Thomsen disease
Thomsen disease also known as “autosomal dominant myotonia congenita”, is an inherited neuromuscular disorder characterized by the inability of muscles to quickly relax after a voluntary contraction causing a stiffness called myotonia 1. There are two types of myotonia congenita: Becker-type myotonia (autosomal recessive myotonia congenita) is the most common form, while Thomsen disease (autosomal dominant myotonia congenita) is a very rare, relatively milder form. In individuals with Thomsen disease, symptoms and findings such as myotonia, associated muscle rigidity, and abnormal muscle enlargement may become apparent from infancy to approximately two to three years of age. Thomsen disease causes more severe muscle stiffness, particularly in males. Unlike people with Becker disease who often experience temporary attacks of muscle weakness, particularly in the arms and hands, brought on by movement after periods of rest, muscle weakness is not seen in people with Thomsen disease 2. In many cases, muscles of the eyelids, hands, and legs may be most affected. Myotonia congenita is caused by mutations in CLCN1 gene is responsible for shutting off electrical excitation in the muscles. Myotonia congenita doesn’t cause muscle atrophy (shrinkage); instead, it sometimes can cause muscle enlargement and increased muscle strength. Thomsen disease is transmitted as an autosomal dominant trait.
Myotonia congenita is estimated to affect 1 in 100,000 people worldwide 3. Myotonia congenita is more common in northern Scandinavia, where it occurs in approximately 1 in 10,000 people 4. This prevalence is ten times higher than the estimated worldwide prevalence 5. A United Kingdom study of 300 myotonia congenita patients demonstrated that only 37% of this cohort has Thomsen disease (autosomal dominant myotonia congenita) 6.
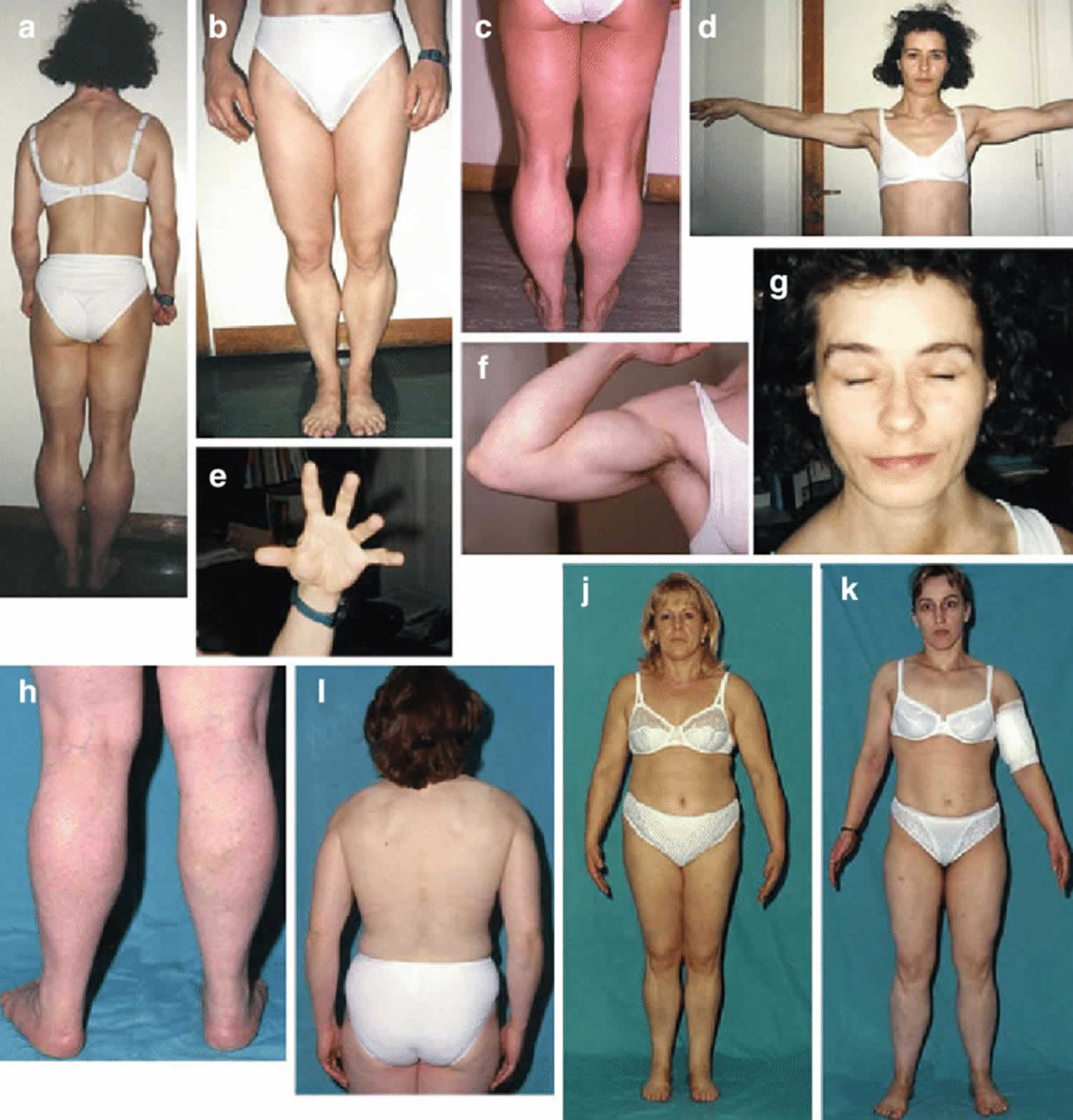
Figure 1. Thomsen disease
Footnote: Thomsen disease in patient 1 (a – g) and the three sisters from Family 2 (h – k). Note a generalized increased muscle bulk in all patients, especially of the calves (a–c, h) and biceps (f), myotonia in the hands (e) and eye (g).
[Source 7 ]Thomsen disease causes
Mutations in the CLCN1 gene mapped to the long arm (q) of chromosome 7 (“7q35” refers to band 35 on the long arm of chromosome 7) cause myotonia congenita. Advanced sequencing techniques have facilitated the identification of over 200 pathogenic mutations throughout the CLCN1 gene 8. The CLCN1 gene provides instructions for making a protein that is critical for the normal function of skeletal muscle cells. For the body to move normally, skeletal muscles must tense (contract) and relax in a coordinated way. Muscle contraction and relaxation are controlled by the flow of charged atoms (ions) into and out of muscle cells. Specifically, the protein produced from the CLCN1 gene forms voltage-gated chloride (CIC-1) channels within the sarcolemmal membrane that controls the flow of negatively charged chlorine atoms (chloride ions) into skeletal muscle cells 9. The main function of voltage-gated chloride (CIC-1) channel is to stabilize the cells’ electrical charge, which prevents muscles from contracting abnormally.
Mutations in the CLCN1 gene alter the usual structure or function of chloride channels. The altered channels cannot properly regulate ion flow, reducing the movement of chloride ions into skeletal muscle cells. This disruption in chloride ion flow triggers prolonged muscle contractions, which are the hallmark of myotonia 10.
Because several CLCN1 mutations can cause either Becker disease or Thomsen disease, doctors usually rely on characteristic signs and symptoms to distinguish the two forms of myotonia congenita.
Thomsen disease inheritance pattern
Thomsen disease is inherited in an autosomal dominant pattern, which means one copy of the altered gene in each cell is sufficient to cause the disorder. In most cases, an affected person has one parent with the condition. The autosomal dominant mutations causative of Thomsen disease are associated with reduced penetrance, and identical mutations inherited through generations can cause markedly different phenotypes 11.
Often autosomal dominant conditions can be seen in multiple generations within the family. If one looks back through their family history they notice their mother, grandfather, aunt/uncle, etc., all had the same condition. In cases where the autosomal dominant condition does run in the family, the chance for an affected person to have a child with the same condition is 50% regardless of whether it is a boy or a girl. These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
- When one parent has the abnormal gene, they will pass on either their normal gene or their abnormal gene to their child. Each of their children therefore has a 50% (1 in 2) chance of inheriting the changed gene and being affected by the condition.
- There is also a 50% (1 in 2) chance that a child will inherit the normal copy of the gene. If this happens the child will not be affected by the disorder and cannot pass it on to any of his or her children.
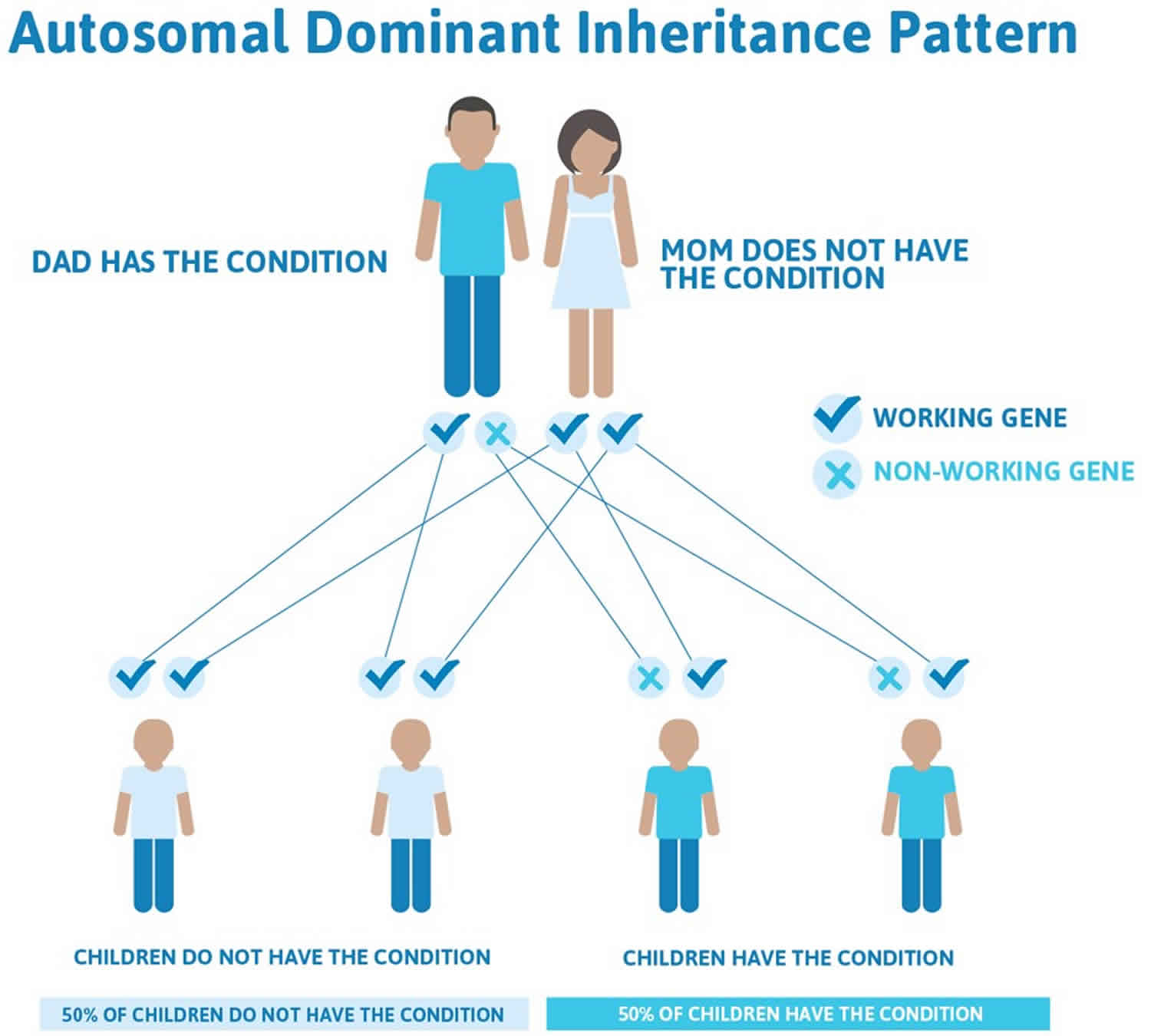
Figure 2 illustrates autosomal dominant inheritance. The example below shows what happens when dad has the condition, but the chances of having a child with the condition would be the same if mom had the condition.
Figure 2. Thomsen disease autosomal dominant inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Thomsen disease symptoms
Thomsen disease is an autosomal dominant myotonia congenita presenting earlier in childhood than Becker disease and may be associated with milder features and are typically nonprogressive 12. In individuals with Thomsen disease, symptoms and findings such as myotonia, associated muscle rigidity, and abnormal muscle enlargement may become apparent from infancy to approximately two to three years of age. Thomsen disease causes more severe muscle stiffness, particularly in males; however, the severity of the myotonia may vary greatly among members of the same family, with males typically more severely affected. Unlike people with Becker disease who often experience temporary attacks of muscle weakness, particularly in the arms and hands, brought on by movement after periods of rest, muscle weakness is not seen in people with Thomsen disease 2. In many cases, muscles of the legs, hands, and/or eyelids are most affected, particularly those of the legs. According to some reports, exposure to cold temperatures may aggravate symptoms in some cases.
Thomsen disease characteristic symptoms may include difficulties starting to walk or run, arising from a bed or chair, relaxing the hand grip, etc 13. In addition, some may experience spasms of other muscles, including certain muscles of the face, trunk, or other regions. For example, involvement of muscles that control movements of the eyeballs (extraocular muscles) may lead to temporary episodes of double vision or abnormal deviation of one eye in relation to the other (strabismus). In addition, in some cases, if myotonia affects muscles of the throat, affected individuals may have difficulties with chewing, swallowing, and/or attempting to talk after a long period of silence.
Individuals with Thomsen disease may develop abnormal enlargement (hypertrophy) of the muscles. Affected individuals typically have normal muscle strength or only minimal muscle weakness.
The hallmark of myotonia congenita is myotonia (muscle stiffness). This means the muscles are unable to quickly relax after contracting. For example, after a handshake, the person is only very slowly able to open and pull away their hand. Muscle stiffness observed in myotonia congenita is often ameliorated by exercise or repetitive movement. This effect is called the “warm-up” phenomenon (though it is quickly lost on cessation of activity) 11. Men appear to be more severely affected by myotonia congenita than women. In addition, symptoms often worsen during pregnancy and menstruation; these observations imply that sex hormones affect CIC-1 channel function 14.
Early symptoms may include:
- Difficulty swallowing
- Gagging
- Stiff movements that improve when they are repeated
- Shortness of breath or tightening of the chest at the beginning of exercise
- Frequent falls
- Difficulty opening eyes after forcing them closed or crying
Children with myotonia congenita often look muscular and well-developed. They may not have symptoms of myotonia congenita until age 2 or 3.
Thomsen disease diagnosis
Myotonia congenita may be diagnosed from infancy or early childhood to adulthood, based upon a thorough clinical evaluation, a detailed patient and family history, various specialized tests, and genetic analysis, if available. Family history consistent with either autosomal dominant or autosomal recessive inheritance.
Myotonia congenita should be suspected in individuals with the following clinical and laboratory findings.
Clinical examination
Episodes of muscle stiffness (myotonia) or cramps beginning in early childhood. Myotonia is defined as impaired relaxation of skeletal muscle after voluntary contraction. Myotonia can be observed by asking patients to repeatedly open and close their eyes or to open and close their fist. Repeated tapping of a muscle similarly induces myotonia 15. Patients may struggle to immediately extend fingers following a handshake. Alleviation of stiffness by brief exercise known as the “warm-up” effect may also be demonstrable.
Laboratory findings
Biochemical investigations are usually unremarkable, although mild elevations of creatinine kinase (CK) have been described up to three to four times the upper limit of normal 15.
Electromyography (EMG) is a useful tool in the diagnosis of myotonia congenita however, it is time-consuming, uncomfortable, and results in an overlap between the different channelopathies 16. It may demonstrate a shower of spontaneous electrical activity in diffuse myotonic discharges or “myotonic bursts”. There is no electromyographical difference between the two types of myotonia congenita. Given the widespread availability of genetic testing, muscle biopsy is now rarely performed, but it may show heterogeneous muscle fibers with increased numbers of nuclei and absent type 2B fibers 15. A muscle biopsy is not necessary to establish a diagnosis of myotonia congenita.
Genetic testing is considered the gold standard 11. However, in many patients, mutations within the CLCN1 gene are not identified despite significant clinical features correlating with a myotonic picture. A multigene panel is usually performed initially, including the CLCN1 gene, as well as other genes of interest such as SCN4A. Genetic testing and selection of techniques used should be undertaken by specialized centers in order to evaluate variants of unknown significance, correlate identified mutations with clinical phenotype, and minimize costs. If a multigene panel does not identify an explanatory mutation, genomic techniques, including exome sequencing and mitochondrial sequencing, may be employed.
Thomsen disease treatment
The treatment of Thomsen and Becker types myotonia congenita is directed toward the specific symptoms that are apparent in each individual. Such treatment may require the coordinated efforts of a team of medical professionals, such as pediatricians; specialists who diagnose and treat disorders of the skeleton, joints, muscles, and related tissues (orthopedists); physical therapists; and/or other health care professionals.
Specific therapies for the treatment of Thomsen and Becker diseases are symptomatic and supportive. In some cases, certain medications may be prescribed to help diminish muscle stiffness and other symptoms resulting from myotonia. In addition, special exercises may be advised to help alleviate myotonic symptoms, since associated muscle rigidity may improve with proper movement and exercise of involved muscle groups.
Pharmacological management of myotonia congenita is not always indicated, and patients should be evaluated by a neurologist to assess the requirement for medication prior to its initiation 17. Lifestyle modifications include avoidance of identified triggers such as stress and cold. Exercise, particularly gymnastics, is anecdotally reported to be beneficial in relieving myotonia; however, the effect needs to be further investigated.
Medications that are used generally aim to reduce hypersensitivity of the muscle membrane by blocking sodium ion flow 18. Mexiletine is the most commonly prescribed medication but requires ECG monitoring of the QT interval prior to and during use 19. It is classified as a class 1b antiarrhythmic and is a derivative of the local anesthetic lidocaine. Mexiletine is primarily used in the management of ventricular arrhythmias by blocking the rapid influx of sodium responsible for phase 0 of the action potential, shortening the action potential, and prolonging the refractory period 20.
Side effects may include tremor, dizziness, ataxia, and gastrointestinal disturbance. These adverse effects are usually dose-dependent and reversible on cessation of medication or dose decrement. Phenytoin and other anticonvulsants are also commonly used 21.
Potassium channels are a novel target. The potassium channel activator retigabine has been investigated in murine myotonia congenita models and demonstrated to significantly improve the severity of myotonia in vivo 18. However, it is recognized that there remains a paucity of evidence guiding the pharmacological management of myotonia congenita 22.
Early intervention is important to ensure that affected children reach their potential. Special services that may be beneficial include special social support, physical therapy, and/or other medical, social, and/or vocational services.
Genetic counseling will be of benefit for affected individuals and their families. Other treatment for this disorder is symptomatic and supportive.
Two sisters with Becker type myotonia congenita demonstrated susceptibility to a malignant hyperthermia-like response. Although the implications of this finding are not fully understood, this potential risk must be taken into consideration by surgeons, anesthesiologists, dentists, and other health care workers when making decisions concerning surgery, the use of particular anesthetics, and the administration of certain medications.
Thomsen disease prognosis
Neither autosomal nor recessively inherited myotonia congenita are associated with systemic effects and do not limit life expectancy 17. This prognosis differs significantly from the disease course of myotonic dystrophy, therefore accurate diagnosis is essential. Once symptoms of myotonia congenita have appeared, this group of diseases do not typically progress. Becker disease is typically considered to be associated with worse symptoms than those seen in Thomsen disease, and permanent weakness may persist over time. Patients with myotonia congenita must have access to genetic counseling services in order to make informed decisions regarding family planning.
References- Coote DJ, Davis MR, Cabrera M, Needham M, Laing NG, Nowak KJ. Clinical Utility Gene Card for: autosomal dominant myotonia congenita (Thomsen Disease). Eur J Hum Genet. 2018;26(7):1072-1077. doi:10.1038/s41431-017-0065-3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6018704
- Richardson RC, Tarleton JC, Bird TD, Gospe SM. Truncating CLCN1 mutations in myotonia congenita: variable patterns of inheritance. Muscle Nerve. 2014 Apr;49(4):593-600.
- Myotonia congenita. https://medlineplus.gov/genetics/condition/myotonia-congenita
- Coote DJ, Davis MR, Cabrera M, Needham M, Laing NG, Nowak KJ. Clinical Utility Gene Card for: autosomal dominant myotonia congenita (Thomsen Disease). Eur. J. Hum. Genet. 2018 Jul;26(7):1072-1077.
- Emery AE. Population frequencies of inherited neuromuscular diseases–a world survey. Neuromuscul. Disord. 1991;1(1):19-29.
- Fialho D, Schorge S, Pucovska U, Davies NP, Labrum R, Haworth A, Stanley E, Sud R, Wakeling W, Davis MB, Kullmann DM, Hanna MG. Chloride channel myotonia: exon 8 hot-spot for dominant-negative interactions. Brain. 2007 Dec;130(Pt 12):3265-74.
- Angelini, Corrado. (2014). Congenital Myotonia, Thomsen Disease. 10.1007/978-3-319-07500-6_41
- Li L, McCall C, Hu X. Editorial: Innate Immunity Programming and Memory in Resolving and Non-Resolving Inflammation. Front Immunol. 2020;11:177.
- Conravey A, Santana-Gould L. Myotonia congenita and myotonic dystrophy: surveillance and management. Curr Treat Options Neurol. 2010 Jan;12(1):16-28.
- Platt D, Griggs R. Skeletal muscle channelopathies: new insights into the periodic paralyses and nondystrophic myotonias. Curr. Opin. Neurol. 2009 Oct;22(5):524-31.
- Bryan ES, Alsaleem M. Myotonia Congenita. [Updated 2020 Sep 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562335
- Sun C, Tranebjaerg L, Torbergsen T, Holmgren G, Van Ghelue M. Spectrum of CLCN1 mutations in patients with myotonia congenita in Northern Scandinavia. Eur. J. Hum. Genet. 2001 Dec;9(12):903-9.
- Myotonia Congenita. https://rarediseases.org/rare-diseases/myotonia-congenita
- Fialho D, Kullmann DM, Hanna MG, Schorge S. Non-genomic effects of sex hormones on CLC-1 may contribute to gender differences in myotonia congenita. Neuromuscul. Disord. 2008 Nov;18(11):869-72.
- Hahn C, Salajegheh MK. Myotonic disorders: A review article. Iran J Neurol. 2016 Jan 05;15(1):46-53.
- Phillips L, Trivedi JR. Skeletal Muscle Channelopathies. Neurotherapeutics. 2018 Oct;15(4):954-965.
- Gutmann L, Phillips LH. Myotonia congenita. Semin Neurol. 1991 Sep;11(3):244-8.
- Dupont C, Denman KS, Hawash AA, Voss AA, Rich MM. Treatment of myotonia congenita with retigabine in mice. Exp. Neurol. 2019 May;315:52-59.
- Roberts K, Kentris M. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jun 30, 2020. Myotonia.
- Singh S, Kerndt CC, Zeltser R. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 23, 2020. Mexiletine.
- Manolis AS, Deering TF, Cameron J, Estes NA. Mexiletine: pharmacology and therapeutic use. Clin Cardiol. 1990 May;13(5):349-59.
- Trip J, Drost G, van Engelen BG, Faber CG. Drug treatment for myotonia. Cochrane Database Syst Rev. 2006 Jan 25;(1):CD004762.