What is Chromium
Chromium is a mineral that humans require in trace amounts, although its mechanisms of action in the body and the amounts needed for optimal health are not well defined. It is found primarily in two forms: 1) trivalent (chromium 3+), which is biologically active and found in food, and 2) hexavalent (chromium 6+), a toxic form that results from industrial pollution. This post focuses exclusively on trivalent (3+) chromium 1. Picolinate, is a by-product of tryptophan that is paired with chromium in supplements (to form Chromium picolate), is said to help the body absorb chromium more efficiently. Chromium dinicocysteinate is a newer supplement complex of trivalent chromium with L-cysteine and niacin 2.
What Does Chromium Do
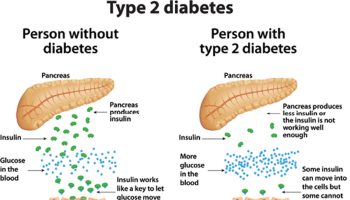
Chromium is known to enhance the action of insulin a hormone critical to the metabolism of glucose reducing insulin resistance 3, 4, 5, 6, improving glucose tolerance 7, 8 and storage of carbohydrate, fat, and protein in the body 9.
Indeed, one of the more common nutrition-related questions posed by patients with or at risk for diabetes to practicing endocrinologists concerns the effectiveness of chromium.
Chromium increases the activity of insulin, and dietary supplementation with chromium has produced improvements in glucose metabolism which may lower blood glucose being important for overweight people with diabetes. In 1957, a compound in brewers’ yeast was found to prevent an age-related decline in the ability of rats to maintain normal levels of sugar (glucose) in their blood 6. Chromium was identified as the active ingredient in this so-called “glucose tolerance factor” in 1959. 10
Chromium is an essential nutrient required for the normal metabolism of carbohydrate, protein and fat (i.e. the chemical reactions involved in breaking down these molecules to a form suitable for absorption by the body), but more research is needed to determine the full range of its roles in the body. The challenges to meeting this goal include:
- Defining the types of individuals who respond to chromium supplementation;
- Evaluating the chromium content of foods and its bioavailability;
- Determining if a clinically relevant chromium-deficiency state exists in humans due to inadequate dietary intakes; and
- Developing valid and reliable measures of chromium status.
What affects chromium levels in the body ?
Absorption of chromium from the intestinal tract is low, ranging from less than 0.4% to 2.5% of the amount consumed 11, 12, 13, 14, 15, 16, 17 and the remainder is excreted in the feces 4, 15. Enhancing the mineral’s absorption are vitamin C (found in fruits and vegetables and their juices) and the B vitamin niacin (found in meats, poultry, fish, and grain products 18. Absorbed chromium is stored in the liver, spleen, soft tissue, and bone 19.
The body’s chromium content may be reduced under several conditions. Diets high in simple sugars (comprising more than 35% of calories) can increase chromium excretion in the urine 20. Infection, acute exercise, pregnancy and lactation, and stressful states (such as physical trauma) increase chromium losses and can lead to deficiency, especially if chromium intakes are already low 21, 22.
Chromium Supplementation
Chromium is sold as a single-ingredient supplement as well as in combination formulas, particularly those marketed for weight loss and performance enhancement. Supplement doses typically range from 50 to 200 mcg. Chromium supplements are available as chromium chloride, chromium nicotinate, chromium picolinate, high-chromium yeast, and chromium citrate. Given the limited data on chromium absorption in humans, it is not clear which forms are best to take.
Furthermore, the safety and efficacy of chromium supplements need more investigation.
Chromium is second only to calcium in most commonly used mineral supplement 23. Its use is especially popular among patients with type 2 diabetes mellitus or those attempting to lose weight 24. Many of these patients have metabolic syndrome, a cluster of metabolic abnormalities characterized by abdominal obesity, impaired fasting glucose, dyslipidemia, and elevated blood pressure 25. It is estimated that 47 million Americans have metabolic syndrome in some form 26. Importantly, metabolic syndrome is associated with a three-fold increase in the risk of developing type 2 diabetes mellitus and also significantly increases the risk of atherosclerotic cardiovascular disease 27. Insulin resistance, a central component of metabolic syndrome, has been identified as a potential therapeutic target of both dietary and pharmacologic interventions. Whereas a few studies have explored the effect of chromium supplementation on insulin sensitivity, the data remain inconclusive 28, 29. Chromium yeast treatment had no effects in patients with type 2 diabetes mellitus 30, whereas chromium picolinate supplementation in subjects with type 2 diabetes mellitus on sulphonylurea (type 2 diabetes medicine) agents significantly improves insulin resistance and glucose control 31. Supplemental trivalent chromium (Cr+3) has been shown to improve insulin sensitivity in some patients with type 2 diabetes mellitus, but its effect in patients at high risk of developing type 2 diabetes mellitus is largely unknown 32.
This randomized, double-blind, placebo-controlled, modified cross-over clinical trial with 6-month sequences of intervention and placebo followed by a 6-month post-intervention assessment by Ali et al. 33 to investigate the effects of daily chromium picolinate supplementation on serum measures of glucose tolerance and insulin sensitivity in patients at high risk for type 2 diabetes mellitus. Adult subjects who have impaired fasting glucose, impaired glucose tolerance, or metabolic syndrome were enrolled. Impaired glucose tolerance, impaired fasting glucose and metabolic syndrome are considered precursors to type 2 diabetes mellitus 34. 59 participants age from 31-88 years enrolled to receive 6-month sequences of chromium picolinate or placebo at 1 of 2 dosages (500 or 1000 mcg daily). After 6 months, the groups crossed over to the alternate assignment (i.e., groups that initially received chromium supplements then received placebo, and vice versa) for another 6 months. Because the time required for chromium to wash out of the body fully is unknown, researchers conducted a 6-month post-treatment assessment following the intervention. After 6 months of of chromium supplementation (500 mcg or 1000 mcg) and after the 6-month post-treatment assessment, there was no significant changes in glycohemoglobin (HbA1c), weight, waist circumference, BMI, blood pressure, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, or urine microalbumin compared with placebo 33. The researchers concluded that the dietary supplement chromium picolinate does not improve insulin resistance or impaired glucose tolerance in people at risk for developing type 2 diabetes 33. The researchers noted that participants’ chromium levels were not assessed upon enrollment or during the intervention, a major limitation of the study. Despite this and other limitations, they concluded that chromium is unlikely to reduce the risk of developing type 2 diabetes and suggested that endocrinologists not recommend this therapy as part of a diabetes prevention strategy.
Does giving Chromium Supplementation help with Weight Loss for Overweight or Obese people ?
Chromium picolinate (200 µg, 400 µg, 500 µg, 1000 µg) is one of several chemical compounds of chromium sold as a nutritional supplement as a potential aid to weight loss. It is generally believed that chromium may help to reduce a person’s weight by decreasing the amount of fat in the body. Chromium is also said to suppress the appetite and stimulate the production of heat by the body, thus increasing energy expenditure. This may contribute to weight loss. However, based on currently available Cochrane review 2013 35 there is inadequate information from which to draw conclusions about the efficacy and safety of chromium picolinate supplementation in overweight or obese adults.
In that review researchers independently screened titles and abstracts of published medical journals and literature as well as other sources including databases of ongoing trials, clinical trials registers 36. In order to find out if chromium picolinate works in general, the researchers also analysed the effect of all pooled chromium picolinate doses versus placebo on body weight only. Across all chromium picolinate doses investigated (200 µg, 400 µg, 500 µg, 1000 µg) they noted an effect on body weight in favour of chromium picolinate of debatable clinical relevance after 12 to 16 weeks of treatment. They found no firm evidence and no dose gradient that could be established when comparing different doses of chromium picolinate with placebo for various weight loss measures (body weight, body mass index, percentage body fat composition, change in waist circumference). The authors concluded the overall quality of evidence was considered low and they have inadequate information from which to draw conclusions about the efficacy and safety of chromium picolinate supplementation in overweight or obese adults 36.
However, in another review by Onakpoya and colleagues 37 on 11 studies showed a statistically significant difference in weight loss favouring chromium over placebo (mean difference: -0.50 kg). There was a high statistical heterogeneity. Adverse events included watery stools, vertigo, headaches and urticaria. The evidence from available randomized clinical trials shows that chromium supplementation generates statistically significant reductions in body weight. The magnitude of the effect is small, and the clinical relevance is uncertain. Future trials should last at least 16 weeks and greater uniformity in the measuring and assessment tools for body composition is recommended 37.
Some Current Issues and Controversies about Chromium Supplementation
Chromium has long been of interest for its possible connection to various health conditions. Among the most active areas of chromium research are its use in supplement form to treat diabetes, lower blood lipid levels, promote weight loss, and improve body composition.
Chromium picolinate on glucose metabolism in patients with metabolic syndrome
About 47 million Americans have some form of metabolic syndrome—a group of conditions that increase the risk of diabetes, heart disease, and related complications 26. Metabolic syndrome is often characterized by abdominal obesity, impaired fasting glucose (an increase in glucose levels), elevated blood pressure, and high cholesterol and triglycerides 38. It is also associated with insulin resistance, a condition in which the body cannot use insulin effectively. Previous studies have suggested that chromium picolinate dietary supplements can help people with type 2 diabetes by improving insulin resistance and increasing the body’s sensitivity to insulin. However, its effects on people with a high risk for developing type 2 diabetes, especially those with metabolic syndrome, are largely unknown.
A double-blind, placebo-controlled, randomized clinical trial was conducted at the University of Pennsylvania to investigate the effects of chromium picolinate on glucose metabolism in obese adults with metabolic syndrome 39. Sixty-three people, age 18 to 75, were randomly assigned to receive either a 500-ug capsule of chromium picolinate or a placebo, twice a day for 16 weeks. During the trial, the researchers looked primarily for improvement in insulin sensitivity, and also for changes in other measurements of glucose metabolism (e.g., insulin secretion), oxidative stress, fasting lipid serums (cholesterol and triglycerides), body weight, and inflammation markers. Sixty participants completed the study.
The results of this small, single-dose study 39 showed that chromium picolinate had no significant effect on insulin sensitivity, or other key features of metabolic syndrome. However, chromium picolinate did appear to increase the early phase of insulin secretion in response to glucose. The researchers concluded that their study does not support the use of chromium picolinate as a treatment for people with metabolic syndrome 39. Instead, they recommend relying on clinically proven alternatives, such as diet and exercise. The researchers also suggest studying the effect of chromium picolinate on insulin secretion in larger study samples to reproduce this finding and more fully understand its mechanism.
Chromium and Type 2 diabetes and Glucose Intolerance
In type 2 diabetes, the pancreas is usually producing enough insulin but, for unknown reasons, the body cannot use the insulin effectively. The disease typically occurs, in part, because the cells comprising muscle and other tissues become resistant to insulin’s action, especially among the obese. Insulin permits the entry of glucose into most cells, where this sugar is used for energy, stored in the liver and muscles (as glycogen), and converted to fat when present in excess. Insulin resistance leads to higher than normal levels of glucose in the blood (hyperglycemia).
Chromium deficiency impairs the body’s ability to use glucose to meet its energy needs and raises insulin requirements. It has therefore been suggested that chromium supplements might help to control type 2 diabetes or the glucose and insulin responses in persons at high risk of developing the disease. A review of randomized controlled clinical trials evaluated this hypothesis 40. This meta-analysis assessed the effects of chromium supplements on three markers of diabetes in the blood: glucose, insulin, and glycated hemoglobin (which provides a measure of long-term glucose levels; also known as hemoglobin A1C). It summarized data from 15 trials on 618 participants, of which 425 were in good health or had impaired glucose tolerance and 193 had type 2 diabetes. Chromium supplementation had no effect on glucose or insulin concentrations in subjects without diabetes nor did it reduce these levels in subjects with diabetes, except in one study. However, that study, conducted in China (in which 155 subjects with diabetes were given either 200 or 1,000 mcg/day of chromium or a placebo) might simply show the benefits of supplementation in a chromium-deficient population.
Overall, the value of chromium supplements for diabetes is inconclusive and controversial 41. Randomized controlled clinical trials in well-defined, at-risk populations where dietary intakes are known are necessary to determine the effects of chromium on markers of diabetes 40. The American Diabetes Association states that there is insufficient evidence to support the routine use of chromium to improve glycemic control in people with diabetes 42. It further notes that there is no clear scientific evidence that vitamin and mineral supplementation benefits people with diabetes who do not have underlying nutritional deficiencies.
Chromium and Lipid metabolism
The effects of chromium supplementation on blood lipid levels in humans are also inconclusive 4, 43, 44. In some studies, 150 to 1,000 mcg/day has decreased total and low-density-lipoprotein (LDL or “bad”) cholesterol and triglyceride levels and increased concentrations of apolipoprotein A (a component of high-density-lipoprotein cholesterol known as HDL or “good” cholesterol) in subjects with atherosclerosis or elevated cholesterol or among those taking a beta-blocker drug 45, 46, 47. These findings are consistent with the results of earlier studies 48, 49, 50, 51.
However, chromium supplements have shown no favorable effects on blood lipids in other studies 52, 53, 54, 55, 56, 57. The mixed research findings may be due to difficulties in determining the chromium status of subjects at the start of the trials and the researchers’ failure to control for dietary factors that influence blood lipid levels 58, 59.
Chromium and Body Weight and Composition
Chromium supplements are sometimes claimed to reduce body fat and increase lean (muscle) mass. Yet a recent review of 24 studies that examined the effects of 200 to 1,000 mcg/day of chromium (in the form of chromium picolinate) on body mass or composition found no significant benefits 60. Another recent review of randomized, controlled clinical trials did find supplements of chromium picolinate to help with weight loss when compared wtth placebos, but the differences were small and of debatable clinical relevance 61. In several studies, chromium’s effects on body weight and composition may be called into question because the researchers failed to adequately control for the participants’ food intakes. Furthermore, most studies included only a small number of subjects and were of short duration 41.
What are the health risks of too much chromium ?
Few serious adverse effects have been linked to high intakes of chromium, so the Institute of Medicine has not established a Tolerable Upper Intake Level for this mineral 59, 62. A tolerable upper intake level is the maximum daily intake of a nutrient that is unlikely to cause adverse health effects 62. It is one of the values (together with the RDA and AI) that comprise the Dietary Reference Intakes (DRIs) for each nutrient.
Several studies have demonstrated that daily doses up to 1000 mcg of chromium are safe. However this study by Onakpoya et al. 37 found adverse events included watery stools, vertigo, headaches and urticaria 37. Some in vitro evidence suggests that chromium picolinate damages chromosomes and may cause cancer 2. However, no clinical studies have shown an association. Some forms of chromium may contribute to gastointestinal irritation and ulcers. Isolated cases of impaired kidney and liver function have been reported; thus, people with pre-existing kidney or liver disorders should avoid supplementation 2. Chromium supplements interfere with iron absorption.

What foods provide chromium ?
Chromium is widely distributed in the food supply, but most foods provide only small amounts (less than 2 micrograms [mcg] per serving). Meat and whole-grain products, as well as some fruits, vegetables, and spices are relatively good sources 63. In contrast, foods high in simple sugars (like sucrose and fructose) are low in chromium 20.
Dietary intakes of chromium cannot be reliably determined because the content of the mineral in foods is substantially affected by agricultural and manufacturing processes and perhaps by contamination with chromium when the foods are analyzed 59, 63, 62. Therefore food-composition databases generally, provide approximate values of chromium in foods that should only serve as a guide.
| Food | Chromium (mcg) |
|---|---|
| Broccoli, ½ cup | 11 |
| Grape juice, 1 cup | 8 |
| English muffin, whole wheat, 1 | 4 |
| Potatoes, mashed, 1 cup | 3 |
| Garlic, dried, 1 teaspoon | 3 |
| Basil, dried, 1 tablespoon | 2 |
| Beef cubes, 3 ounces | 2 |
| Orange juice, 1 cup | 2 |
| Turkey breast, 3 ounces | 2 |
| Whole wheat bread, 2 slices | 2 |
| Red wine, 5 ounces | 1–13 |
| Apple, unpeeled, 1 medium | 1 |
| Banana, 1 medium | 1 |
| Green beans, ½ cup | 1 |
What are recommended intakes of chromium ?
In 1989, the National Academy of Sciences established an “estimated safe and adequate daily dietary intake” range for chromium. For adults and adolescents that range was 50 to 200 mcg 66. In 2001, the Dietary Reference Intakes (DRIs) for chromium were established 62. The research base was insufficient to establish the Recommended Dietary Allowance (RDA), so the Adequate Intake (AI) were developed based on average intakes of chromium from food as found in several studies.
Recommended chromium intakes are provided in the Dietary Reference Intakes (DRIs) developed by the Institute of Medicine of the National Academy of Sciences 62. Dietary Reference Intakes is the general term for a set of reference values to plan and assess the nutrient intakes of healthy people. These values include the Recommended Dietary Allowance (RDA) and the Adequate Intake (AI). The RDA is the average daily intake that meets a nutrient requirement of nearly all (97 to 98%) healthy individuals 62. An AI is established when there is insufficient research to establish an RDA; it is generally set at a level that healthy people typically consume.
Adult women in the United States consume about 23 to 29 mcg of chromium per day from food, which meets their AIs unless they’re pregnant or lactating. In contrast, adult men average 39 to 54 mcg per day, which exceeds their AIs.
The average amount of chromium in the breast milk of healthy, well-nourished mothers is 0.24 mcg per quart, so infants exclusively fed breast milk obtain about 0.2 mcg (based on an estimated consumption of 0.82 quarts per day) 62. Infant formula provides about 0.5 mcg of chromium per quart 67. No studies have compared how well infants absorb and utilize chromium from human milk and formula 59, 62.
| Age | Infants and children (mcg/day) | Males (mcg/day) | Females (mcg/day) | Pregnancy (mcg/day) | Lactation (mcg/day) |
|---|---|---|---|---|---|
| 0 to 6 months | 0.2 | ||||
| 7 to 12 months | 5.5 | ||||
| 1 to 3 years | 11 | ||||
| 4 to 8 years | 15 | ||||
| 9 to 13 years | 25 | 21 | |||
| 14 to 18 years | 35 | 24 | 29 | 44 | |
| 19 to 50 years | 35 | 25 | 30 | 45 | |
| >50 years | 30 | 20 |
mcg = micrograms
When can a chromium deficiency occur ?
In the 1960s, chromium was found to correct glucose intolerance and insulin resistance in deficient animals, two indicators that the body is failing to properly control blood-sugar levels and which are precursors of type 2 diabetes 4. However, reports of actual chromium deficiency in humans are rare. Three hospitalized patients who were fed intravenously showed signs of diabetes (including weight loss, neuropathy, and impaired glucose tolerance) until chromium was added to their feeding solution. The chromium, added at doses of 150 to 250 mcg/day for up to two weeks, corrected their diabetes symptoms 68, 69, 70. Chromium is now routinely added to intravenous solutions.
There is considerable interest in the possibility that supplemental chromium may help to treat impaired glucose tolerance and type 2 diabetes, but the research to date is inconclusive. No large, randomized, controlled clinical trials testing this hypothesis have been reported in the United States. Nevertheless, this is an active area of research.
Who may need extra chromium ?
There are reports of significant age-related decreases in the chromium concentrations of hair, sweat and blood 71, which might suggest that older people are more vulnerable to chromium depletion than younger adults 62. One cannot be sure, however, as chromium status is difficult to determine Gibson RS. Principles of Nutritional Assessment, 2nd Edition. Oxford University Press, New York, 2005.. That’s because blood, urine, and hair levels do not necessarily reflect body stores 58, 62. Furthermore, no chromium-specific enzyme or other biochemical marker has been found to reliably assess a person’s chromium status 58, 72.
There is considerable interest in the possibility that supplemental chromium may help to treat impaired glucose tolerance and type 2 diabetes, but the research to date is inconclusive. No large, randomized, controlled clinical trials testing this hypothesis have been reported in the United States 62. Nevertheless, this is an active area of research.
References- Office of Dietary Supplements – Chromium – https://ods.od.nih.gov/factsheets/Chromium-HealthProfessional/
- Merck Sharp & Dohme Corp., Merck Manual. Chromium. https://www.merckmanuals.com/professional/special-subjects/dietary-supplements/chromium
- A scientific review: the role of chromium in insulin resistance. Diabetes Educ. 2004; Suppl:2-14. https://www.ncbi.nlm.nih.gov/pubmed/15208835/
- Mertz W. Chromium occurrence and function in biological systems. Physiol Rev 1969;49:163-239.
- Mertz W. Chromium in human nutrition: a review. J Nutr 1993;123:626-33.
- Mertz W. Interaction of chromium with insulin: a progress report. Nutr Rev 1998;56:174-7.
- Supplemental-chromium effects on glucose, insulin, glucagon, and urinary chromium losses in subjects consuming controlled low-chromium diets. Anderson RA, Polansky MM, Bryden NA, Canary JJ. Am J Clin Nutr. 1991 Nov; 54(5):909-16. https://www.ncbi.nlm.nih.gov/pubmed/1951165/
- Chromium supplementation of human subjects: effects on glucose, insulin, and lipid variables. Anderson RA, Polansky MM, Bryden NA, Roginski EE, Mertz W, Glinsmann W. Metabolism. 1983 Sep; 32(9):894-9. https://www.ncbi.nlm.nih.gov/pubmed/6350814/
- Porte Jr. D, Sherwin RS, Baron A (editors). Ellengerg & Rifkin’s Diabetes Mellitus, 6th Edition. McGraw-Hill, New York, 2003.
- Schwarz K, Mertz W. Chromium(III) and the glucose tolerance factor. Arch Biochem Biophys 1959;85:292-5.
- Doisy RJ, Streeten DHP, Souma ML, Kalafer ME, Rekant SL, Dalakos TG. Metabolism of 51chromium in human subjects. In: Newer Trace Elements in Nutrition (edited by Mertz W, Cornatzer WE). Dekker, New York, 1971, pp. 155-68.
- Anderson RA, Polansky MM, Bryden NA, Patterson KY, Veillon C, Glinsmann WH. Effects of chromium supplementation on urinary Cr excretion of human subjects and correlation of Cr excretion with selected clinical parameters. J Nutr 1983;113:276-81.
- Bunker VW, Lawson MS, Delves HT, Clayton BE. The uptake and excretion of chromium by the elderly. Am J Clin Nutr 1984;39:797-802.
- Anderson RA, Kolovsky AS. Chromium intake, absorption and excretion of subjects consuming self-selected diets. Am J Clin Nutr 1985;41:1177-83.
- Offenbacher EG, Spencer H, Dowling HJ, Pi-Sunyer FX. Metabolic chromium balances in men. Am J Clin Nutr 1986;44:77-82.
- Anderson RA, Polansky MM, Bryden NA, Canary JJ. Supplemental-chromium effects on glucose, insulin, glucagon, and urinary chromium losses in subjects consuming controlled low-chromium diets. Am J Clin Nutr 1991;54:909-16.
- Anderson RA, Bryden NA, Patterson KY, Veillon C, Andon MB, Moser-Veillon PB. Breast milk chromium and its association with chromium intake, chromium excretion, and serum chromium. Am J Clin Nutr 1993;57:419-23.
- Offenbacher E. Promotion of chromium absorption by ascorbic acid. Trace Elem Elect 1994;11:178-81.
- Lim TH, Sargent T 3rd, Kusubov N. Kinetics of trace element chromium(III) in the human body. Am J Physiol 1983;244:R445-54.
- Kozlovsky AS, Moser PB, Reiser S, Anderson RA. Effects of diets high in simple sugars on urinary chromium losses. Metabolism 1986;35:515-8.
- Anderson R. Stress Effects on Chromium Nutrition in Humans and Animals, 10th Edition. Nottingham University Press, England, 1994.
- Lukaski HC, Bolonchuk WW, Siders WA, Milne DB. Chromium supplementation and resistance training: effects on body composition, strength and trace element status of men. Am J Clin Nutr 1996;63:954-65.
- NBJ’s Supplement Business Report 2005. Nutrition Business Journal. San Diego, CA: Penton Media; 2005.
- Natural products used for diabetes. Shapiro K, Gong WC. J Am Pharm Assoc (Wash). 2002 Mar-Apr; 42(2):217-26. https://www.ncbi.nlm.nih.gov/pubmed/11926665/
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001 May 16; 285(19):2486-97. https://www.ncbi.nlm.nih.gov/pubmed/11368702/
- Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Ford ES, Giles WH, Dietz WH. JAMA. 2002 Jan 16; 287(3):356-9. https://www.ncbi.nlm.nih.gov/pubmed/11790215/
- Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Ford ES. Diabetes Care. 2005 Jul; 28(7):1769-78. https://www.ncbi.nlm.nih.gov/pubmed/15983333/
- Role of chromium in human health and in diabetes. Cefalu WT, Hu FB. Diabetes Care. 2004 Nov; 27(11):2741-51. https://www.ncbi.nlm.nih.gov/pubmed/15505017/
- Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Diabetes Care. 2007 Aug; 30(8):2154-63. https://www.ncbi.nlm.nih.gov/pubmed/17519436/
- Chromium treatment has no effect in patients with type 2 diabetes in a Western population: a randomized, double-blind, placebo-controlled trial. Kleefstra N, Houweling ST, Bakker SJ, Verhoeven S, Gans RO, Meyboom-de Jong B, Bilo HJ. Diabetes Care. 2007 May; 30(5):1092-6. http://care.diabetesjournals.org/content/30/5/1092.long
- Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Martin J, Wang ZQ, Zhang XH, Wachtel D, Volaufova J, Matthews DE, Cefalu WT. Diabetes Care. 2006 Aug; 29(8):1826-32. https://www.ncbi.nlm.nih.gov/pubmed/16873787/
- Glucose and insulin responses to dietary chromium supplements: a meta-analysis. Althuis MD, Jordan NE, Ludington EA, Wittes JT. Am J Clin Nutr. 2002 Jul; 76(1):148-55. https://www.ncbi.nlm.nih.gov/pubmed/12081828/
- Ali A, Ma Y, Reynolds J, Wise JP, Inzucchi SE, Katz DL. Chromium effects on glucose tolerance and insulin sensitivity in persons at risk for diabetes mellitus. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2011;17(1):16-25. doi:10.4158/EP10131.OR. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3118091/
- Diagnosis and classification of diabetes mellitus. American Diabetes Association. Diabetes Care. 2009 Jan; 32 Suppl 1:S62-7. https://www.ncbi.nlm.nih.gov/pubmed/19118289/
- Cochrane Review 29 November 2013 – Chromium picolinate supplementation for overweight or obese people – http://www.cochrane.org/CD010063/ENDOC_chromium_picolinate_supplementation_for_overweight_or_obese_people
- Tian H, Guo X, Wang X, He Z, Sun R, Ge S, Zhang Z. Cochrane Database Syst Rev. 2013 Nov 29;(11):CD010063. doi: 10.1002/14651858.CD010063.pub2. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010063.pub2/abstract
- Onakpoya I, Posadzki P, Ernst E. Obes Rev. 2013 Jun;14(6):496-507. doi: 10.1111/obr.12026. Epub 2013 Mar 18. Chromium supplementation in overweight and obesity: a systematic review and meta-analysis of randomized clinical trials. http://onlinelibrary.wiley.com/doi/10.1111/obr.12026/abstract
- National Institutes of Health, National Heart, Lung and Blood Institute. What Is Metabolic Syndrome ? https://www.nhlbi.nih.gov/health/health-topics/topics/ms/
- Iqbal N, Cardillo S, Volger S, et al. Chromium Picolinate Does Not Improve Key Features of Metabolic Syndrome in Obese Nondiabetic Adults. Metabolic Syndrome and Related Disorders. 2009;7(2):143-150. doi:10.1089/met.2008.0048. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3135886/
- Althuis MD, Jordan NE, Ludington EA, Wittes JT. Glucose and insulin responses to dietary chromium supplements: a meta-analysis. Am J Clin Nutr 2002;76:148-55.
- Cefalu WT, Hu FB. Role of chromium in human health and in diabetes. Diabetes Care 2004;27:2741-51.
- Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL, Urbanski P, Yancy WS Jr. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013;36:3821-42. https://www.ncbi.nlm.nih.gov/pubmed/24107659?dopt=Abstract
- Anderson R. Chromium. In: Trace Elements in Human and Animal Nutrition (edited by Mertz M). Academic Press, San Diego, CA, 1987, pp. 225-244.
- Offenbacher E, Pi-Sunyer F. Chromium. In: Handbook of Nutritionally Essential Mineral Elements (edited by O’Dell B, Sunde R). Marcel Dekker, New York, 1997, pp. 389-411.
- Roeback Jr. JR, Hla KM, Chambless LE, Fletcher RH. Effects of chromium supplementation on serum high-density lipoprotein cholesterol levels in men taking beta-blockers. A randomized, controlled trial. Ann Intern Med 1991;115:917-24.
- Abraham AS, Brooks BA, Eylath U. The effects of chromium supplementation on serum glucose and lipids in patients with and without non-insulin-dependent diabetes. Metabolism 1992;41:768-71.
- Hermann J, Arquitt A. Effect of chromium supplementation on plasma lipids, apolipoproteins, and glucose in elderly subjects. Nutr Res 1994;14: 671-4.
- Doisy RJ, Streeten DHP, Freiberg JM, Schneider AJ. Chromium metabolism in man and biochemical effects. In: Trace Elements in Human Health and Disease, Volume 2: Essential and Toxic Elements (edited by Prasad A, Oberleas D). Academic Press, New York, 1976, pp. 79-104.
- Lifschitz ML, Wallach S, Peabody RA, Verch RL, Agrawal R. Radiochromium distribution in thyroid and parathyroid deficiency. Am J Clin Nutr 1980:33:57-62.
- Riales R, Albrink MJ. Effect of chromium chloride supplementation on glucose tolerance and serum lipids including high-density lipoprotein of adult men. Am J Clin Nutr 1981;34:2670-8.
- Mossop RT. Effects of chromium III on fasting blood glucose, cholesterol and cholesterol HDL levels in diabetics. Cent Afr J Med 1983;29:80-2.
- Anderson RA, Polansky MM, Bryden NA, Roginski EE, Mertz W, Glinsmann W. Chromium supplementation of human subjects: effects on glucose, insulin, and lipid variables. Metabolism 1983;32:894-9.
- Rabinowitz MB, Gonick HC, Levin SR, Davidson MB. Effects of chromium and yeast supplements on carbohydrate and lipid metabolism in diabetic men. Diabetes Care 1983;6:319-27.
- Uusitupa MI, Kumpulainen JT, Voutilainen E, Hersio K, Sarlund H, Pyorala KP, Koivistoinen PE, Lehto JT. Effect of inorganic chromium supplementation on glucose tolerance, insulin response, and serum lipids in noninsulin-dependent diabetics. Am J Clin Nutr 1983;38:404-10.
- Offenbacher EG, Rinko CJ, Pi-Sunyer FX. The effects of inorganic chromium and brewer’s yeast on glucose tolerance, plasma lipids, and plasma chromium in elderly subjects. Am J Clin Nutr 1985;42:454-61.
- Potter JF, Levin P, Anderson RA, Freiberg JM, Andres R, Elahi D. Glucose metabolism in glucose-intolerant older people during chromium supplementation. Metabolism 1985;34:199-204.
- Uusitupa MI, Mykkanen L, Siitonen O, Laakso M, Sarlund H, Kolehmainen P, Rasanen T, Kumpulainen J, Pyorala K. Chromium supplementation in impaired glucose tolerance of elderly: effects on blood glucose, plasma insulin, C-peptide and lipid levels. Br J Nutr 1992;68:209-16.
- Lukaski HC. Chromium as a supplement. Annu Rev Nutr 1999;19:279-302.
- Stoecker BJ. Chromium. In: Present Knowledge in Nutrition, 8th Edition (edited by Bowman B, Russell R). ILSI Press, Washington, DC, 2001, pp. 366-372.
- Vincent JB. The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent. Sports Med 2003;33:213-30.
- Pittler MH, Stevinson C, Ernst E. Chromium picolinate for reducing body weight: meta-analysis of randomized trials. Int J Obes Relat Metab Disord 2003;27:522-9.
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press, Washington, DC, 2001. https://www.nap.edu/read/10026/chapter/1
- Anderson RA, Bryden NA, Polansky MM. Dietary chromium intake: freely chosen diets, institutional diets and individual foods. Biol Trace Elem Res 1992;32:117-21.
- Cabrera-Vique C, Teissedre P-L, Cabanis M-T, Cabinis J-C. Determination and levels of chromium in French wine and grapes by graphite furnace atomic absorption spectrometry. J Agric Food Chem 1997;45:1808-11.
- Dattilo AM, Miguel SG. Chromium in health and disease. Nutr Today 2003;38:121-33.
- National Research Council, Food and Nutrition Board. Recommended Dietary Allowances, 10th Edition. National Academy Press, Washington, DC, 1989.
- Cocho JA, Cervilla JR, Rey-Goldar ML, Fdez-Lorenzo JR, Fraga JM. Chromium content in human milk, cow’s milk, and infant formulas. Biol Trace Elem Res 1992;32:105-7.
- Jeejeebhoy KN, Chu RC, Marliss EB, Greenberg GR, Bruce-Robertson A. Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation in a patient receiving long-term total parenteral nutrition. Am J Clin Nutr 1977;30:531-8.
- Freund H, Atamian S, Fischer JE. Chromium deficiency during total parenteral nutrition. JAMA 1979;241:496-8.
- Brown RO, Forloines-Lynn S, Cross RE, Heizer WD. Chromium deficiency after long-term total parenteral nutrition. Dig Dis Sci 1986;31:661-4.
- Davies S, Howard JM, Hunnisett A, Howard M. Age-related decreases in chromium levels in 51,665 hair, sweat, and serum samples from 40,872 patients — implications for the prevention of cardiovascular disease and type II diabetes mellitus. Metabolism 1997;46:469-73.
- Stoecker BJ. Chromium. In: Modern Nutrition in Health and Disease, 9th Edition (edited by Shils ME, Olson JA, Shike M, Ross AC.) Lippincott Williams and Wilkins, New York, 1999, pp. 277-282.