Chromoendoscopy
Chromoendoscopy is an endoscopic technique that uses stains during endoscopy to highlight differences in mucosa, as well as dysplastic and malignant changes that are not apparent in white light 1. Chromoendoscopy is used to increase the detection rates for various pathologic processes during endoscopy. Chromoendoscopy is often used in surveillance of the esophagus for Barrett esophagus, evaluation of polyps in the colon, and surveillance of dysplasia in inflammatory bowel disease (IBD) 2.
Chromoendoscopy is not routinely performed during general endoscopy. Chromoendoscopy is performed in centers that specialize in this field or on a case-by-case basis as indicated by the clinical situation and dictated by the experience of the endoscopist and the center.
Chromoendoscopy is not an advanced endoscopic technique and is not technically difficult to learn. However, interpretation of the staining patterns requires training and may not always be easy. There is evidence that there is significant intraobserver and interobserver variation 3.
Initial evaluations of computerized virtual chromoendoscopy for screening colonoscopy showed efficacy similar to that of chromoendoscopy, without the logistical difficulties or preparing and applying vital dyes 4. Magnification endoscopy, spectroscopy, confocal laser endomicroscopy and endocytoscopy have important roles in the evaluation of inflammatory bowel disease and surveillance of chronic ulcerative colitis (UC) 5. Use of these techniques in Barrett esophagus has also led better detection of dysplasia, particularly in the absence of discrete lesions 6.
Chromoendoscopy uses
Chromoendoscopy has been used in the evaluation of Barrett esophagus 7, esophageal adenocarcinoma 8, gastric metaplasia and adenocarcinoma 9, colon polyps 10, colon cancer 11 and surveillance in inflammatory bowel disease (IBD) 12.
Barrett esophagus and esophageal adenocarcinoma
The increasing prevalence of Barrett esophagus and its recognition as a premalignant lesion has engendered a great deal of interest in recognizing metaplastic and dysplastic changes 13. The increasing use of endoscopic therapy for dysplasia and adenocarcinoma has given further impetus to this concept. Chromoendoscopy is considered by some as a tool for recognizing high-risk lesions within Barrett esophagus and for facilitating definitive therapy 14. The most commonly used dye is methylene blue.
Magnification chromoendoscopy is an important adjunctive technique that enhances the sensitivity and specificity 15. Evaluating lesions in the absence of biopsies based on staining has also been shown to be useful 16. Unfortunately, the sensitivity and specificity are wide-ranging. Randomized controlled trials have shown conflicting results and also equivalency to routine endoscopy 17.
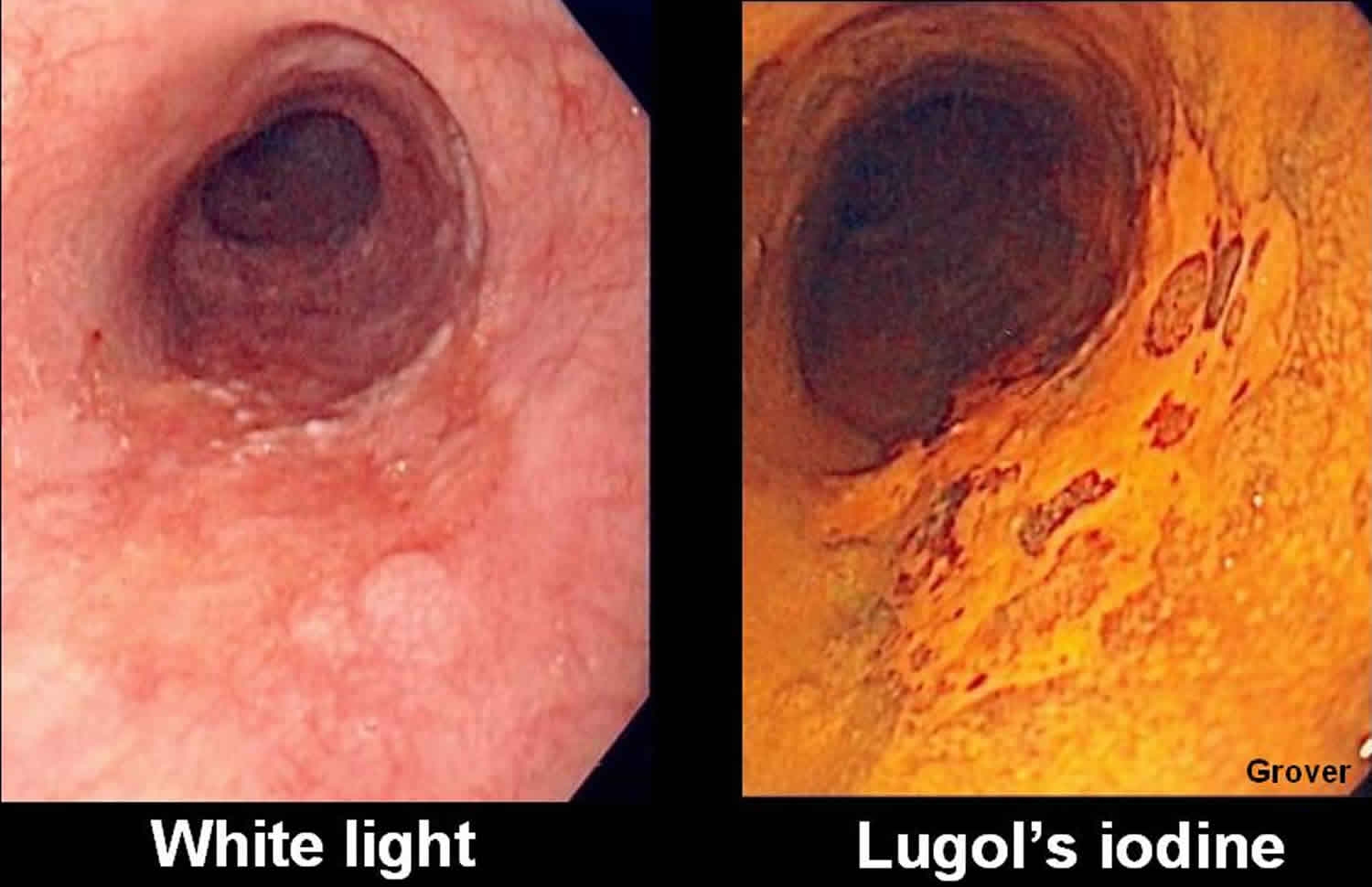
Esophageal squamous cancer
Lugol iodine is most commonly used to survey patients at risk for squamous cell carcinoma, including patients with tobacco abuse, alcohol abuse, or past head and neck cancer 18. The sensitivity exceeds 90%, but the specificity is variable 19. The dye may also be used to guide resection of early lesions.
Gastric metaplasia and cancer
Chromoendoscopy has been used to detect and demarcate dysplasia, intestinal metaplasia, or malignancy 20. Methylene blue and congo red are used in a combination to differentiate abnormal gastric mucosa (which does not stain) from normal mucosa (which stains red or blue) 21. This may be of utility in high-risk groups targeted for intensive surveillance 22.
There is some evidence that Helicobacter pylori detection in the stomach is better with phenol red chromoendoscopy, though this concept has not been evaluated in clinical trials 23. Phenol red staining is being studied in assessing the functional recovery of gastric mucosa after H pylori eradication therapy 24.
Colorectal adenoma
There have been many randomized trials for enhanced adenoma detection with chromoendoscopy using indigo carmine staining 25. Pohl et al 26 conducted a large randomized controlled trial comparing standard endoscopy with pancolonic chromoendoscopy and showed that chromoendoscopy significantly increased the detection rate for adenomas, flat lesions, and serrated lesions. There was an increase in the mean withdrawal time. Other trials have shown mixed results 27.
There may be a advantage to chromoendoscopy in detecting flat lesions 28. An increased cancer detection rate or survival rate has not been demonstrated. Chromoendoscopy is currently not of significant utility in screening or surveillance colonoscopy.
Colorectal cancer
Chromoendoscopy also has a limited and experimental role in endoscopically assessing the depth of known colorectal cancer 29.
Inflammatory bowel disease
The difficulty in identifying dysplasia and carcinoma in chronic ulcerative colitis (ulcerative colitis) has led to the evaluation of chromoendoscopy in this particular high-risk situation. Many trials have shown that dysplasia detection is improved significantly and reproducibly 30, 12. The accumulation of evidence may have an impact on chronic ulcerative colitis surveillance guidelines 31. Evidence with regard to carcinoma detection in this setting is equivocal 32.
Chromoendoscopy contraindications
Contraindications for chromoendoscopy would be any of the usual contraindications for endoscopy or a history of an allergic reaction to the dye or stain used in the specific clinical situation.
Chromoendoscopy technique
The usual preparation prior to upper endoscopy or colonoscopy and sedation administration is undertaken in the standard manner. Thereafter, specialized preparation of mucosa is carried out in accordance with the endoscopic procedure to be done, the dye or stain to be used, the suspected lesion, and the planned therapy for that lesion.
The agents used in chromoendoscopy are commercially available and inexpensive. They are not specifically made for endoscopy. They are prepared and diluted in accordance with the practices of the chromoendoscopists. Common staining agents used are Lugol solution, methylene blue, toluidine blue, crystal violet, indigo carmine, congo red, and phenol red. Special spray catheters are used to spray a fine mist on to the mucosa 19. Table 1 below details the properties and uses of each agent 19.
The agents are classified as absorptive, contrast, or reactive agents. Absorptive stain are absorbed by or diffuse into specific cells. Contrast agents seep between cells and enhance the surface. Reactive agents undergo chemical reactions with specific cell components and undergo color change 19.
Application of some staining agents requires the application of mucolytic agents such as N-acetylcysteine to remove mucus from epithelium to allow optimal results with staining.
The agent may be applied to a specific area or to the whole mucosal surface of interest. The abnormal mucosa may stain positively (ie, taking up the dye) or negatively (ie, remaining unstained or understained). In general, the minimum amount of dye required is used. Excess dye is suctioned or washed. The time required for optimal staining is variable and depends on the targeted tissue and the stain used.
It should be kept in mind that Lugol iodine can cause esophagitis or gastritis in rare cases. Hyperthyroidism or hypersensitivity to iodine should preclude the use of this agent. Methylene blue can cause discoloration of urine and feces. No serious adverse effects of other vital dyes have been described. General precautions should include aspiration and contact precautions 19.
Table 1. Properties of and clinical indications for common stains used in endoscopy
| Stain | Property | Clinical Indication |
| Absorptive | ||
| Lugol solution (iodine and potassium iodide) | Glycogen-containing normal squamous epithelium is stained dark brown; inflammation, columnar mucosa, dysplasia, and cancer remain unstained | Esophageal squamous cell cancer and dysplasia Barrett esophagus |
| Methylene blue (methylthioninium chloride) | Absorptive epithelial cells of the small bowel, colon, and intestinal metaplasia at any site are stained blue; dysplasia and cancer is variably stained or unstained | Barrett esophagus Gastric intestinal metaplasia and cancer Chronic ulcerative colitis |
| Toluidine blue (tolonium chloride) | Nuclei of malignant cells are stained blue | Oral and esophageal squamous cell cancer |
| Crystal violet (methylrosaniline chloride | Absorbed into intestinal and neoplastic cells; nuclear stain | Barrett esophagus Colonic neoplasms |
| Contrast | ||
| Indigo carmine (indigotindisulfonate sodium) | Nonabsorbed dark bluish dye highlighting mucosal topography | Colonic neoplasms Chronic ulcerative colitis |
| Reactive | ||
| Congo red (biphenylenenaphthadene sulfonic acid) | Color change from red to dark blue/black in presence of acid at pH 3 | Ectopic gastric mucosa Gastric cancer Adequacy of vagotomy |
| Phenol red (phenolsulfonephthalein) | Color change from yellow to red in presence of alkali (eg, from hydrolysis of urea to ammonia and carbon dioxide by urease-producing H. pylori) | H. pylori infection |
- Chromoendoscopy. https://emedicine.medscape.com/article/1891483-overview
- [Guideline] Bisschops R, East JE, Hassan C, Hazewinkel Y, Kamiński MF, Neumann H, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – Update 2019. Endoscopy. 2019 Nov 11.
- Huang Q, Fukami N, Kashida H, Takeuchi T, Kogure E, Kurahashi T, et al. Interobserver and intra-observer consistency in the endoscopic assessment of colonic pit patterns. Gastrointest Endosc. 2004 Oct. 60 (4):520-6.
- dos Santos CE, Lima JC, Lopes CV, Malaman D, Salomão AD, Garcia AC, et al. Computerized virtual chromoendoscopy versus indigo carmine chromoendoscopy combined with magnification for diagnosis of small colorectal lesions: a randomized and prospective study. Eur J Gastroenterol Hepatol. 2010 Nov. 22 (11):1364-71.
- Teubner D, Kiesslich R, Matsumoto T, Rey JW, Hoffman A. Beyond standard image-enhanced endoscopy confocal endomicroscopy. Gastrointest Endosc Clin N Am. 2014 Jul. 24 (3):427-34.
- Reddymasu SC, Sharma P. Advances in endoscopic imaging of the esophagus. Gastroenterol Clin North Am. 2008 Dec. 37 (4):763-74, vii.
- Tholoor S, Bhattacharyya R, Tsagkournis O, Longcroft-Wheaton G, Bhandari P. Acetic acid chromoendoscopy in Barrett’s esophagus surveillance is superior to the standardized random biopsy protocol: results from a large cohort study (with video). Gastrointest Endosc. 2014 Sep. 80 (3):417-24.
- Seewald S, Ang TL, Groth S, Zhong Y, Bertschinger P, Altorfer J, et al. Detection and endoscopic therapy of early esophageal adenocarcinoma. Curr Opin Gastroenterol. 2008 Jul. 24 (4):521-9.
- Fujiwara S, Yao K, Nagahama T, Uchita K, Kanemitsu T, Tsurumi K, et al. Can we accurately diagnose minute gastric cancers (≤5 mm)? Chromoendoscopy (CE) vs magnifying endoscopy with narrow band imaging (M-NBI). Gastric Cancer. 2015 Jul. 18 (3):590-6.
- Allen JE, Sharma P. Polyp characterization at colonoscopy: Clinical implications. Best Pract Res Clin Gastroenterol. 2017 Aug. 31 (4):435-440.
- Sakamoto T, Matsuda T, Nakajima T, Saito Y, Fujii T. Impact of clinical experience on type V pit pattern analysis using magnifying chromoendoscopy in early colorectal cancer: a cross-sectional interpretation test. BMC Gastroenterol. 2014 May 30. 14:100.
- Günther U, Kusch D, Heller F, Bürgel N, Leonhardt S, Daum S, et al. Surveillance colonoscopy in patients with inflammatory bowel disease: comparison of random biopsy vs. targeted biopsy protocols. Int J Colorectal Dis. 2011 May. 26 (5):667-72.
- Fock KM, Ang TL. Global epidemiology of Barrett’s esophagus. Expert Rev Gastroenterol Hepatol. 2011 Feb. 5 (1):123-30.
- Panossian AM, Raimondo M, Wolfsen HC. State of the art in the endoscopic imaging and ablation of Barrett’s esophagus. Dig Liver Dis. 2011 May. 43 (5):365-73.
- Canto MI. Chromoendoscopy and magnifying endoscopy for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2005 Jul. 3 (7 Suppl 1):S12-5.
- Sharma P, Marcon N, Wani S, Bansal A, Mathur S, Sampliner R, et al. Non-biopsy detection of intestinal metaplasia and dysplasia in Barrett’s esophagus: a prospective multicenter study. Endoscopy. 2006 Dec. 38 (12):1206-12.
- Ragunath K, Krasner N, Raman VS, Haqqani MT, Cheung WY. A randomized, prospective cross-over trial comparing methylene blue-directed biopsy and conventional random biopsy for detecting intestinal metaplasia and dysplasia in Barrett’s esophagus. Endoscopy. 2003 Dec. 35 (12):998-1003.
- Hori K, Okada H, Kawahara Y, Takenaka R, Shimizu S, Ohno Y, et al. Lugol-voiding lesions are an important risk factor for a second primary squamous cell carcinoma in patients with esosphageal cancer or head and neck cancer. Am J Gastroenterol. 2011 May. 106 (5):858-66.
- ASGE Technology Committee., Wong Kee Song LM, Adler DG, Chand B, Conway JD, Croffie JM, et al. Chromoendoscopy. Gastrointest Endosc. 2007 Oct. 66 (4):639-49.
- Ohnita K, Isomoto H, Shikuwa S, Yamaguchi N, Nakayama T, Nishiyama H, et al. Magnifying chromoendoscopic findings of early gastric cancer and gastric adenoma. Dig Dis Sci. 2011 Sep. 56 (9):2715-22.
- Iishi H, Tatsuta M, Okuda S. Diagnosis of simultaneous multiple gastric cancers by the endoscopic Congo red–methylene blue test. Endoscopy. 1988 Mar. 20 (2):78-82.
- Shaw D, Blair V, Framp A, Harawira P, McLeod M, Guilford P, et al. Chromoendoscopic surveillance in hereditary diffuse gastric cancer: an alternative to prophylactic gastrectomy?. Gut. 2005 Apr. 54 (4):461-8.
- Cho YS, Chae HS, Jang SN, Kim JS, Son HS, Kim HK, et al. Comparison of the 13C-urea breath test and the endoscopic phenol red mucosal pH test in the quantification of Helicobacter pylori infection loading. Korean J Intern Med. 2008 Sep. 23 (3):134-9.
- Graham DY, Shiotani A, El-Zimaity HM. Chromoendoscopy points the way to understanding recovery of gastric function after Helicobacter pylori eradication. Gastrointest Endosc. 2006 Nov. 64 (5):686-90.
- Cha JM, Lee JI, Joo KR, Jung SW, Shin HP. A prospective randomized study on computed virtual chromoendoscopy versus conventional colonoscopy for the detection of small colorectal adenomas. Dig Dis Sci. 2010 Aug. 55 (8):2357-64.
- Pohl J, Schneider A, Vogell H, Mayer G, Kaiser G, Ell C. Pancolonic chromoendoscopy with indigo carmine versus standard colonoscopy for detection of neoplastic lesions: a randomised two-centre trial. Gut. 2011 Apr. 60 (4):485-90.
- Hashimoto K, Higaki S, Nishiahi M, Fujiwara K, Gondo T, Sakaida I. Does chromoendoscopy improve the colonoscopic adenoma detection rate?. Hepatogastroenterology. 2010 Nov-Dec. 57 (104):1399-404.
- Hurlstone DP, George R, Brown S. Novel clinical in vivo roles for indigo carmine: high-magnification chromoscopic colonoscopy. Biotech Histochem. 2007 Apr. 82 (2):57-71.
- Sakamoto T, Saito Y, Nakajima T, Matsuda T. Comparison of magnifying chromoendoscopy and narrow-band imaging in estimation of early colorectal cancer invasion depth: a pilot study. Dig Endosc. 2011 Apr. 23 (2):118-23.
- Cohen-Mekelburg S, Schneider Y, Gold S, Scherl E, Steinlauf A. Advances in the Diagnosis and Management of Colonic Dysplasia in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2017 Jun. 13 (6):357-362.
- Ullman TA. Should chromoendoscopy be the standard of care in ulcerative colitis dysplasia surveillance?. Gastroenterol Hepatol (N Y). 2010 Oct. 6 (10):616-20.
- Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003 Apr. 124 (4):880-8.