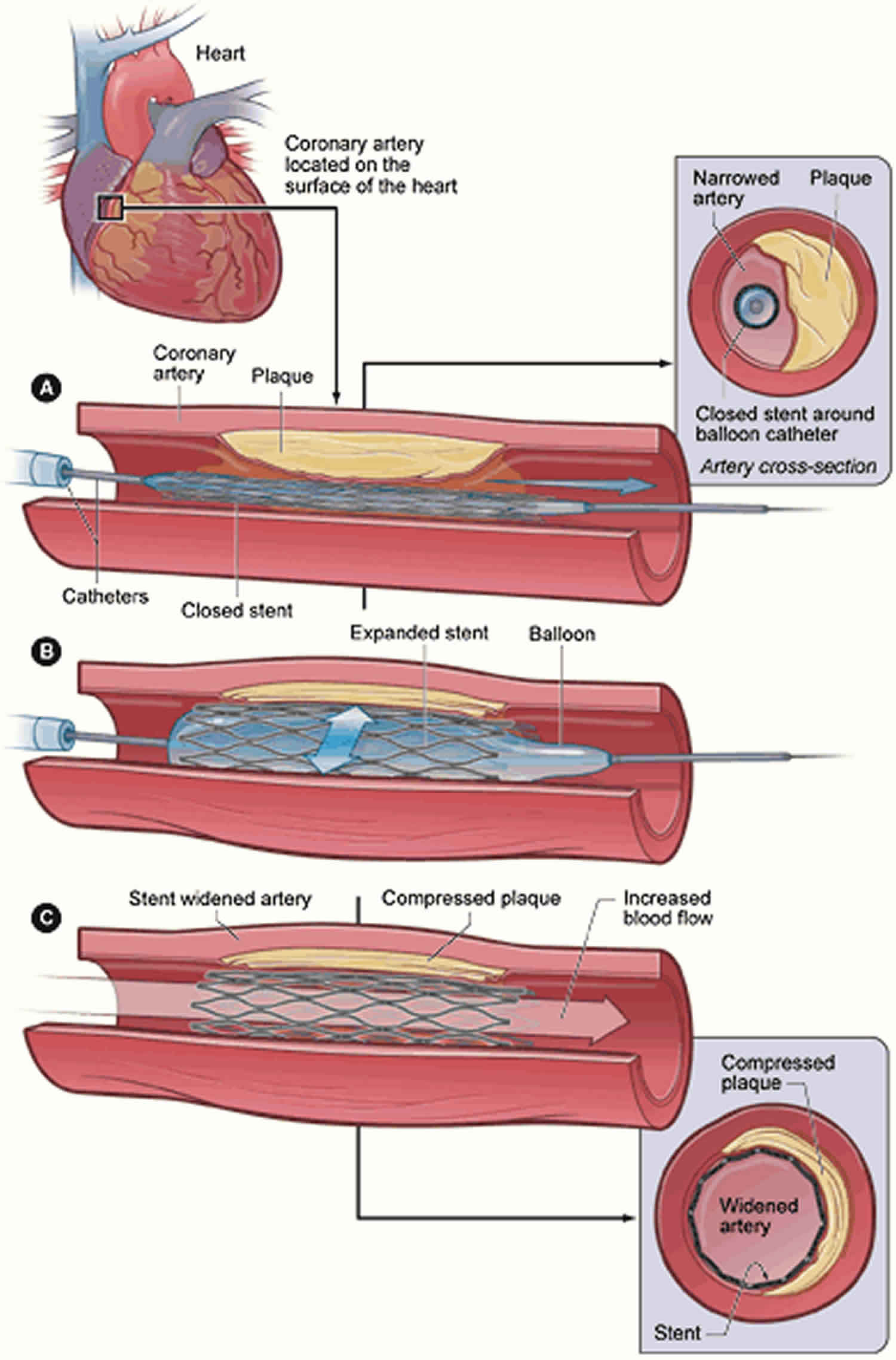
Restenosis
Restenosis is the reduction in the diameter of the vessel lumen after angioplasty 1. Despite advances in stent technology, restenosis continues to be the most frequent cause of target lesion failure following percutaneous coronary intervention 1. Following the introduction of bare-metal stents in the mid-1990s for the treatment of coronary artery disease, a new clinical entity emerged called in-stent restenosis, which is restenosis in an implanted coronary stent. Angiographically, in-stent restenosis is more than 50% stenosis within or immediately adjacent to a previously stented region. Clinical restenosis occurs when there is a recurrence of clinical manifestations of ischemia in the setting of in-stent restenosis, often requiring a repeat revascularization procedure. In-segment restenosis is often defined as restenosis anywhere between 5 mm from the proximal and distal edges of the stent 2. Recurrent in-stent restenosis is defined as the failure of at least two revascularization procedures at the stent segment 3.
In plain-old balloon (no-stent) angioplasty, mechanisms of restenosis involve vessel remodeling and elastic recoil. In contrast, restenosis in-stent angioplasty involves excessive tissue proliferation called neointimal proliferation, or by a new atherosclerotic process called neoatherosclerosis 4. The patterns of in-stent restenosis have been described as either diffuse (lesion over10 mm in length) or focal (lesion less than10 mm in length) according to the Mehran classification criteria 5. Clinically, coronary vessel restenosis will present as recurrence of angina or acute coronary syndrome. In-stent restenosis is associated with significant morbidity. In a prospective cohort study of 10,004 patients who underwent routine control angiography 6 to 8 months after coronary stenting, the presence of restenosis at follow-up angiography was predictive of 4-year mortality 6.
The following conditions increase the risk of restenosis following stent implantation:
- Uncontrolled diabetes mellitus
- Continued cigarette smoking
- High levels of LDL “bad” cholesterol
- Uncontrolled hypertension
- Renal insufficiency
These conditions should be adequately controlled and monitored. Smoking cessation, moderate exercise, and weight loss are advisable lifestyle interventions.
Efforts to reduce the incidence of restenosis and treatment options for in-stent restenosis have evolved remarkably over the last two decades. Advancement in the stent platforms (e.g. thin-strut, and biodegradable), eluting drugs (biolimus A9 and zotarolimus, designed specifically for intracoronary use), and intravascular imaging modalities improving implant technique has led the interventional cardiologist to treat patients who were previously limited to surgical revascularization (i.e. left main stem, multivessel disease, complex bifurcations and complex and severely calcified lesions). As a result, real-world registries, including more complex patients and lesions, show a higher rate of in-stent restenosis when compared to the reports from randomized trials 4.
The metal of the stent scaffold can predispose to blood clot formation within the stent, with severe consequences. Dual antiplatelet therapy (aspirin and a second drug that prevents blood clot formation) can help to prevent it, and adherence to dual antiplatelet therapy must be per the instructions and duration of the treating cardiologist. Any interruption or cessation of dual antiplatelet therapy should be with the approval of a cardiologist.
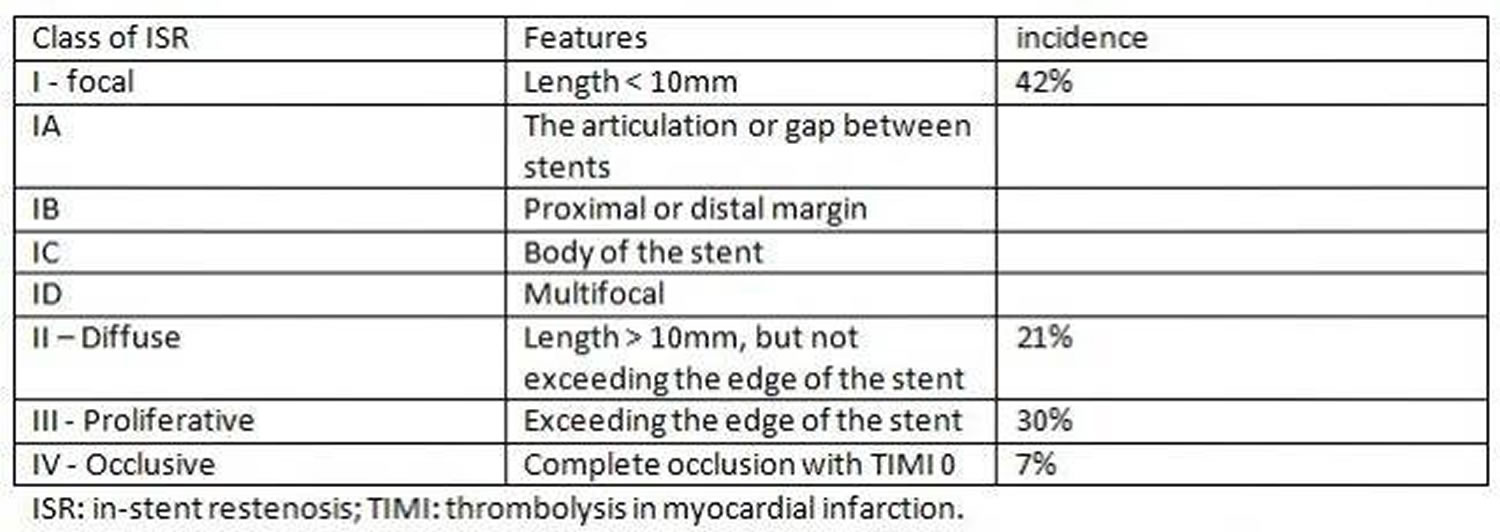
Figure 1. In-stent restenosis types
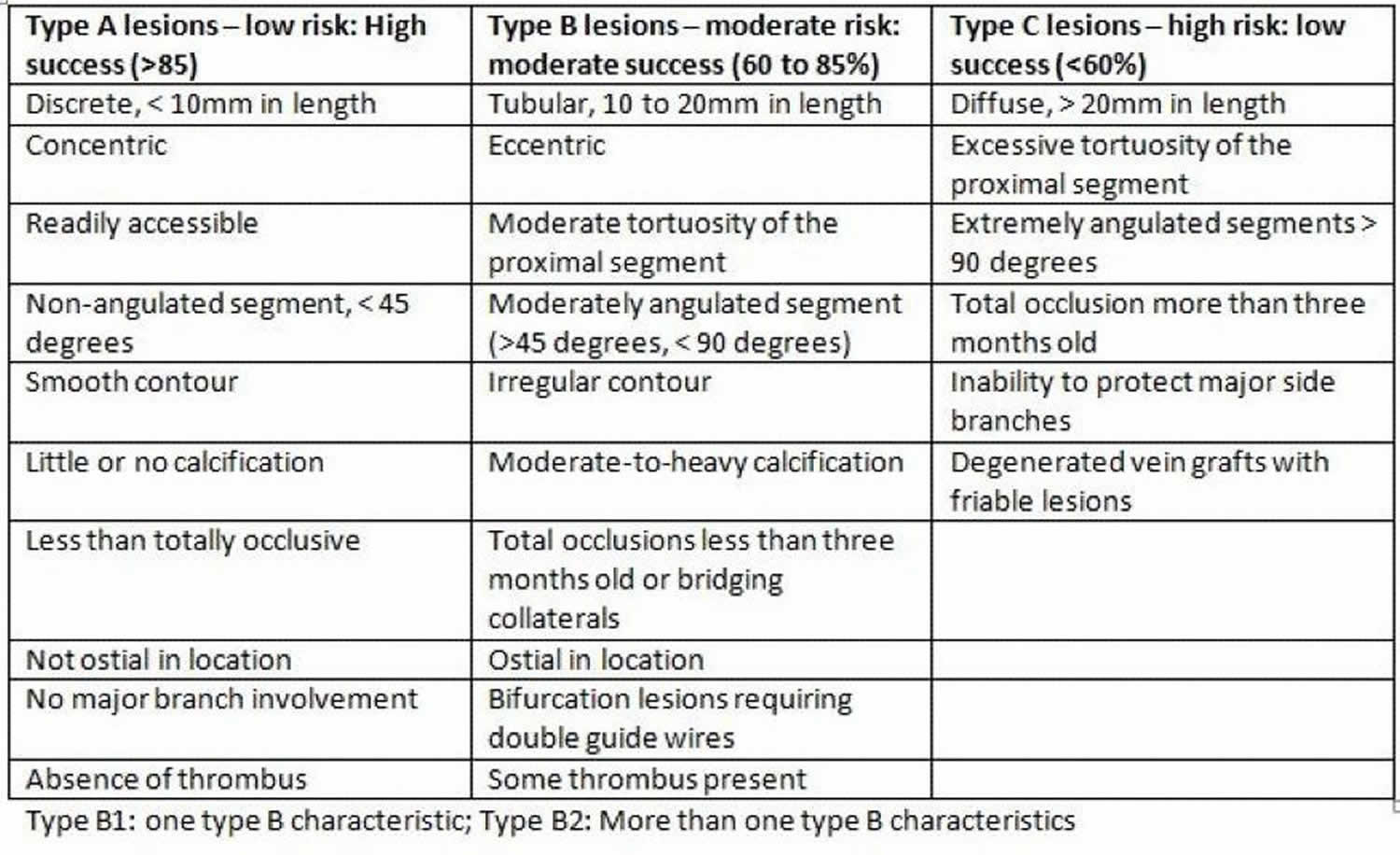
Figure 2. Restenosis lesion types
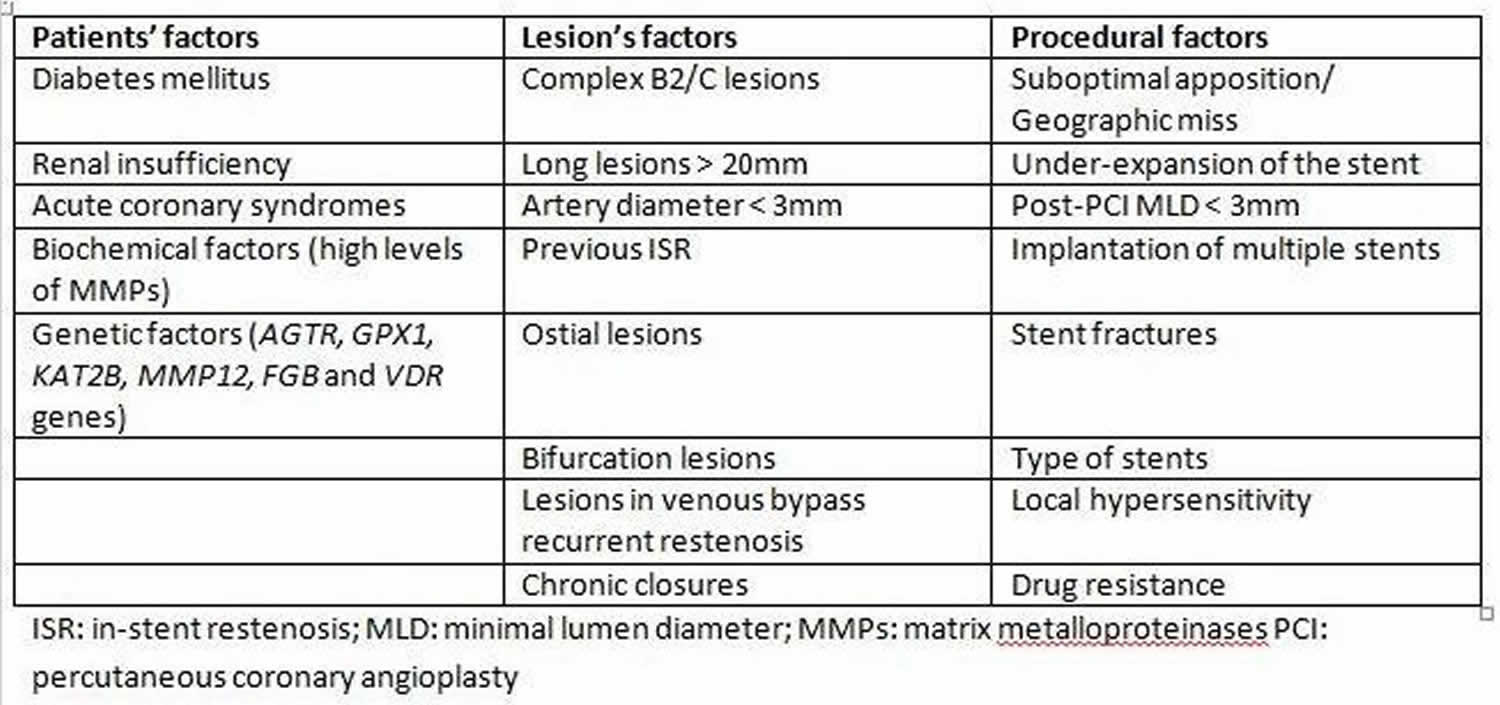
Causes of restenosis
Risk factors for restenosis are classified based on patient features, lesion type, and procedural factors.
Figure 3. Causes of restenosis
Biological factors associated with an increased risk of restenosis include diabetes mellitus and renal insufficiency. In a multivariate regression analysis, diabetes mellitus in itself increases the risk of bare metal in-stent restenosis by 30% to 50% 2. Likewise, the risk of drug-eluting stent in-stent restenosis is increased in diabetic patients when compared to those without diabetes.
Biochemical factors such as elevated serum matrix metalloproteinases, the proteolytic enzymes that degrade the extracellular matrix and facilitate the proliferation and migration of endothelial and vascular smooth muscle cells, has been associated with a higher risk of in-stent restenosis. Hematological indices including neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, red blood cell distribution width, mean platelet volume, and platelet distribution width, have been hypothesized in some studies to predict the occurrence of increased risk for drug-eluting stent in-stent restenosis 7. The GENetic DEterminants of Restenosis (GENDER) study identified single-nucleotide polymorphisms in AGTR, GPX1, KAT2B, MMP12, FGB, and VDR genes as being associated with an increased risk of restenosis 8. Significant mechanical predictors of restenosis include complex lesions American College of Cardiology and American Heart Association Type B2/C (B2 and C lesions are not only more frequently related to suboptimal acute results but also with a higher restenosis rate and poorer long-term clinical outcomes) 3, the length of the lesion and small vessel diameter. A stent length greater than 35 mm correlates with nearly double the in-stent restenosis risk compared to less than 20 mm stents. The major risk factors for recurrent in-stent restenosis are diabetes mellitus, and previous in-stent restenosis, and type according to Mehran’s classification (with an increase from I to IV) 3.
Procedural risk factors
The most known and preventable cause for in-stent restenosis is stent under-expansion. Stent under-expansion can be due to stent under-sizing, low-pressure deployment, inadequate vessel preparation, or extensive vessel calcification leading to an under-expanded stent. However, under-sizing is not the only risk factor for in-stent restenosis. Failure to stent from healthy segment to healthy segment, or geographic miss, is another common risk factor. Geographic miss appears as a characteristic “candy-wrapper” appearance at the edge of a stented segment. Additionally, stent fracture is another possible mechanical precipitant for in-stent restenosis. Finally, the development and use of drug-eluting stent has led to the recognition of drug resistance and local hypersensitivity reactions as a potential cause of in-stent restenosis and drug-eluting stent failure 9.
Restenosis pathophysiology
Restenosis may occur as a result of an excessive reaction to the vascular injury sustained from the percutaneous coronary intervention. The first vascular response following the trauma of percutaneous coronary intervention includes elastic recoil, platelet plug, and thrombus formation, occurring in the initial minutes and hours following intervention. This initial response is followed by a complex inflammatory and reparative process that lasts weeks to months or even years in case of drug-eluting stent-in-stent restenosis. The acute phase is characterized by deposition of platelets and fibrin, with the adhesion of circulating neutrophils and monocytes. Over weeks, chronic inflammatory cells (macrophages and giant cells) will gradually replace the acute inflammatory cells at the site of vascular injury. These processes occur over a relatively short period (weeks to months) unlike the slow progression (years) of atherosclerotic disease in native coronary lesions. The two principal biologic mechanisms of in-stent restenosis are neointimal hyperplasia and neoatherosclerosis 10.
Neointimal hyperplasia is a reparative process whereby various coagulation and inflammatory factors and cells stimulate vascular smooth muscle cell proliferation and extracellular matrix formation at the site of injury. In addition to initial inflammatory response, platelet- and leukocyte-related growth factors stimulate further vascular smooth muscle cell proliferation and migration from the media to the inceptive neointima with the formation of extracellular matrix. At about two weeks after stent implantation, a neointimal layer, composed of vascular smooth muscle cell s and a proteoglycan-rich extracellular matrix are visible above the stent struts. Subsequently, endothelial cells proliferate and cover the extracellular matrix from the luminal side.
A diffuse pattern of in-stent restenosis, characterized by a predominance of vascular smooth muscle cell s surrounded by lesser amount extracellular matrix, is typical of bare metal stent in-stent restenosis. Peak bare metal stent in-stent restenosis is observed at 3 to 6 months and remains relatively stable beyond one year. drug-eluting stent implantation, on the other hand, enables the local release of antiproliferative agents [paclitaxel or “-limus” drug group (sirolimus, everolimus, zotarolimus, biolimus, etc)] resulting in delayed vessel wall healing, characterized by the presence of chronic fibrin deposits, incomplete neoendothelization, and prolonged inflammatory changes that persist for up to 48 months. This process prevents excessive neointimal hyperplasia after drug-eluting stent stent implantation and leads to a reduction in the occurrence of in-stent restenosis 2.
Neoatherosclerosis, on the other hand, is related to incomplete regeneration of the endothelium leading to excessive uptake of circulating lipids and accelerated development of atherosclerotic plaques in the nascent neointima. Intimal thickening, intracellular lipid deposition with thin-cap fibroatheroma or the presence of necrotic tissue have been detected using histopathological or optical coherence tomography evaluations. Nakazawa, et al. 11 found in their histopathological autopsy study that neoatherosclerosis as a result of persistent endothelial dysfunction and incomplete neoendothelization occurs more frequently (31% vs. 16%) and earlier (median: 420 vs. 2160 days) following drug-eluting stent than bare metal stent implantation.
Moreover, neoatherosclerosis in drug-eluting stent shows unstable characteristics (thin-cap fibroatheroma or plaque rupture) earlier (about two years) after implantation, whereas similar features in bare metal stent occur relatively later (about six years). Independent predictors of neoatherosclerosis include younger age, longer implant durations, sirolimus- or paclitaxel-eluting stent implantation, smoking, chronic kidney disease, and LDL-cholesterol greater than 3.9mmol/l. The belief is that neoatherosclerosis, along with the rupturing of the thin-cap fibroatheroma, are among the predominant causes of late stent failure (i.e., delayed in-stent restenosis and late or very late stent thrombosis) 11. Examination of 299 patients post-mortem revealed that, compared to bare metal stent, drug-eluting stent had almost twice the rate of neoatherosclerosis (31 vs. 16 %) 12.
Restenosis symptoms
Restenosis presents with a wide spectrum of clinical features that depict varying levels of cardiac ischemia in the setting of previous coronary intervention. The clinical features of restenosis depend on the individual patient and lesion characteristics as well as the anatomic location and may present as stable angina, unstable angina (26 to 53 % for bare metal stent, 16 to 66 % for drug-eluting stent), or acute myocardial infarction (heart attack) (3.5 to 20 % for bare metal stent, 1 to 20 % for drug-eluting stent) 12. The Mehran’s classification of in-stent restenosis is a predictor of clinical outcome based on bare metal stent-in-stent restenosis, with the necessity of repeated target vessel revascularization being 19% for group I, 35% for group II, 50% for group III and 83% for group IV 2. Depending on the duration since the deployment of the stent, in-stent restenosis can also be classified as acute (within 24 hours), subacute (from 24 hours to 30 days), late (30 days to 1 year), and very late (>1 year) 9.
In a study of 909 patients who had in-stent restenosis after bare metal stent, first-generation drug-eluting stent or second-generation drug-eluting stent, the clinical presentation was acute coronary syndrome in 67.8%, 71.0%, and 66.7% of patients, respectively, whereas myocardial infarction occurred in 10.6%, 10.1%, and 5.2% of patients, respectively. These are not statistically significant and may suggest that patient, and lesion characteristics, rather than the specific initial device deployed, are the major determinants of the clinical presentation of in-stent restenosis 13. Current smoking status and chronic renal failure were found to correlate with presentation of myocardial infarction (odds ratio, 3.02), and (odds ratio, 2.73), respectively; while second-generation drug-eluting stent-in-stent restenosis was attributed to be protective for a presentation with myocardial infarction (odds ratio, 0.35) 13.
Restenosis complications
Complications of restenosis include stable angina, unstable angina, acute coronary syndrome, acute myocardial infarction, or death. Revascularization procedures for in-stent restenosis could also encounter complications from access site bleeding, stent under-expansion, incomplete revascularization, coronary artery dissection, and stent thrombosis.
Restenosis diagnosis
Coronary angiography historically has been a well established diagnostic modality for identifying restenosis and for the guidance of percutaneous coronary intervention. In a study of 288 bare metal stent-in-stent restenosis lesions in 245 patients, Mehran and colleagues classified in-stent restenosis into four angiographic patterns (Figures 1 and 2). Pattern I: focal (length of 10 mm or less) lesions; Pattern II: in-stent restenosis involving more than 10 mm within the stent; Pattern III: in-stent restenosis more than 10 mm and extending outside the stent; and pattern IV is a completely occlusive in-stent restenosis. The incidence was 42%, 21%, 30%, and 7% for pattern I, pattern II, pattern III, and pattern IV respectively 5. Figure 2 highlights the lesion-specific characteristics and their corresponding outcomes with balloon angioplasty.
Intravascular imaging techniques, including intravascular ultrasound and optical coherence tomography (optical coherence tomography), provide additional advantages over angiography, providing more distinct lesion characterization and identification of the cause of in-stent restenosis potentially optimizing the short and long term outcomes of percutaneous coronary intervention for in-stent restenosis 14.
Intravascular imaging with intravascular ultrasound or optical coherence tomography determination of the potential mechanisms of in-stent restenosis can guide appropriate treatment. Both imaging modalities can identify stent under-expansion, presence of neointimal hyperplasia, and borders of the external elastic lamina for appropriate balloon sizing during post-dilation. optical coherence tomography has a superior axial resolution, and can further highlight the morphologic differences between bare metal stent-in-stent restenosis and DES-in-stent restenosis. Findings of homogenous, continuous tissue bands on optical coherence tomography is consistent with neointimal hyperplasia that is typical of bare metal stent-in-stent restenosis, while a focal, heterogeneous and layering appearance represents proteoglycan and fibrin deposition which occurs in the setting of neoatherosclerosis. Other specific findings that are suggestive of neoatherosclerosis include neointimal rupture, thin-cap fibroatheroma formation, microvessels, presence of lipid pools, macrophage accumulation, and evidence of non-occlusive thrombosis 15.
In a meta-analysis of thirteen studies with 1384 stents on double source computed tomography angiography and five studies including 622 stents on 320-row computed tomography angiography (320-row computed tomography angiography), the sensitivity, the specificity, and the area under the curve of double source computed tomography angiography in diagnosing in-stent restenosis were 0.92 (0.87 to 0.96), 0.91 (0.87 to 0.94), and 0.97 (0.95 to 0.98), respectively, and they were 0.91 (0.82 to 0.96), 0.95 (0.88 to 0.98), and 0.96 (0.94 to 0.97) for 320-row computed tomography angiography. This data suggested that both double source computed tomography angiography and 320-row computed tomography angiography had high diagnostic accuracy in detecting in-stent restenosis, especially for stent diameters of more than 3 mm, and may be appropriate tools for further evaluation of in-stent restenosis in the future 16.
Newer data fusion methodologies and hybrid, dual-probe catheters are being developed to allow complete and detailed evaluation of plaque morphology and pathophysiology, and for reliable identification of high-risk and vulnerable lesions. Examples of these dual-probe catheters are combined intravascular ultrasound-optical coherence tomography, near-infrared spectroscopy-intravascular ultrasound, optical coherence tomography-near-infrared spectroscopy, optical coherence tomography-near infrared fluorescence molecular imaging and intravascular ultrasound-near infrared fluorescence (intravascular ultrasound intravascular photoacoustic imaging, and combined fluorescence lifetime-intravascular ultrasound imaging). These multimodal approaches can provide a comprehensive visualization of plaque composition and plaque morphology and are useful for guiding optimal intervention decisions 17.
Several studies have indicated that asymptomatic patients with moderate disease severity in-stent restenosis are unlikely to benefit from routine reintervention. Assessment of fractional flow reserve during cardiac catheterization can assist in determining those that will benefit from revascularization. Prospective studies have shown that deferring revascularization in patients with in-stent restenosis and fractional flow reserve of over 0.75 is safe and appropriate 18.
Restenosis treatment
There are numerous treatment modalities available for the treatment of in-stent restenosis, and treatment often involves more than one technique. The optimal therapy is individualized for each case of in-stent restenosis and often based on the cause of in-stent restenosis and patient and lesion characteristics. Intravascular imaging allows for identification of the mechanism of in-stent restenosis and appropriate tailoring of treatment.
Medical therapy
Secondary prophylaxis with aspirin and statins in persons who have had percutaneous coronary intervention for coronary artery disease should follow current guideline recommendations. However, large clinical trials have not shown any medication with demonstrated efficacy in halting the progression of established in-stent restenosis. Evidence for drug therapy in treating in-stent restenosis is limited. Antiplatelet agents are used as per current guideline recommendations but are ineffective for specifically treating in-stent restenosis. Despite the initial promise, larger trials looking at the use of abciximab to prevent in-stent restenosis recurrence did not suggest a clinical benefit. Limited evidence also suggests that oral sirolimus, when given before re-intervention, may prevent repeat in-stent restenosis. However, the lack of long-term outcomes and the drug side-effect profile have shown sirolimus to be a poor option for the treatment of in-stent restenosis 9.
Plain old balloon angioplasty
Plain old balloon angioplasty (POBA) historically has been one of the primary treatment modalities used in the management of in-stent restenosis. Plain old balloon angioplasty frequently results in an acceptable acute angiographic outcome. Plain old balloon angioplasty is a particularly attractive option in focal areas of restenosis and when there is a documented evidence of stent/native artery size mismatch on intravascular imaging. Nevertheless, plain old balloon angioplasty is not without risk, and stent edge-related injuries and complications are always a concern whenever a stenotic site undergoes dilation. Balloon slippage is another element of Plain old balloon angioplasty which may not only prolong the procedure but can also lead to edge dissection and suboptimal outcomes. Incremental balloon upsizing as well as the use of short, low profile balloons can help avoid balloon slippage and edge-related complications. Although the acute angiographic results may show significant improvement, tissue re-intrusion can occur within minutes of the final balloon inflation; this is one of the factors that contribute to in-stent restenosis recurrence in up to 50% of patients treated with plain old balloon angioplasty 9.
Vascular brachytherapy
Brachytherapy involves temporary intracoronary deposition of a radioactive isotope within the diseased segment. The goal of treatment is to suppress new tissue growth within areas of established in-stent restenosis. This approach has demonstrated greater efficacy in halting in-stent restenosis progression, as well as improving clinical outcomes than debulking techniques or plain old balloon angioplasty. The complexity of the procedure and issues with radioprotection/radiation dosing limited this technique to a few expert centers. Based on the findings of both the Sin-stent restenosis (Sirolimus-Eluting Stents vs Vascular Brachytherapy for In-Stent Restenosis Within Bare-Metal Stents) and TAXUS V in-stent restenosis (Paclitaxel-Eluting Stents vs Vascular Brachytherapy for In-Stent Restenosis Within Bare-Metal Stents) trials, drug-eluting stent was superior in decreasing restenosis rates and need for revascularization as compared to brachytherapy extending out to 5 years. Due in large part to the results of these two studies, vascular brachytherapy has been essentially abandoned for the management of bare-metal stents-in-stent restenosis 19. Vascular brachytherapy, however, continues to play an important role in the treatment of drug-eluting stent in-stent restenosis, especially in lesions with multiple layers of the stent 20.
Cutting and scoring balloons
These balloons work by incising neointimal tissue, thereby allowing for better balloon anchoring during angioplasty. The cutting or scoring action also helps to prep the lesion before balloon angioplasty to allow for better tissue extrusion. However, the largest randomized trial to date to compare cutting balloon angioplasty to Plain old balloon angioplasty in the treatment of in-stent restenosis, Restenosis Cutting Balloon Evaluation (RESCUT) Trial, failed to show improvement in angiographic stenosis or the rate of clinical events during its seven-month follow-up. Cutting balloon angioplasty correlated with a need to use fewer balloons, less additional stenting, and a lower rate of balloon slippage. The ISAR-DESIRE IV trial (Intracoronary Stenting and Angiographic Results: Optimizing Treatment of Drug-Eluting Stent In-Stent Restenosis 4) subsequently evaluated the use of scoring balloons before paclitaxel-coated balloon angioplasty for treatment of drug-eluting stent-in-stent restenosis. The trial showed superior angiographic outcomes at six months in the scoring balloon arm but was underpowered to show any significant difference in clinical outcomes. At this time, there are no large randomized trials to show improvement in clinical outcomes with the use of either cutting or scoring balloons in the treatment of in-stent restenosis. Their use may be a consideration in those situations where balloon migration is of particular concern as well as with the presence of significant tissue 9.
Rotational atherectomy and Laser techniques
The development of debulking technologies such as rotational/directional atherectomy and excimer laser have allowed for a novel treatment approach to in-stent restenosis through physical removal of neointimal tissue or neoatherosclerotic plaque. The technique involves initial removal of excess stenotic tissue by the debulking device followed by low-pressure balloon post-dilation. However, a subsequent multicenter randomized trial comparing plain old balloon angioplasty to rotational atherectomy revealed lower restenosis rates, improved safety profile, and superior clinical outcomes in the plain old balloon angioplasty arm. As a result of this study, debulking technology is not considered to be a routine part of in-stent restenosis management. Instead, it is now a pre-treatment option for heavily calcified lesions which would otherwise compromise balloon expansion 9.
Repeat stenting with bare-metal stents
Although stenting may improve the short-term angiographic appearance of in-stent restenosis, two newly overlapping layers of stents (sandwich technique) with bare-metal stents may predispose to subsequent lumen loss. Patients with large vessel in-stent restenosis (greater than 3 mm) and patients with stent edge disease had better angiographic and clinical outcomes when they received repeat bare-metal stents placement as opposed to plain old balloon angioplasty. To date, no large randomized trials have been performed to evaluate the role of bare-metal stents placement in drug-eluting stent-in-stent restenosis and bare-metal stents should not be used as a primary option 21.
Repeat stenting with drug-eluting stent
Use of drug-eluting stent has thus become an attractive option in the treatment of neointimal hyperplasia seen in bare-metal stents-in-stent restenosis. Indeed, several large scale studies, including ISAR-DESIRE (Intracoronary Stenting and Angiographic Results: Drug-Eluting Stents for In-Stent Restenosis) and RIBS-II (Balloon Angioplasty vs Elective Sirolimus-Eluting Stenting), have shown that the use of drug-eluting stent significantly decreased restenosis rates, reduced neointimal proliferation as seen on intravascular ultrasound imaging, and improved clinical outcomes compared to balloon angioplasty. Some schools of thought have also suggested that using a stent with a different drug (hetero-drug-eluting stent approach) may help overcome the issue of drug resistance. The use of repeat stenting remains a common treatment strategy in the management of drug-eluting stent-in-stent restenosis. However, current data suggest that 10 to 20% of these patients will go on to develop a recurrence of in-stent restenosis. Innovative approaches, including the use of drug-coated balloons (drug-coated balloons) and bioresorbable vascular scaffolds, continue to be developed to avoid the drawback of multiple layers of stent struts; referred to as “onion skinning effect” 9. Despite the higher initial costs, recent meta-analyses have proved the cost-effectiveness of the second generation drug-eluting stent implantations. The cost-reduction in the long term was primarily due to avoidance of secondary revascularisations and absence of myocardial infarction 22.
Drug-coated balloons
The desire to deliver an anti-proliferative drug to the area of in-stent restenosis without leaving behind an additional layer of the stent has led to the development of drug-coated balloons. Although the data does not currently support the use of drug-coated balloons over bare-metal stents or drug-eluting stent for newly diagnosed obstructive atherosclerotic coronary artery disease, the use of drug-coated balloonss may have an important role in the management of both bare metal and drug-eluting in-stent restenosis. Importantly, the overall non-inferior outcomes with drug-coated balloons use as compared with drug-eluting stent placement supports the use of drug-coated balloons for treatment of bare-metal stents-in-stent restenosis, particularly in situations where it is preferable to avoid additional stent layering, or there are concerns for bleeding. The use of drug-coated balloons for the management of drug-eluting stent-in-stent restenosis has also been a topic of research. Similar to other treatment modalities, evidence suggests that drug-coated balloons therapy is likely more effective for bare-metal stents-in-stent restenosis than drug-eluting stent-in-stent restenosis, highlighting the therapeutic challenges presented by drug-eluting stent-in-stent restenosis 23.
As with bare-metal stents-in-stent restenosis, initial data supporting the use of drug-coated balloons in drug-eluting stent-in-stent restenosis came from a small single-center study.[38] Subsequently, two large multicenter randomized trials have provided important comparative data for drug-coated balloons use over both first and second-generation drug-eluting stent. The ISAR-DESIRE 3 (Efficacy Study of Paclitaxel-Eluting Balloon, Stent vs. Plain Angioplasty for Drug-Eluting Stent Restenosis) trial compared drug-coated balloons therapy to both 1st generation drug-eluting stent and Plain old balloon angioplasty. Results of the study revealed that drug-coated balloons application was non-inferior to drug-eluting stent placement, and both were superior to plain old balloon angioplasty 24. Perhaps the more clinically relevant study is the Restenosis Intra-stent of Drug-eluting Stents: Paclitaxel-Eluting Balloon vs. Everolimus-Eluting Stent (RIBS-IV) trial 25, which compared second-generation everolimus-eluting stents to drug-coated balloons angioplasty for treatment of drug-eluting stent-in-stent restenosis. Second-generation drug-eluting stent showed better angiographic and clinical outcomes than drug-coated balloons, thereby suggesting that in situations where there is no clear contraindication to repeat stenting, drug-eluting stent strategy may be the preferred option 9.
Bioresorbable vascular scaffolds
The use of bioabsorbable scaffolds allows for the delivery of an anti-proliferative drug to the area of in-stent restenosis similar to that seen in drug-coated balloons angioplasty. Unlike drug-coated balloons, however, the scaffold also prevents early lumen loss associated with tissue retraction seen in balloon angioplasty. Furthermore, unlike the traditional drug-eluting stent architecture, the bio-absorbable nature of the scaffold avoids permanent deposition of an additional layer of stent strut; potentially reducing the risk of restenosis. These characteristics make bioresorbable vascular scaffolds an attractive option in the management of in-stent restenosis. The minimal lumen diameter after bioresorbable vascular scaffolds was comparable to that obtained with drug-coated balloons (1.88 +/- 0.6 mm) but smaller than that achieved after Everolimus-Eluting Stent (everolimus-eluting stent) (2.16 +/- 0.7 mm). Similarly, target lesion revascularization rates after bioresorbable vascular scaffolds were similar to those seen with drug-coated balloons (10.4%) but higher than with everolimus-eluting stent (3.2%). bioresorbable vascular scaffolds obtained late angiographic and clinical results similar to drug-coated balloons but inferior to everolimus-eluting stent 26.
Comparing all treatment modalities
Treatment options for in-stent restenosis are in constant evolution, and despite the large volume of published data on the various treatment modalities for in-stent restenosis, it is difficult to determine which method is the gold standard as most studies compare one or at most two established modalities to the novel approach. Two recent large network meta-analyses have tried to clarify which strategy has the best evidence behind it.
Siontis et al. 27 identified 27 trials with a total of 5923 patients. The primary outcome of this meta-analysis was percent diameter stenosis at the longest available follow-up (6 months to 1 year). Secondary endpoints included binary restenosis (presence or absence of stenosis), rates of target lesion revascularization, myocardial infarction or death. in-stent restenosis therapies included plain old balloon angioplasty, debulking techniques, brachytherapy, bare-metal stents, drug-eluting stent, and drug-coated balloons. Repeat stenting with everolimus-eluting stents showed statistical superiority over other methods in the primary outcome, binary restenosis rates, and target lesion revascularization. drug-coated balloons was the second most preferable approach but did not achieve a significant difference over sirolimus or paclitaxel-eluting stents.
Giacoppo et al. 28 included 24 trials for a total of 4880 patients. One clinical (target lesion revascularization) and one angiographic (late lumen loss) primary outcome were predefined for this analysis. The included in-stent restenosis-therapies were similar to those evaluated by Siontis. Both drug-coated balloons and drug-eluting stent showed superiority to other approaches based on the predefined clinical outcome, and in a head-to-head comparison, there was no significant difference. Angiographic outcomes again favored drug-coated balloons or drug-eluting stent over all other modalities, however late tissue loss was slightly lower in the drug-coated balloons arm when compared to drug-eluting stent. When compared to available options for treatments of coronary in-stent restenosis, second-generation drug-coated balloons, and drug-eluting stents were associated with superior angiographic and clinical outcomes, with similar comparative efficacy 27.
Restenosis prognosis
The advent of second-generation drug-eluting stents has dramatically improved the outcome of percutaneous coronary intervention, with an in-stent restenosis rate of approximately 5 to 10%. Patients that follow exercise and diet cardiac rehabilitation programs, are medically optimized and are compliant with appropriate dual antiplatelet therapy can help to minimize adverse events. The occurrence of clinical adverse events closely correlates with the final stent area, and maximizing the minimal stent area can help to mitigate adverse events 29.
References- Omeh DJ, Shlofmitz E. Restenosis. [Updated 2019 Aug 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545139
- Pleva L, Kukla P, Hlinomaz O. Treatment of coronary in-stent restenosis: a systematic review. J Geriatr Cardiol. 2018 Feb;15(2):173-184.
- Piraino D, Cimino G, Buccheri D, Dendramis G, Andolina G, Cortese B. Recurrent in-stent restenosis, certainty of its origin, uncertainty about treatment. Int. J. Cardiol. 2017 Mar 01;230:91-96.
- Buccheri D, Piraino D, Andolina G, Cortese B. Understanding and managing in-stent restenosis: a review of clinical data, from pathogenesis to treatment. J Thorac Dis. 2016 Oct;8(10):E1150-E1162
- Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999 Nov 02;100(18):1872-8.
- Cassese S, Byrne RA, Schulz S, Hoppman P, Kreutzer J, Feuchtenberger A, Ibrahim T, Ott I, Fusaro M, Schunkert H, Laugwitz KL, Kastrati A. Prognostic role of restenosis in 10 004 patients undergoing routine control angiography after coronary stenting. Eur. Heart J. 2015 Jan 07;36(2):94-9.
- Wang Z, Liu C, Fang H. Blood Cell Parameters and Predicting Coronary In-Stent Restenosis. Angiology. 2019 Sep;70(8):711-718.
- Verschuren JJ, Trompet S, Postmus I, Sampietro ML, Heijmans BT, Houwing-Duistermaat JJ, Slagboom PE, Jukema JW. Systematic testing of literature reported genetic variation associated with coronary restenosis: results of the GENDER Study. PLoS ONE. 2012;7(8):e42401
- Nicolais C, Lakhter V, Virk HUH, Sardar P, Bavishi C, O’Murchu B, Chatterjee S. Therapeutic Options for In-Stent Restenosis. Curr Cardiol Rep. 2018 Feb 12;20(2):7.
- Kim MS, Dean LS. In-stent restenosis. Cardiovasc Ther. 2011 Jun;29(3):190-8.
- Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J. Am. Coll. Cardiol. 2011 Mar 15;57(11):1314-22.
- Looser PM, Kim LK, Feldman DN. In-Stent Restenosis: Pathophysiology and Treatment. Curr Treat Options Cardiovasc Med. 2016 Feb;18(2):10.
- Magalhaes MA, Minha S, Chen F, Torguson R, Omar AF, Loh JP, Escarcega RO, Lipinski MJ, Baker NC, Kitabata H, Ota H, Suddath WO, Satler LF, Pichard AD, Waksman R. Clinical presentation and outcomes of coronary in-stent restenosis across 3-stent generations. Circ Cardiovasc Interv. 2014 Dec;7(6):768-76.
- Shlofmitz E, Kuku KO, Waksman R, Garcia-Garcia HM. Intravascular ultrasound-guided drug-eluting stent implantation. Minerva Cardioangiol. 2019 Aug;67(4):306-317.
- Liu ZJ, Shi B, Deng CC, Xu GX, Zhao RZ, Shen CY, Wang ZL, Liu HL. [Analysis of optical coherence tomography of early and very late stent restenosis after drug-eluting stent implantation]. Zhonghua Yi Xue Za Zhi. 2017 Jun 20;97(23):1778-1783.
- Liu HF, Wang M, Xu YS, Shrestha MK, Lu XR, Lei JQ. Diagnostic accuracy of dual-source and 320-row computed tomography angiography in detecting coronary in-stent restenosis: a systematic review and meta-analysis. Acta Radiol. 2019 Feb;60(2):149-159.
- Bourantas CV, Jaffer FA, Gijsen FJ, van Soest G, Madden SP, Courtney BK, Fard AM, Tenekecioglu E, Zeng Y, van der Steen AFW, Emelianov S, Muller J, Stone PH, Marcu L, Tearney GJ, Serruys PW. Hybrid intravascular imaging: recent advances, technical considerations, and current applications in the study of plaque pathophysiology. Eur. Heart J. 2017 Feb 07;38(6):400-412.
- Nam CW, Rha SW, Koo BK, Doh JH, Chung WY, Yoon MH, Tahk SJ, Lee BK, Lee JB, Yoo KD, Cho YK, Chung IS, Hur SH, Kim KB, Choi CU, Oh DJ. Usefulness of coronary pressure measurement for functional evaluation of drug-eluting stent restenosis. Am. J. Cardiol. 2011 Jun 15;107(12):1783-6.
- Holmes DR, Teirstein P, Satler L, Sketch M, O’Malley J, Popma JJ, Kuntz RE, Fitzgerald PJ, Wang H, Caramanica E, Cohen SA., SISR Investigators. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. JAMA. 2006 Mar 15;295(11):1264-73.
- Waksman R, Iantorno M. Refractory In-Stent Restenosis: Improving Outcomes by Standardizing Our Approach. Curr Cardiol Rep. 2018 Oct 22;20(12):140.
- Kastrati A, Mehilli J, von Beckerath N, Dibra A, Hausleiter J, Pache J, Schühlen H, Schmitt C, Dirschinger J, Schömig A., ISAR-DESIRE Study Investigators. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005 Jan 12;293(2):165-71.
- Baschet L, Bourguignon S, Marque S, Durand-Zaleski I, Teiger E, Wilquin F, Levesque K. Cost-effectiveness of drug-eluting stents versus bare-metal stents in patients undergoing percutaneous coronary intervention. Open Heart. 2016;3(2):e000445
- Habara S, Iwabuchi M, Inoue N, Nakamura S, Asano R, Nanto S, Hayashi Y, Shiode N, Saito S, Ikari Y, Kimura T, Hosokawa J, Nakamura M, Kotani J, Kozuma K, Mitsudo K. A multicenter randomized comparison of paclitaxel-coated balloon catheter with conventional balloon angioplasty in patients with bare-metal stent restenosis and drug-eluting stent restenosis. Am. Heart J. 2013 Sep;166(3):527-33.
- Byrne RA, Neumann FJ, Mehilli J, Pinieck S, Wolff B, Tiroch K, Schulz S, Fusaro M, Ott I, Ibrahim T, Hausleiter J, Valina C, Pache J, Laugwitz KL, Massberg S, Kastrati A., ISAR-DESIRE 3 investigators. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet. 2013 Feb 09;381(9865):461-7.
- Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, García del Blanco B, García-Touchard A, López-Minguéz JR, Benedicto A, Masotti M, Zueco J, Iñiguez A, Velázquez M, Moreno R, Mainar V, Domínguez A, Pomar F, Melgares R, Rivero F, Jiménez-Quevedo P, Gonzalo N, Fernández C, Macaya C., RIBS IV Study Investigators (under auspices of Interventional Cardiology Working Group of Spanish Society of Cardiology). A Prospective Randomized Trial of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis of Drug-Eluting Stents: The RIBS IV Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015 Jul 07;66(1):23-33.
- Moscarella E, Tanaka A, Ielasi A, Cortese B, Coscarelli S, De Angelis MC, Piraino D, Latib A, Grigis G, Bianchi R, Buccheri D, Calabrò P, Tespili M, Silva Orrego P, Colombo A, Varricchio A. Bioresorbable vascular scaffold versus everolimus-eluting stents or drug eluting balloon for the treatment of coronary in-stent restenosis: 1-Year follow-up of a propensity score matching comparison (the BIORESOLVE-ISR Study). Catheter Cardiovasc Interv. 2018 Oct 01;92(4):668-677.
- Siontis GC, Stefanini GG, Mavridis D, Siontis KC, Alfonso F, Pérez-Vizcayno MJ, Byrne RA, Kastrati A, Meier B, Salanti G, Jüni P, Windecker S. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet. 2015 Aug 15;386(9994):655-64.
- Giacoppo D, Colleran R, Cassese S, Frangieh AH, Wiebe J, Joner M, Schunkert H, Kastrati A, Byrne RA. Percutaneous Coronary Intervention vs Coronary Artery Bypass Grafting in Patients With Left Main Coronary Artery Stenosis: A Systematic Review and Meta-analysis. JAMA Cardiol. 2017 Oct 01;2(10):1079-1088.
- Goto K, Zhao Z, Matsumura M, Dohi T, Kobayashi N, Kirtane AJ, Rabbani LE, Collins MB, Parikh MA, Kodali SK, Leon MB, Moses JW, Mintz GS, Maehara A. Mechanisms and Patterns of Intravascular Ultrasound In-Stent Restenosis Among Bare Metal Stents and First- and Second-Generation Drug-Eluting Stents. Am. J. Cardiol. 2015 Nov 01;116(9):1351-7.