Hashimoto disease
Hashimoto disease also known as Hashimoto’s thyroiditis, chronic lymphocytic thyroiditis or autoimmune thyroiditis, is an autoimmune disease where your body’s immune system attacks your thyroid gland, preventing it from producing enough thyroid hormones (tri-iodothyronine [T3] and thyroxine [T4]). Low thyroid hormone levels may cause hypothyroidism with a range of symptoms, such as tiredness or fatigue, weight gain, intolerance to cold temperatures, dry skin or dry thinning hair, slowed heart rate, heavy or irregular menstrual periods or fertility problems. Rarely, early in the course of the disease, thyroid gland damage may lead to the release of too much thyroid hormone into your blood, causing symptoms of hyperthyroidism 1. Too much thyroid hormone (hyperthyroidism) can cause weight loss, despite an increased appetite. You might also feel anxious and find it difficult to relax.
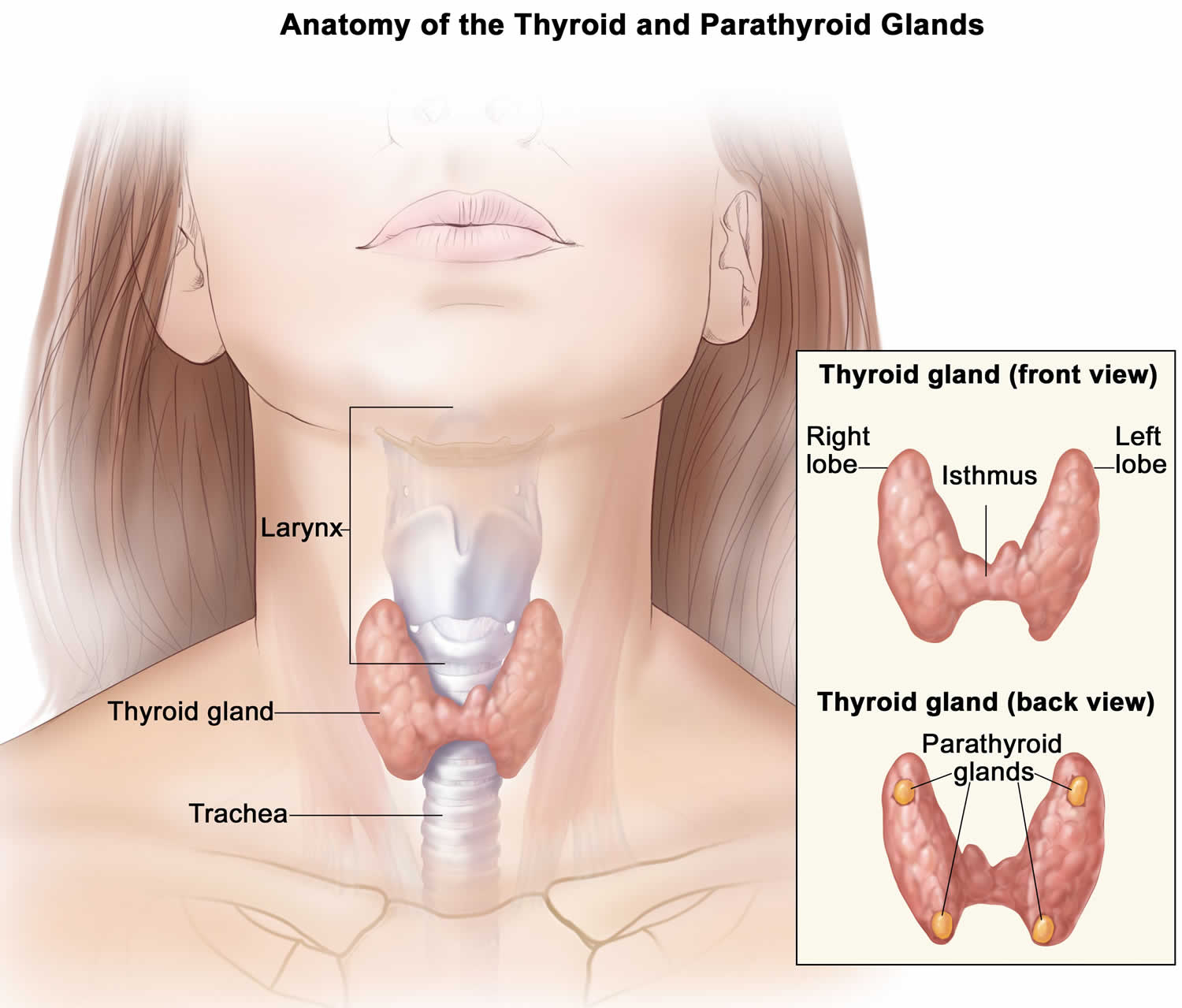
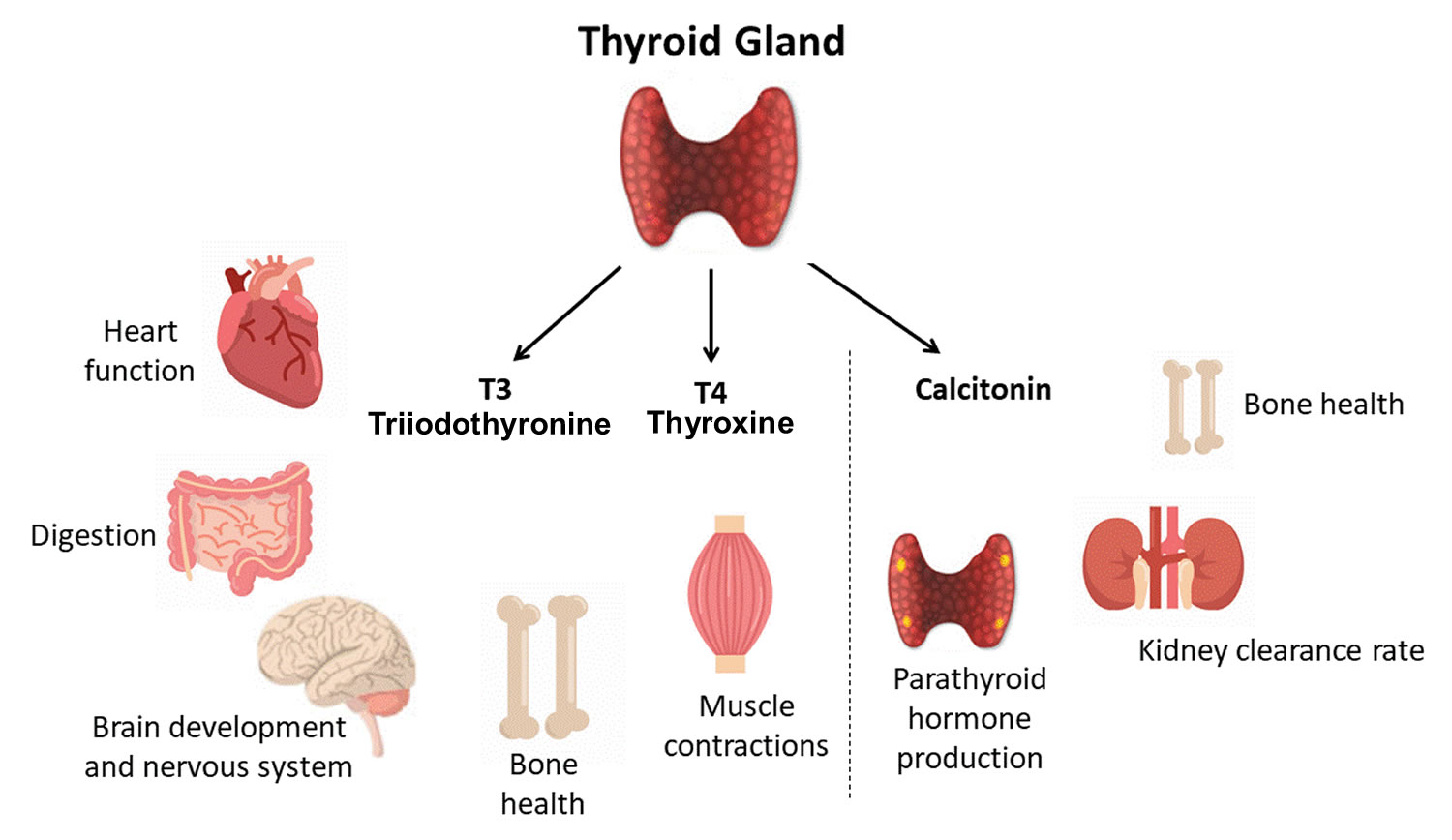
Your thyroid gland is a butterfly-shaped gland with 2 lobes (the right lobe and the left lobe — joined by a narrow piece of the thyroid gland called the isthmus) that is located in front of your neck near the base of your throat, beneath the larynx (voice box or Adam’s apple) (Figure 1). In most people, the thyroid gland cannot be seen or felt. Your thyroid gland produces thyroid hormones, tri-iodothyronine (T3) and thyroxine (T4) (the main hormones that your thyroid gland makes) and calcitonin. The thyroid hormones, T3 (tri-iodothyronine) and T4 (thyroxine) influence important body processes such as body temperature, energy levels, growth, your digestion, muscles and heart. Thyroid hormones are important for how your body uses energy, your metabolism, so thyroid hormones affect nearly every organ in your body even the way your heart beats. You might put on weight and feel very tired and lacking in energy if your thyroid gland doesn’t make enough T3 (tri-iodothyronine) and T4 (thyroxine).
Calcitonin is another hormone produced by the thyroid gland. Calcitonin helps to control the amount of calcium circulating in your blood. Calcitonin works with a hormone called parathyroid hormone (PTH) to do this. Parathyroid hormone is made by parathyroid glands. These sit behind and are attached to the thyroid gland (see Figure 1).
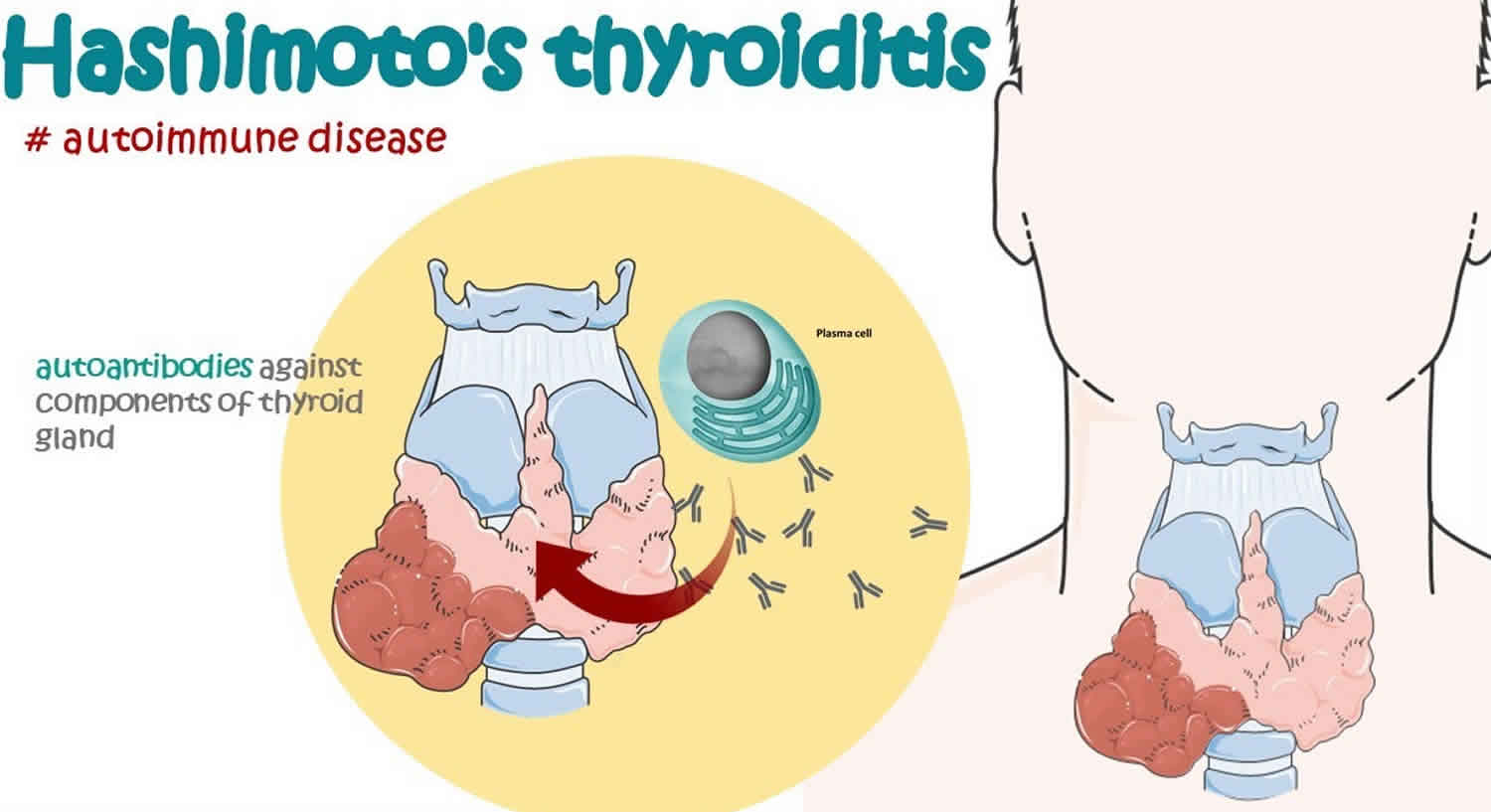
In people with Hashimoto’s disease:
- the immune system makes antibodies that attack the thyroid gland (autoimmune disorder). Usually in Hashimoto’s disease, the immune system produces an antibody to thyroid peroxidase (TPO), a protein that plays an important part in thyroid hormone production. Most people with Hashimoto’s disease will have TPO (thyroid peroxidase) antibodies in their blood. Lab tests for other antibodies associated with Hashimoto’s disease may need to be done.
- large numbers of white blood cells, which are part of the immune system, build up in the thyroid gland
- the thyroid gland becomes damaged and can’t make enough thyroid hormones
Hashimoto’s disease is an autoimmune disorder affecting the thyroid gland. In Hashimoto’s thyroiditis, the immune-system cells lead to the death of the thyroid’s hormone-producing cells. Hashimoto’s disease usually results in a decline in thyroid hormones production (hypothyroidism). The symptoms of hypothyroidism might be mild, or they might be severe. They include:
- fatigue
- being unable to stand the cold
- weight gain
- constipation
- muscle pain
- dry skin, thin hair and / or brittle nails
- low sex drive (libido)
Hashimoto’s disease can also cause cognitive symptoms including:
- depression or low mood
- an inability to concentrate
- poor memory
In some cases, your thyroid gland may become noticeably larger (called a goiter) or it may shrink. Lumps or nodules may also develop in your thyroid gland.
Although Hashimoto’s disease can affect people of all ages, it’s most common in women in their 30s and 40s 2. The female-to-male ratio is at least 10:1 1. If someone in your family has had thyroid disease, you may have an increased risk for Hashimoto’s disease. No one is sure why people get Hashimoto’s disease.
If you have symptoms of hypothyroidism, see your doctor. Your doctor will examine you and may run blood tests, including testing your thyroid hormone levels.
If left untreated, hypothyroidism can lead to problems including goiter (an increase in the size of the thyroid gland), heart problems or mental health problems. Occasionally, it can lead to a potentially life-threatening disorder called myxedema coma.
While there is no cure for Hashimoto’s disease, hypothyroidism can be treated. The primary treatment of Hashimoto’s disease is thyroid hormone replacement. Most people with Hashimoto’s disease take a synthetic thyroid hormone medication called levothyroxine (Levoxyl, Synthroid, others) to treat hypothyroidism. The synthetic thyroid hormone works like the T4 hormone naturally produced by the thyroid. Your hypothyroidism can be well-controlled with thyroid hormone medicine, as long as you take the medicine as instructed by your doctor and have regular follow-up blood tests.
If you have mild hypothyroidism, you may not need to have treatment but get regular thyroid stimulating hormone (TSH) tests to monitor thyroid hormone levels.
How common is Hashimoto’s disease?
The number of people who have Hashimoto’s disease in the United States is unknown. However, Hashimoto’s disease is the most common cause of hypothyroidism the United States, which affects about 5 in 100 Americans 3.
Hashimoto is also the most common cause of hypothyroidism in those areas of the world where iodine intake is adequate. The incidence is estimated to be 0.8 per 1000 per year in men and 3.5 per 1000 per year in women 1. The prevalence of thyroid disease, in general, increases with age.
How does eating, diet, and nutrition affect Hashimoto’s disease?
The thyroid gland uses iodine, a mineral in some foods, to make thyroid hormones. However, if you have Hashimoto’s disease or other types of autoimmune thyroid disorders, you may be sensitive to harmful side effects from iodine. Eating foods that have large amounts of iodine—such as kelp, dulse, or other kinds of seaweed, and certain iodine-rich medicines—may cause hypothyroidism or make it worse. Taking iodine supplements can have the same effect.
Talk with members of your health care team about:
- what foods and beverages to limit or avoid
- whether you take iodine supplements
- any cough syrups you take that may contain iodine
However, if you are pregnant, you need to take enough iodine because the baby gets iodine from your diet. Too much iodine can cause problems as well, such as a goiter in the baby. If you are pregnant, talk with your doctor about how much iodine you need.
Researchers are looking at other ways in which diet and supplements such as vitamin D and selenium may affect Hashimoto’s disease 4. However, no specific guidance is currently available 1.
How much iodine do I need?
The amount of iodine you need each day depends on your age. Average daily recommended amounts are listed below in micrograms (mcg).
Table 1 lists the current Recommended Dietary Allowances (RDA – the average daily level of intake sufficient to meet the nutrient requirements of nearly all [97%–98%] healthy individuals; often used to plan nutritionally adequate diets for individuals) for iodine 5. For infants from birth to 12 months, the Food and Nutrition Board at the Institute of Medicine of the National Academies established an Adequate Intake (AI) for iodine that is equivalent to the mean intake of iodine in healthy, breastfed infants in the United States.
The World Health Organization (WHO), United Nations Children’s Fund (UNICEF), and the International Council for the Control of Iodine Deficiency Disorders (ICCIDD) recommend a slightly higher iodine intake for pregnant women of 250 mcg per day 6, 7.
Table 1. Recommended Dietary Allowances (RDAs) for Iodine
| Age | Male | Female | Pregnancy | Lactation |
| Birth to 6 months | 110 mcg* | 110 mcg* | ||
| 7–12 months | 130 mcg* | 130 mcg* | ||
| 1–3 years | 90 mcg | 90 mcg | ||
| 4–8 years | 90 mcg | 90 mcg | ||
| 9–13 years | 120 mcg | 120 mcg | ||
| 14–18 years | 150 mcg | 150 mcg | 220 mcg | 290 mcg |
| 19+ years | 150 mcg | 150 mcg | 220 mcg | 290 mcg |
Footnote: * Adequate Intake (AI)
What foods are good source for iodine?
Seaweed (such as kelp, nori, kombu, and wakame) is one of the best food sources of iodine 8. Other good sources include fish and other seafood, as well as eggs (see Table 2). Iodine is also present in human breast milk 5 and infant formulas 9. The U.S. Department of Agriculture (USDA) lists the iodine content of numerous foods and beverages 9.
Dairy products contain iodine. However, the amount of iodine in dairy products varies by whether the cows received iodine feed supplements and whether iodophor sanitizing agents were used to clean the cows and milk-processing equipment 10. For example, an analysis of 44 samples of nonfat milk found a range of 38 to 159 mcg per cup (with an average of 85 mcg/cup used for Table 2) 9. Plant-based beverages used as milk substitutes, such as soy and almond beverages, contain relatively small amounts of iodine.
Most commercially prepared bread contains very little iodine unless the manufacturer has used potassium iodate or calcium iodate as a dough conditioner 11. Manufacturers list dough conditioners as an ingredient on product labels but are not required to include iodine on the Nutrition Facts label 12, even though these conditioners provide a substantial amount of iodine. According to 2019 data from the USDA Branded Food Products Database, approximately 20% of ingredient labels for white bread, whole-wheat bread, hamburger buns, and hot dog buns listed iodate. Pasta is not a source of iodine unless it is prepared in water containing iodized salt because it absorbs some of the iodine 13.
Most fruits and vegetables are poor sources of iodine, and the amounts they contain are affected by the iodine content of the soil, fertilizer use, and irrigation practices 11. This variability affects the iodine content of meat and animal products because of its impact on the iodine content of foods that the animals consume 14. The iodine amounts in different seaweed species also vary greatly. For example, commercially available seaweeds in whole or sheet form have iodine concentrations ranging from 16 mcg/g to 2,984 mcg/g 15. For these reasons, the values for the foods listed in Table 2 are approximate but can be used as a guide for estimating iodine intakes.
Table 2. Iodine Content of Selected Foods
| Food | Micrograms (mcg) per serving | Percent DV* |
| Seaweed, nori, dried, 10 g | 232 | 155 |
| Bread, whole-wheat, made with iodate dough conditioner, 1 slice | 198 | 132 |
| Bread, white, enriched, made with iodate dough conditioner, 1 slice | 185 | 123 |
| Cod, baked, 3 ounces | 158 | 106 |
| Yogurt, Greek, plain, nonfat, 1 cup | 116 | 77 |
| Oysters, cooked, 3 ounces | 93 | 62 |
| Milk, nonfat, 1 cup | 85 | 57 |
| Iodized table salt, 1.5 g (approx. ¼ teaspoon) | 76 | 51 |
| Fish sticks, cooked, 3 ounces | 58 | 39 |
| Pasta, enriched, boiled in water with iodized salt, 1 cup | 36 | 24 |
| Egg, hard boiled, 1 large | 26 | 17 |
| Ice cream, chocolate, ½ cup | 21 | 14 |
| Liver, beef, cooked, 3 ounces | 14 | 9 |
| Cheese, cheddar, 1 ounce | 14 | 9 |
| Shrimp, cooked, 3 ounces | 13 | 9 |
| Tuna, canned in water, drained, 3 ounces | 7 | 5 |
| Soy beverage, 1 cup | 7 | 5 |
| Fruit cocktail in light syrup, canned, ½ cup | 6 | 4 |
| Beef, chuck, roasted, 3 ounces | 3 | 2 |
| Chicken breast, roasted, 3 ounces | 2 | 1 |
| Almond beverage, 1 cup | 2 | 1 |
| Apple juice, 1 cup | 1 | 1 |
| Bread, whole-wheat, made without iodate dough conditioner, 1 slice | 1 | 1 |
| Bread, white, enriched, made without iodate dough conditioner, 1 slice | 1 | 1 |
| Raisin bran cereal, 1 cup | 1 | 1 |
| Rice, brown, cooked, ½ cup | 1 | 1 |
| Corn, canned, ½ cup | 1 | 1 |
| Sea salt, non-iodized, 1.5 g (approx. ¼ teaspoon) | <1 | <1 |
| Broccoli, boiled, ½ cup | 0 | 0 |
| Banana, 1 medium | 0 | 0 |
| Lima beans, mature, boiled, ½ cup | 0 | 0 |
| Green peas, frozen, boiled, ½ cup | 0 | 0 |
| Pasta, enriched, boiled in water without iodized salt, 1 cup | 0 | 0 |
Footnotes: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for iodine is 150 mcg for adults and children aged 4 years and older [12]. FDA does not require food labels to list iodine content unless iodine has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 9 ]Hashimoto’s disease and gluten
There is insufficient evidence to support a gluten-free diet for all Hashimoto’s disease patients 16. Few studies have been conducted on the usage of a gluten-free diet in patients with Hashimoto’s disease 17, 18. Some research suggested a relationship between gluten consumption and the development or progression of Hashimoto’s disease. A pilot study of normal thyroid function (euthyroid) women with Hashimoto’s disease showed that gluten-free diet reduced thyroid antibody titers 17. Another study by Velija et al. 18 tested the response of subclinical hypothyroidism patients with Hashimoto’s disease treated with a gluten-free diet and selenium supplementation in restoring a normal thyroid function. After six months, normal thyroid function was restored in more patients who received selenium and had a gluten-free diet compared to the control group who supplemented selenium without any dietary intervention 18. Moreover, the reduction in serum anti-thyroid peroxidase (anti-TPO) antibody levels in the study group was significantly greater than in the control group. The results of that study suggested that the gluten-free diet together with selenium supplementation is more effective compared to only Se supplementation in Hashimoto’s disease women with subclinical hypothyroidism 18. Another study showed no reduction in thyroid autoimmunity after following a gluten-free diet 19. Pobłocki et al. 19 conducted a randomized study with euthyroid women with Hashimoto’s disease receiving levothyroxine. The control group’s diet contained gluten, and the study group was on a gluten-free diet for 12 months. During follow-up, there was a significant reduction in thyroid stimulating hormone (TSH) levels in the study group 19. Only a few studies suggest that gluten elimination may be helpful for some Hashimoto’s disease patients. It should be noted that gluten-free diet is very restrictive and difficult to follow and contributes to the risk of nutritional deficiencies. The studies conducted so far do not confirm that patients with Hashimoto’s disease should be on a gluten-free diet, therefore it is not recommended 20.
Thyroid gland
The thyroid gland is the largest adult gland to have a purely endocrine function, weighing about 25-30 g. The thyroid gland is a small butterfly shaped gland with 2 lobes, the right lobe and the left lobe joined by a narrow piece of the thyroid gland called the isthmus, that is located in front of your neck near the base of your throat, beneath the larynx (voice box or Adam’s apple). About 50% of thyroid glands have a small third lobe, called the pyramidal lobe. It extends superiorly from the isthmus. The thyroid gland makes and releases hormones. You can’t usually feel a thyroid gland that is normal.
The thyroid gland has 2 main types of cells:
- Follicular cells use iodine from the blood to make thyroid hormones, which help regulate a person’s metabolism. Having too much thyroid hormone (hyperthyroidism) can cause a fast or irregular heartbeat, trouble sleeping, nervousness, hunger, weight loss, and a feeling of being too warm. Having too little thyroid hormone (hypothyroidism) causes a person to slow down, feel tired, and gain weight. The amount of thyroid hormone released by the thyroid gland is regulated by the pituitary gland at the base of the brain, which makes a substance called thyroid-stimulating hormone (TSH) (see Figure 5).
- C cells also called parafollicular cells at the periphery of the follicles that make calcitonin, a hormone that helps control how your body uses calcium. The parafollicular cells (C cells) respond to rising levels of blood calcium by secreting the hormone calcitonin. Calcitonin antagonizes (blocks) parathyroid hormone (PTH) and stimulates osteoblast activity, thus promoting calcium deposition and bone formation. It is important mainly in children, having relatively little effect in adults.
Other, less common cells in the thyroid gland include immune system cells (lymphocytes) and supportive (stromal) cells.
Thyroid hormone is secreted or inhibited in response to fluctuations in metabolic rate. The brain monitors the body’s metabolic rate and stimulates thyroid hormone secretion through the action of thyrotropin-releasing hormone (TRH) and thyroid stimulating hormone (TSH) as depicted in figure 5.
The primary effect of thyroid hormone (TH) is to increase one’s metabolic rate. As a result, it raises oxygen consumption and has a calorigenic effect—it increases heat production. To ensure an adequate blood and oxygen supply to meet this increased metabolic demand, thyroid hormone also raises the breathing (respiratory) rate, heart rate, and strength of the heartbeat. It stimulates the appetite and accelerates the breakdown of carbohydrates, fats, and protein for fuel. Thyroid hormone also promotes alertness and quicker reflexes; growth hormone secretion; growth of the bones, skin, hair, nails, and teeth; and development of the fetal nervous system.
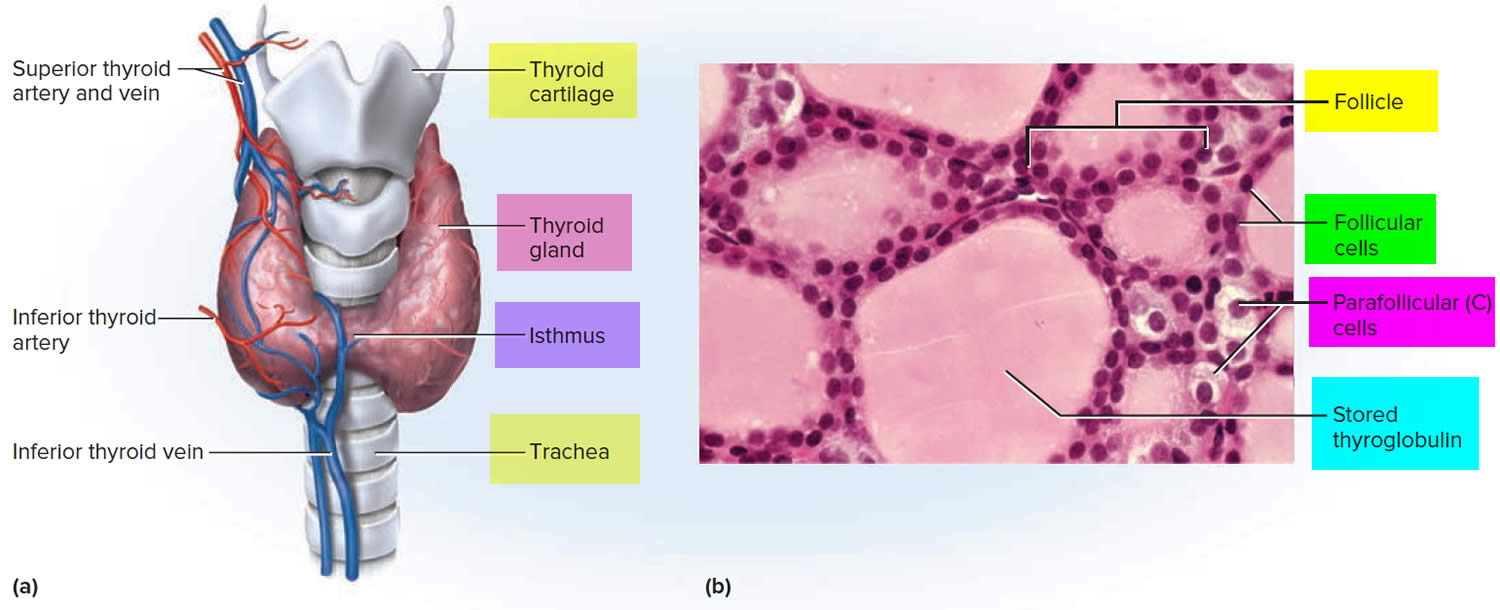
Figure 1. Thyroid gland and parathyroid gland
Footnotes: Anatomy of the thyroid and parathyroid glands. The thyroid gland lies at the base of the throat near the trachea. It is shaped like a butterfly, with the right lobe and left lobe connected by a thin piece of tissue called the isthmus. The parathyroid glands are four pea-sized organs found in the neck near the thyroid. The thyroid and parathyroid glands make hormones.
Figure 2. Thyroid gland location
Figure 3. Thyroid gland anatomy
Footnote: (a) Gross anatomy, anterior view. (b) Histology, showing the saccular thyroid follicles (the source of thyroid hormone) and nests of C cells (the source of calcitonin).
What does the thyroid gland do?
Formation, storage, and release of thyroid hormones
The thyroid gland is the only endocrine gland that stores its secretory product in large quantities—normally about a 100-day supply. Synthesis and secretion of triiodothyronine (T3) and thyroxine or tetraiodothyronine (T4) occurs as follows:
- Iodide trapping. Thyroid follicular cells trap iodide ions (I −) by actively transporting them from the blood into the cytosol. As a result, the thyroid gland normally contains most of the iodide in the body.
- Synthesis of thyroglobulin. While the follicular cells are trapping I −, they are also synthesizing thyroglobulin (TGB), a large glycoprotein that is produced in the rough endoplasmic reticulum, modified in the Golgi complex, and packaged into secretory vesicles. The vesicles then undergo exocytosis, which releases thyroglobulin into the lumen of the follicle.
- Oxidation of iodide. Some of the amino acids in thyroglobulin are tyrosines that will become iodinated. However, negatively charged iodide (I −) ions cannot bind to tyrosine until they undergo oxidation (removal of electrons) to iodine: I −→ I. As the iodide ions are being oxidized, they pass through the membrane into the lumen of the follicle.
- Iodination of tyrosine. As iodine atoms (I) form, they react with tyrosines that are part of thyroglobulin molecules. Binding of one iodine atom yields monoiodotyrosine (T1), and a second iodination produces diiodotyrosine (T2). The thyroglobulin with attached iodine atoms, a sticky material that accumulates and is stored in the lumen of the thyroid follicle, is termed colloid.
- Coupling of monoiodotyrosine (T1) and diiodotyrosine (T2). During the last step in the synthesis of thyroid hormone, two diiodotyrosine (T2) molecules join to form thyroxine (T4) or one T1 and one T2 join to form triiodothyronine (T3).
- Pinocytosis and digestion of colloid. Droplets of colloid reenter follicular cells by pinocytosis and merge with lysosomes. Digestive enzymes in the lysosomes break down thyroglobulin, cleaving off molecules of triiodothyronine (T3) and thyroxine (T4).
- Secretion of thyroid hormones. Because T3 and T4 are lipid soluble, they diffuse through the plasma membrane into interstitial fluid and then into the blood. T4 normally is secreted in greater quantity than T3, but T3 is several times more potent. Moreover, after T4 enters a body cell, most of it is converted to T3 by removal of one iodine.
- Transport thyroid hormones in the blood. More than 99% of both the T3 and the T4 combine with transport proteins in the blood, mainly thyroxine binding globulin (TBG).
Figure 4. Thyroid hormones
Actions of thyroid hormones
Because most body cells have receptors for thyroid hormones, triiodothyronine (T3) and thyroxine (T4) affect tissues throughout the body. Thyroid hormones act on their target cells mainly by inducing gene transcription and protein synthesis. The newly formed proteins in turn carry out the cellular response.
Functions of thyroid hormones include the following:
- Increase basal metabolic rate. Thyroid hormones raise the basal metabolic rate (BMR), the rate of energy expenditure under standard or basal conditions (awake, at rest, and fasting). When basal metabolic rate increases, cellular metabolism of carbohydrates, lipids, and proteins increases. Thyroid hormones increase BMR in several ways: (1) They stimulate synthesis of additional Na+/K+ ATPases, which use large amounts of ATP to continually eject sodium ions (Na+) from cytosol into extracellular fluid and potassium ions (K+) from extracellular fluid into cytosol; (2) they increase the concentrations of enzymes involved in cellular respiration, which increases the breakdown of organic fuels and ATP production; and (3) they increase the number and activity of mitochondria in cells, which also increases ATP production. As cells produce and use more ATP, basal metabolic rate increases, more heat is given off and body temperature rises, a phenomenon called the calorigenic effect. In this way, thyroid hormones play an important role in the maintenance of normal body temperature. Normal mammals can survive in freezing temperatures, but those whose thyroid glands have been removed cannot.
- Enhance actions of catechlolamines. Thyroid hormones have permissive effects on the catecholamines (epinephrine and norepinephrine) because they up-regulate β-adrenergic receptors. Catecholamines bind to β-adrenergic receptors, promoting sympathetic responses. Therefore, symptoms of excess levels of thyroid hormone include increased heart rate, more forceful heartbeats, and increased blood pressure.
- Regulate development and growth of nervous tissue and bones. Thyroid hormones are necessary for the development of the nervous system: They promote synapse formation, myelin production, and growth of dendrites. Thyroid hormones are also required for growth of the skeletal system: They promote formation of ossification centers in developing bones, synthesis of many bone proteins, and secretion of growth hormone (GH) and insulin-like growth factors (IGFs). Deficiency of thyroid hormones during fetal development, infancy, or childhood causes severe mental retardation and stunted bone growth.
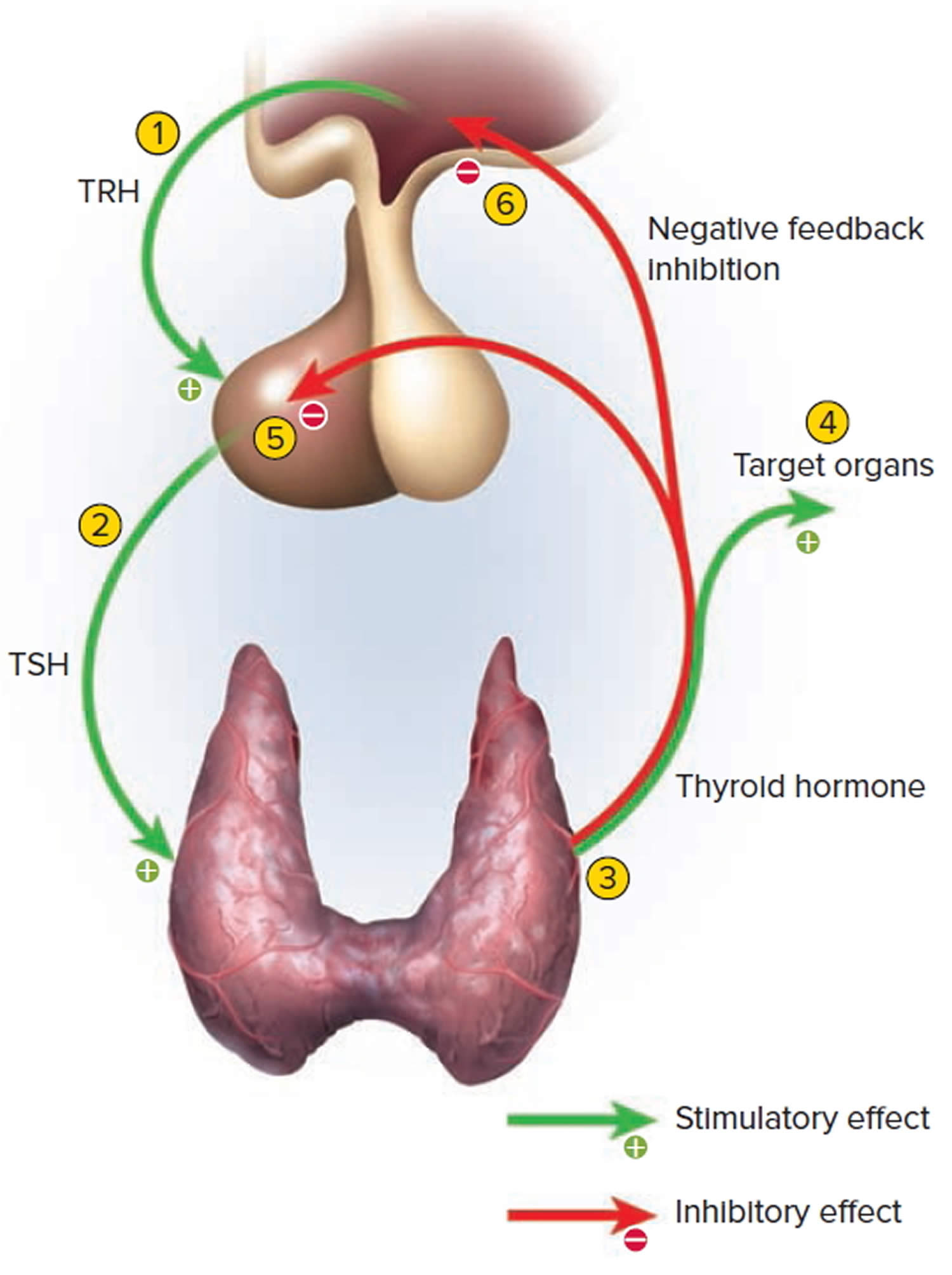
Control of thyroid hormone secretion
Thyrotropin-releasing hormone (TRH) from the hypothalamus and thyroid-stimulating hormone (TSH) from the anterior pituitary stimulate secretion of thyroid hormones, as shown in Figure 5:
- Low blood levels of T3 and T4 or low metabolic rate stimulate the hypothalamus to secrete thyrotropin-releasing hormone (TRH).
- Thyrotropin-releasing hormone (TRH) enters the hypothalamic–hypophyseal portal system and flows to the anterior pituitary, where it stimulates thyrotrophs to secrete thyroid stimulating hormone (TSH).
- Thyroid stimulating hormone (TSH) stimulates virtually all aspects of thyroid follicular cell activity, including iodide trapping, hormone synthesis and secretion, and growth of the follicular cells.
- The thyroid follicular cells release T3 and T4 into the blood until the metabolic rate returns to normal.
- An elevated level of T3 inhibits release of TRH and TSH (negative feedback inhibition).
Conditions that increase ATP demand—a cold environment, hypoglycemia, high altitude, and pregnancy—increase the secretion of the thyroid hormones.
Figure 5. Control of thyroid hormone secretion
Footnote: Negative Feedback Inhibition of the Anterior Pituitary Gland by the Thyroid Gland
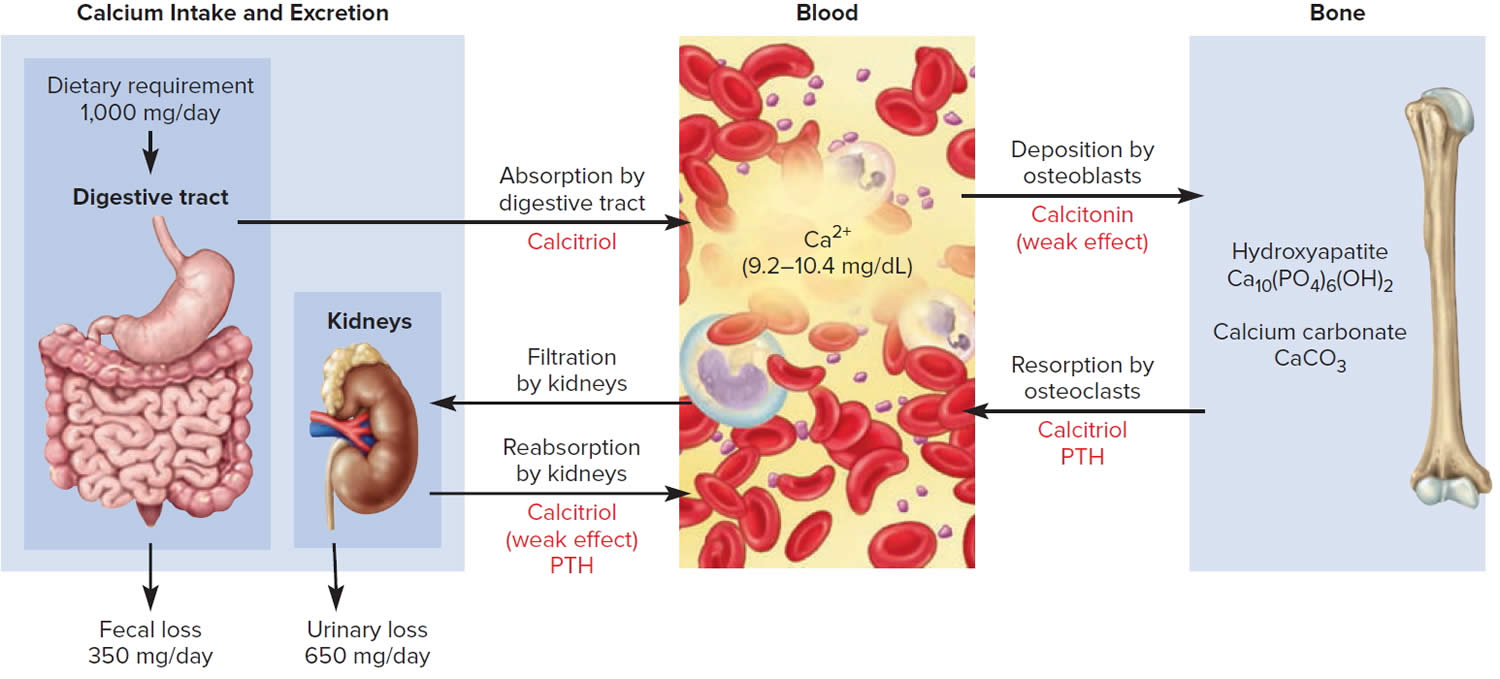
Control of calcium balance
The hormone produced by the parafollicular cells of the thyroid gland is calcitonin. Calcitonin can decrease the level of calcium in the blood by inhibiting the action of osteoclasts, the cells that break down bone extracellular matrix. The secretion of calcitonin is controlled by a negative feedback system (see Figure 7).
Calcitonin is produced by C cells (clear cells) of the thyroid gland. It is secreted when the blood calcium concentration rises too high, and it lowers the concentration by two principal mechanisms:
- Osteoclast inhibition. Within 15 minutes after it is secreted, calcitonin reduces osteoclast activity by as much as 70%, so osteoclasts liberate less calcium from the skeleton.
- Osteoblast stimulation. Within an hour, calcitonin increases the number and activity of osteoblasts, which deposit calcium into the skeleton.
Calcitonin plays an important role in children but has only a weak effect in most adults. The osteoclasts of children are highly active in skeletal remodeling and release 5 g or more of calcium into the blood each day. By inhibiting this activity, calcitonin can significantly lower the blood calcium level in children. In adults, however, the osteoclasts release only about 0.8 g of calcium per day. Calcitonin cannot change adult blood calcium very much by suppressing this lesser contribution. Calcitonin deficiency is not known to cause any adult disease. Calcitonin may, however, inhibit bone loss in pregnant and lactating women. Miacalcin, a calcitonin extract derived from salmon that is 10 times more potent than human calcitonin, is prescribed to treat osteoporosis.
Figure 6. Hormonal control of calcium balance
Footnote: The central panel represents the blood reservoir of calcium and shows its normal (safe) range. Calcitriol and Parathyroid Hormone (PTH) regulate calcium exchanges between the blood and the small intestine and kidneys (left). Calcitonin, calcitriol, and Parathyroid Hormone (PTH) regulate calcium exchanges between blood and bone (right).
Hashimoto disease causes
Hashimoto’s disease is an autoimmune disorder. The immune system creates antibodies that attack thyroid cells as if they were bacteria, viruses or some other foreign body. The immune system wrongly enlists disease-fighting agents that damage cells and lead to cell death. What causes the immune system to attack thyroid cells is not clear. Multiple factors from the external environment and the genetic background contribute to the pathogenesis of Hashimoto’s disease 16. These genetic, environmental, and existential factors provoke the immune system to produce antibodies to thyroid antigens 21, 22, 23, 24, 25, 26, 27, 28. The most important factors associated with Hashimoto’s thyroiditis are summarized in Table 3 below.
The onset of Hashimoto’s disease may be related to 29, 30, 31, 32:
- Genetic factors. Twin studies have shown an increased concordance of autoimmune thyroiditis in monozygotic twins as compared with dizygotic twins. Danish studies have demonstrated concordance rates of 55% in monozygotic twins, compared with only 3% in dizygotic twins 33. This data suggests that 79% of predisposition is due to genetic factors, allotting 21% for environmental and sex hormone influences.
- Environmental triggers, such as infection, stress or radiation exposure
- Interactions between environmental and genetic factors.
Hypothyroidism can also be caused by:
- some medicines used to treat bipolar disorder or other mental health problems
- iodine-containing medicines used to treat abnormal heart rhythm
- exposure to toxins, such as nuclear radiation
- viruses, such as hepatitis C
Several genes have been involved in Hashimoto’s disease pathogenesis, including genes of the immune response (coded in the Human Leukocyte Antigen (HLA) complex) and thyroid function 16. Other immunoregulatory genes are involved in the development of Hashimoto’s disease, including the single nucleotide polymorphisms (SNPs) in cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), protein tyrosine phosphatase non-receptor type 22 (PTPN22), and CD40 34, 21, 26, 35.
Among the environmental factors are inadequate or excessive iodine intake, infections, or the intake of certain medications 21, 26, 25, 27, 36. Several of the currently used anticancer drugs, such as interferon-alpha, may cause autoimmune thyroid dysfunction 37, 28. The role of smoking and alcohol consumption in the etiopathogenesis of Hashimoto’s disease is still not clear 16. The data suggest that moderate alcohol consumption may protect against Hashimoto’s disease and the development of overt hypothyroidism 28, 38, 39. Furthermore, some studies indicate that smoking decreases the levels of thyroid autoantibodies and the risk of hypothyroidism. However, the mechanism for these protective effects of smoking and drinking remains unclear and must be clarified with future studies 28, 38, 39. In recent years, the influence of stress on the development and course of Hashimoto’s disease has also been investigated. Some studies suggest that stress is involved in the pathogenesis of Hashimoto’s disease, while other evidence indicates that it has no effect 28, 40. A randomized controlled trial by Markomanolaki et al. 41 showed that managing stress is also important in treating Hashimoto’s disease patients. After eight weeks of stress management intervention, patients demonstrated a reduction in antithyroglobulin (anti-Tg) titers, decreased levels of stress, depression, anxiety and improved lifestyle 41. Additionally, the adequate levels of vitamin D and selenium may help prevent or delay the onset of Hashimoto’s disease 25, 27, 42, 43. Moreover, the risk of Hashimoto’s disease is increased in other autoimmune diseases 44, 28.
Most Hashimoto’s disease patients develop antibodies to a variety of thyroid antigens, the most common of which is anti-thyroid peroxidase (anti-TPO). Many also form antithyroglobulin (anti-Tg) and TSH receptor-blocking antibodies (TBII) 1. These antibodies attack the thyroid tissue, eventually leading to inadequate production of thyroid hormone. There is a small subset of the population, no more than 10-15% with the clinically evident disease, that are serum antibody-negative 1.
Table 3. Genetic, environmental and existential factors associated with Hashimoto’s thyroiditis
| Genetic Factors | Environmental Factors | Existential Factors |
|---|---|---|
| Histocompatibility genes (HLA class I and II) | Iodine | Sex |
| Immunoregulatory genes (SNPs in HLA, CTLA-4, PTPN22, CD40 genes) | Medications (e.g., interferon-α, lithium, amiodarone) | Associated diseases (e.g., type 1 diabetes mellitus, pernicious anaemia, coeliac disease, myasthenia gravis) |
| Thyroid-specific genes | Infections (e.g., hepatitis C virus) | Age |
| Genes associated with thyroid peroxidase antibody synthesis | Smoking | Pregnancy |
| Selenium | Down’s syndrome | |
| Vitamin D | Microbiome composition | |
| Alcohol | Familial aggregation | |
| Radiation Exposure |
Risk factors for Hashimoto’s disease
The following factors are associated with an increased risk of Hashimoto’s disease 4:
- Sex. Women are much more likely to get Hashimoto’s disease.
- Age. Hashimoto’s disease can occur at any age but more commonly occurs during middle age.
- Other autoimmune disease. Having another autoimmune disease — such as rheumatoid arthritis, type 1 diabetes or lupus — increases your risk of developing Hashimoto’s disease.
- Genetics and family history. You’re at higher risk for Hashimoto’s disease if others in your family have thyroid disorders or other autoimmune diseases.
- Pregnancy. Typical changes in immune function during pregnancy may be a factor in Hashimoto’s disease that begins after pregnancy.
- Excessive iodine intake. Too much iodine in the diet may function as a trigger among people already at risk for Hashimoto’s disease.
- Radiation exposure. People exposed to excessive levels of environmental radiation are more prone to Hashimoto’s disease.
Hashimoto disease pathophysiology
The exact mechanisms underlying Hashimoto’s disease pathogenesis are not fully understood 16. The development of Hashimoto’s disease is thought to be of autoimmune origin with lymphocyte infiltration of T and B cells, especially of CD4+ Th1 and the production of antithyroid antibodies 26, 45, 46, 21, 28. This leads to chronic inflammation and thyroid fibrosis and gradual atrophy of the thyroid tissue 46, 21, 45.
The current diagnosis is based on clinical symptoms correlating with laboratory results of elevated thyroid stimulating hormone (TSH) with normal to low thyroxine (T4) levels. It is interesting to note, however, that there is little evidence demonstrating the role of antithyroid peroxidase (anti-TPO) antibody in the pathogenesis of autoimmune thyroid disease 1. Anti-TPO antibodies can fix complement and, in test tube (in vitro), have been shown to bind and kill thyroid cells (thyrocytes). However, to date, there has been no correlation noted in human studies between the severity of disease and the level of anti-TPO antibody concentration in serum 1. Scientists do, however, know that positive serum anti-TPO antibody concentration is correlated with the active phase of Hashimoto’s disease 47. Other theories implicated immune complexes, containing thyroid directed antibodies, as culprits of thyroid destruction.
Hashimoto disease prevention
At the present time there is no known way to prevent Hashimoto’s disease.
Hashimoto disease signs and symptoms
Signs and symptoms of Hashimoto’s disease vary widely and are not specific to the disorder. Hashimoto’s disease progresses slowly over the years. Many people with Hashimoto’s disease may not notice signs or symptoms of the disease at first. An ordinary blood test may just show a thyroid hormone imbalance. Because the thyroid gland may grow and get larger, you may have a feeling of fullness or tightness in your throat, though it is usually not painful. You may have trouble swallowing food or liquids. You might have a swelling (a bump) in the front of your neck, the enlarged thyroid is called a goiter. After many years, or even decades, damage to the thyroid may cause the gland to shrink and the goiter to disappear.
Some people with Hashimoto’s disease have symptoms such as tiredness, forgetfulness, depression, coarse dry skin, slow heartbeat, weight gain, constipation and intolerance to cold. A blood test can tell if your thyroid gland is underactive. Other blood tests can be done to look for Hashimoto’s disease.
Eventually, the decline in thyroid hormone production can result in hypothyroidism with any of the following:
- Fatigue and sluggishness
- Increased sensitivity to cold
- Increased sleepiness
- Dry skin
- Constipation
- Muscle weakness
- Muscle aches, tenderness and stiffness
- Joint pain and stiffness
- Irregular or excessive menstrual bleeding
- Depression
- Problems with memory or concentration
- Swelling of the thyroid (goiter)
- A puffy face
- Brittle nails
- Hair loss
- Enlargement of the tongue
Because these symptoms could result from any number of disorders, it’s important to see your doctor as soon as possible for a timely and accurate diagnosis.
Hashimoto disease complications
Thyroid hormones are essential for the healthy function of many body systems. Therefore, when Hashimoto’s disease and hypothyroidism are left untreated, many complications can occur. These include:
- Goiter. A goiter is enlargement of the thyroid. As thyroid hormone production declines due to Hashimoto’s disease, the thyroid receives signals from the pituitary gland to make more. This cycle may result in a goiter. It’s generally not uncomfortable, but a large goiter can affect your appearance and may interfere with swallowing or breathing.
- Heart problems. Hypothyroidism can result in poor heart function, an enlarged heart and irregular heartbeats. It can also result in high levels of low-density lipoprotein (LDL) cholesterol — the “bad” cholesterol — that is a risk factor for cardiovascular disease and heart failure.
- Peripheral neuropathy. Hypothyroidism that goes without treatment for a long time can damage the peripheral nerves. These are the nerves that carry information from the brain and spinal cord to the rest of the body. Peripheral neuropathy may cause pain, numbness and tingling in the arms and legs.
- Infertility. Low levels of thyroid hormone can interfere with ovulation, which can limit fertility. Some of the causes of hypothyroidism, such as autoimmune disorders, also can harm fertility.
- Mental health issues. Depression or other mental health disorders may occur early in Hashimoto’s disease and may become more severe over time.
- Sexual and reproductive dysfunction. In women, hypothyroidism can result in a reduced sexual desire (libido), an inability to ovulate, and irregular and excessive menstrual bleeding. Men with hypothyroidism may have a reduced libido, erectile dysfunction and a lowered sperm count.
- Poor pregnancy outcomes. Hypothyroidism during pregnancy may increase the risk of a miscarriage or preterm birth. Babies born to women with untreated hypothyroidism are at risk for decreased intellectual abilities, autism, speech delays and other developmental disorders.
- Birth defects. Babies born to people with untreated thyroid disease may have a higher risk of birth defects compared with babies born to mothers who do not have thyroid disease. Infants with hypothyroidism present at birth that goes untreated are at risk of serious physical and mental development problems. But if the condition is diagnosed within the first few months of life, the chances of typical development are excellent.
- Myxedema coma. This rare, life-threatening condition can develop due to long-term, severe, untreated hypothyroidism. Its signs and symptoms include drowsiness followed by profound lethargy and unconsciousness. A myxedema coma may be triggered by exposure to cold, sedatives, infection or other stress on your body. Myxedema requires immediate emergency medical treatment.
Hashimoto disease diagnosis
A number of conditions may lead to the signs and symptoms of Hashimoto’s disease. If you’re experiencing any of these symptoms, your health care provider will conduct a thorough physical exam, review your medical history and ask questions about your symptoms.
Testing thyroid function
To determine if hypothyroidism is the cause of your symptoms, your doctor will order blood tests that may include the following:
- Thyroid stimulating hormone (TSH) test. Thyroid stimulating hormone (TSH) is produced by the pituitary gland. When the pituitary detects low thyroid hormones in the blood, it sends TSH to the thyroid to prompt an increase in thyroid hormone production. High TSH levels in the blood indicates hypothyroidism.
- Thyroxine (T4) tests. The main thyroid hormone is thyroxine (T4). A low blood level of T4 confirms the findings of a TSH (thyroid stimulating hormone) test and indicates the problem is within the thyroid itself.
Antibody tests
More than one disease process can lead to hypothyroidism. To determine if Hashimoto’s disease is the cause of hypothyroidism, your doctor will order an antibody test.
The intended purpose of an antibody is to flag disease-causing foreign agents that need to be destroyed by other actors in the immune system. In an autoimmune disorder, the immune system produces rogue antibodies that target healthy cells or proteins in the body.
Usually in Hashimoto’s disease, the immune system produces an antibody to thyroid peroxidase (anti-TPO), a protein that plays an important part in thyroid hormone production. Most people with Hashimoto’s disease will have TPO antibodies (anti-TPO) in their blood. Lab tests for other antibodies associated with Hashimoto’s disease may also need to be done.
Thyroglobulin antibodies (Tg) can also be a sign of Hashimoto disease. Most people with Hashimoto disease have high levels of both thyroglobulin antibodies (anti-Tg) and TPO antibodies (anti-TPO).
Circulating antibody to thyroid peroxidase (anti-TPO) are found in about 90% of Hashimoto’s disease patients. Anti-thyroglobulin antibodies (anti-Tg) are less sensitive (positive in about 60–80% of patients) and less specific than antibody to thyroid peroxidase (anti-TPO) 46, 21, 48.
You probably won’t need other tests to confirm you have Hashimoto’s disease. However, if your doctor suspects Hashimoto’s disease but you don’t have antithyroid antibodies in your blood, you may have an ultrasound of your thyroid. The ultrasound images can show the size of your thyroid and other features of Hashimoto’s disease. The ultrasound also can rule out other causes of an enlarged thyroid, such as thyroid nodules—small lumps in the thyroid gland.
Hashimoto disease treatment
How your doctors treat Hashimoto’s disease usually depends on whether your thyroid is damaged enough to cause hypothyroidism. If you don’t have hypothyroidism or you have mild hypothyroidism, your doctor may choose to simply check your symptoms and do regular thyroid stimulating hormone (TSH) tests to monitor your thyroid hormone levels.
Most people with Hashimoto’s disease need take a synthetic thyroid hormone medication called levothyroxine (Levoxyl, Synthroid, others) to treat hypothyroidism. The synthetic thyroid hormone works like the thyroxine (T4) hormone naturally produced by your thyroid. Prescribed in pill form for many years, this medicine is now also available as a liquid and in a soft gel capsule 4. These newer formulas may be helpful to people with digestive problems that affect how the thyroid hormone pill is absorbed.
Some foods and supplements can affect how well your body absorbs levothyroxine. Examples include grapefruit juice, espresso coffee, soy, and multivitamins that contain iron or calcium 3, 49. Taking levothyroxine on an empty stomach can prevent this from happening. Your doctor may ask you to take the levothyroxine in the morning, 30 to 60 minutes before you eat your first meal.
Your doctor will give you a blood test about 6 to 8 weeks after you begin taking levothyroxine and adjust your dose if needed. Each time you change your dose, you’ll have another blood test. Once you’ve reached a dose that’s working for you, your doctor will likely repeat the blood test in 6 months and then once a year.
Never stop taking your levothyroxine or take a higher dose without talking with your doctor first. Taking too much thyroid hormone medicine can cause serious problems, such as atrial fibrillation or osteoporosis 31.
Thyroxine (T4) hormone replacement therapy
Hypothyroidism associated with Hashimoto’s disease is treated with a synthetic hormone called levothyroxine (Levoxyl, Synthroid, others). The recommended dose of levothyroxine is 1.6 to 1.8 mcg/kg/day 1. The synthetic hormone works like the thyroxine (T4) hormone naturally produced by the thyroid. The treatment goal is to restore and maintain adequate thyroxine (T4) hormone levels and improve symptoms of hypothyroidism. You will need this treatment for the rest of your life.
Monitoring the dosage
Your doctor will determine a dosage of levothyroxine that’s appropriate for your age, weight, current thyroid production, other medical conditions and other factors. Your doctor will retest your TSH (thyroid stimulating hormone) levels about 6 to 10 weeks later and adjust the dosage as necessary.
Once the best dosage is determined, you will continue to take the medication once a day. You’ll need follow-up tests once a year to monitor TSH (thyroid stimulating hormone) levels or any time after your doctor changes your dosage.
A levothyroxine pill is usually taken in the morning before you eat. Talk to your doctor if you have any questions about when or how to take the pill. Also, ask what to do if you accidentally skip a dose. If your health insurance requires you to switch to a generic drug or a different brand, talk to your doctor.
Precautions
Because levothyroxine acts like natural thyroxine (T4) in your body, there are generally no side effects as long as the treatment is resulting in “natural” levels of thyroxine (T4) for your body.
Too much thyroid hormone can worsen bone loss that causes weak, brittle bones (osteoporosis) or cause irregular heartbeats (arrhythmias) the most common being atrial fibrillation.
Effects of other substances
Certain medications, supplements and foods may affect your ability to absorb levothyroxine. It may be necessary to take levothyroxine at least four hours before these substances. Talk to your doctor about any of the following:
- Soy products
- High-fiber foods
- Iron supplements, including multivitamins that contain iron
- Cholestyramine (Prevalite), a medication used to lower blood cholesterol levels
- Aluminum hydroxide, which is found in some antacids
- Sucralfate, an ulcer medication
- Calcium supplements
Triiodothyronine (T3) hormone replacement therapy
Naturally produced thyroxine (T4) is converted into another thyroid hormone called triiodothyronine (T3). The thyroxine (T4) replacement hormone is also converted into triiodothyronine (T3), and for most people the thyroxine (T4) replacement therapy results in an adequate supply of triiodothyronine (T3) for the body.
For people who need better symptom control, a doctor also may prescribe a synthetic triiodothyronine (T3) (Cytomel) or a synthetic T4 and T3 combination. Side effects of triiodothyronine (T3) hormone replacement include rapid heartbeat, insomnia and anxiety. These treatments may be tested with a trial period of 3 to 6 months.
Levothyroxine (T4) and triiodothyronine (T3) combination therapy
Several trials using combined levothyroxine (T4)+ triiodothyronine or liothyronine (T3) therapy have been done in the past 15 years 50. Although some studies show a beneficial effect 51, 52, 53, 54, such as patient preference for combination therapy or an improved metabolic profile, patients on combination therapy generally do not have improved outcomes compared with those on levothyroxine monotherapy 55. Possible explanations include inadequate levothyroxine (T4) and liothyronine (T3) doses or frequency of administration 56. Liothyronine (T3) has a short half-life and no previous studies used a slow-release tri-iodothyronine. Additionally, most trials were of short duration (<4 months) and used questionnaires to record patients’ symptoms, which might not have been targeted or sensitive enough.
Alternatively, trials might have failed to identify the appropriate subgroups that would benefit from combination therapy 31. Most trials did not specifically recruit patients who feel unwell on levothyroxine or those with particularly low serum tri-iodothyronine concentrations. Individuals with genetic variations in thyroid hormone metabolism have not been specifically targeted 56. A subgroup that could be targeted is individuals with common genetic variations in DIO2, which encodes the deiodinase 2 enzyme that converts thyroxine to tri-iodothyronine locally in several tissues, including the brain 57. The Thr92Ala polymorphism in DIO2 gives rise to deiodinase 2 with a longer half-life than the wild-type enzyme and ectopic localisation in the Golgi apparatus. It was shown to alter expression profiles in the cerebral cortex in a similar pattern as seen in neurodegenerative disease, without evidence of altered thyroid hormone signalling 58. In a study of 552 people 59, the Thr92Ala polymorphism in DIO2 was associated with lower baseline psychological wellbeing in patients on levothyroxine replacement therapy and with better response to combination therapy, compared with patients without the polymorphism on levothyroxine replacement therapy. However, after appropriate multiple testing correction, the results were not significant. Results from a population-based cohort study 60 showed no effect of the Thr92Ala polymorphism on quality of life or cognitive function measures. Sufficiently powered prospective randomised controlled trials are therefore needed before conclusions can be drawn.
Although the American Thyroid Association 61 and European Thyroid Association guidelines 62 generally recommend against the routine use of combination therapy in patients with hypothyroidism, the recommendations concerning trials in patients with persistent symptoms differ slightly. The European Thyroid Association states that a 3 month trial of levothyroxine–liothyronine combination might be considered experimentally in adherent, biochemically well controlled patients who have persistent complaints despite levothyroxine treatment and provides methods for calculating levothyroxine and liothyronine doses 62. However, treatment should be initiated only by accredited doctors of internal medicine or endocrinologists, closely monitored, and discontinued if no improvement is seen. By contrast, the American Thyroid Association recommends against any routine use of such trials outside of formal research and clinical trials, mainly because of uncertainty regarding benefit and long-term safety 61. Both the European Thyroid Association and American Thyroid Association agree on the need for long-term randomised controlled trials to assess risk-benefit ratios. Such trials would need to incorporate investigation of the ideal thyroid parameters to monitor during combination therapy, and whether tri-iodothyronine concentrations would be an important parameter. The timing of phlebotomy is also important, particularly if liothyronine is being administered more than once daily.
Little evidence exists to support other therapies for hypothyroidism. The use of thyroid extracts or liothyronine monotherapy is generally not recommended because of potential safety concerns associated with the presence of supraphysiological serum tri-iodothyronine concentrations and a paucity of long-term safety outcome data. The use of compounded thyroid hormones, dietary supplements, and any over-the-counter drug for the treatment of hypothyroidism is discouraged.
Alternative medicine
Products with triiodothyronine (T3) and thyroxine (T4) hormones derived from pigs or other animals are available as prescriptions or as dietary supplements, such as Armour Thyroid, in the United States. Concerns about these products include the following:
- The balance of thyroxine (T4) and triiodothyronine (T3) in animals isn’t the same as in humans.
- The exact amount of thyroxine (T4) and triiodothyronine (T3) in each batch of a natural extract product can vary, leading to unpredictable levels of these hormones in your blood.
Anti-inflammatory diet
An anti-inflammatory diet rich in vitamins, minerals and polyphenols is recommended as diet therapy for Hashimoto’s disease 63, 36, 20. The theory behind the inflammation has to do with the leaky gut syndrome, where there is an insult to the gut mucosa, which allows the penetrance of proteins that do not typically enter the bloodstream via transporters in the gut mucosa. It is theorized that a response similar to molecular mimicry occurs, and antibodies are produced against the antigens. Unfortunately, the antigen may be very structurally similar to thyroid peroxidase, leading to antibody formation against this enzyme. The concept of an autoimmune diet is based on healing the gut and decreasing the severity of the autoimmune response.
Natural antioxidants like vitamin A, vitamin C and vitamin E are found in products of plant origin, including a wide variety of vegetables and fruits. Sources of vitamin C include broccoli, peppers, black currant, strawberries, lemons, spinach, kiwifruit, oranges, grapefruit, limes, tomatoes, raspberries, asparagus, pineapples, fennel and parsley. The best source of vitamin E is avocado, nuts, seeds, egg, milk and whole grains. In addition, vitamin A is present in foods such as liver, carrot, broccoli, butter, pumpkin, cheese, egg, mango and milk 64. According to the current findings, the Mediterranean diet may show the most benefits for Hashimoto’s disease patients with its antioxidant properties 65.
One study by Ostrowska et al. 66 assessed the effectiveness of two “reducing diets” and their effect on thyroid parameters in female obese patients with Hashimoto’s disease. All women who received levothyroxine, selenium and zinc were randomly assigned to the study group following individually balanced elimination/reducing diets, in accordance with the previously performer food sensitivity tests, and the control group following reducing diets with the same caloric content, but without product elimination. The anthropometric and thyroid parameters have changed in both groups during the nutritional intervention. This research showed that weight reduction may improve thyroid function in patients suffering from obesity and Hashimoto’s disease 66. Moreover, an individually selected elimination reducing diet was more effective than classic reducing diets with the same energy intake and macronutrient content and can lead to better therapeutic outcomes, which may cause an anti-inflammatory effect 66.
One case report 67 showed a novel approach that led to the improvement of symptoms and a reduction of thyroid antibodies in a 23-year-old woman with Hashimoto’s disease. The woman presented with symptoms of fatigue, hair loss, energy and mood disturbance, problems with insomnia and daytime napping. The thyroid antibodies were strongly positive, with a normal TSH level. Integrative treatment was started, which involved nutritional changes and micronutrient supplementation 67. This supplementation supported the methylation cycle, anti-oxidant capacity and stress management, and included vitamin C, vitamin B1, vitamin B2, vitamin B5, vitamin B6, Pyridoxal-5 Phosphate, zinc picolonate, L-5 methyltetrahydrofolate, magnesium glycinate, selenomethionine, N- Acetyl Cysteine and methylcobalamin (vitamin B12). The patient followed a paleo-style diet without grains and dairy products and increased consumption of bone broth and fermented foods as well as organic animal protein as tolerated. In addition, daily meditation and mindfulness techniques were recommended, and gentle exercise three times a week was added. After 15 months of treatment, there was a reduction in antithyroid antibodies and a significant relief of symptoms. This case demonstrated the potential benefits of an integrative approach to autoimmunity and oxidative stress in Hashimoto’s disease 67.
In a pilot study by Abbott et al. 68, women participated in a 10-week online health coaching program focused on implementing an “autoimmune protocol diet”. They applied a modified paleolithic diet. In the referred study, there were no significant changes in thyroid function markers, as well as serum antithyroid antibody concentrations, although the number of immune cells and an inflammatory processes marker (high sensitivity CRP) were decreased. These results suggest that an “autoimmune protocol” may decrease inflammation and modulate the immune system. Moreover, the therapy improves health-related quality of life (measured by 36-Item Short-Form Health Survey) and reduces symptoms of the diseases (measured by the Medical Symptoms Questionnaire) 68. A case study with a 49-year-old obese Hashimoto’s disease woman indicated that a modified autoimmune paleo low-calorie diet might improve TSH, anti-TPO antibody, body composition and lipid profile 69.
- Mincer DL, Jialal I. Hashimoto Thyroiditis. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459262[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Hashimoto’s Disease: What It Is and How It’s Treated. https://www.aafp.org/pubs/afp/issues/2000/0215/p1054.html[↩]
- Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012 Nov-Dec;18(6):988-1028. doi: 10.4158/EP12280.GL. Erratum in: Endocr Pract. 2013 Jan-Feb;19(1):175.[↩][↩]
- Ragusa F, Fallahi P, Elia G, Gonnella D, Paparo SR, Giusti C, Churilov LP, Ferrari SM, Antonelli A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab. 2019 Dec;33(6):101367. doi: 10.1016/j.beem.2019.101367[↩][↩][↩]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press, 2001. https://www.nap.edu/read/10026/chapter/2[↩][↩]
- World Health Organization. United Nations Children’s Fund & International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. Geneva, Switzerland: WHO, 2007. http://apps.who.int/iris/bitstream/handle/10665/43781/9789241595827_eng.pdf;jsessionid=2E9F56538AEFD33C83934FB34BD4E8C4[↩]
- WHO Secretariat, Andersson M, de Benoist B, Delange F, Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007 Dec;10(12A):1606-11. doi: 10.1017/S1368980007361004. Erratum in: Public Health Nutr. 2008 Mar;11(3):327.[↩]
- Zimmermann MB. Iodine deficiency. Endocr Rev. 2009 Jun;30(4):376-408. doi: 10.1210/er.2009-0011[↩]
- USDA, FDA, and ODS-NIH Database for the Iodine Content of Common Foods Release 1.0. 2020. https://www.ars.usda.gov/ARSUSERFILES/80400535/DATA/IODINE/IODINE_DATABASE_PDFVersion_2020.PDF[↩][↩][↩][↩]
- Pennington JA, Young B. Iron, zinc, copper, manganese, selenium, and iodine in foods from the United States Total Diet Studyexternal link disclaimer. J Food Compost Anal. 1990 June;3(2):166-184. https://www.sciencedirect.com/science/article/abs/pii/088915759090022E[↩]
- Ershow AG, Skeaff SA, Merkel JM, Pehrsson PR. Development of Databases on Iodine in Foods and Dietary Supplements. Nutrients. 2018 Jan 17;10(1):100. doi: 10.3390/nu10010100[↩][↩]
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels[↩]
- Patterson KY, Spungen JH, Roseland JM, Pehrsson PR, Ershow AG, Gahche JJ. USDA-FDA-ODS database for the iodine content of common foods (release one). Iodine database PDF. Methods and Application of Food Composition Laboratory, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture, Beltsville MD. July 2020. https://www.ars.usda.gov/ARSUSERFILES/80400535/DATA/IODINE/IODINE_DATABASE.PDF[↩]
- Pennington JAT, Schoen SA, Salmon GD, Young B, Johnson RD, Marts RW. Composition of Core Foods of the U.S. Food Supply, 1982-1991. III. Copper, Manganese, Selenium, and Iodine. J Food Comp Anal. 1995;8(2):171-217. https://www.sciencedirect.com/science/article/abs/pii/S0889157585710149[↩]
- Teas J, Pino S, Critchley A, Braverman LE. Variability of iodine content in common commercially available edible seaweeds. Thyroid. 2004 Oct;14(10):836-41. doi: 10.1089/thy.2004.14.836[↩]
- Mikulska AA, Karaźniewicz-Łada M, Filipowicz D, Ruchała M, Główka FK. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management-An Overview. Int J Mol Sci. 2022 Jun 13;23(12):6580. doi: 10.3390/ijms23126580[↩][↩][↩][↩][↩][↩]
- Krysiak R., Szkróbka W., Okopień B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp. Clin. Endocrinol. Diabetes. 2019;127:417–422. doi: 10.1055/a-0653-7108[↩][↩]
- Velija A.Z., Hadzovic-Dzuvo A., Al T.D. Endocrine Abstracts. Volume 70 Bioscientifica; Bristol, UK: 2020. The Effect of Selenium Supplementation and Gluten-Free Diet in Patients with Subclinical Hypothyroidism Affected by Autoimmune Thyroiditis.[↩][↩][↩][↩]
- Pobłocki J., Pańka T., Szczuko M., Telesiński A., Syrenicz A. Whether a Gluten-Free Diet Should Be Recommended in Chronic Autoimmune Thyroiditis or Not?—A 12-Month Follow-Up. J. Clin. Med. 2021;10:3240. doi: 10.3390/jcm10153240[↩][↩][↩]
- Szczuko M., Syrenicz A., Szymkowiak K., Przybylska A., Szczuko U., Pobłocki J., Kulpa D. Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease. Nutrients. 2022;14:1727. doi: 10.3390/nu14091727[↩][↩]
- Ragusa F., Fallahi P., Elia G., Gonnella D., Paparo S.R., Giusti C., Churilov L.P., Ferrari S.M., Antonelli A. Hashimotos’ Thyroiditis: Epidemiology, Pathogenesis, Clinic and Therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019;33:101367. doi: 10.1016/j.beem.2019.101367[↩][↩][↩][↩][↩][↩]
- Shukla S.K., Singh G., Ahmad S., Pant P. Infections, Genetic and Environmental Factors in Pathogenesis of Autoimmune Thyroid Diseases. Microb. Pathog. 2018;116:279–288. doi: 10.1016/j.micpath.2018.01.004[↩]
- Weetman A.P. An Update on the Pathogenesis of Hashimoto’s Thyroiditis. J. Endocrinol. Invest. 2021;44:883–890. doi: 10.1007/s40618-020-01477-1[↩]
- Ferrari S.M., Fallahi P., Antonelli A., Benvenga S. Environmental Issues in Thyroid Diseases. Front. Endocrinol. 2017;8:50. doi: 10.3389/fendo.2017.00050[↩]
- Wiersinga W.M. Clinical Relevance of Environmental Factors in the Pathogenesis of Autoimmune Thyroid Disease. Endocrinol. Metab. 2016;31:213–222. doi: 10.3803/EnM.2016.31.2.213[↩][↩][↩]
- Ralli M., Angeletti D., Fiore M., D’Aguanno V., Lambiase A., Artico M., de Vincentiis M., Greco A. Hashimoto’s Thyroiditis: An Update on Pathogenic Mechanisms, Diagnostic Protocols, Therapeutic Strategies, and Potential Malignant Transformation. Autoimmun. Rev. 2020;19:102649. doi: 10.1016/j.autrev.2020.102649[↩][↩][↩][↩]
- Effraimidis G., Wiersinga W.M. Mechanisms in Endocrinology: Autoimmune Thyroid Disease: Old and New Players. Eur. J. Endocrinol. 2014;170:R241–R252. doi: 10.1530/EJE-14-0047[↩][↩][↩]
- Ajjan R.A., Weetman A.P. The Pathogenesis of Hashimoto’s Thyroiditis: Further Developments in Our Understanding. Horm. Metab. Res. 2015;47:702–710. doi: 10.1055/s-0035-1548832[↩][↩][↩][↩][↩][↩][↩]
- Leung AKC, Leung AAC. Evaluation and management of the child with hypothyroidism. World J Pediatr. 2019 Apr;15(2):124-134. doi: 10.1007/s12519-019-00230-w[↩]
- Yuan J, Sun C, Jiang S, Lu Y, Zhang Y, Gao XH, Wu Y, Chen HD. The Prevalence of Thyroid Disorders in Patients With Vitiligo: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2019 Jan 15;9:803. doi: 10.3389/fendo.2018.00803[↩]
- Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017 Sep 23;390(10101):1550-1562. doi: 10.1016/S0140-6736(17)30703-1[↩][↩][↩]
- Ott J, Promberger R, Kober F, Neuhold N, Tea M, Huber JC, Hermann M. Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid. 2011 Feb;21(2):161-7. doi: 10.1089/thy.2010.0191. Epub 2010 Dec 27. Erratum in: Thyroid. 2011 Apr;21(4):467.[↩]
- Brix TH, Hegedüs L, Gardas A, Banga JP, Nielsen CH. Monozygotic twin pairs discordant for Hashimoto’s thyroiditis share a high proportion of thyroid peroxidase autoantibodies to the immunodominant region A. Further evidence for genetic transmission of epitopic “fingerprints”. Autoimmunity. 2011 May;44(3):188-94. doi: 10.3109/08916934.2010.518575[↩]
- Klubo-Gwiezdzinska J., Wartofsky L. Hashimoto Thyroiditis: An Evidence-Based Guide to Etiology, Diagnosis and Treatment. Pol. Arch. Intern. Med. 2022;132:16222. doi: 10.20452/pamw.16222[↩]
- Kust D., Matesa N. The Impact of Familial Predisposition on the Development of Hashimoto’s Thyroiditis. Acta Clin. Belg. 2020;75:104–108. doi: 10.1080/17843286.2018.1555115[↩]
- Ihnatowicz P., Drywień M., Wątor P., Wojsiat J. The Importance of Nutritional Factors and Dietary Management of Hashimoto’s Thyroiditis. Ann. Agric. Environ. Med. 2020;27:184–193. doi: 10.26444/aaem/112331[↩][↩]
- Torino F., Barnabei A., Paragliola R., Baldelli R., Appetecchia M., Corsello S.M. Thyroid Dysfunction as an Unintended Side Effect of Anticancer Drugs. Thyroid. 2013;23:1345–1366. doi: 10.1089/thy.2013.0241[↩]
- Carlé A., Pedersen I.B., Knudsen N., Perrild H., Ovesen L., Rasmussen L.B., Jørgensen T., Laurberg P. Moderate Alcohol Consumption May Protect against Overt Autoimmune Hypothyroidism: A Population-Based Case-Control Study. Eur. J. Endocrinol. 2012;167:483–490. doi: 10.1530/EJE-12-0356[↩][↩]
- Effraimidis G., Tijssen J.G.P., Wiersinga W.M. Alcohol Consumption as a Risk Factor for Autoimmune Thyroid Disease: A Prospective Study. Eur. Thyroid J. 2012;1:99–104. doi: 10.1159/000338920[↩][↩]
- Effraimidis G., Tijssen J.G.P., Brosschot J.F., Wiersinga W.M. Involvement of Stress in the Pathogenesis of Autoimmune Thyroid Disease: A Prospective Study. Psychoneuroendocrinology. 2012;37:1191–1198. doi: 10.1016/j.psyneuen.2011.12.009[↩]
- Markomanolaki ZS, Tigani X, Siamatras T, Bacopoulou F, Tsartsalis A, Artemiadis A, Megalooikonomou V, Vlachakis D, Chrousos GP, Darviri C. Stress Management in Women with Hashimoto’s thyroiditis: A Randomized Controlled Trial. J Mol Biochem. 2019;8(1):3-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6688766[↩][↩]
- Rostami R., Nourooz-Zadeh S., Mohammadi A., Khalkhali H.R., Ferns G., Nourooz-Zadeh J. Serum Selenium Status and Its Interrelationship with Serum Biomarkers of Thyroid Function and Antioxidant Defense in Hashimoto’s Thyroiditis. Antioxidants. 2020;9:1070. doi: 10.3390/antiox9111070[↩]
- Mazokopakis EE, Papadomanolaki MG, Tsekouras KC, Evangelopoulos AD, Kotsiris DA, Tzortzinis AA. Is vitamin D related to pathogenesis and treatment of Hashimoto’s thyroiditis? Hell J Nucl Med. 2015 Sep-Dec;18(3):222-7.[↩]
- Wiebolt J., Achterbergh R., den Boer A., van der Leij S., Marsch E., Suelmann B., de Vries R., van Haeften T.W. Clustering of Additional Autoimmunity Behaves Differently in Hashimoto’s Patients Compared with Graves’ Patients. Eur. J. Endocrinol. 2011;164:789–794. doi: 10.1530/EJE-10-1172[↩]
- Rydzewska M., Jaromin M., Pasierowska I.E., Stożek K., Bossowski A. Role of the T and B Lymphocytes in Pathogenesis of Autoimmune Thyroid Diseases. Thyroid Res. 2018;11:2. doi: 10.1186/s13044-018-0046-9[↩][↩]
- Caturegli P., De Remigis A., Rose N.R. Hashimoto Thyroiditis: Clinical and Diagnostic Criteria. Autoimmun. Rev. 2014;13:391–397. doi: 10.1016/j.autrev.2014.01.007[↩][↩][↩]
- Williams DE, Le SN, Godlewska M, Hoke DE, Buckle AM. Thyroid Peroxidase as an Autoantigen in Hashimoto’s Disease: Structure, Function, and Antigenicity. Horm Metab Res. 2018 Dec;50(12):908-921. doi: 10.1055/a-0717-5514[↩]
- Iddah M.A., Macharia B.N. Autoimmune Thyroid Disorders. ISRN Endocrinol. 2013;2013:e509764. doi: 10.1155/2013/509764[↩]
- Burch HB. Drug Effects on the Thyroid. N Engl J Med. 2019 Aug 22;381(8):749-761. doi: 10.1056/NEJMra1901214[↩]
- Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med. 1999 Feb 11;340(6):424-9. doi: 10.1056/NEJM199902113400603[↩]
- Nygaard B, Jensen EW, Kvetny J, Jarløv A, Faber J. Effect of combination therapy with thyroxine (T4) and 3,5,3′-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur J Endocrinol. 2009 Dec;161(6):895-902. doi: 10.1530/EJE-09-0542[↩]
- Walsh JP, Shiels L, Lim EM, Bhagat CI, Ward LC, Stuckey BG, Dhaliwal SS, Chew GT, Bhagat MC, Cussons AJ. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003 Oct;88(10):4543-50. doi: 10.1210/jc.2003-030249[↩]
- Appelhof BC, Fliers E, Wekking EM, Schene AH, Huyser J, Tijssen JG, Endert E, van Weert HC, Wiersinga WM. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005 May;90(5):2666-74. doi: 10.1210/jc.2004-2111[↩]
- Escobar-Morreale HF, Botella-Carretero JI, Gómez-Bueno M, Galán JM, Barrios V, Sancho J. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005 Mar 15;142(6):412-24. doi: 10.7326/0003-4819-142-6-200503150-00007[↩]
- Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2006 Jul;91(7):2592-9. doi: 10.1210/jc.2006-0448[↩]
- Wiersinga WM. Paradigm shifts in thyroid hormone replacement therapies for hypothyroidism. Nat Rev Endocrinol. 2014 Mar;10(3):164-74. doi: 10.1038/nrendo.2013.258[↩][↩]
- McAninch EA, Bianco AC. New insights into the variable effectiveness of levothyroxine monotherapy for hypothyroidism. Lancet Diabetes Endocrinol. 2015 Oct;3(10):756-8. doi: 10.1016/S2213-8587(15)00325-3[↩]
- McAninch EA, Jo S, Preite NZ, Farkas E, Mohácsik P, Fekete C, Egri P, Gereben B, Li Y, Deng Y, Patti ME, Zevenbergen C, Peeters RP, Mash DC, Bianco AC. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J Clin Endocrinol Metab. 2015 Mar;100(3):920-33. doi: 10.1210/jc.2014-4092[↩]
- Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009 May;94(5):1623-9. doi: 10.1210/jc.2008-1301[↩]
- Wouters HJ, van Loon HC, van der Klauw MM, et al. No effect of the Thr92Ala polymorphism of deiodinase-2 on thyroid hormone parameters, health-related quality of life, and cognitive functioning in a large population-based cohort study. Thyroid 2016; published online Oct 27 DOI: 10.1089/thy.2016.0199[↩]
- Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014 Dec;24(12):1670-751. doi: 10.1089/thy.2014.0028[↩][↩]
- Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA Guidelines: The Use of L-T4 + L-T3 in the Treatment of Hypothyroidism. Eur Thyroid J. 2012 Jul;1(2):55-71. doi: 10.1159/000339444[↩][↩]
- Kawicka A., Regulska-Ilow B., Regulska-Ilow B. Metabolic Disorders and Nutritional Status in Autoimmune Thyroid Diseases. Postepy Hig. Med. Doswiadczalnej Online. 2015;69:80–90. doi: 10.5604/17322693.1136383[↩]
- Landete J.M. Dietary Intake of Natural Antioxidants: Vitamins and Polyphenols. Crit. Rev. Food Sci. Nutr. 2013;53:706–721. doi: 10.1080/10408398.2011.555018[↩]
- Ruggeri R.M., Giovinazzo S., Barbalace M.C., Cristani M., Alibrandi A., Vicchio T.M., Giuffrida G., Aguennouz M.H., Malaguti M., Angeloni C., et al. Influence of Dietary Habits on Oxidative Stress Markers in Hashimoto’s Thyroiditis. Thyroid. 2021;31:96–105. doi: 10.1089/thy.2020.0299[↩]
- Ostrowska L., Gier D., Zyśk B. The Influence of Reducing Diets on Changes in Thyroid Parameters in Women Suffering from Obesity and Hashimoto’s Disease. Nutrients. 2021;13:862. doi: 10.3390/nu13030862[↩][↩][↩]
- Avard N., Grant S. A Case Report of a Novel, Integrative Approach to Hashimoto’s Thyroiditis with Unexpected Results. Adv. Integr. Med. 2018;5:75–79. doi: 10.1016/j.aimed.2018.03.003[↩][↩][↩]
- Abbott R.D., Sadowski A., Alt A.G. Efficacy of the Autoimmune Protocol Diet as Part of a Multi-Disciplinary, Supported Lifestyle Intervention for Hashimoto’s Thyroiditis. Cureus. 2019;11:e4556. doi: 10.7759/cureus.4556[↩][↩]
- Al-Bayyari N.S. Successful Dietary Intervention Plan for Hashimoto’s Thyroiditis: A Case Study. Rom. J. Diabetes Nutr. Metab. Dis. 2020;27:381–385.[↩]