Respiratory alkalosis
Respiratory alkalosis is defined as a disease state where the arterial blood pH rises into the alkaline range greater than 7.45 secondary to alveolar hyperventilation or sustained tachypnea or hyperpnea (breathing more deeply with or without an increased rate of breathing) 1, 2, 3. Respiratory alkalosis is the most common acid-base abnormality observed in patients who are critically ill. Respiratory alkalosis is associated with numerous illnesses and is a common finding in patients on mechanical ventilation. In almost every scenario, respiratory alkalosis is induced by a process involving hyperventilation where carbon dioxide (CO2) is breathed away 4, 5, 6. In its normal state, the body maintains carbon dioxide (arterial partial pressure of carbon dioxide [PaCO2]) in a well-controlled range from 35 to 45 mm Hg by balancing its production and elimination. Alveolar hyperventilation leads to a decreased arterial partial pressure of carbon dioxide (PaCO2) below the normal reference range of 35 mmHg 1, 2. The decrease in arterial partial pressure of carbon dioxide (PaCO2) increases the ratio of plasma bicarbonate (HCO3–) concentration to arterial partial pressure of carbon dioxide (PaCO2) and, thereby, increases the pH level 7.
The pH is a number that shows how acidic or alkaline a substance is. A pH of less than 7 is acidic, and greater than 7 is alkaline. The pH of blood is about 7.4 (a slightly alkaline range of 7.35 to 7.45). Your blood pH is highly stable within a normal range of pH 7.35 to 7.45 and it’s tightly regulated by your kidneys and respiratory system. The primary pH buffering system in the human body is the bicarbonate (HCO3–) and carbon dioxide (CO2). Bicarbonate (HCO3–) functions as an alkalotic substance. Carbon dioxide (CO2) functions as an acidic substance. Therefore, an increase in serum bicarbonate (HCO3–) or a decrease in CO2 (carbon dioxide) will make blood more alkaline. The opposite is also true where decreases in bicarbonate (HCO3–) or an increase in carbon dioxide (CO2) will make blood more acidic. The carbon dioxide (CO2) levels are physiologically regulated by the pulmonary system through respiration, whereas the serum bicarbonate (HCO3–) levels are regulated through your kidneys by two mechanisms: bicarbonate [HCO3–] (a base) reclamation mainly in the proximal tubule and bicarbonate [HCO3–] (a base) generation predominantly in the distal nephron. Elevated pH above 7.45 and a reduced arterial partial pressure of carbon dioxide (PaCO2) level below 35 mmHg characterize respiratory alkalosis. When the arterial partial pressure of carbon dioxide (PaCO2) falls below the normal reference range of 35 mmHg, the bicarbonate (HCO3–) which functions as an alkalotic substance is reduced in order to maintain pH to its normal range. Therefore with respiratory alkalosis, due to a reduction in the arterial partial pressure of carbon dioxide (PaCO2), the compensation is to decrease serum bicarbonate (HCO3–) via the kidneys disposing of excess bicarbonate [HCO3–] (a base) and decrease of hydrogen ions (H+) excretion in order to reduce the pH back to a normal range of pH 7.35 to 7.4; however, this metabolic process occurs over the course of days whereas respiratory disease can adjust carbon dioxide (CO2) levels in minutes to hours. Normally, kidney hydrogen ions (H+) excretion into the urine (as ammonium [NH4+] and/or titratable acid) generates bicarbonate (HCO3–) to replace the quantity decomposed by nonvolatile hydrogen ions (H+) derived from dietary intake and metabolism and any bicarbonate (HCO3–) lost in alkaline stool.
To understand acid-base buffering system, it is important to recall that pH is governed by the ratio bicarbonate [HCO3–] (a base)/arterial partial pressure of carbon dioxide (PaCO2) (an acid). So long as the ratio is normal, pH will be normal.
Normal body functions and metabolism generate large quantities of acids that must be neutralized and/or eliminated to maintain blood pH balance. Most of the acid is carbonic acid (H2CO3), which is created from carbon dioxide (CO2) and water (H2O). Carbon dioxide (CO2) is produced as the body uses glucose (sugar) or fat for energy. In its normal state, the body maintains carbon dioxide (arterial partial pressure of carbon dioxide [PaCO2]) in a well-controlled range from 35 to 45 mm Hg by balancing its production and elimination. Lesser quantities of lactic acid, ketoacids, and other organic acids are also produced.
- Carbon dioxide (CO2) + water (H2O) -> H2CO3 (carbonic acid) -> HCO3– + H+
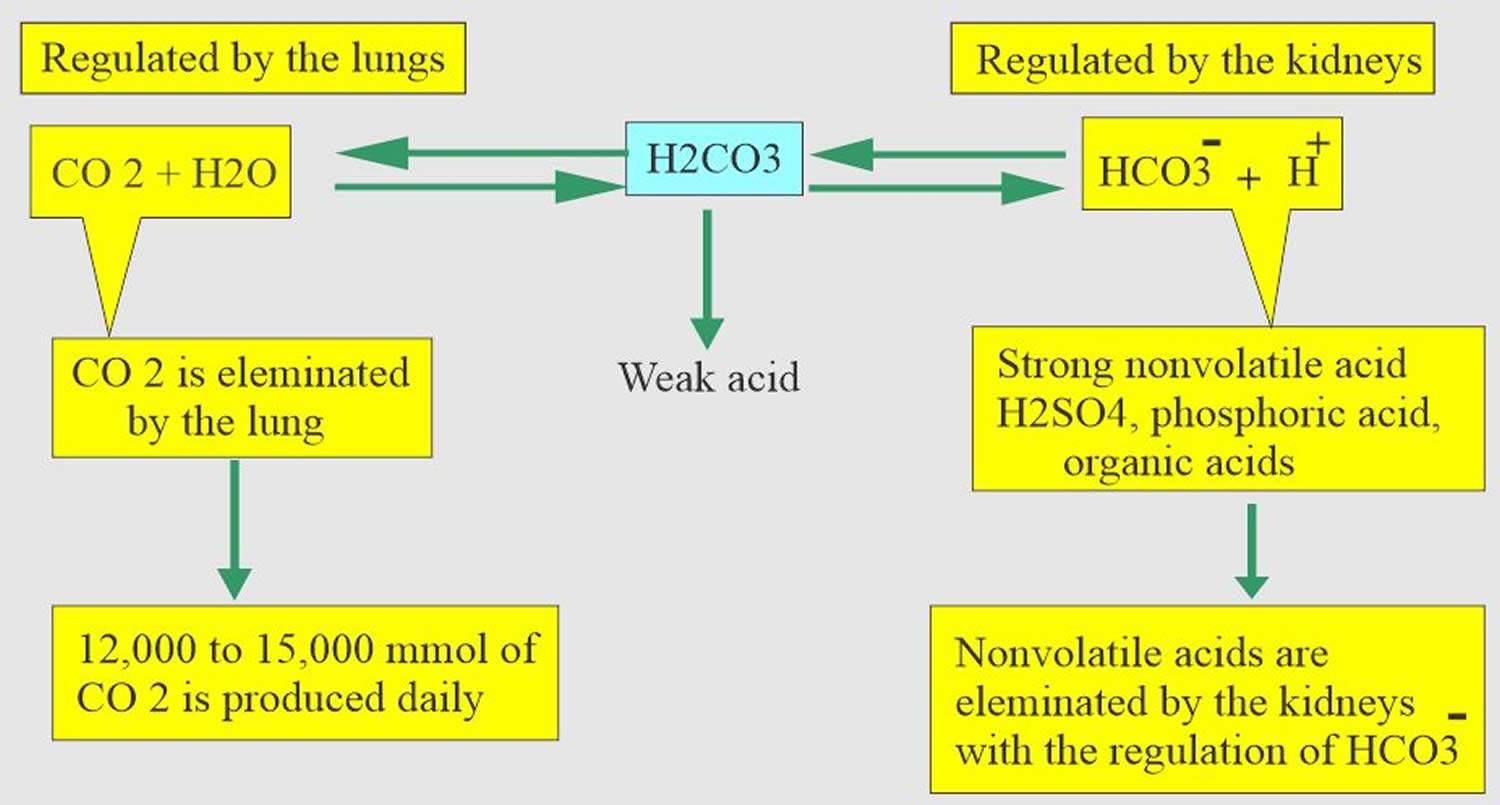
According to the Henderson-Hasselbalch equation (Figure 2), maintaining physiological pH depends on arterial partial pressure of carbon dioxide (PaCO2), which in turn depends on alveolar ventilation (hypoventilation causes acidosis and hyperventilation causes alkalosis). The kidneys participate in maintaining the stable pH by reabsorption of bicarbonate (3,600 mmol of bicarbonate is filtrated in glomeruli during 24 hour) and excretion of hydrogen ions from nonvolatile acids (including sulfur and phosphate) as titratable acidity (0.3 mmol hydrogen ions/kg/day) and in the form of ammonium ion (0.7 mmol hydrogen ions/kg/day) 8, 9.
The lungs and kidneys are the major organs involved in regulating blood pH. And to compensate for the respiratory alkalosis that is due to alveolar hyperventilation, your kidneys will dispose of excess bicarbonate [HCO3–] (a base) 10, 11.
- The lungs flush acid out of your body by exhaling carbon dioxide (CO2). Raising and lowering the respiratory rate alters the amount of carbon dioxide (CO2) that is breathed out, and this can affect blood pH within minutes 2.
- The kidneys excrete acids in the urine, and they regulate the concentration of bicarbonate (HCO3–, a base) in blood. Acid-base changes due to increases or decreases in bicarbonate [HCO3–] concentration occur more slowly than changes in carbon dioxide (CO2), taking hours or days. Bicarbonate (HCO3–) reabsorption occurs in the kidneys in every part of the tubules. About 85–90% of the filtered bicarbonate is reabsorbed in the proximal tubules, 10% in the ascending arms of the Henle loop, 6% in the distal tubules, and 4% in the collecting tubules 8, 9.
Both of these processes are always at work, and they keep the blood pH in healthy people tightly controlled. The absolute quantities of acids or bases are less important than the balance between the two and its effect on blood pH.
Buffering systems that resist changes in pH also contribute to the regulation of acid and base concentrations. The main buffers in blood are hemoglobin (in red blood cells), plasma proteins, carbon dioxide (CO2), bicarbonate (HCO3–) and phosphates.
Carbon dioxide (CO2) plays a remarkable role in the human body mainly through pH regulation of the blood. The pH is the primary stimulus to initiate ventilation. In its normal state, the body maintains carbon dioxide (CO2) in a well-controlled range from 35 to 45 mm Hg by balancing its production and elimination. In a state of hyperventilation (breathing that is too fast or too deep to meet the needs of the body), the body loses more carbon dioxide (CO2) than it can produce, causing a net deficit of carbon dioxide (CO2). The decreased carbon dioxide (CO2) is what leads to an decrease in hydrogen ions (H+) and a slight decrease in bicarbonate (HCO3–), as seen by a right shift in the following equilibrium reaction of carbon dioxide:
- Carbon dioxide (CO2) + water (H2O) -> H2CO3 (carbonic acid) -> HCO3– + H+
The buffer system created by carbon dioxide consists of the following three molecules in equilibrium: carbon dioxide (CO2), H2CO3 (carbonic acid), and bicarbonate (HCO3–). When hydrogen ions (H+) is low, bicarbonate (HCO3–) buffers the high pH. When hydroxide (OH–) is high, H2CO3 (carbonic acid) buffers the high pH.
Respiratory alkalosis can be an acute process or a chronic process. These are determined based on the level of metabolic compensation for the respiratory process 12, 13, 1.
- In acute respiratory alkalosis, the serum pH is alkaline (pH>7.45), the arterial partial pressure of carbon dioxide (PaCO2) level is below the lower limit of normal (PaCO2<35 mm Hg) and the bicarbonate (HCO3–) level is high since there has not been sufficient time to lower the bicarbonate (HCO3–) levels.
- In chronic respiratory alkalosis, the arterial partial pressure of carbon dioxide (PaCO2) level is below the lower limit of normal (PaCO2<35 mm Hg), but the pH level is relatively normal or near normal due to compensatory mechanisms associated with low to normal bicarbonate (HCO3–) levels.
Acute hypocapnia [partial pressure of arterial carbon dioxide (PaCO2) less than 35mmHg] causes a reduction of serum levels of potassium and phosphate secondary to increased intracellular shifts of these ions. A reduction in free serum calcium also occurs. Calcium reduction is secondary to increased binding of calcium to serum albumin due to the change in pH. Many of the symptoms present in persons with respiratory alkalosis are related to hypocalcemia 14. Hyponatremia and hypochloremia may also be present.
Acute hyperventilation with hypocapnia causes a small, early reduction in serum bicarbonate (HCO3–) levels resulting from cellular shift of bicarbonate. Acutely, plasma pH and bicarbonate (HCO3–) concentration vary proportionately with the partial pressure of arterial carbon dioxide (PaCO2) along a range of 15-40 mm Hg. The relationship of partial pressure of arterial carbon dioxide (PaCO2) to arterial hydrogen and bicarbonate is 0.7 mmol/L per mm Hg and 0.2 mmol/L per mm Hg, respectively 15. After 2-6 hours, renal compensation begins via a decrease in bicarbonate reabsorption. The kidneys respond more to the decreased partial pressure of arterial carbon dioxide (PaCO2) rather than the increased pH. Complete kidney compensation may take several days and requires normal kidney function and intravascular volume status 15. The expected change in serum bicarbonate concentration can be estimated as follows:
The expected change in serum bicarbonate concentration can be estimated as follows:
- Acute respiratory alkalosis – Bicarbonate (HCO3–) falls 2 mEq/L for each decrease of 10 mm Hg in the partial pressure of arterial carbon dioxide (PaCO2); that is, Δ(HCO3–) = 0.2(ΔPaCO2); maximum compensation: Bicarbonate (HCO3–) = 12-20 mEq/L
- Chronic respiratory alkalosis – Bicarbonate (HCO3–) falls 5 mEq/L for each decrease of 10 mm Hg in the partial pressure of arterial carbon dioxide (PaCO2); that is, Δ(HCO3–) = 0.5(ΔPaCO2); maximum compensation: Bicarbonate (HCO3–) = 12-20 mEq/L
Note that a plasma bicarbonate concentration of less than 12 mmol/L is unusual in pure respiratory alkalosis alone and should prompt the consideration of a metabolic acidosis as well (ie, the presence of a mixed acid-base disorder) 14.
The expected change in pH with respiratory alkalosis can be estimated with the following equations:
- Acute respiratory alkalosis: Change in pH = 0.008 X (40 – PaCO2)
- Chronic respiratory alkalosis: Change in pH = 0.017 X (40 – PaCO2)
A study by Morel et al 16 suggested that when respiratory alkalosis is present, caution be used in the employment of venous-arterial difference in carbon dioxide (ΔCO2) as an indicator of the adequacy of tissue perfusion (as has been proposed for shock states). Using healthy volunteers in whom either hypocapnia or hypercapnia was induced, the investigators found a significant increase in venous-arterial difference in carbon dioxide (ΔCO2) in the hypocapnic subjects, who also had a significant decrease in skin microcirculatory blood flow 16.
When respiratory alkalosis is present, the cause may be a minor, non–life-threatening disorder. However, more serious disease processes should also be considered in the differential diagnosis. These include central causes, hypoxemic causes, pulmonary causes, and iatrogenic causes (medically induced). Central sources are a head injury, stroke, hyperthyroidism, anxiety-hyperventilation, pain, fear, stress, drugs, medications such as salicylates, and various toxins 2. Hypoxic stimulation leads to hyperventilation in an attempt to correct hypoxia at the expense of a carbon dioxide (CO2) loss. Pulmonary causes include pulmonary embolisms, pneumothorax, pneumonia, and acute asthma or COPD exacerbations. Iatrogenic causes are primarily due to hyperventilation in intubated patients on mechanical ventilation 17, 18.
The causes of respiratory alkalosis is broad; therefore, a thorough history, physical examination, and laboratory evaluation are helpful in arriving at the true diagnosis.
Treatment of respiratory alkalosis is targeted at treating the underlying pathology. Respiratory alkalosis itself is rarely life threatening. Therefore, emergent treatment is usually not indicated unless the pH level is greater than 7.5. In anxious patients, anxiolytics may be necessary. In infectious disease, antibiotics targeting sputum or blood cultures are appropriate. In embolic disease, anticoagulation is necessary. Ventilator support may be necessary for patients with acute respiratory failure, acute asthma, or acute, chronic obstructive pulmonary disease (COPD) exacerbation if they show signs of respiratory fatigue. In ventilator controlled patients, it may be necessary to reevaluate their ventilator settings to reduce respiratory rate. If hyperventilation is intentional, monitor the arterial or venous blood gas values closely. In severe cases, pH may be directly reduced using acidic agents. However, this is not routinely done 19, 20, 21.
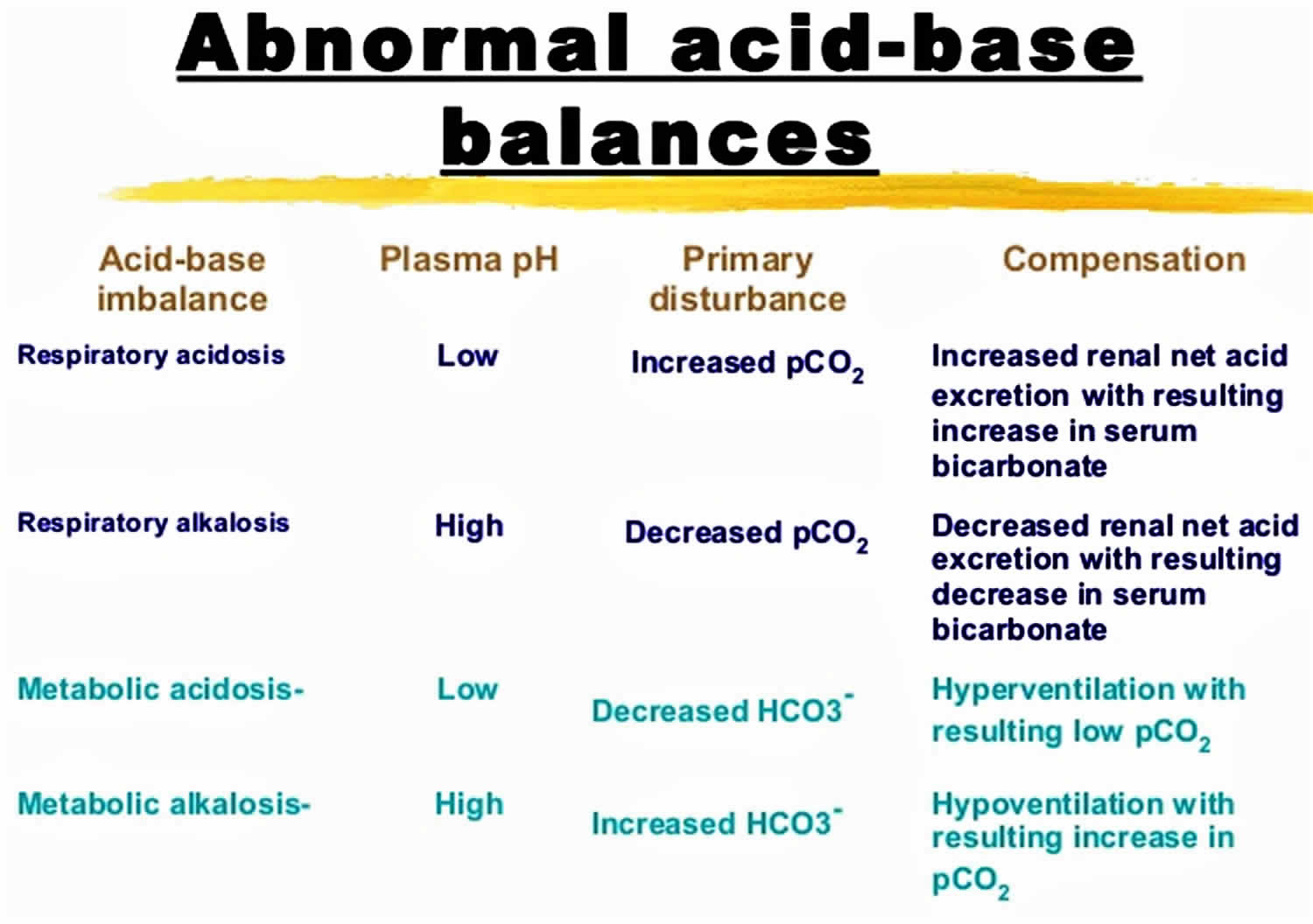
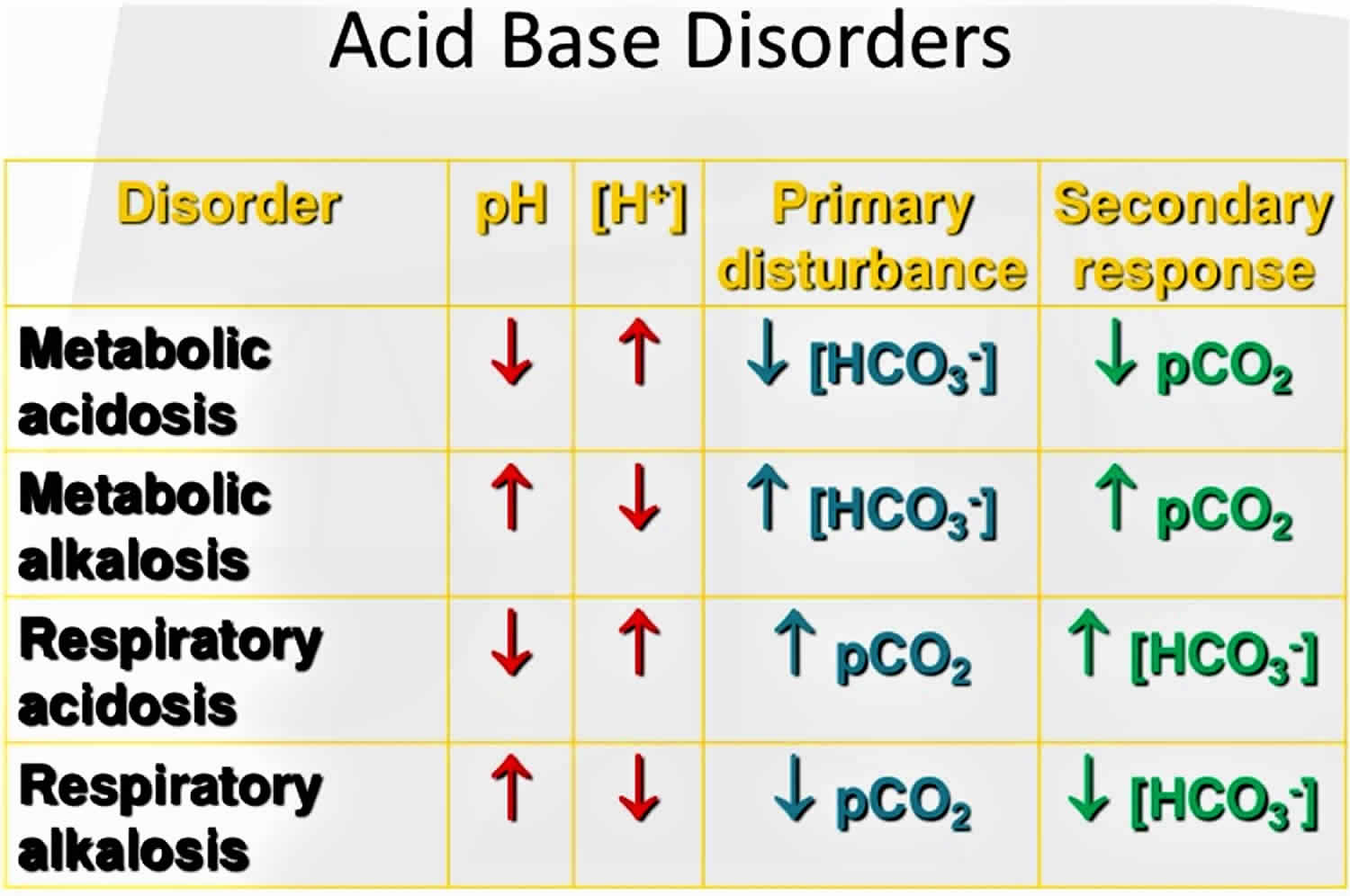
Figure 1. Abnormal acid-base compensation
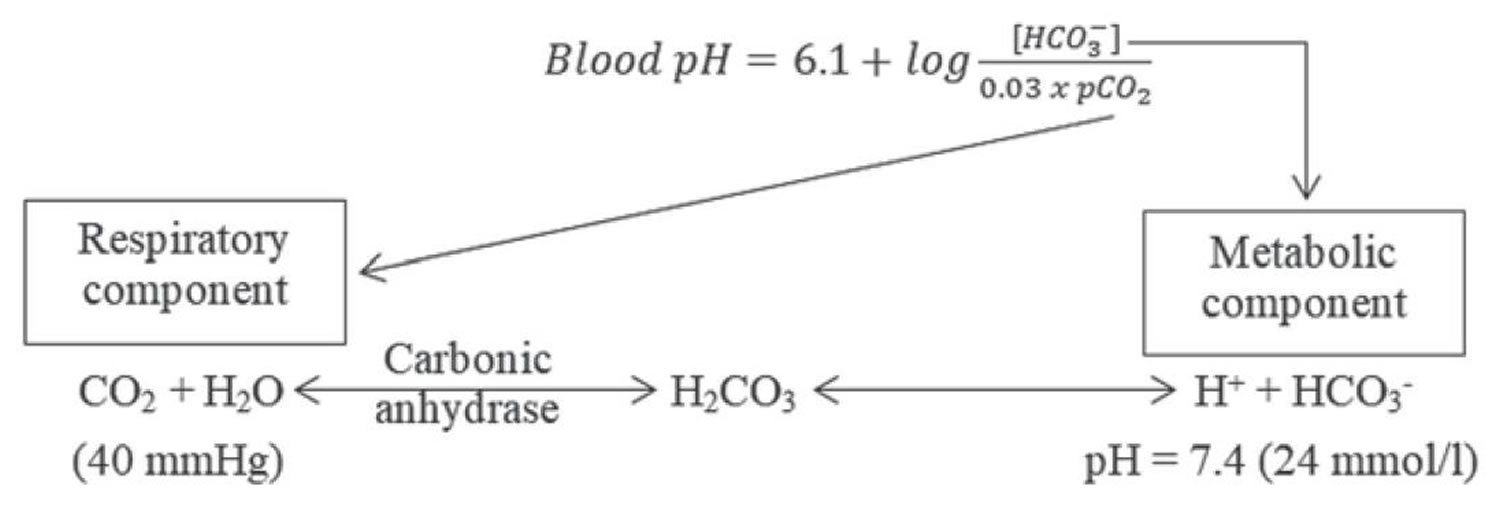
Figure 2. Henderson-Hasselbalch equation
[Source 22 ]Figure 3. Acid-base buffering system
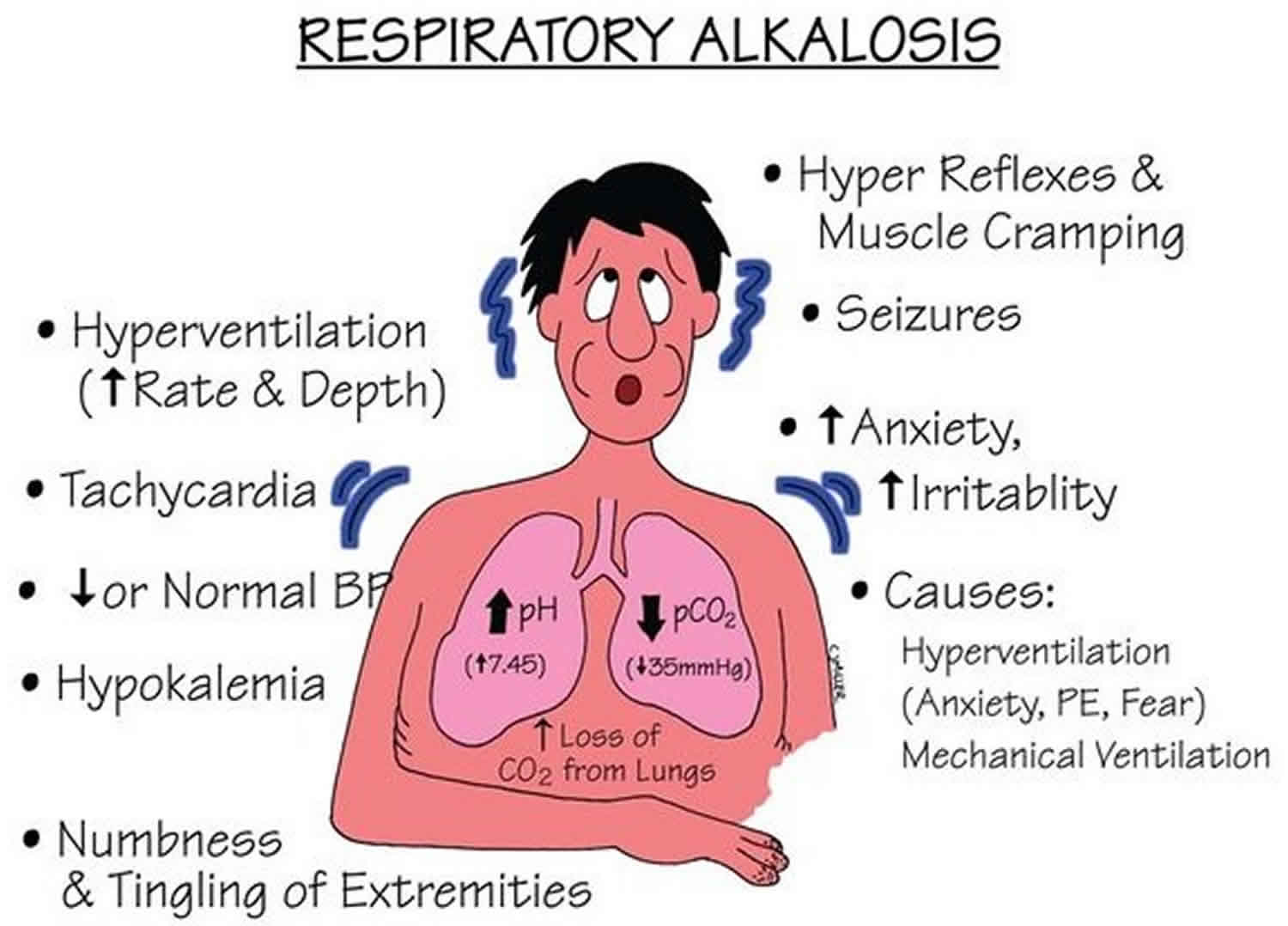
Respiratory alkalosis signs and symptoms
Respiratory alkalosis can mimic many conditions that are more serious. Symptoms may include paresthesia, circumoral numbness, chest pain or tightness, dyspnea, and tetany 23.
Acute onset of decrease in alveolar and blood carbon dioxide (CO2) levels below the normal reference range of 35 mmHg can cause cerebral vasoconstriction. An acute decrease in arterial partial pressure of carbon dioxide (PaCO2) reduces cerebral blood flow and can cause neurologic symptoms, including dizziness, mental confusion, syncope, and seizures. Hypoxemia need not be present for the patient to experience these symptoms 15.
Respiratory alkalosis may impair vitamin D metabolism, which may lead to vitamin D deficiency and cause symptoms such as fibromyalgia 19.
Respiratory alkalosis causes
Respiratory alkalosis is associated with numerous illnesses and is a common finding in patients on mechanical ventilation. In almost every scenario, respiratory alkalosis is induced by a process involving hyperventilation where carbon dioxide (CO2) is breathed away 4, 5, 6.
Central nervous system causes of respiratory alkalosis are as follows:
- Pain
- Hyperventilation syndrome
- Anxiety
- Panic disorders
- Psychosis
- Fever
- Cerebrovascular accident or stroke
- Meningitis
- Encephalitis
- Tumor
- Trauma
Hypoxia-related causes of respiratory alkalosis are as follows:
- High altitude
- Right-to-left shunts
- Drug-related causes are as follows:
- Progesterone
- Methylxanthine toxicity
- Salicylate toxicity
- Catecholamines
- Nicotine
Endocrine-related causes of respiratory alkalosis are as follows:
Pulmonary causes of respiratory alkalosis are as follows:
- Pneumothorax or hemothorax
- Pneumonia
- Pulmonary edema
- Pulmonary embolism
- Aspiration
- Interstitial lung disease
- Asthma
- Emphysema
- Chronic bronchitis
Miscellaneous causes of respiratory alkalosis are as follows:
- Sepsis
- Severe anemia
- Hepatic failure
- Mechanical ventilation
- Heat exhaustion
- Recovery phase of metabolic acidosis
- Congestive heart failure
Respiratory alkalosis pathophysiology
Breathing or alveolar ventilation is the body’s method of providing adequate amounts of oxygen for metabolism while removing carbon dioxide produced in the tissues. By sensing the body’s partial pressure of arterial oxygen (PaO2) and partial pressure of arterial carbon dioxide (PaCO2), the respiratory system adjusts pulmonary ventilation so that oxygen (O2) uptake and carbon dioxide (CO2) elimination at the lungs are balanced with the amount used and produced by the tissues.
The partial pressure of arterial carbon dioxide (PaCO2) must be maintained at a level that ensures that hydrogen ion (H+) concentrations remain in the narrow limits required for optimal protein and enzymatic function. The partial pressure of arterial oxygen (PaO2) is not as closely regulated as the partial pressure of arterial carbon dioxide (PaCO2). Adequate hemoglobin saturation can be achieved over a wide range of partial pressure of arterial oxygen (PaO2) levels. The movement of oxygen from the alveoli to the vascular system is dependent on pressure gradients. On the other hand, carbon dioxide (CO2) diffuses much easier through an aqueous environment.
Metabolism generates a large quantity of volatile acid (carbonic acid excreted as carbon dioxide by the lungs) and nonvolatile acid. The metabolism of fats and carbohydrates leads to the formation of a large amount of carbon dioxide (CO2) 6, which combines with water (H2O) to form carbonic acid (H2CO3). The lungs excrete the volatile fraction through ventilation so that acid accumulation does not occur. Significant alterations in ventilation can affect the elimination of carbon dioxide (CO2) and lead to a respiratory acid-base disorder.
Partial pressure of arterial carbon dioxide (PaCO2) is normally maintained in the range of 35-45 mmHg. Chemoreceptors in the brain (central chemoreceptors) and in the carotid bodies (peripheral chemoreceptors) sense hydrogen ion (H+) concentrations and influence ventilation to adjust the partial pressure of arterial carbon dioxide (PaCO2) and pH. This feedback regulator is how the partial pressure of arterial carbon dioxide (PaCO2) is maintained within its narrow normal range. When these receptors (central chemoreceptors and carotid bodies peripheral chemoreceptors) sense an increase in hydrogen ions (H+), breathing is increased to “blow off” carbon dioxide (CO2) and subsequently reduce the amount of hydrogen ions (H+). Various disease processes may cause stimulation of ventilation with subsequent hyperventilation. If hyperventilation is persistent, it leads to hypocapnia [partial pressure of arterial carbon dioxide (PaCO2) less than 35mmHg].
Hyperventilation refers to an increase in alveolar ventilation that is disproportionate to the rate of metabolic carbon dioxide production, leading to a partial pressure of arterial carbon dioxide (PaCO2) level below the normal range, or hypocapnia. Hyperventilation is often associated with dyspnea, but not all patients who are hyperventilating complain of shortness of breath. Conversely, patients with dyspnea need not be hyperventilating.
Acute hypocapnia [partial pressure of arterial carbon dioxide (PaCO2) less than 35mmHg] causes a reduction of serum levels of potassium and phosphate secondary to increased intracellular shifts of these ions. A reduction in free serum calcium also occurs. Calcium reduction is secondary to increased binding of calcium to serum albumin due to the change in pH. Many of the symptoms present in persons with respiratory alkalosis are related to hypocalcemia 14. Hyponatremia and hypochloremia may also be present.
Acute hyperventilation with hypocapnia causes a small, early reduction in serum bicarbonate (HCO3–) levels resulting from cellular shift of bicarbonate. Acutely, plasma pH and bicarbonate (HCO3–) concentration vary proportionately with the partial pressure of arterial carbon dioxide (PaCO2) along a range of 15-40 mm Hg. The relationship of partial pressure of arterial carbon dioxide (PaCO2) to arterial hydrogen and bicarbonate is 0.7 mmol/L per mm Hg and 0.2 mmol/L per mm Hg, respectively 15. After 2-6 hours, renal compensation begins via a decrease in bicarbonate reabsorption. The kidneys respond more to the decreased partial pressure of arterial carbon dioxide (PaCO2) rather than the increased pH. Complete kidney compensation may take several days and requires normal kidney function and intravascular volume status 15. The expected change in serum bicarbonate concentration can be estimated as follows:
- Acute respiratory alkalosis – Bicarbonate (HCO3–) falls 2 mEq/L for each decrease of 10 mm Hg in the partial pressure of arterial carbon dioxide (PaCO2); that is, Δ(HCO3–) = 0.2(ΔPaCO2); maximum compensation: Bicarbonate (HCO3–) = 12-20 mEq/L
- Chronic respiratory alkalosis – Bicarbonate (HCO3–) falls 5 mEq/L for each decrease of 10 mm Hg in the partial pressure of arterial carbon dioxide (PaCO2); that is, Δ(HCO3–) = 0.5(ΔPaCO2); maximum compensation: Bicarbonate (HCO3–) = 12-20 mEq/L
Note that a plasma bicarbonate concentration of less than 12 mmol/L is unusual in pure respiratory alkalosis alone and should prompt the consideration of a metabolic acidosis as well (ie, the presence of a mixed acid-base disorder) 14.
The expected change in pH with respiratory alkalosis can be estimated with the following equations:
- Acute respiratory alkalosis: Change in pH = 0.008 X (40 – PaCO2)
- Chronic respiratory alkalosis: Change in pH = 0.017 X (40 – PaCO2)
A study by Morel et al 16 suggested that when respiratory alkalosis is present, caution be used in the employment of venous-arterial difference in carbon dioxide (ΔCO2) as an indicator of the adequacy of tissue perfusion (as has been proposed for shock states). Using healthy volunteers in whom either hypocapnia or hypercapnia was induced, the investigators found a significant increase in venous-arterial difference in carbon dioxide (ΔCO2) in the hypocapnic subjects, who also had a significant decrease in skin microcirculatory blood flow 16.
Respiratory alkalosis diagnosis
Your healthcare provider will perform a physical examination and ask about your symptoms. Alkalosis is documented by the presence of an increased pH level (>7.45) on arterial blood gas (ABG) determinations. The presence of a decreased arterial partial pressure of carbon dioxide (PaCO2) level (< 35 mm Hg) indicates a respiratory cause of the alkalemia.
These tests can help diagnose alkalosis. Tests may include:
- Arterial blood gas (ABG). An arterial blood gas is a laboratory test used for the measurement of arterial pH, arterial partial pressure of oxygen (PaO2), arterial partial pressure of carbon dioxide (PaCO2), bicarbonate (HCO3–), base excess, total carbon dioxide (CO2) and oxygen (O2) saturation.
- A venous blood gas test is a laboratory test identical to an arterial blood gas test, except the blood is drawn from a venous site. This results in a slightly more acidic “normal” pH range.
- Basic metabolic panel, (a group of blood tests that measure your sodium, potassium, and chloride levels, kidney function, and other chemicals and functions)
- Urine and stool studies
- Urine chloride is a direct measurement of chloride being excreted into urine. This test is useful to help determine the etiology of metabolic alkalosis 24, 25, 1
- A complete blood count (CBC) to evaluate for an infectious cause with elevated white blood count and fluid body status with hemoglobin and hematocrit values is useful.
- Cultures of blood, sputum, urine, and other sites
- Thyroid testing
- Beta-human chorionic hormone levels
- Drug screens and theophylline and salicylate levels
Other tests that may be needed to determine the cause of the alkalosis include:
- Plasma renin activity and aldosterone level
- Amniocentesis
- Genetic studies
- Physiologic study of renal tubules
- Ultrasonography
- Pulmonary function test to measure breathing and how well the lungs are functioning
- Chest x-ray – Should be performed to help rule out pulmonary disease as a cause of hypocapnia and respiratory alkalosis
- Computerized tomography (CT) scanning of the chest – May be performed if chest radiography findings are inconclusive or a pulmonary disorder is strongly considered as a differential diagnosis
- Ventilation perfusion scanning – Can be considered in patients who are unable to undergo an intravenous contrast injection associated with CT scanning to assess the patient for pulmonary embolism
- Brain magnetic resonance imaging (MRI) – Can be considered if a central cause of hyperventilation and respiratory alkalosis is suggested and the initial brain CT scan findings are negative or inconclusive
Additional studies that may be considered include the following:
- Wrist radiography – This may be performed to determine bone age in infants with growth failure; it may also help assess bone density and the presence of rickets
- Upper gastrointestinal (GI) series – This helps detect gastroesophageal reflux and pyloric stenosis, which are case-dependent conditions 26
- Computed tomography (CT) of the brain – This is useful for evaluation of brain growth and calcifications
- Magnetic resonance imaging (MRI) of the brain – This is helpful in patients who present with seizures
- Electroencephalography (EEG) – This is also helpful in patients who present with seizures
- Renal nuclear scanning – This may facilitate assessment of renal function but is not useful in all patients
- Renal biopsy – This is not usually indicated, but if it is performed, it may reveal interstitial fibrosis and calcium/urate crystal deposition
Physical examination
Physical exam findings are not specific and depend on the cause of the respiratory alkalosis. Physical exam findings can include fever, tachycardia, tachypnea, diaphoresis (excessive sweating), hypertension or hypotension, altered mental status, productive or non-productive cough, wheezing, rales, crackles, cardiac murmur or arrhythmia, jugular venous distension, meningeal signs, focal neurological loss, Trousseau sign, Chvostek sign, jaundice, melena, hematochezia, hepatosplenomegaly, or there may be no definitive signs at all 16, 27.
Arterial blood gas (ABG) analysis
Arterial blood gas (ABG) sampling, is a test often performed in an inpatient setting to assess the acid-base status of a patient. A needle is used to draw blood from an artery, often the radial artery, and the blood is analyzed to determine parameters such as the pH, arterial partial pressure of carbon dioxide (PaCO2), arterial partial pressure of oxygen (PaO2), bicarbonate (HCO3–), oxygen saturation (O2 Sat) and more. This allows the physician to understand the status of the patient better. ABGs are especially important in the critically ill. They are the main tool utilized in adjusting to the needs of a patient on a ventilator.
- Arterial partial pressure of carbon dioxide (PaCO2) as carbon dioxide tension, this measures the level of carbon dioxide in your blood.
- Arterial partial pressure of oxygen (PaO2) also known as oxygen tension, this measures how well oxygen is being transferred into your blood.
- Oxygen saturation (O2 Sat) is an assessment of the amount of oxygen in your blood that is based on measuring levels of hemoglobin. Hemoglobin is a protein found inside red blood cells that is responsible for carrying oxygen throughout the body.
- Bicarbonate (HCO3–) concentration: Bicarbonate (HCO3–) is an electrolyte, which is a type of mineral involved in managing your body’s acid-base balance. Most of the carbon dioxide (CO2) in your blood is stored in the form of bicarbonate, so this measurement helps reflect carbon dioxide (CO2) levels.
- Although not universal, some arterial blood gases tests include measurements of hemoglobin as well as altered forms of the hemoglobin protein. Examples of these potential additional measurements include:
- Methemoglobin: Methemoglobin is a form of hemoglobin that has been oxidized, changing its heme iron configuration from the ferrous (Fe2+) to the ferric (Fe3+) state. Unlike normal hemoglobin, methemoglobin does not bind oxygen and as a result cannot deliver oxygen to the tissues.
- Carboxyhemoglobin: Carboxyhemoglobin is a stable complex of carbon monoxide and hemoglobin that forms in red blood cells upon contact with carbon monoxide. This abnormal form of hemoglobin attaches to carbon monoxide and can interfere with oxygen’s ability to travel in the blood.
- Oxyhemoglobin: Oxyhemoglobin represents the fraction of oxygenated hemoglobin in relation to the total hemoglobin present, including non-oxygen-binding hemoglobins. In healthy individuals, oxyhemoglobin and oxygen saturation are approximately equal.
- Deoxyhemoglobin: This is the form of hemoglobin without oxygen in the blood.
The following are the most important Normal Values on an ABG:
- pH = 7.35 to 7.45
- Arterial partial pressure of carbon dioxide (PaCO2) = 35 to 45 mmHg
- Arterial partial pressure of oxygen (PaO2) = 75 to 100 mmHg
- Bicarbonate (HCO3–) = 22 to 26 mEq/L
- O2 Sat = greater than 95%
The ability to quickly and efficiently read an ABG is paramount to quality patient care.
- Look at the pH. Decide whether it is acidotic, alkalotic, or within the physiological range
- Arterial partial pressure of carbon dioxide (PaCO2) level determines respiratory contribution; a high level means the respiratory system is lowering the pH and vice versa.
- Bicarbonate (HCO3–) level denotes metabolic/kidney effect. An elevated bicarbonate (HCO3–) is raising the pH and vice versa.
- If the pH is acidotic, look for the number that corresponds with a lower pH. If it is a respiratory acidosis, the carbon dioxide (CO2) should be high. If the patient is compensating metabolically, the bicarbonate (HCO3–) should be high as well. A metabolic acidosis will be depicted with an bicarbonate (HCO3–) that is low.
- If the pH is alkalotic, again, determine which value is causing this. A respiratory alkalosis will mean the carbon dioxide (CO2) is low; a metabolic alkalosis should lend an bicarbonate (HCO3–) that is high. Compensation with either system will be reflected oppositely; for a respiratory alkalosis the metabolic response should be a low bicarbonate (HCO3–) and for metabolic alkalosis, the respiratory response should be a high carbon dioxide (CO2).
- If the pH level is in the physiological range but the arterial partial pressure of carbon dioxide (PaCO2) and/or bicarbonate (HCO3–) are not within normal limits, there is likely a mixed disorder. Also, compensation does not always occur; this is when clinical information becomes paramount.
- Sometimes it is difficult to ascertain whether a patient has a mixed disorder.
Other tests that are important to perform when analyzing the acid-base status of a patient include those that measure electrolyte levels and renal function. This helps the clinician gather information that can be used to determine the exact mechanism of the acid-base imbalance as well as the factors contributing to the disorders 28, 29.
Serum Anion Gap
The calculation of the serum anion gap:
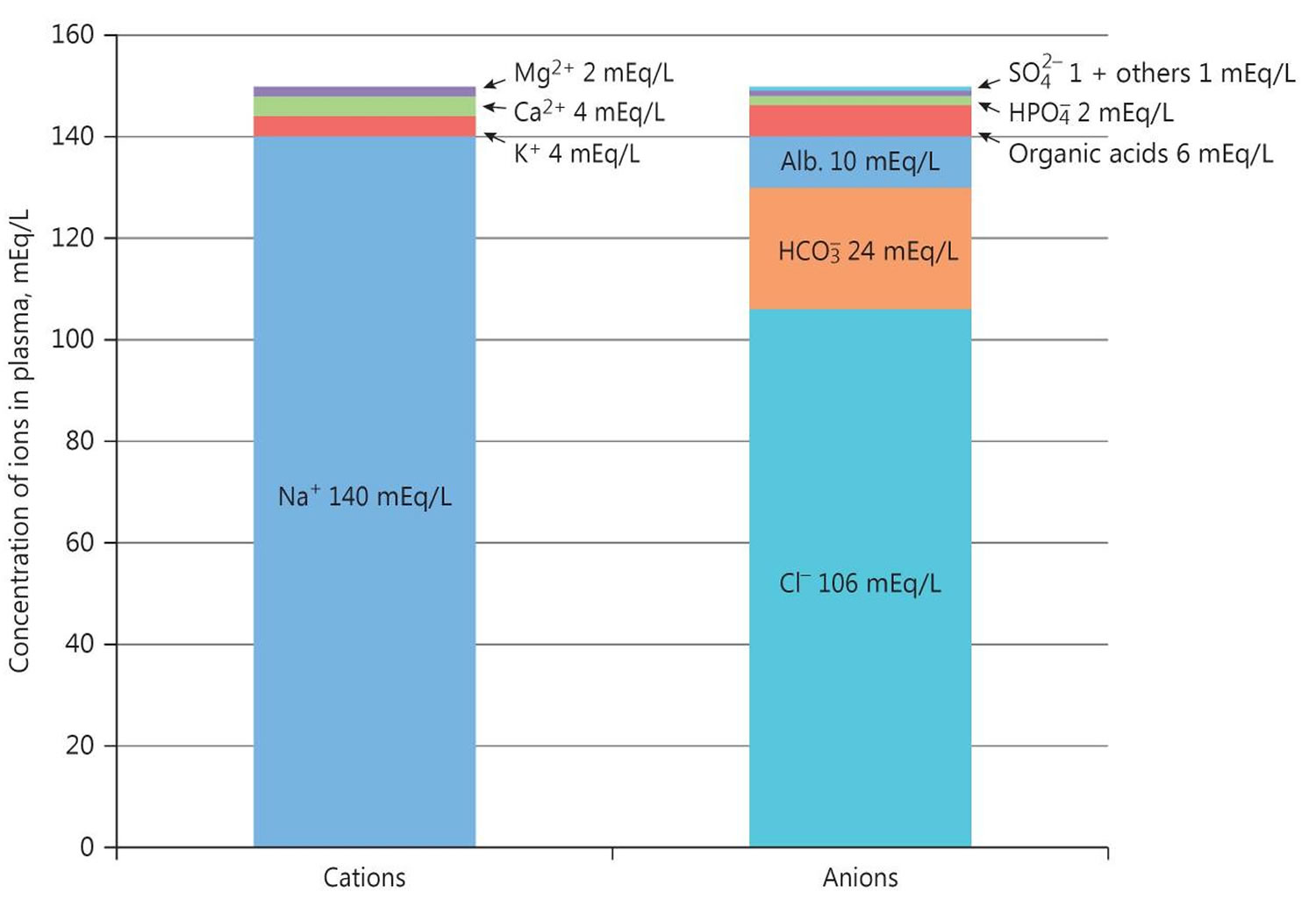
- Serum anion gap = (Na+) – [(HCO3– + Cl–)]
Where Na+ is plasma sodium concentration, HCO3– is plasma bicarbonate concentration, and Cl– is plasma chloride concentration. The anions are negatively charged ions like chloride [Cl–] and bicarbonate [HCO3–]. The anion gap is the difference between measured cations (positively charged ions like sodium [Na+] and potassium [K+]) and measured anions (negatively charged ions like chloride [Cl–] and bicarbonate [HCO3–]) 30. Calculation of the serum anion gap may help to differentiate between primary metabolic alkalosis and metabolic compensation for respiratory acidosis. The anion gap is frequently elevated to a modest degree in metabolic alkalosis because of the increase in the negative charge of albumin and the enhanced production of lactate. A normal serum anion gap is measured to be 5 to 16 mEq/L, with autoanalyzers using an ion-selective electrode. However, the anion gap value is dependent on the type of instrument used to measure its components 31. Therefore, you should know the reference range of the analyzer used and, if known, the patient’s baseline anion gap, too. In any event, the only definitive way to diagnose metabolic alkalosis is with a simultaneous blood gases analysis that shows elevation of both pH and arterial partial pressure of carbon dioxide (PaCO2) and increased calculated bicarbonate.
The anion gap is a calculation to determine the quantity of ionically active components within your blood that are not routinely measured. Serum anion gap is affected by the concentrations of all anions and cations which are not included in its calculations: i.e., albumin, globulin, potassium, calcium, magnesium, and organic and inorganic acids (see Figure 1). Because of the narrow extracellular concentration, most ions are omitted from the anion gap calculation. Since there are always components not directly measured, we expect this value to not equal 0. Most of this number is due to albumin (Alb); this anion is not accounted for in the anion gap formula, which is a large reason why the anion gap is not closer to zero. According to James Gamble 32, electrical neutrality in solution demands that the sum of the cations is equal to the sum of the anions (Figure 1). Sodium, chloride, bicarbonate, and albumin are quantitatively the major ions in the extracellular fluid compartment and are therefore used to calculate the anion gap 31. A true “ion gap,” however, does not exist in vivo which makes the anion gap a fundamental tool to evaluate acid-base disorders 33. Albumin is normally 4 mg/dL. Because of the large effect of albumin on anion gap, if a patient’s albumin level is abnormal, their expected anion gap will not be accurate 1. This can be corrected using simple math. The correction factor for albumin is 2.3–2.5 × [albumin], in g/dL 31. Therefore, each g/dL albumin decline will decrease the anion gap with about 2.5 mEq/L. To appreciate these facts, the anion gap formula should be: [Na+] − [Cl−] − [HCO3−] − 2.5 [albumin, in g/dL]. This equation is about zero in health, to stress the balance of ions, and also shows the relevance of albumin as a negative ion 31.
Serum bicarbonate (HCO3–) concentration can be calculated from a blood gas sample using the Henderson-Hasselbalch equation, as follows (see Figure 2 above):
- pH = 6.10 + log (HCO3– ÷ 0.03 × PaCO2)
- Alternatively, bicarbonate (HCO3–) = 24 × PaCO2 ÷ [H+]
Because pH and arterial partial pressure of carbon dioxide (PaCO2) are directly measured, bicarbonate (HCO3–) can be calculated.
Another means of assessing serum bicarbonate (HCO3–) concentration is with the total carbon dioxide content in serum, which is routinely measured with serum electrolytes obtained from venous blood. In this method, a strong acid is added to serum, which interacts with bicarbonate in the serum sample, forming carbonic acid. Carbonic acid dissociates to carbon dioxide and water; then, carbon dioxide is measured.
Note that the carbon dioxide measured includes bicarbonate and dissolved carbon dioxide. The contribution of dissolved carbon dioxide is quite small (0.03 × PaCO2) and is usually ignored, although it accounts for a difference of 1-3 mEq/L between the measured total carbon dioxide content in venous blood and the calculated bicarbonate in arterial blood. Thus, at an arterial partial pressure of carbon dioxide (PaCO2) of 40, a total carbon dioxide (CO2) content of 25 means a true bicarbonate concentration of 23.8 (ie, 25 – 0.03 × 40).
The Henderson-Hasselbalch equation may fail to account for acid-base findings in critically ill patients. An alternative method of acid-base analysis, known as the quantitative, or strong ion, approach, determines pH on the basis of the following 3 independent variables 34:
- Strong ion difference: Ions almost completely dissociated at physiologic pH (the cations Na+, K+, Ca+, and Mg+, and the anions Cl- and lactate)
- Total weak acid concentration: Ions that can be dissociated or associated at physiologic pH (albumin and phosphate)
- pCO2 (mm Hg)
In a study that compared the conventional Henderson-Hasselbalch equation with the strong ion approach, carried out in 100 patients with trauma who were admitted to a surgical intensive care unit, the investigators concluded that the strong ion approach provides a more accurate means of diagnosing acid-base disorders, including metabolic alkalosis and tertiary disorders 35.
Figure 3. Normal anion gap levels
[Source 31 ]Imaging studies
Imaging studies include the following:
- Chest radiography – Should be performed to help rule out pulmonary disease as a cause of hypocapnia and respiratory alkalosis
- Computerized tomography (CT) scanning of the chest – May be performed if chest radiography findings are inconclusive or a pulmonary disorder is strongly considered as a differential diagnosis
- Ventilation perfusion scanning (VQ scan) – Can be considered in patients who are unable to undergo an intravenous contrast injection associated with CT scanning to assess the patient for pulmonary embolism
- Brain magnetic resonance imaging (MRI) – Can be considered if a central cause of hyperventilation and respiratory alkalosis is suggested and the initial brain CT scan findings are negative or inconclusive
Respiratory alkalosis treatment
Treatment of respiratory alkalosis is targeted at treating the underlying pathology. Respiratory alkalosis itself is rarely life threatening. Therefore, emergent treatment is usually not indicated unless the pH level is greater than 7.5. In anxious patients, anxiolytics may be necessary. In infectious disease, antibiotics targeting sputum or blood cultures are appropriate. In embolic disease, anticoagulation is necessary. Ventilator support may be necessary for patients with acute respiratory failure, acute asthma, or acute, chronic obstructive pulmonary disease (COPD) exacerbation if they show signs of respiratory fatigue. In ventilator controlled patients, it may be necessary to reevaluate their ventilator settings to reduce respiratory rate. If hyperventilation is intentional, monitor the arterial or venous blood gas values closely. In severe cases, pH may be directly reduced using acidic agents. However, this is not routinely done 19, 20, 21.
Respiratory alkalosis prognosis
Respiratory alkalosis in itself is rarely a life-threatening diagnosis. However, the prognosis is variable depending on the underlying disease process and the severity of the underlying illness. Because respiratory alkalosis usually occurs in response to a underlying pathology, treatment is usually unsuccessful unless the underlying disorder is controlled. If the PaCO2 is corrected rapidly in patients with chronic respiratory alkalosis, metabolic acidosis may develop due to the renal compensatory drop in serum bicarbonate.
An Iranian study, by Hamdi et al 36, found primary respiratory alkalosis to be one of the mortality risk factors during hospitalization for poisoning, with the other predictors consisting of age, intensive care unit admission, consciousness level, period of hospitalization, and severe metabolic acidosis.
Lewis et al 19 hypothesized that respiratory alkalosis may interfere with vitamin D production, contributing to the development of fibromyalgia. The investigators suggested that, possibly by suppressing the kidneys’ ability to release phosphate into the urine, alkalotic pH disrupts endogenous 1,25-dihydroxyvitamin D formation 19.
A study by Park et al 27 indicated that in patients with high-risk acute heart failure, respiratory alkalosis is the most frequent acid-base imbalance. However, while acidosis was found to be a significant risk factor for mortality in acute heart failure patients, this was not true for alkalosis 27.
A study by Raphael et al 5 indicated that in healthy older adults, low serum bicarbonate levels can be linked to a higher mortality rate no matter whether respiratory alkalosis or metabolic acidosis is responsible for the bicarbonate reduction. Among the study’s patients (mean age 76 years), the mortality hazard ratio for those with respiratory alkalosis or metabolic acidosis, compared with controls, was 1.21 or 1.17, respectively 5.
A study by Wu et al 37 suggested that an association exists between respiratory alkalosis and the severity of coronavirus disease 2019 (COVID-19). The investigators reported that after adjusting for age, gender, and the presence of comorbidities (specifically, cardiovascular disease and hypertension), the hazard ratio for the development of severe COVID-19 in patients with respiratory alkalosis was 2.445 37.
- Hopkins E, Sanvictores T, Sharma S. Physiology, Acid Base Balance. [Updated 2022 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507807[↩][↩][↩][↩][↩]
- Brinkman JE, Sharma S. Respiratory Alkalosis. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482117[↩][↩][↩][↩]
- Asplund K, Fugl-Meyer AR, Engde M, Eriksson S, Strand T. Respiratory alkalosis early after stroke: its relation to loco-motor function. Scand J Rehabil Med Suppl. 1983;9:103-7.[↩]
- Bae K, Jee D. Hyperventilation Syndrome and Sustained Hyperchloremia After Kidney Transplant: Time-Sequence Swing of Acid-Base Interpretation. Exp Clin Transplant. 2018 Dec;16(6):754-756. doi: 10.6002/ect.2018.0099[↩][↩]
- Raphael KL, Murphy RA, Shlipak MG, Satterfield S, Huston HK, Sebastian A, Sellmeyer DE, Patel KV, Newman AB, Sarnak MJ, Ix JH, Fried LF; Health ABC Study. Bicarbonate Concentration, Acid-Base Status, and Mortality in the Health, Aging, and Body Composition Study. Clin J Am Soc Nephrol. 2016 Feb 5;11(2):308-16. doi: 10.2215/CJN.06200615[↩][↩][↩][↩]
- Kazmaier S, Weyland A, Buhre W, Stephan H, Rieke H, Filoda K, Sonntag H. Effects of respiratory alkalosis and acidosis on myocardial blood flow and metabolism in patients with coronary artery disease. Anesthesiology. 1998 Oct;89(4):831-7. doi: 10.1097/00000542-199810000-00006[↩][↩][↩]
- Respiratory Alkalosis. https://emedicine.medscape.com/article/301680-overview[↩]
- Koeppen BM. The kidney and acid-base regulation. Adv Physiol Educ. 2009 Dec;33(4):275-81. doi: 10.1152/advan.00054.2009[↩][↩]
- Hamm LL, Nakhoul N, Hering-Smith KS. Acid-Base Homeostasis. Clin J Am Soc Nephrol. 2015 Dec 7;10(12):2232-42. doi: 10.2215/CJN.07400715[↩][↩]
- Kisaka T, Cox TA, Dumitrescu D, Wasserman K. CO2 pulse and acid-base status during increasing work rate exercise in health and disease. Respir Physiol Neurobiol. 2015 Nov;218:46-56. doi: 10.1016/j.resp.2015.07.005[↩]
- Brinkman JE, Toro F, Sharma S. Physiology, Respiratory Drive. [Updated 2022 Jun 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482414[↩]
- Piekutowska-Abramczuk D, Rutyna R, Czyżyk E, Jurkiewicz E, Iwanicka-Pronicka K, Rokicki D, Stachowicz S, Strzemecka J, Guz W, Gawroński M, Kosierb A, Ligas J, Puchala M, Drelich-Zbroja A, Bednarska-Makaruk M, Dąbrowski W, Ciara E, Książyk JB, Pronicka E. Leigh syndrome in individuals bearing m.9185T>C MTATP6 variant. Is hyperventilation a factor which starts its development? Metab Brain Dis. 2018 Feb;33(1):191-199. doi: 10.1007/s11011-017-0122-1[↩]
- Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. [Updated 2022 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536919[↩]
- Effros RM, Wesson JA. Acid-Base Balance. Mason RJ, Broaddus VC, Murray JF, Nadel JA, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005. Vol 1: 192-93.[↩][↩][↩][↩]
- DuBose TD, Jr. Acidosis and Alkalosis. Kasper DL, Braunwald E, Fauci AS, Hauser Sl, Longo DL, Jameson JL,eds. Harrison’s Principles of Internal Medicine. 16th. New York, NY: McGraw-Hill; 2005. 270-1.[↩][↩][↩][↩][↩]
- Morel J, Gergelé L, Dominé A, Molliex S, Perrot JL, Labeille B, Costes F. The venous-arterial difference in CO2 should be interpreted with caution in case of respiratory alkalosis in healthy volunteers. J Clin Monit Comput. 2017 Aug;31(4):701-707. doi: 10.1007/s10877-016-9897-6[↩][↩][↩][↩][↩]
- Junod AF. Physiopathologie de l’insuffisance respiratoire chronique [Physiopathology of chronic respiratory insufficiency]. Schweiz Med Wochenschr. 1980 Dec 13;110(50):1896-1901. French.[↩]
- Gardner WN. The pathophysiology of hyperventilation disorders. Chest. 1996 Feb;109(2):516-34. doi: 10.1378/chest.109.2.516[↩]
- Lewis JM, Fontrier TH, Coley JL. Respiratory alkalosis may impair the production of vitamin D and lead to significant morbidity, including the fibromyalgia syndrome. Med Hypotheses. 2017 May;102:99-101. doi: 10.1016/j.mehy.2017.03.013[↩][↩][↩][↩][↩]
- Oppersma E, Doorduin J, van der Hoeven JG, Veltink PH, van Hees HWH, Heunks LMA. The effect of metabolic alkalosis on the ventilatory response in healthy subjects. Respir Physiol Neurobiol. 2018 Feb;249:47-53. doi: 10.1016/j.resp.2018.01.002[↩][↩]
- Mora Carpio AL, Mora JI. Ventilator Management. [Updated 2022 Mar 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448186[↩][↩]
- Adamczak M, Surma S. Metabolic Acidosis in Patients with CKD: Epidemiology, Pathogenesis, and Treatment. Kidney Dis (Basel). 2021 Jun 4;7(6):452-467. doi: 10.1159/000516371[↩]
- Phillipson EA, Duffin J. Hypoventilation and Hyperventilation Syndromes. Mason RJ, Broaddus VC, Murray JF, Nadel JA, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005. Vol 2: 2069-70, 2080-84.[↩]
- Stimson L, Reynolds T. Differential diagnosis for chronic hypokalaemia. BMJ Case Rep. 2018 Jun 5;2018:bcr2017223680. doi: 10.1136/bcr-2017-223680[↩]
- Galla JH. Metabolic alkalosis. J Am Soc Nephrol. 2000 Feb;11(2):369-375. doi: 10.1681/ASN.V112369[↩]
- Hulka F, Campbell TJ, Campbell JR, Harrison MW. Evolution in the recognition of infantile hypertrophic pyloric stenosis. Pediatrics. 1997 Aug. 100(2):E9.[↩]
- Park, J.J., Choi, D.-J., Yoon, C.-H., Oh, I.-Y., Lee, J.H., Ahn, S., Yoo, B.-S., Kang, S.-M., Kim, J.-J., Baek, S.-H., Cho, M.-C., Jeon, E.-S., Chae, S.C., Ryu, K.-H., Oh, B.-H. and (2015), The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail, 17: 601-611. https://doi.org/10.1002/ejhf.276[↩][↩][↩]
- Patel S, Sharma S. Respiratory Acidosis. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482430[↩]
- Rajkumar P, Pluznick JL. Acid-base regulation in the renal proximal tubules: using novel pH sensors to maintain homeostasis. Am J Physiol Renal Physiol. 2018 Nov 1;315(5):F1187-F1190. doi: 10.1152/ajprenal.00185.2018[↩]
- Pandey DG, Sharma S. Biochemistry, Anion Gap. [Updated 2019 Apr 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539757[↩]
- Berend K. Review of the Diagnostic Evaluation of Normal Anion Gap Metabolic Acidosis. Kidney Dis (Basel). 2017 Dec;3(4):149-159. doi: 10.1159/000479279[↩][↩][↩][↩][↩]
- Gamble JL. Extracellular fluid and its maintenance. N Engl J Med. 1936;250:1150–1152.[↩]
- Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid-base disturbances. N Engl J Med. 2015 Jan 8;372(2):195. doi: 10.1056/NEJMc1413880[↩]
- Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983 Dec;61(12):1444-61. doi: 10.1139/y83-207[↩]
- Kaplan LJ, Cheung NH, Maerz L, Lui F, Schuster K, Luckianow G, Davis K. A physicochemical approach to acid-base balance in critically ill trauma patients minimizes errors and reduces inappropriate plasma volume expansion. J Trauma. 2009 Apr;66(4):1045-51. doi: 10.1097/TA.0b013e31819a04be[↩]
- Hamdi H, Hassanian-Moghaddam H, Hamdi A, Zahed NS. Acid-base disturbances in acute poisoning and their association with survival. J Crit Care. 2016 Oct;35:84-9. doi: 10.1016/j.jcrc.2016.05.003[↩]
- Wu C, Wang G, Zhang Q, Yu B, Lv J, Zhang S, Wu G, Wu S, Zhong Y. Association Between Respiratory Alkalosis and the Prognosis of COVID-19 Patients. Front Med (Lausanne). 2021 Apr 26;8:564635. doi: 10.3389/fmed.2021.564635[↩][↩]