What is flu shot
A flu vaccine also called flu shot that is given with a needle, usually in the arm. The seasonal flu shot causes antibodies to develop in your body about two weeks after you get it. These antibodies provide protection against infection with the flu viruses that are in the flu shot. There are many different flu viruses, and they are constantly changing. The composition of U.S. flu vaccines is reviewed annually and updated as needed. The seasonal flu shot protects against the four influenza viruses that research indicates will be most common during the season.
There are 4 types of influenza viruses and they are constantly changing. They are:
- Type A flu virus – this is usually the more serious type. The influenza A virus is most likely to mutate into a new version that people are not resistant to. Influenza A viruses are divided into subtypes based on two proteins on the surface of the virus: hemagglutinin (H) and neuraminidase (N). There are 18 different hemagglutinin (H) subtypes and 11 different neuraminidase (N) subtypes (H1 through H18 and N1 through N11, respectively). While more than 130 influenza A subtype combinations have been identified in nature, primarily from wild birds, there are potentially many more influenza A subtype combinations given the propensity for virus “reassortment.” Reassortment is a process by which influenza viruses swap gene segments. Reassortment can occur when two influenza viruses infect a host at the same time and swap genetic information. Current subtypes of influenza A viruses that routinely circulate in people include A(H1N1) and A(H3N2). Influenza A subtypes can be further broken down into different genetic “clades” and “sub-clades” (Figure 1). Clades and sub-clades can be alternatively called “groups” and “sub-groups,” respectively. An influenza clade or group is a further subdivision of influenza viruses (beyond subtypes or lineages) based on the similarity of their HA gene sequences. The H1N1 (swine flu) strain is a type A virus, and flu pandemics in the past were type A viruses.
- Type B flu virus – this generally causes a less severe illness and is responsible for smaller outbreaks. It mainly affects young children. Influenza B viruses are not divided into subtypes, but instead are further classified into two lineages: B/Yamagata and B/Victoria. Similar to influenza A viruses, influenza B viruses can then be further classified into specific “clades” and “sub-clades”. Influenza B viruses generally change more slowly in terms of their genetic and antigenic properties than influenza A viruses, especially influenza A(H3N2) viruses. Influenza surveillance data from recent years shows co-circulation of influenza B viruses from both lineages in the United States and around the world. However, the proportion of influenza B viruses from each lineage that circulate can vary by geographic location and by season. In recent years, flu B/Yamagata viruses have circulated much less frequently in comparison to flu B/Victoria viruses globally.
- Type C flu virus – this usually causes a mild illness similar to the common cold.
- Type D flu virus – this primarily affect cattle and are not known to infect or cause illness in people.
Most years, 1 or 2 strains of type A flu circulate as well as type B.
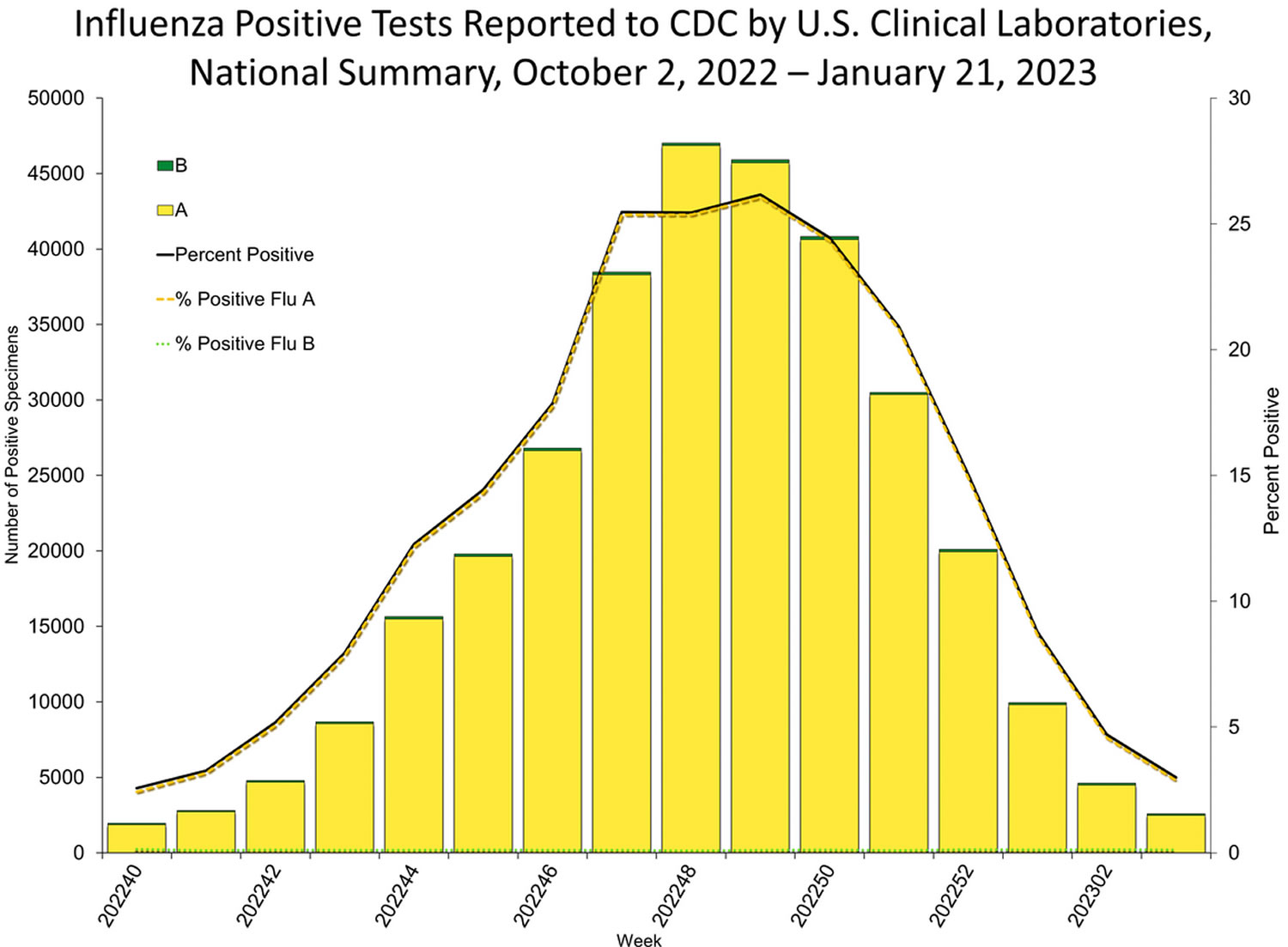
Seasonal influenza (flu) viruses are detected year-round in the United States, furthermore, flu viruses typically circulate in the United States during the fall and winter during what’s known as the “flu season” 1. The “peak month of flu activity” is the month with the highest percentage of respiratory specimens testing positive for influenza virus infection during that flu season. During the peak flu activity in the United States for 1982 to 2022 flu seasons, flu activity most often peaked in February (17 seasons), followed by December (7 seasons), January (6 seasons) and March (6 seasons) 1. However, the exact timing and duration of flu seasons varies, but in general flu activity often begins to increase in October. While influenza viruses spread all year-round, most of the time flu activity peaks between December and February, although significant activity can last as late as May 1. Since the start of the COVID-19 pandemic, the timing and duration of flu activity has been less predictable 1.
In February each year, the World Health Organization (WHO) assesses the strains of flu virus that are most likely to be circulating in the northern hemisphere over the following winter. Based on this assessment, WHO recommends which flu strains the vaccines should contain for the forthcoming winter. Vaccine manufacturers then produce flu vaccines based on WHO’s recommendations. These flu shots are used in all the countries in the northern hemisphere, not just the US. Production of the vaccine starts in March each year after WHO’s announcement.
There are different types of flu shots, including some especially for people 65 and older. Ask your health care provider which one is right for you.
Seasonal influenza (flu) vaccines are designed to protect against the four main groups of flu type A and B viruses that research indicates are most likely to spread and cause illness among people during the upcoming flu season. All current U.S. flu vaccines protect against a flu A(H1) virus, a flu A(H3) virus, a flu B/Yamagata lineage virus and a flu B/Victoria lineage virus.
The recommendations for the 2022-2023 season include two updates compared with the recommended composition of last season’s U.S. flu vaccines. Both the influenza A(H3N2) and the influenza B (Victoria lineage) vaccine virus components were updated.
Current seasonal flu vaccines are formulated to protect against influenza viruses known to cause epidemics, including: one influenza A(H1N1) virus, one influenza A(H3N2) virus, one influenza B/Victoria lineage virus, and one influenza B/Yamagata lineage virus. Seasonal flu vaccines do not protect against influenza C or D viruses or against zoonotic (animal-origin) flu viruses that can cause human infections, such as variant or avian (bird) flu viruses. In addition, flu vaccines will NOT protect against infection and illness caused by other viruses that also can cause influenza-like symptoms. There are many other viruses besides influenza that can result in influenza-like illness (ILI) that spread during flu season.
This year’s seasonal flu shot provides protection against four influenza viruses expected to be the most common during this flu season. This year, the vaccine will be available as an injection and as a nasal spray. There will also be a high-dose flu vaccine for adults age 65 and older.
The nasal spray is approved for people between 2 and 49 years old. It isn’t recommended for some groups, such as:
- Children younger than age 2
- Adults age 50 and older
- Pregnant people
- Children between 2 and 17 years old who are taking aspirin or a salicylate-containing medication
- People with weakened immune systems
- Kids 2 to 4 years old who have had asthma or wheezing in the past 12 months
If you have an egg allergy, you can still get a flu vaccine.
People who can get the flu shot:
- Different flu shots are approved for people of different ages. Everyone should get a vaccine that is appropriate for their age.
- There are standard-dose inactivated flu vaccines that are approved for people as young as 6 months of age.
- Some vaccines are only approved for adults. For example, the recombinant flu vaccine is approved for people aged 18 years and older, and the adjuvanted and high-dose inactivated vaccines are approved for people 65 years and older.
- Pregnant people and people with certain chronic health conditions can get a flu shot.
- People with egg allergy can get a flu shot
People who SHOULD NOT get a flu shot include:
- Children younger than 6 months of age are too young to get a flu shot.
- People with severe, life-threatening allergies to any ingredient in a flu vaccine (other than egg proteins) should not get that vaccine. This might include gelatin, antibiotics, or other ingredients. See Special Considerations Regarding Egg Allergy for more information about egg allergies and flu vaccine.
- People who have had a severe allergic reaction to a dose of influenza vaccine should not get that flu vaccine again and might not be able to receive other influenza vaccines. If you have had a severe allergic reaction to an influenza vaccine in the past, it is important to talk with your health care provider to help determine whether vaccination is appropriate for you.
People who SHOULD NOT get a Nasal Spray Flu Vaccine:
- Children younger than 2 years of age.
- Adults 50 years of age and older.
- People who have had a severe or life-threatening allergic reaction to any ingredient in the nasal spray vaccine (other than egg proteins). See Special Considerations Regarding Egg Allergy for more information about egg allergies and flu vaccine.
- People who have had a severe allergic reaction to any flu vaccine.
- Children and adolescents 2 through 17 years of age who are receiving aspirin- or salicylate-containing medications.
- People with weakened immune systems (immunosuppression) due to any cause, including (but not limited to) immunosuppression from medications, congenital or acquired immune disorders, HIV infection, or asplenia.
- People who care for or are close contacts of severely immunocompromised persons who require a protected environment (or otherwise avoid contact with those persons for 7 days after getting the nasal spray vaccine).
- Pregnant people.
- Children 2 years through 4 years who have asthma or who have had a history of wheezing in the past 12 months.
- People with cerebrospinal fluid (CSF) leaks (communication and leakage of fluid between the space surrounding the brain and the nose, throat, ear, or any other place in the head).
- People with cochlear implants.
- People who have recently taken influenza antiviral drugs. This depends on the specific influenza antiviral medication that was taken, and how recently the last dose was taken.
People who should talk to their health care provider before getting a flu shot:
If you have one of the following conditions, talk with your health care provider. He or she can help decide whether vaccination is right for you, and select the best vaccine for your situation:
- If you have an allergy to eggs or any of the ingredients in the vaccine. Talk to your doctor about your allergy.
- Most flu shots and the nasal spray flu vaccine are manufactured using egg-based technology. Because of this, they contain a small amount of egg proteins, such as ovalbumin. However, studies that have examined the use of both the nasal spray vaccine and flu shots in egg-allergic and non-egg-allergic patients indicate that severe allergic reactions in people with egg allergies are unlikely 2. A recent CDC study found the rate of anaphylaxis after all vaccines is 1.31 per one million vaccine doses given 2.
- People with egg allergies no longer need to be observed for an allergic reaction for 30 minutes after receiving a flu vaccine 2. People with a history of egg allergy of any severity should receive any licensed, recommended, and age-appropriate influenza vaccine. Those who have a history of severe allergic reaction to egg (i.e., any symptom other than hives) should be vaccinated in an inpatient or outpatient medical setting (including but not necessarily limited to hospitals, clinics, health departments, and physician offices), under the supervision of a health care provider who is able to recognize and manage severe allergic conditions.
- If you are someone who has more serious reactions to eating eggs or egg-containing foods, like angioedema, respiratory distress, lightheadedness, or recurrent emesis; or who required epinephrine or another emergency medical intervention, you can get any licensed flu vaccine that is otherwise appropriate for your age and health status, but the vaccine should be given in an inpatient or outpatient medical setting (including but not necessarily limited to hospitals, clinics, health departments, and physician offices), under the supervision of a health care provider who is able to recognize and manage severe allergic conditions 2.
- If you ever had Guillain-Barré Syndrome (a severe paralyzing illness, also called GBS). Some people with a history of GBS should not get a flu vaccine. Talk to your doctor about your GBS history.
- If you had a severe allergic reaction to a previous dose of any other flu vaccine, talk to your health care provider.
- If you are not feeling well, talk to your doctor about your symptoms.
People who should talk to their health care provider before getting a Nasal Spray Flu Vaccine:
If you have one of the following conditions, talk with your health care provider. He or she can help decide whether vaccination is right for you, and select the best vaccine for your situation:
- People with asthma 5 years and older.
- People with other underlying medical conditions that can put them at higher risk of developing serious flu complications. These include conditions such as chronic lung diseases, heart disease (except isolated hypertension), kidney disease, liver disorders, neurologic and neuromuscular disorders, blood disorders, or metabolic disorders (such as diabetes).
- People with moderate or severe acute illness with or without fever.
- People with Guillain-Barré Syndrome after a previous dose of influenza vaccine.
Figure 1. Peak Month of Flu Activity 2015-2016
Figure 2. United States flu season
[Source 3 ]Figure 3. Health impacts and deaths from the flu
Footnotes: While the impact of influenza varies from season to season, it places a substantial burden on the health of people in the United States: millions of people become ill, hundreds of thousands are hospitalized and thousands or tens of thousands of people die from flu every year.
Table 1. Influenza vaccines for 2022–23 influenza season in United States
| Trade name (manufacturer) | Presentations | Age indication | µg HA (IIV4s and RIV4) or virus count (LAIV4) for each vaccine virus (per dose) | Route | Mercury (from thimerosal, if present), µg/0.5 mL |
|---|---|---|---|---|---|
| Inactivated quadrivalent influenza vaccine (standard-dose, egg-based vaccines†) | |||||
| Afluria Quadrivalent (Seqirus) | 0.5-mL prefilled syringe § | ≥3 yrs§ | 15 µg/0.5 mL | Intramuscular ¶ | —** |
| 5.0-mL multidose vial § | ≥6 mos§ (needle/syringe) 18 through 64 yrs (jet injector) | 7.5 µg/0.25 mL 15 µg/0.5 mL | Intramuscular ¶ | 24.5 | |
| Fluarix Quadrivalent (GlaxoSmithKline) | 0.5-mL prefilled syringe | ≥6 mos | 15 µg/0.5 mL | Intramuscular ¶ | — |
| FluLaval Quadrivalent (GlaxoSmithKline) | 0.5-mL prefilled syringe | ≥6 mos | 15 µg/0.5 mL | Intramuscular ¶ | — |
| Fluzone Quadrivalent (Sanofi Pasteur) | 0.5-mL prefilled syringe †† | ≥6 mos†† | 15 µg/0.5 mL | Intramuscular ¶ | — |
| 0.5-mL single-dose vial †† | ≥6 mos†† | 15 µg/0.5 mL | Intramuscular ¶ | — | |
| 5.0-mL multidose vial †† | ≥6 mos†† | 15 µg/0.5 mL 7.5 µg/0.25 mL | Intramuscular ¶ | 25 | |
| Cell culture-based inactivated quadrivalent influenza vaccine (standard-dose, cell culture–based vaccine) | |||||
| Flucelvax Quadrivalent (Seqirus) | 0.5-mL prefilled syringe | ≥6 mos | 15 µg/0.5 mL | Intramuscular ¶ | — |
| 5.0-mL multidose vial | ≥6 mos | 15 µg/0.5 mL | Intramuscular ¶ | 25 | |
| High-Dose inactivated quadrivalent influenza vaccine (high-dose, egg-based vaccine†) | |||||
| Fluzone High-Dose Quadrivalent (Sanofi Pasteur) | 0.7-mL prefilled syringe | ≥65 yrs | 60 µg/0.7 mL | Intramuscular ¶ | — |
| Adjuvanted inactivated quadrivalent influenza vaccine (standard-dose, egg-based† vaccine with MF59 adjuvant). MF59 is an oil-in-water emulsion of squalene oil. Squalene, a naturally occurring substance found in humans, animals, and plants, is highly purified for the vaccine manufacturing process. | |||||
| Fluad Quadrivalent (Seqirus) | 0.5-mL prefilled syringe | ≥65 yrs | 15 µg/0.5 mL | Intramuscular ¶ | — |
| Recombinant quadrivalent influenza vaccine (recombinant HA vaccine) | |||||
| Flublok Quadrivalent (Sanofi Pasteur) | 0.5-mL prefilled syringe | ≥18 yrs | 45 µg/0.5 mL | Intramuscular ¶ | — |
| Live attenuated quadrivalent influenza vaccine (egg-based vaccine†) | |||||
| FluMist Quadrivalent (AstraZeneca) | 0.2-mL prefilled single-use intranasal sprayer | 2 through 49 yrs | 106.5–7.5 fluorescent focus units/0.2 mL | Intranasal | — |
Footnotes:
* Vaccination providers should consult FDA-approved prescribing information for 2022–23 influenza vaccines for the most complete and updated information, including but not limited to indications, contraindications, warnings, and precautions. Package inserts for U.S.-licensed vaccines are available at https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
† Although a history of severe allergic reaction (e.g., anaphylaxis) to egg is a labeled contraindication to the use of egg-based inactivated influenza quadrivalent vaccines and live attenuated influenza quadrivalent vaccine, Advisory Committee on Immunization Practices (ACIP) recommends that persons with a history of egg allergy may receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Those who report having had reactions to egg involving symptoms other than urticaria (e.g., angioedema or swelling, respiratory distress, lightheadedness, or recurrent emesis) or who required epinephrine or another emergency medical intervention should be vaccinated in an inpatient or outpatient medical setting (including but not necessarily limited to hospitals, clinics, health departments, and physician offices) supervised by a health care provider who is able to recognize and manage severe allergic reactions, if a vaccine other than cell culture-based inactivated influenza quadrivalent vaccine or recombinant influenza quadrivalent vaccine is used.
§ The approved dose volume for Afluria Quadrivalent is 0.25 mL for children aged 6 through 35 months and 0.5 mL for persons aged ≥3 years. However, 0.25-mL prefilled syringes are not expected to be available for the 2022–23 season. For children aged 6 through 35 months, a 0.25-mL dose must be obtained from a multidose vial.
¶ Intramuscular-administered influenza vaccines should be given by needle and syringe only, with the exception of the multidose vial presentation of Afluria Quadrivalent, which may alternatively be given by the PharmaJet Stratis jet injector for persons aged 18 through 64 years only. For adults and older children, the recommended site for intramuscular influenza vaccination is the deltoid muscle. The preferred site for infants and young children is the anterolateral aspect of the thigh. Additional specific guidance regarding site selection and needle length for intramuscular administration is available in the ACIP General Best Practice Guidelines for Immunization, available at https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html.
** Not applicable.
†† Fluzone Quadrivalent is currently approved for ages 6 through 35 months at either 0.25 mL or 0.5 mL per dose; however, 0.25-mL prefilled syringes are not expected to be available for the 2022–23 influenza season. If a prefilled syringe of Fluzone Quadrivalent is used for a child in this age group, the dose volume will be 0.5 mL per dose.
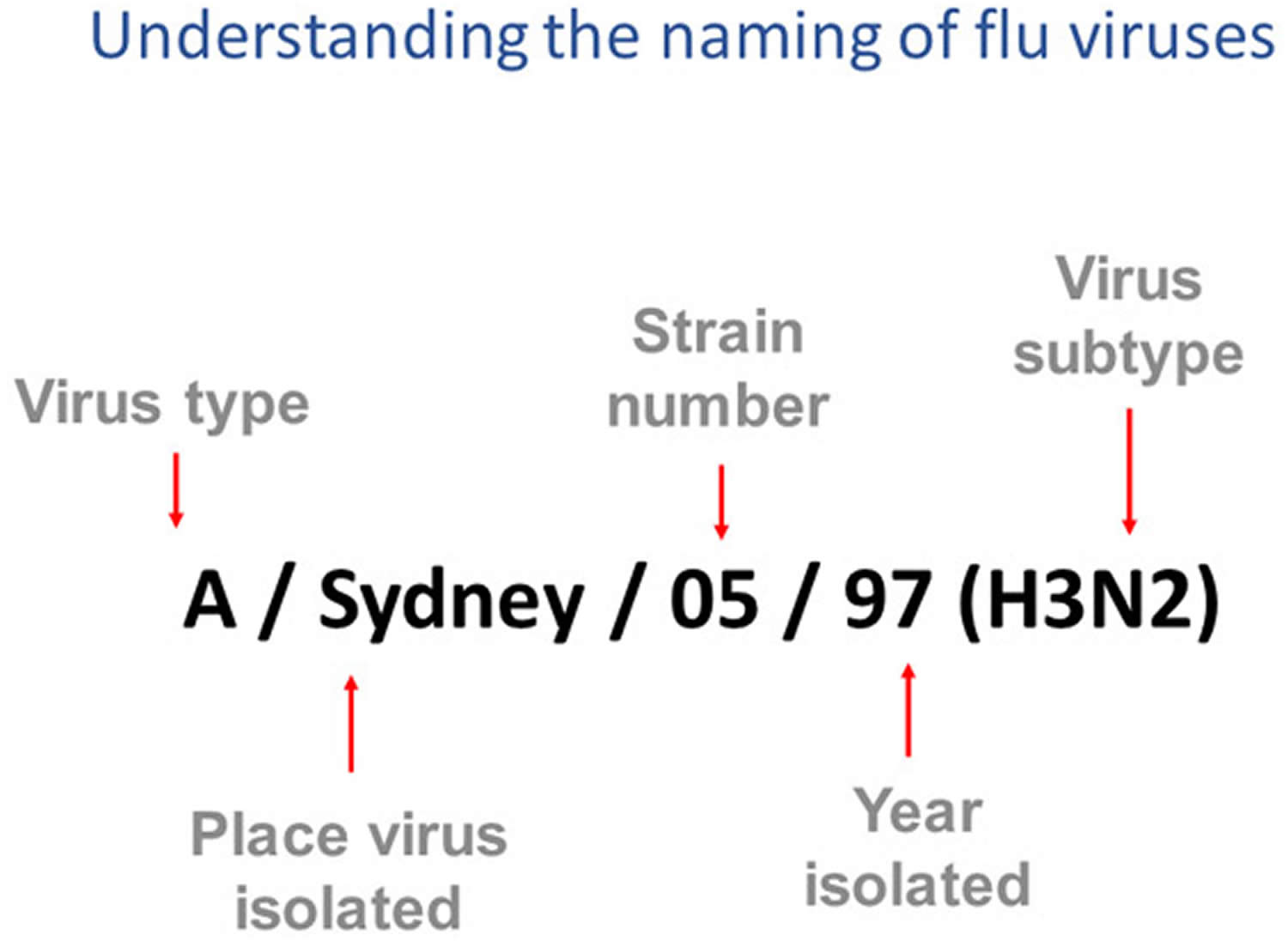
[Source 4 ]How influenza viruses are named
Influenza viruses names follow the internationally accepted World Health Organization (WHO) 1979 naming convention for influenza viruses 5.
The World Health Organization (WHO) 1979 naming convention uses the following components 5:
- The antigenic type (e.g., A, B, C, D)
- The host of origin (e.g., swine, equine, chicken, etc.). For human-origin viruses, no host of origin designation is given. Note the following examples:
- Duck example: avian influenza A(H1N1), A/duck/Alberta/35/76
- Human example: seasonal influenza A(H3N2), A/Perth/16/2019
- Geographical origin (e.g., Denver, Taiwan, etc.)
- Strain number (e.g., 7, 15, etc.)
- Year of collection (e.g., 57, 2009, etc.)
- For influenza A viruses, the hemagglutinin (H) and neuraminidase (N) antigen description are provided in parentheses (e.g., influenza A(H1N1) virus, influenza A(H5N1) virus)
- The 2009 pandemic virus was assigned a distinct name: A(H1N1)pdm09 to distinguish it from the seasonal influenza A(H1N1) viruses that circulated prior to the pandemic.
- When humans are infected with influenza viruses that normally circulate in swine (pigs), these viruses are call variant viruses and are designated with the letter “v” (e.g., an A(H3N2)v virus).
Figure 4. How influenza viruses are named
Footnotes: This image shows how influenza viruses are named. The name starts with the virus type, followed by the place the virus was isolated, followed by the virus strain number (often a sample identifier), the year isolated, and finally, the virus subtype.
[Source 6 ]How flu shot works?
The injected flu vaccine stimulates your body’s immune system to make antibodies to attack the flu virus. Antibodies are proteins that recognize and fight off germs, such as viruses, that have invaded your blood. If you’re exposed to the flu virus after you’ve had the flu vaccine, your immune system will recognize the virus and immediately produce antibodies to fight it.
It may take 10 to 14 days for your immunity to build up fully after you have had the flu shot.
You need to have a flu shot every year, as the antibodies that protect you from flu decline over time, and flu strains can also change from year to year.
Flu vaccines cause antibodies to develop in the body about two weeks after vaccination. These antibodies provide protection against infection with the viruses that are in the vaccine.
The seasonal flu vaccine protects against the influenza viruses that research indicates will be most common during the upcoming season. Traditional flu vaccines (called “trivalent” vaccines) are made to protect against three flu viruses; an influenza A (H1N1) virus, an influenza A (H3N2) virus, and an influenza B virus. There are also flu vaccines made to protect against four flu viruses (called “quadrivalent” vaccines). These vaccines protect against the same viruses as the trivalent vaccine and an additional B virus.
Are there some people who should not receive a flu vaccine?
CDC recommends everyone 6 months of age and older should receive an annual flu vaccination with rare exceptions. Individuals who can’t get the flu shot include 7:
- Children younger than 6 months, since they are too young to get a flu shot.
- Individuals with severe, life-threatening allergies to flu vaccine or any ingredient(s) in the vaccine.
Individuals should talk with their doctor before getting the flu shot if they:
- Have had a severe allergy to eggs or any of the ingredients in the vaccine.
- See Special Considerations Regarding Egg Allergy for more information about egg allergies and flu vaccine.
- Have had Guillain-Barré syndrome (GBS).
- Are not feeling well.
There are multiple flu vaccines available, and not all flu vaccines can be given to people of all ages. Talk to your doctor if you have any questions regarding which flu vaccine options are best for you and your family.
What is in the flu shot?
As there are lots of different flu vaccines produced each year, for more detailed information on ingredients ask your doctor or nurse for the patient information leaflet for the specific vaccine being offered.
Flu shot ingredients: for US-licensed flu vaccines are available at the U.S. Food and Drug Administration https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm
Can egg protein in flu vaccine cause allergic reactions in persons with a history of egg allergy?
Yes, allergic reactions can happen, but they occur very rarely with the flu vaccines available in the United States today 2. Occasional cases of anaphylaxis, a severe life-threatening reaction that involves multiple organ systems and can progress rapidly, in egg-allergic persons have been reported to the Vaccine Adverse Event Reporting System (VAERS) after administration of flu vaccine. Flu vaccines contain various components that may cause allergic reactions, including anaphylaxis. In a Vaccine Safety Datalink study, there were 10 cases of anaphylaxis after more than 7.4 million doses of inactivated flu vaccine, trivalent (IIV3) given without other vaccines, (rate of 1.35 per one million doses) 2. Most of these cases of anaphylaxis were not related to the egg protein present in the vaccine. CDC and the Advisory Committee on Immunization Practices continue to review available data regarding anaphylaxis cases following flu vaccines.
When should I get a flu shot?
You can get the flu shot each autumn when it becomes available, usually sometime in August through November.
- Optimally, vaccination should occur before onset of influenza activity in the community.
- Vaccination should be offered by end of October, if possible.
- Vaccination should be offered as long as influenza viruses are circulating and unexpired vaccine is available.
- Children aged 6 months through 8 years who require 2 doses should receive their first dose as soon as possible after vaccine becomes available, and the second dose ≥4 weeks later.
However, if flu shots are still available and you haven’t yet received a vaccination, you can still benefit from getting a flu shot in January or later. That’s because the flu season doesn’t usually peak until the winter.
Where to get flu shot?
Flu vaccines are offered in many locations, including doctor’s offices, clinics, health departments, pharmacies and college health centers, as well as by many employers, and even in some schools.
Even if you don’t have a regular doctor or nurse, you can get a flu vaccine somewhere else, like a health department, pharmacy, urgent care clinic, and often your school, college health center, or workplace.
The following Vaccine Locator (https://vaccinefinder.org/) is a useful tool for finding vaccine in your area.
How long does the flu shot last?
You need to have a flu shot every year, as the antibodies that protect you from flu decline over time, and flu strains can also change from year to year.
Why do I need a flu vaccine every year?
A flu vaccine is needed every season for two reasons. First, the body’s immune response from vaccination declines over time, so an annual vaccine is needed for optimal protection. Second, because flu viruses are constantly changing, the formulation of the flu vaccine is reviewed each year and sometimes updated to keep up with changing flu viruses. For the best protection, everyone 6 months and older should get vaccinated annually.
Can I get seasonal flu even though I got a flu vaccine this year?
Yes. There is still a possibility you could get the flu even if you got vaccinated. The ability of flu vaccine to protect a person depends on various factors, including the age and health status of the person being vaccinated, and also the similarity or “match” between the viruses used to make the vaccine and those circulating in the community. If the viruses in the vaccine and the influenza viruses circulating in the community are closely matched, vaccine effectiveness is higher. If they are not closely matched, vaccine effectiveness can be reduced. However, it’s important to remember that even when the viruses are not closely matched, the vaccine can still protect many people and prevent flu-related complications. Such protection is possible because antibodies made in response to the vaccine can provide some protection (called cross-protection) against different but related influenza viruses.
Does flu vaccine work right away?
No. It takes about two weeks after vaccination for antibodies to develop in the body and provide protection against influenza virus infection. That’s why it’s better to get vaccinated early in the fall, before the flu season really gets under way.
How effective are flu vaccines?
The CDC conducts studies each year to determine how well influenza (flu) vaccines protect against flu. While vaccine effectiveness can vary, recent studies show that flu vaccination reduces the risk of flu illness by between 40% and 60% among the overall population during seasons when most circulating flu viruses are well-matched to those used to make flu vaccines 8. In general, current flu vaccines tend to work better against influenza B and influenza A(H1N1) viruses and offer lower protection against influenza A(H3N2) viruses 8.
Can I get the flu from the flu vaccine?
No, the flu vaccine cannot cause flu. The vaccines either contain inactivated virus, meaning the viruses are no longer infectious, or a particle designed to look like a flu virus to your immune system. While the nasal spray flu vaccine does contain a live virus, the viruses are changed so that they cannot give you the flu.
Is it safe to get a flu shot during pregnancy?
Yes, it’s safe to get a flu shot during pregnancy. In fact, the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists recommend that all women who are pregnant during flu season get a flu shot, regardless of their trimester.
A flu shot during pregnancy can help:
- Prevent the flu and maternal complications. The flu is more likely to cause severe illness in pregnant women than in women who are not pregnant. Getting the flu during pregnancy increases your risk of becoming hospitalized. A flu shot will decrease your risk of getting the flu during your pregnancy.
- Prevent potential pregnancy problems due to the flu. Some research suggests that having the flu or a flu-like illness during the first trimester of pregnancy is linked with an increased risk of fetal birth defects. Flu during pregnancy is also linked with an increased risk of miscarriage, premature birth, low birth weight and fetal death.
- Protect your baby after birth. Infants are at increased risk of severe flu symptoms, but childhood flu vaccines can’t begin until a baby is 6 months old. If you have a flu shot during pregnancy, the antibodies you develop will pass through the placenta and breast milk, if you’re breast-feeding. These antibodies will help protect your baby from the flu after birth.
When you get vaccinated, request the flu shot — not the nasal spray vaccine. The flu shot is made from an inactivated virus, so it’s safe for both mother and baby during any stage of pregnancy. The nasal spray vaccine isn’t recommended for use in pregnant women.
While all vaccines carry some risk of adverse effects, such as an allergic reaction, no research has shown an increased risk of complications associated with the flu shot for pregnant women. Women can get the flu shot at any time during pregnancy. There also is no evidence that adverse effects occur in the children of women who receive vaccines with the mercury-based preservative thimerosal during pregnancy. In addition, single-dose flu shots don’t contain thimerosal. If you have concerns about the flu shot during pregnancy, talk to your doctor.
Should I get a flu shot?
It depends if you’re in the high-risk group of people who can develop severe flu complications when you have the flu.
Anyone can get the flu (even healthy people), and serious problems related to the flu can happen at any age, but some people are at high risk of developing serious flu-related complications if they get sick. This includes people 65 years and older, people of any age with certain chronic medical conditions (such as asthma, diabetes, or heart disease), pregnant women, and young children. If you have heart disease, flu shots can reduce your risk of flu-related complications. While doctors have long recommended that older adults and other high-risk groups get flu shots, they’re now emphasizing the importance of flu shots for those with heart disease too.
Most people who get the flu will have mild illness, will not need medical care or antiviral drugs, and will recover in less than two weeks. Some people, however, are more likely to get flu complications that can result in hospitalization and sometimes death. Pneumonia, bronchitis, sinus infections and ear infections are examples of flu-related complications. The flu also can make chronic health problems worse. For example, people with asthma may experience asthma attacks while they have the flu, and people with chronic congestive heart failure may experience a worsening of this condition triggered by flu. List below are the groups of people who are more likely to get serious flu-related complications if they get sick with influenza.
Is it safe to get a flu shot if I have heart disease?
Flu shots are safe for most people who have heart disease.
Flu vaccines are usually injected by needle into the upper extremity. Some people develop short-lived side effects, such as mild arm soreness at the injection site, a mild fever or muscle aches.
The flu vaccine that can be given as a nasal spray (FluMist) isn’t recommended for people with heart disease. This type of flu vaccine is made with live virus that can trigger flu symptoms in some people.
Talk to your doctor before getting a flu shot if:
- You’re allergic to eggs
- You’ve had a serious allergic reaction to the flu vaccine in the past
- You have a history of Guillain-Barre syndrome that developed after receiving a flu shot
- You’re sick with a fever at the time you plan to get a flu shot
Why are flu shots important for those with heart disease?
If you have heart disease, you’re at increased risk of complications from the flu — including pneumonia, respiratory failure, heart attack and death. Having the flu can also worsen pre-existing conditions, such as heart failure, diabetes or asthma.
Even if you get the flu despite having a flu shot, you’ll probably have a less severe case of the flu. If you have heart disease, some research suggests that getting a flu shot might even lower your risk of a heart attack or other cardiovascular event, or death from a cardiovascular event. More research is needed to confirm this benefit, however.
It’s also a good idea to get a flu shot if you live with or care for someone who has heart disease. Lowering your risk of getting the flu will lower the risk of those around you.
Influenza Vaccine Composition for 2022–23 United States
Vaccine strains for the 2022–23 influenza vaccines were selected by the Food and Drug Administration’s Vaccines and Related Biologic Products Advisory Committee based on WHO’s recommended Northern Hemisphere 2022–23 influenza vaccine composition 9.
- For the 2022-2023 flu season, there are three flu vaccines that are preferentially recommended for people 65 years and older. These are Fluzone High-Dose Quadrivalent vaccine, Flublok Quadrivalent recombinant flu vaccine and Fluad Quadrivalent adjuvanted flu vaccine.
- The recommended timing of flu shot is similar to last season. For most people who need only one dose for the season, September and October are generally good times to get vaccinated. Vaccination in July and August is not recommended for most adults but can be considered for some groups. While ideally it’s recommended to get vaccinated by the end of October, it’s important to know that vaccination after October can still provide protection during the peak of flu season.
- The age indication for the cell culture-based inactivated flu vaccine, Flucelvax Quadrivalent (ccIIV4), changed from 2 years and older to 6 months and older.
- Pre-filled Afluria Quadrivalent flu shots for children are not expected to be available this season. However, children can receive this vaccine from a multidose vial at the recommended dose.
The recommendations for egg-based and cell-based and recombinant flu vaccines are listed below 10:
- Egg-based vaccine composition recommendations
- an A/Victoria/2570/2019 (H1N1) pdm09-like virus;
- an A/Darwin/9/2021 (H3N2)-like virus (updated);
- a B/Austria/1359417/2021-like virus (B/Victoria lineage) (updated);
- a B/Phuket/3073/2013-like virus (B/Yamagata lineage)
- Cell- or recombinant-based vaccine composition recommendations
- an A/Wisconsin/588/2019 (H1N1) pdm09-like virus;
- an A/Darwin/6/2021 (H3N2)-like virus (updated);
- a B/Austria/1359417/2021-like virus (B/Victoria lineage) (updated);
- a B/Phuket/3073/2013-like virus (B/Yamagata lineage).
This recommendation is the same as the Southern Hemisphere flu vaccine recommendation.
Flu shot side effects
Like any medical product, vaccines can cause side effects. Side effects of the flu vaccine are generally mild and go away on their own within a few days. Most people who get a flu shot do not have any problems with the flu shots.
Common side effects from the flu shot include:
- Soreness, redness, and/or swelling from where the flu shot was given
- Headache
- Fever
- Nausea
- Muscle aches
- Hoarseness
- Sore, red or itchy eyes
- Cough
- Itching
- Fatigue
If these problems occur, they usually begin soon after the shot and last 1 or 2 days.
The flu shot, like other injections, can occasionally cause fainting.
More serious problems following a flu shot can include the following:
- Young children who get the flu shot along with pneumococcal vaccine (PCV13) and/or DTaP vaccine at the same time might be slightly more likely to have a seizure caused by fever. Ask your doctor for more information. Tell your doctor if a child who is getting flu vaccine has ever had a seizure.
Signs of a severe allergic reaction can include:
- Difficulty breathing
- Hoarseness or wheezing
- Swelling around the eyes or lips
- Hives
- Paleness
- Weakness
- A fast heart beat or dizziness
Life threatening allergic reactions to the flu shot are rare. These signs would most likely happen within a few minutes to a few hours after the vaccine is given.
If you think it is a severe allergic reaction or other emergency that can’t wait, call your local emergency services number and get to the nearest hospital. Otherwise, call your doctor.
Afterward, the reaction should be reported to the Vaccine Adverse Event Reporting System. Your doctor might file this report, or you can do it yourself through the Vaccine Adverse Event Reporting System website (https://vaers.hhs.gov/) or by calling 1-800-822-7967.
Some studies have found a possible small association of injectable flu vaccine with Guillain-Barré syndrome (GBS). Overall, these studies estimated the risk for GBS after vaccination as fewer than 1 or 2 cases of GBS per one million people vaccinated. Other studies have not found any association. Guillain-Barré syndrome (GBS) also, rarely, occurs after flu illness. Even though Guillain-Barré syndrome (GBS) following flu illness is rare, Guillain-Barré syndrome (GBS) is more common following flu illness than following flu vaccination. GBS has not been associated with the nasal spray vaccine.
Flu shot allergic reaction
With any vaccine, look for any unusual conditions, such as a high fever, behavior changes, or signs of a severe allergic reaction after vaccination.
Signs of a severe allergic reaction can include:
- Difficulty breathing
- Hoarseness or wheezing
- Swelling around the eyes or lips
- Hives
- Paleness
- Weakness
- A fast heart beat or dizziness
Life threatening allergic reactions to the flu shot are rare. These signs would most likely happen within a few minutes to a few hours after the vaccine is given.
If you think it is a severe allergic reaction or other emergency that can’t wait, call your local emergency services number and get to the nearest hospital. Otherwise, call your doctor.
Afterward, the reaction should be reported to the Vaccine Adverse Event Reporting System. Your doctor might file this report, or you can do it yourself through the Vaccine Adverse Event Reporting System website (https://vaers.hhs.gov/) or by calling 1-800-822-7967.
Flu Vaccine and People with Egg Allergies
CDC and its Advisory Committee on Immunization Practices have not changed their recommendations regarding egg allergy and receipt of influenza (flu) vaccines 11. The recommendations remain the same as last season (2016-2017). Based on those recommendations, people with egg allergies no longer need to be observed for an allergic reaction for 30 minutes after receiving a flu vaccine. People with a history of egg allergy of any severity should receive any licensed, recommended, and age-appropriate influenza vaccine. Those who have a history of severe allergic reaction to egg (i.e., any symptom other than hives) should be vaccinated in an inpatient or outpatient medical setting (including but not necessarily limited to hospitals, clinics, health departments, and physician offices), under the supervision of a health care provider who is able to recognize and manage severe allergic conditions.
Most flu shots and the nasal spray flu vaccine are manufactured using egg-based technology. Because of this, they contain a small amount of egg proteins, such as ovalbumin. However, studies that have examined the use of both the nasal spray vaccine and flu shots in egg-allergic and non-egg-allergic patients indicate that severe allergic reactions in people with egg allergies are unlikely. A recent CDC study found the rate of anaphylaxis after all vaccines is 1.31 per one million vaccine doses given.
Figure 5. Flu Vaccine and People with Egg Allergies – CDC Recommendations
 [Source 11]
[Source 11]
Recommendations for flu vaccination of persons with egg allergy have not changed since the 2016-2017 flu season. CDC recommends:
- Persons with a history of egg allergy who have experienced only hives after exposure to egg should receive flu vaccine. Any licensed and recommended flu vaccine (i.e., any form of IIV or RIV) that is otherwise appropriate for the recipient’s age and health status may be used.
- Persons who report having had reactions to egg involving symptoms other than hives, such as angioedema, respiratory distress, lightheadedness, or recurrent emesis; or who required epinephrine or another emergency medical intervention, may similarly receive any licensed and recommended flu vaccine (i.e., any form of IIV or RIV) that is otherwise appropriate for the recipient’s age and health status. The selected vaccine should be administered in an inpatient or outpatient medical setting (including, but not necessarily limited to hospitals, clinics, health departments, and physician offices). Vaccine administration should be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
- A previous severe allergic reaction to flu vaccine, regardless of the component suspected of being responsible for the reaction, is a contraindication to future receipt of the vaccine.
What is considered an egg allergy?
Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs and egg-containing foods, plus skin and/or blood testing for immunoglobulin E antibodies to egg proteins. Persons who are able to eat lightly cooked egg (e.g., scrambled egg) without reaction are unlikely to be allergic. Egg-allergic persons might tolerate egg in baked products (e.g., bread or cake). Therefore, tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergies can range in severity.
How common is egg allergy in children and adults?
Egg allergy affects about 1.3 % of all children and 0.2 % of all adults.
What vaccine should I get if I am egg allergic, but I can eat lightly cooked eggs?
If you are able to eat lightly cooked egg (e.g., scrambled egg) without reaction, you are unlikely to be allergic and can get any licensed flu vaccine (i.e., any form of inactivated influenza vaccine, live attenuated influenza vaccine, or recombinant influenza vaccine) that is otherwise appropriate for your age and health status.
What flu vaccine should I get if I get hives after eating egg-containing foods?
If you are someone with a history of egg allergy, who has experienced only hives after exposure to egg, you can get any licensed flu vaccine (i.e., any form of inactivated influenza vaccine, live attenuated influenza vaccine, or recombinant influenza vaccine) that is otherwise appropriate for your age and health.
What kind of flu vaccine should I get if I have more serious reactions to eating eggs or egg-containing foods like cardiovascular changes or a reaction requiring epinephrine?
If you are someone who has more serious reactions to eating eggs or egg-containing foods, like angioedema, respiratory distress, lightheadedness, or recurrent emesis; or who required epinephrine or another emergency medical intervention, you can get any licensed flu vaccine (i.e., any form of inactivated influenza vaccine, live attenuated influenza vaccine, or recombinant influenza vaccine) that is otherwise appropriate for your age and health status, but the vaccine should be given by a health care provider who can recognize and respond to a severe allergic response.
Are there still people with egg allergies who should not get flu vaccine?
People with egg allergy can receive flu vaccines according to the recommendations above. A person who has previously experienced a severe allergic reaction to flu vaccine, regardless of the component suspected of being responsible for the reaction should not get a flu vaccine again.
Why do flu vaccines contain egg protein?
Most flu vaccines today are produced using an egg-based manufacturing process and thus contain a small amount of egg protein called ovalbumin.
How much egg protein is in flu vaccine?
While not all manufacturers disclose the amount of ovalbumin in their vaccines, those that did from 2011–12 through 2014–15 reported maximum amounts of ≤1 µg/0.5 mL dose for flu shots and 0.24 µg/0.2 mL dose for the nasal spray vaccine. Cell-based flu vaccine (Flucelvax) likely has a much smaller amount of egg protein since the original vaccine virus is grown in eggs, but mass production of that vaccine does not occur in eggs. Recombinant vaccine (Flublok) is the only vaccine currently available that is completely egg free.
Can egg protein in flu vaccine cause allergic reactions in persons with a history of egg allergy?
Yes, allergic reactions can happen, but they occur very rarely with the flu vaccines available in the United States today. Occasional cases of anaphylaxis, a severe life-threatening reaction that involves multiple organ systems and can progress rapidly, in egg-allergic persons have been reported to the Vaccine Adverse Event Reporting System (VAERS) after administration of flu vaccine. Flu vaccines contain various components that may cause allergic reactions, including anaphylaxis. In a Vaccine Safety Datalink study, there were 10 cases of anaphylaxis after more than 7.4 million doses of inactivated flu vaccine, trivalent (IIV3) given without other vaccines, (rate of 1.35 per one million doses). Most of these cases of anaphylaxis were not related to the egg protein present in the vaccine. CDC and the Advisory Committee on Immunization Practices continue to review available data regarding anaphylaxis cases following flu vaccines.
How long after flu vaccination does a reaction occur in persons with a history of egg allergy?
Allergic reactions can begin very soon after vaccination. However, the onset of symptoms is sometimes delayed. In a Vaccine Safety Datalink study of more than 25.1 million doses of vaccines of various types given to children and adults over 3 years, only 33 people had anaphylaxis. Of patients with a documented time to onset of symptoms, eight cases had onset within 30 minutes of vaccination, while in another 21 cases, symptoms were delayed more than 30 minutes following vaccination, including one case with symptom onset on the following day.
Flu shot effectiveness
The CDC conducts studies each year to determine how well the influenza (flu) vaccine protects against flu illness. While vaccine effectiveness can vary, recent studies show that flu vaccination reduces the risk of flu illness by between 40% and 60% among the overall population during seasons when most circulating flu viruses are well-matched to the flu vaccine. In general, current flu vaccines tend to work better against influenza B and influenza A(H1N1) viruses and offer lower protection against influenza A(H3N2) viruses.
Two general types of studies are used to determine how well flu vaccines work: randomized controlled trials and observational studies. Vaccine effectiveness refers to vaccine protection measured in observational studies that include people with underlying medical conditions who have been administered vaccines by different health care providers under real-world conditions.
The measurement of influenza vaccine efficacy and effectiveness can be affected by virus and host factors as well as the study methodology used. Therefore, vaccine efficacy/effectiveness point estimates have varied among published studies.
Virus factors
The protective benefits of influenza vaccination are generally lower during flu seasons where the majority of circulating influenza viruses differ from the influenza viruses used to make the vaccines. Influenza viruses are continuously changing through a natural process known as antigenic drift. However, the degree of antigenic drift and the frequency of drifted viruses in circulation can vary for each of the three or four viruses included in the seasonal flu vaccine. So even when circulating influenza viruses are mildly or moderately drifted in comparison to the vaccine, it is possible that people may still receive some protective benefit from vaccination; and if other circulating influenza viruses are well matched, the vaccine could still provide protective benefits overall. It is not possible to predict how well the vaccine and circulating strains will be matched in advance of the influenza season, nor is it possible to predict how this match may affect vaccine effectiveness.
Host factors
In addition to virus factors, host factors such as age, underlying medical conditions, history of prior infections and prior vaccinations can affect the benefits received from vaccination.
Adults 65 years or older
Among older adults, annual influenza vaccination was recommended based on the high burden of influenza-related disease and demonstrated vaccine efficacy among younger adults. One randomized controlled trial of adults aged 60 years and older relied on serology for confirmation of influenza and reported a vaccine efficacy of 58% 12. However, it is unknown if infections were missed by serology among the study participants that were vaccinated and if the vaccine efficacy estimate is biased upwards. A meta-analysis of observational studies that used the test-negative design provided vaccine effectiveness estimates for adults aged >60 years against RT-PCR confirmed influenza infection. This meta-analysis reported significant vaccine effectiveness of 52% 13 during seasons when the vaccine and circulating viruses were well-matched. During seasons when the circulating viruses were antigenically drifted (not well matched), reported vaccine effectiveness was 36% 13.
An randomized controlled trial that compared a high-dose, inactivated influenza vaccine (containing four times the standard amount of influenza antigen) to standard dose vaccine in persons aged 65 years or older during the 2011-12 and 2012-13 influenza seasons found that rates of laboratory-confirmed influenza were 24% lower 14 among persons who received high-dose vaccine compared to standard dose influenza vaccine, indicating that high-dose vaccine provided 24% better protection against influenza than standard dose vaccine in this trial.
Several observational studies have reported significant vaccine effectiveness against RT-PCR confirmed influenza-related hospitalization among older adults. A three-year study (2006-07 through 2008-09) in Tennessee that used a test-negative design reported vaccine effectiveness of 61% 15 among hospitalized adults >50 years of age. In an analysis of two additional seasons, including 2010-11 and 2011-2012 (excluding 2009-10), vaccine effectiveness was 58% 16 against RT-PCR confirmed influenza associated hospitalizations for persons >50 years of age for the five seasons combined.
Adults
Several randomized controlled trials have been done in healthy adults aged <65 years 17, 18, 19. These studies have reported vaccine efficacy estimates ranging from 16%-75%; vaccine effectiveness of 16% was reported during a season with few influenza infections. An randomized controlled trial in South Africa among HIV infected adults reported vaccine efficacy of 76% 20. A meta-analysis that included data from randomized controlled trials of licensed inactivated influenza vaccines reported a pooled vaccine effectiveness of 59% 21 against influenza confirmed by RT-PCR or viral culture. In addition, randomized controlled trials of cell-based inactivated influenza vaccines (IIVs) and recombinant trivalent HA protein vaccines have been performed among healthy adults. In general, efficacy estimates for these types of vaccines are similar to other inactivated influenza vaccines that are egg-based 22.
Children
In a four-year randomized controlled trial of inactivated vaccines among children aged 1–15 years, vaccine efficacy was estimated at 77% against influenza A (H3N2) and 91% against influenza A (H1N1) virus infection 23. An randomized controlled trial of children aged 6–24 months reported vaccine efficacy of 66% against laboratory-confirmed influenza in 1999-2000 but no vaccine efficacy during the second year when there was little influenza activity 24. During 2010-11, the vaccine efficacy of a quadrivalent inactivated vaccine among children aged 3-8 years was 59% 25. In addition, a cluster-randomized trial conducted in Hutterite communities in Canada found that vaccinating children aged 3 to 15 years with trivalent inactivated influenza vaccine before the 2008-09 season reduced RT-PCR confirmed influenza in the entire community by 61%, including a 59% reduction in confirmed influenza among non-vaccinated community members, evidence of the “indirect” effect of influenza vaccination on prevention on disease transmission 26.
Several randomized controlled trials of live attenuated influenza vaccines among young children have demonstrated vaccine efficacy against laboratory confirmed influenza with estimates ranging from 74%-94% 27, 28. A study conducted among children aged 12 through 36 months living in Asia during consecutive influenza seasons reported efficacy for live attenuated influenza vaccine of 64%–70% 29.
In 2017, a study in the journal Pediatrics 30 was the first of its kind to show that flu vaccination also significantly reduced a child’s risk of dying from the flu. The study, which looked at data from four flu seasons between 2010 and 2014, found that flu vaccination reduced the risk of flu-associated death by half (51 percent) among children with underlying high-risk medical conditions and by nearly two-thirds (65 percent) among healthy children.
Pregnant women
An randomized controlled trial conducted among pregnant women in South Africa during 2011 and 2012 reported vaccine efficacy against RT-PCR confirmed influenza of 50% among HIV-negative women and 58% among HIV-positive women vaccinated during the third trimester 31. In addition, the trial showed that vaccination reduced the incidence of laboratory-confirmed influenza among infants born to HIV-negative women by 49%; the study was unable to assess vaccine efficacy among infants of HIV infected women. An observational study in the United States during 2010-11 and 2011-12 using a test-negative design reported vaccine effectiveness of 44% against influenza among pregnant woman 32.
A randomized trial in Bangladesh found that babies born to mothers vaccinated during pregnancy with trivalent inactivated influenza vaccines were significantly less likely to be born small for gestational age and weighed an average of 200g more than babies born to unvaccinated mothers 33. No effect of maternal immunization on infant birth weight was reported in the South African trial described above. Some observational studies in developed and developing countries have found lower risk of prematurity or low birth weight in babies born to vaccinated mothers, but the effect has not been consistently demonstrated 34.
How well does the live attenuated influenza vaccine (LAIV) work compared to inactivated influenza vaccine (IIV)?
Children
Three randomized clinical trials comparing live attenuated influenza vaccine to trivalent inactivated influenza vaccine in young children, 2-8 years of age, suggested that live attenuated influenza vaccine had superior efficacy compared to inactivated influenza vaccine 35. Recently, several observational studies suggest that LAIV did not consistently provide better protection against influenza than inactivated vaccine, especially against influenza caused by the 2009 H1N1 pandemic virus 36). However, a randomized, school-based study in Canada reported lower rates of confirmed influenza among students vaccinated with live-attenuated vaccine compared to students vaccinated with inactivated influenza vaccine, as well as decreased influenza transmission among family members of students vaccinated with live-attenuated influenza vaccines 37.
Adults
Clinical trials during 2004-05, 2005-06, and 2007-08 that compared inactivated influenza vaccines and live attenuated influenza vaccines to no vaccine among adults suggested that inactivated influenza vaccines provided better protection against influenza than live attenuated influenza vaccines in adults 38.
What is the flu?
Influenza commonly called the flu, is an infection of the nose, throat and lungs, which are part of the respiratory system, caused by the influenza viruses. Flu (influenza) is not the same as “stomach flu” viruses that cause diarrhea and vomiting.
At first, the flu may seem like a common cold with a runny nose, sneezing and sore throat. Colds usually develop slowly. But the flu tends to come on suddenly. And while a cold can be miserable, you usually feel much worse with the flu.
Flu symptoms come on very quickly and can include:
- sudden high temperature (fever)
- an aching body
- chills and sweats
- feeling tired or exhausted or weak
- dry, persistent cough
- sore throat
- eye pain
- headache
- difficulty sleeping
- loss of appetite
- vomiting and diarrhea or abdominal pain
- feeling sick and being sick
- shortness of breath
- runny or stuffy nose
The symptoms are similar for children, but they can also get pain in their ear and appear less active.
Cold and flu symptoms are similar, but flu tends to be more severe.
Most people with the flu get better on their own. But sometimes, influenza and its complications can be deadly. People at higher risk of developing flu complications include:
- Young children under age 2
- Adults older than age 65
- Residents of nursing homes and other long-term care facilities
- People who are pregnant or plan to be pregnant during flu season
- People with weakened immune systems
- American Indians or Alaska Natives
- People who have chronic illnesses, such as asthma, heart disease, kidney disease, liver disease and diabetes
- People with a body mass index (BMI) of 40 or higher
Most people who get the flu can treat themselves at home and often don’t need to see a health care provider. If you have flu, there are some things you can do to help get better more quickly:
- Do rest and sleep
- Do keep warm
- Do take acetaminophen (paracetamol) or ibuprofen to lower your temperature and treat aches and pains
- Do drink plenty of water to avoid dehydration (your urine should be light yellow or clear)
To help control the spread of influenza in your community, stay home and keep sick children home until the fever has been gone for 24 hours. Avoid being around other people until you’re feeling better, unless you’re getting medical care. If you do need to leave your home and get medical care, wear a face mask. Wash your hands often.
Table 2. How to tell the difference between cold and flu symptoms
| Flu | Cold |
|---|---|
| Appears quickly within a few hours | Appears gradually |
| Affects more than just your nose and throat | Affects mainly your nose and throat |
| Makes you feel exhausted and too unwell to carry on as normal | Makes you feel unwell, but you still feel well enough to do your normal activities |
The emergency warning signs of flu sickness
Complications of flu can include bacterial pneumonia, ear infections, sinus infections, and worsening of chronic medical conditions, such as congestive heart failure, asthma, or diabetes.
In children
- Fast breathing or trouble breathing
- Bluish skin color
- Not drinking enough fluids
- Not waking up or not interacting
- Being so irritable that the child does not want to be held
- Flu-like symptoms improve but then return with fever and worse cough
- Fever with a rash
In adults
- Difficulty breathing or shortness of breath
- Pain or pressure in the chest or abdomen
- Sudden dizziness
- Confusion
- Severe or persistent vomiting
- Flu-like symptoms that improve but then return with fever and worse cough
In addition to the signs above, get medical help right away for any infant who has any of these signs:
- Being unable to eat
- Has trouble breathing
- Has no tears when crying
- Significantly fewer wet diapers than normal
Most people who get the flu can treat themselves at home and often don’t need to see a health care provider.
If you have flu symptoms and are at risk of complications, see your health care provider right away. Taking antiviral medication may shorten the length of your illness and help prevent more-serious problems.
See your doctor right away if you or your child have symptoms of flu and:
- you’re worried about your baby’s or child’s symptoms
- you’re 65 or over
- you’re pregnant
- you have a long-term medical condition – for example, diabetes or a condition that affects your heart, lungs, kidneys, brain or nerves
- you have a weakened immune system – for example, because of chemotherapy or HIV
- your symptoms do not improve after 7 days
If you have emergency symptoms of the flu, get medical care right away. For adults, emergency symptoms can include:
- Difficulty breathing or shortness of breath
- Chest pain
- Ongoing dizziness
- Seizures
- Worsening of existing medical conditions
- Severe weakness or muscle pain
Emergency symptoms in children can include:
- Difficulty breathing
- Pale, gray or blue-colored skin, lips or nail beds — depending on skin color
- Chest pain
- Dehydration
- Severe muscle pain
- Seizures
- Worsening of existing medical conditions
Influenza causes
Influenza commonly called the flu, is an infection of the nose, throat and lungs, caused by the influenza viruses. Influenza viruses travel through the air in droplets when someone with the flu infection coughs, sneezes or talks. You can inhale the droplets directly. Or you can pick up the germs from an object such as a telephone or computer keyboard and then transfer them to your eyes, nose or mouth.
People with the influenza virus are likely contagious from about a day before symptoms appear until about four days after they start. Children and people with weakened immune systems may be contagious for a slightly longer time.
Influenza viruses are constantly changing, with new strains appearing regularly. If you’ve had influenza in the past, your body has already made antibodies to fight that specific strain of the virus. If future influenza viruses are similar to those you’ve encountered before, either by having the disease or by getting vaccinated, those antibodies may prevent infection or lessen its severity. But antibody levels may decline over time.
Also, antibodies against influenza viruses you’ve encountered in the past may not protect you from new influenza strains. New strains can be very different viruses from what you had before.
How Flu Spreads
Most experts believe that flu viruses spread mainly by tiny droplets made when people with flu cough, sneeze or talk. These droplets can land in the mouths or noses of people who are nearby. Less often, a person might also get flu by touching a surface or object that has flu virus on it and then touching their own mouth, nose, or possibly their eyes.
Anyone can get the flu (even healthy people), and serious problems related to the flu can happen at any age, but some people are at high risk of developing serious flu-related complications if they get sick. This includes people 65 years and older, people of any age with certain chronic medical conditions (such as asthma, diabetes, or heart disease), pregnant women, and young children.
Period of Contagiousness
You may be able to pass on the flu to someone else before you know you are sick, as well as while you are sick. Although people with the flu are most contagious in the first 3-4 days after their illness begins, some otherwise healthy adults may be able to infect others beginning 1 day before symptoms develop and up to 5 to 7 days after becoming sick. Some people, especially young children and people with weakened immune systems, might be able to infect others with flu viruses for an even longer time.
Risk factors for getting the flu
Factors that may increase your risk of developing the flu or its complications include:
- Age. Seasonal influenza tends to have worse outcomes in children under age 2, and adults older than age 65.
- Living or working conditions. People who live or work in facilities with many other residents, such as nursing homes or military barracks, are more likely to develop the flu. People who are staying in the hospital also are at higher risk.
- Weakened immune system. Cancer treatments, anti-rejection medications, long-term use of steroids, organ transplant, blood cancer or HIV/AIDS can weaken the immune system. This can make it easier to catch the flu and may increase the risk of developing complications.
- Chronic illnesses. Chronic conditions may increase the risk of influenza complications. Examples include asthma and other lung diseases, diabetes, heart disease, nervous system diseases, metabolic disorders, problems with an airway, and kidney, liver or blood disease.
- Race. American Indians or Alaska Natives people may have an increased risk of influenza complications.
- Aspirin use under age 19. People who are younger than 19 years of age and receiving long-term aspirin therapy are at risk of developing Reye’s syndrome if infected with influenza.
- Pregnancy. Pregnant people are more likely to develop influenza complications, particularly in the second and third trimesters. This risk continues up to two weeks after the baby is born.
- Obesity. People with a body mass index (BMI) of 40 or higher have an increased risk of flu complications.
Flu prevention
The best way to reduce your risk from seasonal flu and its potentially serious complications is to get vaccinated every year.
The U.S. Centers for Disease Control and Prevention (CDC) recommends annual flu vaccination for everyone age 6 months or older. The flu vaccine can lower your risk of getting the flu. It also can lower the risk of having serious illness from the flu and needing to stay in the hospital.
The influenza vaccine isn’t 100% effective, so it’s also important to take several measures to reduce the spread of infection, including:
- Wash your hands. Washing your hands often with soap and water for at least 20 seconds is an effective way to prevent many common infections. Or use alcohol-based hand sanitizers if soap and water aren’t available.
- Avoid touching your face. Avoid touching your eyes, nose and mouth.
- Cover your coughs and sneezes. Cough or sneeze into a tissue or your elbow. Then wash your hands.
- Clean surfaces. Regularly clean often-touched surfaces to prevent spread of infection from touching a surface with the virus on it and then your face.
- Avoid crowds. The flu spreads easily wherever people gather — in child care centers, schools, office buildings, auditoriums and public transportation. By avoiding crowds during peak flu season, you reduce your chances of infection.
Also avoid anyone who is sick. And if you’re sick, stay home for at least 24 hours after your fever is gone so that you lessen your chance of infecting others.
Flu complications
Most people who get influenza will recover in a few days to less than two weeks, but some people will develop complications (such as pneumonia) as a result of the flu, some of which can be life-threatening and result in death.
Children and adults at high risk may develop complications that may include:
- Pneumonia
- Bronchitis
- Asthma flare-ups
- Heart problems
- Ear infections
- Acute respiratory distress syndrome (ARDS)
Pneumonia is one of the most serious complications. For older adults and people with a chronic illness, pneumonia can be deadly.
Sinus and ear infections are examples of moderate complications from flu, while pneumonia is a serious flu complication that can result from either influenza virus infection alone or from co-infection of flu virus and bacteria. Other possible serious complications triggered by flu can include inflammation of the heart (myocarditis), brain (encephalitis) or muscle (myositis, rhabdomyolysis) tissues, and multi-organ failure (for example, respiratory and kidney failure). Flu virus infection of the respiratory tract can trigger an extreme inflammatory response in the body and can lead to sepsis, the body’s life-threatening response to infection. Flu also can make chronic medical problems worse. For example, people with asthma may experience asthma attacks while they have the flu, and people with chronic heart disease may experience a worsening of this condition triggered by flu.
People at High Risk for Developing Flu-Related Complications
- Children younger than 5, but especially children younger than 2 years old
- Adults 65 years of age and older
- Pregnant women (and women up to two weeks postpartum)
- Residents of nursing homes and other long-term care facilities
- Also, American Indians and Alaska Natives
People who have medical conditions including:
- Asthma
- Neurological and neurodevelopmental conditions [including disorders of the brain, spinal cord, peripheral nerve, and muscle such as cerebral palsy, epilepsy (seizure disorders), stroke, intellectual disability, moderate to severe developmental delay, muscular dystrophy, or spinal cord injury].
- Chronic lung disease (such as chronic obstructive pulmonary disease [COPD] and cystic fibrosis)
- Heart disease (such as congenital heart disease, congestive heart failure and coronary artery disease)
- Blood disorders (such as sickle cell disease)
- Endocrine disorders (such as diabetes mellitus)
- Kidney disorders
- Liver disorders
- Metabolic disorders (such as inherited metabolic disorders and mitochondrial disorders)
- Weakened immune system due to disease or medication (such as people with HIV or AIDS, or cancer, or those on chronic steroids)
- People younger than 19 years of age who are receiving long-term aspirin therapy
- People with extreme obesity (body mass index [BMI] of 40 or more).
Note: There is no recommendation for pregnant women or people with pre-existing medical conditions to get special permission or written consent from their doctor or health care professional for influenza vaccination if they get vaccinated at a worksite clinic, pharmacy or other location outside of their physician’s office.
Flu diagnosis
Your doctor will conduct a physical exam, look for signs and symptoms of flu, and possibly order a test that detects influenza viruses. During times when flu is widespread, you may not need to be tested for it. Your health care provider may diagnose you based on your symptoms.
In some cases, your health care provider may suggest that you be tested for influenza. Your doctor may use many tests to diagnose flu. The most common are called “rapid influenza diagnostic tests (RIDTs).” Rapid influenza diagnostic tests work by detecting the parts of the virus (antigens) that stimulate an immune response. The rapid influenza diagnostic tests (RIDTs) can provide results within approximately 10-15 minutes but may not be as accurate as other flu tests. Therefore, you could still have flu, even though your rapid test result is negative. Other flu tests called “rapid molecular assays” detect genetic material of the flu virus. Rapid molecular assays produce results in 15-20 minutes and are more accurate than rapid influenza diagnostic tests (RIDTs).
In addition to rapid influenza diagnostic tests (RIDTs) and rapid molecular assays, there are several more accurate flu tests available that must be performed in specialized laboratories, such as hospital and public health laboratories. These tests include reverse transcription polymerase chain reaction (RT-PCR), viral culture, and immunofluorescence assays. All of these tests require that a health care provider swipe the inside of your nose or the back of your throat with a swab and then send the swab for testing. Results may take one to several hours. The reverse transcription polymerase chain reaction (RT-PCR) testing is more sensitive than other tests and may be able to identify the influenza strain.
Will my health care provider test me for flu if I have flu-like symptoms?
Not necessarily. Most people with flu symptoms are not tested because the test results usually do not change how you are treated.
Your health care provider may diagnose you with flu based on your symptoms and their clinical judgment or they may choose to use an influenza diagnostic test. During an outbreak of respiratory illness, testing for flu can help determine if flu viruses are the cause of the outbreak. Flu testing can also be helpful for some people with suspected flu who are pregnant or have a weakened immune system, and for whom a diagnosis of flu can help their doctor make decisions about their care.
Flu treatment
Usually, you’ll need nothing more than rest and plenty of fluids to treat the flu. But if you have a severe infection or are at higher risk of complications, your health care provider may prescribe an antiviral medication to treat the flu. These drugs can include oseltamivir (Tamiflu), zanamivir (Relenza), peramivir (Rapivab) or baloxavir (Xofluza). These medications may shorten your illness by a day or so and help prevent serious complications.
- Oseltamivir (Tamiflu). Oseltamivir (Tamiflu) for oral administration is FDA-approved for early treatment of uncomplicated influenza in people two weeks and older, and for chemoprophylaxis to prevent influenza in people one year and older. Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of influenza in infants younger than 14 days old, and for chemoprophylaxis in infants 3 months to 1 year, is recommended by CDC and the American Academy of Pediatrics. If a child is younger than 3 months old, use of oseltamivir for chemoprophylaxis is not recommended unless the situation is judged critical, due to limited data in this age group.
- Zanamivir (Relenza). Zanamivir (Relenza) for oral inhalation through a device similar to an asthma inhaler is FDA-approved for early treatment of uncomplicated influenza in people 7 years and older and to prevent influenza in people 5 years and older. It is not recommended for use in people with underlying respiratory disease, including people with and lung disease.
- Peramivir (Rapivab). Peramivir (Rapivab) for intravenous administration is FDA-approved for early treatment of uncomplicated influenza in people 6 months and older.
- Baloxavir (Xofluza). Baloxavir marboxil (Xofluza) for oral administration is FDA-approved for early treatment of uncomplicated influenza in otherwise healthy non-high risk people 5 years to less than 12 years and for all persons 12 years and older, and for post-exposure prophylaxis of influenza in people 5 years and older. Baloxavir is not recommended for pregnant women, immunocompromised persons, breastfeeding mothers, outpatients with complicated or progressive illness, or hospitalized patients.
Antiviral medication side effects may include nausea and vomiting. These side effects may be lessened if the medication is taken with food.
If you get sick with flu, influenza antiviral drugs may be a treatment option. Antiviral drugs work best when started early, such as one to two days after your flu symptoms begin.
Check with your doctor promptly if you are at higher risk of serious flu complications and you get flu symptoms. People at higher risk of flu complications include young children, adults 65 years of age and older, pregnant people, and people with certain medical conditions such as asthma, diabetes and heart disease.
When treatment is started within 1-2 days after flu symptoms begin, influenza antiviral drugs can lessen symptoms and shorten the time you are sick by 1 or 2 days. They might also prevent some flu complications, like pneumonia. For people at higher risk of serious flu complications, treatment with influenza antiviral drugs can mean the difference between milder or more serious illness possibly resulting in a hospital stay.
Yes. Oseltamivir is recommended by the CDC and American Academy of Pediatrics (AAP) for early treatment of flu in people of any age, and for the prevention of flu (i.e., prophylaxis) in people 3 months of age and older. Zanamivir is recommended for early treatment of flu in people 7 years of age and older, and for the prevention of flu in people 5 years of age and older. Peramivir is recommended for early treatment in people 2 years of age and older.
Yes. Oral oseltamivir is recommended for treatment of pregnant women with flu because compared to other recommended antiviral medications it has the most studies available to suggest that it is safe and beneficial during pregnancy.
Flu home remedies
If you do come down with the flu, these measures may help ease your symptoms:
- Drink plenty of liquids. Choose water, juice and warm soups to prevent dehydration.
- Rest. Get more sleep to help your immune system fight infection. You may need to change your activity level, depending on your symptoms.
- Consider pain relievers. Use acetaminophen (Tylenol, others) or ibuprofen (Advil, Motrin IB, others), to combat the achiness associated with influenza. Children and teens recovering from flu-like symptoms should never take aspirin because of the risk of Reye’s syndrome, a rare but potentially fatal condition.
To help control the spread of influenza in your community, stay home and keep sick children home until the fever has been gone for 24 hours. Avoid being around other people until you’re feeling better, unless you’re getting medical care. If you do need to leave your home and get medical care, wear a face mask. Wash your hands often.
References- Flu Season. https://www.cdc.gov/flu/about/season/flu-season.htm
- Flu Vaccine and People with Egg Allergies. https://www.cdc.gov/flu/prevent/egg-allergies.htm
- Weekly U.S. Influenza Surveillance Report. Updated January 27, 2023. https://www.cdc.gov/flu/weekly/index.htm
- TABLE. Influenza vaccines — United States, 2022–23 influenza season*. https://www.cdc.gov/flu/professionals/acip/2022-2023/acip-table.htm
- A revision of the system of nomenclature for influenza viruses: a WHO Memorandum. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2395936/pdf/bullwho00427-0070.pdf
- Types of Influenza Viruses. https://www.cdc.gov/flu/about/viruses/types.htm
- Flu Vaccine Safety Information. https://www.cdc.gov/flu/prevent/general.htm
- Vaccine Effectiveness: How Well Do Flu Vaccines Work? https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm
- Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza Activity and Composition of the 2022–23 Influenza Vaccine — United States, 2021–22 Season. MMWR Morb Mortal Wkly Rep 2022;71:913–919. DOI: http://dx.doi.org/10.15585/mmwr.mm7129a1
- Frequently Asked Influenza (Flu) Questions: 2022-2023 Season. https://www.cdc.gov/flu/season/faq-flu-season-2022-2023.htm
- Flu Vaccine and People with Egg Allergies. https://www.cdc.gov/flu/protect/vaccine/egg-allergies.htm
- Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272(21):1661-5. https://jamanetwork.com/journals/jama/article-abstract/383571
- Darvishian M, Bijlsma MJ, Hak E, van den Heuvel ER. Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case-control studies. Lancet Infect Dis 2014; 14(12): 1228-39.
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, Martin E, Gurunathan S, Nathan R, Greenberg DP, Tornieporth NG, Decker MD, Talbot HK. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635-45.
- Talbot HK, Griffin MR, Chen Q, Zhu Y, Williams JV, Edwards, KM. Effectiveness of seasonal vaccine in preventing confirmed influenza-associated hospitalization in community dwelling older adults. J Infect Dis 2011; 203: 500–8.
- Chen Q, Griffin MR, Nian H, Zhu Y, Williams JV, Edwards, KM, Talbot HK. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥50 years. J Infect Dis 2015; 211: 1045–50.
- Jackson LA, Gaglani MJ, Keyserling HL, et al. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: a randomized, placebo-controlled trial over two influenza seasons. BMC Infect Dis 2010; 10: 71.
- Monto AS, Ohmit SE, Petrie JG, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 2009; 361(13): 1260-7.
- eran J, Vesikari T, Wertzova V, et al. Efficacy of inactivated split-virus influenza vaccine against culture-confirmed influenza in healthy adults: a prospective, randomized, placebo-controlled trial. J Infect Dis 2009; 200(12): 1861-9.
- Madhi SA, Maskew M, Koen A, Kuwanda L, Besselaar TG, Naidoo D, Cohen C, Valette M, Cutland CL, Sanne I. Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clin Infect Dis 2011; 52(1): 128-37.
- Osterholm MT, Kelley NS, Sommer A, et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet ID 2011(12): 36-44. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(11)70295-X/fulltext
- Barrett PN, Berezuk G, Fritsch S, et al. Efficacy, safety, and immunogenicity of a Vero-cell-culture-derived trivalent influenza vaccine: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2011; 377(9767): 751-9.
- Neuzil KM, Dupont WD, Wright PF, Edwards KM. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatr Infect Dis J. 2001;20(8):733-40 https://journals.lww.com/pidj/Abstract/2001/08000/Efficacy_of_inactivated_and_cold_adapted_vaccines.4.aspx
- Hoberman A, Greenberg DP, Paradise JL, Rockette HE, Lave JR, Kearney DH, Colborn DK, Kurs-Lasky M, Haralam MA, Byers CJ, Zoffel LM, Fabian IA, Bernard BS, Kerr JD. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA. 2003;290(12):1608-16. https://jamanetwork.com/journals/jama/fullarticle/197348
- Jain VK, Rivera L, Zaman K, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med 2013; 369(26): 2481-91.
- Loeb M, Russell ML, Moss L, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA 2010; 303(10): 943-50.
- Bracco Neto H, Farhat CK, Tregnaghi MW, et al. Efficacy and safety of 1 and 2 doses of live attenuated influenza vaccine in vaccine-naive children. Pediatr Infect Dis J 2009;28:365–71.
- Vesikari T, Fleming DM, Aristegui JF, et al. Safety, efficacy, and effectiveness of cold-adapted influenza vaccine-trivalent against community-acquired, culture-confirmed influenza in young children attending day care. Pediatrics 2006;118:2298–312.
- Tam JS, Capeding MR, Lum LC, et al. Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatr Infect Dis J 2007;26:619–28.
- Influenza Vaccine Effectiveness Against Pediatric Deaths: 2010–2014. Pediatrics Apr 2017, e20164244; DOI: 10.1542/peds.2016-4244 http://pediatrics.aappublications.org/content/early/2017/03/30/peds.2016-4244
- Madhi SA, Cutland CL, Kuwanda L, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014; 371(10): 918-31.
- Thompson MG, Li DK, Shifflett P, et al. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010-2011 and 2011-2012 influenza seasons. Clin Infect Dis 2014; 58(4): 449-57.
- Steinhoff MC, Omer SB, Roy E, et al. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 2012.
- Richards JL, Hansen C, Bredfeldt C, et al. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin Infect Dis 2013;56:1216-22.
- Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM; CAIV-T Comparative Efficacy Study Group. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356(7):685-96 http://www.columbia.edu/itc/hs/medical/pathophys/immunology/2008/teamA_fluvaccine.pdf
- Gaglani M , Pruszynski J, Murthy K, Clipper L, Robertson A, Reis M, Chung JR, Piedra PA, Avadhanula V, Nowalk MP Zimmerman RK, Jackson ML, Jackson LA, Petrie JG, Ohmit SE, Monto AS, McLean HQ, Belongia EA, Fry AM, Flannery B. Influenza Vaccine Effectiveness against the 2009 Pandemic A (H1N1) Virus Differed by Vaccine-type During 2013-14 in the United States. J Inf Dis (in press
- Kwong J, Pereira J, Quach S, Pellizzari R, Dusome E, Russell M, et al. Randomized evalution of live attenuated vs. trivalent inactivated influenza vaccines in schools (RELATIVES) pilot study: preliminary results from the household surveillance sub-study.
- Ohmit SE, Victor JC, Teich ER, et al. Prevention of symptomatic seasonal influenza in 2005-2006 by inactivated and live attenuated vaccines. J Infect Dis 2008; 198(3): 312-7.