Uremic frost
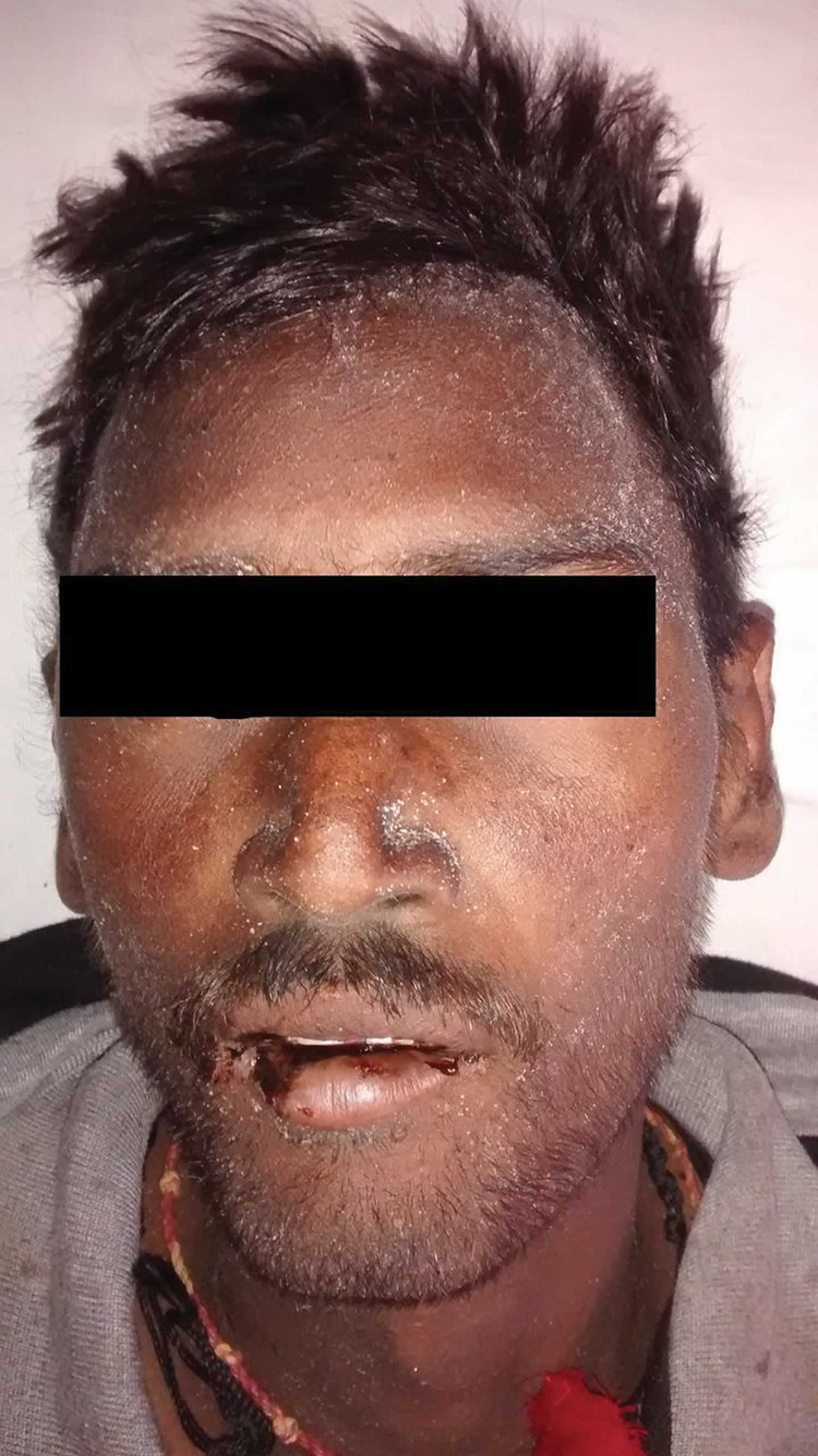
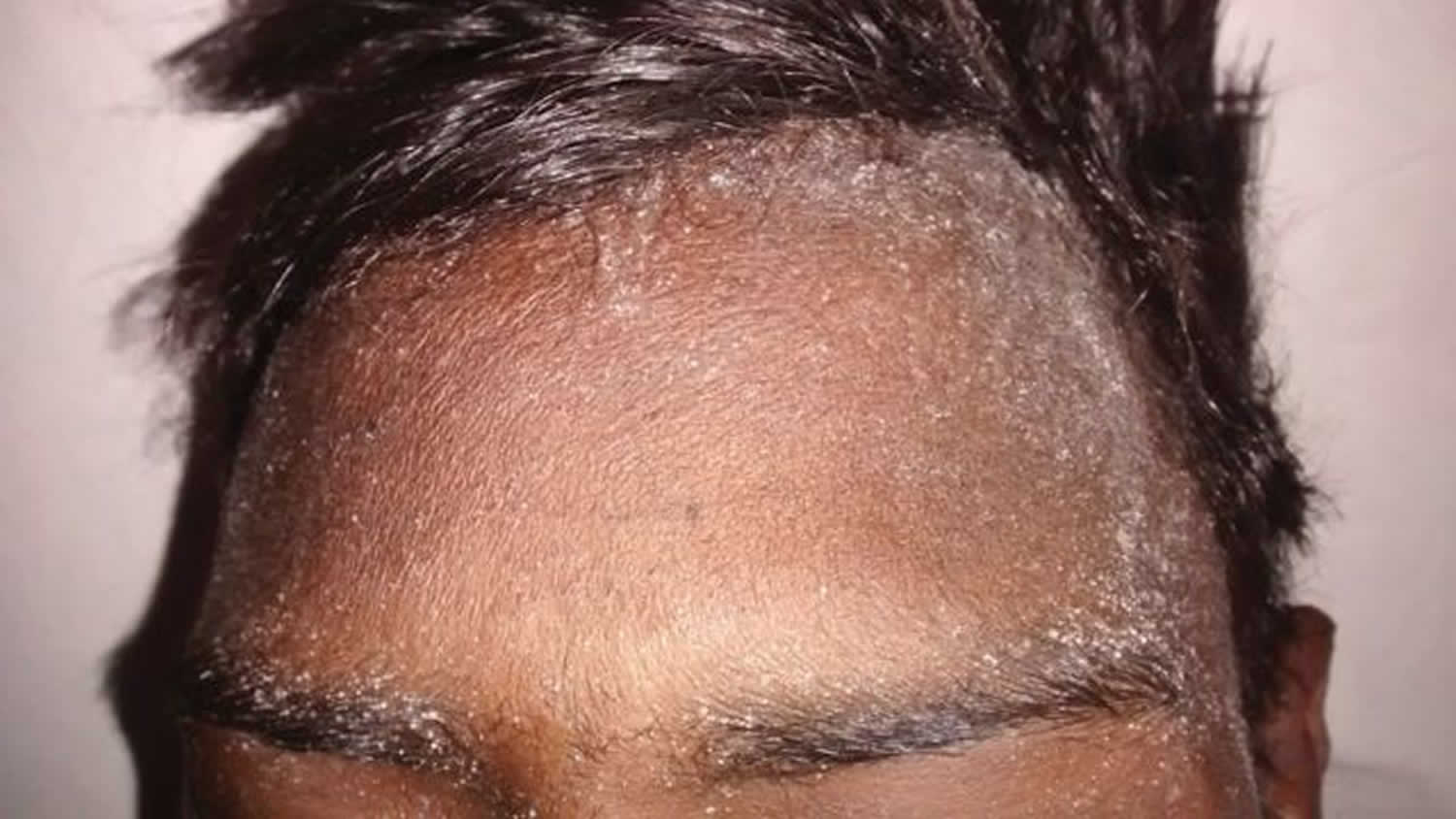
Uremic frost is a dermatological disturbance of advanced chronic kidney disease or end-stage renal disease (ESRD) is believed to result from evaporation of sweat that contains high levels of urea and other nitrogenous waste products 1. A study by Udaykumar et al. 2 on dermatological changes in chronic kidney disease showed the incidence of uremic frost to be 3%. When the blood urea nitrogen (BUN) level is high, the concentration of urea in sweat increases greatly. Evaporation of sweat with high urea concentration causes urea to crystallize and deposit on the skin. The frost consists of a white or yellowish coating of urea crystals on the beard area and other parts of the face, neck and on the trunk 3. It is due to eccrine deposition of urea crystals on the skin surface of patients with severe uremia. These waste products crystallize on the skin, most commonly on the face 4. As a cutaneous manifestation, uremic frost should be differentiated from retention keratosis, eczema and postinflammatory desquamation 5. However, a history of end-stage renal disease and the white, friable, crystalline characteristics of uremic frost can make its diagnosis easy. To verify that the crystals are composed of urea or nitrogenous waste, scrapings of the frost can be diluted in normal saline, which can then be tested for elevated urea nitrogen levels comparable to blood levels 6.
Uremic frost is rarely seen today in developed countries, owing to widespread availability of hemodialysis, but is still reported from resource poor nations.
Figure 1. Uremic frost on skin
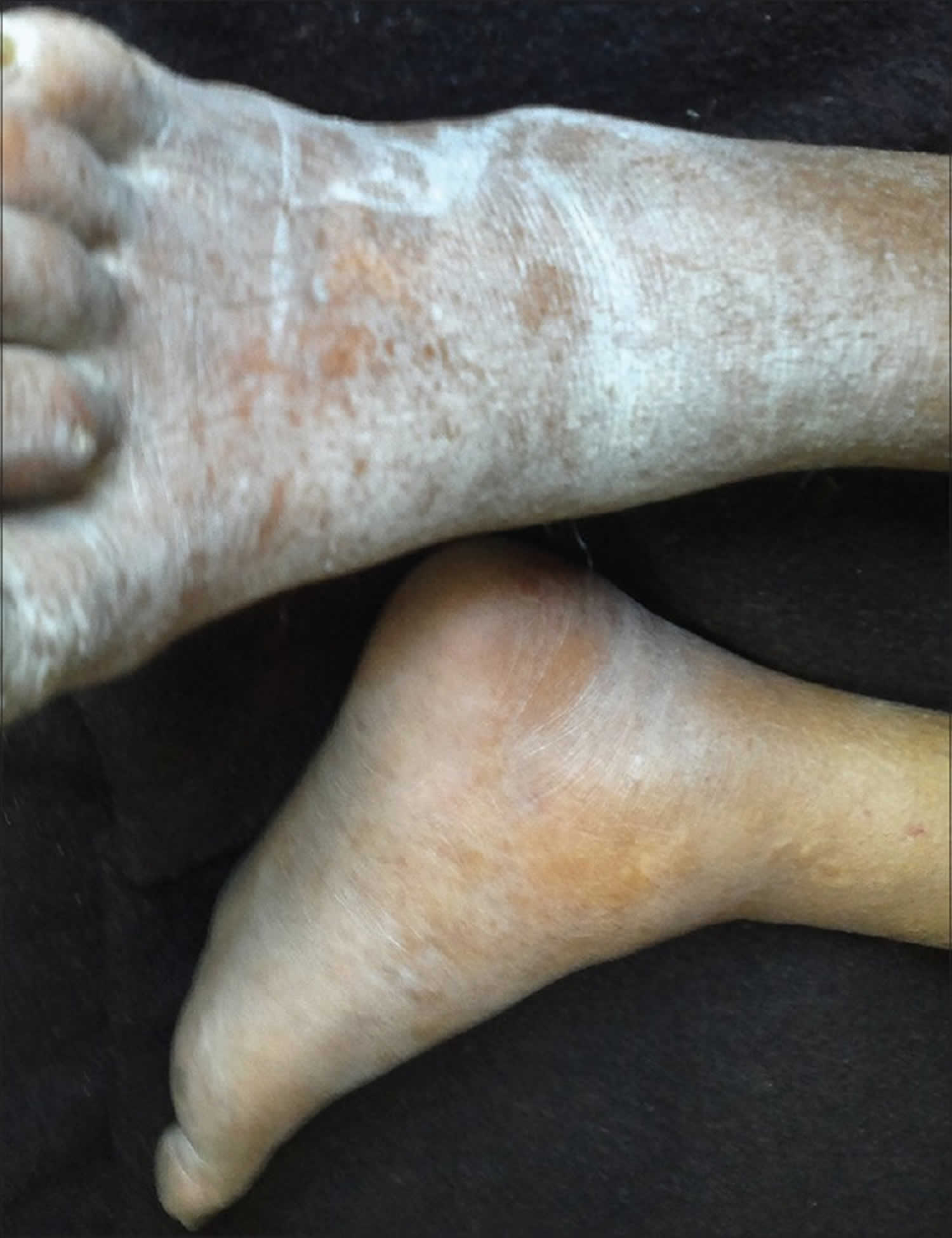
[Source 1 ]Figure 2. Uremic frost on legs
[Source 7 ]Uremic frost causes
Kidney disease can result from some conditions ranging from primary renal disorders, for example IgA nephropathy, focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, polycystic kidney disease) to systemic disorders that can lead to renal damage 8. Systematic disorders can include diabetes mellitus, lupus, multiple myeloma, amyloidosis, Goodpasture disease, Thrombotic thrombocytopenic purpura, or hemolytic uremic syndrome.
The leading cause of end-stage renal disease (ESRD) in the United States is diabetes. Additional causes, listed in order of decreasing incidence, include hypertension, glomerulonephritis, interstitial disease, cystitis, and neoplasms.
Uremia may also result from acute kidney injury if the injury involves a sudden increase in urea or creatinine 9.
Uremic frost symptoms
Symptomatic uremia tends to occur once creatinine clearance decreases below 10 mL/min unless kidney failure develops acutely, in which case, some patients may become symptomatic at higher clearance rates 8.
Patients presenting with uremia typically complain of nausea, vomiting, fatigue, anorexia, weight loss, muscle cramps, pruritus, or changes in mental status. The clinical presentation of uremia can be explained by the metabolic disturbances associated with the condition.
Fatigue as a result of anemia is considered one of the major components of the uremic syndrome.
Patients with a history of diabetes may report improved glycemic control but are at a greater risk of developing hypoglycemic episodes as kidney function worsens.
Hypertension, atherosclerosis, valvular stenosis and insufficiency, chronic heart failure, and angina may all develop as a result of a buildup of uremic toxins and metastatic calcification associated with uremia and end-stage renal disease (ESRD).
Occult gastrointestinal bleeding as a result of platelet abnormalities may present with nausea or vomiting. Uremic fetor, ammonia or urine-like odor of the breath, may also occur in uremic patients.
Uremic frost diagnosis
A history of end-stage renal disease and the white, friable, crystalline characteristics of uremic frost can make its diagnosis easy. To verify that the crystals are composed of urea or nitrogenous waste, scrapings of the frost can be diluted in normal saline, which can then be tested for elevated urea nitrogen levels comparable to blood levels 10.
A diagnosis of renal failure is based on abnormalities in glomerular filtration rate (GFR) or creatinine clearance 11.
It is important to determine whether a patient presenting with uremic symptoms is experiencing acute or chronic renal failure, as acute kidney injury is reversible. Laboratory studies to evaluate for abnormalities in hemoglobin, calcium, phosphate, parathyroid hormone, albumin, potassium, and bicarbonate in addition to urinalysis (with microscopic examination) will help point towards any potential abnormalities.
A 24-hour urine collection may provide insight to both GFR and creatinine clearance, though this method is both burdensome and often inaccurate. Alternatively, a nuclear medicine radioisotope (iothalamate) clearance assay may be used to measure GFR. However, this test is also time-consuming and expensive relative to the Cockcroft-Gault formula [creatinine clearance = Sex times 12 times (weight / 72)] or the Modification of Diet in Renal Disease formula [(GFR (mL/min/1.73 m) = 175 x (S) times (Age) times (0.742 if female) or times (1.212 if African American)] that are often used instead.
As per the National Kidney Foundation, patients presenting with chronic kidney disease are staged based on the estimated GFR (creatinine clearance) as calculated by the Modification of Diet in Renal Disease formula.
- Stage 1 – normal GFR (90 mL/min or greater)
- Stage 2 – mildly reduced GFR (60 mL/min to 90 mL/min)
- Stage 3 – moderately reduced GFR (30 mL/min to 59 mL/min)
- Stage 4 – severely reduced GFR (15 mL/min to -29 mL/min)
- Stage 5 – end-stage renal disease (ESRD) (GFR < 15 mL/min or patient is on dialysis)
Chronic kidney disease classified based on grade:
- Grade 1: GFR greater than 90 mL/min
- Grade 2: 60 to 89 mL/min
- Grade 3a: 45 to 59 mL/min
- Grade 3b: 30 to 44 mL/min
- Grade 4: 15 to 29 mL/min
- Grade 5: Less than 15 mL/min
A renal ultrasound may be useful to determine the size and shape of the kidneys, and to evaluate for hydronephrosis or ureteral and/or bladder obstruction. This may occur as a result of kidney stones, neurologic abnormalities, trauma, pregnancy, prostate enlargement, retroperitoneal fibrosis, abdominal tumors (secondary to cervical or prostate cancers) or additional structural abnormalities. Early diabetic nephropathy, multiple myeloma, polycystic kidney diseases, and glomerulonephritis associated with human immunodeficiency virus (HIV) are all associated with enlarged kidneys on ultrasound. Smaller kidneys are indicative of more chronic, irreversible changes as a result of long-standing kidney disease, ischemic nephropathy, or hypertensive nephrosclerosis.
If a patient presents with significant alterations in mental status, a brain computed tomography (CT) scan may be warranted. Uremic patients with a blood urea nitrogen (BUN) level greater than 150 mg/dL to 200 mg/dL are also at an increased risk of developing spontaneous subdural hematomas. Given the increased risk of bleeding and hemorrhage in uremia (especially in the setting of a fall or trauma), a CT scan of both the brain and abdomen may additionally be considered. An abdominal CT scan might help further elucidate the underlying cause of hydronephrosis if it was found on ultrasound without any obvious etiology.
Finally, magnetic resonance imaging (MRI) may be considered to assess for renal artery stenosis or thrombosis, or aortic and renal artery dissection- all potentially reversible causes of renal failure.
A renal biopsy may be helpful in determining reversibility or treatability of the renal injury, and may ultimately be required to make an accurate diagnosis of acute kidney injury or chronic kidney disease. However, a biopsy should not be performed in the case of small kidneys because of the associated comorbidities and increased risk of bleeding.
Uremic frost treatment
The treatment of uremic frost is largely aimed at correcting the underlying cause of uremia and the other life-threatening conditions associated with renal failure.
Dialysis is indicated in a patient with symptomatic uremia (e.g., nausea, vomiting, hyperkalemia, metabolic acidosis) that is not treatable my medical means, and should be initiated as soon as possible, regardless of the patient’s GFR 13.
Patients presenting with a uremic emergency (e.g., hyperkalemia, acidosis, symptomatic pericardial effusion, or uremic encephalopathy) require emergent dialysis which should be initiated gently to avoid dialysis disequilibrium syndrome (neurologic symptoms secondary to cerebral edema occurring during or shortly after the initiation of dialysis).
Ultimately, the best renal replacement therapy is renal transplantation, although practitioners may also consider long-term hemodialysis and peritoneal dialysis. Renal transplantation is associated with improvements in both survival and quality of life, and should be considered early (before the need for dialysis) as the waiting list for transplantation is often longer than two to three years.
Iron replacement should be initiated in patients with anemia of chronic kidney disease and underlying iron deficiency (as long as serum ferritin is greater than 100 mcg/mL). This can be done with dialysis treatments, or as oral therapy, if dialysis has not yet been initiated. Erythropoietic stimulating agents, such as erythropoietin or darbepoetin, may additionally be used in low doses (due to the increased risk of cardiovascular mortality) once hemoglobin levels reach below 10 g/dL.
Hyperparathyroidism and associated or isolated hypocalcemia and hyperphosphatemia can be treated with oral calcium carbonate or calcium acetate, oral vitamin D therapy, and oral phosphate binders (e.g., calcium carbonate, calcium acetate, sevelamer or lanthanum carbonate).
A dietitian should be consulted if dietary alterations are being considered. Patients with chronic kidney disease should reduce potassium, phosphate and sodium intake to 2 g to 3 g, 2 g, and 2 g per day of each, respectively. Though there is some conflicting evidence regarding protein intake in patients with kidney failure, the current low-protein diet recommendations before initiation of dialysis are 0.8 g to 1 g of protein/kg of weight per day with an added gram of protein for each gram of protein lost in the urine in patients with nephrotic syndrome.
A low-protein diet is not recommended in patients with advanced uremia or malnutrition, as this type of diet can result in worsening of malnutrition and has been associated with increased risk of mortality with the initiation of dialysis.
Patients with a creatinine clearance of less than 20 mL/min should avoid excessive potassium intake and use certain medications with caution (e.g., potassium-sparing diuretics, angiotensin-converting enzymes (ACE) inhibitors, angiotensin-receptor blockers, beta blockers, nonsteroidal anti-inflammatory drugs [NSAIDs]).
Due to the buildup of uremic toxins and potentially increased risk of bleeding and hemorrhage, extra care needs to be taken when prescribing oral anticoagulants or antiplatelet medications to patients who have ESRD.
Finally, nephrotoxic medications (e.g., NSAIDs, aminoglycoside antibiotics) should be avoided in all patients with renal disease. To avoid nephrotoxicity, N-acetylcysteine may be administered before administration of intravenous contrast for radiologic imaging, although alternative modes of imaging like MRI should be considered in these patients, to avoid the risk of acute kidney injury altogether 14.
Uremic frost prognosis
In general, the prognosis for patients with uremia is poor unless they are treated with renal replacement therapy such as transplantation or dialysis. When the cause of uremia is a reversible cause, the prognosis is better than in patients with an irreversible cause. Uremic patients require frequent admission to the hospitals and have high morbidity and mortality without treatment. While dialysis has improved treatment, vascular access is still a major problem in the long run. In addition, there are not enough kidney donors. Patients with uremia are also at a high risk for adverse cardiac events and stroke compared to the general population 15.
References- Mathur M, D’Souza AV, Malhotra V, Agarwal D, Beniwal P. Uremic frost. Clin Kidney J. 2014;7(4):418–419. doi:10.1093/ckj/sfu057 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4377803
- Walsh SR, Parada NA. Uremic frost. N Engl J Med. 2005;352:e13.
- Kuo CC, Hung JB, Tsai CW, et al. Uremic frost. CMAJ. 2010;182:E800
- Udayakumar P, Balasubramanian S, Ramalingam KS, et al. Cutaneous manifestations in patients with chronic renal failure on hemodialysis. Indian J Dermatol Venereol Leprol. 2006;72:119–25.
- Kuo CC, Hung JB, Tsai CW, Chen YM. Uremic frost. CMAJ. 2010;182(17):E800. doi:10.1503/cmaj.091779 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2988568
- Pol-Rodriguez MM, Wanner M, Bhat P, et al. Uremic frost in a critically ill patient. Kidney Int. 2008;73:790.
- Raina S, Chauhan V, Sharma R, Sharma R. Uremic frost. Indian Dermatol Online J. 2014;5(Suppl 1):S58. doi:10.4103/2229-5178.144545 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4252958
- Zemaitis MR, Foris LA, Chandra S, et al. Uremia. [Updated 2019 Jul 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441859
- Martins JM, Magriço R. Uremic Frost in End-Stage Renal Disease. N. Engl. J. Med. 2018 Aug 16;379(7):669.
- Mohan D, Railey M. Uremic frost. Kidney Int. 2012;81:1153.
- Massy ZA, Liabeuf S. Middle-Molecule Uremic Toxins and Outcomes in Chronic Kidney Disease. Contrib Nephrol. 2017;191:8-17.
- 140 – Age) / (serum creatinine
- Leong SC, Sao JN, Taussig A, Plummer NS, Meyer TW, Sirich TL. Residual Function Effectively Controls Plasma Concentrations of Secreted Solutes in Patients on Twice Weekly Hemodialysis. J. Am. Soc. Nephrol. 2018 Jul;29(7):1992-1999.
- Leurs P, Machowska A, Lindholm B. Timing of dialysis initiation: when to start? Which treatment? J Ren Nutr. 2015 Mar;25(2):238-41.
- Vanholder R, Van Laecke S, Glorieux G, Verbeke F, Castillo-Rodriguez E, Ortiz A. Deleting Death and Dialysis: Conservative Care of Cardio-Vascular Risk and Kidney Function Loss in Chronic Kidney Disease (CKD). Toxins (Basel). 2018 Jun 12;10(6).