Glutathione
Glutathione is a tripeptide comprised of three amino acids, cysteine, glycine, and glutamic acid (Figure 1), is a critical component of the body’s primary antioxidant defense mechanism that is found in relatively high concentrations in most cells 1, 2. Glutathione is called an antioxidant (a free radical scavenger and a detoxifying agent) because of its role in protecting cells from the damaging effects of unstable and harmful oxygen-containing molecules that are generated during energy production 3, 4. Glutathione plays a pivotal role against free radicals and oxidative stress, by maintaining redox balance, enhancing metabolic detoxification, and regulating the immune system 1, 5. Glutathione is also important as a cofactor for the enzyme glutathione peroxidase, in the uptake of amino acids, and in the synthesis of leukotrienes. As a substrate for glutathione S-transferase, glutathione reacts with a number of harmful chemical species, such as halides, epoxides and free radicals, to form harmless inactive products 6, 7. In red blood cells, these reactions prevent oxidative damage through the reduction of methemoglobin and peroxides. Glutathione is also involved in the formation and maintenance of disulfide bonds in proteins and in the transport of amino acids across cell membranes 8. Glutathione also plays a role in processing medications and cancer-causing compounds (carcinogens), and building DNA, proteins, and other important cellular components 9, 6, 10.
The largest amount ofglutathione is found in the liver (5 – 10 mM), lining of the lungs, kidneys, heart and brain. Glutathione is necessary for the liver to detoxify toxic substances. Glutathione, especially in the liver, binds with toxic chemicals in order to detoxify them. It is also important as a hydrophilic molecule that is added to lipophilic toxins and waste in the liver during biotransformation before they can become part of the bile. Glutathione is also needed for the detoxification of methylglyoxal, a toxin produced as a by-product of metabolism. This detoxification reaction is carried out by the glyoxalase system. Glyoxalase I catalyzes the conversion of methylglyoxal and reduced glutathione to S-D-Lactoyl-glutathione. Glyoxalase II catalyzes the hydrolysis of S-D-Lactoyl-glutathione to glutathione and D-lactate.
Glutathione is known as a substrate in both conjugation reactions and reduction reactions, catalyzed by glutathione S-transferase enzymes in cytosol, microsomes, and mitochondria. However, it is also capable of participating in non-enzymatic conjugation with some chemicals, as in the case of N-Acetyl-P-Benzoquinone Imine (NAPQI), the reactive cytochrome P450-reactive metabolite formed by acetaminophen, that becomes toxic when glutathione is depleted by an overdose of acetaminophen. Glutathione in this capacity binds to N-Acetyl-P-Benzoquinone Imine (NAPQI) as a suicide substrate and in the process detoxifies it, taking the place of cellular protein thiol groups which would otherwise be covalently modified; when all glutathione has been spent, NAPQI begins to react with the cellular proteins, killing the cells in the process 11. The preferred treatment for an overdose of acetaminophen is the administration (usually in atomized form) of N-acetylcysteine (NAC), which is used by cells to replace spent oxidized glutathione (GSSG) and renew the usable reduced glutathione (GSH) pool.
Glutathione conjugates to drugs to make them more soluble for excretion, is a cofactor for some enzymes, is involved in protein disulfide bond rearrangement and reduces peroxides.
Glutathione is also important in red and white blood cell formation and throughout the immune system.
Glutathione participates in leukotriene synthesis and is a cofactor for the enzyme glutathione peroxidase.
Glutathione is made available in cells in 3 ways (Figures 2, 3 and 4) 1:
- Three conditionally essential amino acids, glycine, cysteine, and glutamic acid combine to form glutathione via a 2-step process catalyzed by the enzymes glutamate cysteine ligase (GCL) and glutathione synthetase (requires ATP). First, cysteine is conjoined with glutamate through the action of glutamate cysteine ligase (GCL) to produce gamma-glutamylcysteine, which proceeds to link with glycine via glutathione synthase 12. Therefore, the human body requires all three amino acids (glycine, cysteine, and glutamic acid) and adequate enzymatic function to make sufficient quantities of glutathione 13. Cysteine is a sulfur amino acid, which might imply that consuming sulfur-rich foods, especially those containing the sulfur amino acids, may also support glutathione de novo synthesis 14, 15.
- Regeneration of oxidized glutathione (GSSG) to reduced glutathione (GSH) by glutathione reductase (requires NADPH).
- Recycling of cysteine from conjugated glutathione via GGTP (requires NADPH).
All 3 glutathione synthesis pathways require energy. The rate of glutathione synthesis, regeneration, and recycling is determined primarily by 3 factors (Figure 3) 16:
- De novo glutathione synthesis is primarily controlled by the cellular level of the amino acid cysteine, the availability of which is the rate-limiting step.
- The enzymes glutamate cysteine ligase (GCL) activity is in part regulated by glutathione feedback inhibition.
- If glutathione is depleted due to oxidative stress, inflammation, or exposure to xenobiotics, de novo synthesis of glutathione is upregulated primarily by increasing availability of cysteine through recycling of oxidized glutathione (GSSG).
Under physiological conditions, glutathione is mainly present in the cytoplasm (inside cells) in the reduced form (GSH), which is also the biologically active form. Reduced glutathione (GSH) is less easily oxidized than its precursors, cysteine and gamma-glutamylcysteine; the fully oxidized form with a disulfide between two identical glutathione molecules (GSSG) represents less than 1% of the total glutathione pool in the cell 17. Reduced glutathione (GSH) concentration in human cells typically ranges from 0.1 to 10 mmol/L, being most focused in the liver (up to 10 mmol/L), spleen, kidney, lens of the eyes, red blood cells, and white blood cells 18, 19, wherein its depletion and/or altered level are associated with various diseases, including cancer, cardiovascular, inflammatory, immune, metabolic, and neurodegenerative diseases 2. Maintaining optimal reduced glutathione (GSH):oxidized glutathione (GSSG) ratios in the cell is critical to survival; hence, tight regulation of this system is important 20.
Natural dietary sources of glutathione include fresh fruits, vegetables, and nuts. Tomatoes, avocados, oranges, walnuts and asparagus are some of the most common foods that help to increase levels of glutathione in the body. Whey protein is another rich source of glutathione and has been used to enhance systemic glutathione levels in cystic fibrosis 21.
Glutathione is synthesized by glutathione synthetase from glutamine and cysteine in a two-step process involving the enzymes glutathione synthatase and glutamate-cysteine ligase 22. Like cysteine, glutathione contains the crucial thiol (-SH) group that makes it an effective antioxidant 23. There are virtually no living organisms on this planet-animal or plant whose cells don’t contain some glutathione 23. Scientists have speculated that glutathione was essential to the very development of life on earth 23. People who are born with glutathione synthetase deficiency prevents their body from producing glutathione 24, 25. People with glutathione synthetase deficiency do not have enough of the molecule called glutathione synthetase, which helps the body produce glutathione. People with glutathione synthetase deficiency can have mild, moderate, or severe disease. The signs and symptoms of glutathione synthetase deficiency may include anemia, the buildup of too much acid in the body (metabolic acidosis), frequent infections, and symptoms caused by problems in the brain including seizures, intellectual disability, and loss of coordination (ataxia).
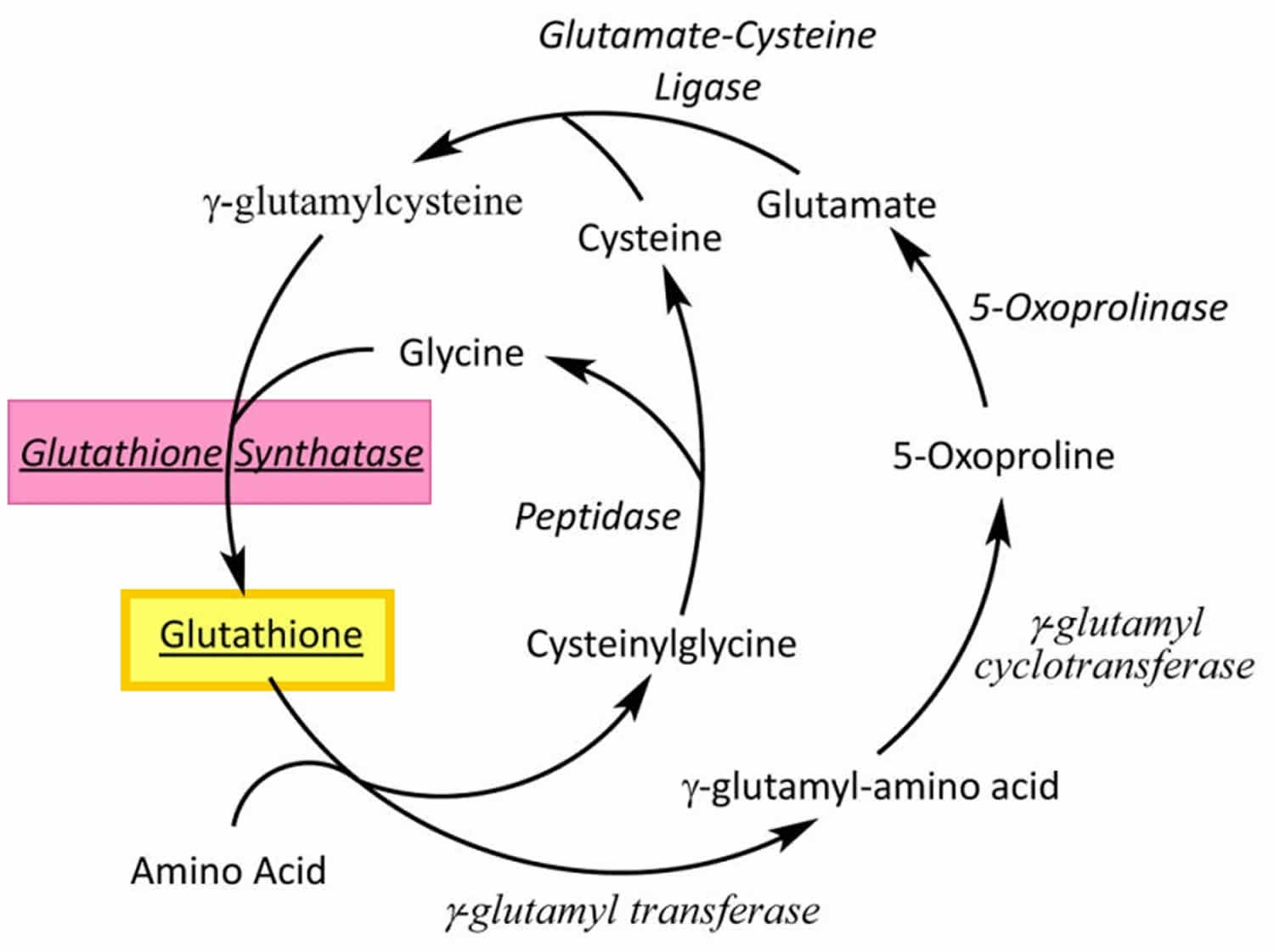
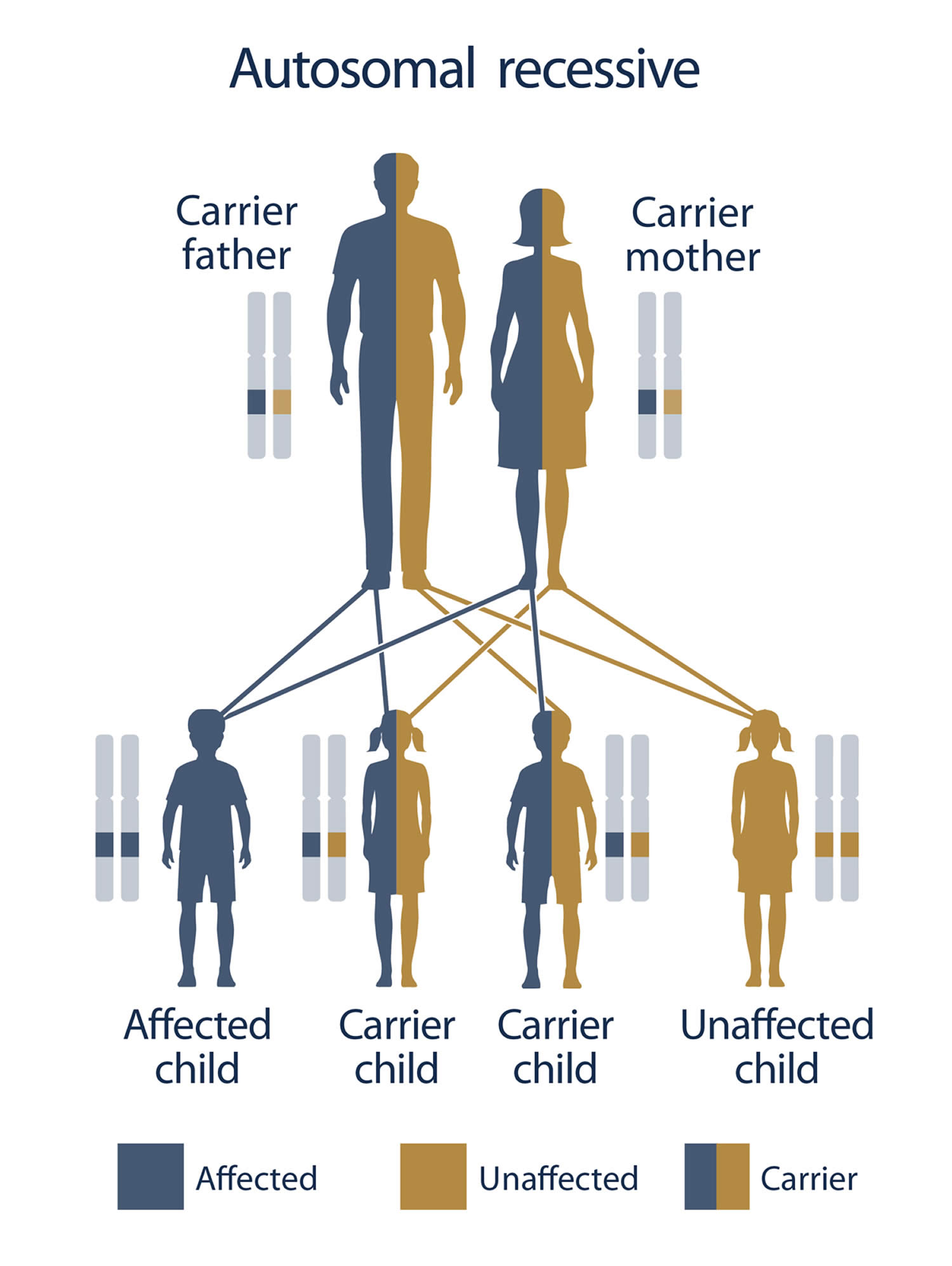
Glutathione synthetase deficiency is a very rare autosomal recessive disorder that has only been described in more than 80 individuals worldwide 26, 27. Glutathione synthetase deficiency is caused by genetic changes (pathogenic variants or mutations) in the GSS gene 9. The GSS gene provides instructions for making glutathione synthetase enzyme. The glutathione synthetase enzyme is involved in a process called the gamma-glutamyl cycle, which takes place in most of the body’s cells (Figure 2). The gamma-glutamyl cycle is necessary for producing a molecule called glutathione (Figure 2). Mutations in the GSS gene prevent cells from making adequate levels of glutathione, leading to the signs and symptoms of glutathione synthetase deficiency.
Glutathione synthetase deficiency is inherited in an autosomal recessive pattern, which means both copies of the GSS gene in each cell have mutations (Figure 5). Each parent is a carrier which means they have a pathogenic variant in only one copy of the GSS gene. The parents (carriers of an autosomal recessive disease) of an individual with glutathione synthetase deficiency each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition. When two carriers of an autosomal recessive disease have children, there is a 25% (1 in 4) chance to have a child who has the disease (Figure 5).
Mild glutathione synthetase deficiency usually results in the destruction of red blood cells (hemolytic anemia) 9, 28. In addition, affected individuals may release large amounts of a compound called 5-oxoproline in their urine (5-oxoprolinuria) 28. This compound builds up when glutathione is not processed correctly in cells. As 5-oxoproline is a highly acidic compound, it causes metabolic acidosis 29.
Individuals with moderate glutathione synthetase deficiency may experience symptoms beginning shortly after birth including hemolytic anemia, 5-oxoprolinuria, and elevated acidity in the blood and tissues (metabolic acidosis) 9.
In addition to the features present in moderate glutathione synthetase deficiency, individuals affected by the severe form of glutathione synthetase deficiency may experience neurological symptoms. These problems may include seizures; pathological electroretinograms and retinal pigmentation; a generalized slowing down of physical reactions, movements, and speech (psychomotor retardation); intellectual disability; spasticity, intention tremors and a loss of coordination (ataxia) 9, 30. Some people with severe glutathione synthetase deficiency also develop recurrent bacterial infections 28.
The diagnosis of a metabolic disorder such as glutathione synthetase deficiency may be suspected when a doctor observes signs of glutathione synthetase deficiency including metabolic acidosis. A doctor may order tests to confirm the diagnosis including enzyme assays, urine analysis, and genetic testing 25. Prenatal diagnosis of glutathione synthetase deficiency is possible by measuring 5-oxoproline in amniotic fluid, or by enzyme analysis in cultured amniocytes or chorionic villi samples 31, 32, 33 or a presumptive diagnosis can be made by detecting elevation of 5-oxoproline in newborn screen blood spots using tandem mass-spectrometry or massive excretion of 5-oxoproline (up to 1 g/kg per day) in the urine 34. Early diagnosis and treatment is thought to correlate with a better long term outcome 35.
Treatment for glutathione synthetase deficiency may include sodium bicarbonate to treat metabolic acidosis and taking high doses of vitamin C and vitamin E for protection against oxidative stress 25, 27, 36. Njalsson et al 37 demonstrated that early initiation of vitamin C and vitamin E could prevent the moderate disease from progressing to severe disease. In a study of 41 patients with glutathione synthetase deficiency, only 1/18 of the severely affected patients were started on vitamin therapy early in life as compared to 6/17 moderately affected patients. With this data and the fact that there is no significant difference between enzymatic activity in the moderate and severe disease, they posit that early initiation of vitamins could prevent or slow down progression of the disease 37. There were no specific comments on length of follow-up of this cohort however.
Selenium is another agent used in glutathione synthetase deficiency patients to prevent oxidative stress. Selenium is found to have strong antioxidant properties through formation of selenoproteins, which are thought to protect against reactive oxygen species 38.
Avoidance of foods and drugs known to cause hemolytic crisis in G6PD deficiency is also important as these same triggers can cause hemolytic crisis in glutathione synthetase deficiency 39. Vitamin E is also used to prevent granulocyte dysfunction, which could cause recurrent infections 40, 41. Patients with moderate and severe glutathione synthetase deficiency disease typically require treatment for metabolic acidosis. In acute crisis, IV bicarbonate is given for immediate correction, and for long term management, citrate (citric acid, potassium citrate, and sodium citrate) or trometamol (THAM) are given to maintain a normal serum bicarbonate level 22.
Glutathione synthetase deficiency prognosis depends on the type of mutation, severity of metabolic acidosis, associated bacterial infections and the quality of supportive therapy. A long-term follow up study of 28 patients with glutathione synthetase deficiency has indicated that the factors most predictive of survival and long-term outcome are early diagnosis and early supplementation with vitamins C and E 28.
Table 1. Glutathione rich fruits and vegetables
| Food | Glutathione | N-Acetylcysteine (NAC) | Cysteine |
|---|---|---|---|
| Asparagus | 349 ± 26 | 46 ± 1 | 122 ± 1 |
| Avocado | 339 ± 10 | ND | 4 ± 1 |
| Banana | ND | ND | 7 ± 0 |
| Broccoli | 4 ± 1 | ND | ND |
| Carrot | 4 ± 0 | ND | ND |
| Cauliflower | 6 ± 1 | ND | 7 ± 1 |
| Cucumber | 123 ± 38 | 6 ± 1 | 11 ± 3 |
| Grapefruit | 13 ± 3 | 4 ± 0 | 15 ± 2 |
| Green Beans | 230 ± 2 | ND | 67 ± 11 |
| Green Pepper | 8 ± 1 | 12 ± 2 | 9 ± 1 |
| Green Squash | 47 ± 11 | ND | 6 ± 1 |
| Lemon | 5 ± 0 | 4 ± 0 | 6 ± 0 |
| Mango | 59 ± 6 | ND | 10 ± 0 |
| Orange | 5 ± 11 | ND | 41 ± 2 |
| Papaya | 136 ± 12 | ND | 58 ± 5 |
| Parsley | 17 ± 9 | 9 ± 1 | 8 ± 1 |
| Potato | 5 ± 0 | ND | ND |
| Red Pepper | 42 ± 2 | 25 ± 4 | 349 ± 18 |
| Spinach | 313 ± 33 | ND | 84 ± 2 |
| Strawberry | 39 ± 8 | 5 ± 1 | 59 ± 5 |
| Tomato | 64 ± 10 | 3 ± 1 | 55 ± 3 |
| Yellow Squash | 39 ± 8 | ND | 27 ± 6 |
Footnote: Numbers represent nM/g wet weight (mean ± SD of three samples)
ND = not detectable
[Source 42 ]Table 2. Nutrients and foods for support of glutathione levels
| Nutrient and Foods | Recommended Dosage |
|---|---|
| Alpha lipoic-acid | 300 mg 3× day; 200–600 mg/day 43 |
| Brassica vegetables | 250 g/day |
| Curcumin | Doses up to 12 g/day safe; 1–2 g/day found to benefit antioxidant capacity; increased bioavailability with piperine 44 |
| Fruit and vegetable juices | 300–400 mL/day |
| Glutathione (Liposomal) | 500–1000 mg/day 45 |
| Glutathione (Oral) | 500–1000 mg/day 46, 47 |
| Glycine | 100 mg/kg/day 48 |
| Green tea | 4 cups/day |
| N-acetylcysteine (NAC) | 600–1200 mg/day in divided doses, but up to 6000 mg/day have been shown effective in studies 49, 50, 51, 52 |
| Omega-3 fatty acids | 4000 mg/day 53 |
| Salmon | 150 g twice a week 54 |
| Selenium | 247 μg/day of selenium enriched yeast; 100–200 ug/day. Anything above 400 ug/day watch for toxicity 55, 52 |
| Vitamin C | 500–2000 mg/day 56, 57 |
| Vitamin E | 100–400 IU/day 58, 59 |
| Whey Protein | 40 g/day 60 |
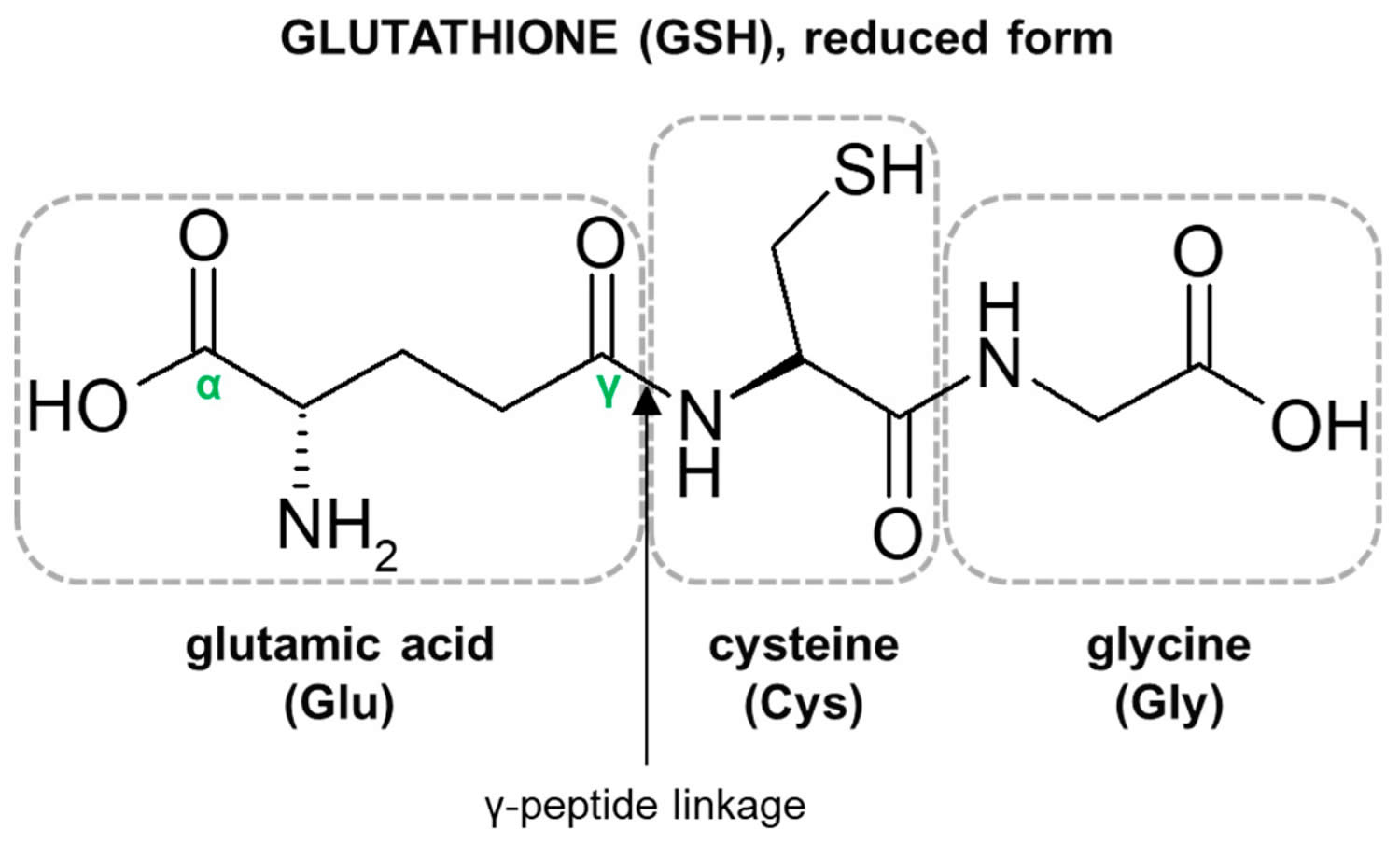
Figure 1. Glutathione structure
Footnote: Structure of the tripeptide glutathione (GSH) (reduced form). Glutamic acid (Glu) is linked in a gamma (γ) peptide linkage (via its gamma-carboxyl group) to cysteine (Cys), which in turn forms an alpah (α) peptide linkage with glycine (Gly).
[Source 61 ]Figure 2. Gamma-glutamyl cycle
Footnotes: Glutathione (GSH) is synthesized by glutathione synthetase from glutamine and cysteine in a two-step process involving the enzymes glutathione synthatase and glutamate-cysteine ligase.
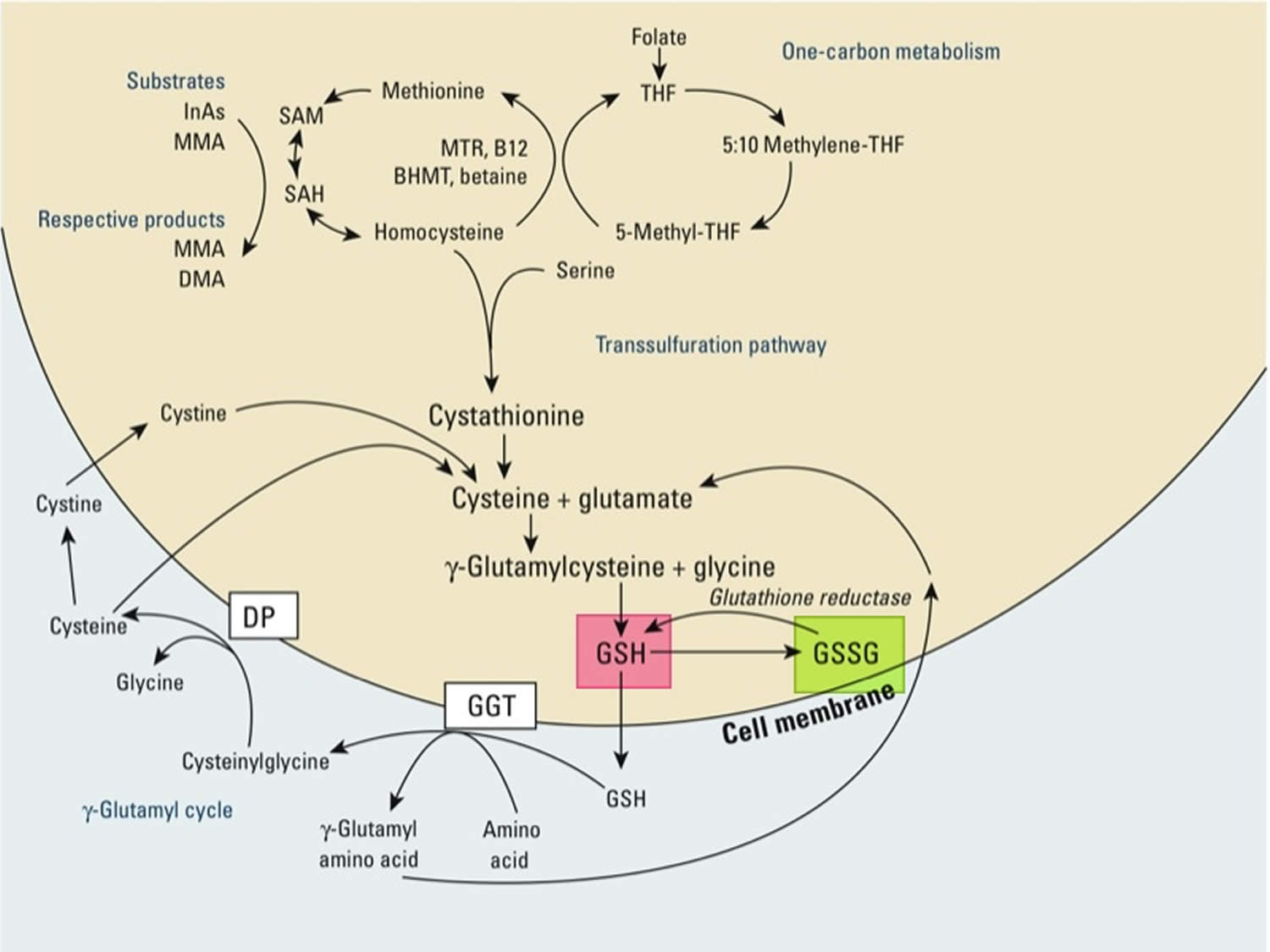
[Source 22 ]Figure 3. Synthesis and recycling of glutathione
Footnotes: One-carbon metabolism and glutathione (GSH) synthesis and metabolism. Folic acid is reduced to tetrahydrofolate (THF) and subsequently converted to 5-methyl THF. In a reaction catalyzed by methionine synthetase (MTR), the methyl group of 5‑methyl-THF can be transferred to homocysteine, generating methionine. Methionine is activated to form S‑adenosylmethionine (SAM), the universal methyl donor. The by-product of methylation reactions, S‑adenosylhomocysteine (SAH), is hydrolyzed to homocysteine. Homocysteine is either used to regenerate methionine or is directed to the transsulfuration pathway. Reduced glutathione (GSH) is a product of the transsulfuration pathway. Reduced glutathione (GSH) can serve as a continuous source of cysteine, which is extremely unstable, via the gamma‑glutamyl cycle. Reduced glutathione (GSH) is exported from the cell, and the enzyme gamma‑glutamyltransferase (GGT) transfers the gamma-glutamyl moiety of glutathione (GSH) to an amino acid, often cystine, producing cysteinylglycine and gamma-glutamyl amino acid. The gamma-glutamyl amino acid can be transported back into the cell and ultimately metabolized to glutamate. Cysteinylglycine is converted to cysteine and glycine by dipeptidase (DP). Cysteine is unstable extracellularly and can oxidize to cystine; both cysteine and cystine can be imported back into the cell for glutathione (GSH) production.
Abbreviations: B12 = vitamin B12; BHMT = betaine homocysteine methyltransferase; DMA = dimethylarsinic acid; InAs = inorganic acids; MMA = monomethylarsonic acid; GSSG = oxidized glutathione; GSH = reduced glutathione
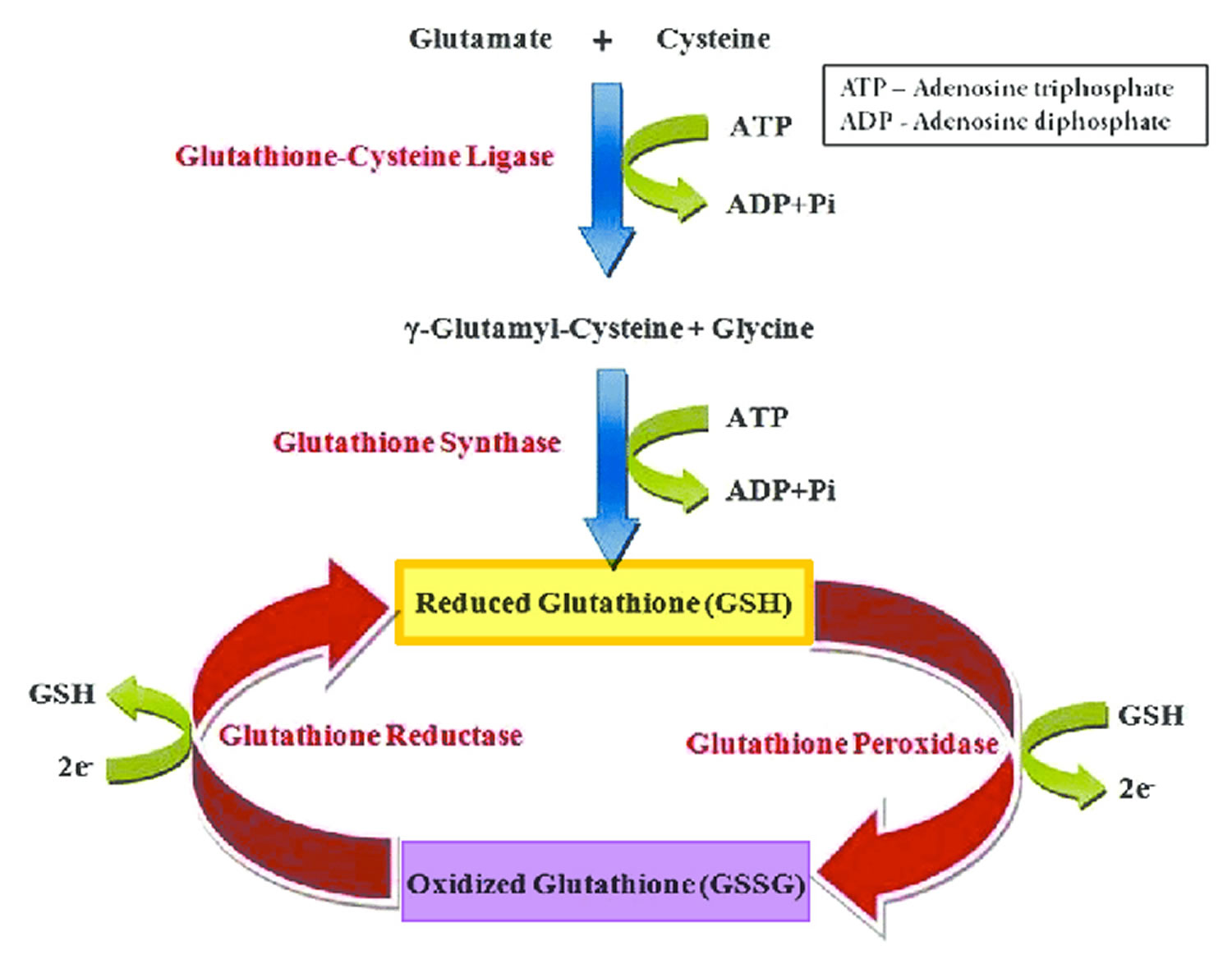
[Source 62 ]Figure 4. The glutathione redox cycle
Footnote: The glutathione redox cycle demonstrating the inter-conversion of oxidized glutathione (GSSG) and reduced glutathione (GSH). Glutathione exists in two interconvertible forms, reduced glutathione (GSH) and oxidized glutathione (GSSG). Reduced glutathione (GSH) is the predominant intracellular form, which acts as a strong antioxidant and defends against toxic compounds and xenobiotics. In this process, reduced glutathione (GSH) is constantly oxidized to GSSG (oxidized glutathione) by the enzyme glutathione peroxidase. To maintain the intracellular redox balance, reduced glutathione (GSH) is replenished through the reduction of GSSG (oxidized glutathione) by glutathione reductase enzyme.
[Source 63 ]Figure 5. Glutathione synthetase deficiency autosomal recessive inheritance
Health benefits of glutathione
It has been found that low levels of glutathione exist in several diseases.
Diseases associated with glutathione dysregulation or deficiency 64, 2:
- Aging 65 and related disorders 2
- Alzheimer’s disease 66
- Cancer 67
- Chronic liver disease 68
- Cognitive impairment 69
- Cystic fibrosis 70
- Diabetes 71, especially uncontrolled diabetes 72
- Human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (AIDS) 73
- High blood pressure (hypertension) 74
- Infertility in both men and women 75
- Systemic lupus erythematosus 76
- Psychosis associated with schizophrenia and bipolar disorder 77
- Multiple sclerosis 78
- Neurodegenerative disorders 79
- Parkinson’s disease 49
Glutathione uses
Glutathione is primarily available as oral formulations (pills, solutions, sublingual tablets, syrups and sprays) and parenteral formulations (intravenous and intramuscular). Glutathione has also been administered by intranasal and intrabronchial routes as well.
Oral glutathione is derived from torula yeast (Candida utilis). It is marketed as a food or dietary supplement, either alone, or in combination with vitamin C, alpha lipoic acid and other antioxidants. In humans, the effectiveness of an oral supplementation with glutathione is very controversial due to the poor absorption by the oral route manly due to the activity of the intestinal enzymes, which degrades glutathione 80. Oral glutathione did not significantly increase the glutathione level in the blood, as demonstrated in oral glutathione supplementation studies 80. A single-dose study conducted by Witschi et al. 81 where 7 healthy volunteers reported no significant increase in plasma glutathione levels for up to 270 minutes. However, Hagen and Jones 82 reported an increase in plasma glutathione levels in four out of five subjects after a single oral dose of 15 mg/kg body weight. In that study, the plasma glutathione levels increased to 300% of baseline levels after one hour, followed by a decrease to approximately 200% of baseline levels within the next three hours. The inadequate absorption of glutathione in humans compared to that in rats has been attributed to a higher hepatic gamma-glutamyl transferase activity in humans. This results in increased hydrolysis of glutathione with resultant low serum levels 83.
A randomized, double-blind, placebo-controlled study on oral glutathione supplementation (500 mg twice daily for four weeks) in 40 healthy adult volunteers failed to show any significant change in serum glutathione levels 84. Another randomized, double-blinded, placebo-controlled trial was conducted in 54 adults which administered oral glutathione for six months, either in a dose of 250 mg or 1000 mg per day 85. Results showed a steady increase in glutathione levels when compared to the baseline 85. There were higher levels in the high-dose group (30–35% increase vs 17% increase in the low-dose group). The raised levels returned to baseline after a one-month washout period 85. In another study 86, glutathione administered at a single dose of 50 mg/kg body weight led to a considerable increase of protein-bound glutathione levels in plasma but not of the deproteinized fraction, measured after two hours of supplementation. Since intracellular glutathione levels can increase only after its amino acid components are transported through the cell membrane after deproteinization, the results of this study remain inconclusive.
In summary, human trials performed before 2013 have shown that over-the-counter oral glutathione supplementation has a negligible effect on raising plasma levels in humans. The only trials that support the concept of oral supplementation to raise glutathione levels in healthy adults have been conducted by Richie et al. 85 and Park et al. 86. It is important to take note of the fact that both studies used a specific brand of glutathione, manufactured by the trial funding company. Therefore, the evidence for the clinically efficacious bioavailability of oral glutathione in humans remains scarce and controversial.
Glutathione has received “Generally Recognized as Safe” (GRAS) status from the US Food and Drug Administration (FDA) for use as food ingredients in food products at levels ranging from 5 to 742.5 milligrams per serving 87. Glutathione as food ingredient is not intended to be used in any meat or meat-containing products 87. There is no restriction on the glutathione availability in United States, Philippines, Japan and India.
There is growing evidence that dysfunctional glutathione homeostasis is implicated in the cause of several diseases. The most well-known conditions associated with glutathione depletion include neurodegenerative disease such as Parkinson’s disease 88, 89, pulmonary diseases 90, liver disease such as liver cirrhosis 91, immune disorder in HIV disease 92, cardiovascular diseases 93, 94 as well as the aging process itself 95. Several studies showed that plasma glutathione levels decrease with age. This deterioration of glutathione homeostasis could participate, with other physiological events, in the ageing process and the appearance of age-related diseases 96, 97. Therefore, dietary supplementation with glutathione has been studied extensively as a potential way to prevent these diseases by countering the negative effects of oxidative stress.
Glutathione clinical uses include the prevention of oxygen toxicity in hyperbaric oxygen therapy, treatment of lead and other heavy metal poisoning, lowering of the toxicity of chemotherapy and radiation in cancer treatments, and reversal of cataracts 23.
Glutathione supplementation has been evaluated in clinical trials in various formulations including oral, intravenous (IV), topical, intranasal, and nebulized for its effects on HIV, Parkinson disease, Alzheimer’s disease, autism, cystic fibrosis, and cardiovascular diseases, among other conditions. N-acetylcysteine (NAC), as the precursor to glutathione, has demonstrated efficacy in raising glutathione levels and is frequently chosen for this purpose.
The oral glutathione formulation has shown mixed results, with some data suggesting it does not increase red blood cell glutathione and other data showing efficacy. Liposomal formulations of glutathione may confer better effects, but further research is needed. N-acetylcysteine (NAC), as the precursor to glutathione, has demonstrated efficacy in raising glutathione levels. In a clinical trial of children with cystic fibrosis, oral reduced glutathione 65 mg/kg/day (divided into 3 doses per day at mealtimes) was administered for 6 months 98. Oral reduced glutathione significantly improves measures of growth status and gut inflammation in cystic fibrosis 98.
A small clinical study in Parkinson’s disease patients used intravenous (IV) glutathione at a dosage of 1,400 mg 3 times per week for 4 weeks 99. There were no significant differences in changes in Unified Parkinson’s Disease Rating Scale (UPDRS) scores between intravenous (IV) glutathione 1,400 mg or placebo 99.
Glutathione for skin
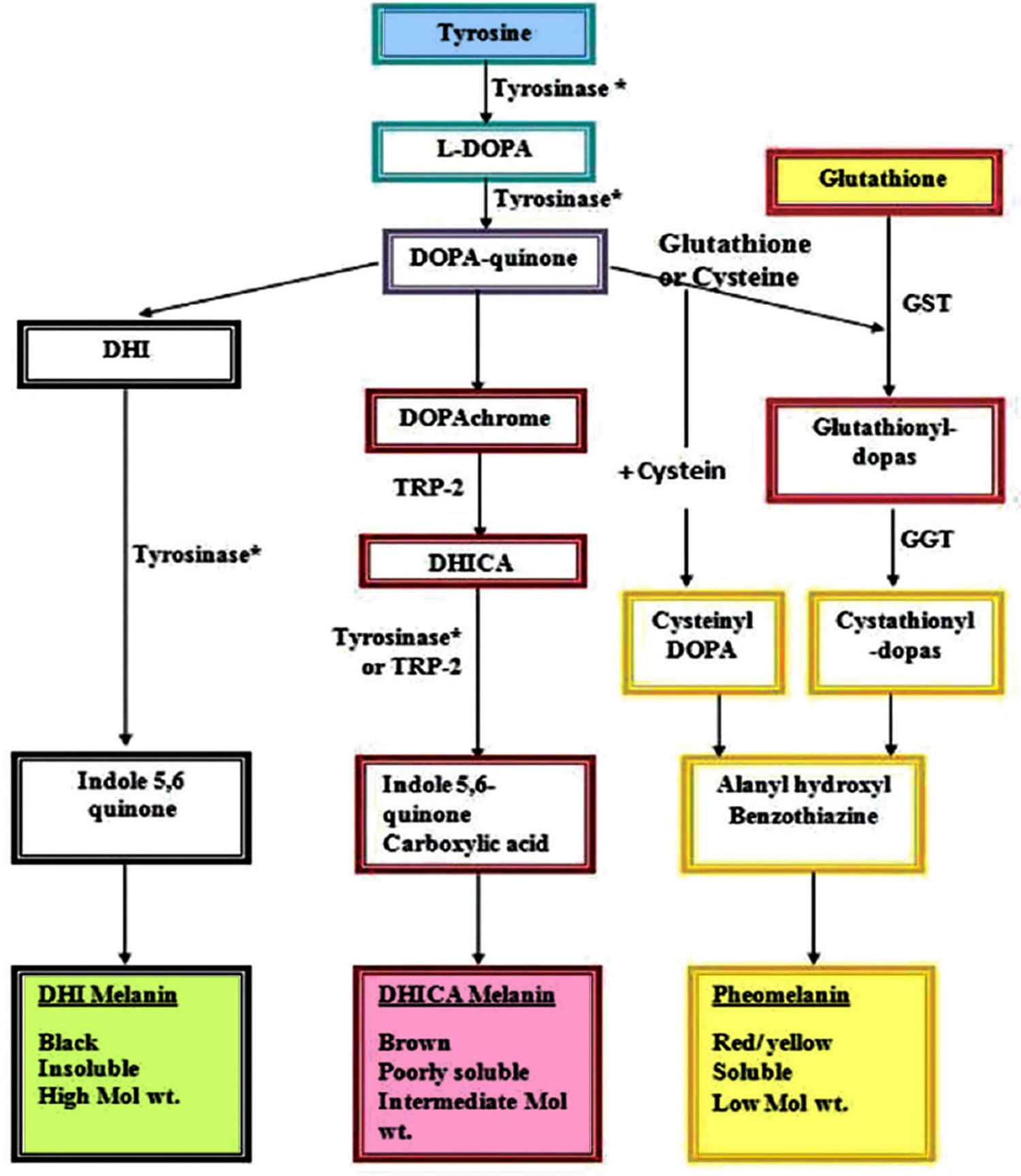
The role of glutathione as a skin-lightening agent was an accidental discovery when skin lightening was noticed as a side effect of large doses of glutathione 100. Various mechanisms for the hypopigmentary effect of glutathione have been proposed, with inhibition of tyrosinase being the most important 63. Glutathione can reduce tyrosinase activity in three different ways 101. Tyrosinase is directly inhibited through chelation of the copper site by the thiol group. Secondly, glutathione interferes with the cellular transfer of tyrosinase to premelanosomes, a prerequisite for melanin synthesis 101. Thirdly, tyrosinase inhibition is effected indirectly via its antioxidant effect. Melanin in human skin is a polymer of various indole compounds synthesized from L-tyrosine by the Raper–Mason pathway of melanogenesis with tyrosinase being the rate limiting enzyme (Figure 6). The ratio of the two different types of melanin found in skin, black-brown colored eumelanin and yellow-red pheomelanin, determines the skin color 102. An increased proportion of pheomelanin is associated with lighter skin color. Glutathione shifts the production of melanin (melanogenesis) from eumelanin to pheomelanin synthesis by reactions between thiol groups and dopaquinone leading to the formation of sulfhydryl-dopa conjugates 103.
Exposure to ultraviolet (UV) radiation is the most important factor that causes undesirable hyperpigmentation. The crucial cellular event is enhanced tyrosinase activity. Exposure to ultraviolet (UV) radiation results in generation of excessive amounts of reactive oxygen and nitrogen species within the cells 104, 105. Oral antioxidants partially reduce melanogenesis by suppressing these free radicals.
Glutathione has potent antioxidant properties and glutathione has been suggested to possess antimelanogenic properties. The free radical scavenging effect of glutathione blocks the induction of tyrosinase activity caused by peroxides 103. Glutathione has been shown to scavenge ultraviolet radiation induced reactive oxygen species generated in epidermal cells 106. A recent study on melasma (a common acquired skin disorder that presents as a bilateral, blotchy, brownish facial pigmentation) patients noted significantly higher levels of glutathione-peroxidase enzyme in patients compared to controls, confirming the role of oxidative stress in melasma 107. Based on these observations, the potential of glutathione as an depigmenting agent in management of melasma and hyperpigmentation seems plausible 108. In the Philippines, glutathione is claimed to produce “magical skin whitening” 109.
The three major routes of glutathione administration used for skin lightening are topical (creams, face washes), oral (capsules and sublingual/buccal tablets) and intravenous injections. Glutathione has also become available in the form of soaps, face washes and creams. Recently, a glutathione based chemical peel has been launched. Although evidence of efficacy is lacking, the manufacturers claim improvement of melasma, hyperpigmentation and skin ageing. Experts including the Food and Drug Administration of the Philippines warned against the to use intravenous glutathione for skin lightening due to the increased risk of adverse events 110, 111. The Food and Drug Administration of the Philippines clearly states the following in an advisory: “Side effects on the use of injectable glutathione for skin lightening include toxic effects on the liver, kidneys, and nervous system. Also of concern is the possibility of Stevens Johnson Syndrome” 110. It is advisable to avoid parenteral (IV) glutathione until more is known about the pharmacodynamics of parenterally administered glutathione. This becomes especially relevant when there are superior options like administration by the orobuccal route, whereby high serum levels are rapidly achieved 112, 113, 114. The orobuccal route has not been seriously considered as a widely prescribed option for skin lightening treatments 115. The effectiveness of the orobuccal route for glutathione administration in rapidly achieving high serum concentrations is well documented 115. The orobuccal administration of glutathione can be standardized by using the hydroxypropyl cellulose oral dispersible film 115. Thirdly, on the basis of published and empirical data, a dose of 100 to 400±50 mg per day, in single or divided doses, for periods of between 10 and 12 weeks can be administered by the orobuccal route, preferably using the oral dispersible film, depending upon severity of hyperpigmentation 115. This treatment advice is based on consideration of all aspects of glutathione absorption and its safety, until results of a more comprehensive trial become available.
Figure 6. Melanin synthesis (Raper–Mason pathway)
Footnote: Role of glutathione in shifting the equation of melanin synthesis from eumelanin to pheomelanin.
Abbreviations: DHI = 5,6-dihydroxyindole; L-DOPA = LevoDOPA; DOPA = 3,4-dihydroxyphenyl alanine; DHICA = 5,6-dihydroxyindole-2-carboxylic acid; GST = glutathione-S-transferase; GGT = gamma glutamyl transpeptidase; TRP2 = tyrosinase-related protein 2.
[Source 115 ]Topical glutathione
Glutathione is commercially available as face washes, solutions and creams. A randomized, double-blind, placebo-controlled clinical trial conducted by Watanabe et al. 104 in 30 healthy Filipino women aged 30–50 years has provided some evidence favouring the efficacy of topical 2% oxidized glutathione (GSSG) lotion in temporary skin lightening 104. Patients were randomized to apply glutathione as 2% oxidized glutathione (GSSG) lotion and a placebo lotion in a split-face protocol, twice daily for ten weeks. GSSG was preferred over GSH, as GSH is unstable in aqueous solutions. GSSG eventually generates GSH after cutaneous absorption. The changes in the melanin index, moisture content of the stratum corneum, skin smoothness, skin elasticity and wrinkle formation were objectively assessed. The reduction of the melanin index with glutathione was statistically significant when compared to placebo 104. Glutathione treated areas had significant improvement in other parameters as well. Topical oxidized glutathione (GSSG) is safe and effective in whitening the skin and improves skin condition in healthy women with no adverse drug effects were reported 104. However, the results of these studies need to be interpreted with caution owing to certain limitations in their study design.
Oral glutathione
Arjinpathana and Asawanonda 116 conducted a randomized double-blind placebo-controlled study on 60 healthy Thai medical students who were given oral glutathione (500 mg/day of glutathione capsule in two divided doses) and studied over a four-week period. They showed some reduction in melanin indices at all the six sites evaluated in the glutathione group subjects, with a statistically significant reduction over placebo at two sites, but the study did not measure blood levels of glutathione. Handog et al 113, in a single-arm study administered glutathione as lozenges (500 mg/day of glutathione in two divided doses) for oral absorption in 30 healthy Filipino women (aged 22–42 years) with Fitzpatrick skin types IV or V over an eight-week period and showed a significant reduction in melanin index at both sun-exposed and sun-protected sites in all the subjects and moderate skin lightening observed by 90% of the subjects on global evaluation. The major limitations of these studies included: small sample size, cohort consisting of healthy volunteers, extremely short study period with an even shorter follow-up, and lack of measurement of blood levels of glutathione 117, 104, 113.
Bruggeman et al 118 conducted a placebo controlled trial using an orobuccal route for glutathione absorption thereby seeking to increase blood levels. Patients were given a solution with 200 mg glutathione or placebo. The solution was held in the mouth for 90 seconds and then swallowed. Blood samples were collected and glutathione levels determined. They found that glutathione absorption from oral mucosa massively and rapidly increased serum concentration. Campolo et al 119 conducted a randomized placebo-controlled trial on 16 male subjects with cardiovascular risk factors. They treated the subjects with 200 mg per day of a sublingual formulation of glutathione 119. They studied peripheral vascular function, liver function, lipid profile and oxidative stress on the subjects after four weeks of treatment. They found that sublingual administration of glutathione significantly increased the blood levels and after four weeks of treatment, beneficial therapeutic effects were discernible 119. They did not study the effects on skin pigmentation, but this study did reaffirm the rapid absorption of reduced glutathione (GSH) from oral mucosa to reach high serum concentrations which can have therapeutic value in many areas.
Safety of glutathione (GSH) when taken by the orobuccal route for an indefinite period of time, needs to be determined in a clinical trial especially using the oral dispersible film formulation.
Glutathione mesotherapy (injection into the subcutaneous fat tissue)
Despite the lack of published literature on the efficacy and methodology of using glutathione solution as mesotherapy (injection via a very fine needle into the subcutaneous fat tissue in the area selected), it is widely practiced by dermatologists for the treatment of melasma and other facial melanosis (abnormal deposition of melanins). Glutathione mesotherapy is used as monotherapy, or in combination with vitamin C (ascorbic acid), vitamin E, tranexamic acid, etc. Although the results are claimed to be very good, use of glutathione as mesotherapy needs more evidence and published data 63.
Glutathione dosage
Dosage of glutathione depends on the uses. Glutathione may be used for treatment of diverse conditions including autism, inborn errors of metabolism, male infertility, cystic fibrosis to improve airway clearance, atherosclerosis, diabetes, immune-stimulation, liver diseases, memory loss, Parkinson’s disease, Alzheimer’s disease, melasma, hyperpigmentation, etc (see above in Health benefits of glutathione). Three commonly used routes for administration of glutathione are parenteral (intravenous and intramuscular), topical, and oral (pills, solutions, sublingual tablets, syrups and sprays). Glutathione has also been administered by intranasal and intrabronchial routes as well. Bioavailability of each route is different.
In a study on the skin whitening effects of glutathione, Weschawalit et al 120 administered 250 mg glutathione daily, both reduced glutathione and oxidized GSSG forms, to 60 volunteers for 12 weeks showing a good depigmenting effect without any adverse effects. Since absorption of glutathione from the gastrointestinal tract is poor, the dose can be lower when administered in an orobuccal formulation. Absorption by this route is significantly higher as recent evidence suggests 112, 114, 121, 113. Therefore, it is safe to say that glutathione can be administered using the orobuccal route at a dose of 100–400 mg daily, variably depending upon severity of hyperpigmentation, for about 10 to 12 weeks. Safety of glutathione glutathione when taken by the orobuccal route for an indefinite period of time, needs to be determined in a clinical trial especially using the oral dispersible film formulation.
Glutathione side effects
Glutathione is quite nontoxic, its lethal dose being quite high, and it is generally well tolerated 122, 120. No serious adverse events were noted in a clinical study of healthy volunteers using oral glutathione 500 mg twice a day for 4 weeks 84. Increased flatulence and loose stools, flushing, and weight gain were reported 84. Furthermore, no significant changes were observed in biomarkers of oxidative stress, including glutathione status, in this clinical trial of oral glutathione supplementation in healthy adults 84.
However, high doses of glutathione for prolonged periods may cause chronic toxicity and carry some risks like zinc depletion, hypersensitivity, drug interactions, teratogenicity, etc. and this is more likely to happen when administered parenterally in an uncontrolled manner 123, 124, 125, 126.
Intravenous (IV) glutathione 1,400 mg given 3 times per week for 4 weeks in Parkinson’s disease patients was well tolerated in a small (N=21) clinical study 99; however, a case report shows reversible, severe hepatic injury related to IV glutathione 1,200 mg given daily, for a cumulative dose of 36,000 mg in 1 month 127.
Nebulized glutathione caused bronchial hyperreactivity, cough, and breathlessness in patients with mild asthma, possibly due to sulfite formation 128 or lack of buffering 2.
References- Pizzorno J. Glutathione! Integr Med (Encinitas). 2014 Feb;13(1):8-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4684116
- Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009 Mar;390(3):191-214. doi: 10.1515/BC.2009.033
- Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009 Feb-Apr;30(1-2):1-12. doi: 10.1016/j.mam.2008.08.006
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001 Jun 1;30(11):1191-212. doi: 10.1016/s0891-5849(01)00480-4
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711-60. doi: 10.1146/annurev.bi.52.070183.003431
- Anderson M.E. Glutathione: An overview of biosynthesis and modulation. Chem. Biol. Interact. 1998;111–112:1–14. doi: 10.1016/S0009-2797(97)00146-4
- Chasseaud LF. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175-274. doi: 10.1016/s0065-230x(08)60848-9
- Lushchak V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids. 2012;2012:736837. doi: 10.1155/2012/736837
- Glutathione synthetase deficiency. https://medlineplus.gov/genetics/condition/glutathione-synthetase-deficiency
- Forman H.J., Zhang H., Rinna A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006
- Ulrich K., Jakob U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019;140:14–27. doi: 10.1016/j.freeradbiomed.2019.05.035
- McCarty MF, O’Keefe JH, DiNicolantonio JJ. Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection. Ochsner J. 2018 Spring;18(1):81-87. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5855430
- Lu S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008
- Jones D.P., Park Y., Gletsu-Miller N., Liang Y., Yu T., Accardi C.J., Ziegler T.R. Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition. 2011;27:199–205. doi: 10.1016/j.nut.2010.01.014
- Parcell S. Sulfur in human nutrition and applications in medicine. Altern Med Rev. 2002 Feb;7(1):22-44.
- Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol Aspects Med. 2009 Feb-Apr;30(1-2):60-76. doi: 10.1016/j.mam.2008.07.001
- Lenton K.J., Therriault H., Wagner J.R. Analysis of glutathione and glutathione disulfide in whole cells and mitochondria by postcolumn derivatization high-performance liquid chromatography with ortho-phthalaldehyde. Anal. Biochem. 1999;274:125–130. doi: 10.1006/abio.1999.4258
- Meister A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988;263:17205–17208. doi: 10.1016/S0021-9258(19)77815-6
- Josephy P.D., Mannervik B. Molecular Toxicology. 2nd ed. Oxford University Press, Inc.; New York, NY, USA: 2006. Biochemistry of Glutathione; pp. 333–364.
- Townsend D.M., Tew K.D., Tapiero H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003;57:145–155. doi: 10.1016/S0753-3322(03)00043-X
- Grey V, Mohammed SR, Smountas AA, Bahlool R, Lands LC. Improved glutathione status in young adult patients with cystic fibrosis supplemented with whey protein. J Cyst Fibros. 2003 Dec;2(4):195-8. doi: 10.1016/S1569-1993(03)00097-3. Erratum in: J Cyst Fibros. 2004 Mar;3(1):62.
- Atwal PS, Medina CR, Burrage LC, Sutton VR. Nineteen-year follow-up of a patient with severe glutathione synthetase deficiency. J Hum Genet. 2016 Jul;61(7):669-72. doi: 10.1038/jhg.2016.20
- National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 124886, Glutathione. https://pubchem.ncbi.nlm.nih.gov/compound/Glutathione
- Al-Jishi E, Meyer BF, Rashed MS, Al-Essa M, Al-Hamed MH, Sakati N, et al. Clinical, biochemical, and molecular characterization of patients with glutathione synthetase deficiency. Clin Genet. 1999;55:444–449. doi: 10.1034/j.1399-0004.1999.550608.x
- Glutathione synthetase deficiency. https://rarediseases.info.nih.gov/diseases/10047/glutathione-synthetase-deficiency
- Xia H, Ye J, Wang L, Zhu J, He Z. A case of severe glutathione synthetase deficiency with novel GSS mutations. Braz J Med Biol Res. 2018 Jan 11;51(3):e6853. doi: 10.1590/1414-431X20176853
- Larsson A, Ristoff E, Anderson ME. Metabolic and molecular bases of inherited disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2005.
- Ristoff E, Mayatepek E, Larsson A. Long-term clinical outcome in patients with glutathione synthetase deficiency. J Pediatr. 2001 Jul;139(1):79-84. doi: 10.1067/mpd.2001.114480
- Al-Jishi E, Meyer BF, Rashed MS, Al-Essa M, Al-Hamed MH, Sakati N, et al. Clinical, biochemical, and molecular characterization of patients with glutathione synthetase deficiency. Clin Genet. 1999;55:444–449. doi: 10.1034/j.1399-0004.1999.550608.x
- Njalsson R. Glutathione synthetase deficiency. Cell Mol Life Sci. 2005;62:1938–1945. doi: 10.1007/s00018-005-5163-7
- Larsson A, Ristoff E, Anderson ME. Glutathione synthetase deficiency and other disorders of the gamma-glutamyl cycle. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Vogelstein B, Childs B, et al., editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2005.
- Erasmus, E., Mienie, L.J., de Vries, W.N., de Wet, W.J., Carlsson, B. and Larsson, A. (1993), Prenatal analysis in two suspected cases of glutathione synthetase deficiency. J Inherit Metab Dis, 16: 837-843. https://doi.org/10.1007/BF00714275
- Manning, N.J., Davies, N.P., Olpin, S.E., Carpenter, K.H., Smith, M.F., Pollitt, R.J., Duncan, S.L.B., Larsson, A. and Carlsson, B. (1994), Prenatal diagnosis of glutathione synthase deficiency. Prenat. Diagn., 14: 475-478. https://doi.org/10.1002/pd.1970140611
- Simon, E., Vogel, M., Fingerhut, R., Ristoff, E., Mayatepek, E. and Spiekerkötter, U. (2009), Diagnosis of glutathione synthetase deficiency in newborn screening. J Inherit Metab Dis, 32: 269-272. https://doi.org/10.1007/s10545-009-1213-x
- Ben Ameur S, Aloulou H, Nasrallah F, Kamoun T, Kaabachi N, Hachicha M. Hemolytic anemia and metabolic acidosis: think about glutathione synthetase deficiency. Fetal Pediatr Pathol. 2015 Feb;34(1):18-20. doi: 10.3109/15513815.2014.947543
- Spielberg SP, Boxer LA, Corash LM, Schulman JD. Improved erythrocyte survival with high-dose vitamin E in chronic hemolyzing G6PD and glutathione synthetase deficiencies. Ann Intern Med. 1979 Jan;90(1):53-4. doi: 10.7326/0003-4819-90-1-53
- Njålsson R, Ristoff E, Carlsson K, Winkler A, Larsson A, Norgren S. Genotype, enzyme activity, glutathione level, and clinical phenotype in patients with glutathione synthetase deficiency. Hum Genet. 2005 Apr;116(5):384-9. doi: 10.1007/s00439-005-1255-6
- Tinggi U. Selenium: its role as antioxidant in human health. Environ Health Prev Med. 2008 Mar;13(2):102-8. doi: 10.1007/s12199-007-0019-4
- Pejaver, R.K. and Watson, A.H. (1992), 5-Oxoprolinuria due to glutathione synthetase deficiency. J Inherit Metab Dis, 15: 937-938. https://doi.org/10.1007/BF01800239
- Boxer LA, Oliver JM, Spielberg SP, Allen JM, Schulman JD. Protection of granulocytes by vitamin E in glutathione synthetase deficiency. N Engl J Med. 1979 Oct 25;301(17):901-5. doi: 10.1056/NEJM197910253011702
- Pejaver, R.K. and Watson, A.H. (1994), High-dose vitamin E therapy in glutathione synthetase deficiency. J Inherit Metab Dis, 17: 749-750. https://doi.org/10.1007/BF00712019
- Minich DM, Brown BI. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients. 2019 Sep 3;11(9):2073. doi: 10.3390/nu11092073
- Jariwalla RJ, Lalezari J, Cenko D, Mansour SE, Kumar A, Gangapurkar B, Nakamura D. Restoration of blood total glutathione status and lymphocyte function following alpha-lipoic acid supplementation in patients with HIV infection. J Altern Complement Med. 2008 Mar;14(2):139-46. doi: 10.1089/acm.2006.6397
- Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8
- Sinha R., Sinha I., Calcagnotto A., Trushin N., Haley J.S., Schell T.D., Richie J.P., Jr. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur. J. Clin. Nutr. 2018;72:105–111. doi: 10.1038/ejcn.2017.132
- Park E.Y., Shimura N., Konishi T., Sauchi Y., Wada S., Aoi W., Nakamura Y., Sato K. Increase in the protein-bound form of glutathione in human blood after the oral administration of glutathione. J. Agric. Food Chem. 2014;62:6183–6189. doi: 10.1021/jf501338z
- Richie J.P., Jr., Nichenametla S., Neidig W., Calcagnotto A., Haley J.S., Schell T.D., Muscat J.E. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur. J. Nutr. 2015;54:251–263. doi: 10.1007/s00394-014-0706-z
- Sekhar R.V., Patel S.G., Guthikonda A.P., Reid M., Balasubramanyam A., Taffet G.E., Jahoor F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am. J. Clin. Nutr. 2011;94:847–853. doi: 10.3945/ajcn.110.003483
- Coles L.D., Tuite P.J., Öz G., Mishra U.R., Kartha R.V., Sullivan K.M., Cloyd J.C., Terpstra M. Repeated-dose oral N-Acetylcysteine in Parkinson’s disease: Pharmacokinetics and effect on brain glutathione and oxidative stress. J. Clin. Pharmacol. 2018;58:158–167. doi: 10.1002/jcph.1008
- Skvarc D.R., Dean O.M., Byrne L.K., Gray L., Lane S., Lewis M., Fernanders B.S., Berk M., Marriott A. The effect of N-acetylcysteine (NAC) on human cognition—A systematic review. Neurosci. Biobehav. Rev. 2017;78:44–56. doi: 10.1016/j.neubiorev.2017.04.013
- Lin C.Y., Wu J.L., Shih T.S., Tsai P.J., Sun Y.M., Ma M.C., Guo Y.L. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res. 2010;269:42–47. doi: 10.1016/j.heares.2010.07.005
- Gaby A. 51: N-Acetylcysteine. In: Concord N.H., editor. Nutritional Medicine. Fritz Perlberg Publishing; Concord, NH, USA: 2011.
- Duffy S.L., Lagopoulos J., Cockayne N., Lewis S.J., Hickie I.B., Hermens D.F., Naismith S.L. The effect of 12-wk ω-3 fatty acid supplementation on in vivo thalamus glutathione concentration in patients “at risk” for major depression. Nutrition. 2015;31:1247–1254. doi: 10.1016/j.nut.2015.04.019
- García-Rodríguez C.E., Mesa M.D., Olza J., Vlachava M., Kremmyda L.S., Diaper N.D., Noakes P.S., Miles E.A., Ramírez-Tortosa M.C., Liaset B., et al. Does consumption of two portions of salmon per week enhance the antioxidant defense system in pregnant women? Antioxid. Redox. Signal. 2012;16:1401–1406. doi: 10.1089/ars.2012.4508
- Richie J.P., Jr., Muscat J.E., Ellison I., Calcagnotto A., Kleinman W., El-Bayoumy K. Association of selenium status and blood glutathione concentrations in blacks and whites. Nutr. Cancer. 2011;63:367–375. doi: 10.1080/01635581.2011.535967
- Lenton K.J., Sané A.T., Therriault H., Cantin A.M., Payette H., Wagner J.R. Vitamin C augments lymphocyte glutathione in subjects with ascorbate deficiency. Am. J. Clin. Nutr. 2003;77:189–195. doi: 10.1093/ajcn/77.1.189
- Johnston C.S., Meyer C.G., Srilakshmi J.C. Vitamin C elevates red blood cell glutathione in healthy adults. Am. J. Clin. Nutr. 1993;58:103–105. doi: 10.1093/ajcn/58.1.103
- Taghizadeh M., Tamtaji O.R., Dadgostar E., Daneshvar Kakhaki R., Bahmani F., Abolhassani J., Aarabi M.H., Kouchaki E., Memarzadeh M.R., Asemi Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017;108:183–189. doi: 10.1016/j.neuint.2017.03.014
- Jain S.K., McVie R., Smith T. Vitamin E supplementation restores glutathione and malondialdehyde to normal concentrations in erythrocytes of type 1 diabetic children. Diabetes Care. 2000;23:1389–1394. doi: 10.2337/diacare.23.9.1389
- Bumrungpert A., Pavadhgul P., Nunthanawanich P., Sirikanchanarod A., Adulbhan A. Whey protein supplementation improves nutritional status, glutathione levels, and immune function in cancer patients: A randomized, double-blind controlled trial. J Med. Food. 2018;21:612–616. doi: 10.1089/jmf.2017.4080
- Potęga A. Glutathione-Mediated Conjugation of Anticancer Drugs: An Overview of Reaction Mechanisms and Biological Significance for Drug Detoxification and Bioactivation. Molecules. 2022 Aug 17;27(16):5252. doi: 10.3390/molecules27165252
- Hall MN, Niedzwiecki M, Liu X, Harper KN, Alam S, Slavkovich V, Ilievski V, Levy D, Siddique AB, Parvez F, Mey JL, van Geen A, Graziano J, Gamble MV. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ Health Perspect. 2013 Sep;121(9):1068-74. doi: 10.1289/ehp.1205727
- Sonthalia S, Daulatabad D, Sarkar R. Glutathione as a skin whitening agent: Facts, myths, evidence and controversies. Indian J Dermatol Venereol Leprol. 2016 May-Jun;82(3):262-72. https://ijdvl.com/glutathione-as-a-skin-whitening-agent-facts-myths-evidence-and-controversies/
- Franco R., Schoneveld O.J., Pappa A., Panayiotidis M.I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198
- Lang C.A., Mills B.J., Lang H.L., Liu M.C., Usui W.M., Richie J., Jr., Mastropaolo W., Murrell S.A. High blood glutathione levels accompany excellent physical and mental health in women ages 60 to 103 years. J Lab. Clin. Med. 2002;140:413–417. doi: 10.1067/mlc.2002.129504
- Saharan S., Mandal P.K. The emerging role of glutathione in Alzheimer’s disease. J. Alzheimers Dis. 2014;40:519–529. doi: 10.3233/JAD-132483
- Traverso N., Ricciarelli R., Nitti M., Marengo B., Furfaro A.L., Pronzato M.A., Marinari U.M., Domenicotti C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell Longev. 2013;2013:972913. doi: 10.1155/2013/972913
- Czuczejko J, Zachara BA, Staubach-Topczewska E, Halota W, Kedziora J. Selenium, glutathione and glutathione peroxidases in blood of patients with chronic liver diseases. Acta Biochim Pol. 2003;50(4):1147-54. http://www.actabp.pl/pdf/4_2003/1147.pdf
- Rae C.D., Williams S.R. Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Anal. Biochem. 2017;529:127–143. doi: 10.1016/j.ab.2016.12.022
- Kettle A.J., Turner R., Gangell C.L., Harwood D.T., Khalilova I.S., Chapman A.L., Winterbourn C.C., Sly P.D., Arest C.F. Oxidation contributes to low glutathione in the airways of children with cystic fibrosis. Eur. Respir. J. 2014;44:122–129. doi: 10.1183/09031936.00170213
- Achari A.E., Jain S.K. l-Cysteine supplementation increases insulin sensitivity mediated by upregulation of GSH and adiponectin in high glucose treated 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2017;630:54–65. doi: 10.1016/j.abb.2017.07.016
- Sekhar R.V., McKay S.V., Patel S.G., Guthikonda A.P., Reddy V.T., Balasubramanyam A., Jahoor F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2011;34:162–167. doi: 10.2337/dc10-1006
- Nguyen D., Hsu J.W., Jahoor F., Sekhar R.V. Effect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J. Clin. Endocrinol. Metab. 2014;99:169–177. doi: 10.1210/jc.2013-2376
- Robaczewska J, Kedziora-Kornatowska K, Kozakiewicz M, Zary-Sikorska E, Pawluk H, Pawliszak W, Kedziora J. Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J Physiol Pharmacol. 2016 Jun;67(3):331-7. https://www.jpp.krakow.pl/journal/archive/06_16/pdf/331_06_16_article.pdf
- Adeoye O., Olawumi J., Opeyemi A., Christiania O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist. Reprod. 2018;22:61–66. doi: 10.5935/1518-0557.20180003
- Shah D., Sah S., Nath S.K. Interaction between glutathione and apoptosis in systemic lupus erythematosus. Autoimmun. Rev. 2013;12:741–751. doi: 10.1016/j.autrev.2012.12.007
- Nucifora L.G., Tanaka T., Hayes L.N., Kim M., Lee B.J., Matsuda T., Nucifora F.C., Jr., Sedlak T., Mojtabai R., Eaton W., et al. Reduction of plasma glutathione in psychosis associated with schizophrenia and bipolar disorder in translational psychiatry. Transl. Psychiatry. 2017;7:e1215. doi: 10.1038/tp.2017.178
- Carvalho A.N., Lim J.L., Nijland P.G., Witte M.E., Van Horssen J. Glutathione in multiple sclerosis: More than just an antioxidant? Mult. Scler. 2014;20:1425–1431. doi: 10.1177/1352458514533400
- Aoyama K., Nakaki T. Impaired glutathione synthesis in neurodegeneration. Int. J. Mol. Sci. 2013;14:21021–21044. doi: 10.3390/ijms141021021
- Schmitt B, Vicenzi M, Garrel C, Denis FM. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015 Dec;6:198-205. doi: 10.1016/j.redox.2015.07.012
- Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43(6):667-9. doi: 10.1007/BF02284971
- Hagen TM, Jones DP. Role of glutathione in extrahepatic detoxification. In: Sakamoto Y, Higashi T, Taniguchi N, Meister A, editors. Glutathione Centennial: Molecular and Clinical Implications. New York Academic Press; 1989. p. 423-33.
- Hagen TM, Wierzbicka GT, Bowman BB, Aw TY, Jones DP. Fate of dietary glutathione: disposition in the gastrointestinal tract. Am J Physiol. 1990 Oct;259(4 Pt 1):G530-5. doi: 10.1152/ajpgi.1990.259.4.G530
- Allen J, Bradley RD. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J Altern Complement Med. 2011 Sep;17(9):827-33. doi: 10.1089/acm.2010.0716
- Richie JP Jr, Nichenametla S, Neidig W, Calcagnotto A, Haley JS, Schell TD, Muscat JE. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur J Nutr. 2015 Mar;54(2):251-63. doi: 10.1007/s00394-014-0706-z
- Park EY, Shimura N, Konishi T, Sauchi Y, Wada S, Aoi W, Nakamura Y, Sato K. Increase in the protein-bound form of glutathione in human blood after the oral administration of glutathione. J Agric Food Chem. 2014 Jul 2;62(26):6183-9. doi: 10.1021/jf501338z
- Glutathione. GRN No. 293. https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=grasnotices&id=293
- Cruz R, Almaguer Melian W, Bergado Rosado JA. El glutatión en la función cognitiva y la neurodegeneración [Glutathione in cognitive function and neurodegeneration]. Rev Neurol. 2003 May 1-15;36(9):877-86. Spanish.
- Smeyne M, Smeyne RJ. Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med. 2013 Sep;62:13-25. doi: 10.1016/j.freeradbiomed.2013.05.001
- Gul M, Kutay FZ, Temocin S, Hanninen O. Cellular and clinical implications of glutathione. Indian J Exp Biol. 2000 Jul;38(7):625-34.
- Loguercio C, Taranto D, Vitale LM, Beneduce F, Del Vecchio Blanco C. Effect of liver cirrhosis and age on the glutathione concentration in the plasma, erythrocytes, and gastric mucosa of man. Free Radic Biol Med. 1996;20(3):483-8. doi: 10.1016/0891-5849(96)02057-6
- Herzenberg LA, De Rosa SC, Dubs JG, Roederer M, Anderson MT, Ela SW, Deresinski SC, Herzenberg LA. Glutathione deficiency is associated with impaired survival in HIV disease. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):1967-72. doi: 10.1073/pnas.94.5.1967
- Giugliano D, Ceriello A, Paolisso G. Diabetes mellitus, hypertension, and cardiovascular disease: which role for oxidative stress? Metabolism. 1995 Mar;44(3):363-8. doi: 10.1016/0026-0495(95)90167-1
- Usal A, Acartürk E, Yüregir GT, Unlükurt I, Demirci C, Kurt HI, Birand A. Decreased glutathione levels in acute myocardial infarction. Jpn Heart J. 1996 Mar;37(2):177-82. doi: 10.1536/ihj.37.177
- Viña J, Sastre J, Anton V, Bruseghini L, Esteras A, Asensi M. Effect of aging on glutathione metabolism. Protection by antioxidants. EXS. 1992;62:136-44. doi: 10.1007/978-3-0348-7460-1_14
- Jones DP, Mody VC Jr, Carlson JL, Lynn MJ, Sternberg P Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002 Nov 1;33(9):1290-300. doi: 10.1016/s0891-5849(02)01040-7
- Thomas NO, Shay KP, Kelley AR, Butler JA, Hagen TM. Glutathione maintenance mitigates age-related susceptibility to redox cycling agents. Redox Biol. 2016 Dec;10:45-52. doi: 10.1016/j.redox.2016.09.010
- Visca A, Bishop CT, Hilton S, Hudson VM. Oral reduced L-glutathione improves growth in pediatric cystic fibrosis patients. J Pediatr Gastroenterol Nutr. 2015 Jun;60(6):802-10. doi: 10.1097/MPG.0000000000000738
- Hauser, R.A., Lyons, K.E., McClain, T., Carter, S. and Perlmutter, D. (2009), Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson’s disease. Mov. Disord., 24: 979-983. https://doi.org/10.1002/mds.22401
- Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002 Nov;973:488-504. https://doi.org/10.1111/j.1749-6632.2002.tb04690.x
- Yamamura T, Onishi J, Nishiyama T. Antimelanogenic activity of hydrocoumarins in cultured normal human melanocytes by stimulating intracellular glutathione synthesis. Arch Dermatol Res. 2002 Nov;294(8):349-54. doi: 10.1007/s00403-002-0345-8
- Nordlund JJ, Boissy RE. The biology of melanocytes. In: Freinkel RK, Woodley DT, editors. The Biology of the Skin. New York: CRC Press; 2001. p. 113-30.
- Karg E, Odh G, Wittbjer A, Rosengren E, Rorsman H. Hydrogen peroxide as an inducer of elevated tyrosinase level in melanoma cells. J Invest Dermatol. 1993 Feb;100(2 Suppl):209S-213S.
- Watanabe F, Hashizume E, Chan GP, Kamimura A. Skin-whitening and skin-condition-improving effects of topical oxidized glutathione: a double-blind and placebo-controlled clinical trial in healthy women. Clin Cosmet Investig Dermatol. 2014 Oct 17;7:267-74. doi: 10.2147/CCID.S68424
- Masaki H. Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci. 2010 May;58(2):85-90. doi: 10.1016/j.jdermsci.2010.03.003
- Maeda K, Hatao M. Involvement of photooxidation of melanogenic precursors in prolonged pigmentation induced by ultraviolet A. J Invest Dermatol. 2004 Feb;122(2):503-9. doi: 10.1046/j.0022-202X.2004.22223.x
- Seçkin HY, Kalkan G, Baş Y, Akbaş A, Önder Y, Özyurt H, Sahin M. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014 Sep;33(3):212-7. doi: 10.3109/15569527.2013.834496
- Villarama, C.D. and Maibach, H.I. (2005), Glutathione as a depigmenting agent: an overview. International Journal of Cosmetic Science, 27: 147-153. https://doi.org/10.1111/j.1467-2494.2005.00235.x
- Sonthalia S, Jha AK, Lallas A, Jain G, Jakhar D. Glutathione for skin lightening: a regnant myth or evidence-based verity? Dermatol Pract Concept. 2018 Jan 31;8(1):15-21. doi: 10.5826/dpc.0801a04
- FDA Advisory No. 2019-182. Unsafe use of glutathione as skin lightening agent. https://www.fda.gov.ph/fda-advisory-no-2019-182-unsafe-use-of-glutathione-as-skin-lightening-agent/
- Juhasz, MLW, Levin, MK. The role of systemic treatments for skin lightening. J Cosmet Dermatol. 2018; 17: 1144– 1157. https://doi.org/10.1111/jocd.12747
- Bruggeman BK, Storo KE, Fair HM, Wommack AJ, Carriker CR, Smoliga JM. The absorptive effects of orobuccal non-liposomal nano-sized glutathione on blood glutathione parameters in healthy individuals: A pilot study. PLoS One. 2019 Apr 30;14(4):e0215815. doi: 10.1371/journal.pone.0215815
- Handog, E.B., Datuin, M.S.L. and Singzon, I.A. (2016), An open-label, single-arm trial of the safety and efficacy of a novel preparation of glutathione as a skin-lightening agent in Filipino women. Int J Dermatol, 55: 153-157. https://doi.org/10.1111/ijd.12999
- Campolo J, Bernardi S, Cozzi L, Rocchiccioli S, Dellanoce C, Cecchettini A, Tonini A, Parolini M, De Chiara B, Micheloni G, Pelosi G, Passino C, Giannattasio C, Parodi O. Medium-term effect of sublingual l-glutathione supplementation on flow-mediated dilation in subjects with cardiovascular risk factors. Nutrition. 2017 Jun;38:41-47. doi: 10.1016/j.nut.2016.12.018
- Sharma DK, Sharma P. Augmented Glutathione Absorption from Oral Mucosa and its Effect on Skin Pigmentation: A Clinical Review. Clin Cosmet Investig Dermatol. 2022 Sep 10;15:1853-1862. doi: 10.2147/CCID.S378470
- Arjinpathana N, Asawanonda P. Glutathione as an oral lightening agent: a randomized, double-blind, placebo controlled study. J Dermatolog Treat. 2012;23:97–102. doi: 10.3109/09546631003801619
- Arjinpathana N, Asawanonda P. Glutathione as an oral whitening agent: a randomized, double-blind, placebo-controlled study. J Dermatolog Treat. 2012 Apr;23(2):97-102. doi: 10.3109/09546631003801619
- Bruggeman BK, Storo KE, Fair HM, Wommack AJ, Carriker CR, Smoliga JM. The absorptive effects of orobuccal non-liposomal nano-sized glutathione on blood glutathione parameters in healthy individuals: a pilot study. PLoS One. 2019;14(4):e0215815. doi: 10.1371/journal.pone.0215815
- Campolo J, Bernardi S, Cozzi L, et al. Medium-term effect of sublingual l-glutathione supplementation on flow-mediated dilation in subjects with cardiovascular risk factors. Nutrition. 2017;38:41–47. doi: 10.1016/j.nut.2016.12.018
- Weschawalit S, Thongthip S, Phutrakool P, Asawanonda P. Glutathione and its antiaging and antimelanogenic effects. Clin Cosmet Investig Dermatol. 2017 Apr 27;10:147-153. doi: 10.2147/CCID.S128339
- Buonocore D, Grosini M, Giardina S, Michelotti A, Carrabetta M, Seneci A, Verri M, Dossena M, Marzatico F. Bioavailability Study of an Innovative Orobuccal Formulation of Glutathione. Oxid Med Cell Longev. 2016;2016:3286365. doi: 10.1155/2016/3286365
- Kovacs-Nolan J, Rupa P, Matsui T, et al. In vitro and ex vivo uptake of glutathione (GSH) across the intestinal epithelium and fate of oral GSH after in vivo supplementation. J Agric Food Chem. 2014;62(39):9499–9506. doi: 10.1021/jf503257w
- Suzuki H, Miki S, Oshima M, Sado T. Chronic toxicity and teratogenicity studies of glutathione sodium salt. Clin Rep. 1972;6:2393.
- Awasthi S, Bajpai KK, Piper JT, Singhal SS, Ballatore A, Seifert WE Jr, Awasthi YC, Ansari GA. Interactions of melphalan with glutathione and the role of glutathione S-transferase. Drug Metab Dispos. 1996 Mar;24(3):371-4. https://dmd.aspetjournals.org/content/24/3/371.long
- Kaplowitz N. Interaction of azathioprine and glutathione in the liver of the rat. J Pharmacol Exp Ther. 1977 Mar;200(3):479-86. https://jpet.aspetjournals.org/content/200/3/479.long
- Kumar A, Singh BK, Ahmad I, Shukla S, Patel DK, Srivastava G, Kumar V, Pandey HP, Singh C. Involvement of NADPH oxidase and glutathione in zinc-induced dopaminergic neurodegeneration in rats: similarity with paraquat neurotoxicity. Brain Res. 2012 Feb 15;1438:48-64. doi: 10.1016/j.brainres.2011.12.028
- Naito, Y., Matsuo, K., Kokubo, Y., Narita, Y. and Tomimoto, H. (2010), Higher-dose glutathione therapy for Parkinson’s disease in Japan: Is it really safe?. Mov. Disord., 25: 962-962. https://doi.org/10.1002/mds.23022
- Reynaert NL. Glutathione biochemistry in asthma. Biochim Biophys Acta. 2011 Nov;1810(11):1045-51. doi: 10.1016/j.bbagen.2011.01.010