Acanthamoeba keratitis
Acanthamoeba keratitis is a rare but potentially sight-threatening eye infection caused by pathogenic species of Acanthamoeba, occurring mostly in contact lens wearers 1. Acanthamoeba are ubiquitous, microscopic, free-living protozoa, present in air, soil, dust, drinking water, sea water and also in nasopharyngeal mucosa of healthy individuals 2. Although contact lens wear is a major risk factor, exposure to contaminated water and eye trauma are also associated with acanthamoeba keratitis. Once a patient develops acanthamoeba keratitis the prognosis is very poor unless an aggressive treatment regimen is initiated early 2. Some of the intriguing features of acanthamoeba keratitis are the lack of immunological memory, resistance of the dormant cyst form to treatment, differences between the pathogenic strains and soil isolates of Acanthamoeba and the unique role of the innate immune system in controlling acanthamoeba keratitis 2.
The life cycle of Acanthamoeba consists of two stages; the infective trophozoite form and the dormant resilient cyst. The Acanthamoeba trophozoites (10 to 25µm) are the vegetative forms which feed on organic matter, other bacteria (which allows for the diagnosis on E. coli plates), algae, and yeasts and divide mitotically. When exposed to harsh conditions such as lack of nutrients or extreme heat or cold, the trophozoites differentiate in to a double walled Acanthamoeba cyst form (8 to 12µm). The outer layer of the cysts is composed of polysaccharides and the inner layer is made up of cellulose. The Acanthamoeba cysts are resistant to repeated cycles of freeze-thawing (for example 15 months at −15 °C) and also remarkably high doses of ultraviolet and gamma irradiation. The Acanthamoeba cysts are metabolically inactive and can remain viable for up to 24 years under dry conditions 1. Pathogenic Acanthamoeba species cause two distinctly different diseases: a) granulomatous amoebic encephalitis and b) amoebic keratitis 2. Granulomatous amoebic encephalitis is most commonly seen in immunocompromised patients whereas the most common disease caused by Acanthamoeba species is amoebic keratitis, which occurs in immunocompetent individuals 3.
Acanthamoeba keratitis is often misdiagnosed and treated as herpetic, bacterial, or mycotic keratitis, as many signs and symptoms may look similar to other kinds of keratitis 1. It is challenging for an ophthalmologist to find the right diagnosis 4; therefore, diagnosis is often delayed and ophthalmologists tend to observe a heterogeneous and protracted clinical course.
Acanthamoeba keratitis can be managed if diagnosed and treated during the early stages. As the Acanthamoeba keratitis progresses an aggressive treatment regimen involving hourly around the clock application of antimicrobials is needed. Corticosteroids are sometimes used to dampen inflammation in severe cases but they often need to be used in combination with antimicrobials such as polyhexamethylene biguanide (PHMB) or propamidine isethionate (Brolene®). However, corticosteroids can cause extensive ocular damage and in some cases, induce the excystment of the cysts to the active trophozoite forms, which necessities coverage with antimicrobials 5. Despite aggressive therapy, some patients fail to respond to treatment and corneal transplantation is needed to restore vision. Although Acanthamoeba species are ubiquitous and the leading risk factor for acanthamoeba keratitis, contact lens wear, is practiced by over 30 million individuals in the United States, acanthamoeba keratitis is extraordinarily rare with less than 150 cases occurring each year in the U.S. 6. Moreover, serological surveys indicate that 90 to 100 percent of the adult population with no history of acanthamoeba keratitis has serum antibodies specific for Acanthamoeba antigens – a finding that suggests that exposure to Acanthamoebae is commonplace, yet the disease is rare. These observations have prompted investigators to search for other risk factors and conditions that contribute to the development of acanthamoeba keratitis.
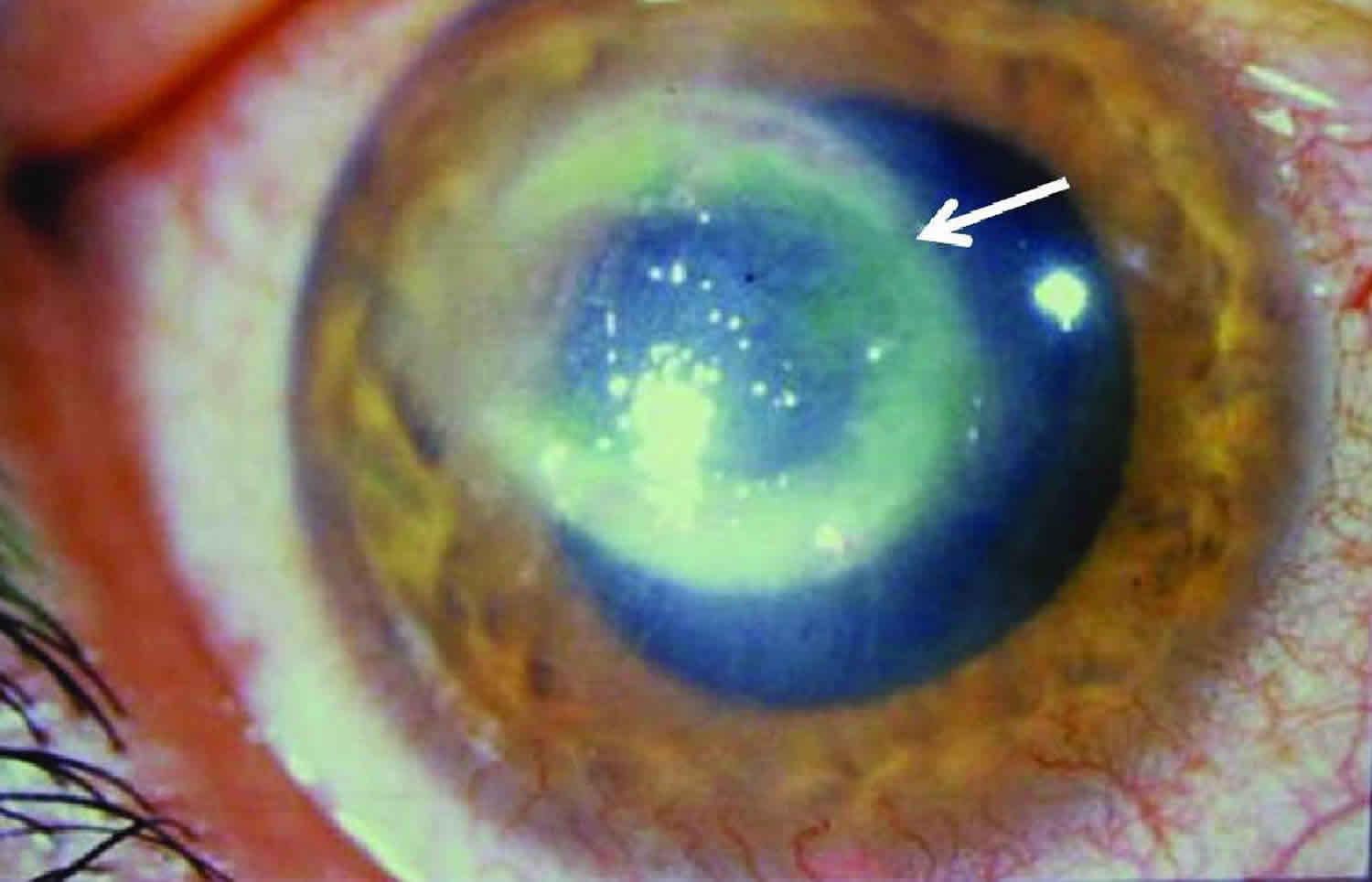
Figure 1. Acanthamoeba keratitis
Footnote: Eye with Acanthamoeba keratitis. The ring-like stromal infiltrate (arrow) is a characteristic feature of Acanthameoba keratitis.
[Source 7 ]Acanthamoeba keratitis causes
Acanthamoeba is ubiquitous. Acanthamoeba are commonly found, free-living amoeba that have been located in various environments including pools, hot tubs, tap water, shower water, and contact lens solution. In May, 2007, the FDA announced an outbreak of Acanthamoeba keratitis that was associated with Complete MoisturePlus Multi Purpose Solution manufactured by Advanced Medical Optics. Corneal trauma, followed by exposure to the parasite (often through a water supply or contact lens solution) in a patient with low tear levels of anti-Acanthamoeba IgA leads to infection.
The Acanthamoeba organism has to make contact directly with the eyes in order to cause acanthamoeba keratitis, so this type of corneal infection cannot occur from drinking or inhaling water that has this ameba in it 8. Acanthamoeba keratitis cannot be spread from person to person.
Acanthamoeba keratitis is a progressive sight-threatening disease caused by at least eight species of Acanthamoeba: Acanthamoeba castellanii, Acanthamoeba culbertsoni, Acanthamoeba polyphaga, Acanthamoeba hatchetti, Acanthamoeba rhysodes,Acanthamoeba lugdunesis, Acanthamoeba quina, and Acanthamoeba griffini 9. Two of the eight known species of Acanthamoeba, Acanthamoeba castellanii and Acanthamoeba polyphaga, are responsible for most infections. Contact lens wear is the most common risk factor and accounts for > 80 percent of the cases of acanthamoeba keratitis 2.
Risk factors for acanthamoeba keratitis
Risk factors for acanthamoeba keratitis include contact lens wear, exposure to organism (often through contaminated water), and corneal trauma. In the United States, an estimated 85% of acanthamoeba keratitis cases occur in contact lens wearers 10, especially extended use of contact lenses (therefore, daily lenses have a lower risk) 11. For people who wear contact lenses, the risk of getting Acanthamoeba keratitis is higher if they:
- use of contact lenses during bath, swim, hot tub, or shower
- using tap water to clean the lenses or lens case 12.
- adding fresh solution to existing used solution in the case instead of using only fresh solution when storing contact lenses 13.
- do not store or handle contact lenses properly. This can include not washing hands before touching contact lenses, not rubbing and rinsing lenses after taking them out, and not storing them in the recommended contact lens solution 14.
- have a history of trauma to the cornea, such as a previous eye injury 15.
Low levels of anti-Acanthamoeba IgA in tears has also been shown to be a risk factor. It is thought that over 80% of Acanthamoeba keratitis appears in contact lens wearers. In one study, 75% of the patients were contact lens wearers; 40% wore daily soft lenses, 22% wore rigid gas permeable lenses, and 38% wore extended wear or other lenses.
Additional risk factors are corneal surface damage, exposition to contaminated water, and low socioeconomic status 16.
A study has proven that only hydrogen-peroxide-containing contact lens cleaners are effective against all acanthamoeba strains 17.
Acanthamoeba keratitis prevention
Since acanthamoeba keratitis treatment is toxic, lengthy, and not necessarily effective, prevention is essential. Contact lens wearers should be taught how to clean their contact lenses properly. They should be instructed never to use tap water or even saline to clean their lenses. They should also be instructed to visit an ophthalmologist at the earliest sign of problems.
How to take proper care of your contact lenses
You must clean and disinfect any contact lens you remove from your eye before you put the lens back in. There are many types of cleansing systems. The choice depends on the type of lens you use, if you have allergies or if your eyes tend to form protein deposits. Ask your eye doctor what kind of cleaning solutions you should use.
Take special care to clean and store your lenses correctly to avoid dangerous eye infections.
Here is what you should do:
- Follow the schedule your eye doctor gives you for wearing and replacing your lenses. You should not wear daily wear lenses while you sleep.
- Remove contact lenses before taking a shower, using a hot tub, swimming, or doing anything where water gets in your eyes.
- Before touching your contact lenses, wash your hands with soap and water and dry them with a lint-free towel.
- Never put contacts in your mouth to wet them. Saliva (spit) is not a sterile solution.
- Do not rinse or store contacts in water (tap or sterile water). Also, never use a homemade saline solution.
- Do not use saline solution or rewetting drops to disinfect your lenses. They are not disinfectants.
- Follow directions from your doctor and from the lens cleaning solution manufacturer to clean and store your lenses.
- No matter what type of lens cleaning solution you buy, use a “rub and rinse” cleaning method. Rub your contact lenses with clean fingers, then rinse the lenses with solution before soaking them. Use this method even if the solution you are using is a “no-rub” type.
- Use new solution each time you clean and disinfect your contact lenses. Never reuse or “top off” with old solution. Also, do not pour contact lens solution into a different bottle. The solution will no longer be sterile.
- Make sure the tip of the solution bottle does not touch any surface. Keep the bottle tightly closed when you are not using it.
- Rinse your contact lens case with sterile contact lens solution (not tap water). Then leave the empty case open to air dry.
- Keep your contact lens case clean. Replace the case at least every 3 months, or right away if it gets cracked or damaged.
- If you store your lenses in the case for a long time, check the contact lens instructions or the lens solution directions to see if you should re-disinfect them before wearing them. Never wear your contact lenses if they have been stored for 30 days or longer without re-disinfecting.
- Contact lenses can warp over time, and your cornea can change shape. To make sure your lenses fit properly and the prescription is right for you, see your eye doctor regularly.
Acanthamoeba keratitis signs and symptoms
In early stages of acanthamoeba keratitis, about 75–90% of all patients are misdiagnosed, as typical acanthamoeba keratitis symptoms are difficult to associate 18. Analysis of the German Acanthamoeba Keratitis Registry have shown that in 47.6% herpetic, in 25.2% mycotic, and in 3.9% bacterial keratitis was erroneously diagnosed by ophthalmologist in acanthamoeba keratitis patients 19. Patients had the correct acanthamoeba keratitis diagnosis not before 2.8 ± 4.0 months (range, 0–23 months) after appearance of the first clinical symptoms, in Germany 19.
In about 23% of the cases, a mixed infection with virus, bacteria, or fungi is present 4.
Acanthamoeba keratitis symptoms
Symptoms of acanthamoeba keratitis include 20:
- Sensation of something in the eye
- Eye pain
- Eye redness
- Blurred vision
- Sensitivity to light
- Excessive tearing
Acanthamoeba keratitis is characterized by pain out of proportion to findings. In one study, 95% of patients complained of pain. Patients may also complain of decreased vision, redness, foreign body sensation, photophobia, tearing, and discharge. Symptoms may wax and wane; they may be quite severe at times.
If you experience any of these symptoms, remove your contact lenses (if you wear them) and call your eye doctor right away. Acanthamoeba keratitis is a rare condition, but if left untreated it can result in vision loss or blindness 21.
Acanthamoeba keratitis signs
Early signs may be mild and non-specific. Possible findings include epithelial irregularities, epithelial or subepithelial infiltrates, and pseudodendrites. Later signs include stromal infiltrates (ring-shaped, disciform, or numular), satellite lesions, epithelial defects, radial keratoneuritis, scleritis, and anterior uveitis (with possible hypopyon). Advanced signs include stromal thinning and corneal perforation.
Clinical signs of acanthamoeba keratitis are the following:
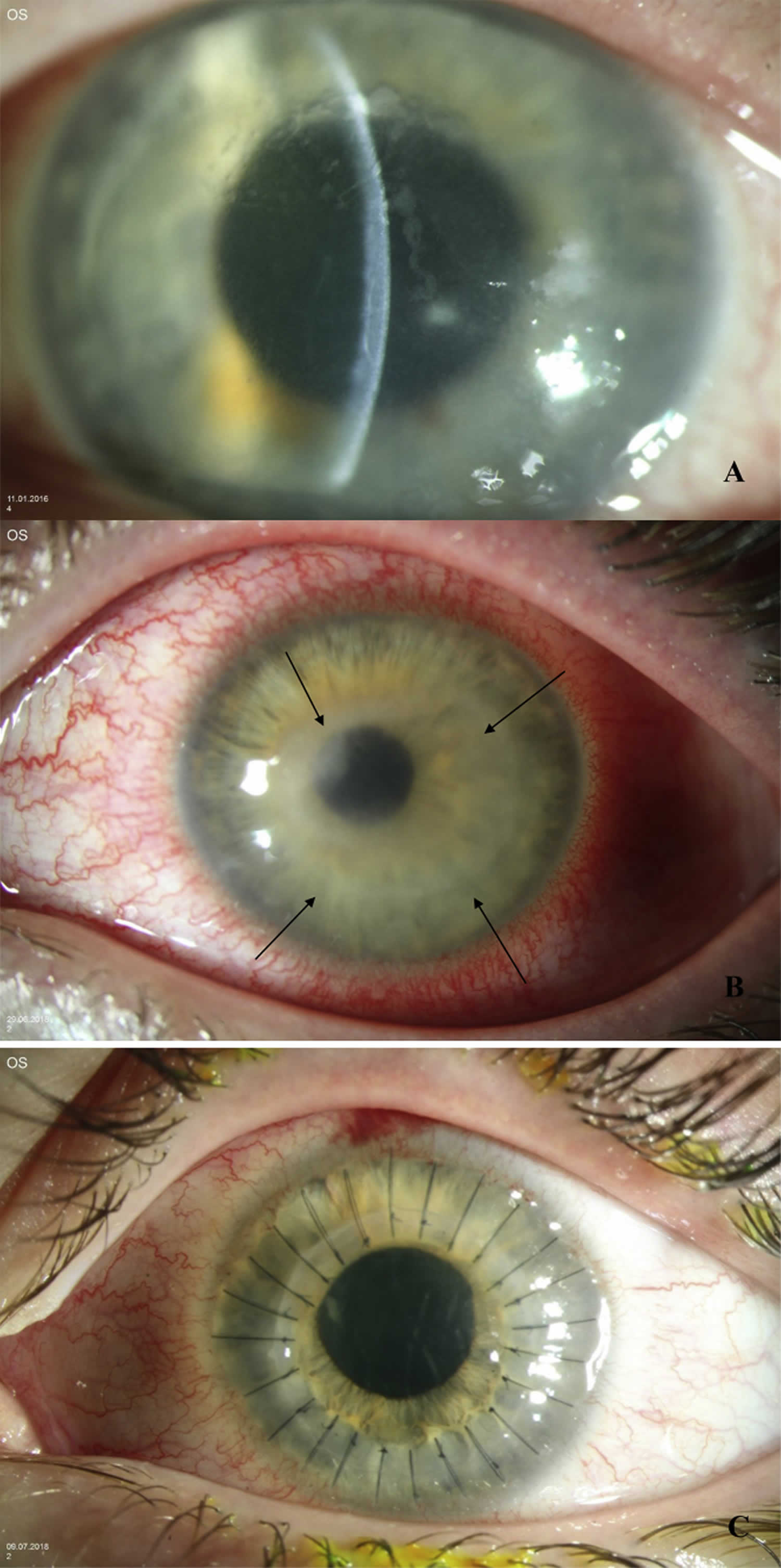
- Chameleon-like epithelial changes (“dirty epithelium”, pseudodendritiformic epitheliopathy, epithelial microerosions, and microcysts) (Figure 2A)
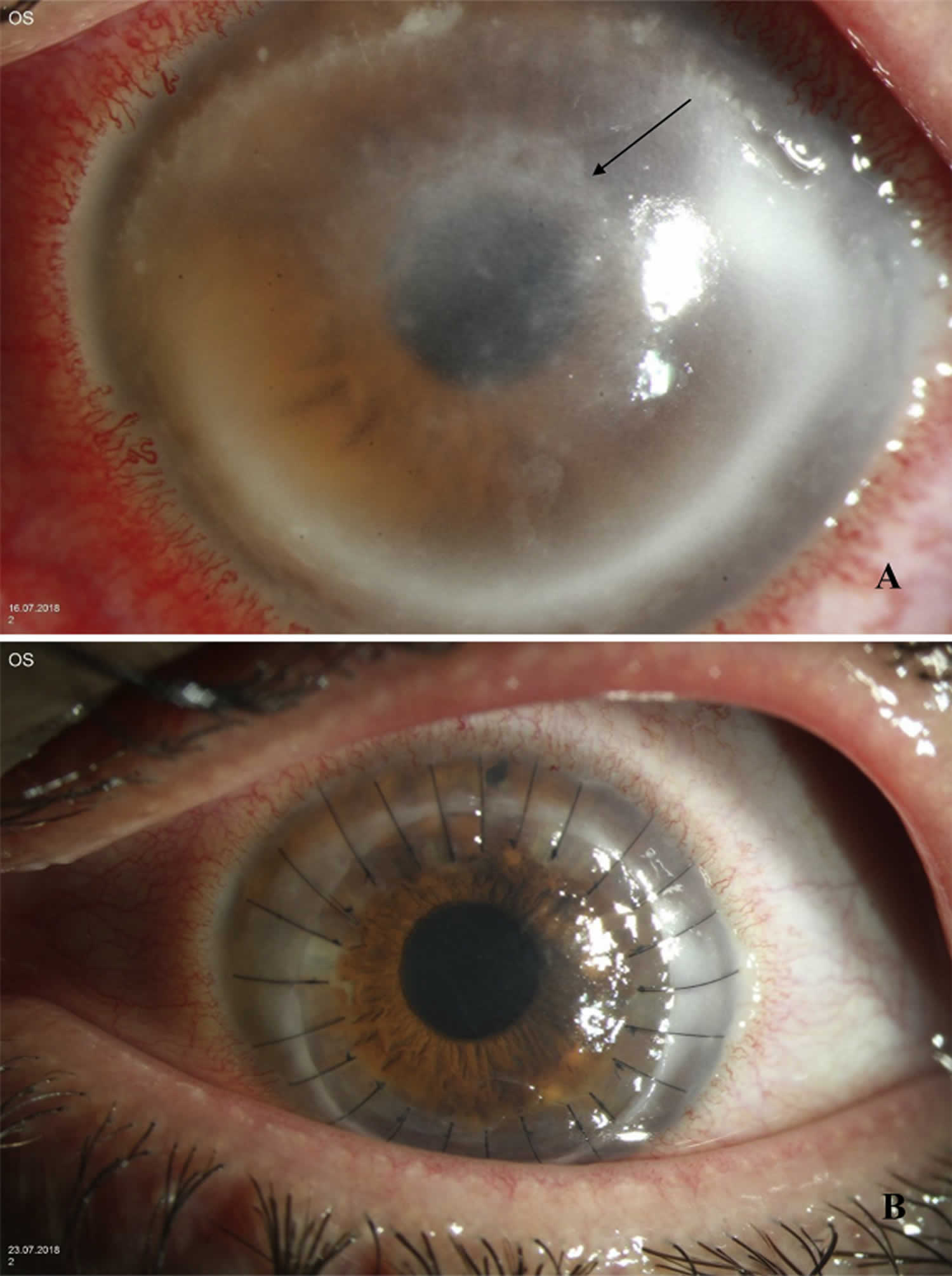
- Multifocal stromal infiltrates (Figure 3A)
- Ring infiltrate (“Wessely immune ring”) (Figure 2 and Figure 3A)
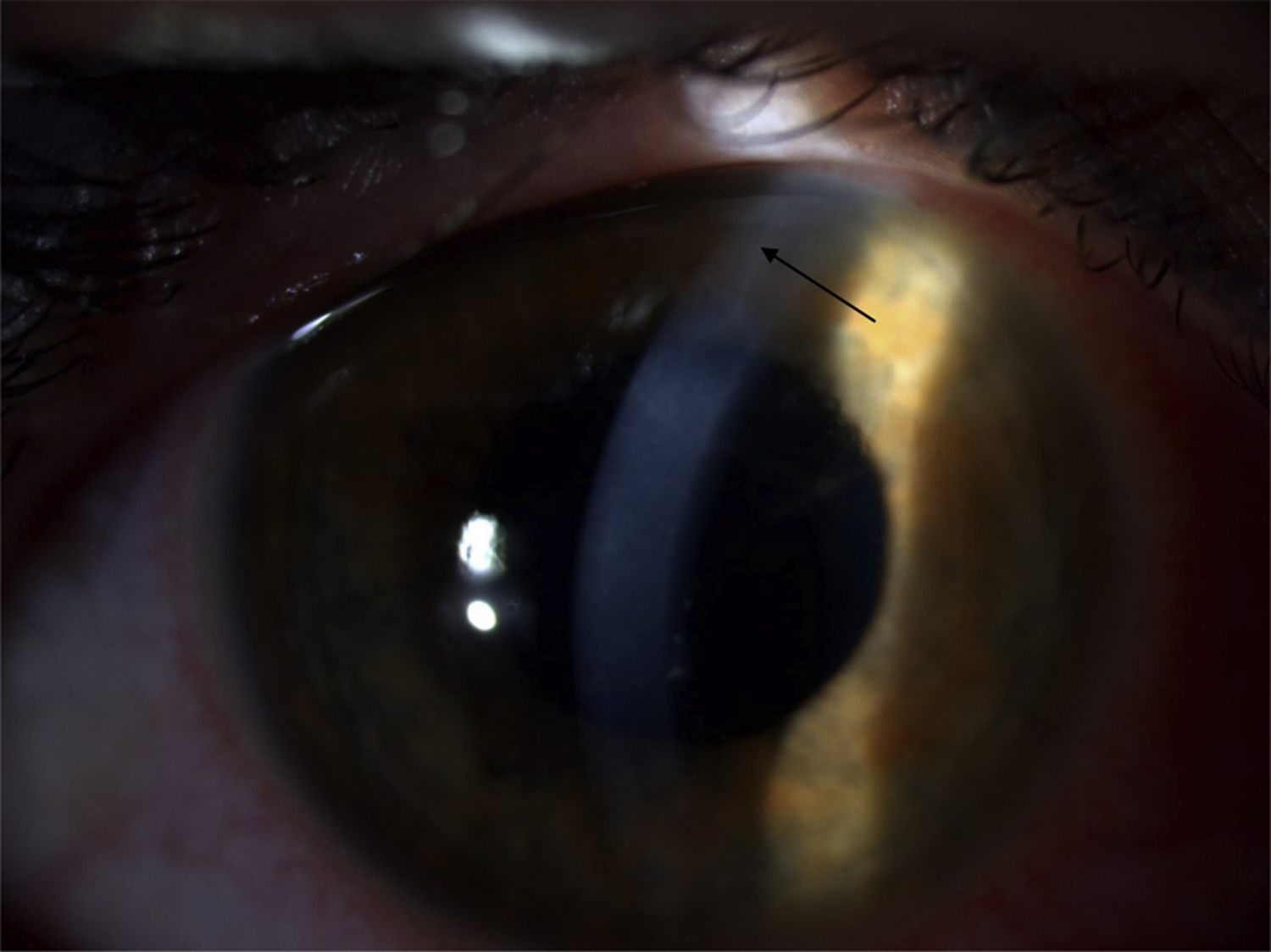
- Peripheral perineurial infiltrate (Figure 4)
Acanthamoeba keratitis presents in early stages with grey-dirty epithelium, pseudodendritiformic epitheliopathy, perineuritis, multifocal stromal infiltrates, ring infiltrates, and in later stages with scleritis, iris atrophy, anterior synechiae, secondary glaucoma, mature cataract, and chorioretinitis.
Table 1. Clinical signs and their timely presentation in acanthamoeba keratitis
| Clinical sign | Time | Special properties |
|---|---|---|
| Chameleon-like epithelial changes (“dirty epithelium”) (Figure 2A) | Within the first 2 weeks in 50% of the patients | Grey epithelial opacities, pseudodendritiformic epitheliopathy, epithelial microerosions or microcysts |
| Multifocal stromal infiltrates (Figure 3A) | Within the first 2 weeks | Mostly central and paracentral |
| Ringinfiltrate/Wessely immune ring (Figure 2 and Figure 3A) | In the first month in 20% of the patients | From polymorphonuclear leukocytes, antigen-antibody-komplex and complement; incidence increases with time |
| Perineural infiltrate (Figure 4) | In the first month of the disease in 2.5–63% of the patients | Radial, from limbus to middle stroma, results in loss of corneal nerve fibers |
| Sterile anterior uveitis, scleritis, broad-based anterior synechiae, secondary glaucoma, iris atrophie, mature cataract (Figure 5), chorioretinitis, retinal vasculitis | Late symptoms, following months | Rare Reason unknown (treatment or disease?). |
Figure 2. Acanthamoeba keratitis dirty epithelium sign
Footnote: (A) “Dirty epithelium”, (B) ring infiltrate (arrows) and (C) six months later excimer laser penetrating keratoplasty with interrupted sutures, in acanthamoeba keratitis.
[Source 1 ]Figure 3. Acanthamoeba keratitis multifocal stromal infiltrates sign
Footnote: (A) Incomplete ring infiltrate (arrow) and multifocal stromal infiltrates in acanthamoeba keratitis. (B) One week later excimer laser penetrating keratoplasty with interrupted sutures.
[Source 1 ]Figure 4. Acanthamoeba keratitis peripheral perineurial infiltrate sign
Footnote: Perineuritis in acanthamoeba keratitis (arrow), 4 weeks after first symptoms (contact lens wearer).
[Source 1 ]Acanthamoeba keratitis complications
Common acanthamoeba keratitis complications include:
- broad-based anterior synechiae,
- secondary glaucoma,
- iris atrophy,
- mature cataract,
- persistent endothelial defect.
Rare acanthamoeba keratitis complications: sterile anterior uveitis and scleritis.
Very rare complications: chorioretinitis and retinal vasculitis
Acanthamoeba keratitis diagnosis
Diagnosis of Acanthamoeba keratitis is difficult and often delayed. Patients should be asked about contact lens wear and hygiene, contact lens solutions, recent corneal trauma, and recent exposure to water sources. If clinical suspicion exists, the involved area of cornea can be scraped with a sterile instrument (blade, spatula, needle, calcium alginate swab, or cotton tip applicator) under topical anesthesia at the slit lamp. The culture specimen can then be inoculated into a dish of E. coli plated over non-nutrient agar. Acanthamoeba trophozoites and cysts can also be identified with the help of Gram, Giemsa-Wright, hematoxylin and eosin, periodic acid-Schiff, calcoflour white, or other stains. If culture results are negative or if the infection appears to be more stromal than epithelial, a small corneal biopsy may be considered. Confocal microscopy has also been used to diagnose Acanthamoeba cysts with some success.
Acanthamoeba keratitis treatment
Medical treatment for Acanthamoeba keratitis is still evolving. Success has been reported with various combinations of antibiotic, antiviral, antifungal, and antiparasitic drugs. Many of these topical treatments are not commercially available in the United States and need to be specially ordered 22. Different regimens include topical preparations of propamidine isethionate (Brolene®), Neomycin-Polymyxin B-Gramicidin, polyhexamethylene biguanide (PHMB), chlorhexadine, and voriconazole. Some eye practitioners recommend oral ketoconazole.
Patients should be followed very closely (daily or almost daily) initially, until clinical response is seen. Since recurrences can occur and Acanthamoeba cysts are so resistant to treatment, medical treatments should be tapered very slowly and, if necessary, continued for many months. Steroids are controversial and may worsen the condition by inhibiting the host immune response. Pain should be addressed.
Medical treatment
Diamidine and biguanide
Diamidines, such as propamidine-isethionate (Brolene), hexamidine-diisethionate (Hexacyl), and dibromopropamidine (Golden Eye) are used in 0.1% concentration 23. Biguanides, such as polyhexamethylene-biguanide (polyhexanid) (Lavasept), and chlorhexidine (Curasept) are applied in 0.02% concentration 4.
The concentration dependent effect of diamidines and biguanides on human epithelial cells, keratocytes, and endothelial cells have already been described, and propamidine-isethionate as diamidine and chlorhexidine as biguanide seem to be the least cytotoxic. However, these may reduce proliferation and migration of human corneal cells more than other diamidines and biguanides 24.
Antibiotics
Neomycin kills trophozoites, prevents bacterial superinfection,62 and reduces bacterial load, as a food source for acanthamoeba 4.
Povidone-iodine and miltefosine
An in vitro experiment reported on a better anticystic effect of 1% povidone-iodine as propamidine-isethionate or polyhexanide. However, clinical studies did not verify these results 25.
Miltefosine was effective against acanthamoeba in vitro 26.
Steroids
Topical use of steroids may mask clinical signs of acanthamoeba keratitis as long as these are used. Their disadvantage is that they support encystment and an increase in number of trophozoites. However, a patient with acanthamoeba keratitis and severe inflammation may also benefit from their use. Steroids should never be used without additional topical antiseptics and should never be applied at early stages of acanthamoeba keratitis treatment (never in the first week even after appropriate diagnosis) 27. In the case of stopping topical steroids, a Wessely immune ring may develop within 2 days in patients with acanthamoeba keratitis.
Antifungals
Miconazole and clotrimazole have been previously used as topical treatment of acanthamoeba keratitis 28. In addition, there are reports on local and systemic voriconazole use in these patients 29. An in vitro study described better anticystic effects using natamycin in contrast to propamidine-isethionate or polyhexamethylene-biguanide 25. However, data on clinical use of natamycin in acanthamoeba keratitis patients is not available.
Szentmáry et al 1 suggest topical application of polyhexamethylene-biguanide, propamidine-isethionate, and neomycin as triple-therapy in case of acanthamoeba keratitis. To date, there is no randomized controlled clinical trial on safety and efficacy of conservative treatment in acanthamoeba keratitis.
During the first two days a “surprise attack” or “flash war” is initiated with polyhexamethylene-biguanide and propamidine-isethionate every quarter to half and hour day and night. Then until the sixth day, polyhexamethylene-biguanide and propamidine-isethionate are applied every hour and only over the day (6:00–24:00). The following 4 weeks, eyedrop use is reduced to every 2 hour. Additionally, neomycin 5× a day is also applied 30. In therapy resistant cases, we may change polyhexamethylene-biguanide to chlorhexidine, or increase concentration (for polyhexamethyleny-biguanidy to 0.06%, for chlorhexidine to 0.2%).
Combination therapy using diamidine, biguanide, and antibiotics should be continued in descending doses for 1 year. However, in case of non-healing epithelial defects after penetrating keratoplasty, Szentmáry et al 1 recommend reducing use of diamidine and biguanide with 1 drop every two months.
Photodynamic therapy
Photodynamic therapy (PDT) may be an alternative treatment option in therapy resistant infectious keratitis 31. The successful use of riboflavin-UVA cross-linking in acanthamoeba keratitis has been summarized in a case series in 2011 32. Nevertheless, in case of stromal infiltrates, UVA-light penetration to the corneal stroma may be reduced. An accelerated cross-linking in acanthamoeba keratitis is not suggested.
Surgical treatment
Cases of corneal perforation may need to be managed with surgical interventions. If possible, penetrating keratoplasty should be reserved for cases of visually significant corneal scarring in quiet eyes. If there are still signs of active infection or even if there are cysts lingering in the cornea, then infection may recur in the graft.
Post-operative complications after penetrating keratoplasty include recurrence of Acanthamoeba infection as well as all of the other possible post-operative complications (such as infection, glaucoma, cataract, wound leak, astigmatism).
If topical conservative treatment does not improve clinical signs and symptoms, a corneal cryotherapy, amniotic membrane transplantation, or penetrating keratoplasty may be performed. In therapy resistant cases, a cross-linking treatment as photodynamic therapy maybe used, in some cases repeatedly.
In the case of acanthamoeba keratitis expansion in direction of the corneoscleral limbus, an early penetrating keratoplasty has to be done in order to perform the excision in uninfected corneal tissue. In the case of progressive, therapy-resistant ulceration over weeks and months with peripheral reparative neovascularization, we suggest an early (<5 months disease course) à chaud penetrating keratoplasty 33. The origin of frequent therapy-resistant epithelial defects at the transplanted tissue after penetrating keratoplasty has not been clarified yet. Potential treatment options of these epithelial defects are (1) autologous serum, (2) amniotic membrane transplantation, (3) Cacicol or, (4) Neurotrophic Growth Factor (NGF).
Following penetrating keratoplasty, Szentmáry et al 1 continue the use of the above-described topical treatment up to 1 year. However, there are also no controlled clinical trials related to this topic. Perhaps local therapy may be stopped earlier, in order to avoid persistent epithelial defects, peripheral anterior synechiae, and mature cataract. Confocal microscopy may be useful in diagnosis of acanthamoeba keratitis recurrences 34.
In the case of perforated corneal ulcers, a non-mechanical, excimer laser keratoplasty is best performed 35. Using an elliptical excimer laser trephination with metal masks, we may remove the infected corneal area with a more homogeneous distance from the limbal vessels, especially in typically elliptical-shaped acanthamoeba keratitis 36.
Some authors suggest at least a 3 month long observation period without inflammatory signs, following discontinuation of conservative therapy, before planning an elective penetrating keratoplasty, following acanthamoeba keratitis. In such elective penetrating keratoplasties, transplantate survival may be 100% after 5 years and 67% after 10 years 37.
Corneal cryotherapy
Corneal cryotherapy is an adjuvant treatment of topical therapy. The infected corneal areas or the recipient area before penetrating keratoplasty will be treated using a Cold Cryoprobe 2–3 times (“freeze-thaw-freeze”) until ice crystals are formed in the corneal stroma 38. As part of a penetrating keratoplasty, cryotherapy is circularly used (about 2 s at −80 °C to the recipient bed) before recipient trephination. The effect of this type of cryotherapy on limbal epithelial stem cells has not been clarified to date.
An amniotic membrane transplantation may be used, especially for persistent epithelial defects or ulcers as “Patch”, “Graft”, or “Sandwich” and may help reach a quiet stage of the eye 39. In many cases, amniotic membrane transplantation has to be repeated several times to reach epithelial closure.
Acanthamoeba keratitis prognosis
The prognosis for Acanthamoeba is worse than for many other types of infectious keratitis and prevention is therefore very important. However, especially if caught early, satisfactory outcomes can certainly be achieved.
References- Szentmáry N, Daas L, Shi L, et al. Acanthamoeba keratitis – Clinical signs, differential diagnosis and treatment. J Curr Ophthalmol. 2018;31(1):16–23. Published 2018 Oct 19. doi:10.1016/j.joco.2018.09.008 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6407156
- Neelam S, Niederkorn JY. Pathobiology and Immunobiology of Acanthamoeba Keratitis: Insights from Animal Models . Yale J Biol Med. 2017;90(2):261–268. Published 2017 Jun 23. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5482302
- Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16(2):273–307.
- Szentmáry N., Göbels S., Matoula P., Schirra F., Seitz B. Acanthamoeba keratitis – a rare and often late diagnosed disease. Klin Monbl Augenheilkd. 2012;229(5):521–528.
- Awwad ST, Parmar DN, Heilman M. et al. Results of penetrating keratoplasty for visual rehabilitation after Acanthamoeba keratitis. Am J Ophthalmol. 2005;140(6):1080–1084.
- Panjwani N. Pathogenesis of acanthamoeba keratitis. Ocul Surf. 2010;8(2):70–79.
- Clarke DW, Niederkorn JY. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006;22(4):175–180.
- van Klink F, Alizadeh H, He Y, Mellon JA, Silvany RE, McCulley JP, Niederkorn JY. The role of contact lenses, trauma, and Langerhans cells in a Chinese hamster model of Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 1993;34(6):1937-44.
- Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10.
- Patel A, Hammersmith K. Contact lens-related microbial keratitis: recent outbreaks. Curr Opin Ophthalmol. 2008;19(4):302-6.
- Chew H.F., Yildiz E.H., Hammersmith K.M. Clinical outcomes and prognostic factors associated with acanthamoeba keratitis. Cornea. 2011;30(4):435–441.
- Hammersmith K.M. Diagnosis and management of Acanthamoeba keratitis. Curr Opin Ophthalmol. 2006;17(4):327–331.
- Verani JR, Lorick SA, Yoder JS, Beach MJ, Braden CR, Roberts JM, Conover CS, Chen S, McConnell KA, Chang DC, Park BJ, Jones DB, Visvesvara GS, Roy SL. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg Infect Dis. 2009;15(8):1236-42.
- Pens CJ, da Costa M, Fadanelli C, Caumo K, Rott M. Acanthamoeba spp. and bacterial contamination in contact lens storage cases and the relationship to user profiles. Parasitol Res. 2008;103(6):1241-5.
- Sharma S, Garg P, Rao GN. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br J Ophthalmol. 2000;84(10):1103-8.
- Sharma S., Garg P., Rao G.N. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br J Ophthalmol. 2000;84(10):1103–1108.
- Johnston S.P., Sriram R., Qvarnstrom Y. Resistance of Acanthamoeba cysts to disinfection in multiple contact lens solutions. J Clin Microbiol. 2009;47(7):2040–2045.
- Mazur T., Hadas E., Iwanicka I. The duration oft he cyst stage and the viability and virulence of Acanthamoeba isolates. Trop Med Parasitol. 1995;46(2):106–108.
- Daas L., Szentmáry N., Eppig T. The German acanthamoeba keratitis register: initial results of a multicenter study. Ophthalmologe. 2015;112(9):752–763.
- Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148(4):487-99e2.
- Tu EY, Joslin CE, Sugar J, Shoff ME, Booton GC. Prognostic factors affecting visual outcome in Acanthamoeba keratitis. Ophthalmology. 2008;115(11):1998-2003.
- Acanthamoeba keratitis. https://eyewiki.org/Acanthamoeba_Keratitis
- Larkin D.F., Kilvington S., Dart J.K. Treatment of Acanthamoeba keratitis with polyhexamethylene biguanide. Ophthalmology. 1992;99(2):185–191.
- Shi L., Stachon T., Seitz B., Wagenpfeil S., Langenbucher A., Szentmáry N. The effect of antiamoebic agents on viability, proliferation and migration of human epithelial cells, keratocytes and endothelial cells, in vitro. Curr Eye Res. 2018;43(6):725–733.
- Sunada A., Kimura K., Nishi K. In vitro evaluations of topical agents to treat acanthamoeba keratitis. Ophthalmology. 2014;121(10):2059–2065.
- Polat Z.A., Walochnik J., Obwaller A., Vural A., Dursun A., Arici M.K. Miltefosine and polyhexamethylene biguanide: a new drug combination for the treatment of Acanthamoeba keratitis. Clin Exp Ophthalmol. 2014;42(2):151–158.
- Carnt N., Optom B., Robaei D., Minassian D.C., Dart J.K. The impact of topical corticosteroids used in conjunction with antiamoebic therapy on the outcome of acanthamoeba keratitis. Ophthalmology. 2016;123(5):984–990.
- Amoils S.P., Heney C. Acanthamoeba keratitis with live isolates treated with cryosurgery and fluconazole. Am J Ophthalmol. 1999;127(6):718–720.
- Oldenburg C.E., Acharya N.R., Tu E.Z. Practice patterns and opinions in the treatment of acanthamoeba keratitis. Cornea. 2011;30(12):1363–1368.
- Elder M.J., Kilvington S., Dart J.K. A clinicopathologic study of in vitro sensitivity testing and Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 1994;35(3):1059–1064.
- Szentmáry N., Goebels S., Bischoff M., Seitz B. Photodynamic therapy (PDT) for infectious keratitis. Ophthalmologe. 2012;109(2):165–170.
- Khan Y.A., Kashiwabuchi R.T., Martins S.A. Riboflavin and ultraviolet light a therapy as an adjuvant treatment for medically refractive Acanthamoeben keratitis: report of 3 cases. Ophthalmology. 2011;118(2):324–331.
- Laurik K.L., Szentmáry N., Daas L. 2016. Penetrating keratoplasty à chaud in acute acanthamoeba keratitis.
- Daas L., Viestenz A., Schnabel P. Confocal microscopy in acanthamoeba keratitis as an early relapse-marker. Clin Anat. 2018;31(1):60–63.
- Seitz B., Langenbucher A., Kus M.M., Küchle M., Naumann G.O. Nonmechanical corneal trephination with the excimer laser improves outcome after penetrating keratoplasty. Ophthalmology. 1999;106(6):1556–1564.
- Szentmáry N., Langenbucher A., Kus M.M., Naumann G.O., Seitz B. Elliptical nonmechanical corneal trephination – intraoperative complications and long-term outcome of 42 consecutive penetrating keratoplasties. Cornea. 2007;26(4):414–420.
- Robaei D., Carnt N., Minassian D.C., Dart J.K. Therapeutic and optical keratoplasty in the management of Acanthamoeba keratitis: risk factors, outcomes, and summary of the literature. Ophthalmology. 2015;122(1):17–24.
- Klüppel M., Reinhard T., Sundmacher R., Daicker B. Therapy of advanced amoeba keratitis with keratoplasty à chaud and adjuvant cryotherapy. Ophthalmologe. 1997;94(2):99–103.
- Seitz B., Resch M.D., Schlötzer-Schrehardt U., Hofmann-Rummelt C., Sauer R., Kruse F.E. Histopathology and ultrastructure of human corneas after amniotic membrane transplantation. Arch Ophthalmol. 2006;124(10):1487–1490.