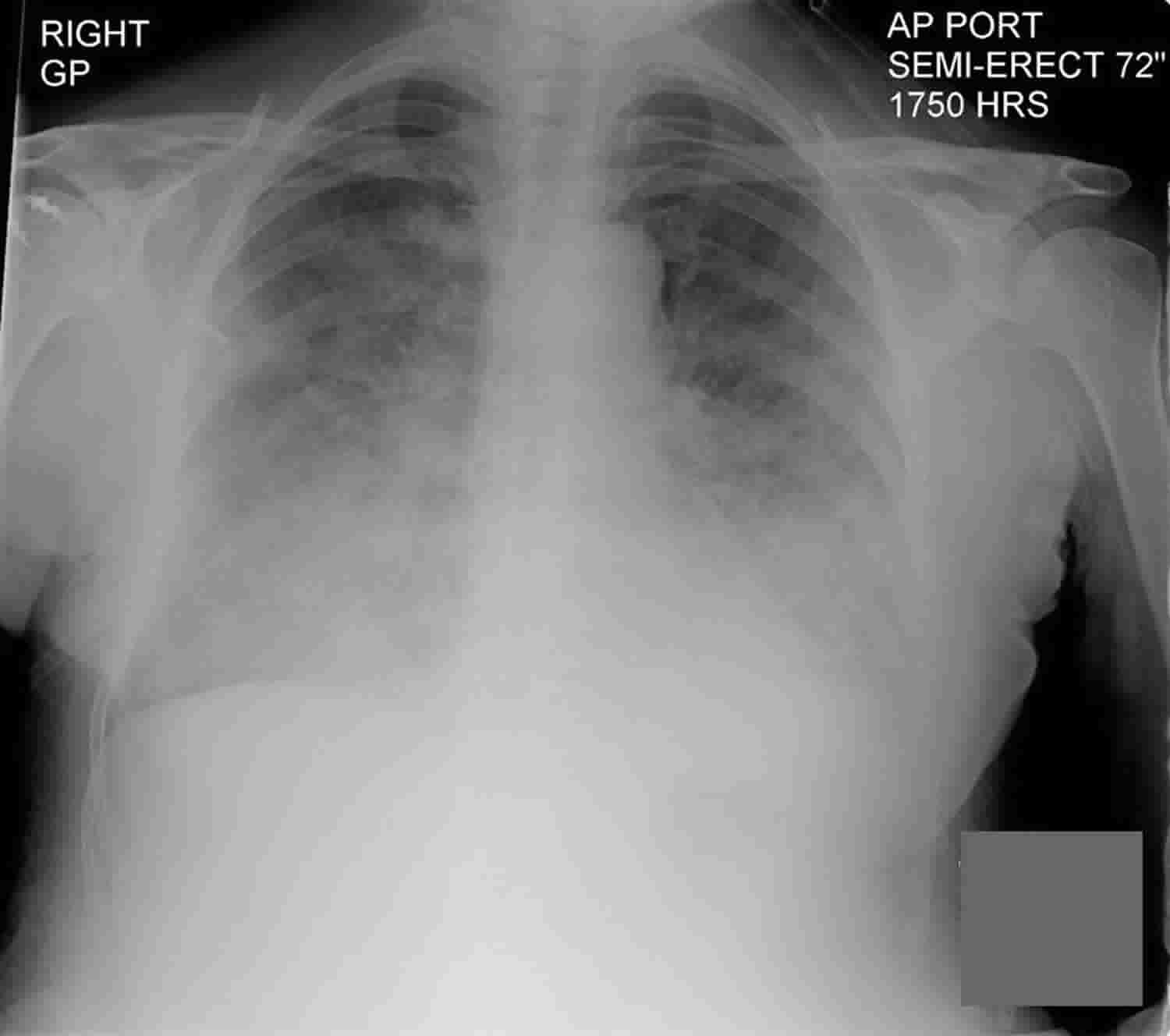
Transfusion-related acute lung injury also called TRALI, is defined as new acute lung injury (ALI) that occurs during or within six hours after transfusing blood products such as whole blood, fresh frozen plasma (FFP), platelets, cryoprecipitate, granulocytes, intravenous immune globulin, allogenic and autologous stem cells, and packed red blood cells, not explained by another acute lung injury (ALI) risk factor 1. Transfusion of part of one unit of any blood product can cause TRALI 2. The mechanism may include factors in unit(s) of blood, such as antibody and biologic response modifiers. In addition, yet to be described factors in a patient’s illness may predispose to transfusion-related acute lung injury. The current incidence is estimated to be 1 in 5,000 units 2. Patients present with acute dyspnea, or froth in the endotracheal tube in intubated patients. Hypertension, hypotension, acute leukopenia have been described. Management is similar to that for acute lung injury (ALI) and is predominantly supportive. When TRALI is suspected, blood banks should be notified to quarantine other components from the same donation 2. No special blood product is required for subsequent transfusion of a patient who has developed TRALI 2.
TRALI was first reported in the 1950s, but the term TRALI was coined by Drs. Popovsky and Moore when they reported a case series at the Mayo Clinic in 1985 3. In this case series, the typical clinical presentation included acute respiratory distress characterized by hypoxemia and fulminant pulmonary edema. The onset was usually within 4 hours of transfusion and was often accompanied by fever, tachycardia, hypotension or hypertension. In most patients (81%), recovery was rapid and complete. The incidence was 1:5,000 units transfused and the TRALI patients were comprised of mainly surgical patients. There is still no consensus on the incidence, pathogenesis or laboratory diagnosis of the transfusion-related acute lung injury syndrome. However, reports of TRALI are increasing due to increasing awareness of the syndrome, although underreporting is still strongly suspected. An analysis of the United States Food and Drug Administration (FDA) fatality reports for the last three fiscal years showed bacterial contamination, TRALI, and ABO hemolytic reactions to be the leading causes of deaths from transfusion 2. TRALI became the leading cause of fatalities reported to the FDA in fiscal 2003. Fatalities were associated with fresh frozen plasma (FFP), red blood cells (RBCs) or platelets 4. Based on these data, it is clear that TRALI is one of the most significant complications of modern blood transfusion.
Diagnostic criteria for TRALI is if the symptoms develop during or within 6 hours of transfusion without any risk factors for developing acute lung injuries such as sepsis from pneumonia, aspiration, and shock 5. Physical symptoms include fever, hypotension, and tachycardia. Clinical findings include exudative bilateral infiltrates on chest radiograph, no evidence of pulmonary vascular overload, and hypoxemia of SpO2 less than 90% on room air with a ratio of partial pressure of oxygen to a fractional inspired oxygen concentration of less than 300 mmHg 6. Possible TRALI is when there are other risk factors for acute lung injury. Delayed TRALI is when transfusion is completed after 6 to 72 hours, and it is associated with higher mortality 7. Transfusion-related circulatory overload (TACO) needs to be ruled out as it can be on differential diagnosis due to the similarity of pulmonary edema picture, but due to actual volume overload 8.
TRALI is the leading cause of transfusion-related mortality in the United States. TRALI occurs in up to 15% of transfused patients 9, but other reports estimate the incidence of TRALI at approximately 0.1% of transfused patients 10.
Although the true incidence of TRALI is unknown, it is likely under-diagnosed and under-estimated, primarily due to poor recognition of the disease 11. Several studies have reported TRALI incidence ranges of 1 in 5000 units of packed red blood cells, 1 in 7,900 units of fresh frozen plasma (FFP), 1 in 2000 plasma-containing components and 1 in 400 units of whole-blood-derived platelet concentrates to 1 in 432 units of whole blood 12. In addition, the incidence of TRALI depends on the patient population, and a higher incidence of TRALI is observed in critically ill patients 13. Historical estimates for TRALI-associated mortality range from 5 to 8% 14, but up to 60% of critically ill patients die from TRALI 15.
According to the 2004 American-European Consensus Conference of acute respiratory distress syndrome (ARDS), TRALI was defined based on clinical and radiological parameters as newly developed acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) within 6 hours of a blood product transfusion (Table 1) 16. The 6 hour time window was chosen based on the opinion of an expert panel. Suspected TRALI was defined as fulfillment of the definition of TRALI without another acute lung injury (ALI) risk factor. Because the characteristics of TRALI are difficult to distinguish from those of acute lung injury (ALI) due to other causes, possible TRALI has been suggested to allow for the presence of other acute lung injury (ALI) risk factors. The conventional definition of TRALI included cases of acute lung injury (ALI) that develop within 6 hours of transfusion, but transfusion is an acute lung injury (ALI) risk factor for 6-72 hours. Thus, delayed TRALI includes delayed onset TRALI after transfusion 17.
Suspected TRALI
- Timing: Acute onset within 6 hours of blood transfusion
- Pulmonary artery wedge pressure (pulmonary arterial occlusion pressure): ≤ 18 mm Hg (or central venous pressure ≤ 15 mmHg) when measured, or a lack of clinical evidence of left atrial hypertension (no sign of hydrostatic pulmonary edema)
- Chest radiograph: Bilateral pulmonary infiltrates on frontal chest radiograph
- Hypoxemia: Ratio of PaO2/FIO2 ≤ 300 mm Hg regardless of PEEP level (Note: In patients in whom an arterial blood gas is not available, an oxygen saturation of <90% when the patient is breathing room air meets the criterion for hypoxemia)
- No other risk factor for acute lung injury (ALI)
Possible TRALI
- Same as for suspected TRALI, but another risk factor present for acute lung injury 16
Delayed TRALI
- Same as for (possible) TRALI and onset within 6-72 hours of blood transfusion 16
Definition of clinical TRALI
The National Heart Lung and Blood Institute Working Group on TRALI developed a definition 18. In patients with no acute lung injury (ALI) immediately before transfusion, and no other acute lung injury (ALI) risk factor (Table 1) is present, a diagnosis of TRALI is made if there is:
- New acute lung injury (ALI) after transfusion, and
- The onset of symptoms or signs is during or within 6 hours after transfusion
The definition includes patients who are massively transfused who develop new acute lung injury (ALI), and such patients may be at greater risk for TRALI as they receive multiple units. The definition excludes patients with acute lung injury (ALI) before transfusion; even though worsening of existing ALI after transfusion could be due to TRALI, defining this form of TRALI is problematic 2.
In patients who have other ALI risk factors can also develop TRALI, and thus TRALI should not be excluded from consideration in these patients. The incidence of ALI in prospective studies of patient groups with ALI risk factors is less than 50% (see Table 1). Thus, the presence of an ALI risk factor does not mean the patient will definitely develop ALI. New ALI in a transfused patient with an ALI risk factor could be mechanistically due to the transfusion and/or the risk factor, i.e. TRALI and/or ALI due to the risk factor. In such patients who have another ALI risk factor, the diagnosis of TRALI can be difficult. The National Heart Lung and Blood Institute Working Group recommended that critical care experts judge whether the new ALI is temporally associated with the transfusion, or whether the new ALI is temporally associated with worsening of the other ALI risk factor. The Canadian Consensus Conference proposed no such judgment evaluation and proposed the term “possible TRALI” for new ALI in a transfused patient who also has another ALI risk factor 19.
Currently there is no definitive laboratory test for the diagnosis of TRALI. Leucopenia or neutropenia has been observed in case reports 20 but has not been studied in small case series 21. Leukocyte antigen-antibody match between donor and recipient (HLA class I or II, granulocytes or monocytes), and neutrophil priming activity in donor blood have been reported but are not diagnostic 22.
Table 1. Risk factors for acute lung injury (ALI) in prospective studies
| Risk Factor | Incidence of acute lung injury (ALI) |
|---|---|
| Septic shock | 47% |
| — Pneumonia source | 35% |
| — Extrapulmonary source | 13% |
| Sepsis syndrome without hypotension | 29 % |
| — Pneumonia source | 24% |
| — Extrapulmonary source | 6% |
| Aspiration of gastric contents | 15%, 22%, 30%, 36% |
| Multiple transfusions | 36%, 36%, 24% |
| Near drowning | 33% |
| Disseminated intravascular coagulation (DIC) | 22% |
| Pulmonary contusion | 17%, 22 % |
| Pneumonia requiring ICU care | 12% |
| Drug overdose requiring ICU care | 9% |
| Fracture of long bones or pelvis | 5%, 8%, 11% |
| Burn, any percent of body surface | 2% |
| Cardiopulmonary bypass | 2% |
TRALI Practice Points:
- TRALI is a clinical diagnosis
- Suspect TRALI when new acute lung injury (ALI) develops during or within six hours of plasma-containing blood products transfusion
- Rule out other acute lung injury (ALI) risk factors such as sepsis and aspiration
- TRALI has been associated with all blood components that contain plasma
- Transfusion of even part of one unit has been associated with TRALI
TRALI causes
TRALI is caused by damage to pulmonary vasculature from neutrophil-mediated in forms of human neutrophil antigen (HNA) or human leukocyte antigen (HLA) antibodies in donor blood which bind to antigens of a recipient 23. Storage of blood products can accumulate proinflammatory mediators that can cause TRALI as well. A two-hit hypothesis applies in this clinical syndrome. Neutrophil sequestration occurs in the pulmonary vasculature, and neutrophils activate to damage the endothelial layer, causing leakage of protein and fluid into alveolar space 24.
Two-hit model
A two-hit hypothesis has been suggested for developing TRALI 25. The first hit involves neutrophil sequestration and priming in the pulmonary endothelium, which precedes transfusion. Endothelial cells are thought to be responsible for both neutrophil sequestration and priming. The second hit is caused by activation of the endothelium and neutrophils by mediators in the transfused blood, resulting in capillary leakage and pulmonary edema. The second hit differs in antibody-mediated TRALI and non-antibody-mediated TRALI. Non-antibody-mediated TRALI is caused by the accumulation of proinflammatory mediators during storage of blood products and possibly by ageing of RBCs and platelets 26, whereas antibody-mediated TRALI or immune TRALI is caused by passive transfusion of HLA or human neutrophil antigen (HNA) and corresponding antibodies from the donor directed against recipient antigens 27. Donor anti-leukocyte antibodies interact with recipient neutrophils, monocytes, and/or the pulmonary endothelium 28. Bioactive lipids and other factors in transfused blood act as biological response modifiers in patients with non-antibodymediated TRALI. The proportions of antibody-mediated vs. non-antibody-mediated TRALI cases remain unclear. A systemic review showed that 86% of TRALI cases are associated with leukocyte antibodies 29.
Threshold model
The two-hit hypothesis explains the occurrence of TRALI in high-risk patients. However, this hypothesis does not fit TRALI that develops in healthy recipients; the threshold model has been proposed to explain these cases 30. This model generally agrees that two hits are needed to develop TRALI. However, the threshold model assumes that TRALI can occur when the second hit is so strong that an initial priming event is not needed. In other words, a large quantity of antibody (second hit) can cause TRALI in a recipient with no previous predisposition, such as in healthy individuals.
Risk factors for TRALI
Specific risk factors for TRALI can be divided conceptually into recipient-related risk factors and transfusion-related risk factors 28.
Many comorbidities have been suggested as TRALI risk factors, including end-stage liver disease, coronary artery bypass graft, hematological malignancies, massive transfusion, mechanical ventilation, sepsis, and heavy alcohol consumption 28. Critical illness is one of the highest risks for TRALI 15. A large database review of 11 million elderly patients in the United States revealed that TRALI rates are higher in patients receiving a transfusion, including platelets or plasma 31. TRALI incidence increase as the number of units transfused increases. A review also identified that elderly female Caucasians 65-79-years-old with a 6-month history of post-inflammatory pulmonary fibrosis and tobacco use have a greater chance of developing TRALI 31.
Immunoglobulin 32 and stem cell preparations 33 are also associated with TRALI. A case-control study enrolled 89 patients with TRALI and 164 controls. The study revealed that a higher TRALI odds ratio is associated with plasma from female donors (odds ratio 4.5) and a greater number of transfusions (odds ratio 4.5) 34. Blood products including high-plasma-volume (platelet concentrates, whole blood, and FFP) have been associated with a higher incidence of TRALI 15. High loading of anti-human neutrophil antigen antibody has also been associated with TRALI 34. In contrast, the presence of anti-human leukocyte antigen (HLA) class II or anti-HLA class I antibodies is not associated with TRALI.
The age of a blood product has been suggested to increase the risk for TRALI 35. Storing red blood cells (RBCs) results in a number of morphological and biochemical alterations known as red blood cell storage lesions. However, two studies failed to reveal blood age as a risk factor for TRALI 36.
TRALI prevention
Cases suspected of TRALI should be promptly reported to the blood bank. The blood bank should investigate all associated donors for the presence of anti-HLA and anti-HNA antibodies to identify donors with these antibodies and prevent them from future donation. This is particularly true if the donor has anti-HNA 3a 37.
In addition, several general blood donor management strategies are in use to reduce the incidence of TRALI 38. First, adherence to current guidelines for utilizing blood components, particularly those for plasma, is mandatory to decrease exposure risk for patients. A restrictive transfusion strategy has been associated with a lower incidence of TRALI compared with that of a liberal transfusion strategy 39. Clifford et al. 40 reported that patients transfused with greater volumes of blood components develop perioperative TRALI/possible TRALI more frequently than that reported previously. He reported that the overall incidence of TRALI was 22.46 per 100,000 stays 40. Optimal transfusion guidelines should provide sufficient blood products to maximize clinical outcomes while avoiding adverse events, such as TRALI. However, no golden transfusion guidelines exist for all patients. Clinicians should consider many factors when deciding to transfuse anemic patients, rather than basing the decision solely on a specified laboratory level. In this respect, Carson et al. 41 demonstrated that liberal blood transfusion approach with a hemoglobin concentration threshold of 90-100 g/L does not affect mortality in a high-risk group of elderly patients with underlying cardiovascular disease or risk factors, compared with that of a restrictive transfusion strategy with a hemoglobin concentration threshold of 70-80 g/L. Therefore, the final decision to transfuse should incorporate the patient’s clinical condition, co-morbidities, and the individual wishes of the patient. Second, donors with little chance to be alloimmunized to leukocytes should receive high plasma volume components (e.g., FFP, plasma frozen within 24 h of phlebotomy [FP-24], plasma, cryo-reduced plasma, apheresis platelets, or whole blood). Third, use pooled solvent detergent-treated plasma as an alternative to FFP. Fourth, test for anti-HLA antibodies in pregnant donors before apheresis of platelets or plasma. In particular, several management strategies including deferral of multiparous female donors are in use to reduce the incidence of TRALI 42.
TRALI symptoms
TRALI presents as sudden onset of respiratory difficulties during or shortly after transfusion. Hypoxemia and lung infiltrates are detected on chest X-rays in almost all patients with TRALI, and half of patients show a pinkish, frothy sputum 43. Tachypnea, tachycardia, and elevated airway pressure are frequently observed. Fever, hypotension, and cyanosis occur in less than one-third of patients with TRALI.
TRALI diagnosis
Confirming hypoxemia, obtaining a chest X-ray (chest radiograph would show bilateral pulmonary infiltrates), and evaluating vital signs are required to diagnose TRALI.
Clinical characteristics of TRALI include acute dyspnea, hypoxemia, fever, hypotension, tachycardia, leukopenia, thrombocytopenia, and normal pulmonary artery occlusion pressure due to noncardiogenic pulmonary edema 44.
No laboratory test is specific for diagnosing TRALI. The greatest change is a transient drop in peripheral neutrophil count (possibly due to neutrophil sequestration in the pulmonary vasculature). Leucopenia occurs in 35% of patients after transfusion with an antibody-containing blood product 45.
TRALI should be distinguished from pulmonary edema due to other causes. Hemolytic and septic transfusion reactions present similarly to TRALI. Anaphylaxis can cause respiratory insufficiency similar to that of TRALI, but airway signs and symptoms are more common in patients with anaphylaxis. ALI/ARDS should be distinguished from TRALI in patients with risk factors for ALI/ARDS prior to a transfusion.
Transfusion-associated circulatory overload (TACO) is another cause of transfusion-related respiratory insufficiency (Table 2) 46. Impaired myocardial function and rapid and aggressive fluid therapy are suggested risk factors for transfusion-associated circulatory overload (TACO). TRALI is more likely to be associated with signs and symptoms of inflammation, including fever, hypotension, and an exudative pulmonary infiltrate. In contrast, TACO is more likely to be associated with findings suggestive of cardiac dysfunction and/or volume overload.
Table 2. Distinguishing TRALI from TACO
| TRALI | TACO | |
|---|---|---|
| Blood pressure | Low-normal | Normal-high |
| Body temperature | Normal-elevated | Normal |
| CXR | No vascular congestion | Vascular congestion, pleural effusion |
| BNP | Low (< 250 pg/ml) | High |
| PAOP | Low-normal | High |
| Ejection fraction | Normal function | Abnormal function |
| Response to diuretics | Inconsistent | Improved |
| Edema fluid | Transudate | Exudate |
Abbreviations: TRALI = transfusion-related acute lung injury; TACO = transfusion-associated cardiac overload; CXR = chest X-ray, BNP = B type natriuretic peptide; PAOP = pulmonary artery occluding pressure.
[Source 16 ]TRALI treatment
Immediate management of TRALI is to stop the transfusion immediately if TRALI is suspected and notify the blood bank to screen the donor unit for antileukocyte antibodies, anti-HLA or anti-neutrophil-specific antibodies to protect further unexpected victims 47. Management of TRALI is supportive measures to improve oxygenation. Providing oxygen supplementation is the central management approach for hypoxemia. Although there is no specific treatment for TRALI, limited evidence is available for managing patients with TRALI using mechanical ventilation 48, and it is logical to apply a lower tidal volume as TRALI’s pathophysiology is similar to acute lung distress syndrome (ARDS) 23. There is a case report of applying extracorporeal membrane oxygenation in a patient with severe TRALI 49. Corticosteroids have also been used for ALI/ARDS, but the results are inconsistent. Routine use of corticosteroids in patients with TRALI is not advocated, although a successful case has been reported 50.
Best practice is prevention. In the United Kingdom, an incidence of TRALI was substantially reduced by using plasma from male donors and screening female donors for HLA and HNA antibodies which are strong risk factors 23.
TRALI prognosis
Mortality due to TRALI is 5-10%, in contrast to ARDS 51. However, 90-day mortality associated with TRALI can reach 47% in critically ill populations 15. Long-term pulmonary function in TRALI survivors seems to be similar to that of patients who have never experienced TRALI, and no apparent late complications, such as fibrosis or other structural damage to the lung parenchyma occur as a result of TRALI 15. The clinical course of patients with TRALI is suggested by quick resolution (~2 days) of hypoxemia.
References- Suddock JT, Crookston KP. Transfusion Reactions. [Updated 2019 Feb 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482202
- Toy P, Lowell C. TRALI–definition, mechanisms, incidence and clinical relevance. Best Pract Res Clin Anaesthesiol. 2007;21(2):183–193. doi:10.1016/j.bpa.2007.01.003 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2767181
- Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577.
- Holness L, Knippen MA, Simmons L, Lachenbruch PA. Fatalities caused by TRALI. Transfus Med Rev. 2004;18:184–188.
- Toy P, Popovsky MA, Abraham E, Ambruso DR, Holness LG, Kopko PM, McFarland JG, Nathens AB, Silliman CC, Stroncek D., National Heart, Lung and Blood Institute Working Group on TRALI. Transfusion-related acute lung injury: definition and review. Crit. Care Med. 2005 Apr;33(4):721-6.
- Kim KN, Kim DW, Jeong MA. The usefulness of a classification and regression tree algorithm for detecting perioperative transfusion-related pulmonary complications. Transfusion. 2015 Nov;55(11):2582-9.
- Khan H, Belsher J, Yilmaz M, Afessa B, Winters JL, Moore SB, Hubmayr RD, Gajic O. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007 May;131(5):1308-14.
- Roubinian N. TACO and TRALI: biology, risk factors, and prevention strategies. Hematology Am Soc Hematol Educ Program. 2018 Nov 30;2018(1):585-594.
- Benson AB, Austin GL, Berg M, McFann KK, Thomas S, Ramirez G, et al. Transfusion-related acute lung injury in ICU patients admitted with gastrointestinal bleeding. Intensive Care Med. 2010;36:1710–1717.
- Rana R, Fernández-Pérez ER, Khan SA, Rana S, Winters JL, Lesnick TG, et al. Transfusion-related acute lung injury and pulmonary edema in critically ill patients: a retrospective study. Transfusion. 2006;46:1478–1483.
- Silliman CC, Boshkov LK, Mehdizadehkashi Z, Elzi DJ, Dickey WO, Podlosky L, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–462.
- Jaworski K, Maślanka K, Kosior DA. Transfusion-related acute lung injury: a dangerous and underdiagnosed noncardiogenic pulmonary edema. Cardiol J. 2013;20:337–344.
- Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176:886–891.
- Looney MR, Gropper MA, Matthay MA. Transfusion-related acute lung injury: a review. Chest. 2004;126:249–258.
- Vlaar AP, Binnekade JM, Prins D, van Stein D, Hofstra JJ, Schultz MJ, et al. Risk factors and outcome of transfusion-related acute lung injury in the critically ill: a nested case-control study. Crit Care Med. 2010;38:771–778.
- Kim J, Na S. Transfusion-related acute lung injury; clinical perspectives. Korean J Anesthesiol. 2015;68(2):101–105. doi:10.4097/kjae.2015.68.2.101 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4384395
- Vlaar AP, Juffermans NP. Transfusion-related acute lung injury: a clinical review. Lancet. 2013;382:984–994.
- Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: Definition and review. Crit Care Med. 2005;33:721–726.
- Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–1789.
- Nakagawa M, Toy P. Acute and transient decrease in neutrophil count in transfusion-related acute lung injury: cases at one hospital. Transfusion. 2004;44:1689–1694.
- Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–462.
- Silliman CC, Dickey WO, Paterson AJ, et al. Analysis of the priming activity of lipids generated during routine storage of platelet concentrates. Transfusion. 1996;36:133–139.
- Cho MS, Sharma S. Transfusion-related Acute Lung Injury (TRALI) [Updated 2019 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507846
- Sachs UJ. Recent insights into the mechanism of transfusion-related acute lung injury. Curr. Opin. Hematol. 2011 Nov;18(6):436-42.
- Silliman CC. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34(5 Suppl):S124–S131.
- Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, et al. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113:1158–1166.
- Curtis BR, McFarland JG. Mechanisms of transfusion-related acute lung injury (TRALI): anti-leukocyte antibodies. Crit Care Med. 2006;34(5 Suppl):S118–S123.
- Sachs UJ. Recent insights into the mechanism of transfusion-related acute lung injury. Curr Opin Hematol. 2011;18:436–442.
- Middelburg RA, van Stein D, Briët E, van der Bom JG. The role of donor antibodies in the pathogenesis of transfusion-related acute lung injury: a systematic review. Transfusion. 2008;48:2167–2176.
- Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI) Br J Haematol. 2007;136:788–799.
- Menis M, Anderson SA, Forshee RA, McKean S, Johnson C, Warnock R, et al. Transfusion-related acute lung injury and potential risk factors among the inpatient US elderly as recorded in Medicare claims data, during 2007 through 2011. Transfusion. 2014;54:2182–2193.
- Rizk A, Gorson KC, Kenney L, Weinstein R. Transfusion-related acute lung injury after the infusion of IVIG. Transfusion. 2001;41:264–268.
- Ganguly S, Carrum G, Nizzi F, Heslop HE, Popat U. Transfusion-related acute lung injury (TRALI) following allogeneic stem cell transplant for acute myeloid leukemia. Am J Hematol. 2004;75:48–51.
- Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–1767
- Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239.
- Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–1767.
- Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–1789.
- Ozier Y, Muller JY, Mertes PM, Renaudier P, Aguilon P, Canivet N, et al. Transfusion-related acute lung injury: reports to the French Hemovigilance Network 2007 through 2008. Transfusion. 2011;51:2102–2110.
- Tuinman PR, Vlaar AP, Cornet AD, Hofstra JJ, Levi M, Meijers JC, et al. Blood transfusion during cardiac surgery is associated with inflammation and coagulation in the lung: a case control study. Crit Care. 2011;15:R59.
- Clifford L, Jia Q, Subramanian A, Yadav H, Wilson GA, Murphy SP, et al. Characterizing the epidemiology of postoperative transfusion-related acute lung injury. Anesthesiology. 2015;122:12–20.
- Carson JL, Sieber F, Cook DR, Hoover DR, Noveck H, Chaitman BR, et al. Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial. Lancet. 2014 [Epub ahead of print].
- Eder AF, Herron RM, Jr, Strupp A, Dy B, White J, Notari EP, et al. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006-2008) Transfusion. 2010;50:1732–1742.
- van Stein D, Beckers EA, Sintnicolaas K, Porcelijn L, Danovic F, Wollersheim JA, et al. Transfusion-related acute lung injury reports in the Netherlands: an observational study. Transfusion. 2010;50:213–220.
- Skeate RC, Eastlund T. Distinguishing between transfusion related acute lung injury and transfusion associated circulatory overload. Curr. Opin. Hematol. 2007 Nov;14(6):682-7.
- Fadeyi EA, De Los Angeles Muniz M, Wayne AS, Klein HG, Leitman SF, Stroncek DF. The transfusion of neutrophil-specific antibodies causes leukopenia and a broad spectrum of pulmonary reactions. Transfusion. 2007;47:545–550.
- Gajic O, Gropper MA, Hubmayr RD. Pulmonary edema after transfusion: how to differentiate transfusion-associated circulatory overload from transfusion-related acute lung injury. Crit Care Med. 2006;34(5 Suppl):S109–S113.
- Marik PE, Corwin HL. Acute lung injury following blood transfusion: expanding the definition. Crit. Care Med. 2008 Nov;36(11):3080-4.
- Goldberg AD, Kor DJ. State of the art management of transfusion-related acute lung injury (TRALI) Curr Pharm Des. 2012;18:3273–3284.
- Worsley MH, Sinclair CJ, Campanella C, Kilpatrick DC, Yap PL. Non-cardiogenic pulmonary oedema after transfusion with granulocyte antibody containing blood: treatment with extracorporeal membrane oxygenation. Br J Anaesth. 1991;67:116–119.
- Jeddi R, Mansouri R, Kacem K, Gouider E, Abid HB, Belhadjali Z, et al. Transfusion-related acute lung injury (TRALI) during remission induction course of acute myeloid leukemia: a possible role for all-transretinoic-acid (ATRA)? Pathol Biol (Paris) 2009;57:500–502.
- Moore SB. Transfusion-related acute lung injury (TRALI): clinical presentation, treatment, and prognosis. Crit Care Med. 2006;34(5 Suppl):S114–S117.