What is glucomannan
Glucomannan also called Konjac glucomannan is a water-soluble polysaccharide or highly viscous fiber commonly separated from Amorphophallus konjac root 1. Glucomannan (Konjac glucomannan) is a dietary fiber with a long history in food and traditional Chinese medicine 2. Glucomannan is also known as E 425 ii when used as food additives 3. Glucomannan is marketed as being helpful in reducing body weight. In otherwise healthy overweight or obese children and adults, there is some evidence that in the short term glucomannan may help to reduce body weight, but not body mass index (BMI). In 2010, the European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies 4 concluded that a cause and effect relationship has been established between the consumption of glucomannan and the maintenance of normal blood cholesterol concentrations and the reduction of body weight. In order to obtain the claimed effect of reduction of body weight, “at least 3 g of glucomannan should be consumed daily in three doses of at least 1 g each, together with 1–2 glasses of water before meals, in the context of an energy‐restricted diet. The target population is overweight adults” 4. The conditions and restrictions of use for the health claims for glucomannan (konjac mannan) to contribute to weight loss and to the maintenance of normal blood cholesterol concentrations are authorized by the Commission Regulation (EU) No 432/2012.
According to European Union Commission Regulation No 231/2012, both konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are defined as water soluble hydrocolloid obtained from konjac flour. Konjac gum is obtained by aqueous extraction, while konjac glucomannan is obtained by washing with water‐containing ethanol. In the European Union Commission Regulation, konjac flour is defined as the unpurified raw product from the tuber of the perennial plant Amorphophallus konjac.
The in vitro degradation and the in vivo digestibility of konjac glucomannan in animals demonstrated that this compound would not be absorbed intact or hydrolyzed by digestive enzymes.
Konjac glucomannan and konjac flour can be regarded as non‐toxic based on the results of acute oral toxicity studies.
Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to prove the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does not certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products.
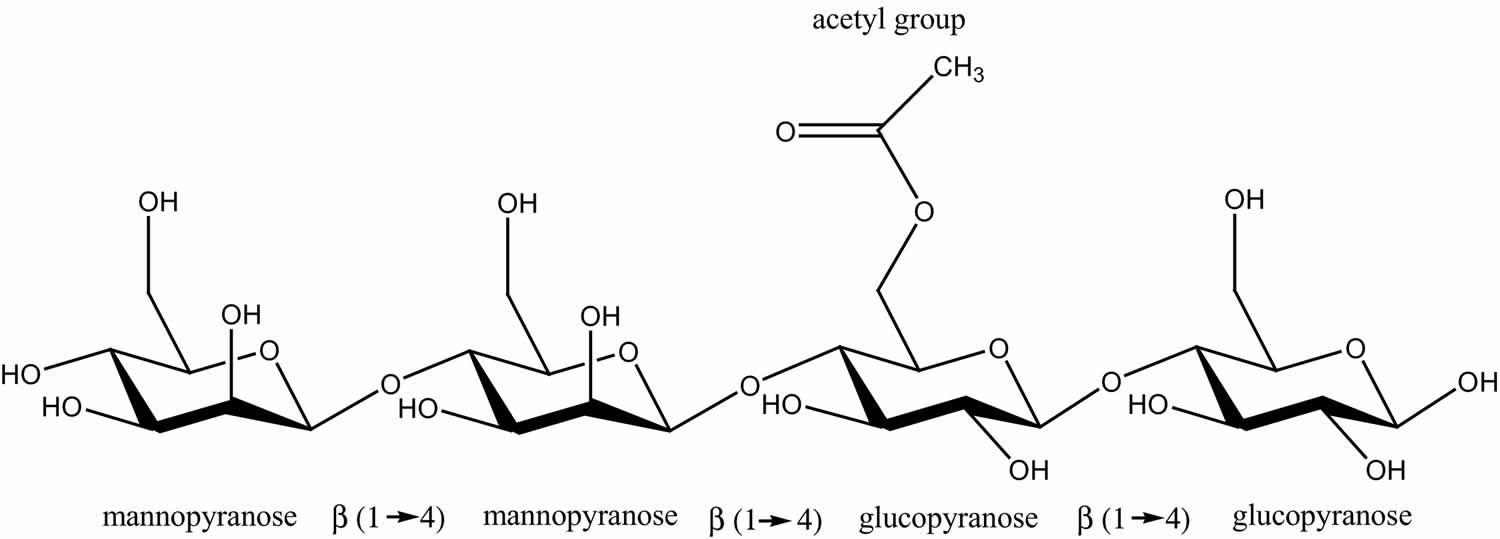
Figure 1. Glucomannan chemical structure
The glucomannan main chain is polymerized by d-mannose and d-glucose with a α-1,4-pyranoside bond and a small amount of acetyl groups at the C–6 position of the side chain. These can only be hydrolyzed by α-mannase at the end of the small intestine and the colon of the human body 5. Glucomannan has good film-forming ability, biocompatibility, biodegradability, and gelation performance, which is one of its most prominent features 6. The preparation methods of glucomannan gel mainly include the alkaline processing 7, borate cross-linking 8, polymer compounding 9, high voltage electric field preparation 10, and metal ion cross-linking after modification 11. The gel microstructure largely determines the performance of the gel, but glucomannan gels prepared through different methods are significantly different in terms of microstructure 12. With good biocompatibility and biodegradability 13, glucomannan gels have been widely used in food 12, pharmaceutical carriers 14, tissue scaffolds 15, absorbing materials 16 and other fields.
Glucomannan as food additives
Konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are authorized as food additives in the European Union in accordance with Annex II and Annex III to Regulation (EU) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012 3. According to this regulation, there are distinct specifications for konjac gum (E 425 i) and konjac glucomannan (E 425 ii). The Joint Food and Agriculture Organization (FAO)/World Health Organisation (WHO) Expert Committee on Food Additives (JECFA) has one specification for konjac flour (INS 425). Konjac gum (E 425 i) and konjac glucomannan (E 425 ii) are distinguished by their grade of purity.
In the European Union, konjac gum (E 425 i) and konjac glucomannan (E 425 ii) have been evaluated by the Scientific Committee on Food in 1996 17, who could not establish an acceptable daily intake (ADI) for both the substances. For konjac gum (E 425 i), the Scientific Committee on Food 17 noted ‘Adequate subchronic and long‐term feeding studies with this material are lacking and a no‐observed‐adverse-effect level (NOAEL) cannot be derived. In addition, it has not been clarified to what extent the main component glucomannan is digested in the human intestine’. For konjac glucomannan (425 ii), the Scientific Committee on Food noted that ‘it was tested adequately in 90‐day feeding studies with rats and beagle dogs. These studies did not reveal any relevant toxic effects and a no‐observed‐effect level of 2.5% glucomannan in the diet can be derived, corresponding to 1.25 g/kg body weight per day. However, a long‐term toxicity/carcinogenicity study is lacking and only gene mutation tests in bacteria were performed with a negative result 17. In addition, it has not been clarified to what extent the glucomannan is digested in the human intestine’. On the other hand, the Scientific Committee on Food concluded, that ‘the existing data (including genotoxicity studies with konjac glucomannan (E 425 ii) as well as human experience did not give reason for concern. Konjac materials have a long history as traditional food in Asian countries. Apart from diarrhea, abdominal pain and an effect on vitamin absorption after ingestion of high doses, no adverse effects of oral ingestion have been reported in humans’. The Scientific Committee on Food considered therefore that ‘the uses of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as additives at the intended levels up to 1% in food are acceptable, provided that the total intake from all sources did not exceed 3 g/day. This upper limit should be taken into account when setting the conditions of use. The Scientific Committee on Food noted that directive included a footnote in relation to similar products which points out that these substances should not be used to produce dehydrated foodstuffs intended to rehydrate on ingestion’. The Scientific Committee on Food considered that a similar remark would be applicable to konjac gum (E 425 i) and konjac glucomannan (E 425 ii).
No relevant studies on short‐term and subchronic toxicity for konjac gum and konjac glucomannan are available. However, additional studies on nutritional effects are described; no relevant substance‐induced adverse effects were observed.
Based on the data available, the European Food Safety Authority Panel noted that there is no concern with respect to the genotoxicity of konjac flour.
No relevant studies on chronic toxicity and carcinogenicity for konjac gum (E 425 i) and konjac glucomannan (E 425 ii) were available 3. The European Food Safety Authority Panel noted that no adverse effects were observed in rats in a long‐term feeding trial over 18 months with 1% refined konjac meal in diet, and in mice receiving 10% of konjac glucomannan with the diet for 10 months.
No reproductive toxicity studies were available 3. The European Food Safety Authority Panel considered that the developmental toxicity studies as referred to by the Joint Food and Agriculture Organization (FAO)/World Health Organisation (WHO) Expert Committee on Food Additives (JECFA) 18 in cats and of Sun Tan et al. 19 in sows were both limited and not sufficient for the evaluation of the developmental toxicity of konjac gum (E 425 i) and konjac glucomannan (E 425 ii).
From both human and animal data, the European Food Safety Authority Panel considered that there was no indication for concern for immunotoxicity or allergenicity with konjac gum (E 425 i) and konjac glucomannan (E 425 ii) used as food additives 3.
In human studies, gastrointestinal discomfort (i.e. laxative effects, flatulence, full stomach, feeling of hungry and abdominal distension) has been reported in several clinical human studies included in two meta‐analyses. In a relevant study, a dosage of 3 g konjac glucomannan (divided in three times 1 g)/person per day corresponding to 33 mg/kg body weight per day based on mean body weight of approximately 90 kg, for 12 weeks, was associated with gastrointestinal effects (diarrhea or constipation).
Konjac (E 425) is authorized in a wide range of foods. Very few reported use levels were made available to the European Food Safety Authority (EFSA). Only three food categories out of 67 were taken into account in the refined scenario. Thus the two refined exposure estimates (brand‐loyal consumer scenario and non‐brand‐loyal scenario) are similarly low (below 0.1 mg/kg body weight per day in any scenario and population).
According to the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation No 257/2010, European Food Safety Authority Panel on Food Additives and Nutrient Sources added to Food 2014 20 and given that:
- Current use of konjac (E 425) was limited in all food categories to maximum permitted level of 10 g/kg;
- An indicative refined exposure assessment has been calculated: for all population groups, it was below 0.1 mg/kg body weight per day for the general population (mean and high level);
- Konjac gum and konjac glucomannan were unlikely to be absorbed intact and were significantly fermented by intestinal microbiota;
- The available database on toxicological studies was considered limited, however no relevant adverse effects were seen in rats and dogs in 90‐day feeding studies according to the Scientific Committee on Food, and the no‐observed‐adverse-effect level (NOAEL) in rats was 1,250 mg konjac glucomannan/kg bw per day;
- Konjac gum and konjac glucomannan would be of no concern with respect to the genotoxicity;
- After a daily dosage of 3,000 mg in adults (corresponding to 33 mg/kg body weight based on mean body weight of approximately 90 kg) for 12 weeks, several individuals experienced abdominal discomfort including diarrhea or constipation,
The European Food Safety Authority Panel concluded that there was no need for a numerical acceptable daily intake (ADI) and that there was no safety concern for the general population at the refined exposure assessment for the reported uses of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as food additives under the current conditions of use at level of 10 g/kg 3.
The European Food Safety Authority Panel agreed with the conclusions of the Scientific Committee on Food (1997) that the uses of konjac (E 425), comprising konjac gum (E 425 i) and konjac glucomannan (E 425 ii), as an additive at the levels up to 10 g/kg in food are acceptable, provided that the total intake from all sources does stay below 3 g/day 3.
The European Food Safety Authority Panel recommended that the European Commission considers harmonizing the microbiological specifications for polysaccharidic thickening agents, such as gums, and to include criteria for total aerobic microbial count and total combined yeasts and molds count into the EU specifications of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) 3.
Although the European Food Safety Authority Panel realized that the exposure to these additives is rather low, the European Food Safety Authority Panel recommended that the European Commission considers revising the current limits for the toxic elements (lead and arsenic) in the European Union specification for konjac gum (E 425 i) and konjac glucomannan (E 425 ii) 3.
Glucomannan supplement
Glucomannan has no specific lactation-related uses. It is most often used to lower cholesterol, to treat constipation and diabetes, and is contained in products to promote weight loss. No data exist on the safety and efficacy of glucomannan in nursing mothers or infants. However, because glucomannan is not absorbable, it will not reach the breastmilk and is very unlikely to affect the nursing infant.
Glucomannan health benefits
Glucomannan and cholesterol
In 2009, the European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies 21 prepared a scientific opinion on the scientific substantiation of health claims in relation to glucomannan (konjac mannan) and the maintenance of normal blood cholesterol concentrations. Eight randomized controlled trials, which investigated the effects of glucomannan on LDL “bad” cholesterol and/or total cholesterol at daily glucomannan doses of 3-15 g/day in either healthy, hypercholesterolemic or diabetic adult human subjects were provided 21. In weighing the evidence, the European Food Safety Authority Panel took into account that a statistically significant effect on either total or LDL “bad” cholesterol was not observed following the consumption of glucomannan in all of these studies, that reduction in total and/or LDL “bad” cholesterol concentrations did not always lead to significant reductions in the total/HDL “good” cholesterol ratio, that the vast majority of these studies had small sample sizes, and that no clear dose-response relationship was established between the consumption of glucomannan and the claimed effect. However, the European Food Safety Authority Panel considers that most studies showed a consistent effect in the reduction of serum total and LDL “bad” cholesterol concentrations at doses of about 4 grams/day of glucomannan, that the effect has been observed not only in hypercholesterolemic subjects but also in healthy individuals, and that the mechanisms by which the consumption of the food may exert the claimed effect (biological plausibility) are established. On the basis of the data available, the European Food Safety Authority Panel concluded that “a cause and effect relationship has been established between the consumption of glucomannan and the reduction of blood cholesterol concentrations. In order to bear the claim, a food should provide at least 4 g/day of glucomannan in one or more servings. The target population is the general population” 21. The following wording reflects the scientific evidence: “Regular consumption of glucomannan helps maintain normal blood cholesterol concentrations”.
Glucomannan and weight loss
In 2010, the European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies 4 prepared another scientific opinion on the scientific substantiation of health claims in relation to glucomannan (konjac mannan) and reduction of body weight (weight loss), reduction of post‐prandial glycemic responses, maintenance of normal blood glucose concentrations, maintenance of normal (fasting) blood concentrations of triglycerides, maintenance of normal blood cholesterol concentrations, maintenance of normal bowel function and decreasing potentially pathogenic gastrointestinal microorganisms. In weighing the evidence, the European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies took into account that most of the intervention studies, which were of adequate sample size and duration, found a statistically significant effect of glucomannan on body weight loss in the context of a hypocaloric diet when administered as a pre-load before meals, and that the mechanism by which glucomannan could exert the claimed effect is established. On the basis of the data presented, the European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies concludes that a cause and effect relationship has been established between the consumption of glucomannan and the reduction of body weight in the context of an energy-restricted diet 4. The Panel considers that in order to obtain the claimed effect, at least 3 g of glucomannan should be consumed daily in three doses of at least 1 g each, together with 1-2 glasses of water before meals, in the context of an energy-restricted diet. The target population is overweight adults. The European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies 4 concluded that a cause and effect relationship has been established between the consumption of glucomannan and the maintenance of normal blood cholesterol concentrations and the reduction of body weight. In order to obtain the claimed effect of reduction of body weight, “at least 3 g of glucomannan should be consumed daily in three doses of at least 1 g each, together with 1–2 glasses of water before meals, in the context of an energy‐restricted diet. The target population is overweight adults” 4. A cause and effect relationship has not been established between the consumption of glucomannan and the other claimed effects.
Glucomannan dosage
For weight loss: At least 3 g of glucomannan should be consumed daily in three doses of at least 1 g each, together with 1–2 glasses of water before meals, in the context of an energy‐restricted diet.
For cholesterol: At least 4 g/day of glucomannan in one or more servings.
Glucomannan side effects
In human studies, gastrointestinal discomfort (i.e. laxative effects, flatulence, full stomach, feeling of hungry and abdominal distension) has been reported in several clinical human studies included in two meta‐analyses. In a relevant study, a dosage of 3 g konjac glucomannan (divided in three times 1 g)/person per day corresponding to 33 mg/kg body weight per day based on mean body weight of approximately 90 kg, for 12 weeks, was associated with gastrointestinal effects (diarrhea or constipation).
References- Merck Index, 2006. Konjac gum and konjac glucomannan. 14th Edition, Merck & Co., Inc., Whitehouse Station, USA.
- Zalewski B.M., Chmielewska A., Szajewska H. The effect of glucomannan on body weight in overweight or obese children and adults: A systematic review of randomized controlled trials. Nutrition. 2015;31:437.e2–442.e2. doi: 10.1016/j.nut.2014.09.004 https://www.nutritionjrnl.com/article/S0899-9007(14)00425-0/fulltext
- Re‐evaluation of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as food additives. EFSA Journal 28 June 2017. https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.4864
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) 2010. Scientific Opinion on the substantiation of health claims related to konjac mannan (glucomannan) and reduction of body weight (ID 854, 1556, 3725), reduction of post‐prandial glycaemic responses (ID 1559), maintenance of normal blood glucose concentrations (ID 835, 3724), maintenance of normal (fasting) blood concentrations of triglycerides (ID 3217), maintenance of normal blood cholesterol concentrations (ID 3100, 3217), maintenance of normal bowel function (ID 834, 1557, 3901) and decreasing potentially pathogenic intestinal microorganisms (ID 1558) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2010;8(10):1798, 27 pp. https://doi.org/10.2903/j.efsa.2010.1798
- Jin W., Song R., Xu W., Wang Y., Li J., Shah B.R., Li Y., Li B. Analysis of deacetylated konjac glucomannan and xanthan gum phase separation by film forming. Food Hydrocoll. 2015;48:320–326. doi: 10.1016/j.foodhyd.2015.02.007
- Behera S.S., Ray R.C. Konjac glucomannan, a promising polysaccharide of Amorphophallus konjac, K. Koch in health care. Int. J. Biol. Macromol. 2016;92:942–956. doi: 10.1016/j.ijbiomac.2016.07.098 https://www.ncbi.nlm.nih.gov/pubmed/27481345
- Du X., Li J., Chen J., Li B. Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannanc. Food Res. Int. 2012;46:270–278. doi: 10.1016/j.foodres.2011.12.015
- Gao S., Guo J., Wu L., Wang S. Gelation of konjac glucomannan crosslinked by organic borate. Carbohydr. Polym. 2008;73:498–505. doi: 10.1016/j.carbpol.2007.12.013
- Li Z.Y., Su Y.L., Haq M.A., Xie B.Q., Wang D.J. Konjac glucomannan/polyacrylamide bicomponent hydrogels: Self-healing originating from semi-interpenetrating network. Polymer. 2016;103:146–151. doi: 10.1016/j.polymer.2016.09.046
- Wang L.X., Jiang Y.P., Lin Y.H., Pang J., Liu X.Y. Rheological properties and formation mechanism of DC electric fields induced konjac glucomannan-tungsten gels. Carbohydr. Polym. 2016;142:293–299. doi: 10.1016/j.carbpol.2016.01.060
- Wu L.P., Lin X.Y., Wu J.J., Zhou X.B., Luo X.G. Adsorption behavior of carboxymethyl konjac glucomannan microspheres for fluoride from aqueous solution. RSC Adv. 2016;6:89417–89429. doi: 10.1039/C6RA17183D
- Ji L., Xue Y., Zhang T., Li Z.J., Xue C.H. The effects of microwave processing on the structure and various quality parameters of Alaska pollock surimi protein-polysaccharide gels. Food Hydrocoll. 2017;63:77–84. doi: 10.1016/j.foodhyd.2016.08.011
- Behera S.S., Ray R.C. Nutritional and potential health benefits of konjac glucomannan, a promising polysaccharide of elephant foot yam, Amorphophallus konjac K. Koch: A review. Food Rev. Int. 2016;33:22–43. doi: 10.1080/87559129.2015.1137310
- Fan L.H., Yi J.Y., Tong J., Zhou X.Y., Ge H.Y., Zou S.Q., Wen H.G., Nie M. Preparation and characterization of Oxidized konjac glucomannan/Carboxymethyl Chitosan/Graphene Oxide hydrogel. Int. J. Biol. Macromol. 2016;91:358–367. doi: 10.1016/j.ijbiomac.2016.05.042
- Weska R.F., Achilli M., Beppu M.M., Mantovani D. Improvement of Collagen Hydrogel Scaffolds Properties by the Addition of konjac glucomannan. Adv. Mater. Res. 2011;409:187–192. doi: 10.4028/www.scientific.net/AMR.409.187
- Chen J.F., Zhang W.Y., Li X. Adsorption of Cu(II) ion from aqueous solutions on hydrogel prepared from konjac glucomannan. Polym. Bull. 2016;73:1965–1984. doi: 10.1007/s00289-015-1588-9
- SCF (Scientific Committee for Food), 1997. Food Science and Techniques, Reports of the Scientific Committee for Food, 41st Series.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1993. Konjac flour, WHO Food Additives Series 32.
- Sun Tan CQ, Wei HK, Zou Y, Long G, Ao JT, Xue HX, Jiang SW and Peng J, 2015. Effects of different amounts of konjac flour inclusion in gestation diets on physio‐chemical properties of diets, postprandial satiety in pregnant sows, lactation feed intake of sows and piglet performance. Animal Reproduction Science, 152, 55–64.
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2014. Statement on a conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. https://doi.org/10.2903/j.efsa.2014.3697
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2009. Scientific Opinion on the substantiation of health claims related to glucomannan and maintenance of normal blood cholesterol concentrations (ID 836, 1560) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 on request from the European Commission. EFSA Journal 2009;7(9):1258, 14 pp. https://doi.org/10.2903/j.efsa.2009.1258