Omenn syndrome
Omenn syndrome also called familial reticuloendotheliosis, is an inherited disorder of the immune system (immunodeficiency). Omenn syndrome is one of several forms of severe combined immunodeficiency, a group of disorders that cause individuals to have virtually no immune protection from bacteria, viruses, and fungi. Individuals with severe combined immunodeficiency are prone to repeated and persistent fungal, bacterial, and viral infections that can be very serious or life-threatening. Infants with Omenn syndrome typically experience pneumonia, chronic diarrhea and failure to thrive. Often the organisms that cause infection in people with Omenn syndrome are described as opportunistic because they ordinarily do not cause illness in healthy people.
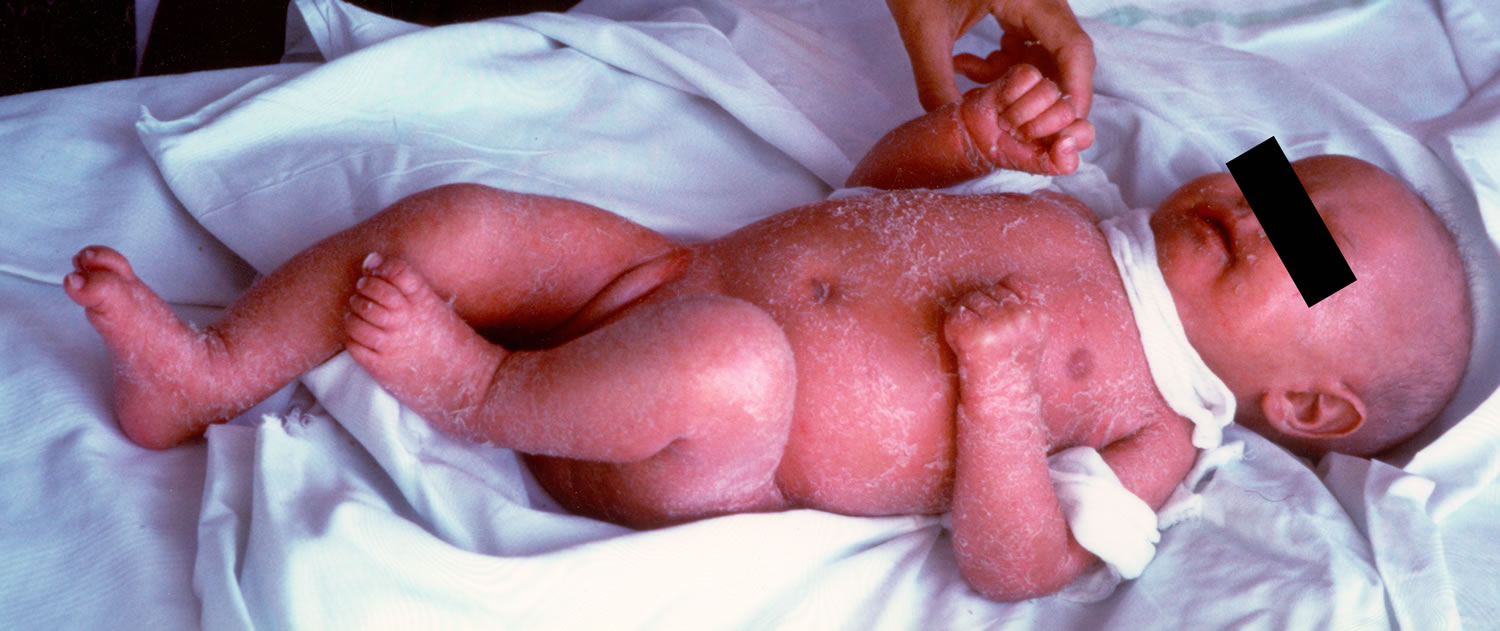
In addition to immunodeficiency, children with Omenn syndrome develop autoimmunity, in which the immune system attacks the body’s own tissues and organs. This abnormal immune reaction can cause very red skin (erythroderma), desquamation (peeling skin), hair loss (alopecia), lymphadenopathy (enlarged lymph nodes), eosinophilia, and an enlarged liver and spleen (hepatosplenomegaly) and elevated serum IgE levels 1. In addition, affected individuals have enlargement of tissues that produce infection-fighting white blood cells called lymphocytes. These include the thymus, which is a gland located behind the breastbone, and lymph nodes, which are found throughout the body.
In Omenn syndrome, the severe combined immunodeficiency is associated with low IgG, IgA, and IgM and the virtual absence of B cells. There is an elevated number of T cells, but their function is impaired. Omenn syndrome has been found to be caused by mutations in the RAG1 or RAG2 genes. Additional causative genes have been identified. The exact prevalence of Omenn syndrome is unknown. Overall, the various forms of severe combined immunodeficiency (SCID) are estimated to affect 1 in 75,000 to 100,000 newborns.
Early recognition of Omenn syndrome is important for genetic counseling and early treatment. If left untreated, Omenn syndrome is fatal, children with Omenn syndrome usually survive only until age 1 or 2. The prognosis may be improved with early diagnosis and treatment with compatible bone marrow or cord blood stem cell transplantation.
Omenn syndrome causes
Mutations in several genes involved in immune system function can cause Omenn syndrome. The two most frequent causes are mutations in the RAG1 and RAG2 genes mapped to chromosome band 11p13. These genes provide instructions for making proteins that are active in two types of lymphocytes called B cells and T cells. To help fight infections, B cells and T cells have special proteins on their surface that help them recognize foreign invaders; these proteins must be somewhat different from each other to be able to recognize a wide variety of substances. The RAG1 and RAG2 proteins help increase the diversity of proteins that are on the surface of these cells.
RAG1 and RAG2 gene mutations that cause Omenn syndrome drastically reduce the respective protein’s function. As a result, the diversity of proteins on the surface of B cells and T cells is severely limited, impairing the cells’ ability to recognize foreign invaders and fight infections. The abnormal B cells and T cells result in the frequent, life-threatening infections of Omenn syndrome. The decrease in lymphocyte function leads to a reduction in the numbers of B cells. The number of T cells is typically normal, although they are highly similar because they are derived from just a few functional precursor cells. The abnormal T cells attack the body’s own cells and tissues, accounting for the autoimmune features of Omenn syndrome.
Although most cases of Omenn syndrome are due to mutations in the RAG genes, recent reports describe Omenn syndrome in the absence of RAG mutations 2. Omenn syndrome caused by mutations in ARTEMIS, ADA, ILRA2, ILRA7, CHD7, and DNA ligase 4 have been described in the medical literature. Some cases of Omenn syndrome have also been found in association with 22q11 microdeletion syndrome 1. Therefore, Omenn syndrome is now defined as a genetically heterogeneous condition in which patients with similar phenotypes may have unidentified genetic defects.
Omenn syndrome inheritance pattern
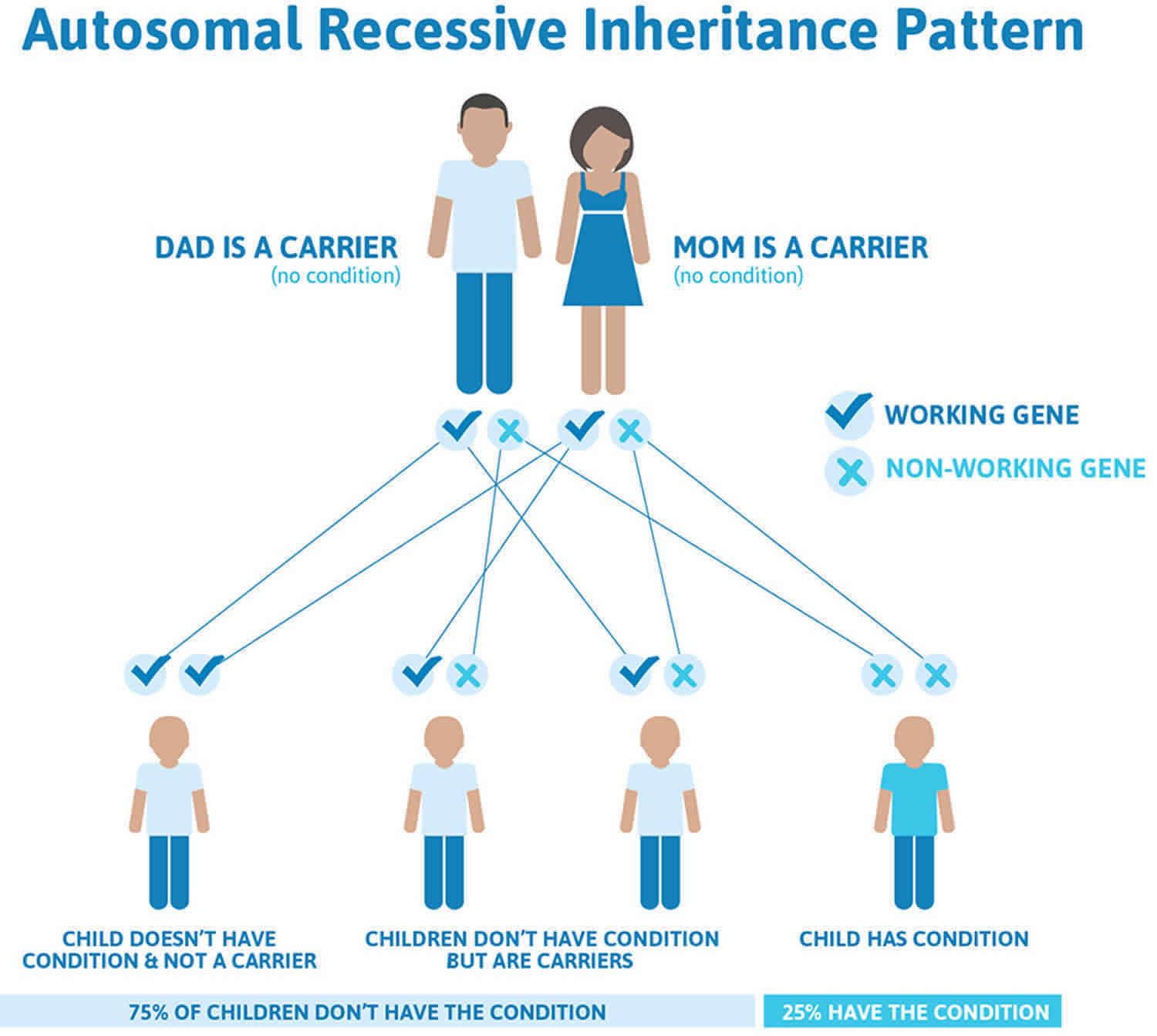
Omenn syndrome is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 1 illustrates autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 1. Omenn syndrome autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Omenn syndrome symptoms
Infants with Omenn syndrome typically present shortly after birth, usually by 3 months of age. This is similar to other types of severe combined immunodeficiency. The characteristic skin findings of red and peeling skin (erythrodermia), chronic diarrhea, and failure to thrive often precede the onset of infections. Life-threatening infections caused by common viral, bacterial, and fungal pathogens occur next. Lymphadenopathy and hepatosplenomegaly, both symptoms unique to Omenn syndrome, develop next 1.
Pneumocystis carinii pneumonia and poliomyelitis due to the attenuated oral poliovirus are classic infections in Omenn syndrome and in other types of severe combined immunodeficiency.
A child with Omenn syndrome and the rare bone marrow disorder of reticular dysgenesis has been described 3.
Omenn syndrome diagnosis
Severe combined immunodeficiency is now diagnosed mainly through from newborn screening in most states. The screen is performed using the dried blood spot from newborn screening (or Guthrie) cards measuring levels T-cell receptor excision circles (or TREC). Although each state has a slightly different methods and thresholds, a low TREC test means the infant has low numbers of lymphocytes in the blood at the time of the test. The result must then be confirmed with additional testing. A complete blood count (CBC) coupled with lymphocyte subset testing may show low levels of B, T, and/or NK cells. Additional tests can show that one or more of these cell types aren’t functioning properly. Genetic and biochemical (protein expression) tests are available for some forms of severe combined immunodeficiency. A combination of these tests may be required to make an accurate diagnosis needed to plan treatment.
Omenn syndrome treatment
The standard treatment for Omenn syndrome is bone marrow transplantation or cord blood stem cell transplantation. General care for any patient with severe combined immunodeficiency, including Omenn syndrome, includes isolation to prevent infection and meticulous skin and mucosal hygienic practices while the patient is awaiting stem cell reconstitution. Patients should avoid crowds in locations such as stores, doctors’ offices, and hospitals, and they and their caregivers should engage in customary hygiene practices such as strict hand washing. Broad-spectrum antibiotics may be administered parenterally while cultures and body fluid analyses are in progress. Parenteral nutrition may also be provided as therapy for diarrhea and failure to thrive.
Bone marrow or other stem cell reconstitution is first-line conventional therapy for most forms of severe combined immunodeficiency, including Omenn syndrome, although the mortality rate is higher when compared to other types of severe combined immunodeficiency. Workup includes major histocompatibility complex (MHC) typing to identify a fully matched sibling, or, in the case of consanguinity, possibly a parent. Reconstitution by using a matched unrelated donor or haploidentical parent has also been successful, although more complications and higher mortality have been reported. Preparatory immunosuppression of malfunctioning activated T cells has decreased the incidence of graft failure in Omenn syndrome. Nutritional support and T-cell suppression prior to bone marrow transplantation may reduce the risk of complications. Pretransplantional evaluation routinely includes testing of the recipient and the donor for infectious agents, such as cytomegalovirus (CMV), HIV, and hepatitis viruses.
Specific therapy for dermatitis and eosinophilia in Omenn syndrome is immunosuppression with cyclosporine. Interferon gamma has been administered in an attempt to down-regulate interleukin 4 (IL-4) and interleukin 5 (IL-5) production by the oligoclonal Th2 cells. Interferon gamma may independently modulate the inflammatory reaction by enhancing phagocytic functions.
Ancillary therapy includes intravenous immunoglobulin (IVIG) replacement. Live viral vaccines should not be administered.
In the future, the identification of the recombinase mutations as the cause of Omenn syndrome should enable gene transfer therapy. At this time, successful gene therapy is available only for the X-linked T-B+ form of severe combined immunodeficiency, in which mutations in the common γ chain are necessary for function of the cell surface receptors of interleukin 2 (IL-2), IL-4, interleukin 7 (IL-7), interleukin 9 (IL-9), and interleukin 15 (IL-15). Thus, gene therapy might represent a good alternative to stem cell transplantation, particularly in those without a matched donor 4.
References- Omenn syndrome. https://emedicine.medscape.com/article/887687-overview
- Zhang, Z. , Zhao, X. , Jiang, L. , Liu, E. , Cui, Y. , Wang, M. , Wei, H. , Yu, J. , An, Y. and Yang, X. (2011), Clinical characteristics and molecular analysis of three Chinese children with Omenn syndrome. Pediatric Allergy and Immunology, 22: 482-487. doi:10.1111/j.1399-3038.2010.01126.x
- Henderson LA, Frugoni F, Hopkins G, Al-Herz W, Weinacht K, Comeau AM, et al. First reported case of Omenn syndrome in a patient with reticular dysgenesis. J Allergy Clin Immunol. 2013 Apr. 131(4):1227-30, 1230.e1-3.
- Capo V, Castiello MC, Fontana E, Penna S, Bosticardo M, Draghici E, et al. Efficacy of lentivirus-mediated gene therapy in an Omenn syndrome recombination-activating gene 2 mouse model is not hindered by inflammation and immune dysregulation. J Allergy Clin Immunol. 2017 Dec 11.