What is parainfluenza
Human parainfluenza viruses commonly cause respiratory illnesses in infants and young children. But anyone can get human parainfluenza virus illness. For example, human parainfluenza viruses are the major cause of croup, which is an inflammation of the voice box (larynx) and windpipe (trachea) that makes breathing more difficult. Human parainfluenza viruses also cause some cases of lower respiratory tract diseases, including pneumonia (a lung infection) and bronchiolitis (an infection of the lung’s small breathing tubes). Human parainfluenza viruses can make the symptoms of chronic lung disease worse in children. Symptoms may include fever, runny nose, and cough. Patients usually recover on their own. However, human parainfluenza viruss can also cause more severe illness, such as pneumonia.
The following symptoms occur in many types of parainfluenza infections, although they may be different from child to child or one kind of infection to another:
- A rough, barking cough
- Rapid, noisy, or labored breathing
- Hoarseness and wheezing
- Redness of the eye
- A runny nose
- Cough
- Fever
- A decline in appetite
- Vomiting
- Diarrhea
There are now two genera of human parainfluenza virus, Respirovirus (human parainfluenza virus-1 and human parainfluenza virus-3) and Rubulavirus (human parainfluenza virus-2 and human parainfluenza virus-4) 1. Both genera (paramyxoviruses) can be separated morphologically from influenza virus (myxoviruses) by their nonsegmented thick nucleocapsids (17 nm versus 9 nm) 2. Other genera of the Paramyxoviridae can be physically distinguished from human parainfluenza virus by the absence of a neuraminidase (morbilliviruses, e.g., measles virus and distemper virus) or a thiner nucleocapsid (pneumoviruses, e.g., respiratory syncytial virus [RSV], or metapneumoviruses). The megamyxoviruses are still being described but appear more closely related to morbilliviruses phylogenetically than to the human parainfluenza virus.
There are four types of human parainfluenza viruss and two subtypes that circulate at different times of the year. People usually get human parainfluenza virus infections in the spring, summer, and autumn.
- Human parainfluenza virus-1 infections often cause croup in children. There are usually more cases in the fall of odd-numbered years.
- Human parainfluenza virus-2 infections can also cause croup. human parainfluenza virus-2 infections occur more commonly in the fall each year. It is less frequently detected than human parainfluenza virus-1 and human parainfluenza virus-3.
- Human parainfluenza virus-3 infections usually occur in spring and early summer months each year. However, human parainfluenza virus-3 infections can occur throughout the year, particularly when human parainfluenza virus-1 and human parainfluenza virus-2 are not in season.
- Human parainfluenza virus-4 (subtypes 4A and 4B) seasonal patterns are not as well characterized.
Human parainfluenza viruses (HPIVs) usually spread from an infected person to others through:
- the air by coughing and sneezing,
- close personal contact, such as touching or shaking hands, and
- touching objects or surfaces that have human parainfluenza viruses on them then touching your mouth, nose, or eyes.
People can get multiple human parainfluenza virus infections in their lifetime. These reinfections usually cause mild upper respiratory tract illness with cold-like symptoms. However, reinfections can cause serious lower respiratory tract illness, such as pneumonia, bronchitis, and bronchiolitis in some people. Older adults and people with compromised immune systems, in particular, have a higher risk for severe infections.
Most children 5 years of age and older have antibodies against human parainfluenza virus-3 and approximately 75% have antibodies against human parainfluenza virus-1 and human parainfluenza virus-2.
Many factors have been found that predispose people to human parainfluenza virus infections, including malnutrition, overcrowding, vitamin A deficiency, lack of breast feeding, and environmental smoke or toxins 3. There are more than 5 million lower respiratory infections each year in the United States in children younger than 5 years 4. Human parainfluenza virus-1 to human parainfluenza virus-3 have been found in as many as one-third of these infections 4. In addition, human parainfluenza virus cause upper respiratory infections in infants, children, and adults and, to a lesser extent, lower respiratory infections in the immunocompromised, those with chronic diseases (e.g., heart and lung disease and asthma) and the elderly 5. Less is known about human parainfluenza virus-4. Young infants and children are clearly infected by this virus, but it is rarely isolated. Serologic surveys have demonstrated that most children between 6 and 10 years of age have evidence of past infection, suggesting mild or asymptomatic primary infections 6. Acute respiratory infections cause 3 to 18% of all admissions to pediatric hospitals, and human parainfluenza virus can be detected in 9 to 30% of these patients depending on the time of year 7. There are between 500,000 and 800,000 lower respiratory infections hospitalizations (in persons younger than 18 years) in the United States each year, with approximately 12% being for human parainfluenza virus-1, human parainfluenza virus-2, and human parainfluenza virus-3 infections 8. This is second only to respiratory syncytial virus (RSV) as a cause of hospitalization for viral lower respiratory infections. Immunity to human parainfluenza virus is incomplete, and infections occur throughout life; however, less is known about infections in adults.
The treatment of viral illnesses, including those caused by parainfluenza viruses, should not involve the use of antibacterials, which are not effective against viruses. Most parainfluenza infections do not require specific treatment other than soothing the symptoms and making your child more comfortable until she feels better. The illness goes away on its own. Antibacterials should only be used if a secondary bacterial infection develops.
Talk to your pediatrician about whether your child with a fever should be given acetaminophen to lower her body temperature. Make sure she drinks lots of liquids.
Some supportive therapies are unique to the specific infection that is present. For croup, which is characterized by a barking cough, your child may feel better if you take her into the bathroom, turn on the hot water in the shower, and let the bathroom fill with steam. The warm, moist air should allow her to breathe easier. Breathing in steam is usually helpful, but if it isn’t, take your child outdoors for a few minutes. Inhaling the moist, cool night air may loosen up her airway, and she will be able to breathe easier.
Your pediatrician may prescribe a dose of corticosteroids for croup. Usually a single dose is all that is needed.
Human parainfluenza virus
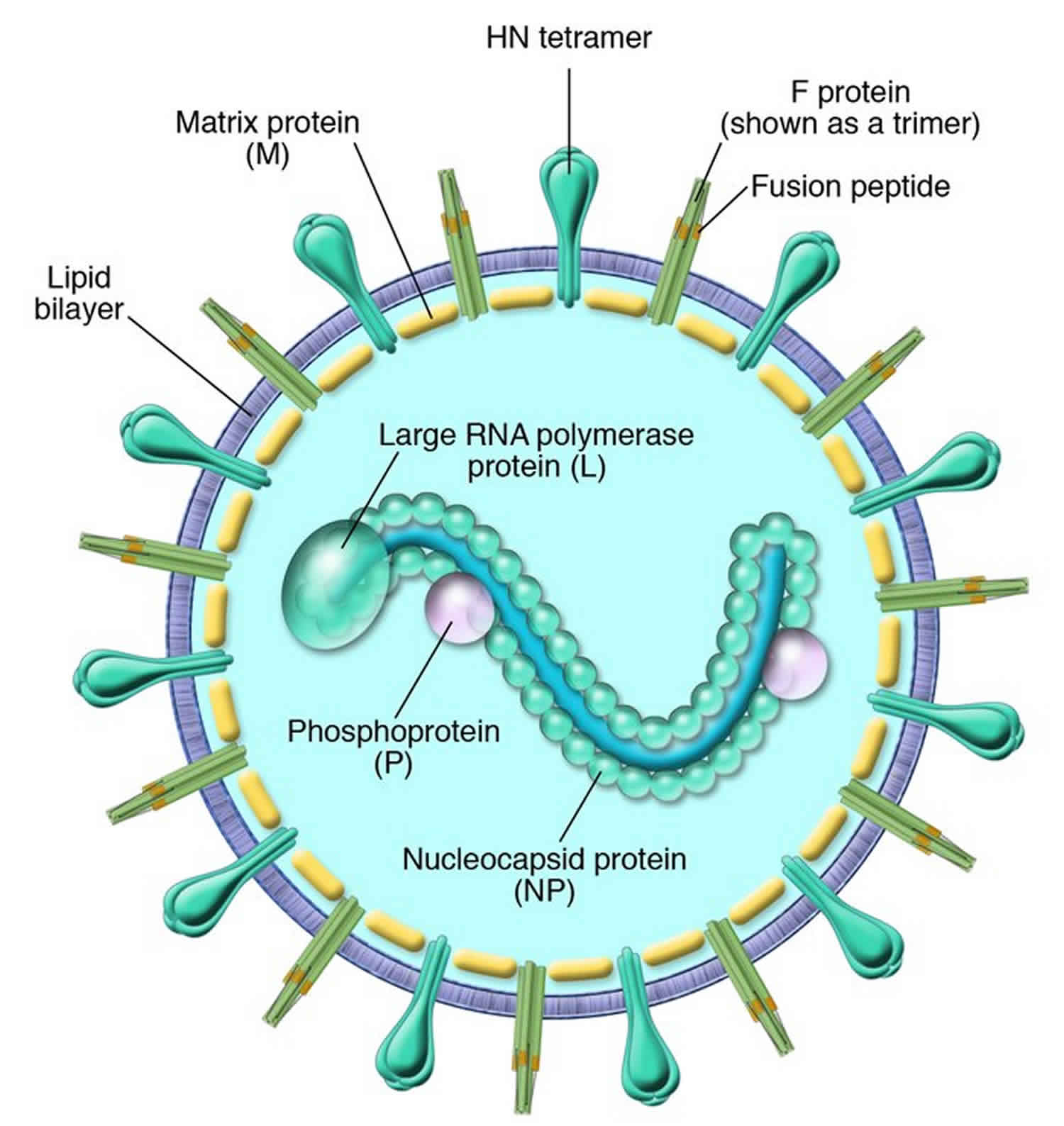
Human parainfluenza viruses were first discovered in the late 1950s 1. Over the last decade, considerable knowledge about their molecular structure and function has been accumulated. Human parainfluenza virus are enveloped and of medium size (150 to 250 nm), and their RNA genome is in the negative sense. Human parainfluenza viruses belong to the Paramyxoviridae family, one of the largest and most rapidly growing groups of viruses causing significant human and veterinary disease. Human parainfluenza virus are closely related to recently discovered megamyxoviruses (Hendra and Nipah viruses) and metapneumovirus 1. There are now two genera of human parainfluenza virus, Respirovirus (human parainfluenza virus-1 and human parainfluenza virus-3) and Rubulavirus (human parainfluenza virus-2 and human parainfluenza virus-4). Both genera (paramyxoviruses) can be separated morphologically from influenza virus (myxoviruses) by their nonsegmented thick nucleocapsids (17 nm versus 9 nm) 2. Other genera of the Paramyxoviridae can be physically distinguished from human parainfluenza virus by the absence of a neuraminidase (morbilliviruses, e.g., measles virus and distemper virus) or a thiner nucleocapsid (pneumoviruses, e.g., respiratory syncytial virus [RSV], or metapneumoviruses). The megamyxoviruses are still being described but appear more closely related to morbilliviruses phylogenetically than to the human parainfluenza virus.
Human parainfluenza virus is genetically and antigenically divided into types 1 to 4. Further major subtypes of human parainfluenza virus-4 (A and B) 9 and subgroups/genotypes of human parainfluenza virus-1 10 and human parainfluenza virus-3 11 have been described. Human parainfluenza virus-1 to human parainfluenza virus-3 are major causes of lower respiratory infections in infants, young children, the immunocompromised, the chronically ill, and the elderly 12. These medium-sized viruses are enveloped, and their genomes are organized on a single negative-sense strand of RNA. The majority of their structural and biological charactertistics are similar, but they each have adapted to infect humans at different ages and cause different diseases. These viruses belong to the Paramyxoviridae family, which is a large rapidly growing group of viruses that cause significant human and veterinary disease. In fact, this virus family is one of the most costly in terms of disease burden and economic impact to our planet. Recently discovered members of the Paramyxoviridae (megamyxoviruses [Hendra and Nipah viruses] and metapneumovirus) emphasize this point 13.
Each subtype can cause somewhat unique clinical diseases in different hosts. In the United States, human parainfluenza viruses commonly cause respiratory tract illnesses. And you can have multiple human parainfluenza virus illnesses in your lifetime.
Different types of human parainfluenza virus
- Human parainfluenza virus-1 and human parainfluenza virus-2 both cause croup, with human parainfluenza virus-1 most often identified as the cause in children. Both can also cause upper and lower respiratory illness, and cold-like symptoms.
- Human parainfluenza virus-3 is more often associated with bronchiolitis, bronchitis, and pneumonia.
- Human parainfluenza virus-4 is recognized less often but may cause mild to severe respiratory illnesses.
Table 1. Taxonomic relationships of human parainfluenza virus types within the family Paramyxoviridae
| Subfamily and genus | Species | |
|---|---|---|
| Human | Animal | |
| Paramyxovirinae | ||
| Respirovirus | Human parainfluenza virus-1, Human parainfluenza virus-3 | Sendai (mouse PIV-1), bovine PIV-3, simian PIV-10 |
| Rubulavirus | Human parainfluenza virus-2, Human parainfluenza virus-4A, Human parainfluenza virus-4B, Mumps virus | NDV (1), Yucaipa virus (2), Kunitachi virus (5), avian PIV-3, avian PIV-4, avian PIV-6 to PIV-9 |
| La-piedad-Michoacan Mexico porcine virus, simian PIV-5 and PIV-41 | ||
| Morbillivirus | Measles virus | Canine distemper virus, rinderpest virus (bovine), pest-des-petits-ruminants virus, dolphine distemper virus, porpoise distemper virus, phocine distemper virus |
| Megamyxovirus | Hendra virus, Nipah virus | HeV (equine, (bats?), NIV (porcine, bats?) |
| Pneumovirinae | ||
| Pneumovirus | Respiratory syncytial virus (RSV) | Bovine RSV, pneumonia virus of mice |
| Metapneumovirus | Human metapneumovirus (hMPV) | Avian pneumovirus (APV) |
Reports from the United States have suggested that a minimum of 50% of croup cases are caused by human parainfluenza virus 14. During each human parainfluenza virus-1 epidemic, an estimated 18,000 to 35,0000 U.S. children younger than 5 years are hospitalized 15. Some of these children have bronchiolitis, tracheobronchitis, pneumonia, and febrile and afebrile wheezing. The majority of infections occur in children aged 7 to 36 months, with a peak incidence in the second and third year of life. Human parainfluenza virus-1 can cause lower respiratory infections in young infants but is rare in those younger than 1 month. The full burden of human parainfluenza virus-1 in adults and the elderly has not been determined, but several studies have shown this virus to cause yearly hospitalizations in healthy adults and perhaps play a role in bacterial pneumonias and deaths in nursing home residents 5. Human parainfluenza virus-1, to human parainfluenza virus-3 have all been found to occur at low levels in most months of the year, similar to respiratory syncytial virus (RSV) and influenza virus 16.
Human parainfluenza virus-2 has been reported to cause infections biennially with human parainfluenza virus-1 or alternate years with human parainfluenza virus-1 or to cause yearly outbreaks 17. Doctors see some human parainfluenza virus-2 activity every year in Milwaukee, Wis. The peak season for this virus is fall to early winter. Human parainfluenza virus-2 causes all of the typical lower respiratory infections syndromes, but in nonimmunocompromised or chronically ill children croup is the most frequent syndrome brought to medical attention. Lower respiratory infections caused by this virus has been reported much less frequently than with human parainfluenza virus-1 and human parainfluenza virus-3. This may be due to difficulties in isolation and detection. As many as 6,000 children younger than 18 years may be hospitalized each year in the United States because of human parainfluenza virus-2. About 60% of all human parainfluenza virus-2 infections occur in children younger than 5 years, and although the peak incidence is between 1 and 2 years of age, significant numbers of infants younger than 1 year are hospitalized each year. Human parainfluenza virus-2 is often overshadowed by human parainfluenza virus-1 or human parainfluenza virus-3 infections, yet in any one year or location it can be the most common cause of parainfluenza lower respiratory infections in young children.
Young infants (younger than 6 months) are particularly vulnerable to infection with human parainfluenza virus-3. Unlike the other human parainfluenza virus, 40% of human parainfluenza virus-3 infections are in the first year of life. Brochiolitis and pneumonia are the most common clinical presentations. Only RSV causes more lower respiratory infections in neonates and young infants. human parainfluenza virus-3 has caused outbreaks within neonatal intensive care units, and the natural history of this virus in the neonate is currently being studied 16. Approximately 18,000 infants and children are hospitalized each year in the United States because of lower respiratory infections caused by human parainfluenza virus-3. This virus causes yearly spring and summer epidemics in North America and Europe and is somewhat endemic, especially in the immunocompromised and chronically ill. Although the exact reasons for the different seasonality of the human parainfluenza virus types is unknown, differences in ambient climate conditions have been proposed as one hypothesis 18.
Only a small number of studies have reported on the isolation or epidemiology of human parainfluenza virus-4 6. These cases and data are distributed fairly equally between infants younger than 1 year, preschool children, and school age children and adults. Seroprevalence studies have demonstrated that 60 to 84% of infants have significant antibody levels after birth (presumably maternal in origin). These levels drop to 7 to 9% by 7 to 12 months of age and stay low for several years before increasing to about 50% by 3 to 5 years of age. Antibody levels to human parainfluenza virus-4 continue to rise throughout childhood until approximately 95% of adults have antibody to human parainfluenza virus-4 A and 75% have antibody to human parainfluenza virus-4 B 6. Interestingly, the majority of human parainfluenza virus-4 clinical isolates appear to be subtype B. All of the different respiratory tract syndromes can be caused by human parainfluenza virus-4. Although hospitalization of infants and young children secondary to human parainfluenza virus-4 lower respiratory infections has been reported, serious disease is either rare or difficult to diagnose based on the seroprevelence 19.
Animal parainfluenza virus have been reported on occasion to infect humans 1. The majority of evidence to support this hypothesis is serologic, and because of the antigenic relatedness of the respirovirus and rubulavirus genera, it is difficult to substantiate. However, closely related megamyxoviruses (Hendra and Nipah viruses), whose natural host appear to be bats, have recently caused serious epidemics of encephalitis in humans 13. Also, Newcastle disease virus clearly causes human disease and is discussed under “Host range.” Other examples of parainfluenza virus infecting outside of their usual host range include bovine parainfluenza virus-3 in humans, human parainfluenza virus-1 in mice, and mouse parainfluenza virus-1 (Sendai virus) in African green monkeys 20. These infections are usually asymptomatic.
Parainfluenza vs Influenza
Influenza (flu) is a contagious respiratory illness caused by influenza viruses that infect the nose, throat, and sometimes the lungs. Influenza (flu) can cause mild to severe illness. Serious outcomes of influenza (flu) infection can result in hospitalization or death. Some people, such as older people, young children, and people with certain health conditions, are at high risk of serious flu complications.
The best way to prevent flu is by getting a flu vaccine each year. Flu vaccine has been shown to reduce flu related illnesses and the risk of serious flu complications that can result in hospitalization or even death. The Centers for Disease Control and Prevention (CDC) also recommends everyday preventive actions (like staying away from people who are sick, covering coughs and sneezes and frequent handwashing) to help slow the spread of germs that cause respiratory (nose, throat, and lungs) illnesses, like flu.
Influenza symptoms
Flu can cause mild to severe illness, and at times can lead to death. Flu is different from a cold. Flu usually comes on suddenly. People who have flu often feel some or all of these symptoms:
- fever. It’s important to note that not everyone with flu will have a fever.
- cough
- sore throat
- runny or stuffy nose
- body aches
- headache
- chills
- fatigue
- sometimes diarrhea and vomiting
How influenza spreads
Most experts believe that flu viruses spread mainly by tiny droplets made when people with influenza (flu) cough, sneeze or talk. These droplets can land in the mouths or noses of people who are nearby. Less often, a person might get influenza (flu) by touching a surface or object that has flu virus on it and then touching their own mouth, nose or possibly their eyes.
Period of Contagiousness
You may be able to pass on flu to someone else before you know you are sick, as well as while you are sick.
- People with influenza (flu) are most contagious in the first 3-4 days after their illness begins.
- Some otherwise healthy adults may be able to infect others beginning 1 day before symptoms develop and up to 5 to 7 days afterbecoming sick.
- Some people, especially young children and people with weakened immune systems, might be able to infect others with flu viruses for an even longer time.
Onset of symptoms
The time from when a person is exposed and infected with flu to when symptoms begin is about 2 days, but can range from about 1 to 4 days.
Complications of influenza
Complications of flu can include bacterial pneumonia, ear infections, sinus infections and worsening of chronic medical conditions, such as congestive heart failure, asthma, or diabetes.
People at high risk from influenza
Anyone can get flu (even healthy people), and serious problems related to flu can happen at any age, but some people are at high risk of developing serious flu-related complications if they get sick. This includes people 65 years and older, people of any age with certain chronic medical conditions (such as asthma, diabetes, or heart disease), pregnant women, and children younger than 5 years.
Influenza diagnosis
It is very difficult to distinguish influenza from other viral or bacterial respiratory illnesses based on symptoms alone. There are tests available to diagnose influenza. A number of flu tests are available to detect influenza viruses in respiratory specimens. The most common are called “rapid influenza diagnostic tests (RIDTs).” RIDTs work by detecting the parts of the virus (antigens) that stimulate an immune response. These tests can provide results within approximately 10-15 minutes, but are not as accurate as other flu tests. Therefore, you could still have the flu, even though your rapid test result is negative. Other flu tests are called “rapid molecular assays” that detect genetic material of the virus. Rapid molecular assays produce results in 15-20 minutes and are more accurate than RIDTs. In addition, there are several more-accurate and sensitive flu tests available that must be performed in specialized laboratories, such as those found in hospitals or state public health laboratories. All of these tests require that a health care provider swipe the inside of your nose or the back of your throat with a swab and then send the swab for testing. Results may take one hour or several hours.
Most people with flu symptoms are not tested because the test results usually do not change how you are treated.
Your health care provider may diagnose you with flu based on your symptoms and their clinical judgment or they may choose to use an influenza diagnostic test. During an outbreak of respiratory illness, testing for flu can help determine if flu viruses are the cause of the outbreak. Flu testing can also be helpful for some people with suspected flu who are pregnant or have a weakened immune system, and for whom a diagnosis of flu can help their doctor make decisions about their care.
Is parainfluenza contagious?
Yes. Human parainfluenza viruss usually spread by direct contact with infectious droplets or by airborne spread when an infected person breathes, coughs, or sneezes. Human parainfluenza viruss may remain infectious in airborne droplets for over an hour and on surfaces for a few hours depending on environmental conditions.
People are most contagious during the early stage of illness.
Human parainfluenza viruses usually spread from an infected person to others through:
- the air by coughing and sneezing,
- close personal contact, such as touching or shaking hands, and
- touching objects or surfaces that have human parainfluenza viruses on them then touching your mouth, nose, or eyes.
The incubation period, the time from exposure to human parainfluenza virus to onset of symptoms, is generally 2 to 7 days.
Parainfluenza duration
Symptoms usually develop between 2 and 7 days from the time of exposure and typically resolve in 7-10 days 21. In developed countries, mortality induced by human parainfluenza virus is unusual, occurring almost exclusively in young infants or immunocompromised or elderly people. In developing countries, however, the preschool population is at considerable risk for human parainfluenza virus-induced death. Whether because of primary viral disease or because of the facilitation of secondary bacterial infections in malnourished children, lower respiratory tract infections causes 25-30% of the death in this age group, and human parainfluenza virus causes at least 10% of lower respiratory tract infections 21.
It was recently reported that detection of the human parainfluenza virus in lungs of infected patients was associated with worse outcomes than viral detection in the upper respiratory tract samples alone. This suggests that viral detection in the lungs of infected patients can be used to predict poor outcome 22.
Human parainfluenza prevention
Currently, there is no vaccine to protect you against human parainfluenza virus infection. However, researchers are trying to develop vaccines.
You may be able to reduce your risk of human parainfluenza virus and other respiratory viral infections by:
- washing your hands often with soap and water,
- avoiding touching your eyes, nose, or mouth, and
- avoiding close contact with people who are sick.
Frequent hand washing is especially important in childcare settings.
Breastfeeding may protect babies from human parainfluenza viruses during their first few months of life. That’s because mothers may have antibodies (protective cells) in their breast milk to fight infection.
If you are sick with human parainfluenza virus illness, you can help protect others by:
- staying home while you are sick,
- avoiding close contact with others,
- covering your mouth and nose when you cough or sneeze, and
- keeping objects and surfaces clean and disinfected.
Parainfluenza symptoms
Human parainfluenza virus have been associated with every kind of upper and lower respiratory tract illness. However, there is a strong relationship between human parainfluenza virus-1, human parainfluenza virus-2, and human parainfluenza virus-3 and specific clinical syndromes, age of child, and time of year, as described above.
Human parainfluenza viruses commonly cause upper and lower respiratory illnesses in infants, young children, older adults, and people with weakened immune systems, but anyone can get infected. After you get infected, it takes about 2 to 7 days before you develop symptoms.
- Human parainfluenza virus-1 and human parainfluenza virus-2 are most often associated with croup. Human parainfluenza virus-1 often causes croup in children, whereas human parainfluenza virus-2 is less frequently detected. Both types can cause upper and lower respiratory tract infections. People with upper respiratory tract illness may have cold-like symptoms.
- Human parainfluenza virus-3 is more often associated with bronchiolitis, bronchitis, and pneumonia.
- Human parainfluenza virus-4 is not recognized as often but may cause mild to severe respiratory tract illnesses.
Symptoms of upper respiratory tract illness may include:
- fever,
- runny nose, and
- cough.
Symptoms of severe lower respiratory tract illness may include:
- croup [infection of the vocal cords (larynx), windpipe (trachea) and bronchial tubes (bronchi)],
- bronchitis (infection of the main air passages that connect the windpipe to the lungs),
- bronchiolitis (infection in the smallest air passages in the lungs), or
- pneumonia (an infection of the lungs).
Other symptoms of human parainfluenza virus illness may include:
- sore throat,
- sneezing,
- wheezing,
- ear pain,
- irritability, and
- decreased appetite.
If your symptoms are severe or do not improve, you should seek medical attention.
Croup (acute laryngotracheobronchitis)
Children present with fever, a hoarse barking cough, laryngeal obstruction, and inspiratory stridor. The incidence peaks between 1 and 2 years of age and is more frequent in boys. In Milwaukee, we have found white children to have had a much higher incidence than black children 23. Approximately 10 to 25% of cases of lower respiratory tract infections in children younger than 5 years presents as croup (depending on age). The causative agent has been identified in about 50% of croup cases (36 to 74%) 14. Human parainfluenza virus have made up between 56 and 74% of these cases 24, with human parainfluenza virus-1 (26 to 74%) being the most frequent subtype 14. human parainfluenza virus-2 and human parainfluenza virus-3 average around 10% each as etiologic agents. Sampling of a national database (0.5% of total) and community-based estimates have suggested between 27,000 and 66,000 hospitalizations each year in the United States for croup 23. In years when human parainfluenza virus-1 is not epidemic, human parainfluenza virus-2 has been found to cause croup outbreaks. Even during human parainfluenza virus-1-epidemic years, human parainfluenza virus-2 has been reported to cause >60% of the croup cases in an individual community 25. Human parainfluenza virus-3 has been demonstrated to cause severe croup in an adult 26. RSV, influenza A and B viruses, adenoviruses, rhinoviruses, and, in school age children, Mycoplasma have all been shown to cause croup. A recent retrospective review suggested that influenza virus croup was more severe than parainfluenza virus croup 27.
Bronchiolitis
The diagnosis of bronchiolitis is unique to pediatrics because of the size of infant’s terminal airways. The predominant symptoms include fever, expiratory wheezing, tachypnea, retractions, rales, and air trapping. The peak incidence of bronchiolitis is in the first year of life (81% of cases occur during this period) and then dramatically declines until it virtually disappears by school age. This syndrome is diagnosed in approximately 25 to 30% of lower respiratory tract infections in childhood but makes up a larger percentage in the first year or two of life. At least 90% of cases of bronchiolitis are thought to be viral in origin, and a viral identification rate as high as 83% has been reported 4. Hospitalizations for bronchiolitis have been steadily increasing over the last 20 years in the United States 8. All four types of human parainfluenza virus can cause bronchiolitis, but human parainfluenza virus-1 and human parainfluenza virus-3 have been reported most commonly. Each of these two groups appears to cause 10 to 15% of cases of bronchiolitis in nonhospitalized children. However, in hospitalized children, human parainfluenza virus-3 causes many more cases than human parainfluenza virus-1 (three or four times as many) and is second only to RSV as a cause of bronchiolitis and pneumonia in young infants.
Pneumonia
Pneumonia is classicly diagnosed by the presence of fever and rales and evidence of pulmonary consolidation on physical examination or X ray. Pneumonia is diagnosed in 29 to 38% of children hospitalized with lower respiratory tract infection and in 23% treated as outpatients 28. However, since several of these classic lower respiratory tract infection syndromes decrease with age, pneumonia causes 83% of hospitalizations of children with lower respiratory tract infection older than 5 years. The peak incidence for pneumonia is in the second and third years of life. Recently, at least one-third of pneumonia hospitalizations have been in children with chronic diseases 29. Viruses have been shown to cause up to 90% of these lower respiratory tract infection, especially in the first year 30, and this percentage decreases to approximately 50% by school age 4. After 9 to 10 years of age, viruses cause a decreasing but still significant amount of pneumonia in immunocompetent individuals. The percentage of pneumonias with a documented viral etiology eventually declines to about 12% by adulthood 31. human parainfluenza virus-1 and human parainfluenza virus-3 each cause about 10% of outpatient pneumonias, but, similar to bronchiolitis, human parainfluenza virus-3 causes a larger percentage of cases in hospitalized patients. Pneumonia can be caused by both human parainfluenza virus-2 and human parainfluenza virus-4, but the incidence of disease is not well described. human parainfluenza virus-1 infection has been associated with secondary bacterial pneumonias in the elderly 32.
Tracheobronchitis
Patients with lower respiratory signs and symptoms who do not fit well into the above three syndromes often receive a diagnosis of tracheobronchitis. The most common symptoms include cough and large-airway noise on auscultation (rhonchi), but patients also may have fever and upper respiratory tract infections. About 20 to 30% of children with lower respiratory tract infection receive this diagnosis. The incidence is lower in the first 5 years of life, but tracheobronchitis is fairly evenly diagnosed throughout school age and adolescence. Viral agents make up the majority of etiologies in children 4. More than 25% of the agents identified to cause tracheobronchitis have been human parainfluenza viruss (human parainfluenza virus-3 is more common than human parainfluenza virus-1 or human parainfluenza virus-2). Several studies have recorded tracheobronchitis as the most common diagnosis in patients with human parainfluenza virus-4 infections. Also, this diagnosis is used more commonly in patients with chronic diseases.
Any single human parainfluenza virus can cause more than one of these lower respiratory tract infection syndromes to occur simultaneously or progressively in the same child. In 5 to 20% of lower respiratory tract infection cases, two viruses can be detected and may be associated with more severe disease 33. human parainfluenza virus routinely cause otitis media, pharyngitis, conjunctivitis, and coryza (common cold). These upper respiratory tract infections can occur singly or in any combination with the above-mentioned lower respiratory tract infections. Otitis media has been shown to be associated with viral respiratory tract infections in 30 to 60% of cases. Viruses may work synergistically with bacteria to initiate otitis media or prolong symptoms, and occasionally they are found to be the only cause of disease 34. Human parainfluenza virus-3 is the most frequently reported human parainfluenza virus associated with otitis media 35. Human parainfluenza virus have been found in 1% of middle ear effusions and in 2% of nasopharyngeal secretions in children with acute otitis media 36.
Immunocompromised hosts
Immunocompromised children and adults appear to be particularly succeptible to developing sever and fatal lower respiratory tract infections with human parainfluenza virus. human parainfluenza virus-2 has caused giant-cell pneumonia in severe combined immunodeficiency syndrome (SCIDS) 37 and has been found along with human parainfluenza virus-3 in severe combined immunodeficiency syndrome 38 and acute myelomonocytic leukemia 39 and after bone marrow transplantation (BMT) 40. It is common for more than one pathogen to be found in these patients on autopsy, but human parainfluenza virus alone is found in two-thirds of the cases. Persistent respiratory tract infection and excretion of human parainfluenza virus-1, human parainfluenza virus-2, and human parainfluenza virus-3 have been described in severe combined immunodeficiency syndrome 41, with human parainfluenza virus-3 in a child with DiGeorge syndrome after a thymic transplant 42, and in human immunodeficiency virus-infected children 43. The natural history of human parainfluenza virus in human immunodeficiency virus-infected children is still largely unknown, but severe disease appears uncommon until they are significantly T-cell deficient 44. Human parainfluenza virus-3 has been associated with acute rejection episodes in renal and liver transplant recipients 45. Solid-organ transplant and bone marrow transplantation patients develop human parainfluenza virus upper respiratory tract infections and lower respiratory tract infections, including consolidated and interstitial pneumonia 46. Human parainfluenza virus-3 has been the most frequent human parainfluenza virus type isolated from immunocompromised patients with lower respiratory tract infections and is a common cause of fever and neutropenia in children with cancer 47. This virus is persistently shed from bone marrow transplantation patients for up to 4 months without accumulating any mutations 48. Between 5 and 7% of adult BMT and leukemia patients become infected with human parainfluenza virus-1, human parainfluenza virus-2, and human parainfluenza virus-3 47; approximately 24 to 50% develop pneumonia and 22 to 75% of these will die. A retrospective study found that 41% of pediatric bone marrow transplantation patients with human parainfluenza virus infection (47% of viral respiratory infections) developed pneumonia and 6% died 49. human parainfluenza virus has been shown to cause serious lower respiratory tract infections and death (8%) in lung transplant recipients up to 5 years after transplantation 50. Human parainfluenza virus-3 has been reported to cause parotitis in children with severe combined immunodeficiency syndrome and “common variable” hypogammaglobulinemia 51. Immunocompromised patients have been found to have human parainfluenza virus infection in other nonrespiratory sites including cerebrospinal fluid (CSF), pericardial fluid, white blood cells, and liver 52, as well as in postmortem cultures of the myocardium and liver 53.
Neurologic disease
For many decades, parainfluenza virus have been associated with acute and chronic neurologic disease. Children hospitalized with human parainfluenza virus have had significant problems with febrile seizures. human parainfluenza virus-4B appears to have the greatest association (up to 62%), followed by human parainfluenza virus-3 (17%) and human parainfluenza virus-1 (7%) 54. Human parainfluenza virus-1 strains were isolated from two patients with multiple sclerosis 55, but evidence of antibody to human parainfluenza virus-1 in the serum or cerebrospinal fluid (CSF) of multiple sclerosis patients has been lacking 56. One report demonstrated that 2 of 25 cerebrospinal fluid (CSF) samples from multiple sclerosis patients had low positive HI titers to human parainfluenza virus-1 but 3 of 25 had positive titers to measles virus 57. Similarly, virus cultures and electron microscopy of other multiple sclerosis patients failed to find evidence of human parainfluenza virus-1. However, human parainfluenza virus-1 and Sendai virus have been detected using PCR in tissue from the ofactory bulbs of mice 58. Animal parainfluenza virus related to human parainfluenza virus-2 have been found in patients with MS and subacute sclerosing panencephalitis 59. Two closely related paramyxoviruses (Hendra and Nipaha viruses) have been shown to cause severe encephalitis and death in children and adults 13. Human parainfluenza virus-3 has been isolated from the CSF of a patient with Guillain-Barré syndrome 60 and adults with demyelinization syndromes 61 and has occasionally been shown to cause meningitis in children 62 and adults 61. Human parainfluenza virus-1, human parainfluenza virus-2, and human parainfluenza virus-3 have been isolated from the respiratory tract of children with acute encephalitis 63. In addition, several diseases have been linked to human parainfluenza virus-3 by serology, including encephalitis, ventriculitis, and cluster headaches in an adult 64. All of these data suggest that some strains of human parainfluenza virus may have neurotropism in certain hosts. Until more studies are done to clarify the role human parainfluenza virus plays in human neurologic disease, this possibility will be vastly overshadowed by their significant contribution to respiratory disease.
Other syndromes
Full-term and premature infants have developed apnea and bradycardia during human parainfluenza virus infection 65. Two children with adult respiratory distress syndrome were infected with human parainfluenza virus-1 66. human parainfluenza virus-3 has been implicated in supraglottitis and bronchiolitis obliterans 67. Human parainfluenza virus parotitis has been diagnosed in otherwise healthy children infected with human parainfluenza virus-1 and human parainfluenza virus-3 68, in immunocompromised children 69, and in a patient with cystic fibrosis 70. Croup caused by human parainfluenza virus has been associated with bacterial tracheitis 71. Exacerbations of nephrotic syndrome in children have been linked to viral respiratory infections in general 72. Nonrespiratory tissues are rarely infected with human parainfluenza virus. However, both immunocompromised patients and children with croup have had documented viremia and disseminated infection 73. Similarly, children and adults with acute non-A, B, C hepatitis have had paramyxovirus-like nucleocapsids found in their liver, giant cells, and syncytia on tissue examination 74. It is possible that human parainfluenza virus may play a role in this disease. human parainfluenza virus-3 has been cultured from a 37-year-old man with myopericarditis, and other human parainfluenza virus types have been diagnosed serologically in patients with myocarditis or peridcarditis 75. Elevated levels of antibodies to human parainfluenza virus-1 have been reported in patients with systemic lupus erythematosus and Reiter’s syndrome 76. However, the differences between the mean antibody titers of the controls and the subjects were only one or two dilutions. Recent work using reverse transcription-PCR (RT-PCR) has demonstrated no evidence of mumps virus P or HN genes in patients with inflammatory bowel disease 77. There does not yet appear to be a clear link established between human parainfluenza virus and inflammatory or connective tissue disease. There is a relationship between infection with common respiratory pathogens and myalgias and rhabdomyolysis. human parainfluenza virus-3 has been cultured from a child who died with rhabdomyolysis 78. Also, human parainfluenza virus-2 has been serologically linked to myalgias and myoglobinuria in an adult 79. human parainfluenza virus-3 has been cultured from throat and rectal cultures in an adult patient with bloody diarrhea, but other now common gastrointestinal pathogens were not sought 80. Likewise, respiratory tract symptoms were found in 11% of young children diagnosed with rotavirus gastroenteritis, but the etiology of the respiratory symptoms was not determined 81. No clear role for human parainfluenza virus in gastroenteritis has been determined.
Nosocomial infections
Doctors’ offices, hospitals, and chronic care facilities are institutions where respiratory viruses are frequently transmitted between patients. The populations at greatest risk for human parainfluenza virus infection are young preschool children, the immunocompromised, and the elderly 48. Children infected with human parainfluenza virus-3 will transmit this virus to a minimum of 20% of uninfected control children residing on the same ward 82. About one-third of these will develop mild respiratory symptoms, but some will experience serious lower respiratory tract infections and even death 54. Serious sequelae are most common in patients with underlying medical problems. The mean length of hospitalization was increased by many days, even for those with only mild symptoms, because of unnecessary tests and therapies due to their new signs and symptoms. If it is not practical to isolate all children admitted to a particular institution with signs of respiratory infection, then strict handwashing between patients must be enforced and high-risk patients must be cohorted away from these possibly infected patients. In addition, strict rules to limit patient exposure to visitors or staff with respiratory symptoms may help to decrease nosocomial human parainfluenza virus infections.
Parainfluenza complications
Complications of human parainfluenza virus infection may include the following:
- Acute respiratory distress syndrome (ARDS) and exacerbation of nephritic syndrome
- Serious morbidity in immunocompromised hosts (eg, transplant recipients) – Posttransplant human parainfluenza virus infection is a cause of serious lower respiratory tract involvement in both adults and children who undergo bone marrow transplant.
- Rare complications, including Guillain-Barré syndrome and meningitis
Parainfluenza diagnosis
Infection with human parainfluenza viruses can be confirmed by—
- Direct detection of viral genome by polymerase chain reaction (PCR) assay,
- Direct detection of viral antigens in respiratory secretions (collected within 1 week of symptom onset) using immunofluorescence or enzyme immunoassay,
- Isolation and identification of the virus in cell culture, or
- Demonstration of a significant rise in human parainfluenza virus-specific IgG antibodies between appropriately collected paired serum specimens or specific IgM antibodies in a single serum specimen.
Parainfluenza treatment
There is no specific antiviral treatment for human parainfluenza virus illness. Most people with human parainfluenza virus illness will recover on their own. However, some things can be done to relieve symptoms, such as:
- taking acetaminophen, ibuprofen, and other over-the-counter medications for pain and fever (Caution: Aspirin should not be given to children.) and
- using a room humidifier or taking a hot shower to help ease a sore throat and cough.
People who are sick should be encouraged to:
- drink plenty of liquids and
- stay home and rest.
If your illness is only caused by human parainfluenza virus, then antibiotics will not make you better. Antibiotics are only effective against bacteria.
If you are concerned about your symptoms, you should contact your healthcare provider.
- Oxygen mist is often helpful. Only 1-5% of patients admitted to the hospital need artificial airway support 21.
- Corticosteroids and nebulizers may be used to treat respiratory symptoms and to help reduce the inflammation and airway edema of croup.
- Antiviral agents are of uncertain benefit. Long-term ribavirin therapy has been helpful in case reports 83.
- Antibiotics are used only if bacterial complications (e.g., otitis and sinusitis) develop.
Management of croup caused by human parainfluenza virus infection depends on the severity of disease.
Mild croup
Management of mild croup consists of cool blow-by oxygen mist, fever control, and observation to determine whether the airway appears compromised.
Moderate croup
Cool oxygen mist and steroids are common therapies for moderate croup. Controlled trials of steroids for the palliation of croup symptoms have yielded conflicting results, and routine use of dexamethasone in this disease remains controversial. Traditionally, dexamethasone was administered intramuscularly (IM); however, some studies have documented the use of oral steroids.
In patients who fail to improve, administration of racemic epinephrine with a nebulizer has been beneficial. If racemic epinephrine alleviates symptoms, observe the patient for a minimum of 3 hours to ensure that his or her condition does not worsen (eg, because of possible rebound laryngospasm as the racemic epinephrine dose wears off). If the patient is asymptomatic at the end of the observation period, he or she can be discharged with proper follow-up care.
In moderate croup, oral intake may be lacking; therefore, it is essential to evaluate the patient’s hydration status. Intravenous (IV) fluids may be required.
Severe croup
The same measures are indicated for severe croup as for moderate croup. Observe the patient for signs of impending respiratory failure.
Repeat racemic epinephrine nebulization may be needed, in addition to intensive care monitoring. Racemic epinephrine nebulizations can be repeated at 1-hour to 2-hour intervals as needed. Endotracheal intubation followed by a tracheotomy may be required in patients with severe respiratory obstruction. Fortunately, fewer than 5% of patients who are admitted require artificial airway support.
Antiviral therapy
Ribavirin is a broad-spectrum antiviral agent that has been shown to be effective against human parainfluenza virus-3 infection in vitro and possibly in vivo. Although results are mixed, ribavirin aerosol or systemic therapy has been used to treat human parainfluenza virus infections in children and adults who are severely immunocompromised. Use at this time is of uncertain clinical benefit.
References- Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev. 2003;16(2):242-64. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC153148/
- Chanock, R. M., and K. McIntosh. 1985. Parainfluenza viruses, p. 1241. In B. N. Fields (ed.), Virology. Raven Press, New York, N.Y.
- Respiratory viral infections among pediatric inpatients and outpatients in Taiwan from 1997 to 1999. Tsai HP, Kuo PH, Liu CC, Wang JR. J Clin Microbiol. 2001 Jan; 39(1):111-8.
- Acute lower respiratory tract infections in nonhospitalized children. Denny FW, Clyde WA Jr. J Pediatr. 1986 May; 108(5 Pt 1):635-46.
- Respiratory syncytial virus and influenza A infections in the hospitalized elderly. Falsey AR, Cunningham CK, Barker WH, Kouides RW, Yuen JB, Menegus M, Weiner LB, Bonville CA, Betts RF. J Infect Dis. 1995 Aug; 172(2):389-94.
- The isolation of parainfluenza 4 subtypes A and B in England and serological studies of their prevalence. Gardner SD. J Hyg (Lond). 1969 Sep; 67(3):545-50.
- Multicentered study of viral acute lower respiratory infections in children from four cities of Argentina, 1993-1994. Carballal G, Videla CM, Espinosa MA, Savy V, Uez O, Sequeira MD, Knez V, Requeijo PV, Posse CR, Miceli I. J Med Virol. 2001 Jun; 64(2):167-74.
- Bronchiolitis-associated hospitalizations among US children, 1980-1996. Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. JAMA. 1999 Oct 20; 282(15):1440-6.
- Canchola, J., A. J. Vargosko, and H. W. Kim. 1964. Antigenic variation among newly isolated strains of parainfluenza type 4 virus. Am. J. Hyg. 79:357-364.
- Henrickson, K. J., and L. L. Savatski. 1996. Two distinct human parainfluenza virus type one genotypes detected during the 1991 epidemic. J. Clin. Microbiol. 34:695-700.
- Prinoski, K., M. J. Cote, C. Y. Kang, and K. Dimock. 1992. Evolution of the fusion protein gene of human parainfluenza virus 3. Virus. Res. 22:55-69.
- Berman, S. 1991. Epidemiology of acute respiratory infections in children of deveioning counties. Rev. Infect. Dis. 13:S454-S462.
- Interferon action against human parainfluenza virus type 3: involvement of a novel antiviral pathway in the inhibition of transcription. Choudhary S, Gao J, Leaman DW, De BP. J Virol. 2001 May; 75(10):4823-31.
- Croup: an 11-year study in a pediatric practice. Denny FW, Murphy TF, Clyde WA Jr, Collier AM, Henderson FW. Pediatrics. 1983 Jun; 71(6):871-6.
- Impact of viral respiratory diseases on infants and young children in a rural and urban area of southern West Virginia. Belshe RB, Van Voris LP, Mufson MA. Am J Epidemiol. 1983 Apr; 117(4):467-74
- Summertime respiratory syncytial virus infection: epidemiology and clinical manifestations. Washburne JF, Bocchini JA Jr, Jamison RM. South Med J. 1992 Jun; 85(6):579-83.
- Seasonal pattern in childhood viral lower respiratory tract infections in Melbourne. Murphy B, Phelan PD, Jack I, Uren E. Med J Aust. 1980 Jan 12; 1(1):22-4.
- Brief report: parainfluenza virus type 3 infections: findings in Sydney and some observations on variations in seasonality world-wide. de Silva LM, Cloonan MJ. J Med Virol. 1991 Sep; 35(1):19-21.
- Parainfluenza virus type 4 infections in pediatric patients. Lindquist SW, Darnule A, Istas A, Demmler GJ. Pediatr Infect Dis J. 1997 Jan; 16(1):34-8.
- Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Hurwitz JL, Soike KF, Sangster MY, Portner A, Sealy RE, Dawson DH, Coleclough C. Vaccine. 1997 Apr; 15(5):533-40.
- Parainfluenza Virus. https://emedicine.medscape.com/article/224708-overview
- Seo S, Xie H, Campbell AP, Kuypers JM, Leisenring WM, Englund JA. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis. 2014 May. 58(10):1357-68.
- Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Henrickson KJ, Kuhn SM, Savatski LL. Clin Infect Dis. 1994 May; 18(5):770-9.
- SURVIVAL OF THE RESPIRATORY SYNCYTIAL VIRUS DURING STORAGE UNDER VARIOUS CONDITIONS. HAMBLING MH. Br J Exp Pathol. 1964 Dec; 45():647-55.
- Pediatric hospitalizations for croup (laryngotracheobronchitis): biennial increases associated with human parainfluenza virus 1 epidemics. Marx A, Török TJ, Holman RC, Clarke MJ, Anderson LJ. J Infect Dis. 1997 Dec; 176(6):1423-7.
- Adult croup: a rare but more severe condition. Woo PC, Young K, Tsang KW, Ooi CG, Peiris M, Yuen K. Respiration. 2000; 67(6):684-8.
- Clinical courses of croup caused by influenza and parainfluenza viruses. Peltola V, Heikkinen T, Ruuskanen O. Pediatr Infect Dis J. 2002 Jan; 21(1):76-8.
- Impact of viral respiratory diseases on infants and young children in a rural and urban area of southern West Virginia. Belshe RB, Van Voris LP, Mufson MA. Am J Epidemiol. 1983 Apr; 117(4):467-74.
- Impact of respiratory virus infections on persons with chronic underlying conditions. Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB.JAMA. 2000 Jan 26; 283(4):499-505.
- Reliability of the chest radiograph in the diagnosis of lower respiratory infections in young children. Davies HD, Wang EE, Manson D, Babyn P, Shuckett B. Pediatr Infect Dis J. 1996 Jul; 15(7):600-4.
- Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. Dowell SF, Anderson LJ, Gary HE Jr, Erdman DD, Plouffe JF, File TM Jr, Marston BJ, Breiman RF. J Infect Dis. 1996 Sep; 174(3):456-62.
- Outbreak of pneumonia in a long-term care facility: antecedent human parainfluenza virus 1 infection may predispose to bacterial pneumonia. Fiore AE, Iverson C, Messmer T, Erdman D, Lett SM, Talkington DF, Anderson LJ, Fields B, Carlone GM, Breiman RF, Cetron MS. J Am Geriatr Soc. 1998 Sep; 46(9):1112-7.
- Dual respiratory virus infections. Drews AL, Atmar RL, Glezen WP, Baxter BD, Piedra PA, Greenberg SB. Clin Infect Dis. 1997 Dec; 25(6):1421-9.
- Respiratory viruses interfere with bacteriologic response to antibiotic in children with acute otitis media. Chonmaitree T, Owen MJ, Howie VM. J Infect Dis. 1990 Aug; 162(2):546-9.
- Wright, P. 1984. Parainfluenza viruses, p. 299-310. In R. B. Belshe (ed.), Textbook of human virology. PSG Publishing, Littleton, Mass.
- Viruses in acute otitis media: increasing evidence for clinical significance. Ruuskanen O, Arola M, Heikkinen T, Ziegler T. Pediatr Infect Dis J. 1991 Jun; 10(6):425-7.
- Parainfluenza virus II and the immunocompromised host. Karp D, Willis J, Wilfert CM. Am J Dis Child. 1974 Apr; 127(4):592-3.
- Monoclonal antibodies to human parainfluenza virus type 1 detect major antigenic changes in clinical isolates. Henrickson KJ. J Infect Dis. 1991 Dec; 164(6):1128-34.
- Giant cell pneumonia caused by parainfluenza type 3 in a patient with acute myelomonocytic leukemia. Weintrub PS, Sullender WM, Lombard C, Link MP, Arvin A. Arch Pathol Lab Med. 1987 Jun; 111(6):569-70.
- Parainfluenza virus respiratory infection after bone marrow transplantation. Wendt CH, Weisdorf DJ, Jordan MC, Balfour HH Jr, Hertz MI. N Engl J Med. 1992 Apr 2; 326(14):921-6.
- Disseminated parainfluenza infection in a child with severe combined immunodeficiency. Frank JA Jr, Warren RW, Tucker JA, Zeller J, Wilfert CM. Am J Dis Child. 1983 Dec; 137(12):1172-4.
- Para-influenza pneumonia in DiGeorge syndrome two years after thymic epithelial transplantation. Beard LJ, Robertson EF, Thong YH. Acta Paediatr Scand. 1980 May; 69(3):403-6.
- Parainfluenza 3 virus and other common respiratory pathogens in children with human immunodeficiency virus infection. Josephs S, Kim HW, Brandt CD, Parrott RH. Pediatr Infect Dis J. 1988 Mar; 7(3):207-9.
- Human immunodeficiency-virus-related pulmonary infections in children. McSherry GD. Semin Respir Infect. 1996 Sep; 11(3):173-83.
- Parainfluenza type 3 in a transplant unit. DeFabritus AM, Riggio RR, David DS, Senterfit LB, Cheigh JS, Stenzel KH. JAMA. 1979 Jan 26; 241(4):384-6.
- Parainfluenza and influenza virus infections in pediatric organ transplant recipients. Apalsch AM, Green M, Ledesma-Medina J, Nour B, Wald ER. Clin Infect Dis. 1995 Feb; 20(2):394-9.
- Respiratory virus infections during anticancer treatment in children. Arola M, Ruuskanen O, Ziegler T, Salmi TT. Pediatr Infect Dis J. 1995 Aug; 14(8):690-4.
- Molecular epidemiology of two consecutive outbreaks of parainfluenza 3 in a bone marrow transplant unit. Zambon M, Bull T, Sadler CJ, Goldman JM, Ward KN. J Clin Microbiol. 1998 Aug; 36(8):2289-93.
- Respiratory virus infections in pediatric hematopoietic stem cell transplantation. Luján-Zilbermann J, Benaim E, Tong X, Srivastava DK, Patrick CC, DeVincenzo JP. Clin Infect Dis. 2001 Oct 1; 33(7):962-8.
- The epidemiology of parainfluenza virus infection in lung transplant recipients. Vilchez RA, McCurry K, Dauber J, Iacono A, Keenan R, Zeevi A, Griffith B, Kusne S. Clin Infect Dis. 2001 Dec 15; 33(12):2004-8.
- Parainfluenza type 3 parotitis in two immunodeficient children. Cullen SJ, Baublis JV. J Pediatr. 1980 Mar; 96(3 Pt 1):437-8.
- Maintenance of viability and comparison of identification methods for influenza and other respiratory viruses of humans. Baxter BD, Couch RB, Greenberg SB, Kasel JA. J Clin Microbiol. 1977 Jul; 6(1):19-22.
- Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. Fishaut M, Tubergen D, McIntosh K. J Pediatr. 1980 Feb; 96(2):179-86.
- Diagnosis and clinical significance of parainfluenza virus infections in children. Downham MA, McQuillin J, Gardner PS. Arch Dis Child. 1974 Jan; 49(1):8-15.
- Analysis of a viral agent isolated from multiple sclerosis brain tissue: characterization as a parainfluenzavirus type 1. Lewandowski LJ, Lief FS, Verini MA, Pienkowski MM, ter Meulen V, Koprowski H. J Virol. 1974 May; 13(5):1037-45.
- Further studies of viral antibodies in the cerebrospinal fluid of patients with multiple sclerosis: vaccinia and parainfluenza type 1. Brown P, Cathala F, Gajdusek DC. Proc Soc Exp Biol Med. 1973 Jul; 143(3):828-9.
- Virological studies with multiple-sclerosis brain tissues. Barbosa LH, Hamilton R. Lancet. 1973 Jun 23; 1(7817):1415-7.
- Parainfluenza virus type 1 infects olfactory neurons and establishes long-term persistence in the nerve tissue. Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. J Gen Virol. 1995 May; 76 ( Pt 5)():1251-4.
- Evidence for the persistence of paramyxoviruses in human bone marrows. Goswami KK, Cameron KR, Russell WC, Lange LS, Mitchell DN. J Gen Virol. 1984 Nov; 65 ( Pt 11)():1881-8.
- Parainfluenza virus type 3: isolation from CSF of a patient with Guillain-Barré syndrome. Román G, Phillips CA, Poser CM. JAMA. 1978 Oct 6; 240(15):1613-5.
- Isolation of parainfluenza virus type 3 from cerebrospinal fluid. Vreede RW, Schellekens H, Zuijderwijk M. J Infect Dis. 1992 Jun; 165(6):1166.
- Parainfluenza type 3 meningitis. Report of two cases and review of the literature. Arguedas A, Stutman HR, Blanding JG. Clin Pediatr (Phila). 1990 Mar; 29(3):175-8.
- Acute childhood encephalitis and Mycoplasma pneumoniae. Bitnun A, Ford-Jones EL, Petric M, MacGregor D, Heurter H, Nelson S, Johnson G, Richardson S. Clin Infect Dis. 2001 Jun 15; 32(12):1674-84.
- Cluster headache associated with parainfluenza virus, preceded and succeeded by migraine. Blanchard BM. Headache. 1998 Feb; 38(2):132-4.
- Parainfluenza type 3 in newborns. McCarthy VP, Carlile JR, Reichelderfer PS, Clark JS. Pediatr Infect Dis J. 1987 Feb; 6(2):217-8.
- Adult respiratory distress syndrome associated with parainfluenza virus type 1 in children. Hotez PJ, Goldstein B, Ziegler J, Doveikis SA, Pasternack MS. Pediatr Infect Dis J. 1990 Oct; 9(10):750-2.
- Viral supraglottitis. Grattan-Smith T, Forer M, Kilham H, Gillis J. J Pediatr. 1987 Mar; 110(3):434-5.
- Bloom, H. H., K. J. Johnson, R. Jacobsen, and R. M. Chanock. 1961. Recovery of parainfluenza viruses from adults with upper respiratory illness. Am. J. Hyg. 74:50-59.
- Whimbey, E., J. A. Englund, and P. Ljungman. 1997. Community respiratory viral infections in the immunocompromised host. Proceedings of a symposium. Am. J. Med. 102:1-80.
- Parotitis and parainfluenza 3 virus. Buckley JM, Poche P, McIntosh K. Am J Dis Child. 1972 Nov; 124(5):789.
- Bacterial tracheitis as a complication of viral croup. Edwards KM, Dundon MC, Altemeier WA. Pediatr Infect Dis. 1983 Sep-Oct; 2(5):390-1.
- Role of respiratory viruses in exacerbations of primary nephrotic syndrome. MacDonald NE, Wolfish N, McLaine P, Phipps P, Rossier E. J Pediatr. 1986 Mar; 108(3):378-82.
- Detection of viraemia in acute respiratory disease of man. Rocchi G, Arangio-Ruiz G, Giannini V, Jemolo AM, Andreoni G, Archetti I. Acta Virol. 1970 Sep; 14(5):405-7.
- Syncytial giant-cell hepatitis. Sporadic hepatitis with distinctive pathological features, a severe clinical course, and paramyxoviral features. Phillips MJ, Blendis LM, Poucell S, offterson J, Petric M, Roberts E, Levy GA, Superina RA, Greig PD, Cameron R. N Engl J Med. 1991 Feb 14; 324(7):455-60.
- Myopericarditis associated with parainfluenza virus type 3 infection. Wilks D, Burns SM. Eur J Clin Microbiol Infect Dis. 1998 May; 17(5):363-5.
- Myxovirus antibody increases in human connective tissue disease. Phillips PE, Christian CL. Science. 1970 May 22; 168(3934):982-4.
- No evidence of persistent mumps virus infection in inflammatory bowel disease. Iizuka M, Saito H, Yukawa M, Itou H, Shirasaka T, Chiba M, Fukushima T, Watanabe S. Gut. 2001 May; 48(5):637-41.
- Fatal rhabdomyolysis associated with parainfluenza type 3 infection. Ueda K, Robbins DA, Iitaka K, Linnemann CC Jr. Hiroshima J Med Sci. 1978 Jun; 27(2):99-103.
- Myoglobinuria associated with parainfluenza type 2 infection. O’Connor JV, Iyer SK. N Y State J Med. 1982 Sep; 82(10):1469-70.
- M. D. Aronson, D. Kaminsky, and C. A. Phillips, Letter, Ann. Intern. Med. 81:856-857, 1974
- Simultaneous infections with different enteric and respiratory tract viruses. Brandt CD, Kim HW, Rodriguez WJ, Arrobio JO, Jeffries BC, Parrott RH. J Clin Microbiol. 1986 Jan; 23(1):177-9.
- Acquisition of parainfluenza 3 virus infection by hospitalized children. I. Frequencies, rates, and temporal data. Mufson MA, Mocega HE, Krause HE. J Infect Dis. 1973 Aug; 128(2):141-7.
- Stankova J, Carret AS, Moore D, McCusker C, Mitchell D, Davis M, et al. Long-term therapy with aerosolized ribavirin for parainfluenza 3 virus respiratory tract infection in an infant with severe combined immunodeficiency. Pediatr Transplant. 2007 Mar. 11(2):209-13.