Stunted growth
Stunted growth also called stunting or stunted development, is when a child fails to meet the expected height or weight for their age. Stunted growth is caused by malnutrition, repeated infections or in some cases, both and/or inadequate psychosocial stimulation 1. The World Health Organization (WHO) categorizes children who are stunted as those whose length or height is lower than average for their age, and at least two standard deviations below the WHO’s Child Growth Standards Median 2. Stunted growth process is thought to begin prior to birth 3. Maternal undernutrition, along with maternal stunting (often due to past undernutrition), infections and other forms of deprivation, contributes to intrauterine growth restriction (IUGR). A recent analysis of Demographic and Health Surveys data from 54 developing countries has shown that the average newborn’s length‐for‐age is close to −0.5 standard deviation of the WHO growth standard (reflecting IUGR) and declines to almost −2 standard deviation by the end of the second year 4.
Stunted growth or significantly impaired growth and development, threatens almost 25% of children around the world. Stunted growth in early life — particularly in the first 1,000 days from conception until the age of two – can lead to long-term health problems, including difficulties affecting both physical and mental development 5. A stunted child may also have a poorer immune system, brain function, and organ development 6. Performing below average in these areas may also limit their future productivity and threaten the health of their future children. Some of those consequences include poor cognition and educational performance, low adult wages, lost productivity and, when accompanied by excessive weight gain later in childhood, an increased risk of nutrition-related chronic diseases in adult life. It’s important to note that stunted growth is different from wasting. If stunted growth is a low height for a child’s weight, wasting is low weight for a child’s height. As of 2018, 151 million children under five suffered from stunted growth and 51 million from wasting 7.
Linear growth in early childhood is a strong marker of healthy growth given its association with morbidity and mortality risk, non-communicable diseases in later life, and learning capacity and productivity. It is also closely linked with child development in several domains including cognitive, language and sensory-motor capacities. According to the World Health Organization, well-nourished children are 33% more likely to escape poverty as adults 8.
Stunted growth is largely irreversible, a child cannot recover height in the same way that they can regain weight. Stunted children fall sick more often, miss opportunities to learn, perform less well in school and grow up to be economically disadvantaged, and more likely to suffer from chronic diseases.
Finally, stunted growth is often intergenerational. Children who are stunted are also more likely as adults to have stunted children. A stunted child is also more prone to becoming overweight as an adult, posing more health risks. Beyond the transgenerational cycle of stunted growth, there are a number of other factors at play: Children who suffer from stunted growth may never reach their full cognitive potential, which can lead to a lower IQ and impaired brain development. This also affects socio-emotional skills, weaker immune systems and overall health. All of these factors ultimately relate to long-term potential, including economic and health potential.
There are measures you can take to prevent stunting, especially in the first 1,000 days between pregnancy and a child’s second birthday. For stunted children, some effects can be reduced and even reversed.
After a child is born, it’s important for both mother and baby receive proper postnatal care. For babies born into poverty, one of the most important strategy to combat stunting is breast milk, which strengthens their immune system and provides the nutrients they need to grow.
If a mother is malnourished, it’s more likely that her baby will be born underweight. This sets off a cycle of stunting: Without proper postnatal care and the proper nutrients, the baby will likely suffer from stunted growth. If the child’s malnutrition goes untreated, they themselves may grow into a young woman who becomes a malnourished mother to a stunted child.
Community programs to ensure household access to proper sanitation, availability of clean water and diversified foods, poverty reduction support for families in need, education on how to feed young children and protect them from infection, and adequate, accessible health services to prevent and treat infections can collectively reduce stunting in populations.
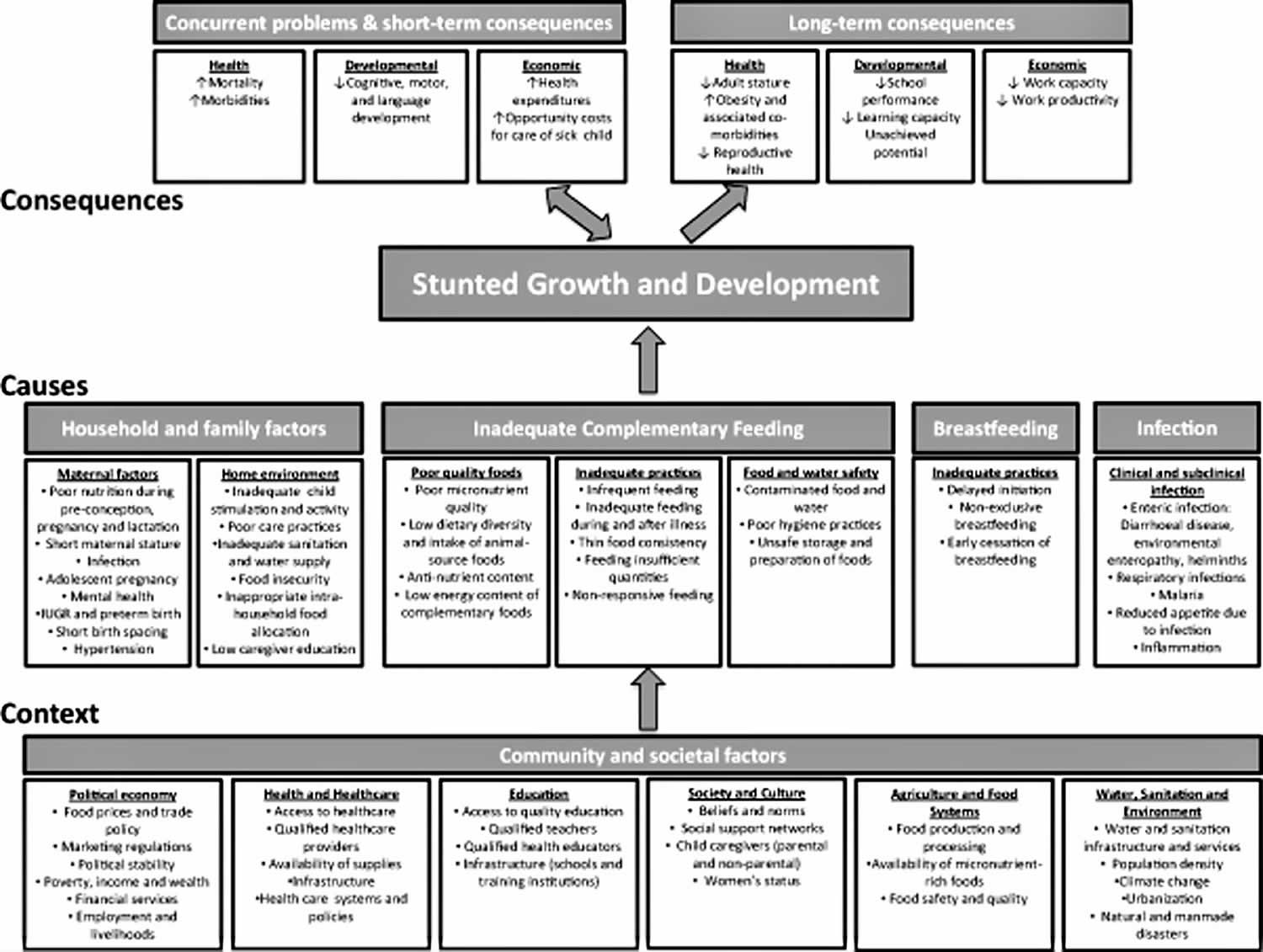
Figure 1. Stunted growth causes
Footnote: World Health Organization (WHO) conceptual framework on Childhood Stunting: Context, Causes, and Consequences, with an emphasis on complementary feeding.
[Source 3 ]Stunted growth
The most direct causes of stunted growth are inadequate nutrition (not eating enough or eating foods that lack growth-promoting nutrients) and recurrent infections or chronic or diseases which cause poor nutrient intake, absorption or utilization. Then there is the lack of care and stimulation for development
There are many factors that contribute to stunted growth in children and these factors are often linked. Some common factors linked to stunting include 9:
- Poor nutrition and a lack of access to diverse foods
- Poor sanitation and no access to clean drinking water
- Lack of proper healthcare for children and their mothers
- Inadequate psychosocial stimulation and/or parent-infant bonding
Stunted growth often begins very early in life, typically in utero, and generally continues during the first two post‐natal years. Most of the decline in length‐for‐age occurs during the complementary feeding period, between 6 and 24 months of age 10. Poor complementary feeding has been identified as a risk factor associated directly with stunted growth in children 11. Dewey & Huffman 10 estimated that the cumulative difference in stature between Malawian children and the WHO growth standard median was 10 cm by age 3 years. Of this, 20% was already present at birth, 20% was added in the first 6 months, 50% occurred at 6–24 months and the remaining 10% in the third year. The 6–24 months age period is important because as the child is introduced to foods other than breast milk and becomes increasingly more independent and mobile, the environmental factors influencing growth and development multiply.
None of these causes exist in a vacuum. Gender equality, male engagement in parenting, and conflict can all contribute to a child’s psychosocial stimulation. Conflict, along with income opportunities, food prices, and climate events, may affect food availability and in turn contribute to undernutrition or malnutrition.
Household and family factors
Periconceptional conditions including the pre‐pregnancy nutritional status of the mother, as well as her energy and nutrient intake, influence the early processes of growth and development 12. The maternal milieu sets physical and biological limits for offspring growth, but may also be signalling an unhealthy environment that adjusts growth trajectories and later reproductive viability 13. In addition to nutrition, other maternal factors play a role in determining offspring growth and development. Maternal infection related to malaria, helminths, HIV/AIDS and other conditions may lead to IUGR and later stunted growth in the infant 14. Adolescent pregnancy interferes with nutrient availability to the fetus due to the competing demands of ongoing maternal growth 15. Short birth spacing increases the risk for depleted maternal reserves in subsequent pregnancies, with negative consequences for both mother and child 16. Hypertension during pregnancy may also lead to adverse nutrition outcomes for the offspring 17. Recent studies have explored the impact of maternal mental health on child growth and development, with mixed findings 18.
Within the home environment, several economic and caring determinants are associated with stunted growth. Some of these factors relate closely to context, but have been included here to highlight the importance of addressing modifiable household factors. Low caregiver education shows a strong and consistent relationship with poor child nutrition outcomes, and likely drives other caring practices associated with stunted development and growth 19.. Dietary intake may be affected by caregiver neglect or absence. Inadequate child stimulation and activity can interact with poor nutrition to impede development through multiple pathways. Household poverty may lead to food insecurity 20., and more specifically micronutrient deficiencies arising from poor quality diets 21. Food may be available in the household but allocated preferentially to certain members, with harmful implications for vulnerable age/gender groups.
Inadequate complementary feeding
At the centre of the conceptual framework, three aspects of complementary feeding have been delineated to represent its contribution to stunted growth and development. Poor quality foods is the first category of determinants negatively impacting infant and young child growth. Inadequacies in micronutrient nutrition may arise from low dietary diversity 22, limited or no intake of animal source foods 23, and high anti‐nutrient content such as phytates and polyphenols in the plant‐based diets of many poor populations 24. The second category is inadequate practices. These include infrequent feeding, excessively dilute feeds with low energy density, inadequate feeding during illness, providing insufficient quantities of food and non‐responsive feeding 25.
The third category, food and water safety, relates primarily to the infection pathway to stunted growth, but may also contribute through inorganic contaminants and environmental pollutants 26. Household‐level hygiene practices such as hand washing, safe water source and storage, and sanitation conditions affect the risk of diarrhoea and other morbidities interfering with growth 27. Complementary foods may be stored in open or contaminated containers or left at temperatures supporting microbial growth 28. Food preparation techniques such as inadequate cleaning or cooking time can also increase the risk of contamination. Recently there has been renewed interest in the role of mycotoxins, such as aflatoxin, in child growth faltering 29. Exposure to these toxins occurs particularly via maize and groundnuts contaminated with fungi during production, storage or food processing. Two studies from West Africa have reported a dose‐response association between serum aflatoxin and height‐for‐age z‐score 30. Smith et al. 29 have proposed that gut inflammation may be one mechanism linking mycotoxin exposure to poor child growth.
Barriers to changing many of the practices just described may exist at many levels and will vary by context. Behavior change messages in the absence of consideration of these barriers may have limited success in changing practices. For example, infrequent feeding may be due to caregiver time constraints. Dilute feeds may be fed due to fears of infant choking. Inadequate feeding during illness may be due to a loss of appetite and food refusals by the infant. Non‐responsive feeding patterns may arise from misinterpretations of infant cues. Unsafe preparation and storage of foods may arise from inadequate access to electricity for refrigeration, poor access to cooking fuel for proper re‐heating of meals or difficulty in accessing sufficient quantities of safe water for proper hygiene practices. Dietary diversity may be limited by access and affordability of higher‐quality foods. In households where both the mother and father work outside of the home, reliance upon other family members and oftentimes older children within the home may limit caregivers’ abilities to carry out their infant feeding intentions.
Inadequate breastfeeding
After birth, breastfeeding practices have an immediate effect on newborn health. Delayed initiation of breastfeeding, not breastfeeding and non‐exclusive breastfeeding all increase the risk of morbidity 31, which may compromise growth in disadvantaged populations 32. Early cessation of breastfeeding can also lead to stunted growth and development through multiple pathways including inadequate energy intake, nutrient deficiencies and removal of passive immunity provided by human milk 33.
Infections
Infection can be a critical proximal cause of both stunted growth and development 34. Diarrheal disease, respiratory illnesses, malaria, fever and helminth infection are known determinants acting variously through inflammation and nutrient diversion, sequestration or loss 35. Checkley et al. 36 have estimated that 25% of the burden of stunting could be attributed to five or more episodes of diarrhoea occurring prior to the age of 2 years. Severe infectious disease can lead to wasting (low weight‐for‐height), which may have longer‐term consequences on linear growth, particularly if there is insufficient food availability to recover after a bout of infection 37. Sub‐clinical infection is also a likely contributor to poor growth and development. Though it occurs without outward cues, it may cause chronic, sustained insults to growth and development over time 38. Environmental enteropathy is one example of a subclinical condition in which repeated exposure to pathogenic microorganisms leads to abnormalities in the structure and function of the small intestine. This condition, first described over 40 years ago as an enteropathy found among individuals living in tropical regions 39, is characterized by villous atrophy, crypt hyperplasia, infiltration of the lamina propria by inflammatory T‐cells and increased permeability to enteric pathogens 40. In studies from The Gambia, children experienced altered intestinal permeability, an indicator of environmental enteropathy, on 76% of observed days. This factor explained 43% variability in linear growth over a 9‐month period and corresponded directly with the peak period of linear growth faltering 41. Studies of children with altered intestinal permeability have suggested poorer absorption of zinc 42 and vitamin A 43. An emerging area of research is focused on understanding how the bacterial communities in the gut contribute to undernutrition in children 44. While a relationship to acute malnutrition has been demonstrated 45, there is not yet evidence of an association between differences in the gut microbiota and child stunting.
Stunted growth prevention
There’s no simple solution for preventing stunted growth in children. However, focusing on what is commonly referred to as the first 1,000 days — the window of time between a mother’s pregnancy and her child’s second birthday — is a key opportunity to ensure the healthy development of children around the world.
While the amount of food is a large piece of the puzzle, a diversified diet can be equally, if not more, important. Treating malnutrition, even in those children who are older than 2, with ready-to-use therapeutic food (RUTF) can have a positive impact on stunting rates.
RUTF (ready-to-use therapeutic food) gives malnourished children the vital nutrients they need to recover. The original and most well-known RUTF, Plumpy’nut, was invented in 1996 by French pediatrician André Briend. As the name suggests, Plumpy’nut is a peanut-based paste served in a foil pouch. This means that it’s portable, non-perishable, and can be eaten by babies who aren’t yet ready for solid foods. The exact ingredients for an RUTF can vary based on the brand, but the standard RUTF has the same features: high in calories, nutrients, and vitamins to help children suffering from acute malnutrition rapidly gain weight.
Consequences of stunted growth and development
There is strong evidence that stunted growth has both immediate and long‐term consequences on health and development. For some outcomes, particularly reproductive health outcomes among women, stunting is a direct risk factor. For other outcomes such as susceptibility to infection, poor schooling, reduced intellectual performance and economic productivity, stunted growth is reflective of and highly correlated with other underlying biologic processes that are likely to be more directly involved in the causal pathway.
Short‐term consequences of stunted growth
Poor nutrition and frequent infection feedback upon each other, leading to a ‘vicious cycle’ 46 that might be more aptly described as a downward spiral of worsening nutritional status and increasing susceptibility to infection. Infection impairs nutritional status through reduced appetite, impaired intestinal absorption, increased catabolism, and direction of nutrients away from growth and towards immune response. In turn, malnutrition increases the risk of infection by its negative impact on the epithelial barrier function and altered immune response 47. Concurrent developmental problems and short‐term consequences consist of poorer psychomotor and mental development 48, while the economic consequences relate to health expenditures and the opportunity costs incurred in caring for sick children. At the immediate level, stunting is associated with infectious diseases that increase household expenditures for the care of a sick child. Although the data on this are sparse, one study from Nepal estimated this to be as large as 4% of per capita annual household expenditures 49.
Long‐term consequences of stunted growth
Individuals who are stunted at 2 years of age are likely to grow up to be stunted adults 50. There may be some opportunity for catch‐up growth during childhood, either due to improved nutrition or through a delay in skeletal maturation and the pubertal growth spurt that results in a longer overall period for growth in height 51. Using pooled data from five birth cohorts in Brazil, Guatemala, India, the Philippines, and South Africa, Adair et al. 50 found that a 1 standard deviation lower height‐for‐age at 2 years was associated with 3.2 cm lower adult height while a 1 standard deviation lower height‐for‐age during mid‐childhood was associated with a 1.9 cm lower adult height. Additionally, these authors estimated that a 1 standard deviation increase in height at age 2 years was associated with a 77% reduction in short adult stature 50.
Among women, shorter adult stature has important implications for pregnancy outcomes. Maternal stunting (<145 cm) is a consistent risk factor for perinatal mortality 52, likely due to an increased risk of obstructed labour and asphyxia at birth. In Nepal, for example, stunted mothers had a 50% increased risk of having a baby with symptoms of birth asphyxia, and larger babies (median 3.3 kg) born to stunted mothers had a near fourfold risk of asphyxia compared with babies of median weight (2.6 kg) born to non‐stunted mothers 53. A pooled analysis of data from 109 Demographic and Health Surveys datasets concluded that children born to stunted women had a nearly 60% increased risk of neonatal mortality compared with those born to women 160 cm or taller 54.
Stunted growth may have effects also on adult health and chronic disease risk 55. Studies of infants born with low birthweight have demonstrated consistent associations with elevated blood pressure, renal dysfunction and altered glucose metabolism 56. The evidence linking stunting with obesity risk or altered energy expenditure has been mixed 50. While it is unclear whether stunting may be a risk factor for obesity per se, rapid weight gain, particularly after the age of 2–3 years among individuals born small at birth, is thought to lead to a particularly high risk of chronic disease in later life 57.
Shorter adult stature has been linked to poorer schooling and economic productivity 58. Data from the COHORTS study showed that controlling for socio‐economic status, gender and maternal education, adults who at age 2 were stunted completed nearly 1 year less of schooling compared with non‐stunted individuals 59. In other analyses, a 1 standard deviation increase in height at age 2 years was associated with a 24% reduced risk of non‐completion of secondary school 50. Stunted growth has important economic consequences at the individual, household and community level, described in more detail in the paper by Hoddinott et al. 60. It has been estimated that stunted children earn 20% less as adults 5 compared with non‐stunted individuals. In World Bank estimates, a 1% loss in adult height due to childhood stunting is associated with a 1.4% loss in economic productivity 61.
Stunted development due to deficiencies of certain micronutrients, such as iodine and iron, can have long‐term and irreversible effects on neural and cognitive development 62, even if growth is not affected. Both iron and iodine are classified as ‘type 1’ nutrients, those that while essential and important for many biologic functions, are not thought to contribute to growth faltering unless deficiencies are severe 63. This is important because population‐level interventions designed to correct iron or iodine deficiency are unlikely to impact the height‐for‐age indicator, yet have potential to impact neurodevelopmental outcomes, which are more difficult to measure 64.
References- Stunting in a nutshell. https://www.who.int/nutrition/healthygrowthproj_stunted_videos/en/
- WHO Multicentre Growth Reference Study Group (2006) WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica (Oslo, Norway : 1992). Supplement 450, 76–85.
- Stewart, C.P., Iannotti, L., Dewey, K.G., Michaelsen, K.F. and Onyango, A.W. (2013), Complementary feeding in stunting prevention. Matern Child Nutr, 9: 27-45. doi:10.1111/mcn.12088 https://doi.org/10.1111/mcn.12088
- Victora C.G., de Onis M., Hallal P.C., Blossner M. & Shrimpton R. (2010) Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 125, e473–e480.
- Grantham‐McGregor S., Cheung Y.B., Cueto S., Glewwe P., Richter L. & Strupp B. (2007) Developmental potential in the first 5 years for children in developing countries. Lancet 369, 60–70.
- Georgieff M.K. (2007) Nutrition and the developing brain: nutrient priorities and measurement. The American Journal of Clinical Nutrition 85 (Suppl.), 614S–620S.
- RUTF and CMAM: How a simple peanut paste became a humanitarian revolution. https://www.concernusa.org/story/rutf-cmam-humanitarian-revolution/
- Improving nutrition, improving potential: Leaving no-one behind in the fight against malnutrition in all its forms. https://www.who.int/nutrition/events/2016_side-event_highlevelpoliticalforum_19jul/en/
- Stunting: What it is and what it means. https://www.concernusa.org/story/what-is-stunting/
- Dewey K.G. & Huffman S.L. (2009) Maternal, infant, and young child nutrition: combining efforts to maximize impacts on child growth and micronutrient status. Food and Nutrition Bulletin 30, S187–S189.
- Bhutta Z.A., Das J.K., Rizvi A., Gaffey M.F., Walker N., Horton S. et al. (2013) Evidence‐based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 382, 452–477.
- Gluckman P.D. & Pinal C.S. (2003) Regulation of fetal growth by the somatotrophic axis. The Journal of Nutrition 133 (5 Suppl. 2), 1741S–1746S.
- Kuzawa C.W. (2007) Developmental origins of life history: growth, productivity, and reproduction. American Journal of Human Biology 19, 654–661.
- Kuzawa C.W., Tallman P.S., Adai L.S., Lee N. & McDade T.W. (2012) Inflammatory profiles in the non‐pregnant state predict offspring birth weight at Cebu: evidence for inter‐generational effects of low grade inflammation. Annals of Human Biology 39, 267–274.
- Prakash R., Singh A., Pathak P.K. & Parasuraman S. (2011) Early marriage, poor reproductive health status of mother and child well‐being in India. The Journal of Family Planning and Reproductive Health Care 37, 136–145.
- Dewey K.G. & Cohen R.J. (2007) Does birth spacing affect maternal or child nutritional status? A systematic literature review. Maternal & Child Nutrition 3, 151–173.
- Thangaratinam S., Rogozinska E., Jolly K., Glinkowski S., Roseboom T., Tomlinson J.W. et al. (2012) Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta‐analysis of randomised evidence. British Medical Journal 344, e2088. doi: 10.1136/bmj.e2088
- Vazir S., Engle P., Balakrishna N., Griffiths P.L., Johnson S.L., Creed‐Kanashiro H. et al. (2013) Cluster‐randomized trial on complementary and responsive feeding education to caregivers found improved dietary intake, growth and development among rural Indian toddlers. Maternal & Child Nutrition 9, 99–117.
- Imdad A., Yakoob M.Y. & Bhutta Z.A. (2011) Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health 11 (Suppl. 3), S25 doi:10.1186/1471‐2458‐11‐S3‐S25
- Hong R. (2007) Effect of economic inequality on chronic childhood undernutrition in Ghana. Public Health Nutrition 10, 371–378.
- Iannotti L.L., Robles M., Pachon H. & Chiarella C. (2012) Food prices and poverty negatively affect micronutrient intakes in Guatemala. The Journal of Nutrition 142, 1568–1576.
- Arimond M. & Ruel M.T. (2004) Dietary diversity is associated with child nutritional status: evidence from 11 Demographic and Health Surveys. The Journal of Nutrition 134, 2579–2585.
- Krebs N.F. (2007) Food choices to meet nutritional needs of breast‐fed infants and toddlers on mixed diets. The Journal of Nutrition 137, 511S–517S.
- Roos N., Sorensen J.C., Sorensen H., Rasmussen S.K., Briend A., Yang Z. et al. (2013) Screening for anti‐nutritional compounds in complementary foods and food aid products for infants and young children. Maternal & Child Nutrition 9 (Suppl. 1), 47–71.
- Aboud F.E. & Akhter S. (2011) A cluster‐randomized evaluation of a responsive stimulation and feeding intervention in Bangladesh. Pediatrics 127, e1191–e1197.
- Weisstaub G. & Uauy R. (2012) Non‐breast milk feeding in developing countries: challenge from microbial and chemical contaminants. Annals of Nutrition & Metabolism 60, 215–219.
- Fink G., Gunther I. & Hill K. (2011) The effect of water and sanitation on child health: evidence from the demographic and health surveys 1986–2007. International Journal of Epidemiology 40, 1196–1204.
- Kimmons J.E., Brown K.H., Lartey A., Collison E., Mensah P.P. & Dewey K.G. (1999) The effects of fermentation and/or vacuum flask storage on the presence of coliforms in complementary foods prepared for Ghanaian children. International Journal of Food Sciences and Nutrition 50, 195–201.
- Smith L.E., Stoltzfus R.J. & Prendergast A. (2012) Food chain mycotoxin exposure, gut health, and impaired growth: a conceptual framework. Advances in Nutrition (Bethesda, Md.) 3, 526–531.
- Gong Y., Hounsa A., Egal S., Turner P.C., Sutcliffe A.E., Hall A.J. et al. (2004) Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environmental Health Perspectives 112, 1334–1338.
- Kramer M.S. & Kakuma R. (2012) Optimal duration of exclusive breastfeeding. Cochrane Database of Systematic Reviews (8), CD003517. doi: 10.1002/14651858.CD003517.pub2
- Engebretsen I.M., Tylleskär T., Wamani H., Karamagi C. & Tumwine J.K. (2008) Determinants of infant growth in Eastern Uganda: a community‐based cross‐sectional study. BMC Public Health 8, 418 doi:10.1186/1471‐2458‐8‐418
- Arpadi S., Fawzy A., Aldrovandi G.M., Kankasa C., Sinkala M., Mwiya M. et al. (2009) Growth faltering due to breastfeeding cessation in uninfected children born to HIV‐infected mothers in Zambia. The American Journal of Clinical Nutrition 90, 344–353.
- Berkman D.S., Lescano A.G., Gilman R.H., Lopez S.L. & Black M.M. (2002) Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow‐up study. Lancet 359, 564–571.
- Hall A., Hewitt G., Tuffrey V. & de Silva N. (2008) A review and meta‐analysis of the impact of intestinal worms on child growth and nutrition. Maternal & Child Nutrition 4 (Suppl. 1), 118–236.
- Checkley W., Buckley G., Gilman R.H., Assis A.M., Guerrant R.L., Morris S.S. et al. (2008) Multi‐country analysis of the effects of diarrhea on childhood stunting. International Journal of Epidemiology 37, 816–830.
- Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M. et al. (2013) Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 382, 427–451.
- Dewey K.G. & Mayers D.R. (2011) Early child growth: how do nutrition and infection interact? Maternal & Child Nutrition 7 (Suppl. 3), 129–142.
- Lindenbaum J., Gerson C.D. & Kent T.H. (1971) Recovery of small‐intestinal structure and function after residence in the tropics. I. Studies in Peace Corps volunteers. Annals of Internal Medicine 74, 218–222.
- Lunn P.G., Northrop‐Clewes C.A. & Downes R.M. (1991) Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 338, 907–910.
- Lunn P.G. (2000) The impact of infection and nutrition on gut function and growth in childhood. The Proceedings of the Nutrition Society 59, 147–154.
- Manary M.J., Abrams S.A., Griffin I.J., Quimper M.M., Shulman R.J., Hamzo M.G. et al. (2010) Perturbed zinc homeostasis in rural 3–5‐y‐old Malawian children is associated with abnormalities in intestinal permeability attributed to tropical enteropathy. Pediatric Research 67, 671–675.
- Chen P., Soares A.M., Lima A.A., Gamble M.V., Schorling J.B., Conway M. et al. (2003) Association of vitamin A and zinc status with altered intestinal permeability: analyses of cohort data from northeastern Brazil. Journal of Health, Population, and Nutrition 21, 309–315.
- Gordon J.I., Dewey K.G., Mills D.A. & Medzhitov R.M. (2012) The human gut microbiota and undernutrition. Science Translational Medicine 4, 137ps12. doi: 10.1126/scitranslmed.3004347
- Smith M.I., Yatsunenko T., Manary M.J., Trehan I., Mkakosya R., Cheng J. et al. (2013) Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339, 548–554.
- Solomons N.W. (2007) Malnutrition and infection: an update. The British Journal of Nutrition 98 (Suppl. 1), S5–10.
- Brown K.H. (2003) Diarrhea and malnutrition. The Journal of Nutrition 133, 328S–332S.
- McDonald C.M., Manji K.P., Kupka R., Bellinger D.C., Spiegelman D., Kisenge R. et al. (2013) Stunting and wasting are associated with poorer psychomotor and mental development in HIV‐exposed Tanzanian infants. The Journal of Nutrition 143, 204–214.
- Pokhrel S. & Sauerborn R. (2004) Household decision‐making on child health care in developing countries: the case of Nepal. Health Policy Plan 19, 218–233.
- Adair L.S., Fall C.H.D., Osmond C., Stein A.D., Martorell R., Ramirez‐Zea M. et al., for the COHORTS Group (2013) Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet published online March 28. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)60103-8/fulltext
- Prentice A.M., Ward K.A., Goldberg G.R., Jarjou L.M., Moore S.E., Fulford A.J. et al. (2013) Critical windows for nutritional interventions against stunting. The American Journal of Clinical Nutrition 95, 911–918.
- Lawn J.E., Cousens S. & Zupan J. (2005) 4 million neonatal deaths: when? where? why? Lancet 365, 891–900.
- Lee A.C., Darmstadt G.L., Khatry S.K., LeClerq S.C., Shrestha S.R. & Christian P. (2009) Maternal‐fetal disproportion and birth asphyxia in rural Sarlahi, Nepal. Archives of Pediatrics & Adolescent Medicine 163, 616–623.
- Ozaltin E., Hill K. & Subramanian S.V. (2010) Association of maternal stature with offspring mortality, underweight, and stunting in low‐ to middle‐income countries. Journal of the American Medical Association 303, 1507–1516.
- Uauy R., Kain J., Mericq V., Rojas J. & Corvalan C. (2008) Nutrition, child growth, and chronic disease prevention. Annals of Medicine 40, 11–20.
- Whincup P.H., Kaye S.J., Owen C.G., Huxley R., Cook D.G., Anazawa S. et al. (2008) Birth weight and risk of type 2 diabetes: a systematic review. Journal of the American Medical Association 300, 2886–2897.
- Gluckman P.D., Hanson M.A. & Beedle A.S. (2007) Early life events and their consequences for later disease: a life history and evolutionary perspective. American Journal of Human Biology : The Official Journal of the Human Biology Council 19, 1–19.
- Martorell R. (1996) The role of nutrition in economic development. Nutrition Reviews 54 (4 Pt 2), S66–S71.
- Martorell R., Horta B.L., Adair L.S., Stein A.D., Richter L., Fall C.H. et al. (2010) Weight gain in the first two years of life is an important predictor of schooling outcomes in pooled analyses from five birth cohorts from low‐ and middle‐income countries. The Journal of Nutrition 140, 348–354.
- Hoddinott J., Alderman H., Behrman J.R., Haddad L. & Horton S. (2013) The economic rationale for investing in stunting reduction. Maternal & Child Nutrition 9 (Suppl. 2), 69–82.
- World Bank (2006) Repositioning Nutrition as Central to Development: A Strategy for Large‐Scale Action. The World Bank: Washington, DC.
- Zimmermann M.B. (2012) The effects of iodine deficiency in pregnancy and infancy. Paediatric and Perinatal Epidemiology 26 (Suppl. 1), 108–117.
- Golden M.H. (1995) Specific deficiencies versus growth failure: type I and type II nutrients. SCN News 12, 10–14.
- Fernald L.C.H., Kariger P., Engle P. & Raikes A. (2009) Examining early child development in low‐income countries: a toolkit for the assessment of children in the first five years of life. The World Bank: Washington, DC.