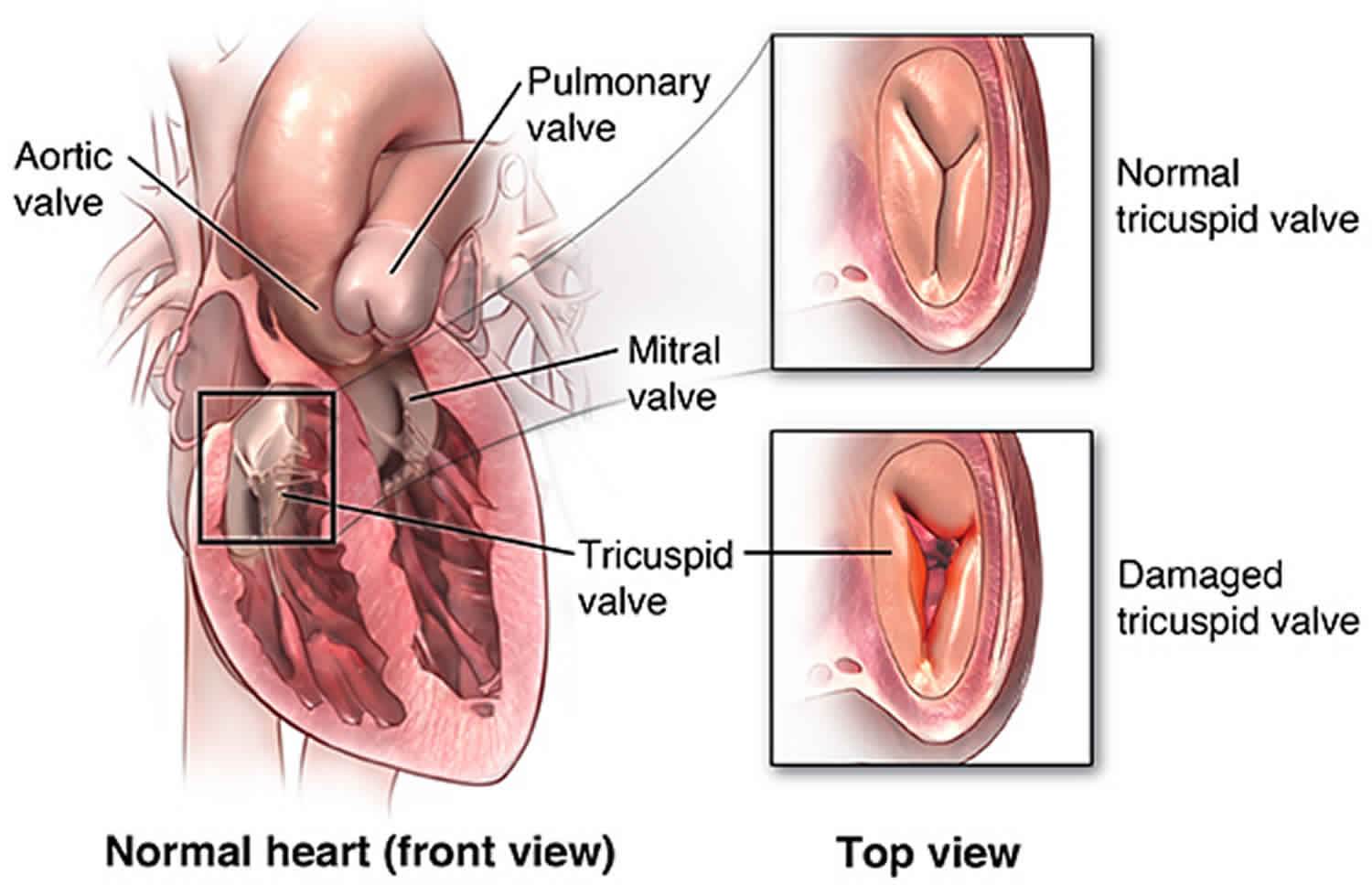
Tricuspid stenosis
Tricuspid valve stenosis is a narrowing or blockage of the tricuspid valve. Tricuspid stenosis restricts blood flow between the upper and lower part of the right side of the heart, or from the right atrium to the right ventricle. Tricuspid stenosis causes the right atrium to become enlarged, while the right ventricle does not get enough blood.
In tricuspid stenosis, the tricuspid valve is narrowed, decreasing the amount of blood that can flow through it from the right atrium to the lower right heart chamber (right ventricle).
Tricuspid stenosis is almost always rheumatic fever in origin and is generally accompanied by mitral and aortic valve involvement 1. Often, people with tricuspid stenosis also have mitral stenosis. You are rarely born with tricuspid stenosis, and it is not passed down through family members.
With rheumatic tricuspid stenosis, the valve leaflets become thickened and sclerotic as the chordae tendineae become shortened. The restricted valve opening hampers blood flow into the right ventricle and, subsequently, to the pulmonary vasculature. Right atrial enlargement is observed as a consequence. The obstructed venous return results in hepatic enlargement, decreased pulmonary blood flow, and peripheral edema. Other rare causes of tricuspid stenosis include carcinoid syndrome, endocarditis, endomyocardial fibrosis, systemic lupus erythematosus, and congenital tricuspid atresia 2.
In the rare instances of congenital tricuspid stenosis, the valve leaflets may manifest various forms of deformity, which can include deformed leaflets, deformed chordae, and displacement of the entire valve apparatus. Other cardiac anomalies are usually present 1.
Most stenotic tricuspid valves are associated with clinical evidence of regurgitation that can be documented by performing a physical examination (murmur), echocardiography, or angiography. Stenotic tricuspid valves are always anatomically abnormal, and the cause is limited to a few conditions. With the exceptions of congenital causes or active infective endocarditis, tricuspid stenosis takes years to develop 3.
Tricuspid stenosis is rare, occurring in less than 1% of the population. While found in approximately 15% of patients with rheumatic heart disease at autopsy, it is estimated to be clinically significant in only 5% of these patients. The incidence of congenital tricuspid stenosis is less than 1%. Tricuspid stenosis accounts for about 2.4% of all cases of organic tricuspid valve disease 4. Tricuspid stenosis is observed more commonly in women than in men, similar to mitral stenosis of rheumatic origin 5. Congenital tricuspid stenosis has a slightly higher male predominance.
Tricuspid stenosis can present as a congenital lesion or later in life when it is due to some other condition. The congenital form accounts for approximately 0.3% of all congenital heart disease cases. The frequency of tricuspid stenosis in the older population, due to secondary causes, ranges from 0.3-3.2%.
Evidence to guide treatment of tricuspid stenosis is scarce. Symptomatic patients not undergoing intervention should receive a low-salt diet, diuretics, and aldosterone antagonists.
Patients with severe tricuspid stenosis should undergo intervention if they are symptomatic or if cardiac surgery is being done for other reasons. Percutaneous balloon tricuspid commissurotomy might be considered for severe tricuspid stenosis without accompanying tricuspid regurgitation.
Tricuspid stenosis key points
- Tricuspid stenosis is almost always due to rheumatic fever; tricuspid regurgitation and mitral stenosis are often also present.
- Heart sounds include a soft opening snap and a mid-diastolic rumble with presystolic accentuation; the murmur becomes louder and longer with maneuvers that increase venous return (eg, exercise, inspiration, leg-raising) and softer and shorter with maneuvers that decrease venous return (standing, Valsalva maneuver).
- Treatment includes diuretics and aldosterone antagonists; surgical repair or replacement is rarely needed.
What problems can result from untreated or advanced tricuspid valve stenosis?
After several years, the right atrium can become enlarged because blood flow through the narrow tricuspid valve opening is partially blocked. An enlarged atrium can affect the pressure and blood flow in the nearby chambers and veins.
It can also cause the right ventricle to shrink because the amount of blood entering from the right atrium is reduced. Eventually, less blood circulates through the lungs to get oxygen.
What are heart valves ?
Your heart is a strong muscle about the size of the palm of your hand. Your body depends on the heart’s pumping action to deliver oxygen- and nutrient-rich blood to the body’s cells. When the cells are nourished properly, the body can function normally. Just like an engine makes a car go, the heart keeps your body running. The heart has two pumps separated by an inner wall called the septum. The right side of the heart pumps blood to the lungs to pick up oxygen. The left side of the heart receives the oxygen-rich blood from the lungs and pumps it to the body.
The heart has four chambers 6, two on the right and two on the left:
- Two upper chambers are called atrium (two is called an atria). The atria collect blood as it flows into the heart.
- Two lower chambers are called ventricles. The ventricles pump blood out of the heart to the lungs or other parts of the body.
The heart also has four valves that open and close to let blood flow from the atria to the ventricles and from the ventricles into the two large arteries connected to the heart in only one direction when the heart contracts (beats). The four heart valves are:
- Tricuspid valve, located between the right atrium and right ventricle
- Pulmonary or pulmonic valve, between the right ventricle and the pulmonary artery. This artery carries blood from the heart to the lungs.
- Mitral valve, between the left atrium and left ventricle
- Aortic valve, between the left ventricle and the aorta. This aorta carries blood from the heart to the body.
Each valve has a set of flaps (also called leaflets or cusps). The mitral valve has two flaps; the others have three. Valves are like doors that open and close. They open to allow blood to flow through to the next chamber or to one of the arteries. Then they shut to keep blood from flowing backward. Blood flow occurs only when there’s a difference in pressure across the valves, which causes them to open. Under normal conditions, the valves permit blood to flow in only one direction.
The heart four chambers and four valves and is connected to various blood vessels. Veins are blood vessels that carry blood from the body to the heart. Arteries are blood vessels that carry blood away from the heart to the body.
The heart pumps blood to the lungs and to all the body’s tissues by a sequence of highly organized contractions of the four chambers. For the heart to function properly, the four chambers must beat in an organized way.
When the heart’s valves open and close, they make a “lub-DUB” sound that a doctor can hear using a stethoscope 7.
- The first sound—the “lub”—is made by the mitral and tricuspid valves closing at the beginning of systole. Systole is when the ventricles contract, or squeeze, and pump blood out of the heart.
- The second sound—the “DUB”—is made by the aortic and pulmonary valves closing at the beginning of diastole. Diastole is when the ventricles relax and fill with blood pumped into them by the atria.
Figure 1. The anatomy of the heart valves
Figure 2. Top view of the 4 heart valves
Figure 3. Normal heart blood flow
Figure 4. Heart valves function
Tricuspid stenosis causes
At least 4 conditions can cause obstruction of the native tricuspid valve. These include 8:
- Rheumatic heart disease. Rheumatic heart disease is one of the most common causes of tricuspid stenosis and almost always occurs in conjunction with mitral stenosis 9.
- Congenital tricuspid stenosis (Ebstein’s anomaly). These lesions are observed more commonly in infants 10. They may manifest as incompletely developed leaflets, shortened or malformed chordae, small annuli, abnormal size and number of the papillary muscles, or any combination of these defects.
- Metabolic or enzymatic abnormalities such as Fabry’s disease, Whipple’s disease 11.
- Active infective endocarditis. Large infected vegetations in infective endocarditis obstructing the orifice of the tricuspid valve can cause relative tricuspid stenosis 11. This condition is relatively uncommon, even in those who abuse intravenous drugs.
Rheumatic tricuspid stenosis: In this entity, diffuse thickening of the leaflets occurs, with or without fusion of the commissures. The chordae tendineae may be thickened and shortened. Calcification of the valve rarely occurs. The leaflet tissue is composed of dense collagen and elastic fibers that produce a major distortion of the normal leaflet layers.
Carcinoid heart disease: Carcinoid syndrome may cause isolated tricuspid stenosis or mixed with the regurgitant lesion 12. Carcinoid valve lesions characteristically manifest as fibrous white plaques located on the valvular and mural endocardium. The valve leaflets are thickened, rigid, and reduced in area. Fibrous tissue proliferation is present on the atrial and ventricular surfaces of the valve structure.
Systemic diseases like systemic lupus erythematosus (SLE), antiphospholipid antibody syndrome and the presence of permanent pacing and fusion of implantable cardioverter defibrillator leads to sub-valvular structures can cause tricuspid stenosis 13. Benign tumors like atrial myxomas can cause functional tricuspid stenosis 14. Blunt trauma has also been described as a risk factor. Renal and ovarian tumors can grow into the tricuspid orifice causing stenosis 15.
Unusual causes: Rare causes of tricuspid stenosis include Fabry disease and giant blood cysts.
Mimickers of tricuspid stenosis: Several conditions may mimic tricuspid stenosis by obstructing flow through the valve. These conditions include supravalvular obstruction from congenital diaphragms, intracardiac or extracardiac tumors, thrombosis or emboli, or large endocarditis vegetations. In addition, conditions that impair right-sided filling can produce similar symptoms and physical findings. These conditions include constrictive pericarditis and restrictive cardiomyopathy.
Other less common causes of tricuspid stenosis include:
- Intravenous leiomyomatosis 16.
- Ventriculoatrial shunts 17.
- Valvulopathy associated with drugs like fenfluramine/phentermine and methysergide is characterized by thickened fibrotic and hypomobile tricuspid leaflets, with various degrees of valve stenosis and regurgitation 18.
Tricuspid valve stenosis symptoms
The only symptoms of tricuspid stenosis are fatigue and exertional syncope due to limited cardiac output 19. The onset is usually gradual, but it may be rapid if atrial fibrillation or flutter develops. Symptoms are rarely severe enough to require valve surgery.
Patients with severe tricuspid stenosis will eventually have right upper quadrant abdominal discomfort (due to an enlarged liver), fluttering discomfort in the neck (due to giant a waves in the jugular pulse), cold skin (due to low cardiac output), abdominal discomfort and swelling, leg edema, ascites, and deterioration of liver function tests and anasarca 20.
Shortness of breath (dyspnea) may be present but is not severe unless concomitant mitral valve disease is present.
Patients may complain about prominent pulsations in the neck. “Giant A waves” classically greater in height than usually perceived in the jugular venous pulse, are seen in tricuspid stenosis. A “slow y descent” due to delayed emptying of the right atrium into the right ventricle can also be seen 21. Jugular venous distention may occur, increasing with inspiration (Kussmaul sign). The face may become dusky and scalp veins may dilate when the patient is recumbent (suffusion sign). Hepatic congestion and peripheral edema may occur.
The lungs are clear in patients with isolated tricuspid stenosis 22. A low frequency presystolic mid-diastolic murmur is heard at the lower left sternal border in the fourth intercostal space. The intensity of the murmur and opening snap in tricuspid stenosis increase with maneuvers that increase blood flow across the tricuspid valve, especially with inspiration and also with leg raising, inhalation of amyl nitrate, squatting, or exercise.
When tricuspid stenosis occurs concomitantly with mitral stenosis, the decrement of cardiac output to the pulmonary bed may paradoxically diminish the dyspnea, hemoptysis, and orthopnea typically seen with mitral stenosis.
Tricuspid stenosis staging
Tricuspid stenosis staging sections into categories A, B, C, D. Stages C (without symptoms) and D (with symptoms). When valve and/or chordal thickening and calcification are evident, there are additional findings indicative of severe tricuspid stenosis, for example, pressure gradient greater than or equal to 5 mm Hg, pressure half-time greater than or equal to 190 milliseconds, valve area less than or equal to 1.0 cm², associated moderate right atrial enlargement, and inferior vena cava dilatation 23.
Tricuspid stenosis diagnosis
Diagnosis of tricuspid stenosis is suspected based on history and physical examination and confirmed by Doppler echocardiography showing a pressure gradient across the tricuspid valve. Two-dimensional echocardiography shows thickened leaflets with reduced movement and right atrial enlargement.
Obtain information regarding preceding rheumatic fever, symptoms of the carcinoid syndrome, and possible congenital abnormalities.
With sinus rhythm (more common with tricuspid stenosis than with mitral stenosis), the jugular venous pulse increases and the “A wave” is prominent (may be confused with an arterial pulse).
If atrial fibrillation occurs, the A wave is lost.
Peripheral edema and ascites are frequent.
Without significant mitral pathology, the patient should not be dyspneic and can probably lie flat without symptoms.
A prominent right atrium may be palpable to the right of the sternum.
On auscultation, tricuspid stenosis is often inaudible but may produce a soft opening snap and a mid-diastolic rumble with presystolic accentuation. The murmur becomes louder and longer with maneuvers that increase venous return (exercise, inspiration, leg-raising, Müller maneuver) and softer and shorter with maneuvers that decrease venous return (standing, Valsalva maneuver).
A diastolic murmur is audible along the left sternal border or at the xiphoid, which increases with inspiration. Often, tricuspid regurgitation is also present, represented by a holosystolic murmur in a similar location.
The first heart sound may be split widely. The second heart sound may be single. This single sound is due to the inaudible closure of the pulmonary valve from the decrease in blood flow through the stenotic tricuspid valve.
Electrocardiogram: In patients with tricuspid stenosis in sinus rhythm, the electrocardiogram may show tall, peaked P waves in leads II, III, and avF consistent with right atrial enlargement 24.
Cardiac MRI: Cardiac MRI is now the preferred method to evaluate right ventricular size and function.
The American College of Cardiology and the American Heart Association guidelines include the following recommendations for diagnostic testing and initial diagnosis of tricuspid stenosis 25:
- Transthoracic echocardiography (TTE) is indicated to assess the anatomy of the valve complex, evaluate severity of stenosis, and characterize any associated regurgitation and/or left-sided valve disease. Tricuspid peak inflow velocity during inspiration of > 1 m/s, Inflow time-velocity integral greater than 60 cm, valve area by the continuity of less than or equal to 1 cm² indicates hemodynamically significant tricuspid stenosis 26. The mean transvalvular pressure gradient derived using the Bernoulli equation is usually lower in tricuspid stenosis than in mitral stenosis, ranging between 2 and 10 mm Hg, and averaging around 5 mmHg. A mean gradient greater than or equal to 5 mm Hg at normal heart rate is considered indicative of clinically significant tricuspid stenosis 26. Higher gradients may be seen with combined stenosis and regurgitation. A longer T 1/2 (pressure halftime by continuous wave Doppler) implies a greater tricuspid stenosis severity with values of greater than or equal to 190 ms being frequently associated with significant (or critical) stenosis 27. Other parameters that are seen include- reduced tricuspid annular plane systolic excursion (TAPSE), dilated IVC, and increased right atrial size 28.
- Consider invasive hemodynamic assessment of severity of tricuspid stenosis in symptomatic patients when clinical and noninvasive data are discordant.
- Cardiac catheterization: In tricuspid stenosis, there is a large right atrial “a” wave of 12 to 20 mm Hg and a diastolic, mean gradient of 4 to 8 mm Hg across the tricuspid valve. The mean gradient across the tricuspid valve is more significant in tricuspid stenosis because an end-diastolic gradient may be absent with significant obstruction. This is because of the lower filling pressures on the right side of the heart 24. Elevated right atrial pressure with a slow fall in early diastole and a diastolic pressure gradient across the tricuspid valve is characteristic of tricuspid stenosis.
Tricuspid stenosis diagnositic criteria:
The circumference of the normal tricuspid valve is 12 to 14 cm, and most pathologists consider a circumference of less than 10 to 11 cm (i.e., diameter less than 3 cm or valve area of less than 7 cm²) as an indication of tricuspid stenosis 29. Normal Area of the tricuspid valve is 4.0 cm² and area less than 1.0 cm² is deemed as severe tricuspid stenosis 30.
Tricuspid stenosis treatment
In the treatment of tricuspid stenosis, medical care consists of assessment and treatment of the underlying cause of the valvular pathology, as follows:
- Treat bacterial endocarditis with the appropriate antibiotics as determined by the sensitivity of the organisms cultured.
- Medically address cardiac arrhythmias depending on their characterization.
- Decreasing right atrial volume overload with diuresis and salt restriction helps decrease symptoms and improve hepatic function.
Consultation with infectious disease specialists may be appropriate if the stenosis is secondary to an infectious process.
An endocrinologist may be of assistance if carcinoid syndrome or an inborn error of metabolism is the cause of the pathology.
Activity
Activity is usually self-limited by the patient because of easy fatigability secondary to oxygen deprivation.
Once the pathology has been corrected, no activity restrictions are necessary.
Medical treatment
Loop diuretics may be useful to relieve systemic and hepatic congestion in patients with severe, symptomatic tricuspid stenosis 23. Caution is advised since diuretics may decrease the preload further in patients who have low output state.
Not every tricuspid stenosis needs invasive intervention. Treatment of SLE and antiphospholipid antibody syndrome may reduce the “coating” over the valves and chordae and reduce stenosis and regurgitation 31. Cessation of fenfluramine or methysergide has been associated with valve normalization 28. Less often, surgical therapy may be needed in addition to medical management.
Tricuspid stenosis surgery
Tricuspid stenosis remains a surgical disease and requires either commissurotomy or replacement of the valve if right heart failure or low cardiac output has resulted. Surgery is rarely performed solely on the tricuspid valve; it is usually performed in combination with mitral and/or aortic valve disease repair.
Valvotomy
Valvotomy is performed using 1, 2, or 3 balloons. While some stenosis may persist, the change in valve area causes a significant reduction in the transvalvular pressure gradient and a decrease in right atrial pressure 32.
Valve surgery
Tricuspid valve surgery includes repair versus replacement. Repair should be attempted when reasonable. When repair is not an option, valve replacement can be done by open versus transcatheter replacement. While replacing the valve, no differences in outcomes have been established between bioprosthetic vs. mechanical valves 33. However, in carcinoid syndrome, a mechanical valve is preferred over bioprosthetic to avoid degeneration.
Surgery for severe tricuspid stenosis is most often performed at the time of operation for left-sided valve disease, chiefly rheumatic mitral stenosis/mitral replacement 34. Tricuspid valve surgery is preferred over percutaneous balloon tricuspid commissurotomy for treatment of symptomatic severe tricuspid stenosis because most cases of severe tricuspid stenosis are accompanied with tricuspid regurgitation (rheumatic, carcinoid), and percutaneous balloon tricuspid commissurotomy may either create or worsen regurgitation 23.
Isolated, symptomatic severe tricuspid stenosis without accompanying tricuspid regurgitation is an extremely rare condition for which percutaneous balloon tricuspid commissurotomy might be considered 23. Valve areas generally increase from less than 1 to almost 2 cm 35. It is also an option for patients considered to be at high surgical risk. Percutaneous balloon valvuloplasty may be done in non-surgical cases.
There is very limited information comparing percutaneous balloon valvotomy with tricuspid valve surgery.
Bioprosthetic valve stenosis can be treated with balloon valvotomy or valve-in-valve replacement.
Note the following:
- With tricuspid valve replacement, the risk of thrombosis is significant and many surgeons advise warfarin therapy for either mechanical or bioprosthetic valve placement.
- Percutaneous balloon valvuloplasty has been used successfully, as long as concomitant regurgitation is not significant 36. Reddy and colleagues report on the successful outcome of percutaneous valvuloplasty to treat severe bioprosthetic tricuspid valve stenosis in the setting of infective endocarditis. Their patient, a 29-year-old male with a history of intravenous drug use and two previous bioprosthetic tricuspid valve placements, was considered extremely high risk for redo valve replacement surgery. The investigators conclude that interventional treatment of prosthetic valvular stenosis in the setting of endocarditis is a reasonable therapy to use when open surgical repair is prohibitively risky with regard to mortality 37.
- The therapy chosen depends on the structure of the valve and the degree of deformity encountered.
- When possible, excise intracavitary pathology, whether it be tumors or other structural abnormalities.
- Redundant portions of the dilated right atrium can be excised during the same procedure for restoring the atrium back to normal size.
- In selected patients with prior tricuspid valve surgery and significant stenosis of a bioprosthetic tricuspid valve or a right atrium to right ventricle (RA-to–RV) conduit, percutaneous tricuspid valve replacement may be an option 38.
- Transcatheter valve-in-valve implantation with either the Melody or Edwards SAPIEN valve may be a potential procedure for patients with significant tricuspid stenosis, significant tricuspid regurgitation, or a mixed lesion and a failing bioprosthesis 39.
Tricuspid stenosis prognosis
The mortality associated with tricuspid stenosis depends on the precipitating cause. The general mortality rate is approximately 5%.
References- Lev M, Liberthson RR, Joseph RH, et al. The pathologic anatomy of Ebstein’s disease. Arch Pathol. 1970 Oct. 90(4):334-43.
- Acikel M, Erol MK, Yekeler I, Ozyazicioglu A. A case of free-floating ball thrombus in right atrium with tricuspid stenosis. Int J Cardiol. 2004 Apr. 94(2-3):329-30.
- Waller BF. Morphological aspects of valvular heart disease: Part II. Curr Probl Cardiol. 1984 Nov. 9(8):1-74.
- Tao G, Kotick JD, Lincoln J. Heart valve development, maintenance, and disease: the role of endothelial cells. Curr. Top. Dev. Biol. 2012;100:203-32.
- Roguin A, Rinkevich D, Milo S, Markiewicz W, Reisner SA. Long-term follow-up of patients with severe rheumatic tricuspid stenosis. Am. Heart J. 1998 Jul;136(1):103-8.
- American Heart Association. About Arrhythmia. http://www.heart.org/HEARTORG/Conditions/Arrhythmia/AboutArrhythmia/About-Arrhythmia_UCM_002010_Article.jsp
- Centers for Disease Control and Prevention. Division of Birth Defects and Developmental Disabilities. Congenital Heart Defects (CHDs). https://www.cdc.gov/ncbddd/heartdefects/index.html
- Tricuspid Stenosis Clinical Presentation. https://emedicine.medscape.com/article/158604-clinical#b5
- Roberts WC, Ko JM. Some observations on mitral and aortic valve disease. Proc (Bayl Univ Med Cent). 2008 Jul;21(3):282-99.
- Khatib N, Blumenfeld Z, Bronshtein M. Early prenatal diagnosis of tricuspid stenosis. Am. J. Obstet. Gynecol. 2012 Nov;207(5):e6-8.
- Waller BF, Howard J, Fess S. Pathology of tricuspid valve stenosis and pure tricuspid regurgitation–Part I. Clin Cardiol. 1995 Feb;18(2):97-102.
- Pellikka PA, Tajik AJ, Khandheria BK, Seward JB, Callahan JA, Pitot HC, Kvols LK. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993 Apr;87(4):1188-96.
- Al-Hijji M, Yoon Park J, El Sabbagh A, Amin M, Maleszewski JJ, Borgeson DD. The Forgotten Valve: Isolated Severe Tricuspid Valve Stenosis. Circulation. 2015 Aug 18;132(7):e123-5.
- Şaşkın H, Düzyol Ç, Özcan KS, Aksoy R. Right atrial myxoma mimicking tricuspid stenosis. BMJ Case Rep. 2015 Aug 13;2015
- Seibert KA, Rettenmier CW, Waller BF, Battle WE, Levine AS, Roberts WC. Osteogenic sarcoma metastatic to the heart. Am. J. Med. 1982 Jul;73(1):136-41.
- Nili M, Liban E, Levy MJ. Tricuspid stenosis due to intravenous leiomyomatosis–a call for caution: case report and review of the literature. Tex Heart Inst J. 1982 Jun;9(2):231-5.
- Akram Q, Saravanan D, Levy R. Valvuloplasty for tricuspid stenosis caused by a ventriculoatrial shunt. Catheter Cardiovasc Interv. 2011 Apr 01;77(5):722-5.
- Muraru D, Badano LP, Sarais C, Soldà E, Iliceto S. Evaluation of tricuspid valve morphology and function by transthoracic three-dimensional echocardiography. Curr Cardiol Rep. 2011 Jun;13(3):242-9.
- Coffey S, Rayner J, Newton J, Prendergast BD. Right-sided valve disease. Int. J. Clin. Pract. 2014 Oct;68(10):1221-6.
- Stapleton JF. Natural history of chronic valvular disease. Cardiovasc Clin. 1986;16(2):105-47.
- Applefeld MM. The Jugular Venous Pressure and Pulse Contour. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Butterworths; Boston: 1990.
- Golamari R, Bhattacharya PT. Tricuspid Stenosis. [Updated 2020 Feb 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499990
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD., American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014 Jun 10;63(22):e57-185.
- Morgan JR, Forker AD, Coates JR, Myers WS. Isolated tricuspid stenosis. Circulation. 1971 Oct;44(4):729-32.
- [Guideline] Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014 Jun 10. 63(22):e57-185.
- Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M., American Society of Echocardiography. European Association of Echocardiography. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009 Jan;22(1):1-23; quiz 101-2.
- Fawzy ME, Mercer EN, Dunn B, al-Amri M, Andaya W. Doppler echocardiography in the evaluation of tricuspid stenosis. Eur. Heart J. 1989 Nov;10(11):985-90.
- Seghatol FF, Rigolin VH. Appetite suppressants and valvular heart disease. Curr. Opin. Cardiol. 2002 Sep;17(5):486-92.
- KITCHIN A, TURNER R. DIAGNOSIS AND TREATMENT OF TRICUSPID STENOSIS. Br Heart J. 1964 May;26:354-79.
- Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS., 2006 Writing Committee Members. American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008 Oct 07;118(15):e523-661.
- Adler DS. Non-functional tricuspid valve disease. Ann Cardiothorac Surg. 2017 May;6(3):204-213.
- Orbe LC, Sobrino N, Arcas R, Peinado R, Frutos A, Blazquez JR, Maté I, Sobrino JA. Initial outcome of percutaneous balloon valvuloplasty in rheumatic tricuspid valve stenosis. Am. J. Cardiol. 1993 Feb 01;71(4):353-4.
- Kunadian B, Vijayalakshmi K, Balasubramanian S, Dunning J. Should the tricuspid valve be replaced with a mechanical or biological valve? Interact Cardiovasc Thorac Surg. 2007 Aug;6(4):551-7.
- Cevasco M, Shekar PS. Surgical management of tricuspid stenosis. Ann Cardiothorac Surg. 2017 May;6(3):275-282.
- Ribeiro PA, Al Zaibag M, Al Kasab S, Idris M, Halim M, Abdullah M, Shahed M. Percutaneous double balloon valvotomy for rheumatic tricuspid stenosis. Am. J. Cardiol. 1988 Mar 01;61(8):660-2.
- Badheka AO, Shah N, Ghatak A, et al. Balloon mitral valvuloplasty in the United States: a 13-year perspective. Am J Med. 2014 Nov. 127(11):1126.e1-12.
- Reddy G, Ahmed M, Alli O. Percutaneous valvuloplasty for severe bioprosthetic tricuspid valve stenosis in the setting of infective endocarditis. Catheter Cardiovasc Interv. 2015 Apr. 85(5):925-9.
- Roberts PA, Boudjemline Y, Cheatham JP, et al. Percutaneous tricuspid valve replacement in congenital and acquired heart disease. J Am Coll Cardiol. 2011 July 5. 58(2):117-22.
- Godart F, Baruteau AE, Petit J, et al. Transcatheter tricuspid valve implantation: A multicentre French study. Arch Cardiovasc Dis. 2014 Nov. 107(11):583-91.