Serum sickness
Serum sickness is a rare allergic reaction to heterologous or foreign protein or serum, usually in response to an injection. Serum sickness is an immune-complex-mediated hypersensitivity reaction or type 3 hypersensitivity reaction, that it is characterized by symptoms such as fever, skin rash, pain and swelling in one or more joints, and kidney damage 1. The symptoms typically occur one to two weeks after exposure to an offending agent and resolve within several weeks of discontinuation 2. The molecular size, charge, structure, amount, and valence of the antigen involved influence the type of immune complexes formed 3. The symptoms of serum sickness arise as a result of the formation of immune complexes between human proteins and heterologous (nonhuman) proteins. Medications containing heterologous antigens are the most common cause of serum sickness and include vaccinations (i.e., Rabies), immune modulating agents (i.e., rituximab, infliximab) and anti-venoms 4. One study of 72,000 patients found that serum sickness was found in less than 0.5 percent of children under ten years who received human and equine anti-rabies globulins 5. Certain patient populations are considered to be at a higher risk for developing serum sickness following rituximab infusion, including those with hypergammaglobulinemia and Hepatitis C-related cryoglobulinemia vasculitis 6.
Plasma is the clear fluid portion of blood. It does not contain blood cells. But it does contain many proteins, including antibodies, which are formed as part of the immune response to protect against infection. Antiserum is produced from the plasma of a person or animal that has immunity against an infection or poisonous substance. Antiserum may be used to protect a person who has been exposed to a germ or toxin. For example, you may receive a certain type of antiserum injection:
- If you have been exposed to tetanus or rabies and have never been vaccinated against these germs. This is called passive immunization.
- If you have been bitten by a snake that produces a dangerous toxin.
During serum sickness, the immune system falsely identifies a protein in antiserum as a harmful substance (antigen). The result is an immune system response that attacks the antiserum. Immune system elements and the antiserum combine to form immune complexes, which cause the symptoms of serum sickness.
Injected proteins such as antithymocyte globulin (used to treat organ transplant rejection) and rituximab (used to treat immune disorders and cancers) can cause serum sickness reactions.
Blood products may also cause serum sickness.
Serum sickness is not to be confused with a serum sickness-like reaction, which results in a similar clinical presentation but does not involve the formation of immune complexes 1. The most common offending medications include penicillins, cephalosporins (most commonly cefaclor), sulfonamides, bupropion, fluoxetine, nonsteroidal anti-inflammatory drugs (NSAIDs) and thiouracil 7. Also, several infections can lead to the development of serum sickness-like reaction, including streptococcus and hepatitis B 8. These reactions typically occur 1 to 3 weeks after exposure to the drug, but may occur as early as 1 to 24 hours afterward. Accelerated reactions are T-cell mediated, although an IgE mechanism cannot always be ruled out 9.
Serum sickness is a self-limited disease process with an excellent prognosis 1. Although occasional reports show mortality resulting from progressive glomerulonephritis or severe neurological complications 10. Withdrawal of the offending agent is the mainstay of treatment in serum sickness. Anti-inflammatory drugs and antihistamines provide symptomatic relief. Severe cases with multisystem involvement with significant symptoms 11 may warrant a brief course of corticosteroids. In some cases, plasmapheresis can attenuate serum sickness 12.
Serum sickness is an example of the type 3 or immune complex–mediated hypersensitivity disease. After the initial exposure to a foreign antigen in the absence of a preexisting antibody, serum sickness can develop within approximately 6-10 days 2. Upon subsequent exposure, however, serum sickness develops sooner. The disease appears as the antibody formation begins, and the pathogenesis of serum sickness is related to protracted interaction between antigen and antibody in the circulation, with antigen-antibody complex formation in an environment of antigen excess.
The immunologic interactions observed in serum sickness occur when antigens capable of remaining in the circulation for long periods incite antibody formation 13. Typically, serum protein molecules are removed from the circulation by nonimmune processes that are not yet completely understood.
Immune complex formation is a common event and does not typically cause symptoms 14. Small complexes usually circulate without triggering inflammation, and large complexes are cleared by the reticuloendothelial system. However, intermediate-sized complexes that develop in the context of slight antigen excess may deposit in blood vessel walls and tissues, where they induce vascular and tissue damage resulting from activation of complement and granulocytes 15.
Endothelial cells increase the expression of adhesion molecules, and monocytes and macrophages release proinflammatory cytokines. Subsequently, additional inflammatory cells are recruited, and necrosis of the small vessels develops. Complement activation promotes chemotaxis and adherence of neutrophils to the site of immune complex deposition. This may be facilitated by increased vascular permeability due to release of vasoactive amines from tissue mast cells 15.
At this point, complement levels fall to half their levels prior to the antibody response 13. This clinicopathological syndrome usually develops within 1-2 weeks of antigen injection.
Free antigen continues to clear from the blood, leading to antibody excess and the formation of large immune complexes, which are quickly removed by circulating macrophages. Finally, the antigen is no longer detectable, and the level of circulating antibodies continues to rise. Clinical recovery is usually apparent after 7-28 days, as intermediate-sized immune complexes are cleared by the reticuloendothelial system.
Secondary serum sickness is the result of antigen recognition by presensitized cells of the immune system. It is characterized by a shorter latent period, exaggerated symptoms, and a brief clinical course.
Why immune complex disease occurs under certain circumstances is not known. Possible factors may include high levels of immune complexes and a relative deficiency of some complement components leading to a decreased ability to eliminate immune complexes 14.
Not all substances that are recognized as foreign by the immune system elicit an immune response. The antigen must be of characteristic size or have specific antigenic determinants and physiological properties to be an effective stimulator of the immune system.
After an appropriate antigen is introduced, an individual’s immune system responds by synthesizing antibodies after 4-10 days. The antibody reacts with the antigen, forming soluble circulating immune complexes that may diffuse into the vascular walls, where they may initiate fixation and activation of complement.
Complement-containing immune complexes generate an influx of polymorphonuclear leukocytes into the vessel wall, where proteolytic enzymes that can mediate tissue damage are released. Immune complex deposition and the subsequent inflammatory response are responsible for the widespread vasculitic lesions seen in serum sickness.
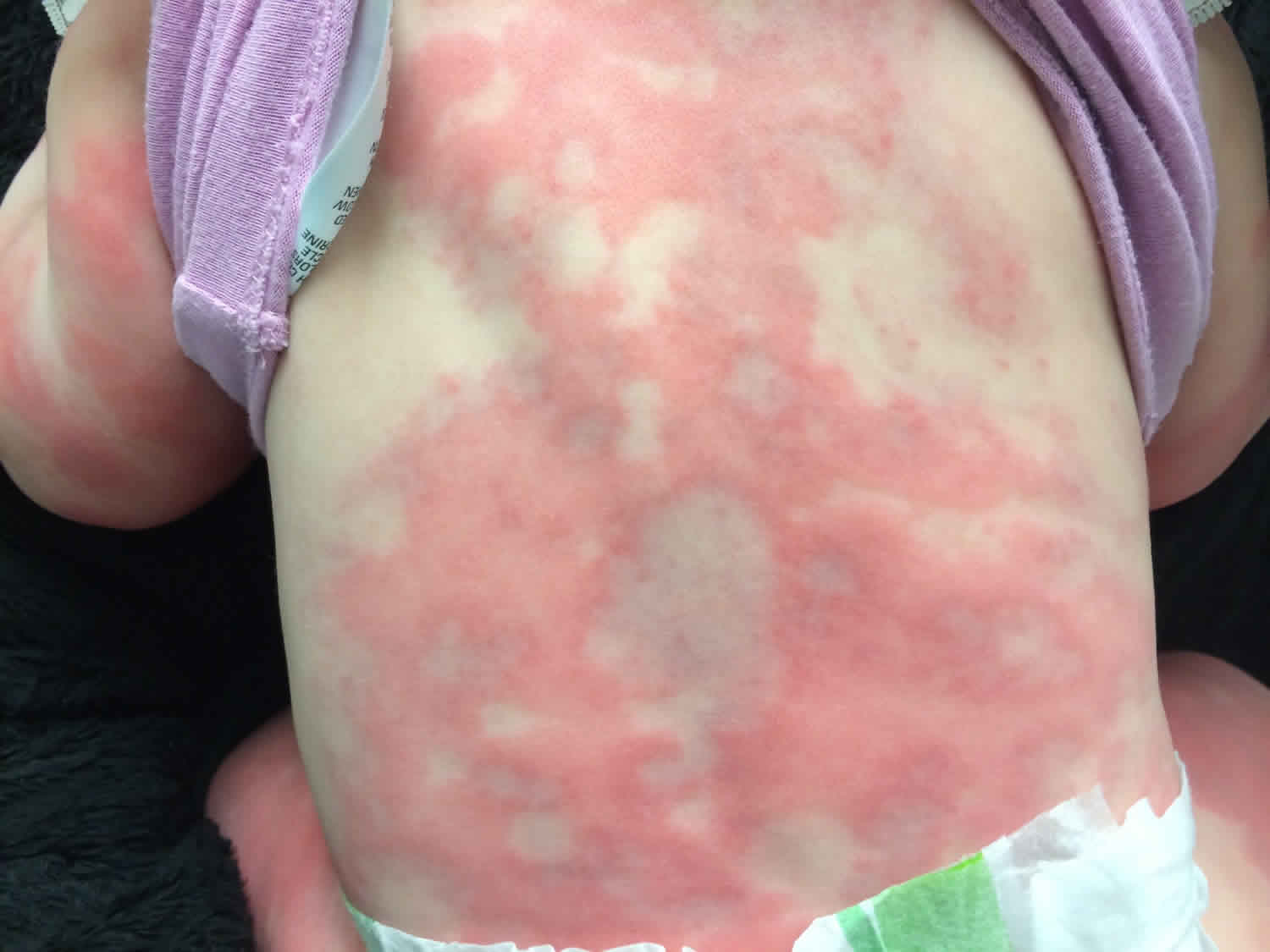
Figure 1. Serum sickness rash
Serum sickness causes
Currently, the most common cause of serum sickness and serum sickness–like is hypersensitivity reaction to drugs 12. Drugs containing proteins of other species include the following:
- Antitoxins
- Antivenoms 16
- Hormones from other species
- Streptokinase
- Vaccines
Polyclonal and monoclonal antibodies prepared from horse, rabbit, or mouse serum (eg, antithymocyte globulin, OKT-3) have also been found to cause serum sickness 17.
Antibiotics and other antimicrobials that can cause serum sickness-like reactions include the following:
- Cephalosporins
- Ciprofloxacin
- Furazolidone (Furoxone)
- Griseofulvin
- Lincomycin
- Metronidazole
- Para-aminosalicylic acid
- Penicillins
- Streptomycin
- Sulfonamides
- Tetracyclines
Other drugs associated with serum sickness include the following:
- Allopurinol
- Barbiturates
- Bupropion (Zyban, Wellbutrin SR) 18
- Captopril (Capoten)
- Carbamazepine
- Fluoxetine
- Gold salts
- Halothane
- Hydantoins (eg, phenytoin)
- Hydralazine (Apresoline)
- Indomethacin
- Iodides
- Iron dextran
- Methimazole
- Methyldopa
- Penicillamine
- Procainamide
- Procarbazine
- Propranolol
- Thiouracil
Monoclonal antibodies have been reported to cause serum sickness–like syndrome. These include infliximab (Remicade), which is used to treat Crohn disease and rheumatoid arthritis 19; omalizumab, which is used to treat allergy-related asthma 20; and rituximab, which is used to treat various diseases, including rheumatologic disorders, mixed cryoglobulinemia, and lymphoma 21.
Stings from insects in the order Hymenoptera (eg, bees, wasps), mosquitoes, and tick bites may cause serum sickness 22.
Infectious diseases involving circulating immune complexes (eg, hepatitis B, infective endocarditis) may cause serum sickness–like reactions. These conditions are often associated with circulating cryoglobulins.
Serum sickness symptoms
Serum sickness develops 1-3 weeks after initial administration of the causative agent (in many cases a medication) but can occur within 12-36 hours in individuals who have been previously sensitized through an antecedent exposure 12.
Symptoms described in serum sickness include the following 12:
- Fever/malaise – 100%
- Cutaneous eruptions (rash) – 93%
- Arthralgias – 77%
- Gastrointestinal complaints – 67%
- Headaches – 57%
- Myalgias – 37%
- Blurred vision – 37%
- Dyspnea/wheezing – 20%
- Lymphadenopathy – 17%
Specific gastrointestinal symptoms may include abdominal pain, nausea, vomiting, or diarrhea 3. Chest pain or breathlessness due to pleuritis, pericarditis, or myocarditis is possible but rare.
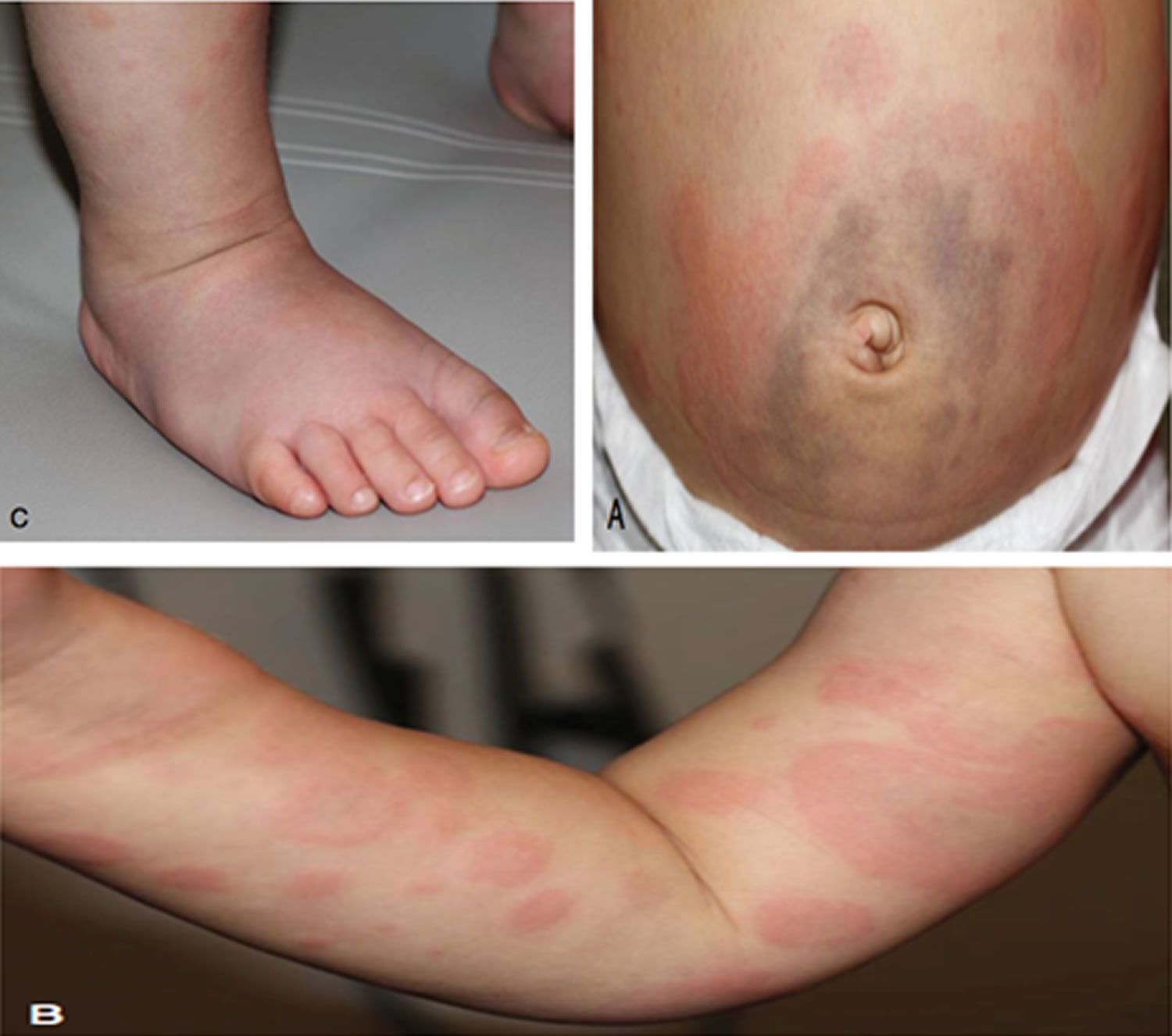
Physical examination should be carefully undertaken to determine the severity of symptoms and signs of systemic illness. A close inspection of the rash may reveal an urticarial, maculopapular or vasculitic (purpuric) eruption. Importantly, the mucous membranes are not involved and can be helpful in distinguishing serum sickness from Stevens-Johnson syndrome or toxic epidermal necrolysis. Most rashes associated with serum sickness are urticarial (92%) and/or serpiginous 3. They typically start on the anterior lower trunk or the periumbilical or axillary regions and spread to the back, upper trunk, and extremities 15. In the extremities, eruptions occur at the junction of the palmar or plantar skin with the dorsolateral surface of the hands, feet, fingers, and toes.
Morbilliform or scarlatiniform rash, palpable purpura, erythema simplex, or erythema multiforme are less common. Pruritus and erythema are possible at injection sites. Edema can be limited to site of injection but can also be observed in the face 3.
If associated with subcutaneous injection, the rash may first appear around the site of the injection. The rash typically takes days to weeks to resolve once the offending agent is discontinued.
Lymphadenopathy (10-20%) may be generalized or may involve tenderness in the nodes that drain the injection site; splenomegaly may occur.
Arthralgias are more common than frank arthritis, but either may occur. The hands, feet, ankles, knees, and shoulders are most commonly affected. Arthritis (10%-50%) is usually in the metacarpophalangeal and knee joints and usually symmetrical 11. Occasionally, small joints, joints of the spine, and the temporomandibular joint may be inflamed. Myalgias or myositis also may occur.
Less common findings on the physical exam include edema, particularly about the face and neck and hands, lymphadenopathy, headache or blurry vision, splenomegaly, anterior uveitis, peripheral neuropathy, nephropathy, and vasculitis 23. Of note, these systemic symptoms are less likely in serum sickness-like reaction, which is usually limited to fever, arthralgias, rash/urticaria, and pruritis.
Renal manifestations include proteinuria, microscopic hematuria, and oliguria; however, significant disease usually does not result.
Cardiovascular findings may include myocardial and pericardial inflammation. Generalized vasculitis occurs rarely.
Neurologic manifestations include the following 3:
- Peripheral neuropathy
- Brachial plexus neuritis
- Optic neuritis
- Cranial nerve palsies
- Guillain-Barré syndrome
- Encephalomyelitis (rare)
Pulmonary manifestations, such as pleurisy, are rare. However, dyspnea and cyanosis are not uncommon.
Serum sickness diagnosis
A thorough history and physical exam are essential in the evaluation of suspected serum sickness. The history should focus on identification of an offending agent within the two weeks before the onset of symptoms, or, in the case of potential repeat exposure, within the few days before presentation. Also, a complete review of systems, with a focus on each organ system and severity of symptoms, should be performed to evaluate for other potential causes of the patient’s presentation.
The evaluation of well-appearing patients with suspected serum sickness can be limited to urinalysis to determine the presence of kidney involvement, which would require close follow-up. However, if the patient is ill-appearing or if there is any degree of diagnostic uncertainty based on the history or physical exam, further testing should be performed. The clinician should consider the following laboratory tests to evaluate for other etiologies and multi-organ system involvement: complete blood count with differential, erythrocyte sedimentation rate, C-reactive protein, total hemolytic complement (CH50), C3, C4, basic metabolic panel, liver transaminases, antinuclear antibody, and rheumatoid factor 24. Depending on the clinical history, testing for infectious diseases may include hepatitis B screen and heterophile antibody testing for Ebstein Barr virus (EBV). If carditis is suspected, an electrocardiogram should be obtained. Stool hematocrit should be obtained in any patient with gastrointestinal symptoms. Neuroimaging with a computed tomography scan should be considered in patients with neurological complaints.
Laboratory results in serum sickness may be widely variable. The complete blood count can show leukopenia or mild leukocytosis. Serum creatinine may be elevated, but typically returns to baseline within days-weeks of discontinuing the offending agent. Inflammatory markers will be elevated in serum sickness. Complement levels including CH50, C3, and C4, will be decreased, reflecting activation and consumption of complement.
Recall that patients with serum sickness-like reaction will generally not manifest with additional multi-organ system symptoms as outlined above. Also, the lab results in a serum sickness-like reaction will not show hypocomplementemia or renal dysfunction 25.
Serum sickness treatment
Serum sickness is a self-limited entity that will usually resolve upon discontinuation of the offending agent, with or without additional treatment 1. Treatment is generally aimed at reducing symptom severity and removing the agent, or reducing exposure to it if complete removal is not possible (for example, potentially life-saving anti-thymocyte globulin in the treatment of aplastic anemia) 26. For mild or moderate presentations, symptomatic relief can be achieved with NSAIDs and/or antihistamines. The patient should be counseled that the rash and pruritis should stop progressing within 48 hours of initiation of these medications. For more severe symptoms, a 7 to 10-day course of 0.5 – 2 mg/kg of systemic glucocorticoids can be helpful 27. Most children can be safely treated as outpatients, but inpatient hospitalization should be considered for those with severe symptoms, multi-organ system involvement or evidence of an underlying infection or more serious cause.
Serum sickness prognosis
Serum sickness is typically self-limited and resolves within within 1-2 weeks following discontinuation of the offending agent 12. The prognosis of serum sickness and serum sickness-like reaction is excellent in patients without internal organ involvement 14. Those with severe symptoms, recurrent serum sickness, or ongoing exposure to the causative agent may experience a prolonged course of illness. Although occasional reports show mortality resulting from progressive glomerulonephritis or severe neurological complications.
Generally, serum sickness resolves on its own and does not lead to any long-term complications.
Complications of serum sickness may include the following 10:
- Vasculitis
- Neuropathy
- Acute renal failure
- Glomerulonephritis (rare)
- Anaphylaxis
- Shock.
To date, there have been no large-scale studies demonstrating any long-standing consequences of serum sickness. However, repeat exposure to a causative agent leading to multiple episodes of serum sickness has caused renal failure and death in animal models 28. However, studies of patients receiving anti-thymocyte globulin in the treatment of aplastic anemia, who developed serum sickness, have demonstrated more favorable outcomes 23.
References- Rixe N, Tavarez MM. Serum Sickness. [Updated 2019 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538312
- Lawley TJ, Bielory L, Gascon P, Yancey KB, Young NS, Frank MM. A prospective clinical and immunologic analysis of patients with serum sickness. N. Engl. J. Med. 1984 Nov 29;311(22):1407-13.
- Mannik M. Serum sickness and pathophysiology of immune complexes. Rich RR, ed. Clinical Immunology Principles and Practice. St. Louis, Mo: Mosby; 1996. 1062-71.
- Schaeffer TH, Khatri V, Reifler LM, Lavonas EJ. Incidence of immediate hypersensitivity reaction and serum sickness following administration of Crotalidae polyvalent immune Fab antivenom: a meta-analysis. Acad Emerg Med. 2012 Feb;19(2):121-31.
- Suwansrinon K, Jaijareonsup W, Wilde H, Benjavongkulchai M, Sriaroon C, Sitprija V. Sex- and age-related differences in rabies immunoglobulin hypersensitivity. Trans. R. Soc. Trop. Med. Hyg. 2007 Feb;101(2):206-8.
- Sène D, Ghillani-Dalbin P, Amoura Z, Musset L, Cacoub P. Rituximab may form a complex with IgMkappa mixed cryoglobulin and induce severe systemic reactions in patients with hepatitis C virus-induced vasculitis. Arthritis Rheum. 2009 Dec;60(12):3848-55.
- Misirlioglu ED, Duman H, Ozmen S, Bostanci I. Serum sickness-like reaction in children due to cefditoren. Pediatr Dermatol. 2012 May-Jun;29(3):327-8.
- Arkachaisri T. Serum sickness and hepatitis B vaccine including review of the literature. J Med Assoc Thai. 2002 Aug;85 Suppl 2:S607-12.
- Torres MJ, Salas M, Ariza A, Fernández TD. Understanding the mechanisms in accelerated drug reactions. Curr Opin Allergy Clin Immunol. 2016 Aug. 16 (4):308-14.
- Serum Sickness. https://emedicine.medscape.com/article/332032-overview
- Erffmeyer JE. Serum sickness. Ann Allergy. 1986 Feb. 56(2):105-9.
- Frank MM, Hester CG. Immune Complex–Mediated Diseases. Adkinson NF Jr, Bochner BS, Burks W, et al, Eds. Middleton’s Allergy Principles and Practice. 8th ed. Philadelphia PA: Saunders; 2014. 602-16.
- Dixon FJ, Cochrane CC. Immune complex injury. Samter M, ed. Immunological Diseases. 4th ed. New York, NY: Little, Brown and Company; 1988. 233.
- Pichler WJ. Drug hypersensitivity. Rich RR, ed. Clinical Immunology Principles and Practice. 4th ed. St Louis, Mo: Elsevier/Saunders; 2013. 564-77.
- Sicherer SH, Leung DYM. Serum sickness. Kliegman, ed. Nelson Textbook of Pediatrics. 18th ed. Online Edition, Chapter 149.
- Ryan NM, Kearney RT, Brown SG, Isbister GK. Incidence of serum sickness after the administration of Australian snake antivenom (ASP-22). Clin Toxicol (Phila). 2016. 54 (1):27-33.
- Lawley TJ, Bielory L, Gascon P, Yancey KB, Young NS, Frank MM. A prospective clinical and immunologic analysis of patients with serum sickness. N Engl J Med. 1984 Nov 29. 311(22):1407-13.
- Wooltorton E. Bupropion (Zyban, Wellbutrin SR): reports of deaths, seizures, serum sickness. CMAJ. 2002 Jan 8. 166(1):68.
- Vermeire S, Van Assche G, Rutgeerts P. Serum sickness, encephalitis and other complications of anti-cytokine therapy. Best Pract Res Clin Gastroenterol. 2009. 23(1):101-12.
- Eapen A, Kloepfer KM. Serum sickness-like reaction in a pediatric patient using omalizumab for chronic spontaneous urticaria. Pediatr Allergy Immunol. 2018 Jun. 29 (4):449-450.
- Cheong J, Ooi K. Rituximab-induced serum sickness in the treatment of idiopathic membranous nephropathy. Clin Kidney J. 2018 Feb. 11 (1):51-53.
- Lazoglu AH, Boglioli LR, Taff ML, Rosenbluth M, Macris NT. Serum sickness reaction following multiple insect stings. Ann Allergy Asthma Immunol. 1995 Dec. 75(6 Pt 1):522-4.
- Bielory L, Gascon P, Lawley TJ, Young NS, Frank MM. Human serum sickness: a prospective analysis of 35 patients treated with equine anti-thymocyte globulin for bone marrow failure. Medicine (Baltimore). 1988 Jan;67(1):40-57.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann. Allergy Asthma Immunol. 2010 Oct;105(4):259-273.
- Zhang Z, Xiang Y, Wang B, Chen H, Cai X, Wang X, Mei L, Zheng Y. Intestinal mucosal permeability of children with cefaclor-associated serum sickness-like reactions. Eur. J. Pediatr. 2013 Apr;172(4):537-43.
- Bayraktar F, Akinci B, Demirkan F, Yener S, Yesil S, Kirmaz C, Comlekci A. Serum sickness-like reactions associated with type III insulin allergy responding to plasmapheresis. Diabet. Med. 2009 Jun;26(6):659-60.
- Clark BM, Kotti GH, Shah AD, Conger NG. Severe serum sickness reaction to oral and intramuscular penicillin. Pharmacotherapy. 2006 May;26(5):705-8.
- Wilson CB, Dixon FJ. Quantitation of acute and chronic serum sickness in the rabbit. J. Exp. Med. 1971 Sep 01;134(3 Pt 2):7s-8s.