Hair loss
Hair loss also known as alopecia, is a common problem and can affect just your scalp or your entire body. There are many types of hair loss with different symptoms and causes. Hair loss can occur in different patterns, depending on the cause. Hair loss (alopecia) can affect both men and women and also children.
Hair loss is a common problem that affects up to 50 percent of men and women throughout their lives 1. Hair loss can occur anywhere on the body, but more commonly affects just the scalp when the patient presents with concerns about the cosmetic effect.
Hair loss is not life threatening, but it is distressing and significantly affects a person’s quality of life. Studies have shown that hair loss can be associated with low self-esteem, anxiety, depression, introversion, and feelings of unattractiveness 2, 3.
This is reinforced by attitudes in Western society, which place great value on youthful appearance and attractiveness. Some studies have shown that based on appearance alone, men with hair loss are seen as less attractive, less assertive, less likeable, and less successful than men without hair loss.
Hair loss can occur in different patterns, depending on the cause. The hair loss can be temporary or permanent.
Hair loss is not usually anything to be worried about, but occasionally it can be a sign of a medical condition.
Some types of hair loss are permanent, like male and female pattern baldness also known as androgenic alopecia or androgenetic alopecia. This type of hair loss usually runs in the family.
Hair loss can be an isolated problem or associated with another disease or condition, including:
- A family history of balding on your mother’s or father’s side. Androgenic alopecia also known as androgenetic alopecia is the modern medical term for either male pattern hair loss or female pattern baldness caused by a combination of genetic and hormonal factors 4. Androgenic alopecia represents close to 95% of all hair loss. This hair loss causes a receding hairline and lack of hair on the top of the head. This type of hair loss can be defined in two parts. First, andro- means to consist of androgens which are various hormones [i.e, dehydroepiandrosterone (DHEA), androstenedione, testosterone, and dihydrotestosterone (DHT)] that control the appearance and development of masculine characteristics such as testosterone. Second is genetics, or the inheritance of genes from either the mother or father. Age added to genetics creates a time clock that signals the hair follicle to produce an enzyme named 5-alpha reductase. When testosterone is present in the hair follicle and it combines with the enzyme 5-alpha-reductase type 2, it produces dihydrotestosterone (DHT). Dihydrotestosterone (DHT) is the most potent hormone among the androgens [i.e, dehydroepiandrosterone (DHEA), androstenedione, testosterone, and dihydrotestosterone (DHT)] and is considered a pure androgen as it cannot be converted into estrogen 5. DHT (dihydrotestosterone) attacks the hair follicle, causing it to shrink, finally causing the hair to fall out and not grow back and is implicated in male-pattern hair loss androgenetic alopecia pathophysiology 6. Hair follicle receptors are sensitive to DHT and thereby start the process of male or female pattern hair loss 7. Upon binding to androgen receptors in the hair follicle, dihydrotestosterone (DHT) promotes the shortening of the anagen phase and elongation of the telogen phase 8, resulting in enhanced apoptosis of hair cells and thus hair loss 9. A mouse-model study found that dihydrotestosterone (DHT) promoted premature hair regression, hair miniaturization, loss of hair density, and altered hair morphology in male mice, with partial reversal with an androgen receptor antagonist, bicalutamide 10. In addition, those with 5-alpha-reductase enzyme deficiencies are less likely to develop male-pattern hair loss androgenetic alopecia. The role of dihydrotestosterone (DHT) in the promotion of transition to telogen and male-pattern hair loss androgenetic alopecia pathophysiology justifies the use of oral 5-alpha-reductase inhibitors, such as finasteride, in the management of hair loss 6.
- Thyroid disease. Hypothyroidism (underactive thyroid) and hyperthyroidism (overactive thyroid) can cause reversible, diffuse hair loss 11 and can promote premature transition from anagen to telogen, potentially resulting in telogen effluvium. In fact, diffuse hair loss may be the only presenting sign of thyroid dysfunction 12. A study published in 2013 12 assessed alopecia patterns related to thyroid dysfunction among all patients presenting to a clinic from December 2007 to December 2009. Thyroid dysfunction, based on a thyroid-stimulating hormone (TSH) reference range, was observed most frequently in alopecia areata and diffuse alopecia patients among those aged 0–20 and 21–40 years, and in alopecia areata and androgenetic alopecia patients among those 40 years and older. A greater association between thyroid dysfunction and alopecia was observed with increasing age 12. The mechanisms of abnormal thyroid hormone levels and hair loss have been described. The deletion of mice thyroid hormone nuclear receptors has been shown to impair epidermal proliferation and hair growth 13. In addition, a 2015 study 13 found that mice with deficient thyroid hormone receptors had increased label-retaining cells in the bulges, the hair follicle stem cell niche, resulting in reduced activation of stem cells and accumulation in bulges. The study authors 13 concluded that thyroid hormone signaling is necessary for proper mobilization of stem cells from the hair bulge, and improper stem cell signaling may mediate hair loss associated with thyroid hormone deficiencies. In addition, prolonged thyroid hormone stimulation has been shown to promote progenitor cell differentiation and subsequent stem cell depletion 13. As such, both deficient and excessive levels of thyroid hormones can contribute to anagen to telogen transition and hair loss. Thyroid-stimulating hormones (TSH) and thyroxine levels should be obtained as part of a standard work-up for non-scarring alopecia.
- Age
- Significant weight loss
- Prolonged fever
- Certain medical conditions, such as diabetes and lupus
- Stressful conditions, physical or emotional, such as illness or surgery. The association of stress and hair loss has been widely documented. Arck et al. 14 suggested that substance P-dependent inflammatory pathways may mediate stress-induced hair loss. In 1998, a case-control study 15 used the Social Readjustment Rating Scale to compare stress among twenty-five women who experienced recent hair loss compared with twenty-five healthy controls. Compared to ten control subjects, twenty-two of those experiencing unexplained hair loss reported high stress, resulting in an odds ratio of eleven; based on this study, authors concluded that women experiencing high stress are eleven times more likely to experience hair loss 15. However, this study is limited by its small sample size and potential recall bias. Still, this study depicts an early documentation of the association of stress and hair loss. Stress can foster anagen to telogen transition and is closely related to telogen effluvium, with resulting telogen elongation 16. Furthermore, cortisol, the primary stress hormone, has been shown to affect cyclic regulation of the hair cycle and proteoglycan synthesis 8. The effects of cortisol on the hair cycle and proteoglycans are important to understand, as elevated cortisol levels have been observed in both men and women with androgenetic alopecia in comparison to healthy controls 17, 18. Research has highlighted the importance of proteoglycans, such as versican and decorin, and glycosaminoglycans in normal hair follicle and hair cycle functioning. For example, while versican functions to protect cells from oxidative stress-induced apoptosis, decorin acts as an anagen inducer, promoting hair growth 8. However, high cortisol levels have been shown to exhibit damaging effects on proteoglycans in the hair follicle, with reduced synthesis and increased breakdown 19. Thus, cortisol inhibition may promote anagen and hair growth via increased proteoglycan concentrations.
- Poor nutrition. Proper nutrition is essential for anagen and telogen balance, and caloric or nutritional deficiency can negatively impact hair structure, growth, and pigmentation 20. Furthermore, telogen effluvium can occur following rapid weight loss or reduced protein intake, and diffuse alopecia may be a presenting sign of nutritional deficiency 21. Studies have found associations between nutritional deficiency and a variety of types of hair loss, including chronic telogen effluvium, androgenetic alopecia, and alopecia areata 21.
- Drug treatment for cancer
- Autoimmune disease
- A localized infection, such as tinea capitis
- Severe local skin disease, such as psoriasis, seborrheic dermatitis, atopic dermatitis, pityriasis rubra pilaris, cutaneous lupus erythematosus, cutaneous T-cell lymphoma
- Generalized skin disease (erythroderma)
- Traumatic causes
- Other causes of hair loss include certain medicines (e.g., chemotherapy drugs, contraceptives, anticoagulants, anticonvulsants), low levels of iron in your blood (iron deficiency), pregnancy (after childbirth), syphilis, systemic lupus erythematosus (SLE) and repeated hair twisting
- Poor sleep. Poor sleep has been associated with increased risk and severity of alopecia subtypes, including alopecia areata and androgenetic alopecia 6. Conversely, those with alopecia have been found to exhibit reduced sleep quality compared to controls. A 2022 study 22 analyzed the prevalence of sleep abnormalities between 223 patients with male-pattern hair loss and 223 control subjects. The authors found a significant association between severe male-pattern hair loss and three sleep profiles: total sleep time less than or equal to six hours; a Pittsburgh Sleep Quality Index (PSQI) score greater than 5; and STOP-Bang score greater than or equal to 5 22. The STOP-Bang score specifically assesses signs of obstructive sleep apnea, and higher STOP-Bang and Pittsburgh Sleep Quality Index (PSQI) scores are negative findings, suggesting an association between sleep disturbances and male-pattern hair loss 22. Similarly, poor sleep habits is associated with increased severity of androgenetic alopecia 23. A similar study assessed the prevalence of sleep disturbances among 51 alopecia areata patients and 51 age- and sex-matched controls 24. As observed among individuals with male-pattern hair loss, the Pittsburgh Sleep Quality Index (PSQI) score was significantly greater among patients with alopecia areata compared to matched controls (7 ± 4.13 vs. 3.53 ± 1.96) 24. A greater number of alopecia areata patients depicted excess daytime sleepiness, measured with the Epworth Sleepiness Scale, than controls. Furthermore, sleep quality was worse among alopecia areata patients also suffering from anxiety or depression, thereby highlighting the importance of addressing both sleep quality and concomitant psychiatric distress in the management of alopecia areata 24. Furthermore, a 2018 study including 25,800 with diagnosed sleep disorders and 129,000 control subjects found those with sleep disorders to have a significantly greater risk for alopecia areata than controls 25. The authors found sleep disorders as an independent risk factor of alopecia areata 25.
- Unknown causes.
Hair loss can be temporary or permanent, depending on the cause.
- Hair loss may be localized or diffuse.
- Hair loss can affect the scalp or other parts of the body.
- Hair loss may be due to hair shedding, poor quality hair, or hair thinning.
- There may be areas of skin that are completely bald.
- There may be associated skin disease or scarring.
Local hair loss in one or more small parts of the scalp can be caused by any of the following:
- Alopecia areata (patchy hair loss; the cause is unknown)
- Traction alopecia (tight hairstyles such as cornrows or pigtails)
- Trichotillomania (repeated hair pulling or nervous hair twisting or twirling)
- Tinea capitis (ringworm or fungal infection)
Since hair loss may be an early sign of a disease, it is important to find the cause so that it can be treated.
If you suspect that you may have excessive hair loss, talk to your doctor. He or she will probably ask you some questions about your diet, any medicines you’re taking, and whether you’ve had a recent illness, and how you take care of your hair. If you’re a woman, your doctor may ask questions about your menstrual cycle, pregnancies, and menopause. Your doctor may want to do a physical exam to look for other causes of hair loss. Finally, your doctor may order blood tests to measure hormone levels, serum ferritin and thyroid function or a biopsy (taking a small sample of cells to examine under a microscope). Your doctor will usually diagnose androgenetic alopecia by examining the pattern of hair loss on the scalp.
Treatment for hair loss depends on the cause. In some cases, treating the underlying cause will correct the problem. With some conditions, such as patchy hair loss (alopecia areata), hair may regrow without treatment within a year.
If a medicine is causing your hair loss, your doctor may be able to prescribe a different medicine. Recognizing and treating an infection may help stop the hair loss. Correcting a hormone imbalance may prevent further hair loss.
Sometimes changing how you style or treat your hair can help. Getting rid of stress in your life can also help. Other treatments include changing your diet, correcting any hormone imbalances, switching medicines, treating infections, or getting shots into your scalp.
Medicines may also help slow or prevent the development of common baldness. One medicine that is used to slow hair loss, minoxidil (brand name: Rogaine), is available without a prescription. It is applied to the scalp. Both men and women can use it. Minoxidil (Rogaine) is the only US Food and Drug Administration (FDA) approved topical treatment for male or female pattern hair loss. Although minoxidil (Rogaine) is not effective in stimulating new hair growth in many males, it appears to be more effective in retarding hair loss in a substantial amount of both male and females.
Another medicine, finasteride (type II 5-alpha-reductase inhibitor) and dutasteride (type I and II 5-alpha-reductase inhibitor), is available with a prescription 26. It comes in pills and is only for men. 5-alpha-reductase type 2 converts testosterone into dihydrotestosterone (DHT) 27. DHT (dihydrotestosterone) binding to the scalp hair follicle androgen receptors produces male pattern hair loss. For men, finasteride tablets reduce levels of dihydrotestosterone (DHT), which may slow hair loss and possibly help regrowth of hair 28. A daily oral finasteride dose of one milligram reduces scalp dihydrotestosterone by 64% and serum dihydrotestosterone by 68% 29. Continuous use for 3 to 6 months is required before a benefit is usually seen. It may take up to 6 months before you can tell if one of these medicines is working. When you stop taking these medicines, any beneficial effects on hair growth will be lost within 6 to 12 months of discontinuing treatment. Two one-year trials encompassing 1,553 men with male-pattern hair loss found 99% of subjects to show decreased progression or the reversal of hair loss with oral finasteride 30. In addition, authors observed clinically significant increases in hair count with oral finasteride treatment compared to placebo 30. Decreased libido and erectile problems are recognized side-effects of treatment with 5-alpha-reductase inhibitors 5.
If treatment with 5-alpha-reductase inhibitor doesn’t work or is not available for your type of hair loss, you may want to consider wearing a wig, hairpiece, hair weave, or artificial hair replacement.
Despite the widespread use of supplements and vitamins for hair growth or hair loss, the safety and effectiveness of available products remain unclear 31. Studies of nutritional interventions with the highest-quality evidence showed the potential benefit of Viviscal, Nourkrin, Nutrafol, Lambdapil, Pantogar, capsaicin and isoflavone, omegas 3 and 6 with antioxidants, apple nutraceutical, total glucosides of paeony and compound glycyrrhizin tablets, zinc, tocotrienol, and pumpkin seed oil 31. Kimchi and cheonggukjang, vitamin D3, and Forti5 had low-quality evidence for hair growth 31.
Figure 1. Male pattern hair loss
Figure 2. Female pattern hair loss
You should seek medical advice for hair loss if:
- you have recently started a new medicine;
- you are a woman and your hair loss is accompanied by excess growth of facial and body hair or you have acne;
- you have been diagnosed with (or think you may have) an autoimmune disorder such as systemic lupus erythematosis (SLE), nutritional deficiency or thyroid disease;
- you have been recently treated with chemotherapy or have used a hormonal medicine;
- the hair loss occurs in discrete patches;
- the hair loss is associated with scaling or inflammation of the scalp;
- you also have loss of body hair; or
- you are aware you have a compulsive hair-pulling habit.
How does your hair grow?
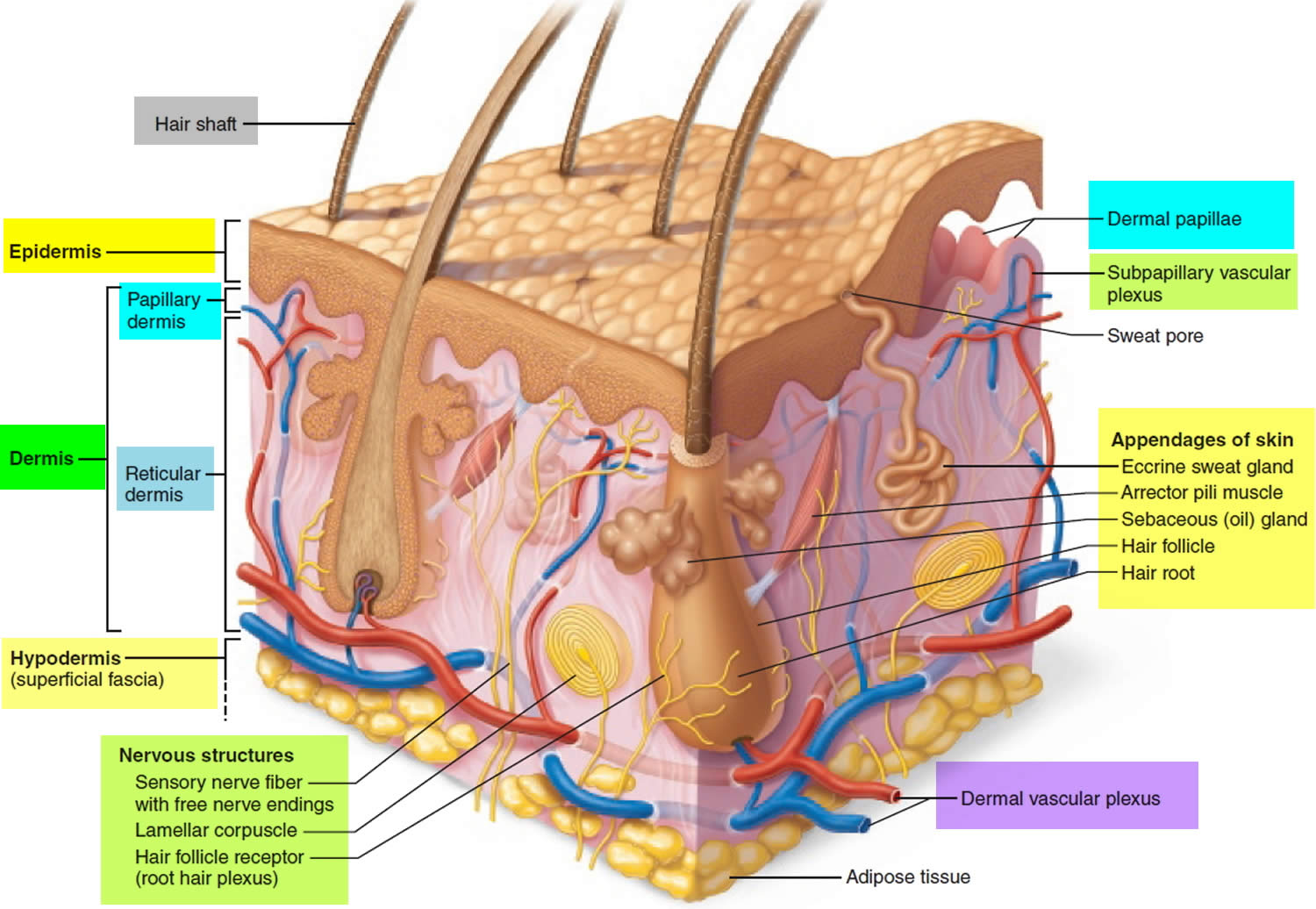
Hair is a slender filament of keratinized cells that grows from an oblique tube in the skin called a hair follicle. Each hair is composed of columns of dead, keratinized epidermal cells bonded together by extracellular proteins. The hair shaft is the superficial portion of the hair, which projects above the surface of the skin. The hair root is the portion of the hair deep to the shaft that penetrates into the dermis, and sometimes into the subcutaneous layer.
Hairs project beyond the surface of the skin almost everywhere except the sides and soles of the feet, the palms of the hands, the sides of the fingers and toes, the lips, and portions of the external genitalia. There are about 5 million hairs on the human body, and 98 percent of them are on the general body surface, not the head. Hairs are nonliving structures that form in organs called hair follicles.
The density of hair does not differ much from one person to another or even between the sexes; indeed, it is virtually the same in humans, chimpanzees, and gorillas. Differences in apparent hairiness are due mainly to differences in texture and pigmentation.
Types of Hairs
Hairs first appear after about three months of embryonic development. These hairs, collectively known as lanugo, are extremely fine and unpigmented. Most lanugo hairs are shed before birth.
The two types of hairs in the adult skin are vellus hairs and terminal hairs:
- Vellus hairs are the fine “peach fuzz” hairs found over much of the body surface.
- Terminal hairs are heavy, more deeply pigmented, and sometimes curly. The hairs on your head, including your eyebrows and eyelashes, are terminal hairs. After puberty, it also forms the armpit and pubic hair, male facial hair, and some of the hair on the trunk and limbs.
Hair follicles may alter the structure of the hairs they produce in response to circulating hormones.
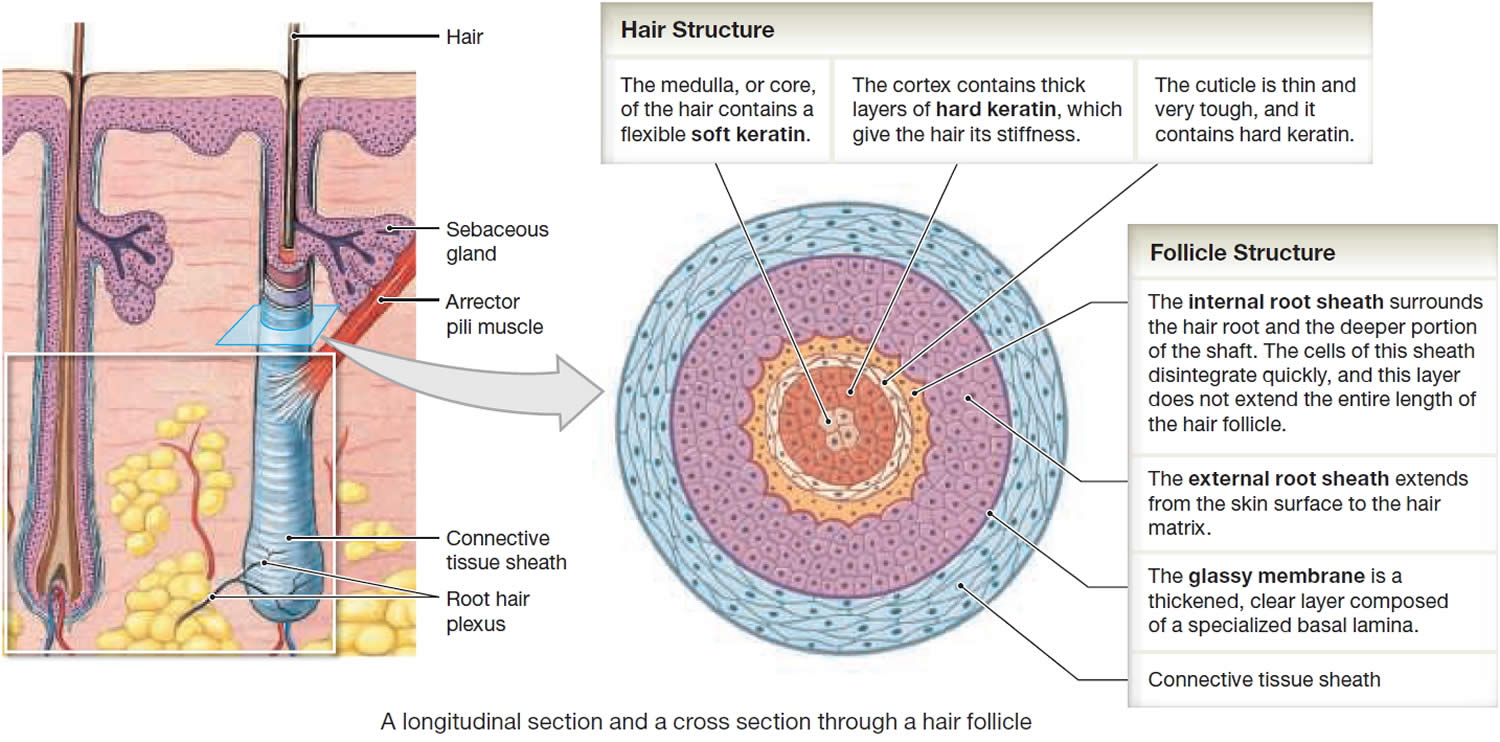
Figure 3. Hair structure
Figure 4. Hair follicle
Structure of Hair Follicle
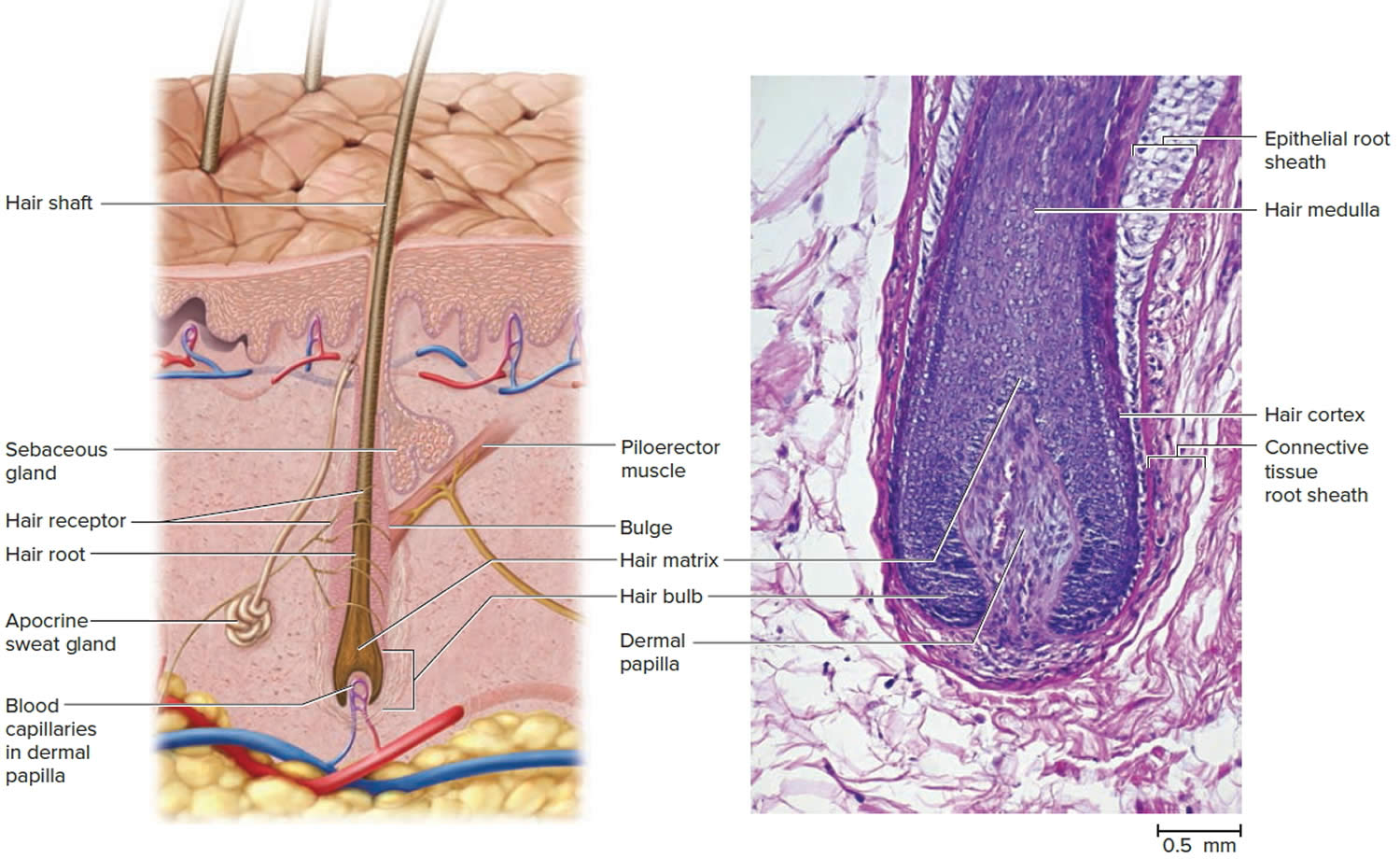
The portion of a hair above the skin is called the shaft, and all that beneath the surface is the root. The root penetrates deeply into the dermis or hypodermis and ends with a dilation called the hair bulb. The only living cells of a hair are in and near the hair bulb. The hair bulb grows around a bud of vascular connective tissue called the dermal papilla, which provides the hair with its sole source of nutrition. Immediately above the papilla is a region of mitotically active cells, the hair matrix, which is the hair’s growth center. All cells higher up are dead.
Figure 5. Hair follicle and hair structure
Hair Structure
In cross section, a hair reveals up to three layers. From the inside out, these are the medulla, cortex, and cuticle.
The medulla is a core of loosely arranged cells and air spaces. It is most prominent in thick hairs such as those of the eyebrows, but narrower in hairs of medium thickness and absent from the thinnest hairs of the scalp and elsewhere.
The cortex constitutes most of the bulk of a hair. It consists of several layers of elongated keratinized cells that appear cuboidal to flattened in cross sections.
The cuticle is composed of multiple layers of very thin, scaly cells that overlap each other like roof shingles with their free edges directed upward.
Hair Follicle
Cells lining the hair follicle are like shingles facing in the opposite direction. They interlock with the scales of the hair cuticle and resist pulling on the hair. When a hair is pulled out, this layer of follicle cells comes with it.
The hair follicle is a diagonal tube that contains the hair root. It has two principal layers: an epithelial root sheath and a connective tissue root sheath. The epithelial root sheath is an extension of the epidermis; it consists of stratified squamous epithelium and lies immediately adjacent to the hair root. Toward the deep end of the follicle, it widens to form a bulge, a source of stem cells for follicle growth. The connective tissue root sheath, which is derived from the dermis and composed of collagenous connective tissue, surrounds the epithelial sheath and is somewhat denser than the adjacent dermis.
Associated with the hair follicle are nerve and muscle fibers. Nerve fibers called hair receptors entwine each hair follicle and respond to hair movements. You can feel their effect by carefully moving a single hair with a pin or by lightly running your finger over the hairs of your forearm without touching the skin.
Each hair has a piloerector muscle—also known as a pilomotor muscle or arrector pili—a bundle of smooth muscle cells extending from dermal collagen fibers to the connective tissue root sheath of the follicle. In response to cold, fear, touch, or other stimuli, the sympathetic nervous system stimulates the piloerector to contract, making the hair stand on end and wrinkling the skin in such areas as the scrotum and areola. In humans, it pulls the follicles into a vertical position and causes “goose bumps,” but serves no useful purpose.
Figure 6. Hair structure
Hair Production
Hair follicles extend deep into the dermis, often projecting into the underlying subcutaneous layer. The epithelium at the follicle base surrounds a small hair papilla, a peg of connective tissue containing capillaries and nerves. The hair bulb consists of epithelial cells that surround the papilla.
Hair production involves a specialized keratinization process. The hair matrix is the epithelial layer involved in hair production. When the superficial basal cells divide, they produce daughter cells that are pushed toward the surface as part of the developing hair. Most hairs have an inner medulla and an outer cortex. The medulla contains relatively soft and flexible soft keratin. Matrix cells closer to the edge of the developing hair form the relatively hard cortex. The cortex contains
hard keratin, which gives hair its stiffness. A single layer of dead, keratinized cells at the outer surface of the hair overlap and form the cuticle that coats the hair.
The hair root anchors the hair into the skin. The root begins at the hair bulb and extends distally to the point where the internal organization of the hair is complete, about halfway to the skin surface. The hair shaft extends from this halfway point to the skin surface, where we see the exposed hair tip.
The size, shape, and color of the hair shaft are highly variable.
Growth and Replacement of Hair
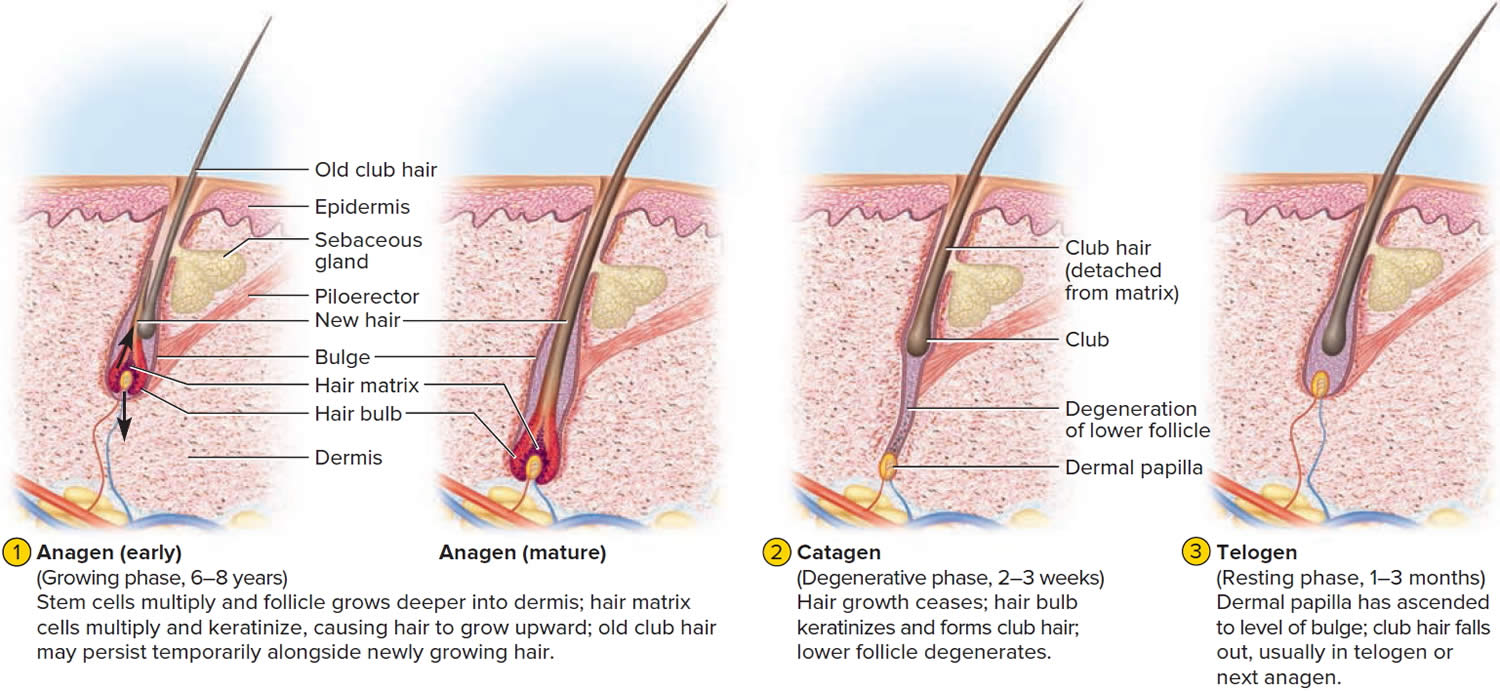
The human scalp contains about 100,000 hair follicles. These anchor the hair to the skin and contain the cells that produce new hairs. A hair in the scalp grows for two to five years, at a rate of around 0.33 mm/day (about 1/64 inch). Variations in hair growth rate and the duration of the hair growth cycle account for individual differences in uncut hair length. Hair grows in 3 developmental stages:
- Anagen. The anagen phase or actively hair growing phase starts the growing of new hair. The anagen phase is genetically determined and can vary from 2 to 6 years (the average is just under 3 years). Most hair follicles on the scalp are in the anagen phase.
- Catagen. The catagen phase is a transition stage (in-between phase) between the growing and resting phases and lasts 2-3 weeks. The catagen phase is when hair growth stops and the hair follicle shrinks. About 1–3% of hairs are in the catagen phase (in-between phase).
- Telogen. The telogen phase or resting phase is a mature hair with a root, which is held very loosely in the follicle. The telogen phase (resting phase) generally lasts about 4-5 months. Up to 10% of hairs in a normal scalp are in the telogen phase (resting phase). About 100 telogen hairs are lost from the human scalp each day.
At any given time, about 90% of the scalp follicles are in the Anagen stage. In this stage, stem cells from the bulge in the hair follicle multiply and travel downward, pushing the dermal papilla deeper into the skin and forming the epithelial root sheath. Root sheath cells directly above the papilla form the hair matrix. Here, sheath cells transform into hair cells, which synthesize keratin and then die as they are pushed upward away from the papilla. The new hair grows up the follicle, often alongside an old club hair left from the previous cycle.
Hair length depends on the duration of anagen stage. Short hairs (eyelashes, eyebrows, hair on arms and legs) have a short anagen phase of around one month. Anagen lasts up to 6 years or longer in scalp hair.
In the Catagen stage, mitosis in the hair matrix ceases and sheath cells below the bulge die. The follicle shrinks and the dermal papilla draws up toward the bulge. The base of the hair keratinizes into a hard club and the hair, now known as a club hair, loses its anchorage. Club hairs are easily pulled out by brushing the hair, and the hard club can be felt at the hair’s end. When the papilla reaches the bulge, the hair goes into a resting period called the Telogen stage. Eventually, anagen begins anew and the cycle repeats itself. A club hair may fall out during catagen or telogen, or as it is pushed out by the new hair in the next anagen phase.
You lose about 50 to 100 scalp hairs daily. In a young adult, scalp follicles typically spend 6 to 8 years in anagen, 2 to 3 weeks in catagen, and 1 to 3 months in telogen. Scalp hairs grow at a rate of about 1 mm per 3 days (10–18 cm/yr) in the anagen phase.
Hair grows fastest from adolescence until the 40s. After that, an increasing percentage of follicles are in the catagen and telogen phases rather than the growing anagen phase. Hair follicles also shrink and begin producing wispy vellus hairs instead of thicker terminal hairs. Thinning of the hair or baldness, is called alopecia. It occurs to some degree in both sexes and may be worsened by disease, poor nutrition, fever, emotional stress, radiation, or chemotherapy. In the great majority of cases, however, it is simply a matter of aging.
Pattern baldness is the condition in which hair is lost unevenly across the scalp rather than thinning uniformly. It results from a combination of genetic and hormonal influences. The relevant gene has two alleles: one for uniform hair growth and a baldness allele for patchy hair growth. The baldness allele is dominant in males and is expressed only in the presence of the high level of testosterone characteristic of men. In men who are either heterozygous or homozygous for the baldness allele, testosterone causes terminal hair to be replaced by vellus hair, beginning on top of the head and later the sides. In women, the baldness allele is recessive. Homozygous dominant and heterozygous women show normal hair distribution; only homozygous recessive women are at risk of pattern baldness. Even then, they exhibit the trait only if their testosterone levels are abnormally high for a woman (for example, because of a tumor of the adrenal gland, a woman’s principal source of testosterone). Such characteristics in which an allele is dominant in one sex and recessive in the other are called sex-influenced traits.
Excessive or undesirable hairiness in areas that are not usually hairy, especially in women and children, is called hirsutism. It tends to run in families and usually results from either masculinizing ovarian tumors or hypersecretion of testosterone by the adrenal cortex. It is often associated with menopause.
Contrary to popular misconceptions, hair and nails do not continue to grow after a person dies, cutting hair does not make it grow faster or thicker, and emotional stress cannot make the hair turn white overnight.
Different causes of hair loss affect the hair follicles in different phases of growth. See below for the different types of hair loss.
Figure 7. Hair growth cycle
Types of Hair loss (Alopecia)
There are different types of alopecia. Listed below are the main types of hair loss:
Androgenetic Alopecia
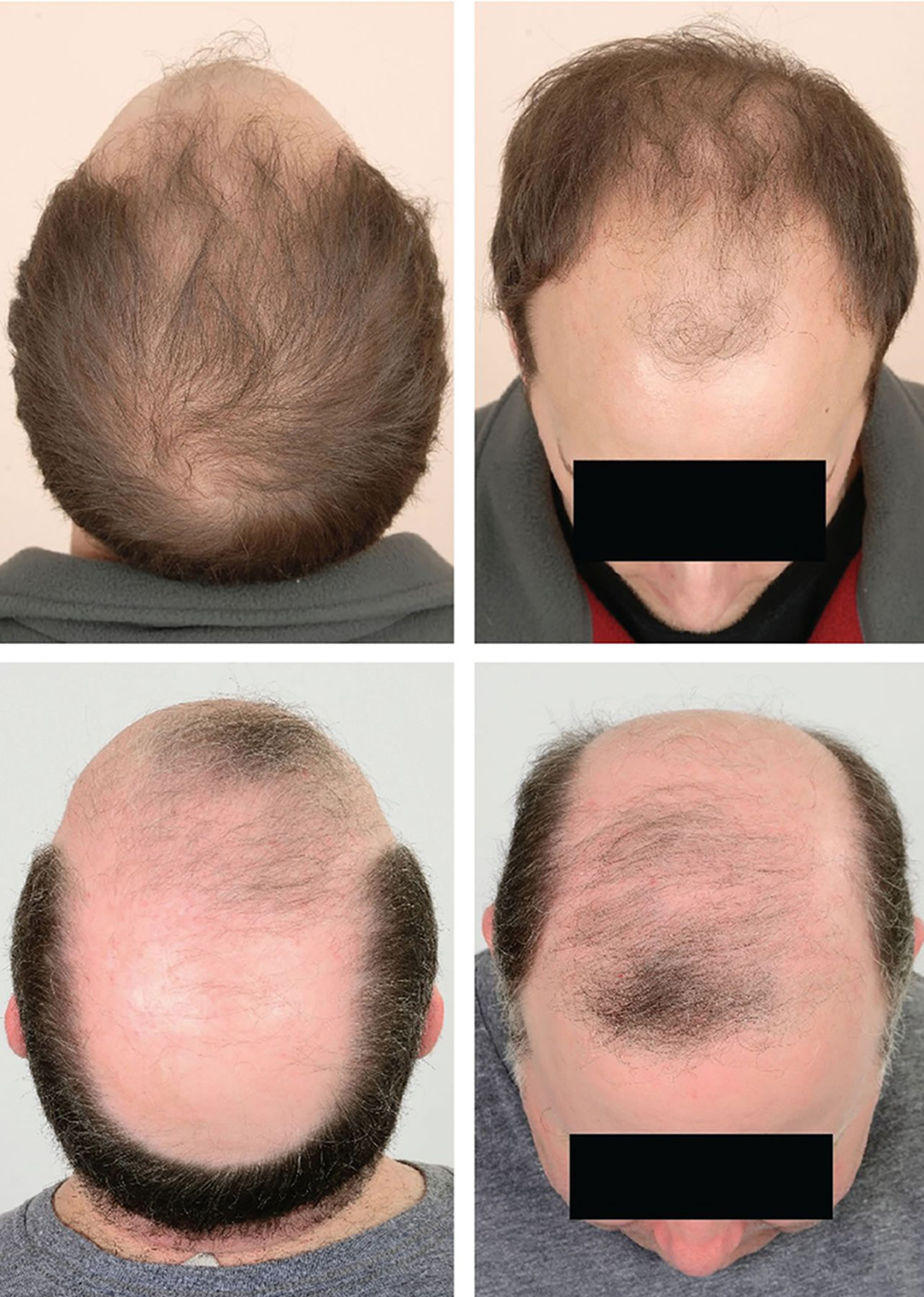
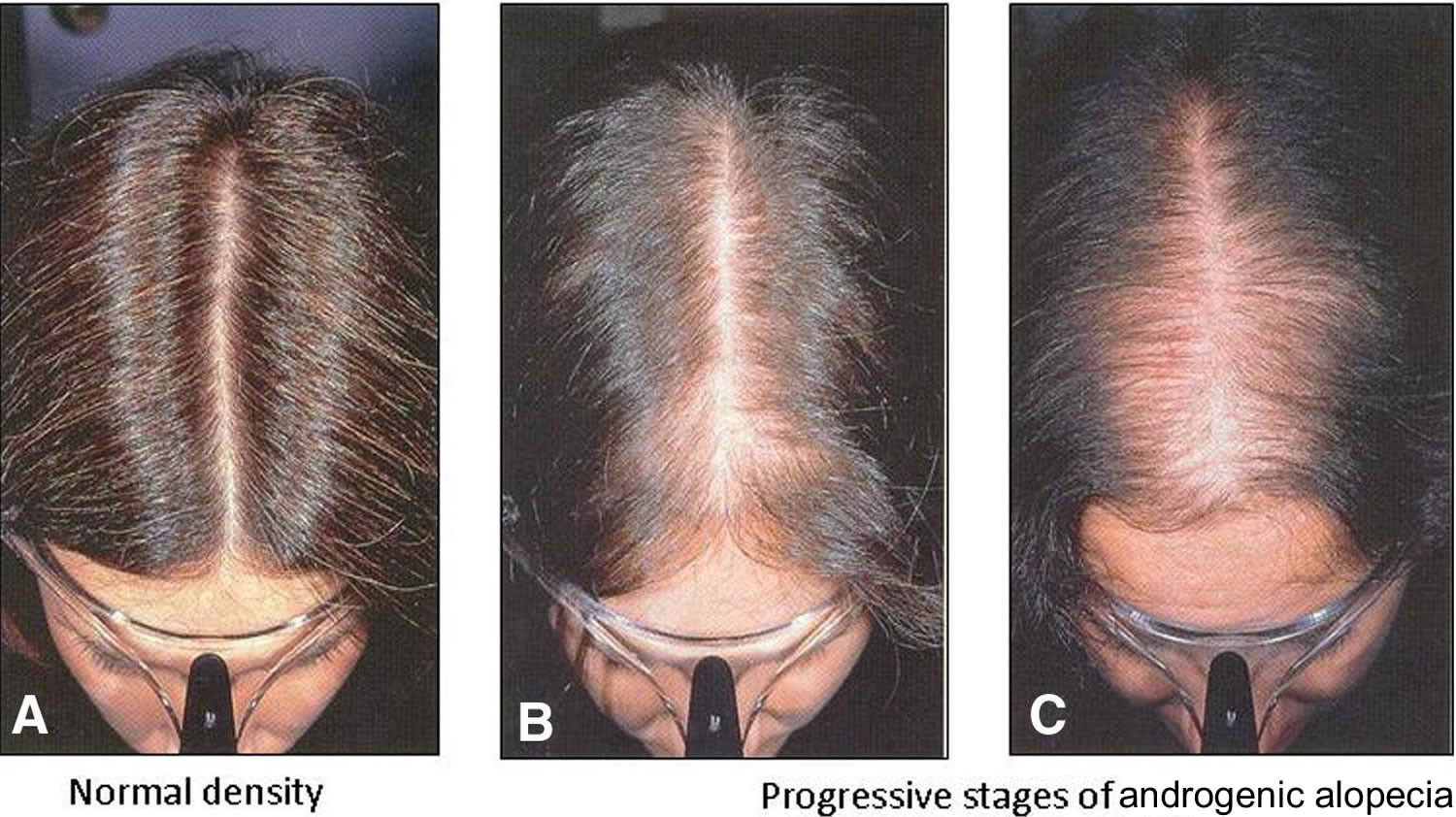
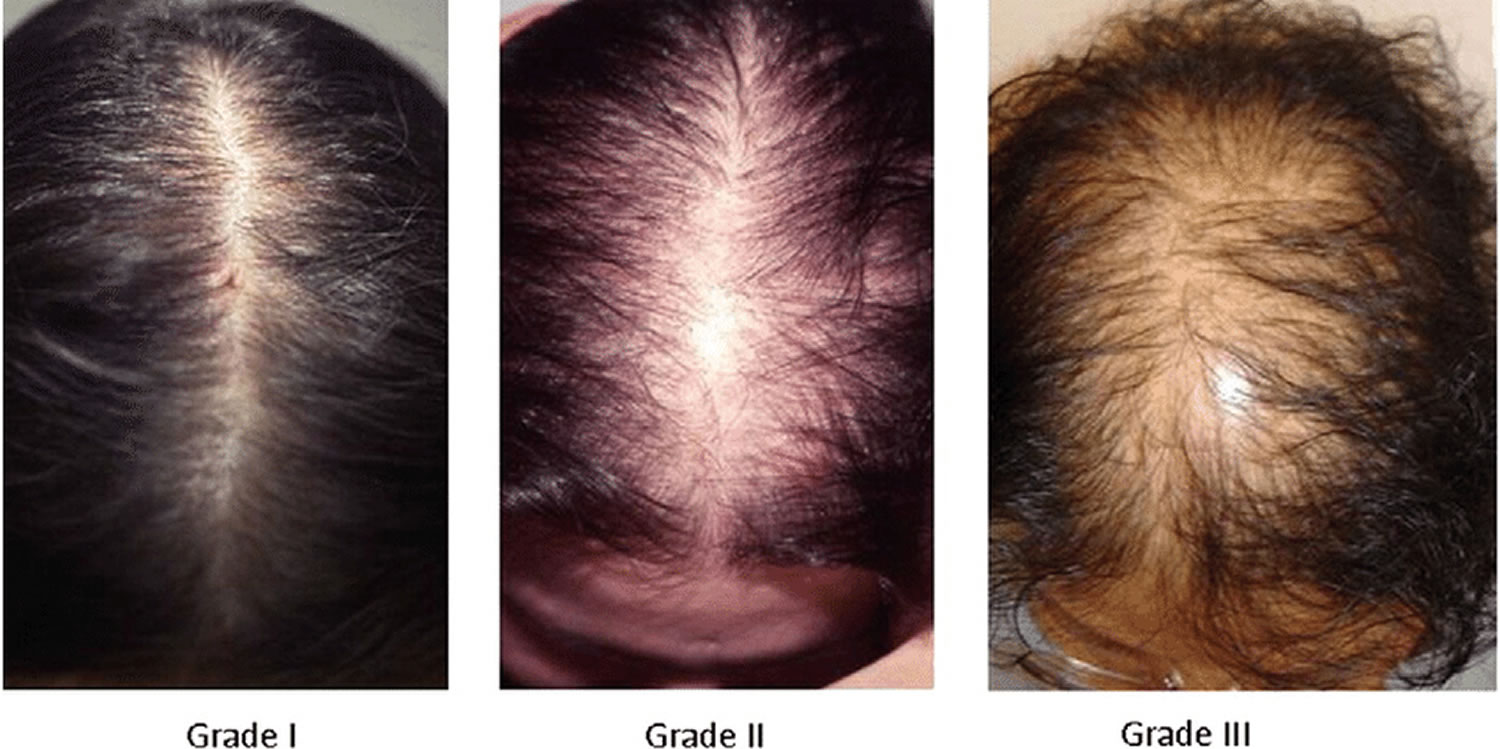
Also known as “male pattern baldness” or “female pattern baldness.” It is a thinning of the hair to an almost transparent state, in both men or women. It is thought to be a hereditary form of hair loss and is the most common type of progressive hair loss.
It is the most common type of hair loss affecting approximately 50% of men over the age of 50 and around 50% of women over the age of 65. Androgenetic Alopecia can also affect younger men and women. It is caused by a number of genetic and hormonal factors. Dihydrotestosterone (DHT) is the main hormone responsible for Androgenetic Alopecia in genetically susceptible individuals. Dihydrotestosterone (DHT) causes hair loss by inducing a change in the hair follicles. The hairs produced by the follicles affected by dihydrotestosterone (DHT) become progressively smaller until eventually the follicles shrink completely and stop producing hair entirely.
What does Androgenetic Alopecia look like?
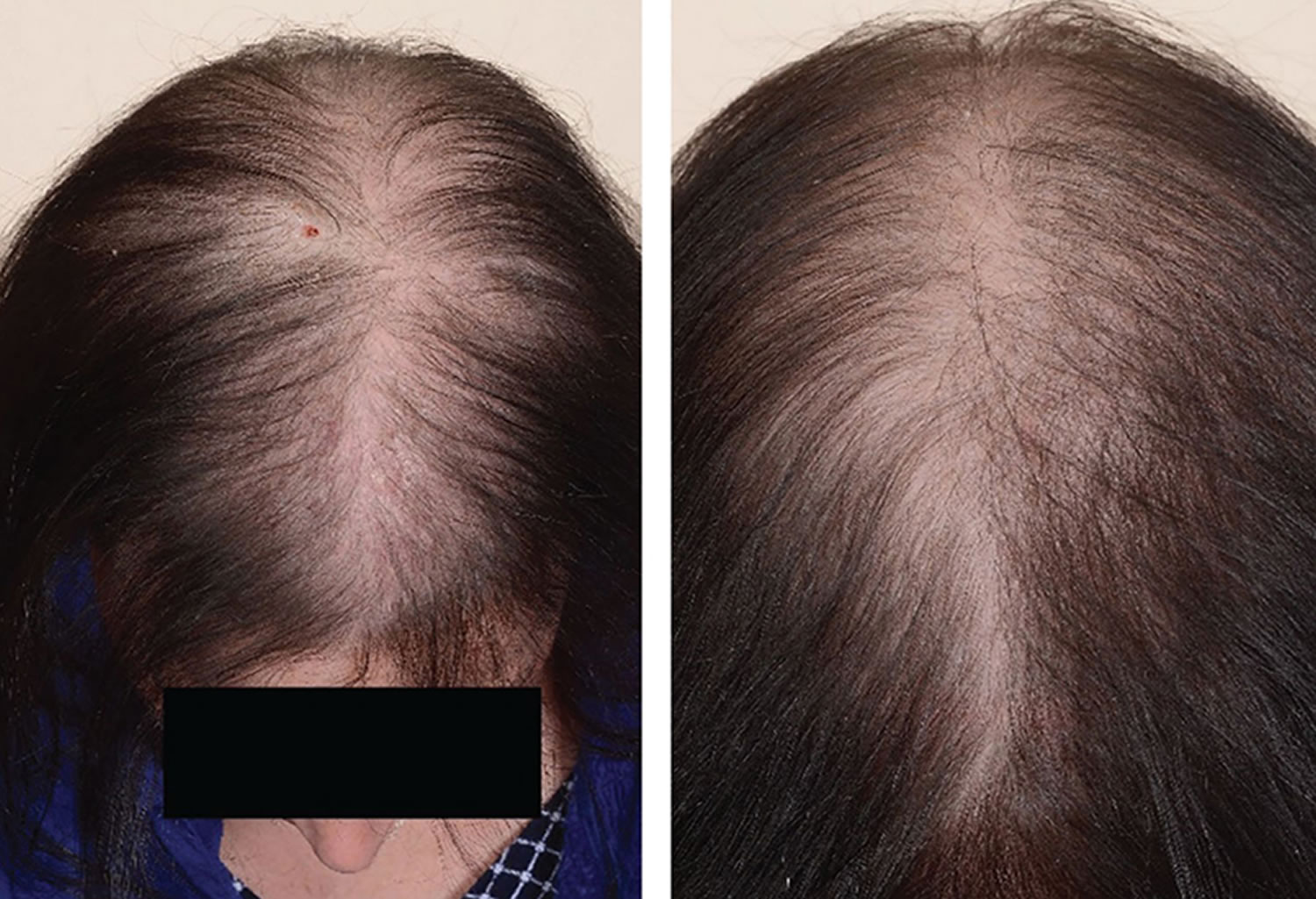
Androgenetic Alopecia tends to look different between males and females. In men, the typical pattern of hair loss is a receding hair line with loss of hair from the top and front of the head. In women, the usual pattern of hair loss is thinning at the crown of the head. Often in women the frontal hairline remains. There are of course exceptions to these patterns but these are typically how Androgenetic Alopecia presents itself in men and women. It is less likely that a woman will experience total baldness as a result of Androgenetic Alopecia.
Figure 8. Androgenetic Alopecia
Figure 9. Androgenetic Alopecia Norwood Classification
Is Androgenetic Alopecia permanent?
The hair follicles affected by Androgenetic Alopecia are permanently damaged and any hair loss as a result is irreversible.
Can Androgenetic Alopecia be treated?
As with other types of alopecia, there is no cure for Androgenetic Alopecia. However, the effects of Androgenetic Alopecia may be slowed down with treatments. Minoxidil is licensed to treat both male and female pattern baldness.
Is Androgenetic Alopecia hereditary?
Yes, it is understood that genetic susceptibility is inherited from either or both parents.
Alopecia Areata
Alopecia areata causes patches of baldness about the size of a large coin. It can result in a single bald patch or extensive patchy hair loss. They usually appear on the scalp but can occur anywhere on the body.
It is not unusual for people with alopecia areata to notice some scalp discomfort. Symptoms may include soreness, itching and tingling sensations, with many describing a sensation similar to that of a very tight ponytail. In cases of alopecia areata, hair loss is caused by inflammation around the hair roots. There are a lot of nerves around the hair follicles so it is not surprising that the inflammation can cause discomfort.
Men and women are equally affected, and, although it can occur at any age, the most common presentation is in children and young adults, with 30 to 48 percent of patients affected before 20 years of age 32. It affects 1 or 2 people in every 1,000 in the US.
The lifetime risk of developing alopecia areata is 1.7 percent, with a prevalence of 0.1 percent 32. There is a genetic predisposition to alopecia areata with a polygenic pattern of inheritance. It’s also believed some people’s genes make them more susceptible to alopecia areata, as 1 in 5 people with the condition have a family history of the condition.
In most studies, 20 to 42 percent of those affected have a family history of the disease 33. Skin biopsy from areas of alopecia may show multiple lymphocytes, which supports the theory for an autoimmune cause.
Alopecia areata is understood to be an autoimmune condition. The immune system which normally protects your body from foreign invaders, such as viruses and bacteria, mistakenly attacks your hair follicles. This is what leads to hair loss. Alopecia Areata typically starts as one or more small, smooth bald patches on the scalp. The hair loss can remain as patchy hair loss or can continue until all hair on the scalp is lost (Alopecia Totalis) or complete loss of hair from the body (Alopecia Universalis). In case series, alopecia totalis and universalis are less common than alopecia areata and account for 4.5 to 30 percent of all alopecia cases. Note that most cases of Alopecia Areata do not develop to the ‘totalis’ or ‘universalis’ stage. Alopecia Barbae is a type of Alopecia Areata which affects facial hair only.
Alopecia areata is more common among people with autoimmune conditions, such as vitiligo, diabetes, an overactive thyroid (hyperthyroidism), rheumatoid arthritis, discoid lupus erythematosus or Down’s syndrome. Patients with a history of atopy are also at an increased risk of developing alopecia.
In most cases of alopecia areata, hair will grow back in a few months. At first, hair may grow back fine and white, but over time it should thicken and regain its normal color.
Figure 10. Alopecia areata
The patient with alopecia areata (Figure 10) typically presents with bald patches on the scalp that often have developed rapidly with sudden loss of hair. In diffuse alopecia, there is more widespread hair loss, often associated with graying of the hair. The classic finding is a smooth, hairless patch surrounded by so-called exclamation point hairs. These are 2- to 3-mm broken hairs that have a club-shaped root with a thinner proximal shaft and a normal caliber distal shaft on microscopic examination.
Some people go on to develop a more severe form of hair loss, such as:
- Alopecia totalis (no scalp hair)
- Alopecia universalis (no hair on the scalp and body).
Is Alopecia Areata permanent?
Alopecia Areata does not cause permanent hair loss. The hair follicles are not destroyed and hair does have the possibility to re-grow. This means that hair can regrow anywhere, even after several years. It doesn’t mean it will happen but the possibility is there. It’s not possible to predict if people will experience full regrowth. Many people with Alopecia Areata do experience full regrowth. However once the condition has developed to Alopecia Totalis or Alopecia Universalis, the chances of full regrowth become smaller. It is quite common for people with Alopecia Areata to experience hair loss on and off throughout their life.
In most cases of regrowth in alopecia areata, the hair regrows the same color as it was before. In some people the regrowing hair is initially lighter in color but then recovers its normal pigmentation. Occasionally, however, the regrowing hair remains white permanently. Some scientists have suggested that alopecia areata specifically affects pigment cells (melanocytes) in the hair root and this may be true in some cases. More commonly it affects the ability of hair cells to take up pigment from melanocytes. Very occasionally the regrowing hair is darker than normal. Regardless, a change in color of any regrowth is nothing to be concerned about.
Can Alopecia Areata be treated?
Treatments may be offered by a Dermatologist but unfortunately none are guaranteed to work. Many people experience spontaneous regrowth without treatment.
Treatments are more likely to be effective in milder cases of alopecia areata with small patches of hair loss. No treatments are universally effective.
Treatment may induce hair growth, but usually does not change the course of the disease. When treatment is stopped, hair loss recurs. Many patients with one or two small patches can be managed without treatment and with reassurance of the benign nature of the condition. A systematic review of 17 randomized controlled trials of topical and oral steroids, topical minoxidil (Rogaine), topical cyclosporine, and photo-dynamic therapy found no long-term benefit of these interventions 34. In patients with persistent hair loss and less than 50 percent scalp involvement, intralesional corticosteroid therapy is the first-line treatment.6 Patients with more than 50 percent hair loss can be treated with topical immunotherapy using diphenyl-cyclopropenone or squaric acid 34. Overall, 34 to 50 percent of patients with alopecia areata will recover within one year; this number is as high as 80 percent in patients with one or two patches 35. The more severe the disease at onset, the worse the prognosis, with fewer than 10 percent of patients recovering from alopecia totalis and alopecia universalis 36.
Is there a cure for Alopecia Areata?
No, currently there is no cure for alopecia areata.
Is Alopecia Areata hereditiary?
It is understood that genetics plays a role in alopecia areata. Around 20% of people with alopecia areata will have a family member with the condition.
Those with alopecia areata are more likely to have family members with other autoimmune or atopic conditions, such as asthma, eczema, hay fever, rheumatoid arthritis, lupus, psoriasis, thyroid disease and vitiligo.
Alopecia Totalis
Alopecia totalis is a more advanced form of alopecia areata which results in total loss of all hair on the scalp.
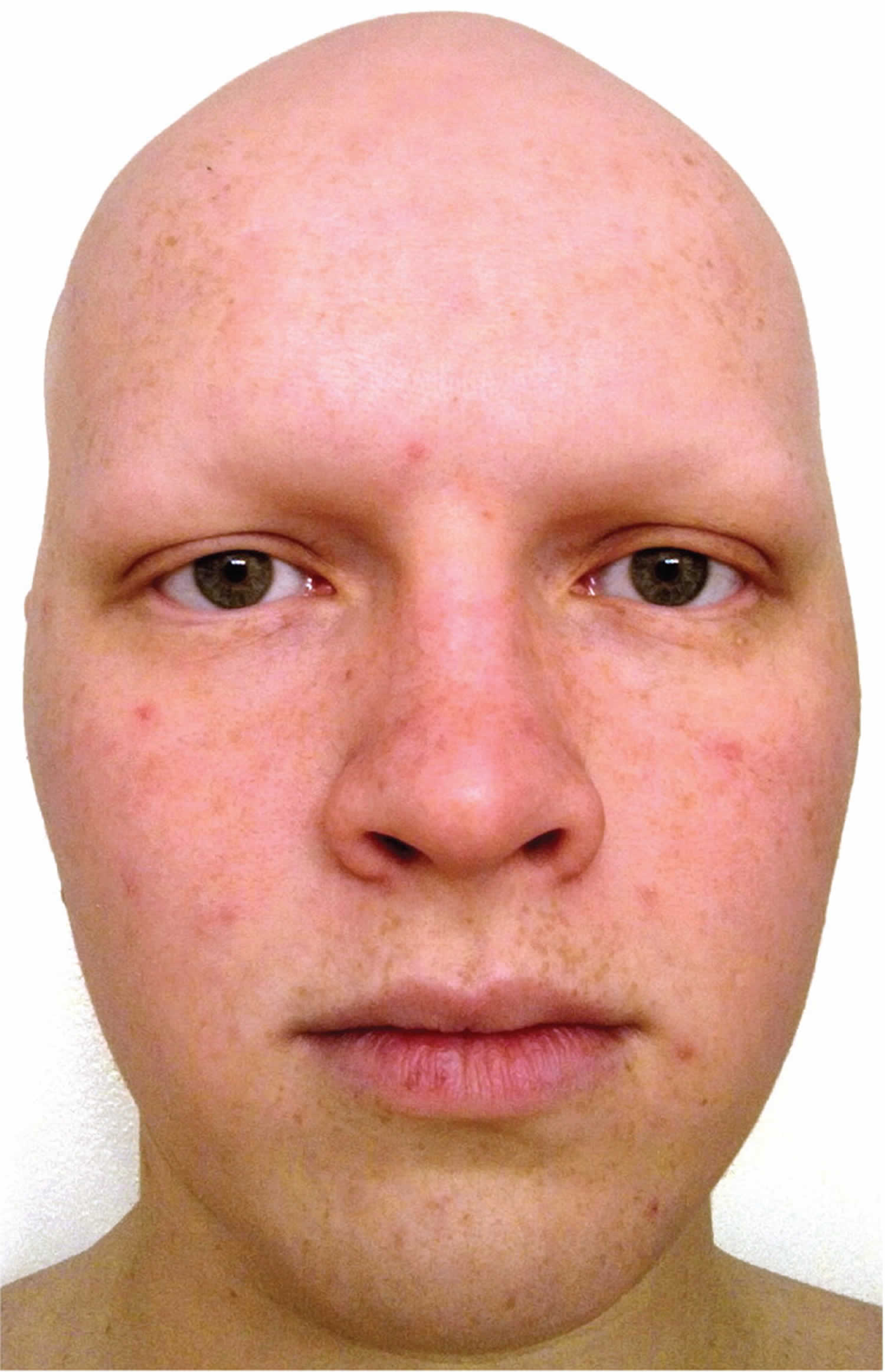
Alopecia Universalis
Alopecia universalis is the most advanced form of alopecia areata which results in total loss of all hair on the body, including eyelashes and eyebrows.
Figure 11. Alopecia universalis
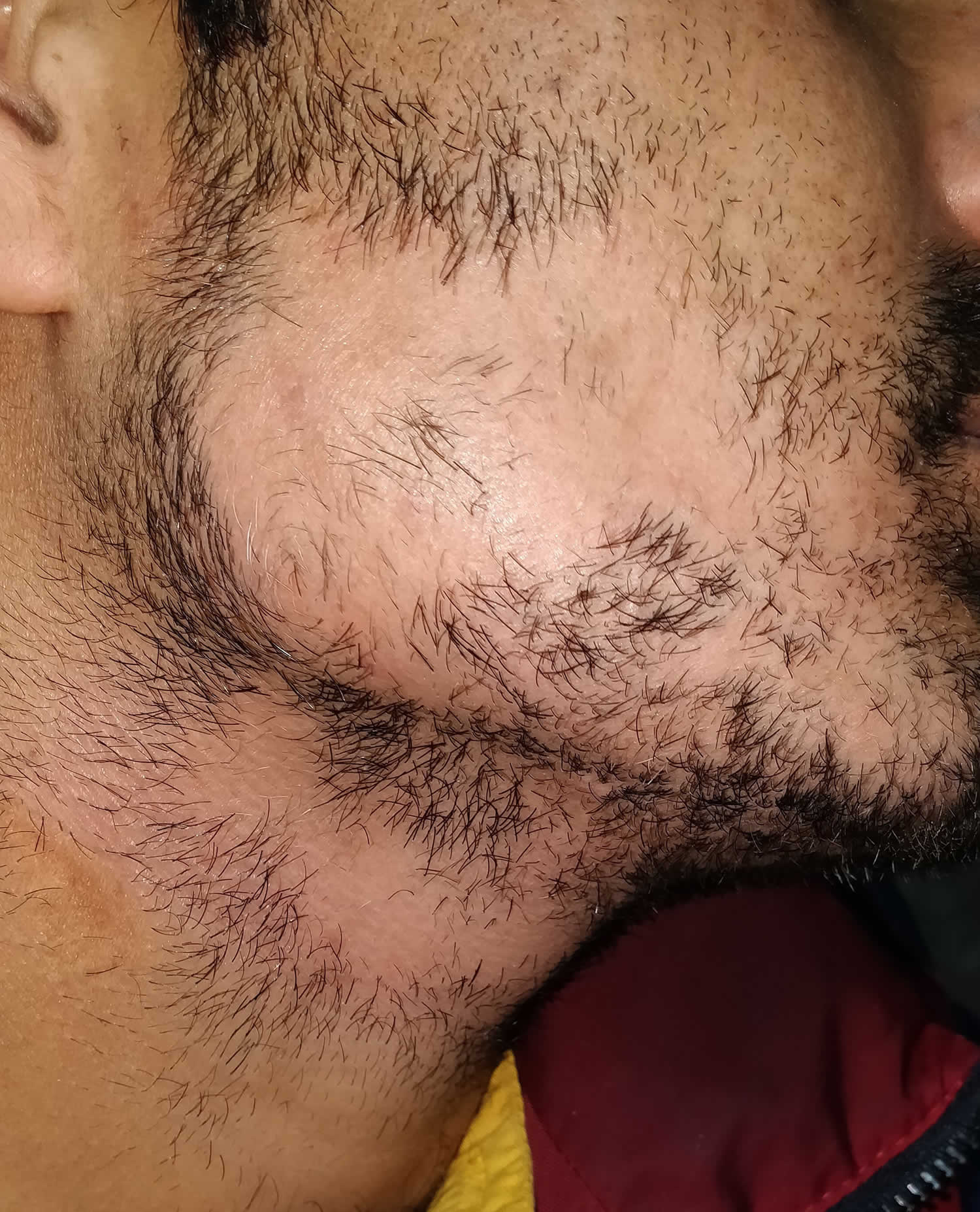
Alopecia Barbae
Alopecia barbae is alopecia areata that is localized to the beard area. It can be a single bald patch or more extensive hair loss across the whole of the beard area.
Figure 12. Alopecia barbae
Scarring Alopecias (Cicatricial Alopecias)
Scarring alopecias, also known as cicatricial alopecias, refers to a group of rare disorders that destroy hair follicles, which cause permanent hair loss.
In this type of alopecia, the hair follicle (the small hole in your skin that an individual hair grows out of) is completely destroyed. This means your hair won’t grow back.
The hair follicles are replaced with scar tissue (hence the name). In some cases the hair loss is without symptoms and can go unnoticed for long periods. In other cases the hair loss is accompanied by burning, itching and pain and is more progressive. It occurs in men and women of all ages but it less common in children. Frontal Fibrosing Alopecia and Lichen Planopilaris are two of the more well known Scarring Alopecias.
Depending on the condition, the skin where the hair has fallen out is likely to be affected in some way.
Conditions that can cause scarring alopecia include:
- scleroderma – a condition affecting the body’s connective (supporting) tissues, resulting in hard, puffy and itchy skin
- lichen planus – an itchy rash affecting many areas of the body
- discoid lupus – a mild form of lupus affecting the skin, causing scaly marks and hair loss
- folliculitis decalvans – a rare form of alopecia that most commonly affects men, causing baldness and scarring of the affected areas
- frontal fibrosing alopecia – a type of alopecia that affects post-menopausal women where the hair follicles are damaged, and the hair falls out and is unable to grow back
Scarring alopecia occurs in both males and females, but is less common in children than adults. It accounts for about 7% of hair loss cases.
What do Scarring Alopecias look like?
Despite the name, there is usually no scar on the scalp as the inflammation that destroys the hair follicle occurs below the skin surface.
Figure 11. Scarring Alopecia
Are Scarring Alopecias permanent?
Unfortunately once hair loss has occurred in cases of Scarring Alopecias, the damage is permanent. The cause of Scarring Alopecias is not well understood. What is known is that the inflammation occurs in the upper part of the hair follicle. This is where the stem cells and sebaceous glands are located. If the stem cells and sebaceous glands are destroyed, the hair follicle cannot regrow and hair loss is permanent.
Can Scarring Alopecias be treated?
Yes, treatments are available for the different kinds of Scarring Alopecias. Some Scarring Alopecias are more treatable than others and an early diagnosis from a Dermatologist will provide the best chance of success.
Infections
Fungal infections (e.g. ringworm, also known as tinea capitis when it affects the scalp) can cause patchy hair loss. This is more common in young children and rarely affects adults.
The scalp is also usually scaly or crusty as a result of the fungal infection.
Hair usually grows back once the fungal infection has been treated with antifungal medicines.
Traction Alopecia
Traction alopecia is usually due to excessive pulling or tension on hair shafts as a result of certain hair styles. Traction alopecia is a form of unintentional hair loss associated with specific social, cultural, and cosmetic practices. It is seen more often in women, particularly those of East Indian and Afro-Caribbean origin. Hair loss depends on the way the hair is being pulled. Prolonged traction alopecia can stop new hair follicles developing and lead to permanent hair loss.
Patients (primarily women) wearing wigs, tight braids, or using curling rollers are at risk. Hair processing including bleaching, coloring, and waving also puts patients at risk. Hair loss usually occurs in the frontotemporal area, although it can vary. Eliminating the stressor or source of traction on the hair commonly cures the problem and returns hair growth to normal. Several small case reports have shown topical minoxidil 2% to be beneficial for treatment 37. In rare circumstances, chronic traction can set in motion a process of folliculitis and subsequent scarring that can result in permanent hair loss to the affected area.
Trichotillomania
Trichotillomania is a condition of compulsive hair picking or pulling that can lead to patches of near complete hair loss. The areas most often affected are the front and side of the scalp.
In children, trichotillomania is usually just a habit and parents can help stop their child from hair pulling and plucking. In adults (and some children) the condition may be related to other psychological issues, and seeing a psychologist or psychiatrist may be recommended.
Telogen Effluvium
It is normal to shed approximately 30-150 hairs from your scalp daily as part of your hair cycle, but this can vary depending on washing and brushing routines. Hair regrows automatically so that the total number of hairs on your head remains constant. Telogen effluvium occurs when an increased number of hairs enter the telogen (resting) phase of the hair cycle and these hairs are lost approximately three months later 38. There is an increased proportion of hairs shift from the growing phase (anagen) to the shedding phase (telogen). Normally only 10% of the scalp hair is in the telogen phase, but in telogen effluvium this increases to 30% or more. This usually happens suddenly and can occur approximately 3 months after a trigger. There is a widespread ‘thinning’ of the hair, rather than specific bald patches. Your hair may feel thinner, but you’re unlikely to lose it all and your other body hair isn’t usually affected. Unlike some other hair and scalp conditions, telogen effluvium is temporary and the hair growth usually recovers.
Common triggers for this change in the usual telogen stage include psychological stress, childbirth, trauma, severe illness, injury, infection, surgery, crash diets, bereavement, sudden weight loss, new medication, hormonal changes, thyroid disorders, iron deficiency, anemia, or drugs. In around a third of those affected, no cause can be found 39.
Hyperthyroidism and hypothyroidism can cause telogen effluvium, which is usually reversible when the thyroid status is corrected (except in long-standing hypothyroidism). Severe iron deficiency anemia may be associated with it, but this remains controversial 40, 41. Drugs that cause telogen effluvium include antithyroid agents, hormones, anticonvulsants, anticoagulants, beta blockers, angiotensin-converting enzyme inhibitors, and lithium.
Patients with telogen effluvium usually present with an increased number of hairs in their hairbrush or shower, and sometimes thinning of the hair in the scalp, axillary, and pubic areas. A detailed history may indicate the cause of the hair loss, which usually has occurred two or three months before the hair falls out. On examination, there is generalized hair loss with a positive hair pull test, indicating active hair shedding, particularly at the vertex and scalp margin. The hair pull test is done by grasping approximately 40 to 60 hairs between the thumb and fore-finger and applying steady traction (slightly stretching the scalp) as you slide your fingers along the length of the hair. Generally, only a few hairs in the telogen phase can be plucked in this fashion. Less than 10 percent is considered normal, whereas greater than this is considered indicative of a pathologic process 42. Treatment of telogen effluvium primarily involves removal of the underlying stressors or correction of any precipitating medical conditions.
Telogen effluvium can be caused by your body reacting to:
- hormonal changes, such as those that take place when a woman is pregnant
- intense emotional stress or psychological stress
- intense physical stress, such as childbirth (this can resolve after a few months or transition into female pattern hair loss)
- physiological neonatal hair loss
- a short-term illness, such as a severe infection or an operation
- acute or chronic illness, especially if there is fever
- a long-term illness, such as cancer or liver disease
- changes in your diet, such as crash dieting or nutritional deficiency (eg, iron deficiency/)
- some medications, such as anticoagulants (medicines that reduce the ability of your blood to clot) or beta-blockers (used to treat a number of conditions, such as high blood pressure)
- endocrine disorders (eg, hypothyroidism, hyperthyroidism)
- discontinuing the contraceptive pill
- overseas travel resulting in jetlag
- skin disease affecting the scalp (eg, erythroderma)
- surgical operation
- accident
- excessive sun exposure.
In most cases, your hair will stop falling out and start to grow back within 6 months.
Is Telogen Effluvium permanent?
Telogen effluvium usually resolves completely without any treatment over several months, as the normal length of the telogen phase is around 100 days (3 to 6 months) after which period the hair begins to grow again (anagen phase). Regrowth usually occurs after removal of the trigger causing telogen effluvium. How long it takes for hair volume to return to normal will vary according to the length of hair. Telogen effluvium can return, especially if the underlying cause is not treated or recurs, and would be called chronic telogen effluvium if lasting more than 6 months. Furthermore, repeated episodes of acute telogen effluvium can sometimes evolve into female pattern hair loss.
What is chronic telogen effluvium?
In some patients, hair shedding continues to be intermittently or continuously greater than normal for long periods of time, sometimes for years. The hair cycle appears to be reset so that the anagen period is shortened.
Chronic telogen effluvium often presents in women that actually continue to have quite thick and moderately long hair – this is because they notice the shed hair more than those with finer or shorter hair. Telogen effluvium does not cause complete baldness, although it may unmask a genetic tendency to genetic balding i.e. female pattern hair loss, or in men, male pattern hair loss.
The mechanism of chronic telogen effluvium is not well understood. Middle-aged women with a long fluctuating course of telogen effluvium, producing widespread thinning lasting many years have normal hormonal studies.
Is telogen effluvium hereditary?
Telogen effluvium is not inherited, and it can affect all age groups and both genders equally.
What does Telogen Effluvium look like?
Hair loss occurs all over the scalp, resulting in a reduced volume of hair.
People with telogen effluvium usually present with an increased number of hairs in their hairbrush or shower, and sometimes thinning of the hair in the scalp, axillary, and pubic areas. A detailed history may indicate the cause of the hair loss, which usually has occurred two or three months before the hair falls out. On examination, there is generalized hair loss with a positive hair pull test, indicating active hair shedding, particularly at the vertex and scalp margin. The hair pull test is done by grasping approximately 40 to 60 hairs between the thumb and fore-finger and applying steady traction (slightly stretching the scalp) as you slide your fingers along the length of the hair. Generally, only a few hairs in the telogen phase can be plucked in this fashion. Less than 10 percent is considered normal, whereas greater than this is considered indicative of a pathologic process 43. Treatment of telogen effluvium primarily involves removal of the underlying stressors or correction of any precipitating medical conditions.
Figure 12. Telogen Effluvium
How is telogen effluvium diagnosed?
Telogen effluvium is usually diagnosed by its clinical features.
- Hair thinning involves the entire scalp +/- loss of other body hair.
- Examination shows diffuse thinning without focal areas of total alopecia and short hairs of normal thickness.
- A gentle hair pull test reveals an increased number of hairs; most are telogen with a typical epithelial sac.
A trichogram can help confirm the diagnosis; more than 25% telogen hairs in a trichogram strongly suggests telogen effluvium.
Light microscopic examination shows club hair
A scalp biopsy is rarely needed; it is expected to show normal terminal/vellus hair ratio, an increased number of telogen follicles, and little to no inflammation and fibrosis.
Can Telogen Effluvium be treated?
There is normally no treatment required for Telogen Effluvium as the hair will start growing once the trigger is removed. Medication is not required. Telogen Efflvium can return if the underlying cause is not treated or if it recurs.
Telogen effluvium management recommendations include:
- Gentle handling of the hair, avoiding over-vigorous combing, brushing and any type of scalp massage
- Treat any underlying scalp disorder or hormonal problem determined, if any
- Ensure a nutritious diet, with plenty of protein, fruit and vegetables.
- Correct any abnormality in thyroid function, or levels of iron, vitamin B12 and folic acid.
Anagen Effluvium
Anagen Effluvium refers to widespread hair loss that can affect your scalp, face and body, that arises during the anagen (growth) stage of the hair cycle. One of the most common causes of this type of hair loss is the cancer treatment chemotherapy. In some cases, other cancer treatments – including immunotherapy and radiotherapy – may also cause hair loss. But not all chemotherapy drugs cause hair loss, and sometimes the hair loss is so small it’s hardly noticeable. It may be possible to reduce hair loss from chemotherapy by wearing a special cap that keeps the scalp cool, but this isn’t always effective or widely available.
The hair loss is usually noticeable within a few weeks of starting treatment. Initially it causes patchy hair loss, which often then becomes total hair loss. The good news is that in most cases, hair loss in anagen effluvium is temporary. Your hair should start to grow back a few months (usually about 6 months later) after chemotherapy has stopped.
Other drugs also can cause hair loss. Many medicines used to treat even common diseases can cause hair loss. Anagen Effluvium can also be due to infection and toxins.
What does Anagen Effluvium look like?
Anagen effluvium presents with abrupt shedding of much of or all of the hair on the scalp, and often from the entire body including eyebrows, eyelashes and body hair. It may leave the scalp partially or completely bald shortly after the traumatic event with up to 90% hair loss over a period of weeks.
Other features depend on the cause of the hair shedding.
Figure 13. Anagen Effluvium
Who gets anagen effluvium?
Anagen effluvium is most common in patients of any age, sex, or race receiving chemotherapy.
Individuals with autoimmune conditions such as alopecia areata and pemphigus vulgaris can be affected.
Is Anagen Effluvium permanent?
In most cases the hair will eventually return once the underlying cause is treated/removed. Anagen effluvium as a result of chemotherapy treatment will normally recover within 3-6 months of the treatment being stopped. Sometimes the hair regrows despite the continuation of chemotherapy.
Anagen effluvium due to alopecia areata may persist; recovery is unpredictable.
What causes anagen effluvium?
Any insult that impairs mitosis of hair follicle keratinocytes can cause anagen effluvium. Disruption to cell division in the hair matrix makes the hair narrowed at its base and susceptible to breakage just above the zone of keratinisation. The necrotic matrix forms plugs consisting of melanin, keratin and inner root sheath which are extruded through the follicular opening. This process is known as trichomalacia.
The main causes of anagen effluvium are an infection, a drug, a toxin, radiation or an autoimmune disease.
- Radiation to the scalp can result in anagen effluvium. Regrowth of hair may be incomplete or may not occur.
- Autoimmune hair loss includes alopecia areata and its variants, alopecia totalis and alopecia universalis.
- Anagen effluvium may also occur in the rare immunobullous disease, pemphigus vulgaris.
An infection may interrupt hair growth in a localised area resulting in a single bald patch or several bald patches. Loose hairs can readily be extracted from the infected area, which may be swollen, boggy and crusted. Examples include:
- Boils and abscesses
- Fungal hair infection: tinea capitis or kerion.
Toxins that can interrupt hair growth include:
- Chemotherapy agents, usually prescribed to treat cancer, especially when multiple drugs are used or they are in high dose. Severe hair loss is reported from doxorubicin, the nitrosoureas, and cyclophosphamide. Other causes are bleomycin, dactinomycin, daunorubicin, systemic fluorouracil, and high-dose methotrexate.
- Other medicines such as colchicine and ciclosporin (ciclosporin more often causes increased hair growth)
- Poisons such as thallium, arsenic, gold and bismuth.
Hair loss develops within 2 to 4 weeks of chemotherapy. It affects most parts of the scalp, but other sites may be affected, such as eyebrows, armpits and genital area.
How is anagen effluvium diagnosed?
The diagnosis of anagen effluvium is usually made by taking a careful history, particularly of recent medicines, and by examining the scalp and shed hair clinically and by dermoscopy/trichoscopy.
In anagen effluvium, the end of the hair that comes from the scalp is tapered, narrowed, irregular, or broken off. Anagen hairs have long roots covered with the inner and outer root sheaths and are pigmented. In contrast, telogen hair is a roundish bulb or club. The follicular openings remain in both conditions.
A trichogram (forcible hair plucking within a unit area to determine the ratio of anagen to telogen hair) will show a large proportion of dystrophic anagen hairs.
Scalp biopsy should reveal a normal anagen-to-telogen ratio of any persisting hairs in anagen effluvium due to chemotherapy.
Other tests may be arranged to rule out other causes of hair loss, including iron deficiency, thyroid disease, systemic lupus, and infections (eg, syphilis).
Anagen effluvium treatment
Anagen effluvium due to chemotherapy is expected to recover fully within 3–6 months of stopping it. The hair nearly always grows back normally, but sometimes patients with straight hair develop curly hair when it regrows. Hair color may also change.
Suggested treatments for anagen effluvium include:
- Topical minoxidil solution
- Scalp cooling during chemotherapy
- Cosmetic camouflage to eyebrows.
Causes of hair loss
The causes of hair loss can be broadly divided into focal or diffuse hair loss (Table 1). Focal hair loss is secondary to an underlying disorder that may cause nonscarring or scarring alopecia. Nonscarring focal alopecia is usually caused by tinea capitis or alopecia areata, although patchy hair loss may also be caused by traction alopecia or trichotillomania. Scarring alopecia is rare and has a number of causes, usually discoid lupus erythematosus. Diffuse hair loss can be further categorized into conditions that cause hair shedding, of which the most common is telogen effluvium, and predominant hair thinning caused by male or female pattern hair loss (previously called androgenetic alopecia).
Table 1. Causes of Hair loss (Alopecia)
| Type of alopecia | Distinguishing characteristics | |
|---|---|---|
| Diffuse | ||
| Female pattern hair loss | Presents with hair thinning; frontal hairline intact; negative pull test away from hair loss | |
| Male pattern hair loss | Presents with hair thinning; M pattern; negative pull test away from hair loss | |
| Diffuse alopecia areata | Distribution more patchy; positive pull test | |
| Alopecia totalis or universalis | Total hair loss on the scalp and/or body | |
| Telogen effluvium | 30 to 50 percent of hair loss three months after precipitating event; positive pull test | |
| Anagen effluvium | Sudden hair loss of up to 90 percent two weeks following chemotherapy | |
| Focal | ||
| Nonscarring | ||
| Alopecia areata | Normal scalp with surrounding exclamation point hairs | |
| Tinea capitis | Scaly scalp with fungus visible on potassium hydroxide examination | |
| Traction alopecia | Patchy; related to hair practices; may have some scarring | |
| Trichotillomania | Patchy; may be some scarring and associated psychological disturbance | |
| Scarring (cicatricial) | Scarring and atrophy of scalp (e.g., discoid lupus erythematosus) | |
Footnote: Listed in order of clinical importance
[Source 44 ]Hair shaft abnormalities
Hair shaft defects can be inherited and congenital, or acquired due to disease or injury (eg excessive brushing, hair pulling [trichotillomania], hair dryer heat, relaxing chemicals, bleach). African hair practices.
Hair shaft abnormalities are diagnosed by dermatoscopy or microscopic examination of the hair, and sometimes by scanning electron microscopy. They include:
- Fractures: trichorrhexis nodosa, trichoschisis, trichoclasis (trichothiodystrophy)
- Irregularities: trichorrhexis invaginata (seen with ichthyosis in Netherton syndrome), Marie-Unna hypotrichosis (uncombable hair), pili bifurcati, pili annulati, pseudopili annulati, monilethrix (beaded hair), pseudomonilethrix
- Coiling and twisting: pili torti (twisted hair), woolly hair, trichonodosis (knotted hair)
Dermatological disease
Conditions resulting in reversible patchy hair thinning, poor hair quality and bald patches include:
- Localized alopecia areata
- Localized infection, such as tinea capitis
- Severe local skin disease, such as psoriasis, seborrheic dermatitis, atopic dermatitis, pityriasis rubra pilaris, cutaneous lupus erythematosus, cutaneous T-cell lymphoma
- Generalized skin disease (erythroderma)
Systemic disease
Systemic diseases resulting in reversible patchy hair thinning, poor hair quality and bald patches include:
- Iron deficiency
- Thyroid hormone deficiency
- Systemic lupus erythematosus
- Syphilis
- Severe acute or chronic illness
Destructive inflammatory skin diseases
Inflammation in the dermis or subcutaneous tissue may injure the hair follicle resulting in localised bald patches in which there are no visible follicles; this is called scarring alopecia or cicatricial alopecia.
Traumatic causes of scarring alopecia may be due to:
- Injury
- Surgery
- Radiation
- Traction (tight curls)
- Central centrifugal cicatricial alopecia
Infections causing scarring alopecia include:
- Bacterial infection: boils and abscesses (Staphylococcus aureus)
- Fungal infection: kerion (inflammatory tinea capitis)
- Viral infection: shingles (herpes zoster)
Inflammatory skin diseases causing scarring alopecia include:
- Folliculitis decalvans
- Dissecting cellulitis
- Lichen planopilaris
- Frontal fibrosing alopecia
- Alopecia mucinosa
- Discoid lupus erythematosus
- Localised scleroderma
Pseudopelade of Brocq is a condition in which there are localised areas of the scalp in which hair follicles have disappeared without visible inflammation.
Zinc and copper
Even though zinc and copper are theoretically considered to play certain roles in the pathogenesis of hair loss, clinical research has shown opposing views on the relationship. Zinc is an essential cofactor for multiple enzymes and it is involved with important functional activities in the hair follicle 21. Furthermore, zinc is a potent inhibitor of hair follicle regression and it accelerates hair follicle recovery 45. More specifically, transient zinc deficiency is a major pathogenesis in acrodermatitis enteropathica, telogen effluvium and brittle hair, resulting in hair loss 46, 21. Arguments that zinc deficiency can be a disturbing factor for the growth of hair have been emerging since the 1990s 47, 21. However, there is no current evidence of the efficacy of zinc supplementation for individuals experiencing hair loss who are not deficient. There is also a contradicting argument that there exists no relationship between zinc and hair loss 48. Even though a few studies have reported that zinc deficiency has correlations with alopecia areata and telogen effluvium 21, no studies have mentioned the relation between zinc and androgenic alopecia so far 49.
Oral zinc compounds have been used for decades for treating disorders such as telogen effluvium 49 and alopecia areata 50. Reports have also been published on oral zinc sulfate therapy with encouraging results for some cases of alopecia areata. In 1976 Wolowa and Jablonska 51 reported that two patients with alopecia areata regrew their hair after treatment with oral zinc sulfate. It has been reported that some alopecia areata patients have zinc deficiency 45. In a recent study 52, alopecia areata patients whose serum zinc was lower than 70 µg/dl were administered zinc gluconate 50 mg every day for 12 weeks. The findings showed that the serum zinc concentration was elevated by 27.6 µg/dl on average, and 60% of the patients administered zinc showed a clinically therapeutic effect, suggest that zinc supplementation could become an adjuvant therapy for the alopecia areata patients with a low serum zinc level and for whom the traditional therapeutic methods have been unsuccessful. Another study examined the blood and urine samples of children with alopecia, and found that the zinc concentrations in blood and urine were lower than in the normal group12. However, another study found that even though there was a difference in serum zinc and copper concentrations between hair loss patients and normal individuals, there was no statistical significance. A similar study also found that there was no difference in zinc and copper, but the concentration of serum magnesium was quite higher 53. Although it is likely that there is a close theoretical relationship between serum copper and hair loss, as with zinc, more studies have shown contradictory results. In a study conducted on Koreans consisting of a control group of 10 normal people and a group of 30 alopecia areata patients, the serum zinc concentration was significantly low in the patients group, but the serum copper concentration was a little higher in the patients group without any statistical significance 54. In a study conducted in Indonesia that measured serum zinc, copper, and magnesium for 50 alopecia areata patients and 50 people in a normal control group, copper and magnesium levels did not show any significant differences, in contrast to the zinc levels 47. However, there was a study that indicated the serum copper concentration in alopecia universalis was lower in the patient group 53.
How is hair loss diagnosed?
Before making a diagnosis, your doctor will likely give you a physical exam and ask about your diet, your hair care routine, and your medical and family history. You might also have tests, such as the following:
- Blood test for hematology, thyroid function and serology. This might help uncover medical conditions that can cause hair loss.
- Hair pull test. Your doctor gently pulls several dozen hairs to see how many come out. This helps determine the stage of the shedding process.
- Scalp biopsy. Your doctor scrapes samples from the skin or from a few hairs plucked from the scalp to examine the hair roots under a microscope. This can help determine whether an infection is causing hair loss.
- Wood lamp examination
- Swabs of pustules for bacterial and/or viral culture
- Skin scrapings and hair clippings for mycology
- Light microscopy. Your doctor uses a special instrument to examine hairs trimmed at their bases. Microscopy helps uncover possible disorders of the hair shaft.
Hair loss in men
Male-pattern hair loss or baldness sometimes referred to as male androgenic alopecia or androgenetic alopecia, is the most common type of hair loss, affecting around half of all men by the age of 50 1.
Male-pattern hair loss usually starts around the late 20s or early 30s and most men have some degree of hair loss by their late 30s.
Male-pattern hair loss generally follows a pattern of a receding hairline, followed by thinning of the hair on the crown and temples, leaving a horseshoe shape around the back and sides of the head. A similar type of hair loss in women, female pattern hair loss, results in thinning hair on the mid-frontal area of the scalp and is generally less severe than occurs in males.
Sometimes it can progress to complete baldness, although this is uncommon.
Male pattern hair loss is due to a combination of hormones (androgens) and a genetic predisposition or hereditary, which means it runs in families. It’s thought to be caused by oversensitive hair follicles, linked to having too much of a certain male hormone.
Although it is not life threatening, it is associated with negative psychological effects, including low self-esteem, depression, and a general dissatisfaction with body appearance 55. Hair loss affects the androgen-sensitive follicles, starting with bitemporal recession, and then spreads to thinning of the vertex and frontal regions in a classic M pattern. Dihydrotestosterone (DHT), the androgen derived from testosterone, plays a key role in male pattern hair loss, and men with lower levels of dihydrotestosterone (DHT) have less hair loss 56.
What causes male pattern baldness?
Male pattern hair loss is an inherited condition, caused by a genetically determined sensitivity to the effects of dihydrotestosterone (DHT) in some areas of the scalp. DHT (dihydrotestosterone) is believed to shorten the growth, or anagen phase of the hair cycle, from a usual duration of 3–6 years to just weeks or months. This occurs together with miniaturisation of the follicles and progressively produces fewer and finer hairs. The production of DHT is regulated by an enzyme called 5-alpha reductase.
Male pattern hair loss occurs in men who are genetically predisposed to be more sensitive to the effects of DHT. Researchers now believe that the condition can be inherited from either side of the family.
Several genes are involved, accounting for differing age of onset, progression, pattern and severity of hair loss in family members. The susceptibility genes are inherited from both mother and father. At this time, genetic testing for prediction of balding is unreliable.
A few women present with male pattern hair loss because they have excessive levels of androgens as well as genetic predisposition. These women also tend to suffer from acne, irregular menses and excessive facial and body hair. These symptoms are characteristic of polycystic ovarian syndrome (PCOS) although the majority of women with PCOS do not experience hair loss. Less often, congenital adrenal hyperplasia may be responsible. Females that are losing their hair with age are more likely to present with female pattern hair loss, in which hormone tests are normal.
Is male pattern hair loss hereditary?
Yes. It is believed male pattern hair loss can be inherited from either or both parents.
How common is male pattern hair loss?
Male pattern hair loss affects nearly all men at some point in their lives. It affects different populations at different rates, probably because of genetics. Up to half of male Caucasians will experience some degree of hair loss by age 50, and possibly as many as 80% by the age of 70 years, while other population groups such as Japanese and Chinese men are far less affected.
Can male pattern hair loss be cured?
No, there is no cure. However, it tends to progress very slowly, from several years to decades. An earlier age of onset may lead to quicker progression.
Hair loss treatment for men
Current male pattern hair loss treatment options include:
- Hair replacement / transplantation
- Cosmetics
- Micropigmentation (tattoo) to resemble shaven scalp
- Hairpieces
- Minoxidil solution
- Finasteride tablets (type II 5-alpha-reductase inhibitor)
- Dutasteride (type I and type II 5-alpha-reductase inhibitor).
Topical minoxidil (2% and 5%) and oral finasteride are the only treatments approved by the FDA for treatment of male pattern hair loss in men older than 18 years. Treatment with finasteride can cause decreased libido, impotence, and ejaculation disorders 57. These adverse effects often abate with continued treatment and happen in less than 2 percent of men younger than 40 years 58. Finasteride may also induce depression 59. Minoxidil is available over-the-counter, and it should be applied to the scalp and not the hair. Its mechanism of action is unclear. Some shedding during the first few months of treatment is common. The minoxidil 5% solution has not been shown to be consistently more effective than the 2% solution, and patients using the higher concentration had more adverse effects, including allergic contact dermatitis, dryness, and itching 60. These typically resolve after treatment is discontinued 61.
Several small studies have shown some increased effectiveness with combined minoxidil and finasteride treatment 62, 63. Starting treatment early can help maximize success, and patients can expect to see results after three to six months, although dense regrowth is not likely. Discontinuation of finasteride or minoxidil results in loss of any positive effects on hair growth within 12 and six months, respectively 64. When switching between treatment with finasteride and minoxidil, it is best to overlap treatments for three months to minimize hair loss 65. Additional treatment options are listed in Table 2: Treatment of Hair Loss (Summary & Evidence) below.
A phase 2 randomized placebo-controlled study of dutasteride versus finasteride showed that the effect of dutasteride was dose dependent and 2.5mg of dutasteride was superior to 5mg finasteride in improving scalp hair growth in men between the ages of 21 and 45 years 66. It was also able to produce hair growth earlier than finasteride. This was evidenced by target area hair counts and clinical assessment at 12 and 24 weeks. In addition, a recent randomized, double blind, placebo-controlled study on the efficacy of dutasteride 0.5mg/day in identical twins demonstrated that dutasteride was able to significantly reduce hair loss progression in men with male pattern hair loss 67. A single case report showed improvement of hair loss with dutasteride 0.5mg in a woman who had failed to show any response to finasteride 68, 69.
In one phase 3 study dutasteride 0.5 mg daily showed significantly higher efficacy than placebo based on subject self-assessment and by investigator and panel photographic assessment 70. There was no major difference in adverse events between two groups the treatment and placebo groups. However, this study was limited to only 6 months. Another more recent phase 3 trial found that dutasteride 0.5 mg was statistically superior to finasteride 1 mg and placebo at 24 weeks 71.
There is some evidence that ketoconazole shampoo, an antifungal cortisol inhibitor shampoo, may also be of benefit, perhaps because it is effective in seborrheic dermatitis and dandruff 72, 73. A study compared shampoo containing 2% ketoconazole with unmedicated shampoo among 39 patients with male-pattern androgenetic alopecia 74. Medicated 2% ketoconazole shampoo increased hair density and the size and proportion of hair follicles residing in the anagen phase, both in isolation and in combination with minoxidil 74. Similarly, a 2007 study 75 including six patients with male-pattern androgenetic alopecia found hair regrowth with 2% ketoconazole topical lotion 76. Interestingly, one patient stopped using the lotion and depicted hair loss recurrence three months later, suggesting continual ketoconazole application is required for maintenance of hair regrowth. In addition, the authors found that ketoconazole may promote hair regrowth via both androgen-dependent and androgen-independent mechanisms 76. A 2019 study 77 compared the efficacy of 2% topical ketoconazole in comparison to 2% minoxidil among patients with female-pattern androgenetic alopecia. Whereas a significant difference between baseline and months 4 and 6 was observed among those receiving topical minoxidil, significant improvement with ketoconazole was observed only at month 6, suggesting delayed treatment efficacy with ketoconazole. However, whereas treatment-related side effects were reported among 55% of those receiving minoxidil, side effects were reported in only 10% receiving ketoconazole, and there was no difference in patient satisfaction between the groups 77. These studies highlight the potential therapeutic role of cortisol inhibition on hair regrowth in patients with both male and female-pattern androgenetic alopecia, although additional large, randomized controlled trials are needed to better assess efficacy.
Low-dose oral minoxidil (off label) can increase hair growth on the scalp, but may also result in generalized hypertrichosis and other adverse effects 78.
Low-level laser therapy (LLLT) is of unproven benefit in male pattern balding; the Capillus® laser cap and Hairmax® Lasercomb/Laserband are two low‐level laser therapy (LLLT) devices have been approved by the FDA for the management of androgenetic alopecia 79, 80. Minimal side effects were reported. Small number of participants reported adverse events of acne, mild paresthesia such as burning sensation, dry skin, headache, and itch 81.
Light‐emitting diode (LED) devices. In contrast with low-level laser therapy (LLLT) that delivers a single, collimated wavelength of light, light‐emitting diode (LED) devices may emit a small band of wavelengths. In particular, an all‐LED device that delivers dual dark orange (620 nm) and red light (660 nm) (Revian Red) to promote blood flow, reduce inflammation, and inhibit DHT via 5-alpha-reductase downregulation 82. In a prospective, randomized, double‐blind, controlled study, 18 male pattern hair loss subjects were treated with Revian Red cap vs. 18 male pattern hair loss subjects were treated with a sham light device for 10 min daily for 16 weeks total 83. Preliminary photographic assessments revealed increased mean hair count in the active group as compared to placebo group. Specifically, active group participants demonstrated approximately 26.3 more hairs per cm² compared to the placebo group. Overall, literature has suggested light therapy to be a safe treatment modality for androgenic alopecia (androgenetic alopecia) in both male and female patients when used independently or in combination with topical/oral therapies 81, 84. Light therapy has an excellent side effect profile, and there are no contraindications for use, although caution may be taken when administering in patients with dysplastic lesions on the scalp 85.
Platelet-rich plasma injections are also under investigation 86. Platelet‐rich plasma treatment can be administered alone or in combination with other therapies for androgenetic alopecia, although better results are obtained if platelet‐rich plasma administration is used in association with topical (such as minoxidil) or oral therapies (finasteride) 86. Further studies are required to determine the magnitude of the benefit if any.
Platelet‐rich plasma is generally indicated for patients with early‐stage androgenic alopecia, as intact hair follicles are present and a more significant hair restorative effect can be achieved 87. During the procedure, approximately 10–30 mL of blood are drawn from the patient’s vein and centrifuged for 10 min in order to separate the plasma from red blood cells. The platelet‐rich plasma, containing numerous growth factors, is then injected into the deep dermis or subcutaneous tissue at a volume of 4–8 mL per session. Mild side effects include scalp pain, headache, and burning sensation, but these effects usually subside in 10–15 minutes post‐injection and do not warrant use of topical anesthesia or pain medications 88. Vibration or cool air is typically sufficient to alleviate any significant pain that a patient may feel from the treatment. Patients can resume regular activities immediately after treatment but should avoid strenuous physical activity 24 hour post‐treatment to allow for optimal absorption of platelet‐rich plasma into tissue.
Hausauer and Jones 89 conducted a single center, blinded, randomized controlled trial investigating the efficacy of two platelet‐rich plasma regimens in 40 androgenic alopecia subjects. Participants received either subdermal platelet‐rich plasma injections with 3 monthly sessions and booster 3 months later (group 1) or 2 sessions every 3 months (group 2). Folliscope hair count and shaft caliber, global photography, and patient satisfaction questionnaires were completed at baseline, 3‐month, and 6‐month visits. The authors reported statistically significant increases in hair count and shaft caliber in both groups at 6 months. Importantly, improvements occurred more rapidly and profoundly in group 1, indicating that platelet‐rich plasma injections should be administered first monthly 89. Alves and Grimalt 90 demonstrated significant differences in mean anagen hair and telogen hair count as well as telogen and overall hair density when compared to baseline. In a review of 16 studies comprising a total of 389 patients with androgenic alopecia, the majority demonstrated efficacy in promoting successful hair growth after 3–4 sessions on a monthly basis, followed by quarterly maintenance sessions 91.
Platelet‐rich plasma is not curative for hair loss and must be continued long term for hair sustenance. However, patient satisfaction is typically very high and 60–70% of patients continue to undergo maintenance treatments. Due to the relatively recent introduction of platelet‐rich plasma injections for androgenic alopecia, there are no long‐term studies evaluating its effectiveness. Additionally, it is difficult to compare the efficacy with other remedies due to the lack of standardization in regard to platelet‐rich plasma kits, treatment fractions, and regimens, including the use of newer multi‐needle injectors.
While platelet‐rich plasma injections are considered safe when performed by a trained medical provider, these treatments are not suitable for everyone. Platelet‐rich plasma may not be appropriate for those with a history of bleeding disorders, autoimmune disease, or active infection, or those currently taking an anticoagulant medication. Although the majority of patients seem to tolerate the pain associated with scalp injections, some patients may prefer to avoid it.
Hair loss in women
As well as affecting men, hair loss can sometimes affect women (female-pattern baldness). It’s estimated, for instance, that around 50% of women over the age of 65 experience female-pattern baldness (also known as androgenic alopecia or androgenetic alopecia)– the most common type of hair loss, which is thought to be inherited. However, it’s not clear if female-pattern baldness is hereditary and the causes are less well understood. Because there is a genetic basis to female pattern baldness, different racial populations are affected at different rates. Almost half of men, and perhaps as many women who are postmenopausal, are affected by hair loss to some degree. Onset of hair loss seems most common at either 20–30 or 40–50 years of age. The incidence is highest in Caucasians followed by Asians and African Americans, and the lowest incidence of hair loss is in Native Americans.
In female-pattern baldness, there is diffuse thinning of hair on the scalp due to increased hair shedding or a reduction in hair volume, or both. It tends to be more noticeable in women who have been through the menopause (when a woman’s periods stop at around age 50-52), perhaps because they have fewer female hormones. Female pattern hair loss presents quite differently from the more easily recognizable male pattern baldness, which usually begins with a receding frontal hairline that progresses to a bald patch on top of the head. It is very uncommon for women to bald following the male pattern unless there is excessive production of androgens in the body.
It is normal to lose up to 50-100 hairs a day. Another condition called chronic telogen effluvium also presents with increased hair shedding and is often confused with female pattern hair loss. It is important to differentiate between these conditions as management for both conditions differ.
Up to 50 percent of women will experience female pattern hair loss during their lifetime 1. Women usually present with hair thinning over the central area of the scalp and widening of the midline part, but with reservation of the frontal hairline (Figure 11). Women who also have abnormal menses, history of infertility, hirsutism, unresponsive cystic acne, virilization, or galactorrhea should have a targeted endocrine work-up for hyperandrogenism (i.e., testosterone, dehydroepiandrosterone sulfate, and prolactin), although most will have normal androgen levels 92. Evaluation for iron deficiency, thyroid disease, and syphilis (a rare cause) should be considered because they can contribute to hair thinning or generalized hair loss.
Figure 14. Female pattern hair loss
Footnote: The pattern of hair loss is different in women than men; the hairline is preserved while there is diffuse thinning of the hair of the crown and frontal scalp. Total hair loss is very rare.
Figure 15. Androgenetic alopecia female (female pattern baldness)
Footnote: Androgenetic alopecia in women with ‘Christmas Tree Pattern’
[Source 93 ]Figure 16. Ludwig classification for female pattern hair loss
Footnote: Ludwig classification for female pattern hair loss (androgenic alopecia in women) Grades 1 to 3 (minimal, moderate, intense)
[Source 94 ]Figure 17. Sinclair’s self-reporting photographic measure of female pattern of hair loss
What causes female pattern hair loss?
Female pattern hair loss has a strong genetic predisposition. The mode of inheritance is polygenic, indicating that there are many genes that contribute to female pattern hair loss, and these genes could be inherited from either parent or both. Genetic testing to assess the risk of balding is currently not recommended, as it is unreliable.
Currently, it is not clear if androgens (male sex hormones) play a role in female pattern hair loss, although androgens have a clear role in male pattern baldness. The majority of women with female pattern hair loss have normal levels of androgens in their bloodstream. Due to this uncertain relationship, the term female pattern hair loss is preferred to ‘female androgenetic alopecia’.
The role of estrogen is uncertain. Female pattern hair loss is more common after the menopause suggesting estrogens may be stimulatory for hair growth. But laboratory experiments have also suggested estrogens may suppress hair growth.
Hair loss treatment for women
Minoxidil 2% is the only treatment approved by the U.S. Food and Drug Administration (FDA) for treating female pattern hair loss in women older than 18 years. Topical minoxidil; the 2% preparation recommended for women is available over the counter. This may help hair to grow in a quarter of the women using it, and it will stop or slow hair loss in the majority of users. A Cochrane systematic review published in 2012 95 concluded that minoxidil solution was effective for female pattern hair loss. This updated 2016 Cochrane review found that minoxidil is more effective than placebo for female pattern hair loss or female androgenic alopecia 96. Minoxidil is available as 2% and 5% solutions; the stronger preparation is more likely to irritate and may cause undesirable hair growth unintentionally on areas other than the scalp. Furthermore, the updated 2016 Cochrane review found no difference in effect between the minoxidil 2% and 5% for female pattern hair loss 96.
Hormonal treatment, i.e. anti-androgen medicines are oral medications that block the effects of androgens (e.g. spironolactone, cyproterone acetate, finasteride and flutamide) is also often tried. These medicines help stop hair loss and may also stimulate hair regrowth. Spironolactone has been shown to stop the loss of hair in 90 per cent women with androgenetic alopecia. In addition, partial hair regrowth occurs in almost half of treated women. The effects of treatment generally only last while you continue to take the medicine – stopping the medicine will mean that your hair loss will return. Spironolactone and cyproterone acetate should not be taken during pregnancy. Effective contraception must be used while you are being treated with these medicines, as it can affect a developing baby. These medicines should also not be taken if you are breast feeding.
A hyperandrogenic state may limit the success of treatment with minoxidil 97 and, in these women, spironolactone (Aldactone) 100 to 200 mg daily may slow the rate of hair loss 98. Women with evidence of a hyperandrogenic state requesting combined oral contraceptives would benefit from using antiandrogenic progesterones, such as drospirenone 99. Finasteride (Propecia) is ineffective in postmenopausal women with female pattern hair loss 100. Finasteride, spironolactone, and cyproterone should not be used in women of childbearing potential.
A combination of low dose oral minoxidil (eg, 2.5 mg daily) and spironolactone (25 mg daily) has been shown to significantly improve hair growth, reduce shedding and improve hair density.
Once started, treatment needs to continue for at least six months before the benefits can be assessed, and it is important not to stop treatment without discussing it with your doctor first. Long term treatment is usually necessary to sustain the benefits.
It takes about 4 months of using minoxidil to see any obvious effect. You might have some hair loss for the first couple of weeks as hair follicles in the resting phase are stimulated to move to the growth phase. You need to keep using minoxidil to maintain its effect – once you stop treatment the scalp will return to its previous state of hair loss within 3 to 4 months. Also, be aware that minoxidil is not effective for all women, and the amount of hair regrowth will vary among women. Some women experience hair regrowth while in others hair loss is just slowed down. If there is no noticeable effect after 6 months, it’s recommended that treatment is stopped.
Always carefully follow the directions for use, making sure you use minoxidil only when your scalp and hair are completely dry. Take care when applying minoxidil near the forehead and temples to avoid unwanted excessive hair growth. Wash your hands after use.
The most common side effects of minoxidil include a dry, red and itchy scalp. Higher-strength solutions are more likely to cause scalp irritation.
Bear in mind that minoxidil is also used in tablet form as a prescription medicine to treat high blood pressure, and there is a small chance that minoxidil solution could possibly affect your blood pressure and heart function. For this reason, minoxidil is generally only recommended for people who do not have heart or blood pressure problems.
Minoxidil should not be used if you are pregnant or breast feeding.
Side effects of spironolactone can include:
- irregular periods and spotting;
- breast tenderness or lumpiness; and
- tiredness.
Side effects of cyproterone acetate can include:
- spotting and irregular periods;
- tiredness;
- weight gain;
- reduced libido; and
- depressed mood.
Cosmetic camouflages include colored hair sprays to cover thinning areas on the scalp, hair bulking fiber powder, and hair wigs. Hair transplantation for female pattern hair loss is becoming more popular although not everyone is suitable for this procedure. Your doctor can refer you to a hair transplant surgeon to assess whether hair transplant surgery may be a suitable option for you.
Hair transplant surgery involves follicular unit transplantation, where tiny clusters of hair-producing tissue (each containing up to 4 hairs) are taken from areas of the scalp where hair is growing well and surgically attached (grafted) onto thinning areas. However, if your hair is very thin all over your scalp, you may not have enough healthy hair to transplant.
Hair transplant surgery can be expensive and painful, and multiple procedures are sometimes needed. Side effects may include infection and scarring.
Low-level laser therapy (LLLT) is of unproven benefit in female pattern balding; the Capillus® laser cap and Hairmax® Lasercomb/Laserband are two low‐level laser therapy (LLLT) devices have been approved by the FDA for the management of androgenetic alopecia 101, 79, 80. In a randomized, double‐blind, placebo‐controlled trial comprising 42 female subjects with androgenetic alopecia, 24 active group subjects were treated with 655 nm low-level laser therapy (LLLT) vs. 18 placebo group subjects were treated with incandescent red lights (sham) 101. Subjects were treated on alternate days for 16 weeks, and photography and hair count assessments revealed a 37% increase in terminal hair counts in the active treatment group as compared to the control group. In a review of 11 trials, 10 demonstrated significant improvement in androgenetic alopecia compared to baseline or controls when treated with low-level laser therapy (LLLT) 81. Two of the trials demonstrated efficacy for low-level laser therapy (LLLT) in combination with topical minoxidil, and one trial showed efficacy in combination with finasteride 87. Small number of participants reported adverse events of acne, mild paresthesia such as burning sensation, dry skin, headache, and itch 81.
Overall, literature has suggested light therapy to be a safe treatment modality for androgenic alopecia (androgenetic alopecia) in both male and female patients when used independently or in combination with topical/oral therapies 81, 84. Light therapy has an excellent side effect profile, and there are no contraindications for use, although caution may be taken when administering in patients with dysplastic lesions on the scalp 85.
Platelet‐rich plasma (PRP) injections are also under investigation 86. Further studies are required to determine the magnitude of the benefit if any.
Platelet‐rich plasma is generally indicated for patients with early‐stage androgenic alopecia, as intact hair follicles are present and a more significant hair restorative effect can be achieved 87. During the procedure, approximately 10–30 mL of blood are drawn from the patient’s vein and centrifuged for 10 min in order to separate the plasma from red blood cells. The platelet‐rich plasma, containing numerous growth factors, is then injected into the deep dermis or subcutaneous tissue at a volume of 4–8 mL per session. Mild side effects include scalp pain, headache, and burning sensation, but these effects usually subside in 10–15 minutes post‐injection and do not warrant use of topical anesthesia or pain medications 88. Vibration or cool air is typically sufficient to alleviate any significant pain that a patient may feel from the treatment. Patients can resume regular activities immediately after treatment but should avoid strenuous physical activity 24 hour post‐treatment to allow for optimal absorption of platelet‐rich plasma into tissue.
Hausauer and Jones 89 conducted a single center, blinded, randomized controlled trial investigating the efficacy of two platelet‐rich plasma regimens in 40 androgenic alopecia subjects. Participants received either subdermal platelet‐rich plasma injections with 3 monthly sessions and booster 3 months later (group 1) or 2 sessions every 3 months (group 2). Folliscope hair count and shaft caliber, global photography, and patient satisfaction questionnaires were completed at baseline, 3‐month, and 6‐month visits. The authors reported statistically significant increases in hair count and shaft caliber in both groups at 6 months. Importantly, improvements occurred more rapidly and profoundly in group 1, indicating that platelet‐rich plasma injections should be administered first monthly 89. Alves and Grimalt 90 demonstrated significant differences in mean anagen hair and telogen hair count as well as telogen and overall hair density when compared to baseline. In a review of 16 studies comprising a total of 389 patients with androgenic alopecia, the majority demonstrated efficacy in promoting successful hair growth after 3–4 sessions on a monthly basis, followed by quarterly maintenance sessions 91. Platelet‐rich plasma is not curative for hair loss and must be continued long term for hair sustenance. However, patient satisfaction is typically very high and 60–70% of patients continue to undergo maintenance treatments. Due to the relatively recent introduction of platelet‐rich plasma injections for androgenic alopecia, there are no long‐term studies evaluating its effectiveness. Additionally, it is difficult to compare the efficacy with other remedies due to the lack of standardization in regard to platelet‐rich plasma kits, treatment fractions, and regimens, including the use of newer multi‐needle injectors.
While platelet‐rich plasma injections are considered safe when performed by a trained medical provider, these treatments are not suitable for everyone. Platelet‐rich plasma may not be appropriate for those with a history of bleeding disorders, autoimmune disease, or active infection, or those currently taking an anticoagulant medication. Although the majority of patients seem to tolerate the pain associated with scalp injections, some patients may prefer to avoid it.
Hair loss treatment
Hair loss treatment depends on the underlying cause and diagnosis. Hair loss can be an isolated problem or associated with another disease or condition.
Hair loss can be due to:
- The decreased growth of the hair: anagen hair loss
- Increased shedding of the hair: telogen hair loss
- Androgenic alopecia or androgenetic alopecia which is the medical term for either male pattern hair loss or female pattern baldness caused by a combination of genetic and hormonal factors
- Congenital or acquired hair shaft abnormalities
- An inflammatory skin disease that damages or destroys the hair bulb
- Stress
- Significant weight loss
- Prolonged fever
- Certain medical conditions, such as diabetes and lupus
- Thyroid disease. Hypothyroidism (underactive thyroid) and hyperthyroidism (overactive thyroid) can cause reversible, diffuse hair loss 11
- Proper nutrition
- Drug treatment for cancer
- Autoimmune disease
- Poor sleep.
- Unknown causes.
There is no universally proven cure for hair loss (alopecia), however, there are treatments. The effectiveness of treatments tends to vary and something that works well for one person may not work well for another. If you find one treatment doesn’t work don’t assume others won’t either. However, bear in mind for some people none of the treatments are effective. Treatments for hair loss are generally divided into two groups:
- People with less than 50% hair loss
- People with over 50% hair loss
All of the therapies have documented side effects, some of which can be unpleasant. Treatments such as topical immunotherapy can be very time consuming. Individuals may decide that some of the adverse effects of treatment and the unpredictable outcome are unacceptable.
Table 2. Treatment of Hair Loss (Summary & Evidence)
| Treatment | Dosing | Strength of evidence | Comments |
|---|---|---|---|
| Alopecia areata | |||
| Topical steroids | Applied twice daily to scalp | B | Evidence for short-term growth, but none for long-term growth 102 |
| Topical minoxidil (Rogaine) | Applied twice daily | B | Evidence for short-term growth; one study showed more hair regrowth with 5% than 1% formulation 34, 34 |
| Topical immunotherapy with diphenylcyclopropenone (DPCP) or squaric acid | Treatment applied by dermatologist every few weeks | B | Unlicensed treatment; may cause severe dermatitis 103 |
| Oral steroids | Six-week tapering course of prednisone starting at 40 mg per day | B | Continued treatment is needed to maintain hair growth; risks of prolonged steroid use outweighs the benefits 35 |
| Intralesional corticosteroids | Triamcinolone acetonide (Kenalog) 5 to 10 mg per mL; 0.1 mL injected with a 30-gauge needle into the dermis 1 cm apart to a maximum of 3 mL; can be repeated every four to six weeks | C | Hair regrowth lasts a few months; effect on long-term outcome is unknown 35 |
| Anthralin cream (Dritho-Creme HP) | 0.5% to 1% cream once daily for 20 to 30 minutes, increasing by 10 to 15 minutes every two weeks | C | Hair staining prevents use in fair-haired patients 35 |
| Female pattern hair loss | |||
| Minoxidil 2% | Apply twice daily to dry scalp | B | 20 percent of women using the drug versus 7 percent of women taking a placebo reported moderate new hair growth after 32 weeks; number needed to treat = 8 104; 7 percent of women using minoxidil experience undesirable hypertrichosis 61 |
| Spironolactone (Aldactone) | 100 to 200 mg orally daily | C | 88 percent of women had a modest decrease in hair loss with treatment 61 |
| Flutamide (formerly Eulexin) | 250 mg orally daily | C | Treatment for one year resulted in a modest improvement in alopecia; 32 percent of participants experienced elevated liver function tests while taking the medication, resulting in some safety concerns 105 |
| Male pattern hair loss | |||
| Finasteride (Propecia) | 1 mg orally daily | A | Promotes hair growth for more than two years, with the effect waning by year three 57, 58 ; does not significantly affect sperm production and poses no risk to a female sex partner; when screening men on finasteride for prostate cancer, the upper limit of normal prostate specific antigen levels should be doubled to ensure appropriate interpretation 106, 107 |
| Minoxidil 2% | 1 mL to scalp twice daily | A | Consistent evidence showing moderate to dense regrowth of hair 60 |
| Minoxidil 5% | 1 mL to scalp twice daily | A | Consistent evidence showing moderate to dense regrowth of hair 60 |
| Ketoconazole (2%) shampoo (Nizoral) | Daily | C | Increased hair density, size, and proportion of anagen follicles after shampooing two to four times per week for 21 weeks 108 |
| Pyrithione zinc (1%) shampoo (Head and Shoulders) | Daily | C | Increased total visible hair count, but 5 percent less than treatment with minoxidil 5% 109 |
Footnotes: A = consistent, good-quality patient-oriented evidence; B = inconsistent or limited-quality patient-oriented evidence; C = consensus, disease-oriented evidence, usual practice, expert opinion, or case series.
[Source 44 ]Table 3. Summary of interventions indicated for hair growth
| Intervention | Support of Hair Cycle | Topical Dose or Dose Range | Evidence (Humans, Animals) |
|---|---|---|---|
| Finasteride | Inhibit 5-alpha-reductase | 1 mg daily | Humans (Men)–FDA Approved |
| Pumpkin seed oil | Inhibit 5-alpha-reductase | 400 mg oral | Humans |
| Herbal based supplement (Nutrafol) | Anti-inflammatory, reduce Stress, and DHT inhibiting properties | Humans | |
| Minoxidil (Topical) | Increase local blood flow | Women—3% or 5% daily Men—5% twice daily | Humans—FDA Approved |
| Morbus alba | Activation of anagen phase | Humans | |
| Low level light therapies | Increased telogen to anagen phase transition | Humans | |
| Latanoprost | Activation of anagen phase | 0.1% latanoprost solution topical | Humans |
| Bimatoprost | Stimulate anagen phase | 0.03% bimatoprost solution topical | Humans |
| Marine protein-based supplement (Viviscal) | Prolongs anagen phase | 450 mg oral | Humans |
| Platelet rich plasma injections | Induces anagen phase | Humans | |
| Bhringaraj (Eclipta alba) | Activation of anagen phase | 5% petroleum ether extract topical | Mice |
| Quercetin | Supports mitochondrial function and anagen phase | Mice |
Treatment for Less than 50% hair loss
Corticosteroids
Today, corticosteroids are the most common treatment for hair loss. In mild cases of hair loss the first choice of treatment would be a corticosteroid cream or lotion which is applied directly to the area(s) of hair loss. Alternatively an injection of corticosteroid can be given directly onto and around the bald area(s).
Side Effects: Side effects are generally rare when corticosteroids are used for a short time. However, when they are taken by mouth and when used ovr a longer period, they may lower the body’s ability to fight off infections or may make infections harder to treat. Other common side effects include changes in appetite (increase or decrease), nervousness, restlessness, sleep problems, and indigestion. These problems usually go away as the body adjusts to the drug and do not require medical treatment. Less common side effects may occur with some forms of corticosteroids. Gels or creams may irritate the skin. Again, these side effects do not need medical attention unless they don’t go away or they interfere with normal activities. More serious side effects are very unlikely, but they may occur.
Effectiveness: This type of treatment can sometimes result in a recovery or can cause hair to grow only for the period during which the treatment occurs, so when the treatment is stopped the hair may fall out again. In the case of corticosteroid injections an average of 4 to 6 monthly injections are usually required for significant improvement. However although some re-growth is common it does not always occur.
Finasteride
Finasteride is marketed under the brand name of Propecia along with other generic names. It is a licensed synthetic type II 5α-reductase inhibitor. The enzyme converts testosterone to dihydrotestosterone. Systemic Finasteride (1mg Propecia) can be used for the treatment of male pattern baldness. It is still in the experimental stages of use for female pattern baldness.
Side Effects: Side effects of finasteride can include impotence and reduced libido, abnormal ejaculation, decreased ejaculatory volume, abnormal sexual function, swelling and tenderness of the breast tissue and testicular pain.
Effectiveness: It has shown a significant improvement for men (80% of men presenting with male pattern baldness, with 60% of those showing some regrowth). The effects do not continue if the treatment is stopped. For women the results have been less successful (if not disappointing) and it must not be used for women likely to become pregnant.
Retin A / Tretinoin
Retin-A was originally used for the treatment of acne and other skin problems. However studies have shown that Retin-A, when used alone in the form of a gel, which is rubbed onto the area of hair loss, or in combination with topical Minoxidil can result in moderate to good hair growth in individuals with hair loss. It is recommended that Minoxidil is used in the morning and Retin-A in the evening as Retin-A increases the skin’s sensitivity to sunlight.
Side Effects: Immediately after applying, the skin may feel warm or mild stinging or redness may occur. Some peeling of the skin may occur. These effects should subside as your skin adjusts to the medication.
Effectiveness: Studies have shown that Retin-A, when used alone in the form of a gel, which is rubbed onto the area of hair loss, or in combination with topical Minoxidil can result in moderate to good hair growth in individuals with alopecia.
Diphenylcyclopropenone (DPCP)
Immunotherapy may be an effective form of treatment for extensive or total hair loss, but less than half of those treated will see worthwhile hair regrowth. Diphenylcyclopropenone (DPCP) is a chemical agent used to treat severe alopecia areata. It is said to be safe although there is no information about the long term side effects when used over many years. Diphenylcyclopropenone (DPCP) comes as a fluid and is applied to the bald areas once a week. Sensitizing is achieved with the application of a 2% solution and is applied to a 4x4cm area on the scalp. One week later the area is examined. If there is a severe response with blisters , intense redness , scaling and itching, treatment is postponed till the following week. If there is only mild redness , scaling and itching, a lower concentrate of diphenylcyclopropenone (DPCP) can be applied on the hairloss areas. After every administration of diphenylcyclopropenone (DPCP), the painted area should not be washed for 48 hours and kept covered because sunlight inhibits the action of diphenylcyclopropenone (DPCP). After DPCP has been applied, you’ll need to wear a hat or scarf over the treated area for 24 hours because light can interact with the chemical.
Side Effects: Eczema, Lymph node enlargement and skin discoloration (vitiligo) are all possible side effects. This can be avoided by increasing the DPCP concentration gradually.
Effectiveness: First evidence of hair growth is expected to occur after 3 months, full growth after 6 months. Once full regrowth occurs, the frequency of application is decreased. Even after full growth of hair, some patients may stop responding to further application of diphenylcyclopropenone (DPCP) and may lose all their hair again. If after 5 months of using diphenylcyclopropenone (DPCP) no hair regrowth is noticed DPCP is discontinued.
Dithranol (also known as Anthralin)
Similar to immunotherapy (DPCP), dithranol cream is applied regularly to the scalp before being washed off. It causes a skin reaction, followed by hair regrowth in some cases. Dithranol is a tar-like ointment that is applied to the scalp and is especially good in the treatment of psoriasis (a skin condition causing red scaly patches). The pharmacist can compound it in various different pastes, ointments, and creams, and the strength may vary from 0.1 to 4%.
Side Effects: It can irritate the skin when applied and can cause burning and redness. It can also stain.
Effectiveness: Dithranol can be effective even if only left on the skin for 10 minutes. This ‘short contact’ method allows stronger concentrations of Dithranol to be used with much less burning and staining. But it hasn’t been proven that dithranol cream is significantly effective in the long term. It can also cause itchiness and scaling of the skin and can stain the scalp and hair. For these reasons, dithranol isn’t widely used.
Topical Minoxidil (Regaine, Rogaine or Headway)
Topical Minoxidil can be successful in hair loss, slowing down hair loss, and bridging the gap until hair starts growing again on its own.
Minoxidil is not available on prescription. Minoxidil comes in a 2% and 5% solution. However the 2% and 5% solutions can be ordered online. A bottle will normally last for about a month. The solution should not be used by women during pregnancy.
A Minoxidil 5% foam is also available, and is shown to be just as effective as the 5% solution. The foam will last about a month.
Side Effects: Side effects may include irritation of the scalp, itching, scaling, a rash, and on the odd occasion excessive hair growth.
Effectiveness: Topical Minoxidil can be effective on patchy alopecia. Unfortunately topical Minoxidil is not normally effective in individuals with extensive, total or universal alopecia.
Zinc
Oral zinc has been shown to be of occasional benefit in alopecia.
Zinc can be bought in any chemist or health food shop. A variety of tablets are sold with different amounts of zinc.
Side Effects: Very high doses are needed for it to be effective and this can result in side effects such as vomiting and diarrhea.
Effectiveness: Prolonged treatment with zinc sulphate is helpful. There is no zinc deficiency noticeable in the body, and short term treatment with zinc is not helpful.
Scalp massage
Developing hair follicles are surrounded by deep dermal vascular plexuses. Associated blood vessels function to supply nutrients to the developing hair follicle and foster waste elimination. As such, proper blood supply is necessary for effective hair follicle growth, further exemplified by the angiogenic properties of the anagen phase 110. A 2016 study 111 assessed the effect of a 4-minute standardized daily scalp massage for 24 weeks among nine healthy men. Authors found scalp massage to increase hair thickness, upregulate 2655 genes, and downregulate 2823 genes; hair cycle-related genes including NOGGIN, BMP4, SMAD4, and IL6ST were among those upregulated, and hair-loss related IL6 was among those downregulated 111. The authors thereby concluded that a standardized scalp massage and subsequent dermal papilla cellular stretching can increase hair thickness, mediated by changes in gene expression in dermal papilla cells 111.
In addition, of 327 survey respondents attempting standardized scalp massages following demonstration video, 68.9% reported hair loss stabilization or regrowth 112. Positive associations existed between self-reported hair changes and estimated daily minutes, months, and total standardized scalp massage effort. This study 112 is limited based on recall bias and reliance on patient adherence and technique, although it suggests promising therapeutic potential for standardized scalp massage, which functions to increase blood flow.
Low-level laser (light) therapy
Low-level laser (light) therapy refers to therapeutic exposure to low levels of red and near infrared light 113. Studies have demonstrated increased hair growth in mice with chemotherapy-induced alopecia and alopecia areata, in addition to both men and women human subjects. Proposed mechanisms of efficacy include stimulation of epidermal stem cells residing in the hair follicle bulge and promoting increased telogen to anagen phase transition 114. Interestingly, while minoxidil and finasteride are the only FDA-approved drugs for androgenetic alopecia, a 2017 study 84 found comparable efficacy among patients receiving low-level light therapy versus topical minoxidil among patients with female-pattern androgenetic alopecia. In addition, combination therapy resulted in the greatest patient satisfaction and lowest Ludwig classification scores of androgenetic alopecia.
A meta-analysis including eleven double-blinded randomized controlled trials found a significant increase in hair density among patients with androgenetic alopecia receiving low level light therapy compared to those in the placebo-controlled group 115. Low level light therapy was effective for men and women. Furthermore, a subgroup analysis observed a more significant increase in hair growth in those receiving low-frequency therapy than receiving high-frequency therapy 115. Despite the limitation of the heterogeneity of included trials, these results suggest low level laser (light) therapy to be a promising therapeutic strategy for androgenetic alopecia 115, although further research is necessary to determine the optimal wavelength and dosimetric parameters for hair growth 114.
Prostaglandins
Latanoprost is a prostaglandin F2 agonist and has been shown to have a direct effect on hair growth and pigmentation in eyelashes and hair around the eyes 116. Clinically used to treat glaucoma, latanoprost was found to affect the hair follicles in the telogen phase and cause them to move to the anagen phase; this was supported by the increased number and length of eyelashes seen in patients using latanoprost 116. Subsequently, the application of latanoprost for patients experiencing alopecia was assessed in clinical studies. One conducted in 2012 studied the effects of 0.1% latanoprost solution applied to the scalp for 24 weeks 117. Participants included 16 males with mild androgenetic alopecia and were instructed to apply placebo on one area of the scalp and the treatment on another area. The results indicated that the area of scalp receiving latanoprost had significantly improved hair density compared to placebo 117.
Another prostaglandin known as bimatoprost, a prostamide-F2 analog, was also found to have a positive effect on hair growth in human and mouse models 118. A study conducted in 2013 also found that bimatoprost, in both humans and mice, stimulated the anagen phase of hair follicles prompting an increase in hair length, i.e., promoting hair growth 118. The study also confirmed the presence of prostanoid receptors in human scalp hair follicles in vivo, opening the strong possibility that scalp follicles can also respond to bimatoprost in a similar fashion 118.
It is important to note, however, that not all prostaglandins induce hair growth 119. In a study analyzing individuals with androgenetic alopecia with a bald scalp versus a haired scalp, it was discovered that there was an elevated level of prostaglandin D2 synthase at the mRNA and protein levels in bald individuals 119. They were also found to have an elevated level of prostaglandin D2 (PGD2). When analyzing the level of prostaglandin D2 synthase presence through the various phases of hair follicular growth, it was found that the level steadily increased throughout the anagen phase with a peak in late anagen, at the time of transition to the catagen (breakdown) phase. Therefore, the study concluded that prostaglandin D2 (PGD2)’s hair loss effect represents a counterbalance to PGE2 and PGF2’s hair growth effects. In conclusion, prostaglandins are a promising treatment option for alopecia that require larger clinical studies; however, clinicians should be aware of which one to recommend for hair growth, as not all prostaglandins are alike 6.
Platelet rich plasma
Platelet rich plasma (PRP) has conventionally been used to supplement a patient’s endogenous platelet supply to promote increased healing 6. However, its prominent supply of growth factors has prompted assessment of platelet rich plasma for alopecia. Growth factors promote hair growth and increase the telogen to anagen transition. For example, a mice study found the fibroblast growth factor (FGF) induced the anagen phase and subsequently promoted hair growth 120. Growth factors prominently included in platelet rich plasma include platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), insulin-like growth factor (IGF) and fibroblast growth factor (FGF) 121.
The growth factors of platelet-rich plasma stimulate the development of new follicles and neovascularization 122. Three meta-analyses have assessed the efficacy of platelet rich plasma injections compared to placebo control on the number of hairs per cm2 among patients with androgenetic alopecia. One meta-analysis involving 177 patients found a mean improvement of platelet rich plasma treatment compared to placebo of 17.9 123; a second meta-analysis with 262 androgenetic alopecia patients observed a mean difference of 38.8 124; and a third meta-analysis including studies with parallel or half-head design found a mean difference of 30.4 125.
Despite the efficacious results described by each meta-analysis for the use in androgenetic alopecia, gender differences have been observed. A 2020 meta-analysis 126 found that while platelet rich plasma significantly increased hair density and hair diameter from baseline in men, platelet rich plasma only increased hair diameter in women, in the absence of significantly increased hair density. Furthermore, hair density in men was only significantly increased by a double spin method, in contrast to a single spin method 126. The authors conclude that platelet rich plasma effectiveness may be improved via higher platelet concentrations 126. Ultimately, platelet rich plasma injections appear to have clinical efficacy in early studies although with slightly different effects in men vs. women. Future research is necessary to establish the optimal treatment protocol for both men and women with androgenetic alopecia. Also, the role of diet in the days prior to collection of the platelet rich plasma has not been assessed in conjunction with hair, although diet influences the quality of the platelet rich plasma 127.
Treatment for Over 50% hair loss
Immunosuppressive drugs
It is thought that Immunosuppressive drugs should be able to suppress the immune system giving the hair a chance to re-grow. The main problem with taking immunosuppressive drugs is that it can lead to a lowered resistance to infection.
Side Effects: Increased chance of infection as the immune system is suppressed and may affect bone marrow, liver or kidneys.
PUVA
PUVA or photochemotherapy is a type of ultraviolet radiation treatment (phototherapy) that involves taking or applying a light sensitive drug and then undergoing a short exposure to UVA (long wave ultraviolet radiation).
PUVA is a combination treatment which consists of Psoralens (P) and then exposing the skin to UVA. It has been available in its present form since 1976.
Psoralens are compounds found in many plants which make the skin temporarily sensitive to UVA. The ancient Egyptians were the first to use psoralens for the treatment of skin diseases thousands of years ago. Medicine psoralens include methoxsalen (8-methoxypsoralen), 5-methoxypsoralen and trisoralen.
Treatment takes place over a three to six week period with sessions two to three times a week. The more recent the hair loss, the more likely it is that there will be a response to treatment.
Side Effects: The likelihood is your skin will burn, as if over tanned, but it’s hoped the body’s immune system gets so busy repairing skin tissue that it lets your hair begin to grow.
PUVA but can be very time consuming involving two or three treatments per week.
Effectiveness: It has not been shown to have a very high success rate (around 6-12%) – and it can be very painful. It is not considered to be an effective long-term treatment.
Systemic Corticosteroids
Oral corticosteroids (Prednisone) are used to try to partially suppress the immune system and allow hair to grow. Corticosteroids taken orally are sometimes prescribed for extensive hair loss or when the condition is rapidly spreading. Corticosteroids taken internally are much more powerful than local injections into the skin. May be used in conjunction with Minoxidil as the dose is being reduced.
Side Effects: The most common side effects reported with the short-term (days to weeks) use of oral corticosteroids include a bigger appetite, weight gain, upset stomach, headache, mood changes, and trouble sleeping. Some people may also have an increase in blood sugar and blood pressure. In general, all of these side effects go away after you stop taking corticosteroids. Side effects associated with long-term (months to years) oral corticosteroid include weakening of the immune system, elevations in cholesterol levels, and weight gain. These side effects also usually improve when you stop taking corticosteroids. Long-term use of corticosteroids may also cause brittle bones (osteoporosis), fat deposits on the face and back, thinning of the skin, and cataracts in the eyes. These side effects may improve but usually don’t go away completely after stopping the drug. In children, long-term use of oral corticosteroids may cause stunted growth.
Effectiveness: When taking oral corticosteroids you are likely to experience some re-growth. However unless your immune system kicks into `normal’ action and allows you to produce hair yourself, the re-growth you have experienced will cease and the hair will unfortunately fall out again after you cease the treatment.
PUVB
This treatment is similar to PUVA but using a different wavelength of light. It is generally used to treat skin conditions but is also used for people with severe hair loss (e.g. more than 90%). It involves standing in a cubicle of ultraviolet lights two to three times a week for an increasing amount of time.
PUVB is the combination of Psoralens and UVB (short wavelength ultraviolet radiation). This is rarely used, as the wavelength that activates the psoralens most effectively is in the UVA range.
Side Effects: Like PUVA you may have redness and burning of the skin, similar to spending too long in the sun.
Effectiveness: Although there is some documented evidence of success, in most cases this treatment does not appear to help with hair growth and the redness and burning can be quite painful. It may also increase your chance of getting skin cancer.
Vitamins and supplements for hair growth
A variety of vitamins and supplements for hair growth have appeared in the market over the past few years 128, 31, 129. The oral regimens are convenient for many patients, but clinical evidence supporting its efficacy is still minimal.
In a randomized controlled trial conducted in 2018 130, 40 women with self-perceived hair thinning were recruited to either take the herbal supplement (brand: Nutrafol) or placebo for 6 months. The supplement was noted to include a variety of ingredients including curcumin, ashwagandha, and saw palmetto 130. By days 90 and 180, the treatment group experienced a significant increase in the terminal and vellus hair counts compared to the placebo 130. Another supplement composed largely of marine protein (brand: Viviscal) were also tested in a separate randomized placebo-controlled trial 131. Participants included 60 women with thinning hair and were asked to take either placebo or the supplement twice daily for 3 months 131. The results showed a significant increase in the terminal hair counts in the treatment group compared to placebo 131.
Pumpkin seed oil supplements have also been shown to be beneficial for hair loss 132. A randomized control trial including 76 males with androgenetic alopecia were instructed to either take 450 mg of pumpkin seed oil supplements or placebo for 24 weeks 132. Hair counts improved by 40% in those taking pumpkin seed oil whereas hair counts only improved 10% in the placebo group 132. The exact mechanism in the hair cycle is not known, however it is thought that pumpkin seed oil is enriched for delta-7-phytosterols and may inhibit 5-alpha-reductase activity 133.
Nutrafol
Nutrafol launched in 2016 and is currently the fastest growing nutraceutical supplement for hair growth on the market 129. Nutrafol is composed of 21 ingredients, including a proprietary Synergen Complex®, which includes standardized phytoactives with clinically tested anti-inflammatory, stress-adaptogenic, antioxidant and dihydrotestosterone (DHT)-inhibiting properties 129. The phytocompounds in this complex include curcumin, piperine, ashwagandha, saw palmetto, and tocotrienols 130, 134. In addition to the components detailed above, Nutrafol also contain amino acids, marine collagen, hyaluronic acid, organic kelp, and vitamins and minerals that have been identified to play a role in the stress response as well as gut, thyroid and hair health 134. There are currently four formulations available: Nutrafol Women, Nutrafol Women’s Balance, Nutrafol Postpartum, and Nutrafol Men, which has a higher concentration of saw palmetto.
Curcumin (a yellow pigment found primarily in turmeric) is a potent anti-inflammatory and immunomodulating agent that has been shown to inhibit NF-kb and decrease tumor necrosis factor (TNF)-alpha and interleukin (IL)-1, inflammatory cytokines involved in follicular regression 134. Curcumin also inhibits androgen receptor expression, which is known to be overexpressed in follicles in androgenetic alopecia 135, 136. Co-administration with botanical piperine, found in black pepper and long pepper, enhances curcumin bioavailability and has been shown to increase plasma levels up to 154 percent after ingestion 134, 137. The stress response is known to play an important role in hair loss pathology and is intrinsically linked to alopecia areata (autoimmune hair loss) and telogen effluvium (hair loss associated with physiologic or emotional stress), with recent studies indicating that cortisol and micro-inflammation at the level of the hair follicle also plays a role in androgenic alopecia 138, 139.
Ashwagandha is a botanical that contains steroidal lactones which modulate and reduce cortisol levels. In a randomized, double-blind, placebo-controlled study of 98 patients, daily supplementation with 10% withanolide ashwaghanda showed statistically significant reductions in serum cortisol, serum C-reactive protein, blood pressure, and subjective feelings of stress compared to placebo 138. Saw palmetto extract is a natural inhibitor of both types I and II 5-alpha reductase which prevents conversion of testosterone to active DHT. A study of 100 men with mild to moderate androgenic alopecia who were treated with either 320 mg of saw palmetto or 1 mg of finasteride daily for two years revealed a significant improvement in 38 percent of patients taking saw palmetto and 68 percent of patients taking finasteride 140. Despite the increased efficacy of finasteride, saw palmetto might be a desirable alternative to avoid side effects of erectile dysfunction and falsely reducing prostate specific antigen (PSA) levels.5 While there are no reports of teratogenicity, saw palmetto is considered functionally related to finasteride, and therefore, it is considered unsafe in pregnancy. Vitamin E isoforms consist of four tocopherols and four tocotrienols, which are potent free radical scavengers 134. A randomized, placebo-controlled study of 38 patients with hair loss showed a statistically significant increase in hair counts of 38 percent from baseline compared to placebo 141. The authors concluded that the effect was most likely due to antioxidant activity, inhibition of lipid peroxidation, and oxidative stress in the scalp 141, 142.
The efficacy of Nutrafol to promote hair growth was studied in a six-month randomized, double-blind, placebo-controlled trial. Forty healthy women between the ages of 21 and 65 years old with self-perceived hair thinning were randomized into two groups, with 26 subjects receiving four capsules of Nutrafol daily and 14 subjects receiving placebo. The number of terminal and vellus hairs was analyzed based on phototrichograms of a 1 cm² area along the frontalis bone at Day 0, Day 90, and Day 180. Subjects taking Nutrafol showed an increased terminal hair count of 6.8 percent and 10.4 percent at 90 and 180 days, respectively, compared to 0.07 percent and 3.5 percent in the placebo group 134. Vellus hair counts increased by 10.1 percent and 15.7 percent at days 90 and 180 in the Nutrafol group compared to a decrease in vellus hair counts of 2.9 percent and 2.2 percent at days 90 and 180 in the placebo group 143. There was no statistically significant difference in mean hair shaft diameter between treatment and placebo groups at any point in the study 143. Investigator scores for hair growth and hair quality increased significantly from baseline to day 180. Eighty percent of subjects in the Nutrafol group reported a significant improvement in hair growth compared with 46.2 percent of placebo-treated subjects. Subjects taking Nutrafol also reported improvement in overall hair volume, noticeable new hair, hair growth rate, stress and anxiety levels, sleep quality, skin smoothness and skin health 143. The majority of subjects taking Nutrafol (73.1%) would recommend it to friends with hair loss.
A recent case series demonstrated clinical improvement in four subjects taking Nutrafol as a monotherapy 134. A 52-year-old woman who had previously failed a several month course of topical minoxidil showed increased hair density after seven months. A 45-year-old woman with early signs of diffuse pattern hair loss showed improved hair density after four months. A 37-year-old man with early pattern hair loss and a strong family history of hair loss who had previously failed minoxidil showed improved hair growth and decreased shedding. Lastly, a 38-year-old woman with early diffuse thinning of the temple areas experienced increased density after three months of daily Nutrafol use. No patient reported any side effects, and all were satisfied with their improvement 129.
Viviscal
Viviscal® is an oral supplement based on a novel marine complex formulation designed to promote hair growth in both men and women 144, 131. The key ingredients in Viviscal include a proprietary blend of shark and mollusk powder derived from sustainable marine sources (AminoMar® C marine complex), Equisteum arvense (natural occurring form of silica), Malpighia glabra (acerola cherry providing vitamin C), biotin (vitamin B7), and zinc. Other ingredients include calcium, iron, horsetail stem extract, millet seed extract, flaxseed extract, procyanidin B-2 (i.e., apple fruit extract), L-cystine and L-methionine, depending on the formulation. In addition to the original formulation, other formulations include Viviscal® Professional Strength and Viviscal® Man. Early studies evaluating a similar oral formulation of marine extracts in women with photodamaged skin demonstrated improvements in skin thickness, elasticity and erythema, as well as improvements in hair and nail brittleness after 90 days of treatment 145, 146. Following an initial open-label pilot study, several randomized, placebo-controlled trials have demonstrated the current oral marine complex supplement to be effective in promoting hair growth in both men and women 131, 147, 148, 149.
Biotin is a water-soluble vitamin that helps turn the carbohydrates, fats, and proteins in the food you eat into the energy you need 150. Biotin (vitamin B7) is a cofactor for five carboxylases (propionyl-CoA carboxylase, pyruvate carboxylase, methylcrotonyl-CoA carboxylase [MCC], acetyl-CoA carboxylase 1, and acetyl-CoA carboxylase 2) that catalyze critical steps in the metabolism of fatty acids, glucose, and amino acids 151, 152. Biotin also plays key roles in histone modifications, gene regulation (by modifying the activity of transcription factors), and cell signaling 151. Foods that contain the most biotin include organ meats, eggs, fish, meat, seeds, nuts, and certain vegetables (such as sweet potatoes) 152. The biotin content of food can vary; for example, plant variety and season can affect the biotin content of cereal grains, and certain processing techniques (e.g., canning) can reduce the biotin content of foods 153. Although overt biotin deficiency is very rare 154, 153 and severe biotin deficiency in healthy individuals eating a normal mixed diet has never been reported 155. The human requirement for dietary biotin has been demonstrated in three different situations: prolonged intravenous feeding (parenteral) without biotin supplementation, infants fed an elemental formula devoid of biotin, and consumption of raw egg white for a prolonged period (many weeks to years) 156. The signs and symptoms of biotin deficiency typically appear gradually and can include thinning hair with progression to loss of all hair on the body; scaly, red rash around body openings (eyes, nose, mouth, and genital area); conjunctivitis; ketolactic acidosis (which occurs when lactate production exceeds lactate clearance) and aciduria (abnormal amounts of acid in urine); seizures; skin infection; brittle nails; neurological findings (e.g., depression, lethargy, hallucinations, and numbness and tingling of the extremities) in adults; and hypotonia, lethargy, and developmental delay in infants 152, 151, 155. The rash and unusual distribution of facial fat in people with biotin deficiency is known as “biotin deficiency facies” 157, 155. Individuals with hereditary disorders of biotin metabolism resulting in functional biotin deficiency often have similar physical findings, as well as seizures and evidence of impaired immune system function and increased susceptibility to bacterial and fungal infections 158, 159.
Therefore, biotin supplements are often promoted for hair, skin, and nail health. However, these claims are supported, at best, by only a few case reports and small studies. Only case reports are available to support claims that biotin supplements can promote hair health, and these reports were only in children 160, 161. These studies found that 3–5 mg/day biotin in children with uncombable hair syndrome (a rare disorder of the hair shaft) significantly improved hair health after 3–4 months. The evidence supporting the use of biotin supplements to support skin health is equally limited to a small number of case reports, all in infants, showing that 100 mcg to 10 mg/day resulted in dramatic improvements in rash or dermatitis as well as hair loss 162, 163.
In the first randomized-controlled trial, 15 healthy women with Fitzpatrick Skin Types I to IV and self-perceived hair thinning were randomized to receive the Viviscal® Maximum Strength oral supplement or placebo twice daily for 180 days 148. A 2 cm² area of the scalp was selected for hair counts, which were performed at baseline and after 90 and 180 days of treatment. Mean number of terminal hairs in the treatment group increased from 271.0 to 571 and 609.6 at 90 and 180 days, respectively. In contrast, the mean number of terminal hairs in the placebo group decreased at 90 and 180 days. The mean number of vellus hairs did not significantly change in either group. After 90 days, more subjects in the treatment group reported improvements in overall hair volume, scalp coverage and hair thickness. Additional improvements noted at 180 days in the treatment group included increased hair shine, skin moisture retention, and skin smoothness. No adverse events were reported.
In another double-blind, placebo-controlled trial, 60 healthy women with Fitzpatrick Skin Types I to IV with self-perceived hair thinning were randomized to receive either the Viviscal® Extra Strength oral supplement or placebo twice daily for 90 days 131. Similar to the prior study, a predesignated 4 cm² area of the scalp was selected for evaluation at baseline and after 90 days. Additionally, ten terminal hairs in the target area were chosen and cut at the surface of the scalp to evaluate hair growth. Digital photographs were obtained to measure hair diameter and the diameter for ten hairs was used to obtain the mean hair diameter within the target area. At 90 days, there was a significant increase in mean number of terminal hairs from 178.3 to 235.8 in the treatment group compared to a smaller increase of 178.2 to 180.9 in the placebo group. The number of vellus hairs also increased in the treatment group from 19.6 to 21.2 compared to 19.8 to 19.9 in the placebo group. A significant decrease in the mean number of shed hairs from 27.1 to 16.5 was also observed in the treatment group (vs. 23.4 to 21.9 in the placebo group). No significant change in terminal hair diameter was observed in either group. In addition to the improvements in objective measures observed, subjects in the treatment group also had higher scores on a self-assessment questionnaire that rated overall hair quality including hair growth, hair volume, hair thickness, hair strength, eyebrow hair growth and scalp coverage as well as overall skin health. No adverse events were reported.
A subsequent double-blind, placebo-controlled trial evaluated the efficacy of the Viviscal® Professional Strength Oral Tablets, an oral formulation of the marine supplement containing procyanidin B-2 (i.e., apple fruit extract), L-cystine and L-methionine for the treatment of hair loss in women 164. Forty healthy women with Fitzpatrick Skin Types I to IV and with self-perceived hair thinning were randomly assigned to receive either the oral supplement or placebo twice daily for 180 days 164. A predesignated target area on the scalp was assessed using phototrichogram analysis to determine change in number of terminal and vellus hairs at 180 days. Terminal hair diameter and response scores to quality of life and self-assessment questionnaires were also evaluated. In the treatment group, the mean number of terminal hairs increased from 189.9 to 297.4 and 341.0 at 90 and 180 days, respectively, and mean number of vellus hairs increased from 19.9 to 20.2 and 22.8 at 90 and 180 days, respectively. Mean hair diameter also significantly improved in the treatment group from 0.06 mm to 0.07 mm and 0.067 mm at 90 and 180 days, respectively, which had not been found in previous studies of the prior formulation. There were no significant improvements in any of these parameters in the placebo group. Subjects in the treatment group reported greater scores on the quality of life and self-assessment questionnaires compared to the placebo group, indicating greater improvements in overall hair volume, scalp coverage, hair strength, nail strength, eyelash growth, skin smoothness, and overall skin health.
In another study, 96 healthy women with Fitzpatrick Skin Types I to III and self-perceived hair thinning were randomly assigned to receive the Viviscal® Oral Supplement or placebo three times daily for 180 days 147. The aim of this study was to add to the results of the initial double-blind, placebo-controlled trial by evaluating shed hair count analysis and hair diameter analysis using a phototrichogram. A predesignated area of the scalp was selected for two-dimensional digital images and trichoanalysis, which was performed at baseline, 90 days and 180 days. Shed hair was collected during shampooing and counted at each visit. Overall, mean hair shedding was significantly reduced in the treatment group from 52.1 to 42.6 and 42.7 at 90 and 180 days, respectively. In the placebo group, an initial increase in mean hair shedding was seen at 90 days, followed by a small decrease at 180 days. Mean vellus hair diameter showed a small but significant increase in the treatment group at 90 and 180 days, but no change was observed in the placebo group at either time point.
The studies discussed thus far consistently demonstrated the effectiveness of Viviscal® in promoting hair growth in women with self-perceived hair thinning 129. However, the efficacy of the oral supplement in men with hair loss had not yet been evaluated. In a double-blind, placebo-controlled trial, Ablon 149 evaluated the efficacy of Viviscal® Man, a reformulation of the original marine complex supplement for use in men. Sixty healthy men with clinically diagnosed male pattern hair loss were randomized to receive the reformulated supplement or placebo twice daily for 180 days 149. A predesignated target area on the midline scalp was chosen for two-dimensional digital images and trichoanalysis at baseline, 90 days and 180 days. A hair pull test on the right and left parietal, frontal and occipital scalp was also performed at baseline and 180 days. After treatment, subjects in the treatment group experienced significant improvement in all efficacy measures. Mean total hair increased from 162.2 to 169.1 and 174.9 at 90 and 180 days, respectively, total hair density increased from 159.7 to 166.5 and 172.2 at 90 and 180 days, respectively, and terminal hair density increased from 121.9 to 127.7 and 130.3 at 90 and 180 days, respectively 149. The hair pull test was also significantly improved in the treatment group at 180 days. No improvements in any of these parameters were observed in the placebo group. Subjects in the treatment group also reported significant improvements in several quality of life measures and self-assessment items including overall hair growth, hair volume, hair and nail strength, and overall skin healthy. No adverse events were reported.
Based on the current literature, Viviscal® appears to be effective in promoting hair growth and decreasing hair shedding in both men and women 149, 131, 164. Studies have also demonstrated that Viviscal® may offer the additional benefit of subjective improvements in the appearance and quality of the skin, nails and eyebrows 149, 164, 148. Theories behind the benefit of Viviscal® primarily involve providing adequate nutrition and vitamins that promote hair growth, as inadequate nutrition and various vitamin deficiencies have been associated with hair loss 165.
Several prior studies have demonstrated vitamins, omegas 3 and 6 fatty acids, and antioxidants to also promote hair growth, suggesting a role of adequate nutrition in hair growth and supporting the use of dietary supplements for hair loss 166, 167, 168. Advantages of Viviscal over current pharmaceutical therapies, such as topical minoxidil and oral finasteride, include additional improvements to skin and nail health and a more favorable side effect profile. In all of the studies performed thus far, no significant adverse events associated with the oral supplement have been reported 129. Therefore, Viviscal would likely be appropriate to use in the majority of patients. This is in contrast to many of the current pharmaceutical treatments for hair loss, such as topical minoxidil, which may cause local irritation at the application site, or oral finasteride, which has been associated in rare instances with sexual dysfunction in men and is often not favorable to use in women. Several Viviscal formulations contain biotin (vitamin B7), which may be a cause for concern. Recently, the FDA issued a warning that biotin might interfere with certain laboratory testing results, including endocrine and cardiovascular laboratory tests. Given this warning, patients taking Viviscal® regularly should be aware of this possibility. However, Viviscal supplement contains about 100 to 240 micrograms of biotin compared to 5,000 micrograms found in most biotin supplements, which might have a smaller impact on laboratory tests compared to supplements containing higher amounts of biotin. Of note, the formulation for men does not contain any biotin 129.
Nourkrin
Nourkrin® is a newly developed hair growth product containing marine proteins extract, vitamins, minerals, acerola cherry extract, silica kieselguhr, horsetail extract and immunoglobulins 169. The active ingredients in Nourkrin are meant tonourish the hair follicles and ‘awaken’ the dormant hair follicles, thereby stopping hairloss, stimulating regrowth of new hair and strengthening the existing hair 169. However, the mechanism of action of Nourkrin is not known, but it might be related to the production of dihydrotestosterone (DHT) in the hair follicle. Dihydrotestosterone (DHT) is known to cause hair loss. Studies have been initiated to investigate this aspect. Nourkrin has been shown, in open pilot studies carried out as part of the product development process, to have a favourable effect on hair loss 169. In a randomized, double-blind, placebo-controlled study involving 60 subjects (55 males and 4 females [there were 5/60 (8.3%) withdrawals/losses to follow‐up: 3/30 (10%) in Nourkrin® group, 2/30 (6.7%) placebo group]) taking 2 capsules/day (< 80 kg in body weight) or 3 capsules/day (> 80 kg) for 6 months, the average hair growth increase was 35.7% in the group using Nourkrin and 1.5% in the placebo group 169, The results of this study show that the subjects reported favourable effects on hair gain with Nourkrin® compared with placebo and the same effect was also detectable in the open long-term (6 months or more) study.
The results obtained in the present study compare favourably with results obtained in studies with the drugs minoxidil and finasteride 169. The positive effect of Nourkrin combined with its excellent tolerability may make this product an attractive alternative treatment for people with hair-loss problems.
Hair loss surgery
Most men and women considering hair loss surgery have male-pattern or female-pattern baldness. But surgery is also sometimes suitable for a range of alopecia conditions.
Surgery for hair loss should only be considered after trying less invasive treatments.
The success of hair loss surgery depends on the skill of the surgeon, as complications can arise. It’s best to speak to your doctor for advice before seeking out a surgeon in the private sector.
The main types of hair loss surgery are explained below.
Hair transplantation
Modern hair transplant techniques involve follicular unit grafting. A follicular unit is the natural grouping of hairs with most hairs grouped as 2, 3, or 4 hairs in a close bunch. There will also be a small number of single hairs that grow on their own. When doctors do surgery they try to keep these natural groupings together and move them surgically by two main techniques. One method involves the removal of a strip of hair (about 1cm wide and 30 to 35cm long) bearing scalp from the back of the head where there’s plenty of hair, then stitching the area. The hair above the surgery site will disguise the stitches until they heal at which point you are left with a scar averaging 1mm in width. The strip of skin and hair is then dissected under microscopes to isolate the follicular units. The other method is called follicular unit extraction (FUE) and is a process of meticulously coring out each grouping with a tiny punch. Usually with this technique the back of the head needs to be shaved. When the scalp heals there are small dot scars that are left behind. Once the follicular unit grafts are prepared these are then inserted into a small incision made in the bald area. Doctors normally place 20-40 of these grafts in each square centimeter of bald scalp. The procedure is done as a day case under local anesthetic.
Stitches are not needed to attach the grafts because they are held in place by the clotting (thickening) action of the blood when the hairs are inserted.
Fine hairs are placed at the front of the scalp and thicker hairs towards the back in a process called grading. This helps achieve a more natural result.
The hair should settle and start to regrow within 6 months.
Hair transplants are carried out over a number of sessions. There should be a break of 9 to 12 months between procedures.
As with any type of surgery, there’s a risk of infection and bleeding, which can lead to hair loss and noticeable scarring.
In a completely bald area with one operation doctors can achieve about 30% of the original density so some patients may require 2 operations to achieve a thicker look. If hair loss is due to genetics then the result should be long lasting. In other situations, if hair is lost due to a medical condition such as Alopecia Areata, then transplantation cannot be guaranteed as the disease process can recur at any time. But for some patients even a temporary return to ‘normal’ hair is acceptable.
Scalp reduction
Scalp reduction involves removing pieces of bald scalp from the crown and the top of the head to move hairy parts of the scalp closer together.
This can be done by cutting out loose skin and stitching the scalp back together, or by tissue expansion.
Tissue expansion is where a balloon is placed underneath the scalp and inflated over several weeks to expand the skin in stages. The balloon is then removed and the excess skin is cut out.
Scalp reductions aren’t suitable for hair loss at the front of the scalp because it can cause scarring. There’s also the risk of infection in the area.
Scalp reduction isn’t usually used for male-pattern baldness, but it’s available to people with scarring alopecia. Surgery should only be carried out after any underlying conditions have cleared up.
Artificial hair
Artificial hair implantation is marketed as a treatment for male-pattern baldness. It involves implanting synthetic fibres into the scalp under local anaesthetic.
Artificial hair implantation carries serious risks of infection and scarring, but clinics may be reluctant to inform people of the possible complications to avoid losing potential clients.
Artificial hair implantation isn’t recommended by dermatologists because of the risk of complications, such as:
- infection
- scarring
- synthetic fibres falling out
People considering hair loss surgery should explore more established treatments, such as hair transplantation and scalp reduction, because the advantages and disadvantages of these techniques are better understood.
Cloning
The latest research into hair loss treatments is studying hair cell cloning.
The technique involves taking small amounts of a person’s remaining hair cells, multiplying them, and injecting them into bald areas.
Cloning is intended to treat both male- and female-pattern baldness. But the science behind the technique is new and more trials are needed before it can be fully assessed.
Tattooing
For many people, it’s possible to replicate hair with a tattoo. This is known as dermatography and generally produces good long-term results, although it’s usually expensive and can only be used to replicate very short hair.
This is usually carried out for eyebrows over a few hourly sessions, and can even be used as a treatment for scalp hair loss caused by male-pattern baldness.
Unproven treatments
There are many treatments suggested for hair loss which have not been shown to be effective. These include jojoba oil, lanolin, wheatgerm oil, nutritional supplements, laser and acupuncture. Again, speak to your doctor before opting for these or any other hair loss treatment options to avoid unwanted effects and spending time or money on a treatment that is unlikely to help.
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341(13):964–973.[↩][↩][↩]
- Elsaie, LT, Elshahid, AR, Hasan, HM, Soultan, FAZM, Jafferany, M, Elsaie, ML. Cross sectional quality of life assessment in patients with androgenetic alopecia. Dermatologic Therapy. 2020; 33:e13799. https://doi.org/10.1111/dth.13799[↩]
- Rajabi, F., Drake, L.A., Senna, M.M. and Rezaei, N. (2018), Alopecia areata: a review of disease pathogenesis. Br J Dermatol, 179: 1033-1048. https://doi.org/10.1111/bjd.16808[↩]
- Chen S, Xie X, Zhang G, Zhang Y. Comorbidities in Androgenetic Alopecia: A Comprehensive Review. Dermatol Ther (Heidelb). 2022 Oct;12(10):2233-2247. doi: 10.1007/s13555-022-00799-7[↩]
- Kinter KJ, Anekar AA. Biochemistry, Dihydrotestosterone. [Updated 2022 Mar 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557634[↩][↩]
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J Clin Med. 2023 Jan 23;12(3):893. doi: 10.3390/jcm12030893[↩][↩][↩][↩][↩][↩]
- Girijala RL, Riahi RR, Cohen PR. Platelet-rich plasma for androgenic alopecia treatment: A comprehensive review. Dermatol Online J. 2018 Jul 15;24(7):13030/qt8s43026c https://doi.org/10.5070/D3247040910[↩]
- Thom E. Stress and the Hair Growth Cycle: Cortisol-Induced Hair Growth Disruption. J Drugs Dermatol. 2016 Aug 1;15(8):1001-4. https://jddonline.com/articles/stress-and-the-hair-growth-cycle-cortisol-induced-hair-growth-disruption-S1545961616P1001X/[↩][↩][↩]
- Bassino E, Gasparri F, Munaron L. Protective Role of Nutritional Plants Containing Flavonoids in Hair Follicle Disruption: A Review. Int J Mol Sci. 2020 Jan 14;21(2):523. doi: 10.3390/ijms21020523[↩]
- Fu D., Huang J., Li K., Chen Y., He Y., Sun Y., Guo Y., Du L., Qu Q., Miao Y., et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. Pharmacother. 2021;137:111247. doi: 10.1016/j.biopha.2021.111247[↩]
- Harrison S, Bergfeld W. Diffuse hair loss: its triggers and management. Cleve Clin J Med. 2009 Jun;76(6):361-7. https://www.ccjm.org/content/ccjom/76/6/361.full.pdf[↩][↩]
- Vincent M., Yogiraj K. A descriptive study of alopecia patterns and their relation to thyroid dysfunction. Int. J. Trichol. 2013;5:57–60. doi: 10.4103/0974-7753.114701[↩][↩][↩]
- Contreras-Jurado C., Lorz C., García-Serrano L., Paramio J.M., Aranda A. Thyroid hormone signaling controls hair follicle stem cell function. Mol. Biol. Cell. 2015;26:1263–1272. doi: 10.1091/mbc.E14-07-1251[↩][↩][↩][↩]
- Arck PC, Handjiski B, Peters EM, Peter AS, Hagen E, Fischer A, Klapp BF, Paus R. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003 Mar;162(3):803-14. doi: 10.1016/S0002-9440(10)63877-1[↩]
- York J., Nicholson T., Minors P., Duncan D.F. Stressful life events and loss of hair among adult women, a case-control study. Psychol. Rep. 1998;82:1044–1046. doi: 10.2466/pr0.1998.82.3.1044[↩][↩]
- Hughes EC, Saleh D. Telogen Effluvium. [Updated 2022 Jun 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430848[↩]
- Schmidt J.B. Hormonal basis of male and female androgenic alopecia: Clinical relevance. Skin Pharmacol. 1994;7:61–66. doi: 10.1159/000211275[↩]
- Schmidt J.B., Lindmaier A., Spona J. Hormonal parameters in androgenetic hair loss in the male. Dermatologica. 1991;182:214–217. doi: 10.1159/000247797[↩]
- Agren U.M., Tammi M., Tammi R. Hydrocortisone regulation of hyaluronan metabolism in human skin organ culture. J. Cell. Physiol. 1995;164:240–248. doi: 10.1002/jcp.1041640204[↩]
- Almohanna H.M., Ahmed A.A., Tsatalis J.P., Tosti A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2018;9:51–70. doi: 10.1007/s13555-018-0278-6[↩]
- Guo E.L., Katta R. Diet and hair loss: Effects of nutrient deficiency and supplement use. Dermatol. Pract. Concept. 2017;7:1. doi: 10.5826/dpc.0701a01[↩][↩][↩][↩][↩][↩]
- Liamsombut S., Pomsoong C., Kositkuljorn C., Leerunyakul K., Tantrakul V., Suchonwanit P. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2022 doi: 10.1007/s11325-022-02618-x[↩][↩][↩]
- Yi Y., Qiu J., Jia J., Djakaya G.D.N., Li X., Fu J., Chen Y., Chen Q., Miao Y., Hu Z. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—A web-based investigation of male pattern hair loss in China. Dermatol. Ther. 2020;33:e13273. doi: 10.1111/dth.13273[↩]
- Shakoei S, Torabimirzaee A, Saffarian Z, Abedini R. Sleep disturbance in alopecia areata: A cross-sectional study. Health Sci Rep. 2022 Apr 1;5(3):e576. doi: 10.1002/hsr2.576[↩][↩][↩]
- Seo H.-M., Kim T.L., Kim J.S. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: A Korean population-based retrospective cohort study. Sleep. 2018;41:zsy111. doi: 10.1093/sleep/zsy111[↩][↩]
- Tacklind J, Fink HA, Macdonald R, Rutks I, Wilt TJ. Finasteride for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2010 Oct 6;2010(10):CD006015. doi: 10.1002/14651858.CD006015.pub3[↩]
- Paus R., Cotsarelis G. The Biology of Hair Follicles. N. Engl. J. Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706[↩]
- Motofei IG, Rowland DL, Tampa M, Sarbu MI, Mitran MI, Mitran CI, et al. Finasteride and androgenic alopecia; from therapeutic options to medical implications. J Dermatolog Treat. 2020;31:415–421. doi: 10.1080/09546634.2019.1595507[↩]
- Cranwell W, Sinclair R. Male Androgenetic Alopecia. [Updated 2016 Feb 29]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278957[↩]
- Kaufman K.D., Olsen E.A., Whiting D., Savin R., DeVillez R., Bergfeld W., Price V.H., Van Neste D., Roberts J.L., Hordinsky M., et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J. Am. Acad. Dermatol. 1998;39:578–589. doi: 10.1016/S0190-9622(98)70007-6[↩][↩]
- Drake L, Reyes-Hadsall S, Martinez J, Heinrich C, Huang K, Mostaghimi A. Evaluation of the Safety and Effectiveness of Nutritional Supplements for Treating Hair Loss: A Systematic Review. JAMA Dermatol. Published online November 30, 2022. doi:10.1001/jamadermatol.2022.4867[↩][↩][↩][↩]
- Shellow WV, Edwards JE, Koo JY. Profile of alopecia areata: a questionnaire analysis of patient and family. Int J Dermatol. 1992;31(3):186–189.[↩][↩]
- McDonagh AJ, Messenger AG. The pathogenesis of alopecia areata. Dermatol Clin. 1996;14(4):661–670.[↩]
- Delamere FM, Sladden MM, Dobbins HM, Leonardi-Bee J. Interventions for alopecia areata. Cochrane Database Syst Rev. 2008;(2):CD004413.[↩][↩][↩][↩]
- MacDonald Hull SP, Wood ML, Hutchinson PE, Sladden M, Messenger AG, for the British Association of Dermatologists. Guidelines for the management of alopecia areata. Br J Dermatol. 2003;149(4):692–699.[↩][↩][↩][↩]
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55(3):438–441.[↩]
- Khumalo NP, Ngwanya RM. Traction alopecia: 2% topical minoxidil shows promise. Report of two cases. J Eur Acad Dermatol Venereol. 2007;21(3):433–434.[↩]
- Diagnosing and Treating Hair Loss. https://www.aafp.org/pubs/afp/issues/2009/0815/p356.html[↩]
- Harrison, S. and Sinclair, R. (2002), Telogen effluvium. Clinical and Experimental Dermatology, 27: 389-395. https://doi.org/10.1046/j.1365-2230.2002.01080.x[↩]
- Kantor J, Kessler LJ, Brooks DG, Cotsarelis G. Decreased serum ferritin is associated with alopecia in women. J Invest Dermatol. 2003 Nov;121(5):985-8. doi: 10.1046/j.1523-1747.2003.12540.x[↩]
- Sinclair, R. (2002), There is no clear association between low serum ferritin and chronic diffuse telogen hair loss. British Journal of Dermatology, 147: 982-984. https://doi.org/10.1046/j.1365-2133.2002.04997.x[↩]
- Piérard GE, Piérard-Franchimont C, Marks R, Elsner P; EEMCO group (European Expert Group on Efficacy Measurement of Cosmetics and other Topical Products). EEMCO guidance for the assessment of hair shedding and alopecia. Skin Pharmacol Physiol. 2004 Mar-Apr;17(2):98-110. doi: 10.1159/000076020[↩]
- Piérard GE, Piérard-Franchimont C, Marks R, Elsner P, for the EEMCO group (European Expert Group on Efficacy Measurement of Cosmetics and Other Topical Products). EEMCO guidance for the assessment of hair shedding and alopecia. Skin Pharmacol Physiol. 2004;17(2):98–110.[↩]
- Diagnosing and Treating Hair Loss. Am Fam Physician. 2009 Aug 15;80(4):356-362. https://www.aafp.org/afp/2009/0815/p356.html[↩][↩]
- Plonka PM, Handjiski B, Popik M, Michalczyk D, Paus R. Zinc as an ambivalent but potent modulator of murine hair growth in vivo-preliminary observations. Exp Dermatol. 2005;14:844–853. https://www.ncbi.nlm.nih.gov/pubmed/16232307[↩][↩]
- Cheshire H, Stather P, Vorster J. Acquired acrodermatitis enteropathica due to zinc deficiency in a patient with pre-existing Darier’s disease. J Dermatol Case Rep. 2009;3:41–43. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3157796/[↩]
- Bhat YJ, Manzoor S, Khan AR, Qayoom S. Trace element levels in alopecia areata. Indian J Dermatol Venereol Leprol. 2009;75:29–31. https://www.ncbi.nlm.nih.gov/pubmed/19172027[↩][↩]
- Rushton DH. Nutritional factors and hair loss. Clin Exp Dermatol. 2002;27:396–404. https://www.ncbi.nlm.nih.gov/pubmed/12190640[↩]
- Arnaud J, Beani JC, Favier AE, Amblard P. Zinc status in patients with telogen defluvium. Acta Derm Venereol. 1995;75:248–249. https://www.ncbi.nlm.nih.gov/pubmed/7653193[↩][↩]
- Camacho FM, Garcia-Hernandez MJ. Zinc aspartate, biotin, and clobetasol propionate in the treatment of alopecia areata in childhood. Pediatr Dermatol. 1999;16:336–338. https://www.ncbi.nlm.nih.gov/pubmed/10515774[↩]
- Wolowa F, Jablonska S. Zinc in the treatment of alopecia areata. In: Kobori T, Montagna W, Toda K, editors. Biology and disease of the hair. 2nd ed. Tokyo: University of Tokyo Press; 1976. pp. 305–308.[↩]
- Park H, Kim CW, Kim SS, Park CW. The Therapeutic Effect and the Changed Serum Zinc Level after Zinc Supplementation in Alopecia Areata Patients Who Had a Low Serum Zinc Level. Annals of Dermatology. 2009;21(2):142-146. doi:10.5021/ad.2009.21.2.142. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2861201/[↩]
- Mussalo-Rauhamaa H, Lakomaa EL, Kianto U, Lehto J. Element concentrations in serum, erythrocytes, hair and urine of alopecia patients. Acta Derm Venereol. 1986;66:103–109. https://www.ncbi.nlm.nih.gov/pubmed/2424231[↩][↩]
- Lee SY, Nam KS, Seo YW, Lee JS, Chung H. Analysis of serum zinc and copper levels in alopecia areata. Ann Dermatol. 1997;9:239–241.[↩]
- Wells PA, Willmoth T, Russell RJ. Does fortune favour the bald? Psychological correlates of hair loss in males. Br J Psychol. 1995;86(pt 3):337–344.[↩]
- Bergfeld WF. Androgenetic alopecia: an autosomal dominant disorder. Am J Med. 1995;98(1A):95S–98S.[↩]
- Leyden J, Dunlap F, Miller B, et al. Finasteride in the treatment of men with frontal male pattern hair loss. J Am Acad Dermatol. 1999;40(6 pt 1):930–937.[↩][↩]
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578–589.[↩][↩]
- Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride induced depression: a prospective study. BMC Clin Pharmacol. 2006;6:7.[↩]
- Olsen EA, Dunlap FE, Funicella T, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47(3):377–385.[↩][↩][↩]
- Dawber RP, Rundegren J. Hypertrichosis in females applying minoxidil topical solution and in normal controls. J Eur Acad Dermatol Venereol. 2003;17(3):271–275.[↩][↩][↩]
- Diani AR, Mulholland MJ, Shull KL, et al. Hair growth effects of oral administration of finasteride, a steroid 5 alpha-reductase inhibitor, alone and in combination with topical minoxidil in the balding stumptail macaque. J Clin Endocrinol Metab. 1992;74(2):345–350.[↩]
- Khandpur S, Suman M, Reddy BS. Comparative efficacy of various treatment regimens for androgenetic alopecia in men. J Dermatol. 2002;29(8):489–498.[↩]
- Olsen EA, Weiner MS. Topical minoxidil in male pattern baldness: effects of discontinuation of treatment. J Am Acad Dermatol. 1987;17(1):97–101.[↩]
- Olsen EA. Female pattern hair loss and its relationship to permanent/cicatricial alopecia: a new perspective. J Investig Dermatol Symp Proc. 2005;10(3):217–221.[↩]
- Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, Wilson T, Rittmaster RS; Dutasteride Alopecia Research Team. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006 Dec;55(6):1014-23. doi: 10.1016/j.jaad.2006.05.007[↩]
- Debruyne F, Barkin J, van Erps P, Reis M, Tammela TL, Roehrborn C; ARIA3001, ARIA3002 and ARIB3003 Study Investigators. Efficacy and safety of long-term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004 Oct;46(4):488-94; discussion 495. doi: 10.1016/j.eururo.2004.05.008[↩]
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005 Sep-Oct;4(5):637-40.[↩]
- Saceda-Corralo D, Moustafa F, Moreno-Arrones Ó, Jaén-Olasolo P, Vañó-Galván S, Camacho F. Mesotherapy With Dutasteride for Androgenetic Alopecia: A Retrospective Study in Real Clinical Practice. J Drugs Dermatol. 2022 Jul 1;21(7):742-747. https://jddonline.com/articles/mesotherapy-with-dutasteride-for-androgenetic-alopecia-a-retrospective-study-in-real-clinical-practice-S1545961622P0742X[↩]
- Eun HC, Kwon OS, Yeon JH, Shin HS, Kim BY, Ro BI, Cho HK, Sim WY, Lew BL, Lee WS, Park HY, Hong SP, Ji JH. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010 Aug;63(2):252-8. doi: 10.1016/j.jaad.2009.09.018[↩]
- Gubelin Harcha W, Barboza Martínez J, Tsai TF, Katsuoka K, Kawashima M, Tsuboi R, Barnes A, Ferron-Brady G, Chetty D. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol. 2014 Mar;70(3):489-498.e3. doi: 10.1016/j.jaad.2013.10.049[↩]
- Inui S, Itami S. Reversal of androgenetic alopecia by topical ketoconzole: relevance of anti-androgenic activity. J Dermatol Sci. 2007 Jan;45(1):66-8. doi: 10.1016/j.jdermsci.2006.08.011[↩]
- Hugo Perez BS. Ketocazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men. Med Hypotheses. 2004;62(1):112-5. doi: 10.1016/s0306-9877(03)00264-0[↩]
- Piérard-Franchimont C., De Doncker P., Cauwenbergh G., Piérard G.E. Ketoconazole shampoo: Effect of long-term use in androgenic alopecia. Dermatology. 1998;196:474–477. doi: 10.1159/000017954[↩][↩]
- [↩]
- Inui S., Itami S. Reversal of androgenetic alopecia by topical ketoconzole: Relevance of anti-androgenic activity. J. Dermatol. Sci. 2007;45:66–68. doi: 10.1016/j.jdermsci.2006.08.011[↩][↩]
- El-Garf A., Mohie M., Salah E. Trichogenic effect of topical ketoconazole versus minoxidil 2% in female pattern hair loss: A clinical and trichoscopic evaluation. Biomed. Dermatol. 2019;3:8. doi: 10.1186/s41702-019-0046-y[↩][↩]
- Jimenez-Cauhe J, Saceda-Corralo D, Rodrigues-Barata R, Hermosa-Gelbard A, Moreno-Arrones OM, Fernandez-Nieto D, Vaño-Galvan S. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J Am Acad Dermatol. 2019 Aug;81(2):648-649. doi: 10.1016/j.jaad.2019.04.054[↩]
- Leavitt M, Charles G, Heyman E, Michaels D. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29(5):283-92. doi: 10.2165/00044011-200929050-00001[↩][↩]
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol. 2017 Jul;77(1):136-141.e5. doi: 10.1016/j.jaad.2017.02.054[↩][↩]
- Darwin E, Heyes A, Hirt PA, Wikramanayake TC, Jimenez JJ. Low-level laser therapy for the treatment of androgenic alopecia: a review. Lasers Med Sci. 2018 Feb;33(2):425-434. doi: 10.1007/s10103-017-2385-5[↩][↩][↩][↩][↩]
- RED light treatment for hair loss ‐ the science behind REVIAN RED. https://revian.com/how-it-works[↩]
- Kernel Networks Inc . Modulated light therapy in participants with pattern hair loss. Case Med Res. 2019. 10.31525/ct1-nct04019795[↩]
- Esmat, S.M., Hegazy, R.A., Gawdat, H.I., Abdel Hay, R.M., Allam, R.S., El Naggar, R. and Moneib, H. (2017), Low level light-minoxidil 5% combination versus either therapeutic modality alone in management of female patterned hair loss: A randomized controlled study. Lasers Surg. Med., 49: 835-843. https://doi.org/10.1002/lsm.22684[↩][↩][↩]
- Frigo L, Luppi JS, Favero GM, Maria DA, Penna SC, Bjordal JM, Bensadoun RJ, Lopes-Martins RA. The effect of low-level laser irradiation (In-Ga-Al-AsP – 660 nm) on melanoma in vitro and in vivo. BMC Cancer. 2009 Nov 20;9:404. doi: 10.1186/1471-2407-9-404[↩][↩]
- Alves R, Grimalt R. Platelet-Rich Plasma and its Use for Cicatricial and Non-Cicatricial Alopecias: A Narrative Review. Dermatol Ther (Heidelb). 2020 Aug;10(4):623-633. doi: 10.1007/s13555-020-00408-5[↩][↩][↩]
- Nestor MS, Ablon G, Gade A, Han H, Fischer DL. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J Cosmet Dermatol. 2021 Dec;20(12):3759-3781. doi: 10.1111/jocd.14537[↩][↩][↩]
- Girijala RL, Riahi RR, Cohen PR. Platelet-rich plasma for androgenic alopecia treatment: A comprehensive review. Dermatol Online J. 2018 Jul 15;24(7):13030/qt8s43026c https://escholarship.org/uc/item/8s43026c[↩][↩]
- Hausauer AK, Jones DH. Evaluating the Efficacy of Different Platelet-Rich Plasma Regimens for Management of Androgenetic Alopecia: A Single-Center, Blinded, Randomized Clinical Trial. Dermatol Surg. 2018 Sep;44(9):1191-1200. doi: 10.1097/DSS.0000000000001567[↩][↩][↩][↩]
- Alves R, Grimalt R. Randomized Placebo-Controlled, Double-Blind, Half-Head Study to Assess the Efficacy of Platelet-Rich Plasma on the Treatment of Androgenetic Alopecia. Dermatol Surg. 2016 Apr;42(4):491-7. doi: 10.1097/DSS.0000000000000665[↩][↩]
- Hesseler MJ, Shyam N. Platelet-Rich Plasma and Its Utilities in Alopecia: A Systematic Review. Dermatol Surg. 2020 Jan;46(1):93-102. doi: 10.1097/DSS.0000000000001965[↩][↩]
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for andro-genetic alopecia. American Academy of Dermatology. J Am Acad Dermatol. 1996;35(3 pt 1):465–469.[↩]
- Monselise A, Cohen DE, Wanser R, Shapiro J. What Ages Hair? Int J Womens Dermatol. 2017 Feb 16;3(1 Suppl):S52-S57. doi: 10.1016/j.ijwd.2017.02.010[↩]
- Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977 Sep;97(3):247-54. https://doi.org/10.1111/j.1365-2133.1977.tb15179.x[↩]
- van Zuuren, E.J., Fedorowicz, Z. and Carter, B. (2012), Evidence-based treatments for female pattern hair loss: a summary of a Cochrane systematic review. British Journal of Dermatology, 167: 995-1010. https://doi.org/10.1111/j.1365-2133.2012.11166.x[↩]
- van Zuuren EJ, Fedorowicz Z, Schoones J. Interventions for female pattern hair loss. Cochrane Database Syst Rev. 2016 May 26;2016(5):CD007628. doi: 10.1002/14651858.CD007628.pub4[↩][↩]
- Vexiau P, Chaspoux C, Boudou P, et al. Effects of minoxidil 2% vs. cyproterone acetate treatment on female androgenetic alopecia: a controlled, 12-month randomized trial. Br J Dermatol. 2002;146(6):992–999[↩]
- Burke BM, Cunliffe WJ. Oral spironolactone therapy for female patients with acne, hirsutism or androgenic alopecia. Br J Dermatol. 1985;112(1):124–125.[↩]
- Raudrant D, Rabe T. Progestogens with antiandrogenic properties. Drugs. 2003;63(5):463–492.[↩]
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768–776.[↩]
- Lanzafame RJ, Blanche RR, Chiacchierini RP, Kazmirek ER, Sklar JA. The growth of human scalp hair in females using visible red light laser and LED sources. Lasers Surg Med. 2014 Oct;46(8):601-7. doi: 10.1002/lsm.22277[↩][↩]
- Delamere FM, Sladden MM, Dobbins HM, Leonardi-Bee J. Interventions for alopecia areata. Cochrane Database Syst Rev. 2008 Apr 16;(2):CD004413. doi: 10.1002/14651858.CD004413.pub2[↩]
- Rokhsar CK, Shupack JL, Vafai JJ, Washenik K. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39(5 pt 1):751–761.[↩]
- DeVillez RL, Jacobs JP, Szpunar CA, Warner ML. Androgenetic alopecia in the female. Treatment with 2% topical minoxidil solution. Arch Dermatol. 1994;130(3):303–307.[↩]
- Carmina E, Lobo RA. Treatment of hyperandrogenic alopecia in women. Fertil Steril. 2003;79(1):91–95.[↩]
- Overstreet JW, Fuh VL, Gould J, et al. Chronic treatment with finasteride daily does not affect spermatogenesis or semen production in young men. J Urol. 1999;162(4):1295–1300.[↩]
- Oesterling JE, Roy J, Agha A, et al. Biologic variability of prostate-specific antigen and its usefulness as a marker for prostate cancer: effects of finasteride. The Finasteride PSA Study Group. Urology. 1997;50(1):13–18.[↩]
- Piérard-Franchimont C, De Doncker P, Cauwenbergh G, Piérard GE. Ketoconazole shampoo: effect of long-term use in androgenic alopecia. Dermatology. 1998;196(4):474–477.[↩]
- Berger RS, Fu JL, Smiles KA, et al. The effects of minoxidil, 1% pyrithione zinc and a combination of both on hair density: a randomized controlled trial. Br J Dermatol. 2003;149(2):354–362.[↩]
- Murphrey MB, Agarwal S, Zito PM. Anatomy, Hair. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513312[↩]
- Koyama T, Kobayashi K, Hama T, Murakami K, Ogawa R. Standardized Scalp Massage Results in Increased Hair Thickness by Inducing Stretching Forces to Dermal Papilla Cells in the Subcutaneous Tissue. Eplasty. 2016 Jan 25;16:e8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4740347[↩][↩][↩]
- English R.S., Barazesh J.M. Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results. Dermatol. Ther. 2019;9:167–178. doi: 10.1007/s13555-019-0281-6[↩][↩]
- Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013 Mar;32(1):41-52. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4126803[↩]
- Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014 Feb;46(2):144-51. doi: 10.1002/lsm.22170[↩][↩]
- Liu KH, Liu D, Chen YT, Chin SY. Comparative effectiveness of low-level laser therapy for adult androgenic alopecia: a system review and meta-analysis of randomized controlled trials. Lasers Med Sci. 2019 Aug;34(6):1063-1069. doi: 10.1007/s10103-019-02723-6[↩][↩][↩]
- Johnstone M.A., Albert D.M. Prostaglandin-induced hair growth. Surv. Ophthalmol. 2002;47:S185–S202. doi: 10.1016/S0039-6257(02)00307-7[↩][↩]
- Blume-Peytavi U., Lönnfors S., Hillmann K., Bartels N.G. A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J. Am. Acad. Dermatol. 2012;66:794–800. doi: 10.1016/j.jaad.2011.05.026[↩][↩]
- Khidhir K.G., Woodward D.F., Farjo N.P., Farjo B.K., Tang E.S., Wang J.W., Picksley S.M., Randall V. The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 2013;27:557–567. doi: 10.1096/fj.12-218156[↩][↩][↩]
- Garza L.A., Liu Y., Yang Z., Alagesan B., Lawson J.A., Norberg S.M., Loy D.E., Zhao T., Blatt H.B., Stanton D.C., et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 2012;4:126ra34. doi: 10.1126/scitranslmed.3003122[↩][↩]
- Lin W., Xiang L.-J., Shi H.-X., Zhang J., Jiang L., Cai P., Lin Z.-L., Lin B.-B., Huang Y., Zhang H.-L., et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. BioMed Res. Int. 2015;2015:730139. doi: 10.1155/2015/730139[↩]
- Pavlovic V., Ciric M., Jovanovic V., Stojanovic P. Platelet Rich Plasma: A short overview of certain bioactive components. Open Med. 2016;11:242–247. doi: 10.1515/med-2016-0048[↩]
- Stevens J., Khetarpal S. Platelet-rich plasma for androgenetic alopecia: A review of the literature and proposed treatment protocol. Int. J. Womens Dermatol. 2018;5:46–51. doi: 10.1016/j.ijwd.2018.08.004[↩]
- Giordano S., Romeo M., Lankinen P. Platelet-rich plasma for androgenetic alopecia: Does it work? Evidence from meta analysis. J. Cosmet. Dermatol. 2017;16:374–381. doi: 10.1111/jocd.12331[↩]
- Mao G., Zhang G., Fan W. Platelet-rich plasma for treating androgenic alopecia: A systematic review. Aesthetic Plast. Surg. 2019;43:1326–1336. doi: 10.1007/s00266-019-01391-9[↩]
- Dervishi G., Liu H., Peternel S., Labeit A., Peinemann F. Autologous platelet-rich plasma therapy for pattern hair loss: A systematic review. J. Cosmet. Dermatol. 2020;19:827–835. doi: 10.1111/jocd.13113[↩]
- Gupta A.K., Renaud H.J., Bamimore M. Platelet-rich plasma for androgenetic alopecia: Efficacy differences between men and women. Dermatol. Ther. 2020;33:e14143. doi: 10.1111/dth.14143[↩][↩][↩]
- Kuffler D.P. Variables affecting the potential efficacy of PRP in providing chronic pain relief. J. Pain Res. 2019;12:109–116. doi: 10.2147/JPR.S190065[↩]
- Hosking AM, Juhasz M, Atanaskova Mesinkovska N. Complementary and Alternative Treatments for Alopecia: A Comprehensive Review. Skin Appendage Disord. 2019 Feb;5(2):72-89. doi: 10.1159/000492035[↩]
- Ring C, Heitmiller K, Correia E, Gabriel Z, Saedi N. Nutraceuticals for Androgenetic Alopecia. J Clin Aesthet Dermatol. 2022 Mar;15(3):26-29. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8944288[↩][↩][↩][↩][↩][↩][↩]
- Ablon G, Kogan S. A Six-Month, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of a Nutraceutical Supplement for Promoting Hair Growth in Women With Self-Perceived Thinning Hair. J Drugs Dermatol. 2018 May 1;17(5):558-565. https://jddonline.com/articles/a-six-month-randomized-double-blind-placebo-controlled-study-evaluating-the-safety-and-efficacy-of-a-S1545961618P0558X/[↩][↩][↩][↩]
- Ablon G. A 3-month, randomized, double-blind, placebo-controlled study evaluating the ability of an extra-strength marine protein supplement to promote hair growth and decrease shedding in women with self-perceived thinning hair. Dermatol Res Pract. 2015;2015:841570. doi: 10.1155/2015/841570[↩][↩][↩][↩][↩][↩][↩]
- Cho YH, Lee SY, Jeong DW, Choi EJ, Kim YJ, Lee JG, Yi YH, Cha HS. Effect of pumpkin seed oil on hair growth in men with androgenetic alopecia: a randomized, double-blind, placebo-controlled trial. Evid Based Complement Alternat Med. 2014;2014:549721. doi: 10.1155/2014/549721[↩][↩][↩]
- Kang XC, Chen T, Zhou JL, Shen PY, Dai SH, Gao CQ, Zhang JY, Xiong XY, Liu DB. Phytosterols in hull-less pumpkin seed oil, rich in ∆7-phytosterols, ameliorate benign prostatic hyperplasia by lowing 5α-reductase and regulating balance between cell proliferation and apoptosis in rats. Food Nutr Res. 2021 Dec 2;65. doi: 10.29219/fnr.v65.7537[↩]
- Farris PK, Rogers N, McMichael A, Kogan S. A Novel Multi-Targeting Approach to Treating Hair Loss, Using Standardized Nutraceuticals. J Drugs Dermatol. 2017 Nov 1;16(11):s141-s148. https://jddonline.com/articles/a-novel-multi-targeting-approach-to-treating-hair-loss-using-standardized-nutraceuticals-S1545961617S0141X[↩][↩][↩][↩][↩][↩][↩]
- Nakamura K, Yasunaga Y, Segawa T, Ko D, Moul JW, Srivastava S, Rhim JS. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int J Oncol. 2002 Oct;21(4):825-30. https://doi.org/10.3892/ijo.21.4.825[↩]
- Shishodia, S. (2013), Molecular mechanisms of curcumin action: Gene expression. BioFactors, 39: 37-55. https://doi.org/10.1002/biof.1041[↩]
- Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998 May;64(4):353-6. doi: 10.1055/s-2006-957450[↩]
- Auddy B, Hazra J, Mitra A et al. A Standardized Withania Somnifera Extract Significantly Reduces Stress-Related Parameters in Chronically Stressed Humans: A Double-Blind, Randomized, Placebo-Controlled Study. Journal of American Nutraceutical Association. 2008;11:50–56.[↩][↩]
- Thom E. Stress and the Hair Growth Cycle: Cortisol-Induced Hair Growth Disruption. J Drugs Dermatol. 2016 Aug 1;15(8):1001-4. https://jddonline.com/articles/stress-and-the-hair-growth-cycle-cortisol-induced-hair-growth-disruption-S1545961616P1001X[↩]
- Rossi A, Mari E, Scarno M, Garelli V, Maxia C, Scali E, Iorio A, Carlesimo M. Comparitive effectiveness of finasteride vs Serenoa repens in male androgenetic alopecia: a two-year study. Int J Immunopathol Pharmacol. 2012 Oct-Dec;25(4):1167-73. doi: 10.1177/039463201202500435[↩]
- Wang Y, Park NY, Jang Y, Ma A, Jiang Q. Vitamin E γ-Tocotrienol Inhibits Cytokine-Stimulated NF-κB Activation by Induction of Anti-Inflammatory A20 via Stress Adaptive Response Due to Modulation of Sphingolipids. J Immunol. 2015 Jul 1;195(1):126-33. doi: 10.4049/jimmunol.1403149[↩][↩]
- Beoy LA, Woei WJ, Hay YK. Effects of tocotrienol supplementation on hair growth in human volunteers. Trop Life Sci Res. 2010 Dec;21(2):91-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3819075[↩]
- Ablon G, Kogan S. A Six-Month, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of a Nutraceutical Supplement for Promoting Hair Growth in Women With Self-Perceived Thinning Hair. J Drugs Dermatol. 2018 May 1;17(5):558-565. https://jddonline.com/articles/a-six-month-randomized-double-blind-placebo-controlled-study-evaluating-the-safety-and-efficacy-of-a-S1545961618P0558X[↩][↩][↩]
- Hornfeldt, CS. Growing evidence of the beneficial effects of a marine protein-based dietary supplement for treating hair loss. J Cosmet Dermatol. 2018; 17: 209– 213. https://doi.org/10.1111/jocd.12400 [↩]
- Costa A, Pereira ESP, Favaro R et al. Treating cutaneous photoaging in women with an oral supplement based on marine protein, concentrated acerola, grape seed extract and tomato extract, for 360 days. Surg Cosmet Dermatol. 2011;3:302–311.[↩]
- Lassus A, Jeskanen L, Happonen HP, Santalahti J. Imedeen for the treatment of degenerated skin in females. J Int Med Res. 1991 Mar-Apr;19(2):147-52. doi: 10.1177/030006059101900208[↩]
- Rizer RL, Stephens TJ, Herndon JH, Sperber BR, Murphy J, Ablon GR. A Marine Protein-based Dietary Supplement for Subclinical Hair Thinning/Loss: Results of a Multisite, Double-blind, Placebo-controlled Clinical Trial. Int J Trichology. 2015 Oct-Dec;7(4):156-66. doi: 10.4103/0974-7753.171573[↩][↩]
- Glynis A. A Double-blind, Placebo-controlled Study Evaluating the Efficacy of an Oral Supplement in Women with Self-perceived Thinning Hair. J Clin Aesthet Dermatol. 2012 Nov;5(11):28-34. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3509882[↩][↩][↩]
- Ablon, G. (2016), A 6-month, randomized, double-blind, placebo-controlled study evaluating the ability of a marine complex supplement to promote hair growth in men with thinning hair. J Cosmet Dermatol, 15: 358-366. https://doi.org/10.1111/jocd.12265[↩][↩][↩][↩][↩][↩]
- Biotin. https://ods.od.nih.gov/factsheets/Biotin-HealthProfessional[↩]
- Zempleni J, Wijeratne SSK, Kuroishi T. Biotin. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:359-74.[↩][↩][↩]
- Mock DM. Biotin. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:390-8.[↩][↩][↩]
- Combs GF, Jr. Biotin. In: Combs GF, Jr., ed. The vitamins: fundamental aspects in nutrition and health. Third ed. Burlington, MA: Elsevier Academic Press; 2008:331-44.[↩][↩]
- Perry CA, West AA, Gayle A, Lucas LK, Yan J, Jiang X, Malysheva O, Caudill MA. Pregnancy and lactation alter biomarkers of biotin metabolism in women consuming a controlled diet. J Nutr. 2014 Dec;144(12):1977-84. doi: 10.3945/jn.114.194472[↩]
- Mock DM. Biotin. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:43-51.[↩][↩][↩]
- Mock DM. Biotin. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed.: Lippincott Williams & Wilkins; 2014:390-398.[↩]
- Food and Nutrition Board, Institute of Medicine. Biotin. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, D.C.: National Academy Press; 1998:374-389.[↩]
- Elrefai S, Wolf B. Disorders of biotin metabolism. In: Rosenberg RN, Pascual JM, eds. Rosenberg’s Molecular and Genetic basis of Neurological and Psychiatric Disease. 5th ed. United States of America: Elsevier; 2015:531-539.[↩]
- Regula Baumgartner, E. and Suormala, T. (1999), Inherited defects of biotin metabolism. BioFactors, 10: 287-290. https://doi.org/10.1002/biof.5520100229[↩]
- Shelley WB, Shelley ED. Uncombable hair syndrome: observations on response to biotin and occurrence in siblings with ectodermal dysplasia. J Am Acad Dermatol. 1985 Jul;13(1):97-102. doi: 10.1016/s0190-9622(85)70150-8[↩]
- Boccaletti, V., Zendri, E., Giordano, G., Gnetti, L. and De Panfilis, G. (2007), Familial Uncombable Hair Syndrome: Ultrastructural Hair Study and Response to Biotin. Pediatric Dermatology, 24: E14-E16. https://doi.org/10.1111/j.1525-1470.2007.00385.x[↩]
- Mock DM, Baswell DL, Baker H, Holman RT, Sweetman L. Biotin deficiency complicating parenteral alimentation: diagnosis, metabolic repercussions, and treatment. J Pediatr. 1985 May;106(5):762-9. doi: 10.1016/s0022-3476(85)80350-4[↩]
- Fujimoto, W., Inaoki, M., Fukui, T., Inoue, Y. and Kuhara, T. (2005), Biotin Deficiency in an Infant Fed with Amino Acid Formula. The Journal of Dermatology, 32: 256-261. https://doi.org/10.1111/j.1346-8138.2005.tb00758.x[↩]
- Ablon G, Dayan S. A Randomized, Double-blind, Placebo-controlled, Multi-center, Extension Trial Evaluating the Efficacy of a New Oral Supplement in Women with Self-perceived Thinning Hair. J Clin Aesthet Dermatol. 2015 Dec;8(12):15-21. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4689507[↩][↩][↩][↩]
- Hornfeldt, CS. Growing evidence of the beneficial effects of a marine protein-based dietary supplement for treating hair loss. J Cosmet Dermatol. 2018; 17: 209– 213. https://doi.org/10.1111/jocd.12400[↩]
- Trueb RM. Berlin Heidelberg: Springer- Verlag; 2008. Diffuse hair loss. In: Blume-Peytavi UTA, Whiting DA, Treub R, eds. Hair Growth and Disorders.[↩]
- Lengg N, Heidecker B, Seifert B, Trueb RM. Dietary supplement increases anagen hair rate in women with telogen effluvium: results of a double-blind, placebo-controlled trial. Therapy. 2007;4:59–65.[↩]
- Le Floc’h, C., Cheniti, A., Connétable, S., Piccardi, N., Vincenzi, C. and Tosti, A. (2015), Effect of a nutritional supplement on hair loss in women. J Cosmet Dermatol, 14: 76-82. https://doi.org/10.1111/jocd.12127[↩]
- Thom E. Nourkrin: objective and subjective effects and tolerability in persons with hair loss. J Int Med Res. 2006 Sep-Oct;34(5):514-9. doi: 10.1177/147323000603400508[↩][↩][↩][↩][↩]