contaminated blood and can be passed to children through breast milk from an infected mother.
- Human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS). Infection with human immunodeficiency virus (HIV), also known as the AIDS virus, can weaken the immune system. HIV infection is a risk factor for developing certain types of non-Hodgkin lymphoma, such as primary central nervous system lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma.
- Human herpes virus 8 (HHV-8) can also infect lymphocytes, leading to a rare type of lymphoma called primary effusion lymphoma. This lymphoma is most often seen in patients who are infected with HIV. HHV-8 infection is also linked to another cancer, Kaposi sarcoma. For this reason, another name for this virus is Kaposi sarcoma-associated herpes virus (KSHV).
- The bacterium Helicobacter Pylori (H Pylori). Helicobacter pylori, a type of bacteria known to cause stomach ulcers, has also been linked to mucosa-associated lymphoid tissue (MALT) lymphoma of the stomach.
- Hepatitis C. Long-term infection with the hepatitis C virus (HCV) seems to be a risk factor for certain types of lymphoma, such as splenic marginal zone lymphoma.
- Chlamydophila psittaci (formerly known as Chlamydia psittaci) is a type of bacteria that can cause a lung infection called psittacosis. It has been linked to MALT lymphoma in the tissues around the eye (called ocular adnexal marginal zone lymphoma).
- Infection with the bacterium Campylobacter jejuni has been linked to a type of MALT lymphoma called immunoproliferative small intestinal disease. This type of lymphoma, which is also sometimes called Mediterranean abdominal lymphoma, typically occurs in young adults in eastern Mediterranean countries.
Other conditions, such as Sjögren syndrome, Wiskott-Aldrich syndrome and Klinefelter syndrome can predispose individuals to later development of non-Hodgkin’s lymphoma.
Age. Getting older is a strong risk factor for lymphoma overall, with most cases occurring in people in their 60s or older. But some types of lymphoma are more common in younger people.
Gender. Overall, the risk of NHL is higher in men than in women, but there are certain types of NHL that are more common in women. The reasons for this are not known.
Race, ethnicity, and geography. In the United States, whites are more likely than African Americans and Asian Americans to develop non-Hodgkin lymphoma. Worldwide, non-Hodgkin lymphoma is more common in developed countries, with the United States and Europe having some of the highest rates. Some types of lymphoma are linked to certain infections that are more common in some parts of the world.
Radiation exposure. Studies of survivors of atomic bombs and nuclear reactor accidents have shown they have an increased risk of developing several types of cancer, including NHL, leukemia,and thyroid cancer. Patients treated with radiation therapy for some other cancers, such as Hodgkin lymphoma, have a slightly increased risk of developing non-Hodgkin lymphoma later in life. This risk is greater for patients treated with both radiation therapy and chemotherapy.
Being overweight or obese. Some studies have suggested that being overweight or obese might increase your risk of non-Hodgkin lymphoma. More research is needed to confirm these findings. In any event, staying at a healthy weight, keeping physically active, and following a healthy eating pattern that includes plenty of fruits, vegetables, and whole grains, and that limits or avoids red and processed meats, sugary drinks, and highly processed foods has many known health benefits outside of the possible effect on lymphoma risk.
Breast implants. Although it is rare, some women with breast implants develop a type of anaplastic large cell lymphoma (ALCL) in their breast. This seems to be more likely with implants that have textured (rough) surfaces (as opposed to smooth surfaces).
Non-Hodgkin lymphoma isn’t infectious and isn’t thought to run in families, although your risk may be slightly increased if a first-degree relative (such as a parent or sibling) has had lymphoma.
Non-Hodgkin lymphoma prevention
There is no sure way to prevent non-Hodgkin lymphoma. Most people with NHL have no risk factors that can be changed, so there is no way to protect against these lymphomas. But there are some things you can do that might lower your risk for non-Hodgkin lymphoma, such as limiting your risk of certain infections and doing what you can to maintain a healthy immune system.
Infection with HIV, the virus that causes AIDS, is known to increase the risk non-Hodgkin lymphoma, so one way to limit your risk is to avoid known risk factors for HIV, such as intravenous drug use or unprotected sex with many partners.
Preventing the spread of the human T-cell lymphotropic virus (HTLV-1) could have a great impact on non-Hodgkin lymphoma in areas of the world where this virus is common, such as Japan and the Caribbean region. The virus is rare in the United States but seems to be increasing in some areas. The same strategies used to prevent HIV spread could also help control HTLV-1.
Helicobacter pylori (H. pylori) infection has been linked to some lymphomas of the stomach. Treating H. pylori infections with antibiotics and antacids may lower this risk, but the benefit of this strategy has not been proven yet. Most people with H. pylori infection have no symptoms, and some have only mild heartburn. More research is needed to find the best way to detect and treat this infection in people without symptoms.
Some lymphomas are caused by treatment of other cancers with radiation and chemotherapy or by the use of immune-suppressing drugs to avoid rejection of transplanted organs. Doctors are trying to find better ways to treat cancer and organ transplant patients without increasing the risk of lymphoma as much. But for now, the benefits of these treatments still usually outweigh the small risk of developing lymphoma many years later.
Some studies have suggested that being overweight or obese may increase your risk of non-Hodgkin lymphoma. Staying at a healthy weight, keeping physically active, and following a healthy eating pattern that includes plenty of fruits, vegetables, and whole grains, and that limits or avoids red and processed meats, sugary drinks, and highly processed foods may help protect against lymphoma, but more research is needed to confirm this.
Non-Hodgkin lymphoma diagnosis
Most people with non-Hodgkin lymphoma (NHL) see their doctor because they have felt a lump that hasn’t gone away, they develop some of the other symptoms of non-Hodgkin’s lymphoma, or they just don’t feel well and go in for a check-up.
Your doctor will likely ask you about your personal and family medical history. He or she may then have you undergo tests and procedures used to diagnose non-Hodgkin’s lymphoma, including:
- Physical exam. Your doctor checks for swollen lymph nodes, including in your neck, underarm and groin, as well as for a swollen spleen or liver.
- Blood and urine tests. Blood and urine tests may help rule out an infection or other disease.
- Imaging tests. Your doctor may recommend imaging tests to look for signs of lymphoma cells elsewhere in your body. Tests may include CT, MRI and positron emission tomography (PET).
- Lymph node test. Your doctor may recommend a lymph node biopsy procedure to remove all or part of a lymph node for laboratory testing. Analyzing lymph node tissue in a lab may reveal whether you have non-Hodgkin’s lymphoma and, if so, which type.
- Bone marrow test. A bone marrow biopsy and aspiration procedure involves inserting a needle into your hipbone to remove a sample of bone marrow. The sample is analyzed to look for non-Hodgkin’s lymphoma cells.
- Lumbar puncture (spinal tap). If there’s a concern that the lymphoma may affect the fluid around your spinal cord, your doctor might recommend a procedure to remove some of the fluid for testing. During a spinal tap, the doctor inserts a small needle into the spinal canal in your lower back.
Other tests and procedures may be used depending on your situation.
The only way to confirm a diagnosis of non-Hodgkin lymphoma is by carrying out a lymph node biopsy. This is a minor surgical procedure where a sample of affected lymph node tissue is removed and studied in a laboratory. But a lymph node biopsy is not always done right away because many symptoms of non-Hodgkin lymphoma can also be caused by other problems, like an infection, or by other kinds of cancer. For example, enlarged lymph nodes are more often caused by infections than by lymphoma. Because of this, doctors often prescribe antibiotics and wait a few weeks to see if the lymph nodes shrink. If the nodes stay the same or continue to grow, the doctor might order a biopsy.
A lymph node biopsy might be needed right away if the size, texture, or location of a lymph node or the presence of other symptoms strongly suggests lymphoma.
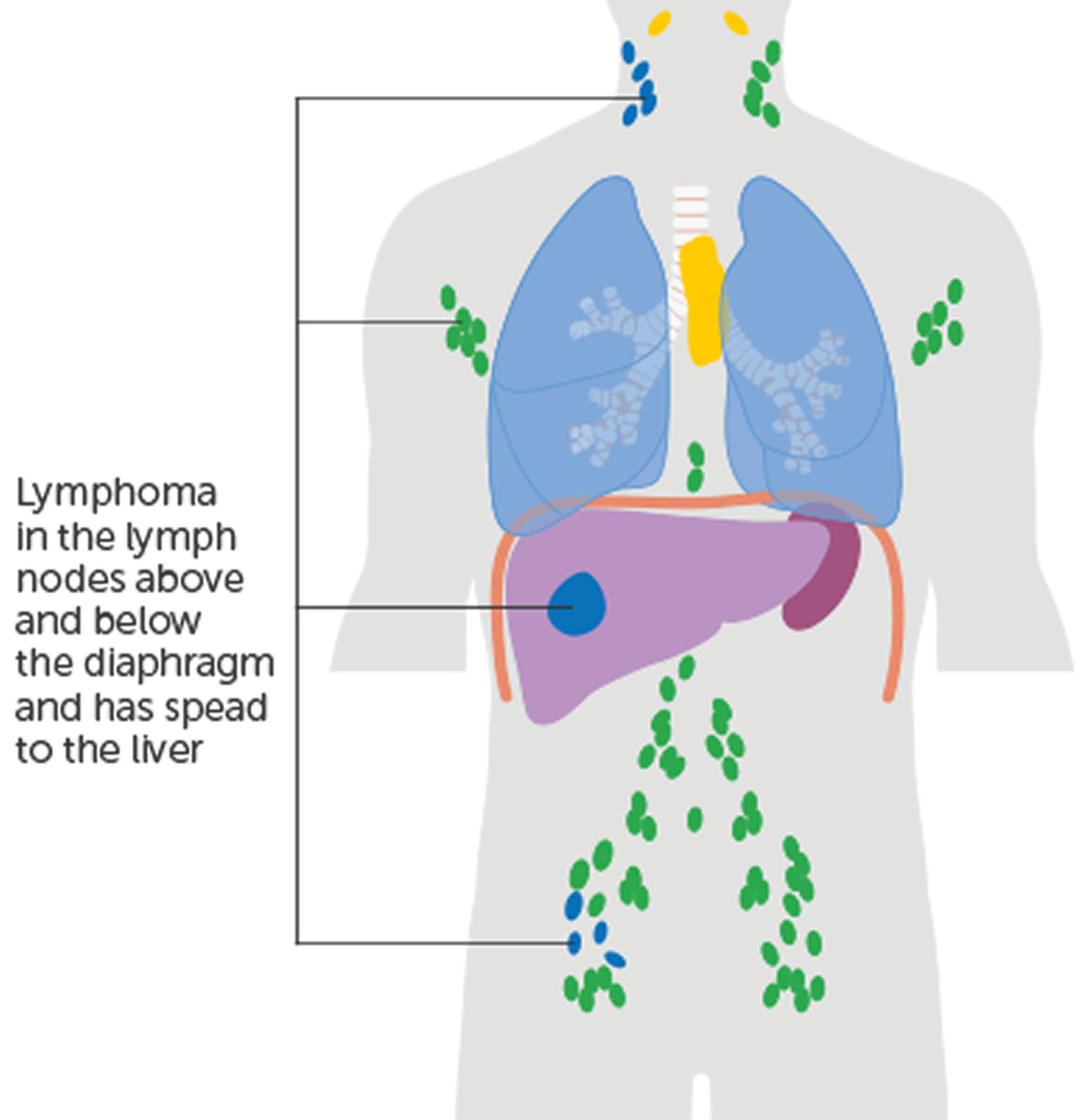
Non-Hodgkin Lymphoma can develop in parts of the body that do not involve lymph nodes, such as the lung or bone. When lymphoma is detected exclusively outside of the lymph nodes, it is called “primary extranodal lymphoma,” and the biopsy specimen is taken from that involved tissue.
Lymph Node Biopsy
Diagnosing non-Hodgkin lymphoma usually involves performing a lymph node biopsy. If the biopsy confirms that you have non-Hodgkin lymphoma, your doctor performs additional tests to stage the lymphoma.
The purpose of a lymph node biopsy is to:
- Confirm a diagnosis
- Identify your non-Hodgkin lymphoma subtype
- Develop a treatment plan.
There are several types of biopsies. Doctors choose which one to use based on each person’s situation.
Excisional or incisional lymph node biopsy
This is the preferred and most common type of lymph node biopsy if lymphoma is suspected, because it almost always provides enough of a sample to diagnose the exact type of non-Hodgkin lymphoma.
In this procedure, a surgeon cuts through the skin to remove the lymph node.
- If the doctor removes the entire lymph node, it is called an excisional biopsy.
- If a small part of a larger tumor or node is removed, it is called an incisional biopsy.
If the enlarged node is just under the skin, this is a fairly simple operation that can often be done with local anesthesia (numbing medicine). But if the node is inside the chest or abdomen, you will be sedated (given drugs to make you drowsy and relaxed) or given general anesthesia (drugs to put you into a deep sleep).
Needle lymph node biopsy
Needle biopsies are less invasive than excisional or incisional biopsies, but the drawback is that they might not remove enough of a sample to diagnose lymphoma (or to determine which type it is). Most doctors do not use needle biopsies to diagnose lymphoma. But if the doctor suspects that your lymph node is enlarged because of an infection or by the spread of cancer from another organ (such as the breast, lungs, or thyroid), a needle biopsy may be the first type of biopsy done. An excisional biopsy might still be needed even after a needle biopsy has been done, to diagnose and classify lymphoma.
There are 2 main types of needle biopsies:
- In a fine needle aspiration (FNA) biopsy, the doctor uses a very thin, hollow needle attached to a syringe to withdraw (aspirate) a small amount of tissue from an enlarged lymph node or a tumor mass.
- For a core needle biopsy, the doctor uses a larger needle to remove a slightly larger piece of tissue.
To biopsy an enlarged node just under the skin, the doctor can aim the needle while feeling the node. If the node or tumor is deep inside the body, the doctor can guide the needle using a computed tomography (CT) scan or ultrasound (see descriptions of imaging tests later in this section).
If lymphoma has already been diagnosed, needle biopsies are sometimes used to check abnormal areas in other parts of the body that might be from the lymphoma spreading or coming back after treatment.
Lab Tests to Confirm a Diagnosis
After your doctor takes samples of your lymph node and tissues, a hematopathologist examines them under a microscope to look for identifying characteristics of non-Hodgkin’s lymphoma. He or she confirms a diagnosis and identifies the NHL subtype. A hematopathologist is a specialist who studies blood cell diseases by looking at samples of blood and bone marrow cells and other tissues.
The hematopathologist uses one or more lab tests such as those below to examine your cells:
- Immunophenotyping and flow cytometry: For both flow cytometry and immunohistochemistry, the biopsy samples are treated with antibodies that stick to certain proteins on cells. The cells are then looked at in the lab (immunohistochemistry) or with a special machine (for flow cytometry), to see if the antibodies attached to them. These tests can help determine whether a lymph node is swollen because of lymphoma, some other cancer, or a non-cancerous disease. The tests can also be used for immunophenotyping – determining which type of lymphoma a person has, based on certain proteins in or on the cells. Different types of lymphocytes have different proteins on their surface, which correspond to the type of lymphocyte and how mature it is.
- Chromosome tests: Normal human cells have 23 pairs of chromosomes (strands of DNA), each of which is a certain size and looks a certain way in the lab. But in some types of lymphoma, the cells have changes in their chromosomes, such as having too many, too few, or abnormal chromosomes. These changes can often help identify the type of lymphoma.
- Cytogenetic analysis: In this lab test, the cells are checked for any abnormalities in the chromosomes.
- Fluorescent in situ hybridization (FISH): This test looks more closely at lymphoma cell DNA using special fluorescent dyes that only attach to specific genes or parts of chromosomes. FISH can find most chromosome changes that can be seen in standard cytogenetic tests, as well as some gene changes too small to be seen with cytogenetic testing. FISH is very accurate and can usually provide results within a couple of days.
- Polymerase chain reaction (PCR): PCR is a very sensitive DNA test that can find gene changes and certain chromosome changes too small to be seen with a microscope, even if very few lymphoma cells are present in a sample.
Since non-Hodgkin’s lymphoma is a difficult disease to diagnose, you may want to get a second medical opinion by an experienced hematopathologist before you begin treatment. Some types of NHL can be confused with each other. The appropriate treatment depends on having the correct diagnosis.
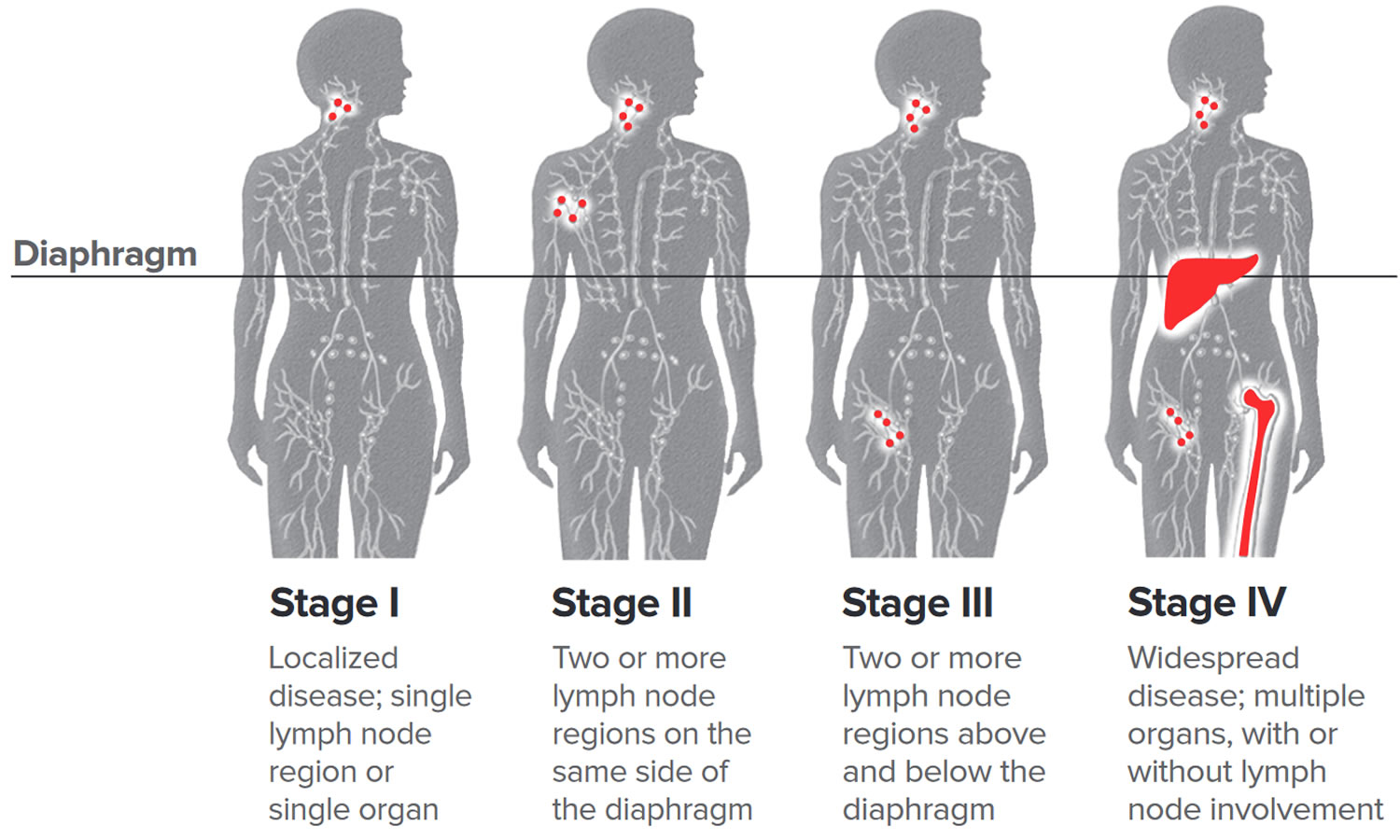
Staging Tests
Once your doctor confirms an non-Hodgkin’s lymphoma diagnosis, he or she runs more tests to stage your disease. Staging identifies the extent of your disease and where it’s located in your body.
Staging tests include:
- Physical exam
- Biopsies of enlarged lymph nodes or other abnormal areas
- Blood tests
- Imaging tests, such as PET and CT scans
- Bone marrow aspiration and biopsy (often but not always done)
- Lumbar puncture (spinal tap – this may not need to be done)
Imaging Tests
Your doctor conducts one or more imaging tests (also called diagnostic radiology), along with a physical exam, to look for:
- The location and distribution of lymph node enlargement
- Whether organs other than the lymph nodes are involved
- If there are very large masses of tumors in one site or another.
Imaging tests may include:
- Chest x-rays. The chest might be x-rayed to look for enlarged lymph nodes in this area.
- CT (computed tomography) scan. A CT scan combines many x-rays to make detailed, cross-sectional images of your body. This scan can help tell if any lymph nodes or organs in your body are enlarged. CT scans are useful for looking for lymphoma in the abdomen, pelvis, chest, head, and neck.
- CT-guided needle biopsy: A CT can also be used to guide a biopsy needle into a suspicious area. For this procedure, you lie on the CT scanning table while the doctor moves a biopsy needle through the skin and toward the area. CT scans are repeated until the needle is in the right place. A biopsy sample is then removed to be looked at in the lab.
- FDG-PET (fluorodeoxyglucose (FDG) positron emission tomography) scan. For a PET scan, you are injected with a slightly radioactive form of sugar (fluorodeoxyglucose [FDG]), which collects mainly in cancer cells. A special camera is then used to create a picture of areas of radioactivity in the body. The picture is not detailed like a CT or MRI scan, but it can provide helpful information about your whole body. If you have lymphoma, a PET scan might be done to:
- See if an enlarged lymph node contains lymphoma.
- Find small areas that might be lymphoma, even if the area looks normal on a CT scan.
- Check if a lymphoma is responding to treatment. Some doctors will repeat the PET scan after 1 or 2 courses of chemotherapy. If the chemotherapy is working, the lymph nodes will no longer absorb the radioactive sugar.
- Help decide whether an enlarged lymph node still contains lymphoma or is just scar tissue after treatment.
- Magnetic resonance imaging (MRI). Like CT scans, MRI scans show detailed images of soft tissues in the body. But MRI scans use radio waves and strong magnets instead of x-rays. This test is not used as often as CT scans for lymphoma, but if your doctor is concerned about spread to the spinal cord or brain, MRI is very useful for looking at these areas.
- PET-CT scan. Some machines can do both a PET scan and a CT scan at the same time. This lets the doctor compare areas of higher radioactivity on the PET scan with the more detailed appearance of that area on the CT scan. PET/CT scans can often help pinpoint the areas of lymphoma better than a CT scan alone.
- Bone scan. Bone scan is usually done if a person is having bone pain or has lab results that suggest the lymphoma may have reached the bones. For bone scans, a radioactive substance called technetium is injected into a vein. It travels to damaged areas of bone, and a special camera can then detect the radioactivity. Lymphoma often causes bone damage, which may be seen on a bone scan. But bone scans can’t show the difference between cancers and non-cancerous problems, such as arthritis and fractures, so further tests might be needed.
Blood Tests
After your blood is taken, it’s sent to a lab for a complete blood count (CBC) and more blood work. Blood tests are used to:
- Determine whether lymphoma cells are present in the blood and if the special proteins (called “immunoglobulins”) made by lymphocytes are either deficient or abnormal
- Check indicators of disease severity by examining blood protein levels, uric acid levels and erythrocyte sedimentation rate (ESR)
- Assess kidney and liver functions
- Measure two important biological markers, lactate dehydrogenase (LDH) and beta2-microglobulin which are helpful prognostic indicators for several non-Hodgkin lymphoma subtypes
- For some types of lymphoma or if certain treatments might be used, your doctor may also advise you to have tests to see if you’ve been infected with certain viruses, such as hepatitis B virus (HBV), hepatitis C virus (HCV), or human immunodeficiency virus (HIV). Infections with these viruses may affect your treatment.
A complete blood count (CBC) may show:
- Anemia (low red blood cell counts)
- Neutropenia (low levels of neutrophils, a type of white blood cells)
- Thrombocytopenia (low platelet levels)
Bone marrow aspiration and biopsy
Most patients diagnosed with NHL undergo a bone marrow biopsy to make sure there is no spread of the disease to the bone marrow and to evaluate the use of specific therapies including radioimmunotherapy (a combination of radiation therapy and immunotherapy). A bone marrow biopsy may not always be required for patients with early-stage disease who also have low-risk features, for example, NHL with no B symptoms and no large masses.
For a bone marrow aspiration, you lie on a table (either on your side or on your belly). After cleaning the skin over the hip, the doctor numbs the area and the surface of the bone with local anesthetic, which can cause a brief stinging or burning sensation. A thin, hollow needle is then inserted into the bone and a syringe is used to suck out a small amount of liquid bone marrow. Even with the anesthetic, most people still have some brief pain when the marrow is removed.
A bone marrow biopsy is usually done just after the aspiration. A small piece of bone and marrow is removed with a slightly larger needle that is pushed into the bone. The biopsy can also cause some brief pain.
Lumbar puncture (spinal tap)
Lumbar puncture (spinal tap) test looks for lymphoma cells in the cerebrospinal fluid (CSF), which is the liquid that bathes the brain and spinal cord. Most people with lymphoma will not need this test. But doctors may order it for certain types of lymphoma or if a person has symptoms that suggest the lymphoma may have reached the brain.
For this test, you may lie on your side or sit up. The doctor first numbs an area in the lower part of your back over the spine. A small, hollow needle is then placed between the bones of the spine to withdraw some of the fluid.
Pleural or peritoneal fluid sampling
Lymphoma that has spread to the chest or abdomen can cause fluid to build up. Pleural fluid (inside the chest) or peritoneal fluid (inside the abdomen) can be removed by placing a hollow needle through the skin into the chest or abdomen.
- When this procedure is used to remove fluid from the area around the lung, it’s called a thoracentesis.
- When it is used to collect fluid from inside the abdomen, it’s known as a paracentesis.
The doctor uses a local anesthetic to numb the skin before inserting the needle. The fluid is then taken out and checked in the lab for lymphoma cells.
Other Tests for Specific Subtypes
Certain tests are performed for specific subtypes only and not necessary for all patients with NHL. They include:
- Full evaluation of the gastrointestinal (GI) tract, including upper and lower endoscopies for patients who have disease involving the gastrointestinal tract, such as mucosa-associated lymphoid tissue (MALT) lymphoma
- Colonoscopy for patients with mantle cell lymphoma (routine colonoscopy is important for all persons beginning at age 50, or earlier if there is a family history of colon cancer)
- Testicular ultrasound for patients who have a testicular mass
- Spinal tap (lumbar puncture) and/or MRI of the brain or spinal column may be required for patients with certain subtypes or symptoms that suggest central nervous system involvement.
Non-Hodgkin’s lymphoma Subtypes
More than 60 specific Non-Hodgkin’s lymphoma subtypes have been identified and assigned names by the World Health Organization (WHO). NHL subtypes are categorized by the characteristics of the lymphoma cells, including their appearance, the presence of proteins on the surface of the cells and their genetic features. It’s important to know your subtype since it plays a large part in determining the type of treatment you’ll receive. A hematopathologist, a doctor who specializes in the diagnosis of blood disorders and blood cancers, should review your biopsy specimens.
Specialists further characterize the Non-Hodgkin’s lymphoma subtypes according to how the disease progresses:
- Aggressive lymphomas also known as high grade lymphomas are fast-moving and account for about 60 percent of all NHL cases. Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive NHL subtype.
- Indolent lymphomas also known as low grade lymphomas are slow-moving and tend to grow more slowly and have fewer signs and symptoms when first diagnosed. Slow-growing or indolent subtypes represent about 40 percent of all NHL cases. Follicular lymphoma (FL) is the most common subtype of indolent Non-Hodgkin’s lymphoma.
The treatments for aggressive and indolent lymphomas are different. When a patient’s rate of disease progression is between indolent and aggressive, he or she is considered to have “intermediate grade” disease. Some cases of indolent Non-Hodgkin’s lymphoma can transform into aggressive Non-Hodgkin’s lymphoma.
Table 1. Most Common Subtypes of Non-Hodgkin’s Lymphoma
| Most Common Subtypes of Non-Hodgkin Lymphoma |
|---|
Aggressive
|
Indolent
|
Below are the Non-Hodgkin’s lymphoma diagnostic designations of the subtypes for non-Hodgkin lymphoma. The descriptive part of the names (eg, follicular, mantle cell or marginal zone) in some disease subtypes refers to the specific areas of the lymph nodes (the follicle, mantle and marginal zones) where the lymphoma appears to have originated.
Table 2. Diagnostic Designations for Non-Hodgkin’s Lymphoma Subtypes
| Diagnostic Designations for Non-Hodgkin Lymphoma (NHL) Subtypes |
|---|
Mature B-cell lymphomas
|
Mature T-cell and NK-cell lymphomas
|
Non-Hodgkin’s lymphoma Staging
After someone is diagnosed with Non-Hodgkin Lymphoma, doctors will try to figure out if it has spread, and if so, how far. This process is called staging. The stage of a cancer describes how much cancer is in the body. It helps determine how serious the cancer is and how best to treat it. Staging helps your doctor predict the disease’s progression and develop a treatment plan.
The current staging system for non-Hodgkin lymphoma in adults is known as the Lugano classification, which is based on the older Ann Arbor system. The stages are described by Roman numerals I through IV (1-4). Limited stage (1 or 2) lymphomas that affect an organ outside the lymph system (an extranodal organ) have an E added (for example, stage 2E).
Non-Hodgkin’s lymphoma doesn’t always begin in stage 1 and spread to more advanced stages. More than half of all patients with intermediate or aggressive disease and more than 80 percent of all patients with indolent disease are diagnosed with stage 3 or 4 non-Hodgkin lymphoma. And if someone is diagnosed in stage 4, it doesn’t mean that the disease is incurable – it may be highly curable depending on the subtype. “Stage 4” does not have the same implications in non-Hodgkin lymphoma as it does for many other cancers.
The Lugano classification is most often used to stage non-Hodgkin lymphoma if it is only in lymph nodes. But if the non-Hodgkin lymphoma is affecting the blood or bone marrow, it is often staged using the systems for Chronic Lymphocytic Leukemia Stages.
Your doctor may also describe your non-Hodgkin lymphoma as being limited (early) or advanced stage.
- Limited stage non-Hodgkin lymphoma. Limited disease generally means you have stage 1 or stage 2 non-Hodgkin lymphoma.
- Advanced stage non-Hodgkin lymphoma. Advanced disease means you have stage 3 or stage 4 lymphoma.
Some people with stage 2 bulky lymphoma might have advanced disease, depending on their circumstances.
You doctor can explain what stage you have and what this means in your situation.
How doctors work out your non-Hodgkin lymphoma stage
Doctors look at whether your non-Hodgkin lymphoma is on one side, or both sides of the diaphragm. And whether it is inside or outside of the lymphatic system. They also measure the size of the lymphoma. They do this by carrying out various tests, such as a CT or PET scan.
- The diaphragm. The diaphragm is the big breathing muscle that separates the chest from the abdominal (belly) area. Doctors use the diaphragm as a guide because it is about halfway down the body.
- Inside or outside of the lymphatic system. Doctors look at whether the lymphoma is affecting:
- the lymph nodes and organs of the lymphatic system – these are called lymphatic sites. Lymphatic sites include a group of lymph nodes or an organ of the lymphatic system, such as the thymus or spleen.
- areas outside of the lymphatic sites – called extranodal (or extralymphatic) sites. Extranodal sites include the lungs, liver, blood, bone marrow, kidneys, brain and spinal cord.
- Your doctor may use the letter E after the stage number if you have lymphoma outside of the lymphatic system. Your doctor or nurse can explain what this means in your situation.
- Bulky disease. This means that you have areas of lymphoma that measure above a certain size. The exact measurement depends on your type of NHL. For example, in follicular lymphoma, bulky disease measures over 6 cm. In diffuse large B cell lymphoma, bulky disease is usually greater than 10 cm.
Ann Arbor Staging System for non-Hodgkin lymphoma
Stage 1 non-Hodgkin lymphoma
Stage 1 non-Hodgkin lymphoma means that you have one of the following:
- lymphoma in a single lymph node or one group of lymph nodes, or an organ of the lymphatic system (such as the thymus or tonsils) (1)
- lymphoma involvement of one organ or area outside the lymph nodes (extranodal site) (1E)
Stage 2 non-Hodgkin lymphoma
Stage 2 non-Hodgkin lymphoma means one of the following:
- Involvement of two or more lymph node regions and both are either above or below the diaphragm. For example, this might include nodes in the underarm and neck area (2) but not the combination of underarm and groin nodes (3).
- Involvement of one or more lymph node groups either above or below the diaphragm and outside the lymph nodes in an organ or area on the same side of the diaphragm as the affected lymph nodes (2E)
- lymphoma involvement of multiple lymph node regions on same side of the diaphragm with “bulky disease” (2 Bulky)
In all cases, the sites of the lymphoma are on the same side of the diaphragm.
Stage 3 non-Hodgkin lymphoma
Stage 3 non-Hodgkin lymphoma means that you have lymphoma on both sides of the diaphragm. One example is that the lymphoma is in lymph nodes on both sides of the diaphragm. Another example (see below) is that the lymphoma is in lymph nodes above the diaphragm, as well as lymphoma in the spleen.
- Stage 3: Involvement of lymph node regions above and below the diaphragm (for example, neck, chest and abdomen)
- Stage 3E: Involvement of lymph node groups above and below the diaphragm and outside of the lymph nodes in a nearby organ or area
- Stage 3S: Involvement of lymph node groups above and below the diaphragm and in the spleen
- Stage 3E+S: Involvement of lymph node groups above and below the diaphragm, outside the lymph nodes in a nearby organ or area, and in the spleen
Stage 4 non-Hodgkin lymphoma
Stage 4 non-Hodgkin lymphoma means one of the following:
- your lymphoma is in one of more organs that area not part of a lymphatic area and in lymph nodes near those organs
- OR
- your lymphoma is in one organ that is not part of a lymphatic area and of organs or lymph nodes far away from that organ
- OR
- your lymphoma is in the liver, bone marrow, cerebrospinal fluid or lungs
Categories
- “E” stands for extranodal. It means the lymphoma extends to an area or organ beyond the lymphatic system
- “S” stands for spleen and it means the lymphoma is found in this organ
- “X” indicates “bulky disease.” This is a nodal mass whose greatest size is usually more than 10 cm or more than one third of the chest diameter by x-ray
Figure 5. Non-Hodgkin Lymphoma Stages
Lugano classification of non-Hodgkin lymphoma
The current staging system for non-Hodgkin lymphoma in adults is known as the Lugano classification, which is based on the older Ann Arbor system.
Table 3. Non-Hodgkin’s lymphoma stages
| Stage | Involvement | Extranodal (E) status |
|---|---|---|
| Limited | ||
| Stage 1 | One node or a group of adjacent nodes | Single extranodal lesions without nodal involvement (1E) |
| Stage 2 | Two or more nodal groups on the same side of the diaphragm | Stage 1 or 2 by nodal extent with limited contiguous extranodal involvement |
| Stage 2 bulky | II as above with “bulky” disease | Not applicable |
| Advanced | ||
| Stage 3 | Nodes on both sides of the diaphragm Nodes above the diaphragm with spleen involvement | Not applicable |
| Stage 4 | Additional non-contiguous extralymphatic involvement | Not applicable |
Categories A, B, X, S and E
The four stages of Non-Hodgkin’s lymphoma can be divided into categories:
- A Category: “A” is put after your stage if you have no additional symptoms other than swollen lymph nodes.
- B Category: “B” is put after your stage if you have additional symptoms of fever, drenching night sweats, loss of more than 10 percent of body weight over the previous six months (without dieting)
- X Category: “Bulky disease”. This term is often used to describe large tumors in the chest. This is a nodal mass whose greatest size is usually more than 10 cm or more than onethird of the chest diameter by x-ray. It is especially important for stage II lymphomas, as bulky disease might need more intensive treatment.
- E Category: Involvement of organs or tissues beyond the lymph system
- S Category: “S” stands for spleen and means the lymphoma is found in this organ.
For example, stage 2B indicates that the patient has
- Two lymph node sites near each other with disease involvement (for example, enlarged lymph nodes in the neck and near the collarbone, or in the neck and the armpit)
- Fever, excessive sweating and/or weight loss (any one of these symptoms).
When all the diagnostic and staging tests are completed, the doctor will evaluate the information, identify the non-Hodgkin’s lymphoma subtype, determine which areas of the body are involved and begin to discuss treatment options with the patient.
Non-Hodgkin’s lymphoma Treatment
It’s important that your doctor is experienced in treating patients with non-Hodgkin lymphoma (NHL) or works in consultation with an non-Hodgkin’s lymphoma specialist. This type of specialist is usually called a hematologist oncologist.
The stage of your non-Hodgkin lymphoma helps your doctor to decide which treatment you need. Your treatment also depends on:
- the type of non-Hodgkin lymphoma (the type of cells the cancer started in)
- the grade of non-Hodgkin lymphoma (the appearance of cells or the number of certain cells in your lymphoma)
- where the non-Hodgkin lymphoma is
- your symptoms
- other health conditions that you have
- your age
Types of Treatment
Doctors use several types of approaches and treatment combinations for non-Hodgkin’s lymphoma, some at different stages:
- Chemotherapy and other drug therapy. Chemotherapy is a widely used treatment for non-Hodgkin lymphoma that involves using medicine to kill cancer cells. Chemotherapy may be used on its own, combined with biological therapy, or combined with radiotherapy. The medication can be given in a number of different ways, depending on the stage of your cancer. You’ll normally get chemotherapy through a drip directly into a vein (intravenous chemotherapy), as tablets taken by mouth, or a combination of both. If there’s a risk of the cancer spreading to your brain, you may have chemotherapy injections directly into the cerebrospinal fluid around your spine. Chemotherapy is usually given over a period of a few months on an outpatient basis, which means you get treatment during the day and shouldn’t have to stay in hospital overnight.
- Steroid medication. Steroid medication is commonly used in combination with chemotherapy to treat non-Hodgkin lymphoma. This is because research has shown that using steroids makes the chemotherapy more effective. The steroid medication is normally given as tablets or injections, usually at the same time as your chemotherapy. You’ll usually take the steroids for a few days or 1 week during each cycle of chemotherapy, and take breaks in between. This helps to reduce the side effects.
- Radiation therapy (radiotherapy), usually combined with chemotherapy. Radiotherapy is most often used to treat early-stage non-Hodgkin lymphoma, where the cancer is only in 1 part of the body. Treatment is normally given in short daily sessions, Monday to Friday, usually for no more than 3 weeks. You shouldn’t have to stay in hospital between appointments. Radiotherapy itself is painless, but it can have some significant side effects. These can vary, depending on which part of your body is being treated.
- Targeted drug therapy. Targeted drug treatments focus on specific abnormalities present within cancer cells. By blocking these abnormalities, targeted drug treatments can cause cancer cells to die. For non-Hodgkin’s lymphoma, targeted drugs can be used alone, but are often combined with chemotherapy. This combination can be used as your initial treatment and as a second treatment if your lymphoma comes back.
- Immunotherapy. Immunotherapy is treatment that either boosts the patient’s own immune system or uses man-made versions of the normal parts of the immune system to kill lymphoma cells or slow their growth. Immunotherapy drugs may be an option for certain types of non-Hodgkin’s lymphoma if other treatments haven’t helped.
- Monoclonal antibody therapy. For some types of non-Hodgkin lymphoma, you may have a type of medication called a monoclonal antibody. These medications attach themselves to both healthy and cancerous cells, and signal to the immune system to attack and kill the cells. You may be given monoclonal antibody therapy as your only treatment, or they’re sometimes given in combination with chemotherapy to make the treatment more effective. For some types of non-Hodgkin lymphoma, you may continue having monoclonal antibody treatment regularly for up to 2 years after initial treatment, in combination with chemotherapy. This can reduce the chances of the cancer coming back in the future.
- Chimeric antigen receptor (CAR)-T cell therapy. A specialized treatment called chimeric antigen receptor (CAR)-T cell therapy takes your body’s germ-fighting T cells, engineers them to fight cancer and infuses them back into your body. CAR-T cell therapy might be an option for certain types of B-cell non-Hodgkin’s lymphoma that haven’t responded to other treatments.
- Stem cell transplantation (bone marrow transplant). Bone marrow transplant or stem cell transplant, involves using high doses of chemotherapy and radiation to suppress your bone marrow and immune system. Then healthy bone marrow stem cells from your body or from a donor are infused into your blood where they travel to your bones and rebuild your bone marrow. For people with non-Hodgkin’s lymphoma, a bone marrow transplant might be an option if other treatments haven’t helped.
- The main types of stem cell transplantation are:
- Allogeneic stem cell transplantation—using stem cells from a matched or partially matched donor, either related or unrelated to the patient.
- Allogeneic transplantation may be considered in the treatment of indolent forms of non-Hodgkin’s lymphoma, particularly for younger patients whose disease behaves more aggressively or has high-risk features.
- Autologous stem cell transplantation—using your own stem cells (taken before the conditioning chemotherapy is given).
- Autologous stem cell transplantation remains a key component of the standard medical care for patients with aggressive forms of non-Hodgkin’s lymphoma. For indolent lymphomas, autologous stem cell transplantation is primarily used to treat patients with relapsed non-Hodgkin’s lymphoma.
- Reduced-intensity stem cell transplantation—a form of allogeneic transplantation in which patients receive lower doses of chemotherapy drugs and/or radiation therapy in
preparation for the transplant.
- Allogeneic stem cell transplantation—using stem cells from a matched or partially matched donor, either related or unrelated to the patient.
- The main types of stem cell transplantation are:
- Watch-and-wait. If the disease is low grade (slow developing) and you’re well, a period of “watch and wait” is often recommended. This is because some people take many years to develop troublesome symptoms and starting treatment immediately is often felt to be unnecessary. If watch and wait is recommended, you’ll be seen regularly for reviews and invited to come back at any stage if you feel your symptoms are getting worse.
- Your doctor may suggest that you participate in a clinical trial. Clinical trials can involve therapy with new drugs and new drug combinations or new approaches to stem cell transplantation.
Some common drug combinations used in the treatment of Non-Hodgkin Lymphoma (NHL)
- CHOP: cyclophosphamide, doxorubicin (hydroxydoxorubicin), Oncovin® (vincristine), prednisone
- B+R: bendamustine hydrochloride (Bendeka®) plus rituximab
- R+ICE: rituximab plus ifosfamide, carboplatin, etoposide
- R-CHOP: rituximab plus cyclophosphamide, doxorubicin (hydroxydoxorubicin), Oncovin® (vincristine), prednisone
- R-HCVAD: rituximab plus cyclophosphamide, vincristine, adriamycin (doxorubicin), dexamethasone
- R-EPOCH: rituximab plus adjusted etoposide, prednisone, vincristine (Oncovin®), cyclophosphamide, doxorubicin
- DHAP: dexamethasone, high-dose cytarabine (ara-C®) and cisplatin (Platinol®)
- ICE: ifosfamide, carboplatin, etoposide
There can also be vast differences between treatment for aggressive non-Hodgkin’s lymphoma and treatment for indolent non-Hodgkin’s lymphoma. Every patient should discuss treatment options with his or her doctor and ask for help to understand the benefits and risks of different treatment approaches. The most effective treatment plan for each patient with non-Hodgkin lymphoma is individualized based on:
- The subtype of non-Hodgkin lymphoma—knowing whether the lymphoma cells are related to T cells, B cells or natural killer (NK) cells gives the doctor important clues about appropriate treatments
- The stage and category of the disease, which is important information that is factored into treatment decisions
- The presence or absence of fever, drenching night sweats and loss of more than 10 percent of body weight over 6 months, referred to as “B symptoms”
- Whether there is lymphoma in areas of the body outside of the lymph nodes (extranodal involvement)
- Other prognostic factors, such as age and any underlying medical conditions.
The patient’s age may be a factor, but older age is no longer a major determinant in treatment decisions for most patients. However, the patient’s overall health status, including other medical problems, and also his or her wishes concerning treatment are significant considerations. When making treatment decisions, it is important to discuss effects on fertility and other possible long-term and late effects of treatment.
Pretreatment Considerations
Adults of childbearing age and parents of children diagnosed with non-Hodgkin’s lymphoma should ask their doctors for information that may lessen the risk for infertility.
Finding the Best Treatment Approach
The goal of non-Hodgkin’s lymphoma treatment is to destroy as many lymphoma cells as possible to induce a complete remission (no trace of the disease). Patients who go into remission are sometimes cured of the disease.
Treatment can keep non-Hodgkin’s lymphoma in check for many years, even if tests show some lingering lymphoma cells. This is called partial remission.
The treatment your doctor recommends is based on several factors, including:
- Your disease subtype
- Whether your disease is aggressive (fast growing) or indolent (slow growing)
- Your disease stage and category
- Whether the lymphoma is in areas of your body other than your lymph nodes (extranodal involvement)
- Your overall health and whether you have any conditions like heart disease, kidney disease, lung disease, diabetes or anemia
As you develop a treatment plan with your doctor, be sure to discuss:
- The results you can expect from treatment
- Potential side effects, including long-term and late-term effects
- The possibility of participating in a clinical trial, where you’ll have access to advanced medical treatment that may be more beneficial to you than standard treatment
You may find it helpful to bring a loved one with you to your doctor’s visits for support, to take notes and ask follow-up questions. It’s a good idea to prepare questions you’d like to ask when you visit your doctor. You can also record your conversations with your doctor and listen more closely when you get home.
Treatment for Aggressive Non-Hodgkin’s lymphoma Subtypes
Aggressive non-Hodgkin lymphoma (NHL) progresses rapidly. It makes up about 60 percent of all non-Hodgkin’s lymphoma cases in the United States. Aggressive subtypes include:
- AIDS-associated lymphoma
- Burkitt lymphoma
- Central nervous system (CNS) lymphoma
- Diffuse large B-cell lymphoma (DLBCL)
- Mantle cell lymphoma (MCL)
- Peripheral T-cell lymphoma (PTCL)
- T-cell lymphoblastic (T-LBL)
Patients with fast-growing non-Hodgkin’s lymphoma are frequently treated with chemotherapy that consists of four or more drugs. In most cases, this is the combination therapy called R-CHOP (rituximab [Rituxan®], cyclophosphamide [Cytoxan®], doxorubicin [hydroxydoxorubicin], Oncovin® [vincristine] and prednisone). This intensive, multidrug chemotherapy can be very effective for aggressive lymphoma, and cures have been achieved. Chemotherapy can be supplemented by radiation therapy in select cases, for instance, when large NHL masses are found during the diagnostic and staging process.
Treating Specific Aggressive Subtypes
AIDS-Associated Lymphoma
The types of non-Hodgkin lymphoma that are most often seen in people with acquired immune deficiency syndrome (AIDS) are diffuse large B-cell lymphoma, Burkitt lymphoma and primary central nervous system (CNS) lymphoma. Treatment outcomes depend on how well the patient with AIDS responds to therapy and manages the effects of chemotherapy on blood counts. Because AIDS already leads to low blood cell counts, chemotherapy must be carefully considered to determine whether the chemotherapy’s additional effects on blood levels can be managed. The number of people developing AIDS-associated non-Hodgkin lymphoma has decreased in the last several years because of improved treatment of HIV, the virus that can lead to AIDS.
The use of highly active anti-retroviral therapy (HAART) to treat HIV has helped patients to better tolerate treatments such as chemotherapy and immunotherapy.
Most experts believe that the prognosis (outlook) for a person with HIV-associated lymphoma relates at least as much to the HIV infection as to the lymphoma. Modern anti-HIV therapy can often control the immune deficiency in patients with AIDS, so the outlook for patients who develop lymphoma has improved.
Burkitt Lymphoma
This B-cell subtype is considered to be highly aggressive. It may involve the jaw, bones of the face, bowel, kidneys, ovaries, bone marrow, blood, central nervous system (CNS) and other organs. Burkitt lymphoma was named after Dr. Dennis Burkitt, a surgeon who first identified the disease in children in Africa. In Africa, the disease usually appears in children as a mass in a facial bone, especially the jaw.
Doctors typically use highly aggressive chemotherapy to treat this subtype of non-Hodgkin’s lymphoma. Commonly used regimens include:
- CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin and high-dose methotrexate) alternating with IVAC (ifosfamide, etoposide and high-dose cytarabine)
- Hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone) alternating with methotrexate and cytarabine
- DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin plus rituximab)
Studies report that Burkitt lymphoma is curable in a significant group of patients when treated with high-dose, multidrug chemotherapy regimens that include central nervous system (CNS) prophylaxis. About 60 to 90 percent of children and young adults with the disease achieve durable remissions if treated timely and appropriately. Older patients with Burkitt lymphoma have less favorable outcomes than younger patients.
Patients with relapsed or refractory Burkitt lymphoma are encouraged to participate in clinical trials. Consolidation treatment with a high-dose conditioning therapy and autologous stem cell transplantation (or allogeneic transplantation, if a donor is available) may be considered for patients who achieve remission after their second-line treatment.
Central Nervous System Lymphoma
There are two types of central nervous system (CNS) lymphoma: primary and secondary. Primary CNS lymphoma starts in the brain and/or the spinal cord. It is often a feature of AIDS-associated lymphoma, although it may be related to other non-Hodgkin’s lymphoma subtypes as well. Secondary CNS lymphoma starts in another area of the body and spreads to the brain and/or spinal cord.
Both primary and secondary CNS lymphomas are uncommon. Standard treatment may include chemotherapy, glucocorticoid drugs and/or radiation therapy. Immunotherapy and high-dose chemotherapy with stem cell transplantation are being studied in clinical trials.
Diffuse Large B-Cell Lymphoma
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin’s lymphoma subtype, making up about 31 percent of all non-Hodgkin’s lymphoma cases in the United States. It affects mostly middle-aged and older adults. DLBCL usually starts in lymph nodes in the neck or abdomen and is characterized by masses of large B cells (lymphocytes). DLBCL grows rapidly and frequently involves the spleen, liver, bone marrow or other organs. In addition, patients with diffuse large B-cell lymphoma (DLBCL) often experience B symptoms (fever, night sweats and loss of more than 10 percent of body weight over 6 months).
For some patients, DLBCL may be the initial diagnosis. For other patients, their indolent lymphomas such as small lymphocytic lymphoma or follicular lymphoma transform and become DLBCL.
DLBCL is frequently treated with a chemotherapy regimen that is made up of four or more drugs. One regimen is R-CHOP. Another more intense regimen is DA-EPOCH-R, which is a combination of dose-adjusted etoposide (Etopophos®, VePesid®, Toposar®), prednisone, vincristine (Oncovin®), cyclophosphamide, hydroxydoxorubicin (doxorubicin) plus rituximab.
Gene expression profiling has been used to categorize patients into groups by diffuse large B-cell lymphoma (DLBCL) subtype. For example, one group of patients may have different responses to therapy than others; another group may have a different clinical presentation based on the number and types of genes that are either more active or less active in the tumor sample. To date, gene expression profiling studies have distinguished three molecular subtypes of DLBCL based on the cell of origin. They are:
- Germinal center B-cell (GCB)
- Activated B-cell (ABC)
- Primary mediastinal B-cell lymphoma (PMBCL).
These distinct DLBCL subtypes arise due to specific genetic changes. Because gene expression profiling is not commercially available, most hematologist-oncologists, working with hematopathologists, will perform immunophenotyping to identify the specific proteins that are associated with either the germinal center B-cell (GCB) or the non-GCB subtypes of DLBCL. Therefore, the disease is most commonly classified into GCB and non-GCB subtypes.
According to some studies, DLBCL patients who have the germinal center B-cell (GCB) subtype experience significantly better treatment outcomes than those with non-GCB subtypes. A number of clinical trials are under way to investigate whether using novel approaches to therapy improves treatment outcomes for patients with non-GCB subtypes of DLBCL.
Primary mediastinal B-cell lymphoma (PMBCL) is a non-GCB subtype of DLBCL characterized by the overgrowth of scarlike lymph tissue. A tumor generally forms behind the breastbone and may cause coughing and difficulty breathing. The tumor is often very large and can cause pressure on the blood vessels or the heart and lungs. It occurs mainly in young adults around age 35 years and affects slightly more women than men.
Patients with primary mediastinal B-cell lymphoma (PMBCL) often need more intensive treatment than other patients with DLBCL. There are two standard combination regimens: EPOCH-R and R-CHOP. EPOCH-R is currently being used more often as a treatment for PMBCL, as there is less need for radiotherapy with this regimen.
For patients who experience a relapsed diffuse large B-cell lymphoma (DLBCL), additional chemotherapy called a “salvage” treatment is given, which may include drugs that were not used previously. The goal of salvage treatment is to achieve a remission so that it is not necessary to use high-dose chemotherapy or to perform autologous stem-cell transplantation. Treatment options for replapsed DLBCL include:
- Axicabtagene ciloleucel (Yescarta®). This treatment, given by IV, is an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy indicated for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. Axicabtagene ciloleucel is not indicated for the treatment of patients who have primary central nervous system (CNS) lymphoma.
- Tisagenlecleucel (Kymriah®). This treatment, given by IV, is a CD19-directed genetically modified autologous T-cell, anti-CD19 chimeric antigen receptor (CAR) immunotherapy indicated for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma and DLBCL arising from follicular lymphoma. Tisagenlecleucel is not indicated for treatment of patients with primary central nervous system lymphoma.
Pembrolizumab (Keytruda®) given by IV, is indicated for the treatment of adult and pediatric patients with refractory primary mediastinal B-cell lymphoma (PMBCL) or for those whose disease has relapsed after two or more prior lines of therapy. Pembrolizumab is not recommended for treating patients with primary mediastinal B-cell lymphoma (PMBCL) who require urgent therapy to reduce the extent of their disease.
Polatuzumab vedotin-piiq (PolivyTM) given by IV, is a CD79b-directed antibody-drug conjugate that is indicated in combination with bendamustine (Bendeka®) and a rituximab product for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, after at least two prior therapies.
High-dose chemotherapy and an autologous stem cell transplant may be used to treat patients who relapse after disease remission, but only a minority of patients achieve long-term remissions with this therapy. Allogeneic stem cell transplant remains a potential cure for relapsed diffuse large B-cell lymphoma (DLBCL), but some patients may not qualify for a transplant due to advanced age or the presence of other medical conditions. The efficacy of reduced-intensity transplantation is being evaluated in clinical trials.
Mantle Cell Lymphoma
Mantle cell lymphoma (MCL) originates from a lymphocyte in the mantle zone of the lymph node. It begins in the lymph nodes and spreads to the spleen, blood, bone marrow and sometimes the esophagus, stomach and intestines. Mantle cell lymphoma usually occurs in people over age 50 and is found in more frequently in men than in women. Some patients do not show signs or symptoms of the disease, so delaying treatment may be an option for them. Most patients need to start treatment after diagnosis.
The standard treatment is a combination chemotherapy regimen, either with or without an autologous stem cell transplant. Common treatment regimens include bendamustine plus rituximab; a form of CHOP in which bortezomib is used instead of vincristine; and various regimens including high-dose cytarabine. The following agents are indicated for relapsed and refractory mantle cell lymphoma: acalabrutinib (Calquence®), given by mouth; bortezomib (Velcade®), given by IV or subcutaneous injection; ibrutinib (Imbruvica®), given by mouth; zanubrutinib (Brukinsa®), given by mouth; lenalidomide (Revlimid®), given by mouth; and brexucabtagene autoleucel (Tecartus™), given by IV. Allogeneic transplantation with a standard or reduced-intensity conditioning regimen may be considered for patients with relapsed and refractory mantle cell lymphoma who achieve remission following second-line therapy.
Peripheral T-Cell Lymphoma
Peripheral T-cell lymphoma (PTCL) refers to a group of aggressive non-Hodgkin’s lymphoma subtypes that originate in mature T-cell lymphocytes and natural killer (NK) cells. Peripheral T-cell lymphoma (PTCL) accountfor approximately 10 percent of non Hodgkin’s lymphoma cases. PTCL generally affects people age 60 and older. The most common subtypes include:
- Peripheral T-cell, not otherwise specified (PTCL NOS) – This is the most common subtype of PTCL accounting for about 30 percent of PTCL cases. It often involves lymph node sites but can also involve other areas such as the liver, bone marrow, gastrointestinal track and skin.
- Anaplastic Large Cell Lymphoma (ALCL) – Anaplastic large-cell lymphoma (ALCL) subtype accounts for about 12 percent of PTCL cases. This subtype usually starts in the lymph nodes and can spread to the skin. There are two main subtypes of ALCL:
- Systemic ALCL ALK-1 positive anaplastic large cell lymphoma – About 80 percent of patients with this subtype are cured. This disease is more common in young people.
- Systemic ALCL ALK-1 negative anaplastic large cell lymphoma – This subtype occurs mainly in older patients. Treatment with chemotherapy or radiation therapy is often less successful and a stem cell transplant may be discussed.
- Primary cutaneous anaplastic large cell lymphoma (pcALCL) – This subtype mostly affects the skin, but other parts of the body may be involved.
- Angioimmunoblastic T-cell lymphoma (AITL) – One of the most common types in the US and Europe. It is associated with B cells infected with the Epstein-Barr virus. This type of T-cell lymphoma often involves lymph nodes and the bone marrow. Many patients have “paraneoplastic symptoms” including fevers, rashes and abnormal protein levels in their blood.
- Extranodal natural killer/T-cell lymphoma (ENK/TCL)—This is an uncommon type of lymphoma that can occur in the nasal sinuses or in other parts of the body. It is usually a very aggressive lymphoma that requires both chemotherapy and radiation.
- Adult T-Cell Leukemia/ Lymphoma (ATLL)—This is a rare and aggressive type of PTCL that is associated with the human T-cell lymphotropic virus-1 (HTLV-1).
- Monomorphic Epitheliotropic Intestinal T-cell Lymphoma (MEITL)—This is a lymphoma of the gastrointestinal tract that is NOT associated with celiac disease.
- Hepatosplenic T-cell lymphoma (HSTCL) – This subtype of PTCL starts in the liver and spleen and usually affects young men.
- Enteropathy-associated T-cell lymphoma (EATL)—This T-cell lymphoma frequently develops in the small bowel of patients with untreated celiac disease.
- Subcutaneous Panniculitis-like T-cell Lymphoma (SPTCL)— This is a very rare form of skin lymphoma that occurs primarily in the subcutaneous fat tissue, where it causes nodules to form.
- Primary Cutaneous gamma/delta T-cell lymphoma (PCGDTCL)—This is a very rare and aggressive skin lymphoma.
Peripheral T-cell lymphoma is commonly treated with the regimens used for diffuse large B-cell lymphoma (DLBCL). Chemotherapy with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or other drug combinations is the standard treatment for newly diagnosed peripheral T-cell lymphoma. For early-stage disease, radiation therapy may be added. Another option for some of these lymphomas might be the chemo combination of cyclophosphamide, doxorubicin, and prednisone, along with the monoclonal antibody brentuximab vedotin (Adcetris). A stem cell transplant may be recommended when possible.
If other treatments are no longer working, newer chemo drugs such as pralatrexate (Folotyn), targeted drugs such as bortezomib (Velcade) or belinostat (Beleodaq), or immunotherapy drugs such as alemtuzumab (Campath) and denileukin diftitox (Ontak) may be tried.
The outlook for these lymphomas is usually not as good as in diffuse large B-cell lymphoma (DLBCL), so taking part in a clinical trial of newer treatments is often a good option.
Treatment for Indolent Non-Hodgkin’s lymphoma Subtypes
Indolent non-Hodgkin lymphoma (NHL) subtypes progress slowly. They make up about 40 percent of all NHL cases in the United States. Indolent subtypes include:
- Cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome)
- Follicular lymphoma (FL)
- Lymphoplasmacytic lymphoma and Waldenström macroglobulinemia
- Marginal zone lymphoma and mucosa-associated lymphoid tissue (MALT) lymphoma
- Small cell lymphocytic lymphoma (SLL) and chronic lymphocytic leukemia (CLL)
Treatment for indolent NHL ranges from observation with careful monitoring (the watch-and-wait approach) to aggressive therapy. Indolent NHL management or treatment is highly individual. Your doctor considers a number of factors such as:
- Prognostic factors
- Stage of disease
- Age and other medical conditions.
Treating Specific Indolent Subtypes
Cutaneous T-Cell Lymphoma (Mycosis Fungoides and Sézary Syndrome)
Cutaneous T-cell lymphomas (CTCLs) are a group of non-Hodgkin’s lymphomas that develop primarily in the skin and may grow to involve lymph nodes, blood and other organs. This type of lymphoma originates in a T-cell. Mycosis fungoides is the most common type of CTCL, and is characterized by prominent skin involvement. When the malignant lymphocytes enter and accumulate in the blood, the disease is called Sézary syndrome.
Therapy for CTCL depends on the nature of the skin lesions and whether disease is present in the lymph nodes. Topical therapies are among the approaches used to treat the skin lesions. These include drugs applied directly to the skin combined with either ultraviolet light therapy or electron beam therapy. Ultraviolet light is used in conjunction with psoralen (a drug that becomes active when it is exposed to light); the combination therapy is often referred to as “PUVA” (psoralen and ultraviolet A) therapy.
If there is widespread involvement of lymph nodes and other sites, single- or multi-drug chemotherapy or extracorporeal photopheresis can be used. Extracorporeal photopheresis (ECP) is a method of removing blood from the body and treating it with ultraviolet light before returning it to the body. This treatment is recommended for patients either with, or at risk for, blood involvement such as that seen in Sézary syndrome.
Two histone deacetylase (HDAC) inhibitors, romidepsin (Istodax®), given by IV infusion and vorinostat (Zolinza®), given by mouth, as well as one monoclonal antibody, mogamulizumab (Poteligeo®), given by IV, are indicated for the treatment of adult patients with either relapsed or refractory cutaneous T-cell lymphomas (CTCLs) who have received previous systemic therapy.
Follicular Lymphoma (FL)
Follicular lymphoma (FL) is the second most common subtype of non-Hodgkin lymphoma (NHL), making up about 22 percent of all NHL cases. Most patients with FL are age 50 or older at diagnosis. Most follicular lymphoma cells have a specific chromosomal abnormality (a translocation between parts of chromosomes 14 and 18) that causes the production (overexpression) of the gene, BCL-2, which can make the cells resistant to therapy.
Follicular lymphoma is a very slow-growing disease. Some patients may not need treatment for several years, whereas others may have extensive lymph node or organ involvement and need treatment right away. In a small percentage of patients, FL may transform into a more aggressive disease.
Stage 1 or stage 2 follicular lymphoma may be treated with:
- Watch-and-wait approach; patients with less advanced disease can be observed with periodic examinations and imaging tests.
- Radiation therapy
- Chemotherapy with rituximab (Rituxan®) followed by radiation therapy
For patients with stage 2 follicular lymphoma who have large lymph nodes, stage 3 or stage 4 follicular lymphoma or advanced-stage relapsed follicular lymphoma, treatment will be based on symptoms, the patient’s age and health status and the extent of the patient’s disease. Taking part in a clinical trial can also be a good treatment option. Other treatment options include:
- The watch-and-wait approach
- Radiation therapy to lymph nodes that are causing symptoms, or to a large localized mass, if one is present
- Chemotherapy plus immunotherapy (rituximab)
- Single chemotherapy drugs in combination with rituximab. Examples of drugs used for treatment include cyclophosphamide, chlorambucil or bendamustine hydrochloride (Bendeka®)
- Chemotherapy combinations plus rituximab, such as R-CVP (rituximab plus cyclophosphamide [Cytoxan®], hydroxydoxorubicin [doxorubicin], vincristine [Oncovin®] and prednisone) or R-CHOP (rituximab [Rituxan®] plus cyclophosphamide [Cytoxan®], doxorubicin [hydroxydoxorubicin], Oncovin® [vincristine] and prednisone)
- Maintenance rituximab after completion of initial therapy with either rituximab alone or rituximab in combination with chemotherapy. This involves a single dose of rituximab administered on a prescribed schedule (generally every 2 to 3 months). Rituximab maintenance may be continued for 2 years.
- Autologous and allogeneic stem cell transplantation for selected patients
- Targeted therapy, using kinase inhibitors
- Copanlisib (Aliqopa®) to treat adult patients with relapsed follicular lymphoma who have received at least two prior systemic therapies.
- Duvelisib (Copiktra)
- Lenalidomide (Revlimid®)
- A radioactive monoclonal antibody, such as yttrium-90+ibritumomab tiuxetan (Zevalin®)
- Obinutuzumab (Gazyva®)
- Rituximab and the endoglycosidase hyaluronidase human (Rituxan Hycela)
- Tazemetostat (Tazverik) works by targeting EZH2, a protein known as a methyltransferase that normally helps some cancer cells grow. This drug can be used to treat follicular lymphomas with an EZH2 gene mutation, after other treatments have been tried. Tazemetostat can also be used to treat follicular lymphomas without an EZH2 mutation, if there are no other good treatment options available. This drug is taken as pills, typically twice a day.
- Idelalisib (Zydelig®) to treat patients who have relapsed follicular lymphoma and who have received at least two prior therapies
Transformed B-Cell Follicular Lymphoma. Follicular lymphoma has a small risk of transforming into an aggressive large B-cell lymphoma, such as DLBCL. Patients with transformed B-cell follicular lymphoma appear to benefit from rituximab therapy, either alone or in combination with chemotherapy. Other options include
- Axicabtagene ciloleucel (Yescarta®)
- Tisagenlecleucel (Kymriah®)
Doctors have created a scoring system called the Follicular Lymphoma International Prognostic Index (FLIPI) that is used to predict which patients have a higher risk of the disease returning (recurring). Doctors design a treatment plan based upon the patients’ score. Factors that raise the risk for follicular lymphoma patients include:
- Older than 60
- Stage 3 or 4 disease
- More than five lymph nodes involved
- An elevated level of lactic dehydrogenase (a type of protein) in the blood
- Hemoglobin concentration less than 12 g/dL.
Lymphoplasmacytic Lymphoma and Waldenström Macroglobulinemia
Lymphoplasmacytic lymphoma and Waldenström macroglobulinemia are both slow-growing types of lymphoma that originate in a B-lymphocyte precursor. Both subtypes can become more advanced and involve the lungs, the gastrointestinal tract and other organs.
One option for patients without symptoms is to take a watch-and-wait approach. Active treatment begins for patients only if symptoms develop. Therapy regimens may then include a combination of biological agents (monoclonal antibodies such as rituximab), signaling inhibitors (drugs that block cell growth and survival signals), and chemotherapy with alkylating agents such as chlorambucil (Leukeran®), melphalan (Alkeran®) and cyclophosphamide (Cytoxan®). The Bruton tyrosine kinase (BTK) inhibitor ibrutinib (Imbruvica®) has been approved by the FDA for the treatment of patients with symptomatic Waldenström macroglobulinemia.
A serious complication of both diseases is called hyperviscosity syndrome. Hyperviscosity syndrome may occur when the malignant cells secrete an abnormal protein that can thicken the blood. This leads to inadequate blood flow, causing such symptoms as headache, blurred vision and mental confusion. Hyperviscosity syndrome can be treated with plasmapheresis, a process of removing plasma from the blood. Plasmapheresis can reverse acute symptoms and signs, but long-term control requires a reduction in the mass of lymphoma cells that make the protein. This is followed by treatment to control the lymphoma.
Marginal Zone Lymphoma and Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma
Marginal zone lymphoma can develop in either the lymph nodes (nodal) or outside the lymph nodes (extranodal). It begins in B lymphocytes in a part of the lymph tissue called the “marginal zone.” The disease tends to remain localized.
Marginal zone lymphoma includes several subtypes, each categorized by the type of tissue where the lymphoma forms.
- Gastric mucosa-associated lymphoid tissue (MALT) lymphoma usually develops in the stomach. Patients with MALT lymphoma may have a history of an autoimmune disease such as Hashimoto thyroiditis or Sjögren syndrome. A higher incidence of MALT lymphoma involving the stomach is seen in patients who have been infected with the bacterium H. pylori. Bacteria have also been implicated in other forms of MALT lymphoma. Treatment often includes potent combinations of antibiotics, which both eradicate the H. pylori infection and cause the lymphoma to regress. Many patients with H. pylori have been cured of MALT lymphoma without radiation or chemotherapy. If remission is not achieved following antibiotic treatment, radiotherapy can be a curative option. For a small subset of patients, MALT lymphoma can transform into diffuse large B-cell lymphoma (DLBCL). These patients can benefit from treatments used for Diffuse Large B-cell Lymphoma.
- Extragastric MALT lymphoma begins outside the stomach and may form in almost any part of the body including other areas of the gastrointestinal tract, salivary glands, thyroid, lung, skin and around the eye.
- Monocytoid B-cell lymphoma, also known as “nodal marginal zone B-cell lymphoma,” may be found in the spleen and blood. This form of NHL is generally treated like follicular lymphoma.
- Splenic marginal zone lymphoma (SMZL) begins in the spleen and may spread to the blood and bone marrow. One of the first signs of SMZL is an enlarged spleen; however, symptoms can be slow to develop. Splenic marginal zone lymphoma has been associated with hepatitis C infection. Treatment for hepatitis C with interferon (either alone or in combination with ribavirin) may result in a remission of the patient’s lymphoma.
For patients with splenic marginal zone lymphoma (SMZL) who do not have hepatitis C or any symptoms of lymphoma, the first treatment may be the watch-and-wait approach. Treatment is generally started when an enlarged spleen starts to cause symptoms or produces low white blood cell counts. For symptomatic patients who are hepatitis-C negative, treatment may include:
- Removal of the spleen
- Single-agent chemotherapy
- Combination chemotherapy plus rituximab (Rituxan)
- R-CVP (rituximab, cyclophosphamide, vincristine and prednisone)
- R-CHOP (rituximab [Rituxan®] plus cyclophosphamide [Cytoxan®],doxorubicin [hydroxydoxorubicin], Oncovin® [vincristine] and prednisone)
- BR (bendamustine hydrochloride (Bendeka), rituximab)
For relapsed or refractory cases, treatment may include:
- Ibrutinib (Imbruvica®), a Bruton tyrosine kinase (BTK) inhibitor given by mouth, is indicated for the treatment of patients with MZL who require systemic therapy and have received at least one prior anti-CD20-based therapy.
- Lenalidomide (Revlimid®), given by mouth, is indicated in combination with a rituximab product for MZL patients who have been previously treated.
Small Cell Lymphocytic Lymphoma (SLL) and Chronic Lymphocytic Leukemia (CLL)
Small cell lymphocytic lymphoma (SLL) and chronic lymphocytic leukemia (CLL) are similar NHL subtypes. They tend to affect the same age groups (median age of patients is 65 years), have common signs and symptoms and be slow-growing. The differences lie in where they develop: SLL mostly affects the lymph nodes and lymphoid tissue; CLL mostly affects blood and bone marrow but can spread to the lymph nodes.
Rituximab or a rituximab-containing regimen is used as first-line therapy for CLL/SLL . The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib (Imbruvica®) has also been approved as first-line therapy. The PI3K inhibitor idelalisib (Zydelig®) in combination with rituximab and the BCL-2 inhibitor venetoclax (Venclexta®) have been approved for use in patients with relapsed CLL/SLL. Bendamustine hydrochloride is a chemotherapy agent that has been approved for the treatment of CLL patients who have progressed during or within six months of treatment with rituximab or a rituximab-containing regimen.
The following drugs are used for cases of relapsed chronic lymphocytic leukemia (CLL) or small cell lymphocytic lymphoma (SLL):
- Idelalisib (Zydelig®), in combination with rituximab or in patients who received at least two prior systemic therapies
- Duvelisib (Copiktra™), after at least two prior therapies.
The FCR (fludarabine, cyclophosphamide and rituximab) regimen is a potentially curative option for some patients with chronic lymphocytic leukemia (CLL) or small cell lymphocytic lymphoma (SLL). Recent reports from clinical studies indicate that chimeric antigen receptor (CAR) T-cell therapy can induce durable remissions in patients with refractory disease. This therapy is under investigation in clinical trials.
Survival rates for non-Hodgkin lymphoma
The numbers below come from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER) database, looking at people diagnosed with non-Hodgkin lymphoma between 2012–2018 12:
- Stage 1 non-Hodgkin lymphoma, the 5-year survival rate is about 86.5%.·
- Stage 2 non-Hodgkin lymphoma, the 5-year survival rate is about 78.1%.
- Stage 3 non-Hodgkin lymphoma, the 5-year survival rate is about 72.3%.
- Stage 4 non-Hodgkin lymphoma has a 5-year survival rate of about 63.9%.
Based on data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results program (SEER) 2012–2018, percent surviving 5 Years is 73.8% 12.
5-year relative survival rates for Non-Hodgkin’s lymphoma
Footnote: A relative survival rate compares people with the same type and stage of non-Hodgkin’s lymphoma to people in the overall population. For example, if the 5-year survival rate for a specific stage of non-Hodgkin lymphoma is 73.8%, it means that people who have that cancer are, on average, about 73.8% as likely as people who don’t have that cancer to live 5 years after being diagnosed.
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, how well the cancer responds to treatment, and other prognostic factors (described below) can also affect your outlook.
- People now being diagnosed with non-Hodgkin lymphoma may have a better outlook than these numbers show. Treatments improve over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
Non-Hodgkin’s lymphoma Survival by Stage
Cancer stage at diagnosis, which refers to extent of a cancer in the body, determines treatment options and has a strong influence on the length of survival. The earlier non-Hodgkin lymphoma is caught, the better chance a person has of surviving five years after being diagnosed. The National Cancer Institute’s Surveillance, Epidemiology, and End Results program (SEER) database tracks 5-year relative survival rates for non-Hodgkin lymphoma in the United States, based on how far the cancer has spread. The SEER database, however, does not group cancers by the Lugano classification (stage 1, stage 2, stage 3, etc.). Instead, it groups cancers into localized, regional, and distant stages:
- Localized: The cancer is limited to one lymph node area, one lymphoid organ, or one organ outside the lymph system.
- Regional: The cancer reaches from one lymph node area to a nearby organ, is found in two or more lymph node areas on the same side of the diaphragm, or is considered bulky disease.
- Distant: The cancer has spread to distant parts of the body, such as the lungs, liver, or bone marrow, or to lymph node areas above and below the diaphragm.
For non-Hodgkin lymphoma, 22.7% are diagnosed at stage 1. The 5-year relative survival for stage 1 non-Hodgkin lymphoma is 86.5% 12.
The overall 5-year relative survival rate for people with non-Hodgkin lymphoma is 73.8%. But it’s important to keep in mind that survival rates can vary widely for different types and stages of lymphoma. Furthermore, these survival rates are only estimates – they can’t predict what will happen to any individual person. We understand that these statistics can be confusing and may lead you to have more questions. Talk to your doctor to better understand your specific situation.
Below are the 5-year relative survival rates for two common types of non-Hodgkin lymphoma – diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) – based on people diagnosed between 2011 and 2017.
Diffuse large B-cell non-Hodgkin lymphoma 5-year relative survival rates
| Surveillance, Epidemiology, and End Results program (SEER) Stage | 5-Year Relative Survival Rate |
|---|---|
| Localized | 74% |
| Regional | 73% |
| Distant | 57% |
| All SEER stages combined | 64% |
Footnotes:
- 5-year relative survival rates based on people diagnosed between 2011 and 2017.
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- People now being diagnosed with non-Hodgkin lymphoma may have a better outlook than these numbers show. Treatments improve over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, the type of non-Hodgkin lymphoma, how well the cancer responds to treatment, and other factors can also affect your outlook.
Follicular non-Hodgkin lymphoma 5-year relative survival rates
| Surveillance, Epidemiology, and End Results program (SEER) Stage | 5-Year Relative Survival Rate |
|---|---|
| Localized | 97% |
| Regional | 91% |
| Distant | 86% |
| All SEER stages combined | 90% |
Footnotes:
- 5-year relative survival rates based on people diagnosed between 2011 and 2017.
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- People now being diagnosed with non-Hodgkin lymphoma may have a better outlook than these numbers show. Treatments improve over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, the type of non-Hodgkin lymphoma, how well the cancer responds to treatment, and other factors can also affect your outlook.
Non Hodgkin’s lymphoma prognosis
Non-Hodgkin lymphoma prognosis depends on the histologic type, stage, patient factors and treatment 14. Patients with aggressive T- or NK cell lymphomas usually have a worse prognosis. Patients with low-grade lymphomas have increased survival, which is usually 6 to 10 years. However, they can have the transformation into high-grade lymphomas.
Non-Hodgkin lymphoma can be divided into two prognostic groups: the indolent non-Hodgkin lymphomas and the aggressive non-Hodgkin lymphomas.
- Indolent non-Hodgkin lymphoma types have a relatively good prognosis with a median survival as long as 20 years, but they usually are not curable in advanced clinical stages 15. Early-stage (stage 1 and stage 2) indolent NHL can be effectively treated with radiation therapy alone. Most of the indolent types are nodular (or follicular) in morphology.
- Aggressive non-Hodgkin lymphoma has a shorter natural history, but a significant number of these patients can be cured with intensive combination chemotherapy regimens.
In general, with modern treatment of patients with non-Hodgkin lymphoma, the overall survival rate at 5 years is over 60%. Of patients with aggressive NHL, more than 50% can be cured 14. Most relapses occur in the first 2 years after therapy. The risk of late relapse is higher in patients who manifest both indolent and aggressive histologies 16.
While indolent NHL is responsive to immunotherapy, radiation therapy, and chemotherapy, a continuous rate of relapse is usually seen in advanced stages. Patients, however, can often be re-treated with considerable success if the disease histology remains low grade. Patients who present with or convert to aggressive forms of NHL may have sustained complete remissions with combination chemotherapy regimens or aggressive consolidation with marrow or stem cell support 17.
For some types of lymphoma the stage isn’t too helpful in determining a person’s prognosis (outlook). In these cases, other factors can give doctors a better idea about a person’s prognosis. The International Prognostic Index (IPI) and its variant are used as the main prognostic tools in patients with non-Hodgkin lymphoma (see Table 1 below) 8. The International Prognostic Index (IPI) helps to determine overall survival after standard of care treatment. The International Prognostic Index (IPI) is assessed by factors including age>60 years, serum lactate dehydrogenase (LDH) more than normal, Eastern Cooperative oncology group (ECOG) performance status more than or equal to 2, clinical stage 3 or 4 and >1 extranodal involvement. One point is given for each factor, a total score from (0 to 5), which determines the degree of risk. These are classified as low risk (0-1 adverse factor), Intermediate risk (2 adverse factors), and Poor risk (three or more adverse factors) 18. Congenital or acquired immunodeficiency states are associated with an increased risk of lymphoma, and response to therapy is poor 19. The International Prognostic Index (IPI) has been modified for most of the NHL for better assessment of prognosis, e.g., FLIPI for follicular lymphoma, MIPI for mantle cell lymphoma, and so on.
Prognostic factors for non-Hodgkin lymphoma
The International Prognostic Index (IPI) was first developed to help doctors determine the outlook (prognosis) for people with fast-growing (aggressive) lymphomas (see Table 1). However, it has proven useful for most other lymphomas as well (other than slow-growing [indolent] follicular lymphomas). The International Prognostic Index (IPI) allows doctors to plan treatment better than they could just based on the type and stage of the lymphoma. This has become more important as new, more effective treatments have been developed that sometimes have more side effects. The index helps doctors figure out whether these treatments are needed.
The International Prognostic Index (IPI) depends on 5 factors:
- The patient’s age
- The stage of the lymphoma
- Whether or not the lymphoma is in organs outside the lymph system
- Performance status (PS) – how well a person can complete normal daily activities
- The blood level of lactate dehydrogenase (LDH), which goes up with the amount of lymphoma in the body
Table 4. International Prognostic Index Non-Hodgkin’s lymphoma
| Good prognostic factors | Poor prognostic factors |
|---|---|
| Age 60 or below | Age above 60 |
| Stage 1 or 2 | Stage 3 or 4 |
| No lymphoma outside of lymph nodes, or lymphoma in only 1 area outside of lymph nodes | Lymphoma is in more than 1 organ of the body outside of lymph nodes |
| Performance status (PS): Able to function normally | Performance status (PS): Needs a lot of help with daily activities |
| Serum lactate dehydrogenase (LDH) is normal | Serum lactate dehydrogenase (LDH) is high |
Footnotes: Each poor prognostic factor is assigned 1 point. People without any poor prognostic factors would have a score of 0, while those with all poor prognostic factors would have a score of 5. The index divides people with lymphomas into 4 risk groups:
- Low risk (0 or 1 poor prognostic factors)
- Low-intermediate risk (2 poor prognostic factors)
- High-intermediate risk (3 poor prognostic factors)
- High risk (4 or 5 poor prognostic factors)
International Prognostic Index for Follicular Lymphoma
The International Prognostic Index (IPI) is useful for most lymphomas, but it’s not as helpful for follicular lymphomas, which tend to be slower growing. Doctors have developed the Follicular Lymphoma International Prognostic Index (FLIPI) specifically for this type of lymphoma. It uses slightly different prognostic factors than the International Prognostic Index (IPI).
Table 5. Follicular Lymphoma International Prognostic Index (FLIPI)
| Good prognostic factors | Poor prognostic factors |
|---|---|
| Age 60 or below | Age above 60 |
| Stage 1 or 2 | Stage 3 or 4 |
| Blood hemoglobin 12 g/dL or above | Blood hemoglobin level below 12 g/dL |
| 4 or fewer lymph node areas affected | More than 4 lymph node areas affected |
| Serum lactate dehydrogenase (LDH) is normal | Serum lactate dehydrogenase (LDH) is high |
Footnotes: Patients are assigned a point for each poor prognostic factor. People without any poor prognostic factors would have a score of 0, while those with all poor prognostic factors would have a score of 5. The index then divides people with follicular lymphoma into 3 groups:
- Low risk (no or 1 poor prognostic factor[s])
- Intermediate risk (2 poor prognostic factors)
- High risk (3 or more poor prognostic factors).
- Non-Hodgkin Lymphoma. https://www.lls.org/lymphoma/non-hodgkin-lymphoma
- Swerdlow, S. H., Campo, E., Pileri, S. A., Harris, N. L., Stein, H., Siebert, R., Advani, R., Ghielmini, M., Salles, G. A., Zelenetz, A. D., & Jaffe, E. S. (2016). The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood, 127(20), 2375–2390. https://doi.org/10.1182/blood-2016-01-643569
- Non-Hodgkin Lymphoma — Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/nhl.html
- Key Statistics for Non-Hodgkin Lymphoma. https://www.cancer.org/cancer/non-hodgkin-lymphoma/about/key-statistics.html
- Non-Hodgkin Lymphoma. https://www.lls.org/sites/default/files/file_assets/PS58_NHL_Booklet_2020FINAL_rev.pdf
- Ghobrial IM, Wolf RC, Pereira DL, Fonseca R, White WL, Colgan JP, Habermann TM, Inwards DJ, Markovic SN, Ansell SM, Micallef IN, Porrata LF, Witzig TE. Therapeutic options in patients with lymphoma and severe liver dysfunction. Mayo Clin Proc. 2004 Feb;79(2):169-75. doi: 10.4065/79.2.169
- Zhou X, Teegala S, Huen A, Ji Y, Fayad L, Hagemeister FB, Gladish G, Vadhan-Raj S. Incidence and risk factors of venous thromboembolic events in lymphoma. Am J Med. 2010 Oct;123(10):935-41. doi: 10.1016/j.amjmed.2010.05.021
- Sapkota S, Shaikh H. Non-Hodgkin Lymphoma. [Updated 2022 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559328
- Eriksson, M., Hardell, L., Carlberg, M. and Åkerman, M. (2008), Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int. J. Cancer, 123: 1657-1663. https://doi.org/10.1002/ijc.23589
- Zhang, Y., Sanjose, S. D., Bracci, P. M., Morton, L. M., Wang, R., Brennan, P., Hartge, P., Boffetta, P., Becker, N., Maynadie, M., Foretova, L., Cocco, P., Staines, A., Holford, T., Holly, E. A., Nieters, A., Benavente, Y., Bernstein, L., Zahm, S. H., & Zheng, T. (2008). Personal use of hair dye and the risk of certain subtypes of non-Hodgkin lymphoma. American journal of epidemiology, 167(11), 1321–1331. https://doi.org/10.1093/aje/kwn058
- Anderson, L. A., Gadalla, S., Morton, L. M., Landgren, O., Pfeiffer, R., Warren, J. L., Berndt, S. I., Ricker, W., Parsons, R., & Engels, E. A. (2009). Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. International journal of cancer, 125(2), 398–405. https://doi.org/10.1002/ijc.24287
- Cancer Stat Facts: Non-Hodgkin Lymphoma. https://seer.cancer.gov/statfacts/html/nhl.html
- Survival Rates and Factors That Affect Prognosis (Outlook) for Non-Hodgkin Lymphoma. https://www.cancer.org/cancer/non-hodgkin-lymphoma/detection-diagnosis-staging/factors-prognosis.html
- PDQ Adult Treatment Editorial Board. Adult Non-Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version. 2022 Jan 18. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK66057
- Tan, D., Horning, S. J., Hoppe, R. T., Levy, R., Rosenberg, S. A., Sigal, B. M., Warnke, R. A., Natkunam, Y., Han, S. S., Yuen, A., Plevritis, S. K., & Advani, R. H. (2013). Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: the Stanford University experience. Blood, 122(6), 981–987. https://doi.org/10.1182/blood-2013-03-491514
- Cabanillas F, Velasquez WS, Hagemeister FB, McLaughlin P, Redman JR. Clinical, biologic, and histologic features of late relapses in diffuse large cell lymphoma. Blood. 1992 Feb 15;79(4):1024-8. https://doi.org/10.1182/blood.V79.4.1024.1024
- Bastion Y, Sebban C, Berger F, Felman P, Salles G, Dumontet C, Bryon PA, Coiffier B. Incidence, predictive factors, and outcome of lymphoma transformation in follicular lymphoma patients. J Clin Oncol. 1997 Apr;15(4):1587-94. doi: 10.1200/JCO.1997.15.4.1587
- Armitage JO, Gascoyne RD, Lunning MA, et al. Non-Hodgkin lymphoma. Lancet. 2017;390(10091):298–310.
- International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993 Sep 30;329(14):987-94. https://www.nejm.org/doi/full/10.1056/nejm199309303291402