Chickenpox
Chickenpox also known as varicella is a highly contagious viral infection caused by the primary infection (first time infection) with the varicella-zoster virus (VZV) of the herpes family 1. The varicella-zoster virus (VZV) is sometimes called herpesvirus type 3. Most chickenpox cases are in children under age 10, but older children and adults of all races and sex can get it. Adult with chickenpox are more likely to have life-threatening complications than children with chickenpox, such as thrombocytopenia (low blood platelets), viral pneumonia, disseminated primary varicella infection, Reye syndrome, Guillain-Barré syndrome and encephalitis, especially in adults with weakened immune system 2. Careful follow-up is required when an adult is diagnosed with adult chickenpox 3.
Chickenpox is highly contagious and the varicella-zoster virus (VZV) spreads very easily from person to person by breathing in airborne respiratory droplets from an infected person’s coughing or sneezing or through direct contact with the fluid from the open sores or fluid from shingles lesions. Chickenpox is acquired through contact with respiratory droplets or fluid from shingles lesions. A person who is not immune to the varicella-zoster virus (VZV) has a 70–90% chance of being infected with the virus if exposed to someone in the early stages of the disease. Once a person has had the chickenpox infection, it is unlikely he or she will get it again, as it confers lifelong immunity.
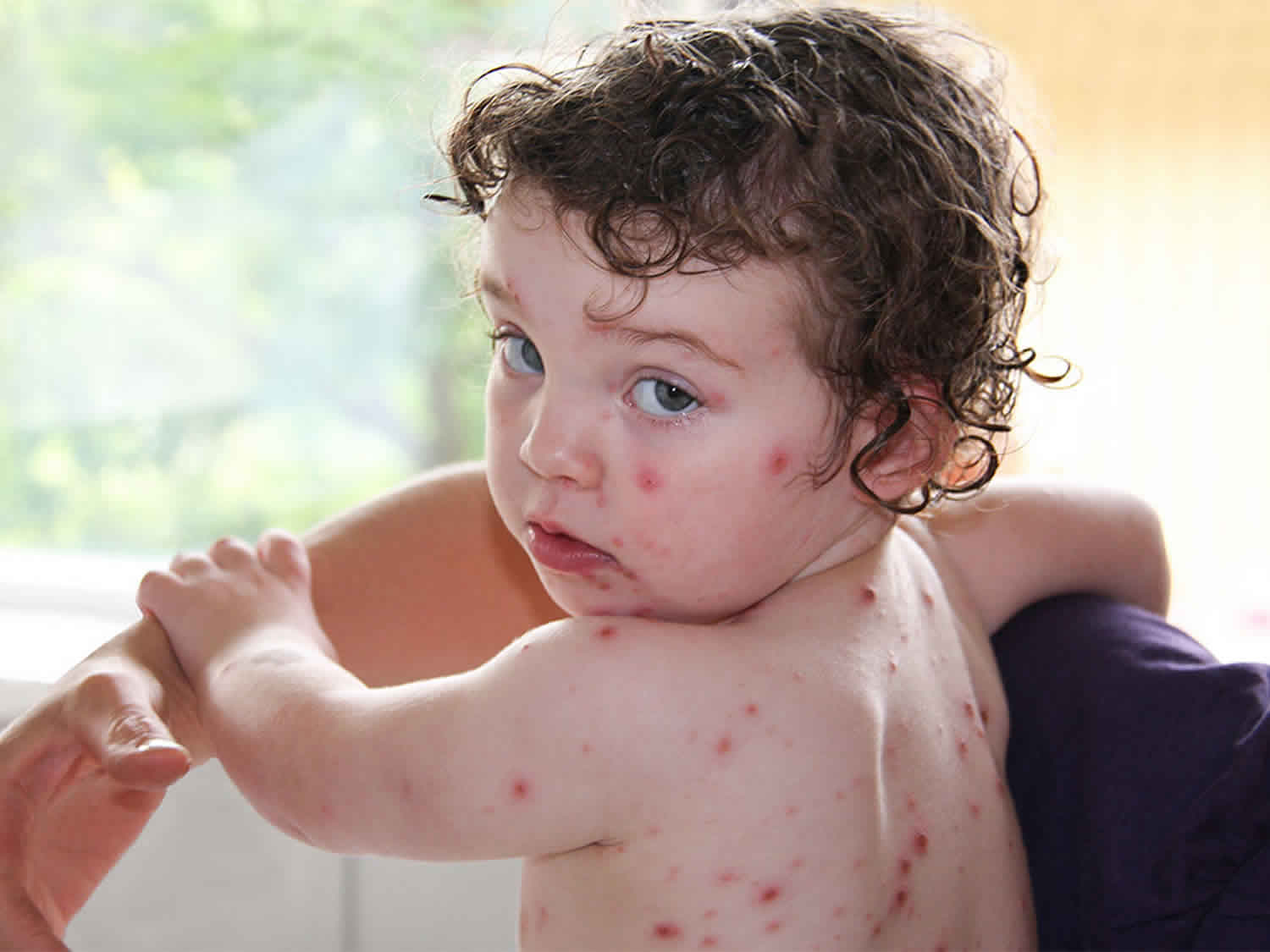
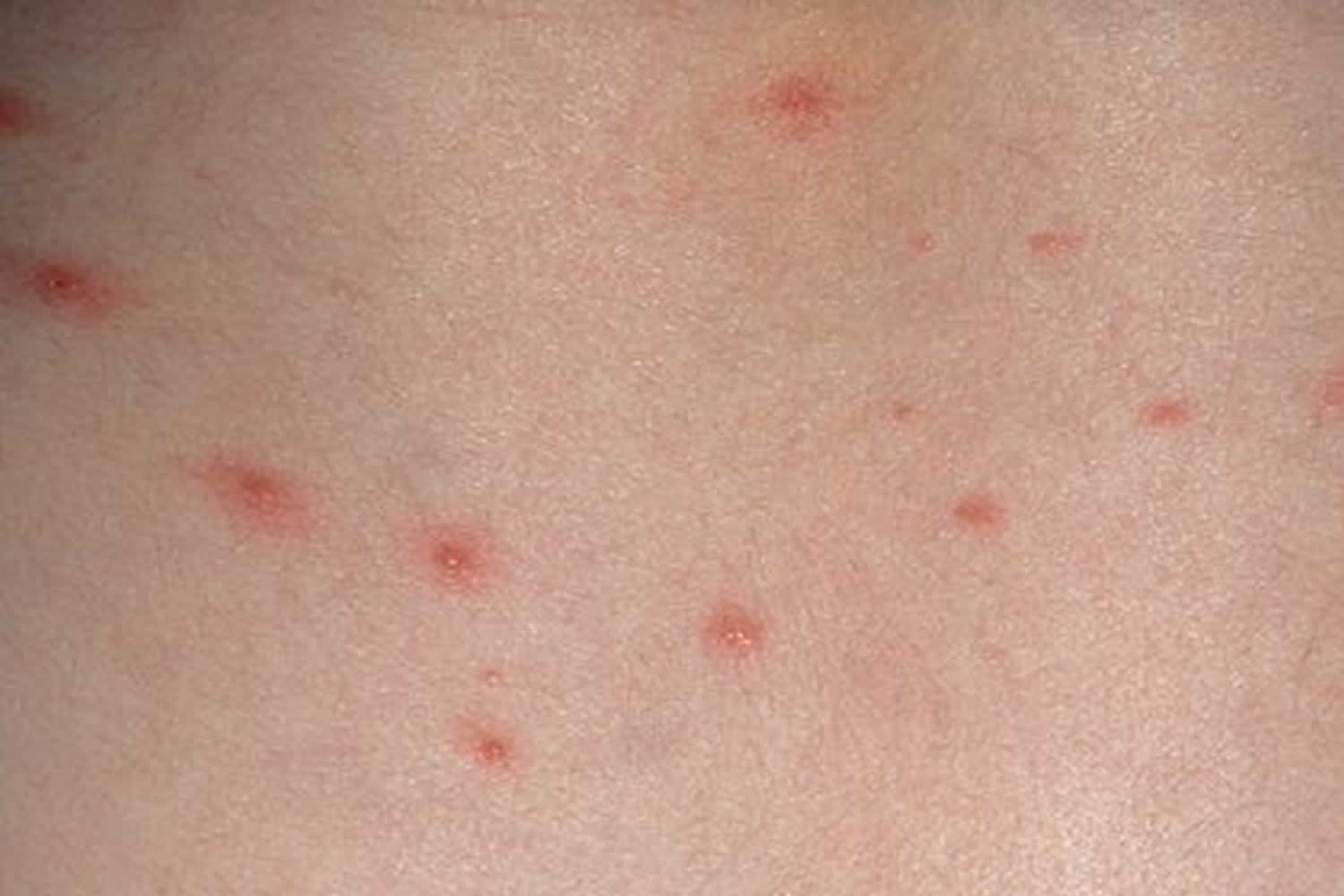
People develop symptoms about 7-21 days after being exposed to chickenpox. The classic symptom of chickenpox is an uncomfortable, itchy rash. The rash turns into fluid-filled blisters (vesicles) and eventually into scabs. It usually shows up on the face, chest, and back and then spreads to the rest of the body. Other symptoms include:
- Fever
- Headache
- Tiredness
- Loss of appetite
Sometimes children who have been vaccinated develop chickenpox. In these children, the rash is typically milder, fever is less common, and the illness is shorter. However, contact with the sores can spread the infection.
When exposed to the rash, a person can develop the disease in 14-21 days. Generally, the symptoms that are seen are an itchy rash with vesicular (fluid filled) lesions. Prior to chickenpox vaccine, the average was 250-500 lesions in varying stages of development during the course of illness. The lesions go though papules (flattened lesions) to vesicles (fluid filled), and then to resolution (crusting). Generally, these are seen with a low grade fever. The lesions put the person at higher risk for local infection to the affected areas on their skin. People can develop more symptoms that affect the whole body and can cause major illness. Prior to widespread vaccinating, each year, chickenpox caused about 4 million cases, about 10,600 hospitalizations and 100 to 150 deaths in the US from varicella. During the first 25 years of the U.S. chickenpox vaccination program, the vaccine has prevented an estimated 91 million cases, 238,000 hospitalizations, and 2,000 deaths.
In otherwise healthy children, chickenpox typically needs no medical treatment. Chickenpox in children is usually mild and lasts 5 to 10 days. Calamine lotions and oatmeal baths can help with itching. Your doctor may prescribe an antihistamine to relieve itching. Acetaminophen can treat the fever. Do not use aspirin for chickenpox; that combination can cause Reye syndrome.
Chickenpox can sometimes cause serious problems. Adults, babies, teenagers, pregnant women, and those with weak immune systems tend to get sicker from it. For people who are at high risk of complications from chickenpox, doctors sometimes prescribe antiviral medicines to shorten the length of the infection and to help reduce the risk of complications.
If you or your child are at high risk of complications, your doctor may suggest an antiviral drug such as acyclovir (Zovirax, Sitavig). This medication might lessen the severity of chickenpox when given within 24 hours after the rash first appears. Other antiviral drugs, such as valacyclovir (Valtrex) and famciclovir, also may lessen the severity of the disease, but might not be approved or appropriate for everyone.
In some instances, your doctor may recommend getting the chickenpox vaccine within three to five days after you’ve been exposed to the virus. This can prevent the disease or lessen its severity.
If complications develop, your doctor will determine the appropriate treatment. He or she may prescribe antibiotics for skin infections and pneumonia. Brain inflammation (encephalitis) is usually treated with antiviral drugs. You may need to be hospitalized.
Once you catch chickenpox, the virus usually stays in your body. You probably will not get chickenpox again, but the varicella-zoster virus (VZV) can cause shingles (herpes zoster) in adults. A chickenpox vaccine can help prevent most cases of chickenpox, or make it less severe if you do get it.
Figure 1. Chickenpox rash (varicella rash) on a child
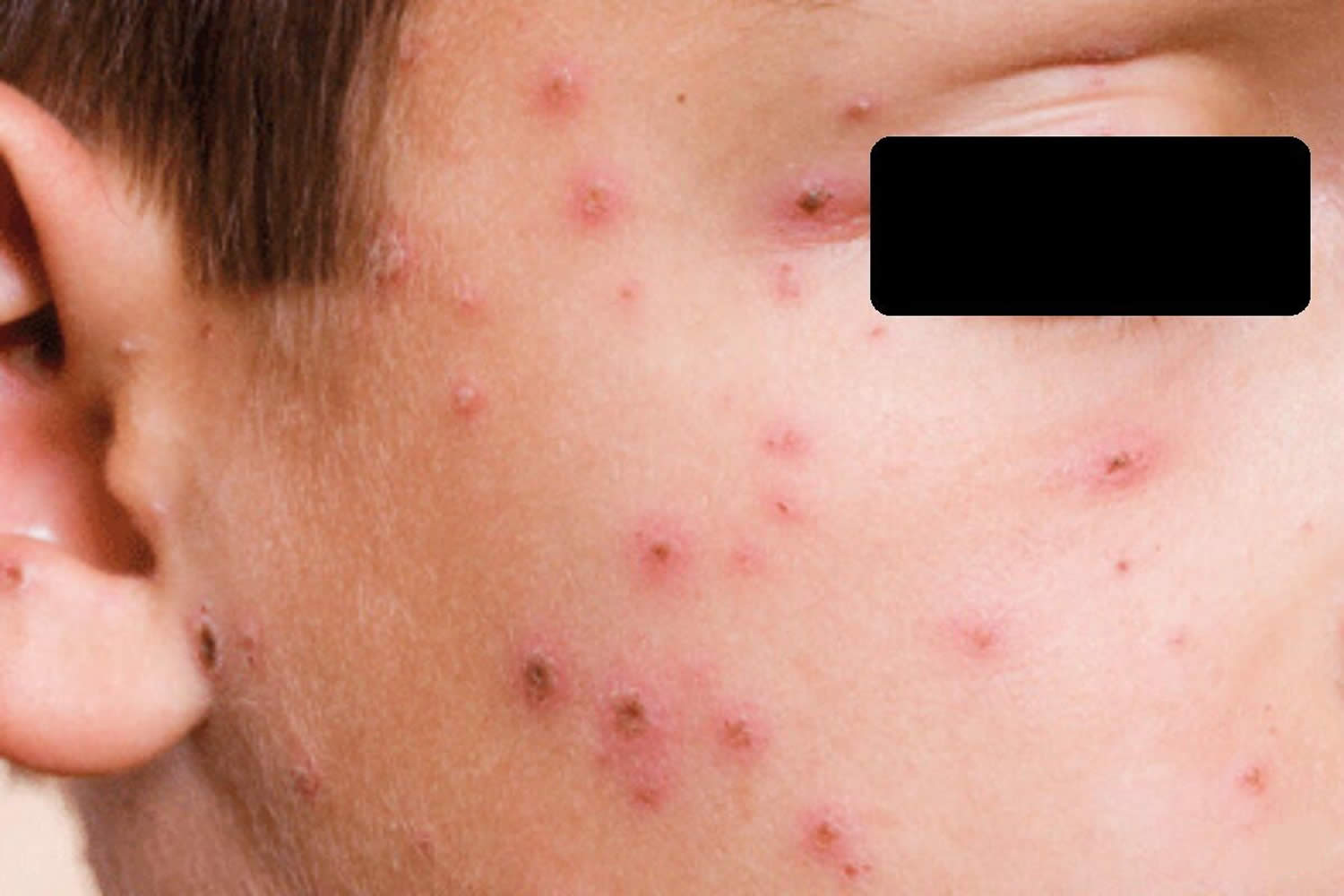
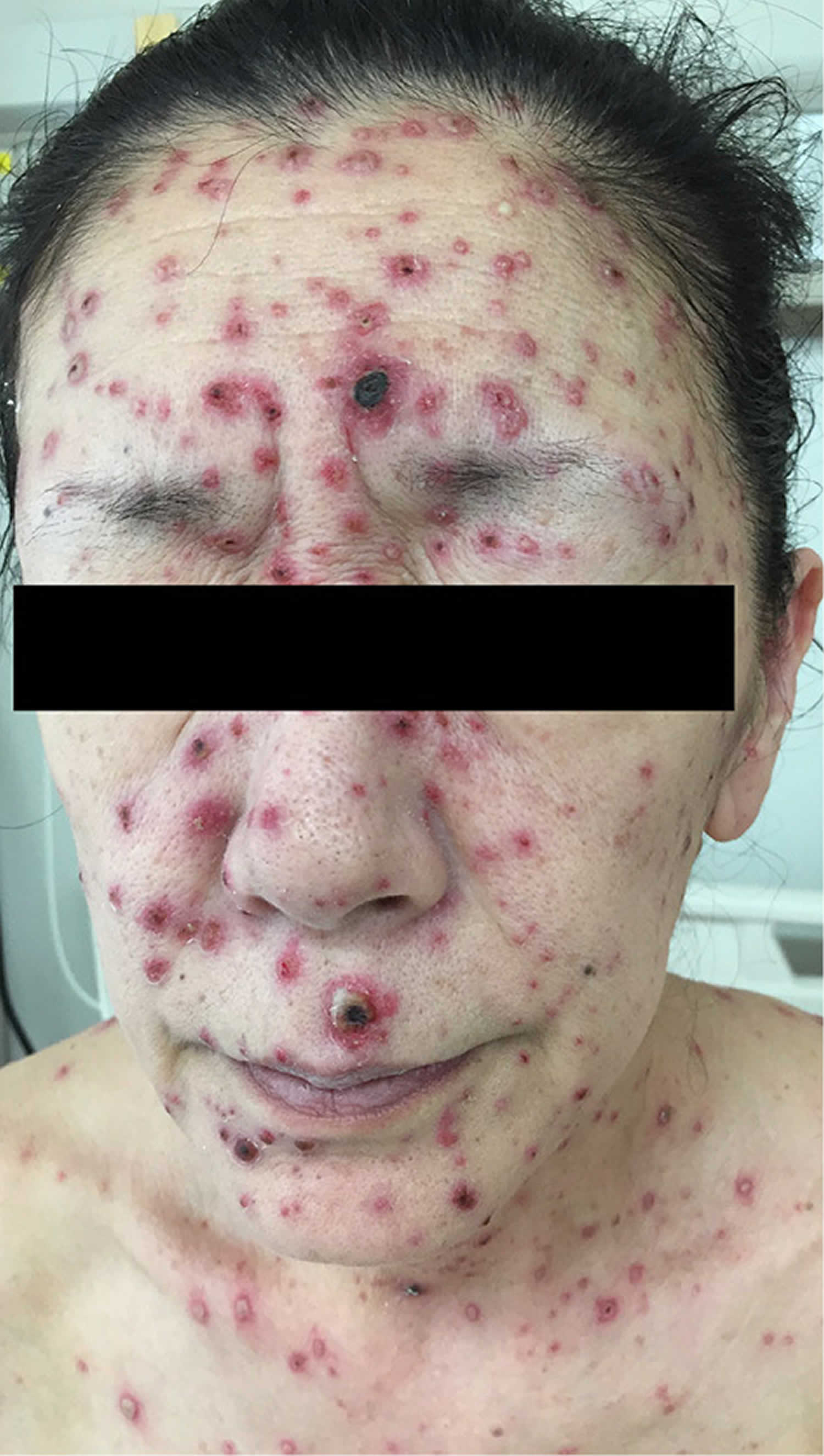
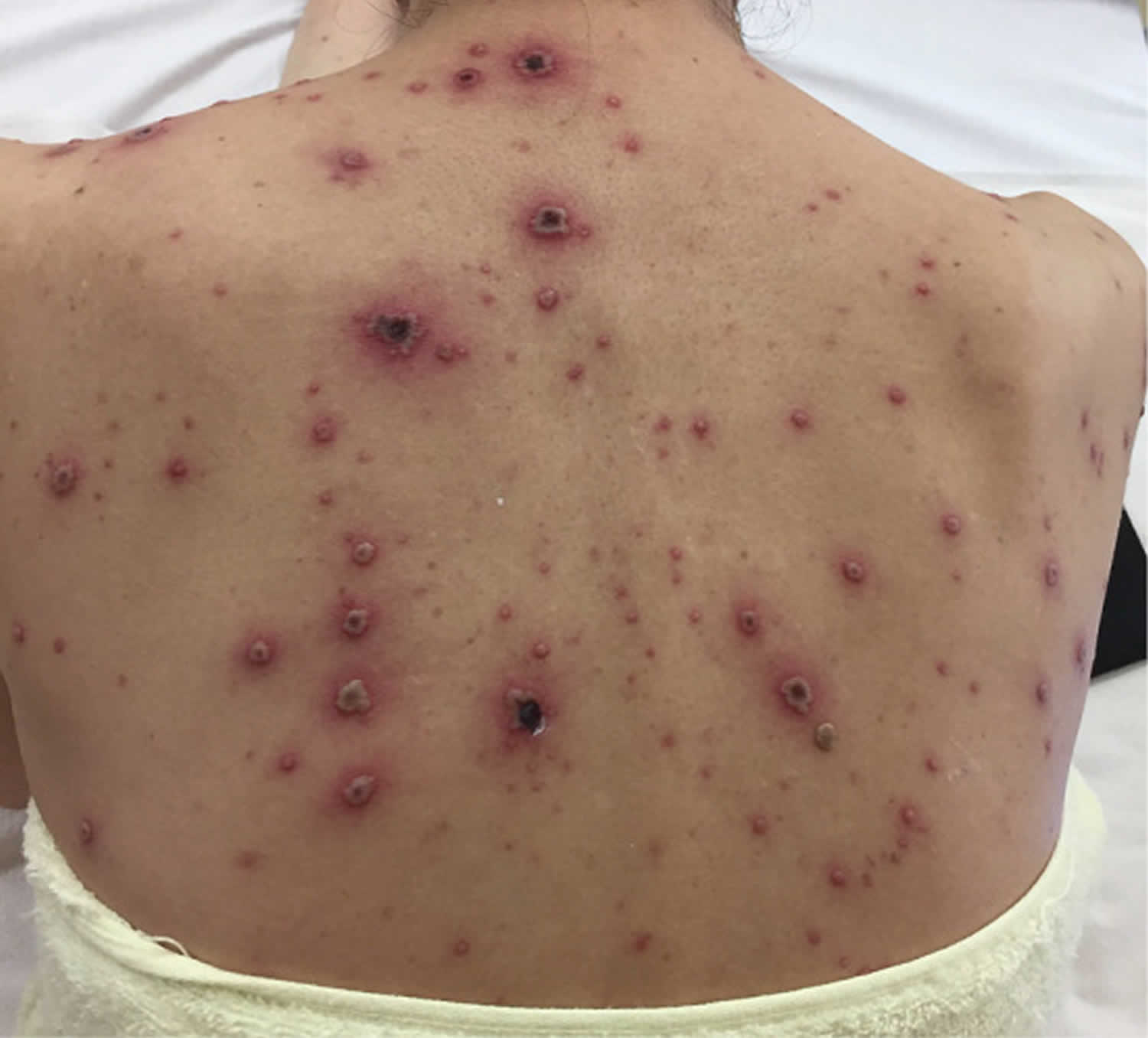
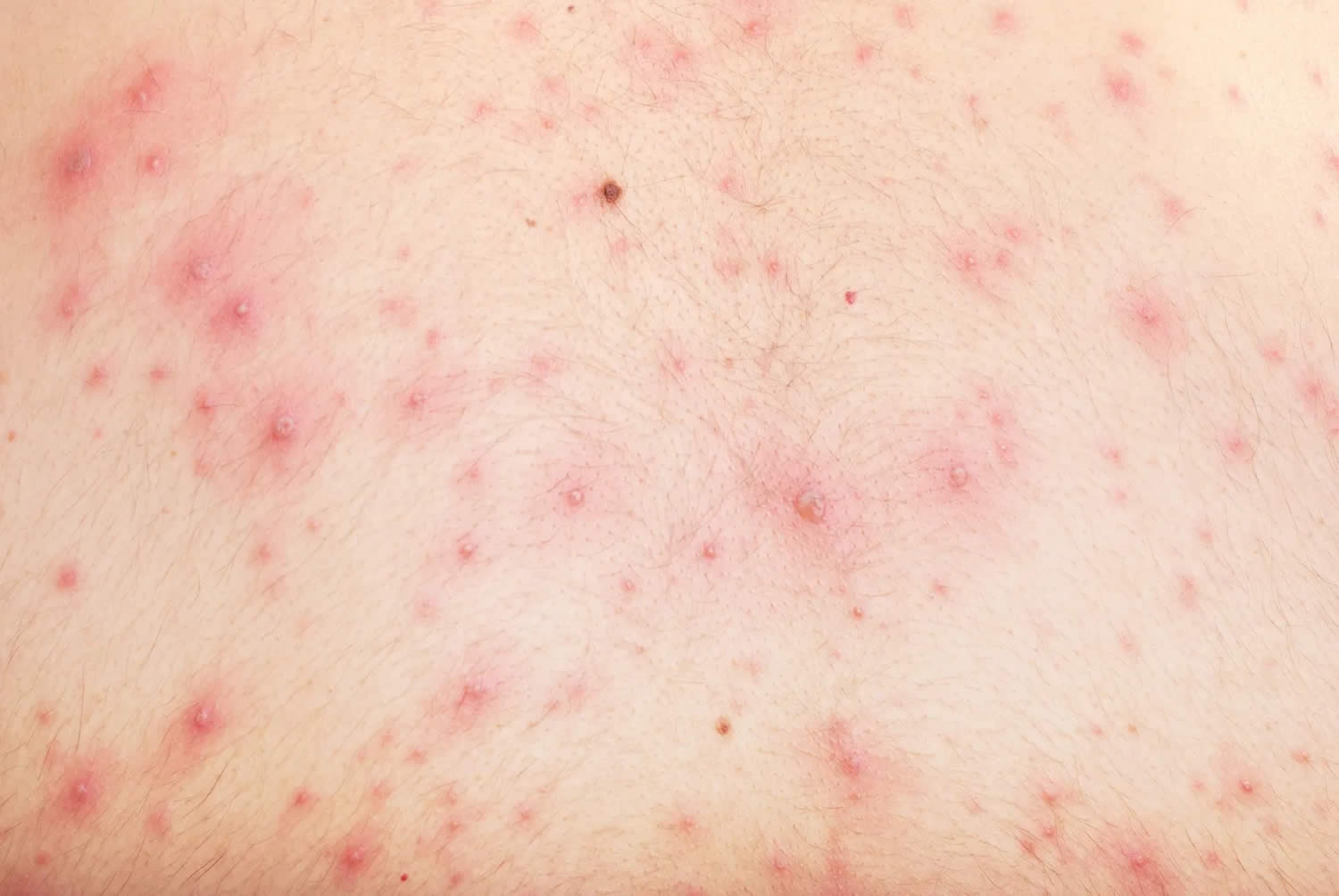
Figure 2. Chickenpox in adults
[Source 3 ]Chicken pox incubation period
The incubation period for varicella-zoster virus (VZV) is typically between 10 to 21 days. Patients with chickenpox are considered contagious from 1 to 2 days before developing the rash until all skin lesions have crusted over. This may take 5–10 days. Varicella infection in pregnant women can spread via the placenta and infect the fetus 4, 5.
Chickenpox contagious period
A person with chickenpox is contagious 1–2 days before the rash appears and until all the blisters have formed scabs. This may take 5–10 days. Children should stay away from school or childcare facilities throughout this contagious period. Adults with chickenpox who work among children should also remain home.
It can take 10–21 days after contact with an infected person for someone to develop chickenpox. This is how long it takes for the virus to replicate and come out in the characteristic rash in the new host.
As chickenpox may cause complications in immunocompromised individuals and pregnant women, these people should avoid visiting friends or family when there is a known case of chickenpox. In cases of inadvertent contact, see your doctor who may prescribe special preventive treatment.
How can I find out if I am infected with varicella?
Your doctor can do a blood test to find out if you have a current infection with chickenpox. They can also test to see if you have ever had chickenpox in the past. When a person has chickenpox, they make antibodies to the virus. These antibodies usually last a long time and keep a person from getting chickenpox again. (The person becomes immune.) People who are immune are not likely to develop chickenpox if they are exposed to the virus again.
The most sensitive method for confirming a diagnosis of varicella is the use of polymerase chain reaction (PCR) to detect varicella-zoster virus (VZV) in skin lesions (vesicles, scabs, maculopapular lesions). Vesicular lesions or scabs, if present, are the best for sampling. Adequate collection of specimens from maculopapular lesions in vaccinated people can be challenging. However, one study comparing a variety of specimens from the same patients vaccinated with one dose suggests that maculopapular lesions collected with proper technique can be highly reliable specimen types for detecting varicella-zoster virus (VZV). Other sources such as nasopharyngeal secretions, saliva, urine, bronchial washings, and cerebrospinal fluid are less likely to provide an adequate sample and can often lead to false negative results.
Other viral isolation techniques for confirming varicella are direct fluorescent antibody assay (DFA) and viral culture. However, these techniques are generally not recommended because they are less sensitive than PCR and, in the case of viral culture, will take longer to generate results.
IgM serologic testing is considerably less sensitive than PCR testing of skin lesions. IgM serology can provide evidence for a recent active varicella-zoster virus (VZV) infection, but cannot discriminate between a primary infection and reinfection or reactivation from latency since specific IgM antibodies are transiently produced on each exposure to varicella-zoster virus (VZV). IgM tests are also inherently prone to poor specificity.
Paired acute and convalescent sera showing a four-fold rise in IgG antibodies have excellent specificity for varicella, but are not as sensitive as PCR of skin lesions for diagnosing varicella. People with prior history of vaccination or disease history may have very high baseline titers, and may not achieve a four-fold increase in the convalescent sera. The usefulness of this method for diagnosing varicella is further limited as it requires two office visits. A single positive IgG ELISA result cannot be used to confirm a varicella case.
If you have recently been around someone with chickenpox and you do not have immunity, talk to your doctor about what steps you can take to avoid getting chickenpox, or to reduce the severity of the symptoms.
Chickenpox in pregnancy
Non-immune pregnant women should take care to avoid contact with people who have chickenpox and to wash their hands frequently when handling food, animals, and children. Exposure to the varicella-zoster virus (VZV) in pregnancy (chickenpox in the first half of pregnancies) may cause viral pneumonia, premature labor and preterm delivery (delivery before 37 weeks of pregnancy) and rarely maternal death. About 2% of babies (1 in 50) of a pregnant person with chickenpox will have 1 or more birth defects due to the infection 6. 1% (1/100) of pregnancies that are infected with chickenpox in the first trimester have birth defects related to chickenpox (and 99% do not). When chickenpox occurs between 13 and 20 weeks of pregnancy, the chance for birth defects related to the chickenpox infection is to 2% (98% of pregnancies do not have related birth defects) 6. The chance for birth defects is greatest when chickenpox develops between 7 and 20 weeks of pregnancy 6.
Babies with birth defects from exposure to chickenpox in pregnancy are said to have congenital varicella syndrome. The birth defects include scars on the skin, eye problems (fetal chorioretinitis & cataracts), poor growth, underdevelopment of an arm or leg (limb atrophy), small head size (microcephaly), or delayed development and/or intellectual disability. Some babies may have only one of these problems while others have some or all. However, most babies born to women who have chickenpox in pregnancy are healthy 6.
If a mother develops chickenpox just before delivery or during the 28 days after delivery, her baby is at risk of severe infection. Chickenpox infection in this time period is called neonatal varicella. For infants with neonatal varicella infection, if untreated, the mortality rate is as high as 31%. Death typically occurs from varicella pneumonia. The mortality rate decreases to 7% when varicella-zoster immune globulin (VariZIG) is administered 4, 7.
Approximately 25% of fetuses of mothers with chickenpox become infected. It is harmless to most of them. Offspring may remain asymptomatic, or develop herpes zoster (shingles) at a young age without a previous history of primary chickenpox infection. They may also develop congenital varicella syndrome, one of the TORCH infections.
Congenital varicella syndrome
Congenital varicella syndrome occurs in up to 2% of fetuses exposed to varicella-zoster virus (VZV) in the first 20 weeks of gestation. It can result in spontaneous abortion, fetal chorioretinitis, cataracts, limb atrophy (underdevelopment of an arm or leg), cerebral cortical atrophy and microcephaly (small head size), skin scars, and delayed development and/or intellectual disability. The overall prognosis of infants born with congenital varicella syndrome is poor, often fatal in roughly 30% of infected babies within the first month of life 8. Death in infancy often results from intractable gastrointestinal reflux, severe recurrent aspiration pneumonia, and respiratory failure 5, 9.
My due date is in 3 weeks, and I have just been exposed to chickenpox. Is there any risk to my baby if I develop chickenpox at this stage of pregnancy?
If you already had chickenpox or had the varicella vaccine, your past infection or vaccine should protect you. If you have not had chickenpox or the varicella vaccine, talk to your healthcare provider right away. If you develop chickenpox 5 days or less before delivery or 1-2 days after delivery, there is a 20-25% chance your newborn could also develop chickenpox. Chickenpox infection in this time period is called neonatal varicella. For infants with neonatal varicella infection, if untreated, the mortality rate is as high as 31%. Death typically occurs from varicella pneumonia. The mortality rate decreases to 7% when varicella-zoster immune globulin (VariZIG) is administered 4, 7.
If you develop chickenpox between 6 and 21 days before delivery, there is still a chance your newborn could develop neonatal varicella. However, because your baby will get some of your newly-made chickenpox antibodies, the neonatal varicella is more likely to be mild.
Does getting chickenpox in pregnancy cause long-term problems in behavior or learning for the baby?
Most babies born to a person who had chickenpox in pregnancy are healthy. However, some children could have eye problems, small head size, or delayed development and/or intellectual disability.
Can I breastfeed while sick with chickenpox?
The chickenpox virus has not been found in breast milk of people with a chickenpox infection. Breast milk might contain antibodies that can help to protect your baby from getting chickenpox. However, because chickenpox is very contagious, talk to your child’s pediatrician right away if you come down with chickenpox. It is important to prevent your baby from coming into direct contact with the rash or the affected areas in order lower the chances of your baby from getting the virus. If you suspect your baby has any symptoms that could be due to chickenpox, contact your child’s healthcare provider.
What is the cause of chickenpox?
Chickenpox is caused by primary infection with the varicella-zoster virus (VZV), of the Herpesviridae family. The varicella-zoster virus (VZV) is sometimes called herpesvirus type 3. Humans are the only source of this disease. Chickenpox is highly contagious and the varicella-zoster virus (VZV) spreads very easily from person to person by breathing in airborne respiratory droplets from an infected person’s coughing or sneezing or through direct contact with the fluid from the open sores or fluid from shingles lesions. Chickenpox is acquired through contact with respiratory droplets or fluid from shingles lesions. A person who is not immune to the varicella-zoster virus (VZV) has a 70–80% chance of being infected with the virus if exposed to someone in the early stages of the disease.
The varicella-zoster virus (VZV) is a human alphaherpesvirus with worldwide distribution and is highly contagious 10. The varicella-zoster virus (VZV) is responsible for chickenpox (primary varicella infection) and herpes zoster or shingles infection (which is a reactivation of latent varicella infection).
Initially, viral replication occurs in the respiratory tract. The varicella-zoster virus (VZV) then invades local lymph nodes, leading to viremia and widespread dissemination. As the viremia increases, new skin vesicular lesions erupt, with individuals typically having 250 to 500 lesions in varying evolution stages 8.
The incubation period for varicella-zoster virus (VZV) is typically between 10 to 21 days. Individuals with primary varicella infection (chickenpox) may also experience a prodrome of fever, malaise, abdominal pain, and headaches. Patients with chickenpox are considered contagious from 1 to 2 days before developing the rash until all skin lesions have crusted over. Varicella infection in pregnant women can spread via the placenta and infect the fetus 4, 5.
After chickenpox (primary varicella infection), the varicella-zoster virus (VZV) becomes latent in the dorsal root ganglia (a collection of neuronal cell bodies of sensory neurons) 11. It can later reactivate as herpes zoster or shingles (reactivation of latent varicella infection). The specific mechanisms of varicella-zoster virus (VZV) reactivation are not fully understood 12. Scientists theorized that when the varicella-zoster virus (VZV)-specific immunity decreases, the latent virus in the ganglions reactivates, replicates, and then various herpes zoster (shingles) symptoms appear 13. Many factors affecting cellular immunity may cause the reactivation of the varicella-zoster virus (VZV) 14. These risk factors for varicella-zoster virus (VZV) reactivation include old age, cellular immunodeficiency, genetic susceptibility, trauma, systemic diseases (such as diabetes, kidney disease, fever, hypertension, etc.), mental stress and fatigue 15, 16, 17.
The clinical manifestation of herpes zoster (shingles) includes a painful vesicular rash in a dermatomal distribution, followed by postherpetic neuralgia. Typically, a single dermatome is involved, although two or three adjacent dermatomes may be affected 18. The lesions usually do not cross the midline 18. Direct contact with the vesicles may be contagious, especially in non-immune or immunocompromised hosts. Herpes zoster (shingles) does not pose a risk to a developing fetus due to circulating protective maternal antibodies transmitted through placental transfer 9.
Chickenpox signs and symptoms
In children, chickenpox usually begins as itchy red papules progressing to vesicles (fluid filled blisters) on the stomach, back and face, and then spreading to other parts of the body. Blisters can also arise inside the mouth. The itchy blister rash caused by chickenpox infection appears 7 to 21 days after exposure to the virus and usually lasts about five to 10 days.
Once the chickenpox rash appears, it goes through three phases:
- Raised pink or red bumps (papules), which break out over several days. About 24 to 36 hours after the first symptoms begin, a rash of small, flat, red spots appears. The spots usually begin on the trunk and face, later appearing on the arms and legs. Some people have only a few spots. Others have them almost everywhere, including on the scalp and inside the mouth.
- Small fluid-filled blisters (vesicles), which form in about one day and then break and leak. Within 6 to 8 hours, each spot becomes raised. It forms an itchy, round, fluid-filled blister against a red background and finally crusts. Spots continue to develop and crust for several days. A hallmark of chickenpox is that the rash develops in crops so that the spots are in various forms of development at any affected area. Very rarely, the spots become infected by bacteria, which can cause a severe skin infection (cellulitis or necrotizing fasciitis).
- Crusts and scabs, which cover the broken blisters and take several more days to heal. New spots usually stop appearing by the fifth day, the majority are crusted by the sixth day, and most disappear in fewer than 20 days.
New bumps continue to appear for several days, so you may have all three stages of the rash — bumps, blisters and scabbed lesions — at the same time. You can spread the virus to other people for up to 48 hours before the rash appears, and the virus remains contagious until all broken blisters have crusted over.
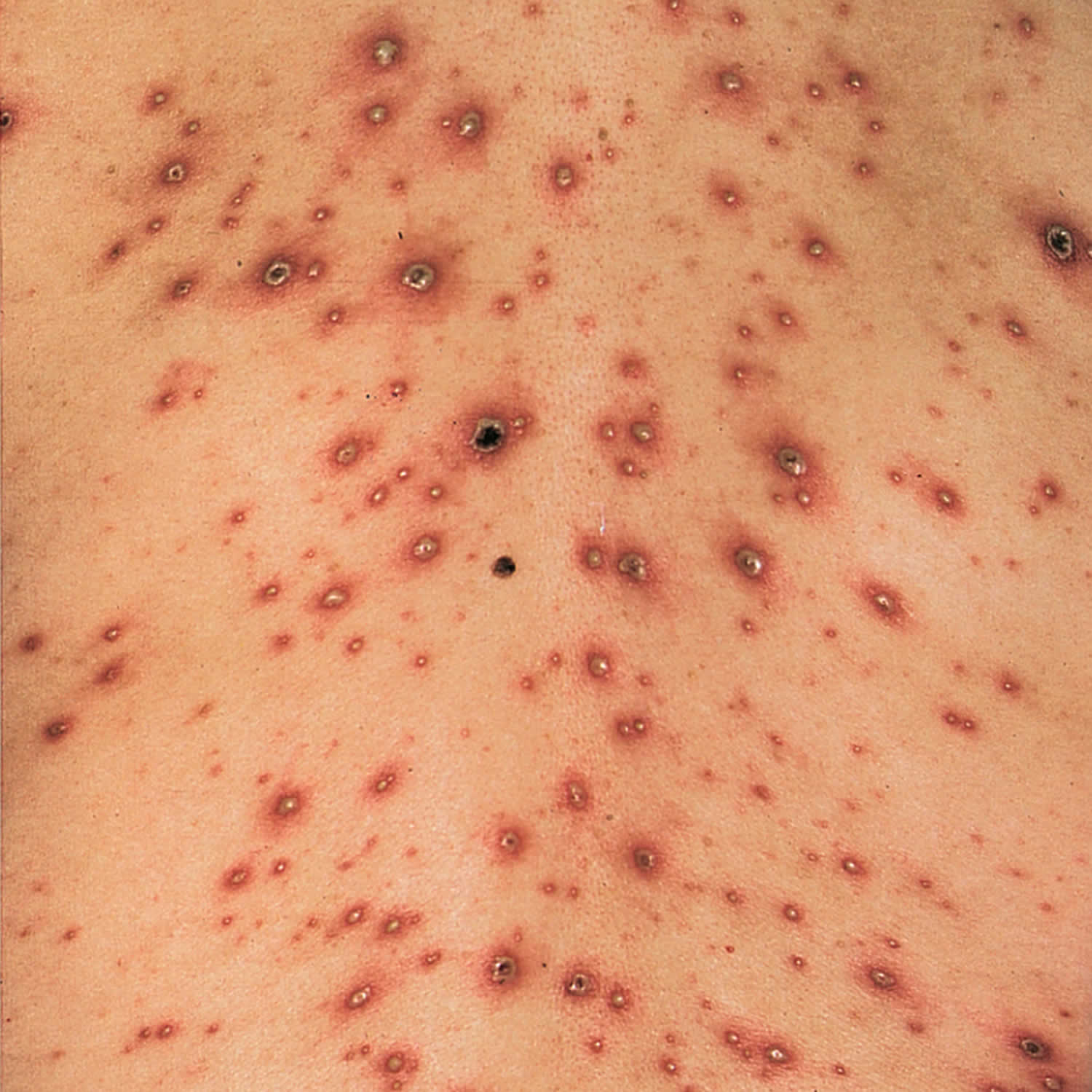
The rash spread pattern can vary from child to child. There may be only a scattering of vesicles, or the entire body may be covered with up to 500 vesicles. In severe cases, the rash can cover the entire body, and lesions may form in the throat, eyes, and mucous membranes of the urethra, anus and vagina. The vesicles tend to be very itchy and uncomfortable.
Some children may also experience additional symptoms such as high fever, headache, cold-like symptoms, loss of appetite, vomiting and diarrhea and tiredness and a general feeling of being unwell (malaise). Younger children often do not have these symptoms, but symptoms are often severe in adults.
Spots in the mouth quickly rupture and form raw sores (ulcers), which often make swallowing painful. Raw sores may also occur on the eyelids and in the upper airways, rectum, and vagina. The worst part of the illness usually lasts 4 to 7 days.
Chickenpox symptoms in adults
Chickenpox is usually more severe in adults and can be life-threatening in complicated cases. Most adults who get chickenpox experience prodromal symptoms for up to 48 hours before breaking out in the rash. These include fever, malaise, headache, loss of appetite and abdominal pain. Chickenpox is usually more severe in adults and can be life-threatening in complicated cases.
The blisters clear up within one to three weeks but may leave a few scars. These are most often depressed (anetoderma), but they may be thickened (hypertrophic scars). Scarring is prominent when the lesions get infected with bacteria.
Chickenpox stages
Chickenpox happens in 3 stages. But new spots can appear while others are becoming blisters or forming a scab.
An itchy, spotty rash is the main symptom of chickenpox. It can be anywhere on the body. Once the chickenpox rash appears, it goes through three phases:
- Raised pink or red bumps (papules), which break out over several days. About 24 to 36 hours after the first symptoms begin, a rash of small, flat, red spots appears. The spots usually begin on the trunk and face, later appearing on the arms and legs. Some people have only a few spots. Others have them almost everywhere, including on the scalp and inside the mouth.
- Small fluid-filled blisters (vesicles), which form in about one day and then break and leak. Within 6 to 8 hours, each spot becomes raised. It forms an itchy, round, fluid-filled blister against a red background and finally crusts. Spots continue to develop and crust for several days. A hallmark of chickenpox is that the rash develops in crops so that the spots are in various forms of development at any affected area. Very rarely, the spots become infected by bacteria, which can cause a severe skin infection (cellulitis or necrotizing fasciitis).
- Crusts and scabs, which cover the broken blisters and take several more days to heal. New spots usually stop appearing by the fifth day, the majority are crusted by the sixth day, and most disappear in fewer than 20 days.
Stage 1 small spots appear
The pink or red spots (papules) can:
- be anywhere on the body, including inside the mouth and around the genitals, which can be painful
- spread or stay in a small area
- be red, pink, darker or the same colour as surrounding skin, depending on your skin tone
- be harder to see on brown and black skin
Figure 3. Chickenpox stage 1
Stage 2 small fluid-filled blisters (vesicles)
- The spots fill with fluid and become blisters. The blisters are very itchy and may burst.
- The blisters are pink and shiny. The skin around some spots appears slightly pink.
Figure 4. Chickenpox stage 2
Stage 3 crusts and scabs
- The spots form a scab. Some scabs are flaky while others leak fluid.
- Most of the spots have scabs over them. The scabs are pink, purple or grey. There are also a few spots without scabs, which look like small blisters. These are slightly darker in colour than the surrounding skin.
Figure 5. Chickenpox stage 3
Complications of chickenpox
In healthy children, chickenpox infection is usually an uncomplicated, self-limiting disease. Risk of complications from chickenpox is increased for newborns, adults, and people who have a weakened immune system or certain disorders.
Pregnant women who get varicella are at risk of serious complications, such as pneumonia, and may die as a result. Chickenpox can also be transmitted to the fetus, especially if chickenpox develops during the 1st or early 2nd trimester. Such an infection can result in scars on the skin, birth defects, and a low birth weight.
Chickenpox complications may include:
- Secondary bacterial infection of skin lesions caused by scratching
- Infection may lead to abscess, cellulitis, necrotizing fasciitis and gangrene
- Dehydration from vomiting and diarrhea
- Worsening of asthma
- Viral pneumonia. Lung infection (pneumonia) occurs in about 1 out of 400 adults, resulting in cough and difficulty breathing. Pneumonia rarely develops in young children who have a normal immune system.
- Chickenpox lesions may heal with scarring.
Some complications are more commonly seen in immunocompromised and adult patients with chickenpox:
- Disseminated primary varicella infection; this carries high morbidity
- Central nervous system complications such as Reye syndrome, Guillain-Barré syndrome and encephalitis
- Brain infection (encephalitis) is less common and causes unsteadiness in walking, headache, dizziness, confusion, and seizures. In adults, encephalitis can be life threatening. It occurs in 1 to 2 of 1,000 cases of chickenpox.
- Reye syndrome is a rare but very severe complication that occurs almost only in those younger than 18 following the use of aspirin. Therefore, aspirin should not be given to children with chickenpox. Reye syndrome may begin 3 to 8 days after the rash begins.
- Thrombocytopenia and purpura.
- Inflammation of the liver and bleeding problems may also occur.
Shingles (herpes zoster)
- The varicella-zoster virus remains dormant in sensory ganglia after infection.
- It may reactivate after many years as shingles. Shingles presents with grouped vesicular lesions, which usually affect a single dermatome.
- Other infections occurring as a result of reactivation of virus include post-herpetic neuralgia, vasculopathy (any disease affecting the blood vessels), myelopathy (injury to the spinal cord), acute retinal necrosis (inflammatory condition of the retina), meningitis or meningoencephalitis, cerebellitis and zoster sine herpete (pain without rash) 19, 20, 21, 22, 23. Among them, varicella-zoster virus (VZV) cerebrovasculopathy is caused by viral infection of the arteries, leading to pathological vascular remodeling and an ischemic or hemorrhagic stroke, with a mortality rate of 25%, and one-third of patients with virology-proven varicella-zoster virus (VZV) vascular disease had no previous rash 24. In addition, by reactivating and infecting the meninges, brain parenchyma, and nerve roots, the varicella-zoster virus (VZV) can cause varicella-zoster meningitis, varicella-zoster encephalitis, and varicella-zoster radiculitis 25. Varicella-zoster virus (VZV) meningoencephalitis can manifest as mild aseptic meningitis but also as severe encephalitis with edema, brain necrosis, and demyelination 21. The mortality rate of varicella-zoster virus (VZV) encephalitis is as high as 9% 26. Besides, varicella-zoster virus (VZV) meningoradiculitis can be lethal as reported 27. Varicella-zoster virus (VZV) myelopathy can present as a fatal myelitis, mostly in immunocompromised individuals. Postmortem analyses of the spinal cord from fatal cases have revealed a apparent invasion of the varicella-zoster virus (VZV) in the parenchyma with associated inflammation and, in some instances, spread of the virus to adjacent nerve roots 28.
- Evidence suggests that persistent radicular pain from herpes zoster can be caused by chronic activation of the varicella-zoster virus (VZV) and ganglionitis. In a patient who had experienced chronic trigeminal neuralgia for more than 1 year, the trigeminal ganglionic mass was removed and the pathological and virological analyses discovered active varicella-zoster virus (VZV) ganglionitis 29. In another study, active varicella-zoster virus (VZV) ganglionitis was found in the trigeminal ganglia of a patient who experienced chronic trigeminal neuralgia for months before death 30. Using human dorsal root ganglia xenografts in immunodeficient mice, Zerboni and Arvin 31 demonstrated varicella-zoster virus (VZV) replication in mechanoreceptive neurons; They also found that the VZV in the dorsal root ganglia triggers release of pro-inflammatory cytokines that cause neuronal damage. These findings may help to explain the neurologic sequelae in herpes zoster, zoster sine herpete (pain without rash) and post-herpetic neuralgia patients.
Prevention of chickenpox
Chickenpox is highly preventable by vaccination with live attenuated varicella vaccine. Chickenpox vaccine became available in the United States in 1995. The Centers for Disease Control and Prevention (CDC) recommends two doses of chickenpox (varicella) vaccine for children, adolescents, and adults who have never had chickenpox and were never vaccinated. Children are routinely recommended to receive the first dose at 12 through 15 months of age and the second dose at 4 through 6 years of age. During the first 25 years of the U.S. chickenpox vaccination program, the vaccine has prevented an estimated 91 million cases, 238,000 hospitalizations, and 2,000 deaths 32.
Chickenpox vaccination is especially important for:
- Healthcare professionals
- People who care for or are around other people whose body is less able to fight germs and sickness (weakened immune system)
- Teachers
- Childcare workers
- Residents and staff in nursing homes and other residential settings
- College students
- Inmates and staff of correctional institutions
- Military personnel
- Non-pregnant women of child-bearing age
- Premature infants born to susceptible mothers
- Infants born at less than 28 weeks gestation or who weigh ≤1000 grams, regardless of maternal immune status
- Immunocompromised people, including those who are undergoing immunosuppressive therapy, have malignant disease, or are immunodeficient
- Pregnant women
- Adolescents and adults living with children
- International travelers
Some people with a weakened immune system who do not have immunity against chickenpox may be considered for vaccination after talking with their doctor, including:
- People with HIV infection
- People with cancer, but whose disease is in remission
- People on low dose steroids
Two doses of varicella vaccine are recommended for all children, adolescents, and adults without evidence of immunity to varicella. Those who previously received one dose of varicella vaccine should receive their second dose for best protection against the disease.
Chickenpox vaccine
Two vaccines containing varicella virus are licensed for use in the United States 33.
- Varivax® is the single-antigen varicella vaccine 34.
- Contains only chickenpox vaccine.
- Is licensed for use in people 12 months of age or older.
- Can be given to children for their routine two doses of chickenpox vaccine at 12 through 15 months old and age 4 through 6 years old.
- ProQuad® is a combination measles, mumps, rubella, and varicella (MMRV) vaccine 35.
- Contains a combination of measles, mumps, rubella, and varicella (chickenpox) vaccines, which is also called MMRV.
- Is only licensed for use in children 12 months through 12 years of age.
Both vaccines contain live, attenuated varicella-zoster virus derived from the Oka strain.
Children 12 months through 12 years old
- 2 doses (0.5 ml each) of varicella vaccine should be given subcutaneously, separated by at least 3 months.
- Measles, mumps, rubella, and varicella (MMRV) vaccine is approved for healthy children in this age group.
Single-antigen vaccine and MMRV vaccine can be used for the routine 2-dose varicella vaccination.
- First dose: age 12 through 15 months
- Second dose: age 4 through 6 years
For the first dose, CDC recommends that MMR and varicella (MMRV) vaccines be given separately in children 12 through 47 months old unless the parent or caregiver expresses a preference for MMRV vaccine. For the second dose of measles, mumps, rubella, and varicella (MMRV) vaccines at any age (15 months–12 years) and for the first dose at age ≥48 months, use of MMRV vaccine generally is preferred over separate injections of MMR and varicella vaccines.
Both vaccines may be given at the same time as other vaccines for children age 12 through 15 months and age 4 through 6 years.
People 13 years or older
- 2 doses (0.5 ml each) of the single-antigen varicella vaccine subcutaneously 4 to 8 weeks apart
Measles, mumps, rubella, and varicella (MMRV) vaccine is NOT approved for people in this age group.
Post exposure chickenpox vaccination
Getting vaccinated after you are exposed to someone with chickenpox can:
- Prevent the disease or make it less serious.
- Protect you from chickenpox if you are exposed again in the future.
A healthcare provider can also prescribe a medicine to make chickenpox less severe if you:
- Are exposed to chickenpox.
- Do not have immunity against the disease.
- Are not eligible for vaccination.
How effective is the varicella vaccine?
One dose varicella vaccine
- 1 dose of single-antigen varicella vaccine is:
- 82% effective at preventing any form of varicella
- Almost 100% effective against severe varicella
Two doses of varicella vaccine
- In a pre-licensure clinical trial, 2 doses of vaccine were:
- 98% effective at preventing any form of varicella
- 100% effective against severe varicella
- In post-licensure studies, two doses of vaccine were:
- 92% (range 88% to 98%) effective at preventing all varicella
In children with HIV-infection
- 1 dose of single-antigen varicella vaccine is:
- 82% effective at preventing any form of varicella
Who should NOT get chickenpox vaccine
You do not need to get the chickenpox vaccine if you have evidence of immunity against the disease.
Some people should NOT get chickenpox vaccine or they should wait.
- People should check with their doctor about whether they should get chickenpox vaccine if they:
- Have HIV and AIDS or another disease that affects the immune system.
- Are being treated with drugs that affect the immune system, such as steroids, for 2 weeks or longer.
- Have any kind of cancer.
- Are getting cancer treatment with radiation or drugs.
- Recently had a transfusion or were given other blood products.
Duration of chickenpox vaccine protection
It is not known how long a vaccinated person is protected against varicella. But, live vaccines in general provide long-lasting immunity.
- Several studies have shown that people vaccinated against varicella had antibodies for at least 10 to 20 years after vaccination. But, these studies were done before the vaccine was widely used and when infection with wild-type varicella was still very common.
- A case-control study conducted from 1997 to 2003 showed that 1 dose of varicella vaccine was 97% effective in the first year after vaccination and 86% effective in the second year. From the second to eighth year after vaccination, the vaccine effectiveness remained stable at 81 to 86%. Most vaccinated children who developed varicella during the 8 years after vaccination had mild disease 36.
- A clinical trial showed that children with 2 doses of varicella vaccine were protected 10 years after being vaccinated. Fewer people had breakthrough varicella after 2 doses compared with 1 dose. The risk of breakthrough varicella did not increase over time 37.
- A meta-analysis that included 1-dose vaccine effectiveness reported through 2015 found a pooled estimate of 82% within the first decade. Considering the age of participants in the studies and vaccine recommendations in each country, the median time since vaccination is likely lower than 10 years. Four studies reported decline in vaccine effectiveness with time since vaccination; however, the differences did not reach statistical significance 38.
- Two doses of varicella vaccine add improved protection, with a pooled estimate of 92% (assessed ~5 years after vaccination).
Varicella vaccine cost
Most health insurance plans cover the cost of vaccines. However, you may want to check with your insurance provider before going to the doctor. If you don’t have health insurance or if your insurance does not cover vaccines for your child, the Vaccines for Children Program (https://www.cdc.gov/vaccines/programs/vfc/index.html) may be able to help. This program helps families of eligible children who might not otherwise have access to vaccines. To find out if your child is eligible, visit the VFC website or ask your child’s doctor. You can also contact your state VFC coordinator here (https://www.cdc.gov/vaccines/imz-managers/awardee-imz-websites.html).
Chickenpox diagnosis
Diagnosis of chickenpox is usually made on the presence of its characteristic rash and the presence of different stages of lesions simultaneously. A clue to the diagnosis is in knowing that the patient has been exposed to an infected contact within the 10–21 day incubation period. Patients may also have prodromal signs and symptoms.
If there’s any doubt about the diagnosis, chickenpox can be confirmed with lab tests, including blood tests or a culture of lesion samples.
Laboratory tests are often undertaken to confirm the diagnosis:
- Polymerase chain reaction (PCR) detects the varicella virus in skin lesions and is the most accurate method for diagnosis.
- The culture of blister fluid is time-consuming and is less frequently performed.
- Serology (IgM and IgG) is most useful in pregnant women, or before prescribing immune suppression medication to determine the need for pre-treatment immunization.
Chickenpox treatment
For most healthy patients with chickenpox, symptomatic therapy is usually all that is required.
- Trim children’s fingernails to minimize scratching.
- Take a warm bath and apply moisturizing cream.
- Acetaminophen (paracetamol) can reduce fever and pain
- Avoid non-steroidal anti-inflammatory drug (NSAID) such as ibuprofen (Advil, Motrin IB, others) use outside of hospital settings due to the increased risk of severe cutaneous complications such as invasive group A streptococcal superinfections.
- Do not use aspirin in children as this is associated with Reye syndrome.
- Calamine lotion and oral antihistamines may relieve itching.
- Consider oral aciclovir (antiviral agent) in people older than 12 years, which reduces the number of days with a fever.
Immunocompromised patients with chickenpox need intravenous treatment with the antiviral aciclovir.
In cases of unintentional exposure to the varicella-zoster virus (VZV), varicella-zoster immune globulin if given within 96 hours of initial contact can reduce the severity of the disease though not prevent it. This is used where there is no previous history of chickenpox (or the patient has no antibodies to the varicella-zoster virus on blood testing) in pregnancy, in the first 28 days after delivery, and in immune deficient or immune-suppressed patients.
Varicella-zoster immune globulin
For people exposed to varicella or herpes zoster who cannot receive varicella vaccine, varicella-zoster immune globulin can prevent varicella from developing or lessen the severity of the disease 39. Varicella-zoster immune globulin is recommended for people who cannot receive the vaccine and 1) who lack evidence of immunity to varicella, 2) whose exposure is likely to result in infection, and 3) are at high risk for severe varicella 39.
The varicella-zoster immune globulin product licensed for use in the United States is VariZIG. Varicella-zoster immune globulin (VariZIG) should be given as soon as possible after exposure to varicella-zoster virus (VZV); it can be given within 10 days of exposure. VariZIG is commercially available from a broad network of specialty distributors in the United States (list available at https://varizig.com).
Timing of varicella-zoster immune globulin (VariZIG) administration. CDC recommends administration of VariZIG as soon as possible after exposure to varicella-zoster virus and within 10 days 40.
Patient groups for whom varicella-zoster immune globulin (VariZIG) is recommended. Patients without evidence of immunity to varicella who are at high risk for severe varicella and complications, who have been exposed to varicella or herpes zoster, and for whom varicella vaccine is contraindicated, should receive varicella-zoster immune globulin (VariZIG). Patient groups recommended by CDC to receive VariZIG include the following 40, 41:
- Immunocompromised patients without evidence of immunity.
- Newborn infants whose mothers have signs and symptoms of varicella around the time of delivery (i.e., 5 days before to 2 days after).
- Hospitalized premature infants born at ≥28 weeks of gestation whose mothers do not have evidence of immunity to varicella.
- Hospitalized premature infants born at <28 weeks of gestation or who weigh ≤1,000 g at birth, regardless of their mothers’ evidence of immunity to varicella.
- Pregnant women without evidence of immunity.
Varicella-zoster immune globulin administration
Varicella-zoster immune globulin (VariZIG) is supplied in 125-IU vials and should be administered intramuscularly as directed by the manufacturer. The recommended dose is 125 IU/10 kg of body weight, up to a maximum of 625 IU (five vials). The minimum dose is 62.5 IU (0.5 vial) for patients weighing ≤2.0 kg and 125 IU (one vial) for patients weighing 2.1–10.0 kg 42.
Unchanged from previous recommendations 41, for patients who become eligible for vaccination, varicella vaccine should be administered ≥5 months after VariZIG administration. Because varicella zoster immune globulin might prolong the incubation period by ≥1 week, any patient who receives VariZIG should be observed closely for signs and symptoms of varicella for 28 days after exposure. Antiviral therapy should be instituted immediately if signs or symptoms of varicella occur. Most common adverse reactions following VariZIG administration were pain at injection site (2%) and headache (2%) 42.
Contraindications for varicella-zoster immune globulin (VariZIG) administration include a history of anaphylactic or severe systemic reactions to human immune globulins and IgA-deficient patients with antibodies against IgA and a history of hypersensitivity 42.
The American Academy of Pediatrics (AAP) recommends that certain groups at increased risk for moderate to severe varicella be considered for oral acyclovir or valacyclovir treatment. These high risk groups include:
- Healthy people older than 12 years of age
- People with chronic skin disorders such as eczema or pulmonary disorders
- People receiving long-term salicylate therapy
- People receiving short, intermittent, or aerosolized courses of corticosteroids
Some healthcare providers may elect to use oral acyclovir or valacyclovir for secondary cases within a household. For maximum benefit, oral acyclovir or valacyclovir therapy should be given within the first 24 hours after the varicella rash starts.
Oral acyclovir or valacyclovir therapy is not recommended by American Academy of Pediatrics for use in otherwise healthy children experiencing typical varicella without complications. Acyclovir is a category B drug based on the U.S. Food and Drug Administration’s (FDA) Drug Risk Classification in pregnancy. Some experts recommend oral acyclovir or valacyclovir for pregnant women with varicella, especially during the second and third trimesters. Intravenous acyclovir is recommended for the pregnant patient with serious, viral-mediated complications of varicella, such as pneumonia.
Intravenous acyclovir therapy is recommended for severe disease (e.g., disseminated varicella-zoster virus (VZV) such as pneumonia, encephalitis, thrombocytopenia, severe hepatitis) and for varicella in immunocompromised patients (including patients being treated with high-dose corticosteroid therapy for >14 days).
Famciclovir is available for treatment of varicella-zoster virus (VZV) infections in adults, but its efficacy and safety have not been established for children. In cases of infections caused by acyclovir-resistant varicella-zoster virus (VZV) strains, which usually occur in immunocompromised people, Foscarnet should be used to treat the varicella-zoster virus (VZV) infection, but consultation with an infectious disease specialist is recommended.
Chickenpox treatment at home
To help ease the symptoms of an uncomplicated case of chickenpox, follow these self-care measures.
Avoid scratching
Scratching can cause scarring, slow healing and increase the risk that the sores will become infected. If your child can’t stop scratching:
- Put gloves on his or her hands, especially at night
- Trim his or her fingernails
Relieve the itch and other symptoms
The chickenpox rash can be very itchy, and broken vesicles sometimes sting. These discomforts, along with fever, headache and fatigue, can make anyone miserable. For relief, try:
- A cool bath with added baking soda, aluminum acetate (Domeboro, others), uncooked oatmeal or colloidal oatmeal — a finely ground oatmeal that is made for soaking.
- Calamine lotion dabbed on the spots.
- A soft, bland diet if chickenpox sores develop in the mouth.
- Antihistamines such as diphenhydramine (Benadryl, others) for itching. Check with your doctor to make sure your child can safely take antihistamines.
- Acetaminophen (Tylenol, others) for a mild fever.
If fever lasts longer than four days and is higher than 102, call your doctor. And don’t give aspirin to children and teenagers who have chickenpox because it can lead to a serious condition called Reye’s syndrome.
Chickenpox prognosis
Healthy children nearly always recover from chickenpox without problems. Before routine immunization, about 4 million people developed chickenpox annually in the United States, and about 100 to 150 of them died each year because of complications of chickenpox 43.
In adults, chickenpox is more severe, and the risk of dying is higher.
Chickenpox is particularly severe in people with a weakened immune system.
When people who have been vaccinated develop chickenpox, the disease is less severe, and fewer of these people die.
References- Chickenpox/Varicella Vaccination. https://www.cdc.gov/vaccines/vpd/varicella/index.html
- Bernal JL, Hobbelen P, Amirthalingam G. Burden of varicella complications in secondary care, England, 2004 to 2017. Euro Surveill. 2019 Oct;24(42):1900233. doi: 10.2807/1560-7917.ES.2019.24.42.1900233
- Yokota K, Tamiya A, Sahara T, Nakamura-Uchiyama F. Adult Chickenpox. Intern Med. 2020 Aug 1;59(15):1923. doi: 10.2169/internalmedicine.4478-20
- Cobelli Kett J. Perinatal varicella. Pediatr Rev. 2013 Jan;34(1):49-51. doi: 10.1542/pir.34-1-49
- Smith CK, Arvin AM. Varicella in the fetus and newborn. Semin Fetal Neonatal Med. 2009 Aug;14(4):209-17. doi: 10.1016/j.siny.2008.11.008
- Mother To Baby | Fact Sheets [Internet]. Brentwood (TN): Organization of Teratology Information Specialists (OTIS); 1994-. Varicella Infection (Chickenpox) 2021 Apr. Available from: https://www.ncbi.nlm.nih.gov/books/NBK583011
- Miller E, Cradock-Watson JE, Ridehalgh MK. Outcome in newborn babies given anti-varicella-zoster immunoglobulin after perinatal maternal infection with varicella-zoster virus. Lancet. 1989 Aug 12;2(8659):371-3. doi: 10.1016/s0140-6736(89)90547-3
- Bhavsar SM, Mangat C. Congenital Varicella Syndrome. [Updated 2022 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK568794
- Blumental S, Lepage P. Management of varicella in neonates and infants. BMJ Paediatr Open. 2019 May 30;3(1):e000433. doi: 10.1136/bmjpo-2019-000433
- ROSS AH. Modification of chicken pox in family contacts by administration of gamma globulin. N Engl J Med. 1962 Aug 23;267:369-76. doi: 10.1056/NEJM196208232670801
- Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48(Suppl 1):S2–7. doi: 10.1016/S1386-6532(10)70002-0
- Laemmle L, Goldstein RS, Kinchington PR. Modeling varicella zoster virus persistence and reactivation – closer to resolving a perplexing persistent state. Front Microbiol. 2019;10:1634. doi: 10.3389/fmicb.2019.01634
- Jeon YH. Herpes zoster and postherpetic neuralgia: practical consideration for prevention and treatment. Korean J Pain. 2015;28:177–84. doi: 10.3344/kjp.2015.28.3.177
- Koshy E, Mengting L, Kumar H, Jianbo W. Epidemiology, treatment and prevention of herpes zoster: a comprehensive review. Indian J Dermatol Venereol Leprol. 2018;84:251–62. doi: 10.4103/ijdvl.ijdvl_1021_16
- Sauerbrei A. Diagnosis, antiviral therapy, and prophylaxis of varicella-zoster virus infections. Eur J Clin Microbiol Infect Dis. 2016;35:723–34. doi: 10.1007/s10096-016-2605-0
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016. doi: 10.1038/nrdp.2015.16
- McDonald JR, Zeringue AL, Caplan L, Ranganathan P, Xian H, Burroughs TE, et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis. 2009;48:1364–71. doi: 10.1086/598331
- Dayan RR, Peleg R. Herpes zoster – typical and atypical presentations. Postgrad Med. 2017 Aug;129(6):567-571. doi: 10.1080/00325481.2017.1335574
- Nagel MA, Gilden DH. The protean neurologic manifestations of varicella-zoster virus infection. Cleve Clin J Med. 2007 Jul;74(7):489-94, 496, 498-9 passim. doi: 10.3949/ccjm.74.7.489
- Ibraheem M, Marin M, Leung J, Bryce CH, Schmid DS, Zaki SR, et al. Fatal wild-type varicella-zoster virus encephalitis without a rash in a vaccinated child. Pediatr Infect Dis J. 2013;32:183–5. doi: 10.1097/INF.0b013e318273e43d
- Pollak L, Mehta SK, Pierson DL, Sacagiu T, Avneri Kalmanovich S, Cohrs RJ. Varicella-zoster DNA in saliva of patients with meningoencephalitis: a preliminary study. Acta Neurol Scand. 2015;131:417–21. doi: 10.1111/ane.12335
- Yamada S, Atsuta N, Tokunaga S, Motegi Y. Ipsilateral truncal sensory deficit in a patient with ophthalmic zoster sine herpete. Neurology. 2003;60:1049–50. doi: 10.1212/01.WNL.0000052999.43687.7B
- Mantero V, De Toni Franceschini L, Lillia N, Guccione A, Santilli I, Agostoni E. Varicella-zoster meningoencephaloradiculoneuropathy in an immunocompetent young woman. J Clin Virol. 2013;57:361–2. doi: 10.1016/j.jcv.2013.04.006
- Chen WH, Chui C, Yin HL. Zoster sine herpete, vertebral artery stenosis, and ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:e234–7. doi: 10.1016/j.jstrokecerebrovasdis.2012.07.017
- Nagel MA, Gilden D. Neurological complications of varicella zoster virus reactivation. Curr Opin Neurol. 2014;27:356–60. doi: 10.1097/WCO.0000000000000092
- Persson A, Bergström T, Lindh M, Namvar L, Studahl M. Varicella-zoster virus CNS disease–viral load, clinical manifestations and sequels. J Clin Virol. 2009;46:249–53. doi: 10.1016/j.jcv.2009.07.014
- Dueland AN, Devlin M, Martin JR, Mahalingam R, Cohrs R, Manz H, et al. Fatal varicella-zoster virus meningoradiculitis without skin involvement. Ann Neurol. 1991;29:569–72. doi: 10.1002/ana.410290520
- Kleinschmidt-DeMasters BK, Gilden DH. Varicella-Zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med. 2001 Jun;125(6):770-80. doi: 10.5858/2001-125-0770-VZVIOT
- Hevner R, Vilela M, Rostomily R, Cohrs R, Mahalingam R, Wellish M, et al. An unusual cause of trigeminal-distribution pain and tumour. Lancet Neurol. 2003;2:567–71. doi: 10.1016/S1474-4422(03)00506-4
- Birlea M, Nagel MA, Khmeleva N, Choe A, Kleinschmidt-Demasters B, Hevner R, et al. Varicella-zoster virus trigeminal ganglioneuritis without rash. Neurology. 2014;82:90–2. doi: 10.1212/01.wnl.0000438228.48470.86
- Zerboni L, Arvin A. Neuronal subtype and satellite cell tropism are determinants of varicella-zoster virus virulence in human dorsal root ganglia xenografts in vivo. PLoS Pathog. 2015;11:e1004989. doi: 10.1371/journal.ppat.1004989
- Chickenpox Vaccination: What Everyone Should Know. https://www.cdc.gov/vaccines/vpd/varicella/public/index.html
- About the Varicella Vaccines. https://www.cdc.gov/vaccines/vpd/varicella/hcp/about-vaccine.html
- VARIVAX (refrigerated and frozen formulations). https://www.fda.gov/vaccines-blood-biologics/vaccines/varivax-refrigerated-and-frozen-formulations
- PROQUAD. https://www.fda.gov/vaccines-blood-biologics/vaccines/proquad
- Vázquez M, LaRussa PS, Gershon AA, Niccolai LM, Muehlenbein CE, Steinberg SP, Shapiro ED. Effectiveness over time of varicella vaccine. JAMA. 2004 Feb 18;291(7):851-5. doi: 10.1001/jama.291.7.851
- Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, Reisinger K, Kim LL, Lupinacci L, Hartzel J, Chan I; Study Group for Varivax. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004 Feb;23(2):132-7. doi: 10.1097/01.inf.0000109287.97518.67
- Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global Varicella Vaccine Effectiveness: A Meta-analysis. Pediatrics. 2016 Mar;137(3):e20153741. doi: 10.1542/peds.2015-3741
- Chickenpox (Varicella). https://www.cdc.gov/chickenpox/hcp/index.html#managing-high-risk
- Updated Recommendations for Use of VariZIG — United States, 2013. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6228a4.htm
- FDA Approval of an Extended Period for Administering VariZIG for Postexposure Prophylaxis of Varicella. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6112a4.htm?s_cid=mm6112a4_w
- Cangene Corporation. VariZIG: varicella zoster immune globulin (human). [package insert]. Winnipeg, Canada: Cangene Corporation; 2013.
- Bozzola E, Guolo S, Macchiarulo G, Festa L, Spina G, Krzysztofiak A, Grandin A, Bozzola M, Raponi M, Villani A. Hospitalization for acute cerebellitis in children affected by varicella: how much does it cost? Ital J Pediatr. 2020 Aug 6;46(1):114. doi: 10.1186/s13052-020-00875-8