Plasmacytoma
Plasmacytoma is a type of blood cancer consisting of abnormal plasma cells (white blood cells that produce antibodies) that grows within the soft tissue or bony skeleton. Plasma cells are a type of white blood cell that develops from mature B-lymphocytes in the bone marrow. Plasma cells play an important role in protecting your body against infection and disease by producing proteins called immunoglobulins (Ig), also known as antibodies. A plasmacytoma may turn into multiple myeloma. The prognosis and treatment of solitary plasmacytomas is very different to myeloma.
Plasmacytoma can be present as a discreet solitary mass of abnormal monoclonal plasma cells (solitary plasmacytoma) that occurs either inside the bone marrow, in which case it is termed a solitary bone plasmacytoma or outside bone marrow where it’s called extramedullary plasmacytoma.
Plasmacytoma and multiple myeloma are cytologically and immunophenotypically identical. However, unlike multiple myeloma, plasmacytoma is not a systemic disease and lacks the characteristic CRAB abnormalities such as hypercalcemia, renal failure, anemia, and bone lesions with the exception of solitary extramedullary plasmacytoma 1. Recent studies have shown that there might be small cytogenetic differences as well 2.
Plasmacytoma mainly affects people in the 5th to 6th decade of their lives, and the incidence of the disease is higher in men than in women 1. Incidence rates anywhere from 2 to 3 times higher in men than in women have been reported 3. Plasmacytoma also affects African Americans more often than Caucasians, with the lowest incidence being reported in Asian populations. Unlike multiple myeloma, plasmacytoma is not a systemic disease and lacks the characteristic CRAB abnormalities such as hypercalcemia, renal failure, anemia, and bone disease, excluding the plasmacytoma itself. The median time to progression of plasmacytoma to multiple myeloma is 2-3 years, with progression more likely to occur in solitary bone plasmacytoma than extramedullary plasmacytoma 1. Solitary bone plasmacytoma commonly presents in the axial skeleton and mostly manifests as local pain, pathological fractures nerve compression, while extramedullary plasmacytoma is usually seen in the head and neck and typically manifests as space-occupying lesions. Therapeutically, local therapy such as radiotherapy is the gold standard and is able to achieve long-term disease-free survival in approximately 30% and 65% of patients with solitary bone plasmacytoma and extramedullary plasmacytoma respectively. Surgery and adjuvant radiation therapy, on the other hand, is mostly reserved for easily resectable plasmacytomas.
According to the WHO classification in 2017, plasma cell neoplasms includes non-Ig-M monoclonal gammopathy of undetermined significance (MGUS), plasmacytoma, monoclonal immunoglobulin deposition diseases and plasma cell myeloma and plasma cell neoplasms with the associated paraneoplastic syndromes 4, 5.
Table 1. International Myeloma Working Group Diagnostic Criteria for Multiple Myeloma and Related Plasma Cell Disorders
| Disorder | Disease Definition |
|---|---|
| Non-IgM monoclonal gammopathy of undetermined significance (MGUS) | All 3 criteria must be met: • Serum monoclonal protein (non-IgM type) <3gm/dL • Clonal bone marrow plasma cells <10%* • Absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, and bone lesions (CRAB) that can be attributed to the plasma cell proliferative disorder |
| Smoldering multiple myeloma (SMM) | Both criteria must be met: • Serum monoclonal protein (IgG or IgA) ≥3gm/dL, or urinary monoclonal protein ≥500 mg per 24h and/or clonal bone marrow plasma cells 10-60% • Absence of myeloma defining events or amyloidosis |
| Multiple Myeloma (plasma cell myeloma) | Both criteria must be met: • Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or extramedullary plasmacytoma • Any one or more of the following myeloma defining events:
|
| Plasma cell leukemia | Both criteria must be met: • Meets diagnostic criteria for multiple myeloma • Presence of 5% or more plasma cells in conventional peripheral blood smear white blood cell differential count |
| IgM Monoclonal gammopathy of undetermined significance (IgM MGUS) | All 3 criteria must be met: • Serum IgM monoclonal protein <3gm/dL • Bone marrow lymphoplasmacytic infiltration <10% • No evidence of anemia, constitutional symptoms, hyperviscosity, lymphadenopathy, or hepatosplenomegaly that can be attributed to the underlying lymphoproliferative disorder. |
| Light Chain MGUS | All criteria must be met: • Abnormal serum free light chain (FLC) ratio (<0.26 or >1.65) • Increased level of the appropriate involved light chain (increased kappa FLC in patients with ratio > 1.65 and increased lambda FLC in patients with ratio < 0.26) • No immunoglobulin heavy chain expression on immunofixation • Absence of end-organ damage that can be attributed to the plasma cell proliferative disorder • Clonal bone marrow plasma cells <10% • Urinary monoclonal protein <500 mg/24h |
| Solitary Plasmacytoma | All 4 criteria must be met • Biopsy proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells • Normal bone marrow with no evidence of clonal plasma cells • Normal skeletal survey and MRI (or CT) of spine and pelvis (except for the primary solitary lesion) • Absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, or bone lesions (CRAB) that can be attributed to a lympho-plasma cell proliferative disorder |
| Solitary Plasmacytoma with minimal marrow involvement** | All 4 criteria must be met • Biopsy proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells • Clonal bone marrow plasma cells <10% • Normal skeletal survey and MRI (or CT) of spine and pelvis (except for the primary solitary lesion) • Absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, or bone lesions (CRAB) that can be attributed to a lympho-plasma cell proliferative disorder |
Footnotes:
* A bone marrow can be deferred in patients with low risk MGUS (IgG type, M protein <15 gm/L, normal free light chain ratio) in whom there are no clinical features concerning for myeloma
** Solitary plasmacytoma with 10% or more clonal plasma cells is considered as multiple myeloma
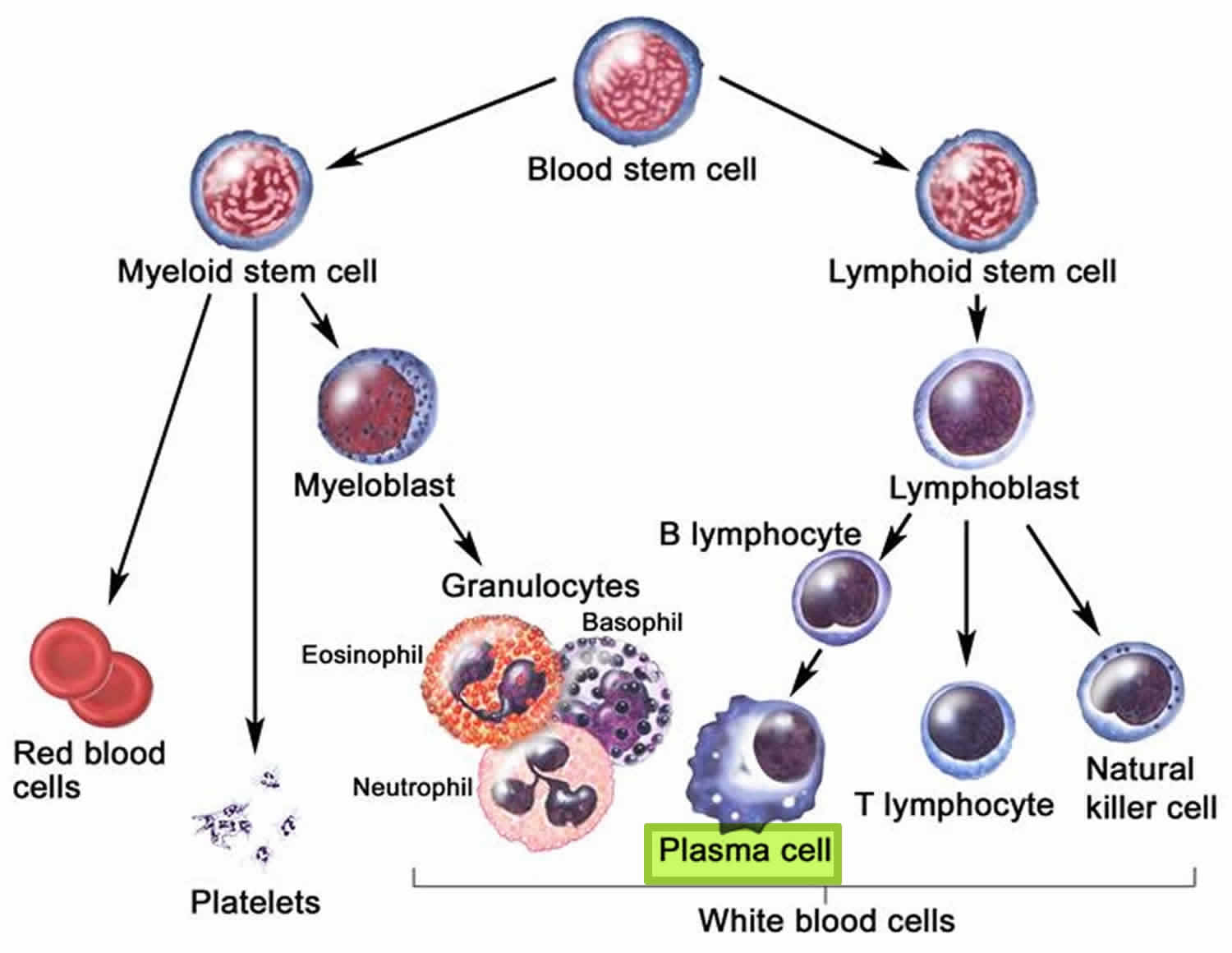
Figure 1. Plasma cells
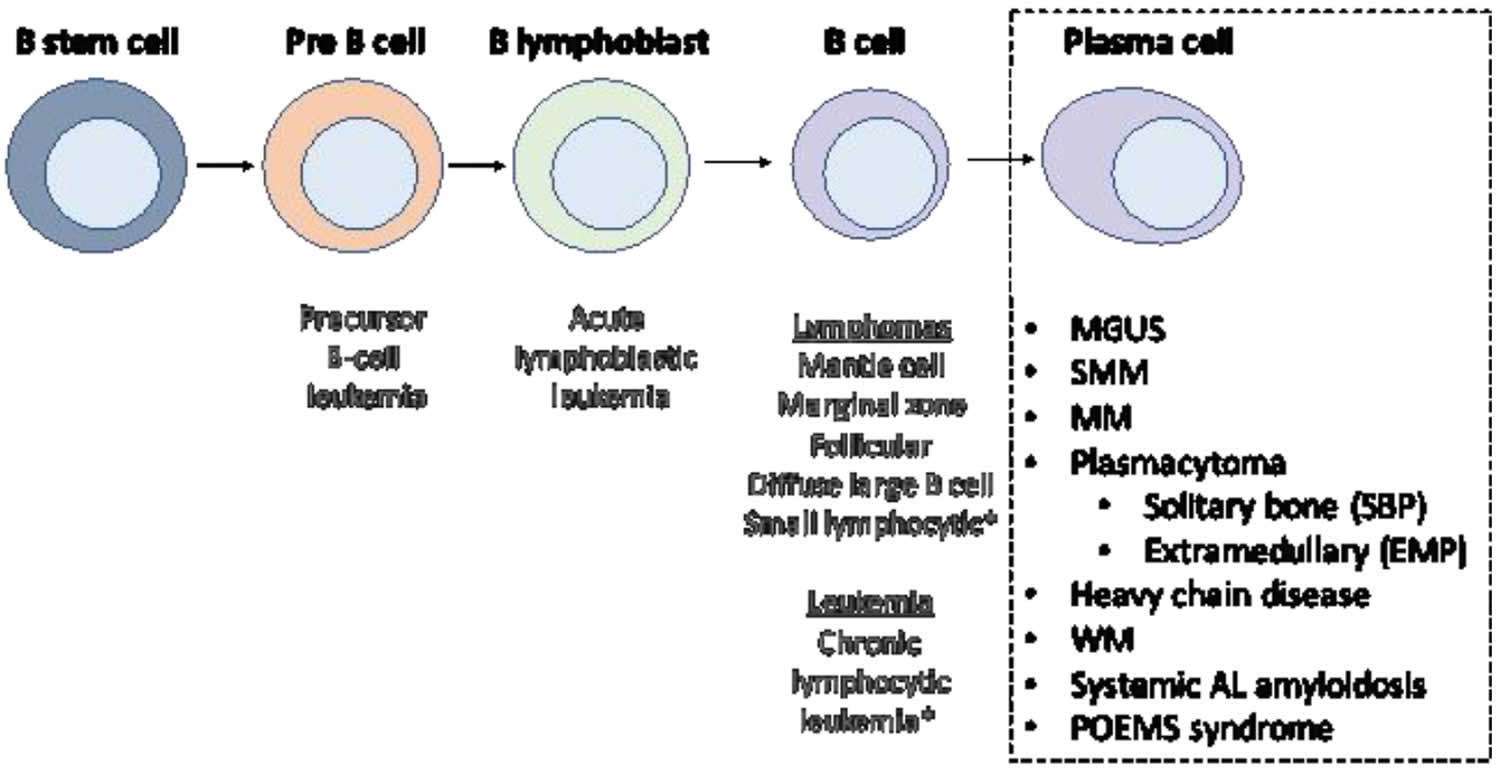
Figure 2. Stem cell origin of plasmacytoma
Footnote: This figure demonstrates the origin of the monoclonal proliferation of plasma cells that characterizes plasmacytoma and all other plasma cell neoplasms.
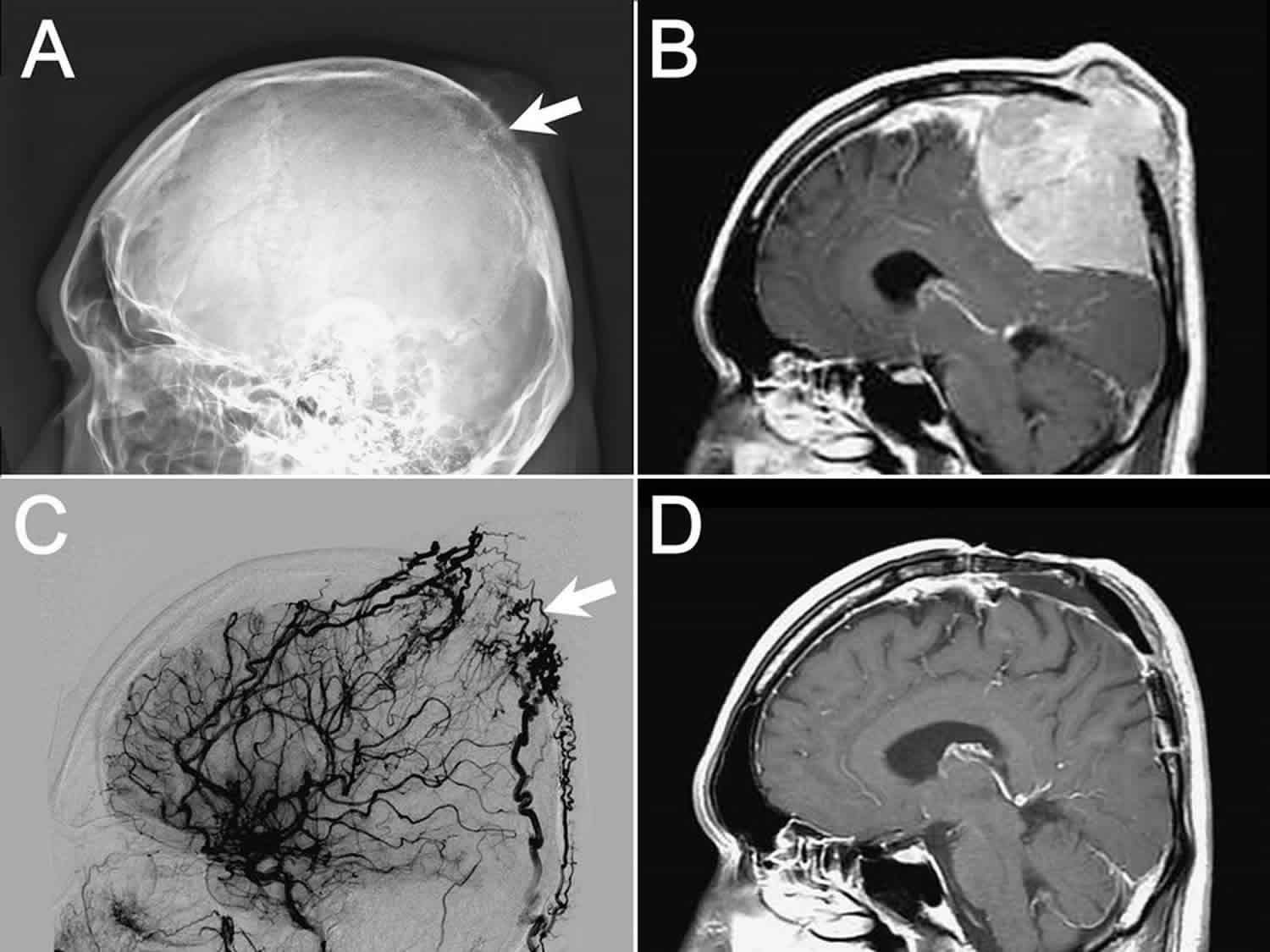
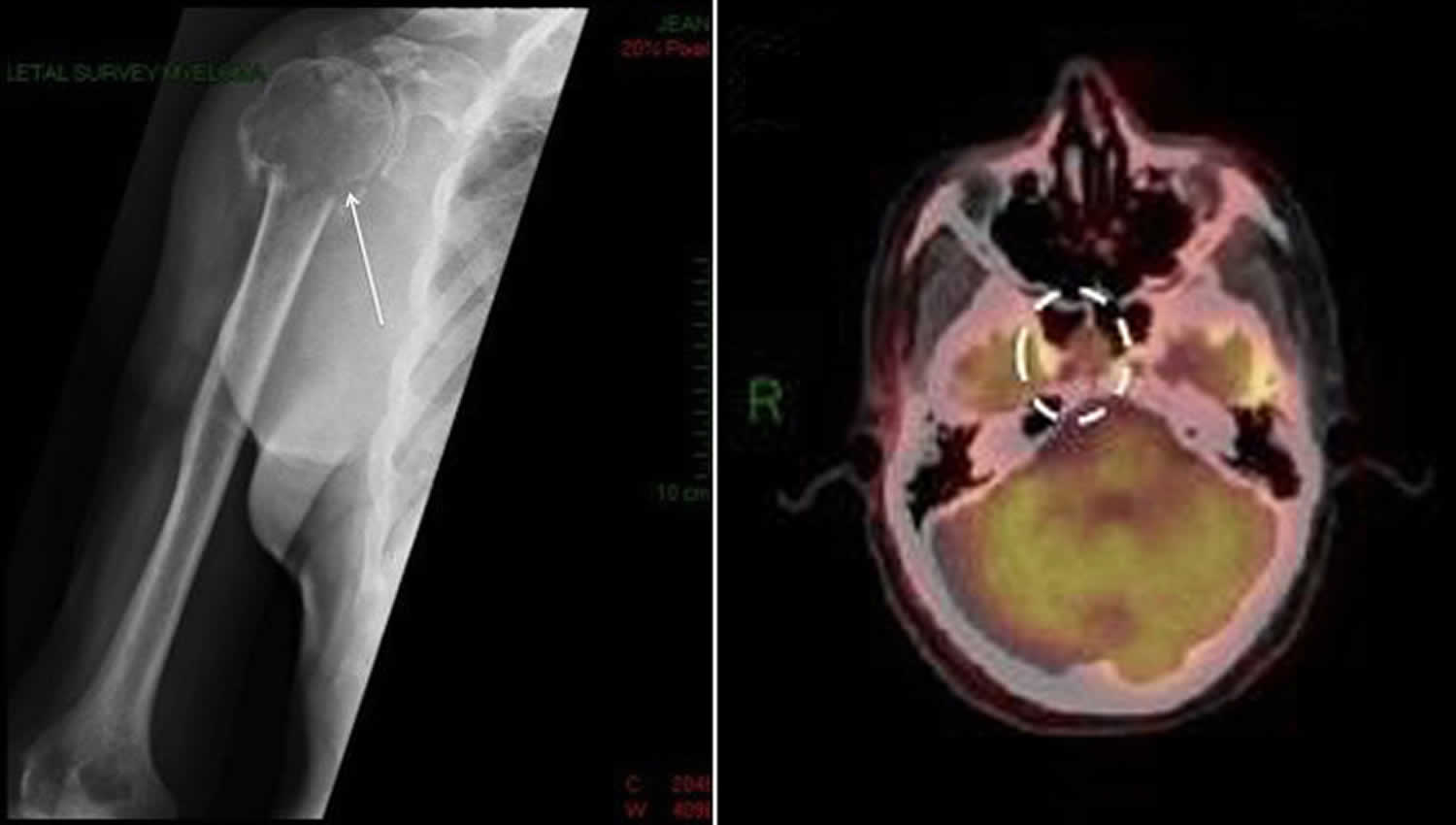
[Source 1 ]Figure 3: Solitary plasmacytoma
Footnote: Left: Shoulder X-ray showing solitary bone plasmacytoma located in the right shoulder complicated by pathological fracture of the head of humerus. Right: Positron emission tomography-computed tomography (PET-CT) scan showing a mass occupying the anterior clivus, later identified to be an solitary extramedullary plasmacytoma tumor.
[Source 1 ]Solitary plasmacytoma
Solitary plasmacytomas are rare, comprising less than 5% of all plasma cell neoplasms 8. Solitary extramedullary plasmacytomas are even less common. Solitary plasmacytomas occur more commonly in men than women.
The International Working Myeloma Group has defined two discrete categories of solitary plasmacytoma 9:
- Solitary bone plasmacytoma: Solitary bone plasmacytoma where there is localized build-up of abnormal monoclonal plasma cells in the bone marrow. Most commonly, these tumors develop in the spinal column but they may also develop in the pelvis, ribs, arms, face, skull, femur (thigh), and sternum (breast bone). The diagnosis of solitary bone plasmacytoma requires a solitary bone lesion, a biopsy of which shows infiltration by plasma cells; negative imaging results for other bone lesions; absence of clonal plasma cells in a random sample of bone marrow; and no evidence of anemia, hypercalcemia, or renal involvement suggesting systemic myeloma. Some people with solitary bone plasmacytoma may go on to develop multiple myeloma – around 50-70% over 10 years – so you’ll be regularly monitored with blood tests and x-rays and/or MRI scans.
- Solitary extramedullary plasmacytoma: Solitary extramedullary plasmacytoma is where the clump of abnormal plasma cells occurs outside the bone marrow and separate from bone in soft tissue. Solitary extramedullary plasmacytomas most commonly occur in the head and neck region, particularly in the upper airways (nose, throat and sinuses), but may also be found in the gastrointestinal tract, lymph nodes, bladder, lung or other organs. There is less than a 10% chance of this disease progressing to myeloma.
They are further distinguished by the location of their occurrence:
- Solitary bone plasmacytoma mainly presents as a single, typically painful bone lesion mainly occurring in the axial skeleton (83%), in such places as the vertebrae, with the other 17% of cases occurring in the appendicular skeleton, in such places as the pelvic bones.
- Solitary extramedullary plasmacytoma involves the soft tissue and usually manifests itself in the head and neck region (80% of cases; commonly of respiratory origin), and in the mouth and pharynx 1.
The symptoms of each vary depending on the site of the malignancy. For solitary bone plasmacytoma, the most common symptom is pain, with motor and sensory deficits occurring if the spine is involved. For solitary extramedullary plasmacytoma, the symptoms are nonspecific and usually secondary to space-occupying lesions 10. Patients may present with bony pain, neurological symptoms and/or pathological fracture in the case of a solitary plasmacytoma or epistaxis, rhinorrhoea and nasal obstruction in the case of an upper respiratory tract extramedullary plasmacytomas, which is the most commonly affected site.
Solitary plasmacytomas do not have the typical features of myeloma, which include low red blood cell counts, elevated calcium levels in the blood, or reduced kidney function. And although 75% of people with solitary bone plasmacytoma and 25% of people with solitary extramedullary plasmacytoma have an M-protein (abnormal proteins produced by the cancerous plasma cells), they are usually small and disappear following treatment.
A solitary plasmacytoma most commonly occurs in middle-aged or elderly people and is very rare under the age of 30. The median age at diagnosis is about a decade younger than that of people with myeloma, 55 to 65 years, compared to a median age of 71 years for patients diagnosed with multiple myeloma.
Risk of progression to myeloma
Patients with solitary plasmacytoma can progress to overt multiple myeloma at a rate of approximately 40% to 50% over 5 years, with lower rates of progression reported for solitary extramedullary plasmacytoma 11. The Rare Cancer Network described the natural history of 258 patients with solitary plasmacytomas and found the median time to multiple myeloma progression was 21 months (2–135) 12. One possible explanation for this wide variation in progression free survival is that this study was reported in 2006, when the primary imaging modality was skeletal survey, and some patients with multiple myeloma may have been wrongly classified as plasmacytoma resulting in a relatively short progression free survival. Despite this, the study still reflects the fact that solitary plasmacytoma patients are a heterogeneous group, with some progressing early to multiple myeloma, whilst others remain disease free for many years or are effectively cured.
A number of risk factors are postulated as predictors of disease progression. Warsame et al. 13 identified the presence of excess clonal plasma cells in bone marrow greater than or equal to 5%, and delivered radiotherapy dose as prognostic factors for risk of progression. Whilst Knobel et al. 14 reported patient age as a risk factor for progression and Tasang et al. 15 reported tumor size to be a predictive factor. Other recognized risk factors for progression to multiple myeloma include abnormal serum free light chains (sFLC) ratio at diagnosis, and M-protein persistence post radiation therapy 16. Both Spanish and UK groups have independently demonstrated that the presence of aberrant plasma cells on flow cytometry of the bone marrow can identify patients with solitary plasmacytomas at high risk for progression to active multiple myeloma 17.
The use of imaging methods to assess patients with solitary plasmacytoma at risk of progression has also been explored 18. Fouquet et. al 19 identified baseline FDG PET-CT features as a strong predictive factor for risk of progression, but some of these features would now be classified as multiple myeloma. Whether functional imaging findings post-therapy reliably predict risk of progression remains unknown, and whether maximum standardised uptake (SUVmax) can be used as a biomarker of risk remains unknown.
Plasmacytoma causes
It is not known what causes plasmacytoma. Radiation, industrial solvents and airborne toxins have been identified as possible risk factors. Factors such as viral pathogenesis and irritation from inhaled irritants have been noted. Genetic factors may also play a role; however, no specific loci for the origin of this disease have been identified 20.
Plasmacytoma symptoms
Solitary bone plasmacytomas may cause bone pain or fractures. Symptoms depend on where the tumour is located. Generally speaking, a common yet nonspecific clinical symptom of solitary bone plasmacytoma is pain. Motor and sensory deficits can also occur, secondary to nerve impingement from compression fractures 10. As space occupying lesions, the symptoms of plasmacytoma vary based on the location of the tumor. Plasmacytomas located in the brain (e.g. solitary craniocerebral plasmacytoma or extramedullary plasmacytoma of the brain) can cause headaches, seizures, and paralysis while plasmacytomas in the rib may cause pain while breathing.
Complications of solitary bone plasmacytoma include pathological fractures due to lytic bone disease. extramedullary plasmacytoma, on the other hand, usually manifests itself in the upper respiratory tract and oral cavity; symptoms often include epistaxis, sore throat, rhinorrhea, and hemoptysis 21.
Plasmacytoma diagnosis
Plasmacytoma is a diagnosis of exclusion, as active multiple myeloma must be ruled out. The diagnostic criteria for solitary bone plasmacytoma and solitary extramedullary plasmacytoma are as follows 22.
- Biopsy proven, single infiltrate of clonal plasma cells in bone (solitary bone plasmacytoma) or soft tissue (extramedullary plasmacytoma).
- A bone marrow biopsy showing no evidence of infiltration by clonal plasma cells.
- Negative skeletal survey plus MRI/CT spine and pelvis except for the solitary lesion.
- Lack of CRAB, which would suggest multiple myeloma.
- Increased Calcium
- Renal failure
- Anemia
- Bone lesions (with the exception of solitary bone plasmacytoma)
The diagnosis of a solitary plasmacytoma relies upon histological evidence of a tumor consisting of monoclonal plasma cells, with a plasma cell percentage in the bone marrow of <10%, along with imaging confirmation of a solitary tumor with no other evidence of organ or tissue damage. It is known that conventional staging with skeletal X-ray survey will understage some patients 23. As a result, some patients will be miscategorized as solitary plasmacytoma and may receive radical radiotherapy, the treatment of choice for solitary plasmacytoma, rather than benefitting from systemic chemotherapy to treat symptomatic myeloma. To improve the accuracy of diagnosis of solitary plasmacytoma, there has been a shift towards whole body imaging techniques. If bone marrow aspirate/biopsy shows less than 10% involvement by clonal plasma cells, then the disease is defined as solitary plasmacytoma with minimal marrow involvement, which carries a 60% (solitary bone plasmacytoma) or 20% (extramedullary plasmacytoma) risk of transformation in multiple myeloma within 3 years.
Magnetic resonance imaging (MRI) remains the gold standard imaging modality for detecting bone marrow infiltration at an early stage, prior to bone destruction, particularly in the axial skeleton. Newer MRI protocols enable whole body imaging techniques (WB-MRI) to improve coverage of the appendicular skeleton, whilst diffusion weighted sequences (DW-MRI) improve specificity 24. 2-deoxy-2-[18F]fluoro-D-glucose (FDG) positron emission tomography-computed tomography (PET-CT) has emerged as a powerful hybrid imaging tool both for diagnosis of plasmacytomas, and also for monitoring treatment response, with a growing body of data suggesting the benefit over conventional MRI in the setting of myeloma (multiple myeloma) 25. In countries where access to FDG PET-CT is not widely available, and Technetium (99mTc) sestamibi (99mTc-MIBI) scans are used for diagnosis and monitoring of some patients. Although not as accurate as FDG PET-CT for detecting focal lesions, 99mTc-MIBI can be better than FDG PET-CT at detecting diffuse marrow involvement 26. Technetium (99mTc) sestamibi is an inexpensive and rapid technique for whole-body evaluation enabling its wider availability in developing countries 26.
Plasmacytoma treatment
The treatment used most commonly for both types of plasmacytoma is radiotherapy. This is possible because by definition, “solitary plasmacytomas” are localized. Radiotherapy involves focusing radiation (similar to x-rays) on the plasmacytoma to kill the abnormal cells. The treatment is generally given over several days to reduce side-effects. Although chemotherapy is generally not used in addition to radiotherapy, there are times when the types of medications used to treat myeloma are considered.
Radiotherapy generally provides excellent local and often durable control of the plasmacytoma. However, there is a risk that plasmacytomas may recur or progress to myeloma (particularly with solitary bone plasmacytoma). All people with plasmacytomas require life-long follow-up. This generally involves physical examination, blood and urine tests, and x-rays, MRI or PET scans at regular intervals for at least the first five years after treatment has been completed.
While the most effective dose has been debated in the literature, usually a dose of 40-45 Gray (Gy) is required for the best local control without adverse toxicity 27. However, it has also been reported that there is no dose-response relationship for radiation therapy of doses greater than 30 Gy 28. Plasmacytoma is a particularly radiosensitive tumor, and rates of local control in solitary extramedullary plasmacytoma from 90-100% have been reported for this treatment alone 20.
Surgery is rarely necessary but may be required in situations where plasmacytoma involvement of the bone causes skeletal instability and high risk of fracture. In these cases, radiation therapy may be delayed until after surgery.
Surgical resection has proven to be beneficial in some cases, though surgery alone is usually not recommended as it results in lower rates of local control and higher rates of recurrence 3. It shows promise in localized tumors that are easily resectable, but is a treatment that is often avoided in cases of extramedullary plasmacytoma, as a result of the majority of tumors being located in the head and neck region with mutilation from surgery being a significant risk 3. Orthopedic surgery for the stabilization or reduction of pathological fractures (e.g. open reduction, internal fixation) or the restoration of vertebral structure (e.g. kyphoplasty) may also be of benefit.
The use of chemotherapy for this disease has also been reported, but is usually reserved for cases in which the plasmacytoma has progressed 29. Particular promise for chemotherapy is seen in antiangiogenic compounds such as thalidomide. Angiogenesis is highly correlated to progression of plasmacytoma to multiple myeloma, so compounds such as thalidomide may play an important role in halting this progression 28. Bortezomib has also been reported to stimulate bone formation and could be of benefit in patients with bone disease 30.
Plasmacytoma life expectancy
The median survival time for plasmacytoma averages 10 years 27. There are a variety of factors that affect the prognosis for plasmacytoma, and either shorten or lengthen the survival time. Higher-grade angiogenesis has been shown to lead to a higher rate of progression to multiple myeloma as well as a shorter progression free survival 3. A major factor in the prognosis of plasmacytoma is also which type of plasmacytoma is present in the patient. There is a large body of literature that shows that extramedullary plasmacytoma carries with it a favorable prognosis over solitary bone plasmacytoma 21 and that the overall and median survival rate for extramedullary plasmacytoma is higher than for solitary bone plasmacytoma 31. It has been demonstrated that extramedullary plasmacytoma progresses to multiple myeloma at a much lower rate than solitary bone plasmacytoma. Galieni et al 21 notes that this favorable prognosis might point to extramedullary plasmacytoma having a different origin and biological aspects than multiple myeloma or even solitary bone plasmacytoma despite the diseases sharing multiple histologic and immunohistochemical similarities. Other factors that affect prognosis are age and size of tumor. People over the age of 60 with plasmacytoma have the least favorable prognosis, whereas those under the age of 40 have the most favorable 32, and tumors larger than 5 cm were shown to be an unfavorable prognostic factor 28. Interestingly, one study shows that tumors were larger in the observed solitary bone plasmacytoma group than in the extramedullary plasmacytoma group, pointing to a possible explanation for the higher rates of progression in solitary bone plasmacytoma 10. Another study also notes that, while the occurrence of plasmacytoma is higher in men than in women, men have a significantly higher chance of survival. Race, on the other hand, did not affect survival in this study, despite playing a role in the incidence of the disease 32.
References- Rowell, Sean & Ho, Matthew & Anderson, Kenneth. (2019). Plasmacytoma (Solitary bone plasmacytoma, extramedullary plasmacytoma). Atlas of Genetics and Cytogenetics in Oncology and Haematology. 23. 10.4267/2042/69762
- Bink K, Haralambieva E, Kremer M, et al. Primary extramedullary plasmacytoma: similarities with and differences from multiple myeloma revealed by interphase cytogenetics. Haematologica. 2008;93(4):623–626. doi:10.3324/haematol.12005
- Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, Haydaroglu A. A review for solitary plasmacytoma of bone and extramedullary plasmacytoma. ScientificWorldJournal. 2012;2012:895765. doi:10.1100/2012/895765 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3354668
- Rajkumar SV. Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2012 Jan;87(1):78-88.
- Kaseb H, Durer C, Fazal S, et al. Plasma Cell Cancer. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507913
- Rajkumar, V., Dimopoulos, M., Palumbo, A., Blade, J., Merlini, G., Mateos, M.-V., Kumar, S., Hillengass, J., Kastritis, E., Richardson, P., Landgren, O., Paiva, B., Dispenzieri, A., Weiss, B., Leleu, X., Zweegman, S., Lonial, S., Rosinol, L., Zamagni, E., & San Miguel, J. (2014). International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncology, 538-548. doi:10.1016/S1470-2045(14)70442-5
- Rajkumar, SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020; 95: 548– 567. https://doi.org/10.1002/ajh.25791
- Sharpley FA, Neffa P, Panitsas F, et al. Long-term clinical outcomes in a cohort of patients with solitary plasmacytoma treated in the modern era [published correction appears in PLoS One. 2019 Nov 7;14(11):e0225184]. PLoS One. 2019;14(7):e0219857. Published 2019 Jul 23. doi:10.1371/journal.pone.0219857 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6650037
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. doi:10.1016/S1470-2045(14)70442-5 https://doi.org/10.1016/S1470-2045(14)70442-5
- Guo SQ, Zhang L, Wang YF, et al. Prognostic factors associated with solitary plasmacytoma. Onco Targets Ther. 2013;6:1659–1666. Published 2013 Nov 14. doi:10.2147/OTT.S53248 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3833869
- Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000;96(6):2037–44. Epub 2000/09/09
- Ozsahin M, Tsang RW, Poortmans P, Belkacemi Y, Bolla M, Dincbas FO, et al. Outcomes and patterns of failure in solitary plasmacytoma: a multicenter Rare Cancer Network study of 258 patients. International journal of radiation oncology, biology, physics. 2006;64(1):210–7. Epub 2005/10/19. 10.1016/j.ijrobp.2005.06.039
- Warsame R, Gertz MA, Lacy MQ, Kyle RA, Buadi F, Dingli D, et al. Trends and outcomes of modern staging of solitary plasmacytoma of bone. American journal of hematology. 2012;87(7):647–51. Epub 2012/05/03. 10.1002/ajh.23201
- Knobel D, Zouhair A, Tsang RW, Poortmans P, Belkacemi Y, Bolla M, et al. Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC cancer. 2006;6:118 Epub 2006/05/09. 10.1186/1471-2407-6-118
- Tsang RW, Gospodarowicz MK, Pintilie M, Bezjak A, Wells W, Hodgson DC, et al. Solitary plasmacytoma treated with radiotherapy: impact of tumor size on outcome. International journal of radiation oncology, biology, physics. 2001;50(1):113–20. Epub 2001/04/24. 10.1016/s0360-3016(00)01572-8
- Dingli D, Kyle RA, Rajkumar SV, Nowakowski GS, Larson DR, Bida JP, et al. Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood. 2006;108(6):1979–83. Epub 2006/06/03. 10.1182/blood-2006-04-015784
- Hill QA, Rawstron AC, de Tute RM, Owen RG. Outcome prediction in plasmacytoma of bone: a risk model utilizing bone marrow flow cytometry and light-chain analysis. Blood. 2014;124(8):1296–9. Epub 2014/06/19. 10.1182/blood-2014-04-566521
- Schirrmeister H, Buck AK, Bergmann L, Reske SN, Bommer M. Positron emission tomography (PET) for staging of solitary plasmacytoma. Cancer biotherapy & radiopharmaceuticals. 2003;18(5):841–5. Epub 2003/11/25. 10.1089/108497803770418382
- Fouquet G, Guidez S, Herbaux C, Van de Wyngaert Z, Bonnet S, Beauvais D, et al. Impact of initial FDG-PET/CT and serum-free light chain on transformation of conventionally defined solitary plasmacytoma to multiple myeloma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(12):3254–60. Epub 2014/04/10. 10.1158/1078-0432.ccr-13-2910
- Jiang CZ, Lin QS, Wu XY, Wang CY, Kang DZ. Sellar solitary plasmacytoma progressing to multiple myeloma: a case report and literature review. Medicine (Baltimore). 2014;93(11):e58. doi:10.1097/MD.0000000000000058 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4616275
- Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85(1):47–51.
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014 Nov;15(12):e538-48. https://doi.org/10.1016/S1470-2045(14)70442-5
- Chargari C, Vennarini S, Servois V, Bonardel G, Lahutte M, Fourquet A, et al. Place of modern imaging modalities for solitary plasmacytoma: toward improved primary staging and treatment monitoring. Critical reviews in oncology/hematology. 2012;82(2):150–8. Epub 2011/05/31. 10.1016/j.critrevonc.2011.04.006
- Chantry A, Kazmi M, Barrington S, Goh V, Mulholland N, Streetly M, et al. Guidelines for the use of imaging in the management of patients with myeloma. 2017;178(3):380–93. 10.1111/bjh.14827
- Zamagni E, Nanni C, Patriarca F, Englaro E, Castellucci P, Geatti O, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92(1):50–5. Epub 2007/01/19. 10.3324/haematol.10554
- Fonti R, Salvatore B, Quarantelli M, Sirignano C, Segreto S, Petruzziello F, et al. 18F-FDG PET/CT, 99mTc-MIBI, and MRI in evaluation of patients with multiple myeloma. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2008;49(2):195–200. Epub 2008/01/18. 10.2967/jnumed.107.045641
- Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000;96(6):2037–2044.
- Knobel D, Zouhair A, Tsang RW, et al. Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC Cancer. 2006;6:118. Published 2006 May 5. doi:10.1186/1471-2407-6-118
- Corvo MA, Granato L, Ikeda F, de Próspero JD. Extramedullary nasal plasmacytoma: Literature review and a rare case report. Int Arch Otorhinolaryngol. 2013;17(2):213–217. doi:10.7162/S1809-97772013000200016 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4423260
- Pennisi A, Li X, Ling W, et al. Inhibitor of DASH proteases affects expression of adhesion molecules in osteoclasts and reduces myeloma growth and bone disease. Br J Haematol. 2009;145(6):775–787. doi:10.1111/j.1365-2141.2009.07696.x https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2748971
- Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144(1):86–94. doi:10.1111/j.1365-2141.2008.07421.x
- Thumallapally N, Meshref A, Mousa M, Terjanian T. Solitary plasmacytoma: population-based analysis of survival trends and effect of various treatment modalities in the USA [published correction appears in BMC Cancer. 2017 Jun 23;17 (1):443]. BMC Cancer. 2017;17(1):13. Published 2017 Jan 5. doi:10.1186/s12885-016-3015-5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5216567