Skin infection
Your skin is your body’s largest organ. It has many different functions, including covering and protecting your body. It helps keep germs out. But sometimes the germs can cause a skin infection. This often happens when there is a break, cut, or wound on your skin. It can also happen when your immune system is weakened, because of another disease or a medical treatment.
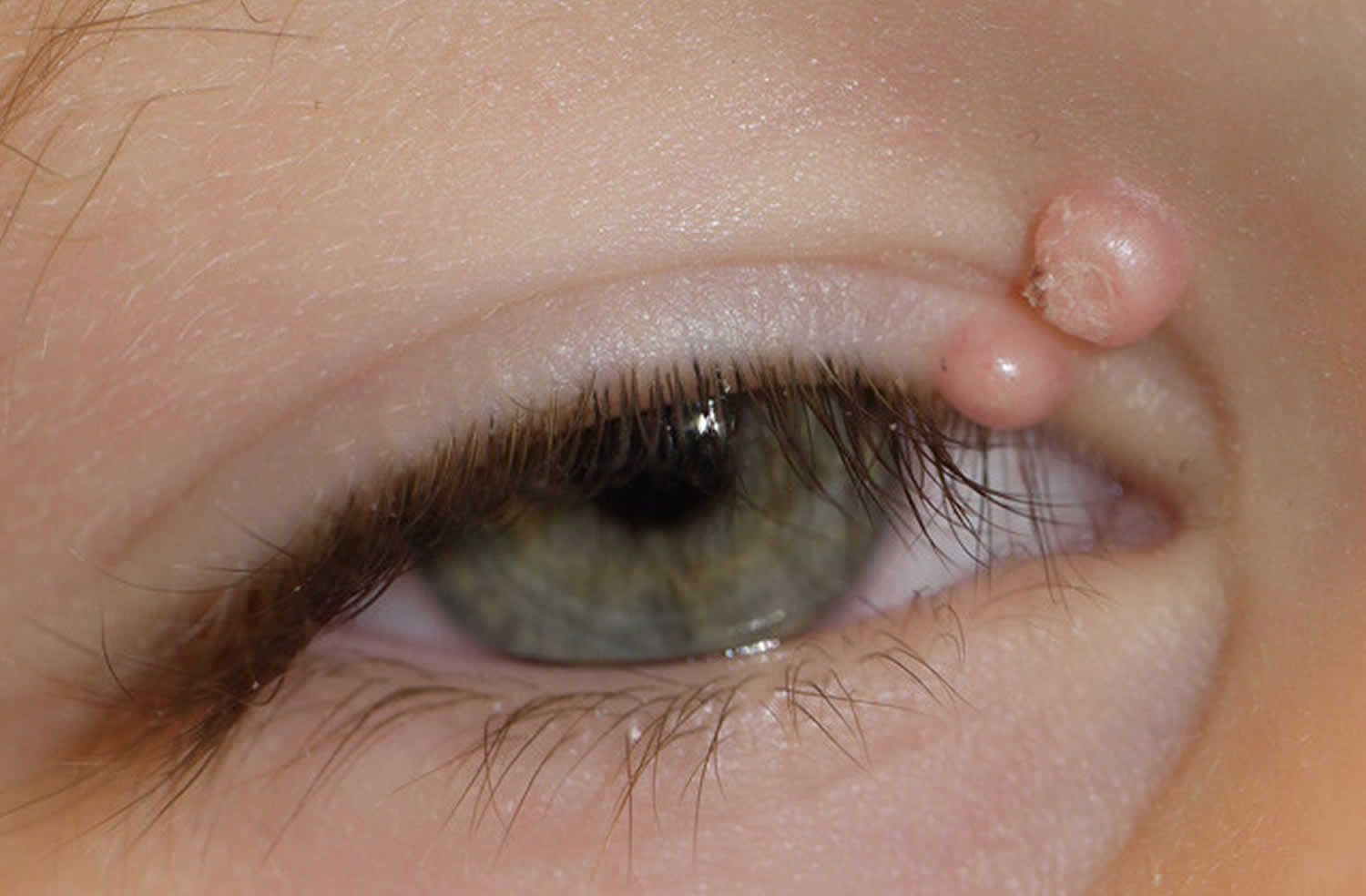
Skin infections are caused by different kinds of germs ranging from the commonest bacterial Staph infection caused by the bacteria Staphylococcus aureus (S. aureus) causing cellulitis, impetigo (Streptococcus pyogenes) to fungi and yeast infections (Pityriasis versicolor [Tinea versicolor], ringworm fungus called Tinea, Athlete’s foot, Jock itch), parasites (body lice, head lice, pubic lice and scabies) and viruses (Molluscum contagiosum, shingles, warts, and herpes simplex)
Some skin infections cover a small area on the top of your skin. Other infections can go deep into your skin or spread to a larger area.
You are at a higher risk for a skin infection if you:
- Have poor circulation
- Have diabetes
- Are older
- Have an immune system disease, such as HIV and AIDS
- Have a weakened immune system because of chemotherapy or other medicines that suppress your immune system
- Have to stay in one position for a long time, such as if you are sick and have to stay in bed for a long time or you are paralyzed
- Are malnourished
- Have excessive skinfolds, which can happen if you have obesity
- Injury to the skin
- Have skin conditions, such as dermatitis or eczema
- Have chronic swelling of the legs or arms (lymphedema)
Skin infection symptoms depend on the type of infection. Some symptoms that are common to many skin infections include rashes, swelling, redness, pain, pus, and itching.
To diagnose a skin infection, your doctor will perform a physical exam and ask about your symptoms. You may have lab tests, such as a skin or wound swabs and culture. This is a test to identify what type of infection you have, using a sample from your skin. Your doctor may take the sample by swabbing or scraping your skin, or removing a small piece of skin (biopsy). Sometimes doctors use other tests, such as blood tests.
The treatment of a skin infection depends on the type of infection, the severity and location of the infection and your overall health and immune status and how serious it is. Some skin infections will go away on their own. When you do need treatment, it may include a cream or lotion to put on the skin. Other possible treatments include medicines(antibiotics, anti-fungals or anti-virals) and a procedure to drain pus.
Bacterial skin infection
Some bacteria live on normal skin and cause no harm, but some bacteria (listed below) invade normal skin, broken skin from eczema or dermatitis or wounds causing infection. Staphylococcus aureus or Staphylococci (‘staph’) are a common type of bacteria that live on your skin and mucous membranes (for example, in the nostrils) of humans. Staphylococcus aureus (S. aureus) is the most important of these bacteria in human diseases. Other staphylococci, including Staphylococcus epidermidis, are considered commensals or normal inhabitants of the skin surface.
About 15–40 per cent of healthy humans are carriers of Staphylococcus aureus (S. aureus), that is, they have the bacteria on their skin without any active infection or disease (colonization). The carrier sites are usually the nostrils and skin folds, where the bacteria may be found intermittently or every time they are looked for.
Bacterial skin infections develop when bacteria enter through the hair follicles or through small breaks in your skin that result from scrapes, punctures, surgery, burns, sunburn, animal or insect bites, wounds, and preexisting skin disorders. People can develop bacterial skin infections after participating in a variety of activities, for example, gardening in contaminated soil or swimming in a contaminated pond, lake, or ocean.
Some bacterial skin infections involve just the skin, and others also involve the soft tissues under the skin. Cellulitis is a common type bacterial of skin infection that involves the deeper layers of the skin that causes redness, swelling, and pain in the infected area of the skin. If untreated, cellulitis can spread and cause serious health problems. Different types of bacteria can cause cellulitis. The most common bacteria causing cellulitis are Streptococcus pyogenes (two-thirds of cases) and Staphylococcus aureus (one third). Good wound care and hygiene are important for preventing cellulitis. Another type of common bacterial skin infection is skin abscess, which is a collection of pus under the skin.
Relatively minor skin infections include:
- Carbuncles
- Ecthyma
- Erythrasma
- Folliculitis
- Furuncles
- Impetigo
- Lymphadenitis
- Small skin abscesses (pus-filled pockets in the skin)
More serious bacterial skin and skin structure infections include:
- Cellulitis
- Erysipelas
- Large skin abscesses
- Lymphangitis
- Necrotizing skin infections
- Wound infections
Bacterial skin infections can be classified as simple (uncomplicated) or complicated (necrotizing or nonnecrotizing), or as suppurative or nonsuppurative. Most community-acquired infections are caused by methicillin-resistant Staphylococcus aureus (MRSA) and beta-hemolytic streptococcus. Every year, community-acquired methicillin-resistant Staphylococcus aureus (MRSA) accounts for 59% of skin and soft tissue infections presenting to the emergency department 1. Simple skin infections are usually monomicrobial (one bacteria) and present with localized clinical findings. In contrast, complicated skin infections can be mono- or polymicrobial and may present with systemic inflammatory response syndrome (SIRS). Staphylococcal scalded skin syndrome, Scarlet fever, and toxic shock syndrome are skin-related consequences of bacterial infections.
Bacteria that cause skin infection
Many types of bacteria can infect the skin. The most common are Staphylococcus and Streptococcus. Methicillin-resistant Staphylococcus aureus (also known as MRSA) is a common bacteria causing skin infections in the United States. MRSA is resistant to many commonly used antibiotics because it has undergone genetic changes that allow it to survive despite exposure to some antibiotics. Because MRSA is resistant to several antibiotics that used to kill it, doctors tailor their treatment based on how often MRSA is found in the local area and whether or not it has been found to be resistant to commonly used antibiotics.
Staphylococcus aureus
- Folliculitis
- Furunculosis (boils) and abscesses
- Impetigo (school sores) and ecthyma
- Methicillin-resistant Staphylococcus aureus (also known as MRSA)
- Staphylococcal scalded skin syndrome
- Toxic shock syndrome
- Tropical pyomyositis
- Botryomycosis (pyoderma vegetans)
Streptococcus pyogenes
- Cellulitis
- Erysipelas
- Impetigo
- Necrotising fasciitis
- Infectious gangrene
- Scarlet fever
- Rheumatic fever, erythema marginatum
Corynebacterium species
Less common bacteria
Less common bacteria that may also cause infection include:
- Neisseria species, the cause of gonorrhea and meningococcal disease
- Erysipelothrix insidiosa, the cause of erysipeloid (usually an animal infection)
- Haemophilus species, the cause of chancroid and cellulitis in young children
- Helicobacter pylori, a stomach infection, which may be associated with some cases of chronic urticaria and rosacea
- Klebsiella rhinoscleromatis, the cause of rhinoscleroma
- Mycoplasma pneumoniae, a cause of pneumonia, causes non-specific erythema, bullous eruptions, urticarial rashes, erythema multiforme, mucositis and rarely, Stevens–Johnson syndrome and toxic epidermal necrolysis (TEN)
- Pseudomonas aeruginosa causes wound infections, athlete’s foot, gram-negative folliculitis, chronic paronychia (green nail syndrome), spa pool folliculitis and ecthyma gangrenosum
- Calymmatobacterium granulomatis, the cause of granuloma inguinale
- Bacillus anthracis, the cause of anthrax
- Clostridium perfringens and other species cause gas gangrene
- Listeria monocytogenes rarely causes cutaneous listeriosis.
- Treponema species cause syphilis, yaws and pinta
- Bartonella species cause cat scratch fever, bacillary angiomatosis, Carrion disease, and bartonellosis
- Mycobacterium species cause tuberculosis, leprosy and atypical mycobacterial infections including Buruli ulcer
- Leptospira, the cause of leptospirosis, which may cause bleeding into the skin (purpura)
- Nocardia, the cause of nocardiosis
- Yersinia pestis, the cause of bubonic plague, which causes swollen lymph glands and pustules, ulcers and scabs on the skin
- Serratia marcescens, a facultative anaerobic gram-negative bacillus that may rarely cause skin infections such as cellulitis, abscesses and ulcers; usually in patients with immunodeficiency.
- Fusobacterium species, Bacillus fusiformis, Treponema vincenti and other bacteria may result in tropical ulcer
- Burkholderia species, the cause of melioidosis and glanders, in which abscesses may be associated with systemic symptoms.
- Actinomyces species, the cause of actinomycosis, in which granular bacteriosis occurs, with abscesses and sinus tracts draining sulphur-yellow granules.
- Vibrio vulnificus, a cause of septic shock characterized by blood-filled blisters.
- Brucella species, the cause of brucellosis, a febrile illness caught from unvaccinated animals or their unpasteurized milk.
- Salmonella species, particularly S. typhi (typhoid fever)
- Aeromonas species, found in water, rarely cause skin and soft tissue infections
Tick-borne bacterial infections
Tick-borne bacterial infections include:
- Lyme disease, due to Borrelia burgdorferi
- Relapsing fever, due to Babesia microti
- Tularemia, due to Francisella tularensis
- Rickettsial diseases (some of these also transmitted by body louse, fleas, mosquitoes and mites).
- Ehrlichiosis and anaplasmosis.
Conditions sometimes caused by or associated with bacterial infection
Other conditions sometimes caused by or associated with bacterial infection include:
- Kawasaki disease (mucocutaneous lymph node syndrome)
- Folliculitis barbae (shaving bumps)
- Sarcoidosis
- Scalp folliculitis
- Osler nodes and Janeway lesions (bacterial endocarditis)
- Vaginitis and bacterial vaginosis.
Risk factor for bacterial skin infection
Some people are at particular risk of developing bacterial skin infections:
- People with diabetes, who are likely to have poor blood flow (especially to the hands and feet), have a high level of sugar (glucose) in their blood, which decreases their ability to fight infections
- People who are hospitalized or living in a nursing home
- People who are older
- People who have human immunodeficiency virus (HIV), AIDS or other immune disorders, or hepatitis
- People who are undergoing chemotherapy or treatment with other drugs that suppress the immune system
Skin that is inflamed (dermatitis) or damaged is more likely to become infected. In fact, any break in the skin predisposes a person to infection.
Bacterial skin infection prevention
Preventing bacterial skin infections involves keeping the skin undamaged and clean. When the skin is cut or scraped, the injury should be washed with soap and water and covered with a sterile bandage.
Petrolatum may be applied to open areas to keep the tissue moist and to try to prevent bacterial invasion. Doctors recommend that people do not use antibiotic ointments (prescription or nonprescription) on uninfected minor wounds because of the risk of developing an allergy to the antibiotic.
Bacterial skin infection diagnosis
Laboratory tests for bacterial skin infections may include:
- A swab of the inflamed site, such as throat, skin lesions for culture
- Complete blood count. bacterial infection often raises the white cell count with increased neutrophils
- C-reactive protein (CRP). Elevated CRP > 50 in serious bacterial infections
- Procalcitonin. Procalcitonin blood test marker for generalized sepsis due to bacterial infection
- Serology. Serology tests ten days apart to determine immune response to a particular organism
- Polymerase chain reaction (PCR) and ELISA tests for specific organisms
- Blood culture. Blood culture if high fever > 38°C
Bacterial skin infection treatment
The treatment of bacterial skin infection depends on the bacteria that is causing the infection, the severity and location of the infection and your overall health and immune status. When bacterial skin infections do occur, they can range in size from a tiny spot to the entire body surface. Bacterial skin infection can range in seriousness as well, from harmless to life threatening. Minor bacterial infections may resolve without treatment. However, persistent and serious bacterial infections are treated with antibiotics. These are available for localized topical use (creams, gels, solutions), such as antibiotics for acne, or as systemic treatment as tablets, capsules and intramuscular or intravenous injections.
It is best to take samples or swabs to test which organism is responsible for the skin infection before commencing antibiotics. If the infection is serious (e.g, if meningococcal disease is suspected), immediate treatment with a broad-spectrum antibiotic may commence. Once the specific organism causing infection has been determined, the antibiotic may be changed to a narrow-spectrum antibiotic directed against that organism.
Antibiotics have important side effects and societal impact and should not be prescribed or taken if they are not required or if they are unlikely to be of benefit, for example, if the infection is viral in origin. Antibiotic side effects can range from minor issues, like a rash, to very serious health problems, such as antibiotic-resistant infections and Clostridioides difficile infection, which causes diarrhea that can lead to severe colon damage and death. See your doctor if you develop any side effects while taking your antibiotic.
Abscesses should be cut open by a doctor and allowed to drain, and any dead tissue must be surgically removed. Antibiotics are sometimes needed for abscesses after the pus has been drained.
In some cases, severe infections need to be treated in the hospital.
Cellulitis
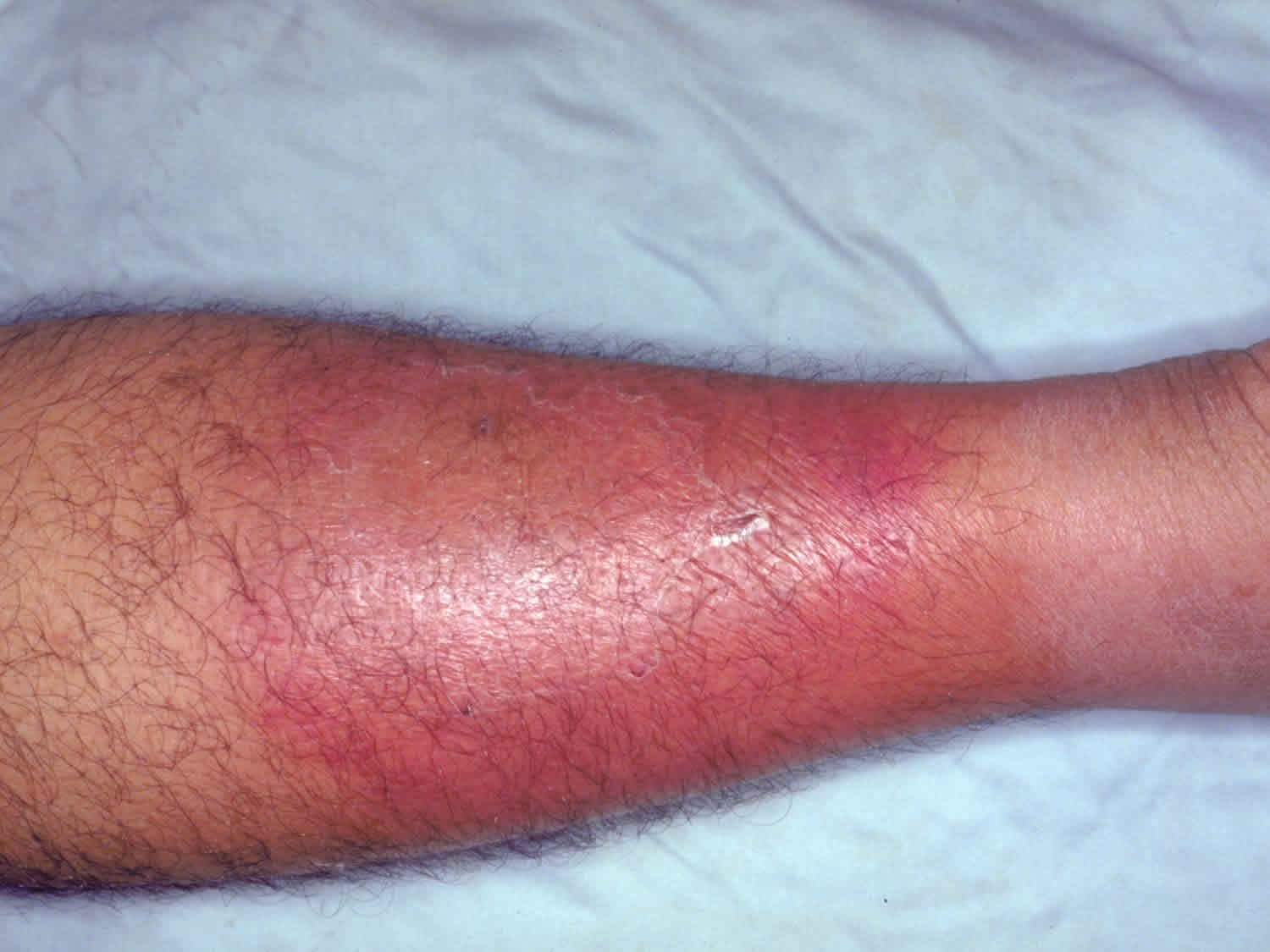
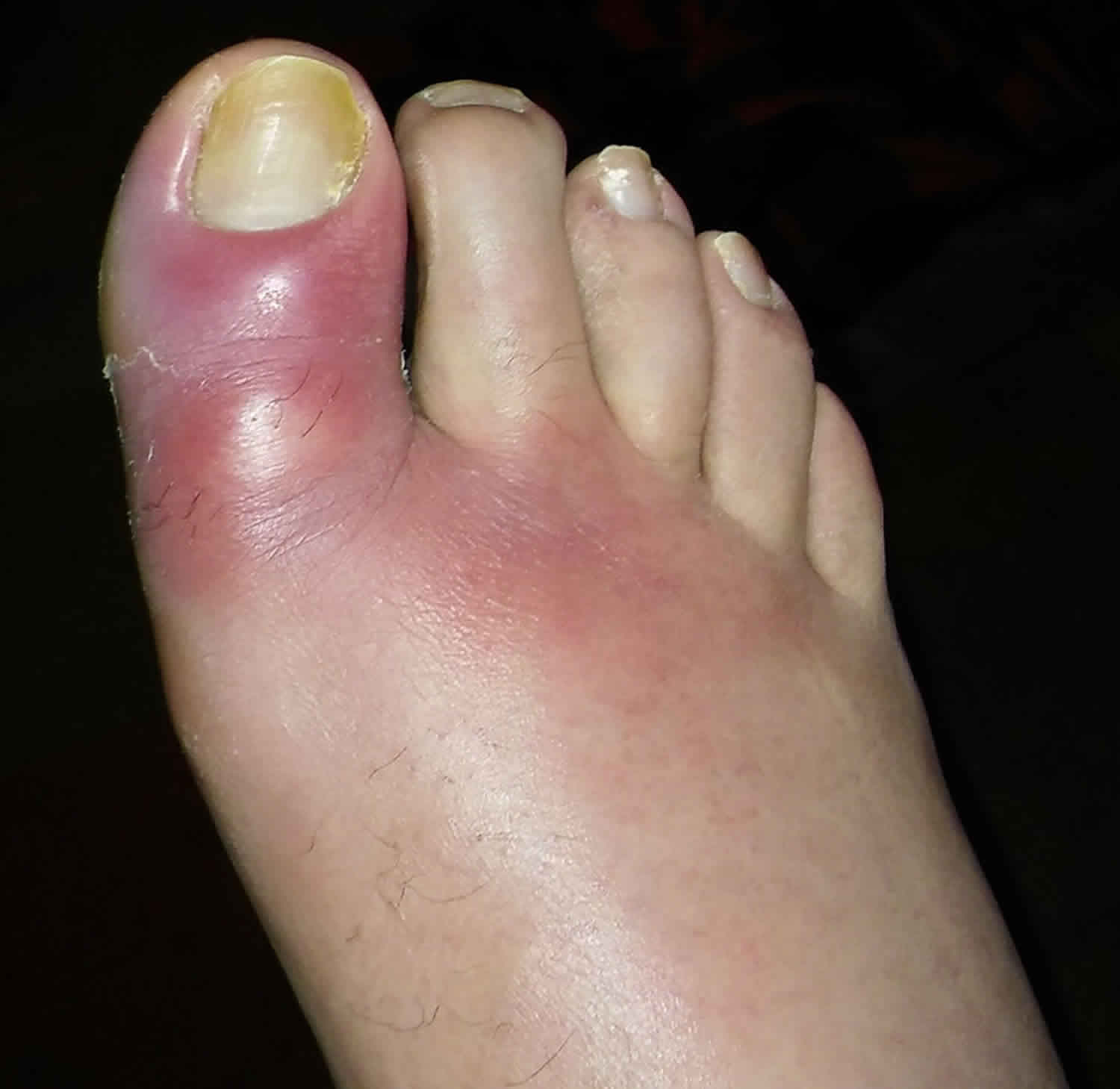
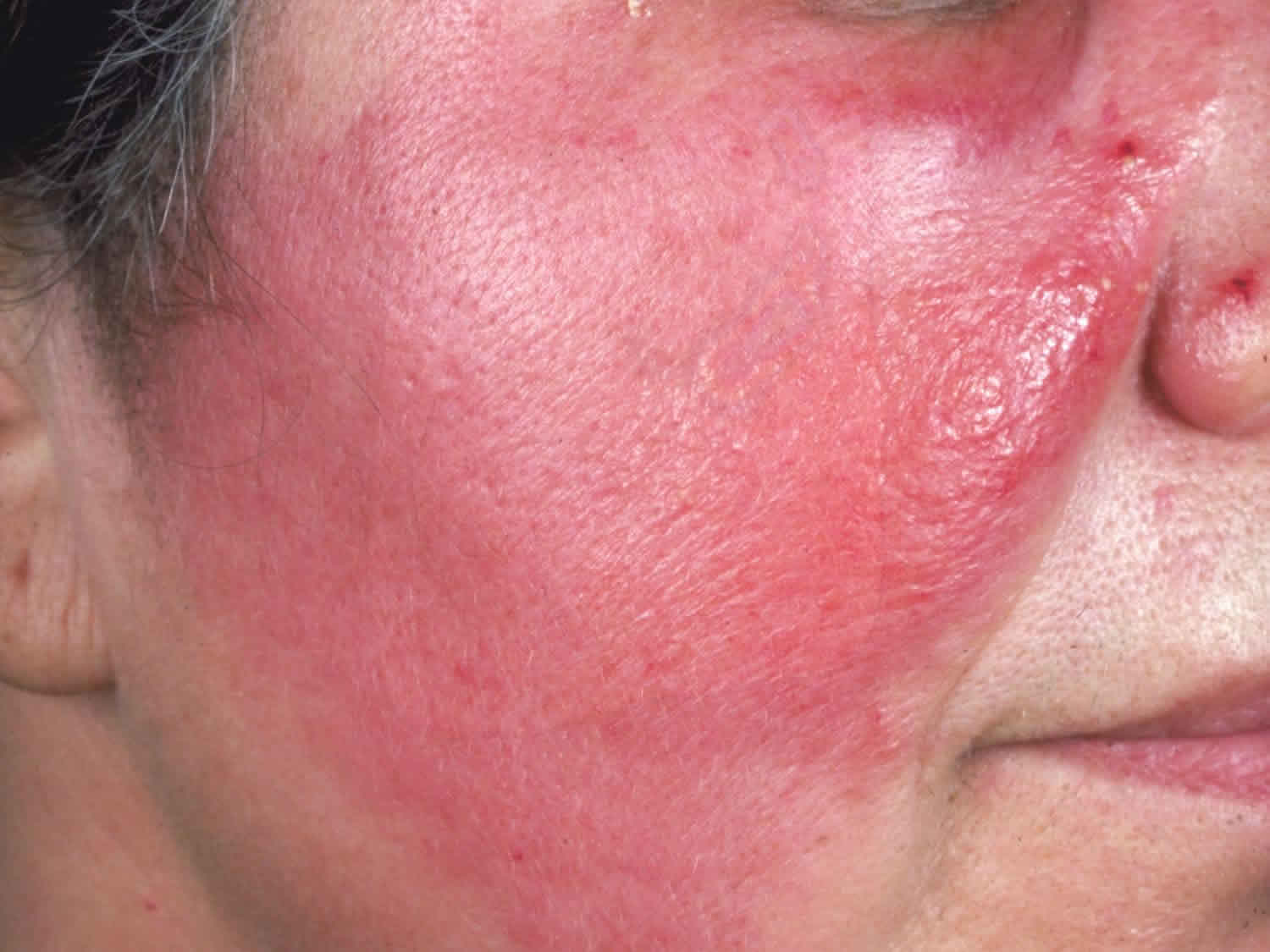
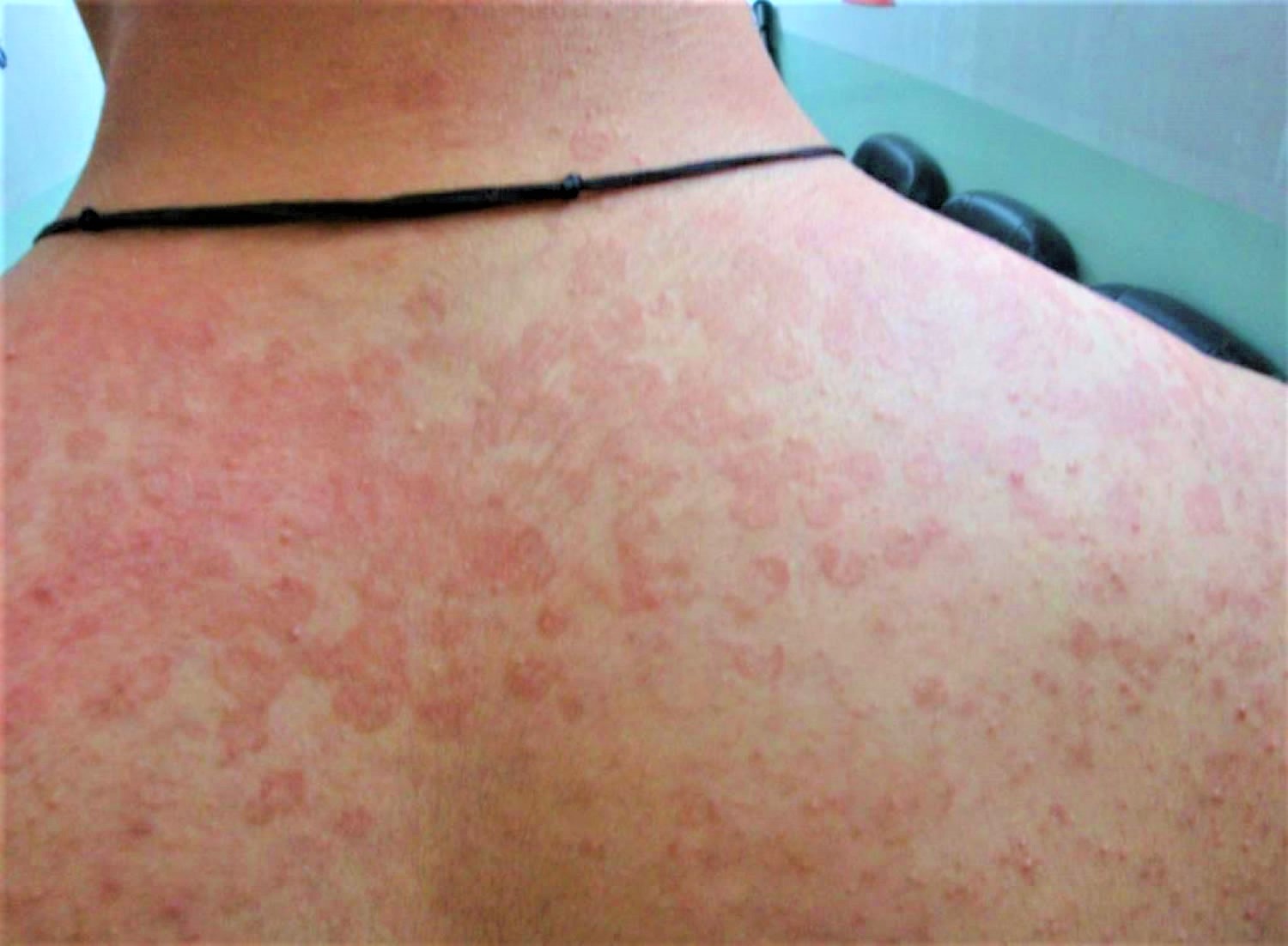
Cellulitis is a common, potentially serious bacterial infection of the skin (the lower dermis) and subcutaneous tissues (just under the skin). The affected skin appears swollen and red (but this may be less obvious on brown or black skin) and is typically painful and warm to the touch 2, 3, 4, 5, 6, 7. The skin may look pitted, like the peel of an orange, or blisters may appear on the affected skin 8. Cellulitis is sometimes accompanied by fever, chills, and general fatigue. Cellulitis can appear anywhere on the body, but it is most common on the feet and legs 8. Cellulitis is usually caused by a bacterial infection and can become serious if not treated with antibiotics. The most common bacteria are Staphylococcus aureus (golden Staph) and group A beta-hemolytic Streptococcus (Streptococcus pyogenes). These bacteria are able to enter the skin through small cracks (fissures) or normal skin, and can spread easily to the tissue under the skin.
Cellulitis can affect almost any part of your body (e.g., face, eye, arms and other areas). Most commonly, cellulitis occurs on the lower legs and in areas where the skin is damaged or inflamed allowing bacteria to enter. Anyone, at any age, can develop cellulitis. However, you are at increased risk if you smoke, have diabetes or poor circulation.
Left untreated, cellulitis can spread to your lymph nodes (lymphadenitis) and can result in pockets of pus (abscesses) or the bacteria can spread into your bloodstream (bacteremia) and rapidly become life-threatening. Prior to the development of antibiotics, cellulitis was fatal. With the introduction of antibiotics, most people recover fully within a week.
Cellulitis isn’t usually spread from person to person.
If you think you or someone in your care has cellulitis, it’s important to get medical attention soon as possible!
The main signs of cellulitis are skin that is red, painful, swollen, tender and warm to touch. People with severe cellulitis can get fever, chills, sweating and nausea, and might feel generally unwell.
Cellulitis often affects the lower leg, but can occur on any part of the body including the face. The infection may occur when bacteria enter the skin through an ulcer, cut or a scratch or an insect bite. However it can occur without any visible damage to the skin.
Sometimes bacteria from cellulitis spreads into the blood stream, which is called sepsis and this is a medical emergency.
People with cellulitis can quickly become very unwell and a small number of people may develop serious complications.
Antibiotics are the main treatment, usually orally at home. Some people need treatment in hospital with intravenous (IV) antibiotics. Rest and elevation (raising) of the limb are also very important. In some cases the affected limb may need compression.
After successful treatment, the skin may flake or peel off as it heals. This can be itchy.
Figure 1. Cellulitis leg
Figure 2. Cellulitis foot
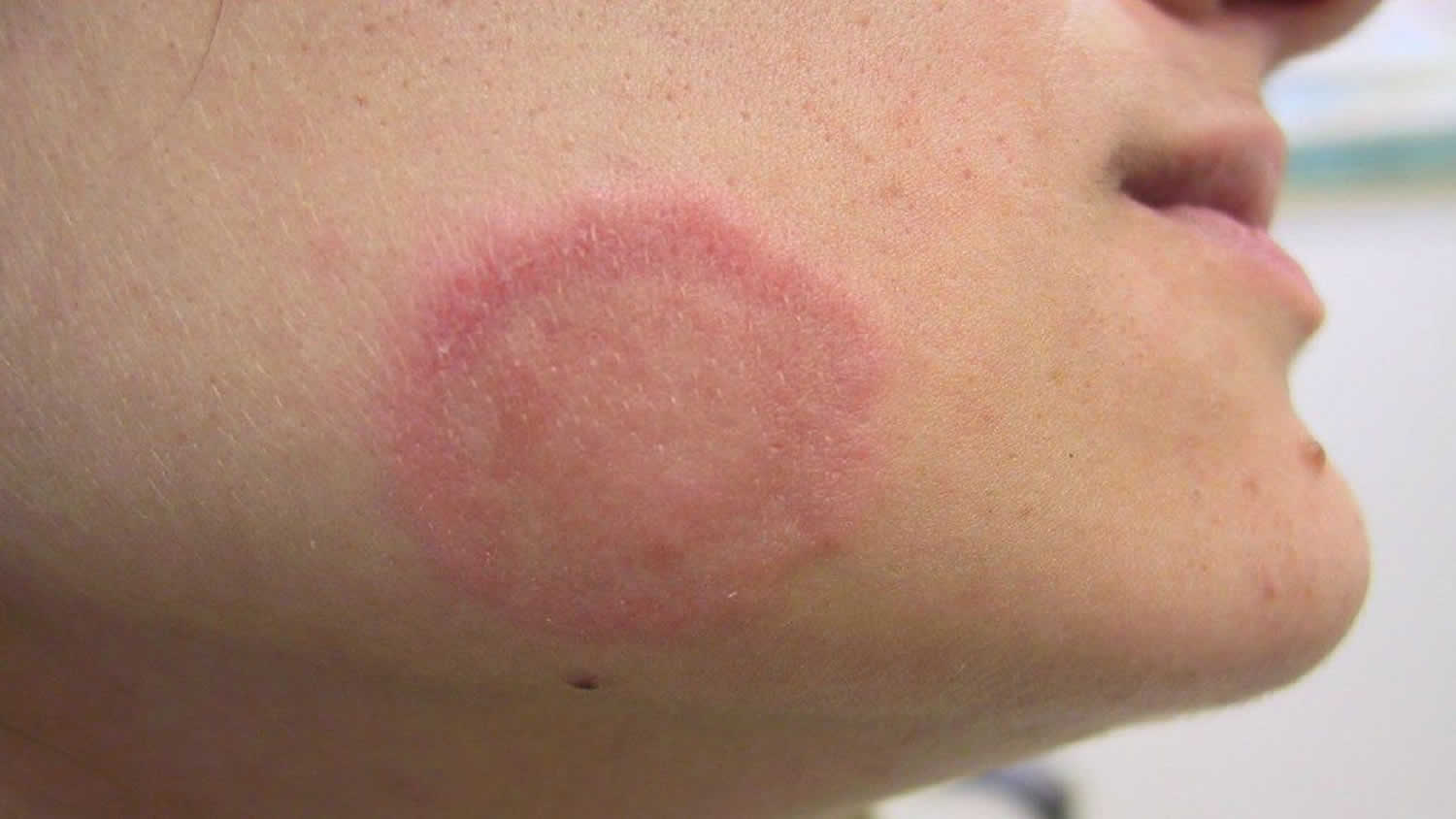
Figure 3. Facial cellulitis
It’s important to identify and treat cellulitis early because the condition can spread rapidly throughout your body.
Seek emergency care if:
- You have a red, swollen, tender rash or a rash that’s changing rapidly
- You have a fever
See your doctor, preferably that day, if:
- You have a rash that’s red, swollen, tender and warm — and it’s expanding — but without fever
Who gets cellulitis?
Cellulitis affects people of all ages and races. Factors that increase the risk of developing cellulitis include:
- Previous episode(s) of cellulitis
- Skin wounds or injuries that cause a break in the skin (like cuts, ulcers, bites, puncture wounds, tattoos, piercings)
- Fissuring of toes or heels, eg due to athlete’s foot, tinea pedis or cracked heels
- Poor circulation in the legs (peripheral vascular disease)
- Venous disease, eg gravitational eczema, leg ulceration, and/or lymphedema
- Having limbs (feet, legs, hands, and arms) that stay swollen (chronic edema), including swelling due to:
- Lymphedema (problems with the lymphatic system so it does not drain the way it should); the lymphatic system is a part of the body’s immune system that helps move fluid that contains infection-fighting cells throughout the body
- Coronary artery bypass grafting (having a healthy vein removed from the leg and connected to the coronary artery to improve blood flow to the heart)
- Bites from insects, animals, or other humans
- Chronic lower leg swelling (edema)
- Current or prior injury, eg trauma, surgical wounds, radiotherapy
- Immunodeficiency, eg human immunodeficiency virus infection (HIV)
- Intravenous drug abuse
- Weakened immune system due to underlying illness or medication
- Diabetes
- Chronic kidney disease
- Chronic liver disease
- Obesity
- Pregnancy
- Alcoholism
- Athlete’s foot (tinea pedis)
- Chickenpox
- Shingles
- Eczema
Many people falsely attribute an episode of cellulitis to an unseen spider bite. Documented spider bites have not led to cellulitis.
Is cellulitis contagious?
Cellulitis isn’t usually spread from person to person. Cellulitis is an infection of the deeper layers of the skin most commonly caused by bacteria that normally live on the skin’s surface. You have an increased risk of developing cellulitis if you:
- Have an injury, such as a cut, fracture, burn or scrape
- Have a skin condition, such as eczema, athlete’s foot or shingles
- Participate in contact sports, such as wrestling
- Have diabetes or a weakened immune system
- Have a chronic swelling of your arms or legs (lymphedema)
- Use intravenous drugs
However, direct person-to-person transmission of group A strep (Streptococcus pyogenes) can occur through contact with skin lesions or exposure to respiratory droplets 7. People with active infection are more likely to transmit group A strep (Streptococcus pyogenes) compared to asymptomatic carriers. Local dermatophyte (fungal) infection (e.g., athlete’s foot) may serve as portal of entry for group A strep (Streptococcus pyogenes) 2.
The spread of all types of group A strep (Streptococcus pyogenes) infection can be reduced by good hand hygiene, especially after coughing and sneezing, and respiratory etiquette (e.g., covering your cough or sneeze). Early identification and management of superficial skin lesions is also key to cellulitis prevention. Patients with recurrent cellulitis on their leg or foot should be inspected for tinea pedis and should be treated if present. Traumatic or bite wounds should be cleaned and managed appropriately (e.g., antibiotic prophylaxis, surgical debridement if indicated) to prevent secondary infections 5.
Reduce the risk of transmission
Cellulitis is spread by skin-to-skin contact or by touching infected surfaces. Stop the spread by:
- Washing your hands often.
- Bathing or showering daily.
- Do not let dressing become wet. If they do get wet they will need to be changed. Do not swim until infection clears up.
- Covering the wound with a gauze dressing (not a Band-Aid)
- Washing your bed linen, towels and clothing separately from other family members while the infection is healing.
Cellulitis may arise when skin injury or inflammation is not adequately treated.
How to clean a wound
You can look after most cuts and wounds yourself. You can:
- Stop any bleeding by holding a clean cloth or bandage on it and apply firm pressure. Use a clean towel to apply light pressure to the area until bleeding stops (this may take a few minutes). Minor cuts and scrapes usually stop bleeding on their own. Be aware that some medicines (e.g. aspirin and warfarin) will affect bleeding, and may need pressure to be applied for a longer period of time. If needed, apply gentle pressure with a clean bandage or cloth and elevate the wound until bleeding stops.
- Wash your hands well. Prior to cleaning or dressing the wound, ensure your hands are washed to prevent contamination and infection of the wound.
- Clean the wound by rinsing it with clean water and picking out any dirt (e.g. gravel) or debris with tweezers (don’t use antiseptic cream), as this will reduce the risk of infection. Keeping the wound under running tap water will reduce the risk of infection. Wash around the wound with soap.
- Dry the wound. Gently pat dry the surrounding skin with a clean pad or towel.
- Replace any skin flaps if possible. If there is a skin flap and it is still attached, gently reposition the skin flap back over the wound as much as possible using a moist cotton bud or pad.
- To help the injured skin heal, use an antibiotic ointment (e.g., Neosporin, Polysporin) or petroleum jelly to keep the wound moist. Petroleum jelly prevents the wound from drying out and forming a scab; wounds with scabs take longer to heal. This will also help prevent a scar from getting too large, deep or itchy. As long as the wound is cleaned daily, it is not necessary to use anti-bacterial ointments. However, several studies have supported the use of prophylactic topical antibiotics for minor wounds. An randomized controlled trial of 426 patients with uncomplicated wounds found significantly lower infection rates with topical bacitracin, neomycin/bacitracin/polymyxin B, or silver sulfadiazine (Silvadene) compared with topical petrolatum (5.5%, 4.5%, 12.1%, and 17.6%, respectively) 9. Certain ingredients in some ointments can cause a mild rash in some people. If a rash appears, stop using the ointment.
- Cover the wound (small wounds can be left uncovered) 10. Use a non-stick or gentle dressing and lightly bandage in place; try to avoid using tape on fragile skin to prevent further trauma on dressing removal. Dressings protect the wound by acting as a barrier to infection and absorbing wound fluid. A moist wound bed stimulates epithelial cells to migrate across the wound bed and resurface the wound 11. A dry environment leads to cell desiccation and causes scab formation, which delays wound healing. Older studies in animals and humans suggest that moist wounds had faster rates of re-epithelialization compared with dry wounds 12.
- Manage pain. Wounds can be painful, so consider pain relief while the wound heals. Talk to your doctor or pharmacist about options for pain relief.
- Change the dressing every day or whenever the bandage becomes wet or dirty.
- Get a tetanus shot. Get a tetanus shot if you haven’t had one in the past five years and the wound is deep or dirty.
- Watch for signs of infection. See a doctor if you see signs of infection on the skin or near the wound, such as redness, increasing pain, drainage, warmth or swelling.
- It’s also important to care for yourself, as this helps wounds heal faster. So eat fresh food, get some exercise, avoid smoking, and avoid drinking too much and drink plenty of water (unless you have liquid intake restrictions) to maintain supple, healthy skin.
See a doctor or nurse for a tetanus immunization within a day if you have had any cut or abrasion and any of the following apply:
- It is more than 10 years since your last tetanus shot or you can’t remember when you last had a tetanus shot 13. The Centers for Disease Control and Prevention (CDC) recommends that tetanus toxoid be administered as soon as possible to patients who have no history of tetanus immunization, who have not completed a primary series of tetanus immunization (at least three tetanus toxoid–containing vaccines), or who have not received a tetanus booster in the past 10 years.
- It is more than five years since your last tetanus shot and there was dirt in in the cut or abrasion, or the cut is deep.
- You should have the tetanus booster shot within 48 hours of the injury.
- Besides a tetanus shot, your doctor may also give you an injection of something called tetanus immune globulin, which acts fast to prevent infection 14. There is a small window of opportunity for the tetanus immune globulin to work, so don’t delay seeking medical care.
- Be aware of the first signs of tetanus infection. Also known as lockjaw, tetanus causes stiffness of the neck, difficulty swallowing, rigidity of abdominal muscles, spasms, sweating and fever. Symptoms usually begin eight days after the infection, but occur anywhere within three days to three weeks.
Cellulitis causes
Cellulitis occurs when bacteria, most commonly Streptococcus pyogenes (two thirds of cases) and Staphylococcus aureus (one third of cases), enter through a crack or break in your skin. The incidence of a more serious staphylococcus infection called methicillin-resistant Staphylococcus aureus (MRSA) is increasing.
For many people who get cellulitis, experts do not know how the bacteria get into the body. Sometimes the bacteria get into the body through openings in the skin, like an injury or surgical wound. In general, people cannot catch cellulitis from someone else; it is not contagious.
Rare causes of cellulitis include 15:
- Pseudomonas aeruginosa, usually in a puncture wound of foot or hand
- Haemophilus influenzae, in children with facial cellulitis
- Anaerobes, Eikenella, Streptococcus viridans, due to human bite
- Pasteurella multocida, due to cat or dog bite
- Vibrio vulnificus, due to salt water exposure, e.g., coral injury
- Aeromonas hydrophila from fresh or salt water exposure, e.g., following leech bites
- Erysipelothrix (erysipeloid), in butchers
Cellulitis usually occurs in skin areas that have been damaged or inflamed for other reasons, including:
- trauma, such as an insect bite, burn, abrasion or cut
- a surgical wound
- skin problems, such as eczema, psoriasis, scabies or acne
- a foreign object in the skin, such as metal or glass.
Often, it is not possible to find a cause for cellulitis.
Although cellulitis can occur anywhere on your body, the most common location is the lower leg. Bacteria are most likely to enter disrupted areas of skin, such as where you’ve had recent surgery, cuts, puncture wounds, an ulcer, athlete’s foot or dermatitis.
Animal bites can cause cellulitis. Bacteria can also enter through areas of dry, flaky skin or swollen skin.
Risk factors for developing cellulitis
Several factors put you at increased risk of cellulitis:
- Injury. Any cut, fracture, burn or scrape gives bacteria an entry point.
- Weakened immune system. Conditions that weaken your immune system — such as diabetes, leukemia and HIV/AIDS — leave you more susceptible to infections. Certain medications also can weaken your immune system.
- Skin conditions. Conditions such as eczema, athlete’s foot and shingles can cause breaks in the skin, which give bacteria an entry point.
- Chronic swelling of your arms or legs (lymphedema). This condition sometimes follows surgery.
- History of cellulitis. Having had cellulitis before makes you prone to develop it again.
- Obesity. Being overweight or obese increases your risk of developing cellulitis.
Cellulitis prevention
If your cellulitis recurs, your doctor may recommend preventive antibiotics. To help prevent cellulitis and other infections, take these precautions when you have a skin wound:
- Wash your wound daily with soap and water. Do this gently as part of your normal bathing.
- Apply a protective cream or ointment. For most surface wounds, an over-the-counter ointment (Vaseline, Polysporin, others) provides adequate protection.
- Cover your wound with a bandage. Change bandages at least daily.
- Watch for signs of infection. Redness, pain and drainage all signal possible infection and the need for medical evaluation.
People with diabetes and those with poor circulation need to take extra precautions to prevent skin injury. Good skin care measures include the following:
- Inspect your feet daily. Regularly check your feet for signs of injury so you can catch infections early.
- Moisturize your skin regularly. Lubricating your skin helps prevent cracking and peeling. Do not apply moisturizer to open sores.
- Trim your fingernails and toenails carefully. Take care not to injure the surrounding skin.
- Protect your hands and feet. Wear appropriate footwear and gloves.
- Promptly treat infections on the skin’s surface (superficial), such as athlete’s foot. Superficial skin infections can easily spread from person to person. DON’T wait to start treatment.
How to prevent recurrent episodes of cellulitis
To help prevent recurrent episodes of cellulitis — a bacterial infection in the deepest layer of skin — keep skin clean and well-moisturized. Prevent cuts and scrapes by wearing appropriate clothing and footwear, using gloves when necessary, and trimming fingernails and toenails with care.
Factors that may increase your risk of cellulitis include:
- Pre-existing skin diseases, such as athlete’s foot
- Puncture injuries, such as insect or animal bites
- Surgical incisions or pressure sores
- Immune system problem, such as diabetes
- Injuries that occur when you’re in a lake, river or ocean
- Hot tub use
Cellulitis usually makes the affected skin hot, red, swollen and painful. Your skin may look pebbled, like an orange peel. Seek prompt medical attention at the first sign of a skin infection. Treatment is usually with antibiotics. Some people who frequently develop cellulitis may benefit from long-term low-dose antibiotic treatment to prevent recurrent infections. Prophylactic antibiotics, such as oral penicillin or erythromycin oral twice daily for 4–52 weeks, or intramuscular benzathine penicillin every 2–4 weeks, should be considered in patients who have 3–4 episodes of cellulitis per year despite attempts to treat or control predisposing factors 5. This antibiotic program should be continued so long as the predisposing factors persist 5.
Cellulitis symptoms
Possible signs and symptoms of cellulitis, which usually occur on one side of the body, include:
- Redness, swelling and tenderness of skin and subcutaneous tissue underneath it that tends to expand (spread)
- Warmth of the affected skin
- Fever and chills
- Swollen glands or lymph nodes
- Pain
- Red spots
- Blisters
- Dimpled skin (peau d’orange)
- Weeping or leaking of yellow clear fluid or pus
- Erosions and ulceration
- Abscess formation
- Purpura: petechiae, ecchymoses, or hemorrhagic bullae
Cellulitis may be associated with lymphangiitis and lymphadenitis, which are due to bacteria within lymph vessels and local lymph glands. A red line tracks from the site of infection to nearby tender, swollen lymph glands.
Left untreated, cellulitis can rapidly turn into a life-threatening condition. Treatment usually includes antibiotics. In severe cases, you may need to be hospitalized and receive antibiotics through your veins (intravenously).
Cellulitis complications
The infection can spread to the rest of the body. The lymph nodes may swell and be noticed as a tender lump in the groin and armpit. You may also have fevers, sweats and vomiting.
Severe or rapidly progressive cellulitis may lead to:
- Necrotizing fasciitis (a more serious soft tissue infection recognized by severe pain, skin pallor, loss of sensation, purpura, ulceration and necrosis). Necrotizing fasciitis is an example of a deep-layer infection. It’s an extreme emergency.
- Gas gangrene
- Severe sepsis (blood poisoning)
- Infection of other organs, e.g., pneumonia, osteomyelitis, meningitis
- Endocarditis (heart valve infection).
Sepsis is recognized by fever, malaise, loss of appetite, nausea, lethargy, headache, aching muscles and joints. Serious infection leads to hypotension (low blood pressure, collapse), reduced capillary circulation, heart failure, diarrhea, gastrointestinal bleeding, renal failure and loss of consciousness.
Recurrent episodes of cellulitis may damage the lymphatic drainage system and cause chronic swelling of the affected limb.
Cellulitis diagnosis
Your doctor will likely be able to diagnose cellulitis by looking at your skin. In some cases, he or she may suggest blood tests or other tests to help rule out other conditions.
Investigations may reveal:
- Leukocytosis (raised white cell count).
- Elevated C-reactive protein (CRP)
- Causative organism, on culture of blood or of pustules, crusts, erosions or wound
Imaging may be performed. For example:
- Chest X-ray in case of heart failure or pneumonia
- Doppler ultrasound to look for blood clots (deep vein thrombosis)
- MRI in case of necrotizing fasciitis.
Cellulitis treatment
Antibiotics are used to treat the infection. Oral antibiotics may be adequate, but in the severely ill person, intravenous antibiotics will be needed to control and prevent further spread of the infection. This treatment is given in hospital or sometimes, at home by a local doctor or nurse. Your doctor also might recommend elevating the affected area, which may speed recovery.
Within three days of starting an antibiotic, let your doctor know whether the infection is responding to treatment. You’ll need to take the antibiotic for as long as your doctor directs, usually five to 10 days but possibly as long as 14 days. After successful treatment, the skin may flake or peel off as it heals. This can be itchy.
As the infection improves, you may be able to change from intravenous to oral antibiotics, which can be taken at home for a further week to 10 days. Most people respond to antibiotics in two to three days and begin to show improvement. In some cases, antibiotics are continued until all signs of infection have cleared (redness, pain and swelling), sometimes for several months.
In most cases, signs and symptoms of cellulitis disappear after a few days. You may need to be hospitalized and receive antibiotics through your veins (intravenously) if:
- Signs and symptoms don’t respond to oral antibiotics
- Signs and symptoms are extensive
- You have a high fever
In rare cases, the cellulitis may progress to a serious illness by spreading to deeper tissues. In addition to broad spectrum antibiotics, surgery is sometimes required.
Treatment should also include:
- Analgesia to reduce pain
- Adequate water/fluid intake
- Management of co-existing skin conditions like venous eczema or tinea pedis
Cellulitis antibiotics
The management of cellulitis is becoming more complicated due to rising rates of methicillin-resistant Staphylococcus aureus (MRSA) and macrolide- or erythromycin-resistant Streptococcus pyogenes.
Usually, doctors prescribe a drug that’s effective against both streptococci and staphylococci. It’s important that you take the medication as directed and finish the entire course of medication, even after you feel better.
Treatment of cellulitis with systemic illness
More severe cellulitis and systemic symptoms should be treated with fluids, intravenous antibiotics and oxygen. The choice of antibiotics depends on local protocols based on prevalent organisms and their resistance patterns, and may be altered according to culture/susceptibility reports.
- Penicillin-based antibiotics are often chosen (eg penicillin G or flucloxacillin)
- Amoxicillin and clavulanic acid provide broad-spectrum cover if unusual bacteria are suspected
- Cephalosporins are also commonly used (eg ceftriaxone, cefotaxime or cefazolin)
- Clindamycin, sulfamethoxazole/trimethoprim, doxycycline and vancomycin are used in patients with penicillin or cephalosporin allergy, or where infection with methicillin-resistant Staphylococcus aureus is suspected
- Broad-spectrum antibiotics may also include linezolid, ceftaroline, or daptomycin
Sometimes oral probenecid is added to maintain antibiotic levels in the blood.
Treatment may be switched to oral antibiotics when fever has settled, cellulitis has regressed, and CRP is reducing.
Cellulitis home treatment
Self-care at home to help ease any pain and swelling include:
- Get plenty of rest. This gives your body a chance to fight the infection.
- Raise the area of the body involved as high as possible. This will ease the pain, help drainage and reduce swelling.
- Take pain-relieving medication such as paracetamol. Check the label for how much to take and how often. The pain eases once the infection starts getting better.
- If you are not admitted to hospital, you will require a follow-up appointment with your doctor within a day or two to make sure the cellulitis is improving. This appointment is important to attend.
Management of recurrent cellulitis
Patients with recurrent cellulitis should:
- Avoid trauma, wear long sleeves and pants in high risk activities, eg gardening
- Keep skin clean and well moisturized, with nails well tended
- Avoid having blood tests taken from the affected limb
- Treat fungal infections of hands and feet early
- Keep swollen limbs elevated during rest periods to aid lymphatic circulation. Those with chronic lymphedema may benefit from compression garments.
Patients with 2 or more episodes of cellulitis may benefit from chronic suppressive antibiotic treatment with low-dose penicillin V or erythromycin, for one to two years.
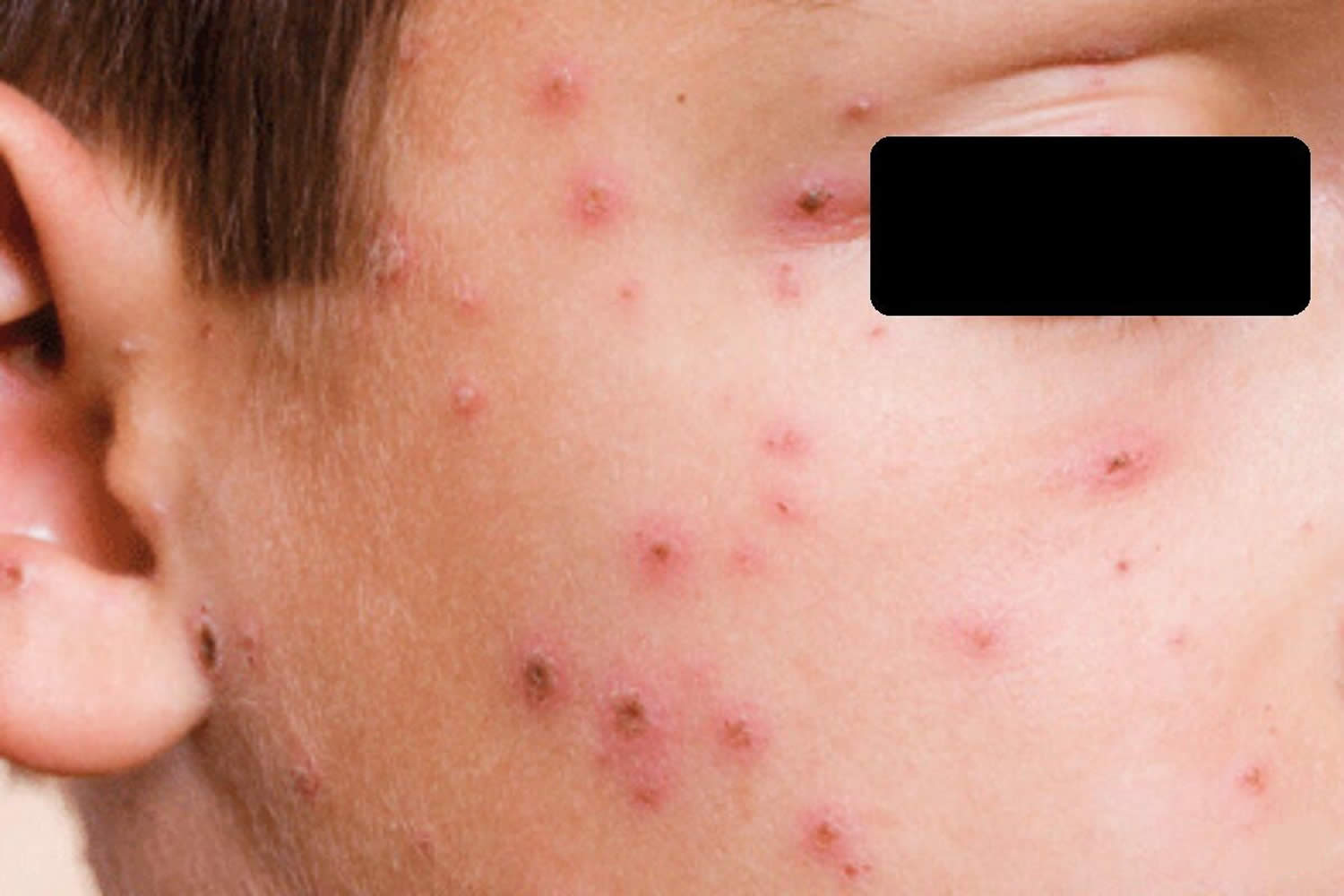
Impetigo
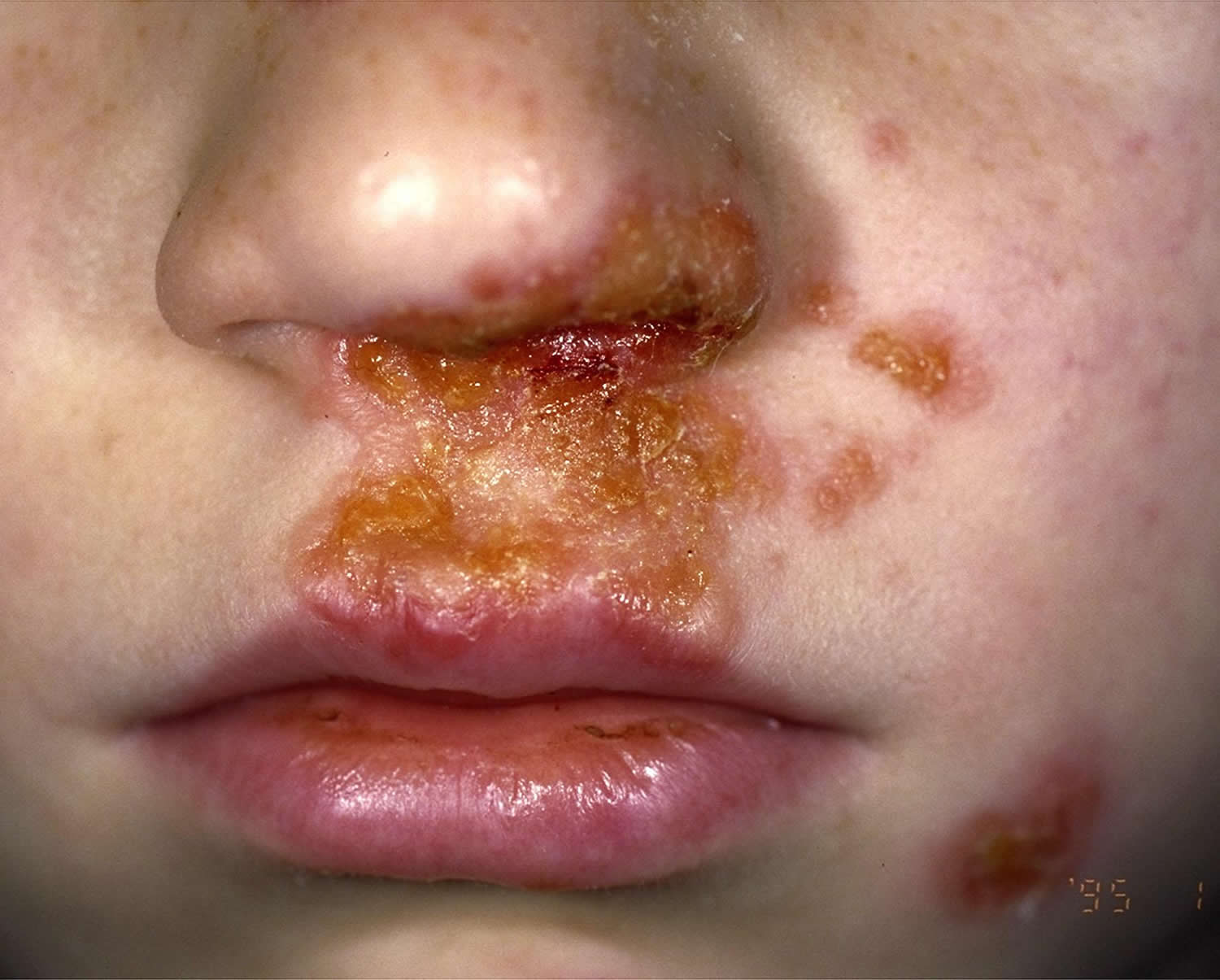
Impetigo also called “school sores” or pyoderma, is a highly contagious skin infection caused by bacteria 16. Impetigo is characterized by pustules and honey-colored crusted erosions (“school sores”). More than 90 percent of impetigo cases are caused by Staphylococcus aureus or “golden staph” bacteria, while the rest are caused by Streptococcus pyogenes (group A beta-hemolytic Streptococcus) bacteria (which also are responsible for “strep throat” and “scarlet fever“) 17, 18, 19. Generally, those who are affected are carriers of these bacteria, Staphylococcus aureus and Streptococcus pyogenes, meaning that their nostrils are colonized by the bacteria. If Staphylococcus aureus bacteria are to blame, the infection may cause blisters filled with clear fluid (bullous impetigo). These can break easily, leaving a raw, glistening area that soon forms a scab with a honey colored crust. By contrast, infections with Streptococcus pyogenes (group A beta-hemolytic Streptococcus) bacteria usually are not associated with blisters (non bullous impetigo or impetigo contagiosa), but they do cause crusts over larger sores and ulcers. Methicillin-resistant Staphylococcus aureus (MRSA) bacteria is also becoming an important cause of impetigo.
Impetigo is most common in children between the ages of two and six; however, adults can be affected by impetigo too. Impetigo usually starts when bacteria get into a break in the skin, such as a cut, scratch, or insect bite.
Young children often develop impetigo around the nose or mouth as a result of colonization of the nostrils, but the disease can occur anywhere – particularly if the skin barrier is disrupted in another part of the body such as with eczema or a dermatitis.
Symptoms start with red or pimple-like sores surrounded by red skin. These sores can be anywhere, but usually they occur on your face (around the nose, mouth, and ears), arms and legs. The sores fill with pus, then break open after a few days and form a thick crust. They are often itchy, but scratching them can spread the sores to other parts of their body. Impetigo can spread to anyone who touches infected skin or items that have been touched by infected skin (such as clothing, towels, and bed linens).
In the United States, impetigo is more common in the summer 20. There are more than 3 million cases of impetigo in the United States every year. Doctors typically see impetigo with kids 2 to 6 years old, probably because they get more cuts and scrapes and scratch more. The World Health Organization (WHO) estimates that 111 million children in less developed countries have streptococcal impetigo at any one time 21. Higher rates of impetigo are found in crowded and impoverished settings, in warm and humid conditions, and among populations with poor hygiene.
Complications related to impetigo can include deeper skin infection (cellulitis), meningitis, or a kidney inflammation (post streptococcal glomerulonephritis, which is not prevented by treatment) with few people going to develop kidney failure 22. The kidney dysfunction appears 7-14 days after the infection 22. The transient blood in urine (hematuria) and protein in urine (proteinuria) may last a few weeks or months. Other complications include septic arthritis, scarlet fever, sepsis, and staphylococcal scalded skin syndrome.
Impetigo is contagious and it can spread by contact with sores or nasal discharge from an infected person. You can treat impetigo with antibiotics.
Your doctor will look at your skin to determine if you have impetigo. Your doctor may take a sample of bacteria from your skin to grow in the lab. This can help determine if MRSA is the cause. Specific antibiotics are needed to treat this type of bacteria.
Impetigo will generally resolve on its own in a matter of weeks, but the use of topical or oral antibiotics prescribed by your doctor can hasten resolution of the infection.
Who gets impetigo?
Impetigo is most common in children (especially boys), but may also affect adults if they have low immunity to the bacteria. It is prevalent worldwide. Peak onset is during summer and it is more prevalent in developing countries.
The following factors predispose to impetigo.
- Atopic dermatitis or eczema
- Scabies
- Skin trauma: chickenpox, insect bite, abrasion, laceration, thermal burn, dermatitis, surgical wound.
How does impetigo spread?
Streptococcal impetigo is most commonly spread through direct contact with other people with impetigo, including through contact with drainage from impetigo lesions. Lesions can be spread (by fingers and clothing) to other parts of the body. People with impetigo are much more likely to transmit the bacteria than asymptomatic carriers. Crowding, such as found in schools and daycare centers, increases the risk of disease spread from person to person.
Humans are the primary reservoir for Streptococcus pyogenes (group A beta-hemolytic Streptococcus). There is no evidence to indicate that pets can transmit the bacteria to humans.
What is the incubation period for impetigo?
The incubation period of impetigo, from colonization of the skin to development of the characteristic lesions, is about 10 days 20. It is important to note not everyone who becomes colonized will go on to develop impetigo.
How long is impetigo contagious?
Impetigo is contagious until the rash clears, or until at least two days of antibiotics have been given and there is evidence of improvement 17. Your child should avoid close contact with other children during this period, and you should avoid touching the rash. If you or other family members do come in contact with it, wash your hands and the exposed site thoroughly with soap and water. Also, keep the infected child’s washcloths and towels separate from those of other family members.
Types of impetigo
The three types of impetigo are non-bullous (crusted), bullous (large blisters) and ecthyma (ulcers):
Non-bullous impetigo
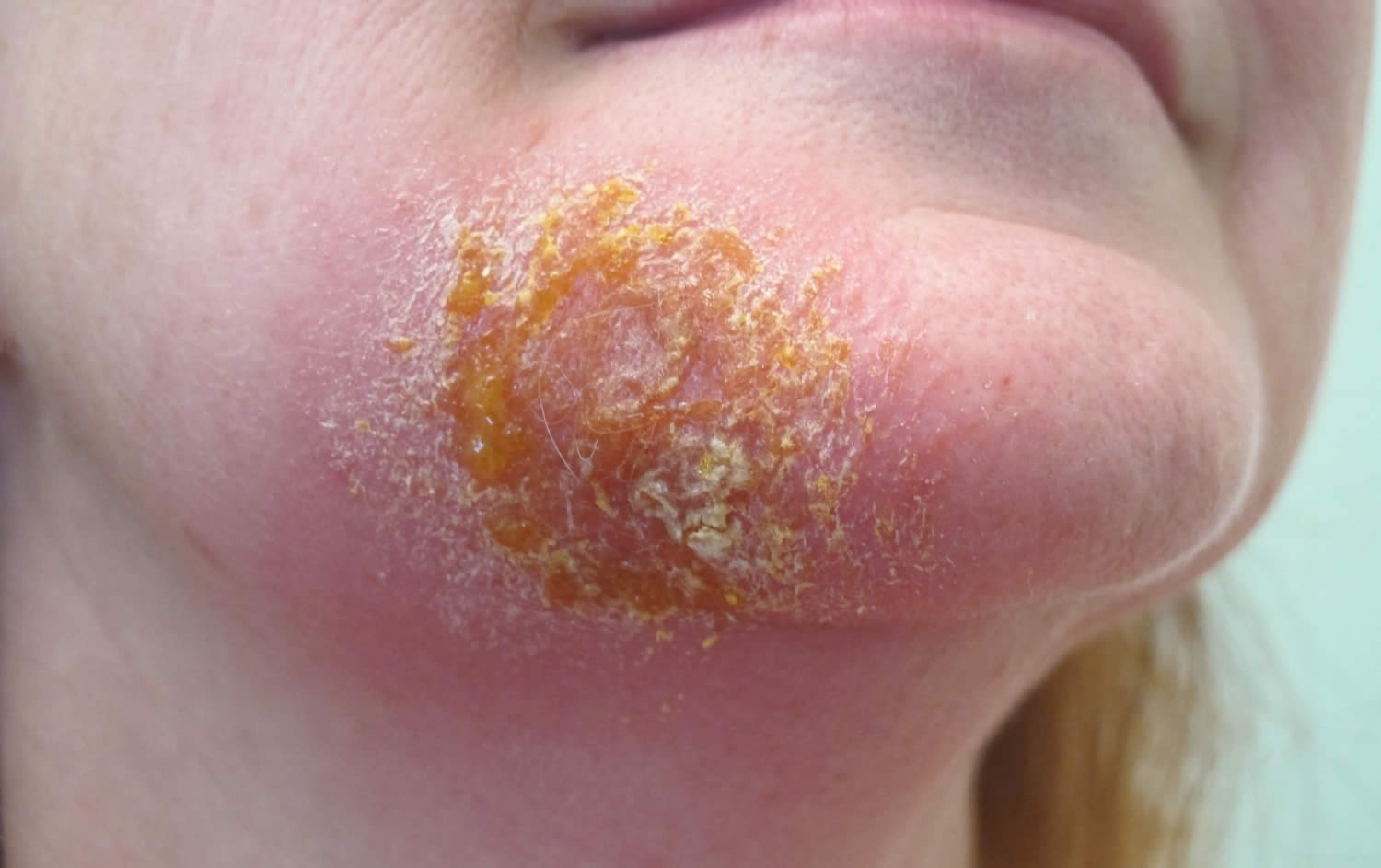
Non-bullous also known as crusted impetigo or impetigo contagiosa is most common. Non-bullous impetigo is caused by either Staphylococcus aureus, Streptococcus pyogenes, or both bacteria conjointly. It begins as tiny blisters that eventually burst and leave small wet patches of red skin that may weep fluid. Gradually, a yellowish-brown or tan crust covers the area, making it look like it has been coated with honey or brown sugar.
Intact skin is usually resistant to colonization from bacteria. Disruption in skin integrity allows for invasion of bacteria via the interrupted surface.
Bullous impetigo
Bullous impetigo causes larger fluid-containing blisters that look clear, then cloudy. Bullous impetigo is caused by Staphylococcus aureus bacteria which produces exfoliative toxins (exfoliatins A and B). Exfoliative toxins target intracellular adhesion molecules (desmoglein – 1) present in the epidermal granular layer. Results in dissociation of epidermal cells which causes blister formation. These blisters are more likely to stay longer on the skin without bursting.
Bullous impetigo can occur on areas of intact skin.
Ecthyma or ulcerated impetigo
Ecthyma or ulcerated impetigo is usually due to Streptococcus pyogenes, but co-infection with Staphylococcus aureus may occur. Ecthyma is a deep form of impetigo, as the same bacteria causing the infection are involved. Ecthyma causes deeper erosions of the skin into the dermis. Ecthyma is a skin infection characterized by crusted sores beneath which ulcers form.
Impetigo causes
Impetigo is caused by Streptococcus pyogenes (group A beta-hemolytic Streptococcus) or Staphylococcus aureus (staph) bacteria 18, 19. Methicillin-resistant staph aureus (MRSA) is becoming a common cause. Impetigo can be “bullous impetigo” or “non-bullous impetigo”. Toxin-producing Staphylococcus aureus cause bullous impetigo, Streptococcus pyogenes (group A beta-hemolytic Streptococcus) or both cause non-bullous impetigo, which is also called “impetigo contagiosa.”
Streptococcus pyogenes (group A beta-hemolytic Streptococcus) are Gram-positive cocci that grow in chains. They exhibit beta-hemolysis (complete hemolysis) when grown on blood agar plates. Streptococcus pyogenes belong to group A in the Lancefield classification system for beta-hemolytic Streptococcus, and thus are also called group A streptococci.
Skin normally has many types of bacteria on it; Streptococcus pyogenes (group A beta-hemolytic Streptococcus) and Staphylococcus aureus, intermittently colonizing the nasal, armpit, throat, or perineal areas 23, 24. When there is a break in the skin, the bacteria can enter the body and grow there. This causes inflammation and infection. Breaks in the skin may occur from injury or trauma to the skin or from insect (e.g., infected mosquito bites), animal, or human bites 25, 26. However, impetigo may also occur on skin where there is no visible break.
Many bacteria inhabit healthy skin; some types, such as Streptococcus pyogenes (group A beta-hemolytic Streptococcus) or Staphylococcus aureus (staph) can lead to infection of susceptible skin 24. Other factors that predispose to impetigo are skin trauma; hot, humid climates; poor hygiene; day care settings; crowding; malnutrition; and diabetes mellitus or other medical comorbidities 23, 24. Autoinoculation via fingers, towels, or clothing often leads to the formation of satellite lesions in adjacent areas 24. The highly contagious nature of impetigo also allows spread from patients to close contacts. Although impetigo is considered a self-limited infection, antibiotic treatment is often initiated for a quicker cure and to prevent the spread to others 23. This can help decrease the number of school and work days lost 24. Hygienic practices such as cleaning minor injuries with soap and water, handwashing, regular bathing, and avoiding contact with infected children can help prevent infection 27.
Impetigo is most common in children who live in unhealthy conditions.
In adults, it may occur following another skin problem. It may also develop after a cold or other virus.
Impetigo can spread to others. You can catch the infection from someone who has it if the fluid that oozes from their skin blisters touches an open area on your skin.
Because impetigo spreads by skin-to-skin contact, there often are small outbreaks within a family or a classroom. Avoid touching objects that someone with impetigo has used, such as utensils, towels, sheets, clothing and toys. If you have impetigo, keep your fingernails short so the bacteria can’t live under your nails and spread. Also, don’t scratch the sores.
See your health care provider if the symptoms don’t go away or if there are signs the infection has worsened, such as fever, pain, or increased swelling.
Risk factors for impetigo
Factors that increase the risk of impetigo include:
- Age. Impetigo most commonly occurs in children ages 2 to 5.
- Poor personal hygiene. Lack of proper handwashing, body washing, and facial cleanliness can increase someone’s risk of getting impetigo.
- People with weakened immune system or immunosuppressed (eg, diabetes, neutropenia, immunosuppressive medication, cancer, HIV and AIDS)
- Crowded conditions. Impetigo spreads easily in schools and child care settings. Close contact with another person with impetigo is the most common risk factor for infection.
- Warm, humid weather. Impetigo infections are more common in areas with hot, humid summers and mild winters (subtropics), or wet and dry seasons (tropics).
- Certain sports. Participation in sports that involve skin-to-skin contact, such as football or wrestling, increases your risk of developing impetigo.
- Skin trauma. The bacteria that cause impetigo often enter your skin through a small skin injury, thermal burns, abrasions, insect bite or rash.
- Skin conditions such as atopic dermatitis, contact dermatitis and chickenpox
- People with scabies infection are at increased risk for impetigo 28, 29.
Adults and people with diabetes or a weakened immune system are more likely to develop ecthyma. Ecthyma is a deep form of impetigo, as the same bacteria causing the infection are involved. Ecthyma causes deeper erosions of the skin into the dermis. Ecthyma is a skin infection characterized by crusted sores beneath which ulcers form.
Impetigo prevention
The bacteria that cause impetigo thrive in breaks in the skin. The best ways to prevent impetigo rash are to keep your child’s fingernails clipped and clean and to teach him not to scratch minor skin irritations. When he does have a scrape, cleanse it with soap and water, and apply an antibiotic cream or ointment. Be careful not to use washcloths or towels that have been used by someone else who has an active skin infection.
When certain types of strep bacteria cause impetigo, a rare but serious complication called glomerulonephritis can develop. This disease injures the kidney and may cause high blood pressure and blood to pass in the urine. Therefore, if you notice any blood or dark brown color in your child’s urine, let your pediatrician know so he can evaluate it and order further tests if needed.
Prevent the spread of impetigo.
- If you have impetigo, always use a clean washcloth and towel each time you wash.
- DO NOT share towels, clothing, razors, and other personal care products with anyone.
- Avoid touching blisters that are oozing.
- Wash your hands thoroughly after touching infected skin.
Keep your skin clean to prevent getting the infection. Wash minor cuts and scrapes well with soap and clean water. You can use a mild antibacterial soap.
Impetigo symptoms
Symptoms of impetigo are:
- One or many blisters that are filled with pus and easy to pop. In infants, the skin is reddish or raw-looking where a blister has broken.
- Blisters that itch, are filled with yellow or honey-colored fluid, and ooze and crust over. Rash that may begin as a single spot, but spreads to other areas with scratching.
- Skin sores on the face, lips, arms, or legs that spread to other areas.
- Swollen lymph nodes near the infection.
- Patches of impetigo on the body (in children).
In general, impetigo is a mild infection that can occur anywhere on the body. Impetigo most often affects exposed skin, such as:
- Around the nose and mouth
- On the arms or legs
Impetigo symptoms include red, itchy sores that break open and leak a clear fluid or pus for a few days. Next, a crusty yellow or “honey-colored” scab forms over the sore, which then heals without leaving a scar.
Primary impetigo mainly affects exposed areas such as the face and hands, but may also affect trunk, perineum and other body sites. It presents with single or multiple, irregular crops of irritable superficial plaques. These extend as they heal, forming annular or arcuate lesions.
Although many children are otherwise well, lymphadenopathy (enlarged lymph gland), mild fever and malaise may occur.
Figure 4. Impetigo on nose
Figure 5. Impetigo on face
Nonbullous impetigo
Nonbullous impetigo starts as a pink macule that evolves into a vesicle or pustule. Pustule or vesicle ruptures releasing serous contents which dries leaving a typical honey-colored crust. There’s minimal or no surrounding redness (erythema). Can spread rapidly with satellite lesions due to autoinoculation. “Kissing lesions” arise where two skin surfaces are in contact. People with non-bullous impetigo are typically otherwise well; they may experience some itching and regional enlarged lymph node (lymphadenopathy).
Non-bullous impetigo most commonly found on the face or extremities but skin on any part of the body can be involved.
Untreated nonbullous impetigo usually resolves within 2 to 4 weeks without scarring.
Bullous impetigo
Bullous impetigo presents with small vesicles that evolve quickly into flaccid transparent superficial, small or large thin roofed bullae which tend to spontaneously rupture and ooze yellow fluid leaving a scaley rim (collarette). It heals without scarring. People with bullous impetigo are more likely to have systemic symptoms of malaise, fever, and enlarged lymph node (lymphadenopathy).
Bullous impetigo is usually found on the face, trunk, extremities, buttocks, and perineal regions. Can spread distally due to autoinoculation.
Ecthyma
Ecthyma is a deep form of impetigo, as the same bacteria causing the infection are involved. Ecthyma causes deeper erosions of the skin into the dermis. Ecthyma starts as nonbullous impetigo but develops into a punched-out necrotic ulcer. This heals slowly, leaving a scar.
Streptococcus pyogenes and Staphylococcus aureus are the bacteria responsible for ecthyma.
Impetigo complications
Impetigo is usually a self-limited condition, and rarely, complications can occur after impetigo. These include cellulitis (nonbullous form), septicemia, osteomyelitis, septic arthritis, lymphangitis, lymphadenitis, guttate psoriasis, staphylococcal scalded skin syndrome, and acute poststreptococcal glomerulonephritis, with post streptococcal glomerulonephritis being the most serious 30. Post streptococcal glomerulonephritis is thought to be the result of an immune response that is triggered by Streptococcus pyogenes (group A beta-hemolytic Streptococcus) infection. The number of possible causes, incidence, and clinical severity of acute post streptococcal glomerulonephritis have decreased, because the causative organism of impetigo has shifted from Streptococcus pyogenes (group A beta-hemolytic Streptococcus) to Staphylococcus aureus 31. Most cases of poststreptococcal glomerulonephritis in the United States are associated with pharyngitis. The strains of Streptococcus pyogenes (group A beta-hemolytic Streptococcus) implicated in impetigo are thought to have minimal nephritogenic potential 31. There are no data to indicate that antibiotic treatment of impetigo has any effect on preventing the development of acute poststreptococcal glomerulonephritis, which can occur in up to 5% of patients with nonbullous impetigo 32, 33, 3, 34. Rheumatic fever does not appear to be a complication of impetigo 3.
Impetigo may lead to:
- Spread of the infection to other parts of the body (common)
- Kidney inflammation or failure (post-streptococcal glomerulonephritis) (rare). Acute kidney condition following infection with Streptococcus pyogenes (group A streptococcus). This is due to a type III hypersensitivity reaction and presents 2–6 weeks post-skin infection.
- Cellulitis. This potentially serious infection affects the tissues underlying your skin and eventually may spread to your lymph nodes and bloodstream. Untreated cellulitis can quickly become life-threatening.
- Wider spread infection: lymphangitis, and bacteremia.
- Staphylococcal scalded skin syndrome.
- Scarlet fever.
- Streptococcal toxic shock syndrome: a rare complication causing diffuse erythematous rash, hypotension, and pyrexia.
- Postinflammatory pigmentation.
- Permanent skin damage and scarring, particularly with ecthyma (very rare)
Soft tissue infection
The bacteria causing impetigo can become invasive, leading to cellulitis and lymphangitis; subsequent bacteremia might result in osteomyelitis, septic arthritis or pneumonia.
Staphylococcal scalded skin syndrome
In infants under 6 years of age or adults with renal insufficiency, localized bullous impetigo due to certain Staphylococcal serotypes can lead to a sick child with generalized staphylococcal scalded skin syndrome. Superficial crusting then tender cutaneous denudation on face, in skin folds, and elsewhere is due to circulating exfoliatin/epidermolysin, rather than direct skin infection. It does not scar.
Toxic shock syndrome
Post-streptococcal glomerulonephritis
Group A streptococcal infection may rarely lead to acute post-streptococcal glomerulonephritis 3–6 weeks after the skin infection. It is associated with anti-DNase B and antistreptolysin O (ASO) antibodies.
Rheumatic fever
Group A streptococcal skin infections have rarely been linked to cases of rheumatic fever and rheumatic heart disease. It is thought that this occurs because strains of group A streptococci usually found on the skin have moved to the throat (the more usual site for rheumatic fever-associated infection).
Impetigo diagnosis
Impetigo is usually diagnosed clinically but can be confirmed by bacterial swabs sent for microscopy (Gram positive cocci are observed), culture and sensitivity.
Blood count may reveal neutrophil leucocytosis when impetigo is widespread.
Skin biopsy is rarely necessary. Histological features are characteristic.
Non-bullous impetigo
- Gram-positive cocci
- Intraepidermal neutrophilic pustules,
- Dense inflammatory infiltrate in upper dermis
Bullous impetigo
- Split through granular layer of epidermis without inflammation or bacteria
- Acantholytic cells
- Minimal inflammatory infiltrate in upper dermis
- Resembles pemphigus foliaceus
Ecthyma
- Full thickness skin ulceration
- Gram stain shows cocci within dermis
Impetigo treatment
Impetigo needs to be treated with antibiotics, either topically (antibiotics rubbed onto the sores) or by mouth (oral antibiotics), and your doctor may order a culture in the lab to determine which bacteria are causing the rash. Gram stain and culture of the pus or exudates from skin lesions of impetigo and ecthyma are recommended to help identify whether Staphylococcus aureus and/or a Streptococcus pyogenes (group A beta-hemolytic Streptococcus) is the cause 33.
Make sure your child takes the medication for the full prescribed course or the impetigo could return.
- When it just affects a small area of the skin (and especially if it’s the non-bullous form), impetigo is treated with antibiotic ointment. Treatment of bullous and nonbullous impetigo should be with either mupirocin or retapamulin twice daily for 5 days 33.
- If the infection has spread to other areas of the body or the antibiotic ointment isn’t working, the doctor may prescribe an antibiotic pill or liquid to be taken for 7–10 days. Oral antibiotic therapy for ecthyma or impetigo should be a 7-day regimen with an agent active against Staphylococcus aureus unless cultures yield streptococci alone (when oral penicillin is the recommended agent) 33. Because Staphylococcus aureus isolates from impetigo and ecthyma are usually methicillin susceptible, dicloxacillin or cephalexin is recommended. When MRSA is suspected or confirmed, doxycycline, clindamycin, or sulfamethoxazole-trimethoprim (SMX-TMP) is recommended 33.
There is no over-the-counter (OTC) treatment for impetigo 35.
Quality evidence-based research for the most effective treatment of impetigo is lacking 36. In 2012, an updated Cochrane review on impetigo interventions evaluated 68 randomized controlled trials, including 26 on oral treatments and 24 on topical treatments. There was no clear evidence as to which intervention is most effective 37. Topical antibiotics are more effective than placebo and preferable to oral antibiotics for limited impetigo 36. Systemic antibiotics are often reserved for more generalized or severe infections in which topical therapy is not practical. Clinicians sometimes may choose both topical and systemic therapy 37. The ideal treatment should be effective, be inexpensive, have limited adverse effects, and should not promote bacterial resistance 38.
Untreated, impetigo often clears up on its own after a few days or weeks 39. The key is to keep the infected area clean with soap and water and not to scratch it. The downside of not treating impetigo is that some people might develop more lesions that spread to other areas of their body.
And you can infect others. To spread impetigo, you need fairly close contact — not casual contact — with the infected person or the objects they touched. Avoid spreading impetigo to other people or other parts of your body by:
- Cleaning the infected areas with soap and water (DO NOT scrub).
- Loosely covering scabs and sores until they heal.
- Gently removing crusty scabs.
- Washing your hands with soap and water after touching infected areas or infected persons.
- To prevent impetigo from spreading among family members, make sure everyone uses their own clothing, sheets, razors, soaps, and towels. Separate the bed linens, towels, and clothing of anyone with impetigo, and wash them in hot water. Keep the surfaces of your kitchen and household clean.
Table 1. Topical antibiotics for impetigo
| Medication | Instructions | Cost* |
|---|---|---|
| Fusidic acid 2% ointment† | Apply to affected skin three times daily for seven to 12 days | Available in Canada and Europe |
| Mupirocin 2% cream (Bactroban)‡ | Apply to affected skin three times daily for seven to 10 days; reevaluate after three to five days if no clinical response Approved for use in persons older than three months | 15-g tube: $48 ($89) 30-g tube: $50 ($144) |
| Mupirocin 2% ointment‡ | Apply to affected skin three times daily for seven to 14 days Dosing in children is same as adults Approved for use in persons older than two months | 22-g tube: $14 ($103) |
| Retapamulin 1% ointment (Altabax)§ | Apply to affected skin twice daily for five days Total treatment area should not exceed 100 cm2 in adults or 2% of total body surface area in children Approved for use in persons nine months or older | 15-g tube: NA ($130) 30-g tube: NA ($245) |
Footnotes: NA = not available
*Estimated retail price
† Coverage for Staphylococcus aureus (methicillin-susceptible) and streptococcus
‡ Coverage for Staphylococcus aureus (methicillin-susceptible) and streptococcus. Mupirocin resistant streptococcus has now been documented.
§ First member of the pleuromutilin class of antibiotics. Coverage for Staphylococcus aureus (methicillin-susceptible) and streptococcus.
[Source 40 ]Table 2. Oral antibiotics for impetigo
| Drug | Adult seven-day dose | Cost (for a typical course of treatment)* | Children seven-day dose | Cost* |
|---|---|---|---|---|
| Amoxicillin/clavulanate (Augmentin)† | 875/125 mg every 12 hours | $19 ($193) | Younger than three months: 30 mg per kg per day Three months or older: 25 to 45 mg per kg per day for those weighing less than 40 kg (88 lb); 875/125 mg every 12 hours for those weighing 40 kg or more Based on mg per kg per day of the amoxicillin component in divided doses every 12 hours | 1 bottle, 400/57 mg per 5 mL (100-mL oral suspension): $30 ($125) |
| Cephalexin (Keflex) | 250 mg every six hours or 500 mg every 12 hours | $5 ($90) | 25 to 50 mg per kg per day in divided doses every six to 12 hours | 1 bottle, 250 mg per 5 mL (100-mL oral suspension): $14 (NA) |
| Clindamycin‡ | 300 to 600 mg every six to eight hours | $18 ($200) | 10 to 25 mg per kg per day in divided doses every six to eight hours | 1 bottle, 75 mg per 5 mL (100-mL oral solution): $47 (pricing varies by region) |
| Dicloxacillin | 250 mg every six hours | $14 (NA) | 12.5 to 25 mg per kg per day in divided doses every six hours | See adult pricing: no liquid formulation available |
| Doxycycline§ | 50 to 100 mg every 12 hours | $15 ($95) | 2.2 to 4.4 mg per kg per day in divided doses every 12 hours Not recommend in children younger than eight years | 1 bottle, 25 mg per 5 mL (60-mL oral suspension): $20 (pricing varies by region) |
| Minocycline (Minocin)§ | 100 mg every 12 hours | $36 ($185) | Loading dose of 4 mg per kg for first dose (maximum dose of 200 mg), then 4 mg per kg per day in divided doses every 12 hours Maximum of 400 mg per day Not recommend in children younger than eight years | See adult pricing: no liquid formulation available |
| Trimethoprim/sulfamethoxazole§ | 160/800 mg every 12 hours | $4 (NA) | 8 to 10 mg per kg per day based on the trimethoprim component in divided doses every 12 hours | 1 bottle, 40/200 mg per 5 mL (100-mL oral suspension): $4 (pricing varies by region) |
Footnote: Because of emerging resistance, penicillin and erythromycin are no longer recommended treatments.
NA = not available
* Estimated retail price based on information obtained at http://www.goodrx.com. Generic price listed first; brand listed in parentheses.
† Good coverage for Staphylococcus aureus (methicillin-susceptible) and streptococcus.
‡ If methicillin-resistant Staphylococcus aureus (MRSA) is suspected or proven.
§ If methicillin-resistant Staphylococcus aureus (MRSA) is suspected or proven. There is no activity against streptococcus.
[Source 40 ]Home remedies for impetigo
Topical disinfectants
There are some studies on the benefits of nonantibiotic treatments, such as disinfectant soaps, but they lack statistical power 36. Disinfectants appear to be less effective than topical antibiotics and are not recommended 37. Studies comparing hexachlorophene (not available in the United States) with bacitracin and hydrogen peroxide with topical fusidic acid found the topical antibiotic to be more effective 41.
Natural therapies
The evidence is insufficient to recommend or dismiss popular herbal treatments for impetigo 42. Natural remedies such as tea tree oil; tea effusions; olive, garlic, and coconut oils; and Manuka honey have been anecdotally successful. The fact that impetigo is self-limited means that many “cures” could appear to be helpful without being superior to placebo. In one study, tea leaf ointment and oral cephalexin (Keflex) were similarly effective, with a cure rate of 81% vs. 79% 43. Tea tree oil (derived from Melaleuca alternifolia) appeared to be equivalent to mupirocin 2% for topical decolonization of MRSA 44.
Impetigo prognosis
Without treatment, impetigo heals in 14-21 days 22. About 20% of cases resolve spontaneously. Scarring is rare but some patients may develop pigmentation changes. Some patients may develop ecthyma. With treatment, cure occurs within 10 days 22. Neonates may develop meningitis. A rare complication is acute post streptococcal glomerulonephritis, which occurs 2-3 weeks after the skin infection 22.
Fungal skin infections
Fungal infections of the skin are also known as ‘mycoses’. They are common and generally mild. However, in very sick or otherwise immune suppressed people, fungi can sometimes cause severe disease.
Fungi usually make their homes in moist areas of the body where skin surfaces meet: between the toes, in the genital area, and under the breasts. Common fungal skin infections are caused by yeasts (such as Candida or Malassezia furfur) or dermatophytes, such as Epidermophyton, Microsporum, and Trichophyton.
Many fungi live only in the topmost layer of the epidermis (stratum corneum) and do not penetrate deeper. Obese people are more likely to get these infections because they have excessive skinfolds, especially if the skin within a skinfold becomes irritated and broken down (intertrigo). People with diabetes tend to be more susceptible to fungal infections as well.
Strangely, fungal infections on one part of the body can cause rashes on other parts of the body that are not infected. For example, a fungal infection on the foot may cause an itchy, bumpy rash on the fingers. These eruptions (dermatophytids, or identity or id reactions) are allergic reactions to the fungus. They do not result from touching the infected area.
- Superficial fungal infections. These affect the outer layers of the skin, the nails and hair. The main groups of fungi causing superficial fungal infections are:
- Dermatophytes (tinea)
- Yeasts: Candida, including non-albicans candida species, Malassezia, Piedra. Yeasts form a subtype of fungus characterized by clusters of round or oval cells. These bud out similar cells from their surface to divide and propagate. In some circumstances, they form a chain of cells called a pseudomycelium.
- Molds.
- Subcutaneous fungal infections. These involve the deeper layers of the skin (the dermis, subcutaneous tissue and even bone). The causative organisms live in the soil on rotting vegetation. They can get pricked into the skin as a result of an injury but usually stay localized at the site of implantation. Deeper skin infections include:
Fungus that cause skin infection
Candida
- diaper dermatitis (diaper rash)
- Non-albicans candida infections
- Oral candidiasis (oral thrush)
- Vulvovaginal candidiasis (vaginal thrush)
- Candida intertrigo (skin fold infection)
- Paronychia (nail fold infections)
- Chronic mucocutaneous candidiasis
- Cyclic vulvovaginitis (cyclical symptoms due to vulvovaginal candidiasis)
Malassezia
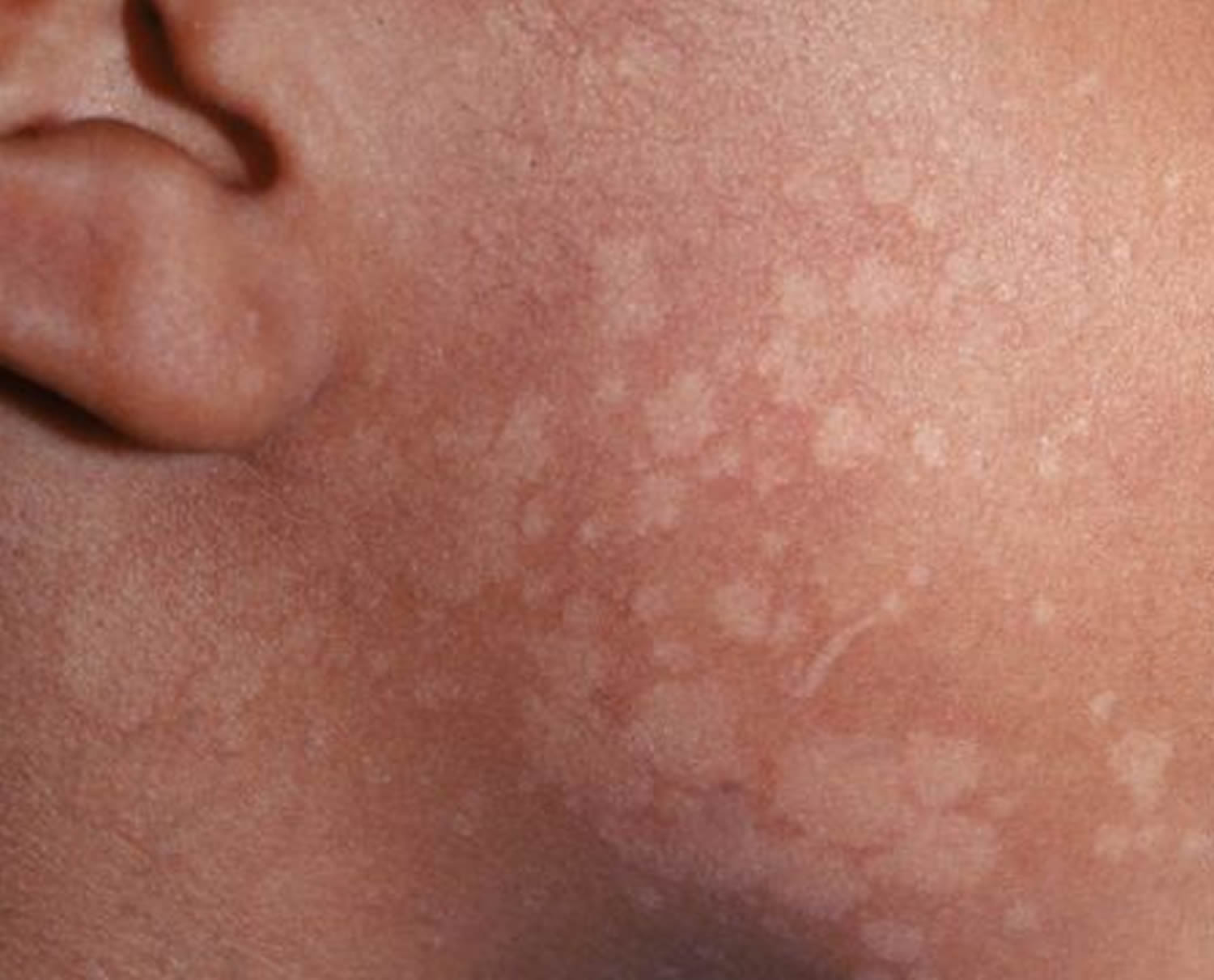
- Pityriasis versicolor (tinea versicolor)
- Malassezia (pityrosporum) folliculitis
- Seborrheic dermatitis
Dermatophyte infections
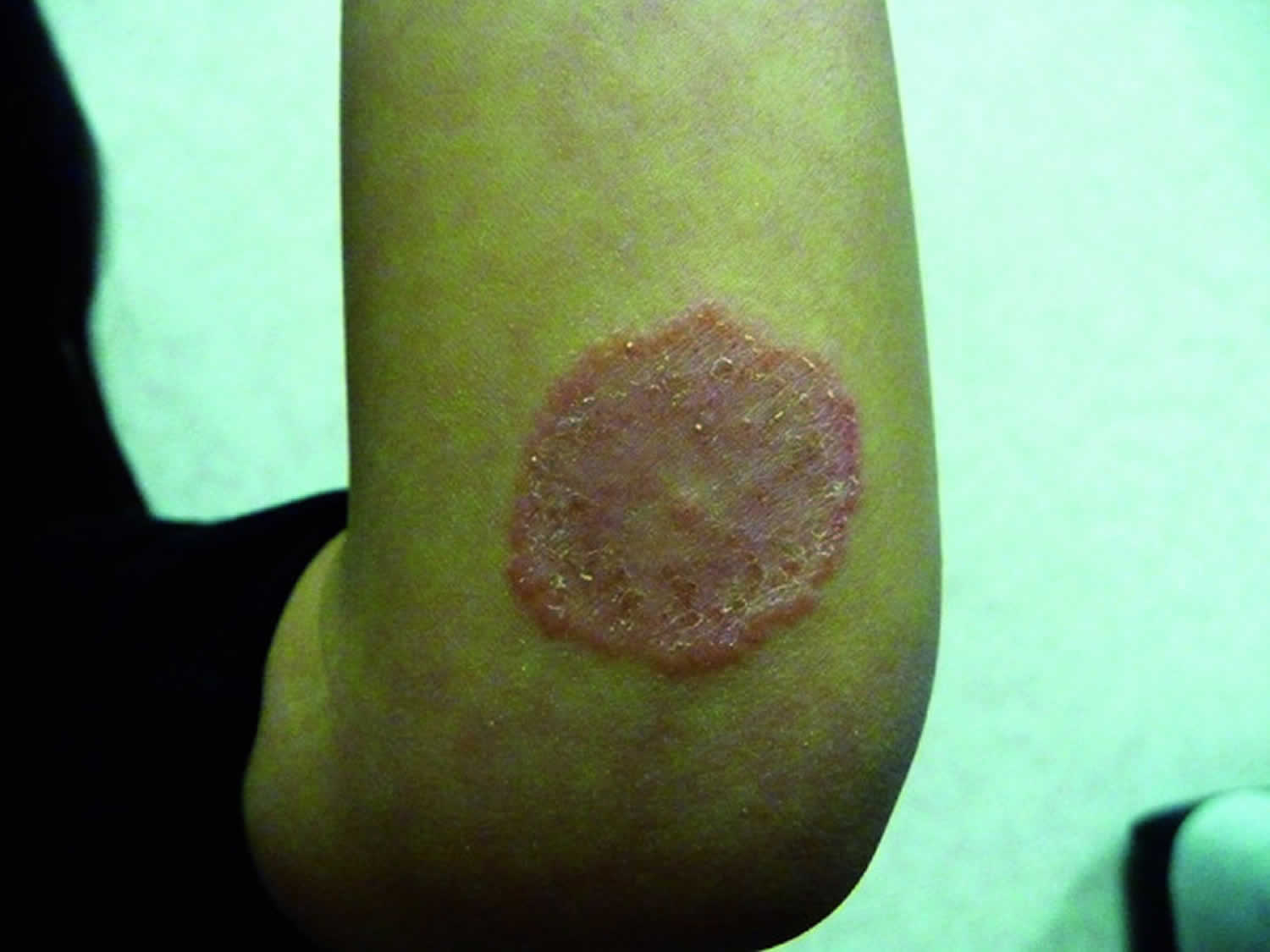
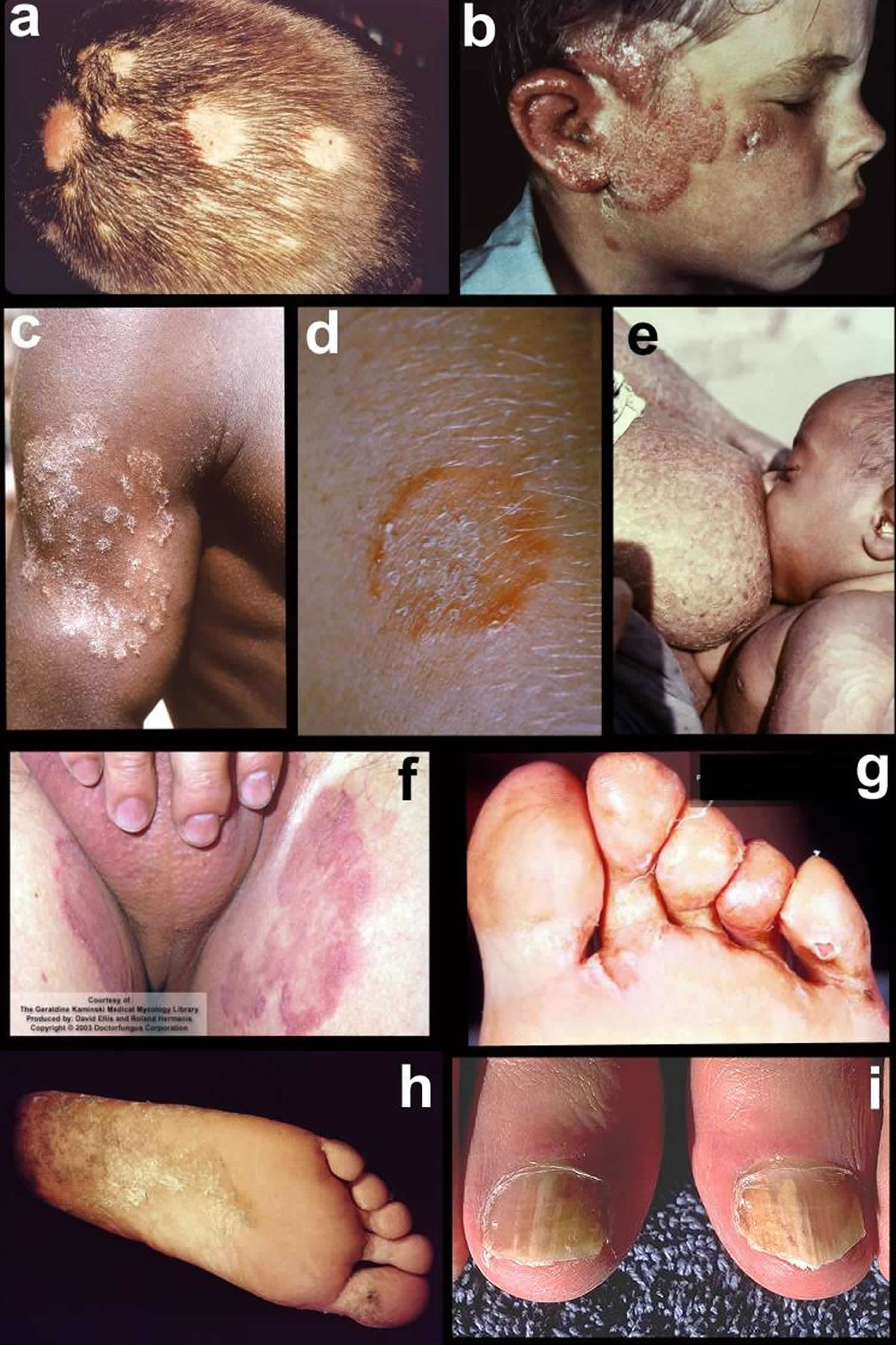
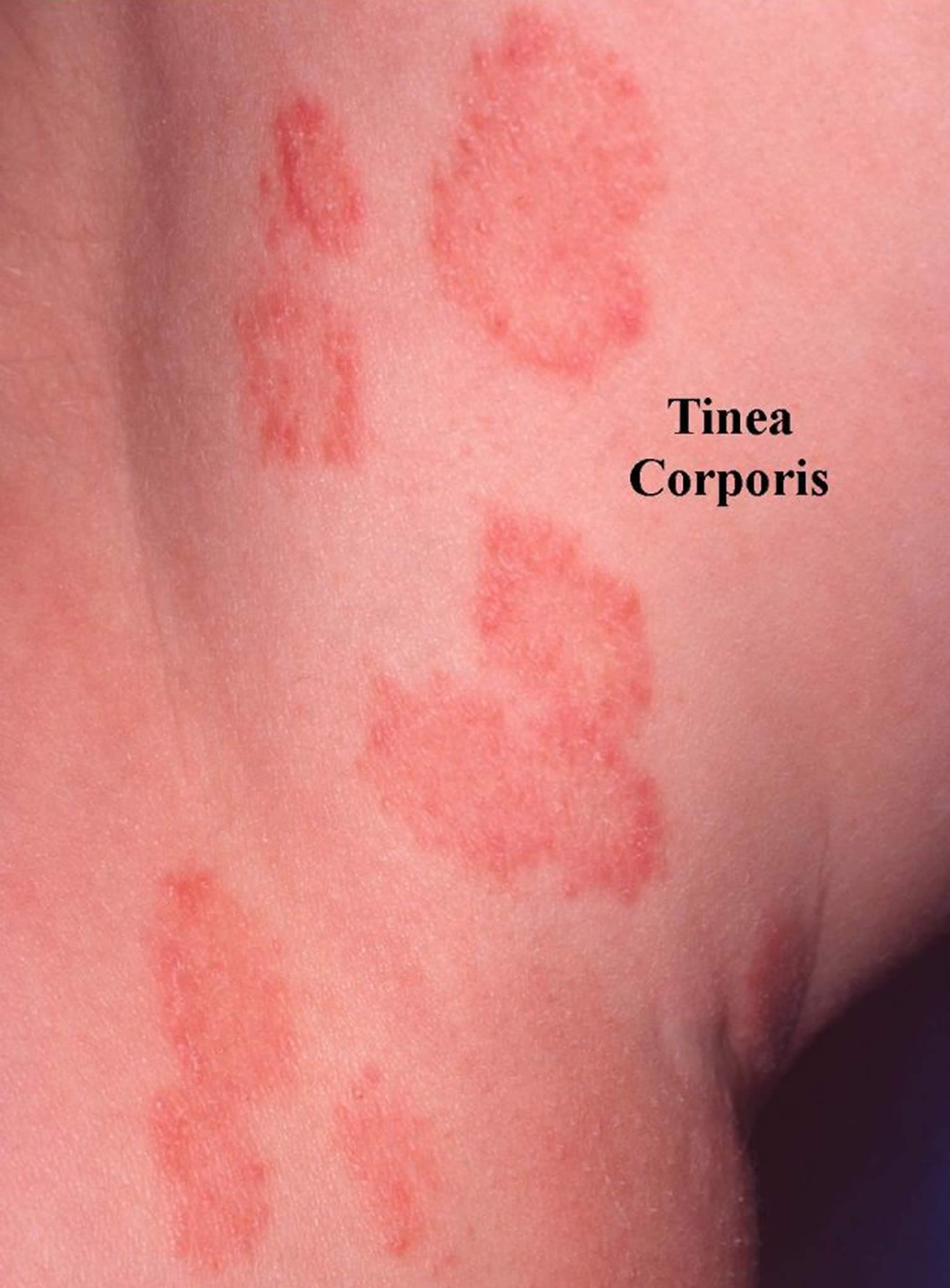
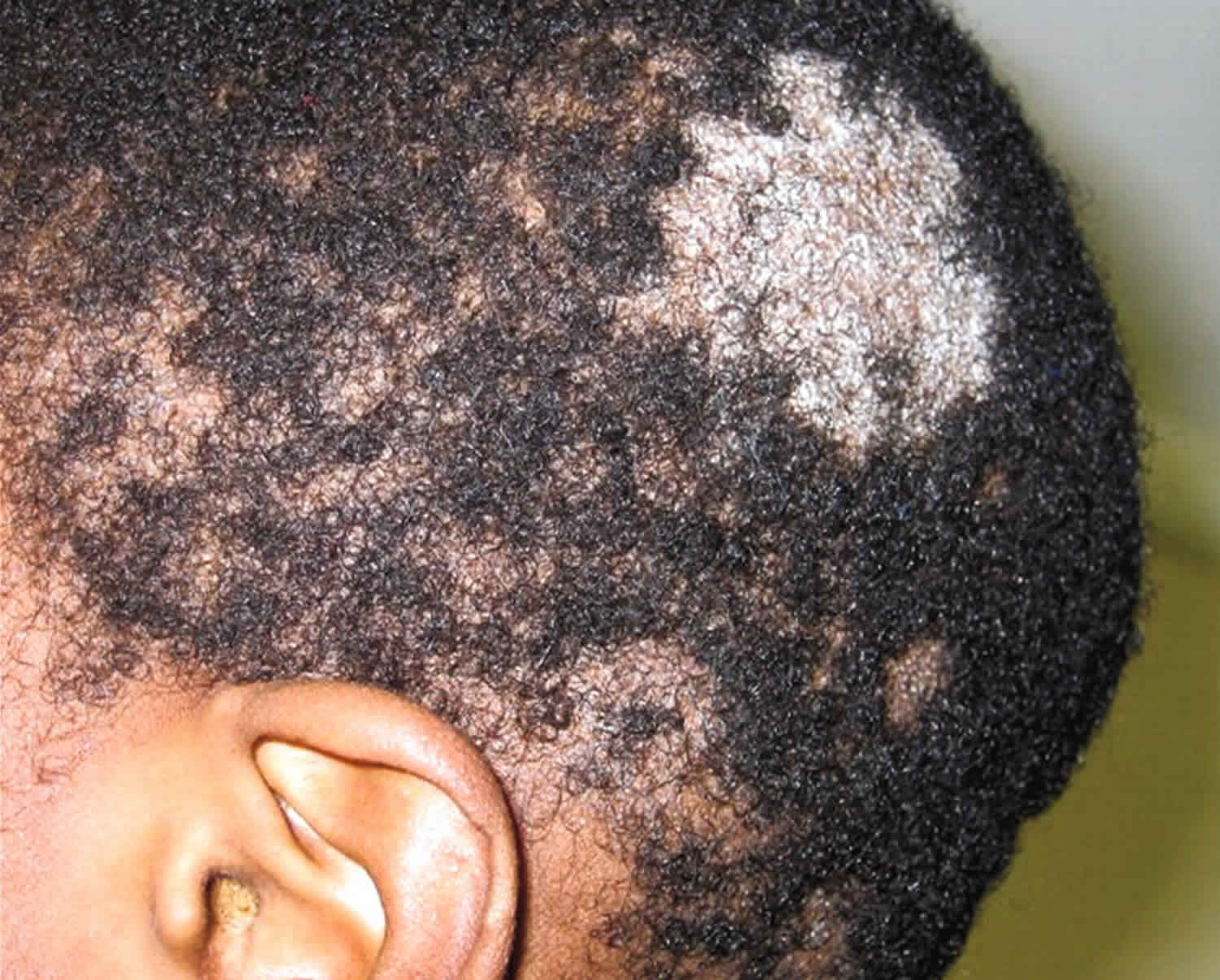
- Tinea infections. Tinea is a skin infection with a dermatophyte ringworm fungus. Depending on which part of the body is affected, it is given a specific name.
- Tinea barbae (fungal infection of the beard)
- Tinea capitis (fungal infection of the scalp)
- Tinea corporis (fungal infection of the trunk and limbs)
- Tinea cruris (fungal infection of the groin)
- Tinea faciei (fungal infection of the face)
- Tinea incognito (steroid-treated fungal infection)
- Tinea manuum (fungal infection of the hand)
- Tinea pedis (fungal infection of the foot)
- Tinea unguium (onychomycosis, fungal nail infection)
- Majocchi granuloma (deep fungal infection of hair follicles)
- Kerion (fungal abscess)
- Dermatophytide (id reactions) dermatitis in reaction to tinea infection
Deep fungal infections
Systemic mycoses may result from breathing in the spores of fungi, which live in the soil or rotting vegetation, or present as an opportunistic disease in immunocompromised individuals.
- Aspergillosis
- Blastomycosis
- Cryptococcosis
- Chromoblastomycosis
- Histoplasmosis
- Mycetoma
- Sporotrichosis
- Sporotrichoid spread
- Systemic mycoses
- Talaromycosis
- Zygomycosis
Fungal skin infection diagnosis
Your doctor may suspect a fungal infection when they see a red, irritated, or scaly rash in one of the commonly affected areas.
Your doctor can usually confirm the diagnosis of a fungal skin infection by scraping off a small amount of skin and having it examined under a microscope or placed in a culture medium where the specific fungus can grow and be identified.
Fungal skin infection treatment
Fungal skin infections are typically treated with antifungal drugs, usually with antifungal drugs that are applied directly to the affected area (called topical drugs). Topical drugs may include creams, gels, lotions, solutions, or shampoos. Antifungal drugs may also be taken by mouth.
Many antifungal medications are suitable for both dermatophyte and yeast infections. Others are more specific to one or the other type of fungus. Those unsuitable for dermatophyte fungal infections are marked with an asterisk (*) in the list that follows.
- Whitfield ointment (3% salicylic acid, 6% benzoic acid in petrolatum)
- Undecylenic alkanolamide
- Ciclopirox olamine
- Polyenes *
- Nystatin
- Imidazoles
- Bifonazole
- Clotrimazole
- Econazole
- Efinaconazole
- Ketoconazole
- Luliconazole
- Miconazole
- Sulconazole
- Tioconazole
- Allylamine
- Terbinafine
- Thiocarbamates
- Tolciclate
- Tolnaftate
- Benzoxaborole
- Tavaborole
Topical antifungals can be obtained over the counter without a doctor’s prescription. They are generally applied to the affected area twice daily for two to four weeks, including a margin of several centimetres of normal skin. Treatment should continue for one or two weeks after the last visible rash has cleared. They can often cure a localized infection, although recurrence is common so repeated treatment is often necessary.
In addition to antifungal drugs, people may use measures to keep the affected areas dry, such as applying powders or wearing open-toed shoes.
Corticosteroids can help relieve inflammation and itching caused by some infections, but these should be used only when prescribed by a doctor.
Oral antifungal medications may be required for a fungal infection if:
- It is extensive or severe
- It resists topical antifungal therapy
- It affects hair-bearing areas (tinea capitis and tinea barbae).
Scalp antifungal agents
Antifungal shampoos are mainly used to treat dandruff or seborrheic dermatitis but are used as an adjunct for tinea capitis and scalp psoriasis. The most effective ingredients are ketoconazole, miconazole and ciclopirox (Stieprox® liquid), but many other shampoos marketed for dandruff have antifungal properties.
Nail fold antifungal agents
There are many antiseptic and antifungal preparations to control nail fold fungal infections (paronychia). They should be applied two or three times daily for several months.
- Clotrimazole solution
- Econazole solution
- Miconazole
- Sulfacetamide 15% in spirit
Nail plate antifungal agents
Distal onychomycosis can be treated with an antifungal lacquer applied once or twice weekly. The medication should be applied to the surface of the cleaned nail plate after it has been roughened using an emery board. Extra lacquer should be applied under the edge of the nail.
Available preparations are:
- Amorolfine
- Ciclopirox
- Bifonazole cream + urea ointment
- Efinaconazole solution
- Tavaborole solution.
These can be expected to reduce and sometimes cure the infection, provided that:
- No more than 50% of the nail plate is infected
- The growing part of the nail plate (the matrix) is not involved
- There is no complicating internal disease (such as diabetes) or skin condition (such as psoriasis).
Treatment needs to be undertaken for long periods (a year or longer) because nails take a long time to grow, especially in older individuals. Nail polish is not recommended, in case it interferes with the efficacy of the product, although this is not proven.
Oral fungal infections medicines
Oral candidiasis can be treated with:
- Nystatin *
- Amphotericin B *
- Miconazole.
Note: miconazole oral gel should not be used in patients who are taking warfarin because it has been reported to cause a dangerous interaction, which could result in serious bleeding.
Vaginal antifungal agents
Vulvovaginal candidiasis can be treated with:
- Nystatin * (unsuitable for dermatophyte fungal infections)
- Clotrimazole
- Econazole
- Isoconazole
- Miconazole
- Tioconazole.
Antifungal combination products
Topical antifungals may be sold with an oral antifungal medication, for example, fluconazole capsule in combination with clotrimazole cream.
Antifungal creams are sometimes combined with:
- Hydrocortisone or another topical steroid
- Antibacterial agent
- Both topical steroid and antibacterial agent.
A strong topical steroid can mask the fungal infection, and, as they are not curative, they can result in more extensive infection (tinea incognito) and adverse effects such as cutaneous atrophy.
To reduce reinfection
Fungal spores can survive long periods. The following measures can be used to reduce the chance of reinfection with the fungus.
- Do not share towels, sheets or personal clothing.
- Avoid walking bare foot where others may tread – wear jandals, sandals or aquasocks at the public pools and sports changing rooms.
- Avoid long periods wearing the same clothing, or wearing occlusive clothing such as wet weather gear and nylon pantyhose.
- Wear open-toed sandals when possible. Avoid long periods in occlusive footwear such as gum boots or tramping boots.
- Use antifungal foot powder containing ciclopirox, econazole, miconazole, tolciclate, tolnaftate or undecylenic acid. Sprinkle it in your shoes.
- In the case of zoophilic fungal infections, infected animals should be identified and treated.
Antifungal drug resistance
The acquired resistance of dermatophyte fungi to antifungal medication (both topical and oral) was previously thought to be exceptionally rare. However, in recent years, both topical and oral allylamine and triazole antifungal drug resistance has become a problem, particularly in the Indian subcontinent 45, 46. An epidemic-like spread of recalcitrant and terbinafine-resistant dermatophytosis has been reported across the Indian subcontinent since 2015. Antifungal medication resistant fungal infection management has yet to be clarified 47. Sensitivity testing is becoming standardized, more widely available in some countries, and should guide onward therapy choice. For terbinafine resistance, itraconazole seems to be the favored agent, and long courses at higher than standard doses have been advocated 47.
Ringworm
Ringworm also known as “tinea” or “dermatophytosis”, is a common fungal skin infection that is caused by a fungus. It’s called “ringworm” because it can cause a circular rash (shaped like a ring) that is usually red, scaly and itchy. There are approximately 40 different species of fungi can cause ringworm; the scientific names for the types of fungi that cause ringworm are Trichophyton, Microsporum, and Epidermophyton 48. No worm is involved. Anyone can get ringworm. The fungi that cause this infection can live on skin, surfaces, and on household items such as clothing, towels, and bedding.
Ringworm rash is itchy and is highly contagious being easily spread by touching an infected person or animal. Ringworm can also be spread by touching objects or surfaces that had contact with the infection. If infected, people often begin itching four to fourteen days after contact. The rash may be scaly, reddened, and circular. Ringworm on the scalp usually makes a bald patch of scaly skin.
Ringworm can affect skin on almost any part of your body as well as fingernails and toenails. Ringworm goes by different names depending on which body part it affects. Ringworm on your body is called tinea corporis. This type of ringworm affects your arms, legs, torso and face.
Areas of the body that can be affected by ringworm include:
- Feet (tinea pedis, commonly called “athlete’s foot”)
- Groin, inner thighs, or buttocks (tinea cruris, commonly called “jock itch”)
- Scalp (tinea capitis)
- Face (tinea faciei)
- Beard (tinea barbae)
- Hands (tinea manuum)
- Toenails or fingernails (tinea unguium, also called “onychomycosis”)
- Other parts of the body such as arms or legs (tinea corporis)
Sometimes, the name gives a different meaning:
- Tinea versicolor is more accurately called pityriasis versicolor. This is a common yeast infection on the trunk.
- Tinea incognito refers to a tinea infection in which the clinical appearance has changed because of inappropriate treatment, usually a topical steroid cream. It is also known as steroid-modified tinea 49.
- Tinea nigra is a mold infection (not a dermatophyte). It affects the palms or soles, which appear brown (on white skin) or black (on dark skin).
The symptoms of ringworm often depend on which part of the body is infected, but they generally include:
- Itchy skin
- Ring-shaped rash
- Red, scaly, cracked skin
- Hair loss
Symptoms typically appear between 4 and 14 days after the skin comes in contact with the fungi that cause ringworm.
Symptoms of ringworm by location on the body:
- Ringworm on the feet (tinea pedis or “athlete’s foot”): The symptoms of ringworm on the feet include red, swollen, peeling, itchy skin between the toes (especially between the pinky toe and the one next to it). The sole and heel of the foot may also be affected. In severe cases, the skin on the feet can blister.
- Ringworm on the scalp (tinea capitis): Ringworm on the scalp usually looks like a scaly, itchy, red, circular bald spot. The bald spot can grow in size and multiple spots might develop if the infection spreads. Ringworm on the scalp is more common in children than it is in adults.
- Ringworm on the groin (tinea cruris or “jock itch”): Ringworm on the groin looks like scaly, itchy, red spots, usually on the inner sides of the skin folds of the thigh.
- Ringworm on the beard (tinea barbae): Symptoms of ringworm on the beard include scaly, itchy, red spots on the cheeks, chin, and upper neck. The spots might become crusted over or filled with pus, and the affected hair might fall out.
- Fungal nail infections also known as “onychomycosis”. Fungal nail infections may cause nails to become discolored, thick, fragile, or cracked. The nail may also become separated from the nail bed. Fungal toenail infections are more common than fungal fingernail infections 50.
Your doctor might suspect you have ringworm by looking at your affected skin and asking questions about your symptoms. Your doctor will generally take a small skin scraping or nail sample to examine under a microscope or send to a laboratory for further testing.
Ringworm is treated with antifungal medication available either over the counter or as a prescription. Mild ringworm often responds to antifungal medications applied to the skin. For more-severe infections, you may need to take prescription-strength oral antifungal medication for several weeks.
Most ringworm infections improve with antifungal treatment, but treatment failure may occur. Common reasons for treatment failure may include incorrect diagnosis and inadequate treatment.
Figure 6. Ringworm rash
Figure 7. Ringworm (tineas) throughout the body
Footnote: From top to bottom, left to right: fungal infections of (A) ringworm on the scalp (tinea capitis); (B) ringworm on the face (tinea faciei); (C) ringworm on the arm (tinea corporis); (D) close-up of ringworm; (E) ringworm on the torso with concentric rings (tinea corporis); (F) ringworm on the groin (tinea cruris); (G) ringworm on between the toe webbing (tinea pedis); (H) ringworm on the foot (tinea pedis / “moccasin” type); and (I) ringworm in the nails (onycomycosis).
[Source 52 ]Figure 8. Ringworm on face
Figure 9. Ringworm on body (tinea corporis)
Figure 10. Ringworm on hands
Footnote: (A) Tinea manuum clinical examination shows diffuse white scaling involving the palmar surface and the volar aspect of the fingers of both hands. (B) Polarized light dermoscopy reveals white scaling mainly located in the creases. Direct microscopic examination of 10% potassium hydroxide (KOH) preparation of the scales scraped from the palmar surface showed septate branching hyphae. Cultures of specimens on conventional Sabouraud’s dextrose agar medium showed Trichophyton rubrum growth after three weeks, thus confirming the diagnosis of tinea manuum.
[Source 53 ]Figure 11. Ringworm on foot
Figure 12. Ringworm of the scalp
Footnote: Ringworm of the scalp appears as round patches where the hair has broken off at or just above the scalp. These bald-looking patches slowly grow larger.
Is ringworm contagious?
Yes. Ringworm is a highly contagious fungal skin infection that is spread easily and through close contact with an infected person, animal or object.
Many different kinds of animals can transmit ringworm to people. Dogs and cats, especially kittens or puppies, can have ringworm that can be passed to people. Cows, goats, pigs, and horses can also pass ringworm to people.
Animals, like people, get infected through touching an infected animal’s skin or hair or by touching things that are infected with the fungus, like blankets and towels.
Adult animals, especially long-haired cats, do not always show signs of ringworm infection. Puppies and kittens most often have patches that are hairless, circular, or irregularly shaped areas of scaling, crusting, and redness that may or may not be itchy. The area may not be completely hairless, and instead have brittle, broken hairs. If the claws are affected, they may have a whitish, opaque appearance with shredding of the claw’s surface. If you suspect your pet has ringworm, make sure it is seen by a veterinarian.
Ringworm is also fairly common in show lambs, and is referred to as “club lamb fungus” 54. Lambs usually have circular, hairless areas with thick scabs on their head and face.
How does ringworm spread?
The fungi (Trichophyton, Microsporum, and Epidermophyton) that cause ringworm can live on your skin and in the environment. There are three main ways that ringworm can spread:
- From a person who has ringworm. People can get ringworm after contact with someone who has the infection. To avoid spreading the infection, people with ringworm shouldn’t share clothing, towels, combs, or other personal items with other people.
- From an animal that has ringworm. People can get ringworm after touching an animal that has ringworm. Many different kinds of animals can spread ringworm to people, including dogs and cats, especially kittens and puppies. Other animals, like cows, goats, pigs, and horses can also spread ringworm to people.
- From the environment. The fungi that cause ringworm can live on surfaces, particularly in damp areas like locker rooms and public showers. For that reason, it’s a good idea not to walk barefoot in these places.
Ringworm causes
Ringworm also called “tinea” or “dermatophytosis,” is a common infection of the epidermis (skin, hair, or nails) caused by dermatophyte fungi (the fungal organisms that cause tinea) 55. There are approximately 40 different species of fungi can cause ringworm; the scientific names for the types of fungi that cause ringworm are Trichophyton (abbreviated as “T”), Microsporum (“M”), and Epidermophyton (“E”) 48. Dermatophytes (the fungal organisms that cause tinea) have the ability to invade keratinized tissue (skin, hair and nails). Infection is normally restricted to the outermost layer of the skin (epidermis).
People can acquire ringworm through direct skin contact with people and animals who are infected. People can also acquire ringworm by sharing personal items (e.g., towels, clothing, bedding) or through contact with surfaces found in moist areas (e.g., shower stalls, locker room floors, pool areas). Ringworm can also spread from one part of the body to another.
Dermatophytes are grouped into three general categories based on their natural environment: anthropophilic (live exclusively on humans), zoophilic (live on an animal host), and geophilic (live in the soil) 56. The majority of human infections are caused by anthropophilic dermatophytes; however, dermatophytes from all three groups have been associated with human disease. For example, pets with ringworm can transmit the infection to their owners, and stray cats carrying dermatophytes are considered to be a vector for infection in several eastern and southern European countries 57.
Anthropophilic dermatophytes are so well adapted to living on human skin that they provoke a minimal inflammatory reaction. Zoophilic or geophilic dermatophytes will often provoke a more vigorous inflammatory reaction when they attempt to invade human skin.
Anthropophilic dermatophytes (live exclusively on humans) include:
- Trichophyton rubrum
- Trichophyton interdigitale
- Trichophyton tonsurans (very common in the USA)
- Microsporum audouinii
- Trichophyton violaceum
- Microsporum ferrugineum
- Trichophyton schoenleinii
- Trichophyton megninii
- Trichophyton soudanense
- Trichophyton yaoundei
Zoophilic dermatophytes (live on an animal host) include:
- Microsporum canis (originating from cats and dogs)
- Trichophyton equinum (originating from horses)
- Trichophyton erinacei (originating from hedgehogs and other animals)
- Trichophyton verrucosum (originating from cattle)
- Microsporum nanum (originating from pigs)
- Microsporum distortum (a variant of M. canis).
Geophilic dermatophytes (live in the soil) include:
- Nannizzia gypsea
- Microsporum fulvum.
Although dermatophytes are found throughout the world, the most prevalent strains and the most common sites of infection vary by region 57, 48. Hot, humid climates and overcrowding predispose populations to skin diseases, including tinea infections 58. Developing countries have high rates of tinea capitis, while developed countries have high rates of tinea pedis and onychomycosis 57.
Low socioeconomic conditions are strongly linked to higher prevalence rates for skin infections, including tinea infections. A review of 18 studies representing large geographical areas determined that tinea capitis is present in up to 19.7% of the general population in developing countries 58. A recent study found tinea capitis present in more than 30% of children at certain grade levels in some urban areas of the United States 59.
High prevalence rates of tinea pedis and onychomycosis have been linked to increased urbanization, community showers, sports, and the use of occlusive footwear 57, 48, 60. These factors are thought to contribute to the high prevalence of tinea pedis in certain occupational groups, including marathon runners (22%–31% prevalence), miners (21%–72.9% prevalence), and soldiers (16.4%–58% prevalence) 57. Several of these studies also found high rates of onychomycosis presenting with tinea pedis. Although tropical and subtropical climates have a higher overall prevalence of skin mycoses, tinea pedis and onychomycosis are rare in India and rural Africa 60.
Ringworm prevention
- Keep your skin clean and dry.
- Wear shoes that allow air to circulate freely around your feet.
- Don’t walk barefoot in areas like locker rooms or public showers.
- Clip your fingernails and toenails short and keep them clean.
- Change your socks and underwear at least once a day.
- Don’t share clothing, towels, sheets, or other personal items with someone who has ringworm.
- Wash your hands with soap and running water after playing with pets. If you suspect that your pet has ringworm, take it to see a veterinarian. If your pet has ringworm, follow the steps below to prevent spreading the infection.
- If you’re an athlete involved in close contact sports, shower immediately after your practice session or match, and keep all of your sports gear and uniform clean. Don’t share sports gear (helmet, etc.) with other players.
Ringworm signs and symptoms
Ringworm typically begins as a flat, discolored patch, which may appear red in lighter complexions and brown in darker complexions. The patch has a ring-like or circular shape with a raised, scaly border.
Ringworm has different names based on where it appears on your body and it can appear just about anywhere.
Symptoms of ringworm by location on the body:
- Ringworm on the feet (tinea pedis or “athlete’s foot”): The symptoms of ringworm on the feet include red, swollen, peeling, itchy skin between the toes (especially between the pinky toe and the one next to it). The sole and heel of the foot may also be affected. In severe cases, the skin on the feet can blister.
- Ringworm on the scalp (tinea capitis): Ringworm on the scalp usually looks like a scaly, itchy, red, circular bald spot. The bald spot can grow in size and multiple spots might develop if the infection spreads. Ringworm on the scalp is more common in children than it is in adults.
- Ringworm on the face (tinea faciei): Ringworm on the face does not include infection of the beard and moustache area, which is called tinea barbae. Tinea faciei resembles tinea corporis (ringworm). It may be acute (sudden onset and rapid spread) or chronic (slow extension of a mild, barely inflamed, rash). There are round or oval red scaly patches, often less red and scaly in the middle or healed in the middle. It is frequently aggravated by sun exposure. It may also present as a kerion (fungal abscess). Tinea faciei is uncommon and often misdiagnosed at first.
- Ringworm on the body (tinea corporis): Ringworm on the body (tinea corporis) is a superficial fungal infection of the skin that can affect any part of the body, excluding the hands and feet, scalp, face and beard, groin, and nails. Ringworm on the body (tinea corporis) is most commonly seen in children and young adults, however all age groups can be infected including newborns. Ringworm on the body (tinea corporis) initially presents as a solitary circular red patch with a raised scaly leading edge. A lesion spreads out from the centre forming a ring-shape with central hypopigmentation and a peripheral scaly red rim (ringworm). The border can be papular or pustular. Itch is common. With time, multiple lesions can develop which may coalesce to form a polycyclic pattern. The distribution of lesions is typically asymmetrical.
- Ringworm on the groin (tinea cruris or “jock itch”): Ringworm on the groin looks like scaly, itchy, red spots, usually on the inner sides of the skin folds of the thigh.
- Ringworm on the beard (tinea barbae): Symptoms of ringworm on the beard include scaly, itchy, red spots on the cheeks, chin, and upper neck. The spots might become crusted over or filled with pus, and the affected hair might fall out.
- Fungal nail infections also known as “onychomycosis”. Fungal nail infections may cause nails to become discolored, thick, fragile, or cracked. The nail may also become separated from the nail bed. Fungal toenail infections are more common than fungal fingernail infections 50.
Ringworm diagnosis
Ringworm may be difficult to distinguish from other skin conditions 61. Physical examination with the help of dermatoscopy and clinical history alone may not be enough to diagnose ringworm. Doctors often use a diagnostic test to confirm suspected ringworm, especially before prescribing antifungal treatment. Clinicians should generally confirm the diagnosis of scalp fungus (tinea capitis) using a laboratory test 55.
The presence of a dermatophyte infection is confirmed by:
- Microscopy and culture of skin scrapings
- Histopathological examination of skin or nail biopsy using periodic acid-Schiff (PAS) stains to reveal fungal elements
- Specific antigen tests using molecular biology techniques such as polymerase chain reaction (PCR).
Potassium hydroxide preparation
Doctors often use a potassium hydroxide (KOH) preparation of skin scrapings or nail clippings to confirm a diagnosis of ringworm. This test can provide rapid results, but the test’s accuracy depends on clinician experience and technique 62.
Fungal culture
Fungal culture can be used to diagnose ringworm. Fungal culture is more specific than potassium hydroxide (KOH) stain, but results may take several weeks 63, 64.
Note: Antifungal-resistant fungal nail infections (onychomycosis) is a growing problem 65, 66, 67 Therefore, antifungal susceptibility testing may be considered based on the fungus or fungi identified and the patient’s clinical course.12,16,17
Histopathologic examination with a periodic acid-Schiff stain
Histopathologic examination of nail clippings with a periodic acid-Schiff (PAS) stain is a method for confirming the diagnosis for patients with suspected onychomycosis, a fungal nail infection most often caused by dermatophytes.
Polymerase chain reaction (PCR)
Ultraviolet light (Wood’s lamp)
Ultraviolet light can be useful for diagnosing ringworm caused by Microsporum canis and Microsporum audouinii. Although both species fluoresce blue-green under a Wood’s lamp, these species are uncommon causes of ringworm infections in people.
Ringworm treatment
best medicine for ringworm
Ringworm on foot treatment
Tinea pedis or athlete’s foot can usually be treated with over-the-counter topical antifungal products. Chronic or extensive tinea pedis may require treatment with oral antifungal medications such as terbinafine, itraconazole, or fluconazole 68. In addition, chronic tinea pedis may require adjunctive therapy such as foot powder or talcum powder to prevent skin maceration.
Scalp fungus treatment
Scalp fungus or tinea capitis requires prescription-strength oral antifungal medication, as topical antifungal products are ineffective for treatment of tinea capitis 69. Many experts consider griseofulvin to be the drug of choice 68. Terbinafine is also FDA-approved for the treatment of tinea capitis in patients four years of age and older. Itraconazole (Spoanox, Tolsura) and fluconazole (Diflucan) have been shown to be safe and effective, but are not FDA-approved for this indication 68. You or your child might need to take one of these medications for six weeks or more until the hair regrows. Typically, with successful treatment, the bald spots will grow hair again and the skin will heal without scarring.
Your doctor might recommend that you or your child also wash you or your child’s hair with a prescription-strength medicated shampoo such as selenium sulfide shampoos 68, 70. The shampoo removes fungus spores and helps prevent spreading the infection to others or to other areas of your body.
There is no need to shave the head or cut the hair as part of the treatment.
Tinea corporis and tinea cruris treatment
Tinea corporis and tinea cruris can usually be treated with topical antifungal products 68. Patients who have tinea cruris should be advised to keep the groin area clean and dry and to wear cotton underwear. Patients who have extensive or recurrent infections may require prescription-strength oral antifungal medication 68.
Fungal nail treatment
Fungal nail infections can be difficult to cure, and treatment is most successful when started early. Fungal nail infections typically don’t go away on their own, and the best treatment is usually prescription antifungal pills taken by mouth. Oral antifungal therapy should be prescribed only after confirmation of fungal infection. Oral terbinafine is typically the first-line treatment for confirmed fungal nail infections (onychomycosis). The treatment course is generally 6 weeks for fingernails and 12 weeks for toenails 71. Given increasing reports of terbinafine resistance in fungal nail infections (onychomycosis), the possibility of resistance should be considered if patients do not improve with therapy. Identification and susceptibility testing of the causative fungus or fungi may help guide therapy. Note, however, that antimicrobial resistance is not the only possible reason for treatment failure 72. Clinicians should also consider the possibility of incorrect diagnosis or inadequate patient adherence to therapy.
Laser treatments for onychomycosis appear to be a promising area for future study 73.
In severe cases, your doctor might remove the affected nail completely. It can take several months to a year for the infection to go away.
Fungal nail infections can be closely associated with fungal skin infections. If a fungal infection is not treated, it can spread from one place to the other. You should discuss all skin concerns with their doctor to ensure that all fungal infections are properly treated.
Even after treatment, fungal nail infections can come back 74. This is more common in people who have conditions like diabetes that make them more likely to get a fungal nail infection. If you suspect an infection has returned, contact your healthcare provider.
Tinea versicolor
Tinea versicolor also known as Pityriasis versicolor, is a common long-term (chronic) fungal infection of the skin. Tinea versicolor is a superficial infection of the stratum corneum (the top layer of your skin) by the lipophilic fungus known as Malassezia furfur, formerly known as Pityrosporum, that interferes with the normal pigmentation of your skin, resulting in small, discolored patches. People with tinea versicolor develop white, yellow, red, pink or brown spots that can be mildly itchy. These patches may be lighter or darker in color than the surrounding skin and most commonly affect your shoulders, back and upper chest. The most common predisposing factor is excessive sweating but others include application of oils and systemic steroids. Hot weather, humidity and sun exposure can make tinea versicolor worse 75.
Tinea versicolor (pityriasis versicolor) is a skin reaction to overgrowth of normally occurring yeast organisms Malassezia furfur living on the skin 76. About 90% of healthy people have these yeast organisms growing on their skin but they grow in larger numbers with sweating and humidity and where the sebaceous (oil) glands in the skin are very active. Some people may have an inherited predisposition to overgrowth of these organisms. The Malassezia furfur yeast produces chemicals which may affect the normal pigment production in the skin.
Tinea versicolor is more common in warm humid environments and may be seasonal. Tinea versicolor affects about 1% of people who live in mild or moderate climates. Tinea versicolor affects up to 40% of people who live in tropical, humid climates.
Tinea versicolor occurs most frequently in teens and young adults. Sun exposure may make tinea versicolor more apparent.
Tinea versicolor is characterized by mildly scaly lighter (hypopigmented) or darker (hyperpigmented) round or oval shaped patches, most commonly affecting areas of skin that are rich in sebum production such as the trunk (especially the upper part), neck, shoulders and upper arms 77, 78, 79, 80, 81.
Facial involvement is less common in adults. On the other hand, facial involvement is common in children and may be the only site involved 77, 82. The forehead is the usual site of facial involvement 83, 84. Other sites of involvement, such as forearms and thighs, are less common 85. Unusual sites of involvement include the scalp, eyelid, armpit, areola, periareolar area, antecubital fossae(anterior surface of the elbow), popliteal fossa (behind the knee), pubis, groin, perineum, penile shaft and vulva 86, 85, 87, 88, 89, 90.
Most people with tinea versicolor or Pityriasis versicolor are concerned about the appearance of the rash. Sometimes it may cause minor symptoms such as scaling, itch or irritation. Tinea versicolor can be much more widespread if there is a problem with your immune system. This may happen if you take medications like corticosteroids or have medical conditions like diabetes. People who are pregnant are more susceptible to tinea versicolor because of hormonal changes.
Antifungal creams, lotions or shampoos can help treat tinea versicolor. But even after successful treatment, skin color may remain uneven for several weeks or months. Tinea versicolor often recurs, especially in warm, humid weather.
What does tinea versicolor look like?
The typical appearance of the rash is of a discolored round or oval shaped eruption most commonly affecting areas of skin that are rich in sebum production such as the upper trunk region, sometimes extending to the neck, shoulders and upper arms 77, 78, 79, 80, 81. Occasionally, the rash may affect other areas of your body. The color of the eruption may appear lighter (hypopigmented) or darker (hyperpigmented) than the surrounding normal skin, hence the name “versicolor”. It’s more common for your skin to get lighter. The rash itself may be slightly raised with a fine scale. The spots can appear white, pink, red, brown, light tan or yellow.
On darker skin, tinea versicolor appears white or light tan. On lighter or paler skin, tinea versicolor looks light red or pink.
Some patches or spots can become scaly and dry. Over time, the patches get larger and start to connect, covering larger areas of your skin.
The patches might be more noticeable after sun exposure because the rest of your skin tans (or gets darker) but the infected area won’t. This makes them stand out a little more.
Figure 13. Tinea versicolor on face
Figure 14. Tinea versicolor upper back
Is tinea versicolor contagious?
Tinea versicolor, which is also called pityriasis versicolor, is not painful or contagious. But it can lead to emotional distress or self-consciousness.
Tinea versicolor is caused by a type of yeast called Malassezia furfur. This yeast is found on the skin of more than 90% of adults, where it normally lives without causing any problems. But tinea versicolor can develop if this yeast starts to multiply more than usual. It’s not clear exactly why this happens in some people and not in others.
Who gets tinea versicolor?
Tinea versicolor affects many people worldwide. It’s unclear why the Malassezia furfur yeast overgrows on some people’s skin and not others. People living in tropical or subtropical regions are most at risk for tinea versicolor 91, 92, 93. Tinea versicolor occurs most frequently in teens and young adults presumably because of increased skin oil production 94, 95, 96, 97. It’s common during the summer months in temperate climates and around puberty when your skin’s oil glands are more active.
Tinea versicolor is slightly more common in men than in women presumably due to increased sebaceous (oil gland) activity in men 98, 99. A positive family history of tinea versicolor is present in approximately 17% of affected individuals 100, 101. The incidence of tinea versicolor appears to be the same in all races, though the change in skin pigmentation is more visually apparent in dark-skinned individuals 99.
You may be at higher risk if you have a weak immune system. This may happen if you take medications like corticosteroids or have medical conditions like diabetes. People who are pregnant are more susceptible to tinea versicolor because of hormonal changes.
What happens if tinea versicolor is left untreated?
Tinea versicolor tends to persist for years if left untreated 102, 103, 104. Tinea versicolor doesn’t typically cause any serious side effects. If left untreated, you may experience worsening symptoms like increased discoloration or itching.
How long does tinea versicolor take to go away?
Tinea versicolor is generally easy to treat. Your skin may stay lighter or darker for several weeks or months, but it should return to its usual color eventually. Mild cases of tinea versicolor respond well to over-the-counter treatments such as using a dandruff shampoo containing selenium. Apply the shampoo to your skin in the shower and let it sit for 10 minutes before rinsing. But some people need stronger medication from their doctor. Your doctor may recommend a topical antifungal medication such as:
- Ketoconazole (Nizoral® or Extina®).
- Ciclopirox (Loprox® or Penlac®).
If your symptoms are severe, your docto may also prescribe oral antifungals such as:
- Fluconazole (Diflucan®).
- Itraconazole (Onmel® or Sporanox®).
Tinea versicolor can return, especially in the summer months. Some people may need to use medication several times a year to manage skin discoloration.
Can tinea versicolor come back?
Tinea versicolor isn’t a harmful infection and only affects the top layer of your skin. People often have more than one episode of tinea versicolor. Because the yeast grows naturally on your skin, it can recur (come back). When the air outdoors is warm and humid, the yeast can quickly grow out of control. Using medicated soap a couple of times each week or month can reduce recurrences of tinea versicolor.
Tinea versicolor outlook (prognosis)
Tinea versicolor prognosis is good. Tinea versicolor is easy to treat. Mycological cure is usually achieved soon after antifungal treatment 105. Changes in skin color may last for months. The condition may come back during warm weather, especially in patients with a positive family history of tinea versicolor 106, 107. Framil et al. 107 followed 102 patients with clinical and laboratory diagnosis of tinea versicolor for one year. After appropriate treatment, 33 (33.35%) patients did not have any relapsing episodes, 54 (52.94%) patients had one to four relapsing episodes and 15 (14.7%) patients had more than four relapsing episodes. Patients with a positive family history of tinea versicolor also have a longer duration of the disease 108. Relapse rates as high as 80% following treatment have been reported 109.
Several factors can increase your risk of developing tinea versicolor, including:
- living or staying in a warm, moist environment, in the summer
- sweating excessively (hyperhidrosis)
- having naturally oily skin
- being a teenager or in your early 20s
Tinea versicolor isn’t related to poor hygiene. The condition can’t be spread from person to person because most people already have the Malassezia yeast on their skin.
Tinea versicolor causes
Tinea versicolor is caused by dimorphic lipophilic and lipid-dependent fungus in the genus Malassezia (formerly known as Pityrosporum) species, notably Malassezia furfur, Malassezia globosa and Malassezia sympodialis 105. Other Malassezia species that have been implicated include Malassezia restricta, Malassezia obtuse, Malassezia slooffiae, Malassezia pachydermatis and Malassezia japonica 110, 111, 112, 113. These fungi are normal inhabitants on the skin surface 91, 114. Skin colonization increases with age; 25% of children and almost 100% of adults are affected 77.
Tinea versicolor occurs when the saprophytic yeast or budding form of the organism converts to the pathogenic hyphal or mycelial form. The fungal infection is localized to the stratum corneum.
A number of factors may trigger this conversion, including 105 , 115, 78, 79, 116, 117, 118:
- Hot, humid weather
- Oily skin
- Application of oily lotion or cream to the skin
- Hormonal changes
- Weakened immune system
- Hyperhidrosis (excessive sweating)
- Wearing of masks
- Excessive lipid-containing sebaceous secretions
- Malnutrition
- Poor general health
- Use of oral contraceptives
- Pregnancy
- Diabetes mellitus
- Use of topical or systemic corticosteroids
- Cushing disease
- Helicobacter pylori infection
- Immunodeficiency
- Genetic predisposition .
A recent study showed oxidative stress has no role in the pathogenesis of tinea versicolor 116.
Hypopigmented lesions (more commonly noted in darker skin tones) seen in tinea versicolor are thought to result from damage to melanocytes (skin cells that produce the skin pigment melanin) and inhibition of tyrosinase by azelaic acid (a dicarboxylic acid) produced by the Malassezia species, small melanosomes and accumulation of lipid-like material in the stratum corneum blocking ultraviolet light 119, 120, 121, 122. On the other hand, hyperpigmented lesions (more commonly noted in lighter skin tones) may result from a hyperemic inflammatory response elicited by Malassezia species, more tonofilaments in the granulosum, a thicker stratum corneum and abnormally large melanosomes 99, 120, 123, 124. Keratinase, produced by the Malassezia fungi, causes loosening of the stratum corneum with subsequent scale formation 125, 126.
Tinea versicolor prevention
To help prevent tinea versicolor from returning, your doctor can prescribe a skin or oral treatment that you use once or twice a month. You may need to use these just during warm and humid months. Preventive treatments include 127:
- Selenium sulfide (Selsun) 2.5 percent lotion or shampoo
- Ketoconazole (Ketoconazole, Nizoral, others) cream, gel, shampoo
- Itraconazole (Onmel, Sporanox) tablets, capsules or oral solution
- Fluconazole (Diflucan) tablets or oral solution.
The Malassezia furfur yeast that causes tinea versicolor occurs naturally on your skin. Doctors aren’t sure why some people develop tinea versicolor and others don’t.
If you have a history of tinea versicolor, your doctor may recommend you use soap containing zinc pyrithione (like Vanicream™ Z-Bar or DermaZinc™ Zinc Therapy Soap), ketoconazole (Nizoral®) or selenium sulfide (Selsun Blue®). This type of soap may help prevent future infections and yeast overgrowth. Your doctor may also recommend using prescriptions medications during summer months when tinea versicolor is more likely to return.
Some other things you can do to lower your risk for repeat tinea versicolor infections are:
- Avoid excessive sweating, exposure to sunlight and heat.
- Wear sunscreen or avoid sun exposure.
- Wear loose-fitting, cotton clothing to reduce sweating.
Tinea versicolor symptoms
The main symptom of tinea versicolor is patches of discolored skin that:
- Have sharp borders (edges) and fine scales
- Tinea versicolor skin spots may be lighter (or darker) than your surrounding skin. The color of the spots can be white, pink, salmon, red, tan, or brown.
- Are often dark reddish to tan in color
- Are found on your back, underarms, upper arms, chest, and neck (can appear anywhere on the body).
- Do not darken in the sun so may appear lighter than the surrounding healthy skin. The yeast prevents the skin from tanning.
- Grow together, forming patches of lighter (or darker) skin.
- Grow slowly.
- Disappear when the temperature drops and return in the spring or summer when the air turns warm and humid.
Sometimes the spots are so faint that people do not realize they have tinea versicolor. If tinea versicolor causes light spots on the skin, it can be mistaken for vitiligo. Vitiligo is a skin disease that causes the skin to lose its natural color.
But there are many ways to tell the difference:
- Vitiligo often develops symmetrically (on both sides of your body at the same time), whereas tinea versicolor may not.
- Skin affected by vitiligo usually has a normal texture, whereas areas affected by tinea versicolor are usually slightly scaly or flaky.
- Vitiligo is more common around the mouth, eyes, fingers, wrists, armpits and groin, whereas tinea versicolor tends to develop on the chest, tummy, back and upper arms.
African Americans may have a loss of skin color or an increase in skin color.
Other symptoms include:
- Increased sweating
- Cause the affected skin to itch.
Tinea versicolor complications
For some people, tinea versicolor causes skin discoloration that lasts for months to years. In most cases, this discoloration fades away gradually after treatment is complete. A preliminary study showed that topical application of cycloserine, a transaminase 1 inhibitor, to the hyperpigmented lesions of tinea versicolor twice a day for 5 days resulted in complete clearing of the hyperpigmentation 128. Well-designed, large-scale, multicenter, randomized, placebo-controlled trials are needed to confirm this finding.
Hair thinning and/or hair loss within the tinea versicolor lesions has been reported 129. In a study of 39 patients with tinea versicolor, hair thinning and/or hair loss within the tinea versicolor lesions occurred in 24 (61.5%) patients 129. Hair thinning and/or hair loss occurred most commonly on the forearms, abdomen, neck and, in men, the beard area 129.
Tinea versicolor diagnosis
The diagnosis of tinea versicolor (pityriasis versicolor) is based on a clinical examination and the typical appearance of the eruption. Your doctor can diagnose tinea versicolor by looking at it. The affected skin may show yellowish fluorescence under long wave ultraviolet light (Wood’s lamp) examination. If there’s any doubt, he or she may take skin scrapings from the infected area and view them under a microscope. In rare cases, a skin biopsy taken under local anesthetic may be required for examination under a microscope.
Tinea versicolor treatment
Treatment may be required if the rash is causing concern. Treatment is directed towards reducing the number of yeast organisms living in the skin of the affected areas, using an anti-fungal preparation applied directly to the skin.
Treatment options for tinea versicolor 105:
- Topical antifungals
- Azoles (for example, ketoconazole, econazole, eberconazole, efinaconazole, bifonazole, luliconazole, clotrimazole, miconazole, sertaconazole, sulconazole, oxiconazole, fenticonazole, tioconazole, fluconazole and dapaconazole)
- Terbinafine
- Naftifine
- Butenafine
- Ciclopirox olamine
- Non-specific topical antifungal agents (for example, selenium sulfide, zinc pyrithione, propylene glycol, Whitfield ointment, sulfur plus salicylic acid and benzoyl peroxide)
- Oral antifungals
- Itraconazole
- Fluconazole
- Laser and photodynamic therapies
- Alternative therapies
Tinea versicolor treatment over the counter
Examples include imidazole lotions or creams (such as ketoconazole, clotrimazole, econazole, miconazole) and anti-dandruff shampoos (such as ketoconazole, zinc pyrithione, selenium sulphide, ciclopirox olamine). With regard to topical therapy, ketaconazole is the most studied and seemingly the most effective. Topical antifungal, e.g., clotrimazole cream daily twice daily for 10 days is an alternative.
For smaller areas, one of the imidazole creams or lotions may be suitable and may be applied once or twice daily for 1 to 4 weeks.
For larger areas, one of the above anti-dandruff shampoos may be used as a body wash. The shampoo is applied to the whole affected area (after wetting the skin) and left on for 10 minutes before washing off. This is to allow time for the active ingredient to penetrate into the skin and hair follicles to reduce the number of yeast organisms. The treatment is performed daily for 2 to 4 weeks and subsequent maintenance therapy once or twice a week may be required to prevent recurrence. The effectiveness is generally improved with a longer course of treatment and a higher concentration of the active ingredient. If skin irritation occurs, treatment should be stopped and medical advice obtained.
Tinea versicolor home remedies
For a mild case of tinea versicolor, you can apply an over-the-counter antifungal lotion, cream, ointment or shampoo. Most fungal infections respond well to these topical agents, which include:
- Clotrimazole (Lotrimin AF) cream or lotion
- Miconazole (Micaderm) cream
- Selenium sulfide (Selsun Blue) 1 percent lotion. Apply the shampoo to your skin in the shower and let it sit for 10 minutes before rinsing.
- Terbinafine (Lamisil AT) cream or gel
- Zinc pyrithione soap
When using creams, ointments or lotions, wash and dry the affected area. Then apply a thin layer of the product once or twice a day for at least two weeks. If you’re using shampoo, rinse it off after waiting five to 10 minutes. If you don’t see an improvement after four weeks, see your doctor. You may need a stronger medication.
It also helps to protect your skin from the sun and artificial sources of UV light. Usually, the skin tone evens out eventually.
Some people need stronger medicine, so they see a doctor. Whether you decide to self-treat or see a doctor, these tips can help you get better results:
- Stop using skin care products that are oily. Use products that are oil-free. The label may also read “non-comedogenic.”
- Wear loose clothes. Nothing should feel tight.
- Protect your skin from the sun. A tan makes tinea versicolor easier to see.
- Do not use a tanning bed or sun lamp. Again, a tan makes tinea versicolor easier to see.
How to protect your skin from the sun
To get the best results, you need to protect your skin from the sun. To do this, you should apply sunscreen every day. Be sure to apply the sunscreen 20 minutes before you go outside. And apply it to all skin that will not be covered by clothing. Make sure to use a sunscreen that offers:
- UVA and UVB protection (label may say broad-spectrum).
- Sun Protection Factor (SPF) of 30+ or higher.
- Non-greasy formula (label may read “oil-free” or “non-comedogenic”).
Tinea versicolor natural treatment
A wide variety of alternative medicines have been shown to have some therapeutic effects on tinea versicolor. However, none of these treatments have yet been subjected to rigorous studies nor randomized clinical trials 105.
Tinea versicolor natural treatments include:
- topical application of beeswax and honey 130,
- essential oils of Cymbopogon citratus 131,
- quince seed mucilage hydrogel decorated with essential oils of Nigella sativa, Citrus sinensis and Cinnamon verum 132,
- polyherbal Unani formulation 133,
- Pentas longiflora leaf extract 134,
- Acalypha wilkesiana leaf extract 135,
- Artemisia sieberi shrub extract 136,
- nitric oxide-liberating cream 137,
- irradiated human amniotic membrane in combination with tea tree oil 138.
Prescription-strength medication
If tinea versicolor is severe or doesn’t respond to over-the-counter antifungal medicine, you may need a prescription-strength medication. Some of these medications are topical preparations that you rub on your skin. Others are drugs that you swallow. Examples include:
- Ciclopirox (Loprox, Penlac) cream, gel or shampoo
- Fluconazole (Diflucan) tablets or oral solution. Fluconazole 300 mg once and repeat in one week.
- Itraconazole (Onmel, Sporanox) tablets, capsules or oral solution. Itraconazole 200 mg/day for 5-7 days.
- Pramiconazole 200 mg/day for 2 days.
- Ketoconazole (Ketoconazole, Nizoral, others) cream, gel or shampoo. Ketoconazole 2% shampoo, lather scalp and trunk for 10 minutes daily for 7 days.
- Selenium sulfide (Selsun) 2.5 percent lotion or shampoo
Oral anti-fungal treatment may be considered in unusually severe or resistant cases but there is a risk of side effects and drug interactions occurring with these oral medications. Your doctor will discuss these issues.
Side effects of these tablets are uncommon, although some people experience problems such as rashes, feeling sick and abdominal (tummy) pain while taking them.
With regard to oral anti fungal agents, itraconazole and fluconazole seem equally effective for the treatment of tinea versicolor. For simplicity, fluconazole 300 mg orally once and repeat in one week is recommended.
The discoloration of the skin may take several weeks, or even months to return to normal, even after successful treatment.
Also, the infection may return in hot, humid weather. In persistent cases, you may need to take a medication once or twice a month to prevent the infection from recurring.
Tinea versicolor recurrences
It’s common for tinea versicolor to come back after treatment, particularly during the summer or during holidays to warm and humid countries.
But you can reduce this likelihood by regularly using the antifungal shampoos mentioned above.
For example, continuing to use the shampoo once every 2 to 4 weeks after the initial treatment, or once a day for a few days before going on holiday, can help prevent tinea versicolor recurring.
As these shampoos are available to buy from pharmacies, you don’t need to see your doctor for a prescription if you run out.
If you develop tinea versicolor again after treatment, you can try treating it yourself with antifungal shampoo, or see your doctor for advice and alternative treatments.
If you have frequent and severe episodes of tinea versicolor, your doctor may consider prescribing antifungal tablets to take a few times a month to prevent the condition recurring.
Viral skin infections
Viral skin infections are common and some of the most common viral skin infections include varicella zoster (chickenpox), herpes zoster (shingles), molluscum contagiosum, roseola, Herpes simplex virus (HSV type 1 aka cold sores mainly affects the mouth and lip and HSV type 2 aka genital herpes mainly affects genital skin) and human papilloma virus (HPV) warts.
Molluscum contagiosum
Molluscum contagiosum is an infection caused by a Poxvirus (molluscum contagiosum virus) 139. Molluscum contagiosum is transmitted mainly by direct contact with infected skin 140. The result of the infection is usually a benign, mild skin disease characterized by umbilicated pink or skin-colored papules (growths) that may appear anywhere on the body 141. Within 6-12 months, Molluscum contagiosum typically resolves without scarring but may take as long as 4 years 142.
Children aged 2–5 years are most commonly affected with molluscum contagiosum, although it can occur in adolescents and adults 143. Molluscum contagiosum is rare in children aged younger than one year 142. In adolescents and adults, molluscum contagiosum could occur either as a sexually transmitted disease (STD) or in relation to contact sports 144. Molluscum contagiosum in adults is more common in immunosuppressed patients: in the 1980s, the number of reported cases of molluscum contagiosum increased, apparently associated with the onset of the acquired immune deficiency virus (HIV) epidemic 145. It is estimated that in human immunodeficiency virus (HIV) patients the prevalence is close to 20% 146. Besides HIV, molluscum contagiosum may be associated with medically induced immunosuppression or primary immunodeficiencies (eg, DOCK8 immunodeficiency syndrome) 147.
Molluscum contagiosum is more prevalent in warm climates than cool ones, and in overcrowded environments 148. In the United States, it is estimated that the prevalence of molluscum contagiosum in children is less than 5% 149.
Children with atopic eczema (atopic dermatitis) may be more severely affected by molluscum contagiosum due to the skin barrier breaks and immune cell dysfunction in atopic skin. In addition, these patients may be more likely to autoinoculate (excoriation of primary lesions and spread to areas of normal skin) new areas of skin because of the underlying itch (pruritus) from their atopy. However, studies on atopic dermatitis are controversial. Some studies have found an increased risk of molluscum contagiosum in patients with atopic dermatitis 150, 151, with prevalence rates of atopic dermatitis in patients with molluscum contagiosum of up to 62% 152, 153. It has even been hypothesized that an increased risk of molluscum contagiosum virus infection in patients with atopic dermatitis and filaggrin mutation 154. Other studies have shown no significant differences 155.
The lesions, known as Mollusca, are small, raised, and usually white, pink, or flesh-colored with a dimple or pit in the center. They often have a pearly appearance. They’re usually smooth and firm. In most people, the lesions range from about the size of a pinhead to as large as a pencil eraser (2 to 10 millimeters in diameter). They may become itchy, sore, red, and/or swollen.
Whenever you can see the bumps on the skin, molluscum contagiosum is contagious.
Most people get about 10 to 20 bumps on their skin. If a person has a weakened immune system, many bumps often appear.
Mollusca may occur anywhere on the body including the face, neck, arms, legs, abdomen, and genital area, alone or in groups. The lesions are rarely found on the palms of the hands or the soles of the feet.
Molluscum contagiosum can be very extensive and troublesome in patients with human immunodeficiency virus (HIV) infection or that have other reasons for poor immune function.
Patients with HIV/AIDS and other immunocompromising conditions (e.g., solid organ transplant recipients) can develop “giant” lesions (≥15 mm in diameter), larger numbers of lesions (100 or more molluscum contagiosum bumps) and lesions that are more resistant to standard therapy 156, 157, 158. The following diseases should be considered in the differential diagnosis of molluscum contagiosum: cryptococcosis, basal cell carcinoma, keratoacanthoma, histoplasmosis, coccidioidomycosis, and verruca vulgaris. For genital lesions, condyloma acuminata and vaginal syringomas should be considered.
Molluscum contagiosum lesions have recently come to be classified in one of three ways:
- The commonly seen skin lesions found largely on the faces, trunks, and limbs of children;
- The sexually transmitted lesions found on the abdomen, inner thighs, and genitals of sexually active adults; and
- The diffuse and recalcitrant eruptions of patients with AIDS or other immunosuppressive disorders.
Treatment of mild molluscum contagiosum infections is often not required because lesions will eventually resolve on their own. However, you can decrease the chance of spreading the infection to other parts of your body or to other people with the following guidelines:
- Do not scratch or shave the affected areas.
- Avoid sharing clothing, towels, and bedding with others.
- If the affected area is small, keep it covered.
The duration of molluscum contagiosum lesions is variable, but in most cases, they are self-limiting in a period of 6 to 9 months; however, some cases may persist for more than 3 or 4 years 159. It has been described a phenomenon called “beginning of the end” (BOTE) sign which refers to clinical erythema and swelling of an molluscum contagiosum skin lesion when the regression phase begins (Figure 3). This phenomenon is likely due to an immune response towards the molluscum contagiosum infection rather than a bacterial superinfection 140, 153, 160.
Figure 15. Molluscum contagiosum in children
Footnote: Each molluscum contagiosum starts out as a very small spot, about the size of a pinhead, and grows over several weeks into a larger bump that might become as large as a pea or pencil eraser. A tiny dimple (indentation) often develops on the top of each molluscum contagiosum.
Figure 16. Molluscum contagiosum papules
Footnote: Firm, rounded, skin-colored papules with a shiny and umbilicated surface.
What causes molluscum contagiosum?
Molluscum contagiosum is caused by molluscum contagiosum virus (MCV), a double-strand DNA virus which belongs to the Poxviridae family; humans are molluscum contagiosum virus only host 142. Molluscum contagiosum virus has 4 different genotypes: molluscum contagiosum virus 1 (MCV 1), molluscum contagiosum virus 2 (MCV 2), molluscum contagiosum virus 3 (MCV 3) and molluscum contagiosum virus 4 (MCV 4). Molluscum contagiosum virus 1 (MCV 1) is the most common genotype (75–96%), followed by molluscum contagiosum virus 2 (MCV 2), while molluscum contagiosum virus 3 (MCV 3) and molluscum contagiosum virus 4 (MCV 4) are extremely infrequent 140, 161, 162. A Slovenian study 161 showed that in children molluscum contagiosum virus 1 (MCV 1) infection is more frequent than in adults, and in adult women, molluscum contagiosum virus 2 (MCV 2) infection is more frequent than molluscum contagiosum virus 1 (MCV 1).
Molluscum contagiosum virus infects the epidermis and replicates in the cytoplasm of cells with a variable incubation period between two and six weeks 152. Different studies have been developed to sequence the genome of molluscum contagiosum virus (MCV) and determine possible genes involved in the evasion of the host immune response, a hypothesis that arose based on the absence of inflammation observed in histopathological samples of infected skin 163, 164. To date, four viral genes have been identified that code proteins that would alter the activation of the nuclear factor kB (NF-kB): MC159, MC160, MC132, and MC005 164, 165, 166, 167. Nuclear factor kB (NF-kB) is a nuclear protein complex present in dendritic cells that regulate the transcription of DNA and facilitate the synthesis of pro-inflammatory cytokines (TNF, IL-1, IL-6, among others) and activation of innate and acquired immune response 168. Brady et al 164 have seen that MC132 and MC005 proteins would alter the activation of NF-kB by inhibiting pattern recognition receptors (PRRs). Added to this, MC132 would bind and stimulate the degradation of the p65 subunit of NF-kB and MC005 would inhibit the activation of the IKK complex (IkB kinase) binding to active NEMO subunit (essential modulator of NF-kB).
Molluscum contagiosum virus is transmitted by direct contact with infected skin, which can be sexual, non-sexual, or by autoinoculation 142. Additionally, it can be transmitted by contaminated fomites like bath sponges or towels 140. It has been associated with the use of the swimming pool 152.
There are several ways the molluscum contagiosum virus can spread 169:
- Direct skin-to-skin contact
- Indirect contact via shared towels or other items
- Auto-inoculation into another site by scratching or shaving
- Sexual transmission in adults.
Transmission of molluscum contagiosum appears to be more likely in wet conditions, such as when children bathe or swim together. The incubation period is usually about 2 weeks but can be as long as 6 months.
How do people get molluscum contagiosum?
The virus that causes molluscum spreads from direct person-to-person physical contact and through contaminated fomites. Fomites are inanimate objects that can become contaminated with virus; in the instance of molluscum contagiosum this can include linens such as clothing and towels, bathing sponges, pool equipment, and toys. Although the virus might be spread by sharing swimming pools, baths, saunas, or other wet and warm environments, this has not been proven. Researchers who have investigated this idea think it is more likely the virus is spread by sharing towels and other items around a pool or sauna than through water.
Someone with molluscum contagiosum can spread it to other parts of their body by touching or scratching a lesion and then touching their body somewhere else. This is called autoinoculation. Shaving and electrolysis can also spread mollusca to other parts of the body.
Molluscum contagiosum can spread from one person to another by sexual contact 139. Many, but not all, cases of molluscum in adults are caused by sexual contact 139.
Conflicting reports make it unclear whether the disease may be spread by simple contact with seemingly intact lesions or if the breaking of a lesion and the subsequent transferring of core material is necessary to spread the virus.
Molluscum contagiosum lesions can also be congenital when transmitted vertically from mother to baby by contact with molluscum contagiosum virus in the birth canal 170, 171. In this case, molluscum contagiosum lesions are typically located on the scalp and have a circular arrangement 171. Other sites of atypical location, in addition to the oral mucosa 140, include the palms and soles, the areola/nipple 172, 173, the conjunctiva 174, lips 175, eyelids 176, among others 177. Clinical presentation of periocular lesions has been described as erythematous, nodular umbilicated, big/giant, conglomerated, inflamed, or pedunculated 178. The periocular presentation has also been associated with conjunctivitis 179.
The molluscum contagiosum virus remains in the top layer of skin (epidermis) and does not circulate throughout the body; therefore, it cannot spread through coughing or sneezing 139.
Since the virus lives only in the top layer of skin, once the lesions are gone the virus is gone and you cannot spread it to others 139. Molluscum contagiosum is not like herpes viruses, which can remain dormant (“sleeping”) in your body for long periods and then reappear.
Who is at risk for molluscum contagiosum infection?
Molluscum contagiosum is common enough that you should not be surprised if you see someone with it or if someone in your family becomes infected. Although not limited to children, it is most common in children 1 to 10 years of age.
People at increased risk for getting the disease include:
- People with weakened immune systems (i.e., HIV-infected persons or persons being treated for cancer) are at higher risk for getting molluscum contagiosum. Their growths may look different, be larger, and be more difficult to treat.
- Atopic dermatitis may also be a risk factor for getting molluscum contagiosum due to frequent breaks in the skin. People with this condition also may be more likely to spread molluscum contagiousm to other parts of their body for the same reason.
- People who live in warm, humid climates where living conditions are crowded.
In addition, there is evidence that molluscum contagiosum infections have been on the rise in the United States since 1966, but these infections are not routinely monitored because they are seldom serious and routinely disappear without treatment.
How can you prevent molluscum contagiosum from spreading?
The best way to avoid getting molluscum contagiosum is by following good hygiene habits. Remember that the virus lives only in the skin and once the lesions are gone, the virus is gone and you cannot spread the virus to others.
- Wash your hands. There are ways to prevent the spread of molluscum contagiosum. The best way is to follow good hygiene (cleanliness) habits. Keeping your hands clean is the best way to avoid molluscum infection, as well as many other infections. Hand washing removes germs that may have been picked up from other people or from surfaces that have germs on them.
- Don’t scratch or pick at molluscum contagiosum lesions. It is important not to touch, pick, or scratch skin that has lesions, that includes not only your own skin but anyone else’s. Picking and scratching can spread the virus to other parts of the body and makes it easier to spread the disease to other people too.
- Keep molluscum contagiosum lesions covered. It is important to keep the area with molluscum lesions clean and covered with clothing or a bandage so that others do not touch the lesions and become infected. Do remember to keep the affected skin clean and dry. Any time there is no risk of others coming into contact with your skin, such as at night when you sleep, uncover the lesions to help keep your skin healthy.
- Be careful during sports activities. Do not share towels, clothing, or other personal items. People with molluscum contagiosum should not take part in contact sports like wrestling, basketball, and football unless all lesions can be covered by clothing or bandages. Activities that use shared gear like helmets, baseball gloves and balls should also be avoided unless all lesions can be covered. Swimming should also be avoided unless all lesions can be covered by watertight bandages. Personal items such as towels, goggles, and swim suits should not be shared. Other items and equipment such as kick boards and water toys should be used only when all lesions are covered by clothing or watertight bandages.
- Swimming Pools. Parents and others often ask if molluscum virus can spread in swimming pools. There is also concern that it can spread by sharing swimming equipment, pool toys, or towels. Some investigations report that spread of molluscum contagiosum is increased in swimming pools. However, it has not been proved how or under what circumstances swimming pools might increase spread of the virus. Activities related to swimming might be the cause. For example, the virus might spread from one person to another if they share a towel or toys. More research is needed to understand if and for how long the molluscum virus can live in swimming pool water and if such water can infect swimmers. Open sores and breaks in the skin can become infected by many different germs. Therefore, people with open sores or breaks from any cause should not go into swimming pools. If a person with molluscum contagiosum is going swimming, they should:
- Cover all visible lesions with watertight bandages
- Dispose of all used bandages at home
- Not share towels, kick boards, other equipment, or toys.
- Day Care Centers and Schools. There is no reason to keep a child with molluscum infection home from day care or school. If you notice bumps on a child’s skin, it is reasonable to inform the child’s parents and to request a doctor’s note. Only a healthcare professional can diagnose molluscum contagiosum because there are many other causes of growths on the skin, both infectious and non-infectious. Lesions not covered by clothing should be covered with a watertight bandage. Change the bandage daily or when obviously soiled. If a child with lesions in the underwear/diaper area needs assistance going to the bathroom or needs diaper changes, then lesions in this area should be bandaged too if possible. Covering the lesions will protect other children and adults from getting molluscum contagiosum and will also keep the child from touching and scratching the lesions, which could spread the infection to other parts of his/her body or cause secondary (bacterial) infections. Children should be reminded to wash their hands frequently. If day care or school employees with regular physical contact with others are normally required to have skin examinations during pre-employment physicals, then infection molluscum contagiosum should be noted.
- Work. Although there has been only one reported case of healthcare provider-to-patient transmission of molluscum contagiosum, if an employee who comes in physical contact with clients regularly, such as an aesthetician or health care provider, is diagnosed with molluscum contagiosum by a health care professional, it would be reasonable to require that he/she cover visible lesions with a watertight dressing while at work. Otherwise, no special precautions are needed. Other precautions can prevent or reduce spread to uninfected people:
- Frequent and correct hand hygiene practices.
- Recommending that all visible lesions be covered by a watertight dressing, if molluscum contagiosum is diagnosed by a healthcare professional during a physical examination.
- Routinely disinfecting shared equipment (e.g., kick boards, wrestling mats). The molluscum contagiosum virus is not particularly difficult to kill and usual sanitation procedures should be sufficient.
- Other ways to avoid sharing your molluscum contagiosum infection.
- Do not shave or have electrolysis on areas with lesions.
- Don’t share personal items such as unwashed clothes, hair brushes, wrist watches, and bar soap with others.
- If you have lesions on or near the penis, vulva, vagina, or anus, avoid sexual activities until you see a health care provider.
Molluscum contagiosum signs and symptoms
Molluscum contagiosum presents as clusters of small round dome-shaped papules (bumps). The firm rounded papules range in size from 2 to 10 mm and may be white, skin-colored, pink, pearly or brown and occur in clusters and sometimes in a straight line. They often have a waxy, shiny look with a small central pit (this appearance is sometimes described as umbilicated). Each papule contains white cheesy material. The molluscum contagiosum papules (bumps) may be single, multiple or clustered, and occasionally they may have an erythematous halo or be pediculated. Pruritus (itch) may be present.
There may be few or hundreds of papules on one individual. They mostly arise in warm moist places, such as the armpit, behind the knees, groin or genital areas. They can arise on the lips or rarely inside the mouth. They do not occur on palms or soles.
The bumps are usually painless and may occasionally itch. When molluscum contagiosum is autoinoculated by scratching, the papules often form a row. Individual bumps may get bigger over the course of 6–12 weeks. Usually the bumps do not grow larger than 10 mm, but in patients with weak immune systems, they can be larger than a nickel.
Molluscum contagiosum frequently induces dermatitis around them and affected skin becomes pink, dry and itchy. As the papules resolve, they may become inflamed, crusted or scabby for a week or two.
Molluscum contagiosum infections may be:
- Mild – under 10 lesions
- Moderate – about 10–50 lesions
- Severe – over 50 lesions
Common areas for molluscum contagiosum lesions are the chest, abdomen, back, armpits, groin, or backs of the knees. Occasionally, they can be seen on the face and genital region. Because the incubation period for molluscum contagiosum is 2 weeks to 6 months, lesions may not immediately be seen after contracting the virus. Molluscum contagiosum infection is self-limited, and lesions will go away on their own in 6–9 months, although they rarely can persist for a few years. As the bumps begin to resolve, they may initially appear more inflamed, with pus and crusting of the lesions, before they eventually fade. They usually do not leave a scar.
In children, the main affected areas are sites of exposed skin, such as the trunk, extremities, intertriginous regions, genitals, and face, except the palms and soles 180. The involvement of the oral mucosa is rare 181. In adults, lesions are most frequently located in the lower abdomen, thighs, genitals, and perianal area, most of the cases transmitted by sexual contact 142. In children, genital lesions are mainly due to autoinoculation and are not pathognomonic of sexual abuse 182.
Molluscum contagiosum complications
Complications of Molluscum contagiosum can include:
- Secondary bacterial infection from scratching (impetigo)
- Conjunctivitis when the eyelid is infected
- Disseminated secondary eczema; this represents an immunological reaction or ‘id’ to the virus
- Numerous and widespread molluscum contagiosum that are larger than usual may occur in immune-deficient patients (such as uncontrolled HIV infection or in patients on immune
- suppressing drugs), and often affect the face
- Spontaneous, pitted scarring
- Scarring due to surgical treatment
Molluscum contagiosum diagnosis
Your doctor will most likely be able to diagnose molluscum contagiosum by its appearance. Molluscum is usually recognized by its characteristic clinical appearance or on dermatoscopy. White molluscum bodies can often be expressed from the center of the papules.
Very rarely, a biopsy is required. Histopathology shows characteristic intracytoplasmic inclusion bodies.
Molluscum contagiosum treatment
Molluscum contagiosum viral skin infection will resolve on its own within a few months. Talk to your child’s doctor about whether he or she recommends treatment or watchful waiting. No treatment is 100% effective since scientists are currently unable to kill the virus, and most can have side effects such as pain or irritation of the skin.
There is a consensus among experts that molluscum contagiosum treatment should be indicated in patients with extensive disease, secondary complications (bacterial superinfection, molluscum dermatitis, conjunctivitis), or aesthetic complaints 140.
Treatment for molluscum is usually recommended if lesions are in the genital area (on or near the penis, vulva, vagina, or anus) 183. If lesions are found in this area it is a good idea to visit your healthcare provider as there is a possibility that you may have another disease spread by sexual contact.
A retrospective study 184 evaluated the resolution rate of molluscum contagiosum lesions in treated and untreated patients, showing a resolution at 12 months of 45.6% in the treated group and 48.8% in the untreated group. At 18 months, they found a resolution rate of 69.5% and 72.6% in the treated versus the untreated group, respectively. From this cardinal study 184, it appears that active treatment does not improve the resolution rate when compared to observation alone.
- Be aware that some treatments available through the internet may not be effective and may even be harmful.
Because molluscum contagiosum is self-limited in healthy individuals, treatment may be unnecessary. Nonetheless, issues such as lesion visibility, underlying atopic disease, and the desire to prevent transmission may prompt therapy.
Active treatments can be classified as phyiscal removal, chemical, immunomodulatory, and antiviral 140.
Possible treatments include the following:
- Cantharidin 0.7% or 0.9% liquid – This is an extract from the blister beetle. It is applied to the lesions and then washed off in 2–6 hours. It is not for use on the face or genitals.
- Removal with freezing (cryosurgery), scraping (curettage), or burning (electrocautery) – All of these options may be painful.
- Salicylic acid
- Podofilox (Condylox®)
- Tretinoin (Retin-A®)
- Trichloroacetic acid
- Silver nitrate paste
- Imiquimod cream (Aldara®) – This may be useful for widespread, difficult-to-treat lesions.
Physical removal
Physical removal of lesions may include cryotherapy (freezing the lesion with liquid nitrogen), curettage (the piercing of the core and scraping of caseous or cheesy material), and laser therapy. These options are rapid and require a trained health care provider, may require local anesthesia, and can result in post-procedural pain, irritation, and scarring.
- It is not a good idea to try and remove lesions or the fluid inside of lesions yourself. By removing lesions or lesion fluid by yourself you may unintentionally autoinoculate other parts of your body or risk spreading it to others. By scratching or scraping your skin you could cause a bacterial infection.
Cryotherapy is an effective treatment. It can be applied with a cotton-tipped swab or by portable sprayers, 1 or 2 cycles of 10 to 20 seconds are typically used 141. A prospective, randomized and comparative trial 185 evaluated the efficacy of cryotherapy in molluscum contagiosum treatment. The study demonstrated a complete clearance in 70.7% of the patients at 3 weeks and in 100% of them at 16 weeks 185. Another study 186 showed full clearance in 83.3% of 60 patients (average age of 20 years) at 6 weeks. In both, the application of cryotherapy was administered weekly. The disadvantages of cryotherapy are the possibility of blistering, scarring, and post-inflammatory hypo or hyperpigmentation 141.
Curettage is also an effective method and involves the physical removal of skin lesions 141. One study showed that of 1,879 children, 70% were cured with one session while 26% needed two sessions, with overall satisfaction of 97% in both parents and children 187. A randomized, controlled trial 188 showed a complete clearance with only one curettage session in 80.3% of the patients and without recurrences at 6 months of follow-up. It can be done with a curette, punch biopsy or with an ear speculum 189, 190. To reduce pain, topical application of EMLA (eutectic mixture of local anesthetics), a combination of 2.5% lidocaine and 2.5% prilocaine, may be required 1 hour before the procedure 187, 191. Curettage can cause pain, bleeding, and scarring.
After curettage, topical povidone iodine can be applied. This is an antiseptic useful in the treatment of molluscum contagiosum based on case reports 192. A Cochrane systematic review of 2017 showed that povidone-iodine 10% potentiates the effect of 50% salicylic acid, without adverse effects reported 159. For topical povidone iodine, an application scheme of 3 times a day until the resolution of the cutaneous lesions.
Another useful mechanical method is pulse dye laser therapy, which due to its costs and limited availability is suggested to be left for refractory cases 193, 140. It is an effective, safe, and well-tolerated treatment, with infrequent adverse effects 193. Cases of successful use in immunosuppressed patients have also been reported 194.
Oral therapy
Gradual removal of lesions may be achieved by oral therapy. This technique is often desirable for children because it is generally less painful and may be performed by parents at home in a less threatening environment. Oral cimetidine has been used as an alternative treatment for small children who are either afraid of the pain associated with cryotherapy, curettage, and laser therapy or because the possibility of scarring is to be avoided. Oral cimetidine is an H2 (histamine-2) receptor antagonist that would stimulate the delayed hypersensitivity response 141.
While cimetidine is safe, painless, and well tolerated and the recommended dose is 25–40 mg/kg/day, facial molluscum contagiosum do not respond as well as lesions elsewhere on the body 140.
Topical therapy
Topical therapy destroy skin lesions through the inflammatory response they produce. Podophyllotoxin cream (0.5%) is reliable as a home therapy for men but is not recommended for pregnant women because of presumed toxicity to the fetus. Each lesion must be treated individually as the therapeutic effect is localized. Other options for topical therapy include iodine and trichloroacetic acid, salicylic acid, lactic acid, glycolic acid, benzoyl peroxide, potassium hydroxide (KOH), tretinoin, cantharidin (a blistering agent usually applied in an office setting), and imiquimod (T cell modifier) 140, 141, 192. These treatments must be prescribed by a health care professional.
Cantharidin is a topical agent, an inhibitor of phosphodiesterase, which produces an intraepidermal blister, followed by resolution of the lesion and healing without a scar in some cases 195. The efficacy of cantharidin in the treatment of molluscum contagiosum is variable, with cure rates varying between 15.4% and 100% among the different studies 196, 197. It is recommended to apply cantharidin 0.7–0.9% at the site of the lesion, with or without occlusion, and to wash with soap and water 2–4 hours later, each every 2–4 weeks until the resolution of lesions 141, 188, 195. In the face and anogenital region it should be used with precaution due to the risk of bacterial superinfection of blisters that form after 24–48 hours 141, 198.
Potassium hydroxide (KOH) is an alkaline compound that dissolves keratin 141. Potassium hydroxide (KOH) has been used in concentrations and therapeutic schemes that vary from 5% to 20%, two times a day or every other day for 1 week or until inflammation develops 199. A recent study 200 showed that 10% and 15% potassium hydroxide clear lesions of molluscum contagiosum entirely in 58.8% and 64.3% of the patients, respectively. It would be a safe and effective treatment that could be applied by patients, and its effectiveness has been compared with cryotherapy and imiquimod without significant differences 199, 201.
Immunomodulatory methods
Immunomodulatory methods stimulate the patient’s immune response against the infection. Imiquimod is an immune-stimulatory agent agonist of the toll-like receptor 7 that activates the innate and acquired immune response 141. Imiquimod is a useful alternative in the treatment of molluscum contagiosum based on case reports and uncontrolled studies 160, 202. However, imiquimod has not been proven effective for the treatment of molluscum contagiosum in children and is not recommended for children due to possible adverse events 203.
A prospective, randomized trial 185 compared the efficacy of cryotherapy with 5% imiquimod, demonstrating a complete clearance in 100% of the patients at 16 weeks for cryotherapy versus 92% for imiquimod 5% (difference not statistically significant). Cutaneous adverse effects were more frequent in the cryotherapy group. However, a recent Cochrane systematic review showed that it is not better than placebo in short-term improvement (3 months) or long-term cure (more than 6 months) and may produce adverse effects at the application site such as pain, blistering, scars and/or pigmentary changes 159. The current evidence positions imiquimod as a controversial therapeutic alternative 204.
Other immunomodulatory methods are oral cimetidine, interferon alfa, candidin, and diphencyprone.
Candidin is intralesional immunotherapy derived from the purified extract of Candida albicans. It is an alternative in the treatment of molluscum contagiosum, being applied purely or diluted at 50% with lidocaine in a dose of 0.2–0.3 mL intralesional every 3 weeks 141. A retrospective study evaluating the efficacy of candidin in the treatment of molluscum contagiosum showed a complete resolution rate of 55% and a partial resolution of 37.9%, with an overall response rate of 93% 205.
Diphencyprone is a topical immunomodulator used in multiple skin diseases. Cases of successful treatment with diphencyprone have been reported in immunosuppressed and immunocompetent patients 206, 207.
Therapy for immunocompromised persons
Most therapies are effective in immunocompetent patients; however, patients with HIV and AIDS or other immunosuppressing conditions often do not respond to traditional treatments. In addition, these treatments are largely ineffective in achieving long-term control in HIV patients.
Low CD4 cell counts have been linked to widespread facial mollusca and therefore have become a marker for severe HIV disease. Thus far, therapies targeted at boosting the immune system have proven the most effective therapy for molluscum contagiosum in immunocompromised persons. In extreme cases, intralesional interferon alfa has been used to treat facial lesions in these patients. Interferon alfa is a proinflammatory cytokine that is used in the treatment of molluscum contagiosum in immunosuppressed patients with severe or refractory disease. It can be administered subcutaneously or intralesionally 208, 209, 210. However, the severe and unpleasant side effects of interferon, such as influenza-like symptoms, site tenderness, depression, and lethargy, make it a less-than-desirable treatment. Furthermore, interferon therapy proved most effective in otherwise healthy persons.
Another method used in immunosuppressed patients with extensive or refractory disease is cidofovir, an antiviral drug initially used in cytomegalovirus retinitis in HIV patients. It can be used topically at a concentration of 1–3% or intravenously 211, 212, 213. The major problem with intravenous administration is nephrotoxicity 214.
Radiation therapy is also of little benefit.
New therapies
New molluscum contagiosum treatments include topical sinecatechins 215, intralesional 5-fluorouracil (5 FU) 216, hyperthermia 217 and zoster immune globulin (ZIG) 218. Evidence is preliminary to determine the effectiveness of these therapies.
Chickenpox
Chickenpox also known as varicella is a highly contagious viral infection caused by the primary infection (first time infection) with the varicella-zoster virus (VZV) of the herpes family 219. The varicella-zoster virus (VZV) is sometimes called herpesvirus type 3. Most chickenpox cases are in children under age 10, but older children and adults of all races and sex can get it. Adult with chickenpox are more likely to have life-threatening complications than children with chickenpox, such as thrombocytopenia (low blood platelets), viral pneumonia, disseminated primary varicella infection, Reye syndrome, Guillain-Barré syndrome and encephalitis, especially in adults with weakened immune system 220. Careful follow-up is required when an adult is diagnosed with adult chickenpox 221.
Chickenpox is highly contagious and the varicella-zoster virus (VZV) spreads very easily from person to person by breathing in airborne respiratory droplets from an infected person’s coughing or sneezing or through direct contact with the fluid from the open sores or fluid from shingles lesions. Chickenpox is acquired through contact with respiratory droplets or fluid from shingles lesions. A person who is not immune to the varicella-zoster virus (VZV) has a 70–90% chance of being infected with the virus if exposed to someone in the early stages of the disease. Once a person has had the chickenpox infection, it is unlikely he or she will get it again, as it confers lifelong immunity.
People develop symptoms about 7-21 days after being exposed to chickenpox. The classic symptom of chickenpox is an uncomfortable, itchy rash. The rash turns into fluid-filled blisters (vesicles) and eventually into scabs. It usually shows up on the face, chest, and back and then spreads to the rest of the body. Other symptoms include:
- Fever
- Headache
- Tiredness
- Loss of appetite
Sometimes children who have been vaccinated develop chickenpox. In these children, the rash is typically milder, fever is less common, and the illness is shorter. However, contact with the sores can spread the infection.
When exposed to the rash, a person can develop the disease in 14-21 days. Generally, the symptoms that are seen are an itchy rash with vesicular (fluid filled) lesions. Prior to chickenpox vaccine, the average was 250-500 lesions in varying stages of development during the course of illness. The lesions go though papules (flattened lesions) to vesicles (fluid filled), and then to resolution (crusting). Generally, these are seen with a low grade fever. The lesions put the person at higher risk for local infection to the affected areas on their skin. People can develop more symptoms that affect the whole body and can cause major illness. Prior to widespread vaccinating, each year, chickenpox caused about 4 million cases, about 10,600 hospitalizations and 100 to 150 deaths in the US from varicella. During the first 25 years of the U.S. chickenpox vaccination program, the vaccine has prevented an estimated 91 million cases, 238,000 hospitalizations, and 2,000 deaths.
In otherwise healthy children, chickenpox typically needs no medical treatment. Chickenpox in children is usually mild and lasts 5 to 10 days. Calamine lotions and oatmeal baths can help with itching. Your doctor may prescribe an antihistamine to relieve itching. Acetaminophen can treat the fever. Do not use aspirin for chickenpox; that combination can cause Reye syndrome.
Chickenpox can sometimes cause serious problems. Adults, babies, teenagers, pregnant women, and those with weak immune systems tend to get sicker from it. For people who are at high risk of complications from chickenpox, doctors sometimes prescribe antiviral medicines to shorten the length of the infection and to help reduce the risk of complications.
If you or your child are at high risk of complications, your doctor may suggest an antiviral drug such as acyclovir (Zovirax, Sitavig). This medication might lessen the severity of chickenpox when given within 24 hours after the rash first appears. Other antiviral drugs, such as valacyclovir (Valtrex) and famciclovir, also may lessen the severity of the disease, but might not be approved or appropriate for everyone.
In some instances, your doctor may recommend getting the chickenpox vaccine within three to five days after you’ve been exposed to the virus. This can prevent the disease or lessen its severity.
If complications develop, your doctor will determine the appropriate treatment. He or she may prescribe antibiotics for skin infections and pneumonia. Brain inflammation (encephalitis) is usually treated with antiviral drugs. You may need to be hospitalized.
Once you catch chickenpox, the virus usually stays in your body. You probably will not get chickenpox again, but the varicella-zoster virus (VZV) can cause shingles (herpes zoster) in adults. A chickenpox vaccine can help prevent most cases of chickenpox, or make it less severe if you do get it.
Figure 17. Chickenpox rash (varicella rash) on a child
Chicken pox incubation period
The incubation period for varicella-zoster virus (VZV) is typically between 10 to 21 days. Patients with chickenpox are considered contagious from 1 to 2 days before developing the rash until all skin lesions have crusted over. This may take 5–10 days. Varicella infection in pregnant women can spread via the placenta and infect the fetus 222, 223.
Chickenpox contagious period
A person with chickenpox is contagious 1–2 days before the rash appears and until all the blisters have formed scabs. This may take 5–10 days. Children should stay away from school or childcare facilities throughout this contagious period. Adults with chickenpox who work among children should also remain home.
It can take 10–21 days after contact with an infected person for someone to develop chickenpox. This is how long it takes for the virus to replicate and come out in the characteristic rash in the new host.
As chickenpox may cause complications in immunocompromised individuals and pregnant women, these people should avoid visiting friends or family when there is a known case of chickenpox. In cases of inadvertent contact, see your doctor who may prescribe special preventive treatment.
How can I find out if I am infected with varicella?
Your doctor can do a blood test to find out if you have a current infection with chickenpox. They can also test to see if you have ever had chickenpox in the past. When a person has chickenpox, they make antibodies to the virus. These antibodies usually last a long time and keep a person from getting chickenpox again. (The person becomes immune.) People who are immune are not likely to develop chickenpox if they are exposed to the virus again.
The most sensitive method for confirming a diagnosis of varicella is the use of polymerase chain reaction (PCR) to detect varicella-zoster virus (VZV) in skin lesions (vesicles, scabs, maculopapular lesions). Vesicular lesions or scabs, if present, are the best for sampling. Adequate collection of specimens from maculopapular lesions in vaccinated people can be challenging. However, one study comparing a variety of specimens from the same patients vaccinated with one dose suggests that maculopapular lesions collected with proper technique can be highly reliable specimen types for detecting varicella-zoster virus (VZV). Other sources such as nasopharyngeal secretions, saliva, urine, bronchial washings, and cerebrospinal fluid are less likely to provide an adequate sample and can often lead to false negative results.
Other viral isolation techniques for confirming varicella are direct fluorescent antibody assay (DFA) and viral culture. However, these techniques are generally not recommended because they are less sensitive than PCR and, in the case of viral culture, will take longer to generate results.
IgM serologic testing is considerably less sensitive than PCR testing of skin lesions. IgM serology can provide evidence for a recent active varicella-zoster virus (VZV) infection, but cannot discriminate between a primary infection and reinfection or reactivation from latency since specific IgM antibodies are transiently produced on each exposure to varicella-zoster virus (VZV). IgM tests are also inherently prone to poor specificity.
Paired acute and convalescent sera showing a four-fold rise in IgG antibodies have excellent specificity for varicella, but are not as sensitive as PCR of skin lesions for diagnosing varicella. People with prior history of vaccination or disease history may have very high baseline titers, and may not achieve a four-fold increase in the convalescent sera. The usefulness of this method for diagnosing varicella is further limited as it requires two office visits. A single positive IgG ELISA result cannot be used to confirm a varicella case.
If you have recently been around someone with chickenpox and you do not have immunity, talk to your doctor about what steps you can take to avoid getting chickenpox, or to reduce the severity of the symptoms.
Chickenpox in pregnancy
Non-immune pregnant women should take care to avoid contact with people who have chickenpox and to wash their hands frequently when handling food, animals, and children. Exposure to the varicella-zoster virus (VZV) in pregnancy (chickenpox in the first half of pregnancies) may cause viral pneumonia, premature labor and preterm delivery (delivery before 37 weeks of pregnancy) and rarely maternal death. About 2% of babies (1 in 50) of a pregnant person with chickenpox will have 1 or more birth defects due to the infection 224. 1% (1/100) of pregnancies that are infected with chickenpox in the first trimester have birth defects related to chickenpox (and 99% do not). When chickenpox occurs between 13 and 20 weeks of pregnancy, the chance for birth defects related to the chickenpox infection is to 2% (98% of pregnancies do not have related birth defects) 224. The chance for birth defects is greatest when chickenpox develops between 7 and 20 weeks of pregnancy 224.
Babies with birth defects from exposure to chickenpox in pregnancy are said to have congenital varicella syndrome. The birth defects include scars on the skin, eye problems (fetal chorioretinitis & cataracts), poor growth, underdevelopment of an arm or leg (limb atrophy), small head size (microcephaly), or delayed development and/or intellectual disability. Some babies may have only one of these problems while others have some or all. However, most babies born to women who have chickenpox in pregnancy are healthy 224.
If a mother develops chickenpox just before delivery or during the 28 days after delivery, her baby is at risk of severe infection. Chickenpox infection in this time period is called neonatal varicella. For infants with neonatal varicella infection, if untreated, the mortality rate is as high as 31%. Death typically occurs from varicella pneumonia. The mortality rate decreases to 7% when varicella-zoster immune globulin (VariZIG) is administered 222, 225.
Approximately 25% of fetuses of mothers with chickenpox become infected. It is harmless to most of them. Offspring may remain asymptomatic, or develop herpes zoster (shingles) at a young age without a previous history of primary chickenpox infection. They may also develop congenital varicella syndrome, one of the TORCH infections.
Congenital varicella syndrome
Congenital varicella syndrome occurs in up to 2% of fetuses exposed to varicella-zoster virus (VZV) in the first 20 weeks of gestation. It can result in spontaneous abortion, fetal chorioretinitis, cataracts, limb atrophy (underdevelopment of an arm or leg), cerebral cortical atrophy and microcephaly (small head size), skin scars, and delayed development and/or intellectual disability. The overall prognosis of infants born with congenital varicella syndrome is poor, often fatal in roughly 30% of infected babies within the first month of life 226. Death in infancy often results from intractable gastrointestinal reflux, severe recurrent aspiration pneumonia, and respiratory failure 223, 227.
What is the cause of chickenpox?
Chickenpox is caused by primary infection with the varicella-zoster virus (VZV), of the Herpesviridae family. The varicella-zoster virus (VZV) is sometimes called herpesvirus type 3. Humans are the only source of this disease. Chickenpox is highly contagious and the varicella-zoster virus (VZV) spreads very easily from person to person by breathing in airborne respiratory droplets from an infected person’s coughing or sneezing or through direct contact with the fluid from the open sores or fluid from shingles lesions. Chickenpox is acquired through contact with respiratory droplets or fluid from shingles lesions. A person who is not immune to the varicella-zoster virus (VZV) has a 70–80% chance of being infected with the virus if exposed to someone in the early stages of the disease.
The varicella-zoster virus (VZV) is a human alphaherpesvirus with worldwide distribution and is highly contagious 228. The varicella-zoster virus (VZV) is responsible for chickenpox (primary varicella infection) and herpes zoster or shingles infection (which is a reactivation of latent varicella infection).
Initially, viral replication occurs in the respiratory tract. The varicella-zoster virus (VZV) then invades local lymph nodes, leading to viremia and widespread dissemination. As the viremia increases, new skin vesicular lesions erupt, with individuals typically having 250 to 500 lesions in varying evolution stages 226.
The incubation period for varicella-zoster virus (VZV) is typically between 10 to 21 days. Individuals with primary varicella infection (chickenpox) may also experience a prodrome of fever, malaise, abdominal pain, and headaches. Patients with chickenpox are considered contagious from 1 to 2 days before developing the rash until all skin lesions have crusted over. Varicella infection in pregnant women can spread via the placenta and infect the fetus 222, 223.
After chickenpox (primary varicella infection), the varicella-zoster virus (VZV) becomes latent in the dorsal root ganglia (a collection of neuronal cell bodies of sensory neurons) 229. It can later reactivate as herpes zoster or shingles (reactivation of latent varicella infection). The specific mechanisms of varicella-zoster virus (VZV) reactivation are not fully understood 230. Scientists theorized that when the varicella-zoster virus (VZV)-specific immunity decreases, the latent virus in the ganglions reactivates, replicates, and then various herpes zoster (shingles) symptoms appear 231. Many factors affecting cellular immunity may cause the reactivation of the varicella-zoster virus (VZV) 232. These risk factors for varicella-zoster virus (VZV) reactivation include old age, cellular immunodeficiency, genetic susceptibility, trauma, systemic diseases (such as diabetes, kidney disease, fever, hypertension, etc.), mental stress and fatigue 233, 234, 235.
The clinical manifestation of herpes zoster (shingles) includes a painful vesicular rash in a dermatomal distribution, followed by postherpetic neuralgia. Typically, a single dermatome is involved, although two or three adjacent dermatomes may be affected 236. The lesions usually do not cross the midline 236. Direct contact with the vesicles may be contagious, especially in non-immune or immunocompromised hosts. Herpes zoster (shingles) does not pose a risk to a developing fetus due to circulating protective maternal antibodies transmitted through placental transfer 227.
Chickenpox signs and symptoms
In children, chickenpox usually begins as itchy red papules progressing to vesicles (fluid filled blisters) on the stomach, back and face, and then spreading to other parts of the body. Blisters can also arise inside the mouth. The itchy blister rash caused by chickenpox infection appears 7 to 21 days after exposure to the virus and usually lasts about five to 10 days.
Once the chickenpox rash appears, it goes through three phases:
- Raised pink or red bumps (papules), which break out over several days. About 24 to 36 hours after the first symptoms begin, a rash of small, flat, red spots appears. The spots usually begin on the trunk and face, later appearing on the arms and legs. Some people have only a few spots. Others have them almost everywhere, including on the scalp and inside the mouth.
- Small fluid-filled blisters (vesicles), which form in about one day and then break and leak. Within 6 to 8 hours, each spot becomes raised. It forms an itchy, round, fluid-filled blister against a red background and finally crusts. Spots continue to develop and crust for several days. A hallmark of chickenpox is that the rash develops in crops so that the spots are in various forms of development at any affected area. Very rarely, the spots become infected by bacteria, which can cause a severe skin infection (cellulitis or necrotizing fasciitis).
- Crusts and scabs, which cover the broken blisters and take several more days to heal. New spots usually stop appearing by the fifth day, the majority are crusted by the sixth day, and most disappear in fewer than 20 days.
New bumps continue to appear for several days, so you may have all three stages of the rash — bumps, blisters and scabbed lesions — at the same time. You can spread the virus to other people for up to 48 hours before the rash appears, and the virus remains contagious until all broken blisters have crusted over.
The rash spread pattern can vary from child to child. There may be only a scattering of vesicles, or the entire body may be covered with up to 500 vesicles. In severe cases, the rash can cover the entire body, and lesions may form in the throat, eyes, and mucous membranes of the urethra, anus and vagina. The vesicles tend to be very itchy and uncomfortable.
Some children may also experience additional symptoms such as high fever, headache, cold-like symptoms, loss of appetite, vomiting and diarrhea and tiredness and a general feeling of being unwell (malaise). Younger children often do not have these symptoms, but symptoms are often severe in adults.
Spots in the mouth quickly rupture and form raw sores (ulcers), which often make swallowing painful. Raw sores may also occur on the eyelids and in the upper airways, rectum, and vagina. The worst part of the illness usually lasts 4 to 7 days.
Chickenpox symptoms in adults
Chickenpox is usually more severe in adults and can be life-threatening in complicated cases. Most adults who get chickenpox experience prodromal symptoms for up to 48 hours before breaking out in the rash. These include fever, malaise, headache, loss of appetite and abdominal pain. Chickenpox is usually more severe in adults and can be life-threatening in complicated cases.
The blisters clear up within one to three weeks but may leave a few scars. These are most often depressed (anetoderma), but they may be thickened (hypertrophic scars). Scarring is prominent when the lesions get infected with bacteria.
Complications of chickenpox
In healthy children, chickenpox infection is usually an uncomplicated, self-limiting disease. Risk of complications from chickenpox is increased for newborns, adults, and people who have a weakened immune system or certain disorders.
Pregnant women who get varicella are at risk of serious complications, such as pneumonia, and may die as a result. Chickenpox can also be transmitted to the fetus, especially if chickenpox develops during the 1st or early 2nd trimester. Such an infection can result in scars on the skin, birth defects, and a low birth weight.
Chickenpox complications may include:
- Secondary bacterial infection of skin lesions caused by scratching
- Infection may lead to abscess, cellulitis, necrotizing fasciitis and gangrene
- Dehydration from vomiting and diarrhea
- Worsening of asthma
- Viral pneumonia. Lung infection (pneumonia) occurs in about 1 out of 400 adults, resulting in cough and difficulty breathing. Pneumonia rarely develops in young children who have a normal immune system.
- Chickenpox lesions may heal with scarring.
Some complications are more commonly seen in immunocompromised and adult patients with chickenpox:
- Disseminated primary varicella infection; this carries high morbidity
- Central nervous system complications such as Reye syndrome, Guillain-Barré syndrome and encephalitis
- Brain infection (encephalitis) is less common and causes unsteadiness in walking, headache, dizziness, confusion, and seizures. In adults, encephalitis can be life threatening. It occurs in 1 to 2 of 1,000 cases of chickenpox.
- Reye syndrome is a rare but very severe complication that occurs almost only in those younger than 18 following the use of aspirin. Therefore, aspirin should not be given to children with chickenpox. Reye syndrome may begin 3 to 8 days after the rash begins.
- Thrombocytopenia and purpura.
- Inflammation of the liver and bleeding problems may also occur.
Shingles (herpes zoster)
- The varicella-zoster virus remains dormant in sensory ganglia after infection.
- It may reactivate after many years as shingles. Shingles presents with grouped vesicular lesions, which usually affect a single dermatome.
- Other infections occurring as a result of reactivation of virus include post-herpetic neuralgia, vasculopathy (any disease affecting the blood vessels), myelopathy (injury to the spinal cord), acute retinal necrosis (inflammatory condition of the retina), meningitis or meningoencephalitis, cerebellitis and zoster sine herpete (pain without rash) 237, 238, 239, 240, 241. Among them, varicella-zoster virus (VZV) cerebrovasculopathy is caused by viral infection of the arteries, leading to pathological vascular remodeling and an ischemic or hemorrhagic stroke, with a mortality rate of 25%, and one-third of patients with virology-proven varicella-zoster virus (VZV) vascular disease had no previous rash 242. In addition, by reactivating and infecting the meninges, brain parenchyma, and nerve roots, the varicella-zoster virus (VZV) can cause varicella-zoster meningitis, varicella-zoster encephalitis, and varicella-zoster radiculitis 243. Varicella-zoster virus (VZV) meningoencephalitis can manifest as mild aseptic meningitis but also as severe encephalitis with edema, brain necrosis, and demyelination 239. The mortality rate of varicella-zoster virus (VZV) encephalitis is as high as 9% 244. Besides, varicella-zoster virus (VZV) meningoradiculitis can be lethal as reported 245. Varicella-zoster virus (VZV) myelopathy can present as a fatal myelitis, mostly in immunocompromised individuals. Postmortem analyses of the spinal cord from fatal cases have revealed a apparent invasion of the varicella-zoster virus (VZV) in the parenchyma with associated inflammation and, in some instances, spread of the virus to adjacent nerve roots 246.
- Evidence suggests that persistent radicular pain from herpes zoster can be caused by chronic activation of the varicella-zoster virus (VZV) and ganglionitis. In a patient who had experienced chronic trigeminal neuralgia for more than 1 year, the trigeminal ganglionic mass was removed and the pathological and virological analyses discovered active varicella-zoster virus (VZV) ganglionitis 247. In another study, active varicella-zoster virus (VZV) ganglionitis was found in the trigeminal ganglia of a patient who experienced chronic trigeminal neuralgia for months before death 248. Using human dorsal root ganglia xenografts in immunodeficient mice, Zerboni and Arvin 249 demonstrated varicella-zoster virus (VZV) replication in mechanoreceptive neurons; They also found that the VZV in the dorsal root ganglia triggers release of pro-inflammatory cytokines that cause neuronal damage. These findings may help to explain the neurologic sequelae in herpes zoster, zoster sine herpete (pain without rash) and post-herpetic neuralgia patients.
Prevention of chickenpox
Chickenpox is highly preventable by vaccination with live attenuated varicella vaccine. Chickenpox vaccine became available in the United States in 1995. The Centers for Disease Control and Prevention (CDC) recommends two doses of chickenpox (varicella) vaccine for children, adolescents, and adults who have never had chickenpox and were never vaccinated. Children are routinely recommended to receive the first dose at 12 through 15 months of age and the second dose at 4 through 6 years of age. During the first 25 years of the U.S. chickenpox vaccination program, the vaccine has prevented an estimated 91 million cases, 238,000 hospitalizations, and 2,000 deaths 250.
Chickenpox vaccination is especially important for:
- Healthcare professionals
- People who care for or are around other people whose body is less able to fight germs and sickness (weakened immune system)
- Teachers
- Childcare workers
- Residents and staff in nursing homes and other residential settings
- College students
- Inmates and staff of correctional institutions
- Military personnel
- Non-pregnant women of child-bearing age
- Premature infants born to susceptible mothers
- Infants born at less than 28 weeks gestation or who weigh ≤1000 grams, regardless of maternal immune status
- Immunocompromised people, including those who are undergoing immunosuppressive therapy, have malignant disease, or are immunodeficient
- Pregnant women
- Adolescents and adults living with children
- International travelers
Some people with a weakened immune system who do not have immunity against chickenpox may be considered for vaccination after talking with their doctor, including:
- People with HIV infection
- People with cancer, but whose disease is in remission
- People on low dose steroids
Two doses of varicella vaccine are recommended for all children, adolescents, and adults without evidence of immunity to varicella. Those who previously received one dose of varicella vaccine should receive their second dose for best protection against the disease.
Chickenpox vaccine
Two vaccines containing varicella virus are licensed for use in the United States 251.
- Varivax® is the single-antigen varicella vaccine 252.
- Contains only chickenpox vaccine.
- Is licensed for use in people 12 months of age or older.
- Can be given to children for their routine two doses of chickenpox vaccine at 12 through 15 months old and age 4 through 6 years old.
- ProQuad® is a combination measles, mumps, rubella, and varicella (MMRV) vaccine 253.
- Contains a combination of measles, mumps, rubella, and varicella (chickenpox) vaccines, which is also called MMRV.
- Is only licensed for use in children 12 months through 12 years of age.
Both vaccines contain live, attenuated varicella-zoster virus derived from the Oka strain.
Children 12 months through 12 years old
- 2 doses (0.5 ml each) of varicella vaccine should be given subcutaneously, separated by at least 3 months.
- Measles, mumps, rubella, and varicella (MMRV) vaccine is approved for healthy children in this age group.
Single-antigen vaccine and MMRV vaccine can be used for the routine 2-dose varicella vaccination.
- First dose: age 12 through 15 months
- Second dose: age 4 through 6 years
For the first dose, CDC recommends that MMR and varicella (MMRV) vaccines be given separately in children 12 through 47 months old unless the parent or caregiver expresses a preference for MMRV vaccine. For the second dose of measles, mumps, rubella, and varicella (MMRV) vaccines at any age (15 months–12 years) and for the first dose at age ≥48 months, use of MMRV vaccine generally is preferred over separate injections of MMR and varicella vaccines.
Both vaccines may be given at the same time as other vaccines for children age 12 through 15 months and age 4 through 6 years.
People 13 years or older
- 2 doses (0.5 ml each) of the single-antigen varicella vaccine subcutaneously 4 to 8 weeks apart
Measles, mumps, rubella, and varicella (MMRV) vaccine is NOT approved for people in this age group.
Post exposure chickenpox vaccination
Getting vaccinated after you are exposed to someone with chickenpox can:
- Prevent the disease or make it less serious.
- Protect you from chickenpox if you are exposed again in the future.
A healthcare provider can also prescribe a medicine to make chickenpox less severe if you:
- Are exposed to chickenpox.
- Do not have immunity against the disease.
- Are not eligible for vaccination.
Who should NOT get chickenpox vaccine
You do not need to get the chickenpox vaccine if you have evidence of immunity against the disease.
Some people should NOT get chickenpox vaccine or they should wait.
- People should check with their doctor about whether they should get chickenpox vaccine if they:
- Have HIV and AIDS or another disease that affects the immune system.
- Are being treated with drugs that affect the immune system, such as steroids, for 2 weeks or longer.
- Have any kind of cancer.
- Are getting cancer treatment with radiation or drugs.
- Recently had a transfusion or were given other blood products.
Chickenpox diagnosis
Diagnosis of chickenpox is usually made on the presence of its characteristic rash and the presence of different stages of lesions simultaneously. A clue to the diagnosis is in knowing that the patient has been exposed to an infected contact within the 10–21 day incubation period. Patients may also have prodromal signs and symptoms.
If there’s any doubt about the diagnosis, chickenpox can be confirmed with lab tests, including blood tests or a culture of lesion samples.
Laboratory tests are often undertaken to confirm the diagnosis:
- Polymerase chain reaction (PCR) detects the varicella virus in skin lesions and is the most accurate method for diagnosis.
- The culture of blister fluid is time-consuming and is less frequently performed.
- Serology (IgM and IgG) is most useful in pregnant women, or before prescribing immune suppression medication to determine the need for pre-treatment immunization.
Chickenpox treatment
For most healthy patients with chickenpox, symptomatic therapy is usually all that is required.
- Trim children’s fingernails to minimize scratching.
- Take a warm bath and apply moisturizing cream.
- Acetaminophen (paracetamol) can reduce fever and pain
- Avoid non-steroidal anti-inflammatory drug (NSAID) such as ibuprofen (Advil, Motrin IB, others) use outside of hospital settings due to the increased risk of severe cutaneous complications such as invasive group A streptococcal superinfections.
- Do not use aspirin in children as this is associated with Reye syndrome.
- Calamine lotion and oral antihistamines may relieve itching.
- Consider oral aciclovir (antiviral agent) in people older than 12 years, which reduces the number of days with a fever.
Immunocompromised patients with chickenpox need intravenous treatment with the antiviral aciclovir.
In cases of unintentional exposure to the varicella-zoster virus (VZV), varicella-zoster immune globulin if given within 96 hours of initial contact can reduce the severity of the disease though not prevent it. This is used where there is no previous history of chickenpox (or the patient has no antibodies to the varicella-zoster virus on blood testing) in pregnancy, in the first 28 days after delivery, and in immune deficient or immune-suppressed patients.
Varicella-zoster immune globulin
For people exposed to varicella or herpes zoster who cannot receive varicella vaccine, varicella-zoster immune globulin can prevent varicella from developing or lessen the severity of the disease 254. Varicella-zoster immune globulin is recommended for people who cannot receive the vaccine and 1) who lack evidence of immunity to varicella, 2) whose exposure is likely to result in infection, and 3) are at high risk for severe varicella 254.
The varicella-zoster immune globulin product licensed for use in the United States is VariZIG. Varicella-zoster immune globulin (VariZIG) should be given as soon as possible after exposure to varicella-zoster virus (VZV); it can be given within 10 days of exposure. VariZIG is commercially available from a broad network of specialty distributors in the United States (list available at https://varizig.com).
Timing of varicella-zoster immune globulin (VariZIG) administration. CDC recommends administration of VariZIG as soon as possible after exposure to varicella-zoster virus and within 10 days 255.
Patient groups for whom varicella-zoster immune globulin (VariZIG) is recommended. Patients without evidence of immunity to varicella who are at high risk for severe varicella and complications, who have been exposed to varicella or herpes zoster, and for whom varicella vaccine is contraindicated, should receive varicella-zoster immune globulin (VariZIG). Patient groups recommended by CDC to receive VariZIG include the following 255, 256:
- Immunocompromised patients without evidence of immunity.
- Newborn infants whose mothers have signs and symptoms of varicella around the time of delivery (i.e., 5 days before to 2 days after).
- Hospitalized premature infants born at ≥28 weeks of gestation whose mothers do not have evidence of immunity to varicella.
- Hospitalized premature infants born at <28 weeks of gestation or who weigh ≤1,000 g at birth, regardless of their mothers’ evidence of immunity to varicella.
- Pregnant women without evidence of immunity.
Varicella-zoster immune globulin administration
Varicella-zoster immune globulin (VariZIG) is supplied in 125-IU vials and should be administered intramuscularly as directed by the manufacturer. The recommended dose is 125 IU/10 kg of body weight, up to a maximum of 625 IU (five vials). The minimum dose is 62.5 IU (0.5 vial) for patients weighing ≤2.0 kg and 125 IU (one vial) for patients weighing 2.1–10.0 kg 257.
Unchanged from previous recommendations 256, for patients who become eligible for vaccination, varicella vaccine should be administered ≥5 months after VariZIG administration. Because varicella zoster immune globulin might prolong the incubation period by ≥1 week, any patient who receives VariZIG should be observed closely for signs and symptoms of varicella for 28 days after exposure. Antiviral therapy should be instituted immediately if signs or symptoms of varicella occur. Most common adverse reactions following VariZIG administration were pain at injection site (2%) and headache (2%) 257.
Contraindications for varicella-zoster immune globulin (VariZIG) administration include a history of anaphylactic or severe systemic reactions to human immune globulins and IgA-deficient patients with antibodies against IgA and a history of hypersensitivity 257.
The American Academy of Pediatrics (AAP) recommends that certain groups at increased risk for moderate to severe varicella be considered for oral acyclovir or valacyclovir treatment. These high risk groups include:
- Healthy people older than 12 years of age
- People with chronic skin disorders such as eczema or pulmonary disorders
- People receiving long-term salicylate therapy
- People receiving short, intermittent, or aerosolized courses of corticosteroids
Some healthcare providers may elect to use oral acyclovir or valacyclovir for secondary cases within a household. For maximum benefit, oral acyclovir or valacyclovir therapy should be given within the first 24 hours after the varicella rash starts.
Oral acyclovir or valacyclovir therapy is not recommended by American Academy of Pediatrics for use in otherwise healthy children experiencing typical varicella without complications. Acyclovir is a category B drug based on the U.S. Food and Drug Administration’s (FDA) Drug Risk Classification in pregnancy. Some experts recommend oral acyclovir or valacyclovir for pregnant women with varicella, especially during the second and third trimesters. Intravenous acyclovir is recommended for the pregnant patient with serious, viral-mediated complications of varicella, such as pneumonia.
Intravenous acyclovir therapy is recommended for severe disease (e.g., disseminated varicella-zoster virus (VZV) such as pneumonia, encephalitis, thrombocytopenia, severe hepatitis) and for varicella in immunocompromised patients (including patients being treated with high-dose corticosteroid therapy for >14 days).
Famciclovir is available for treatment of varicella-zoster virus (VZV) infections in adults, but its efficacy and safety have not been established for children. In cases of infections caused by acyclovir-resistant varicella-zoster virus (VZV) strains, which usually occur in immunocompromised people, Foscarnet should be used to treat the varicella-zoster virus (VZV) infection, but consultation with an infectious disease specialist is recommended.
Chickenpox treatment at home
To help ease the symptoms of an uncomplicated case of chickenpox, follow these self-care measures.
Avoid scratching
Scratching can cause scarring, slow healing and increase the risk that the sores will become infected. If your child can’t stop scratching:
- Put gloves on his or her hands, especially at night
- Trim his or her fingernails
Relieve the itch and other symptoms
The chickenpox rash can be very itchy, and broken vesicles sometimes sting. These discomforts, along with fever, headache and fatigue, can make anyone miserable. For relief, try:
- A cool bath with added baking soda, aluminum acetate (Domeboro, others), uncooked oatmeal or colloidal oatmeal — a finely ground oatmeal that is made for soaking.
- Calamine lotion dabbed on the spots.
- A soft, bland diet if chickenpox sores develop in the mouth.
- Antihistamines such as diphenhydramine (Benadryl, others) for itching. Check with your doctor to make sure your child can safely take antihistamines.
- Acetaminophen (Tylenol, others) for a mild fever.
If fever lasts longer than four days and is higher than 102, call your doctor. And don’t give aspirin to children and teenagers who have chickenpox because it can lead to a serious condition called Reye’s syndrome.
Chickenpox prognosis
Healthy children nearly always recover from chickenpox without problems. Before routine immunization, about 4 million people developed chickenpox annually in the United States, and about 100 to 150 of them died each year because of complications of chickenpox 258.
In adults, chickenpox is more severe, and the risk of dying is higher.
Chickenpox is particularly severe in people with a weakened immune system.
When people who have been vaccinated develop chickenpox, the disease is less severe, and fewer of these people die.
Cold sores
Cold sores also called fever blisters, are caused by a contagious virus called herpes simplex virus (HSV). Cold sores are tiny, fluid-filled blisters on and around your lips. These blisters are often grouped together in patches. After the blisters break, a crust forms over the resulting sore. Cold sores usually heal in two to four weeks without leaving a scar. There are two types of herpes simplex virus. Type 1 herpes simplex virus (HSV-1) usually causes oral herpes, or cold sores. Type 1 herpes virus infects more than half of the U.S. population by the time they reach their 20s. Type 2 herpes simplex virus (HSV-2) usually affects the genital area.
Herpes simplex is caused by one of two types of herpes simplex virus (HSV), members of the Herpesvirales family of double-stranded DNA viruses.
- Type 1 herpes simplex virus is mainly associated with oral and facial infections
- Type 2 herpes simplex virus is mainly associated with genital and rectal infections (anogenital herpes)
However, either virus can affect almost any area of skin or mucous membrane.
After the primary episode of infection, herpes simplex virus (HSV) resides in a latent state in spinal dorsal root nerves that supply sensation to the skin. During a recurrence, the virus follows the nerves onto the skin or mucous membranes, where it multiplies, causing the clinical lesion. After each attack and lifelong, it enters the resting state.
During an attack, the herpes simplex virus (HSV) can be inoculated into new sites of skin, which can then develop blisters as well as the original site of infection.
Some people have no symptoms from the infection. But others develop painful and unsightly cold sores. Cold sores usually occur outside the mouth, on or around the lips. When they are inside the mouth, they are usually on the gums or the roof of the mouth. They are not the same as canker sores, which are not contagious.
There is no cure for cold sores. They normally go away on their own in a few weeks. Antiviral medicines can help them heal faster. They can also help to prevent cold sores in people who often get them. Other medicines can help with the pain and discomfort of the sores. These include ointments that numb the blisters, soften the crusts of the sores, or dry them out. Protecting your lips from the sun with sunblock lip balm can also help.
Cold sores generally clear up without treatment. See your doctor if:
- You have a weakened immune system
- The cold sores don’t heal within two weeks
- Symptoms are severe
- You have frequent recurrences of cold sores
- You experience irritation in your eyes
How do you get cold sores?
Primary attacks of type 1 herpes simplex virus (HSV-1) infections occur mainly in infants and young children. In crowded, underdeveloped areas of the world, nearly all children have been infected by the age of 5. In less crowded places, the incidence is lower; for example, less than half of university entrants in Britain have been infected. Type 2 herpes simplex virus (HSV-2) infections occur mainly after puberty and are often transmitted sexually.
Herpes simplex virus (HSV) is transmitted by direct or indirect contact with someone with active herpes simplex, which is infectious for 7–12 days. Asymptomatic shedding of the virus in saliva or genital secretions can also lead to transmission of herpes simplex virus (HSV), but this is infrequent, as the amount shed from inactive lesions is 100 to 1000 times less than when it is active. The incubation period is 2–12 days.
Minor injury helps inoculate herpes simplex virus (HSV) into the skin. For example:
- A thumb sucker may transmit the virus from their mouth to their thumb.
- A health-care worker may develop herpetic whitlow (paronychia)
- A rugby player may get a cluster of blisters on one cheek (‘scrumpox’).
Are cold sores contagious?
Yes. Cold sores are contagious even if you don’t see the sores. Cold sores are spread from person to person by close contact, such as kissing. Cold sores are caused by a herpes simplex virus (HSV-1) closely related to the one that causes genital herpes (HSV-2). Both of these viruses can affect your mouth or genitals and can be spread by oral sex.
Can you get genital herpes from a cold sore?
Yes — it is possible to get genital herpes from oral sex.
Genital herpes is caused by the herpes simplex virus (HSV). There are two types of herpes viruses — HSV-1 and HSV-2. Genital herpes is usually caused by HSV-2; oral herpes (cold sores) is usually caused by HSV-1.
However, genital herpes can also be caused by HSV-1. Someone with HSV-1 can transmit the virus through oral contact with another person’s genitals, anus, or mouth, even if they don’t have sores that are visible at the time.
Other than abstinence (not having sex) the best way to help prevent herpes is to use a condom during any type of sex (oral, vaginal, or anal). Girls should have their partners use a dental dam every time they receive oral sex to help protect against genital herpes. And if either partner has a sore, it’s best to not have sex until the sore has cleared up.
What causes cold sores?
Cold sores are caused by certain strains of the herpes simplex virus (HSV). Type 1 herpes simplex virus (HSV-1) usually causes cold sores. Type 2 herpes simplex virus (HSV-2) is usually responsible for genital herpes. However, either type can cause sores in the facial area or on the genitals. Most people who are infected with the virus that causes cold sores never develop signs and symptoms.
Cold sores are most contagious when oozing blisters are present. But you can transmit the virus to others even if you don’t have blisters. Shared eating utensils, razors and towels, as well as kissing, may spread HSV-1. Oral sex can spread HSV-1 to the genitals and HSV-2 to the lips.
Once you’ve had an episode of herpes infection, the virus lies dormant in nerve cells in your skin and may emerge as another cold sore at the same place as before. Recurrence may be triggered by:
- Viral infection or fever
- Hormonal changes, such as those related to menstruation
- Stress
- Fatigue
- Exposure to sunlight and wind
- Changes in the immune system
Risk factors for getting cold sores
About 90 percent of adults worldwide — even those who’ve never had symptoms of an infection — test positive for evidence of the herpes simplex virus (HSV) that causes cold sores.
People who have weakened immune systems are at higher risk of complications from the herpes simplex virus (HSV). Medical conditions and treatments that increase your risk of complications include:
- HIV/AIDS
- Severe burns
- Eczema
- Cancer chemotherapy
- Anti-rejection drugs for organ transplants
Cold sores prevention
Sun exposure often triggers facial herpes simplex, sun protection using high protection factor sunscreens and other measures are important.
Your doctor may prescribe an antiviral medication for you to take on a regular basis, if you develop cold sores frequently or if you’re at high risk of serious complications. Antiviral drugs will stop herpes simplex virus (HSV) multiplying once it reaches the skin or mucous membranes but cannot eradicate the virus from its resting stage within the nerve cells. They can, therefore, shorten and prevent attacks but a single course cannot prevent future attacks. Repeated courses may be prescribed, or the medication may be taken continuously to prevent frequent attacks.
To help avoid spreading cold sores to other people or to other parts of your body, you might try some of the following precautions:
- Avoid skin-to-skin contact with others while blisters are present. The virus spreads most easily when there are moist secretions from the blisters.
- Avoid sharing items. Utensils, towels, lip balm and other items can spread the virus when blisters are present.
- Keep your hands clean. When you have a cold sore, wash your hands carefully before touching yourself and other people, especially babies.
Cold sores diagnosis
Your doctor can usually diagnose cold sores just by looking at them. To confirm the diagnosis, he or she may take a sample from the blister for testing in a laboratory by culture or PCR of a viral swab taken from fresh vesicles. Herpes simplex virus (HSV) serology is not very informative, as it’s positive in most individuals and thus not specific for the lesion with which they present.
Cold sores treatment
Cold sores generally clear up without treatment in two to four weeks. Blisters may be covered if desired, for example with a hydrocolloid patch. Severe infection may require treatment with an antiviral agent.
Several types of prescription antiviral drugs may speed the healing process. Examples include:
- Acyclovir (Xerese, Zovirax) – 200 mg 5 times daily for five days
- Valacyclovir (Valtrex) – 1 g 3 times daily for seven days
- Famciclovir (Famvir) – as a single dose of 3 x 500 mg
- Penciclovir (Denavir)
Some of these products are packaged as pills to be swallowed. Others are creams to be applied to the sores several times a day. In general, the pills work better than the creams. For very severe infections, some antiviral drugs can be given with an injection.
Topical aciclovir or penciclovir may shorten attacks of recurrent herpes simplex, provided the cream is started early enough.
Higher doses of antiviral drugs are used for eczema herpeticum or for disseminated herpes simplex.
Home remedies for cold sores
The over-the-counter cold sore ointment docosanol (Abreva) may shorten the healing time of a cold sore. At the first sign of symptoms, apply it to the affected skin as directed on the package.
To ease the discomfort of a cold sore:
- Try other cold sore remedies. Some over-the-counter preparations contain a drying agent, such as alcohol, that may speed healing.
- Use lip balms and cream. Protect your lips from the sun with a zinc oxide cream or lip balm with sunblock. If your lips become dry, apply a moisturizing cream.
- Apply a cool compress. A cool, damp cloth may reduce redness, help remove crusting and promote healing.
- Apply pain-relieving creams. Over-the-counter creams with lidocaine or benzocaine may offer some pain relief.
Alternative medicine
Although study results have been mixed, alternative medicine treatments for cold sores include:
- Lysine. An amino acid, lysine is available as an oral supplement and as a cream.
- Propolis. Also known as synthetic beeswax, this is available as a 3 percent ointment. When applied early and often, it may shorten the duration of the breakout.
- Rhubarb and sage. A cream combining rhubarb and sage may be about as effective as acyclovir (Zovirax) cream.
- Stress reduction. If your cold sores are triggered by stress, you might want to try relaxation techniques, such as deep-breathing exercises and meditation.
Genital herpes
Genital herpes is a common sexually transmitted disease (STD) that is caused by the herpes simplex virus (HSV) – the same type of virus that causes cold sores.
Most people with the herpes simplex virus (HSV) don’t have symptoms. Even without signs of the disease, herpes can still be spread to sex partners.
Genital herpes is common in the United States. At least 50 million people in the United States—about one in six adults—are infected with HSV. More than one out of every six people aged 14 to 49 years have genital herpes. Genital herpes is more common in women than in men.
Genital herpes can cause outbreaks of blisters or sores on the genitals and anus. Once infected, you can continue to have recurrent episodes of symptoms throughout your life.
There are 2 types of herpes simplex virus (HSV), both viruses can affect either the lips, mouth, genital or anal areas, however:
- Herpes simplex virus type 1 (HSV1) commonly causes cold sores on the lips, mouth or face. However, most people do not have any symptoms. Most people with oral herpes were infected during childhood or young adulthood from non-sexual contact with saliva. Oral herpes caused by HSV-1 can be spread from the mouth to the genitals through oral sex. This is why some cases of genital herpes are caused by HSV-1.
- Herpes simplex virus type 2 (HSV2) causes most genital herpes.
It is most easily spread when there are blisters or sores, but can still be passed even if a person has no current blisters or sores or other symptoms. HSV also can be present on the skin even if there are no sores. If a person comes into contact with the virus on an infected person’s skin, he or she can become infected.
- If you think you have genital herpes, it is important to see a doctor as soon as possible. Your doctor can confirm the diagnosis with testing and start treatment. Anti-viral medication may help to prevent transmission. Talk to your doctor about this in more detail.
- If you have genital herpes, it is important to always use condoms and dental dams when having sex, even when you have no symptoms. A dental dam is a square of thin latex that can be placed over the vulva or anal area during oral sex. It is safest to avoid sex when you have blisters, sores or symptoms.
- It is also important to tell your sexual partners that you have genital herpes. Your doctor can help you decide who to tell and how to tell them.
- If you’re pregnant: It’s important to tell your obstetrician that you or a partner have had genital herpes, so that they can monitor you for symptoms and manage your pregnancy safely. There is a risk you can pass the virus on to your baby if you have a vaginal delivery during a first attack of genital herpes. If this happens you may be recommended to have a caesarean delivery.
How is genital herpes spread?
You can get genital herpes by having vaginal, anal, or oral sex with someone who has the disease.
If you do not have herpes, you can get infected if you come into contact with the herpes virus in:
- A herpes sore;
- Saliva (if your partner has an oral herpes infection) or genital secretions (if your partner has a genital herpes infection);
- Skin in the oral area if your partner has an oral herpes infection, or skin in the genital area if your partner has a genital herpes infection.
You can get herpes from a sex partner who does not have a visible sore or who may not know he or she is infected. It is also possible to get genital herpes if you receive oral sex from a sex partner who has oral herpes.
- You will not get herpes from toilet seats, bedding, or swimming pools, or from touching objects around you such as silverware, soap, or towels. If you have additional questions about how herpes is spread, consider discussing your concerns with a healthcare provider.
Risk factors for genital herpes
Your risk of becoming infected with genital herpes may increase if you:
- Are a woman. Women are more likely to have genital herpes than are men. The virus is sexually transmitted more easily from men to women than it is from women to men.
- Have multiple sexual partners. Each additional sexual partner raises your risk of being exposed to the virus that causes genital herpes.
Genital herpes complications
Complications associated with genital herpes may include:
- Other sexually transmitted infections. Having genital sores increases your risk of transmitting or contracting other sexually transmitted infections, including AIDS.
- Newborn infection. Babies born to infected mothers can be exposed to the virus during the birthing process. This may result in brain damage, blindness or death for the newborn.
- Bladder problems. In some cases, the sores associated with genital herpes can cause inflammation around the tube that delivers urine from your bladder to the outside world (urethra). The swelling can close the urethra for several days, requiring the insertion of a catheter to drain your bladder.
- Meningitis. In rare instances, HSV infection leads to inflammation of the membranes and cerebrospinal fluid surrounding your brain and spinal cord.
- Rectal inflammation (proctitis). Genital herpes can lead to inflammation of the lining of the rectum, particularly in men who have sex with men.
Genital herpes prevention
The only way to avoid sexually transmitted diseases (STDs) is to not have vaginal, anal, or oral sex.
If you are sexually active, you can do the following things to lower your chances of getting genital herpes:
- Be in a long-term mutually monogamous relationship with a partner who is not infected with an STD (e.g., a partner who has been tested and has negative STD test results);
- Using latex condoms the right way every time you have sex.
Be aware that not all herpes sores occur in areas that are covered by a latex condom. Also, herpes virus can be released (shed) from areas of the skin that do not have a visible herpes sore. For these reasons, condoms may not fully protect you from getting herpes.
If you are in a relationship with a person known to have genital herpes, you can lower your risk of getting genital herpes if:
- Your partner takes an anti-herpes medication every day. This is something your partner should discuss with his or her doctor.
- You avoid having vaginal, anal, or oral sex when your partner has herpes symptoms (i.e., when your partner is having an outbreak).
Genital herpes diagnosis
Your doctor usually can diagnose genital herpes based on a physical exam and the results of certain laboratory tests:
- Viral culture. This test involves taking a tissue sample or scraping of the sores for examination in the laboratory.
- Polymerase chain reaction (PCR) test. PCR is used to copy your DNA from a sample of your blood, tissue from a sore or spinal fluid. The DNA can then be tested to establish the presence of HSV and determine which type of HSV you have.
- Blood test. This test analyzes a sample of your blood for the presence of HSV antibodies to detect a past herpes infection.
Genital herpes treatment
There’s no cure for genital herpes. Treatment with prescription antiviral medications may:
- Help sores heal sooner during an initial outbreak
- Lessen the severity and duration of symptoms in recurrent outbreaks
- Reduce the frequency of recurrence
- Minimize the chance of transmitting the herpes virus to another
Genital herpes anti-viral medications include:
- Acyclovir (Zovirax)
- Valacyclovir (Valtrex)
- Famciclovir
These antiviral drugs will stop the herpes simplex virus multiplying once it reaches the skin or mucous membranes but cannot eradicate the virus from its resting stage within the nerve cells. They can therefore shorten and prevent episodes while the drug is being taken, but a single course cannot prevent future episodes.
Your doctor may prescribe anti-viral medication to help reduce the severity of genital herpes symptoms. This is most effective when started within 72 hours of the first symptoms.
If you have frequent or severe recurrent episodes there are medications available to help control them.
Other treatments being studied include:
- Imiquimod cream, an immune enhancer
- Human leukocyte interferon alpha cream.
Both appear less beneficial than conventional antiviral drugs.
Your doctor may recommend that you take the medicine only when you have symptoms of an outbreak or that you take a certain medication daily, even when you have no signs of an outbreak. These medications are usually well-tolerated, with few side effects.
- Different formulations of topical antiviral creams are available. They are not generally recommended for genital herpes.
Treatment regimen
The dose and length of treatment depends upon whether the outbreak is the first episode or is a recurrence.
Initial episode — The first episode of genital herpes is generally treated with 7 to 10 days of one antiviral medication, taken by mouth.
Episodic therapy — Episodic therapy is a treatment strategy of taking antiviral medicines only when outbreaks occur. Episodic therapy may be recommended if you have fewer than six outbreaks each year. Unfortunately, episodic treatment does not reduce the frequency of outbreaks.
The advantage of episodic therapy is that it can decrease the duration and severity of the illness by hours to a few days.
Treatment is most likely to be effective if it is started within 72 hours of the first symptoms. People with a history of recurrent genital herpes are often advised to keep a supply of antiviral medication in their home, which they can initiate at the first signs of a recurrence (eg, pain or tingling symptoms or at the sign of their first blister).
Suppressive therapy — Suppressive therapy is low dose antiviral treatment that is taken every day to prevent outbreaks.
The advantage of suppressive therapy is that it decreases the frequency and duration of recurrences, and can reduce the risk of transmitting HSV to an uninfected sex partner.
Suppressive therapy may be recommended if you have six or more recurrences each year or have a weakened immune system due to the human immunodeficiency virus (HIV), use of immune-suppressing drugs, or other factors.
Suppressive therapy may also be an option if you are in a sexual relationship with a partner who does not have a history of genital herpes or antibodies to HSV-1 or 2 (as determined by blood testing). One study of valacyclovir showed that taking suppressive therapy can reduce the chances of transmitting the virus by approximately one-half.
It is not clear how long suppressive therapy should continue. Some experts recommend taking a break from treatment periodically (every few years) to determine if suppressive therapy is still needed. If recurrent outbreaks develop, suppressive therapy may be restarted.
No treatment — It is not necessary to treat a recurrent episode of genital herpes. No treatment may be appropriate for some patients, particularly those with infrequent outbreaks or minimal symptoms. It also may be appropriate if the patient is not currently sexually active, so transmission of HSV is not a consideration.
Home remedies for genital herpes
The symptoms can also be helped by:
- gently bathing the area with a warm salt solution (also called a sitz bath) (1 teaspoon to 2 cups water, or 1 cup of salt in a bath)
- pain medication, such as paracetamol or ibuprofen may also help relieve the pain of genital ulcers.
- local anesthetic ointment
- urinating while sitting in a warm bath, if urination is painful
- soaps and bubble baths should be avoided. It is important to keep the genital area clean and dry, and to avoid tight or irritating underwear and clothing.
- over-the-counter creams and ointments are generally not recommended.
Shingles
Anyone who has had chickenpox (varicella-zoster virus infection) can get shingles also known as herpes zoster. Varicella-zoster virus is also called herpesvirus 3 and is a member of the Herpesvirales order of double-stranded DNA viruses. Shingles is a varicella-zoster virus infection also known as zoster or herpes zoster, that reactivates (wakes up) causing a painful blistering rash. Most cases of chickenpox occur in children under age 15, but older children and adults can get it too. Chickenpox (varicella-zoster virus infection) spreads very easily from one person to another. After the chickenpox clears, the varicella-zoster virus stays inside your body (inside the nerves within dorsal root ganglia and lie dormant for years). The varicella-zoster virus may not cause problems for many years. Eventually, it may reactivates (wakes up) and travels along nerve pathways to your skin — producing shingles (herpes zoster) — a painful, blistering rash. But, not everyone who’s had chickenpox will develop shingles. Shingles tends to cause more pain and less itching than chickenpox. As you get older, the varicella-zoster virus may reappear as shingles. Although shingles is most common in people over age 50, anyone who has had chickenpox is at risk.
Shingles (herpes zoster) often affects people with weak immunity. People with various kinds of cancer have a 40% increased risk of developing shingles. People who have had shingles rarely get it again; the chance of getting a second episode is about 1%.
Shingles generally lasts between two and six weeks. Most people get shingles only once, but it is possible to get it two or more times.
There is no cure for shingles. Early treatment with medicines that fight the virus may help. These medicines may also help prevent lingering pain.
- If you think you might have shingles, talk to your doctor as soon as possible. It’s important to see your doctor no later than 3 days after the rash starts. The doctor will confirm whether or not you have shingles and can make a treatment plan. Although there is no cure for shingles, early treatment with drugs that fight the virus can help the blisters dry up faster and limit severe pain. Shingles can often be treated at home. People with shingles rarely need to stay in a hospital.
The risk of getting shingles increases with age. A vaccine can reduce your risk of getting shingles or lessen its effects. Your doctor may recommend getting this vaccine after your 50th birthday or once you reach 60 years of age. There’s another — and maybe even more important — reason for getting the shingles vaccine. If you’ve had chickenpox, you can still get shingles after getting shingles vaccine. The vaccine also lessens your risk of developing serious complications from shingles, such as life-disrupting nerve pain.
The nerve pain can last long after the shingles rash goes away. Some people have this nerve pain, called post-herpetic neuralgia (PHN), for many years. The pain can be so bad that it interferes with your everyday life. The shingles vaccine reduces your risk of developing this nerve pain, even more than it reduces your risk of getting shingles.
An anti-viral medicine may also prevent long-lasting nerve pain if your get shingles. It’s most effective when started within 3 days of seeing the rash. The anti-viral medicine can also make shingles symptoms milder and shorter.
You can’t catch shingles from someone. However, if you have a shingles rash, you can pass the virus to someone who has not had chickenpox (or the chickenpox vaccine) can get this virus. This would usually be a child, when this happens, the person develops chickenpox, not shingles. The virus spreads through direct contact with the rash and cannot spread through the air. Chickenpox can be dangerous for some people. Until your shingles blisters scab over, you are contagious and should avoid physical contact with anyone who hasn’t yet had chickenpox or the chickenpox vaccine, especially people with weakened immune systems, pregnant women and newborns.
If you have shingles, what can you do to prevent spreading the virus?
When you have shingles, you’re only contagious while you have blisters. To prevent spreading the virus while you have blisters, the Centers for Disease Control and Prevention (CDC) recommends that you:
- Cover the rash
- Wash your hands often and try to avoid touching the rash
- Avoid being around people for whom catching the virus could be dangerous
The varicella-zoster virus is especially dangerous for these people
- Catching this virus and getting chickenpox can be dangerous for women who are pregnant and have not had chickenpox or gotten the chickenpox vaccine. In this situation, the virus can harm the woman’s unborn baby.
Babies less than 1 month old and people who have a weak immune system can also have complications if they catch the virus. People who have a weak immune system include those who are:
- HIV positive
- Taking medicine that weakens their immune system
- Receiving chemotherapy or radiation treatments
You’re not contagious before you develop blisters or after the blisters scab over. Be sure to take precautions while you have blisters.
A person must have had chickenpox to get shingles. Some people who have had chickenpox have a higher risk of getting shingles. But, not everyone who’s had chickenpox will develop shingles.
Most people who develop shingles have only one episode during their lifetime. However, a person can have a second or even a third episode.
- One out of every three people 60 years old or older will get shingles.
- One out of six people older than 60 years who get shingles will have severe pain. The pain can last for months or even years.
- The most common complication of shingles is severe pain where the shingles rash was. This pain can be debilitating. There is no treatment or cure from this pain. As people get older, they are more likely to develop long-term pain as a complication of shingles and the pain is likely to be more severe.
- Shingles may also lead to serious complications involving the eye.
- Very rarely, shingles can also lead to pneumonia, hearing problems, blindness, brain inflammation (encephalitis), or death.
Some illnesses and medical treatments can weaken a person’s immune system and increase the risk of getting shingles. These include:
- Are 50 years of age or older
- Have an illness or injury
- Are under great stress
- Have medical conditions that keep their immune systems from working properly:
- Cancers like leukemia and lymphoma
- Human immunodeficiency virus (HIV)/AIDS
- Some cancer treatments, such as chemotherapy or radiation
- Medicine taken to prevent rejection of a transplanted organ. Immunosuppressive drugs, such as steroids and drugs that are given after organ transplantation.
- Cortisone when taken for a long time
What causes shingles?
Shingles is caused by the varicella zoster virus, the same virus that causes chickenpox. After a person recovers from chickenpox, the virus stays dormant (inactive) in the body (in the nervous system). Shingles appears when the virus wakes up. Scientists aren’t sure why the virus can reactivates or “wakes up” years later, causing shingles. A short-term weakness in immunity may cause this. Shingles is more common in older adults and in people who have weakened immune systems.
Shingles cannot be passed from one person to another. However, the virus that causes shingles, the varicella zoster virus, can spread from a person with active shingles to cause chickenpox in someone who had never had chickenpox or received chickenpox vaccine.
The virus is spread through direct contact with fluid from the rash blisters caused by shingles.
A person with active shingles can spread the virus when the rash is in the blister-phase. A person is not infectious before the blisters appear. Once the rash has developed crusts, the person is no longer infectious.
Shingles is less contagious than chickenpox and the risk of a person with shingles spreading the virus is low if the rash is covered.
If you have shingles, you should:
- Cover the rash.
- Avoid touching or scratching the rash.
- Wash your hands often to prevent the spread of varicella zoster virus.
- Avoid contact with the people below until your rash has developed crusts
- pregnant women who have never had chickenpox or the chickenpox vaccine;
- premature or low birth weight infants; and
- people with weakened immune systems, such as people receiving immunosuppressive medications or undergoing chemotherapy, organ transplant recipients, and people with human immunodeficiency virus (HIV) infection.
Risk factors for shingles
Anyone who has ever had chickenpox can develop shingles. Most adults in the United States had chickenpox when they were children, before the advent of the routine childhood vaccination that now protects against chickenpox.
Factors that may increase your risk of developing shingles include:
- Being older than 50. Shingles is most common in people older than 50. The risk increases with age. Some experts estimate that half the people age 80 and older will have shingles.
- Having certain diseases. Diseases that weaken your immune system, such as HIV/AIDS and cancer, can increase your risk of shingles.
- Undergoing cancer treatments. Radiation or chemotherapy can lower your resistance to diseases and may trigger shingles.
- Taking certain medications. Drugs designed to prevent rejection of transplanted organs can increase your risk of shingles — as can prolonged use of steroids, such as prednisone.
Triggering factors for shingles
Shingles triggering factors are sometimes recognized, such as:
- Pressure on the nerve roots
- Radiotherapy at the level of the affected nerve root
- Spinal surgery
- An infection
- An injury (not necessarily to the spine)
- Contact with someone with varicella (chickenpox) or herpes zoster (shingles)
Shingles prevention
Adults 60 years old or older should talk to their healthcare professional about getting a one-time dose of the shingles vaccine.
- The shingles vaccine can reduce your risk of shingles and the long-term pain it can cause 259.
- Persons who have already had shingles or who have a chronic medical condition can receive the shingles vaccine.
- In a clinical trial involving thousands of adults 60 years old or older, the vaccine reduced the risk of shingles by about half. Even if the shingles vaccine doesn’t prevent you from getting shingles, it can still reduce the chance of having long-term pain or post-herpetic neuralgia.
Talk with your healthcare professional for more information and to find out if the shingles vaccine is right for you.
Adults can get vaccines at doctors’ offices, pharmacies, workplaces, community health clinics, and health departments. To find a place to get a vaccine near you, go to https://vaccinefinder.org/
Shingles complications
The most common complication of shingles is a condition called post-herpetic neuralgia (PHN). People with post-herpetic neuralgia have severe pain in the areas where they had the shingles rash, even after the rash clears up. Post-herpetic neuralgia is diagnosed in people who have pain that persists after their rash has resolved. Some define post-herpetic neuralgia as any duration of pain after the rash resolves; others define it as duration of pain for more than 30 days, or for more than 90 days after rash onset.
The pain from post-herpetic neuralgia may be severe and debilitating, but it usually resolves in a few weeks or months. Some people can have pain from post-herpetic neuralgia for many years and can interfere with daily life.
A person’s risk of having post-herpetic neuralgia after herpes zoster increases with age. As people get older, they are more likely to develop post-herpetic neuralgia, and the pain is more likely to be severe. Post-herpetic neuralgia occurs rarely among people under 40 years of age but can occur in approximately 13% (and possibly more) of untreated people who are 60 years of age and older with herpes zoster. Older adults are more likely to have post-herpetic neuralgia and to have longer lasting and more severe pain. Other predictors of post-herpetic neuralgia include the level of pain a person has when they have the rash and the size of their rash.
Other complications of herpes zoster include:
- Shingles may also lead to serious complications involving the eye (herpes zoster ophthalmicus, when the ophthalmic division of the fifth cranial nerve is involved) may result in vision loss.
- Bacterial superinfection of the lesions, usually due to Staphylococcus aureus and, less commonly, due to group A beta hemolytic streptococcus.
- Cranial and peripheral nerve palsies. Depending on which nerves are affected, shingles can cause an inflammation of the brain (encephalitis), facial paralysis, or hearing or balance problems. Muscle weakness in about one in 20 patients. Facial nerve palsy is the most common result (Ramsay Hunt syndrome). There is a 50% chance of complete recovery, but some improvement can be expected in nearly all cases
- Deep blisters that take weeks to heal followed by scarring.
- Very rarely, shingles can also lead to pneumonia, hearing problems, blindness, brain inflammation (meningoencephalitis), hepatitis, acute retinal necrosis or death.
People with compromised or suppressed immune systems are more likely to have complications from herpes zoster. They are more likely to have severe rash that lasts longer. Also, they are at increased risk of developing disseminated herpes zoster.
Shingles (herpes zoster) in the early months of pregnancy can harm the fetus, but luckily this is rare. Shingles in late pregnancy can cause chickenpox in the fetus or newborn. Herpes zoster may then develop as an infant.
Shingles diagnosis
Shingles is usually diagnosed based on the history of pain on one side of your body, along with the telltale rash and blisters. Your doctor may also take a tissue scraping or culture of the blisters for examination in the laboratory.
Shingles treatment
There’s no cure for shingles, but prompt treatment with prescription antiviral drugs can speed healing and reduce your risk of complications. These medications include:
- Acyclovir (Zovirax)
- Valacyclovir (Valtrex)
- Famciclovir
Antiviral treatment can reduce pain and the duration of symptoms if started within one to three days after the onset of herpes zoster. Aciclovir 800 mg 5 times daily for seven days is most often prescribed. Valaciclovir and famciclovir are also useful.
In healthy children shingles is often relatively mild and may not require treatment, however, children who take courses of systemic steroids, e.g., for asthma, are rendered vulnerable for up to three months after treatment is complete – if the child develops chickenpox in this period parents should seek urgent competent advice as treatment with oral antiviral therapy will be required.
Immunocompromised patients
- Should continue on with oral antiviral therapy for two days after crusting of the lesions
- If the patient is unwell / develops widespread (disseminated) zoster, hospital admission will be needed
Management of acute herpes zoster may include:
- Rest and pain relief
- Protective ointment applied to the rash, such as petroleum jelly.
- Oral antibiotics for secondary infection
Shingles can cause severe pain, so your doctor also may prescribe:
- Capsaicin topical patch (Qutenza)
- Anticonvulsants, such as gabapentin (Neurontin)
- Tricyclic antidepressants, such as amitriptyline
- Numbing agents, such as lidocaine, delivered via a cream, gel, spray or skin patch
- Medications that contain narcotics, such as codeine
- An injection including corticosteroids and local anesthetics
Shingles generally lasts between two and six weeks. Most people get shingles only once, but it is possible to get it two or more times.
Shingles medication
It is best to get treatment immediately. Treatment can include:
- Pain relievers to help ease the pain: The pain can be very bad, and prescription painkillers may be necessary. Your doctor also may prescribe:
- Capsaicin topical patch (Qutenza)
- Anticonvulsants, such as gabapentin (Neurontin)
- Tricyclic antidepressants, such as amitriptyline
- Numbing agents, such as lidocaine, delivered via a cream, gel, spray or skin patch
- Medications that contain narcotics, such as codeine
- An injection including corticosteroids and local anesthetics
Post-herpetic neuralgia may be difficult to treat successfully. It may respond to any of the following.
- Local anesthetic applications
- Topical capsaicin
- Tricyclic antidepressant medications such as amitriptyline
- Anti-epileptic medications gabapentin and pregabalin
- Transcutaneous electrical nerve stimulation or acupuncture
- Botulinum toxin into the affected area
- Early use of antiviral medication
Nonsteroidal anti-inflammatories (NSAIDs) and opioids are generally unhelpful.
Anti-viral medicine: This medicine may be prescribed when a doctor diagnoses shingles within 72 hours of the rash first appearing. The earlier anti-viral treatment is started, the better it works. Anti-viral medicines include famciclovir, valacyclovir, and acyclovir. These can lessen the pain and the amount of time the pain lasts.
Nerve blocks: Given for intense pain, these injections (shots) contain a numbing anesthetic and sometimes a corticosteroid.
Corticosteroids: To lower swelling and pain, some patients may get corticosteroid pills with their anti-viral medicine. This treatment is not common because it can make the rash spread.
Treatments for pain after the rash clears: Certain anti-depressants, pain relievers, anesthetic creams and patches, and anti-seizure medicines can help.
Ophthalmic zoster
- All patients with ophthalmic zoster, irrespective of age or severity of symptoms, should be prescribed oral antiviral drugs at the first sign of disease
- Patients with a red eye or visual complaints must be referred to an ophthalmologist on an urgent basis
- Those not needing referral must be reviewed after at most one week
Ramsay-Hunt syndrome
- Oral antiviral therapy must be started immediately and contact the on-call ENT (ear nose and throat) specialist
- Some advocate the use of systemic steroids – refer to local guidelines for management
Home remedies for shingles
- Get plenty of rest and eat well-balanced meals.
- Try simple exercises like stretching or walking. Check with your doctor before starting a new exercise routine.
- Apply a cool washcloth to your blisters to ease the pain and help dry the blisters.
- Cool compresses, baths or ice packs may help with the discomfort. Do not apply ice packs directly to the skin. Wrap the ice pack in a light towel and place it gently over the dressing. Wash the towel in hot water after use.
- Apply calamine lotion to the blisters.
- Cover the rash with loose, non-stick, sterile bandages.
- Wear loose cotton clothes around the body parts that hurt.
- Do things that take your mind off your pain. For example, watch TV, read, talk with friends, listen to relaxing music, or work on a hobby you like.
- Avoid stress. It can make the pain worse.
- Try not to scratch the rash. Scratching may cause scarring and infection of the blisters.
- If the blisters are open, applying creams or gels is not recommended because they might increase the risk of a secondary bacterial infection.
- After a bath or shower, gently pat yourself dry with a clean towel. Do not rub or use the towel to scratch yourself and do not share towels.
- Do not use antibiotic creams or sticking plasters on the blisters since they may slow down the healing process.
- Take an oatmeal bath or use calamine lotion to see if it soothes your skin.
- Avoid contact with people who may be more at risk, such as pregnant women who are not immune to chickenpox, people who have a weak immune system and babies less than 1 month old.
- Do not share towels, play contact sports, or go swimming.
- Share your feelings about your pain with family and friends. Ask for their understanding.
Also, you can limit spreading the virus by:
- Keeping the rash covered
- Not touching or scratching the rash
- Washing your hands often
Warts
Warts are very common growths of the skin caused by infection with human papillomavirus (HPV). A wart is also called a verruca or papilloma and warty lesions may be described as verrucous. Warts usually go away on their own but may take months or even years.
A wart will usually have a flesh colored appearance and the skin forming the wart will be rough.
Warts don’t cause you any harm but some people find them itchy, painful or embarrassing. Warty lesions (verrucas) are more likely to be painful – like standing on a needle.
There are several different types of warts, each with a slightly different appearance:
Cutaneous warts have a hard, keratinous surface. A tiny black dot may be observed in the middle of each scaly spot, due to a thrombosed capillary blood vessel.
- Common warts – these are small, raised areas of skin, usually round (papules), with a rough surface of skin often looking like the top of a cauliflower (known as butcher’s warts) — papillomatous and hyperkeratotic surface ranging in size from 1 mm to larger than 1 cm. These warts often appear on backs of fingers or toes, your hands, around the nails —where they can distort nail growth, elbows and knees.
- Flat or Plane warts – these are flat warts that are usually yellow in color (see Figure 6 below). They are often numerous. They are most common in children and can often spread and group together. Multiple flat-topped, flesh-colored papules are typical. Flat warts distribution is grouped and assymetric, helping to distinguish them from seborrheic keratosis in the adult. Flat warts tend to occur on sun-exposed skin, e.g. the back of the hands, the forehead and shins. It appears the immune suppressive nature of sunlight predisposes to flat wart infection. Shaving (or scratching) the legs or beard area can cause multiple lesions through auto-innoculation. Linear flat warts distribution (pseudo-Koebner response) are common. In some cases, the lesions are brown and resemble nevi (freckles). Flat warts are mostly caused by HPV types 3 and 10.
- Plantar warts (verrucas) are warts that appear on your feet, usually on the sole, heel or toes — include tender inwardly growing and painful ‘myrmecia’ on the sole of the foot, and clusters of less painful mosaic warts. The weight of your body causes the wart to be pushed into the skin so a verruca will usually not be raised like other warts and may even cause some discomfort when walking. You may notice a white area of skin with a tiny black dot in the center. Plantar epidermoid cysts are associated with warts. Persistent plantar warts may rarely be complicated by the development of verrucous carcinoma.
- Filiform warts – filiform warts (finger like) are on a long stalk like a thread that usually appear on your eyelids, armpits or neck. Filiform warts commonly appear on the face. Filiform warts are also described as digitate warts.
- Mosaic warts – these grow in clusters and are most common on your hands and feet.
- Mucosal warts – Oral warts can affect the lips and even inside the cheeks, where they may be called squamous cell papillomas. They are softer than cutaneous warts.
Most warts will usually disappear within two years if left untreated, but they can sometimes cause discomfort and can look unpleasant. Warts are also contagious.
You can treat warts if they bother you, keep coming back or are painful. There are treatments available to buy over-the-counter without prescription from pharmacies and some supermarkets. Please speak to your doctor about other treatment options for warts and verrucas.
Warts causes
Common warts are caused by an infection with the human papillomavirus (HPV). More than 200 types of human papillomavirus (HPV) exist, but only a few cause warts or papillomas on your hands, which are non-cancerous tumors. The most common subtypes of human papillomavirus (HPV) are types 2, 3, 4, 27, 29, and 57. Human papillomavirus (HPV) is spread by direct skin-to-skin contact or autoinoculation. This means if a wart is scratched or picked, the viral particles may be spread to another area of skin. The incubation period can be as long as twelve months.
Other types of human papillomavirus (HPV) are more likely to cause warts on your feet and other areas of your skin and mucous membranes. Most types of HPV cause relatively harmless conditions such as common warts, while others about 40 of those human papillomavirus (HPV) types affect the genitals and may cause serious disease such as cancer of the cervix. Genital warts are spread through sexual contact (sexually transmitted infections) with an infected partner. Some of those can put you at risk or known to cause cancer, including cancers of the cervix (the base of the womb at the top of the vagina), vagina, vulva (the area around the outside of the vagina), penis, anus, and parts of the mouth and throat. Women are somewhat more likely than men to develop genital warts. Like warts that appear elsewhere on your body, genital warts are caused by the human papillomavirus (HPV). Some strains of genital HPV can cause genital warts, while others can cause cancer. About 14 million new genital HPV infections occur each year 260. In fact, the Centers for Disease Control and Prevention (CDC) estimates that more than 90% and 80%, respectively, of sexually active men and women will be infected with at least one type of HPV at some point in their lives 261. Around one-half of these infections are with a high-risk HPV type 262. Vaccines can help protect against certain strains of genital HPV.
You can get warts from skin-to-skin contact with people who have warts. If you have warts, you can spread the virus to other places on your own body. You can also get the wart virus indirectly by touching something that another person’s wart touched, such as a towel or exercise equipment. The virus usually spreads through breaks in your skin, such as a hangnail or a scrape. Biting your nails also can cause warts to spread on your fingertips and around your nails.
Each person’s immune system responds to the HPV virus differently, so not everyone who comes in contact with HPV develops warts.
Warts prevention
To reduce your risk of common warts:
- Avoid direct contact with warts. This includes your own warts.
- Don’t pick at warts. Picking may spread the virus.
- Don’t use the same emery board, pumice stone or nail clipper on your warts as you use on your healthy skin and nails.
- Don’t bite your fingernails. Warts occur more often in skin that has been broken. Nibbling the skin around your fingernails opens the door for the virus.
- Groom with care. Use a disposable emery board. And avoid brushing, clipping or shaving areas that have warts. If you must shave, use an electric razor.
- Wash your hands carefully after touching your warts or surfaces such as shared exercise equipment.
HPV is very common, so the only way to keep from becoming infected may be to completely avoid any contact of the areas of your body that can become infected (like the mouth, anus, and genitals) with those of another person. This means not having vaginal, oral, or anal sex, but it also means not allowing those areas to come in contact with someone else’s skin.
HPV vaccines can prevent infection with the types of HPV most likely to cause cancer and genital warts, although the vaccines are most effective when given at a younger age (in older children and teens). The Food and Drug Administration (FDA) has approved three vaccines to prevent HPV infection: Gardasil®, Gardasil® 9, and Cervarix®. These vaccines provide strong protection against new HPV infections, but they are not effective at treating established HPV infections or disease caused by HPV 263. Anecdotally, HPV vaccines have been reported to result in clearance of non-genital warts in some people.
If you are sexually active, limiting the number of sex partners and avoiding sexual activity with people who have had many other sex partners can help lower your risk of exposure to genital HPV. But again, HPV is very common, so having sexual contact with even one other person can put you at risk.
Condoms can offer some protection from HPV infection, but HPV might be on skin that’s not covered by the condom. And condoms must be used every time, from start to finish. The virus can spread during direct skin-to-skin contact before the condom is put on, and male condoms don’t protect the entire genital area, especially for women. The female condom covers more of the vulva in women, but hasn’t been studied as carefully for its ability to protect against HPV. Condoms are very helpful, though, in protecting against other infections that can be spread through sexual activity.
It’s usually not possible to know who has a mucosal HPV infection, and HPV is so common that even using these measures doesn’t guarantee that a person won’t get infected, but they can help lower the risk.
Warts diagnosis
Tests are rarely needed to diagnosis viral warts, as they are so common and have a characteristic appearance.
- Pinpoint dots (clotted capillaries) are revealed when the top of the wart is removed.
- Dermatoscopic examination is sometimes helpful to distinguish viral warts from other verrucous lesions such as seborrhoeic keratosis and skin cancer.
- Sometimes, viral warts are diagnosed on skin biopsy. The histopathological features of verruca vulgaris differ from that of plane warts.
Warts treatment
Many people don’t bother to treat viral warts because treatment can be more uncomfortable than the warts—they are hardly ever a serious problem. Warts that are very small and not troublesome can be left alone and in some cases they will regress on its own.
However, warts may be painful, and they often look ugly so cause embarrassment.
To get rid of them, you have to stimulate your body’s own immune system to attack the wart virus. Persistence with the treatment and patience is essential!
Topical treatment
Topical treatment includes wart paints containing salicylic acid or similar compounds, which work by removing the dead surface skin cells. Podophyllin is a cytotoxic agent used in some products, and must not be used in pregnancy or in women considering pregnancy.
The paint is normally applied once daily. Treatment with wart paint usually makes the wart smaller and less uncomfortable; 70% of warts resolve within twelve weeks of daily applications.
- Soften the wart by soaking in a bath or bowl of hot soapy water.
- Rub the wart surface with a piece of pumice stone or emery board.
- Apply wart paint or gel accurately, allowing it to dry.
- Covered with plaster or duct tape.
If the wart paint makes the skin sore, stop treatment until the discomfort has settled, then recommence as above. Take care to keep the chemical off normal skin.
Cryotherapy
Cryotherapy is normally repeated at one to two–week intervals. It is uncomfortable and may result in blistering for several day or weeks. Success is in the order of 70% after 3-4 months of regular freezing.
A hard freeze using liquid nitrogen might cause a permanent white mark or scar. It can also cause temporary numbness.
An aerosol spray with a mixture of dimethyl ether and propane (DMEP) can be purchased over the counter to freeze common and plantar warts. It is important to read and follow the instructions carefully.
Combining Immunotherapy with cryotherapy reduces the number of cryotherapy sessions.
Electrosurgery
Electrosurgery (curettage and cautery) is used for large and resistant warts. Under local anaesthetic, the growth is pared away and the base burned. The wound heals in two weeks or longer; even then 20% of warts can be expected to recur within a few months. This treatment leaves a permanent scar.
Other treatments
Other experimental treatments for recurrent, resistant or extensive warts include:
- Topical retinoids, such as tretinoin cream or adapalene gel
- The immune modulator, imiquimod cream
- Fluorouracil cream
- Bleomycin injections
- Oral retinoids
- Pulsed dye laser destruction of feeding blood vessels
- Photodynamic therapy
- Laser vaporisation
- H2 receptor antagonists
- Oral zinc oxide and zinc sulfate
- Applications of raw garlic or tea tree oil
- Immune stimulation using diphencyprone, squaric acid
- Immunotherapy with Candida albicans or tuberculin PPD
- Hypnosis
- Hyperthermia
- Occlusion with duct tape.
- Lawrence J. Eron, Benjamin A. Lipsky, Donald E. Low, Dilip Nathwani, Alan D. Tice, Gregory A. Volturo, Managing skin and soft tissue infections: expert panel recommendations on key decision points, Journal of Antimicrobial Chemotherapy, Volume 52, Issue suppl_1, November 2003, Pages i3–i17, https://doi.org/10.1093/jac/dkg466
- Stevens DL, Bryant AE. Impetigo, Erysipelas and Cellulitis. 2016 Feb 10. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes : Basic Biology to Clinical Manifestations [Internet]. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK333408
- Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996 Jan 25;334(4):240-5. doi: 10.1056/NEJM199601253340407
- Sigrídur Björnsdóttir, Magnús Gottfredsson, Anna S. Thórisdóttir, Gunnar B. Gunnarsson, Hugrún Ríkardsdóttir, Már Kristjánsson, Ingibjörg Hilmarsdóttir, Risk Factors for Acute Cellulitis of the Lower Limb: A Prospective Case-Control Study, Clinical Infectious Diseases, Volume 41, Issue 10, 15 November 2005, Pages 1416–1422, https://doi.org/10.1086/497127
- Dennis L. Stevens, Alan L. Bisno, Henry F. Chambers, E. Patchen Dellinger, Ellie J. C. Goldstein, Sherwood L. Gorbach, Jan V. Hirschmann, Sheldon L. Kaplan, Jose G. Montoya, James C. Wade, Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America, Clinical Infectious Diseases, Volume 59, Issue 2, 15 July 2014, Pages e10–e52, https://doi.org/10.1093/cid/ciu296
- Karppelin M, Siljander T, Vuopio-Varkila J, Kere J, Huhtala H, Vuento R, Jussila T, Syrjänen J. Factors predisposing to acute and recurrent bacterial non-necrotizing cellulitis in hospitalized patients: a prospective case-control study. Clin Microbiol Infect. 2010 Jun;16(6):729-34. doi: 10.1111/j.1469-0691.2009.02906.x
- Efstratiou A, Lamagni T. Epidemiology of Streptococcus pyogenes. 2016 Feb 10 [Updated 2017 Apr 3]. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes : Basic Biology to Clinical Manifestations [Internet]. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK343616
- Cellulitis: All You Need to Know. https://www.cdc.gov/groupastrep/diseases-public/cellulitis.html
- Dire DJ, Coppola M, Dwyer DA, Lorette JJ, Karr JL. Prospective evaluation of topical antibiotics for preventing infections in uncomplicated soft-tissue wounds repaired in the ED. Acad Emerg Med. 1995;2(1):4–10.
- Sibbald RG, Goodman L, Woo KY, et al. Special considerations in wound bed preparation 2011: an update. Adv Skin Wound Care. 2011;24(9):415–436.
- Okan D, Woo K, Ayello EA, Sibbald G. The role of moisture balance in wound healing. Adv Skin Wound Care. 2007;20(1):39–53.
- Agren MS, Karlsmark T, Hansen JB, Rygaard J. Occlusion versus air exposure on full-thickness biopsy wounds. J Wound Care. 2001;10(8):301–304.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13–15.
- Immunization Action Coalition. Ask the experts: diseases & vaccines. Diphtheria, tetanus, and pertussis. http://www.immunize.org/askexperts/experts_per.asp
- Cellulitis. https://dermnetnz.org/topics/cellulitis
- Impetigo. Medline Plus. https://medlineplus.gov/impetigo.html
- Impetigo Care. American Academy of Pediatrics. https://www.healthychildren.org/English/health-issues/conditions/skin/pages/Impetigo-Care.aspx
- Bowen AC, Mahé A, Hay RJ, Andrews RM, Steer AC, Tong SY, Carapetis JR. The Global Epidemiology of Impetigo: A Systematic Review of the Population Prevalence of Impetigo and Pyoderma. PLoS One. 2015 Aug 28;10(8):e0136789. doi: 10.1371/journal.pone.0136789
- Pereira LB. Impetigo – review. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9. doi: 10.1590/abd1806-4841.20142283
- Bryant AE, Stevens DL. Streptococcus pyogenes. In Bennett J, Dolin R, Blaser M, editors. 8th Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia (PA): Elsevier; 2015:2:2285–300.
- Carapetis JR. The current evidence for the burden of group A streptococcal diseases. World Health Organization. Geneva. 2005. https://apps.who.int/iris/handle/10665/69063
- Nardi NM, Schaefer TJ. Impetigo. [Updated 2022 Oct 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430974
- Feaster T, Singer JI. Topical therapies for impetigo. Pediatr Emerg Care. 2010 Mar;26(3):222-7; quiz 228-31. doi: 10.1097/PEC.0b013e3181d1e71b
- Bangert, S., Levy, M. and Hebert, A.A. (2012), Bacterial Resistance and Impetigo Treatment Trends: A Review. Pediatric Dermatology, 29: 243-248. https://doi.org/10.1111/j.1525-1470.2011.01700.x
- Cole C, Gazewood J. Diagnosis and treatment of impetigo. Am Fam Physician. 2007 Mar 15;75(6):859-64. https://www.aafp.org/pubs/afp/issues/2007/0315/p859.html
- Hochedez P, Canestri A, Lecso M, Valin N, Bricaire F, Caumes E. Skin and soft tissue infections in returning travelers. Am J Trop Med Hyg. 2009 Mar;80(3):431-4.
- Luby SP, Agboatwalla M, Feikin DR, Painter J, Billhimer W, Altaf A, Hoekstra RM. Effect of handwashing on child health: a randomised controlled trial. Lancet. 2005 Jul 16-22;366(9481):225-33. doi: 10.1016/S0140-6736(05)66912-7
- Taiaroa G, Matalavea B, Tafuna’i M, Lacey JA, Price DJ, Isaia L, Leaupepe H, Viali S, Lee D, Gorrie CL, Williamson DA, Jack S. Scabies and impetigo in Samoa: A school-based clinical and molecular epidemiological study. Lancet Reg Health West Pac. 2020 Dec 29;6:100081. doi: 10.1016/j.lanwpc.2020.100081
- Mitchell E, Bell S, Thean LJ, Sahukhan A, Kama M, Koroivueti A, Kaldor J, Steer A, Romani L. Community perspectives on scabies, impetigo and mass drug administration in Fiji: A qualitative study. PLoS Negl Trop Dis. 2020 Dec 4;14(12):e0008825. doi: 10.1371/journal.pntd.0008825
- Weinberg JM, Tyring SK. Retapamulin: an antibacterial with a novel mode of action in an age of emerging resistance to Staphylococcus aureus. J Drugs Dermatol. 2010 Oct;9(10):1198-204.
- Ilyas M, Tolaymat A. Changing epidemiology of acute post-streptococcal glomerulonephritis in Northeast Florida: a comparative study. Pediatr Nephrol. 2008 Jul;23(7):1101-6. doi: 10.1007/s00467-008-0778-1
- George A, Rubin G. A systematic review and meta-analysis of treatments for impetigo. Br J Gen Pract. 2003 Jun;53(491):480-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1314624/pdf/12939895.pdf
- Dennis L. Stevens, Alan L. Bisno, Henry F. Chambers, E. Patchen Dellinger, Ellie J. C. Goldstein, Sherwood L. Gorbach, Jan V. Hirschmann, Sheldon L. Kaplan, Jose G. Montoya, James C. Wade, Executive Summary: Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America, Clinical Infectious Diseases, Volume 59, Issue 2, 15 July 2014, Pages 147–159, https://doi.org/10.1093/cid/ciu444
- Brown, J., Shriner, D.L., Schwartz, R.A. and Janniger, C.K. (2003), Impetigo: an update. International Journal of Dermatology, 42: 251-255. https://doi.org/10.1046/j.1365-4362.2003.01647.x
- How to Treat Impetigo and Control This Common Skin Infection. U.S. Food and Drug Administration. https://www.fda.gov/forconsumers/consumerupdates/ucm048837.htm
- George A, Rubin G. A systematic review and meta-analysis of treatments for impetigo. Br J Gen Pract. 2003;53(491):480–487.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;(1):CD003261.
- Feaster T, Singer JI. Topical therapies for impetigo. Pediatr Emerg Care. 2010;26(3):222–227, quiz 228–231.
- How to Treat Impetigo and Control This Common Skin Infection. U.S. Food and Drug Administration. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm048837.htm
- Impetigo: Diagnosis and Treatment. Am Fam Physician. 2014 Aug 15;90(4):229-235. http://www.aafp.org/afp/2014/0815/p229.html
- Christensen OB, Anehus S. Hydrogen peroxide cream: an alternative to topical antibiotics in the treatment of impetigo contagiosa. Acta Derm Venereol. 1994;74(6):460–462.
- Martin KW, Ernst E. Herbal medicines for treatment of bacterial infections: a review of controlled clinical trials. J Antimicrob Chemother. 2003;51(2):241–246.
- Sharquie KE, al-Turfi IA, al-Salloum SM. The antibacterial activity of tea in vitro and in vivo (in patients with impetigo contagiosa). J Dermatol. 2000;27(11):706–710.
- Caelli M, Porteous J, Carson CF, Heller R, Riley TV. Tea tree oil as an alternative topical decolonization agent for methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2000;46(3):236–237.
- Ebert, A, Monod, M, Salamin, K, et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicentre study. Mycoses. 2020; 63: 717– 728. https://doi.org/10.1111/myc.13091
- Pai V, Ganavalli A, Kikkeri NN. Antifungal Resistance in Dermatology. Indian J Dermatol. 2018 Sep-Oct;63(5):361-368. doi: 10.4103/ijd.IJD_131_17
- Antifungal drug resistance. https://dermnetnz.org/topics/antifungal-drug-resistance
- Havlickova, B., Czaika, V.A. and Friedrich, M. (2008), Epidemiological trends in skin mycoses worldwide. Mycoses, 51: 2-15. https://doi.org/10.1111/j.1439-0507.2008.01606.x
- Verma S. Steroid modified tinea. BMJ. 2017 Mar 8;356:j973. doi: 10.1136/bmj.j973
- Gupta AK, Jain HC, Lynde CW, Macdonald P, Cooper EA, Summerbell RC. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter canadian survey of 15,000 patients. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):244-8. doi: 10.1067/mjd.2000.104794
- Leung AK, Lam JM, Leong KF, Hon KL. Tinea corporis: an updated review. Drugs Context. 2020 Jul 20;9:2020-5-6. doi: 10.7573/dic.2020-5-6
- Achterman RR, White TC. A foot in the door for dermatophyte research. PLoS Pathog. 2012;8(3):e1002564. doi: 10.1371/journal.ppat.1002564
- Errichetti E, Stinco G. Dermoscopy in tinea manuum. An Bras Dermatol. 2018 Jun;93(3):447-448. doi: 10.1590/abd1806-4841.20186366
- Ringworm. Healthy Pets, Healthy People. https://www.cdc.gov/healthypets/diseases/ringworm.html
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and Management of Tinea Infections. Am Fam Physician. 2014;90(10):702-711. https://www.aafp.org/pubs/afp/issues/2014/1115/p702.html
- Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev. 1995 Apr;8(2):240-59. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC172857/pdf/080240.pdf
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008 Nov-Dec;166(5-6):335-52. doi: 10.1007/s11046-008-9100-9
- World Health Organization. Epidemiology and management of common skin diseases in children in developing countries. Department of Child and Adolescent Health and Development. 2005. 54 WHO reference number WHO/FCH/CAH/05.12.
- Abdel-Rahman SM, Farrand N, Schuenemann E, Stering TK, Preuett B, Magie R, Campbell A. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010 May;125(5):966-73. doi: 10.1542/peds.2009-2522
- Ameen M. Epidemiology of superficial fungal infections. Clin Dermatol. 2010 Mar 4;28(2):197-201. doi: 10.1016/j.clindermatol.2009.12.005
- Yadgar RJ, Bhatia N, Friedman A. Cutaneous fungal infections are commonly misdiagnosed: A survey-based study. J Amer Acad Dermatol. 2017 March; 76(3): 562-563. https://doi.org/10.1016/j.jaad.2016.09.041
- Levitt JO, Levitt BH, Akhavan A, Yanofsky H. The Sensitivity and Specificity of Potassium Hydroxide Smear and Fungal Culture Relative to Clinical Assessment in the Evaluation of Tinea Pedis: A Pooled Analysis. Derma Res and Prac. 2010 Jun; 2010. https://doi.org/10.1155/2010/764843
- Hainer BL. Dermatophyte infections. Am Fam P 2003 Jan 1;67(1):101-8. https://www.aafp.org/pubs/afp/issues/2003/0101/p101.html
- Crawford F, Hollis S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst Rev. 2007 Jul 18;2007(3):CD001434. doi: 10.1002/14651858.CD001434.pub2
- Saunte DML, Hare RK, Jorgensen KM, Jorgensen R, Deleuran M, Zachariae CO, et al. Emerging Terbinafine Resistance in Trichophyton: Clinical Characteristics, Squalene Epoxidase Gene Mutations, and a Reliable EUCAST Method for Detection. Antimic Agent and Chemo. 2019 Sept; 63(10). https://doi.org/10.1128/AAC.01126-19
- Gupta AK, Renaud HJ, Quinlan EM, Shear NH, Piguet V. The Growing Problem of Antifungal Resistance in Onychomycosis and Other Superficial Mycoses. Am J Clin Dermatol. 2021 Mar;22(2):149-157. doi: 10.1007/s40257-020-00580-6
- Astvad KMT, Hare RK, Jørgensen KM, Saunte DML, Thomsen PK, Arendrup MC. Increasing Terbinafine Resistance in Danish Trichophyton Isolates 2019–2020. Journal of Fungi. 2022; 8(2):150. https://doi.org/10.3390/jof8020150
- Michaels BD, Del Rosso JQ. Tinea capitis in infants: recognition, evaluation, and management suggestions. J Clin Aesthet Dermatol. 2012 Feb;5(2):49-59. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3315884
- Ringworm Information for Healthcare Professionals. https://www.cdc.gov/fungal/diseases/ringworm/health-professionals.html
- Allen HB, Honig PJ, Leyden JJ, McGinley KJ. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1982 Jan;69(1):81-3.
- Onychomycosis: current and future therapies. Cutis. 2014 Feb;93(2):60-3.
- Gupta AK, Venkataraman M, Renaud HJ, Summerbell R, Shear NH, Piguet V. A Paradigm Shift in the Treatment and Management of Onychomycosis. Skin Append Disord. 2021;7(5). https://doi.org/10.1159/000516112
- Ghannoum MA, Hajjeh RA, Scher R, Konnikov N, Gupta AK, Summerbell R, Sullivan S, Daniel R, Krusinski P, Fleckman P, Rich P, Odom R, Aly R, Pariser D, Zaiac M, Rebell G, Lesher J, Gerlach B, Ponce-De-Leon GF, Ghannoum A, Warner J, Isham N, Elewski B. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000 Oct;43(4):641-8. doi: 10.1067/mjd.2000.107754
- Tosti A, Elewski BE. Onychomycosis: Practical Approaches to Minimize Relapse and Recurrence. Skin Appendage Disord. 2016 Sep;2(1-2):83-87. doi: 10.1159/000448056
- Renati S, Cukras A, Bigby M. Pityriasis versicolor. BMJ. 2015;350:h1394. doi: 10.1136/bmj.h1394
- Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel) 2015;1(1):13–29. doi: 10.3390/jof1010013
- Leung AKC. Pityriasis versicolor. In: Lang F, editor. The Encyclopedia of Molecular Mechanisms of Disease. Berlin: Springer-Verlag; 2009. pp. 1652–1654.
- Alam HS, Ward JM, Davis LS. Generalized tinea versicolor following initiation of ixekizumab therapy. JAAD Case Rep. 2021;18:54–56. doi: 10.1016/j.jdcr.2021.10.008
- Brandi N, Starace M, Alessandrini A, Piraccini BM. Tinea versicolor of the neck as side effect of topical steroids for alopecia areata. J Dermatolog Treat. 2019;30(8):757–759. doi: 10.1080/09546634.2019.1573308
- Cohen L, Seminario-Vidal L, Lockey RF. Dermatologic problems commonly seen by the allergist/immunologist. J Allergy Clin Immunol Pract. 2020;8(1):102–112. doi: 10.1016/j.jaip.2019.07.019
- Tan C, Zhu WY, Min ZS. Blaschkoid pityriasis versicolor. Mycoses. 2010;53(4):366–368. doi: 10.1111/j.1439-0507.2009.01721.x
- Terragni L, Lasagni A, Oriani A, Gelmetti C. Pityriasis versicolor in the pediatric age. Pediatr Dermatol. 1991;8(1):9–12. doi: 10.1111/j.1525-1470.1991.tb00831.x
- Sandhu K, Kanwar AJ. Extensive pityriasis versicolor of the face. J Dermatol. 2004;31(3):258–259. doi: 10.1111/j.1346-8138.2004.tb00670.x
- Terragni L, Lasagni A, Oriani A. Pityriasis versicolor of the face. Mycoses. 1991;34(7–8):345–347. doi: 10.1111/j.1439-0507.1991.tb00674.x
- Romano C, Feci L, Mancianti F, Fimiani M. Perineal and genital pityriasis versicolor due to Malassezia globosa. J Eur Acad Dermatol Venereol. 2015;29(9):1857–1858. doi: 10.1111/jdv.12547
- Greco V, Megna M, Luciano MA, Fabbrocini G. Pityriasis versicolor with uncommon localizations. G Ital Dermatol Venereol. 2020;155(6):786–787. doi: 10.23736/S0392-0488.18.06120-5
- Cinelli E, Vastarella M, Fabbrocini G, Gallo L. Erythrasmoid pityriasis versicolor with exclusive involvement of the pubis and inguinal region. Ital J Dermatol Venerol. 2021;156(Suppl 1 to 6):11–12. doi: 10.23736/S2784-8671.18.06208-9
- Nevas J. Tinea versicolor: understanding effective treatment options. Nurse Pract. 2012;37(1):11–13. doi: 10.1097/01.NPR.0000409912.87769.f0
- Huang WW, Tharp MD. A case of tinea versicolor of the eyelids. Pediatr Dermatol. 2013;30(6):e242–e243. doi: 10.1111/j.1525-1470.2012.01753.x
- Khaddar RK, Cherif F, Ben Hadid R, Mokni M, Ben Osman A. Penile shaft involvement in pityriasis versicolor. Acta Dermatovenerol Alp Pannonica Adriat. 2008 Jun;17(2):86-9. https://s3-eu-west-1.amazonaws.com/thejournalhub/10.15570/archive/acta-apa-08-2/9.pdf
- Gupta AK, Bluhm R, Summerbell R. Pityriasis versicolor. J Eur Acad Dermatol Venereol. 2002;16(1):19–33. doi: 10.1046/j.1468-3083.2002.00378.x
- Gupta AK, Batra R, Bluhm R, Faergemann J. Pityriasis versicolor. Dermatol Clin. 2003;21(3):413–429. v–vi. doi: 10.1016/s0733-8635(03)00039-1
- Hellgren L, Vincent J. The incidence of tinea versicolor in central Sweden. J Med Microbiol. 1983;16(4):501–502. doi: 10.1099/00222615-16-4-501
- Ghosh SK, Dey SK, Saha I, Barbhuiya JN, Ghosh A, Roy AK. Pityriasis versicolor: a clinicomycological and epidemiological study from a tertiary care hospital. Indian J Dermatol. 2008;53(4):182–185. doi: 10.4103/0019-5154.44791
- Karakaş M, Turaç-Biçer A, Ilkit M, Durdu M, Seydaoğlu G. Epidemiology of pityriasis versicolor in Adana, Turkey. J Dermatol. 2009;36(7):377–382. doi: 10.1111/j.1346-8138.2009.00663.x
- Leung AKC, Barankin B. Tinea versicolor in a 69-year-old man: an uncommon finding. Sch J Med Case Rep. 2015;3(10A):993–994.
- Rao GS, Kuruvilla M, Kumar P, Vinod V. Clinico-epidermiological studies on tinea versicolor. Indian J Dermatol Venereol Leprol. 2002 Jul-Aug;68(4):208-9.
- Bélec L, Testa J, Bouree P. Pityriasis versicolor in the Central African Republic: a randomized study of 144 cases. J Med Vet Mycol. 1991;29(5):323–329. doi: 10.1080/02681219180000491
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137–141. doi: 10.1111/ijd.12345
- Burke RC. Tinea versicolor: susceptibility factors and experimental infection in human beings. J Invest Dermatol. 1961;36:389–402. doi: 10.1038/jid.1961.60
- Gupta AK, Kogan N, Batra R. Pityriasis versicolor: a review of pharmacological treatment options. Expert Opin Pharmacother. 2005;6(2):165–178. doi: 10.1517/14656566.6.2.165
- Gupta AK, Lyons DC. Pityriasis versicolor: an update on pharmacological treatment options. Expert Opin Pharmacother. 2014;15(12):1707–1713. doi: 10.1517/14656566.2014.931373
- Gupta AK, Lane D, Paquet M. Systematic review of systemic treatments for tinea versicolor and evidence-based dosing regimen recommendations. J Cutan Med Surg. 2014;18(2):79–90. doi: 10.2310/7750.2013.13062
- Pantazidou A, Tebruegge M. Recurrent tinea versicolor: treatment with itraconazole or fluconazole? Arch Dis Child. 2007;92(11):1040–1042. doi: 10.1136/adc.2007.124958
- Leung AK, Barankin B, Lam JM, Leong KF, Hon KL. Tinea versicolor: an updated review. Drugs Context. 2022 Nov 14;11:2022-9-2. doi: 10.7573/dic.2022-9-2
- Dyląg M, Leniak E, Gnat S, Szepietowski JC, Kozubowski L. A case of anti-pityriasis versicolor therapy that preserves healthy mycobiome. BMC Dermatol. 2020;20:9. doi: 10.1186/s12895-020-00106-x
- Framil VM, Melhem MS, Szeszs MW, Zaitz C. New aspects in the clinical course of pityriasis versicolor. An Bras Dermatol. 2011;86(6):1135–1140. doi: 10.1590/s0365-05962011000600011
- Jena DK, Sengupta S, Dwari BC, Ram MK. Pityriasis versicolor in the pediatric age group. Indian J Dermatol Venereol Leprol. 2005;71(4):259–261. doi: 10.4103/0378-6323.16618
- Faergemann J. Pityrosporum species as a cause of allergy and infection. Allergy. 1999;54(5):413–419. doi: 10.1034/j.1398-9995.1999.00089.x
- Duy Nguyen B, Thi Thanh Vo H, Dinh Thi Thanh M, et al. Epidemiological characterization of pityriasis versicolor and distribution of Malassezia species among students in Hai Phong city, Vietnam. Curr Med Mycol. 2020;6(2):11–17. doi: 10.18502/CMM.6.2.2838
- Ogunbiyi AO, George AO. Pityriasis versicolor: Current concepts in Aetiology and Management. Niger Postgrad Med J. 2005 Sep;12(3):183-8.
- Petry V, Tanhausen F, Weiss L, Milan T, Mezzari A, Weber MB. Identification of Malassezia yeast species isolated from patients with pityriasis versicolor. An Bras Dermatol. 2011;86(4):803–806. doi: 10.1590/s0365-05962011000400032
- Romero-Sandoval K, Costa AA, Teixeira Sousa MG, et al. Recurrent and disseminated pityriasis versicolor: a novel clinical form consequent to Malassezia-host interaction? Med Hypotheses. 2017;109:139–144. doi: 10.1016/j.mehy.2017.10.013
- Kurniadi I, Hendra Wijaya W, Timotius KH. Malassezia virulence factors and their role in dermatological disorders. Acta Dermatovenerol Alp Pannonica Adriat. 2022 Jun;31(2):65-70. https://acta-apa.mf.uni-lj.si/journals/acta-dermatovenerol-apa/papers/10.15570/actaapa.2022.8/actaapa.2022.8.pdf
- Aghaei Gharehbolagh S, Kordbacheh P, Hashemi SJ, et al. MGL_3741 gene contributes to pathogenicity of Malassezia globosa in pityriasis versicolor. Mycoses. 2018;61(12):938–944. doi: 10.1111/myc.12840
- Kilinc F, Akbas A, Sener S, Ergin M, Baran P, Metin A. The effect of tinea versicolor on thiol/disulphide homeostasis. Postepy Dermatol Alergol. 2018;35(3):299–303. doi: 10.5114/ada.2018.76227
- Kutlu Ö, Doğan Z, Ekşioğlu HM, Kekilli M. Relationship between helicobacter pylori infection and pityriasis versicolor: can helicobacter pylori infection be a new etiologic factor for pityriasis versicolor? Turk J Med Sci. 2020;50(4):771–775. doi: 10.3906/sag-1910-48
- Miotto IZ, De Oliveira WRP. Epidermodysplasia verruciformis: report of two patients with autosomal dominant inheritance. Dermatol Online J. 2021;27(2) 13030/qt53t469nn
- Charles CR, Sire DJ, Johnson BL, Beidler JG. Hypopigmentation in tinea versicolor: a histochemical and electronmicroscopic study. Int J Dermatol. 1973;12(1):48–58. doi: 10.1111/j.1365-4362.1973.tb00212.x
- Galadari I, el Komy M, Mousa A, Hashimoto K, Mehregan AH. Tinea versicolor: histologic and ultrastructural investigation of pigmentary changes. Int J Dermatol. 1992;31(4):253–256. doi: 10.1111/j.1365-4362.1992.tb03565.x
- Karaoui R, Bou-Resli M, Al-Zaid NS, Mousa A. Tinea versicolor: ultrastructural studies on hypopigmented and hyperpigmented skin. Dermatologica. 1981;162(2):69–85. doi: 10.1159/000250253
- Nazzaro-Porro M, Passi S. Identification of tyrosinase inhibitors in cultures of Pityrosporum. J Invest Dermatol. 1978;71(3):205–208. doi: 10.1111/1523-1747.ep12547184
- Aljabre SH, Alzayir AA, Abdulghani M, Osman OO. Pigmentary changes of tinea versicolor in dark-skinned patients. Int J Dermatol. 2001;40(4):273–275. doi: 10.1046/j.1365-4362.2001.01201.x
- Dotz WI, Henrikson DM, Yu GS, Galey CI. Tinea versicolor: a light and electron microscopic study of hyperpigmented skin. J Am Acad Dermatol. 1985;12(1 Pt 1):37–44. doi: 10.1016/s0190-9622(85)70006-0
- Han A, Calcara DA, Stoecker WV, Daly J, Siegel DM, Shell A. Evoked scale sign of tinea versicolor. Arch Dermatol. 2009;145(9):1078. doi: 10.1001/archdermatol.2009.203
- Rivard SC. Pityriasis versicolor: avoiding pitfalls in disease diagnosis and therapy. Mil Med. 2013;178(8):904–906. doi: 10.7205/MILMED-D-13-00057
- Choi FD, Juhasz MLW, Atanaskova Mesinkovska N. Topical ketoconazole: a systematic review of current dermatological applications and future developments. J Dermatolog Treat. 2019;30(8):760–771. doi: 10.1080/09546634.2019.1573309
- Mayser P, Rieche I. Rapid reversal of hyperpigmentation in pityriasis versicolor upon short-term topical cycloserine application. Mycoses. 2009;52(6):541–543. doi: 10.1111/j.1439-0507.2009.01784.x
- Mostafa WZ, Assaf MI, Ameen IA, El Safoury OS, Al Sulh SA. Hair loss in pityriasis versicolor lesions: a descriptive clinicopathological study. J Am Acad Dermatol. 2013;69(1):e19–e23. doi: 10.1016/j.jaad.2012.03.004
- Cherniack EP. Bugs as drugs, Part 1: Insects: the “new” alternative medicine for the 21st century? Altern Med Rev. 2010 Jul;15(2):124-35.
- Carmo ES, Pereira Fde O, Cavalcante NM, Gayoso CW, Lima Ede O. Treatment of pityriasis versicolor with topical application of essential oil of Cymbopogon citratus (DC) Stapf – therapeutic pilot study. An Bras Dermatol. 2013;88(3):381–385. doi: 10.1590/abd1806-4841.20131800
- Mirzaii M, Yaeghoobi M, Afzali M, Amirkhalili N, Mahmoodi M, Sajirani EB. Antifungal activities of quince seed mucilage hydrogel decorated with essential oils of Nigella sativa, Citrus sinensis and Cinnamon verum. Iran J Microbiol. 2021;13(3):352–359. doi: 10.18502/ijm.v13i3.6398
- Lone AH, Ahmad T, Anwar M, Sofi G. Clinical efficacy and safety of a pharmacopial polyherbal Unani formulation in pityriasis versicolor: a comparative randomized single-blind study. J Altern Complement Med. 2012;18(10):978–982. doi: 10.1089/acm.2011.0520
- Kagisha V, Djang’eing’a RM, Muganga R, et al. Pentas longiflora Oliv. (Rubiaceae), a plant used in the treatment of pityriasis versicolor in Rwanda: chemical composition and standardization of leaves and roots. Fitoterapia. 2021;153:104974. doi: 10.1016/j.fitote.2021.104974
- Sherifat KO, Itohan AM, Adeola SO, Adeola KM, Aderemi OL. Antifungal activity of Acalypha wilkesiana: a preliminary study of fungal isolates of clinical significance. Afr J Infect Dis. 2021;16(1):21–30. doi: 10.21010/Ajid.v16i1.4
- Rad F, Aala F, Reshadmanesh N, Yaghmaie R. Randomized comparative clinical trial of Artemisia sieberi 5% lotion and clotrimazole 1% lotion for the treatment of pityriasis versicolor. Indian J Dermatol. 2008;53(3):115–118. doi: 10.4103/0019-5154.43209
- Jowkar F, Jamshidzadeh A, Pakniyat S, Namazi MR. Efficacy of nitric oxide-liberating cream on pityriasis versicolor. J Dermatolog Treat. 2010;21(2):93–96. doi: 10.3109/09546630902887229
- Nashwa RK, Ahmed EB, Nemr WA. Comparative study between topically applied irradiated human amniotic membrane in combination with tea tree oil versus topical tioconazole in pityraisis versicolor treatment. Cell Tissue Bank. 2020;21(2):313–320. doi: 10.1007/s10561-020-09824-5
- Molluscum Contagiosum. https://www.cdc.gov/poxvirus/molluscum-contagiosum/index.html
- Leung AKC, Barankin B, Hon KLE. Molluscum contagiosum: an update. Recent Pat Inflamm Allergy Drug Discov. 2017;11(1):22–31. doi: 10.2174/1872213X11666170518114456
- Gerlero P, Hernandez-Martin A. Update on the treatment of molluscum contagiosum in children. Actas Dermosifiliogr. 2018;109(5):408–415. doi: 10.1016/j.ad.2018.01.007
- Meza-Romero R, Navarrete-Dechent C, Downey C. Molluscum contagiosum: an update and review of new perspectives in etiology, diagnosis, and treatment. Clin Cosmet Investig Dermatol. 2019 May 30;12:373-381. doi: 10.2147/CCID.S187224
- Rogers M, Barnetson RSC. Diseases of the skin. In: Campbell AGM, McIntosh N, et al. editor(s). Forfar and Arneil’s Textbook of Pediatrics. 5th Edition. New York: Churchill Livingstone, 1998:1633–5.
- Peterson AR, Nash E, Anderson BJ. Infectious disease in contact sports. Sports Health. 2019;11(1):47–58. doi: 10.1177/1941738118789954
- Koopman RJ, van Merriënboer FC, Vreden SG, Dolmans WM. Molluscum contagiosum; a marker for advanced HIV infection. Br J Dermatol. 1992 May;126(5):528-9. doi: 10.1111/j.1365-2133.1992.tb11835.x
- Czelusta A, Yen-Moore A, Van der Straten M, Carrasco D, Tyring SK. An overview of sexually transmitted diseases. Part III. Sexually transmitted diseases in HIV-infected patients. J Am Acad Dermatol. 2000;43(3):409–432; quiz 33-6 DOI: 10.1067/mjd.2000.105158
- Kaufman WS, Ahn CS, Huang WW. Molluscum contagiosum in immunocompromised patients: AIDS presenting as molluscum contagiosum in a patient with psoriasis on biologic therapy. Cutis. 2018 Feb;101(2):136-140. https://cdn.mdedge.com/files/s3fs-public/Document/February-2018/CT101002136.PDF
- Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31(2):130–136. doi: 10.1093/fampra/cmt075
- Dohil MA, Lin P, Lee J, Lucky AW, Paller AS, Eichenfield LF. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54(1):47–54. doi: 10.1016/j.jaad.2005.08.035
- Olsen JR, Piguet V, Gallacher J, Francis NA. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66(642):e53–e58. doi: 10.3399/bjgp15X688093
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018 Sep;102(3):191-194. https://cdn.mdedge.com/files/s3fs-public/Document/August-2018/CT102003191.PDF
- Braue A, Ross G, Varigos G, Kelly H. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr Dermatol. 2005;22(4):287–294. doi: 10.1111/j.1525-1470.2005.22401.x
- Berger EM, Orlow SJ, Patel RR, Schaffer JV. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148(11):1257–1264. doi: 10.1001/archdermatol.2012.2414
- Manti S, Amorini M, Cuppari C, et al. Filaggrin mutations and Molluscum contagiosum skin infection in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(5):446–451. doi: 10.1016/j.anai.2017.07.019
- Hayashida S, Furusho N, Uchi H, et al. Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci. 2010;60(3):173–178. doi: 10.1016/j.jdermsci.2010.09.003
- Basu S, Kumar A. Giant molluscum contagiosum – a clue to the diagnosis of human immunodeficiency virus infection. J Epidemiol Glob Health. 2013;3(4):289–291. doi: 10.1016/j.jegh.2013.06.002
- Vora RV, Pilani AP, Kota RK. Extensive giant molluscum contagiosum in a HIV positive patient. J Clin Diagn Res. 2015;9(11):Wd01–wd02. doi: 10.7860/JCDR/2015/15107.6797
- Husak R, Garbe C, Orfanos CE. [Mollusca contagiosa in HIV infection. Clinical manifestation, relation to immune status and prognostic value in 39 patients]. Hautarzt. 1997;48(2):103–109. doi: 10.1007/s001050050554
- van der Wouden JC, van der Sande R, Kruithof EJ, Sollie A, van Suijlekom-Smit LW, Koning S. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2017 May 17;5(5):CD004767. doi: 10.1002/14651858.CD004767.pub4
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131(5):e1650–e1653. doi: 10.1542/peds.2012-2933
- Trcko K, Hosnjak L, Kusar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum. Open Forum Infect Dis. 2018;5(11):ofy298. doi: 10.1093/ofid/ofy298
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13(10):877–888. doi: 10.1016/S1473-3099(13)70109-9
- Zorec TM, Kutnjak D, Hosnjak L, et al. New Insights into the evolutionary and genomic landscape of Molluscum Contagiosum Virus (MCV) based on nine MCV1 and six MCV2 complete genome sequences. Viruses. 2018;10(11). doi: 10.3390/v10110586
- Brady G, Haas DA, Farrell PJ, Pichlmair A, Bowie AG. Molluscum contagiosum virus protein MC005 inhibits NF-kappaB activation by targeting NEMO-regulated IkappaB kinase activation. J Virol. 2017;91(15). doi: 10.1128/JVI.00955-17
- Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201–252. doi: 10.1016/bs.aivir.2014.11.004
- Biswas S, Smith GL, Roy EJ, Ward B, Shisler JL. A comparison of the effect of molluscum contagiosum virus MC159 and MC160 proteins on vaccinia virus virulence in intranasal and intradermal infection routes. J Gen Virol. 2018;99(2):246–252. doi: 10.1099/jgv.0.001006
- Brady G, Haas DA, Farrell PJ, Pichlmair A, Bowie AG. Poxvirus protein MC132 from molluscum contagiosum virus inhibits NF-B activation by targeting p65 for degradation. J Virol. 2015;89(16):8406–8415. doi: 10.1128/JVI.00799-15
- Gurtler C, Bowie AG. Innate immune detection of microbial nucleic acids. Trends Microbiol. 2013;21(8):413–420. doi: 10.1016/j.tim.2013.04.004
- Molluscum contagiosum. https://dermnetnz.org/topics/molluscum-contagiosum
- Berbegal-DeGracia L, Betlloch-Mas I, DeLeon-Marrero FJ, Martinez-Miravete MT, Miralles-Botella J. Neonatal Molluscum contagiosum: five new cases and a literature review. Australas J Dermatol. 2015;56(2):e35–e38. doi: 10.1111/ajd.12127
- Mira-Perceval Juan G, Alcala Minagorre PJ, Betlloch Mas I, Sanchez Bautista A. [Molluscum contagiosum due to vertical transmission]. An Esp Pediatr. 2017;86(5):292–293. doi: 10.1016/j.anpedi.2015.12.014
- Ives C, Green M, Wright T. Molluscum contagiosum: a rare nipple lesion. Breast J. 2017;23(1):107–108. doi: 10.1111/tbj.12693
- Hoyt BS, Tschen JA, Cohen PR. Molluscum contagiosum of the areola and nipple: case report and literature review. Dermatol Online J. 2013 Jul 14;19(7):18965. https://doi.org/10.5070/D3197018965
- Ringeisen AL, Raven ML, Barney NP. Bulbar conjunctival molluscum contagiosum. Ophthalmology. 2016;123(2):294. doi: 10.1016/j.ophtha.2015.11.022
- Ma H, Yang H, Zhou Y, Jiang L. Molluscum contagiosum on the lip. J Craniofac Surg. 2015;26(7):e681–e682. doi: 10.1097/SCS.0000000000002187
- Serin S, Bozkurt Oflaz A, Karabagli P, Gedik S, Bozkurt B. Eyelid molluscum contagiosum lesions in two patients with unilateral chronic conjunctivitis. Turk Oftalmol Derg. 2017;47(4):226–230. doi: 10.4274/tjo.52138
- Kim HK, Jang WS, Kim BJ, Kim MN. Rare manifestation of giant molluscum contagiosum on the scalp in old age. Ann Dermatol. 2013;25(1):109–110. doi: 10.5021/ad.2013.25.1.109
- Rosner M, Zloto O. Periocular molluscum contagiosum: six different clinical presentations. Acta Ophthalmol (Copenh). 2018;96(5):e600–e5. doi: 10.1111/aos.13717
- Schornack MM, Siemsen DW, Bradley EA, Salomao DR, Lee HB. Ocular manifestations of molluscum contagiosum. Clin Exp Optom. 2006;89(6):390–393. doi: 10.1111/cxo.2006.89.issue-6
- Schaffer JV, Berger EM. Molluscum contagiosum. JAMA Dermatol. 2016;152(9):1072. doi: 10.1001/jamadermatol.2016.2344
- Fornatora ML, Reich RF, Gray RG, Freedman PD. Intraoral molluscum contagiosum: a report of a case and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(3):318–320. doi: 10.1067/moe.2001.117299
- Brown, J., Janniger, C.K., Schwartz, R.A. and Silverberg, N.B. (2006), Childhood molluscum contagiosum. International Journal of Dermatology, 45: 93-99. https://doi.org/10.1111/j.1365-4632.2006.02737.x
- Molluscum Contagiosum. https://www.cdc.gov/poxvirus/molluscum-contagiosum/treatment.html
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? Experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32(3):353–357. doi: 10.1111/pde.12504
- Al-Mutairi N, Al-Doukhi A, Al-Farag S, Al-Haddad A. Comparative study on the efficacy, safety, and acceptability of imiquimod 5% cream versus cryotherapy for molluscum contagiosum in children. Pediatr Dermatol. 2010;27(4):388–394. doi: 10.1111/j.1525-1470.2009.00974.x
- Qureshi A, Zeb M, Jalal-Ud-Din M, Sheikh ZI, Alam MA, Anwar SA. Comparison Of Efficacy Of 10% Potassium Hydroxide Solution Versus Cryotherapy In Treatment Of Molluscum Contagiosum. J Ayub Med Coll Abbottabad. 2016 Apr-Jun;28(2):382-385. https://jamc.ayubmed.edu.pk/jamc/index.php/jamc/article/view/1103/303
- Harel A, Kutz AM, Hadj-Rabia S, Mashiah J. To treat molluscum contagiosum or not-curettage: an effective, well-accepted treatment modality. Pediatr Dermatol. 2016;33(6):640–645. doi: 10.1111/pde.12968
- Hanna D, Hatami A, Powell J, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23(6):574–579. doi: 10.1111/j.1525-1470.2006.00313.x
- Kelly V, Coulombe J, Lavoie I. Use of a disposable ear speculum: an alternative technique for molluscum contagiosum curettage. Pediatr Dermatol. 2018;35(3):418–419. doi: 10.1111/pde.13453
- Navarrete-Dechent C-M-M, Droppelmann N, González S. Actualización en el uso de la biopsia de piel por punch. Revista Chilena De Cirugía. 2016;68:467–473. doi: 10.1016/j.rchic.2016.05.008
- Gobbato AA, Babadopulos T, Gobbato CA, Moreno RA, Gagliano-Juca T, De Nucci G. Tolerability of 2.5% lidocaine/prilocaine hydrogel in children undergoing cryotherapy for molluscum contagiosum. Pediatr Dermatol. 2016;33(3):e214–e215. doi: 10.1111/pde.12842
- Capriotti K, Stewart K, Pelletier J, Capriotti J. Molluscum Contagiosum Treated with Dilute Povidone-Iodine: A Series of Cases. J Clin Aesthet Dermatol. 2017 Mar;10(3):41-45. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5367881
- Griffith RD, Yazdani Abyaneh MA, Falto-Aizpurua L, Nouri K. Pulsed dye laser therapy for molluscum contagiosum: a systematic review. J Drugs Dermatol. 2014 Nov;13(11):1349-52. https://jddonline.com/articles/pulsed-dye-laser-therapy-for-molluscum-contagiosum-a-systematic-review-S1545961614P1349X/
- Fisher C, McLawhorn JM, Adotama P, Stasko T, Collins L, Levin J. Pulsed dye laser repurposed: treatment of refractory molluscum contagiosum in renal transplant patient. Transpl Infect Dis. 2019;21(2):e13036. doi: 10.1111/tid.2019.21.issue-2
- Moye V, Cathcart S, Burkhart CN, Morrell DS. Beetle juice: a guide for the use of cantharidin in the treatment of molluscum contagiosum. Dermatol Ther. 2013;26(6):445–451. doi: 10.1111/dth.12105
- Vakharia PP, Chopra R, Silverberg NB, Silverberg JI. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19(6):791–803. doi: 10.1007/s40257-018-0375-4
- Ting, P.T. and Dytoc, M.T. (2004), Therapy of external anogenital warts and molluscum contagiosum: a literature review. Dermatologic Therapy, 17: 68-101. https://doi.org/10.1111/j.1396-0296.2004.04009.x
- Rush J, Dinulos JG. Childhood skin and soft tissue infections: new discoveries and guidelines regarding the management of bacterial soft tissue infections, molluscum contagiosum, and warts. Curr Opin Pediatr. 2016;28(2):250–257. doi: 10.1097/MOP.0000000000000334
- Can B, Topaloglu F, Kavala M, Turkoglu Z, Zindanci I, Sudogan S. Treatment of pediatric molluscum contagiosum with 10% potassium hydroxide solution. J Dermatolog Treat. 2014;25(3):246–248. doi: 10.3109/09546634.2012.697988
- Teixido C, Diez O, Marsal JR, et al. Efficacy and safety of topical application of 15% and 10% potassium hydroxide for the treatment of Molluscum contagiosum. Pediatr Dermatol. 2018;35(3):336–342. doi: 10.1111/pde.13438
- Metkar A, Pande S, Khopkar U. An open, nonrandomized, comparative study of imiquimod 5% cream versus 10% potassium hydroxide solution in the treatment of molluscum contagiosum. Indian J Dermatol Venereol Leprol. 2008 Nov-Dec;74(6):614-8. doi: 10.4103/0378-6323.45104
- Skinner RB Jr. Treatment of molluscum contagiosum with imiquimod 5% cream. J Am Acad Dermatol. 2002 Oct;47(4 Suppl):S221-4. doi: 10.1067/mjd.2002.126578
- Molluscum Contagiosum Treatment Options. https://www.cdc.gov/poxvirus/molluscum-contagiosum/treatment.html
- Katz KA, Williams HC, van der Wouden JC. Imiquimod cream for molluscum contagiosum: neither safe nor effective. Pediatr Dermatol. 2018;35(2):282–283. doi: 10.1111/pde.13398
- Enns LL, Evans MS. Intralesional immunotherapy with Candida antigen for the treatment of molluscum contagiosum in children. Pediatr Dermatol. 2011;28(3):254–258. doi: 10.1111/j.1525-1470.2011.01492.x
- Chularojanamontri L, Tuchinda P, Kulthanan K, Manuskiatti W. Generalized molluscum contagiosum in an HIV patient treated with diphencyprone. J Dermatol Case Rep. 2010;4(4):60–62. doi: 10.3315/jdcr.2010.1059
- Kang SH, Lee D, Hoon Park J, Cho SH, Lee SS, Park SW. Treatment of molluscum contagiosum with topical diphencyprone therapy. Acta Derm Venereol. 2005;85(6):529–530. doi: 10.1080/00015550510034948
- Bohm M, Luger TA, Bonsmann G. Disseminated giant molluscum contagiosum in a patient with idiopathic CD4+ lymphocytopenia successful eradication with systemic interferon. Dermatology. 2008;217(3):196–198. doi: 10.1159/000141649
- Kilic SS, Kilicbay F. Interferon-alpha treatment of molluscum contagiosum in a patient with hyperimmunoglobulin E syndrome. Pediatrics. 2006;117(6):e1253–e1255. doi: 10.1542/peds.2005-2706
- Nelson MR, Chard S, Barton SE. Intralesional interferon for the treatment of recalcitrant molluscum contagiosum in HIV antibody positive individuals–a preliminary report. Int J STD AIDS. 1995;6(5):351–352. doi: 10.1177/095646249500600509
- Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147(6):652–654. doi: 10.1001/archdermatol.2011.20
- Foissac M, Goehringer F, Ranaivo IM, et al. [Efficacy and safety of intravenous cidofovir in the treatment of giant molluscum contagiosum in an immunosuppressed patient]. Ann Dermatol Venereol. 2014;141(10):620–622. doi: 10.1016/j.annder.2014.04.114
- Toro JR, Wood LV, Patel NK, Turner ML. Topical cidofovir: a novel treatment for recalcitrant molluscum contagiosum in children infected with human immunodeficiency virus 1. Arch Dermatol. 2000 Aug;136(8):983-5. doi: 10.1001/archderm.136.8.983. Erratum in: Arch Dermatol 2002 Feb;138(2):257.
- Vora SB, Brothers AW, Englund JA. Renal toxicity in pediatric patients receiving cidofovir for the treatment of adenovirus infection. J Pediatric Infect Dis Soc. 2017;6(4):399–402. doi: 10.1093/jpids/pix011
- Padilla Espana L, Mota-Burgos A, Martinez-Amo JL, Benavente-Ortiz F, Rodriguez-Bujaldon A, Hernandez-Montoya C. Recalcitrant molluscum contagiosum successfully treated with sinecatechins. Dermatol Ther. 2016;29(4):217–218. doi: 10.1111/dth.12338
- Viswanath V, Shah RJ, Gada JL. Intralesional 5-fluorouracil: novel therapy for extensive molluscum contagiosum in an immunocompetent adult. Indian J Dermatol Venereol Leprol. 2017;83(2):265–266. doi: 10.4103/0378-6323.193626
- Gao YL, Gao XH, Qi RQ, et al. Clinical evaluation of local hyperthermia at 44 degrees C for molluscum contagiosum: pilot study with 21 patients. Br J Dermatol. 2017;176(3):809–812. doi: 10.1111/bjd.14849
- Maiolo C, Marshman G. Zoster immunoglobulin-VF: a potential treatment for molluscum contagiosum in immunosuppressed children. Pediatr Dermatol. 2015;32(4):e193. doi: 10.1111/pde.2015.32.issue-4
- Chickenpox/Varicella Vaccination. https://www.cdc.gov/vaccines/vpd/varicella/index.html
- Bernal JL, Hobbelen P, Amirthalingam G. Burden of varicella complications in secondary care, England, 2004 to 2017. Euro Surveill. 2019 Oct;24(42):1900233. doi: 10.2807/1560-7917.ES.2019.24.42.1900233
- Yokota K, Tamiya A, Sahara T, Nakamura-Uchiyama F. Adult Chickenpox. Intern Med. 2020 Aug 1;59(15):1923. doi: 10.2169/internalmedicine.4478-20
- Cobelli Kett J. Perinatal varicella. Pediatr Rev. 2013 Jan;34(1):49-51. doi: 10.1542/pir.34-1-49
- Smith CK, Arvin AM. Varicella in the fetus and newborn. Semin Fetal Neonatal Med. 2009 Aug;14(4):209-17. doi: 10.1016/j.siny.2008.11.008
- Mother To Baby | Fact Sheets [Internet]. Brentwood (TN): Organization of Teratology Information Specialists (OTIS); 1994-. Varicella Infection (Chickenpox) 2021 Apr. Available from: https://www.ncbi.nlm.nih.gov/books/NBK583011
- Miller E, Cradock-Watson JE, Ridehalgh MK. Outcome in newborn babies given anti-varicella-zoster immunoglobulin after perinatal maternal infection with varicella-zoster virus. Lancet. 1989 Aug 12;2(8659):371-3. doi: 10.1016/s0140-6736(89)90547-3
- Bhavsar SM, Mangat C. Congenital Varicella Syndrome. [Updated 2022 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK568794
- Blumental S, Lepage P. Management of varicella in neonates and infants. BMJ Paediatr Open. 2019 May 30;3(1):e000433. doi: 10.1136/bmjpo-2019-000433
- ROSS AH. Modification of chicken pox in family contacts by administration of gamma globulin. N Engl J Med. 1962 Aug 23;267:369-76. doi: 10.1056/NEJM196208232670801
- Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48(Suppl 1):S2–7. doi: 10.1016/S1386-6532(10)70002-0
- Laemmle L, Goldstein RS, Kinchington PR. Modeling varicella zoster virus persistence and reactivation – closer to resolving a perplexing persistent state. Front Microbiol. 2019;10:1634. doi: 10.3389/fmicb.2019.01634
- Jeon YH. Herpes zoster and postherpetic neuralgia: practical consideration for prevention and treatment. Korean J Pain. 2015;28:177–84. doi: 10.3344/kjp.2015.28.3.177
- Koshy E, Mengting L, Kumar H, Jianbo W. Epidemiology, treatment and prevention of herpes zoster: a comprehensive review. Indian J Dermatol Venereol Leprol. 2018;84:251–62. doi: 10.4103/ijdvl.ijdvl_1021_16
- Sauerbrei A. Diagnosis, antiviral therapy, and prophylaxis of varicella-zoster virus infections. Eur J Clin Microbiol Infect Dis. 2016;35:723–34. doi: 10.1007/s10096-016-2605-0
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016. doi: 10.1038/nrdp.2015.16
- McDonald JR, Zeringue AL, Caplan L, Ranganathan P, Xian H, Burroughs TE, et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis. 2009;48:1364–71. doi: 10.1086/598331
- Dayan RR, Peleg R. Herpes zoster – typical and atypical presentations. Postgrad Med. 2017 Aug;129(6):567-571. doi: 10.1080/00325481.2017.1335574
- Nagel MA, Gilden DH. The protean neurologic manifestations of varicella-zoster virus infection. Cleve Clin J Med. 2007 Jul;74(7):489-94, 496, 498-9 passim. doi: 10.3949/ccjm.74.7.489
- Ibraheem M, Marin M, Leung J, Bryce CH, Schmid DS, Zaki SR, et al. Fatal wild-type varicella-zoster virus encephalitis without a rash in a vaccinated child. Pediatr Infect Dis J. 2013;32:183–5. doi: 10.1097/INF.0b013e318273e43d
- Pollak L, Mehta SK, Pierson DL, Sacagiu T, Avneri Kalmanovich S, Cohrs RJ. Varicella-zoster DNA in saliva of patients with meningoencephalitis: a preliminary study. Acta Neurol Scand. 2015;131:417–21. doi: 10.1111/ane.12335
- Yamada S, Atsuta N, Tokunaga S, Motegi Y. Ipsilateral truncal sensory deficit in a patient with ophthalmic zoster sine herpete. Neurology. 2003;60:1049–50. doi: 10.1212/01.WNL.0000052999.43687.7B
- Mantero V, De Toni Franceschini L, Lillia N, Guccione A, Santilli I, Agostoni E. Varicella-zoster meningoencephaloradiculoneuropathy in an immunocompetent young woman. J Clin Virol. 2013;57:361–2. doi: 10.1016/j.jcv.2013.04.006
- Chen WH, Chui C, Yin HL. Zoster sine herpete, vertebral artery stenosis, and ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:e234–7. doi: 10.1016/j.jstrokecerebrovasdis.2012.07.017
- Nagel MA, Gilden D. Neurological complications of varicella zoster virus reactivation. Curr Opin Neurol. 2014;27:356–60. doi: 10.1097/WCO.0000000000000092
- Persson A, Bergström T, Lindh M, Namvar L, Studahl M. Varicella-zoster virus CNS disease–viral load, clinical manifestations and sequels. J Clin Virol. 2009;46:249–53. doi: 10.1016/j.jcv.2009.07.014
- Dueland AN, Devlin M, Martin JR, Mahalingam R, Cohrs R, Manz H, et al. Fatal varicella-zoster virus meningoradiculitis without skin involvement. Ann Neurol. 1991;29:569–72. doi: 10.1002/ana.410290520
- Kleinschmidt-DeMasters BK, Gilden DH. Varicella-Zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med. 2001 Jun;125(6):770-80. doi: 10.5858/2001-125-0770-VZVIOT
- Hevner R, Vilela M, Rostomily R, Cohrs R, Mahalingam R, Wellish M, et al. An unusual cause of trigeminal-distribution pain and tumour. Lancet Neurol. 2003;2:567–71. doi: 10.1016/S1474-4422(03)00506-4
- Birlea M, Nagel MA, Khmeleva N, Choe A, Kleinschmidt-Demasters B, Hevner R, et al. Varicella-zoster virus trigeminal ganglioneuritis without rash. Neurology. 2014;82:90–2. doi: 10.1212/01.wnl.0000438228.48470.86
- Zerboni L, Arvin A. Neuronal subtype and satellite cell tropism are determinants of varicella-zoster virus virulence in human dorsal root ganglia xenografts in vivo. PLoS Pathog. 2015;11:e1004989. doi: 10.1371/journal.ppat.1004989
- Chickenpox Vaccination: What Everyone Should Know. https://www.cdc.gov/vaccines/vpd/varicella/public/index.html
- About the Varicella Vaccines. https://www.cdc.gov/vaccines/vpd/varicella/hcp/about-vaccine.html
- VARIVAX (refrigerated and frozen formulations). https://www.fda.gov/vaccines-blood-biologics/vaccines/varivax-refrigerated-and-frozen-formulations
- PROQUAD. https://www.fda.gov/vaccines-blood-biologics/vaccines/proquad
- Chickenpox (Varicella). https://www.cdc.gov/chickenpox/hcp/index.html#managing-high-risk
- Updated Recommendations for Use of VariZIG — United States, 2013. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6228a4.htm
- FDA Approval of an Extended Period for Administering VariZIG for Postexposure Prophylaxis of Varicella. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6112a4.htm?s_cid=mm6112a4_w
- Cangene Corporation. VariZIG: varicella zoster immune globulin (human). [package insert]. Winnipeg, Canada: Cangene Corporation; 2013.
- Bozzola E, Guolo S, Macchiarulo G, Festa L, Spina G, Krzysztofiak A, Grandin A, Bozzola M, Raponi M, Villani A. Hospitalization for acute cerebellitis in children affected by varicella: how much does it cost? Ital J Pediatr. 2020 Aug 6;46(1):114. doi: 10.1186/s13052-020-00875-8
- What You Need to Know About Shingles and the Shingles Vaccine. https://www.cdc.gov/vaccines/hcp/adults/downloads/fs-shingles.pdf
- Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sexually Transmitted Diseases 2013; 40(3):187-193. https://www.ncbi.nlm.nih.gov/pubmed/23403598
- Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sexually Transmitted Diseases 2014; 41(11):660-664. https://www.ncbi.nlm.nih.gov/pubmed/25299412
- Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003–2006. Journal of Infectious Diseases 2011; 204(4):566–573. https://www.ncbi.nlm.nih.gov/pubmed/21791659
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012; 30 Suppl 5:F123-138. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4636904/