Sweet potato
Sweet potato also known as Ipomoea batatas is a delicious vegetable of the member of the Convolvulaceae family; sweet potato is a dicotyledonous perennial plant found in the tropical and subtropical belts 1. Most sweet potato varieties are edible, and all parts of the plants – shoots, leaves, vine and tubers – are consumed 2. The edible tuberous root is long and tapered, with a smooth skin whose color ranges between yellow, orange, red, brown, purple, and beige. Sweet potato flesh ranges from beige through white, red, pink, violet, yellow, orange, and purple. Sweet potato cultivars with white or pale yellow flesh are less sweet and moist than those with red, pink or orange flesh 3. The large, sweet-tasting tuberous roots, young leaves and shoots are common market vegetables. In fact, sweet potato (Ipomoea batata) is the seventh most produced crop worldwide after wheat, rice, maize, potato, barley, and cassava, and the fifth in developing countries 4. Although the soft, orange sweet potato is often called a “yam” in parts of North America, the sweet potato is botanically very distinct from a genuine yam (Dioscorea), which is native to Africa and Asia and belongs to the monocot family Dioscoreaceae 5. A true yam is a starchy edible root of the Dioscorea genus, and is generally imported to America from the Caribbean. It is rough and scaly and very low in beta carotene 6.
The center of origin of sweet potato is Central America, but the crop is widely grown in many tropical and subtropical countries. Sweet potatoes are ranked seventh in world staple food production (expressed on a dry matter basis), after wheat, maize, rice, potato, barley and cassava. The crop is particularly important in South-East Asia, Oceania and Latin America. China still accounts for over 90% of total production; the other major sweet potato producing countries in Asia are: Indonesia, India, Japan, Vietnam, The Philippines and The Republic of Korea. Rwanda and Uganda are Africa’s largest producing countries. Sweet potato production in Latin America and The Caribbean is relatively small.
Sweet potato is rich in nutrients and ranked highest in nutritional value amongst vegetables available in the United States of America 7. Among the important nutrients found in sweet potato tubers are monosaccharides 8, complex carbohydrates 9, dietary fiber, and protein, in addition to an extensive range of micronutrients, including beta-carotene (a source of vitamin A) 10, vitamin C, vitamin B6, vitamin E, anthocyanins (purple sweet potatoes) 11, minerals (manganese, copper, potassium, and iron) 12, flavonoids, and coumarins 13.
Compared to other root and tuber crops, the sweet potato contains more carbohydrates and proteins, as well as certain vitamins and minerals 14 and it has higher levels of provitamin A (as carotenoids), vitamin C, and minerals than wheat or rice 15.
The color of sweet potato is linked to its beneficial health effects 16. Lighter fleshed sweet potato varieties are reported to have higher levels of phenolic compounds, whereas a more intense yellow color is associated with a higher content of carotenoids, mainly ß-carotene 17. Additionally, yellow- and orange-fleshed sweet potatoes are rich in phenolic acids, while those varieties that are purple have very high levels of anthocyanins 18.
The leaves of sweet potato are rich in antioxidants, protein, fiber, fat, vitamins, and minerals 19, 12. Linoleic and alpha-linolenic acids 20, galactolipids 21, and bioactive substances (e.g. dietary antioxidants, including anthocyanins 22, polyphenols 23, flavonoids 24, and caffeic acid derivatives 25 are also present. These compounds have been extensively investigated for their role in health promotion in many countries.
Many studies on functional compounds of sweet potato leaves indicate that their health benefits are related to high levels of polyphenols, flavonoids, and carotenoids 26. These compounds exhibit various bioactivities, such as antioxidant 27, 28, 29, anti-cancer 30, 31, 32, anti-mutagenic activities 33, immune modulation 34, and liver-protection (see Table 3 below) 35.
Figure 1. Sweet potato plant

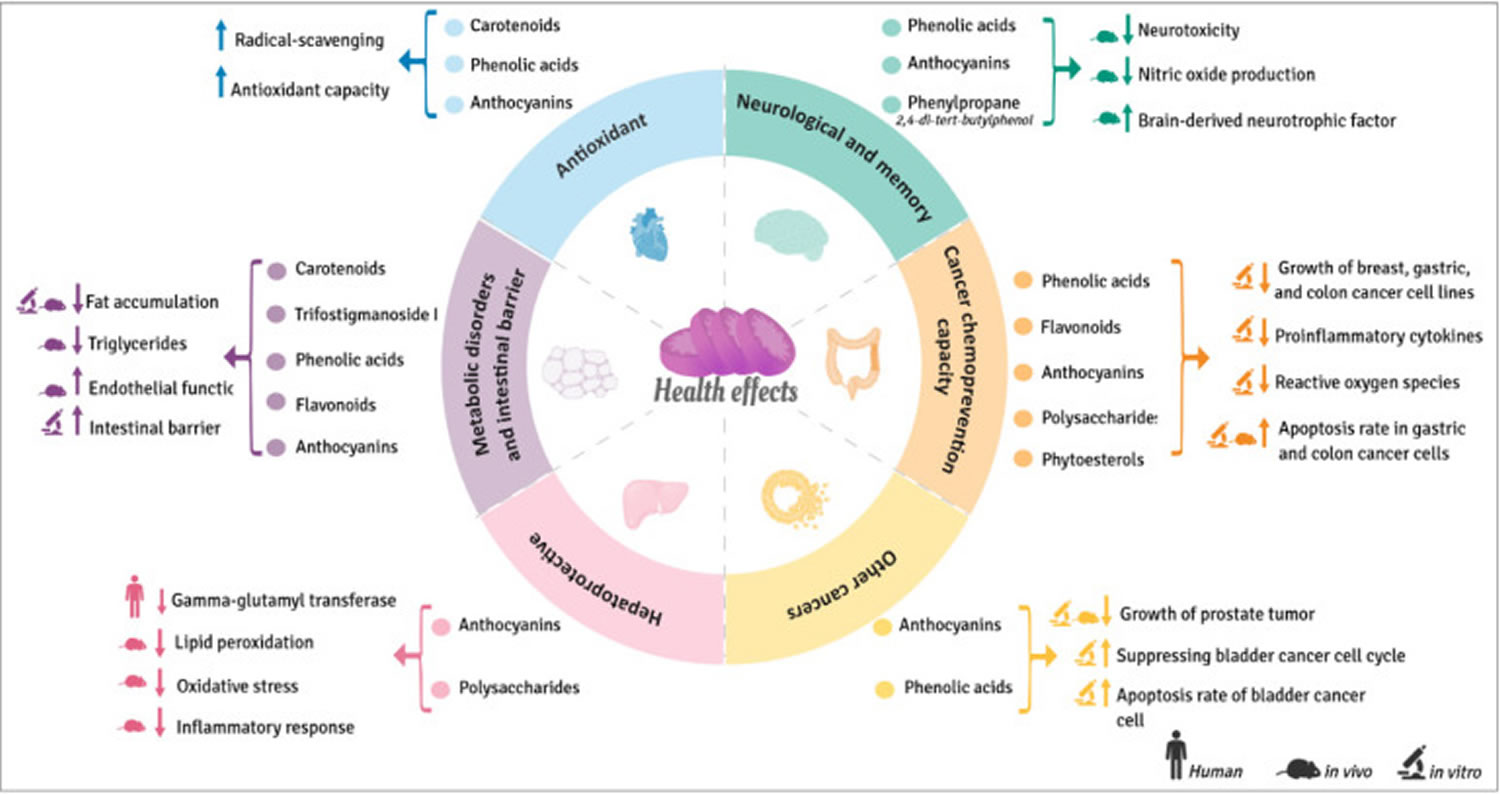
Figure 2. Sweet potato health benefits (beneficial health effects of bioactive compounds from sweet potatoes)
What is anthocyanin?
Anthocyanins are members of the flavonoid group of phytochemicals, a group predominant in teas, honey, wines, fruits, vegetables, nuts, olive oil, cocoa, and cereals 37. Anthocyanins occur ubiquitously in the plant kingdom and confer the bright red, blue and purple colors to fruits and vegetables such as purple sweet potato, berries, grapes, apples, purple cabbage and corn 38. Total anthocyanin concentrations are higher in purple sweet potato varieties than those with orange flesh, with reported values of 14–182 mg/100 g fresh weight 39, 40. More than 20 anthocyanins have been identified in sweet potatoes 41, 42, 43. The main ones in purple varieties are 3-sophoroside-5-glucoside derivatives of peonidin, cyanidin, and pelargonidin aglycones, almost all of them mono- or di-acylated with p-hydroxybenzoic acid, ferulic acid, or caffeic acid 44, 45, 46. According to Fossen et al. 47, these acylated forms represent more than 98% of the total anthocyanin content in purple sweet potato. However, the anthocyanin composition differs widely between varieties. Thus, Im et al 48, reported that ‘Sinjami’, ‘Danjami’, and ‘Yeonjami’ contained 72–77% di-acylated anthocyanins compared to 90–95% in the Korean varieties ‘Jami’ and ‘Borami’. Conversely, a higher proportion of mono-acylated anthocyanins (21–24%) was found in ‘Sinjami’, ‘Danjami’, and ‘Yeonjami’ compared to ‘Jami’ and ‘Borami’. Acylation with the phenolic acids p-coumaric, ferulic, or caffeic acids enhances the stability of anthocyanins in conditions of heat, pH, and ultraviolet radiation, which facilitates their application in the food industry as natural colorants 49. Compared to non- and di-acylated anthocyanins, mono-acylated forms have a higher resistance to heat, especially those derived from cyanidin 3- -hydroxybenzoylsophoroside-5-glucoside 42. Therefore, these acylated forms could contribute to the higher antioxidant activity of purple sweet potatoes compared to those of other colours 42.
Epidemiologic studies suggest that the consumption of anthocyanins lowers the risk of cardiovascular disease, diabetes, arthritis and cancer due, at least in part, to their anti-oxidant and anti-inflammatory activities 50. The daily intake of anthocyanins in residents of the United States is is estimated to be between 180 and 215 mg or about 9-fold higher than that of other dietary flavonoids 38.
In both in vitro and in vivo research trials, anthocyanins have demonstrated marked ability to reduce cancer cell proliferation and to inhibit tumor formation 51, 52, 53, 54. The capacity of anthocyanin pigments to interfere with the process of carcinogenesis seems to be linked to multiple potential mechanisms of action including inhibition of cyclooxygenase enzymes and potent antioxidant potential. Hou et al. 55 revealed that anthocyanins inhibit tumorigenesis by blocking activation of a mitogen-activated protein kinase pathway. This report provided the first indication of a molecular basis for why anthocyanins demonstrate anticarcinogenic properties. In other research, fruit extracts with significant anthocyanin concentrations proved to be effective against various stages of carcinogenesis 56, 52, 57, 58, but the individual role of anthocyanins versus other components was not determined, in part because the anthocyanins were too easily degraded during bioassays if separated from stabilizing cofactors such as other phenolic constituents 59. In vivo studies have shown that dietary anthocyanins inhibit cancers of the gastrointestinal tract and topically applied anthocyanins inhibit skin cancer. Although experimental studies have clearly demonstrated the anti-cancer activity of anthocyanins, epidemiological studies have not revealed protective effects of anthocyanin consumption on cancer risk in humans, and their antioxidant activity in humans remains questionable 38.
The roles of anthocyanin pigments as medicinal agents have been well-accepted dogma in folk medicine throughout the world, and, in fact, these pigments are linked to an amazingly broad-based range of health benefits. For example, visual acuity can be markedly improved through administration of anthocyanin pigments to animal and human subjects, and the role of these pigments in enhancing night vision or overall vision has been particularly well documented 60. Oral intake of anthocyanosides from black currants resulted in significantly improved night vision adaptation in human subjects 61, and similar benefits were gained after administration of anthocyanins from bilberries 62. Three anthocyanins from black currant stimulated regeneration of rhodopsin (a G-protein-coupled receptor localized in the retina of the eye), and formation of a regeneration intermediate was accelerated by cyanidin 3-rutinoside 63. These studies strongly suggest that enhancement of rhodopsin regeneration is at least one mechanism by which anthocyanins enhance visual acuity.
Sweet potato nutrition facts
Besides simple starches, raw sweet potatoes are rich in complex carbohydrates, are a good source of potassium, dietary fiber and is a rich source of beta-carotene (a provitamin A carotenoid). In a 100 gram amount, raw sweet potato provides 88 calories and is a rich source of beta-carotene (a provitamin A carotenoid) that meets 100% of women and 80% of men Recommended Dietary Allowance 64. Vitamin A deficiency can erode the immune system and contribute to malnutrition, most seriously affecting pregnant women and young children in low-income countries. Somalia, like many developing countries, has a high prevalence of Vitamin A deficiency and malnutrition among its population. The use of orange-fleshed sweet potatoes – containing high levels of Vitamin A precursor – as animal fodder and food for humans has been successfully tested in southern Somalia 65.
While having moderate contents of other micronutrients, including vitamin B5, vitamin B6 and manganese (Table 1. Sweet potato nutrition facts) 66.
Sweet potato is 79% water, 20% carbohydrates, 1.6% protein, 3% dietary fiber and contains negligible amount of fat.
When cooked by baking, small variable changes in micronutrient density occur to include a higher content of vitamin C at 24% of the Daily Value per 100 g serving 67, 68.
The Center for Science in the Public Interest ranked the nutritional value of sweet potatoes as highest among several other foods 69.
Sweet potato cultivars with dark orange flesh have more beta-carotene than those with light-colored flesh, and their increased cultivation is being encouraged in Africa where vitamin A deficiency is a serious health problem. A 2012 study of 10,000 households in Uganda found that children eating beta-carotene enriched sweet potatoes suffered less vitamin A deficiency than those not consuming as much beta-carotene 70.
Table 1. Sweet potato (raw with skin) nutrition facts
| Nutrient | Unit | Sweet potato 130 g | Value per 100 g | |
|---|---|---|---|---|
| Approximates | ||||
| Water | g | 102.78 | 79.06 | |
| Energy | kcal | 114 | 88 | |
| Protein | g | 2.09 | 1.61 | |
| Total lipid (fat) | g | 0.07 | 0.05 | |
| Carbohydrate, by difference | g | 26.75 | 20.58 | |
| Fiber, total dietary | g | 4 | 3.1 | |
| Sugars, total | g | 5.56 | 4.28 | |
| Minerals | ||||
| Calcium, Ca | mg | 40 | 31 | |
| Iron, Fe | mg | 0.81 | 0.62 | |
| Magnesium, Mg | mg | 32 | 25 | |
| Phosphorus, P | mg | 62 | 48 | |
| Potassium, K | mg | 448 | 345 | |
| Sodium, Na | mg | 73 | 56 | |
| Zinc, Zn | mg | 0.4 | 0.31 | |
| Vitamins | ||||
| Vitamin C, total ascorbic acid | mg | 3.2 | 2.5 | |
| Thiamin | mg | 0 | 0 | |
| Riboflavin | mg | 0.081 | 0.062 | |
| Niacin | mg | 0.741 | 0.57 | |
| Vitamin B-6 | mg | 0.278 | 0.214 | |
| Folate, DFE | µg | 16 | 12 | |
| Vitamin B-12 | µg | 0 | 0 | |
| Vitamin A, RAE | µg | 942 | 725 | |
| Vitamin A, IU | IU | 18870 | 14515 | |
| Vitamin D (D2 + D3) | µg | 0 | 0 | |
| Vitamin D | IU | 0 | 0 | |
| Vitamin K (phylloquinone) | µg | 2.3 | 1.8 | |
| Lipids | ||||
| Fatty acids, total saturated | g | 0.026 | 0.02 | |
| Fatty acids, total monounsaturated | g | 0 | 0 | |
| Fatty acids, total polyunsaturated | g | 0.013 | 0.01 | |
| Fatty acids, total trans | g | 0 | 0 | |
| Cholesterol | mg | 0 | 0 | |
| Other | ||||
| Caffeine | mg | 0 | 0 | |
Table 2. Sweet potato leaves mineral and vitamin compositions
| Elements | Quantity (mg/100 g dry weight) |
|---|---|
| Sodium (Na) | 8.06−832.31 |
| Magnesium (Mg) | 220.2−910.5 |
| Phosphorus (P) | 131.1−2639.8 |
| Calcium (Ca) | 229.7−1958.1 |
| Potassium (K) | 479.3−4280.6 |
| Copper (Cu) | 0.7−1.9 |
| Zinc (Zn) | 1.2−3.2 |
| Manganese (Mn) | 1.7−10.9 |
| Iron (Fe) | 1.9−21.8 |
| Niacine (vitamin B3) | 0.856−1.498 |
| Vitamin B6 | 0.12−0.329 |
| Vitamin B2 | 0.248−0.254 |
| Vitamin B1 | 0.053−0.128 |
| Vitamin C | 0.0627−0.081 |
| Vitamin E | 0.00139−0.00284 |
| Pantothenic acid (Vitamin B5) | 0.32−0.66 |
| Beta-carotene | 0.273−0.4 |
| Biotin | 0.003−0.008 |
Benefits of sweet potato
Traditionally, sweet potatoes have been used as an important source of carbohydrates and energy for both human beings and livestock because of their high content of starch 71. Nowadays, sweet potatoes are recognized as a highly nutritious and useful food for the prevention of chronic diseases, mainly due to its content of dietary fiber, naturally occurring sugars, protein content, vitamins A and C, potassium, iron, and calcium, and its low amount of fat (mainly saturated fat), sodium, and cholesterol 72. Sweet potatoes are a good source of several bioactive compounds, above all polyphenols, terpenoids, tannins, saponins, glycosides, alkaloids, and phytosterols 36. The diversity of skin and flesh color in this root vegetable arises from the different levels of polyphenols and carotenoids 73. Purple sweet potato, a special sweet potato cultivar, has been extensively investigated because large amounts of anthocyanin accumulate in its tuberous roots. Anthocyanin is well known for its free radical-scavenging activity and beneficial effects on human health. Its biosynthetic pathway has been well characterized in model plants. Although large-scale systematic studies have been performed to identify the anthocyanin proteins present in sweet potato, information on the regulation of anthocyanin synthesis in sweet potato is insufficient 74. In yellow and orange-fleshed sweet potatoes, the dominant pigments are phenolic acids, flavonoids, and carotenoids 75. In addition to genetics, the concentration and bioavailability of bioactive compounds in sweet potatoes and derived products are affected by external factors such as environment and cultivar, storage conditions, and processing; moreover, the available data can be influenced by the extraction and analytical methods 76.
As well as being popular in cooking in countries in Asia-Pacific, Africa and North America, sweet potato is also used in traditional medicine for the treatment of diabetes mellitus. Research in animal and human models suggests a possible role of sweet potato in glycaemic control 77.
Antioxidant properties
The potent antioxidant capacity of purple sweet potatoes is mainly attributed to their anthocyanin and carotenoid content. These compounds exhibit free radical scavenging activity, and consequently, the consumption of anthocyanin-rich products is associated with a lower risk of diabetes cardiovascular disease, cancer, and cognitive performance 78. Anthocyanins from purple sweet potato showed stronger 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging capacity than those from red cabbage, grape skin, elderberry, or purple corn, and some were also more active than either vitamin C or vitamin E 79.
The overall antioxidant capacity of white-fleshed cultivars was attributed to their high content of phenolic acids and carotenoids, and it was concluded that their consumption might protect the human body from oxidative stress 80. Rather than anthocyanins, chlorogenic acid was the main 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenger in extracts of purple-fleshed varieties “Miyanou-36” and “Bise” 81 On the other hand, in yellow or orange sweet potatoes, the carotenoid β-carotene, which is the main pigment, has a strong antioxidant capacity due to its conjugated double bonds 82 and acts as a source of provitamin A 17.
Anti-cancer activity
Most of the studies on protective cancer effects derived from sweet potato consumption have been carried out using in-vitro and animal models. Further research is needed in this field, especially in randomized clinical trials in humans.
Extracts of purple-fleshed sweet potato Tainung 73 (PFSP TNG 73) are reported to have in vitro anti-inflammatory and anticancer activities, attributed to a high content of antioxidative compounds, including phenolics, flavonoids, and the pigment anthocyanin 83. Anthocyanin-rich extracts of PFSP TNG 73 suppressed the production of nitric oxide and some proinflammatory cytokines, such as NF𝜅-𝛽, TNF-𝛼, and IL-6, in macrophage cells stimulated by lipopolysaccharides. They also inhibited the growth of some in vitro cancer cell lines, including human breast cancer (MCF-7), gastric cancer (SNU-1), and colon adenocarcinoma (WiDr), in a concentration- and time-dependent manner. Additionally, PFSP TNG 73 extracts induced apoptosis in MFC-7 cells. Thus, this variety of sweet potato is a source of metabolites with potential application in the development of drugs, nutritional foods, and health supplements.
Purple sweet potatoes also contain polysaccharides with promising antitumor properties. Three beta-type polysaccharides, PSPP1-1, PSPP2-1, and PSPP3, with low amounts of proteins and uronic acids, were isolated from crude purple sweet potato polysaccharides. In an in vitro antitumor assay, PSPP1-1 exhibited strong activities against gastric cancer SGC7901 cells and colon cancer SW620 cells, whereas PSPP2-1 and PSPP3-1 had moderate activities. Furthermore, PSPP1-1 was found to induce apoptosis in both types of cancer cells 16.
Breast cancer
Breast cancer is a prominent cause of mortality in women throughout the world. In a study by Kato et al. 84 using E0771 murine breast cancer cells, lipid-soluble polyphenols (mainly caffeic acid derivatives) from fermented sweet potato were found to accumulate in cell cytoplasm due to their high lipophilicity and reduce ROS through their strong antioxidant activity. These metabolites also arrested the cell cycle at G0/G1 by suppressing Akt activity and enhancing the cytotoxicity of anti-cancer agents. Thus, lipid-soluble polyphenols from sweet potatoes inhibited tumor growth and improved the efficacy of chemotherapy drugs, suggesting they have application as a functional food to support cancer therapy.
Another study revealed that three phytosterols from sweet potato, daucosterol linolenate (DLA), daucosterol linoleate (DL), and daucosterol palmitate (DP), had a stronger inhibitory effect against the MCF-7 than the MDA-MB-231 breast cancer cell line, and had no impact on non-tumorigenic MCF-10A cells 85. In vivo experiments demonstrated that daucosterol linolenate (DLA), daucosterol linoleate (DL), and daucosterol palmitate (DP) suppressed MCF-7 xenografts in nude mice. In another study, sitosterol-d-glucoside (β-SDG), a recently isolated phytosterol from sweet potato, also displayed potent anticancer activity against MCF7 and MDA-MB-231 cell lines and suppressed the growth of MCF7 xenografts in nude mice 86. This effect of β-SDG was due to the up-regulation of miR-10a expression and inactivation of the PI3K–Akt signaling pathway.
A component of the new sweet potato variety Zhongshu NO. 1, the glycoprotein SPG-56, was reported to inhibit proliferation and promote apoptosis of MCF-7 cells in mice in a dose- and time-dependent manner 87. The serum tumor markers CEA, CA125, and CA153 were reduced by 54.8%, 91.8%, and 90.3%, respectively, in mice orally administered 240 mg/kg/d of SPG-56, with a significant difference compared with the untreated control 87. The inhibitory effect of SPG-56 against MCF-7 cells was found to be mediated by the altered expression of specific genes. It was concluded that SPG-56 merits further research as a novel anti-tumor agent for breast cancer treatment.
Colon cancer
Colon cancer is responsible for a high proportion of cancer mortality throughout the world. A new small molecule, glycoprotein SPG-8700, isolated from Zhongshu-1 sweet potatoes 88 was found to promote apoptosis in HCT-116 colon cancer cells by regulating the expression of Bcl-2 and Bax genes, with no effect on normal cell growth. Sporamin, another molecule isolated from sweet potato, has promising effects against colorectal cancer in vitro and in vivo 89. This proteinase inhibitor was able to modify the gene expression profile of colon cancer cells, up-regulating genes involved in the homeostasis of intracellular metal ions and the activities of essential enzymes and DNA damage repair.
A study by Lim et al. 90 on the anthocyanin-enriched sweet potato (clone P40) found it offered protection against colorectal cancer by inducing cell cycle arrest, inhibiting proliferation, and apoptotic mechanisms. The anticancer activity of this clone was demonstrated in both in vitro cell culture and an in vivo animal model. Treatment of human colon SW480 cancer cells with a P40 anthocyanin extract resulted in a dose–dependent inhibition of cell proliferation due to a cytostatic but not cytotoxic mechanism.
Other cancers
Phenolic phytochemicals present in fruits and vegetables indisputably confer anticancer benefits upon regular consumption. The protective effect of purple sweet potato anthocyanin and polyphenol-rich sweet potato extract in other types of cancer has been assessed in studies with cell culture and in vivo.
purple sweet potato anthocyanin has been shown to have in vitro antitumor effects in bladder cancer, a common malignant disease. Li et al. 91 reported that purple sweet potato anthocyanin reduced bladder cancer cell viability in a dose-dependent manner, increasing the apoptosis rate and suppressing the cell cycle. The mechanism of action underlying the anticancer effects of purple sweet potato anthocyanin includes upregulation of pro-apoptosis genes and a lower expression of the anti-apoptotic gene Bcl-2. Thus, the results of this study provide new insights into the treatment of bladder cancer and the potential role that purple sweet potato anthocyanin plays in cancer prevention.
The effect of purple sweet potato anthocyanin on the bladder cancer cell line BIU87 was also investigated 91. Compared with the control, the proliferation of BIU87 cells was significantly inhibited in groups treated with this phenolic compound, which induced cell apoptosis in a dose-dependent manner.
The growth-inhibitory and apoptosis-inducing properties of polyphenol-rich sweet potato extract were recently demonstrated in cell culture and in vivo prostate cancer xenograft models 92. Thus, the extract is a candidate for use as a dietary supplement for prostate cancer management. Despite the growth and apoptosis inhibitory properties of phenolic compounds, future studies should be carried out in humans to support the findings detected in vitro.
Liver protective effects
To date, most of the studies on liver protective effects have been carried out using animal models and only one study has been carried out in human studies. In addition, it should be noted that all these studies have focused on evaluating the protective effect of anthocyanins from sweet potatoes.
The anthocyanins offer protection from injury induced by hepatotoxins mainly by inhibition of lipid peroxidation and scavenging free radicals. In healthy men with borderline hepatitis, purple sweet potato beverages significantly decreased the serum levels of some liver enzymes, particularly gamma-glutamyl transferase (GGT) 93.
In animal models, a protective effect against CCl4-induced acute liver damage has been extensively reported after the intake of anthocyanin-rich purple sweet potato extract 94, as well as sweet potato polysaccharides 95. Inhibition of lipid peroxidation was also observed in male rats fed a high-cholesterol diet after the administration of anthocyanin-rich sweet potato flakes 96 and in the acetaminophen-induced hepatotoxicity mouse model 97. Purple sweet potato anthocyanin attenuated the oxidative stress and inflammatory response induced by D-galactose in mouse liver 98, whereas an extract from Shinzami, a variety of purple sweet potato, prevented ischaemia–reperfusion-induced liver damage in rats by improvement of the antioxidant status 99.
The fact that the protective effect has been demonstrated in animal studies indicates that human studies are needed to show whether there is strong evidence for the hepatoprotective effects of anthocyanins in purple sweet potato.
Cognitive and memory improvement
The bioactive compounds from purple sweet potato exhibit memory-enhancing effects. According to Isoda et al. 100, 101, caffeoylquinic acid-rich purple sweet potato extracts improved spatial learning and memory in a mouse model of aging. In addition, D-galactose-induced impairment of memory and spatial learning was repaired through the regulation of synaptic protein expression in the hippocampus and cerebral cortex of mice by different purple sweet potato anthocyanin treatments 102. Protection against Aβ-induced neurotoxicity by caffeoylquinic acids has also been reported in a mouse model 103, 104. Similarly, in mice injected with Aβ₁₋₄₂, attenuation of cognitive dysfunction and neuronal cell damage was observed after the administration of 2,4-di-tert-butylphenol extracts from sweet potatoes 105.
A neuroprotective effect of purple sweet potato anthocyanins in a Wistar rat model with ischemic stroke was reported by Adnyana et al. 106, 107, which was attributed to the inhibition of damaging effects of reactive oxygen species (ROS) 106. The treated rats showed an enhanced neurological score between day-3 and day-7 post-stroke, an increase in the brain-derived neurotrophic factor level, and a reduced apoptosis rate 107.
Cognitive deterioration is also associated with obesity, a growing public health concern. In this context, purple sweet potato anthocyanins were observed to improve cognitive function in high-fat diet-fed mice via the activation of AMP-activated protein kinase, which protects against hippocampal apoptosis by restoring impaired autophagy 108. The protective role of this pigment in high-fat diet-associated neuroinflammation in the mouse brain was also examined by Li 109, who found significant improvement in impaired memory function and behavior, as well as suppression of the increment in body weight, hyperlipemia, fat content, and endotoxin levels.
In summary, numerous studies have been carried out attempting to explain the neuroprotective effect of sweet potato. However, all studies have only been conducted in animals, which leaves the need to confirm this protective effect in human studies. In addition, future studies are needed to evaluate the brain-protective effect of other sweet potato varieties (yellow- and orange-fleshed sweet potatoes).
Sweet potato for type 2 diabetes mellitus
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycemia (elevated blood sugar levels) with disturbances in carbohydrate, fat and protein metabolism. Long-term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy (i.e. problems with the eyes, kidneys and peripheral nerves). Diabetes mellitus also increases the risk of cardiovascular disease.
Type 2 diabetes mellitus is a global public health issue 110, 111. The increase in numbers of people with type 2 diabetes mellitus across the age spectrum is of concern. Given the progressive nature of the disease, and the multiple pathophysiological (disease) abnormalities associated with it, accelerated ageing is suspected. This is supported by evidence both at molecular and functional levels 112.
Although full evidence from clinical trials is still lacking, and the long-term effects have not been studied yet, sweet potato has proved effective in treating hyperglycemia, as concluded in a recent systematic review based on in vitro and in vivo studies 113. The blood glucose-lowering activities of sweet potato were demonstrated in animal studies. A number of bioactive compounds were isolated from the leaves 114 and also the tubers 115, 116. These compounds, together with dietary fiber, contribute to blood glucose-lowering activitiy. Besides glycemic control, sweet potato has shown anti-sclerotic activity and inhibition of glycation in test tube studies 11, 117, as well as antihypertensive 118, antioxidative 119, 120, antimutagenic 121, chemopreventive 122, and cardioprotective properties 10. Finally, there is a suggestion that sweet potato may delay amyloid formation and prevent neuronal damage in the brain of mice 123, 124, 125, 126, 127.
This review 77 of randomised controlled trials found only three studies (with a total of 140 participants) that evaluated the effects of sweet potato for type 2 diabetes mellitus compared with a fake medicine (placebo). All these trials were of very low quality. Two studies with 122 participants showed improved long-term metabolic control of blood sugar levels as measured by glycosylated haemoglobin A1c (HbA1c) which was moderately lowered by 0.3% in participants who were given 4 g sweet potato tablets a day for three to five months. The duration of treatment ranged from six weeks to five months. No study investigated diabetic complications, death from any cause, health-related quality of life, well-being, functional outcomes or costs. Adverse effects were mostly mild, and included abdominal distension and pain. There are many varieties of sweet potatoes and sweet potato preparations. More trials are needed to assess the quality of the various sweet potato preparations as well as to evaluate further the use of different varieties of sweet potato in the diet of diabetic people. In conclusion there is insufficient evidence about the use of sweet potato for type 2 diabetes mellitus 77.
Other health benefits
There is a great deal of interest in using potent dietary antioxidants in foods and pharmaceuticals to prevent oxidative reactions and chronic degenerative diseases 128, 129. Sweet potato leaves are a good source of nutrients, enhancing dietary protein, amino acid intake, and growth performance 130. Furthermore, these major nutrients play a role in reducing the risks associated with certain diseases 131. It was reported that sweet potato leaves consumption can decrease the risks associated with cardiovascular disease due to the availability of complex carbohydrates, low-fat content, high dietary fiber 132, 133. In a randomized crossover study, daily oral administration of purple sweet potato leaves (200 g) can modulate various immune functions in human including elevated lytic activity of NK cells, secretion of cytokines IL-2 and IL-4, and increased proliferation responsiveness of peripheral blood mononuclear cells 34. Since numerous health-promoting phytochemicals are found in sweet potato leaves, regular intake of sweet potato leaves provides various health benefits. Among them, polyphenol constituents show various physiological functions and promote human health 132. Leaves of sweet potato are rich in chlorogenic acid, a caffeoylquinic acid derivative, which is well-known for its health benefits, including protection against cancers 134, 135, high blood pressure 136, bacteria 137, diabetes 138 and heart disease 19. Caffeoylquinic acids in sweet potato leaf is an angiotensin-converting enzyme inhibitor, controlling hypertension and congestive heart failure 136. Among the health benefits of sweet potato leaves, anti-cancer activity, liver-protection, anti-inflammatory activity, antidiabetic activity, and antimicrobial activity are recognized as the major effects of sweet potato leaves (see Table 3 below).
Sweet potato leaves health benefits
Leaves of various sweet potato varieties (red, yellow, purple, green, white flesh) have their unique bioactive compositions, and polyphenols and flavonoids are considered as major constituents 19. Due to high content of such bioactive compounds, many great potential health-promoting benefits, including anti-oxidation, anti-diabetics, anti-cancer, anti-hepatotoxicity, anti-inflammation, and anti-bacteria have been observed in these sweet potato leaves. However, more human health studies and clinical trials should be performed to validate the health-promoting benefits of various sweet potato leaves. In addition, identification of the complete profiles of phytochemicals of sweet potato varieties in relation to their bio-activity is also needed. Generally, sweet potato leaves can also serve as a promising natural dietary resource and can be further developed as a sustainable crop for use in the food, food supplement and nutraceutical industries.
Sweet potato leaves are recognized as one of the most important sources of polyphenols 139 with various constituents 140. Among them, caffeic acid and caffeoylquinic acid derivatives, such as 4,5-di-O-caffeoylquinic acid, 3,4,5-tri-O-caffeoylquinic acid, 3-mono-O-caffeoylquinic acid, 3,4-di-O-caffeoylquinic acid, and 3,5-di-O-caffeoylquinic acid, are indicated as the main phenolic constituents in sweet potato leaves 141. These constituents are associated with the specific genotypes and the stages of sweet potato leaf development 140. The level of polyphenols in sweet potato leaves varies among their varieties ranging from 0.3−13.5 g gallic acid equivalent (GAE)/100 g dry weight, which is 7–9 times higher than that levels found in grape seeds 142. The leaf total phenolic compounds of eight sweet potato varieties from Japan ranged from 6.3–13.5 g gallic acid equivalent (GAE)/100 g dry weight 27, whereas the total phenolic compounds in leaves of four Taiwanese varieties and/or cultivars (‘TNG10’, ‘TNG57’, ‘TNG66’, and ‘YSP’) is relatively low 143. The total phenolic compounds in sweet potato leaves cultivated in China and Portugal was 0.9–2.7 g chlorogenic acid equivalent (CAE)/100 g dry weight 32 and 1.20−1.32 g gallic acid equivalent (GAE)/100 g dry weight 144, respectively.
The phenolic and flavonoid contents of sweet potato leaves are affected by the level of light exposure. The content of hydroxybenzoic acids (p-anisic acids and benzoic), hydroxycinnamic acids (sinapic acid and p-coumaric acid conjugates), anthocyanins, catechins, and flavonols in sweet potato leaves are dramatically increased under a long day photoperiod 145.
Sweet potato leaves are harvested several times during the growth of sweet potato, and the phenolic composition and antioxidant activities of sweet potato leaves may vary among the stages of sweet potato leaves development. Therefore, selection a suitable period for harvesting sweet potato leaves is important to obtain sweet potato leaves with high level of phenolic constituents and antioxidant activities. Suárez et al. 146 compared the nutritional and phenolic compositions of sweet potato leaves harvested in three different periods—August 21 (T1), September 6 (T2), and September 21 (T3)—and found that sweet potato leaves harvested in September 21 (T3) had the highest total phenolic compounds (9.1 ± 0.3 g/100 g dry weight), vitamin E content (5.8 ± 0.4 mg/100 g dry weight), vitamin C content (104.6 ± 4.9 mg/100 g dry weight), and the antioxidant activity, compared to those in August 21 (T1) and September 6 (T2). Furthermore, among the phenolic compositions, 2 flavonoids (quercetin and isoquercetin), 1 caffeic acid, and 6 caffeoylquinic acids (3,4,5-tri-O-caffeoylquinic acid, 3,4-di-O-caffeoylquinic acid, 3-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, 4,5-di-O-caffeoylquinic acid, and 5-O-caffeoylquinic acid) were significantly different in those three harvest periods 146.
Similar to other leafy vegetables, fresh sweet potato leaves are easily decayed after harvesting, which lowers thier nutritional values and bioactive compounds. Therefore, sweet potato leaves are proposed to be dried to prolong their shelf-life for industrial applications. The effect of drying method (freeze drying and drying at different temperatures) on the content of caffeoylquinic acid derivatives in sweet potato leaves was investigated by Jeng et al. 28. The freeze-drying treatment resulted in the highest amount of caffeoylquinic acid derivatives (147.84 mg/g), whereas the caffeoylquinic acid derivatives contents of sweet potato leaves were significantly reduced by using drying methods at both 70 °C (58.26 mg/g) and 100 °C (20.53 mg/g) 28. These results imply that drying at low temperatures (<30 °C) may be a suitable method to maintain the nutritional value and bioactive compounds in sweet potato leaves 30. In addition, Sui et al. 147 investigated the influences of vacuum-freeze, hot-air, and microwave-vacuum drying methods on the nutritional composition of sweet potato leaves, and indicated that the vacuum-freeze drying method maintained the highest vitamins (B1, B2, C, and E), minerals (Zn, P, and Mg), total dietary fiber, and total phenolic compounds.
Sweet potato leaves are commonly used for human diet by domestic cooking; therefore, it is crucial to understand the effect of domestic cooking on the the level of polyphenols and antioxidant activity of sweet potato leaves. Sun et al. 148 studied the influences of different cooking methods, including baking, steaming, boiling, frying, and microwaving, on individual phenolic compound, total phenolic compounds and antioxidant activity of sweet potato leaves. Among these tested cooking methods, steaming showed the highest total phenolic compounds, whereas boiling resulted in the lowest total phenolic compounds in sweet potato leaves, indicating that steaming is the most efficient cooking method to maintain levels of polyphenols and antioxidant activity in sweet potato leaves 148.
Extraction is an initial step in the separation and purification process to obtain bioactive compounds from biomass materials for further applications. To maximize the level of a target component and biological activities, the most suitable solvent should be selected for the extraction. Fu et al. 139 investigated the influence of ten different solvents (water, aqueous ethanol, aqueous methanol, and aqueous acetone) on the recovery of polyphenols from sweet potato leaves, and showed that sweet potato leaves extract produced by using 50% acetone resulted in the highest total phenolic compounds (43.8 mg chlorogenic acid equivalent (CAE)/g dry weight) and the strongest antioxidant activities, whereas sweet potato leaves extract using 70% ethanol contained the highest total flavonoid (3.4 mg quercetin equivalents (QE)/g dry weight) and total anthocyanin content (36.5 mgcyanidin-3-glucoside equivalents (C3GE)/100 g dry weight). Fourteen phenolic compounds were identified in 50% acetone extract with quercetin derivatives and caffeoylquinic acids being the most abundant components 139. Moreover, Zhang et al. 149 reported that 37 constituents, including 20 phenolic acids, 12 flavonoids, three organic acids, one ester, and one nucleoside, were identified in the ethyl acetate fraction of sweet potato leaves extract, and 20 of them, such as caffeic acid ethyl ester, trans-N-feruloyltyramine, cis-N-feruloyltyramine, trans-N-(p-coumaroyl) tyramine, 4,5-feruloylcourmaoylquinic acid, indole-3- carboxaldehyde, 7,3′-dimethylquercetin, and 7-hydroxy-5-methoxycoumarin, were initially detected in sweet potato leaves.
Flavonoid content of sweet potato leaves also varies among sweet potato varieties, ranging from 18.0–72.7 quercetin equivalents (QE) mg/g 143. Fu et al. 139 demonstrated that the total phenolic compounds in sweet potato leaves extracts ranged from 23.3–43.8 mg chlorogenic acid equivalent (CAE)/g dry weight, and 70% ethanol extract had the highest total anthocyanin (36.5 mg C3GE/100 g dry weight) and total flavonoid (3.4 mg quercetin equivalents (QE)/g DW) contents. The flavonoid compositions also vary greatly, depending on leaf color. Purple leaves of sweet potatoes contain cyanidin, quercetin, myricetin, and luteolin, while green leaves include apigenin 144. Among flavonoid constituents, anthocyanins are the major compound occurring in substantial amounts in sweet potato leaves 134, levels which are 2.5-fold higher than those in spinach 134. The anthocyanin amount of sweet potato leaves varies among sweet potato varieties. Ji et al. 150 found that purple sweet potato leaves had much higher anthocyanin content than red-, yellow-, and green-colored sweet potato leaves. sweet potato leaves are also a source of carotenoids, and lutein is the major constituent occurring in sweet potato leaves, ranging from 34–68 mg/100 g among sweet potato varieties 145. Moreover, sweet potato leaves also contain other phytochemicals, such as alkaloids, anthraquinones, oxalates, and steroids, at concentrations of 345.7, 328.4, 1.66, and 0.375 mg/100 g dry weight 139, respectively, whereas sweet potato leaves contain lower amounts of phytic acid, cyanide, saponins, and tannins 151.
Since sweet potato leaves are a significant dietary source of bioactive compounds, a comprehensive assessment of the compositions in leaves of sweet potatoes under various treatment methods (e.g., harvesting, cooking, drying, extraction methods) and cultivation conditions is warranted.
Table 3. Health benefits of bioactive compounds in sweet potato leaves
| Compounds | Function | |
|---|---|---|
| Phenolic acids | Caffeic acid derivatives | Antioxidant activity |
| Anti-mutagenic | ||
| Antidiabetic | ||
| Anticancer | ||
| Anti-inflammatory | ||
| Caffeoylquinic acid derivatives | Antioxidant activity | |
| Anticancer | ||
| Anti-hypertension | ||
| Antidiabetic | ||
| Heart protection | ||
| Chlorogenic acid | Antidiabetic | |
| Anticancer | ||
| Quinic acid | Anticancer | |
| Flavonoids | Anthocyanins | Antioxidant activity |
| Anti-mutagenic activity | ||
| Anticancer | ||
| Hypoglycemic activity | ||
| Hepato-protection | ||
| Anti-inflammatory | ||
| Quercetin | Antioxidant activity | |
| Anticancer | ||
| Anti-inflammatory | ||
| Apigenin | Anticancer | |
| Kaempferol | Anticancer | |
| Myricetin | Anticancer | |
| Antidiabetic | ||
| Fisetin | Anticancer | |
| Anti-inflammatory | ||
| Morin | Anticancer | |
| Anti-inflammatory | ||
| Isorhamnetin | Cardioprotection | |
| Luteolin | Anticancer | |
| Anti-inflammatory | ||
| Mono-and di-galactosyldiacylglycerol | Anti-inflammatory | |
| Carotenoids | Anti-cancer | |
| Cardioprotection | ||
| Dietary fiber | Antioxidant activity | |
| Cardioprotection | ||
| Anticancer | ||
| Antidiabetic | ||
| Dietary protein | Growth performance enhancement | |
| 16-amino acid-peptide (IbAcp) | Anticancer | |
| Polysaccharides | Antibacterial activity | |
| Antifungal activity | ||
| Omega-3 fatty acids | Cardioprotection | |
| Anti-inflammatory | ||
| Alkaloids | Antioxidant activity | |
| Saponins | Antioxidant activity | |
| Coumarins | Antioxidant activity | |
| Tannins | Antioxidant activity | |
Antioxidant activities
Various edible sweet potato leaves are valuable sources of antioxidants in the diet. sweet potato leaves marketed in different countries or areas widely vary in their antioxidant activities and may provide different health-promoting values. Leaves of sweet potato varieties with high antioxidant values can be processed for developing products with high nutraceutical values, providing good nutrition and improving human health. sweet potato leaves contain various antioxidants 152, which contribute to the physiological defense against oxidative and free-radical-mediated reactions, leading to an increase in antioxidant defense and the suppression of low-density lipoprotein (LDL) oxidation and DNA damage in human lymphocytes 153. Polyphenol antioxidants, especially caffeoylquinic acid derivatives (3,4,5-tri-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, 3,4-di-O-caffeoylquinic acid, and 4,5-di-O-caffeoylquinic acid), exhibit strong antioxidant capacity 149. In a human study, Chang et al. 154 reported that the consumption of a high-polyphenol diet (200 g cooked purple sweet potato leaves containing 5.7 mg GAE/g) for seven days modulated antioxidative status through dramatically enhancing the plasma total polyphenol level and ferric reducing ability of plasma, lowering the plasma IL-6 concentration, the thiobarbituric acid-reactive substance and protein carbonyl concentrations. Chang et al. 155 revealed that consumption of a purple sweet potato leaves diet for two weeks modulated the antioxidant status of basketball players during the training periods through reducing lipid and DNA oxidation. In addition, purple sweet potato leaves consumption influenced erythrocyte glutathione, plasma total antioxidant capacity, and plasma α-tocopherol 156. Polyphenols occurring in sweet potato leaves bring about an increase in glutathione by facilitating the expression of γ-glutamylcysteine synthetase 157 and inhibiting glutathione reductase 158.
Sweet potato leaves also can contain high levels of flavonoid antioxidants and flavonoids can significantly differ in their antioxidant capacity 159. Green sweet potato leaves are a rich source of quercetin, which was reported to exhibit three-fold more antioxidant capacity than eridictyol, and kaempferol 160. Furthermore, anthocyanin was also considered as one of the most potent antioxidants of purple sweet potato leaves 161. Islam et al. 161 revealed the identification and characterization of 15 anthocyanins in sweet potato leaves exhibiting both antioxidant and anti-mutagenic activities. In addition to anthocyanins, other phytochemicals, including triterpenes, alkaloids, saponins, anthraquinones, coumarins, and tannins also displayed the antioxidant activity in yellow sweet potato leaves 139. The antioxidant capacity of sweet potato leaves was found to vary with the color of sweet potato leaves and to be higher than that of other leafy vegetables. Purple sweet potato leaves exhibited higher antioxidant capacity than celosia (Celosia argentea L.), gynura (Gynura bicolor DC.), perilla (purple-leaved and bicolored-leaved) (Perilla frutescens (L.) Britton), edible amaranth (Amaranthus tricolor L.), heart leaf houttuynia (Houttuynia cordata Thumb.), and other commercial leafy vegetables, due to higher antioxidant content 162, 163. Ji et al. 150 found that the antioxidant capacity of purple sweet potato leaves was significantly higher than other colored sweet potato leaves (red, yellow, and white sweet potato leaves). In a comparison of the oxidative capacity of six sweet potato leaves varieties (‘Indon’, ‘Vitato’, ‘Oren’, ‘Biru-Putih’, ‘Batu-Biasa’, and ‘Batu-Kelantan’), ‘Biru-Putih’ and ‘Indon’ had the lowest and highest scavenging activities with IC50 of 597.61 µg/mL and 372.4 µg/mL, respectively 164. In addition, Truong et al. 165 found a higher level of radical scavenging activity in leaves than in other plant parts in variety of sweet potato cultivars, including ‘Covington’, ‘Hernandez’, and ‘Beauregard’.
Therefore, sweet potato leaves possess antioxidant properties that hold promise for applications of diet-mediated disease treatment and prevention. Further investigation focusing on the optimization of the sweet potato leaves processing techniques (e.g., drying and extraction) in order to maintain the maximum content of antioxidant compounds is warranted 19.
Anti-cancer activity
Leaves of sweet potato have been recognized as a potent anti-cancer food source against various cancer cells, including HCT-116 colon cancer 166, HeLa cancer 166, MCF-7 breast cancer 30, prostate cancer 31, colorectal cancer 167, and lung cancer 135 cells due to high content of anthocyanins 168, and polyphenols 169. Methanol extracts of sweet potato leaves inhibit proliferation of all human prostate cancer cells (PC-3, C4-2B, C4-2, DU145, and LNCaP) with IC50 values of 145–315 μg/mL due to modulations of cell cycle, inductions of apoptosis, and reductions of clonogenic survival 134. The anti-prostate cancer activity of sweet potato leaves is attributed to the presence of 3,4-di-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, 4,5-di-O-caffeoylquinic acid, quinic acid, isochlorogenic acids, caffeic acid, and ester chlorogenic acid 31. Chen et al. 170 showed that polyphenols in purple sweet potato leaves depressed proliferation, migration, and tube formation of vascular endothelial growth factor-treated human umbilical vascular endothelial cells (HUVECs). Chlorogenic acid was reported as a strong and selective inhibitor of matrix metalloproteinase-2 170 and matrix metalloproteinase-9 171, which are angiogenic enzymes responsible for tumor metastasis and invasion, so that it can demonstrate several desirable anti-carcinogenic properties including inhibitory activity against A549 human lung cancer cells 135. Caffeoylquinic acid derivatives also have potential for cancer prevention through apoptosis induction by increasing caspase-3 activity and expression of c-Jun (apoptosis-related gene) 169. Among these compounds, 3,4,5-tri-O-caffeoylquinic acid effectively inhibits the development of human colon cancer DLD-1 cells, promyelocytic leukemia HL-60 cells, and stomach Kato III cancer cells, whereas caffeic acid demonstrates higher inhibitory activity against HL-60 cells than other di- and tri-O-caffeoylquinic acids 169. Recently, a 16-amino-acid peptide, named the peptide Ipomoea batatas anti-cancer peptide (IbACP) from sweet potato leaves, showed the inhibition of pancreatic cancer line 172. Several studies have also been performed to evaluate the in vivo anti-cancer activity of sweet potato leaves 173. Gundala et al. 31 reported that the daily consumption of polyphenol-rich sweet potato leaves extract (400 mg/kg) inhibited the growth and induced the apoptosis in both human prostate cancer cell and in vivo prostate cancer xenograft models. Similarly, the consumption of Okinawan sweet potato leaves extract (200 ppm and 1000 ppm) for 12 weeks potentially inhibited the progression and development of neoplasms in mouse colon carcinogenesis model 173.
Liver protection
The activity of anthocyanins of purple sweet potato leaves was tested on carbon tetrachloride-treated human normal hepatocyte HL7702 cells 35, tert-butyl hydroperoxide-treated HepG2 cells, and rat hepatic stellate HSC-T6 cells 174. The results demonstrate that anthocyanins of purple sweet potato leaves (100–400 μg/mL) reduced the accumulation of reactive oxygen species (ROS) in TC-HL7702 cells 35, and inhibited the proliferation of HSC-T6 cells by inhibiting α-smooth muscle actin (SMA) expression, extracellular signal-regulated kinases 1 and 2 (ERK1/2), and the serine-threonine kinase Akt activation, and blocking platelet-derived growth factor receptor (PDGFR)-β signaling 174. Furthermore, anthocyanins also reduced the cell death in t-BHP-treated HepG2 cells by lowering the levels of intracellular ROS, caspases-3 activity, lipid peroxidation, and by enhancing the levels of cytoprotective enzymes in HepG2 cells via ERK1/2/Nrf2 and Akt signaling pathways 174.
Anti-inflammatory activity
Extract of sweet potato leaves and its constituents, cyanidin and quercetin, were observed to show anti-inflammatory effects via reducing the mitogen-activated protein kinase (MAPK), ERK1/2 expression, and nuclear factor kappa B (NFκB), inhibiting tumor necrosis factor-α (TNF-α)-induced monocyte-endothelial cell adhesion, and attenuating interleukin-8 (IL-8), vascular cell adhesion molecule-1 (VCAM-1), and CD40 (a member of TNF receptor family of cell surface proteins) expression 175. The consumption of purple sweet potato leaves can modulate various immune functions, including secretion of cytokines IL-2 and IL-4 of NK cells and can induce an increase in proliferation responsiveness of peripheral blood mononuclear cells due to the high polyphenol content of the leaves 34. Moreover, the purple sweet potato leaves extracts depress neuroinflammatory responses in lipopolysaccharide-activated BV-2 microglia cells by inhibiting production of pro-inflammatory mediators, such as inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), nitric oxide (NO), and TNF-α. The anti-neuroinflammatory potential of sweet potato leaves extract was considered to be related to its strong antioxidant properties 176.
Antidiabetic activity
Sweet potato leaves contain several constituents that show a potential activity against diabetes. Chlorogenic acid reduces the release of glucose into the blood-stream, lowering the glycemic index, thereby benefitting diabetic patients and reducing the risk of type 2 diabetes 177. In addition, the polyphenol contents in leaves of 116 sweet potato cultivars grown in China showed anti-diabetic activity 178. In an lab animal study, the extract of sweet potato leaves can reduce the blood glucose levels of both STZ-induced diabetic and healthy rats, indicating hypoglycemic and anti-hyperglycemic activities of sweet potato leaves by stimulating glucagon-like peptide-1 (GLP-1) secretion [48]. The maximum hypoglycemic activity of the extracts in both STZ-induced hyperglycemic and healthy rats were obtained at the dose of 400 mg/kg with non-cytotoxicity 174. In another in vivo study, the consumption of sweet potato leaves (3% in diet) for 5 weeks modulated the hypoglycemic activity in type-2 diabetic mice 179. Phenolic acids, caffeoylquinic acid derivatives, and anthocyanins were found to be one of the key hypoglycemic contributors in sweet potato leaves 179. In addition, Zhang et al. 178 reported that phenethylcinnamides and 3,4,5-tri-O-caffeoylquinic acid from sweet potato leaves manifested strong α-glucosidase inhibition. Lin et al. 180 described that purple, yellow, and red sweet potato leaves extracts in 70% ethanol considerably promoted expressions of glucose transporter (GLUT)-2 relative to that of a tumor necrosis factor-α-treated group in insulin-resistant FL83B hepatocyte cells, and lowered risk of diseases such as type 2 diabetes.
Antimicrobial activity
Sweet potato leaves also exhibit potential for antimicrobial activity. Islam 137 reported the potent antibacterial activity of the leaf extract of three sweet potato cultivars against Staphylococcus aureus, Bacillus cereus, and E. coli O157:H7. Polysaccharides are considered to be the major anti-bacterial agents in sweet potato leaves extract 137. However, ethanol leaf extract of Brazilian sweet potato did not show any anti-bacterial and anti-fungal activities against S. aureus, S. mutans, S. mitis, and Candida albicans 181. This result could be attributed to differences in the methodology of antimicrobial tests and phytochemical compositions in the sweet potato leaves 181. There are still limited studies on the antimicrobial activity of sweet potato leaves, thus, further studies are needed to clarify sweet potato leaves antimicrobial activity and the mechanisms involved.
References- Woolfe, Jennifer A. (5 March 1992). Sweet Potato: An Untapped Food Resource. Cambridge, UK: Cambridge University Press and the International Potato Center (CIP). ISBN 9780521402958.
- Purseglove, John Williams (1968). Tropical crops: Dicotyledons. Longman Scientific and Technical. New York: John Wiley and Sons. ISBN 978-0-582-46666-1
- Gad Loebenstein; George Thottappilly (2009). The sweetpotato. pp. 391–425. ISBN 978-1-4020-9475-0
- Jung J.-K., Lee S.-U., Kozukue N., Levin C.E., Friedman M. Distribution of Phenolic Compounds and Antioxidative Activities in Parts of Sweet Potato (Ipomoea batata L.) Plants and in Home Processed Roots. J. Food Compos. Anal. 2011;24:29–37. doi: 10.1016/j.jfca.2010.03.025
- Wikipedia. Sweet potato. https://en.wikipedia.org/wiki/Sweet_potato
- Food Reference. THE CONFUSION BETWEEN SWEET POTATOES & YAMS. http://www.foodreference.com/html/art-sweet-potato-yam.html
- USDA National Nutrient Database for Standard Reference 2010, Release 23. U.S. Department of Agriculture, Agricultural Research Service; Nutrient Data Laboratory Home Page. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/
- Salvador LD, Suganuma T, Kitahara K, Tanoue H, Ichiki M. Monosaccharide composition of sweet potato fiber and cell wall polysaccharides from sweet potato, cassava, and potato analyzed by the high-performance anion exchange chromatography with pulsed amperometric detection method. Journal of Agricultural and Food Chemistry 2000;48(8):3448-54. https://www.ncbi.nlm.nih.gov/pubmed/10956132
- Sabater-Molina M, Larque E, Torrella F, Zamora S. Dietary fructo-oligosaccharides and potential benefits on health. Journal of Physiology and Biochemistry 2009;65(3):315-28. https://www.ncbi.nlm.nih.gov/pubmed/20119826
- Maiani G, Caston MJ, Catasta G, Toti E, Cambrodon IG, Bysted A, et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Molecular Nutrition & Food Research 2009;53 Suppl 2:S194-218. https://www.ncbi.nlm.nih.gov/pubmed/19035552
- Miyazaki K, Makino K, Iwadate E, Deguchi Y, Ishikawa F. Anthocyanins from purple sweet potato Ipomoea batatas cultivar Ayamurasaki suppress the development of atherosclerotic lesions and both enhancements of oxidative stress and soluble vascular cell adhesion molecule-1 in apolipoprotein E-deficient mice. Journal of Agricultural and Food Chemistry 2008;56(23):11485-92. https://www.ncbi.nlm.nih.gov/pubmed/18986148
- Antia BS, Akpan EJ, Okon PA, Umoren IU. Nutritive and anti-nutritive evaluation of sweet potatoes (Ipomoea batatas) leaves. Pakistan Journal of Nutrition 2006;5(2):166-8.
- Bovell-Benjamin A.C. Sweet Potato: A Review of Its Past, Present, and Future Role in Human Nutrition. Adv. Food Nutr. Res. 2007;52:1–59. doi: 10.1016/S1043-4526(06)52001-7
- Shih P.-H., Yeh C.-T., Yen G.-C. Anthocyanins Induce the Activation of Phase II Enzymes through the Antioxidant Response Element Pathway against Oxidative Stress-Induced Apoptosis. J. Agric. Food Chem. 2007;55:9427–9435. doi: 10.1021/jf071933i
- Wang H., Cao G., Prior R.L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agric. Food Chem. 1997;45:304–309. doi: 10.1021/jf960421t
- Mohanraj R., Sivasankar S. Sweet Potato (Ipomoea batatas [L.] Lam)-A Valuable Medicinal Food: A Review. J. Med. Food. 2014;17:733–741. doi: 10.1089/jmf.2013.2818
- Tang Y., Cai W., Xu B. Profiles of Phenolics, Carotenoids and Antioxidative Capacities of Thermal Processed White, Yellow, Orange and Purple Sweet Potatoes Grown in Guilin, China. Food Sci. Hum. Wellness. 2015;4:123–132. doi: 10.1016/j.fshw.2015.07.003
- Li L., Aldini G., Carini M., Chen C.-Y.O., Chun H.-K., Cho S.-M., Park K.-M., Correa C.R., Russell R.M., Blumberg J.B. Characterisation, Extraction Efficiency, Stability and Antioxidant Activity of Phytonutrients in Angelica Keiskei. Food Chem. 2009;115:227–232. doi: 10.1016/j.foodchem.2008.12.015
- Nguyen HC, Chen CC, Lin KH, Chao PY, Lin HH, Huang MY. Bioactive Compounds, Antioxidants, and Health Benefits of Sweet Potato Leaves. Molecules. 2021 Mar 24;26(7):1820. doi: 10.3390/molecules26071820
- Almazan AM, Adeyeye SO. Fat and fatty acid concentrations in some green vegetables. Journal of Food Composition and Analysis 1998;11(4):375-80.
- Napolitano A, Carbone V, Saggese P, Takagaki K, Pizza C. Novel galactolipids from the leaves of Ipomoea batatas L.: characterization by liquid chromatography coupled with electrospray ionization-quadrupole time-of-flight tandem mass spectrometry. Journal of Agricultural and Food Chemistry 2007;55(25):10289-97. https://www.ncbi.nlm.nih.gov/pubmed/17988089
- Islam MS, Yoshimoto M, Terahara N, Yamakawa O. Anthocyanin compositions in sweet potato (Ipomoea batatas L.) leaves. Bioscience, Biotechnology, and Biochemistry 2002;66(11):2483-6. https://www.jstage.jst.go.jp/article/bbb/66/11/66_11_2483/_pdf
- Thu NN, Sakurai C, Uto H, Van Chuyen N, Lien DT, Yamamoto S, et al. The polyphenol content and antioxidant activities of the main edible vegetables in northern Vietnam. Journal of Nutritional Science and Vitaminology 2004;50(3):203-10. https://www.ncbi.nlm.nih.gov/pubmed/15386933
- Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. Journal of Agricultural and Food Chemistry 2001;49(6):3106-12. https://www.ncbi.nlm.nih.gov/pubmed/11410016
- Islam MS, Yoshimoto M, Yamakawa O. Distribution and physiological functions of caffeoylquinic acid derivatives in leaves of sweet potato genotypes. Journal of Food Science 2003;68(1):111-6. http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2621.2003.tb14124.x/pdf
- Sasaki K., Oki T., Kai Y., Nishiba Y., Okuno S. Effect of repeated harvesting on the content of caffeic acid and seven species of caffeoylquinic acids in sweet potato leaves. Biosci. Biotechnol. Biochem. 2015;79:1308–1314. doi: 10.1080/09168451.2015.1025032
- Nagai M., Tani M., Kishimoto Y., Iizuka M., Saita E., Toyozaki M., Kamiya T., Ikeguchi M., Kondo K. Sweet potato (Ipomoea batatas L.) leaves suppressed oxidation of low density lipoprotein (LDL) in vitro and in human subjects. J. Clin. Biochem. Nutr. 2011;48:203–208. doi: 10.3164/jcbn.10-84
- Jeng T.L., Lai C.C., Liao T.C., Lin S.Y., Sung J.M. Effects of drying on caffeoylquinic acid derivative content and antioxidant capacity of sweet potato leaves. J. Food Drug Anal. 2015;23:701–708. doi: 10.1016/j.jfda.2014.07.002
- Kwak C.S., Lee K.J., Chang J.H., Park J.H., Cho J.H., Park J.H., Kim K.M., Lee M.S. In vitro antioxidant, anti-allergic and anti-inflammatory effects of ethanol extracts from Korean sweet potato leaves and stalks. J. Korean Soc. Food Sci. Nutr. 2013;42:369–377. doi: 10.3746/jkfn.2013.42.3.369
- Ezekiel R., Singh N., Sharma S., Kaur A. Beneficial phytochemicals in potato—A review. Food Res. Int. 2013;50:487–496. doi: 10.1016/j.foodres.2011.04.025
- Gundala S.R., Yang C., Lakshminarayana N., Asif G., Gupta M.V., Shamsi S., Aneja R. Polar biophenolics in sweet potato greens extract synergize to inhibit prostate cancer cell proliferation and in vivo tumor growth. Carcinogenesis. 2013;34:2039–2049. doi: 10.1093/carcin/bgt141
- Taira J., Uehara M., Tsuchida E., Ohmine W. Inhibition of the β-catenin/Tcf signaling by caffeoylquinic acids in sweet potato leaf through down regulation of the Tcf-4 transcription. J. Agric. Food Chem. 2014;62:167–172. doi: 10.1021/jf404411r
- Yoshimoto M., Yahara S., Okuno S., Islam M.S., Ishiguro K., Yamakawa O. Antimutagenicity of mono-, di-, and tricaffeoylquinic acid derivatives isolated from sweetpotato (Ipomoea batatas L.) leaf. Biosci. Biotechnol. Biochem. 2002;66:2336–2341. doi: 10.1271/bbb.66.2336
- Chen C.M., Li S.C., Lin Y.L., Hsu C.Y., Shieh M.J., Liu J.F. Consumption of purple sweet potato leaves modulates human immune response: T-lymphocyte functions, lytic activity of natural killer cell and antibody production. World J. Gastroenterol. 2005;11:5777–5781. doi: 10.3748/wjg.v11.i37.5777
- Wang W., Li J., Wang Z., Gao H., Su L., Xie J., Chen X., Liang H., Wang C., Han Y. Oral hepatoprotective ability evaluation of purple sweet potato anthocyanins on acute and chronic chemical liver injuries. Cell Biochem. Biophys. 2014;69:539–548. doi: 10.1007/s12013-014-9829-3
- Laveriano-Santos EP, López-Yerena A, Jaime-Rodríguez C, González-Coria J, Lamuela-Raventós RM, Vallverdú-Queralt A, Romanyà J, Pérez M. Sweet Potato Is Not Simply an Abundant Food Crop: A Comprehensive Review of Its Phytochemical Constituents, Biological Activities, and the Effects of Processing. Antioxidants (Basel). 2022 Aug 25;11(9):1648. doi: 10.3390/antiox11091648
- Lila MA. Anthocyanins and Human Health: An In Vitro Investigative Approach. Journal of Biomedicine and Biotechnology. 2004;2004(5):306-313. doi:10.1155/S111072430440401X. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1082894/
- Wang L-S, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer letters. 2008;269(2):281-290. doi:10.1016/j.canlet.2008.05.020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2582525/
- Teow C.C., Truong V.-D., McFeeters R.F., Thompson R.L., Pecota K.V., Yencho G.C. Antioxidant Activities, Phenolic and β-Carotene Contents of Sweet Potato Genotypes with Varying Flesh Colours. Food Chem. 2007;103:829–838. doi: 10.1016/j.foodchem.2006.09.033
- Ji H., Zhang H., Li H., Li Y., Ji H., Zhang H., Li H., Li Y. Analysis on the Nutrition Composition and Antioxidant Activity of Different Types of Sweet Potato Cultivars. Food Nutr. Sci. 2015;6:161–167. doi: 10.4236/fns.2015.61017
- Wang S., Nie S., Zhu F. Chemical Constituents and Health Effects of Sweet Potato. Food Res. Int. 2016;89:90–116. doi: 10.1016/j.foodres.2016.08.032
- Xu J., Su X., Lim S., Griffin J., Carey E., Katz B., Tomich J., Smith J.S., Wang W. Characterisation and Stability of Anthocyanins in Purple-Fleshed Sweet Potato P40. Food Chem. 2015;186:90–96. doi: 10.1016/j.foodchem.2014.08.123
- He W., Zeng M., Chen J., Jiao Y., Niu F., Tao G., Zhang S., Qin F., He Z. Identification and Quantitation of Anthocyanins in Purple-Fleshed Sweet Potatoes Cultivated in China by UPLC-PDA and UPLC-QTOF-MS/MS. J. Agric. Food Chem. 2016;64:171–177. doi: 10.1021/acs.jafc.5b04878
- Sun H., Mu B., Song Z., Ma Z., Mu T. The In Vitro Antioxidant Activity and Inhibition of Intracellular Reactive Oxygen Species of Sweet Potato Leaf Polyphenols. Oxid. Med. Cell. Longev. 2018;2018:9017828. doi: 10.1155/2018/9017828
- Sun H., Zhang P., Zhu Y., Lou Q., He S. Antioxidant and Prebiotic Activity of Five Peonidin-Based Anthocyanins Extracted from Purple Sweet Potato (Ipomoea batatas (L.) Lam.) Sci. Rep. 2018;8:5018. doi: 10.1038/s41598-018-23397-0
- Vishnu V.R., Renjith R.S., Mukherjee A., Anil S.R., Sreekumar J., Jyothi A.N. Comparative Study on the Chemical Structure and in Vitro Antiproliferative Activity of Anthocyanins in Purple Root Tubers and Leaves of Sweet Potato (Ipomoea batatas) J. Agric. Food Chem. 2019;67:2467–2475. doi: 10.1021/acs.jafc.8b05473
- Fossen T., Andersen Ø.M. Anthocyanins from Tubers and Shoots of the Purple Potato, Solanum tuberosum. J. Hortic. Sci. Biotechnol. 2000;75:360–363. doi: 10.1080/14620316.2000.11511251
- Im Y.R., Kim I., Lee J. Phenolic Composition and Antioxidant Activity of Purple Sweet Potato (Ipomoea batatas (L.) Lam.): Varietal Comparisons and Physical Distribution. Antioxidants. 2021;10:462. doi: 10.3390/antiox10030462
- Zhao C.L., Yu Y.Q., Chen Z.J., Wen G.S., Wei F.G., Zheng Q., Wang C., Xiao X.L. Stability-Increasing Effects of Anthocyanin Glycosyl Acylation. Food Chem. 2017;214:119–128. doi: 10.1016/j.foodchem.2016.07.073
- Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Prior RL, Wu X. Free Radic Res. 2006 Oct; 40(10):1014-28. https://www.ncbi.nlm.nih.gov/pubmed/17015246/
- Hou D.X. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr Mol Med. 2003;3(2):149–159. https://www.ncbi.nlm.nih.gov/pubmed/12630561
- Kang S, Seeram N, Nair M, Bourquin L. Tart cherry anthocyanins inhibit tumor development in Apc(Min) mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003;194(1):13–19. https://www.ncbi.nlm.nih.gov/pubmed/12706854
- Koide T, Hashimoto Y, Kamei H, Kojima T, Hasegawa M, Terabe K. Antitumor effect of anthocyanin fractions extracted from red soybeans and red beans in vitro and in vivo. Cancer Biother Radiopharm. 1997;12(4):277–280. https://www.ncbi.nlm.nih.gov/pubmed/10851476
- Meiers S, Kemeny M, Weyand U, Gastpar R, von Angerer E, Marko D. The anthocyanidins cyanidin and delphinidin are potent inhibitors of the epidermal growth-factor receptor. J Agric Food Chem. 2001;49(2):958–962. https://www.ncbi.nlm.nih.gov/pubmed/11262056
- Hou D.X, Kai K, Li J.J, et al. Anthocyanidins inhibit activator protein 1 activity and cell transformation: structure-activity relationship and molecular mechanisms. Carcinogenesis. 2004;25(1):29–36. https://www.ncbi.nlm.nih.gov/pubmed/14514663
- Smith M, Marley K, Seigler D, Singletary K, Meline B. Bioactive properties of wild blueberry fruits. J Food Sci. 2000;65:352–356.
- Bomser J, Madhavi D, Singletary K, Smith M.A. In vitro anticancer activity of fruit extracts from Vaccinium species. Planta Med. 1996;62(3):212–216. https://www.ncbi.nlm.nih.gov/pubmed/8693031
- Kandil F, Song L, Pezzuto J, Seigler D, Smith M.A. Isolation of oligomeric proanthocyanidins from flavonoid-producing cell cultures. In Vitro Cell Dev Biol Plant. 2000;36:492–500.
- Mazza G, Miniati E. Boca Raton, Fla: CRC Press; 1993. Anthocyanins in Fruits, Vegetables, and Grains.
- Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. Matsumoto H, Inaba H, Kishi M, Tominaga S, Hirayama M, Tsuda T. J Agric Food Chem. 2001 Mar; 49(3):1546-51. https://www.ncbi.nlm.nih.gov/pubmed/11312894/
- Effects of black current anthocyanoside intake on dark adaptation and VDT work-induced transient refractive alteration in healthy humans. Nakaishi H, Matsumoto H, Tominaga S, Hirayama M. Altern Med Rev. 2000 Dec; 5(6):553-62. https://www.ncbi.nlm.nih.gov/pubmed/11134978/
- The effect of bilberry nutritional supplementation on night visual acuity and contrast sensitivity. Muth ER, Laurent JM, Jasper P. Altern Med Rev. 2000 Apr; 5(2):164-73. https://www.ncbi.nlm.nih.gov/pubmed/10767671/
- Stimulatory effect of cyanidin 3-glycosides on the regeneration of rhodopsin. Matsumoto H, Nakamura Y, Tachibanaki S, Kawamura S, Hirayama M. J Agric Food Chem. 2003 Jun 4; 51(12):3560-3. https://www.ncbi.nlm.nih.gov/pubmed/12769524/
- Institute of Medicine, US Panel on Micronutrients. Dietary reference intakes for vitamin A, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academies Press. Washington, DC, 2001. PMID: 25057538 www.ncbi.nlm.nih.gov/pubmed/25057538
- Food and Agriculture Organization of the United Nations. Sweet potatoes – cultivating resilience, fighting malnutrition in Somalia. http://www.fao.org/emergencies/fao-in-action/stories/stories-detail/en/c/266043/
- United States Department of Agriculture Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/search/list
- “Sweet potato, cooked, baked in skin, without salt”. Nutritiondata.com. Conde Nast. 2013. http://nutritiondata.self.com/facts/vegetables-and-vegetable-products/2667/2
- Dincer, C; Karaoglan, M; Erden, F; Tetik, N; Topuz, A; Ozdemir, F (Nov 2011). “Effects of baking and boiling on the nutritional and antioxidant properties of sweet potato [Ipomoea batatas (L.) Lam.] cultivars”. Plant Foods for Human Nutrition. 66 (4): 341–7. https://link.springer.com/article/10.1007%2Fs11130-011-0262-0
- Center for Science in the Public Interest. 10 Best Foods. https://cspinet.org/eating-healthy/what-eat/10-best-foods
- “Nutrient-boosted foods protect against blindness”. New Scientist, Health. https://www.newscientist.com/article/mg21528784-200-nutrient-boosted-foods-protect-against-blindness/
- Tian S.J., Rickard J.E., Blanshard J.M.V. Physicochemical Properties of Sweet Potato Starch. J. Sci. Food Agric. 1991;57:459–491. doi: 10.1002/jsfa.2740570402
- Willcox D.C., Willcox B.J., Todoriki H., Suzuki M. The Okinawan Diet: Health Implications of a Low-Calorie, Nutrient-Dense, Antioxidant-Rich Dietary Pattern Low in Glycemic Load. J. Am. Coll. Nutr. 2009;28:500S–516S. doi: 10.1080/07315724.2009.10718117
- Luo D., Mu T., Sun H. Profiling of Phenolic Acids and Flavonoids in Sweet Potato (Ipomoea batatas L.) Leaves and Evaluation of Their Anti-Oxidant and Hypoglycemic Activities. Food Biosci. 2021;39:100801. doi: 10.1016/j.fbio.2020.100801
- Proteomic approach reveals that starch degradation contributes to anthocyanin accumulation in tuberous root of purple sweet potato. J Proteomics. 2016 Jun 30;143:298-305. doi: 10.1016/j.jprot.2016.03.010. Epub 2016 Mar 6. https://www.ncbi.nlm.nih.gov/pubmed/26957144
- Kurata R., Sun H.N., Oki T., Okuno S., Ishiguro K., Sugawara T. Sweet Potato Chemistry, Processing and Nutrition. Elsevier Inc.; Amsterdam, The Netherlands: 2019. Sweet Potato Polyphenols; pp. 177–222.
- Abong’ G.O., Muzhingi T., Okoth M.W., Ng’ang’a F., Emelda Ochieng P., Mbogo D.M., Malavi D., Akhwale M., Ghimire S. Processing Methods Affect Phytochemical Contents in Products Prepared from Orange-fleshed Sweetpotato Leaves and Roots. Food Sci. Nutr. 2021;9:1070–1078. doi: 10.1002/fsn3.2081
- Ooi CP, Loke SC. Sweet potato for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 9. Art. No.: CD009128. DOI: 10.1002/14651858.CD009128.pub3. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD009128.pub3/full
- Philpott M., Lim C.C., Ferguson L.R. Dietary Protection against Free Radicals: A Case for Multiple Testing to Establish Structure-Activity Relationships for Antioxidant Potential of Anthocyanic Plant Species. Int. J. Mol. Sci. 2009;10:1081–1103. doi: 10.3390/ijms10031081
- Kano M., Takayanagi T., Harada K., Makino K., Ishikawa F. Antioxidative Activity of Anthocyanins from Purple Sweet Potato, Ipomoera Batatas Cultivar Ayamurasaki. Biosci. Biotechnol. Biochem. 2005;69:979–988. doi: 10.1271/bbb.69.979
- Padda M.S., Picha D.H. Quantification of Phenolic Acids and Antioxidant Activity in Sweetpotato Genotypes. Sci. Hortic. 2008;119:17–20. doi: 10.1016/j.scienta.2008.07.008
- Oki T., Masuda M., Furuta S., Nishiba Y., Terahara N., Suda I. Involvement of Anthocyanins and Other Phenolic Compounds in Radical-scavenging Activity of Purple-fleshed Sweet Potato Cultivars. J. Food Sci. 2002;67:1752–1756. doi: 10.1111/j.1365-2621.2002.tb08718.x
- Fu H., Xie B., Ma S., Zhu X., Fan G., Pan S. Evaluation of Antioxidant Activities of Principal Carotenoids Available in Water Spinach (Ipomoea aquatica) J. Food Compos. Anal. 2011;24:288–297. doi: 10.1016/j.jfca.2010.08.007
- Sugata M., Lin C.-Y., Shih Y.-C. Anti-Inflammatory and Anticancer Activities of Taiwanese Purple-Fleshed Sweet Potatoes (Ipomoea batatas L. Lam) Extracts. BioMed Res. Int. 2015;2015:768093. doi: 10.1155/2015/768093
- Kato K., Nagane M., Aihara N., Kamiie J., Miyanabe M., Hiraki S., Luo X., Nakanishi I., Shoji Y., Matsumoto K. Lipid-Soluble Polyphenols from Sweet Potato Exert Antitumor Activity and Enhance Chemosensitivity in Breast Cancer. J. Clin. Biochem. Nutr. 2021;68:193–200. doi: 10.3164/jcbn.20-73
- Jiang P., Han B., Jiang L., Li Y., Yu Y., Xu H., Li Z., Zhou D., Jia X., Li X., et al. Simultaneous Separation and Quantitation of Three Phytosterols from the Sweet Potato, and Determination of Their Anti-Breast Cancer Activity. J. Pharm. Biomed. Anal. 2019;174:718–727. doi: 10.1016/j.jpba.2019.06.048
- Xu H., Li Y., Han B., Li Z., Wang B., Jiang P., Zhang J., Ma W., Zhou D., Li X. Anti-Breast-Cancer Activity Exerted by β-Sitosterol-d-Glucoside from Sweet Potato via Upregulation of Microrna-10a and via the Pi3k–Akt Signaling Pathway. J. Agric. Food Chem. 2018;66:9704–9718. doi: 10.1021/acs.jafc.8b03305
- Li Z., Yu Y., Wang M., Xu H., Han B., Jiang P., Ma H., Li Y., Tian C., Zhou D. Anti-Breast Cancer Activity of SPG-56 from Sweet Potato in MCF-7 Bearing Mice in Situ through Promoting Apoptosis and Inhibiting Metastasis. Sci. Rep. 2019;9:146. doi: 10.1038/s41598-018-29099-x
- Tian C., Wang M., Liu S., Ma H., He K., Zhou D., Li Y., Ye X., Li X. A New Glycoprotein SPG-8700 Isolated from Sweet Potato with Potential Anti-Cancer Activity against Colon Cancer. Nat. Prod. Res. 2019;33:2322–2328. doi: 10.1080/14786419.2018.1446007
- Yang C., Chen S.-J., Chen B.-W., Zhang K.-W., Zhang J.-J., Xiao R., Li P.-G. Gene Expression Profile of the Human Colorectal Carcinoma LoVo Cells Treated With Sporamin and Thapsigargin. Front. Oncol. 2021;11:2003. doi: 10.3389/fonc.2021.621462
- Lim S., Xu J., Kim J., Chen T., Su X., Standard J., Carey E., Griffin J., Herndon B., Katz B. Role of Anthocyanin-enriched Purple-fleshed Sweet Potato P40 in Colorectal Cancer Prevention. Mol. Nutr. Food Res. 2013;57:1908–1917. doi: 10.1002/mnfr.201300040
- Li W.-L., Yu H.-Y., Zhang X.-J., Ke M., Hong T. Purple Sweet Potato Anthocyanin Exerts Antitumor Effect in Bladder Cancer. Oncol. Rep. 2018;40:73–82. doi: 10.3892/or.2018.6421
- Gundala S.R., Yang C., Lakshminarayana N., Asif G., Gupta M.V., Shamsi S., Aneja R. Polar Biophenolics in Sweet Potato Greens Extract Synergize to Inhibit Prostate Cancer Cell Proliferation and in Vivo Tumor Growth. Carcinogenesis. 2013;34:2039–2049. doi: 10.1093/carcin/bgt141
- Suda I., Ishikawa F., Hatakeyama M., Miyawaki M., Kudo T., Hirano K., Ito A., Yamakawa O., Horiuchi S. Intake of Purple Sweet Potato Beverage Affects on Serum Hepatic Biomarker Levels of Healthy Adult Men with Borderline Hepatitis. Eur. J. Clin. Nutr. 2008;62:60–67. doi: 10.1038/sj.ejcn.1602674
- Wang L., Zhao Y., Zhou Q., Luo C.-L., Deng A.-P., Zhang Z.-C., Zhang J.-L. Characterization and Hepatoprotective Activity of Anthocyanins from Purple Sweet Potato (Ipomoea batatas L. Cultivar Eshu No. 8) J. Food Drug Anal. 2017;25:607–618. doi: 10.1016/j.jfda.2016.10.009
- Sun J., Zhou B., Tang C., Gou Y., Chen H., Wang Y., Jin C., Liu J., Niu F., Kan J. Characterization, Antioxidant Activity and Hepatoprotective Effect of Purple Sweetpotato Polysaccharides. Int. J. Biol. Macromol. 2018;115:69–76. doi: 10.1016/j.ijbiomac.2018.04.033
- Han K.-H., Shimada K., Sekikawa M., Fukushima M. Anthocyanin-Rich Red Potato Flakes Affect Serum Lipid Peroxidation and Hepatic SOD MRNA Level in Rats. Biosci. Biotechnol. Biochem. 2007;71:1356–1359. doi: 10.1271/bbb.70060
- Choi J.H., Choi C.Y., Lee K.J., Hwang Y.P., Chung Y.C., Jeong H.G. Hepatoprotective Effects of an Anthocyanin Fraction from Purple-Fleshed Sweet Potato against Acetaminophen-Induced Liver Damage in Mice. J. Med. Food. 2009;12:320–326. doi: 10.1089/jmf.2007.0691
- Zhang Z.-F., Fan S.-H., Zheng Y.-L., Lu J., Wu D.-M., Shan Q., Hu B. Purple Sweet Potato Color Attenuates Oxidative Stress and Inflammatory Response Induced by D-Galactose in Mouse Liver. Food Chem. Toxicol. 2009;47:496–501. doi: 10.1016/j.fct.2008.12.005
- Jung S., Shin J., Kim J.Y., Kwon O. Shinzami Korean Purple-fleshed Sweet Potato Extract Prevents Ischaemia–Reperfusion-induced Liver Damage in Rats. J. Sci. Food Agric. 2015;95:2818–2823. doi: 10.1002/jsfa.7021
- Han J., Miyamae Y., Shigemori H., Isoda H. Neuroprotective Effect of 3, 5-Di-O-Caffeoylquinic Acid on SH-SY5Y Cells and Senescence-Accelerated-Prone Mice 8 through the up-Regulation of Phosphoglycerate Kinase-1. Neuroscience. 2010;169:1039–1045. doi: 10.1016/j.neuroscience.2010.05.049
- Sasaki K., Han J., Shimozono H., Villareal M.O., Isoda H. Caffeoylquinic Acid-Rich Purple Sweet Potato Extract, with or without Anthocyanin, Imparts Neuroprotection and Contributes to the Improvement of Spatial Learning and Memory of SAMP8 Mouse. J. Agric. Food Chem. 2013;61:5037–5045. doi: 10.1021/jf3041484
- Wu D., Lu J., Zheng Y., Zhou Z., Shan Q., Ma D. Purple Sweet Potato Color Repairs D-Galactose-Induced Spatial Learning and Memory Impairment by Regulating the Expression of Synaptic Proteins. Neurobiol. Learn. Mem. 2008;90:19–27. doi: 10.1016/j.nlm.2008.01.010
- Matthews D.G., Caruso M., Alcazar Magana A., Wright K.M., Maier C.S., Stevens J.F., Gray N.E., Quinn J.F., Soumyanath A. Caffeoylquinic Acids in Centella Asiatica Reverse Cognitive Deficits in Male 5XFAD Alzheimer’s Disease Model Mice. Nutrients. 2020;12:3488. doi: 10.3390/nu12113488
- Choi S.J., Kim J.K., Suh S.H., Kim C.R., Kim H.K., Kim C.-J., Park G.G., Park C.-S., Shin D.-H. Ligularia Fischeri Extract Protects against Oxidative-Stress-Induced Neurotoxicity in Mice and Pc12 Cells. J. Med. Food. 2014;17:1222–1231. doi: 10.1089/jmf.2013.3014
- Choi S.J., Kim J.K., Kim H.K., Harris K., Kim C.-J., Park G.G., Park C.-S., Shin D.-H. 2, 4-Di-Tert-Butylphenol from Sweet Potato Protects against Oxidative Stress in PC12 Cells and in Mice. J. Med. Food. 2013;16:977–983. doi: 10.1089/jmf.2012.2739
- Adnyana I.M.O., Sudewi A.A.R., Samatra D.P.G.P., Suprapta D.N. Neuroprotective Effects of Purple Sweet Potato Balinese Cultivar in Wistar Rats with Ischemic Stroke. Open Access Maced. J. Med. Sci. 2018;6:1959. doi: 10.3889/oamjms.2018.435
- Adnyana I.M.O., Sudewi R., Samatra P., Suprapta S. Balinese Cultivar of Purple Sweet Potato Improved Neurological Score and BDNF and Reduced Caspase-Independent Apoptosis among Wistar Rats with Ischemic Stroke. Open Access Maced. J. Med. Sci. 2019;7:38–44. doi: 10.3889/oamjms.2019.019
- Zhuang J., Lu J., Wang X., Wang X., Hu W., Hong F., Zhao X., Zheng Y. Purple Sweet Potato Color Protects against High-Fat Diet-Induced Cognitive Deficits through AMPK-Mediated Autophagy in Mouse Hippocampus. J. Nutr. Biochem. 2019;65:35–45. doi: 10.1016/j.jnutbio.2018.10.015
- Li J., Shi Z., Mi Y. Purple Sweet Potato Color Attenuates High Fat-Induced Neuroinflammation in Mouse Brain by Inhibiting MAPK and NF-ΚB Activation. Mol. Med. Rep. 2018;17:4823–4831. doi: 10.3892/mmr.2018.8440
- Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. The Lancet 2007;370(9603):1929-38. https://www.ncbi.nlm.nih.gov/pubmed/18063029
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27(5):1047-53. http://care.diabetesjournals.org/content/27/5/1047.long
- Monnier VM, Mustata GT, Biemel KL, Reihl O, Lederer MO, Zhenyu D, et al. Cross-linking of the extracellular matrix by the Maillard reaction in aging and diabetes: an update on “a puzzle nearing resolution”. Annals of the New York Academy of Sciences 2005;1043(Jun):533-44. https://www.ncbi.nlm.nih.gov/pubmed/16037276
- Naomi R., Bahari H., Yazid M.D., Othman F., Zakaria Z.A., Hussain M.K. Potential Effects of Sweet Potato (Ipomoea batatas) in Hyperglycemia and Dyslipidemia—A Systematic Review in Diabetic Retinopathy Context. Int. J. Mol. Sci. 2021;22:10816. doi: 10.3390/ijms221910816
- Li F, Li Q, Gao D, Peng Y. The optimal extraction parameters and anti-diabetic activity of flavonoids from Ipomoea batatas leaf. African Journal of Traditional, Complementary, and Alternative Medicines : AJTCAM / African Networks on Ethnomedicines 2009;6(2):195-202.
- Kusano S, Abe H, Tamura H. Isolation of antidiabetic components from white-skinned sweet potato (Ipomoea batatas L.). Bioscience, Biotechnology, and Biochemistry 2001;65(1):109-14. https://www.jstage.jst.go.jp/article/bbb/65/1/65_1_109/_pdf
- Sakuramata Y, Oe H, Kusano S, Aki O. Effects of combination of Caiapo with other plant-derived substance on anti-diabetic efficacy in KK-Ay mice. BioFactors (Oxford, England) 2004;22(1-4):149-52. https://www.ncbi.nlm.nih.gov/pubmed/15630271
- Park KH, Kim JR, Lee JS, Lee H, Cho KH. Ethanol and water extract of purple sweet potato exhibits anti-atherosclerotic activity and inhibits protein glycation. Journal of Medicinal Food 2010;13(1):91-8. https://www.ncbi.nlm.nih.gov/pubmed/20136441
- Egert S, Bosy-Westphal A, Seiberl J, Kurbitz C, Settler U, Plachta-Danielzik S, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. British Journal of Nutrition 2009;102(7):1065-74. https://www.ncbi.nlm.nih.gov/pubmed/19402938
- Huang Z, Wang B, Eaves DH, Shikany JM, Pace RD. Phenolic compound profile of selected vegetables frequently consumed by African Americans in the southeast United States. Food Chemistry 2007;103(4):1395-402.
- Islam I, Shaikh AU, Shahidul IM. Antioxidative and antimutagenic potentials of phytochemicals from Ipomoea batatas (L.) lam. International Journal of Cancer Research 2009;5(3):83-94.
- Kurata R, Adachi M, Yamakawa O, Yoshimoto M. Growth suppression of human cancer cells by polyphenolics from sweet potato (Ipomoea batatas L.) leaves. Journal of Agricultural and Food Chemistry 2007;55(1):185-90. https://www.ncbi.nlm.nih.gov/pubmed/17199331
- Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Letters 2008;269(2):281-90. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2582525/
- Kim JK, Choi SJ, Cho HY, Kim YJ, Lim ST, Kim CJ, et al. Ipomoea batatas attenuates amyloid beta peptide-induced neurotoxicity in ICR mice. Journal of Medicinal Food 2011;14:1-6. https://www.ncbi.nlm.nih.gov/pubmed/21244238
- Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF. Purple sweet potato color alleviates D-galactose-induced brain aging in old mice by promoting survival of neurons via PI3K pathway and inhibiting cytochrome C-mediated apoptosis. Brain Pathology (Zurich, Switzerland) 2010;20(3):598-612. https://www.ncbi.nlm.nih.gov/pubmed/19863544
- Wang YJ, Zheng YL, Lu J, Chen GQ, Wang XH, Feng J, et al. Purple sweet potato colour suppresses lipopolysaccharide-induced acute inflammatory response in mouse brain. Neurochemistry International 2010;56(3):424-30. https://www.ncbi.nlm.nih.gov/pubmed/19941923
- Wu DM, Lu J, Zheng YL, Zhou Z, Shan Q, Ma DF. Purple sweet potato colour repairs d-galactose-induced spatial learning and memory impairment by regulating the expression of synaptic proteins. Neurobiology of Learning and Memory 2008;90(1):19-27. https://www.ncbi.nlm.nih.gov/pubmed/18316211
- Ye J, Meng X, Yan C, Wang C. Effect of purple sweet potato anthocyanins on beta-amyloid-mediated PC-12 cells death by inhibition of oxidative stress. Neurochemical Research 2010;35(3):357-65. https://www.ncbi.nlm.nih.gov/pubmed/19771514
- Nguyen H.C., Nguyen H.N.T., Huang M.Y., Lin K.H., Pham D.C., Tran Y.B., Su C.H. Optimization of aqueous enzyme—Assisted extraction of rosmarinic acid from rosemary (Rosmarinus officinalis L.) leaves and the antioxidant activity of the extract. J. Food Process. Preser. 2021;45:e15221. doi: 10.1111/jfpp.15221
- Do T.H., Truong H.B., Nguyen H.C. Optimization of extraction of phenolic compounds from Ocimum basilicum leaves and evaluation of their antioxidant activity. Pharm. Chem. J. 2020;54:162–169. doi: 10.1007/s11094-020-02181-3
- Abonyi F., Iyi E., Machebe N. Effects of feeding sweet potato (Ipomoea batatas) leaves on growth performance and nutrient digestibility of rabbits. Afr. J. Biotechnol. 2012;11:3709–3712. doi: 10.5897/AJB11.3103
- Sun H., Mu T., Xi L., Zhang M., Chen J. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 2014;156:380–389. doi: 10.1016/j.foodchem.2014.01.079
- Islam S. Sweetpotato (Ipomoea batatas L.) leaf: Its potential effect on human health and nutrition. J. Food Sci. 2006;71:R13–R121. doi: 10.1111/j.1365-2621.2006.tb08912.x
- Shintani T.T., Hughes C.K., Beckham S., O’connor H. Obesity and cardiovascular risk intervention through the ad libitum feeding of traditional Hawaiian diet. Am. J. Clin. Nutr. 1991;53:1647S–1651S. doi: 10.1093/ajcn/53.6.1647S
- Karna P., Gundala S.R., Gupta M.V., Shamsi S.A., Pace R.D., Yates C., Narayan S., Aneja R. Polyphenol-rich sweet potato greens extract inhibits proliferation and induces apoptosis in prostate cancer cells in vitro and in vivo. Carcinogenesis. 2011;32:1872–1880. doi: 10.1093/carcin/bgr215
- Feng R., Lu Y., Bowman L.L., Qian Y., Castranova V., Ding M. Inhibition of activator protein-1, NF-κB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J. Biol. Chem. 2005;280:27888–27895. doi: 10.1074/jbc.M503347200
- Ishiguro K., Yoshimoto M., Tsubata M., Takagaki K. Hypotensive effect of sweetpotato [Ipomoea batatas] tops. J. Jpn. Soc. Food Sci. Technol. 2007;54:45–49. doi: 10.3136/nskkk.54.45
- Islam S. Antimicrobial activities of Ipomoea batatas (L.) leaf. J. Food Agric. Environ. 2008;6:14.
- Weickert M.O., Pfeiffer A.F. Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 2008;138:439–442. doi: 10.1093/jn/138.3.439
- Fu Z.F., Tu Z.C., Zhang L., Wang H., Wen Q.H., Huang T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016;15:11–18. doi: 10.1016/j.fbio.2016.04.004
- Zhang C., Liu D., Wu L., Zhang J., Li X., Wu W. Chemical characterization and antioxidant properties of ethanolic extract and its fractions from sweet potato (Ipomoea batatas L.) leaves. Foods. 2020;9:15. doi: 10.3390/foods9010015
- Luo C., Wang X., Gao G., Wang L., Li Y., Sun C. Identification and quantification of free, conjugate and total phenolic compounds in leaves of 20 sweetpotato cultivars by HPLC–DAD and HPLC–ESI–MS/MS. Food Chem. 2013;141:2697–2706. doi: 10.1016/j.foodchem.2013.05.009
- Xi L., Mu T., Sun H. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015;172:166–174. doi: 10.1016/j.foodchem.2014.09.039
- Liao W.C., Lai Y.C., Yuan M.C., Hsu Y.L., Chan C.F. Antioxidative activity of water extract of sweet potato leaves in Taiwan. Food Chem. 2011;127:1224–1228. doi: 10.1016/j.foodchem.2011.01.131
- Anastácio A., Carvalho I.S. Spotlight on PGI sweet potato from e urope: Study of plant part, time and solvent effects on antioxidant activity. J. Food Biochem. 2013;37:628–637. doi: 10.1111/jfbc.12017
- Wang S., Nie S., Zhu F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016;89:90–116. doi: 10.1016/j.foodres.2016.08.032
- Suárez S., Mu T., Sun H., Añón M.C. Antioxidant activity, nutritional, and phenolic composition of sweet potato leaves as affected by harvesting period. Int. J. Food Prop. 2020;23:178–188. doi: 10.1080/10942912.2020.1716796
- Sui W., Mu T., Sun H., Yang H. Effects of different drying methods on nutritional composition, physicochemical and functional properties of sweet potato leaves. J. Food Process. Preser. 2019;43:e13884. doi: 10.1111/jfpp.13884
- Sun H., Mu T., Xi L., Song Z. Effects of domestic cooking methods on polyphenols and antioxidant activity of sweet potato leaves. J. Agric. Food Chem. 2014;62:8982–8989. doi: 10.1021/jf502328d
- Zhang L., Tu Z.C., Wang H., Fu Z.F., Wen Q.H., Chang H.X., Huang X.Q. Comparison of different methods for extracting polyphenols from Ipomoea batatas leaves, and identification of antioxidant constituents by HPLC-QTOF-MS2. Food Res. Int. 2015;70:101–109. doi: 10.1016/j.foodres.2015.01.012
- Ji H., Zhang H., Li H., Li Y. Analysis on the nutrition composition and antioxidant activity of different types of sweet potato cultivars. Food Nutr. Sci. 2015;6:161–167. doi: 10.4236/fns.2015.61017
- Mbaeyi-Nwaoha I., Emejulu V. Evaluation of phytochemical composition and antimicrobial activity of sweet potato (Ipomoea batatas) leaf. Pak. J. Nutr. 2013;12:575–586. doi: 10.3923/pjn.2013.575.586
- Jang Y., Koh E. Antioxidant content and activity in leaves and petioles of six sweet potato (Ipomoea batatas L.) and antioxidant properties of blanched leaves. Food Sci. Biotechnol. 2019;28:337–345. doi: 10.1007/s10068-018-0481-3
- Chao P.Y., Huang W.Y., Hu S.P., Lo H.F., Lin K.H., Huang M.Y., Chang T.R., Yang C.M. Indigenous purple vegetable extracts protect against hydrogen peroxide-induced DNA damage in human lymphocytes. Food Nutr. Sci. 2013;4:62–70. doi: 10.4236/fns.2013.48A008
- Chang W.H., Hu S.P., Huang Y.F., Yeh T.S., Liu J.F. Effect of purple sweet potato leaves consumption on exercise-induced oxidative stress and IL-6 and HSP72 levels. J. Appl. Physiol. 2010;109:1710–1715. doi: 10.1152/japplphysiol.00205.2010
- Chang WH, Chen CM, Hu SP, Kan NW, Chiu CC, Liu JF. Effect of purple sweet potato leaf consumption on the modulation of the antioxidative status in basketball players during training. Asia Pac J Clin Nutr. 2007;16(3):455-61. https://apjcn.nhri.org.tw/server/APJCN/16/3/455.pdf
- Ameho C.K., Chen C.Y.O., Smith D., Sánchez-Moreno C., Milbury P.E., Blumberg J.B. Antioxidant activity and metabolite profile of quercetin in vitamin-E-depleted rats. J. Nutr. Biochem. 2008;19:467–474. doi: 10.1016/j.jnutbio.2007.06.004
- Moskaug J.Ø., Carlsen H., Myhrstad M.C., Blomhoff R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005;81:277S–283S. doi: 10.1093/ajcn/81.1.277S
- Zhang K., Yang E.B., Tang W.Y., Wong K.P., Mack P. Inhibition of glutathione reductase by plant polyphenols. Biochem. Pharmacol. 1997;54:1047–1053. doi: 10.1016/S0006-2952(97)00315-8
- Pietta P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000;63:1035–1042. doi: 10.1021/np9904509
- Chu Y.H., Chang C.L., Hsu H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000;80:561–566. doi: 10.1002/(SICI)1097-0010(200004)80:5<561::AID-JSFA574>3.0.CO;2-#
- Islam M.S., Yoshimoto M., Terahara N., Yamakawa O. Anthocyanin compositions in sweetpotato (Ipomoea batatas L.) leaves. Biosci. Biotechnol. Biochem. 2002;66:2483–2486. doi: 10.1271/bbb.66.2483
- Tang S.C., Lo H.F., Lin K.H., Chang T.J., Yang C.M., Chao P.Y. The antioxidant capacity of extracts from Taiwan indigenous purple-leaved vegetables. J. Taiwan Soc. Hort. Sci. 2013;59:43–57.
- Lako J., Trenerry V.C., Wahlqvist M., Wattanapenpaiboon N., Sotheeswaran S., Premier R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007;101:1727–1741. doi: 10.1016/j.foodchem.2006.01.031
- Hue S.M., Boyce A.N., Somasundram C. Antioxidant activity, phenolic and flavonoid contents in the leaves of different varieties of sweet potato (‘ipomoea batatas’) Aust. J. Crop Sci. 2012;6:375.
- Truong V.D., McFeeters R., Thompson R., Dean L., Shofran B. Phenolic acid content and composition in leaves and roots of common commercial sweetpotato (Ipomea batatas L.) cultivars in the United States. J. Food Sci. 2007;72:C343–C349. doi: 10.1111/j.1750-3841.2007.00415.x
- Vishnu V.R., Renjith R.S., Mukherjee A., Anil S.R., Sreekumar J., Jyothi A.N. Comparative study on the chemical structure and in vitro antiproliferative activity of anthocyanins in purple root tubers and leaves of sweet potato (Ipomoea batatas) J. Agric. Food Chem. 2019;67:2467–2475. doi: 10.1021/acs.jafc.8b05473
- Lim S., Xu J., Kim J., Chen T.Y., Su X., Standard J., Carey E., Griffin J., Herndon B., Katz B. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013;57:1908–1917. doi: 10.1002/mnfr.201300040
- Reddivari L., Vanamala J., Chintharlapalli S., Safe S.H., Miller J.C., Jr. Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways. Carcinogenesis. 2007;28:2227–2235. doi: 10.1093/carcin/bgm117
- Kurata R., Adachi M., Yamakawa O., Yoshimoto M. Growth suppression of human cancer cells by polyphenolics from sweetpotato (Ipomoea batatas L.) leaves. J. Agric. Food Chem. 2007;55:185–190. doi: 10.1021/jf0620259
- Chen C.M., Li S.C., Chen C.Y.O., Au H.K., Shih C.K., Hsu C.Y., Liu J.F. Constituents in purple sweet potato leaves inhibit in vitro angiogenesis with opposite effects ex vivo. Nutrition. 2011;27:1177–1182. doi: 10.1016/j.nut.2011.01.005
- Jin U.H., Lee J.Y., Kang S.K., Kim J.K., Park W.H., Kim J.G., Moon S.K., Kim C.H. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: Isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005;77:2760–2769. doi: 10.1016/j.lfs.2005.02.028
- Chang V.H.S., Yang D.H.A., Lin H.H., Pearce G., Ryan C.A., Chen Y.C. IbACP, a sixteen-amino-acid peptide isolated from Ipomoea batatas leaves, induces carcinoma cell apoptosis. Peptides. 2013;47:148–156. doi: 10.1016/j.peptides.2013.02.005
- Nakachi S., Tokeshi A., Takamatsu R., Arakaki K., Uehara M., Iguchi A., Taira J., Yoshimi N. The modifying effects of the extract from Okinawan sweet potato leaves in mouse colon carcinogenesis. Cancer Res. 2016;76:840.
- Olowu A.O., Adeneye A.A., Adeyemi O.O. Hypoglycaemic effect of Ipomoea batatas aqueous leaf and stem extract in normal and streptozotocin-induced hyperglycaemic rats. J. Nat. Pharm. 2011;2:56–61.
- Lee S.L., Chin T.Y., Tu S.C., Wang Y.J., Hsu Y.T., Kao M.C., Wu Y.C. Purple sweet potato leaf extract induces apoptosis and reduces inflammatory adipokine expression in 3T3-L1 differentiated adipocytes. Evid. Based Complement. Alternat. Med. 2015;2015:126302. doi: 10.1155/2015/126302
- Kang H., Kwak Y., Koppula S. Protective effect of purple sweet potato (Ipomoea batatas Linn, Convolvulaceae) on neuroinflammatory responses in lipopolysaccharide-stimulated microglial cells. Trop. J. Pharm. Res. 2014;13:1257–1263. doi: 10.4314/tjpr.v13i8.9
- Bassoli B.K., Cassolla P., Borba-Murad G.R., Constantin J., Salgueiro-Pagadigorria C.L., Bazotte R.B., da Silva R.S.D.S.F., de Souza H.M. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: Effects on hepatic glucose release and glycaemia. Cell Biochem. Funct. 2008;26:320–328. doi: 10.1002/cbf.1444
- Zhang L., Tu Z.C., Yuan T., Wang H., Xie X., Fu Z.F. Antioxidants and α-glucosidase inhibitors from Ipomoea batatas leaves identified by bioassay-guided approach and structure-activity relationships. Food Chem. 2016;208:61–67. doi: 10.1016/j.foodchem.2016.03.079
- Nagamine R., Ueno S., Tsubata M., Yamaguchi K., Takagaki K., Hira T., Hara H., Tsuda T. Dietary sweet potato (Ipomoea batatas L.) leaf extract attenuates hyperglycaemia by enhancing the secretion of glucagon-like peptide-1 (GLP-1) Food Funct. 2014;5:2309–2316. doi: 10.1039/C4FO00032C
- Lin K.H., Low P.Y., Chao P.Y., Shih M.C., Chiang M.C., Lai Y.C., Wu S.B. Antioxidant properties and glucose uptake effect of ethanol extracts from different sweet potato leaves prepared by lyophilization and oven-drying at 40 °C. Curr. Nutr. Food Sci. 2017;13:227–236. doi: 10.2174/1573401313666170222120700
- Pochapski MT, Fosquiera EC, Esmerino LA, Dos Santos EB, Farago PV, Santos FA, Groppo FC. Phytochemical screening, antioxidant, and antimicrobial activities of the crude leaves’ extract from Ipomoea batatas (L.) Lam. Pharmacogn Mag. 2011 Apr;7(26):165-70. doi: 10.4103/0973-1296.80682