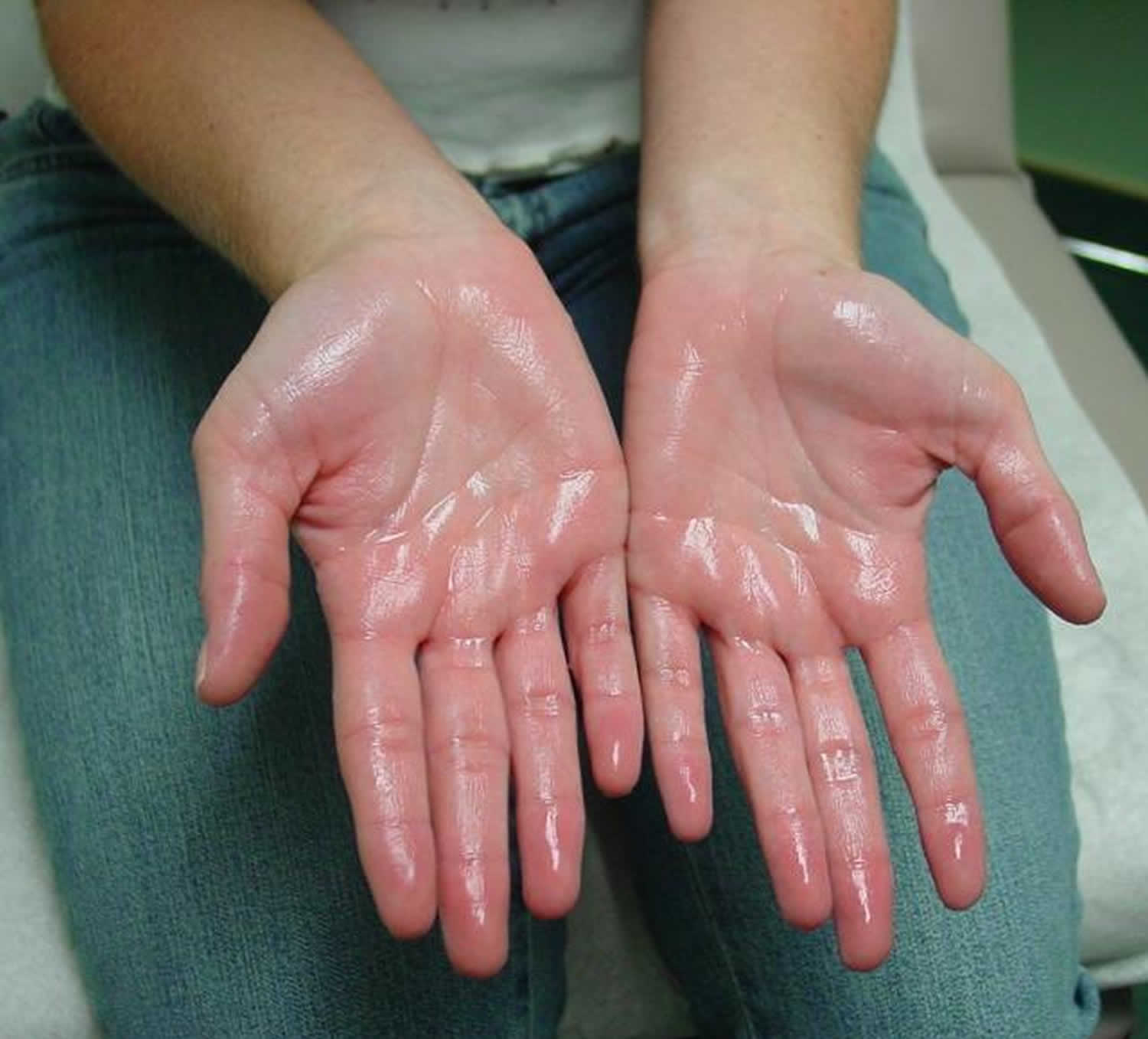
Palmoplantar hyperhidrosis
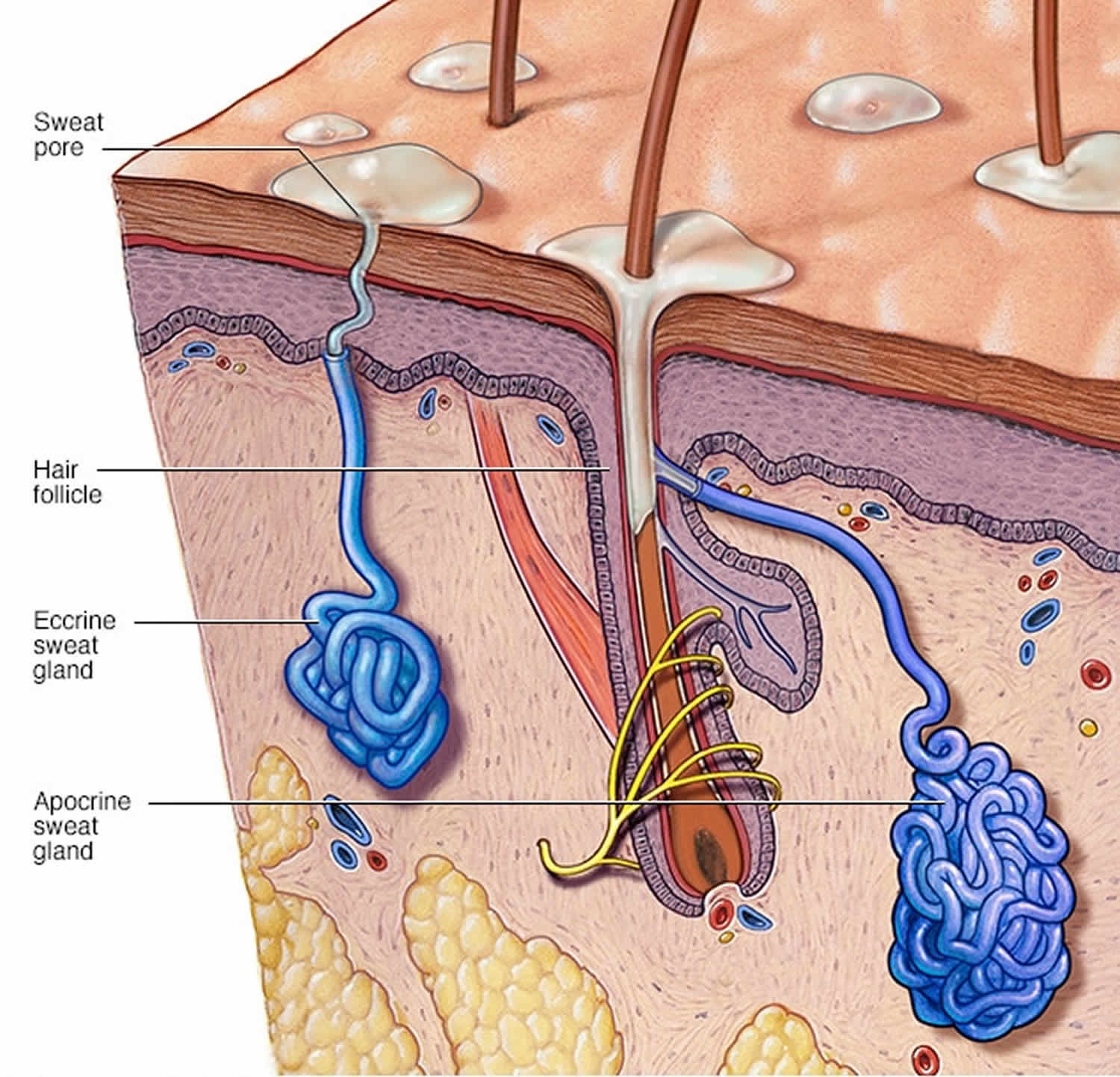
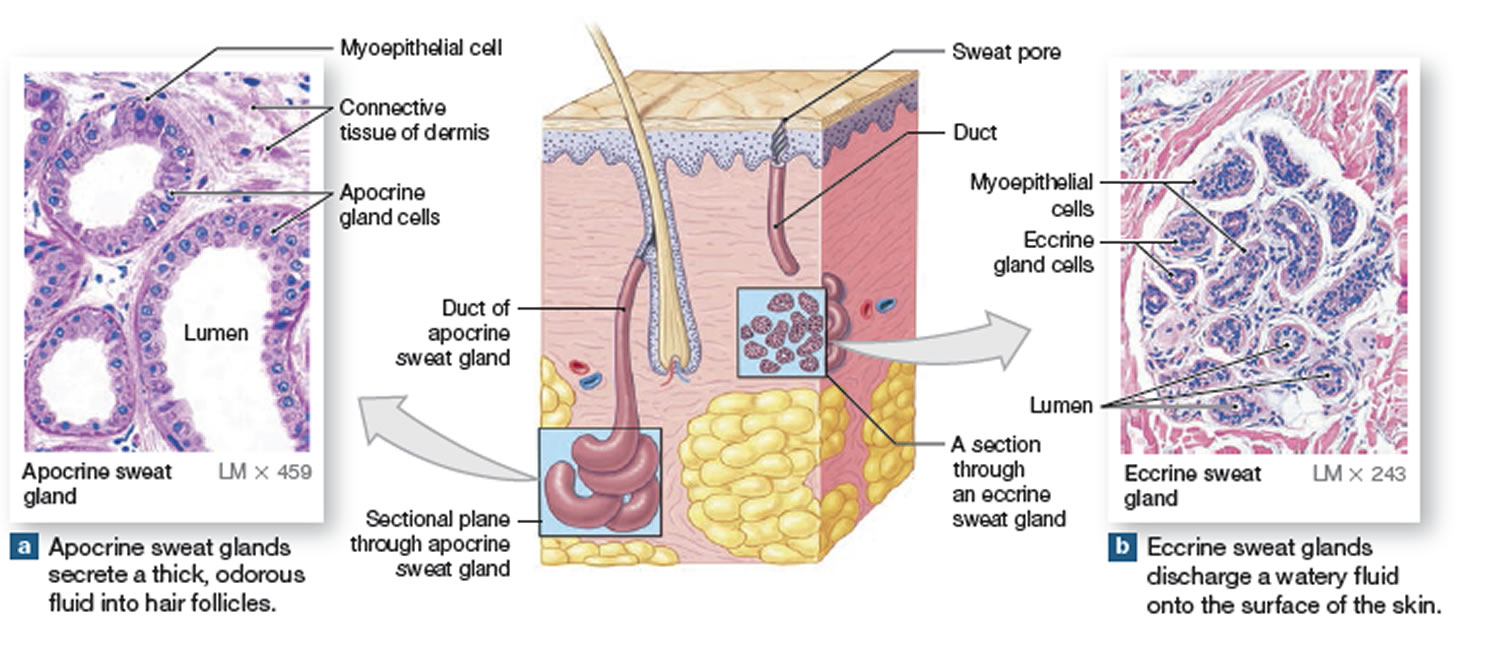
Palmoplantar hyperhidrosis is the name given to excessive and uncontrollable sweating of the palms and soles that affects both children and adults 1. Palmoplantar hyperhidrosis is a common condition in which the eccrine (sweat) glands of the palms and soles secrete inappropriately large quantities of sweat. The palms of your hands and the soles of your feet have more eccrine (sweat) glands than any other part of your body. Sweat is a weak salt solution produced by the eccrine sweat glands. These are distributed over the entire body but are most numerous on the palms and soles with about 700 glands per square centimeter. Individuals with palmoplantar hyperhidrosis have morphologically and functionally normal eccrine glands 2. Eccrine glands are distributed over almost all of the body surface but are most dense in the palms and soles. These glands are morphologically and functionally normal in patients with palmoplantar hyperhidrosis 3. Because the hyperhidrosis is stimulated by emotion and stress, it does not occur during sleep or sedation. Conversely, normal sweating is controlled primarily by thermoregulation and, thus, occurs independently of level of consciousness. The primary defect in patients with hyperhidrosis may be hypothalamic hypersensitivity to emotional stimuli from the cerebral cortex 3.
Hyperhidrosis may be idiopathic (unknown cause) or secondary to other diseases, chronic alcoholism, metabolic disorders, febrile illnesses, or medication use. Hyperhidrosis exists in 3 forms: emotionally induced hyperhidrosis (in which it affects the palms, soles, and armpits), localized hyperhidrosis, and generalized hyperhidrosis 4. Palmoplantar hyperhidrosis usually is idiopathic 5. Idiopathic palmoplantar hyperhidrosis begins in childhood and frequently runs in families 6. Palmoplantar hyperhidrosis may be inherited in an autosomal dominant manner 7.
Hyperhidrosis often causes great emotional distress and occupational disability for the patient, regardless of the form 8.
Primary hyperhidrosis is reported to affect 1–3% of the U.S. population and nearly always starts during childhood or adolescence. The tendency may be inherited, and it is reported to be particularly prevalent in Japanese people.
Secondary hyperhidrosis is less common and can present at any age.
Palmoplantar hyperhidrosis treatment depends on how much you sweat, how much the sweating interferes with your daily activities, and how well a treatment works for you. Therapy for hyperhidrosis remains a challenge for both the patient and the physician. Both topical and systemic medications have been used in the treatment of hyperhidrosis. Other treatment options for hyperhidrosis include iontophoresis and botulinum toxin type A injections, with surgical sympathectomy as a last resort.
Several treatments are available 9:
- An aluminum chloride solution (brand name: Drysol) can be applied to the skin on the palms of your hands and the soles of your feet.
- Another treatment is tap-water iontophoresis. In this treatment, a mild electrical current is passed through water and applied to the skin.
- People with severe sweating might be treated with injections of botulinum toxin type A (brand name: Botox).
- If all other treatments do not work, surgery can be done to cut the nerves that cause the sweating.
All of these treatments can have side effects or associated complications. You and your doctor can decide which treatment is best for you.
Topical aluminum chloride hexahydrate therapy and iontophoresis are simple, safe, and inexpensive therapies; however, continuous application is required because results are often short-lived, and they may be insufficient. Systemic agents such as anticholinergic drugs are tolerated poorly at the dosages required for efficacy and usually are not an option because of their associated toxicity. While botulinum toxin can be used in treatment-resistant cases, numerous painful injections are required, and effects are limited to a few months. Surgical sympathectomy should be reserved for the most severe cases and should be performed only after all other treatments have failed. Although the safety and reliability of treatments for palmoplantar hyperhidrosis have improved dramatically, side effects and compensatory sweating are still common, potentially severe problems 5.
Figure 1. Sweat glands
Figure 2. Sweat glands anatomy
Palmoplantar hyperhidrosis causes
Primary palmoplantar hyperhidrosis appears to be due to overactivity of the hypothalamic thermoregulatory center in the brain and is transmitted via the sympathetic nervous system to the eccrine sweat gland. Palmoplantar hyperhidrosis may be inherited in an autosomal dominant manner 7.
Triggers to attacks of sweating may include:
- Hot weather
- Exercise
- Fever
- Anxiety
- Spicy food
Hyperhidrosis may be idiopathic or secondary to other diseases, metabolic disorders, febrile illnesses, or medication use.
Generalized hyperhidrosis may be secondary to numerous conditions including the following.
Causes of secondary localized hyperhidrosis include:
- Gustatory stimuli (associated with Frey syndrome, encephalitis, syringomyelia, diabetic neuropathies, herpes zoster parotitis, and parotid abscess)
- Stroke
- Spinal nerve damage
- Peripheral nerve damage, when it may be associated with cutaneous dysaesthesia
- Surgical sympathectomy
- Chronic alcoholism
- Neuropathy
- A brain tumor
- Chronic anxiety disorder
- Eccrine nevus: This may be associated with severe localized hyperhidrosis 10
- Eccrine angiomatous hamartoma: This is a rare, benign malformation characterized by both eccrine and vascular components 11. It is usually first evident at birth or during early infancy and childhood as a nodule or a plaque, usually solitary, involving acral skin. Although often asymptomatic, it may be associated with focal hyperhidrosis, hypertrichosis, and pain.
- Contralateral hyperhidrosis in the left ophthalmic trigeminal division was documented on the forehead after a lateral medullary infarction 12.
- Paroxysmal sympathetic hyperactivity after a spontaneous intracerebral hemorrhage may also produce hyperhidrosis 13.
- Blue rubber-bleb nevus
- Glomus tumor
- Peripheral neuropathy, organomegaly, endocrinopathy, monoclonal plasma-proliferative disorder, and skin changes (POEMS) syndrome
- Burning feet syndrome
- Pachydermoperiostosis
- Pretibial myxedema
- Spinal cord tumors: Rarely, hyperhidrosis may be an initial symptom 14.
Causes of secondary generalized hyperhidrosis include:
- Obesity
- Diabetes
- Hypoglycemia
- Menopause
- Overactive thyroid (thyrotoxicosis)
- Cardiovascular disorders
- Respiratory failure
- Gout
- Other endocrine tumors, such as phaeochromocytoma
- Parkinson disease
- Hodgkin lymphoma
- Febrile illnesses
- Tuberculosis (in nocturnal hyperhidrosis)
- Drugs: alcohol, caffeine, corticosteroids, cholinesterase inhibitors, tricyclic antidepressants, propranolol, physostigmine, pilocarpine, selective serotonin reuptake inhibitors, nicotinamide and opioids. Efavirenz was recently described to induce excessive nocturnal sweating that resolved after dose reduction 15.
- Spontaneous periodic hypothermia and hyperhidrosis: This is postulated to be a rare cerebral neurotransmitter disorder 16.
Localized unilateral or segmental hyperhidrosis is rare and of unknown origin. The condition usually presents on the forearm or forehead in otherwise healthy individuals, without evidence of the typical triggering factors found in essential hyperhidrosis. Primary focal hyperhidrosis tends to arise on the palms, plantar feet, and armpits, as well as from the face and scalp of children and young adults; there seems to have a distinct subtype of craniofacial hyperhidrosis in postmenopausal women 17.
Unilateral hyperhidrosis with accompanying contralateral anhidrosis is also rare 18. Unilateral hyperhidrosis has been described on the right sides of the forehead, the nose, and the palmar surface of the right hand, with anhidrosis on the left hand.
Palmoplantar hyperhidrosis symptoms
Hyperhidrosis can be localized or generalized.
- Localized hyperhidrosis affects armpits, palms, soles, face or other sites
- Palmar hyperhidrosis
- Slippery hands lead to avoidance of handshaking
- Marks left on paper and fabrics
- Difficulty in writing neatly
- Malfunction of electronic equipment such as keypads and trackpads
- Prone to a blistering type of hand dermatitis (pompholyx)
- Plantar hyperhidrosis
- Affects soles of the feet
- Unpleasant smell
- Ruined footwear
- Prone to a blistering type of dermatitis (pompholyx)
- Prone to secondary infection (tinea pedis, pitted keratolysis)
- Palmar hyperhidrosis
- Generalized hyperhidrosis affects most or all of the body
Hyperhidrosis is can be embarrassing and interferes with many daily activities.
Hyperhidrosis can be primary or secondary.
Primary hyperhidrosis
- Starts in childhood or adolescence
- May persist lifelong or improve with age
- There may be a family history
- Tends to involve armpits, palms and or soles symmetrically
- Usually, sweating reduces at night and disappears during sleep
Secondary hyperhidrosis
- Less common than primary hyperhidrosis
- More likely to be unilateral and asymmetrical, or generalized
- Can occur at night or during sleep.
- Due to endocrine or neurological conditions or drugs
Palmoplantar hyperhidrosis diagnosis
Hyperhidrosis is usually diagnosed clinically. Tests relate to the potential underlying cause of hyperhidrosis and are rarely necessary for primary hyperhidrosis.
The precise site of localized hyperhidrosis can be revealed using the Minor test.
- Iodine (orange) is painted onto the skin and air-dried.
- Starch (white) is dusted on the iodine.
- Sweating is revealed by a change to dark blue/black color.
Screening tests in secondary generalized hyperhidrosis depend on other clinical features but should include as a minimum:
- Blood sugar / glycosylated hemoglobin (HbA1C) may reveal diabetes mellitus or hypoglycemia.
- Thyroid function tests may reveal underlying hyperthyroidism or thyrotoxicosis.
- Urinary catecholamines may reveal a possible pheochromocytoma.
- Uric acid levels may reveal gout.
- A purified protein derivative (PPD) test can be performed to screen for tuberculosis.
Imaging studies
Chest radiography may be used to rule out tuberculosis or a neoplastic cause of the hyperhidrosis.
Palmoplantar hyperhidrosis treatment
Therapy for hyperhidrosis can be challenging for both the patient and the physician. Both topical and systemic medications have been used in the treatment of hyperhidrosis. Other treatment options for hyperhidrosis include iontophoresis and botulinum toxin injections.
Topical agents for hyperhidrosis therapy include topical anticholinergics, boric acid, 2-5% tannic acid solutions, resorcinol, potassium permanganate, formaldehyde (which may cause sensitization), glutaraldehyde, and methenamine 19. All of these agents are limited by staining, contact sensitization, irritancy, or limited effectiveness. These agents reduce perspiration by denaturing keratin and thereby occluding the pores of the sweat glands. They have a short-lasting effect.
The US Food and Drug Administration (FDA) approved glycopyrronium tosylate topical cloth in June 2018 for primary axillary hyperhidrosis in adults and children aged 9 years or older. It is an anticholinergic agent that inhibits the action of acetylcholine on sweat glands. Approval was based on results from two phase 3 clinical trials, ATMOS-1 and ATMOS-2 (n=697). Of these, 44 were pediatric patients aged 9-16 years. The proportions of patients experiencing a reduction of at least 50% in sweat production at 4 weeks for pediatric versus adult patients were 79.9% versus 74.3% of glycopyrronium tosylate–treated patients compared with 54.8% versus 53% of vehicle-treated patients, respectively 20.
Contact sensitization is increased, especially with formalin. Aldehydes are used to treat the palms and soles; they are not as effective in the axillae. Glutaraldehyde solution 2% is sold as Cidex. It is not as effective but less staining. The 20-50% solution can be diluted to 10% (more effective, especially for feet, but still staining occurs).
Because of the limitations of other agents, Drysol (20% aluminum chloride hexahydrate in absolute anhydrous ethyl alcohol) is more commonly used as the first-line topical agent. Drysol should be applied nightly on dry skin with or without occlusion until a positive result is obtained, after which the intervals between applications may be lengthened. To minimize irritation, the remainder of the medication should be washed off when the patient awakes, and the area may be neutralized with the topical application of baking soda 21.
Axillary hyperhidrosis may be treated with aluminium chloride gel, although the gel may cause mild cutaneous irritation 22. Its antiperspirant action for treatment of palmar hyperhidrosis and its low risk of systemic adverse effects from absorption and accumulation of aluminium in visceral organs are noteworthy 23. Use of a novel microwave device has been suggested for axillary hyperhidrosis 24. A single high-energy microwave treatment may be efficacious for selected patients with primary axillary hyperhidrosis 25.
Systemic agents used to treat hyperhidrosis include anticholinergic medications. Anticholinergics such as propantheline bromide, glycopyrrolate, oxybutynin 26 and benztropine are effective because the preglandular neurotransmitter for sweat secretion is acetylcholine (although the sympathetic nervous system innervates the eccrine sweat glands) 27. The use of anticholinergics may be unappealing because their adverse effect profile includes mydriasis, blurry vision, dry mouth and eyes, difficulty with micturition, and constipation. In addition, other systemic medications, such as sedatives and tranquilizers, indomethacin, and calcium channel blockers, may be beneficial in the treatment of palmoplantar hyperhidrosis.
Iontophoresis was introduced in 1952 and consists of passing a direct current across the skin 28. The mechanism of action remains under debate. In palmoplantar hyperhidrosis, the daily treatment of each palm or sole for 30 minutes at 15-20 mA with tap water iontophoresis is effective 29. Intact skin can endure 0.2-mA/cm2 galvanic current without negative consequences, and as much as 20-25 mA per palm may be tolerated 29. Numerous agents have been used to induce hypohidrosis, including tap water and anticholinergics; however, treatment with anticholinergic iontophoresis is more effective than tap water iontophoresis 30. However, the latter is safe and effective when used on Monday, Wednesday, and Friday for 4 weeks, with continued treatment maintaining the effect 31. Noncompliance is common with tap water iontophoresis, as it can be time-consuming 32. This technique merits consideration prior to systemic or aggressive surgical intervention.
Botulinum toxin injections are effective because of their anticholinergic effects at the neuromuscular junction and in the postganglionic sympathetic cholinergic nerves in the sweat glands 33.
In palmar hyperhidrosis, 50 subepidermal injections of 2 mouse units per palm (total 100 mouse units per palm) results in anhydrosis lasting 4-12 months 34. Each injection produces an area of anhydrosis approximately 1.2 cm in diameter. The only adverse effect is mild transient thumb weakness that resolves within 3 weeks. Adverse effects of intradermal injections of botulinum A toxin may result from diffusion into underlying muscles 35. A substantial increase in the duration of efficacy may be produced by repetitive injections in those with primary palmar hyperhidrosis 36.
In a similar study, the effects of sodium chloride solution injections in one palm were compared with botulinum toxin injections in the other palm 37. Treatment with 120 mouse units of botulinum toxin (injected into 6 sites in the palm) resulted in a 26% reduction in sweat production after 3 and 8 weeks and a 31% reduction after 13 weeks. Noted adverse effects included minor muscle weakness at the toxin-treated sites, which resolved after 2-5 weeks. Injections of botulinum toxin must be repeated at varying intervals to maintain long-term results.
Treatment of axillary hyperhidrosis with botulinum toxin type A reconstituted in lidocaine or in normal saline was described in a randomized, side-by-side, double-blind study 38. The results were the same; however, injections of botulinum toxin A reconstituted in lidocaine are associated with significantly reduced pain, thus, lidocaine-reconstituted botulinum toxin A may be preferable for treating axillary hyperhidrosis.
A 2008 study 39 found botulinum toxin type A to be more effective than topical 20% aluminum chloride for the treatment of moderate-to-severe primary focal axillary hyperhidrosis.
Woolery-Lloyd et al 40 reported on successful treatment of inguinal hyperhidrosis with botulinum toxin A. The condition was initially misdiagnosis as urinary incontinence.
Bromhidrosis may be treated with a glycine-soja sterocomplex topical agent, which has shown encouraging results on both the intensity and quality of odor in patients with bromhidrosis 41.
General measures
- Wear loose-fitting, stain-resistant, sweat-proof garments.
- Change clothing and footwear when damp.
- Socks containing silver or copper reduce infection and odour.
- Use absorbent insoles in shoes and replace them frequently.
- Use a non-soap cleanser.
- Apply corn starch powder after bathing.
- Avoid caffeinated food and drink.
- Discontinue any drug that may be causing hyperhidrosis.
- Apply antiperspirant.
Topical antiperspirants
- Deodorants are fragrances or antiseptics to disguise unpleasant smells; on their own, they do not reduce perspiration.
- Antiperspirants contain 10–25% aluminium salts to reduce sweating; “clinical strength” aluminium zirconium salts are more effective than aluminium chloride.
- Topical anticholinergics such as glycopyrrolate and oxybutynin gel have been successful in reducing sweating; cloths containing glycopyrronium tosylate (Qbrexza™) were approved by the FDA in July 2018 for axillary hyperhidrosis in adults and children 9 years of age and older. Dusting powder is available containing the anticholinergic drug, diphemanil 2%.
- Antiperspirants are available as a cream, aerosol spray, stick, roll-on, wipe, powder, and paint.
- Specific products are available for different body sites such as underarms, other skin folds, face, hands and feet.
- They are best applied when the skin is dry, after a cool shower just before sleep.
- Wash off in the morning if tending to irritate.
- Use from once weekly to daily if necessary.
- If irritating, apply hydrocortisone cream short-term.
The most effective topical treatment for palmoplantar hyperhidrosis is 20 percent aluminum chloride hexahydrate in absolute anhydrous ethyl alcohol (Drysol) 3. Less satisfactory results have been achieved with other topical agents, including boric acid, anticholinergic drugs, resorcinol, tannic acid (2 to 5 percent solutions), potassium permanganate, formaldehyde, methenamine, and glutaraldehyde.
Aluminum chloride is thought to obstruct sweat pores and induce atrophy of secretory cells within the sweat glands. The only contraindication to this treatment is documented hypersensitivity, and aluminum chloride should not be used on irritated, broken, or recently shaven skin.
Patients should apply the agent to dry skin nightly until clinical relief is achieved, at which point maintenance therapy is instituted and frequency of applications can be spread out over time in some patients. The morning after an overnight treatment, patients should wash away residual aluminum chloride and apply topical baking soda to limit skin irritation.
Topical therapy has some drawbacks. Compliance may become an issue because the daily applications necessary for efficacy may be considered too time-consuming for patients 42. Topical therapy also may fail to adequately control hyperhidrosis.
Iontophoresis
Iontophoresis is used for hyperhidrosis of palms, soles and armpits. Iontophoresis is a procedure in which an electrical current is passed through skin soaked in tap water (not distilled water), normal saline (0.9%), or a solution containing an anticholinergic medication, which allows ionized (charged) particles to cross the normal skin barrier 43. Iontophoresis with saline is less effective than tap-water iontophoresis. Iontophoresis reduces sweating and enhances the delivery of drugs and macromolecules into and through the skin. Iontophoresis is safe and inexpensive 44. A galvanic current of 0.2 milliamperes (mA) per cm² on intact skin has no adverse side effects, and a rate of up to 20 to 25 mA per surface generally is tolerable 45.
Successful induction of hypohidrosis by tap-water iontophoresis requires the application of 15 to 20 mA to each palm or sole for 30 minutes per session for 10 consecutive days, followed by one or two maintenance sessions per week. Initially, many patients experience an aggravation of their symptoms, but this problem resolves after three to five treatments. Without maintenance therapy, symptoms recur in one to two weeks 46. No side effects have been reported from the use of 20-mA iontophoresis in pregnant women or patients with pacemakers.
- Mains and battery-powered units are available.
- The affected area is immersed in water, or, with a more significant effect, glycopyrronium solution.
- A gentle electrical current is passed across the skin surface for 10–20 minutes.
- Repeated daily for several weeks then less frequently as required
- Iontophoresis may cause discomfort, irritation or irritant contact dermatitis.
- The treatment requires a long-term commitment.
- It is not always effective.
The mechanism of action of iontophoresis in reducing sweating is not completely understood 47. According to one hypothesis, iontophoresis may induce hyperkeratosis of the sweat pores and obstruct sweat flow and secretion (although no plugging of the pores has been found) 47. Other proposed mechanisms include impairment of the electrochemical gradient of sweat secretion and a biofeedback mechanism.
Sweat forms in response to an electrical gradient produced by sympathetic nerve activity on the cells of the sweat gland. There are several theories as to how a change in the electrical gradient reduces sweat production.
- Ions produced by iontophoresis may physically block the sweat ducts in the stratum corneum.
- The external electrical current may disrupt normal sympathetic nerve transmission.
- The pH drops in the sweat gland due to an accumulation of hydrogen ions.
Iontophoresis for hyperhidrosis is usually carried out with ordinary tap water, however, sodium chloride electrolyte (saline) solution or an anticholinergic agent such as glycopyrronium bromide can be added if the water alone is not effective.
Use of the anticholinergic drug atropine during tap-water iontophoresis sometimes is helpful, but extreme caution is necessary to avoid toxicity from atropine overdose. No more than 1 mg of atropine should be added to 30 mL of tap water. The solution is poured over thin gauze placed on a stainless steel anodal plate. Atropine–tap-water iontophoresis should be used only by physicians well trained in this method.
Use of combined aluminum chloride, an anticholinergic drug, and tap-water iontophoresis for one hour each day resulted in the remission of symptoms for 20 days, compared with 3.5 days for the use of iontophoresis alone; this combination also was more effective in reducing the severity of symptoms 48. Employing a device for home use makes this treatment relatively affordable and accessible.
Iontophoresis side effects and risks
Iontophoresis is generally a safe procedure. It is important to avoid direct contact with the electrodes during treatment, as it may cause a mild electric shock.
A feeling of pins and needles or burning sensation is experienced by most people. Adverse effects may include:
- Redness of treated skin
- Small blisters(vesicles) or pompholyx
- Dry and cracked skin or dermatitis.
Although these side effects from iontophoresis are expected to resolve within a few days, emollients/moisturisers should be applied several times daily to reduce symptoms. Topical corticosteroids can be applied.
If used, anticholinergic drugs such as glycopyrronium may be absorbed into the body and produce systemic side effects such as dizziness, dry eyes and dry mouth.
Iontophoresis contraindications
Iontophoresis should not be used by:
- A patient who is epileptic or has a history of seizures
- A patient with a heart condition or a pacemaker
- A patient with a metal implant
- A pregnant woman.
Patients should delay treatment if they have a recent wound, skin graft, or scar in the area requiring treatment, as iontophoresis may be painful and the treatment less effective.
Oral medications
Oral anticholinergic drugs
- Available drugs are propantheline 15–30 mg up to three times daily, oxybutynin 2.5–7.5 mg daily, benztropine, glycopyrrolate (unapproved).
- They can cause dry mouth, and less often, blurred vision, constipation, dizziness, palpitations and other side effects.
- People with glaucoma or urinary retention should not take them.
- Caution in older patients: increased risk of side effects is reported, including dementia.
- Oral anticholinergics may interact with other medications.
Beta-blockers
- Beta-blockers block the physical effects of anxiety.
- They may aggravate asthma or symptoms of peripheral vascular disease.
Calcium channel blockers, alpha-adrenergic agonists (clonidine), nonsteroidal anti-inflammatory drugs (NSAIDs) and anxiolytics may also be useful for some patients.
Botulinum toxin injections
- Botulinum toxin injections are approved for hyperhidrosis affecting the armpits.
- The injections reduce or stop sweating for three to six months.
- Botulinum toxins are used off-license for localized hyperhidrosis in other sites such as palms.
- Topical botulinum toxin gel is under investigation for hyperhidrosis.
Injections of botulinum toxin type A (Botox) are safe and effective, and often improve quality of life in patients with hyperhidrosis 49. The toxin inhibits the release of acetylcholine at the neuromuscular junction and affects the post-ganglionic sympathetic innervation of the sweat glands 50.
An area about 1.2 cm in diameter is made anhidrotic around each injection site; therefore, multiple injections spaced 1 to 2.5 cm apart are necessary over the hyper-hidrotic areas. Efficacy can be observed within one week. Anhidrosis induced by botulinum toxin injections persists for four to 13 months 51. For successful long-term therapy, injections must be repeated regularly.
Intracutaneous injections are recommended rather than subepidermal injections, which are too close to nerve endings 52. Botulinum toxin injections are painful and require the use of an anesthetic. Ulnar and median nerve blocks or intravenous regional anesthesia is more effective in preventing pain than is topical application of a local anesthetic 53.
Potential side effects of botulinum toxin injections include transient, slight weakness in the muscles of the hand and the formation of small hematomas at the injection sites 54. Cost also should be considered. It may take 100 units (acquisition cost: approximately $426) for each hand. Many physicians charge $1,400 to $1,600 for both palms, and the injections have to be repeated every four to six months.
Surgical removal of axillary sweat glands
Overactive sweat glands in the armpits may be removed by several methods, usually under local anaesthetic.
- Tumescent liposuction (sucking them out)
- Subcutaneous curettage (scraping them out)
- Microwave thermolysis (the MiraDry® system approved by FDA in 2011)
- Subdermal Nd:YAG laser
- High-intensity micro-focused ultrasound (experimental)
- Surgery to cut out the sweat gland-bearing skin of the armpits. If a large area needs to be removed, it may be repaired using a skin graft
Sympathectomy
Sympathectomy has been used as a permanent effective treatment since 1920. Consult a neurosurgeon if sympathectomy is necessary in severe cases of hyperhidrosis that are refractory to all other treatments. Division of the sympathetic spinal nerves by chemical or surgical endoscopic thoracic sympathectomy may reduce sweating of face, armpit or palmar hyperhidrosis, but is reserved for the most severely affected individuals due to potential risks and complications 55. Sympathectomy involves the surgical destruction of the ganglia responsible for hyperhidrosis 56. The second (T2) and third (T3) thoracic ganglia are responsible for palmar hyperhidrosis, the fourth (T4) thoracic ganglia controls axillary hyperhidrosis, and the first (T1) thoracic ganglia controls facial hyperhidrosis. Sympathectomy for hyperhidrosis treatment requires an inpatient stay.
- Hyperhidrosis may recur in up to 15% of cases.
- Sympathectomy is often accompanied by undesirable skin warmth and dryness.
- New-onset hyperhidrosis of other sites occurs in 50–90% of patients and is severe in 2%. It is reported to be less frequent after T4 ganglion sympathectomy compared with T2 ganglion sympathectomy.
- Serious complications include Horner syndrome, pneumothorax (in up to 10%), pneumonia and persistent pain (in fewer than 2%).
Lumbar sympathectomy is not recommended for hyperhidrosis affecting the feet, as it can interfere with sexual function.
Two surgical approaches are available: an open approach and a newer endoscopic approach. The endoscopic approach has become favored because of its improvements in terms of complications, surgical scars, and surgical times. Endoscopic thoracic sympathectomy is an effective treatment for hyperhidrosis; in one study, immediate positive results occurred in 832 (98%) of 850 patients 57. After a 31-month average follow-up, symptoms recurred in 17 patients. Improved quality of life has been described for upper limb hyperhidrosis after treatment with limited endoscopic thoracic sympathetic block at T4 58.
Numerous complications are associated with this endoscopic treatment option; these include compensatory sweating (induction of sweating in previously unaffected areas of the body), gustatory sweating, pneumothorax, intercostal neuralgia, Horner syndrome, recurrence of hyperhidrosis, and the complications of general anesthetic use.
Of 850 patients who underwent endoscopic transthoracic sympathectomy, 55% had compensatory sweating (mostly on the trunk), and 36% had gustatory sweating 57. In a similar study of 72 patients who underwent transthoracic endoscopic sympathectomy (T2 or T2 and T3) for palmar hyperhidrosis, the success rate was 93%; compensatory sweating occurred in an overwhelming 99% of patients within 1 month after surgery, and gustatory sweating occurred in 17% 59. The overall occurrence of severe compensatory hyperhidrosis was reduced after T3 ganglionectomy as opposed to ganglionectomies performed at all other levels 60.
T4 ganglion interruption for palmar hyperhidrosis is an effective approach that can simultaneously minimize the rate of compensatory hyperhidrosis 61. Thus, T4 sympathectomy may be an effective cure. Its rate of compensatory hyperhidrosis appears to be remarkably low compared with T2 sympathetic ganglionic interruption. An effective treatment for such compensatory sweating is the intradermal injection of botulinum toxin 62.
Li et al 63 reported on minimizing endoscopic thoracic sympathectomy for hyperhidrosis of the palms using the skin temperature of the palms and Doppler-guided blood flow analysis as aids.
Video-assisted thoracic sympathectomy may be preferable to no treatment for children with palmar hyperhidrosis and a poor quality of life 64.
Future treatments for hyperhidrosis
Several research projects are underway to find safer and more effective treatments for hyperhidrosis. These include:
- Topical anticholinergic DRM04
- Combination of oxybutynin and pilocarpine (to counteract the adverse effects of the anticholinergic, oxybutynin) THVD-102
Palmoplantar hyperhidrosis prognosis
Palmoplantar hyperhidrosis tends to improve with age. The outlook for secondary localized or generalized hyperhidrosis depends on the cause.
References- Palmoplantar hyperhidrosis. In: Levine N., Levine C.C. (eds) Dermatology Therapy 2004. A to Z Essentials. Springer, Berlin, Heidelberg. https://doi.org/10.1007/3-540-29668-9_2010
- Wenzel FG, Horn TD. Nonneoplastic disorders of the eccrine glands. J Am Acad Dermatol. 1998;38:1–17.
- Stolman LP. Treatment of hyperhidrosis. Dermatol Clin. 1998; 16:863–9.
- Ruchinskas R. Hyperhidrosis and anxiety: chicken or egg?. Dermatology. 2007. 214(3):195-6.
- Palmoplantar Hyperhidrosis: A Therapeutic Challenge. Am Fam Physician. 2004 Mar 1;69(5):1117-1121. https://www.aafp.org/afp/2004/0301/p1117.html
- Tugnoli V, Eleopra R, De Grandis D. Hyperhidrosis and sympathetic skin response in chronic alcoholic patients. Clin Auton Res. 1999; 9:17–22.
- Yamashita N, Tamada Y, Kawada M, Mizutani K, Watanabe D, Matsumoto Y. Analysis of family history of palmoplantar hyperhidrosis in Japan. J Dermatol. 2009 Dec. 36(12):628-31.
- Kumagai K, Kawase H, Kawanishi M. Health-related quality of life after thoracoscopic sympathectomy for palmar hyperhidrosis. Ann Thorac Surg. 2005 Aug. 80(2):461-6.
- Hyperhidrosis Treatment & Management. https://emedicine.medscape.com/article/1073359-treatment
- Lera M, España A, Idoate MÁ. Focal hyperhidrosis secondary to eccrine naevus successfully treated with botulinum toxin type A. Clin Exp Dermatol. 2015 Mar 28.
- Sanusi T, Li Y, Sun L, Wang C, Zhou Y, Huang C. Eccrine Angiomatous Hamartoma: A Clinicopathological Study of 26 Cases. Dermatology. 2015 Apr 14.
- Thirugnanachandran T, Ma H, Phan T. Contralateral hyperhidrosis following lateral medullary infarction. Pract Neurol. 2020 Jan 30.
- Li Z, Chen W, Zhu Y, Han K, Wang J, Chen J, et al. Risk factors and clinical features of paroxysmal sympathetic hyperactivity after spontaneous intracerebral hemorrhage. Auton Neurosci. 2020 Jan 27. 225:102643
- Shi W, Zhao B, Yao J, Zhou Y, Tong M, Jing L, et al. Intramedullary Spinal Cord Ganglioglioma Presenting as Hyperhidrosis: A Rare Case Report and literature Review. World Neurosurg. 2019 Mar 20.
- Martín AF, Figueroa SC, Merino Mde L, Hurlee AD. Hyperhidrosis in association with efavirenz. AIDS Patient Care STDS. 2009 Mar. 23(3):143-5.
- Mehta S, Ralot T, Masatkar V, Agarwal N, Rana A. A curious case of hourly attacks of disabling episodic spontaneous hypothermia with hyperhidrosis. Indian J Dermatol Venereol Leprol. 2015 Mar-Apr. 81(2):185-6.
- Eustace K, Wilson NJ. Postmenopausal craniofacial hyperhidrosis. Clin Exp Dermatol. 2018 Mar. 43 (2):180-182.
- Kocyigit P, Akay BN, Saral S, Akbostanci C, Bostanci S. Unilateral hyperhidrosis with accompanying contralateral anhidrosis. Clin Exp Dermatol. 2009 Dec. 34(8):e544-6.
- Shelley WB, Laskas JJ, Satanove A. Effect of topical agents on planter sweating. AMA Arch Derm Syphilol. 1954 Jun. 69(6):713-6.
- Hebert A. Glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: Pediatric subgroup analyses from the ATMOS-1 and ATMOS-2 Phase 3 randomized controlled trials (Abstract 6659). Presented at the 76th Annual Meeting of the American Academy of Dermatology (AAD). February 16-20, 2018. San Diego, Calif:
- Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol. 1989 May. 20(5 Pt 1):713-26.
- Streker M, Reuther T, Verst S, Kerscher M. [Axillary hyperhidrosis–efficacy and tolerability of an aluminium chloride antiperspirant. Prospective evaluation on 20 patients with idiopathic axillary hyperhidrosis]. Hautarzt. 2010 Feb. 61(2):139-44.
- Yanagishita T, Tamada Y, Ohshima Y, Ito K, Akita Y, Watanabe D. Histological localization of aluminum in topical aluminum chloride treatment for palmar hyperhidrosis. J Dermatol Sci. 2012 Mar 3.
- Sánchez-Carpintero I, Martín-Gorgojo A, Ruiz-Rodríguez R. Microwave Treatment for Axillary Hyperhidrosis and Bromhidrosis. Actas Dermosifiliogr. 2017 Mar 8.
- Kaminaka C, Mikita N, Inaba Y, Kunimoto K, Okuhira H, Jinnin M, et al. Clinical and histological evaluation of a single high energy microwave treatment for primary axillary hyperhidrosis in Asians: A prospective, randomized, controlled, split-area comparative trial. Lasers Surg Med. 2019 Feb 27.
- Del Boz J, Millán-Cayetano JF, Blázquez-Sánchez N, de Troya M. Individualized Dosing of Oral Oxybutynin for the Treatment of Primary Focal Hyperhidrosis in Children and Teenagers. Pediatr Dermatol. 2016 May. 33 (3):327-31.
- Wozniacki L, Zubilewicz T. Primary hyperhidrosis controlled with oxybutynin after unsuccessful surgical treatment. Clin Exp Dermatol. 2009 Dec. 34(8):e990-1.
- Karakoç Y, Aydemir EH, Kalkan MT, Unal G. Safe control of palmoplantar hyperhidrosis with direct electrical current. Int J Dermatol. 2002 Sep. 41(9):602-5.
- Sato K, Ohtsuyama M, Samman G. Eccrine sweat gland disorders. J Am Acad Dermatol. 1991 Jun. 24(6 Pt 1):1010-4.
- Abell E, Morgan K. The treatment of idiopathic hyperhidrosis by glycopyrronium bromide and tap water iontophoresis. Br J Dermatol. 1974 Jul. 91(1):87-91.
- Siah TW, Hampton PJ. The effectiveness of tap water iontophoresis for palmoplantar hyperhidrosis using a Monday, Wednesday, and Friday treatment regime. Dermatol Online J. 2013 Mar 15. 19(3):14.
- Ozcan D, Güleç AT. Compliance with tap water iontophoresis in patients with palmoplantar hyperhidrosis. J Cutan Med Surg. 2014 Mar 1. 18(2):109-13.
- Kang A, Burns E, Glaser DA. Botulinum toxin A for palmar hyperhidrosis: associated pain, duration, and reasons for discontinuation of therapy. Dermatol Surg. 2015 Feb. 41(2):297-8.
- Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998 Feb. 38(2 Pt 1):227-9.
- Swartling C, Farnstrand C, Abt G, Stalberg E, Naver H. Side-effects of intradermal injections of botulinum A toxin in the treatment of palmar hyperhidrosis: a neurophysiological study. Eur J Neurol. 2001 Sep. 8(5):451-6.
- Lecouflet M, Leux C, Fenot M, Célerier P, Maillard H. Duration of efficacy increases with the repetition of botulinum toxin A injections in primary palmar hyperhidrosis: A study of 28 patients. J Am Acad Dermatol. 2014 Mar 12.
- Schnider P, Binder M, Auff E, Kittler H, Berger T, Wolff K. Double-blind trial of botulinum A toxin for the treatment of focal hyperhidrosis of the palms. Br J Dermatol. 1997 Apr. 136(4):548-52.
- Vadoud-Seyedi J, Simonart T. Treatment of axillary hyperhidrosis with botulinum toxin type A reconstituted in lidocaine or in normal saline: a randomized, side-by-side, double-blind study. Br J Dermatol. 2007 May. 156(5):986-9.
- Flanagan KH, King R, Glaser DA. Botulinum toxin type a versus topical 20% aluminum chloride for the treatment of moderate to severe primary focal axillary hyperhidrosis. J Drugs Dermatol. 2008 Mar. 7(3):221-7.
- Woolery-Lloyd H, Elsaie ML, Avashia N. Inguinal hyperhidrosis misdiagnosed as urinary incontinence: treatment with botulinum toxin A. J Drugs Dermatol. 2008 Mar. 7(3):293-5.
- Gregoriou S, Rigopoulos D, Chiolou Z, Papafragkaki D, Makris M, Kontochristopoulos G. Treatment of bromhidrosis with a glycine-soja sterocomplex topical product. J Cosmet Dermatol. 2011 Mar. 10(1):74-7.
- Leung AK, Chan PY, Choi MC. Hyperhidrosis. Int J Dermatol. 1999; 38:561–7.
- Boumann HD, Grunewald-Lentzer EM. The treatment of hyperhidrosis of the hands and feet with a constant current. Am J Phys Med. 1952;31:158–69.
- Jain S, Dey VK, Agrawal N. A Pocket-Friendly and Sustainable Iontophoresis Apparatus for Palmoplantar Hyperhidrosis: Advancement over a Previously Described Homemade Design. J Cutan Aesthet Surg. 2018;11(3):153-156. doi:10.4103/JCAS.JCAS_58_17 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6243828
- Sato K, Ohtsuyama M, Samman G. Eccrine sweat gland disorders. J Am Acad Dermatol. 1991;24(6 pt 1):1010–4.
- Karakoc Y, Aydemir EH, Kalkan MT, Unal G. Safe control of palmoplantar hyperhidrosis with direct electrical current. Int J Dermatol. 2002;41:602–5.
- Hill AC, Baker GF, Jansen GT. Mechanism of action of iontophoresis in the treatment of palmar hyperhidrosis. Cutis. 1981;28:69–70,72.
- Shen JL, Lin GS, Li WM. A new strategy of iontophoresis for hyperhidrosis. J Am Acad Dermatol. 1990;22(2 pt 1):239–41.
- Tan SR, Solish N. Long-term efficacy and quality of life in the treatment of focal hyperhidrosis with botulinum toxin A. Dermatol Surg. 2002;28:495–9.
- Rusciani L, Severino E, Rusciani A. Type A botulinum toxin: a new treatment for axillary and palmar hyperhidrosis. J Drugs Dermatol. 2002;1:147–51.
- Vadoud-Seyedi J, Heenen M, Simonart T. Report of idiopathic palmar hyper-hidrosis with botulinum toxin. Review of 23 cases and review of the literature. Dermatology. 2001;203:318–21.
- Wollina U, Karamfilov T. Botulinum toxin A for palmar hyperhidrosis. J Eur Acad Dermatol Venereol. 2001;15:555–8.
- Blaheta HJ, Vollert B, Zuder D, Rassner G. Intravenous regional anesthesia (Bier’s block) for botulinum toxin therapy of palmar hyperhidrosis is safe and effective. Dermatol Surg. 2002;28:666–71.
- Schnider P, Binder M, Auff E, Kittler H, Berger T, Wolff K. Double-blind trial of botulinum A toxin for the treatment of focal hyperhidrosis of the palms. Br J Dermatol. 1997;136:548–52.
- Kotzareff A. Resection partielle de trone sympathetique cervical droit pour hyperhidrose unilaterale. Rev Med Suisse Romande. 1920. 40:111-3.
- Kim BY, Oh BS, Park YK, Jang WC, Suh HJ, Im YH. Microinvasive video-assisted thoracoscopic sympathicotomy for primary palmar hyperhidrosis. Am J Surg. 2001 Jun. 181(6):540-2.
- Drott C, Gothberg G, Claes G. Endoscopic transthoracic sympathectomy: an efficient and safe method for the treatment of hyperhidrosis. J Am Acad Dermatol. 1995 Jul. 33(1):78-81.
- Panhofer P, Zacherl J, Jakesz R, Bischof G, Neumayer C. Improved quality of life after sympathetic block for upper limb hyperhidrosis. Br J Surg. 2006 May. 93(5):582-6.
- Lai YT, Yang LH, Chio CC, Chen HH. Complications in patients with palmar hyperhidrosis treated with transthoracic endoscopic sympathectomy. Neurosurgery. 1997 Jul. 41(1):110-3; discussion 113-5.
- Chwajol M, Barrenechea IJ, Chakraborty S, Lesser JB, Connery CP, Perin NI. Impact of compensatory hyperhidrosis on patient satisfaction after endoscopic thoracic sympathectomy. Neurosurgery. 2009 Mar. 64(3):511-8; discussion 518.
- Chou SH, Kao EL, Li HP, Lin CC, Huang MF. T4 sympathectomy for palmar hyperhidrosis: an effective approach that simultaneously minimzes compensatory hyperhidrosis. Kaohsiung J Med Sci. 2005 Jul. 21(7):310-3.
- Heckmann M. Complications in patients with palmar hyperhidrosis treated with transthoracic endoscopic sympathectomy. Neurosurgery. 1998 Jun. 42(6):1403-4.
- Li X, Tu YR, Lin M, Lai FC, Chen JF, Miao HW. Minimizing endoscopic thoracic sympathectomy for primary palmar hyperhidrosis: guided by palmar skin temperature and laser Doppler blood flow. Ann Thorac Surg. 2009 Feb. 87(2):427-31.
- Neves S, Uchoa PC, Wolosker N, Munia MA, Kauffman P, de Campos JR, et al. Long-Term Comparison of Video-Assisted Thoracic Sympathectomy and Clinical Observation for the Treatment of Palmar Hyperhidrosis in Children Younger Than 14. Pediatr Dermatol. 2012 Apr 4.