Purple glove syndrome
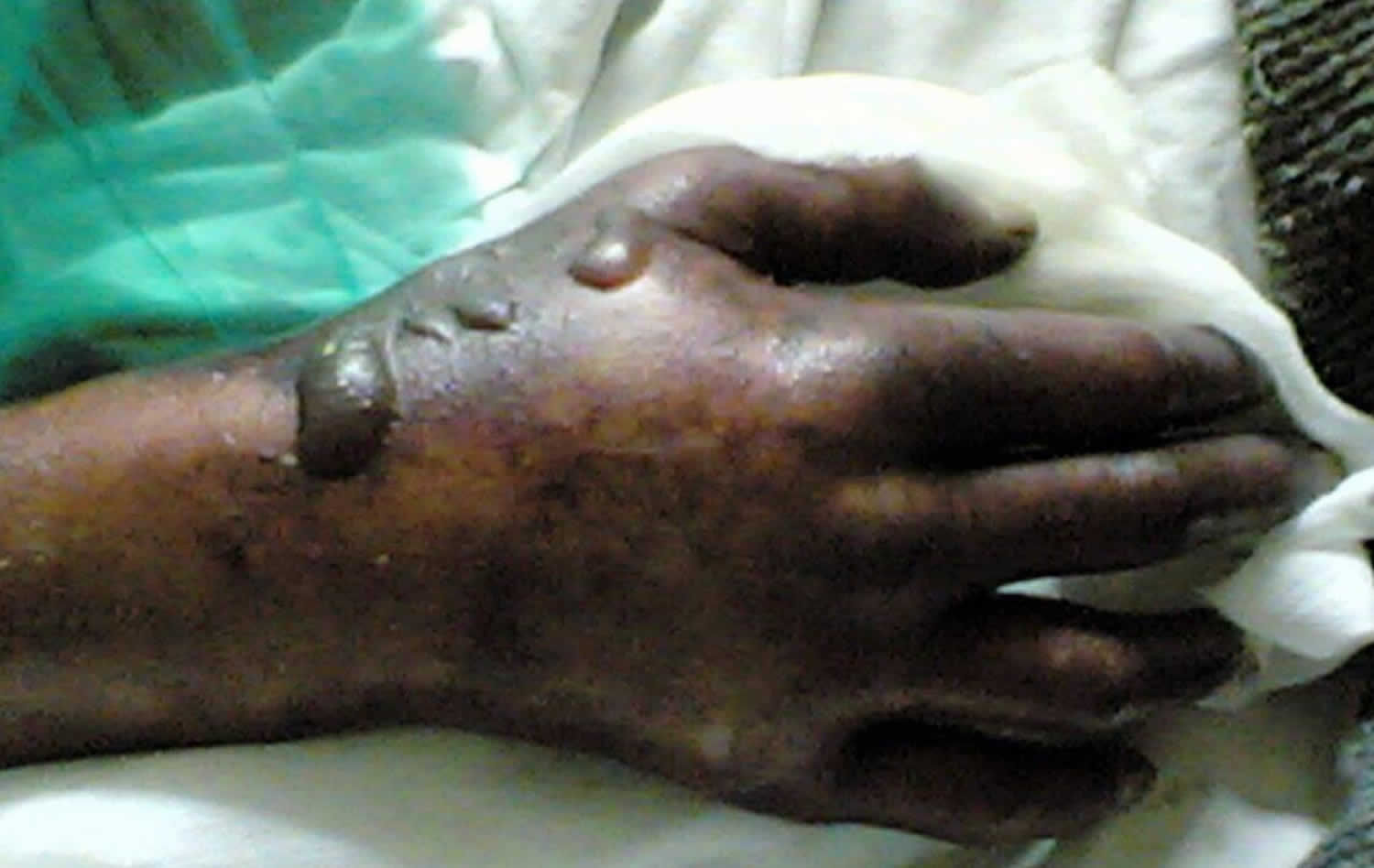
Purple glove syndrome also known as dilantin purple glove syndrome, is an uncommon complication of intravenous phenytoin (dilantin) administration characterized by pain, edema and purple-blue discoloration of the limb distal to the site of injection 1. The evolution of purple glove syndrome may range from simple discoloration to development of blisters and necrosis of the involved area (see Figure 1 below) 2. Purple glove syndrome is a dreaded complication of intravenous phenytoin use and may account for 1.7–5.9% of all phenytoin administrations depending upon the recognition as well as adverse drug event reporting 3. Purple glove syndrome has been described to evolve in three stages: in the initial stage, 2–12 hours after injection, there is pain and purple-blue discoloration around the site of administration (IV catheter site). The second stage involves development of edema, varying degrees of discoloration, blister formation or necrosis (epidermal sloughing, ulceration) and neuromuscular symptoms such as paresthesias or weakness. The third stage is the phase of resolution of pain, edema and discoloration 4. Timing of these stages appears quite variable, with initial discoloration occurring from minutes to days after phenytoin administration and tissue recovery spanning days to months 5. There are also a subset of more severe cases in which the second stage of expanding edema and superficial tissue damage is followed by dermal and subcutaneous tissue necrosis and ischemia leading to permanent injury, surgical debridement, or amputation 6. Tissue samples obtained both early and late in the course of symptoms have shown the common presence of epidermal necrosis, subcutaneous edema, perivascular lymphoid infiltration, and local vascular thrombosis 7.
The vast majority of cases described a combination of purple discoloration (93.8 %) and edema (92.6 %), with other common findings including erythema (51.9 %), pain (34.6 %), and coldness (21 %) 6. Physical findings included blistering (39.2 %), necrosis (18.9 %), and ulceration (17.6 %). One third (32.7 %) noted a decrease in amplitude or lack of palpable distal pulses in the affected extremity 8.
In terms of site, purple glove syndrome is most commonly associated with upper extremities; however, it has been also reported in lower extremities and referred to as purple sock syndrome 9.
Purple glove syndrome needs to be differentiated from local infiltration or extravasation, cellulitis and manifestations of peripheral vascular disease or a collagen vascular disorder 2. Characteristic purple-blue discoloration and evolution of the features beyond the infusion time, and absence of fever or purulent discharge clinically negate the possibility of local effect and cellulitis, respectively. Prior history of a vascular illness in the form of Raynaud’s phenomenon, acrocyanosis, multiple organ afflictions or other cutaneous changes may help to provide contributory evidence of such an illness 2.
Administration of higher than recommended phenytoin (dilantin) concentration or dose, extravasation, chemical irritation by solvents (sodium hydroxide, propylene glycol and ethanol), drug-induced vasculitis and drug-induced vasoconstriction are the important pathophysiological mechanisms theorized to explain the occurrence of purple glove syndrome. However, administration of phenytoin in accordance with the established guidelines may help in preventing the development of purple glove syndrome 2.
The treatment of purple glove syndrome involves stopping the phenytoin infusion, elevation of the affected limb, avoiding further venous access through the limb, and preventing secondary infection through the site of initial access. Nitroglycerin application to the area and brachial plexus block may reduce vasospasm and edema and promote intravascular absorption of phenytoin 10. Severe purple glove syndrome may need fasciotomy to relieve the pressure if vascular compromise is evident or amputation 11.
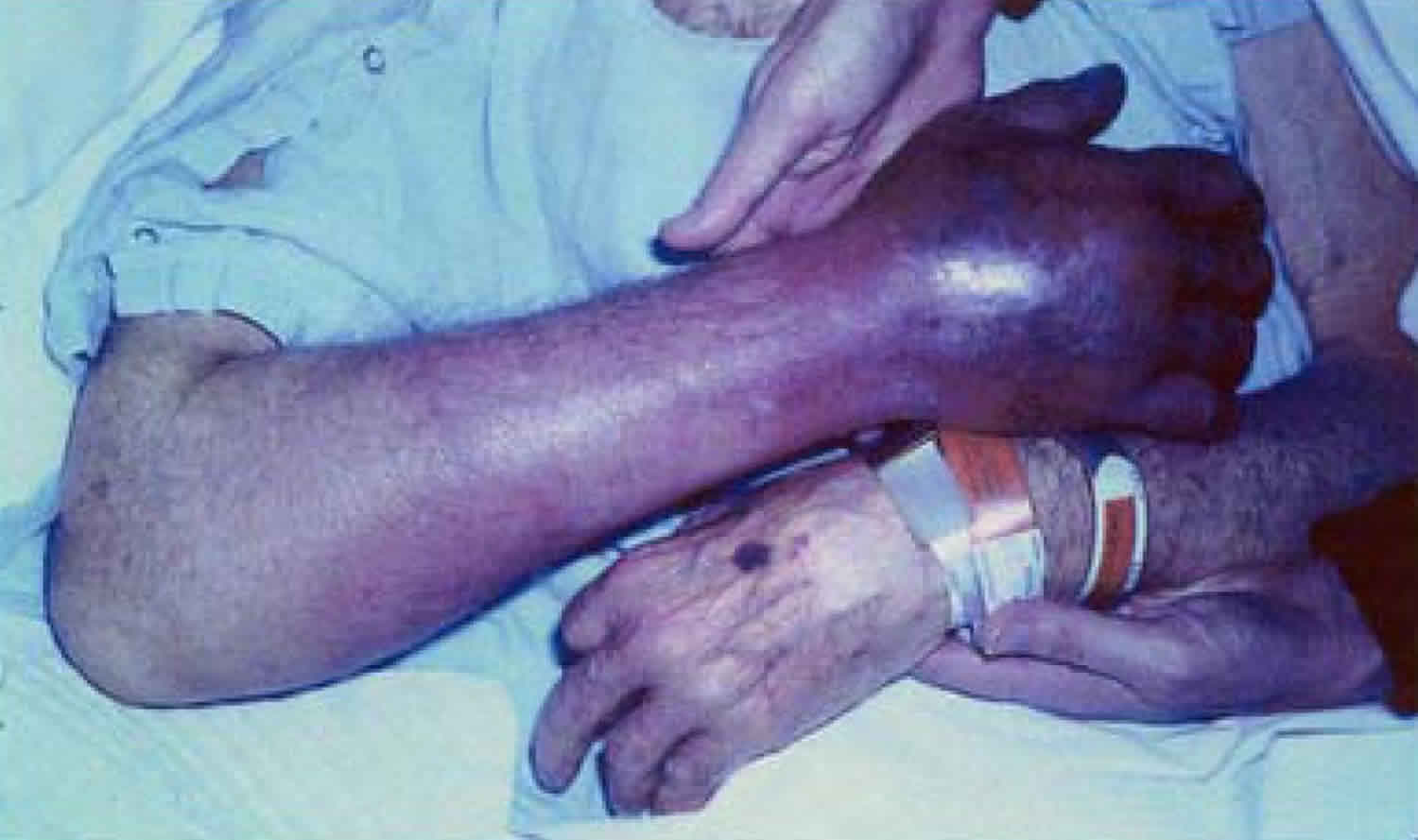
Figure 1. Dilantin purple glove syndrome
Footnote: Photograph of the right distal forearm and hand depicts swollen and congested skin with purple-blue discoloration; multiple blisters are observed over the anatomical snuff box, the site of venous access.
[Source 2 ]Purple glove syndrome causes
Purple glove syndrome is a rare adverse reaction to intravenous phenytoin (dilantin). Its occurrence is increased when small peripheral lines are used for drug administration. As phenytoin infusion is most often used in emergency settings, primarily in cases of refractory status epilepticus, it should be borne in mind that safe use is through larger lines in more proximal veins. Several pathophysiological mechanisms have been proposed to explain the occurrence of purple glove syndrome. The most plausible ones include administration of higher than recommended phenytoin (dilantin) concentration or dose, extravasation, chemical irritation by solvents (sodium hydroxide, propylene glycol and ethanol), drug-induced vasculitis and drug-induced vasoconstriction 2. Not all patients who receive intravenous phenytoin develop purple glove syndrome and therefore a patient’s susceptibility such as genetic predisposition seems to be a possibility.
Purple glove syndrome has also been associated with fosphenytoin (Cerebyx), the water-soluble pro-drug of phenytoin approved by the U.S. Food and Drug Administration (FDA) in 1996, although at a lower estimated incidence 12. Use of fosphenytoin has been limited, in part due to cost, however now a less expensive generic formulation has led to further debate about the continued role of phenytoin in the treatment of seizures, particularly status epilepticus 13.
Phenytoin sodium (Dilantin) is an anticonvulsant drug used for the treatment and prevention of seizures since 1956 14. It is recommended by the Epilepsy Foundation of America and European Federation of Neurological Societies practice guidelines as the second-line agent for treatment of status epilepticus after IV benzodiazepines 15.
Phenytoin is commonly utilized in several clinical settings within the emergency department. Patients with a history of seizures who are on daily phenytoin therapy can present with uncomplicated seizures after noncompliance or subtherapeutic administration of the drug. In this situation, an oral or parenteral loading dose is often administered. Patients presenting with status epilepticus refractory to benzodiazepine administration require prompt IV phenytoin administration 16. Finally, patients with traumatic intracranial injury or symptomatic mass lesions are frequently prescribed with phenytoin for prophylaxis against seizure activity in either oral or parenteral form 17. While the dosing of the drug is similar for all of the above situations, the route and timing of administration vary and influence the predisposition to possible adverse reactions such as purple glove syndrome.
Risk factors for developing purple glove syndrome
Several authors have attempted to identify risk factors for purple glove syndrome after intravenous phenytoin administration. Spengler et al. 18 identified 11 patients with 17 cases of purple glove syndrome at a single hospital over a 2-year period and performed a case-control study using unaffected patients that received IV phenytoin over the same period. They identified older age, female gender, and history of cardiovascular disease, number of doses, infusion rate, and smaller gauge IV catheters as factors associated with purple glove syndrome in a univariate analysis. O’Brien et al. 19 performed a retrospective cohort study that identified 152 consecutive patients who had received intravenous phenytoin over a 3-month period at several hospitals, of whom 9 developed purple glove syndrome. Generally patients who are 60 years old and older, who receive phenytoin for acute seizure indications, or who receive large doses and multiple doses of IV phenytoin are more predisposed to developing purple glove syndrome 19. Along with the more established risk factors for purple glove syndrome, Spengler et al 20 performed a case-control study that revealed that women, patients receiving high phenytoin infusion rates (>25 mg/min), and the use of IV catheter bore sizes smaller than 20 gauge (20-G) (ie, smaller bore diameter and higher gauge [>20-G] value) are associated with an increased risk of purple glove syndrome. It is important to note that there is conflicting evidence with regard to the role of IV catheter bore size as a risk factor for purple glove syndrome. Spengler et al9 reported higher risk of purple glove syndrome with IV catheter bore sizes smaller than 20-G (smaller bore diameter and higher gauge value), and O’Brien et al 19 reported the use of IV catheter bore sizes greater than 20-G (larger bore diameter and lower gauge value) in most of the patients (89%) developing purple glove syndrome in his study. Another less empirically supported risk factor that predisposes patients to purple glove syndrome is the presence of conditions that weaken vascular and skin integrity 21.
Purple glove syndrome pathophysiology
The mechanism by which purple glove syndrome occurs remains speculative. Many reports have proposed that the combination of small vascular tearing or micro-extravasation at the site of IV catheter insertion allow for leakage of the severely alkaline (pH = 12) phenytoin solution into surrounding tissue, causing necrosis, disruption of endothelial barriers, and vasoconstriction 19; however, recent data have shown that purple glove syndrome can occur with or without clinically apparent extravasation 22. Most recently, Yoshikawa et al 23 reported a case of purple glove syndrome following oral administration of phenytoin that provides supporting evidence that purple glove syndrome may occur with or without phenytoin extravasation. This case of purple glove syndrome following oral phenytoin was observed in a patient with supratherapeutic serum levels of phenytoin 23. This has raised the question as to whether phenytoin itself, irrespective of route of administration, may induce purple glove syndrome in patients due to phenytoin doses and serum levels that are higher than recommended therapeutic doses or levels.
An additional theory regarding the unique alkalinity of IV phenytoin solution argues that contact with blood leads to precipitation of certain compounds, leading to catheter obstruction and solution extravasation 24. The observation that multiple cases of purple glove syndrome have been reported without IV catheter extravasation suggests the presence of an additional underlying pathophysiology 25. The repeated finding of microvascular thrombosis on histopathology and the association between documented large vessel thromboses in the most severe cases of purple glove syndrome suggests that an unidentified procoagulant mechanism may be responsible for the extent of tissue destruction 7.
Purple glove syndrome prevention
The single most important preventable factor determining the development of purple glove syndrome appears to be the lack of adherence to the guidelines for intravenous administration of phenytoin 2. Use of an appropriate catheter (20-gauge or more), dedicated access through a large-bore vein preventing admixture with other injectables especially glucose, correct dose (15–20 mg/kg body weight) and rate (25–50 mg/min) and postinfusion flush with normal saline can help in preventing this dreaded adverse event associated with intravenous phenytoin 2. The infusion should be discontinued immediately at the very first appearance of purple glove syndrome 26. Nursing staff and the other personnel involved should be advised to follow the guidelines to minimize avoidable errors.
Purple glove syndrome symptoms
Purple glove syndrome affects a wide spectrum of patients receiving intravenous phenytoin, from the very young to elderly, and occurs in any of the more frequent scenarios in which phenytoin is administered in the acute care setting 6. The hallmark of purple glove syndrome is purplish discoloration and edema of the distal extremity, seen in greater than 90 % of reported cases 6. Pain is also likely to be present, but its presence may often be obscured by pathologic or iatrogenic-induced alteration in consciousness after a seizure. Alterations in appearance including blistering, erythema, or necrosis are more common than deficits in function, such as paralysis or sensory changes, potentially allowing for a more rapid diagnosis 7. Time to onset of symptoms was generally rapid, consistent with a potentially ischemic pathology, though cases were reported up to 5 days after the last dose of phenytoin 27.
Just over half of cases reported symptoms after one dose of IV phenytoin. Four cases of purple glove syndrome were associated with documented or strongly suspected intra-arterial administration of phenytoin and tended to display more severe injury and worse outcomes, with one amputation and one death directly associated with purple glove syndrome 28. While previous studies have emphasized an association between extravasation of drug and purple glove syndrome, only 37.9 % of cases we reported had documented or suspected extravasation, and more than one half (62.1 %) specifically described a lack of extravasation on inspection of the affected limb. Nonetheless, visible and tactile inspection cannot completely exclude any contact between the highly alkaline IV phenytoin solution and surrounding tissue, as microscopic vascular injury after intravenous catheter placement leading to extravasation is still possible.
Purple glove syndrome diagnosis
Purple glove syndrome is diagnosed clinically based on history of recent phenytoin use. Risk factors for purple glove syndrome after intravenous phenytoin administration include older age, female sex, peripheral vascular disease, sepsis, history of chronic debilitating disease, number of doses, higher dosage (>15-20 mg/kg body weight), higher infusion rate (>25 mg/min), and use of an intravenous catheter <20G 29. Some expert recommend immediate limb angiography for patients with any change in signs or symptoms of altered perfusion of the affected extremity, as this finding may progress rapidly in a proportion of patients. If vascular imaging is not performed, frequent neurovascular assessments must be initiated to identify ischemic progression to prevent subsequent tissue compromise. Once vascular compromise is suspected, consultation or transfer for evaluation by a hand or vascular surgery expert is highly recommended.
Purple glove syndrome treatment
Purple glove syndrome treatment includes pain management, elevation of the affected limb, gentle heat compression, massage, and local application of nitroglycerin and brachial plexus block 30. Anecdotal reports have documented beneficial outcomes with the use of a low concentration of IV 0.1% bupivacaine and fentanyl (2 mcg/mL) to localize and alleviate pain 31. Rarely, purple glove syndrome may progress to necrosis, ischemia, vascular compression, or compartment syndrome and may require systemic anticoagulant therapy and surgical interventions including fasciotomy, skin grafting or amputation 32.
References- Hanna DR. Purple glove syndrome: a complication of intravenous phenytoin. J Neurosci Nurs 1992;24:340–5.
- Lalla R, Malhotra HS, Garg RK, Sahu R. Purple glove syndrome: a dreadful complication of intravenous phenytoin administration. BMJ Case Rep. 2012;2012:bcr2012006653. Published 2012 Aug 24. doi:10.1136/bcr-2012-006653 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4543991
- O’Brien TJ, Cascino GD, So EL, et al. Incidence and clinical consequence of the purple glove syndrome in patients receiving intravenous phenytoin. Neurology 1998;51:1034–9.
- Bhattacharjee P, Glusac EJ. Early histopathologic changes in purple glove syndrome. J Cutan Pathol. 2004;31(7):513-515. doi:10.1111/j.0303-6987.2004.00224.x
- Santoshi J, Justin A, Jacob J, Pallapati S, Thomas B. Purple glove syndrome: a case report. Hand surgeons and physicians be aware. J Plast Reconstr Aesthet Surg: JPRAS. 2010;63(3):e340–2. doi: 10.1016/j.bjps.2009.06.021
- Garbovsky LA, Drumheller BC, Perrone J. Purple Glove Syndrome after Phenytoin or Fosphenytoin Administration: Review of Reported Cases and Recommendations for Prevention. J Med Toxicol. 2015;11(4):445-459. doi:10.1007/s13181-015-0490-z https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4675605
- Bhattacharjee P, Glusac E. Early histopathologic changes in purple glove syndrome. J Cutan Pathol. 2004;31(7):513–5. doi: 10.1111/j.0303-6987.2004.00224.x
- McLean C, Cheng K, Clifton M. Fatal case of accidental intra-arterial phenytoin injection. Eur J Vasc Endovasc Surg. 2002;23(4):378–9. doi: 10.1053/ejvs.2001.1586
- Jain RS, Nagpal K, Kumar S, Prakash S, Handa R. Purple glove syndrome occurring after oral administration of phenytoin in therapeutic doses: mechanism still a dilemma. Am J Emerg Med. 2015;33(1):123.e5–123.e6. doi: 10.1016/j.ajem.2014.05.039
- Meek PD, Davis SN, Collins DM, et al. Guidelines for nonemergency use of parenteral phenytoin products: proceedings of an expert panel consensus process. Panel on Nonemergency Use of Parenteral Phenytoin Products. Arch Intern Med 1999;159:2639–44.
- Edwards JJ, Bosek V. Extravasation injury of the upper extremity by intravenous phenytoin. Anesth Analg. 2002;94(3):. doi:10.1097/00000539-200203000-00035
- Intravenous Phenytoin and Fosphenytoin Safety Concerns Background Package. https://www.fda.gov/media/103872/download
- Brophy G, Bell R, Claassen J, Alldredge B, Bleck T, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23. doi: 10.1007/s12028-012-9695-z
- Parenteral Dilantin (Phenytoin Sodium Injection, USP) [prescribing information]. Revised February 2013. Parke Davis: New York. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/010151s037lbl.pdf
- Meierkord H, Boon P, Engelsen B, Gocke K, Shorvon S, Tinuper P, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17(3):348–55. doi: 10.1111/j.1468-1331.2009.02917.x
- Huff J, Melnick E, Tomaszewski C, Thiessen M, Jagoda A, Fesmire F. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med. 2014;63(4):437–447.e15. doi: 10.1016/j.annemergmed.2014.01.018
- Birbeck GL, French JA, Perucca E, Simpson DM, Fraimov H, George JM, et al. Evidence-based guidelins: antiepileptic drug selection for people with HIV/AIDS: report of the quality standards subcommittee of the American Academy of Neurology and the Ad Hoc Task Force of the Commission on Therapeutic Strategies of the International League Against Epilepsy. Neurology. 2012;78(2):139–45. doi: 10.1212/WNL.0b013e31823efcf8
- Spengler R, Arrowsmith J, Kilarski D, Buchanan C, Von Behren L, Graham D. Severe soft-tissue injury following intravenous infusion of phenytoin. Patient and drug administration risk factors. Arch Intern Med. 1988;148(6):1329–33. doi: 10.1001/archinte.1988.00380060093019
- O’Brien T, Cascino G, So E, Hanna D. Incidence and clinical consequence of the purple glove syndrome in patients receiving intravenous phenytoin. Neurology. 1998;51(4):1034–9. doi: 10.1212/WNL.51.4.1034
- Spengler RF, Arrowsmith JB, Kilarski DJ, Buchanan C, Von Behren L, Graham DR. Severe soft-tissue injury following intravenous infusion of phenytoin. Patient and drug administration risk factors. Arch Intern Med. 1988;148(6):1329–1333.
- Hanna DR. Purple glove syndrome: A complication of intravenous phenytoin. J Neurosci Nurs. 1992;24(6): 340–345.
- O’Brien TJ, Cascino GD, So EL, Hanna DR. Incidence and clinical consequence of the purple glove syndrome in patients receiving intravenous phenytoin. Neurology. 1998;51(4):1034–1039.
- Yoshikawa H, Abe T, Oda Y. Purple glove syndrome caused by oral administration of phenytoin. J Child Neurol. 2000;15(11):762.
- Hannon M, Lee S. Extravasation injuries. J Hand Surg. 2011;36A:2060–5. doi: 10.1016/j.jhsa.2011.10.001
- Singh G, Cherian V, Thomas B. Low-concentration, continuous brachial plexus block in the management of purple glove syndrome: a case report. J Med Case Rep. 2010
- Chokshi R, Openshaw J, Mehta NN, et al. Purple glove syndrome following intravenous phenytoin administration. Vasc Med 2007;12:29–31.
- Rajabally H, Nageshwaran S, Russell S. An atypical case of purple glove syndrome: an avoidable adverse event. BMJ Case Reports. 2012
- Prasad R, Mishra O, Gupta A. Gangrene of left hand following accidental intra-arterial injection of phenytoin sodium. Pediatric Oncall [serial online] 2012. doi: 10.7199/ped.oncall.2012.28
- Garbovsky LA, Drumheller BC, Perrone J. Purple glove syndrome after phenytoin or fosphenytoin administration: review of reported cases and recommendations for prevention. J Med Toxicol 2015;11:445-59
- Shah AM, Dahodwala A. Purple glove syndrome following intravenous phenytoin. Indian Pediatr. 2014;51:763–4.
- Singh G, Cherian VT, Thomas BP. Low-concentration, continuous brachial plexus block in the management of purple glove syndrome: A case report. J Med Case Rep. 2010;4:48-1947-4-48.
- Chokshi R, Openshaw J, Mehta NN, Mohler E., 3rd Purple glove syndrome following intravenous phenytoin administration. Vasc Med. 2007;12(1):29–31.