What is citrulline
Citrulline is a non-essential amino acid made in your liver from ornithine and carbamoyl phosphate in one of the central reactions in the urea cycle. L-citrulline is synthesized in the urea cycle by the addition of carbon dioxide and ammonia to ornithine. L-citrulline is converted into L-arginine (arginine) by the enzymes argininosuccinate synthetase and argininosuccinate lyase in the presence of L-aspartate and ATP. L-arginine (arginine) is in turn responsible for citrulline’s therapeutic affects. Many of L-arginine’s activities, including its possible anti-atherogenic actions, may be accounted for by its role as the precursor to nitric oxide (NO). Nitric oxide (NO) is produced by all tissues of the body and plays very important roles in the cardiovascular system, immune system and nervous system. Nitric oxide (NO) is formed from L-arginine via the enzyme nitric oxide synthase or synthetase (NOS), and the effects of nitric oxide (NO) are mainly mediated by 3′,5′ -cyclic guanylate or cyclic GMP. Nitric oxide (NO) activates the enzyme guanylate cyclase, which catalyzes the synthesis of cyclic GMP from guanosine triphosphate or GTP. Cyclic GMP is converted to guanylic acid via the enzyme cyclic GMP phosphodiesterase.
Citrulline is also produced from L-arginine as a by-product of the reaction catalyzed by the enzyme nitric oxide synthase. Your kidneys convert most citrulline into arginine. L-arginine is converted to nitric oxide by nitric oxide synthase and L-citrulline is regenerated as a by-product. Citrulline is a protein building block in your body that your body then transforms the arginine into nitric oxide (NO), which expands blood vessels. This expansion increases blood flow and the delivery of oxygen and nutrients to exercising muscles and speeds up the removal of waste products that cause muscle fatigue. Citrulline supplements have been claimed to promote energy levels, stimulate the immune system and help detoxify ammonia (a cell toxin).
Besides the kidney, citrulline is readily converted into Arginine in nearly all cell types, including adipocytes, endothelial cells, enterocytes, macrophages, neurons, and myocytes 1. Studies with macrophages 2 and endothelial cells 3 demonstrated that citrulline is transported into cells by the N system which is selective for amino acids with a side-chain amide group (e.g., glutamine and asparagine). Inside cells, conversion of citrulline into Arginine via argininosuccinate synthase and lyase is the only pathway for citrulline utilization 1. Humans 4 with functional kidneys have high rates of Arginine synthesis from endogenous and exogenous citrulline.
L-citrulline, while being an amino acid, is not involved in protein synthesis and is not one of the amino acids coded for by DNA. Although citrulline cannot be incorporated in proteins during protein synthesis, several proteins are known to contain citrulline as an amino acid. These citrulline residues are generated by a family of enzymes called peptidylarginine deiminases, which convert the amino acid arginine into citrulline. Proteins that contain citrulline residues include myelin basic protein, fillagrin and several histone proteins.
Citrulline, the limiting factor in the arginine de novo synthesis 5, exhibits a low dietary intake (approximately 13% of total arginine) 6. However, the main precursor of citrulline is glutamine, which is converted in the enterocytes of the proximal small bowel, accounting for 60%–80% of the total citrulline 7. The small intestine releases the produced citrulline into the circulation, of which approximately 80% is taken up by the proximal tubular cells of the kidney for the arginine de novo synthesis 7. This is also known as the intestinal-renal axis. The balance between gut synthesis and kidney degradation determines the plasma citrulline concentration 8. Since the intestine is the main site of citrulline production, plasma citrulline concentration has been suggested as a biomarker of the functionality of the small bowel enterocyte mass 9, although the plasma concentration does not provide exact information concerning the balance between production and disposal. For example, renal failure is associated with an impairment of citrulline metabolism, because the kidney is the main organ that metabolizes citrulline into arginine 10.
In addition, citrulline can also be derived from the conversion of ornithine by ornithine transcarbamylase, which is present in the enterocytes and hepatocytes 11. This produced citrulline is also exported and enters the portal circulation to bypass the liver and serve as arginine precursor. Furthermore, the dietary intake of arginine account for 40% of the circulating citrulline 11.
Citrulline’s name is derived from citrullus, the Latin word for watermelon, from which it was first isolated.
In a randomized, double-blind, cross-over study involving 12 healthy adults 12, compared with placebo, oral citrulline supplementation increased plasma citrulline, arginine and ornithine concentrations, but failed to affect albumin, transthyretin, free insulin and insulin-like growth factor (IGF)-1 plasma concentrations, urinary nitrate excretion, or nitrogen balance. Citrulline supplementation did not alter leucine Ra, leucine oxidation, nor whole-body protein synthesis.
The research on citrulline as a performance supplement is limited. There’s not much scientific evidence to support taking citrulline supplements to improve exercise or athletic performance. In these studies, participants took up to 9 grams of citrulline for 1 day or 6 grams per day for up to 16 days.
Nitric oxide synthase or synthetase (NOS) is a heme-containing enzyme with some sequences similar to cytochrome P-450 reductase. Several isoforms of NOS exist, two of which are constitutive and one of which is inducible by immunological stimuli. The constitutive NOS found in the vascular endothelium is designated eNOS and that present in the brain, spinal cord and peripheral nervous system is designated nNOS. The form of NOS induced by immunological or inflammatory stimuli is known as iNOS. iNOS may be expressed constitutively in select tissues such as lung epithelium.
All the nitric oxide synthases use NADPH (reduced nicotinamide adenine dinucleotide phosphate) and oxygen (O2) as cosubstrates, as well as the cofactors FAD (flavin adenine dinucleotide), FMN (flavin mononucleotide), tetrahydrobiopterin and heme. Interestingly, ascorbic acid appears to enhance NOS activity by increasing intracellular tetrahydrobiopterin. eNOS and nNOS synthesize NO in response to an increased concentration of calcium ions or in some cases in response to calcium-independent stimuli, such as shear stress. In vitro studies of NOS indicate that the Km of the enzyme for L-arginine is in the micromolar range. The concentration of L-arginine in endothelial cells, as well as in other cells, and in plasma is in the millimolar range. What this means is that, under physiological conditions, NOS is saturated with its L-arginine substrate. In other words, L-arginine would not be expected to be rate-limiting for the enzyme, and it would not appear that supraphysiological levels of L-arginine which could occur with oral supplementation of the amino acid would make any difference with regard to NO production. The reaction would appear to have reached its maximum level. However, in vivo studies have demonstrated that, under certain conditions, e.g. hypercholesterolemia, L-arginine could enhance endothelial-dependent vasodilation and NO production.
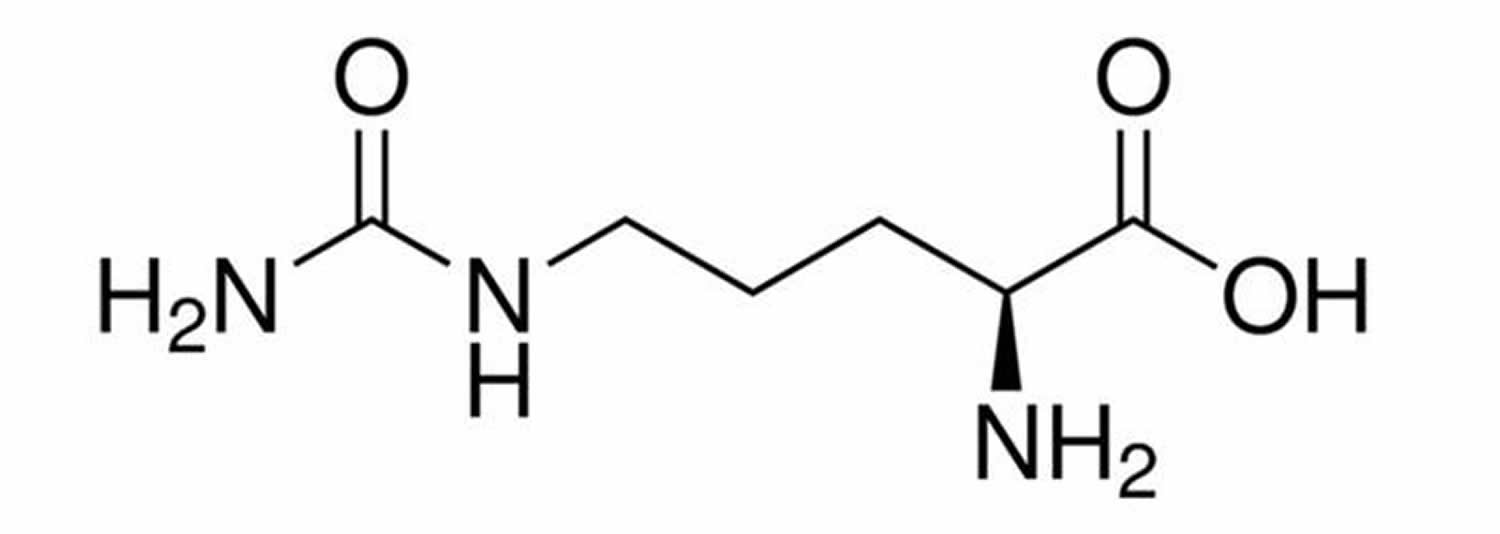
Figure 1. Citrulline
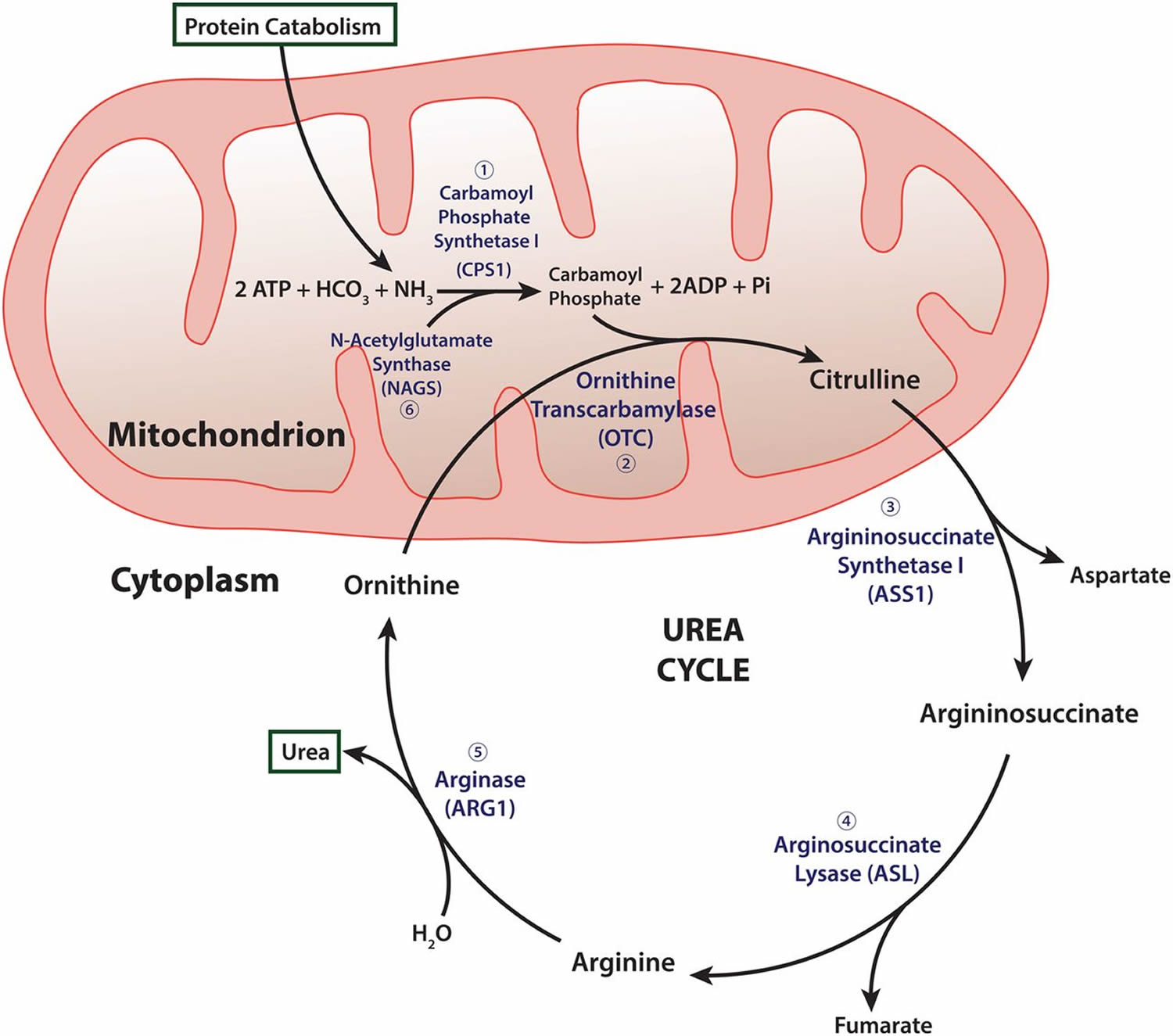
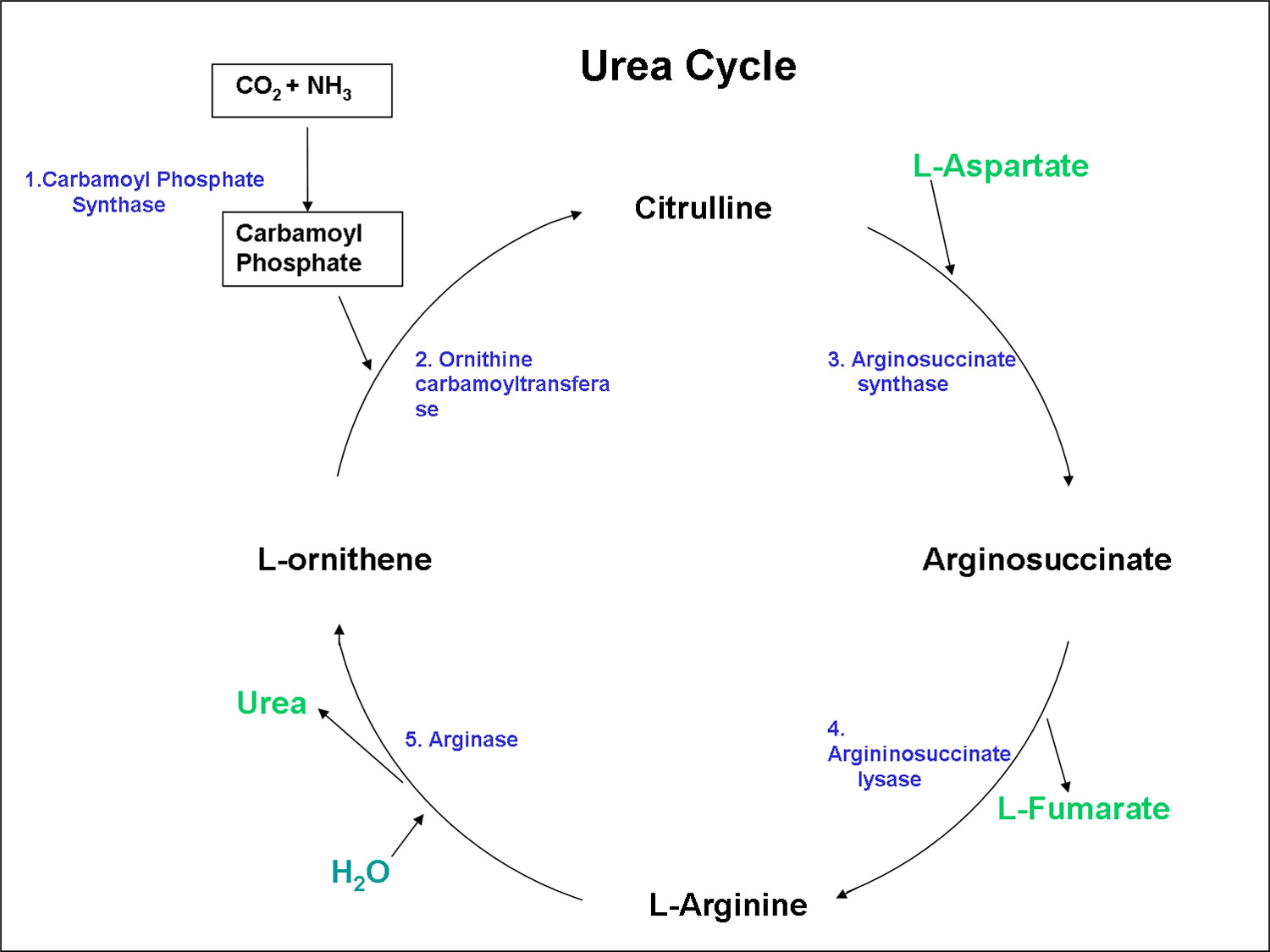
Figure 2. Urea cycle
Footnotes: Carbamyl phosphate synthetase I (CPSI) is rate-limiting enzyme of urea cycle. Polymorphisms in its gene alter availability of nitric oxide precursors, citrulline, and arginine. Citrulline is metabolized into arginine, which is then metabolized into either nitric oxide (NO) by nitric oxide synthetase (NOS) or urea by arginase (ARG).
Abbreviations: HCO3 = Bicarbonate; NH4 = ammonium; ATP = adenosine triphosphate; OTC = ornithine transcarbamylase; ASS = argininosuccinate synthetase; ASL = argininosuccinate lyase.
Citrulline supplementation during inflammatory conditions
Since reduced citrulline bioavailability in sepsis and endotoxemia, resulting in low plasma arginine 13 is associated with higher mortality rates 14, the supplementation of citrulline could be another therapeutic intervention restoring the balance between arginine production and metabolism as well as improving nitric oxide (NO) production and related functions. During endotoxemia and inflammatory conditions, supplementation of l-citrulline enhances plasma citrulline and arginine concentrations 15. Oral supplementation of l-citrulline in endotoxemic rats resulted in higher plasma arginine concentrations compared to l-arginine supplementation 16, making l-citrulline a more efficient supplement than l-arginine during inflammation. A study 17 previously showed that l-citrulline supplementation resulted in an enhanced nitric oxide (NO) concentration and improved microcirculation during endotoxemia in the jejunal villi, which was not observed following l-arginine supplementation.
Study of Qualls and colleagues 18 showed no enhancement of nitric oxide (NO) synthesis under arginine overflowing conditions in LPS + IFN-γ stimulated rat fetal liver-derived macrophages after addition of citrulline. However, under conditions of arginine scarcity, addition of citrulline contributed to an enhanced nitric oxide (NO) production in that same model 18. Interestingly, the effect of citrulline supplementation on increased NO production was only seen in arginine depleted macrophages, suggesting citrulline metabolism contributes to macrophage arginine biosynthesis and NO production only under conditions of arginine scarcity 18.
However, citrulline supplementation requires a functional NOS3 complex, and thus also a functional intracellular argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL) complex. As previously demonstrated, in tissue specific arginase-deficient mice, an enhanced NOS2 pathway was present due to the absence of the inhibitory effects of arginase in the macrophage 19. Citrulline supplementation in these animals did not lead to an improved outcome, based on the absence of a functional NOS3 complex.
L-citrulline dosage
Citrulline, the endogenous precursor for the synthesis of arginine, has been proposed as an alternative to arginine supplementation 20. This extensive extraction by the splanchnic organs limits the ability of arginine supplementation to increase arginine availability. Because of the reduced first-pass metabolism of citrulline and the fact that the only known fate of this amino acid is its conversion to arginine, citrulline has the potential to be a more efficient option to increase arginine availability. Only ∼30% of the supplemented arginine escaped splanchnic first-pass metabolism (that is 70% of the supplemental arginine disappeared before reaching the peripheral circulation) and reached peripheral circulation, increasing plasma arginine concentrations 21.
L-Citrulline dose-dependently increased plasma L-arginine concentration more effectively than L-arginine 22. The highest dose of citrulline (3 g twice daily) increased the plasma L-arginine and improved the L-arginine/asymmetric dimethylarginine (ADMA) ratio from 186 ± 8 (baseline) to 278 ± 14 and augments NO-dependent signalling in a dose-dependent manner.
The appropriate dose of L-citrulline depends on several factors such as the user’s age, health, and several other conditions. At this time there is not enough scientific information to determine an appropriate range of doses for L-citrulline. However doses of arginine used in clinical research have varied considerably, from as little as 500 mg per day for oligospermia to as much as 30 g per day for cancer, preeclampsia, and premature uterine contractions. Typical doses fall into either the 1-3 g per day range, or the 7-15 g per day range, depending on the condition being treated 23.
Citrulline vs Arginine
Arginine (L-Arginine) is a conditionally essential amino acid that can be derived from dietary intake (approximately 4–6 g of arginine per day) 24, from de novo synthesis from citrulline (10%–15% of the total arginine production) [see urea cycle] 25 and through protein breakdown (approximately 80%) 26. Arginine is synthesized in mammals from glutamine via pyrroline 5-carboxylate (P5C) synthetase and proline oxidase in a multi-step metabolic conversion 27. In adults, most endogenous arginine is derived from citrulline, a by-product of glutamine metabolism in the gut or liver. Citrulline is released into the circulation and taken up primarily by the kidney for conversion into arginine 28.
Approximately 15%–20% of arginine enters the urea cycle 29 where the enzyme arginase converts arginine into urea and ornithine 29. There are two different isotypes of arginase; arginase-I and arginase-II. The cytosolic arginase-I is highly expressed constitutively in the liver, which accounts for 20% of the whole body conversion of arginine into ornithine 30. Furthermore, arginase-I is also expressed in macrophages 31 and endothelial cells 19. Mitochondrial arginase-II is expressed in low concentrations in extrahepatic tissue, such as kidney, brain, small intestine 32, mammary gland and macrophages 33. Type II arginase is mainly involved in the syntheses of ornithine as precursor for proline and polyamines synthesis, such as spermine, spermidine, and putrescine 34.
In endothelial cells, both arginase isoforms are expressed constitutively, although the expression of the specific isoforms differs between the species 33. Arginase activity can consequently regulate NO synthesis, promote the production of glutamate and proline due to expression of ornithine aminotransferase and enhance cellular polyamine levels 34. In the presence of arginase-I overexpression in the small intestine, as present in transgenic mice, an impaired growth and development was observed as a result of a decreased arginine availability 35.
L-Arginine is synthesized from glutamine, glutamate, and proline via the intestinal-renal axis in humans and most other mammals (including pigs, sheep and rats). L-Arginine degradation occurs via multiple pathways that are initiated by arginase, nitric-oxide synthase, arginine:glycine amidinotransferase, and arginine decarboxylase. These pathways produce nitric oxide, polyamines, proline, glutamate, creatine, and agmatine with each having enormous biological importance. L-Arginine is also required for the detoxification of ammonia, which is an extremely toxic substance for the central nervous system. There is compelling evidence that l-Arginine regulates interorgan metabolism of energy substrates and the function of multiple organs. The results of both experimental and clinical studies indicate that l-Arginine is a nutritionally essential amino acid for spermatogenesis, embryonic survival, fetal and neonatal growth, as well as maintenance of vascular tone and hemodynamics 36. Moreover, a growing body of evidence clearly indicates that dietary supplementation or intravenous administration of l-Arginine is beneficial in improving reproductive, cardiovascular, pulmonary, renal, gastrointestinal, liver and immune functions, as well as facilitating wound healing, enhancing insulin sensitivity, and maintaining tissue integrity 36. Additionally, l-Arginine or l-citrulline may provide novel and effective therapies for obesity, diabetes, and the metabolic syndrome 36. The effect of l-Arginine in treating many developmental and health problems is unique among amino acids, and offers great promise for improved health and wellbeing of humans and animals.
Table 1. Roles of arginine in growth, health and disease
| Roles of arginine | Effect | Mediators |
|---|---|---|
| Cardiovascular disorders | ||
| Coronary and peripheral arterial diseases | ↓ | NO |
| Heart failure, stroke, and ischemia/reperfusion injury | ↓ | NO |
| Sickle cell anemia and vasculopathy | ↓ | NO |
| Endothelial dysfunction in patients with CVRF | ||
| Aging and hyperhomocysteinemia | ↓ | NO |
| Diabetes, hypertension, and smoking | ↓ | NO |
| Hypercholesterolemia and high fat feeding | ↓ | NO |
| Hormone secretion | ||
| Growth hormone, glucagon, insulin, and prolactin | ↑ | NO and ornithine |
| Placental lactogen and progesterone | ↑ | NO and ornithine |
| Immune function | ||
| B-cell maturation and antibody production | ↑ | NO, PA, and PS |
| Killing pathogens (bacteria, fungi, parasites and virus) | ↑ | NO |
| T-cell proliferation and cytokine production | ↑ | NO, PA, and PS |
| Metabolism | ||
| BAT growth and energy-substrate oxidation | ↑ | cGMP, PA, cAMP, and NO |
| Cell signaling (AMPK, mTOR, and cGMP) | ↑ | NO and Arg |
| Lactogenesis and neonatal growth and development | ↑ | Arg, NO, mTOR, PA, and proline |
| Mitochondrial biogenesis and function | ↑ | cGMP, PA, and NO |
| Protein synthesis and muscle growth | ↑ | mTOR and PA |
| Ammonia detoxification via the urea cycle | ↑ | NAG and ornithine |
| Obesity, insulin resistance, and dyslipidemia | ↓ | AMPK signaling, Arg, and NO |
| Orotic aciduria and gouts | ↓ | NAG and ornithine |
| Production of ROS and oxidative stress | ↓ | Arg, creatine, PA, and NO |
| Protein degradation and apoptosis | ↓ | mTOR, NO, and autophagy |
| Reproduction | ||
| Embryo implantation, survival, and growth | ↑ | NO, PA, and PS |
| Fetal survival, growth, and health | ↑ | NO, PA, and PS |
| Ovulation, ovarian steroidogenesis and oocyte quality | ↑ | NO and PA |
| Placental angiogenesis, growth, and function | ↑ | NO, PA, and PS |
| Spermatogenesis, sperm quality, and male fertility | ↑ | NO, PA, and PS |
| Uterine contractility and preterm labor | ↓ | NO |
| Erectile dysfunction | ↓ | NO |
| Preeclampsia in human pregnancy and animal models | ↓ | NO |
| Skeletal muscle and brain function | ↑ | Creatine, NO, and PS |
| Tissue injury and repair | ||
| Cystic fibrosis and lung injury | ↓ | NO, PA, and proline |
| Gastrointestinal, liver and vessel injury | ↓ | NO, PA, and proline |
| Necrotizing enterocolitis in infants | ↓ | NO and PA |
| Renal disease with systemic hypertension | ↓ | NO |
| Severe malaria, ulcers, and mitochondrial myopathy | ↓ | NO |
| Tissue integrity, wound healing, and angiogenesis | ↑ | NO, PA, proline, and PS |
| Tumor growth | ||
| Tumorigenesis at early stages | ↓ | NO |
| Tumorigenesis at late stages | ↑ | PA, proline, ornithine, and PS |
Footnotes:
The symbols “↑” and “↓” denote enhancement and inhibition (or prevention), respectively
Abbreviations: AMPK = AMP-activated protein kinase; BAT= brown adipose tissue; CVRF = cardiovascular risk factors; mTOR mammalian target of rapamycin (protein kinase); NAG = N-acetylglutamate; NO = nitric oxide; PA = polyamines; PS = protein synthesis; ROS = reactive oxygen species
Arginine content is relatively high in seafood, watermelon juice, nuts, seeds, algae, meats, rice protein concentrate, and soy protein isolate 38, but low in the milk of most mammals (including cows, humans, and pigs) 39. Results of the third National Health and Nutrition Examination Survey (NHANES III) indicate that mean arginine intake for the US adult population is 4.4 g/day, with 25, 20 and 10% of people consuming <2.6 (suboptimal), 5–7.5, and >7.5 g/day, respectively 40. In addition, preterm infants, who represent 10–12% of newborns, exhibit L-Arginine deficiency 41, resulting in hyperammonemia and multiorgan dysfunction 42.
L-arginine deficiency is also linked to a variety of inflammatory and oxidative processes in the vascular endothelium, and may be crucial in the development of atherosclerosis 43. The normal function of the vascular system depends on nitric oxide (NO) production by vascular endothelial cells. However, under conditions associated with oxidative vascular injury, such as obesity or hypertension, excess formation of reactive oxygen species (ROS) can lead to an accumulation of asymmetric dimethyl arginine 44. This accumulation of asymmetric dimethyl arginine competitively inhibits NO synthase (NOS), which decreases nitric oxide (NO) production. With limited L-arginine, NOS forms superoxide (O-), which causes vascular endothelial injury and further inflammation. The deleterious effects of low L-arginine availability may lead to a pro-atherosclerotic environment and subsequent cardiovascular disease 45.
Thus, dietary intake of L-arginine may be critically important in the context of how the human body responds to inflammation and oxidative stress. Lower intake of dietary L-arginine has recently been associated with higher levels of C-reactive protein (CRP), an inflammatory biomarker 46. Recent studies suggest that supplemental L-arginine may be helpful in preventing harmful oxidation and reversing endothelial dysfunction 47. In addition, supplementation with L-arginine may reverse the endothelial dysfunction associated with hypercholesterolemia, smoking, and hypertension 48. Some of L-arginine’s antioxidant and anti-inflammatory effects are independent of nitric oxide (NO) production 49.
Arginine is poorly absorbed in the intestine, with the jejunum as major absorption site, and exhibits a significant liver uptake 50. The synthesized arginine from citrulline accounts for 60% of the de novo whole-body arginine synthesis, however this only represents 5%–15% of the total circulating arginine. This indicates that most of the plasma arginine is derived from proteolysis and food intake 51.
Regulation of Arginine metabolism
Arginine metabolism is regulated by multiple factors that include dietary components (e.g., lysine, manganese, omega-3 fatty acids), hormones (e.g., glucocorticoids, growth hormone, and leptin), cytokines, endotoxins, and endogenously generated substances (e.g., creatine, lactate, ornithine, P5C, and methylarginines) 52. Lysine competes with Arginine for entry into cells and also inhibits arginase activity 1. Therefore, the dietary arginine:lysine ratio is a critical factor influencing the effect of arginine supplementation. Under normal feeding conditions, the total amount of Arginine in the diet should not be 150% greater than that of lysine (namely, arginine/lysine <2.5).
Glucocorticoids (e.g. cortisol) play a major role in upregulating Arginine metabolism via the arginase pathway in many cell types, particularly hepatocytes and enterocytes 53. In contrast, these hormones inhibit NO generation by suppressing NOS expression and BH4 synthesis 54. During weaning, the glucocorticoid surge induces expression of intestinal P5C synthase and arginase, resulting in enhanced citrulline production, Arginine hydrolysis, and polyamine synthesis 55. Consequently, there is a metabolically significant urea cycle for ammonia detoxification in enterocytes of postweaning pigs 56. Interestingly, a high level of circulating cortisol in the porcine fetus during late gestation and in the newborn does not induce arginase expression in their small intestines 57. Thus, intestinal arginase expression is unresponsive to cortisol during the fetal and early neonatal periods, possibly due to limited expression of glucocorticoid receptors or inactive signal transduction in enterocytes 53.
Cytokines (e.g., interleukin 4 and interferon-γ), other inflammatory stimuli (e.g., lipopolysaccharide), and cAMP can greatly stimulate expression of arginase I, arginase II, and ornithine decarboxylase in many cell types 58. Inflammatory cytokines and endotoxins also strongly induce the expression of NOS2 and GTP cyclohydrolase I (the first and rate-controlling enzyme in de novo BH4 synthesis) in almost all cell types 54. Therefore, these substances upregulate Arginine metabolism for the synthesis of urea, ornithine, proline, polyamines and NO in a cell-specific manner. For example, in response to intraperitoneal administration of lipopolysaccharide, whole-body NO production increases by 10- to 20-fold within 24 h 59. In contrast, lactate decreases intestinal citrulline synthesis by inhibiting proline oxidase via a noncompetitive mechanism 60. Therefore, concentrations of Arginine in plasma are reduced markedly in response to infection or inflammation 61. Impaired synthesis from proline in enterocytes results in a deficiency of citrulline and arginine in neonates or adults with hyperlactacidemia 60.
NG-monomethy-l-arginine (NMMA) and asymmetric dimethylarginine (ADMA) are competitive inhibitors of all NOS isoforms. However, concentrations of NMMA and ADMA are very low in plasma of healthy subjects (0.5–1 μM) compared with those of arginine (100–250 μM) depending on nutritional state and developmental stages) 62. Kohli et al. 63 reported that 1 μM NMMA or ADMA did not affect NO synthesis in endothelial cells. Although much higher concentrations of NMMA and ADMA (e.g., 5–10 μM) can inhibit NO synthesis by these cells 64, the physiological significance of endogenous methylarginines in the regulation of NO production remains to be defined.
Arginine benefits
Arginine and Fertility
On the basis of nitrogen balance, Arg was traditionally not considered as a nutritionally essential AA for healthy adult humans (Flynn et al. 2002) or livestock species (Wu et al. 2007c). However, this notion is not supported by studies on fertility in both males and females. Seminal fluid is particularly abundant in polyamines (putrescine, spermidine and spermine), polycationic products of Arg degradation, that are essential for cell growth and differentiation. For example, concentrations of polyamines are relatively high in porcine seminal fluid (∼90 μM), in comparison with plasma (3–5 μM) (Fig. 2). Dietary supplementation with 1% Arg-HCl to sexually active boars for 30 days had no effect on the volume of ejaculated semen (Wu et al. 2007b), but enhanced concentrations of Arg, proline, ornithine, and polyamines in seminal fluid by 43, 41, 56, and 63%, respectively, compared with the control group (Fig. 2). In addition, dietary Arg supplementation to boars increased sperm counts by 18% and sperm motility by 8% (Wu et al. 2007b). Notably, Holt and Albanese (1944) reported that feeding an Arg-deficient diet to adult men for 9 days decreased both the number and motility of sperm cells by 90%. This striking observation underlines a critical role for Arg in spermatogenesis and argues that functional needs beyond tissue protein synthesis should be important criteria for the classification of AA as nutritionally essential or nonessential. Thus, enhancing Arg provision may improve fertility in males. For example, Tanimura (1967) reported that oral administration of Arg-HCl (0.5 g/day) to infertile men for 6–8 weeks markedly increased sperm counts and motility in most patients and resulted in successful pregnancies. The underlying mechanism(s) may include (1) enhanced synthesis of polyamines and Arg-rich basic proteins in sperm cells; and (2) a regulatory role for NO in sperm motility and capacitation (Balercia et al. 2004) as well as the sustenance of good-quality fertilized oocytes (Goud et al. 2008). Because men with high body mass indexes (BMI) are more likely to be infertile than their normal weight counterparts (Nguyen et al. 2007), it would be important to determine if dietary Arg supplementation will improve fertility in obese males.
Female fertility is also a significant problem in human medicine and animal agriculture. For example, a high BMI is associated with lower success rates in women for live births following the use of assisted reproductive technologies (Veleva et al. 2008). Also, studies of dairy cows and pigs indicate that up to 60% embryonic losses occur during early pregnancy, particularly under an adverse environment (e.g., hot and humid conditions) (Starbuck et al. 2004; Wu et al. 2006). Recognizing an important role for Arg in embryonic and conceptus survival and growth (Wu et al. 1996a, 2004a, b, c), we have developed Arg treatment protocols to enhance pregnancy outcome. For example, dietary supplementation with 1% Arg-HCl between days 30 and 114 of gestation increased fetal survival in gilts (Mateo et al. 2007). In addition, supplementing 1.3% Arg-HCl to the diet for female rats either throughout the entire pregnancy (21 days) or between days 1 and 7 of gestation increased embryonic survival and birth litter size by 30% (Zeng et al. 2008). It remains to be determined whether dietary Arg supplementation can ameliorate embryonic deaths in women and livestock species.
Arginine and fetal growth and development
Polyamines and NO are essential to placental growth and angiogenesis (the growth of new vessels from the existing vasculature) and, therefore, for increasing uterine and placental-fetal blood flow 65. Feeding Arginine-free diets to pregnant rats or inhibiting NO synthesis resulted in increased fetal resorptions, intrauterine growth retardation (IUGR), increased perinatal mortality, and decreased number of live fetuses 66. Thus, endogenous synthesis of Arginine is insufficient for pregnant dams (female dog, rat or horse) and must be provided from diets to support fetal survival and growth. Accordingly, dietary Arginine supplementation (0.2 or 2% Arginine in drinking water) prevented hypoxia-induced fetal growth retardation in rats 67. Similarly, dietary supplementation with Arginine to gilts or rats fed conventional diets during pregnancy increased the live-born litter weight by 24 and 30%, respectively 68. Similarly, intravenous administration of Arginine-HCl (3 × 27 mg/kg body weight per day) enhanced fetal growth in sheep models of both undernutrition-induced and naturally-occurring IUGR 69. Finally, during late (week 33) gestation, daily intravenous infusion of Arginine (20 g/day) for 7 days to women with unknown causes of IUGR increased birth weight at term (week 39) by 6.4% 70. These findings provide a strong experimental basis for the use of Arginine to prevent and treat IUGR in animals and humans.
Neonates supported by total parenteral nutrition
Preterm infants are primarily supported by total parenteral nutrition (TPN) in the first weeks of life due to their inability to tolerate oral feeding. Past and current commercial TPN solutions do not contain glutamine, although preterm infants have underdeveloped Arginine-synthetic pathways and reduced intestinal mass for citrulline (and hence Arginine) production 57. As an allosteric activator of N-acetylglutamate synthase and a substrate of ornithine, Arginine is required to maintain the urea cycle in an active state. Consequently, life-threatening hyperammonemia can occur in preterm infants because of Arginine deficiency (≤32 μM in plasma) 71. Importantly, increasing Arginine provision may prevent hyperammonemia 71 and necrotizing enterocolitis 72 in preterm infants, as well as persistent pulmonary hypertension of neonates 73.
Because the small intestine prefers to utilize enteral amino acid for citrulline synthesis 1, dietary Arginine requirements estimated on the basis of enteral feeding cannot be applied to TPN feeding. Brunton et al. 74 reported that intravenous infusion of neonatal pigs with an amino acid solution (containing proline but no glutamine, ornithine, citrulline or Arginine) elicited Arginine deficiency, which resulted in hyperammonemia and death. However, enteral feeding of the same amino acid solution to neonatal pigs ameliorated Arginine deficiency 74, which further supports an important role for the small intestine in endogenous synthesis of Arginine from proline 75.
Neonates fed enteral diets
There are only a few reports of studies regarding Arginine nutrition in enterally fed infants. On the basis of a nitrogen balance study involving only two term infants (1.5–3 week-old) and one preterm infant (3.5 month-old), Snyderman et al. 76 concluded that Arginine was not an essential amino acid for neonates. However, these authors did not measure either metabolic indicators of an Arginine deficiency (e.g., plasma concentrations of Arginine and ammonia, as well as urinary excretion of orotic acid and ammonia) or physiological parameters (e.g., cardiovascular, pulmonary, intestinal, muscular, immunological, and neurological functions). Feeding Arginine-deficient diets to infants likely results in low Arginine concentrations in their plasma 57, which may affect all of the above metabolic and physiological parameters. Clearly, much research is needed to define Arginine requirements of infants.
The relative contributions of milk versus endogenous synthesis to meet Arginine requirements of the suckling neonate can be estimated on the basis of Arginine intake and Arginine accretion plus catabolism in the body. Published studies with the neonatal pig indicate that endogenous synthesis must provide at least 60% of Arginine for a 7-day-old suckling pig 77. Accordingly, inhibition of intestinal synthesis of citrulline and Arginine for 12 h decreased plasma concentrations of citrulline and Arginine by 52 and 76%, respectively, in the 4-day-old suckling pig 78. Both metabolic and growth data indicate that Arginine deficiency, due to a low availability of mitochondrial NAG in enterocytes, is a major factor limiting maximal growth of milk-fed piglets 77. Thus, either dietary supplementation with Arginine 79 or activation of endogenous Arginine synthesis 80 is effective in increasing Arginine availability and growth performance in milk-fed piglets. Additionally, dietary Arginine supplementation promotes lactogenesis in sows and the growth of suckling piglets with normal- or low-birth weights 81. Interestingly, the effect of Arginine provision was greater for low-birth-weight piglets than for their normal-birth-weight littermates 82, suggesting a lower rate of endogenous Arginine synthesis in IUGR piglets. Similarly, oral administration of Arginine (3 × 55–70 mg/kg body weight per day) for 3–18 months prevented ammonia toxicity and enhanced growth of infants with an inborn deficiency of Arginine synthesis 83.
Arginine and preeclampsia and preterm labor
There are both clinical and biochemical evidence for endothelial dysfunction in preeclamptic women 84. Additionally, plasma levels of Arginine and placental NOS3 abundance are reduced in preeclamptic compared to healthy pregnant women 85. In a rat model of preeclampsia induced by chronic inhibition of NO synthesis, intravenous administration of Arginine (0.16 g/kg body wt/day) from gestational day 10 through term reversed the disease-associated lesions (hypertension, IUGR, proteinuria, and renal glomerulus injury) 86. Beginning at 29 weeks of gestation, oral administration of Arginine (3 g daily for 4 weeks) to women with preeclampsia increased NO synthesis, reduced blood pressure, prolonged pregnancy, improved fetal wellbeing, and enhanced fetal growth 87. Also, administration of Arginine (20 g/day intravenously for 5 days followed by 4 g/day orally for 2 weeks) to women with gestational hypertension prolonged pregnancy, reduced blood pressure, and decreased the frequency of babies with low birth weights 88.
Preterm births account for 5–10% of all births and are a major cause of neonatal morbidity and mortality 57. Thus, prevention of preterm labor has a profound impact on improving neonatal survival and reducing the high costs of intensive care. Some evidence from human and animal studies indicates that NO inhibits uterine contractility and may play an important role in maintaining uterine quiescence during pregnancy 89. Thus, inhibition of NO synthesis resulted in preterm delivery in mice, which was reversed by infusion of sodium nitroprusside (an NO donor) 90. Importantly, intravenous infusion of Arginine (30 g over 30 min) into women with the preterm onset of uterine contractions reduced spontaneous uterine contractility 91. Similarly, oral administration of Arginine (3 g/day for 7 days) to women with threatened preterm labor beneficially increased pulsatility indexes of middle cerebral arteries and altered fetal-placental blood flow distribution 92. These findings suggest that Arginine administration may be a novel means to prevent preterm birth.
Arginine and obesity and the metabolic syndrome
Obesity in humans (and animals) occurs because of a chronic imbalance between energy intake and expenditure. This disease is a major health crisis worldwide and is a leading risk factor for insulin resistance, type 2 diabetes, atherosclerosis, stroke, hypertension, and some types of cancer (including colon and breast cancers). Growing evidence indicates that Arginine supplementation may be a novel therapy for obesity and the metabolic syndrome. First, dietary supplementation with Arginine decreased plasma levels of glucose, homocysteine, fatty acids, and triglycerides, and improved insulin sensitivity in chemically induced diabetic rats 63, genetically obese Zucker diabetic fatty rats 93, and diet-induced obese rats 94. Similar results have been reported for obese humans with type-II diabetes receiving oral (Lucotti et al. 2006) or intravenous 95 administration of Arginine. Second, citrulline or Arginine supplementation retarded the progression of high fat diet-induced atherosclerosis in obese rabbits 96. Third, supplementing conventional diets for growing-finishing pigs with Arginine reduced body-fat accretion, enhanced muscle gain, and improved the metabolic profile 97. A distinct advantage of Arginine over drugs (e.g., metformin and thiazolidinediones) is that dietary Arginine supplementation reduces adiposity, while improving insulin sensitivity 98. The possible underlying mechanisms for the effect of Arginine may involve multiple NO-dependent pathways that favor the whole-body oxidation of fatty acids and glucose 99.
Arginine and endothelial dysfunction
An NO deficiency is a major factor contributing to endothelial dysfunction, which occurs in a variety of metabolic disorders, including diabetes, hypercholesterolemia, hypertension, tobacco smoking, and malaria (Wu and Meininger 2000). Several lines of evidence demonstrate that Arg administration is effective in reversing endothelial dysfunction under these conditions. First, oral administration of Arg (1.25% Arg in drinking water) to diabetic rats reversed the defective endothelium-dependent relaxation (Pieper 1997). Also, dietary supplementation with Arg or watermelon (rich in citrulline) increased circulating levels of Arg, endothelial BH4 availability and NO synthesis, and enhanced endothelium-dependent relaxation in Zucker diabetic fatty rats (Wu et al. 2007d). Second, the impaired endothelium-dependent relaxation in hypercholesterolemic humans could be improved by intravenous infusion of Arg (10 mg/kg body weight per min for 20 min) or oral administration of Arg (7 g × 3/day) (Clarkson et al. 1996; Creager et al. 1992). Third, acute supplementation of 15 g Arg ameliorated endothelial dysfunction and oxidative stress in young men fed a high fat diet (Lin et al. 2008). Fourth, hypertension in adult rats with intestinal resection (Wakabayashi et al. 1994) or patients with lysinuric protein intolerance (Kamada et al. 2001) could be prevented by parenteral administration of Arg. Fifth, intravenous infusion of Arg (30 g Arg-HCl over 45 min) normalized coronary vasomotion in long-term smokers (Campisi et al. 1999), and 3-day oral administration of Arg (7 g/day) prevented smoking-induced impairment of endothelial function in young adults (Siasos et al. 2008). Finally, intravenous infusion of Arg reversed endothelial dysfunction in patients with severe falciparum malaria, which is characterized by the cytoadherence of parasitized erythrocytes to the microvascular endothelium, hemolysis and impaired blood flow (Yeo et al. 2007). The mechanisms by which Arg administration may prevent cardiovascular dysfunction include: (1) restoring endothelial NO synthesis and decreasing superoxide production; (2) reducing vascular oxidative damage; and (3) inhibiting platelet adherence and aggregation, leukocyte adherence to the endothelium, and the proliferation of vascular smooth muscle cells (Wu and Meininger 2000, 2009).
Arginine and sickle cell disease vasculopathy
Sickle cell disease is associated with hemolysis or premature destruction of red blood cells. This, along with the injury to other cell types, results in elevated levels of Arginineinase and reduced concentrations of Arginine in plasma, impaired NO synthesis by endothelial cells 100, as well as microvascular vaso-occlusion, vasoculopathy, and multiorgan injury 101. Studies involving a transgenic-knockout mouse model with sickle cell disease showed that dietary Arginine supplementation reduced red blood cell density and Gardos channel activity, while increasing systemic NO synthesis, reducing oxidative stress, and improving microvascular function (Kaul et al. 2008). Importantly, oral administration of l-citrulline (0.1 g/kg body weight per day) normalized circulating levels of Arginine and total leukocyte counts, and improved wellbeing in patients with sickle cell disease 102.
Arginine and immunity
Arginine regulates NO synthesis by NOS2, production of antibodies by B-cells, as well as T-cell receptor expression and B-cell development 103. Thus, Arginine plays an important role in both innate and acquired immunity. Inadequate intake of dietary Arginine impairs NO synthesis by both constitutive and inducible NOS in mammals 104, indicating a role for adequate Arginine nutrition in immune function. Available evidence shows that Arginine is required for defense against viruses, bacteria, fungi, malignant cells, intracellular protozoa, and parasites in mammals, birds, terrestrial animals, lower vertebrates, and invertebrates 61. For example, adequate provision of Arginine is required for lymphocyte proliferation and development, and dietary Arginine supplementation enhances immune responses in various models of immunological challenges 61. Moreover, dietary supplementation with 1 or 2% Arginine (approximately 1 or 2 times the Arginine content of the regular diet) to tumor-bearing or septic rats increased thymic weight, the number of thymic lymphocytes, T-lymphocyte proliferation, the cytotoxicity of specific cells (T lymphocyte, macrophages, and NK cells), IL-2 production, IL-2 receptor expression on T lymphocytes, and the delayed type hypersensitivity response 61. Further, dietary supplementation with 1% Arginine-HCl enhanced the immune status of pregnant sows and neonatal pigs, thereby reducing morbidity and mortality in response to infectious pathogens 105.
Arginine and cancers
Elevated levels of polyamines and NO promote and inhibit tumor growth, respectively 106. Thus, whether Arginine suppresses or enhances tumor growth depends on the relative activities of NOS and arginase pathways, whose expression may vary with the stage of carcinogenesis. This may explain apparently conflicting findings in the literature that Arginine both stimulated and inhibited the growth of tumors 107. The majority of in vivo studies have shown that dietary Arginine supplementation from the time of tumor induction or inoculation resulted in protection against the growth of transplantable tumors and the tumorigenicity of carcinogens 107. For example, low-dose oral supplementation of Arginine (50 mg/kg body wt/day) for 1 year decreased the total number of tumors and increased survival in mice, via NO-mediated cytotoxicity against tumor cells and blocking the formation of lipid peroxidation products 108. Furthermore, Arginine supplementation (1% Arginine in drinking water) during the initiation stage of carcinogenesis decreased colorectal tumor production and crypt cell hyperproliferation, but Arginine supplementation during the promotion stage stimulated colorectal tumor growth 109. New knowledge about the regulation of Arginine metabolism in tumor tissues is key to designing sound therapeutic means to effectively prevent and treat cancers.
Arginine and skeletal muscle function
Skeletal muscle represents 40–45% of the adult body weight and is crucial for whole-body homeostasis of protein and amino acids. Recent studies have demonstrated that Arginine plays a cell signaling role in this tissue. For example, Arginine activates the mTOR cell signaling pathway in skeletal muscle to enhance protein synthesis and whole-body growth 110. There is also indirect evidence that Arginine inhibits proteolysis in skeletal muscle 80. Thus, regulation of intracellular protein turnover by Arginine favors muscle gain 111, which may have important implications for health. In support of this view, oral administration of Arginine enhanced exercise endurance and muscle force generation in humans 112. Moreover, Arginine administration reduced inflammation and maintained muscle integrity in a mouse model of Duchenne muscular dystrophy 113, the most common muscle wasting disease. Because muscle wasting occurs in astronauts or patients on bed rest, it is important to determine whether this disorder can be prevented by dietary Arginine supplementation.
Safety of arginine supplement
Intravenous Arginine infusion (up to 0.5 g Arg-HCl/kg body weight for infants, or 30 g Arg-HCl for adults over 30–60 min) or oral Arginine (9 g Arg-HCl/day for adults) has no adverse effects on humans 114. However, higher oral doses of Arg-HCl (>9 g/day) are occasionally associated with nausea, gastrointestinal discomfort, and diarrhea for some subjects 115, which may result from a rapid and excess production of nitric oxide (NO) by the gastrointestinal tract and from impaired intestinal absorption of other dietary basic amino acid (lysine and histidine). A solution to this potential problem may be the alternative use of L-citrulline, a precursor for arginine synthesis 116. As a neutral amino acid, L-citrulline does not compete with basic amino acid for transport by cells, its conversion to Arginine consumes one mole of ammonia in the form of aspartate, and its administration does not require equimolar HCl. Thus, enteral or parenteral L-citrulline may be particularly useful for patients with elevated ammonia concentrations, impaired Arginine transport, enhanced intestinal Arginine catabolism, or a relatively high activity of constitutively expressed arginase. Because excessive production of nitric oxide (NO) is destructive to cells, it would not be advisable to administer Arginine alone to patients with severe infections, active inflammatory or autoimmune disorders, active malignancy (e.g., late stages of tumorigenesis), or pathological angiogenesis 37. Finally, as with other nutrients (e.g., glucose, fatty acids, vitamins and minerals), improper use of Arginine (e.g., high dose and disturbance of acid-base balance) may yield an undesirable effect and, thus, should be avoided in dietary supplementation and clinical therapy. Therefore, it is important that arginine be taken in divided doses on each day of administration (1) to prevent gastrointestinal tract disorders due to abrupt production of large amounts of NO; (2) to increase the availability of circulating arginine over a longer period of time; and (3) to avoid a potential imbalance among amino acids 117.
Citrulline and Arginine summary
Arginine and its precursor citrulline play an important role in the immune response during inflammation and sepsis 118. Maintaining the arginine availability during inflammatory conditions is of crucial importance, probably best by enhancing the citrulline concentration, preventing NOS uncoupling and maintaining adequate enzyme function during these conditions. These findings underscore the complexity of the interaction between substrate metabolism and the functional enzyme complex. Future research is essential in this field to unravel the complexity of the arginine-NO metabolism during endotoxemia and sepsis and most importantly to prevent arginine deficiency. Future studies are warranted to determine the presence of arginine deficiency in other inflammatory conditions, the safety of supplementation and also to find the best supplement to enhance the arginine-NO pathway in these situations.
Arginine (L-arginine) is a component of dietary protein and body fluids. In humans (and animals), this amino acid fulfills versatile physiological functions. Based on nitrogen balance (or growth) and functional needs beyond tissue protein synthesis, Arginine is classified as a nutritionally essential amino acid for: (1) the conceptuses of mammals; (2) neonates; (3) birds, cats, ferrets, and fish; and (4) adults under certain conditions (e.g., intestinal resection or dysfunction, burns, or renal dysfunction associated with NO deficiency). Compelling evidence shows that Arginine plays an important role in reproduction, fetal and postnatal development, wound healing, immune function, and tissue integrity, as well as prevention and treatment of endothelial dysfunction. Additionally, Arginine or L-Citrulline may provide novel and effective therapies for obesity, diabetes, and the metabolic syndrome. The beneficial effect of Arginine in treating many developmental and health problems is unique among amino acid, and Arginine may become a useful “nutraceutical”. Appropriate use of Arginine is safe for humans in dietary supplementation and clinical therapy.
Previous research has established an association between lower L-arginine intake and inflammatory markers associated with cardiovascular risk 119. Further, the L-arginine/asymmetric dimethylarginine (ADMA) pathway is emerging as critical to endothelial function 120 and insulin resistance 121. Because of the emerging evidence, interest in L-arginine intake and supplementation is likely to grow.
The most common dietary sources of L-arginine are meat, poultry and fish, dairy products, and nuts 122. Lower intake of L-arginine-rich foods such as fish and nuts has been consistently shown to be associated with future cardiovascular risk 122. The association of lower L-arginine intake with inflammation and subsequent cardiovascular risk is bolstered by the fact that dietary intake of L-arginine is directly related to serum arginine levels 123. In light of the recent finding that asymmetric dimethylarginine (ADMA), a chemical that blocks the proper metabolism of L-arginine, is an independent predictor of cardiovascular risk, it is particularly important to characterize L-arginine intake patterns. Correspondingly, the lower intake of L-arginine in women and smokers documented in this study 124 may be a possible contributing factor to their cardiovascular risk. Only future research can answer this important question.
References- Arginine metabolism: nitric oxide and beyond. Wu G, Morris SM Jr. Biochem J. 1998 Nov 15; 336 ( Pt 1)():1-17.
- Macrophages can convert citrulline into arginine. Wu GY, Brosnan JT. Biochem J. 1992 Jan 1; 281 ( Pt 1)():45-8.
- Regulation of L-arginine synthesis from L-citrulline by L-glutamine in endothelial cells. Wu G, Meininger CJ. Am J Physiol. 1993 Dec; 265(6 Pt 2):H1965-71.
- Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: the Citrudose pharmacokinetic study. Moinard C, Nicolis I, Neveux N, Darquy S, Bénazeth S, Cynober L. Br J Nutr. 2008 Apr; 99(4):855-62.
- Dhanakoti S.N., Brosnan J.T., Herzberg G.R., Brosnan M.E. Renal arginine synthesis: Studies in vitro and in vivo. Am. J. Physiol. 1990;259:E437–E442.
- Van de Poll M.C., Ligthart-Melis G.C., Boelens P.G., Deutz N.E., van Leeuwen P.A., Dejong C.H. Intestinal and hepatic metabolism of glutamine and citrulline in humans. J. Physiol. 2007;581:819–827. doi: 10.1113/jphysiol.2006.126029
- Windmueller H.G., Spaeth A.E. Source and fate of circulating citrulline. Am. J. Physiol. 1981;241:E473–E480.
- Piton G., Manzon C., Monnet E., Cypriani B., Barbot O., Navellou J.C., Carbonnel F., Capellier G. Plasma citrulline kinetics and prognostic value in critically ill patients. Intensive Care Med. 2010;36:702–706. doi: 10.1007/s00134-010-1751-6.
- Jianfeng G., Weiming Z., Ning L., Fangnan L., Li T., Nan L., Jieshou L. Serum citrulline is a simple quantitative marker for small intestinal enterocytes mass and absorption function in short bowel patients. J. Surg. Res. 2005;127:177–182. doi: 10.1016/j.jss.2005.04.004
- Lau T., Owen W., Yu Y.M., Noviski N., Lyons J., Zurakowski D., Tsay R., Ajami A., Young V.R., Castillo L. Arginine, citrulline, and nitric oxide metabolism in end-stage renal disease patients. J. Clin. Investig. 2000;105:1217–1225. doi: 10.1172/JCI7199
- Marini J.C. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J. Nutr. 2012;142:572–580. doi: 10.3945/jn.111.153825
- Oral citrulline does not affect whole body protein metabolism in healthy human volunteers: Results of a prospective, randomized, double-blind, cross-over study. Clinical Nutrition December 2011; Volume 30, Issue 6, Pages 807–811. https://www.clinicalnutritionjournal.com/article/S0261-5614(11)00106-3/fulltext
- Luiking Y.C., Poeze M., Ramsay G., Deutz N.E. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am. J. Clin. Nutr. 2009;89:142–152. doi: 10.3945/ajcn.2007.25765
- Piton G., Manzon C., Monnet E., Cypriani B., Barbot O., Navellou J.C., Carbonnel F., Capellier G. Plasma citrulline kinetics and prognostic value in critically ill patients. Intensive Care Med. 2010;36:702–706. doi: 10.1007/s00134-010-1751-6
- Wijnands K.A., Vink H., Briede J.J., van Faassen E.E., Lamers W.H., Buurman W.A., Poeze M. Citrulline a more suitable substrate than arginine to restore no production and the microcirculation during endotoxemia. PLoS One. 2012;7:e37439. doi: 10.1371/journal.pone.0037439
- Elwafi F., Curis E., Zerrouk N., Neveux N., Chaumeil J.C., Arnaud P., Cynober L., Moinard C. Endotoxemia affects citrulline, arginine and glutamine bioavailability. Eur. J. Clin. Investig. 2012;42:282–289. doi: 10.1111/j.1365-2362.2011.02581.x
- Wijnands K.A., Vink H., Briede J.J., van Faassen E.E., Lamers W.H., Buurman W.A., Poeze M. Citrulline a more suitable substrate than arginine to restore no production and the microcirculation during endotoxemia. PLoS One. 2012;7:e37439. doi: 10.1371/journal.pone.0037439 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3362574/
- Qualls J.E., Subramanian C., Rafi W., Smith A.M., Balouzian L., DeFreitas A.A., Shirey K.A., Reutterer B., Kernbauer E., Stockinger S., et al. Sustained generation of nitric oxide and control of mycobacterial infection requires argininosuccinate synthase 1. Cell Host Microbe. 2012;12:313–323. doi: 10.1016/j.chom.2012.07.012
- Wijnands K.A., Hoeksema M.A., Meesters D.M., van den Akker N.M., Molin D.G., Briede J.J., Ghosh M., Kohler S.E., van Zandvoort M.A., de Winther M.P., et al. Arginase-1 deficiency regulates arginine concentrations and NOS2-mediated no production during endotoxemia. PLoS One. 2014;9:e86135. doi: 10.1371/journal.pone.0086135
- El-Hattab AW, Hsu JW, Emrick LT, Wong LJC, Craigen WJ, Jahoor F, Scaglia F. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Mol Genet Metab 2012;105:607–14
- Agarwal U, Didelija IC, Yuan Y, Wang X, Marini JC. Supplemental Citrulline Is More Efficient Than Arginine in Increasing Systemic Arginine Availability in Mice. The Journal of Nutrition. 2017;147(4):596-602. doi:10.3945/jn.116.240382. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5368575/
- Schwedhelm E, Maas R, Freese R, et al. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. British Journal of Clinical Pharmacology. 2008;65(1):51-59. doi:10.1111/j.1365-2125.2007.02990.x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2291275/
- Arginine: Clinical Potential of a Semi-Essential Amino Acid. http://archive.foundationalmedicinereview.com/publications/7/6/512.pdf
- Heys S.D., Gardner E. Nutrients and the surgical patient: Current and potential therapeutic applications to clinical practice. J. R. Coll. Surg. Edinb. 1999;44:283–293
- Windmueller H.G., Spaeth A.E. Source and fate of circulating citrulline. Am. J. Physiol. 1981;241:E473–E480. https://www.physiology.org/doi/pdf/10.1152/ajpendo.1981.241.6.E473
- Yu Y.M., Burke J.F., Tompkins R.G., Martin R., Young V.R. Quantitative aspects of interorgan relationships among arginine and citrulline metabolism. Am. J. Physiol. 1996;271:E1098–E1109. https://www.physiology.org/doi/pdf/10.1152/ajpendo.1996.271.6.E1098
- Wu G, Davis PK, Flynn NE, et al. Endogenous synthesis of arginine plays an important role in maintaining arginine homeostasis in postweaning growing pigs. J Nutr 1997;127:2342-2349
- Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME. Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol 1990;259:E437-E442.
- Castillo L., Beaumier L., Ajami A.M., Young V.R. Whole body nitric oxide synthesis in healthy men determined from [15N]arginine-to-[15N]citrulline labeling. Proc. Natl. Acad. Sci. USA. 1996;93:11460–11465. doi: 10.1073/pnas.93.21.11460
- Poeze M., Bruins M.J., Kessels F., Luiking Y.C., Lamers W.H., Deutz N.E. Effects of l-arginine pretreatment on nitric oxide metabolism and hepatosplanchnic perfusion during porcine endotoxemia. Am. J. Clin. Nutr. 2011;93:1237–1247. doi: 10.3945/ajcn.110.007237
- Yeramian A., Martin L., Arpa L., Bertran J., Soler C., McLeod C., Modolell M., Palacin M., Lloberas J., Celada A. Macrophages require distinct arginine catabolism and transport systems for proliferation and for activation. Eur. J. Immunol. 2006;36:1516–1526. doi: 10.1002/eji.200535694
- Ozaki M., Gotoh T., Nagasaki A., Miyanaka K., Takeya M., Fujiyama S., Tomita K., Mori M. Expression of arginase II and related enzymes in the rat small intestine and kidney. J. Biochem. 1999;125:586–593. doi: 10.1093/oxfordjournals.jbchem.a022324
- Morris S.M., Jr. Recent advances in arginine metabolism: Roles and regulation of the arginases. Br. J. Pharmacol. 2009;157:922–930. doi: 10.1111/j.1476-5381.2009.00278.x
- Li H., Meininger C.J., Hawker J.R., Jr., Haynes T.E., Kepka-Lenhart D., Mistry S.K., Morris S.M., Jr., Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2001;280:E75–E82
- De Jonge W.J., Hallemeesch M.M., Kwikkers K.L., Ruijter J.M., de Gier-de Vries C., van Roon M.A., Meijer A.J., Marescau B., de Deyn P.P., Deutz N.E., et al. Overexpression of arginase I in enterocytes of transgenic mice elicits a selective arginine deficiency and affects skin, muscle, and lymphoid development. Am. J. Clin. Nutr. 2002;76:128–140.
- Wu G, Bazer FW, Davis TA, et al. Arginine metabolism and nutrition in growth, health and disease. Amino acids. 2009;37(1):153-168. doi:10.1007/s00726-008-0210-y. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2677116/
- Wu G, Bazer FW, Davis TA, et al. Arginine metabolism and nutrition in growth, health and disease. Amino acids. 2009;37(1):153-168. doi:10.1007/s00726-008-0210-y https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2677116/
- Hou ZP, Yin YL, Huang RL, et al. Rice protein concentrate partially replaces dried whey in the diet for early-weaned piglets and improves their growth performance. J Sci Food Agric. 2008;88:1187–1193.
- Amino acid composition of human milk is not unique. Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Lewis DS, Lee DR, Reeds PJ. J Nutr. 1994 Jul; 124(7):1126-32. https://www.ncbi.nlm.nih.gov/pubmed/8027865/
- Variation in L-arginine intake follow demographics and lifestyle factors that may impact cardiovascular disease risk. King DE, Mainous AG 3rd, Geesey ME. Nutr Res. 2008 Jan; 28(1):21-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2245877/
- Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. Becker RM, Wu G, Galanko JA, Chen W, Maynor AR, Bose CL, Rhoads JM. J Pediatr. 2000 Dec; 137(6):785-93. https://www.ncbi.nlm.nih.gov/pubmed/11113834/
- Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. Wu G, Jaeger LA, Bazer FW, Rhoads JM. J Nutr Biochem. 2004 Aug; 15(8):442-51. https://www.ncbi.nlm.nih.gov/pubmed/15302078/
- Mechanisms of disease: L-arginine in coronary atherosclerosis–a clinical perspective. Tousoulis D, Böger RH, Antoniades C, Siasos G, Stefanadi E, Stefanadis C. Nat Clin Pract Cardiovasc Med. 2007 May; 4(5):274-83. https://www.ncbi.nlm.nih.gov/pubmed/17457351/
- Relationship between obesity, smoking, and the endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine. Eid HM, Arnesen H, Hjerkinn EM, Lyberg T, Seljeflot I. Metabolism. 2004 Dec; 53(12):1574-9. https://www.ncbi.nlm.nih.gov/pubmed/15562402/
- Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Böger RH. Ann Med. 2006; 38(2):126-36. https://www.ncbi.nlm.nih.gov/pubmed/16581698/
- Association between dietary arginine and C-reactive protein. Wells BJ, Mainous AG 3rd, Everett CJ. Nutrition. 2005 Feb; 21(2):125-30. https://www.ncbi.nlm.nih.gov/pubmed/15723738/
- ADMA and oxidative stress. Sydow K, Münzel T. Atheroscler Suppl. 2003 Dec; 4(4):41-51. https://www.ncbi.nlm.nih.gov/pubmed/14664902/
- Kawano H, Motoyama T, Hirai N, Kugiyama K, Yasue H, Ogawa H. Endothelial dysfunction in hypercholesterolemia is improved by Larginine administration: possible role of oxidative stress. Atherosclerosis. 2002;161:375–80. https://www.ncbi.nlm.nih.gov/pubmed/11888520
- Appleton J. Clinical potential of a semi-essential amino acid. Altern Med Rev. 2002;7:512–22. http://archive.foundationalmedicinereview.com/publications/7/6/512.pdf
- Grimble G.K. Adverse gastrointestinal effects of arginine and related amino acids. J. Nutr. 2007;137:1693S–1701S. https://www.ncbi.nlm.nih.gov/pubmed/17513449
- Thibault R., Flet L., Vavasseur F., Lemerle M., Ferchaud-Roucher V., Picot D., Darmaun D. Oral citrulline does not affect whole body protein metabolism in healthy human volunteers: Results of a prospective, randomized, double-blind, cross-over study. Clin. Nutr. 2011;30:807–811. doi: 10.1016/j.clnu.2011.06.005 https://www.ncbi.nlm.nih.gov/pubmed/21733603
- IL-4 and IL-13 upregulate ornithine decarboxylase expression by PI3K and MAP kinase pathways in vascular smooth muscle cells. Wei LH, Yang Y, Wu G, Ignarro LJ. Am J Physiol Cell Physiol. 2008 May; 294(5):C1198-205.
- Glucocorticoid regulation of amino acid and polyamine metabolism in the small intestine. Flynn NE, Bird JG, Guthrie AS. Amino Acids. 2009 May; 37(1):123-9.
- Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Cell Biochem Biophys. 2004; 41(3):415-34.
- A cortisol surge mediates the enhanced expression of pig intestinal pyrroline-5-carboxylate synthase during weaning. Wu G, Meininger CJ, Kelly K, Watford M, Morris SM Jr. J Nutr. 2000 Aug; 130(8):1914-9.
- Urea synthesis in enterocytes of developing pigs. Wu G. Biochem J. 1995 Dec 15; 312 ( Pt 3)():717-23.
- Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. Wu G, Jaeger LA, Bazer FW, Rhoads JM. J Nutr Biochem. 2004 Aug; 15(8):442-51.
- Arginine metabolism: boundaries of our knowledge. Morris SM Jr. J Nutr. 2007 Jun; 137(6 Suppl 2):1602S-1609S.
- Dietary protein or arginine deficiency impairs constitutive and inducible nitric oxide synthesis by young rats. Wu G, Flynn NE, Flynn SP, Jolly CA, Davis PK. J Nutr. 1999 Jul; 129(7):1347-54.
- Lactate inhibits citrulline and arginine synthesis from proline in pig enterocytes. Dillon EL, Knabe DA, Wu G. Am J Physiol. 1999 May; 276(5 Pt 1):G1079-86.
- Amino acids and immune function. Li P, Yin YL, Li D, Kim SW, Wu G. Br J Nutr. 2007 Aug; 98(2):237-52.
- A new selective pre-column ninhydrin-based derivatization for a RP-HPLC determination of plasma asymmetric dimethyl-L-arginine (ADMA) by fluorescence detection. Sotgia S, Zinellu A, Pinna GA, Deiana L, Carru C. Amino Acids. 2008 May; 34(4):677-82.
- Dietary L-arginine supplementation enhances endothelial nitric oxide synthesis in streptozotocin-induced diabetic rats. Kohli R, Meininger CJ, Haynes TE, Yan W, Self JT, Wu G. J Nutr. 2004 Mar; 134(3):600-8.
- Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. J Biol Chem. 2007 Jan 12; 282(2):879-87.
- Wu G, Bazer FW, Datta S, et al. Intrauterine growth retardation in livestock: implications, mechanisms and solutions. Arch Fur Tierzucht-Arch Anim Breed. 2008b;51(Special Issue 1):4–10.
- Effects of NO synthase inhibitors, arginine-deficient diet, and amiloride in pregnant rats. Greenberg SS, Lancaster JR, Xie J, Sarphie TG, Zhao X, Hua L, Freeman T, Kapusta DR, Giles TD, Powers DR. Am J Physiol. 1997 Sep; 273(3 Pt 2):R1031-45.
- Dietary L-arginine prevents fetal growth restriction in rats. Vosatka RJ, Hassoun PM, Harvey-Wilkes KB. Am J Obstet Gynecol. 1998 Feb; 178(2):242-6.
- Dietary L-arginine supplementation enhances the reproductive performance of gilts. Mateo RD, Wu G, Bazer FW, Park JC, Shinzato I, Kim SW. J Nutr. 2007 Mar; 137(3):652-6.
- Lassala A. PhD dissertation. Texas A&M University; College Station, Texas: 2008. Arginine and fetal growth in ovine models of intrauterine growth restriction.
- L-Arginine treatment for asymmetric fetal growth restriction. Xiao XM, Li LP. Int J Gynaecol Obstet. 2005 Jan; 88(1):15-8.
- Hyperammonemia resulting from intravenous alimentation using a mixture of synthetic l-amino acids: a preliminary report. Heird WC, Nicholson JF, Driscoll JM Jr, Schullinger JN, Winters RW. J Pediatr. 1972 Jul; 81(1):162-5.
- Arginine supplementation prevents necrotizing enterocolitis in the premature infant. Amin HJ, Zamora SA, McMillan DD, Fick GH, Butzner JD, Parsons HG, Scott RB. J Pediatr. 2002 Apr; 140(4):425-31.
- Effect of L-arginine infusion on infants with persistent pulmonary hypertension of the newborn. McCaffrey MJ, Bose CL, Reiter PD, Stiles AD. Biol Neonate. 1995; 67(4):240-3.
- Proline ameliorates arginine deficiency during enteral but not parenteral feeding in neonatal piglets. Brunton JA, Bertolo RF, Pencharz PB, Ball RO. Am J Physiol. 1999 Aug; 277(2 Pt 1):E223-31.
- Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Wu G. Am J Physiol. 1997 Jun; 272(6 Pt 1):G1382-90.
- The arginine requirement of the infant. SNYDERMAN SE, BOYER A, HOLT LE Jr. AMA J Dis Child. 1959 Feb; 97(2):192-5.
- Arginine nutrition in neonatal pigs. Wu G, Knabe DA, Kim SW. J Nutr. 2004 Oct; 134(10 Suppl):2783S-2790S; discussion 2796S-2797S.
- An important role for endogenous synthesis of arginine in maintaining arginine homeostasis in neonatal pigs. Flynn NE, Wu G. Am J Physiol. 1996 Nov; 271(5 Pt 2):R1149-55.
- Dietary L-arginine supplementation enhances the immune status in early-weaned piglets. Tan B, Li XG, Kong X, Huang R, Ruan Z, Yao K, Deng Z, Xie M, Shinzato I, Yin Y, Wu G. Amino Acids. 2009 Jul; 37(2):323-31.
- Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. Frank JW, Escobar J, Nguyen HV, Jobgen SC, Jobgen WS, Davis TA, Wu G. J Nutr. 2007 Feb; 137(2):315-9.
- Effects of dietary arginine supplementation during gestation and lactation on the performance of lactating primiparous sows and nursing piglets. Mateo RD, Wu G, Moon HK, Carroll JA, Kim SW. J Anim Sci. 2008 Apr; 86(4):827-35.
- Regulatory role for amino acids in mammary gland growth and milk synthesis. Kim SW, Wu G. Amino Acids. 2009 May; 37(1):89-95.
- Effects of arginine treatment on nutrition, growth and urea cycle function in seven Japanese boys with late-onset ornithine transcarbamylase deficiency. Nagasaka H, Yorifuji T, Murayama K, Kubota M, Kurokawa K, Murakami T, Kanazawa M, Takatani T, Ogawa A, Ogawa E, Yamamoto S, Adachi M, Kobayashi K, Takayanagi M. Eur J Pediatr. 2006 Sep; 165(9):618-24.
- Objective evidence of endothelial dysfunction in preeclampsia. Roberts JM. Am J Kidney Dis. 1999 May; 33(5):992-7.
- Reduced L-arginine level and decreased placental eNOS activity in preeclampsia. Kim YJ, Park HS, Lee HY, Ha EH, Suh SH, Oh SK, Yoo HS. Placenta. 2006 Apr-May; 27(4-5):438-44.
- L-arginine reverses the adverse pregnancy changes induced by nitric oxide synthase inhibition in the rat. Helmbrecht GD, Farhat MY, Lochbaum L, Brown HE, Yadgarova KT, Eglinton GS, Ramwell PW. Am J Obstet Gynecol. 1996 Oct; 175(4 Pt 1):800-5.
- Effects of oral L-arginine on the foetal condition and neonatal outcome in preeclampsia: a preliminary report. Rytlewski K, Olszanecki R, Lauterbach R, Grzyb A, Basta A. Basic Clin Pharmacol Toxicol. 2006 Aug; 99(2):146-52.
- L-arginine supplementation in patients with gestational hypertension: a pilot study. Facchinetti F, Saade GR, Neri I, Pizzi C, Longo M, Volpe A. Hypertens Pregnancy. 2007; 26(1):121-30.
- The nitric oxide pathway in pre-eclampsia: pathophysiological implications. Buhimschi IA, Saade GR, Chwalisz K, Garfield RE. Hum Reprod Update. 1998 Jan-Feb; 4(1):25-42.
- Inhibition of nitric oxide synthesis causes preterm delivery in the mouse. Tiboni GM, Giampietro F. Hum Reprod. 2000 Aug; 15(8):1838-42.
- L-arginine infusion reduces preterm uterine contractions. Facchinetti F, Neri I, Genazzani AR. J Perinat Med. 1996; 24(3):283-5.
- Effects of oral L-arginine on the pulsatility indices of umbilical artery and middle cerebral artery in preterm labor. Rytlewski K, Olszanecki R, Lauterbach R, Grzyb A, Kiec-Wilk B, Dembinska-Kiec A, Basta A. Eur J Obstet Gynecol Reprod Biol. 2008 May; 138(1):23-8.
- Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G. J Nutr. 2005 Apr; 135(4):714-21.
- Jobgen WS. PhD dissertation. Texas A&M University; College Station, Texas: 2007. Dietary l-arginine supplementation reduces fat mass in diet-induced obese rats
- Effects of low-dose L-arginine on insulin-mediated vasodilatation and insulin sensitivity. Wascher TC, Graier WF, Dittrich P, Hussain MA, Bahadori B, Wallner S, Toplak H. Eur J Clin Invest. 1997 Aug; 27(8):690-5.
- l-Citrulline and l-arginine supplementation retards the progression of high-cholesterol-diet-induced atherosclerosis in rabbits. Hayashi T, Juliet PA, Matsui-Hirai H, Miyazaki A, Fukatsu A, Funami J, Iguchi A, Ignarro LJ. Proc Natl Acad Sci U S A. 2005 Sep 20; 102(38):13681-6.
- Metabolomic analysis of the response of growing pigs to dietary L-arginine supplementation. He Q, Kong X, Wu G, Ren P, Tang H, Hao F, Huang R, Li T, Tan B, Li P, Tang Z, Yin Y, Wu Y. Amino Acids. 2009 May; 37(1):199-208.
- Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. Wu G, Collins JK, Perkins-Veazie P, Siddiq M, Dolan KD, Kelly KA, Heaps CL, Meininger CJ. J Nutr. 2007 Dec; 137(12):2680-5.
- Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. J Nutr Biochem. 2006 Sep; 17(9):571-88.
- Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM Jr, Gladwin MT. JAMA. 2005 Jul 6; 294(1):81-90.
- Sickle cell disease vasculopathy: a state of nitric oxide resistance. Wood KC, Hsu LL, Gladwin MT. Free Radic Biol Med. 2008 Apr 15; 44(8):1506-28.
- Oral citrulline as arginine precursor may be beneficial in sickle cell disease: early phase two results. Waugh WH, Daeschner CW 3rd, Files BA, McConnell ME, Strandjord SE. J Natl Med Assoc. 2001 Oct; 93(10):363-71.
- Arginine deficiency affects early B cell maturation and lymphoid organ development in transgenic mice. de Jonge WJ, Kwikkers KL, te Velde AA, van Deventer SJ, Nolte MA, Mebius RE, Ruijter JM, Lamers MC, Lamers WH. J Clin Invest. 2002 Nov; 110(10):1539-48.
- Regulation of nitric oxide synthesis by dietary factors. Wu G, Meininger CJ. Annu Rev Nutr. 2002; 22():61-86.
- Dietary L-arginine supplementation alleviates immunosuppression induced by cyclophosphamide in weaned pigs. Han J, Liu YL, Fan W, Chao J, Hou YQ, Yin YL, Zhu HL, Meng GQ, Che ZQ. Amino Acids. 2009 Oct; 37(4):643-51.
- Regulation of apoptosis by protein S-nitrosylation. Mannick JB. Amino Acids. 2007; 32(4):523-6.
- Eremin O. l-Arginine: biological aspects and clinical applications. RG Landes Company; Georgetown: 1997
- Decreased tumor incidence and increased survival by one year oral low dose arginine supplementation in the mouse. Lubec B, Hoeger H, Kremser K, Amann G, Koller DY, Gialamas J. Life Sci. 1996; 58(25):2317-25.
- The effects of l-arginine on crypt cell hyperproliferation in colorectal cancer. Ma Q, Williamson KE, O’rourke D, Rowlands BJ. J Surg Res. 1999 Feb; 81(2):181-8.
- Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. Yao K, Yin YL, Chu W, Liu Z, Deng D, Li T, Huang R, Zhang J, Tan B, Wang W, Wu G. J Nutr. 2008 May; 138(5):867-72.
- Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Tan B, Yin Y, Liu Z, Li X, Xu H, Kong X, Huang R, Tang W, Shinzato I, Smith SB, Wu G. Amino Acids. 2009 May; 37(1):169-75.
- The effect of L-arginine administration on muscle force and power in postmenopausal women. Fricke O, Baecker N, Heer M, Tutlewski B, Schoenau E. Clin Physiol Funct Imaging. 2008 Sep; 28(5):307-11.
- L-arginine decreases inflammation and modulates the nuclear factor-kappaB/matrix metalloproteinase cascade in mdx muscle fibers. Hnia K, Gayraud J, Hugon G, Ramonatxo M, De La Porte S, Matecki S, Mornet D. Am J Pathol. 2008 Jun; 172(6):1509-19.
- Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Shao A, Hathcock JN. Regul Toxicol Pharmacol. 2008 Apr; 50(3):376-99. https://www.ncbi.nlm.nih.gov/pubmed/18325648/
- Adverse gastrointestinal effects of arginine and related amino acids. Grimble GK. J Nutr. 2007 Jun; 137(6 Suppl 2):1693S-1701S. https://www.ncbi.nlm.nih.gov/pubmed/17513449/
- Arginine nutrition and cardiovascular function. Wu G, Meininger CJ. J Nutr. 2000 Nov; 130(11):2626-9. https://www.ncbi.nlm.nih.gov/pubmed/11053497/
- Pharmacokinetics and safety of arginine supplementation in animals. Wu G, Bazer FW, Cudd TA, Jobgen WS, Kim SW, Lassala A, Li P, Matis JH, Meininger CJ, Spencer TE. J Nutr. 2007 Jun; 137(6 Suppl 2):1673S-1680S. https://www.ncbi.nlm.nih.gov/pubmed/17513446/
- Wijnands KAP, Castermans TMR, Hommen MPJ, Meesters DM, Poeze M. Arginine and Citrulline and the Immune Response in Sepsis. Nutrients. 2015;7(3):1426-1463. doi:10.3390/nu7031426. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4377861/
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511
- Wojciak-Stothard B, Torondel B, Tsany LY, et al. The ADMA/DDAH pathway is a critical regulator of endothelial cell motility. J Cell Sci. 2007;120(Pt 6):929–42. Epub. http://jcs.biologists.org/content/120/6/929.long
- Anderson JL, Carlquist JF, Roberts WL, et al. Asymmetric dimethylarginine, cortisol/cortisone ratio, and C-peptide: markers for diabetes and cardiovascular risk?
- Hu FB, Stampfer MJ, Manson JE. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ. 1998;317:1341–5.
- Venho B, Voutilainen S, Valkonen VP, Virtanen J, Lahka TA, Rissanen TH, et al. Arginine intake, blood pressure, and the incidence of acute coronary events in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2002;76:359–364
- King DE, Mainous AG, Geesey ME. VARIATION IN L-ARGININE INTAKE ACCORDING TO DEMOGRAPHIC CHARACTERISTICS AND CARDIOVASCULAR RISK. Nutrition research (New York, NY). 2008;28(1):21-24. doi:10.1016/j.nutres.2007.11.003. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2245877/