Permissive hypercapnia
Permissive hypercapnia also called “gentle ventilation” or deliberate hypoventilation, is a respiratory treatment strategy used to reduce the risk of ventilator-associated lung injury (VALI) in adults and bronchopulmonary dysplasia (BPD) in premature infants by permitting relatively high levels of partial pressure of arterial carbon dioxide (PaCO2) above normal (range between 45–65 mmHg), thus allowing less aggressive respiratory care and, as a result, less trauma to the lung 1. Mechanical ventilation is normally adjusted so that PaCO2 is maintained within normal limits (35-45 mmHg). Hypercapnia also known as hypercarbia, is defined by an increase in carbon dioxide in the bloodstream or the elevation in the partial pressure of carbon dioxide (PaCO2) above 45 mm Hg 2. Permissive hypercapnia can be achieved by reductions in respiratory rate (RR) or tidal volume (TV) or both. The resulting reduction in minute ventilation leads to higher Pco2 values and reduces the propensity for auto-PEEP (positive end-expiratory pressure). As a result of expiratory flow limitation, the amount of gas that can exit the lung in a given amount of time is fixed; therefore, reductions in inspired tidal volume lead to less gas trapping. Early in management, some compromise in ventilation and rise in carbon dioxide (CO2) is acceptable to maintain either lower tidal volumes for a lung-protective ventilation strategy for acute respiratory distress syndrome (ARDS) or to allow more time for expiration on the ventilator (severe asthma).
Deliberate hypoventilation was initially described in 1984 by Darioli and Perret 3, wherein a series of 29 mechanically ventilated patients (34 episodes of ventilation) with status asthmaticus were managed with deliberate hypoventilation (resulting in severe hypercapnia) with the goal of reducing barotrauma. There were no fatalities in the series, which was very unusual at the time for intubated patients with status asthmaticus. The following year, Wung and colleagues described a series of 15 neonates with persistent pulmonary hypertension of the newborn. This case series was remarkable given that, up to then, vigorous hyperventilation (to achieve hypocapnia in an attempt to reduce the pulmonary vascular resistance) was considered a vital aspect of management; in such cases, death was common and chronic lung disease the rule. There were no deaths in the series of Wung and colleagues and only a single case of chronic lung disease.
Hickling et al. 4 introduced the concept of permissive hypercapnia, reporting that reducing the peak inspiratory airway pressure to a maximum of 20–30 cm H2O while allowing PaCO2 to increase resulted in a decreased mortality rate of 16% for 50 consecutive patients with acute respiratory distress syndrome (ARDS). Amato et al. 5 reported similar results in the first controlled study on the use of permissive hypercapnia in patients with acute respiratory distress syndrome (ARDS).
Permissive hypercapnia aims to avoid hyperinflation-induced lung trauma, as described initially by limiting the plateau airway pressure (as a surrogate of static alveolar pressure) to approximately 30–35 cm H2O while allowing partial pressure of arterial carbon dioxide (PaCO2) to increase absent any contraindications (such as increased intracranial pressure).
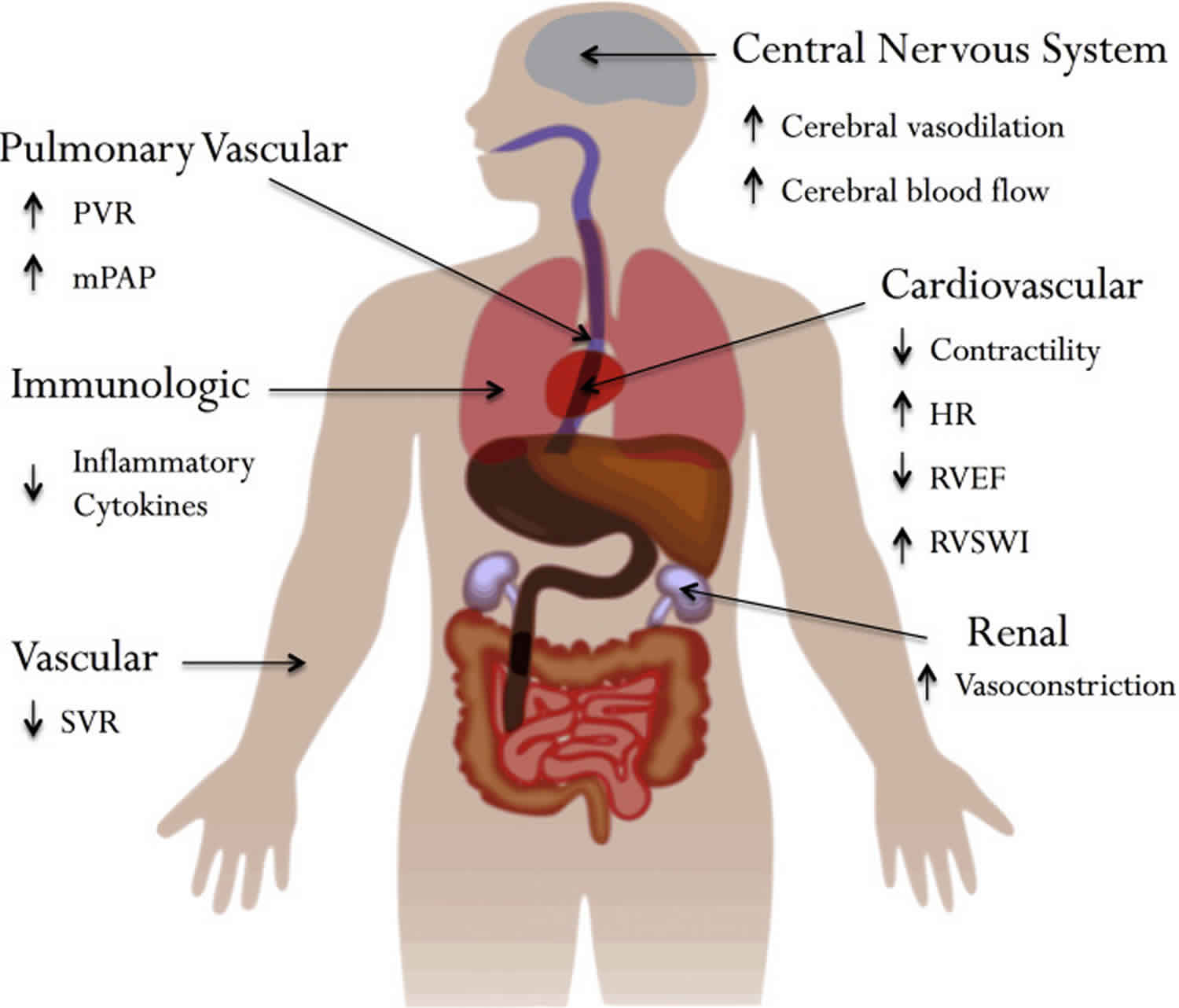
Contraindications and adverse effects of permissive hypercapnia include cerebral edema or high intracranial pressure, convulsions, depressed cardiac function, arrhythmias, increased pulmonary vascular resistance, tachypnea, increased work of breathing, dyspnea, respiratory distress, headache, sweating, and biochemical disturbances related to acidosis.
- PaCO2 = 0.863 x Metabolic production of CO2 (VCO2) / Alveolar ventilation (VA)
- Alveolar ventilation (VA) = Minute ventilation (VE) – Dead space ventilation (VD)
- Minute ventilation (VE) = Respiratory rate (RR) x Tidal volume (TV)
- Tidal volume (TV) = Respiratory rate (RR) x dead-space volume
These relationships indicate that respiratory rate (RR) and tidal volume (TV) are the two components of ventilation that are physiologically or artificially controlled to moderate CO2 elimination. Therefore, a failure in either of these fields will induce hypercapnia. It is important also to note that as partial pressure of arterial carbon dioxide (PaCO2) increases, oxygenation decreases. This is explained using the alveolar gas equation:
- PaO2 = FiO2 (Patm – PH2O) – PaCO2 / R
Where FiO2 is the fraction of inspired oxygen (0.21 at room air), Patm is atmospheric pressure (760 mm Hg), PH2O (47 mm Hg), PaCO2 as calculated from the arterial blood gas, and R (0.8 standard value) 6.
To establish the diagnosis of hypercapnia, blood gas analysis is essential. While a venous blood gas can provide information about the pH and an estimate of CO2 retention, the CO2 content in venous blood is naturally higher than arterial blood as it has not passed through the lungs. Arterial blood gas analysis is the preferred technique for diagnosing hypercapnia, with any partial pressure of CO2 (PaCO2) level greater than 45 mmHg qualifying. The next step in evaluation is to refer to the pH from the same sample. If it is within a normal range, there is either a mixed acid-base disorder or the hypercapnia is chronic. If the pH is less than 7.35, there is respiratory acidosis 7.
Permissive hypercapnia is an important concept in the management of both acute respiratory distress syndrome (ARDS) and severe asthma. To avoid overdistention and ventilator-induced lung injury, a lung-protective ventilation strategy is employed in treating ARDS. Tidal volumes are limited to 6-8 mL/kg of ideal body weight, which can slightly compromise ventilation. The resultant respiratory acidosis is typically well-tolerated, so long as the pH is greater than 7.25. Guidelines for acute respiratory distress syndrome (ARDS) recommend allowing for permissive hypercapnia up to a pH of 7.2. If the pH drops below 7.2, the initiation of a bicarbonate infusion should be a therapeutic consideration to correct the pH to 7.2 8. There is not a specific cut off for the CO2 level 9.
Permissive hypercarbia is also allowed for severe asthma exacerbation, though for a different reason. Due to bronchospasm, full exhalation is not possible with higher respiratory rates, which can lead to progressive air-trapping with worsening respiratory and hemodynamic consequences. Therefore, while treating the exacerbation with bronchodilators and steroids, a lower respiratory rate is set on the ventilator, which will lead to an increase in CO2, but enhance airflow dynamics 10.
Lung-protective ventilation with volumes limited to 6–8 mL/kg of predicted body weight or lower, and plateau pressure < 30 cm H2O, dramatically increases survival 11. As a consequence of reducing alveolar ventilation with lower tidal volumes, hypercapnia results. Recognizing that low tidal volume ventilation confers survival benefits by reducing lung stretch and the cyclical collapse of alveoli, clinicians have accepted hypercapnia, giving rise to the concept of permissive hypercapnia 12. However, it remains unclear whether hypercapnic acidosis carries survival benefits independent of using low tidal volumes 13.
In a retrospective analysis of the ARDS network, hypercapnic acidosis was associated with lower mortality in the group of patients receiving tidal volumes of 12 mL/kg predicted body weight. However, there was no survival benefit in patients ventilated at tidal volumes of 6 mL/kg predicted body weight 14. It was hypothesized that lung-protective ventilation reduced lung injury caused by the ventilator to a point where the protective effect of hypercapnic acidosis could not be detected.
In a multicenter randomized clinical trial comparing low (7 mL/kg predicted body weight) with conventional tidal volumes (10 mL/kg predicted body weight), a trend towards higher mortality was observed in patients who developed hypercapnia and acidosis 15. These findings resulted in a premature stop of that trial, making interpretation of the results difficult.
Recently, a post hoc analysis of three prospective non-interventional international studies in ARDS patients was published 16. In this analysis, severe hypercapnia (PaCO2 > 50 mmHg) was associated with higher mortality and more organ failures compared to patients with normocapnia. Acidosis or the combination of hypercapnia and acidosis independently increased the risk of mortality in the intensive care unit. The incidence of severe hypercapnia increased significantly with the time (1998, 2004, and 2010) as a consequence of the diverse respiratory strategies practiced over the years, which may reflect the feeling of many intensivists that hypercapnia could be beneficial 16.
One retrospective analysis including over 250,000 ARDS patients receiving mechanical ventilation showed that patients who developed hypercapnic acidosis (pH < 7.35 PaCO2 > 65 mmHg) during the first 24 hour of mechanical ventilation had significantly higher mortality than those who had compensated hypercapnia or normocapnia 17.
In recent years, a lot of experimental and clinical studies have been conducted to determine if permissive hypercapnia is effective and safe in preterm infants 18. However, these trials that compare permissive hypercapnia with conventional ventilation have shown conflicting results.
It is known that both extremes of partial pressure of arterial carbon dioxide (PaCO2) could adversely affect the premature brain. Hypocapnia could increase the risk of periventricular leukomalacia (periventricular leukomalacia) by impairing cerebral blood flow 19. While severe hypercapnia is associated with an increased risk of intraventricular hemorrhage 20. However, the effect of mild hypercapnia on premature brain during permissive hypercapnic ventilation in extremely low birth weight infants is not clear. In this study 21, the authors found that the rates of intraventricular haemorrhage, intraventricular haemorrhage (grade 3–4), periventricular leukomalacia did not differ in permissive hypercapnia group and control group. Besides, Ma et al 21 found that permissive hypercapnia did not increase the rates of mortality, necrotising enterocolitis, retinopathy of prematurity or air leaks.
Some previous studies have found that both extreme fluctuations in PaCO2 and higher max PaCO2 were associated with worse neurodevelopmental outcomes 22. While it is unknown if permissive hypercapnia was associated with neurodevelopmental impairment. In this meta-analysis 21, the authors found that permissive hypercapnia did not reduce the rate of bronchopulmonary dysplasia in ventilated extremely low birth weight infants. The rates of mortality, intraventricular haemorrhage, intraventricular haemorrhage (grade 3–4), periventricular leukomalacia, necrotising enterocolitis, retinopathy of prematurity and air leaks did not decrease in permissive hypercapnia group. There was no difference in the risk of cerebral palsy, Mental Developmental Index < 70, Psychomotor Developmental Index < 70, visual deficit and hearing deficit in extremely low birth weight infants between the permissive hypercapnia and control group.
References- Varughese M, Patole S, Shama A, Whitehall J. Permissive hypercapnia in neonates: The case of the good, the bad, and the ugly. Pediatr Pulmonol. 2002;33(1):56–64.
- Rawat D, Modi P, Sharma S. Hypercapnea. [Updated 2020 Jun 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500012
- Darioli R, Perret C. Mechanical controlled hypoventilation in status asthmaticus. Am Rev Respir Dis. 1984;129(3):385-387. doi:10.1164/arrd.1984.129.3.385
- Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 1990;16(6):372-377. doi:10.1007/BF01735174
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338: 347–354. doi: 10.1056/NEJM199802053380602
- Sharma S, Hashmi MF. Hypocarbia. [Updated 2020 Jun 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493167
- Pisani L, Corcione N, Nava S. Management of acute hypercapnic respiratory failure. Curr Opin Crit Care. 2016 Feb;22(1):45-52.
- Feihl F, Perret C. Permissive hypercapnia. How permissive should we be? Am. J. Respir. Crit. Care Med. 1994 Dec;150(6 Pt 1):1722-37.
- Barnes T, Zochios V, Parhar K. Re-examining Permissive Hypercapnia in ARDS: A Narrative Review. Chest. 2018 Jul;154(1):185-195.
- Laher AE, Buchanan SK. Mechanically Ventilating the Severe Asthmatic. J Intensive Care Med. 2018 Sep;33(9):491-501.
- The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801
- Curley GF, Laffey JG, Kavanagh BP. CrossTalk proposal: there is added benefit to providing permissive hypercapnia in the treatment of ARDS. J Physiol. 2013;591(11):2763–2765. doi: 10.1113/jphysiol.2013.252601
- Morales-Quinteros L, Camprubí-Rimblas M, Bringué J, Bos LD, Schultz MJ, Artigas A. The role of hypercapnia in acute respiratory failure. Intensive Care Med Exp. 2019;7(Suppl 1):39. Published 2019 Jul 25. doi:10.1186/s40635-019-0239-0 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6658637
- Kregenow DA, Rubenfeld GD, Hudson LD, et al. Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med. 2006;34:1–7. doi: 10.1097/01.CCM.0000194533.75481.03
- Brochard L, Roudot-Thoraval F, Roupie E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;158:1831–1838. doi: 10.1164/ajrccm.158.6.9801044
- Nin N, Muriel A, Peñuelas O, et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017;43:200–208. doi: 10.1007/s00134-016-4611-1
- Tiruvoipati R, Pilcher D, Buscher H, et al. Effects of hypercapnia and hypercapnic acidosis on hospital mortality in mechanically ventilated patients. Crit Care Med. 2017;45:e649–e656. doi: 10.1097/CCM.0000000000002332
- Tapia JL, Urzua S, Bancalari A, Meritano J, Torres G, Fabres J, et al. Randomized trial of early bubble continuous positive airway pressure for very low birth weight infants. J Pediatr. 2012;161(1):75–80. doi: 10.1016/j.jpeds.2011.12.054
- Wiswell TE, Graziani LJ, Kornhauser MS, Stanley C, Merton DA, McKee L, et al. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high-frequency jet ventilation. Pediatrics. 1996;98(5):918–924.
- Kaiser JR, Gauss CH, Pont MM, Williams DK. Hypercapnia during the first 3 days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. J Perinatol. 2006;26(5):279–285. doi: 10.1038/sj.jp.7211492
- Ma J, Ye H. Effects of permissive hypercapnia on pulmonary and neurodevelopmental sequelae in extremely low birth weight infants: a meta-analysis. Springerplus. 2016;5(1):764. Published 2016 Jun 17. doi:10.1186/s40064-016-2437-5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4912505
- McKee LA, Fabres J, Howard G. PaCO2 and neurodevelopment in extremely low birth weight infants. J Pediatr. 2009;155(2):217–221. doi: 10.1016/j.jpeds.2009.02.024