Trichomoniasis
Trichomoniasis is a very common sexually transmitted disease (STD) caused by a tiny protozoan parasite called Trichomonas vaginalis. Trichomoniasis is usually spreads during sex, but can also be spread by sharing wet towels or washers. Trichomoniasis mostly affects women, but men can get it too. Most people who have trichomoniasis do not have any symptoms.
Trichomoniasis is the most common curable STD (sexually transmitted disease). In the United States, an estimated 3.7 million people have the infection 1. However, only about 30% develop any symptoms of trichomoniasis. Trichomoniasis is more common in women than in men. Older women are more likely than younger women to have been infected with trichomoniasis.
Although symptoms of trichomoniasis vary, most people who have the Trichomonas vaginalis parasite cannot tell they are infected. Your doctor or sexual health clinic can test for and treat trichomoniasis.
Clinical manifestations in symptomatic pubertal or postpubertal females may include a diffuse foul-smelling (smelly fishy odor) vaginal discharge and vulvovaginal itch and irritation. Pain with urination or sexual intercourse and, less often, lower abdominal pain can occur. Vaginal discharge may be any color, but classically is yellow-green, frothy, and malodorous. The vulva and vaginal mucosa can be reddish and swollen. The cervix can be inflamed and sometimes is covered with numerous punctate cervical hemorrhages and swollen papillae, referred to as “strawberry” cervix. This finding occurs in less than 5% of infected females but is highly suggestive of trichomoniasis. Clinical manifestations in symptomatic men include urethritis (urethra inflammation) and, rarely, epididymitis or prostatitis. Reinfection is common, and resistance to treatment is rare but increasing. Rectal infections are uncommon, and oral infections have not been described.
The prevalence of Trichomonas vaginalis infection in the United States is estimated to be 2.3 million (3.1%) among women ages 14-49, based on a nationally representative sample of women who participated in National Health and Examination Survey (NHANES) 2001–2004. The following are other findings from this study 2:
- An estimated 3.7 million women and men are infected with Trichomonas vaginalis in the United States 3
- Most women found to have trichomoniasis (85%) reported no symptoms.
- Women with no history of sexual intercourse can still be affected by trichomoniasis (1.0%), as can pregnant women (3.2%), and women who have ever been pregnant (4.1%).
- African American women had a prevalence of 13.3%, white women prevalence of 1.3%, and Mexican American women prevalence of 1.8%.
- Prevalence of trichomoniasis increases with age and lifetime number of sexual partners among African American women.
It is not possible to diagnose trichomoniasis based on your symptoms alone. For both men and women, your doctor or sexual health care provider can examine you and get a laboratory test to diagnose trichomoniasis.
Trichomoniasis can be cured with a single dose of prescription antibiotic medication (either metronidazole or tinidazole or or secnidazole), pills which can be taken by mouth. It is okay for pregnant women to take this medication. Some people who drink alcohol within 24 hours after taking this kind of antibiotic can have uncomfortable side effects.
People who have been treated for trichomoniasis can get it again. About 1 in 5 people get infected again within 3 months after treatment. To avoid getting reinfected, make sure that all of your sex partners get treated too, and wait to have sex again until all of your symptoms go away (about a week). Get checked again if your symptoms come back. You can reduce your risk of infection by using condoms correctly every time you have sex.
If your symptoms don’t go away after treatment, talk to your doctor or sexual health worker.
Without treatment, trichomoniasis can last months, or even years. Trichomoniasis can cause premature labor, low birth weight, or increase your risk of getting HIV, the virus that causes AIDS.
Trichomoniasis key facts
- Trichomoniasis can be asymptomatic in men and women, and may persist silently for years.
- Untreated trichomoniasis is associated with adverse pregnancy outcomes such as premature rupture of the membranes, preterm delivery, and low birthweight infants.
- Douching may worsen vaginal discharge in patients with trichomoniasis.
- Alcohol consumption is contraindicated with metronidazole and tinidazole.
- Transmission Issues:
- Trichomoniasis is almost always sexually transmitted.
- Sex partners should be treated.
- Patients should abstain from intercourse until they and their sex partners are cured (about 7 days).
- Trichomoniasis has been associated with increased susceptibility to HIV acquisition and transmission.
- Risk Reduction:
- Individualize risk-reduction plans with each patient.
- Prevention strategies include abstinence, mutual monogamy with an uninfected partner, use of condoms, and limiting the number of sex partners.
- Latex condoms, when used consistently and correctly, can reduce the risk of transmission of Trichomonas vaginalis.
- Douching should be avoided since it increases the risk for trichomoniasis.
- Male circumcision reduces the risk of trichomoniasis.
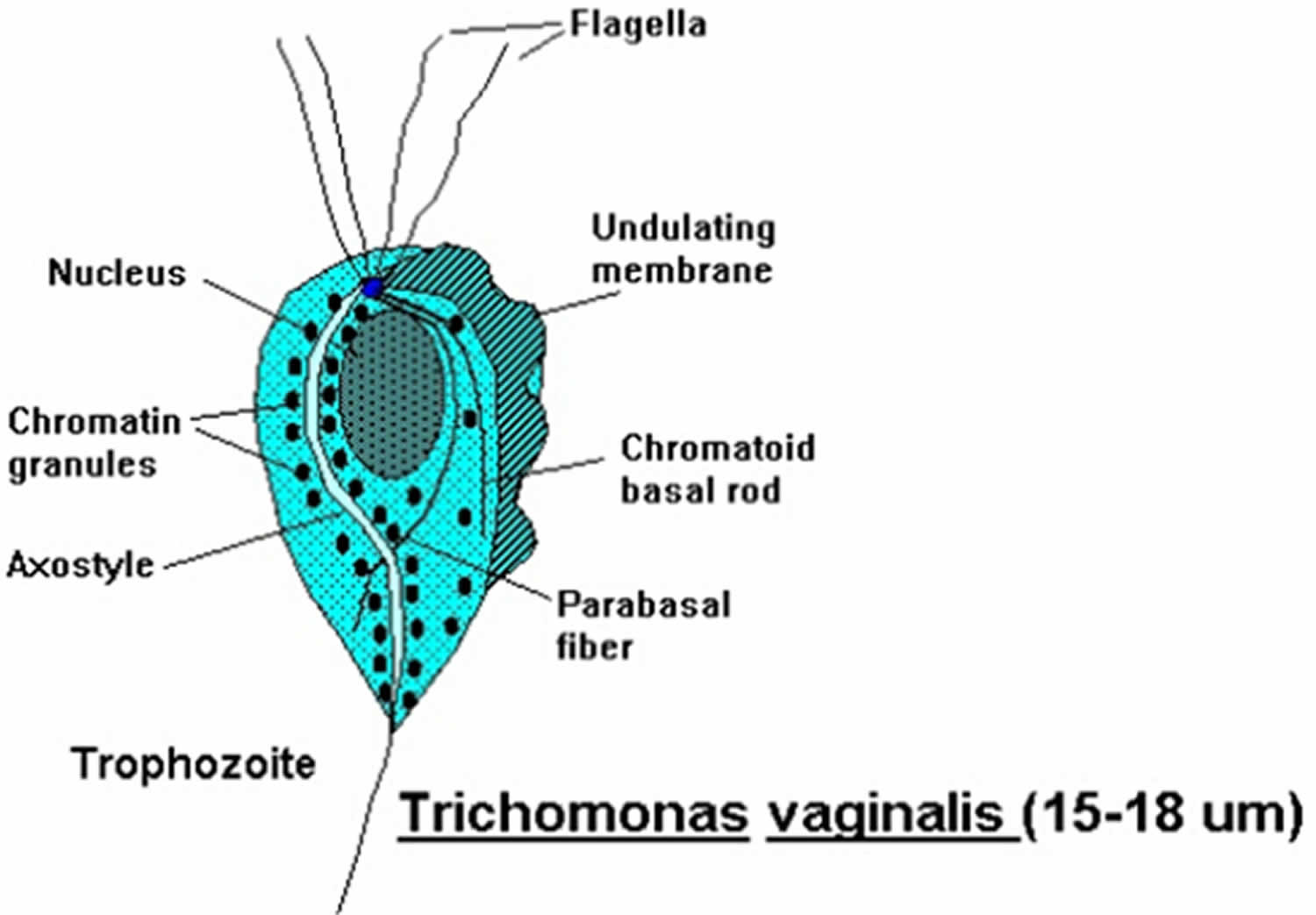
Figure 1. Trichomonas vaginalis
Footnote: Trichomonas vaginalis is a pear-shaped flagellated protozoan parasitic organism that is approximately 10 by 7 micrometers. The organism achieves a quivering motion via the anterior flagella and the undulating membrane. After attaching to vaginal epithelial cells, the organism takes on a more ameboid-like appearance.
[Source 4]Trichomoniasis in men
Trichomonas vaginalis may cause up to 11% to 13% of nongonococcal urethritis (NGU) in males, but urethral infection in males is frequently asymptomatic 5. Men with Trichomonas vaginalis infection may also present with prostatitis or epididymitis 6.
Trichomoniasis rarely causes symptoms in men. When men do have signs and symptoms, however, they might include:
- Irritation inside the penis
- Burning with urination or after ejaculation
- Discharge from the penis
How does trichomoniasis affect a pregnant woman and her baby?
Pregnant women with trichomoniasis are more likely to have their babies too early (preterm delivery) 7. Also, babies born to infected mothers are more likely to have a low birth weight (less than 5.5 pounds) 8.
How do you get trichomoniasis?
The Trichomonas vaginalis parasite passes from an infected person to an uninfected person during sex. In women, the most commonly infected part of the body is the lower genital tract (vulva, vagina, cervix, or urethra). In men, the most commonly infected body part is the inside of the penis (urethra). During sex, the parasite usually spreads from a penis to a vagina, or from a vagina to a penis. Trichomoniasis can also spread from a vagina to another vagina. It is not common for the Trichomonas vaginalis parasite to infect other body parts, like the hands, mouth, or anus. It is unclear why some people with the trichomoniasis infection get symptoms while others do not. It probably depends on factors like a person’s age and overall health. Infected people without symptoms can still pass the infection on to others.
Resumption of Sexual Activity
Patients should be instructed to avoid sex until they and their sex partners have been treated, and until they no longer have any symptoms of trichomoniasis. This usually takes about 7 days.
Post-Treatment Follow-Up
All sexually active women who are diagnosed and treated for Trichomonas vaginalis infection (including pregnant women and women with HIV infection) should be retested 3 months after initial treatment to evaluate the possibility of reinfection. Retesting in men is not routinely recommended.
Trichomoniasis symptoms
About 70% of infected people with trichomoniasis do not have any signs or symptoms. When trichomoniasis does cause symptoms, they can range from mild irritation to severe inflammation. Some people with symptoms get them within 5 to 28 days after being infected. Others do not develop symptoms until much later. Symptoms can come and go.
Trichomoniasis symptoms in men
Men don’t usually get symptoms from trichomoniasis, but there may be a discharge from the penis, or pain when urinating 9.
Men with trichomoniasis may notice:
- Itching or irritation inside the penis;
- Burning after urination or ejaculation;
- Discharge from the penis.
Trichomoniasis in women
Women with trichomoniasis may notice:
- Itching, burning, redness or soreness of the genitals;
- Vaginal itching or burning;
- Pain during sex;
- Discomfort with urination;
- Pain low in the tummy;
- A change in their vaginal discharge (i.e., thin discharge or increased volume) that can be clear, frothy, white, yellowish, greyish or greenish with an unusual fishy smell.
Having trichomoniasis can make it feel unpleasant to have sex, but many women are asymptomatic 10. Without treatment, the infection can last for months or even years.
Chronic Trichomonas vaginalis infection may be associated with minimal vaginal discharge, mild itch and/or pain during sex (dyspareunia) 10. The presence of cervical punctate hemorrhages (Figure 2), often referred to as a “strawberry cervix,” strongly suggests a diagnosis of trichomoniasis, but this occurs in fewer than 5% of women with trichomoniasis 10, 11.
Figure 2. Trichomoniasis and Cervical Petechiae (“strawberry cervix”)
Footnote: This photograph shows multiple petechiae on the cervix of a women with trichomoniasis. This manifestation in a woman with vaginal discharge strongly suggests a diagnosis of trichomoniasis and is often referred to as a ‘strawberry cervix‘.
[Source 4 ]Trichomoniasis in pregnancy
Infection with Trichomonas vaginalis in pregnant women is associated with both obstetrical and gynecologic adverse outcomes, including low birth weight, premature rupture of membranes (PPROM) and preterm labor; trichomoniasis in pregnancy increases the risk of preterm birth by about 30% 12, 13, 14. Neonatal trichomoniasis is unusual but can occur 15, 16.
Trichomoniasis in people with HIV
Among women with HIV, more than half are coinfected with Trichomonas vaginalis, and they have been shown to have an increased risk for pelvic inflammatory disease (PID) and for shedding of HIV in the genital tract 17, 18. Antiretroviral therapy appears to lessen the potentiating effects of trichomoniasis infections on HIV transmission risk 17. Infection with HIV does not make a woman more likely to have persistent or recurrent trichomoniasis 19.
Trichomoniasis causes
Trichomoniasis is caused by Trichomonas vaginalis, which is a single-celled flagellated anaerobic protozoan parasite, a type of tiny parasite that travels between people during sexual intercourse. The incubation period between exposure and infection is unknown, but it’s thought to range from five to 28 days.
Trichomonas vaginalis is the only known protozoan parasite that infects the genital tract. Trichomonas vaginalis has four anterior flagella and one flagellum embedded in an undulating membrane (Figure 1) 20. The flagella are responsible for the jerky motility of this organism that is seen under a microscope. After attaching to vaginal epithelial cells, this globular, pear-shaped organism transforms into a thin, flat, ameboid shape 21. Trichomoniasis is almost always sexually transmitted; fomite transmission is extremely rare. T. vaginalis may persist for months to years in epithelial crypts and periglandular areas of the genital tract 20. Distinguishing persistent, subclinical infection from remote sexual acquisition is not always possible.
Risk factors for trichomoniasis
Investigators have identified multiple risk factors associated with trichomoniasis that include the following 22:
- Older age
- Multiple sex partners
- Drug use (marijuana, crack cocaine, alcohol, cigarettes)
- Sex without a condom
- Presence of sexually transmitted infections (STIs) at baseline
- Low socioeconomic status
- Douching
- Black race.
Trichomoniasis complications
Trichomoniasis can increase the risk of getting or spreading other sexually transmitted infections (STIs). For example, trichomoniasis can cause genital inflammation that makes it easier to get infected with human immunodeficiency virus (HIV), the virus that causes acquired immunodeficiency syndrome (AIDS), or to pass the HIV virus on to a sex partner 23.
Pregnant women who have trichomoniasis might:
- Deliver too early (prematurely)
- Have a baby with a low birth weight
- Give the infection to the baby as the baby passes through the birth canal
Trichomoniasis is also associated with an increased risk of cervical or prostate cancer.
Untreated, trichomoniasis infection can last for months to years.
Trichomoniasis prevention
The only way to avoid STDs (sexually transmitted diseases) is to not have vaginal, anal, or oral sex. The best way to avoid trichomoniasis is by using condoms every time you have sex. Safe sex also helps stop the spread of other sexually transmitted diseases (STDs).
To prevent re-infection with trichomoniasis, make sure any partner is treated too. It is best to avoid having sex for seven days after your or your partner’s treatment to lower the chance of you both getting the infection again.
If you are sexually active, you can do the following things to lower your chances of getting trichomoniasis:
- Be in a long-term mutually monogamous relationship with a partner who has been tested and has negative STD test results;
- Use latex condoms the right way every time you have sex. This can lower your chances of getting trichomoniasis. But the parasite can infect areas that are not covered by a condom – so condoms may not fully protect you from getting trichomoniasis.
Another approach is to talk about the potential risk of STDs before you have sex with a new partner. That way you can make informed choices about the level of risk you are comfortable taking with your sex life.
If you or someone you know has questions about trichomoniasis or any other STD, talk to a health care provider.
Trichomoniasis diagnosis
Your doctor is likely to take a sample from inside the vagina to test for trichomoniasis. Men can be checked with a urine test or a swab from the opening of the penis.
If the Trichomonas vaginalis parasite can be seen under the microscope, no further tests are needed.
If the test doesn’t show the parasite, but your doctor thinks you may have trichomoniasis, other tests may be done. Your doctor may order tests done on a sample of vaginal fluid, a penis uretheral swab or sometimes urine. Tests include a rapid antigen test and nucleic acid amplification test (NAAT).
If you have trichomoniasis, your doctor may also want to test you for other sexually transmitted infections (STIs) so they can also be treated.
Trichomoniasis test
In clinical practice, wet mount preparation has been the most commonly used method for diagnosing trichomoniasis, primarily because of the low cost, convenience, and point-of-care diagnosis 24, 25. The wet mount preparation however has a sensitivity (44 to 68%) that is significantly lower than with newer molecular nucleic acid amplification tests (NAATs) 26. Papanicolaou (PAP) testing is not considered an appropriate diagnostic tool for trichomoniasis; if Trichomonas vaginalis infection is identified on routine Papanicolaou (PAP) testing, a standard trichomonas diagnostic test should be used to verify infection 27. The following summarizes the major methods used to diagnose trichomoniasis.
Wet Mount Preparation
The traditional diagnostic method for trichomoniasis has been wet mount with microscopic visualization of motile Trichomonas vaginalis parasites on slide preparations from vaginal or urethral secretions. Ideally, specimens should be examined within 10 minutes to observe motile parasites, which are diagnostic. Wet mount is an inexpensive diagnostic test; however, sensitivity is estimated at 51-65%, and varies based on the individual performing the test and how promptly the slide is interpreted 28.
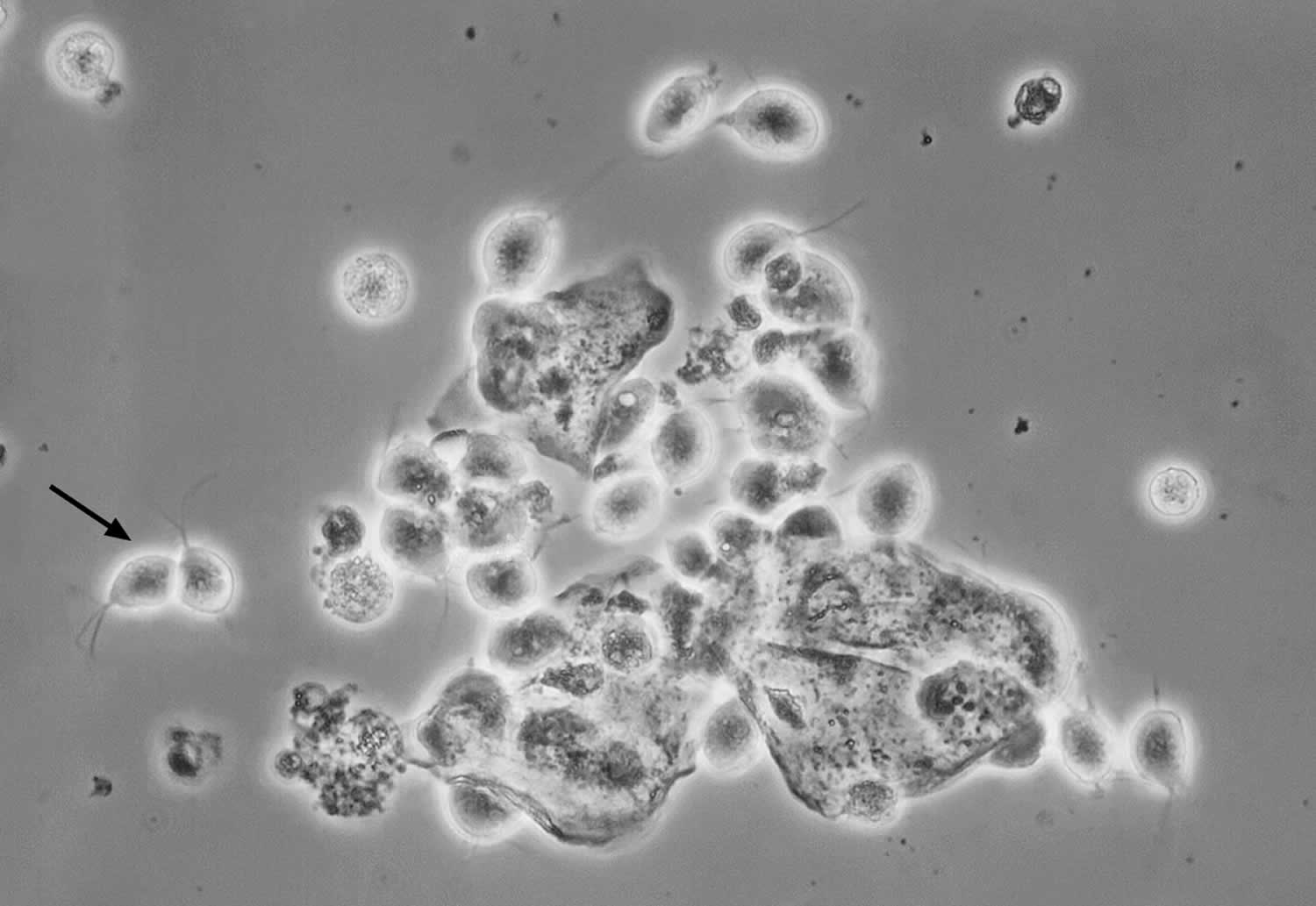
Figure 3. Trichomonas vaginalis on Wet Mount preparation
Footnote: This photomicrograph taken of a vaginal discharge wet mount sample shows numerous oval Trichomonas vaginalis protozoan parasites; the black arrow on left indicates two characteristic Trichomonas vaginalis organisms (the thin flagellum can be faintly seen).
[Source 29 ]Culture
Obtaining a culture using modified Diamond’s medium was the previous gold standard for diagnosis of trichomoniasis prior to the availability of highly sensitive NAATs. Culture has been considered the gold standard for diagnosis of trichomoniasis with a specificity approaching 100%, but it is not widely used and its sensitivity can be as low as 75–96% 28. Culture is a more sensitive diagnostic tool than wet mount alone, but results are not immediately available. Clinical specimens can be inoculated into transport systems such as Amies gel medium to maintain viability for up to 24 hours at room temperature 30. Specialized culture systems (i.e. InPouch System [Biomed Diagnostics]) are available to allow for transport of cultures when shipping to an off-site laboratory. Additionally, these systems can be used to transport specimens after inoculation. Such systems are useful when immediate transportation of specimens to the laboratory is not available. The specimen should be inoculated as soon as possible (within an hour of collection) to maintain viability of the organism.
Culture may be used for diagnosing Trichomonas vaginalis in both men and women. Culture in men may be performed on samples of urethral secretions, urine sediment, or semen, but testing in women requires sampling of vaginal secretions, as the sensitivity is low in urine culture 31. Trichomonas vaginalis culture is categorized by the Clinical Laboratory Improvement Amendments (CLIA) as moderately complex as it is time-consuming and requires incubation 26. If Trichomonas vaginalis is isolated in culture, drug susceptibility testing can be performed, particularly in cases of persistent infection.
Nucleic Acid Amplification Testing (NAAT)
Several NAAT-based methods are available for diagnosis of Trichomonas vaginalis, including transcription-mediated amplification and polymerase chain reaction (PCR). There are no data to suggest Trichomonas vaginalis causes anorectal infection, and therefore use of NAAT to detect Trichomonas vaginalis anorectal infection is not recommended 31, 32, 33.
- Aptima Trichomonas vaginalis Assay (Becton Dickinson): This assay uses transcription-mediated amplification for detection of Trichomonas vaginalis RNA 9. This test is FDA-cleared for detection of Trichomonas vaginalis in symptomatic and asymptomatic women 34. The test can be performed on clinician-collected vaginal swabs, clinician-collected endocervical swabs, female urine specimens, or liquid endocervical Pap smear specimens collected in PreservCyt Solution 35. The Aptima Trichomonas vaginalis assay has a sensitivity of 95.3 to 100% and specificity of 95.2 to 100%, which are considerably higher than wet mount or culture 36, 9, 37. The Aptima Trichomonas vaginalis assay is not FDA-cleared for use in men, but it may be used to test urine or urethral swabs from men if the assay is internally validated in accordance with CLIA regulations 34.
- Probe Tec TV Qx Amplified DNA Assay (Becton-Dickinson): This assay uses Strand Displacement Amplification technology and is FDA-cleared for detection of Trichomonas vaginalis from vaginal swabs (clinician-collected or self-collected), endocervical swabs (clinician-collected), and female urine specimens 34. This assay can be used to detect Trichomonas vaginalis in symptomatic and asymptomatic females. The Probe Tec TV Qx Amplified DNA assay has a sensitivity of 98.3% and specificity of 99.6% for detecting Trichomonas vaginalis 38.
- GeneXpert TV (Cepheid): This PCR-based NAAT is FDA-cleared for detection of Trichomonas vaginalis genomic DNA using self-collected or clinician-collected vaginal specimens, female urine specimens, clinician-collected endocervical swab specimens, and male urine specimens 34, 39. With this assay, the results are available within 63 minutes; for samples that have a clear positive result after 45 PCR cycles have been completed, the Early Assay Termination function will provide the positive result earlier, typically within 40 minutes 39. The GeneXpert TV has a sensitivity of 99.5 to 100% and a specificity of 99.4 to 99.9% 40.
- Max CTGCTV2 Assay (Becton Dickinson): This FDA-cleared PCR assay for gonorrhea and chlamydia has been modified to test concurrently for Trichomonas vaginalis 41. This test is FDA-cleared for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis from vaginal specimens (self-collected or clinician-collected), female urine specimens, and male urine specimens 34. The Max CTGCTV2 Assay has a sensitivity of 96.2 to 100% and specificity of 99.1 to 100% for detecting Trichomonas vaginalis 40.
- Cobas TV/MG (Roche Diagnostics): This PCR-based NAAT is FDA-cleared for detection of Trichomonas vaginalis and M. genitalium using vaginal specimens (self-collected or clinician-collected), clinician-collected endocervical swab specimens, female urine specimens, male urine specimens, and clinician-collected meatal specimens 42. This assay is FDA-cleared for both symptomatic and asymptomatic patients. The Cobas TV/MG has a sensitivity and specificity greater than 99.5% 42, 43.
Point-of-Care Testing
There are multiple point-of-care tests available for diagnosing trichomoniasis in women 44:
- Osom Trichomonas Rapid Test (Sekisui Diagnostics): This is an antigen-detection point-of-care test for use with clinician-collected vaginal samples. The test requires about 10 to 15 minutes for test results to become available; this test has a sensitivity of 82 to 95% and a specificity of 97 to 100% 45. The Osom Trichomonas Rapid Test is not FDA-cleared for use in men.
- Solana Trichomonas Assay (Quidel): This point-of-care test uses isothermal Helicase-Dependent Amplification technology to detect Trichomonas vaginalis DNA from asymptomatic and symptomatic female urine specimens or clinician-collected vaginal specimens, with a sensitivity greater than 92% for urine specimens and greater than 98% for vaginal samples 46. Results are available within 40 minutes. This test is not FDA-cleared for use in men.
- Sexual Health Click Test (Visby Medical): This point-of-care PCR test is a single-use, disposable test that can detect chlamydia, gonorrhea, and trichomonas using self-collected vaginal swabs 47. The compact test device provides results within 30 minutes. In an analysis of self-collected vaginal swabs obtained in 1,449 women, this test had a sensitivity of 99.2% and specificity of 96.9% for detection of Trichomonas vaginalis 47. This assay received FDA clearance and a CLIA waiver in August 2021.
Trichomoniasis treatment
Trichomoniasis can be treated with oral antibiotic (either metronidazole or tinidazole or secnidazole). These pills are taken by mouth. It is safe for pregnant women to take this medication. Don’t drink alcohol for 24 hours after taking metronidazole, 48 hours after taking secnidazole or 72 hours after taking tinidazole. Drinking alcohol during and for a few days after treatment can cause severe nausea and vomiting.
Oral antibiotic options may include:
- Megadose. Your doctor may recommend one large dose (megadose) of either metronidazole (Flagyl), tinidazole (Tindamax) or secnidazole (Solosec). You only take these oral medications one time.
- Multiple doses. Your provider might recommend several lower doses of metronidazole or tinidazole. You take the pills two times a day for seven days. To help clear up the infection completely, keep taking this medicine for the full time your provider prescribed the drug, even if you begin to feel better after a few days. If you stop using this medicine too soon, your infection may not go away completely.
Recommended oral antibiotic regimen for Women 34:
- Metronidazole 500 mg orally twice a day for 7 days
Recommended oral antibiotic regimen for Men 34:
- Metronidazole 2 g orally in a single dose
Alternative oral antibiotic regimen for Women and Men 34:
- Tinidazole2 g orally in a single dose
People who have been treated for trichomoniasis can get it again. About 1 in 5 people get infected again within 3 months after receiving treatment. To avoid getting reinfected, make sure that all of your sex partners get treated. Also, wait 7- 10 days after you and your partner have been treated to have sex again. This usually takes about a week after finishing the last antibiotic dose. Get checked again if your symptoms come back.
Your doctor will typically retest you for trichomoniasis after treatment. A retest two weeks to three months after treatment can check to be sure the infection is gone and that you haven’t been reinfected.
Even if you’ve had treatment that gets rid of trichomoniasis, it’s possible to get it again if you’re exposed to someone with the infection.
Trichomoniasis cure
Recommended oral antibiotic regimen for Women 34:
- Metronidazole 500 mg orally twice a day for 7 days
Recommended oral antibiotic regimen for Men 34:
- Metronidazole 2 g orally in a single dose
Alternative oral antibiotic regimen for Women and Men 34:
- Tinidazole2 g orally in a single dose
The nitroimidazoles are the only class of antimicrobial medications known to be effective against Trichomonas vaginalis infections. Of these drugs, metronidazole and tinidazole have been cleared by FDA for the oral or parenteral treatment of trichomoniasis 48. Tinidazole is generally more expensive, reaches higher levels in serum and the genitourinary tract, has a longer half-life than metronidazole (12.5 hours versus 7.3 hours), and has fewer gastrointestinal side effects 49. In randomized clinical trials, recommended metronidazole regimens have resulted in cure rates of approximately 84%–98% 49, and the recommended tinidazole regimen has resulted in cure rates of approximately 92%–100% 50. Randomized controlled trials comparing single 2 g doses of metronidazole and tinidazole suggest that tinidazole is equivalent or superior to metronidazole in achieving parasitologic cure and resolution of symptoms 51.
Metronidazole gel does not reach therapeutic levels in the urethra and perivaginal glands. Because it is less efficacious than oral metronidazole, it is not recommended.
Treatment of persistent or recurrent trichomoniasis
The most likely reasons for persistent or recurrent trichomoniasis are reinfection from an untreated partner or lack of adherence with treatment, but in some individuals, antimicrobial-resistant Trichomonas vaginalis infection can occur. Currently, rates of metronidazole resistance range from 4 to 10%, and the rate of tinidazole resistance is about 1% 52, 53. Tinidazole retains activity against many metronidazole-refractory strains. The following summarizes the approach to treatment in persons with persistent or recurrent trichomoniasis 34.
- Treatment Failure with Reexposure: For women and men who received standard treatment for trichomoniasis and have treatment failure due to reexposure from an untreated partner, retreatment should consist of the same regimen they initially received 34.
- Treatment Failure without Reexposure: For men who have treatment failure after receiving an initial single-dose therapy of metronidazole 2 grams orally (and reexposure has not occurred), the recommended retreatment is metronidazole 500 mg orally twice daily for 7 days 34. For women who have failed the initial regimen of metronidazole 500 mg twice daily for 7 days and have not been reexposed, repeat treatment should be given with a 7-day regimen of either metronidazole 2 grams given once per day or tinidazole 2 grams once per day 34. Tinidazole should not be used in pregnant women.
- Treatment Failure after Second-Line Treatment without Reexposure: If an individual experiences persistent infection after receiving treatment with a second-line regimen (and reexposure to a partner with trichomoniasis has not occurred), the clinician should request a special kit from the Centers for Disease Control and Prevention for 5-nitroimidazole drug-resistance testing (Trichomonas Susceptibility Testing) 34.
- Treatment of 5-Nitroimidazole-Resistant Trichomonas: If drug-resistance testing reveals nitroimidazole resistance and treatment with a 7-day regimen of either metronidazole 2 grams given once per day or tinidazole 2 grams once per day has been unsuccessful, then the next option is oral tinidazole 2 grams daily plus intravaginal tinidazole 500 mg twice daily for 14 days 34. If this option fails, then consider using high-dose oral tinidazole (1 gram 3 times daily) plus intravaginal paromomycin (4 grams of 6.25% intravaginal paromomycin cream nightly) for 14 days 34. Note that tinidazole should not be used in pregnant women. Intravaginal boric acid has been used to treat trichomoniasis in women allergic to nitroimidazole and thus could be considered as an option for women with treatment-refractory nitroimidazole-resistant trichomoniasis 34.
Other Management Considerations
You should abstain from sex until you and your sex partners are treated and until you and your partners no longer have any symptoms of trichomoniasis. This usually takes about 7 days after completion of treatment 34.
Testing for other STDs including HIV should be performed in people infected with Trichomonas vaginalis.
Follow-up
Because of the high rate of reinfection among women treated for trichomoniasis (17% within 3 months in one study) 54, retesting for T. vaginalis is recommended for all sexually active women within 3 months following initial treatment regardless of whether they believe their sex partners were treated. Testing by nucleic acid amplification can be conducted as soon as 2 weeks after treatment 55. Data are insufficient to support retesting men 34.
Management of Sex Partners
Concurrent treatment of all sex partners is critical for symptomatic relief, microbiologic cure, and prevention of transmission and reinfections. Current partners should be referred for presumptive therapy to avoid reinfection. Partners should be advised to abstain from intercourse until they and their sex partners have been adequately treated and any symptoms have resolved. EPT might have a role in partner management for trichomoniasis 56 and can be used in states where permissible by law; however, no one partner management intervention has been shown to be superior in reducing reinfection rates. Though no definitive data exist to guide treatment for partners of persons with persistent or recurrent trichomoniasis in whom nonadherance and reinfection are unlikely, partners benefit from undergoing evaluation and receiving the same regimen as the patient (see Persistent or Recurrent Trichomoniasis).
Treatment of women during pregnancy
Trichomonas vaginalis infection in pregnant women is associated with adverse pregnancy outcomes, particularly premature rupture of membranes, preterm delivery, and delivery of a low birthweight infant 57. Pregnant women with symptomatic trichomoniasis, in any trimester, should receive treatment with metronidazole 500 mg orally twice daily for 7 days 34. Treatment of asymptomatic trichomoniasis in pregnancy, however, has not been shown to reduce preterm birth 58. One trial suggested the possibility of increased preterm delivery in women with Trichomonas vaginalis infection who received metronidazole treatment 58, yet study limitations prevented definitive conclusions regarding the risks of treatment. More recent, larger studies have shown no positive or negative association between metronidazole use during pregnancy and adverse outcomes of pregnancy 59. Symptomatic pregnant women, regardless of pregnancy stage, should be tested and considered for treatment. Treatment of Trichomonas vaginalis infection can relieve symptoms of vaginal discharge in pregnant women and reduce sexual transmission to partners. Although perinatal transmission of trichomoniasis is uncommon, treatment also might prevent respiratory or genital infection of the newborn 60. Clinicians should counsel symptomatic pregnant women with trichomoniasis regarding the potential risks for and benefits of treatment and about the importance of partner treatment and condom use in the prevention of sexual transmission.
Although metronidazole crosses the placenta, data suggest that it poses a low risk to pregnant women 61. No evidence of teratogenicity or mutagenic effects in infants has been found in multiple cross-sectional and cohort studies of pregnant women 62.
Both metronidazole and tinidazole are secreted in breast milk (https://www.ncbi.nlm.nih.gov/books/NBK501922). Metronidazole is secreted in breast milk. With maternal oral therapy, breastfed infants receive metronidazole in doses that are lower than those used to treat infections in infants, although the active metabolite adds to the total infant exposure. Plasma levels of the drug and metabolite are measurable, but remain less than maternal plasma levels. Although several reported case series found no evidence of adverse effects in infants exposed to metronidazole in breast milk, some clinicians advise deferring breastfeeding for 12–24 hours after taking a dose of metronidazole 34, 63. Tinidazole is not recommended during pregnancy due to limited animal studies suggesting fetal risk; women who are breastfeeding should wait 72 hours after taking tinidazole before breastfeeding 34.
Treatment of women with HIV
Up to 53% of women with HIV infection also are infected with Trichomonas vaginalis 64. Trichomonas vaginalis infection in these women is significantly associated with pelvic inflammatory disease 65, and treatment of trichomoniasis is associated with significant decreases in genital-tract HIV viral load and viral shedding 66. For these reasons, routine screening and prompt treatment are recommended for all women with HIV infection; screening should occur at entry to care and then at least annually thereafter. A randomized clinical trial involving women with HIV infection and Trichomonas vaginalis infection demonstrated that a single dose of metronidazole 2 g orally was less effective than 500 mg twice daily for 7 days 67. Thus, to improve cure rates, women with HIV infection who receive a diagnosis of Trichomonas vaginalis infection should be treated with a 7-day treatment course of metronidazole 500 mg twice daily (rather than with a 2-g single dose of metronidazole) 34. This longer course of therapy has been shown to have higher cure rates than single-dose metronidazole therapy in women with HIV 18, 68, 69.
Factors that might interfere with standard single-dose treatment for trichomoniasis in these women include high rates of asymptomatic bacterial vaginosis co-infections, use of antiretroviral therapy, changes in vaginal ecology, and impaired immunity 70.
In women with HIV infection who receive a diagnosis of T. vaginalis infection, retesting is recommended within 3 months following initial treatment; NAAT is encouraged because of higher sensitivity of these tests. Data are insufficient to recommend routine screening, alternative treatment regimens of longer duration, or retesting in men.
Pregnant women with HIV who are treated for Trichomonas vaginalis infection should be retested 3 months after treatment.
Allergy, Intolerance, and Adverse Reactions
Metronidazole and tinidazole are both nitroimidazoles. Patients with an IgE mediated-type allergy to a nitroimidazole can be managed by metronidazole desensitization according to a published regimen 71 and in consultation with a specialist.
References- Trichomoniasis – CDC Fact Sheet. https://www.cdc.gov/std/trichomonas/stdfact-trichomoniasis.htm
- Madeline Sutton, Maya Sternberg, Emilia H. Koumans, Geraldine McQuillan, Stuart Berman, Lauri Markowitz; The Prevalence of Trichomonas vaginalis Infection among Reproductive-Age Women in the United States, 2001–2004, Clinical Infectious Diseases, Volume 45, Issue 10, 15 November 2007, Pages 1319–1326, https://doi.org/10.1086/522532
- Sutton MY, Sternberg M, Nsuami M, Behets F, Nelson AM, St Louis ME. Trichomoniasis in pregnant human immunodeficiency virus-infected and human immunodeficiency virus-uninfected Congolese women: prevalence, risk factors, and association with low birth weight, Am J Obstet Gynecol , 1999, vol. 181, pg. 656-62
- Vaginitis. https://www.std.uw.edu/go/syndrome-based/vaginal-discharge/core-concept/all#trichomoniasis
- Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular Testing for Trichomonas vaginalis in Women: Results from a Prospective U.S. Clinical Trial . Journal of Clinical Microbiology. 2011;49(12):4106-4111. doi:10.1128/JCM.01291-11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3232944/
- Trichomoniasis. https://www.cdc.gov/std/tg2015/trichomoniasis.htm
- Cotch MF, Pastorek JG2nd, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group, Sex Transm Dis , 1997, vol. 24, pg. 353-60
- French JI, McGregor JA, Parker R. Readily treatable reproductive tract infections and preterm birth among black women, Am J Obstet Gynecol , 2006, vol. 194 (pg. 1717-26) discussion 1726-7
- Schwebke JR, Hobbs MM, Taylor SN, Sena AC, Catania MG, Weinbaum BS, Johnson AD, Getman DK, Gaydos CA. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol. 2011 Dec;49(12):4106-11. doi: 10.1128/JCM.01291-11
- Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998 Apr;11(2):300-17. doi: 10.1128/CMR.11.2.300
- Anthony C. Fouts, Stephen J. Kraus, Trichomonas vaginalis: Reevaluation of Its Clinical Presentation and Laboratory Diagnosis, The Journal of Infectious Diseases, Volume 141, Issue 2, February 1980, Pages 137–143, https://doi.org/10.1093/infdis/141.2.137
- Van Gerwen OT, Craig-Kuhn MC, Jones AT, Schroeder JA, Deaver J, Buekens P, Kissinger PJ, Muzny CA. Trichomoniasis and adverse birth outcomes: a systematic review and meta-analysis. BJOG. 2021 Nov;128(12):1907-1915. doi: 10.1111/1471-0528.16774
- Mann JR, McDermott S, Gill T. Sexually transmitted infection is associated with increased risk of preterm birth in South Carolina women insured by Medicaid. J Matern Fetal Neonatal Med. 2010 Jun;23(6):563-8. doi: 10.3109/14767050903214574
- Mann JR, McDermott S, Zhou L, Barnes TL, Hardin J. Treatment of trichomoniasis in pregnancy and preterm birth: an observational study. J Womens Health (Larchmt). 2009 Apr;18(4):493-7. doi: 10.1089/jwh.2008.0964
- Carter JE, Whithaus KC. Neonatal respiratory tract involvement by Trichomonas vaginalis: a case report and review of the literature. Am J Trop Med Hyg. 2008 Jan;78(1):17-9.
- Trintis J, Epie N, Boss R, Riedel S. Neonatal Trichomonas vaginalis infection: a case report and review of literature. Int J STD AIDS. 2010 Aug;21(8):606-7. doi: 10.1258/ijsa.2010.010174
- Masese LN, Graham SM, Gitau R, Peshu N, Jaoko W, Ndinya-Achola JO, Mandaliya K, Richardson BA, Overbaugh J, McClelland RS. A prospective study of vaginal trichomoniasis and HIV-1 shedding in women on antiretroviral therapy. BMC Infect Dis. 2011 Nov 3;11:307. doi: 10.1186/1471-2334-11-307
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013 Sep;89(6):426-33. doi: 10.1136/sextrans-2012-051005
- Susan Cu-Uvin, Ko Hyejin, Denise J. Jamieson, Joseph W. Hogan, Paula Schuman, Jean Anderson, S. Klein Robert, HIV Epidemiology Research Study (HERS) Group, Prevalence, Incidence, and Persistence or Recurrence of Trichomoniasis among Human Immunodeficiency Virus (HIV)–Positive Women and among HIV-Negative Women at High Risk for HIV Infection, Clinical Infectious Diseases, Volume 34, Issue 10, 15 May 2002, Pages 1406–1411, https://doi.org/10.1086/340264
- Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and Microbiological Aspects of Trichomonas vaginalis. Clinical Microbiology Reviews. 1998;11(2):300-317. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC106834/
- Signalling of Trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol Microbiol. 1993 Jan;7(2):299-309. https://www.ncbi.nlm.nih.gov/pubmed/8446032
- Swartzendruber A, Sales JM, Brown JL, DiClemente RJ, Rose ES. Correlates of Incident Trichomonas vaginalis Infections Among African American Female Adolescents. Sexually transmitted diseases. 2014;41(4):240-245. doi:10.1097/OLQ.0000000000000094. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4313569/
- Guenthner PC, Secor WE, Dezzutti CS. Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for the sexual transmission of HIV-1, Infect Immun , 2005, vol. 73, pg. 4155-60
- Kingston MA, Bansal D, Carlin EM. ‘Shelf life’ of Trichomonas vaginalis. Int J STD AIDS. 2003 Jan;14(1):28-9. doi: 10.1258/095646203321043228
- Bachmann LH, Hobbs MM, Seña AC, Sobel JD, Schwebke JR, Krieger JN, McClelland RS, Workowski KA. Trichomonas vaginalis genital infections: progress and challenges. Clin Infect Dis. 2011 Dec;53 Suppl 3(Suppl 3):S160-72. doi: 10.1093/cid/cir705
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019 Sep 20;8:F1000 Faculty Rev-1666. doi: 10.12688/f1000research.19972.1
- Lobo TT, Feijo G, Carvalho SE, Costa PL, Chagas C, Xavier J, et al. A comparative evaluation of the Papanicolaou test for the diagnosis of trichomoniasis. Sex Transm Dis 2003;30(9):694-9.
- Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009;200(2):188 e1-7.
- Centers for Disease Control and Prevention Public Health Image Library (Joe Miller, 1975).
- Beverly AL, Venglarik M, Cotton B, Schwebke JR. Viability of Trichomonas vaginalis in transport medium. J Clin Microbiol 1999;37(11):3749-50.
- Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021 Jul 23;70(4):1-187. doi: 10.15585/mmwr.rr7004a1
- Cosentino LA, Campbell T, Jett A, Macio I, Zamborsky T, Cranston RD, Hillier SL. Use of nucleic acid amplification testing for diagnosis of anorectal sexually transmitted infections. J Clin Microbiol. 2012 Jun;50(6):2005-8. doi: 10.1128/JCM.00185-12
- Francis SC, Kent CK, Klausner JD, Rauch L, Kohn R, Hardick A, Gaydos CA. Prevalence of rectal Trichomonas vaginalis and Mycoplasma genitalium in male patients at the San Francisco STD clinic, 2005-2006. Sex Transm Dis. 2008 Sep;35(9):797-800. doi: 10.1097/OLQ.0b013e318177ec39
- Trichomoniasis. https://www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
- https://www.aphl.org/AboutAPHL/publications/Documents/ID_2013August_Advances-in-Laboratory-Detection-of-Trichomonas-vaginalis.pdf
- Hollman D, Coupey SM, Fox AS, Herold BC. Screening for Trichomonas vaginalis in high-risk adolescent females with a new transcription-mediated nucleic acid amplification test (NAAT): associations with ethnicity, symptoms, and prior and current STIs. J Pediatr Adolesc Gynecol. 2010 Oct;23(5):312-6. doi: 10.1016/j.jpag.2010.03.004
- Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol. 2011 Mar;49(3):866-9. doi: 10.1128/JCM.02367-10
- Van Der Pol B, Williams JA, Taylor SN, Cammarata CL, Rivers CA, Body BA, Nye M, Fuller D, Schwebke JR, Barnes M, Gaydos CA. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper system. J Clin Microbiol. 2014 Mar;52(3):885-9. doi: 10.1128/JCM.02966-13
- Schwebke JR, Gaydos CA, Davis T, Marrazzo J, Furgerson D, Taylor SN, Smith B, Bachmann LH, Ackerman R, Spurrell T, Ferris D, Burnham CA, Reno H, Lebed J, Eisenberg D, Kerndt P, Philip S, Jordan J, Quigley N. Clinical Evaluation of the Cepheid Xpert TV Assay for Detection of Trichomonas vaginalis with Prospectively Collected Specimens from Men and Women. J Clin Microbiol. 2018 Jan 24;56(2):e01091-17. doi: 10.1128/JCM.01091-17
- Gaydos CA, Beqaj S, Schwebke JR, Lebed J, Smith B, Davis TE, Fife KH, Nyirjesy P, Spurrell T, Furgerson D, Coleman J, Paradis S, Cooper CK. Clinical Validation of a Test for the Diagnosis of Vaginitis. Obstet Gynecol. 2017 Jul;130(1):181-189. doi: 10.1097/AOG.0000000000002090
- Van Der Pol B, Torres-Chavolla E, Kodsi S, Cooper CK, Davis TE, Fife KH, Taylor SN, Augenbraun MH, Gaydos CA. Clinical Performance of the BD CTGCTV2 Assay for the BD MAX System for Detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis Infections. Sex Transm Dis. 2021 Feb 1;48(2):134-140. doi: 10.1097/OLQ.0000000000001280
- Van Der Pol B. A profile of the cobas® TV/ MG test for the detection of Trichomonas vaginalis and Mycoplasma genitalium. Expert Rev Mol Diagn. 2020 Apr;20(4):381-386. doi: 10.1080/14737159.2020.1714440
- Van Der Pol B, Rao A, Nye MB, Chavoustie S, Ermel A, Kaplan C, Eisenberg D, Chan PA, Mena L, Pacheco S, Waites KB, Xiao L, Krishnamurthy S, Mohan R, Bertuzis R, McGowin CL, Arcenas R, Marlowe EM, Taylor SN. Trichomonas vaginalis Detection in Urogenital Specimens from Symptomatic and Asymptomatic Men and Women by Use of the cobas TV/MG Test. J Clin Microbiol. 2021 Sep 20;59(10):e0026421. doi: 10.1128/JCM.00264-21
- Gaydos CA, Manabe YC, Melendez JH. A Narrative Review of Where We Are With Point-of-Care Sexually Transmitted Infection Testing in the United States. Sex Transm Dis. 2021 Aug 1;48(8S):S71-S77. doi: 10.1097/OLQ.0000000000001457
- Sheele JM, Crandall CJ, Arko BL, Vallabhaneni M, Dunn CT, Chang BF, Fann P, Bigach M. The OSOM® Trichomonas Test is unable to accurately diagnose Trichomonas vaginalis from urine in men. Am J Emerg Med. 2019 May;37(5):1002-1003. doi: 10.1016/j.ajem.2018.10.022
- Gaydos CA, Schwebke J, Dombrowski J, Marrazzo J, Coleman J, Silver B, Barnes M, Crane L, Fine P. Clinical performance of the Solana® Point-of-Care Trichomonas Assay from clinician-collected vaginal swabs and urine specimens from symptomatic and asymptomatic women. Expert Rev Mol Diagn. 2017 Mar;17(3):303-306. doi: 10.1080/14737159.2017.1282823
- Morris SR, Bristow CC, Wierzbicki MR, Sarno M, Asbel L, French A, Gaydos CA, Hazan L, Mena L, Madhivanan P, Philip S, Schwartz S, Brown C, Styers D, Waymer T, Klausner JD. Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: a cross-sectional study. Lancet Infect Dis. 2021 May;21(5):668-676. doi: 10.1016/S1473-3099(20)30734-9
- Trichomoniasis. 2015 Sexually Transmitted Diseases Treatment Guidelines. https://www.cdc.gov/std/tg2015/trichomoniasis.htm
- Spence MR, Harwell TS, Davies MC, et al. The minimum single oral metronidazole dose for treating trichomoniasis: a randomized, blinded study. Obstet Gynecol 1997;89(5 Pt 1):699–703.
- Prasertsawat PO, Jetsawangsri T. Split-dose metronidazole or single-dose tinidazole for the treatment of vaginal trichomoniasis. Sex Transm Dis 1992;19:295–7.
- Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003;2:CD000218.
- Kirkcaldy RD, Augostini P, Asbel LE, Bernstein KT, Kerani RP, Mettenbrink CJ, Pathela P, Schwebke JR, Secor WE, Workowski KA, Davis D, Braxton J, Weinstock HS. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD Surveillance Network, 2009-2010. Emerg Infect Dis. 2012 Jun;18(6):939-43. doi: 10.3201/eid1806.111590
- Schwebke JR, Barrientes FJ. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob Agents Chemother. 2006 Dec;50(12):4209-10. doi: 10.1128/AAC.00814-06
- Peterman TA, Tian LH, Metcalf CA, et al. High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: a case for rescreening. Ann Intern Med 2006;145:564–72.
- Williams JA, Van Der Pol B, Ofner S, et al. Time from treatment to negative PCR results for C. trachomatis, N. gonorrhoeae and T. vaginalis. National STD Prevention Conference; March 10-13, 2008, 2008; Chicago, IL.
- Schwebke JR, Desmond RA. A randomized controlled trial of partner notification methods for prevention of trichomoniasis in women. Sex Transm Dis 2010;37:392–6.
- Mann JR, McDermott S. Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? J Atten Disord 2011;15:667–73.
- Klebanoff MA, Carey JC, Hauth JC, Hillier SL, Nugent RP, Thom EA, Ernest JM, Heine RP, Wapner RJ, Trout W, Moawad A, Leveno KJ, Miodovnik M, Sibai BM, Van Dorsten JP, Dombrowski MP, O’Sullivan MJ, Varner M, Langer O, McNellis D, Roberts JM; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001 Aug 16;345(7):487-93. doi: 10.1056/NEJMoa003329
- Stringer E, Read JS, Hoffman I, et al. Treatment of trichomoniasis in pregnancy in sub-Saharan Africa does not appear to be associated with low birth weight or preterm birth. South African Medical Journal 2010;100:58–64.
- Trintis J, Epie N, Boss R, et al. Neonatal Trichomonas vaginalis infection: a case report and review of literature. International journal of STD and AIDS 2010;21:606–7.
- Briggs GC, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation, 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
- Goldenberg RL, Mwatha A, Read JS, et al. The HPTN 024 Study: the efficacy of antibiotics to prevent chorioamnionitis and preterm birth. Am J Obstet Gynecol 2006;194:650–61.
- Erickson SH, Oppenheim GL, Smith GH. Metronidazole in breast milk. Obstet Gynecol 1981;57:48–50.
- Miller M, Liao Y, Wagner M, et al. HIV, the clustering of sexually transmitted infections, and sex risk among African American women who use drugs. Sex Transm Dis 2008;35:696–702.
- Moodley P, Wilkinson D, Connolly C, et al. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis 2002;34:519–22.
- Anderson BL, Firnhaber C, Liu T, et al. Effect of trichomoniasis therapy on genital HIV viral burden among African women. Sex Transm Dis 2012;39:638–42.
- Kissinger P, Mena L, Levison J, et al. A randomized treatment trial: single versus 7-day dose of metronidazole for the treatment of Trichomonas vaginalis among HIV-infected women. J Acquir Immune Defic Syndr 2010;55:565–71.
- Kissinger P, Adamski A, Clark RA, Mena L, Levison J, Martin DH. Does Antiretroviral Therapy Interfere With the Treatment of Trichomonas vaginalis Among HIV+ Women? Sex Transm Dis. 2013 Jun;40(6):506-7. doi: 10.1097/OLQ.0b013e31829335fe
- Balkus JE, Richardson BA, Mochache V, Chohan V, Chan JD, Masese L, Shafi J, Marrazzo J, Farquhar C, McClelland RS. A prospective cohort study comparing the effect of single-dose 2 g metronidazole on Trichomonas vaginalis infection in HIV-seropositive versus HIV-seronegative women. Sex Transm Dis. 2013 Jun;40(6):499-505. doi: 10.1097/OLQ.0b013e31828fce34
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect 2013;89:426–33.
- Helms DJ, Mosure DJ, Secor WE, et al. Management of Trichomonas vaginalis in women with suspected metronidazole hypersensitivity. Am J Obstet Gynecol 2008;198:e371–7.