What vitamin is good for bones
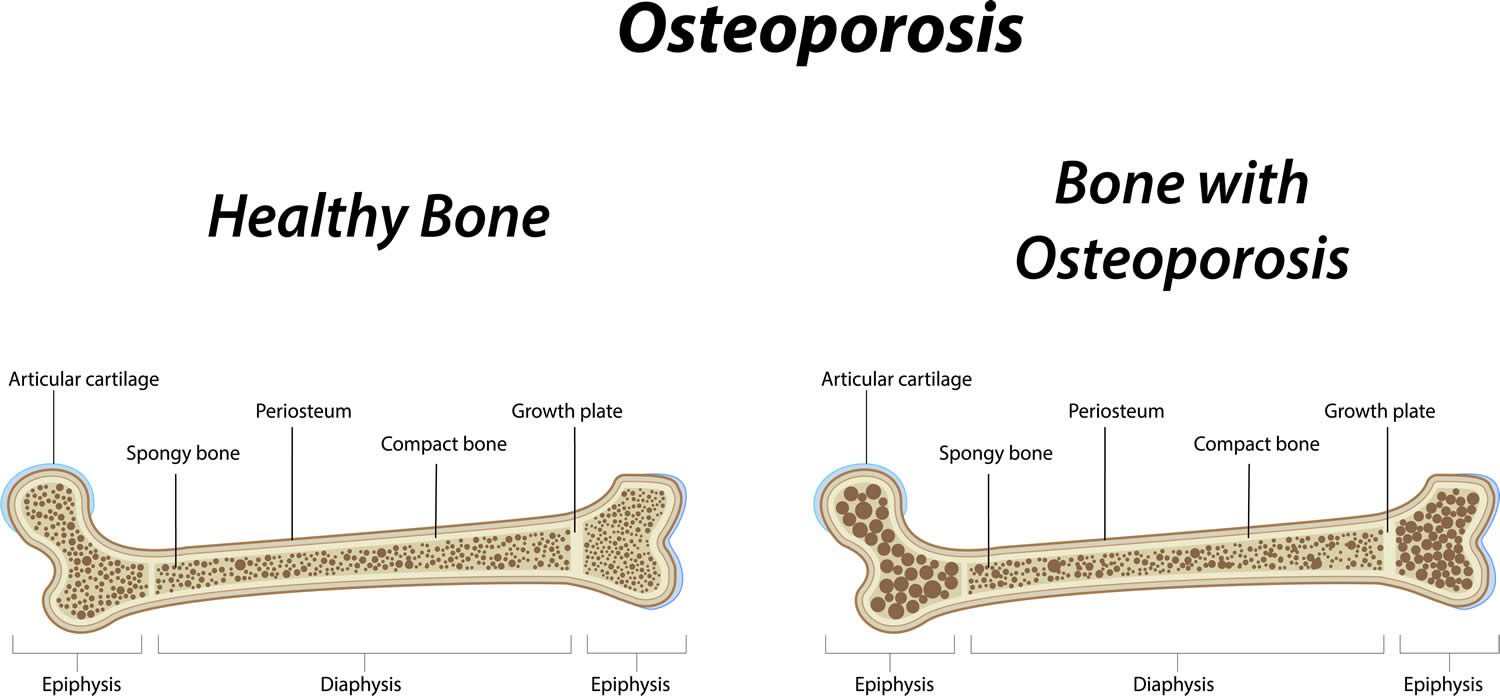
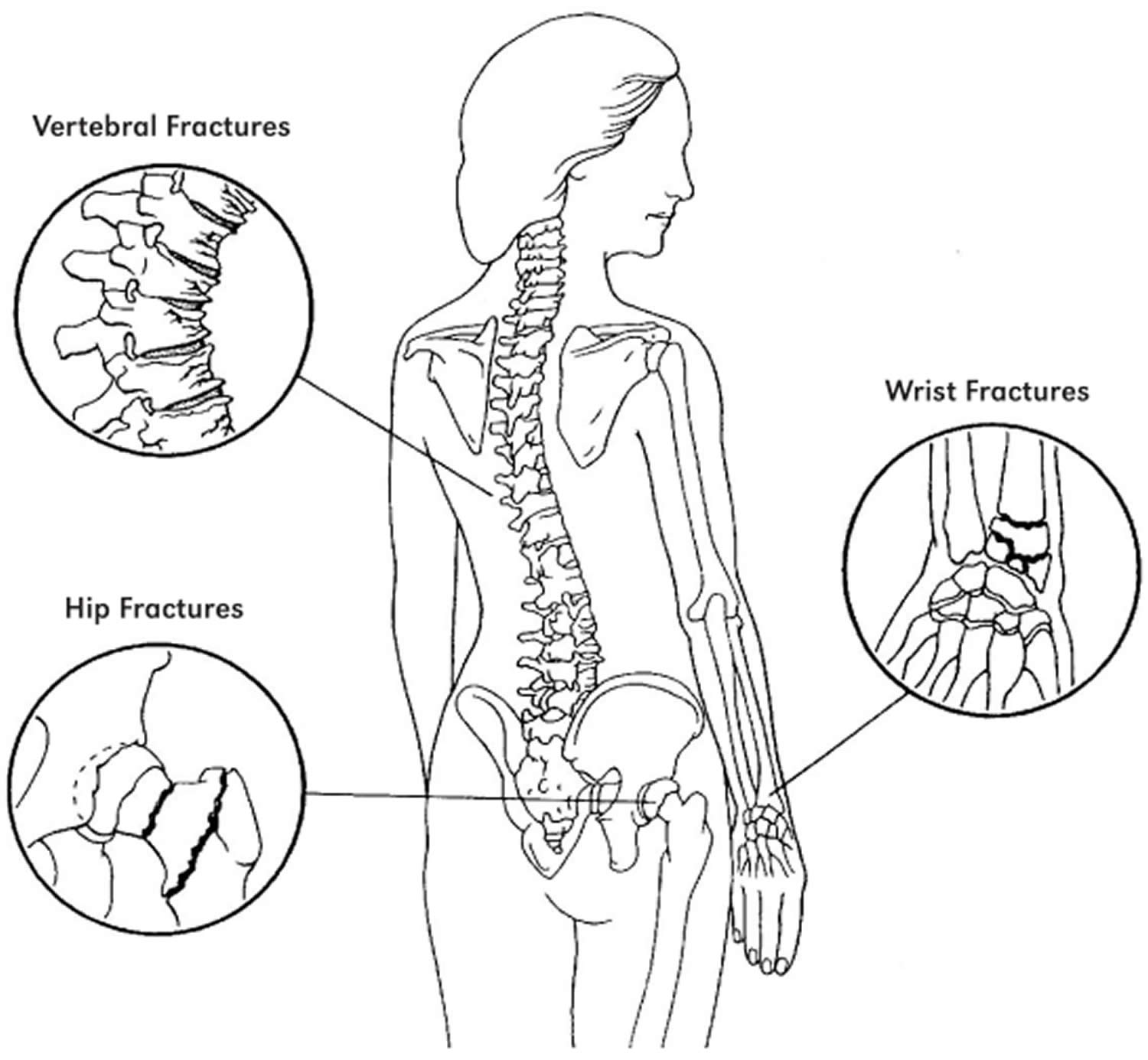
Osteoporosis is a long-term (chronic) bone disorder that causes your bones to become thin, weak and break easily. In osteoporosis your bones become fragile and fracture (break) easily, especially the bones in your hip, spine, and wrist. Other sites where broken bones occur include the ankle, leg, forearm, upper arm and ribs. These fractures typically occur after a minor trip, fall or similar incident. Your bone is living tissue that is constantly being broken down and replaced. Bones increase in size and mass during periods of growth in childhood and adolescence, reaching peak bone mass around age 30. The greater your peak bone mass, the longer you can delay serious bone loss with increasing age. Peak bone mass is determined largely by genetic factors, with contributions from nutrition, endocrine status, physical activity, and health during growth 1. Everyone should therefore consume adequate amounts of calcium and vitamin D throughout childhood, adolescence, and early adulthood.
Bone mass in older adults equals the peak bone mass achieved by age 18–25 minus the amount of bone subsequently lost 2. Osteoporosis occurs when the creation of new bone doesn’t keep up with the loss of old bone. Broken bones can occur in patients with either osteoporosis or osteopenia (low bone density). Once a fracture occurs the person is considered to be at much higher risk of another fracture. The operational definition of osteoporosis is based on the T-score for bone mineral density (BMD) assessed by dual energy x-ray absorptiometry (DEXA) at the femoral neck or spine and is defined as a value for BMD 2.5 standard deviation (SD) or more below the young female adult mean. World Health Organization (WHO) criteria define a normal T-score value as within 1 standard deviation (SD) of the mean BMD value in a healthy young adult. Secondary osteoporosis is due to the presence of underlying disease or medications, can also lead to low bone mass, resulting in increased risk of fractures 3. Osteoporosis is called a “silent” disease, because you may have bone loss for many years without any symptoms until you break a bone. Osteoporotic fractures can cause severe pain and lead to a significant decrease in quality of life, with increased morbidity, mortality, and disability 4. Osteoporotic fracture can make it harder to do daily tasks on your own, such as walking. Over 50 percent of postmenopausal white women will have an osteoporotic-related fracture, and only 33 percent of senior women who have a hip fracture will be able to return to independent living 5. In white men, the risk of an osteoporotic fracture is 20 percent, however, the one-year mortality in men who have a hip fracture is twice that of women. Black males and females have a decreased incidence of osteoporosis compared to white, however, those diagnosed with osteoporosis have similar fracture risks 5. The aging of the American population is expected to triple the number of osteoporotic fractures 6.
Anyone can develop osteoporosis, but it is more common in older women. Osteoporosis is caused by a decrease in bone density, which makes your bones more fragile and easily broken. Everyone’s bones become weaker as they age, but in some people this process happens too quickly. You are more likely to develop osteoporosis if you have risk factors for the disease. Some of the risk factors can be reduced through lifestyle changes or medications but others, such as your age, cannot be changed.
Research has identified common risk factors for developing osteoporosis. Your doctor should investigate these risk factors. This mainly applies to patients 50 years and over but can also apply to younger adults. A bone density scan is the most common test to help diagnose osteoporosis.
Risk factors that cannot be changed include:
- Being over 70 years of age. Your chances of getting osteoporosis increase as you get older.
- Being female. You have a greater chance of getting osteoporosis if you are a woman. Women have smaller bones than men and lose bone faster than men do because of hormone changes that happen after menopause.
- Having fallen in the past
- Your parents having had hip fractures. Having a close family member who has osteoporosis or has broken a bone may also increase your risk.
- Early menopause
- Ethnicity. White women and Asian women are most likely to get osteoporosis. Hispanic women and African American women are also at risk, but less so.
Risk factors that can be reduced include:
- Not being physically active. Not exercising and not being active for long periods of time can increase your chances of getting osteoporosis. Like muscles, bones become stronger and stay stronger with regular exercise.
- Low muscle mass and strength
- Low body weight (BMI below 20kg/m²). Being too thin makes you more likely to get osteoporosis.
- Smoking. Smoking cigarettes can keep your body from using the calcium in your diet. Also, women who smoke go through menopause earlier than those who don’t smoke. These things can increase your risk for osteoporosis.
- High alcohol intake. People who drink a lot are more likely to get osteoporosis.
- Not eating enough energy-rich foods or proteins.
- Getting too little calcium can increase your chances of getting osteoporosis. Not getting enough vitamin D can also increase your risk for the disease. Vitamin D is important because it helps the body use the calcium in your diet.
If you suffer from certain diseases, you are more likely to develop osteoporosis. These include:
- Hypogonadism or early menopause
- Diseases that cause bone loss, such as rheumatoid arthritis
- Hyperthyroidism or hyperparathyroidism
- Chronic liver or kidney disease
- Celiac disease and inflammatory bowel disease
- Cushing’s syndrome
- Certain types of cancer
- HIV/AIDS
- Anorexia nervosa
Some medications can also increase your risk of developing osteoporosis, including:
- Steroids also called glucocorticoids — when used for more than 3 months. Glucocortiocoids are given to people who have arthritis, asthma, and many other diseases.
- Anti-androgen therapy — drugs that block testosterone from working and which are sometimes used to treat prostate cancer
- Aromatase inhibitors — drugs that block oestrogen from being produced and working and which are sometimes used to treat or prevent ovarian or breast cancer
- Thyroid hormone replacement therapy — which can be a risk factor when used for too long
- Antidepressant medications — particularly medicines from the selective serotonin reuptake inhibitors (SSRIs) group
- Proton pump inhibitors (PPIs) — medicines which make your stomach less acidic
- Thiazolidinedione — a medicine sometimes used to treat type 2 diabetes
- Antipsychotic medications — some medicines used in mental illnesses such as schizophrenia
- Anti-epileptic medications — some medicines used to control epilepsy
Osteoporosis is a silent disease. You may not know you have osteoporosis until your symptoms are severe or until you break a bone. Signs and symptoms of osteoporosis include frequent broken bones or fractures, low back pain, loss of height over time or a hunched back. You may get shorter over time due to osteoporosis. The condition can cause your vertebrae (the bones in your spine) to collapse. These problems tend to occur after a lot of bone calcium has already been lost. A bone mineral density (BMD) test is the best way to check your bone health.
You cannot always avoid osteoporosis. However, there are some changes you can make to prevent or reduce your risk. The best way to prevent weak bones is to work on building strong ones. No matter how old you are, it is never too late to start. Building strong bones during childhood and the teen years is one of the best ways to keep from getting osteoporosis later. As you get older, your bones don’t make new bone fast enough to keep up with the bone loss. And after menopause, bone loss happens more quickly. But there are things you can do to slow the natural bone loss with aging and to prevent your bones from becoming weak and brittle. These include getting regular exercise, quitting smoking and getting enough calcium and vitamin D in your diet. They help keep your bones healthy as you age. Dietary supplements can be used as an additional source of calcium and vitamin D if you are not getting enough in your diet.
The National Academy of Medicine recommends 600 IU daily of vitamin D from food in patients up to 70 years of age and 800 IU of vitamin D in those older than 70 years. For calcium, they recommend 1,000 mg daily for adults up to 50 years of age, increasing to 1,200 mg daily for those older than 50 years. However, because supplements do not reduce fractures, the U.S. Preventive Services Task Force (USPSTF) recommends against supplementing with 1,000 mg calcium and 400 IU vitamin D in postmenopausal women. The evidence is insufficient for larger doses and supplementation in premenopausal women.
- Calcium. Women 50 years of age and younger and men 70 years of age and younger should get 1,000 mg of calcium per day. Women older than 50 years of age and men older than 70 years of age should get 1,200 mg of calcium per day. Women who are post-menopausal may need 1,500 mg of calcium per day. It is best to get your calcium from food. Nonfat and low-fat dairy products are good sources of calcium. Other options include dried beans, salmon, spinach, and broccoli. If you don’t get enough calcium from the food you eat, your doctor may suggest taking a calcium supplement.
- Vitamin D. Most people need about 800 International Units (IU) of vitamin D each day. It helps your body absorb calcium. You can get vitamin D from sunlight, food, and supplements. Your skin makes vitamin D when it is exposed to sunlight. However, you should be careful of sun exposure. Too much can cause skin cancer. Your doctor can test your blood to measure your vitamin D level. If your vitamin D level is low, your doctor may suggest taking a supplement.
Table 1. Recommended calcium and vitamin D intakes
| Life-stage group | Calcium mg/day | Vitamin D (IU/day) |
| Infants 0 to 6 months | 200 | 400 |
| Infants 6 to 12 months | 260 | 400 |
| 1 to 3 years old | 700 | 600 |
| 4 to 8 years old | 1000 | 600 |
| 9 to 13 years old | 1300 | 600 |
| 14 to 18 years old | 1300 | 600 |
| 19 to 30 years old | 1000 | 600 |
| 31 to 50 years old | 1000 | 600 |
| 51- to 70-year-old males | 1000 | 600 |
| 51- to 70-year-old females | 1200 | 600 |
| >70 years old | 1200 | 800 |
| 14 to 18 years old, pregnant/lactating | 1300 | 600 |
| 19 to 50 years old, pregnant/lactating | 1000 | 600 |
Abbreviations: mg = milligrams; IU = International Units
[Source 7 ]Calcium
Ninety-nine percent of the calcium in your human body is stored in your bones and teeth, where it supports their structure and hardness. Your body needs calcium to maintain strong bones and to carry out many important functions. Calcium is required for muscles to move and for nerves to carry messages between the brain and every body part. In addition, calcium is used to help blood vessels move blood throughout the body and to help release hormones and enzymes that affect almost every function in the human body, though less than 1% of total body calcium is needed to support these critical metabolic functions 7. Serum calcium is very tightly regulated and does not fluctuate with changes in dietary intakes; the body uses bone tissue as a reservoir for, and source of calcium, to maintain constant concentrations of calcium in blood, muscle, and intercellular fluids 7. The remaining 99% of your body’s calcium supply is stored in the bones and teeth where it supports their structure and function 7. Bone itself undergoes continuous remodeling, with constant resorption and deposition of calcium into new bone. The balance between bone resorption and deposition changes with age. Bone formation exceeds resorption in periods of growth in children and adolescents, whereas in early and middle adulthood both processes are relatively equal. In aging adults, particularly among postmenopausal women, bone breakdown exceeds formation, resulting in bone loss that increases the risk of osteoporosis over time 7.
The body gets the calcium it needs in two ways. One is by eating foods or supplements that contain calcium. Good sources include dairy products, which have the highest concentration per serving of highly absorbable calcium, and dark leafy greens or dried beans, which have varying amounts of absorbable calcium. Calcium supplements often contain vitamin D; taking calcium paired with vitamin D seems to be more beneficial for bone health than taking calcium alone 8.
The other way the body gets calcium is by pulling it from bones. This happens when blood levels of calcium drop too low, usually when it’s been awhile since having eaten a meal containing calcium. Ideally, the calcium that is “borrowed” from the bones will be replaced at a later point. But, this doesn’t always happen. Most important, this payback can’t be accomplished simply by eating more calcium 8.
Not all calcium consumed is actually absorbed in the gut. Humans absorb about 30% of the calcium in foods, but this varies depending upon the type of food consumed 7. Other factors also affect calcium absorption including the following:
- Amount consumed: the efficiency of absorption decreases as calcium intake increases 7.
- Age and life stage: net calcium absorption is as high as 60% in infants and young children, who need substantial amounts of the mineral to build bone 9. Absorption decreases to 15%–20% in adulthood (though it is increased during pregnancy) and continues to decrease as people age; compared with younger adults, recommended calcium intakes are higher for females older than 50 years and for both males and females older than 70 years 10.
- Vitamin D intake: this nutrient, obtained from food and produced by skin when exposed to sunlight of sufficient intensity, improves calcium absorption 7.
- Other components in food: phytic acid and oxalic acid, found naturally in some plants, bind to calcium and can inhibit its absorption. Foods with high levels of oxalic acid include spinach, collard greens, sweet potatoes, rhubarb, and beans. Among the foods high in phytic acid are fiber-containing whole-grain products and wheat bran, beans, seeds, nuts, and soy isolates 7. The extent to which these compounds affect calcium absorption varies. Research shows, for example, that eating spinach and milk at the same time reduces absorption of the calcium in milk 11. In contrast, wheat products (with the exception of wheat bran) do not appear to lower calcium absorption 12. For people who eat a variety of foods, these interactions probably have little or no nutritional consequence and, furthermore, are accounted for in the overall calcium DRIs, which factor in differences in absorption of calcium in mixed diets.
Some absorbed calcium is eliminated from the body in urine, feces, and sweat. This amount is affected by such factors as the following:
- Sodium (salt) and protein intakes: high sodium intake increases urinary calcium excretion 13. High protein intake also increases calcium excretion and was therefore thought to negatively affect calcium status 13. However, more recent research suggests that high protein intake also increases intestinal calcium absorption, effectively offsetting its effect on calcium excretion, so whole body calcium retention remains unchanged 14.
- Caffeine intake: this stimulant in coffee and tea can modestly increase calcium excretion and reduce absorption 15. One cup of regular brewed coffee, for example, causes a loss of only 2–3 mg of calcium 13. Moderate caffeine consumption (1 cup of coffee or 2 cups of tea per day) in young women has no negative effects on bone 16.
- Alcohol intake: alcohol intake can affect calcium status by reducing its absorption 17 and by inhibiting enzymes in the liver that help convert vitamin D to its active form 18. However, the amount of alcohol required to affect calcium status and whether moderate alcohol consumption is helpful or harmful to bone is unknown.
- Phosphorus intake: the effect of this mineral on calcium excretion is minimal. Several observational studies suggest that consumption of carbonated soft drinks with high levels of phosphate is associated with reduced bone mass and increased fracture risk. However, the effect is probably due to replacing milk with soda rather than the phosphorus itself 19.
- Fruit and vegetable intakes: metabolic acids produced by diets high in protein and cereal grains increase calcium excretion 20. Fruits and vegetables, when metabolized, shift the acid/base balance of the body towards the alkaline by producing bicarbonate, which reduces calcium excretion. However, it is unclear if consuming more fruits and vegetables affects bone mineral density. These foods, in addition to reducing calcium excretion, could possibly reduce calcium absorption from the gut and therefore have no net effect on calcium balance.
It is important to get plenty of calcium in the foods you eat. Foods rich in calcium include:
- Dairy products such as milk, cheese, and yogurt
- Leafy, green vegetables
- Fish with soft bones that you eat, such as canned sardines and salmon
- Calcium-enriched foods such as breakfast cereals, fruit juices, soy and rice drinks, and tofu. Check the product labels.
The exact amount of calcium you need depends on your age and other factors. Growing children and teenagers need more calcium than young adults. Older women need plenty of calcium to prevent osteoporosis. People who do not eat enough high-calcium foods should take a calcium supplement.
Lifelong adequate calcium intake is necessary for the acquisition of peak bone mass and subsequent maintenance of bone health. The skeleton contains 99 % of the body’s calcium stores; when the exogenous supply is inadequate, bone tissue is resorbed from the skeleton to maintain serum calcium at a constant level.
Americans obtain most of their calcium from dairy products. Most Americans above age 9 on average do not consume recommended levels of calcium 21. In fact, approximately three 8-ounce glasses of milk each day, combined with the calcium from the rest of a normal diet, is enough to meet the recommended daily requirements for most adults.
For postmenopausal women, the recommended total daily calcium intake is 1,200 mg per day in two or more doses. These levels of intake can be achieved through dietary sources of calcium, including both dairy and non-dairy products. In addition, calcium supplements (e.g., calcium carbonate, calcium citrate, other calcium salts) are available in the form of pills, chewable tablets, and liquids 22.
Average daily recommended amounts are listed below in milligrams (mg). The Food and Nutrition Board (FNB) at the Institute of Medicine of the National Academies established Recommended Dietary Allowances (RDAs) for the amounts of calcium required for bone health and to maintain adequate rates of calcium retention in healthy people. They are listed in Table 2 in milligrams (mg) per day. Here’s how much calcium you need each day 23:
Table 2. Recommended Dietary Allowances (RDAs) for Calcium
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 200 mg |
| Infants 7–12 months | 260 mg |
| Children 1–3 years | 700 mg |
| Children 4–8 years | 1,000 mg |
| Children 9–13 years | 1,300 mg |
| Teens 14–18 years | 1,300 mg |
| Adults 19–50 years | 1,000 mg |
| Adult men 51–70 years | 1,000 mg |
| Adult women 51–70 years | 1,200 mg |
| Adults 71 years and older | 1,200 mg |
| Pregnant and breastfeeding teens | 1,300 mg |
| Pregnant and breastfeeding adults | 1,000 mg |
Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
* Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA).
[Source 24 ]Pregnant or nursing women need the same amount of calcium as other women of the same age.
There is no evidence that calcium intake in excess of these amounts confers additional bone strength. Some research suggests that high calcium intakes might increase the risk of heart disease and prostate cancer. The Tolerable Upper Intake Levels (ULs) for calcium established by the Food and Nutrition Board are listed in Table 2. They are based on observational evidence from the Women’s Health Initiative (WHI) showing a link between higher intakes of supplemental calcium (1,000 mg/day for 7 years) and a greater risk of kidney stones 25, 26. However, two subsequent systematic reviews of the evidence from 10 studies in more than 8,000 adults with osteoporosis who took 120 to 1,500 mg supplemental calcium daily for 3 days to 3 years 27 and 11 randomized controlled trial in 51,419 adults 50 years and older who took 1,000 to 1,600 mg calcium with or without vitamin D for 2 to 7 years 28 found no such association.
High levels of calcium in the blood and urine can cause poor muscle tone, poor kidney function, low phosphate levels, constipation, nausea, weight loss, extreme tiredness, frequent need to urinate, abnormal heart rhythms, and a high risk of death from heart disease. However, high levels of calcium in the blood and urine are usually caused by a health condition such as high levels of parathyroid hormone or cancer, not by high calcium intakes 29.
The daily upper limits for calcium include intakes from all sources—food, beverages, and supplements—and are listed below.
Table 3. Tolerable Upper Intake Levels (ULs) for Calcium
| Life Stage | Upper Limit |
|---|---|
| Birth to 6 months | 1,000 mg |
| Infants 7–12 months | 1,500 mg |
| Children 1–8 years | 2,500 mg |
| Children 9–18 years | 3,000 mg |
| Adults 19–50 years | 2,500 mg |
| Adults 51 years and older | 2,000 mg |
| Pregnant and breastfeeding teens | 3,000 mg |
| Pregnant and breastfeeding adults | 2,500 mg |
Calcium Rich Foods
Calcium is found in many foods. You can get recommended amounts of calcium by eating a variety of foods, including the following:
- Milk, yogurt, and cheese are the main food sources of calcium for most people in the United States.
- Canned sardines and salmon with bones contain calcium.
- Certain vegetables, such as kale, broccoli, and Chinese cabbage (bok choi) also contain calcium.
- Calcium is added to some beverages, including many fruit juices and milk substitutes such as soy and almond beverages, as well as some brands of tofu and ready-to-eat cereals. To find out whether these foods have calcium added, check the product labels.
- Most grains (such as breads, pastas, and unfortified cereals) do not have high amounts of calcium. However, because people eat them often, what they contribute adds up.
The U.S. Department of Agriculture’s (USDA’s) Nutrient Database website (https://fdc.nal.usda.gov) lists the nutrient content of many foods with Calcium arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Calcium-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/Calcium-Food.pdf).
Milk, yogurt, and cheese are rich natural sources of calcium and are the major food contributors of this nutrient to people in the United States 7. Nondairy sources include vegetables, such as Chinese cabbage, kale, and broccoli. Spinach provides calcium, but its bioavailability is poor. Most grains do not have high amounts of calcium unless they are fortified; however, they contribute calcium to the diet because they contain small amounts of calcium and people consume them frequently. Foods fortified with calcium include many fruit juices and drinks, tofu, and cereals. Selected food sources of calcium are listed in Table 3.
In its food guidance system, MyPlate, the U.S. Department of Agriculture recommends that persons aged 9 years and older eat 3 cups of foods from the milk group per day 30. A cup is equal to 1 cup (8 ounces) of milk, 1 cup of yogurt, 1.5 ounces of natural cheese (such as Cheddar), or 2 ounces of processed cheese (such as American).
Table 4. Calcium content of selected foods
| Food* | Milligrams (mg) per serving | Percent DV* |
|---|---|---|
| Yogurt, plain, low fat, 8 ounces | 415 | 32 |
| Orange juice, calcium fortified, 1 cup | 349 | 27 |
| Yogurt, fruit, low fat, 8 ounces | 344 | 27 |
| Mozzarella, part skim, 1.5 ounces | 333 | 26 |
| Sardines, canned in oil, with bones, 3 ounces | 325 | 25 |
| Milk, nonfat, 1 cup** | 299 | 23 |
| Soymilk, calcium fortified, 1 cup | 299 | 23 |
| Milk, whole (3.25% milk fat), 1 cup** | 276 | 21 |
| Tofu, firm, made with calcium sulfate, ½ cup*** | 253 | 19 |
| Salmon, pink, canned, solids with bones, 3 ounces | 181 | 14 |
| Cottage cheese, 1% milk fat, 1 cup | 138 | 11 |
| Tofu, soft, made with calcium sulfate, ½ cup*** | 138 | 11 |
| Soybeans, cooked, ½ cup | 131 | 10 |
| Breakfast cereals, fortified with 10% of the DV for calcium, 1 serving | 130 | 10 |
| Spinach, boiled, drained, ½ cup | 123 | 9 |
| Frozen yogurt, vanilla, soft serve, ½ cup | 103 | 8 |
| Turnip greens, fresh, boiled, ½ cup | 99 | 8 |
| Kale, fresh, cooked, 1 cup | 94 | 7 |
| Chia seeds, 1 tablespoon | 76 | 6 |
| Chinese cabbage (bok choi), raw, shredded, 1 cup | 74 | 6 |
| Beans, pinto, canned, drained, ½ cup | 54 | 4 |
| Tortilla, corn, one, 6” diameter | 46 | 4 |
| Sour cream, reduced fat, 2 tablespoons | 31 | 2 |
| Bread, whole-wheat, 1 slice | 30 | 2 |
| Kale, raw, chopped, 1 cup | 24 | 2 |
| Broccoli, raw, ½ cup | 21 | 2 |
| Apple, golden delicious, with skin, 1 medium | 10 | 0 |
Footnotes:
* DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for calcium is 1,300 mg for adults and children age 4 years and older [13]. FDA requires food labels to list calcium content. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
** Calcium content varies slightly by fat content; the more fat in the food, the less calcium it contains.
*** Calcium content is for tofu processed with a calcium salt. Tofu processed with other salts does not provide significant amounts of calcium.
Calcium supplements
It is best to obtain calcium from your diet. However when adequate calcium intake is not possible a supplement may be required as directed by your doctor or pharmacist. The two main forms of calcium in supplements are calcium carbonate and calcium citrate. Calcium carbonate is more commonly available and is both inexpensive and convenient 32. Due to its dependence on stomach acid for absorption, calcium carbonate is absorbed most efficiently when taken with food, whereas calcium citrate is absorbed equally well when taken with or without food 33. Calcium citrate is also useful for people with achlorhydria, inflammatory bowel disease, or absorption disorders 7. Other calcium forms in supplements or fortified foods include gluconate, lactate, and phosphate. Calcium citrate malate is a well-absorbed form of calcium found in some fortified juices 34. Calcium supplements are available as oral tablets, effervescent tablets or soluble powder.
Calcium supplements contain varying amounts of elemental calcium. For example, calcium carbonate is 40% calcium by weight, whereas calcium citrate is 21% calcium. Fortunately, elemental calcium is listed in the Supplement Facts panel, so consumers do not need to calculate the amount of calcium supplied by various forms of calcium supplements.
The percentage of calcium absorbed depends on the total amount of elemental calcium consumed at one time; as the amount increases, the percentage absorption decreases. Absorption is highest in doses ≤500 mg 7. So, for example, one who takes 1,000 mg/day of calcium from supplements might split the dose and take 500 mg at two separate times during the day.
Some individuals who take calcium supplements might experience gastrointestinal side effects including gas, bloating, constipation, or a combination of these symptoms. Calcium carbonate appears to cause more of these side effects than calcium citrate 7, so consideration of the form of calcium supplement is warranted if these side effects are reported. Other strategies to alleviate symptoms include spreading out the calcium dose throughout the day and/or taking the supplement with meals.
Calcium supplements are sometimes combined with vitamin D, as adequate vitamin D levels are important to assist the absorption of calcium in the body. Take supplements as directed and talk to your doctor or pharmacist if you have any queries.
Calcium phosphate supplement
The beneficial effects of calcium phosphate mainly focus on the intestinal metabolism, e.g., bile acid metabolism, fatty acid (cholesterol) excretion, and modulation of the gut microbiota 35, 36, 37, 38. Calcium from tricalcium phosphate (CaP, a water-insoluble compound at neutral pH value), is partly absorbed in the human gut; but the main part of the calcium and phosphorus is precipitated to amorphous calcium phosphate in the gut, and thus, not absorbed 39. Nevertheless, supplementation with vitamin D3 and calcium reduces the risk of hip fractures and other nonvertebral fractures among elderly women 40. Supplementation with daily 10 μg vitamin D3 significantly increases plasma 25-(OH)D concentration. The combination with daily 1 g calcium (as CaP) has a further increasing effect on the 25-(OH)D concentration. Both CaP alone and in combination with vitamin D3 have no beneficial effect on bone remodelling markers and on the metabolism of calcium, phosphorus, magnesium and iron 41.
Is it safe to take calcium supplements?
For most people, it is safe to eat foods containing calcium and to take calcium supplements that together do not exceed the tolerable upper intake level of 2.5 grams of calcium per day 42. This upper level for daily calcium intake in adults is the highest level that likely will not pose risks of unwanted side effects in the general population. The upper level of 2.5 grams a day is an average recommendation for all healthy people who are older than a year, regardless of gender 42.
Excessively high levels of calcium in the blood known as hypercalcemia can cause renal insufficiency, vascular and soft tissue calcification, hypercalciuria (high levels of calcium in the urine) and kidney stones 7. Although very high calcium intakes have the potential to cause hypercalcemia 43, it is most commonly associated with primary hyperparathyroidism or malignancy 7.
High calcium intake can cause constipation 31. It might also interfere with the absorption of iron and zinc, though this effect is not well established 7. High intake of calcium from supplements, but not foods, has been associated with increased risk of kidney stones 44.
Consuming too much calcium—in excess of 5 grams a day, or 3 grams a day in people with existing kidney problems 45 can lead to several harmful side effects. The milk-alkali syndrome, a triad of hypercalcemia, metabolic alkalosis, and renal insufficiency, was identified in 1923 as an adverse effect of peptic ulcer disease therapies involving the use of dairy products and alkaline powders 46. Most of these side effects result from people taking too many calcium supplements. Recent trends in the prevention and treatment of osteoporosis using widely available over-the-counter (OTC) calcium supplements appear to be contributing to its return 47. Rare harmful side effects from excess calcium include kidney stones 48, hypercalcemia (too much calcium in the blood), and kidney failure 49. In addition, excessive consumption of milk (which is high in calcium) and some types of antacids, especially antacids containing calcium carbonate or sodium bicarbonate (baking soda), over a long period of time can cause milk-alkali syndrome, a condition that can also lead to calcium deposits in the kidneys and other tissues and to kidney failure 45, 50, 51.
Some evidence links higher calcium intake with increased risk of prostate cancer, but this effect is not well understood, in part because it is challenging to separate the potential effect of dairy products from that of calcium 7. Some studies also link high calcium intake, particularly from supplements, with increased risk of cardiovascular disease 52, 53.
The Tolerable Upper Intake Levels (ULs) for calcium established by the Food and Nutrition Board are listed in Table 3 above in milligrams (mg) per day. Getting too much calcium from foods is rare; excess intakes are more likely to be caused by the use of calcium supplements. National Health and Nutrition Examination Survey (NHANES) data from 2003–2006 indicate that approximately 5% of women older than 50 years have estimated total calcium intakes (from foods and supplements) that exceed the UL by about 300–365 mg 54.
How does your body control blood calcium levels?
Normally, your body controls blood calcium by adjusting the levels of several hormones. When blood calcium levels are low, your parathyroid glands (four pea-sized glands in your neck) secrete a hormone called parathyroid hormone (PTH). PTH helps your bones release calcium into the blood.
Vitamin D is also important in keeping calcium levels in the normal range. Vitamin D, which is actually a hormone, helps your body absorb calcium and move it from your intestines into your blood.
Together, PTH and vitamin D, along with other hormones and minerals, help move calcium in or out of body tissues to keep your blood calcium at a normal level.
The regulation of both calcium and phosphate balance is greatly influenced by concentrations of circulating parathyroid hormone (PTH), vitamin D, and, to a lesser extent, calcitonin. Calcium and phosphate concentrations are also linked by their ability to chemically react to form calcium phosphate. The product of concentrations of calcium and phosphate (in mEq/L) is estimated to be < 60 normally; when the product exceeds 70, precipitation of calcium phosphate crystals in soft tissue is much more likely. Calcification of vascular tissue accelerates arteriosclerotic vascular disease and may occur when the calcium and phosphate product is even lower (> 55), especially in patients with chronic kidney disease.
Calcium is absorbed passively (no cellular energy required) in the intestines by diffusing through the spaces between cells. It is also absorbed actively (cellular energy required) through intestinal cells by binding to a transport protein known as calbindin. The production of calbindin is dependent on vitamin D 55.
Not all calcium consumed is actually absorbed in the gut. Humans absorb about 30% of the calcium in foods, but this varies depending upon the type of food consumed 7. Other factors also affect calcium absorption including the following:
- Amount consumed: the efficiency of absorption decreases as calcium intake increases 7.
- Age and life stage: net calcium absorption is as high as 60% in infants and young children, who need substantial amounts of the mineral to build bone 9. Absorption decreases to 15%–20% in adulthood (though it is increased during pregnancy) and continues to decrease as people age; compared with younger adults, recommended calcium intakes are higher for females older than 50 years and for both males and females older than 70 years 10.
- Vitamin D intake: this nutrient, obtained from food and produced by skin when exposed to sunlight of sufficient intensity, improves calcium absorption 7.
- Other components in food: phytic acid and oxalic acid, found naturally in some plants, bind to calcium and can inhibit its absorption. Foods with high levels of oxalic acid include spinach, collard greens, sweet potatoes, rhubarb, and beans. Among the foods high in phytic acid are fiber-containing whole-grain products and wheat bran, beans, seeds, nuts, and soy isolates 7. The extent to which these compounds affect calcium absorption varies. Research shows, for example, that eating spinach and milk at the same time reduces absorption of the calcium in milk 11. In contrast, wheat products (with the exception of wheat bran) do not appear to lower calcium absorption 12. For people who eat a variety of foods, these interactions probably have little or no nutritional consequence and, furthermore, are accounted for in the overall calcium Dietary Reference Intakes (DRIs), which factor in differences in absorption of calcium in mixed diets.
Some absorbed calcium is eliminated from the body in urine, feces, and sweat. This amount is affected by such factors as the following:
- Sodium (salt) and protein intakes: high sodium intake increases urinary calcium excretion 13. High protein intake also increases calcium excretion and was therefore thought to negatively affect calcium status 13. However, more recent research suggests that high protein intake also increases intestinal calcium absorption, effectively offsetting its effect on calcium excretion, so whole body calcium retention remains unchanged 14.
- Caffeine intake: this stimulant in coffee and tea can modestly increase calcium excretion and reduce absorption 15. One cup of regular brewed coffee, for example, causes a loss of only 2–3 mg of calcium 13. Moderate caffeine consumption (1 cup of coffee or 2 cups of tea per day) in young women has no negative effects on bone 16.
- Alcohol intake: alcohol intake can affect calcium status by reducing its absorption 17 and by inhibiting enzymes in the liver that help convert vitamin D to its active form 18. However, the amount of alcohol required to affect calcium status and whether moderate alcohol consumption is helpful or harmful to bone is unknown.
- Phosphorus intake: the effect of this mineral on calcium excretion is minimal. Several observational studies suggest that consumption of carbonated soft drinks with high levels of phosphate is associated with reduced bone mass and increased fracture risk. However, the effect is probably due to replacing milk with soda rather than the phosphorus itself 19.
- Fruit and vegetable intakes: metabolic acids produced by diets high in protein and cereal grains increase calcium excretion 20. Fruits and vegetables, when metabolized, shift the acid/base balance of the body towards the alkaline by producing bicarbonate, which reduces calcium excretion. However, it is unclear if consuming more fruits and vegetables affects bone mineral density. These foods, in addition to reducing calcium excretion, could possibly reduce calcium absorption from the gut and therefore have no net effect on calcium balance.
Parathyroid hormone (PTH) is secreted by the parathyroid glands. It has several actions, but perhaps the most important is to defend against hypocalcemia. Parathyroid cells sense decreases in serum calcium and, in response, release preformed PTH into the circulation. PTH increases serum calcium within minutes by increasing renal and intestinal absorption of calcium and by rapidly mobilizing calcium and phosphate from bone (bone resorption). Renal calcium excretion generally parallels sodium excretion and is influenced by many of the same factors that govern sodium transport in the proximal tubule. However, PTH enhances distal tubular calcium reabsorption independently of sodium.
PTH also decreases renal phosphate reabsorption and thus increases renal phosphate losses. Renal phosphate loss prevents the solubility product of calcium and phosphate from being exceeded in plasma as calcium concentrations rise in response to PTH.
PTH also increases serum calcium by stimulating conversion of vitamin D to its most active form, calcitriol. This form of vitamin D increases the percentage of dietary calcium absorbed by the intestine. Despite increased calcium absorption, long-term increases in PTH secretion generally result in further bone resorption by inhibiting osteoblastic function and promoting osteoclastic activity. PTH and vitamin D both function as important regulators of bone growth and bone remodeling (see Vitamin D Deficiency and Dependency).
Radioimmunoassays for the intact PTH molecule are still the recommended way to test for PTH. Second-generation assays for intact PTH are available. These tests measure bioavailable PTH or complete PTH. They give values equal to 50 to 60% of those obtained with the older assay. Both types of assays can be used for diagnosing primary hyperparathyroidism or monitoring hyperparathyroidism secondary to renal disease, as long as normal ranges are noted.
PTH increases urinary cAMP. Sometimes total or nephrogenous cAMP excretion is measured in diagnosis of pseudohypoparathyroidism.
Calcitoninis secreted by the thyroid parafollicular cells (C cells). Calcitonin tends to lower serum calcium concentration by enhancing cellular uptake, renal excretion, and bone formation. The effects of calcitonin on bone metabolism are much weaker than those of either PTH or vitamin D.
Vitamin D
Vitamin D also called calciferol, plays a major role in calcium absorption, health of bone, bone mineralization (hardening), muscle performance, balance and risk of falling. Vitamin D is produced in your skin when it is exposed to sunlight. You need 10 to 15 minutes of sunlight to the hands, arms, and face, two to three times a week to make enough vitamin D. The amount of time depends on how sensitive your skin is to light. It also depends on your use of sunscreen, your skin color, and the amount of pollution in the air. You can also get vitamin D by eating foods, such as milk, or by taking vitamin pills. In foods and dietary supplements, vitamin D has two main forms, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol), that differ chemically only in their side-chain structures. Vitamin D2 (ergocalciferol) is synthesized from ergosterol and found in yeast, sun dried and ultraviolet irradiated mushrooms, and plants 56. Vitamin D3 (cholecalciferol) is synthesized endogenously from 7-dehydrocholesterol in the skin and found naturally in cod liver oil and oily fish 56. Both forms are well absorbed in the small intestine and raise serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels, and they seem to have equivalent ability to cure rickets 57. However, most evidence indicates that vitamin D3 (cholecalciferol) increases serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels to a greater extent and maintains these higher levels longer than vitamin D2 (ergocalciferol), even though both forms are well absorbed in the gut 58.
- Vitamin D2 (ergocalciferol): Vitamin D2 is created in plants, such as yeast or mushrooms. Vitamin D2 is also available as a supplement and in fortified foods like breakfast cereals, milk, and other dairy items.
- Vitamin D3 (cholecalciferol): Vitamin D3 is generated in the skin when it is exposed to sunlight. Vitamin D3 is also found in some animal-based foods (eggs and fatty fish, such as salmon, tuna, and mackerel) and may be consumed in certain fortified foods or dietary supplements.
Vitamin D taken in the diet by food or pills is measured in international units (IU). Look at the pill bottle or food label for the IU amount.
The National Osteoporosis Foundation recommends an intake of 800 to 1000 international units (IU) of vitamin D per day for adults age 50 and older. Vitamin D is synthesized in the skin through sunlight exposure, or it may be taken as a supplement. However, the skin of older individuals does not synthesize vitamin D as well as the skin of younger individuals, and in some parts of the country, the winter sun does not produce vitamin D in the skin of all individuals. In addition, vitamin D is not available in many foods other than fortified milk, which contains 100 IU (international units) per cup. Thus, many individuals will need to take a supplement, especially those who avoid sun exposure, use sun block, or do not drink milk. The recommended dose of vitamin D is 200 to 600 IU daily, with the dose dependent on age, as shown in the table below 59. However, many experts are recommending more vitamin D for the frail elderly 60.
Institute of Medicine Dietary Reference Intakes for vitamin D are 600 IU/day until age 70 and 800 IU/day for adults age 71 years and older.
The main function of vitamin D is to help your body absorb calcium from the gut and maintains adequate serum calcium and phosphate concentrations to enable normal mineralization of bone and to prevent hypocalcemic tetany. Vitamin D also helps maintain proper levels of calcium, phosphate, and parathyroid hormone in your blood. Calcium is one of the main building blocks of bones and teeth. Vitamin D is needed for bone growth and bone remodeling by osteoblasts and osteoclasts 61. Maintaining adequate levels of vitamin D supports healthy bones. Vitamin D deficiency can lead to bone diseases such as osteoporosis, rickets and osteomalacia 62. In addition, vitamin D has other roles in the body, including anti-inflammatory and other properties that play a role in maintaining normal muscle, immune, and nervous system functions and glucose metabolism 63. Many genes encoding proteins that regulate cell proliferation, differentiation, and apoptosis are modulated in part by vitamin D 64. The major source of vitamin D is sunlight (exposure to ultraviolet B radiation). Vitamin D deficiency is typically due to limited sunlight exposure. However, too much sun exposure can lead to skin aging and skin cancer. So many people try to get their vitamin D from other sources. Vitamin D-rich foods include egg yolks, saltwater fish, and liver. Some other foods, like milk and cereal, often have added vitamin D. You can also take vitamin D supplements. Check with your health care provider to see how much vitamin D you should take.
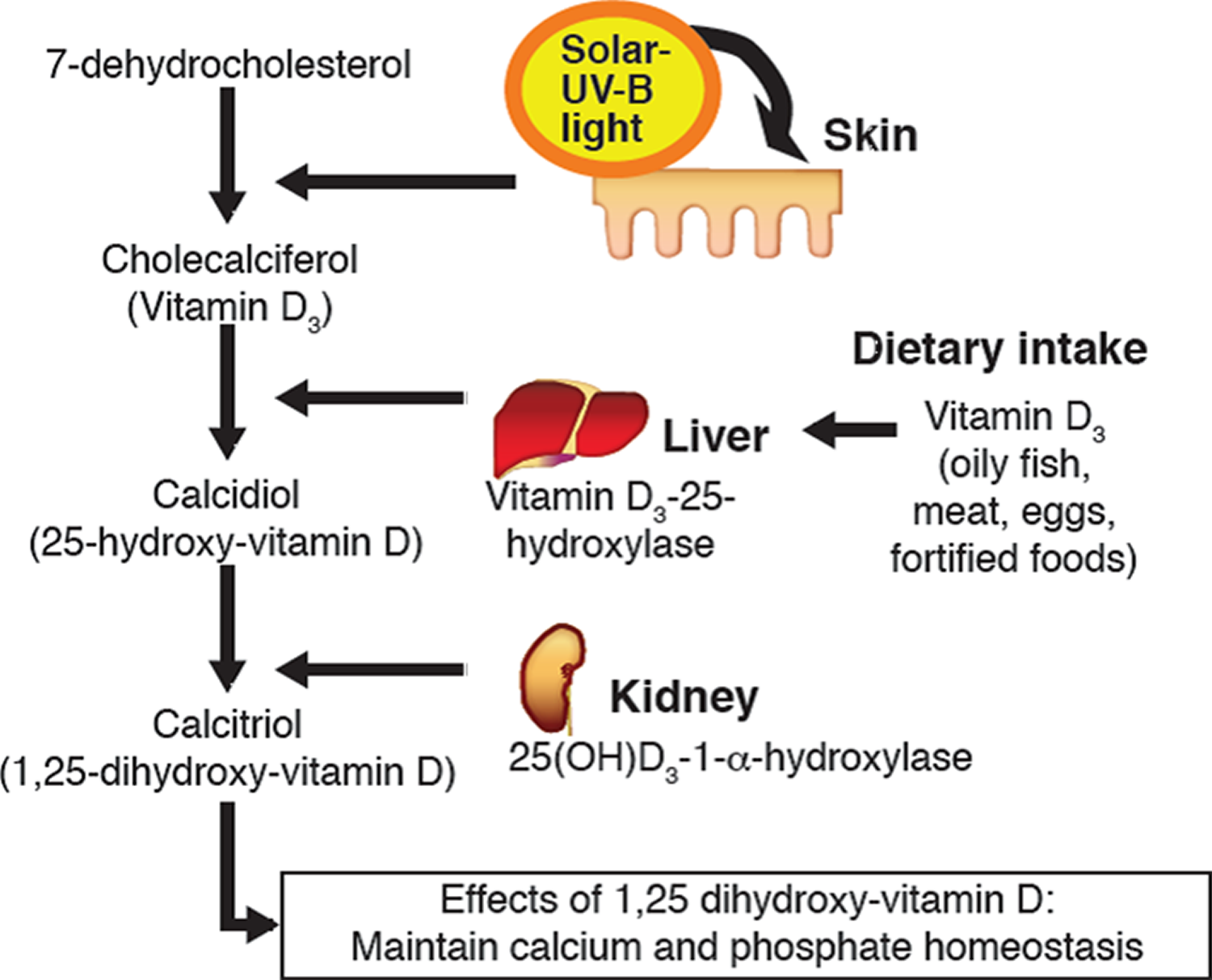
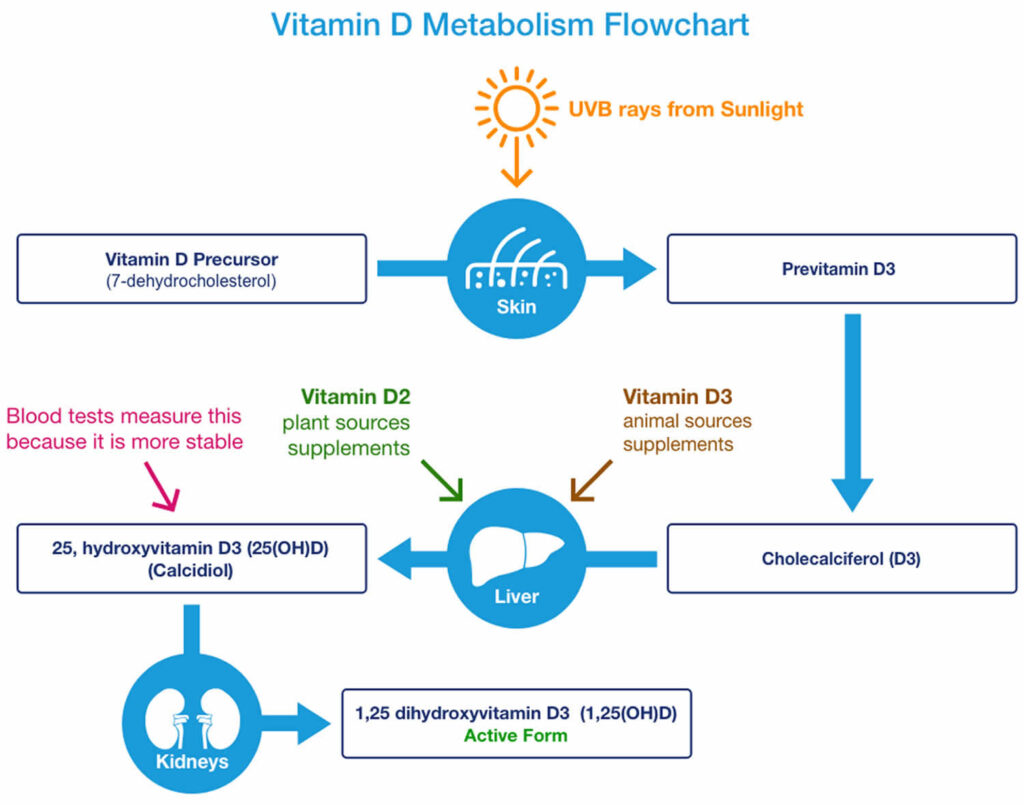
You can get vitamin D in three ways: through your skin, from your diet, and from supplements. Vitamin D obtained from sun exposure, food, and supplements is biologically inert and must undergo two hydroxylations in the body for activation before being able to be used by your body (see Figure 1 below) 65. Both vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) need to go through chemical changes in your liver and kidneys before being able to be used by your body. The first occurs in your liver where vitamin D is converted by vitamin D-25-hydroxylase (CYP2R1) enzyme into measurable substance called 25-hydroxyvitamin D [25(OH)D], also known as “calcidiol” 66. The second hydroxylation occurs primarily in your kidneys where the enzyme 25-hydroxyvitamin D-1-alpha-hydroxylase (CYP27B1) convert 25-hydroxyvitamin D [25(OH)D] into a hormone called active vitamin D or 1,25-dihydroxyvitamin D [1,25(OH)2D], also known as “calcitriol” (active vitamin D) 67. The enzyme 25-hydroxyvitamin D-1-alpha-hydroxylase (CYP27B1) is also expressed by many other tissues including activated macrophages, parathyroid glands, microglia, breast, colon, and keratinocytes where 1,25-dihydroxyvitamin D [calcitriol or 1,25(OH)2D] is produced and exerts its autocrine and paracrine functions 68. 1,25-dihydroxyvitamin D [1,25(OH)2D or calcitriol] exerts its physiologic functions in the target tissue by binding to the vitamin D receptor in the nucleus where it leads to up- or down-regulation of a multitude of genes 69. A manufactured calcitriol (1,25-dihydroxyvitamin D3) is used to treat kidney disease with low blood calcium, hyperparathyroidism due to kidney disease, low blood calcium due to hypoparathyroidism, osteoporosis, osteomalacia, and familial hypophosphatemia. It is taken by mouth or by injection into a vein.
Vitamin D absorption occurs by simple passive diffusion and by a mechanism that involves intestinal membrane carrier proteins 57. The concurrent presence of fat in the gut enhances vitamin D absorption, but some vitamin D is absorbed even without dietary fat. Neither aging nor obesity alters vitamin D absorption from the gut 57.
Vitamin D (calciferol) is also produced in your body when ultraviolet (UV) rays from sunlight strike your skin and trigger vitamin D synthesis (see Figure 1). Sunlight exposure is the primary source of vitamin D for most people. Solar ultraviolet-B radiation (UVB; wavelengths of 290 to 315 nanometers) stimulates the production of vitamin D3 (cholecalciferol) from 7-dehydrocholesterol in the epidermis of your skin. Hence, vitamin D is actually more like a hormone than a vitamin, a substance that is required from the diet.
Vitamin D enters the circulation and is transported to the liver, where it is hydroxylated to form 25-hydroxyvitamin D (calcidiol; the major circulating form of vitamin D). In the kidneys, the 1-alpha-hydroxylase enzyme catalyzes a second hydroxylation of 25-hydroxyvitamin D, resulting in the formation of 1,25-dihydroxyvitamin D [calcitriol or 1,25(OH)2D] — the most potent form of vitamin D 70. Most of the physiological effects of vitamin D in the body are related to the activity of 1,25-dihydroxyvitamin D (calcitriol or 1,25(OH)2D).
Most of the time, vitamin D levels will be tested by measuring blood levels of 25-hydroxyvitamin D [25(OH)D or calcidiol]. Testing 25-hydroxyvitamin D [25(OH)D or calcidiol] is considered the most accurate way to measure how much vitamin D is in your body because 25-hydroxyvitamin D [25(OH)D or calcidiol] is the major form of vitamin D circulating in your bloodstream. Sometimes, doctors may check your blood level of 1,25 dihydroxyvitamin D (active vitamin D), which is also called calcitriol. However, 1,25 dihydroxyvitamin D (calcitriol) is generally not used to detect inadequate vitamin D levels, but it may be measured in patients with abnormal calcium levels or kidney problems 71.
Vitamin D testing measures the level of this essential substance in your blood. Vitamin D blood testing is used to diagnose vitamin D deficiencies or to monitor treatment for a known vitamin D deficiency. Less commonly, vitamin D testing may be used to detect vitamin D toxicity, a condition in which there is an excess of vitamin D in the body.
There is a bit of controversy regarding what is considered a low vitamin D level between different expert organizations. A vitamin D level measures levels of 25-hydroxyvitamin D (25(OH)D) also known as calcidiol, in the blood.
Most experts recommend:
- Levels of 20-50 nanograms/milliliter (ng/ml) of 25-hydroxyvitamin D (calcidiol): Sufficient (good)
- Levels of 12-19 ng/ml of 25-hydroxyvitamin D (calcidiol): Borderline
- Levels of less than 12 ng/ml of 25-hydroxyvitamin D (calcidiol): Deficient (low)
However, not everybody agrees, and some organizations suggest different cut-off values.
The Institute of Medicine states:
- Levels above 20 ng/ml of 25-hydroxyvitamin D (calcidiol): Sufficient
- Levels below 20 ng/ml of 25-hydroxyvitamin D (calcidiol): Deficient
Note that several members of the Institute of Medicine committee publicly stated that over screening for vitamin D deficiency was a problem which typically resulted in unnecessary treatment. They were not in agreement with a cut-off level of 20 ng/ml for deficiency and recommended a lower level of 12.5 ng/ml.
The Endocrine Society states:
- Levels above 30 ng/ml of 25-hydroxyvitamin D (calcidiol): Sufficient; however, some assays are inaccurate and levels of 40-60 ng/ml better guarantee sufficiency
- Levels of 21-29 ng/ml of 25-hydroxyvitamin D (calcidiol): Insufficient
- Levels below 20 ng/ml pf 25-hydroxyvitamin D (calcidiol): Deficient
Other medical institution states 72:
- Levels below 20 ng/mL of 25-hydroxyvitamin D (calcidiol): Mild deficiency
- Levels below 10 ng/mL of 25-hydroxyvitamin D (calcidiol): Moderate deficiency
- Levels below 5 ng/mL of 25-hydroxyvitamin D (calcidiol): Severe deficiency
Talk to your doctor about what he/she considers to be a low vitamin D level. Abnormal levels of vitamin D can indicate bone disorders, nutrition problems, organ damage, or other medical conditions.
Screening for vitamin D status is becoming a more common part of the routine laboratory bloodwork ordered by primary-care physicians, irrespective of any indications for this practice 73. No studies have examined whether such screening for vitamin D deficiency results in improved health outcomes 74. The U.S. Preventive Services Task Force (USPSTF) found insufficient evidence to assess the benefits and harms of screening for vitamin D deficiency in asymptomatic adults 75. It added that no national professional organization recommends population screening for vitamin D deficiency.
Figure 1. Production of vitamin D3 in the skin
The plasma calcitriol (1,25-dihydroxyvitamin D or 1,25(OH)2D) or vitamin D concentration depends on the availability of calcidiol (25-hydroxyvitamin D or 25(OH)D) and the activities of the renal enzymes 1-α-hydroxylase and 24-α-hydroxylase 76. The renal 1-alpha-hydroxylase enzyme (CYP27B1) is primarily regulated by parathyroid hormone (PTH), serum calcium and phosphate concentrations, and fibroblast growth factor 23 (FGF23) 77. Increased parathyroid hormone (PTH), calcitonin, and hypophosphatemia (low blood phosphate) stimulate the renal enzymes 25-hydroxyvitamin D-1-alpha-hydroxylase (CYP27B1) and enhance calcitriol [1,25-dihydroxyvitamin D or 1,25(OH)2D] production, while high calcium, hyperphosphatemia (high blood phosphate) and calcitriol [1,25(OH)2D] inhibit the renal enzymes 1-alpha-hydroxylase (CYP27B1) 78. Calcitriol [1,25-dihydroxyvitamin D or 1,25(OH)2D] inhibits the synthesis and secretion of parathyroid hormone (PTH), providing negative feedback regulation of calcitriol production. Calcitriol [1,25-dihydroxyvitamin D or 1,25(OH)2D] synthesis may also be modulated by vitamin D receptors on the cell surface; downregulation of these receptors may play an important role in regulating vitamin D activation 79. Fibroblast growth factor 23 (FGF23), a newly described phosphaturic hormone, inhibits renal production of calcitriol [1,25-dihydroxyvitamin D or 1,25(OH)2D] by inhibiting 1-α-hydroxylase in the renal proximal tubule and by simultaneously increasing the expression of 24-α-hydroxylase and production of 24,25(OH)2D (an inactive metabolite) 80. Calcitriol [1,25-dihydroxyvitamin D or 1,25(OH)2D] stimulates fibroblast growth factor 23 (FGF23), creating a feedback loop. Fibroblast growth factor 23 (FGF23) decreases renal reabsorption of phosphate, and thereby counteracts the increased gastrointestinal phosphate reabsorption induced by Calcitriol, maintaining phosphate homeostasis 81.
When hypocalcemia (low blood calcium) occurs, serum parathyroid hormone (PTH) concentration increases and enhances renal tubular reabsorption of calcium, as well as the activity of 1-α-hydroxylase in the kidney. This results in increased Calcitriol [1,25-dihydroxyvitamin D or 1,25(OH)2D] production, and in turn, intestinal calcium absorption. Parathyroid hormone (PTH) also stimulates bone osteoclast activity to mobilize bone calcium stores, thereby increasing serum calcium. Both Calcitriol [1,25-dihydroxyvitamin D or 1,25(OH)2D] and Calcidiol [25-hydroxyvitamin D or 25(OH)D] are degraded in part by being hydroxylated at the 24 position by a 24-hydroxylase. The activity of the 24-hydroxylase gene is increased by calcitriol (which therefore promotes its own inactivation) and reduced by parathyroid hormone (thereby allowing more active hormone to be formed) 77. Estrogen, placental growth hormone, and prolactin may also regulate vitamin D metabolism, playing a role during pregnancy to meet increased calcium demands. Calcitriol is also formed in some other tissues, but is used only within the tissues and not circulated. Parathyroid hormone (PTH)- independent extrarenal production of Calcitriol from Calcidiol is by activated macrophages in the lung and lymph nodes. The 1-α-hydroxylase enzyme is also expressed at other extrarenal sites, including the gastrointestinal tract, skin, vasculature, mammary epithelial cells, and in osteoblasts and osteoclasts 82.
People can become deficient in vitamin D because they don’t consume enough or absorb enough from food, their exposure to sunlight is limited, or their kidneys cannot convert vitamin D to its active form in the body. In children, vitamin D deficiency causes rickets, a disease where the bones become soft and bend due to a failure of bone tissue to properly mineralize 83. The fortification of milk and other staples, such as breakfast cereals and margarine, with vitamin D beginning in the 1930s has made rickets a rare disease in the United States, although it is still reported periodically, particularly among African American infants and children, immigrants from African, Middle-Eastern, and Asian countries 84, 85, 86. Rickets is also more prevalent among immigrants from Asia, Africa, and the Middle East, possibly because of genetic differences in vitamin D metabolism, dietary preferences, and behavioral differences that lead to less sun exposure 87.
Prolonged exclusive breastfeeding without the American Academy of Pediatrics-recommended vitamin D supplementation is a significant cause of rickets, particularly in dark-skinned infants breastfed by mothers who are not vitamin D replete 88. Additional causes of rickets include extensive use of sunscreens and placement of children in daycare programs, where they often have less outdoor activity and sun exposure 83, 89.
In adults and adolescents, vitamin D deficiency can lead to osteomalacia, in which existing bone is incompletely or defectively mineralized during the remodeling process, resulting in weak bones causing bone pain and muscle weakness 90. Signs and symptoms of osteomalacia are similar to those of rickets and include bone deformities and pain, hypocalcemic seizures, tetanic spasms, and dental abnormalities 91.
A lack of vitamin D has been associated with:
- An impairment in memory and thinking skills in older adults
- Bone, back, or muscle pain
- Cancer (particularly colon cancer)
- Cardiovascular disease, and an increased risk of dying from a stroke or a heart attack
- Constant fatigue and tiredness
- Frequent infections (such as colds and flu)
- Hair loss
- Kidney disease
- Low mood or depression
- Osteomalacia
- Osteoporosis
- Poor dental health
- Rickets
- Severe asthma in children
- Skin wounds that take a long time to heal.
Research also suggests low vitamin D may be a factor in several other conditions such as type 2 diabetes, high blood pressure, and multiple sclerosis.
Vitamin D has other roles in the body, including modulation of cell growth, neuromuscular and immune function, and reduction of inflammation 62, 84, 92. Many genes encoding proteins that regulate cell proliferation, differentiation, and apoptosis are modulated in part by vitamin D 62. Many cells have vitamin D receptors, and some convert 25-hydroxyvitamin D (25(OH)D or calcidiol) to calcitriol [1,25-dihydroxyvitamin D or 1,25(OH)2D].
Serum concentration of 25-hydroxyvitamin D (25(OH)D or calcidiol) is the best indicator of vitamin D status. It reflects vitamin D produced cutaneously and that obtained from food and supplements 62 and has a fairly long circulating half-life of 15 days 93. 25-hydroxyvitamin D (25(OH)D or calcidiol) functions as a biomarker of exposure, but it is not clear to what extent 25-hydroxyvitamin D (25(OH)D or calcidiol) levels also serve as a biomarker of effect (i.e., relating to health status or outcomes) 62. Serum 25-hydroxyvitamin D (25(OH)D or calcidiol) levels do not indicate the amount of vitamin D stored in body tissues.
In contrast to 25-hydroxyvitamin D [25(OH)D or calcidiol], circulating Calcitriol [1,25-dihydroxyvitamin D or 1,25(OH)2D] is generally not a good indicator of vitamin D status because it has a short half-life of 15 hours and serum concentrations are closely regulated by parathyroid hormone, calcium, and phosphate 93. Levels of 1,25(OH)2D do not typically decrease until vitamin D deficiency is severe 94, 95.
Researchers have not definitively identified serum concentrations of 25-hydroxyvitamin D [25(OH)D] associated with deficiency (e.g., rickets), adequacy for bone health, and overall health. After reviewing data on vitamin D needs, an expert committee of the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine concluded that people are at risk of vitamin D deficiency at serum 25-hydroxyvitamin D [25(OH)D] concentrations less than 30 nmol/L (12 ng/mL; see Table 1 for definitions of “deficiency” and “inadequacy”) 67. Some people are potentially at risk of inadequacy at 30 to 50 nmol/L (12–20 ng/mL). Levels of 50 nmol/L (20 ng/mL) or more are sufficient for most people. In contrast, the Endocrine Society stated that, for clinical practice, a serum 25(OH)D concentration of more than 75 nmol/L (30 ng/mL) is necessary to maximize the effect of vitamin D on calcium, bone, and muscle metabolism 96. The Food and Nutrition Board committee also noted that serum concentrations greater than 125 nmol/L (50 ng/mL) can be associated with adverse effects (Table 1).
Optimal serum concentrations of 25-hydroxyvitamin D [25(OH)D] for bone and general health have not been established because they are likely to vary by stage of life, by race and ethnicity, and with each physiological measure used 97. In addition, although 25-hydroxyvitamin D [25(OH)D] levels rise in response to increased vitamin D intake, the relationship is nonlinear 62. The amount of increase varies, for example, by baseline serum levels and duration of supplementation.
An additional complication in assessing vitamin D status is in the actual measurement of 25-hydroxyvitamin D (25(OH)D or calcidiol) concentrations. Considerable variability exists among the various assays available (the two most common methods being antibody based and liquid chromatography based) and among laboratories that conduct the analyses 62, 98, 99. This means that compared with the actual concentration of 25-hydroxyvitamin D (25(OH)D or calcidiol) in a sample of blood serum, a falsely low or falsely high value may be obtained depending on the assay or laboratory used 100. A standard reference material for 25-hydroxyvitamin D (25(OH)D or calcidiol) became available in July 2009 that permits standardization of values across laboratories and may improve method-related variability 62, 101.
Table 5. Serum 25-Hydroxyvitamin D [25(OH)D] Concentrations and Health
| nmol/L** | ng/mL* | Health status |
|---|---|---|
| <30 | <12 | Associated with vitamin D deficiency, leading to rickets in infants and children and osteomalacia in adults |
| 30 to <50 | 12 to <20 | Generally considered inadequate for bone and overall health in healthy individuals |
| ≥50 | ≥20 | Generally considered adequate for bone and overall health in healthy individuals |
| >125 | >50 | Emerging evidence links potential adverse effects to such high levels, particularly >150 nmol/L (>60 ng/mL) |
Footnotes:
* Serum concentrations of 25(OH)D are reported in both nanomoles per liter (nmol/L) and nanograms per milliliter (ng/mL).
** 1 nmol/L = 0.4 ng/mL and 1 ng/mL = 2.5 nmol/L.
What is a low vitamin D level?
There is a bit of controversy regarding what is considered a low vitamin D level between different expert organizations. A vitamin D level measures levels of 25-hydroxyvitamin D [25(OH)D] also known as calcidiol, in the blood.
Most experts recommend:
- Levels of 20-50 nanograms/milliliter (ng/ml) of 25(OH)D: Sufficient (good)
- Levels of 12-19 ng/ml: Borderline
- Levels of less than 12 ng/ml: Deficient (low)
However, not everybody agrees, and some organizations suggest different cut-off values.
The Institute of Medicine states:
- Levels above 20 ng/ml: Sufficient
- Levels below 20 ng/ml: Deficient
Note that several members of the Institute of Medicine committee publicly stated that over screening for vitamin D deficiency was a problem which typically resulted in unnecessary treatment. They were not in agreement with a cut-off level of 20 ng/ml for deficiency and recommended a lower level of 12.5 ng/ml.
The Endocrine Society states:
- Levels above 30 ng/ml: Sufficient; however, some assays are inaccurate and levels of 40-60 ng/ml better guarantee sufficiency
- Levels of 21-29 ng/ml: Insufficient
- Levels below 20 ng/ml: Deficient
Talk to your doctor about what he/she considers to be a low vitamin D level.
How much vitamin D do you need?
Intake reference values for vitamin D and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by the Food and Nutrition Board (FNB) at the Institute of Medicine of The National Academies 62. DRI is the general term for a set of reference values used to plan and assess nutrient intakes of healthy people. These values, which vary by age and gender, include:
- Recommended Dietary Allowance (RDA): average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy people.
- Adequate Intake (AI): established when evidence is insufficient to develop an RDA and is set at a level assumed to ensure nutritional adequacy.
- Tolerable Upper Intake Level (UL): maximum daily intake unlikely to cause adverse health effects 62.
The Food and Nutrition Board (FNB) established an RDA for vitamin D representing a daily intake that is sufficient to maintain bone health and normal calcium metabolism in healthy people. RDAs for vitamin D are listed in both International Units (IUs) and micrograms (mcg); the biological activity of 40 IU is equal to 1 mcg (Table 5). Even though sunlight may be a major source of vitamin D for some, the vitamin D RDAs are set on the basis of minimal sun exposure 62.
Table 5. Recommended Dietary Allowances (RDAs) for Vitamin D
| Life Stage | Recommended Amount |
|---|---|
| Birth to 12 months | 10 mcg (400 IU) |
| Children 1–13 years | 15 mcg (600 IU) |
| Teens 14–18 years | 15 mcg (600 IU) |
| Adults 19–70 years | 15 mcg (600 IU) |
| Adults 71 years and older | 20 mcg (800 IU) |
| Pregnant and breastfeeding teens and women | 15 mcg (600 IU) |
Footnotes: The amount of vitamin D contained in supplements is sometimes expressed in international units (IU) where 40 IU is equal to one microgram (1 mcg) of vitamin D.
The total daily vitamin D intake of persons who are not vitamin D deficient should not exceed 2,000 IU 102. Many calcium supplements contain vitamin D. Most multivitamins contain 400 IU of vitamin D. Vitamin D supplements can be taken on their own, or with calcium or food.
The upper limit of safety for vitamin D is 4000 IU/day. There are currently differing recommendations regarding the optimal 25-hydroxyvitamin D (25-OHD) level for bone health with the Institute of Medicine committee recommending a 25-hydroxyvitamin D (25-OHD) level ≥20-29 ng/mL while several other societies recommend a 25-OHD level ≥30 ng/mL 96.
Adults who are vitamin D deficient require treatment with higher doses of vitamin D, may be treated with 50,000 IU of vitamin D2 or vitamin D3 once a week or the equivalent daily dose (7000 IU vitamin D2 or vitamin D3) for 8–12 weeks to achieve a 25(OH)D blood level of approximately 30 ng/ml 103. This regimen should be followed by maintenance therapy of 1500–2000 IU/day or whatever dose is needed to maintain the target blood level 104, 105.
In the presence of vitamin D deficiency, it is safe to normalize vitamin D levels to a 25-hydroxyvitamin D (25-OHD) level of 30 ng/ml to prevent the compensatory rise in parathyroid hormone (PTH) level 107 . This may be done in a variety of ways. One approach is shown in Table 6 below. High doses of vitamin D are needed [e.g., 50,000 IU of vitamin D2 (ergocalciferol) weekly for 8 weeks or according to the 25-hydroxyvitamin D level] 96. Individuals with malabsorption often require very high doses of supplemental vitamin D.
Table 6. Vitamin D repletion to achieve a 25-hydroxy (25-OH) vitamin D level of 30 to 32 ng/ml
| 25-(OH) Vitamin D | Recommended Treatment Dose |
| < 10 ng/ml | Evaluation by a bone specialist. |
| < 20 ng/ml | 50,000 IU Vitamin D2 weekly for 8 weeks and then recheck level. Once sufficient level is reached, consider maintenance with 800-1000 IU of Vitamin D3 daily or 50,000 IU Vitamin D2 once or twice monthly as needed. |
| ≤ 25 ng/ml | 50,000 IU of Vitamin D2 every 2 weeks for 4 weeks and then consider maintenance with 800-1000 IU of Vitamin D3 daily or 50,000 IU Vitamin D2 once or twice monthly as needed. (opinion based) |
What foods provide vitamin D?
The flesh of fatty fish (such as salmon, tuna, and mackerel) and fish liver oils are among the best sources of vitamin D 62, 108. An animal’s diet affects the amount of vitamin D in its tissues. Small amounts of vitamin D are found in beef liver, cheese, and egg yolks. Vitamin D in these foods is primarily in the form of vitamin D3 and its metabolite 25(OH)D3 109. Mushrooms provide variable amounts of vitamin D2 110. Some mushrooms available on the market have been treated with UV light to increase their levels of vitamin D2. In addition, the Food and Drug Administration (FDA) has approved UV-treated mushroom powder as a food additive for use as a source of vitamin D2 in food products 111. Very limited evidence suggests no substantial differences in the bioavailability of vitamin D from various foods 112.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin D arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminD-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminD-Food.pdf). However, FoodData Central does not include the amounts of 25(OH)D in foods. A variety of foods and their vitamin D levels per serving are listed in Table 3.
Animal-based foods typically provide some vitamin D in the form of 25-hydroxyvitamin D (25(OH)D or calcidiol) in addition to vitamin D3 (cholecalciferol). The impact of this form on vitamin D status is an emerging area of research. Studies show that 25-hydroxyvitamin D (25(OH)D or calcidiol) appears to be approximately five times more potent than the parent vitamin D for raising serum 25(OH)D concentrations 110. One study found that when the 25-hydroxyvitamin D (25(OH)D or calcidiol) content of beef, pork, chicken, turkey, and eggs is taken into account, the total amount of vitamin D in the food is 2 to 18 times higher than the amount in the parent vitamin D alone, depending on the food 113.
Fortified foods provide most of the vitamin D in the American diet 62, 114. For example, almost all of the U.S. milk supply is voluntarily fortified with about 3 mcg/cup (120 IU), usually in the form of vitamin D3 115. In the 1930s, a milk fortification program was implemented in the United States to combat rickets, then a major public health problem 62. In Canada, milk must be fortified with 0.88–1.0 mcg/100 mL (35–40 IU), and the required amount for margarine is at least 13.25 mcg/100 g (530 IU). Other dairy products made from milk, such as cheese and ice cream, are not usually fortified in the United States or Canada. Plant milk alternatives (such as beverages made from soy, almond, or oats) are often fortified with similar amounts of vitamin D to those in fortified cow’s milk (about 3 mcg [120 IU]/cup); the Nutrition Facts label lists the actual amount 116. Ready-to-eat breakfast cereals often contain added vitamin D, as do some brands of orange juice, yogurt, margarine, and other food products.
Both the United States and Canada mandate the fortification of infant formula with vitamin D: 1–2.5 mcg/100 kcal (40–100 IU) vitamin D in the United States and 1–2 mcg/100 kcal (40–80 IU) in Canada 62.
Fortified foods provide most of the vitamin D in American diets 62:
- Fatty fish such as salmon, tuna, and mackerel are among the best sources.
- Beef liver, cheese, and egg yolks provide small amounts.
- Mushrooms provide some vitamin D. In some mushrooms that are newly available in stores, the vitamin D content is being boosted by exposing these mushrooms to ultraviolet light.
- Almost all of the U.S. milk supply is fortified with 400 IU of vitamin D per quart. But foods made from milk, like cheese and ice cream, are usually not fortified.
- Vitamin D is added to many breakfast cereals and to some brands of orange juice, yogurt, margarine, and soy beverages; check the labels.
A variety of foods and their vitamin D levels per serving are listed in Table 7.
Table 7. Vitamin D content of selected foods
| Food | Micrograms (mcg) per serving | International Units (IU) per serving | Percent DV* |
|---|---|---|---|
| Cod liver oil, 1 tablespoon | 34 | 1360 | 170 |
| Trout (rainbow), farmed, cooked, 3 ounces | 16.2 | 645 | 81 |
| Salmon (sockeye), cooked, 3 ounces | 14.2 | 570 | 71 |
| Mushrooms, white, raw, sliced, exposed to UV light, ½ cup | 9.2 | 366 | 46 |
| Milk, 2% milkfat, vitamin D fortified, 1 cup | 2.9 | 120 | 15 |
| Soy, almond, and oat milks, vitamin D fortified, various brands, 1 cup | 2.5-3.6 | 100-144 | 13-18 |
| Ready-to-eat cereal, fortified with 10% of the DV for vitamin D, 1 serving | 2 | 80 | 10 |
| Sardines (Atlantic), canned in oil, drained, 2 sardines | 1.2 | 46 | 6 |
| Egg, 1 large, scrambled** | 1.1 | 44 | 6 |
| Liver, beef, braised, 3 ounces | 1 | 42 | 5 |
| Tuna fish (light), canned in water, drained, 3 ounces | 1 | 40 | 5 |
| Cheese, cheddar, 1.5 ounce | 0.4 | 17 | 2 |
| Mushrooms, portabella, raw, diced, ½ cup | 0.1 | 4 | 1 |
| Chicken breast, roasted, 3 ounces | 0.1 | 4 | 1 |
| Beef, ground, 90% lean, broiled, 3 ounces | 0 | 1.7 | 0 |
| Broccoli, raw, chopped, ½ cup | 0 | 0 | 0 |
| Carrots, raw, chopped, ½ cup | 0 | 0 | 0 |
| Almonds, dry roasted, 1 ounce | 0 | 0 | 0 |
| Apple, large | 0 | 0 | 0 |
| Banana, large | 0 | 0 | 0 |
| Rice, brown, long-grain, cooked, 1 cup | 0 | 0 | 0 |
| Whole wheat bread, 1 slice | 0 | 0 | 0 |
| Lentils, boiled, ½ cup | 0 | 0 | 0 |
| Sunflower seeds, roasted, ½ cup | 0 | 0 | 0 |
| Edamame, shelled, cooked, ½ cup | 0 | 0 | 0 |
Footnotes:
* DV = Daily Value. The FDA developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for vitamin D is 20 mcg (800 IU) for adults and children aged 4 years and older 117. The labels must list vitamin D content in mcg per serving and have the option of also listing the amount in IUs in parentheses. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
** Vitamin D is in the yolk.
[Source 118 ]What kinds of vitamin D dietary supplements are available?
Vitamin D is found in supplements (and fortified foods) in two different forms: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Both increase vitamin D in the blood.
In supplements and fortified foods, vitamin D is available in two forms, D2 (ergocalciferol) and D3 (cholecalciferol) that differ chemically only in their side-chain structure. Vitamin D2 is manufactured by the UV irradiation of ergosterol in yeast, and vitamin D3 is manufactured by the irradiation of 7-dehydrocholesterol from lanolin and the chemical conversion of cholesterol 95. The two forms have traditionally been regarded as equivalent based on their ability to cure rickets and, indeed, most steps involved in the metabolism and actions of vitamin D2 and vitamin D3 are identical. Both forms (as well as vitamin D in foods and from cutaneous synthesis) effectively raise serum Calcidiol [25-hydroxyvitamin D or 25(OH)D] levels 94. Firm conclusions about any different effects of these two forms of vitamin D cannot be drawn. However, it appears that at nutritional doses vitamins D2 and D3 are equivalent, but at high doses vitamin D2 is less potent. Some studies suggest that cholecalciferol (Vitamin D3) increases serum Calcidiol [25(OH) D] more efficiently than does ergocalciferol (Vitamin D2) 76.
- Vitamin D3 (cholecalciferol) is available in 400, 800, 1000, 2000, 5000, 10,000, and 60,000 IU capsules. It is available in some countries as an intramuscular injection (Arachital 600,000 IU, which maintains vitamin D levels for 1 year). However, it can be extremely painful 76.
- Vitamin D2 (ergocalciferol) is available for oral use in 400 and 50,000 unit capsules or in a liquid form (8000 IU/mL) 76.
The American Academy of Pediatrics (AAP) recommends that exclusively and partially breastfed infants receive supplements of 400 IU/day of vitamin D shortly after birth and continue to receive these supplements until they are weaned and consume ≥1,000 mL/day of vitamin D-fortified formula or whole milk 119. Similarly, all non-breastfed infants ingesting <1,000 mL/day of vitamin D-fortified formula or milk should receive a vitamin D supplement of 400 IU/day 119. The American Academy of Pediatrics also recommends that older children and adolescents who do not obtain 400 IU/day through vitamin D-fortified milk and foods should take a 400 IU vitamin D supplement daily. However, this latter recommendation (issued November 2008) needs to be reevaluated in light of the Food and Nutrition Board’s vitamin D RDA of 600 IU/day for children and adolescents (issued November 2010 and which previously was an AI of 200 IU/day).
Am I getting enough vitamin D?
Because you get vitamin D from food, sunshine, and dietary supplements, one way to know if you’re getting enough is a blood test that measures the amount of vitamin D in your blood. In the blood, a form of vitamin D known as 25-hydroxyvitamin D is measured in either nanomoles per liter (nmol/L) or nanograms per milliliter (ng/mL). One nmol/L is the same as 0.4 ng/mL.
- Levels of 50 nmol/L (20 ng/mL) or above are adequate for most people for bone and overall health.
- Levels below 30 nmol/L (12 ng/mL) are too low and might weaken your bones and affect your health.
- Levels above 125 nmol/L (50 ng/mL) are too high and might cause health problems.
In the United States, most people have adequate blood levels of vitamin D. However, almost one out of four people have vitamin D blood levels that are too low or inadequate for bone and overall health.
Some people are more likely than others to have trouble getting enough vitamin D:
- Breastfed infants. Breast milk alone does not provide infants with an adequate amount of vitamin D. Breastfed infants should be given a supplement of 10 mcg (400 IU) of vitamin D each day.
- Older adults. As you age, your skin’s ability to make vitamin D when exposed to sunlight declines.
- People who seldom expose their skin to sunshine because they do not go outside or because they keep their body and head covered. Sunscreen also limits the amount of vitamin D your skin produces.
- People with dark skin. The darker your skin, the less vitamin D you make from sunlight exposure.
- People with conditions that limit fat absorption, such as Crohn’s disease, celiac disease, or ulcerative colitis. This is because the vitamin D you consume is absorbed in the gut along with fat, so if your body has trouble absorbing fat, it will also have trouble absorbing vitamin D.
- People who are obese or have undergone gastric bypass surgery. They may need more vitamin D than other people.
Can you get vitamin D from the sun?
Most people meet at least some of their vitamin D needs through exposure to sunlight 67, 94. Ultraviolet (UV) B radiation with a wavelength of 290–320 nanometers penetrates uncovered skin and converts cutaneous 7-dehydrocholesterol to previtamin D3, which in turn becomes vitamin D3 67. Season, time of day, length of day, cloud cover, smog, skin melanin content, and sunscreen are among the factors that affect UV radiation exposure and vitamin D synthesis 67. Perhaps surprisingly, geographic latitude does not consistently predict average serum 25(OH)D levels in a population. Ample opportunities exist to form vitamin D (and store it in the liver and fat) from exposure to sunlight during the spring, summer, and fall months even in the far north latitudes 67.
Complete cloud cover reduces UV energy by 50%; shade (including that produced by severe pollution) reduces it by 60% 120. UVB radiation does not penetrate glass, so exposure to sunshine indoors through a window does not produce vitamin D 121. Sunscreens with a sun protection factor (SPF) of 8 or more appear to block vitamin D-producing UV rays, although in practice people generally do not apply sufficient amounts, cover all sun-exposed skin, or reapply sunscreen regularly 67, 122. Therefore, skin likely synthesizes some vitamin D even when it is protected by sunscreen as typically applied.
The factors that affect UV radiation exposure and research to date on the amount of sun exposure needed to maintain adequate vitamin D levels make it difficult to provide general guidelines. It has been suggested by some vitamin D researchers, for example, that approximately 5–30 minutes of sun exposure between 10 AM and 3 PM at least twice a week to the face, arms, legs, or back without sunscreen usually lead to sufficient vitamin D synthesis and that the moderate use of commercial tanning beds that emit 2%–6% UVB radiation is also effective 95, 123. Individuals with limited sun exposure need to include good sources of vitamin D in their diet or take a supplement to achieve recommended levels of intake.
Despite the importance of the sun for vitamin D synthesis, it is prudent to limit exposure of skin to sunlight 122 and UV radiation from tanning beds 124. UV radiation is a carcinogen responsible for most of the estimated 1.5 million skin cancers and the 8,000 deaths due to metastatic melanoma that occur annually in the United States 122. Lifetime cumulative UV damage to skin is also largely responsible for some age-associated dryness and other cosmetic changes. The American Academy of Dermatology advises that photoprotective measures be taken, including the use of sunscreen, whenever one is exposed to the sun 86. Assessment of vitamin D requirements cannot address the level of sun exposure because of these public health concerns about skin cancer, and there are no studies to determine whether UVB-induced synthesis of vitamin D can occur without increased risk of skin cancer 67.
The body makes vitamin D when skin is directly exposed to the sun, and most people meet at least some of their vitamin D needs this way. Skin exposed to sunshine indoors through a window will not produce vitamin D. Cloudy days, shade, and having dark-colored skin also cut down on the amount of vitamin D the skin makes.
However, despite the importance of the sun to vitamin D synthesis, it is prudent to limit exposure of skin to sunlight in order to lower the risk for skin cancer. When out in the sun for more than a few minutes, wear protective clothing and apply sunscreen with an SPF (sun protection factor) of 15 or more. Tanning beds also cause the skin to make vitamin D, but pose similar risks for skin cancer.
People who avoid the sun or who cover their bodies with sunscreen or clothing should include good sources of vitamin D in their diets or take a supplement. Recommended intakes of vitamin D are set on the assumption of little sun exposure.
How long should you spend in the sun?
Most people can make enough vitamin D from being out in the sun daily for short periods with their forearms, hands or lower legs uncovered and without sunscreen from late March or early April to the end of September, especially from 11am to 3pm.
It’s not known exactly how much time is needed in the sun to make enough vitamin D to meet your body’s requirements. This is because there are a number of factors that can affect how vitamin D is made, such as your skin color or how much skin you have exposed. But you should be careful not to burn in the sun, so take care to cover up, or protect your skin with sunscreen, before your skin starts to turn red or burn.
Your risk of sunburn depends on 2 things. How sun-sensitive your skin is, and how strong the UV rays are you’re exposed to. Different people will have a different risk of sunburn on the same day, so it’s a good idea to know when your risk is high, so you can protect your skin.
In general people who have one or more of the following are at more risk:
- skin that burns easily
- light or fair colored skin, hair, or eyes
- lots of moles or freckles
- a history of sunburn
- a personal or family history of skin cancer
People with dark skin, such as those of African, African-Caribbean or south Asian origin, will need to spend longer in the sun to produce the same amount of vitamin D as someone with lighter skin.
- Children aged under six months should be kept out of direct strong sunlight. To ensure they get enough vitamin D, babies and children aged under five years should be given vitamin D supplements even if they do get out in the sun.
How long it takes for your skin to go red or burn varies from person to person. You’re the best person to know how your skin reacts in the sun. The more easily you get sunburnt, the more careful you need to be. Remember, you don’t need to peel – if your skin’s gone red or pink in the sun, that’s sunburn, and it’s dangerous. For people with darker skin it may feel irritated, tender or itchy. The longer you stay in the sun, especially for prolonged periods without sun protection, the greater your risk of skin cancer. Using sunbeds is not a recommended way of making vitamin D.
Other things that affect the strength of UV rays are the:
- Time of year – the highest risk months in the US are April to September. Near the equator, there are strong UV rays all year round.
- Altitude – UV rays are stronger the higher you go. So skiers and mountaineers can easily get caught out.
- Cloud cover – over 90% of UV can pass through light cloud.
- Reflection – up to 80% of UV rays are reflected back from snow, 15% from sand, 10% from concrete and up to 30% from water (depending on how choppy it is).
Other Nutrients Important to Bone
The Institute of Medicine 102 recently provided recommended intakes for other bone-related nutrients, including phosphorus and magnesium (see Table 6: Adequate Intakes (Al) or Recommended Daily Allowances (RDA) and Tolerable Upper Intake Levels (UL) for Calcium, Vitamin D, Phosphorus, and Magnesium by Life-Stage Group for United States and Canada). Most Americans consume adequate quantities of phosphorus through their regular intake of meats, cereals, milk, and processed foods. While some beverages such as soft drinks also contain phosphorus, they are not a preferred source of phosphorus because they may displace calcium-rich beverages like milk 125.
Magnesium intakes may be suboptimal in those who do not eat enough green leafy vegetables, whole grains, nuts, and dairy products. Fortunately, most diets contain adequate levels of other bone-related micronutrients, such as vitamins K and C, copper, manganese, zinc, and iron, to promote bone health.
Some dietary components may potentially have negative effects on bone health, especially if calcium intakes are not adequate. For example, high levels of sodium or caffeine intake can increase calcium excretion in the urine. The effects of these factors can be overcome by increasing the amount of calcium in the diet 126. Studies have linked excessive amounts of phosphorus to altered calcium metabolism, but it appears that the typical level of phosphorus consumed by most individuals in the United States should not negatively affect bone health 127. Excessive amounts of preformed vitamin A (e.g., retinol) can also have negative effects on bone, so individuals should not consume more than the recommended dietary allowance for this vitamin 128. The vitamin A precursor (beta carotene) found in many fruits and vegetables does not have negative effects on bone, however.
Table 8. Adequate Intakes (Al) or Recommended Daily Allowances (RDA) and Tolerable Upper Intake Levels (UL) for Calcium, Vitamin D, Phosphorus, and Magnesium by Life-Stage Group for United States and Canada
| Life-stage group | Calcium (mg/day) | Vitamin D (IU/day) | Phosphorous (mg/day) | Magnesium (mg/day) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Al | UL | Al | UL | RDA | UL | RDA | UL† | ||
| Male | Female | ||||||||
| 0–6 months | 210 | ND* | 200 | 1000 | 100 | ND* | 30 | 30 | ND* |
| 7–12 months | 270 | ND* | 200 | 1000 | 275 | ND* | 75 | 75 | ND* |
| 1–3 years | 500 | 2500 | 200 | 2000 | 460 | 3000 | 80 | 80 | 65 |
| 4–8 years | 800 | 2500 | 200 | 2000 | 500 | 3000 | 130 | 130 | 110 |
| 9–13 years | 1300 | 2500 | 200 | 2000 | 1250 | 4000 | 240 | 240 | 350 |
| 14–18 years | 1300 | 2500 | 200 | 2000 | 1250 | 4000 | 410 | 360 | 350 |
| 19–30 years | 1000 | 2500 | 200 | 2000 | 700 | 4000 | 400 | 310 | 350 |
| 31–50 years | 1000 | 2500 | 200 | 2000 | 700 | 4000 | 420 | 320 | 350 |
| 51–70 years | 1200 | 2500 | 400 | 2000 | 700 | 4000 | 420 | 320 | 350 |
| > 70 years | 1200 | 2500 | 600 | 2000 | 700 | 3000 | 420 | 320 | 350 |
| Pregnancy: | |||||||||
| <18 years | 1300 | 2500 | 200 | 2000 | 1250 | 3500 | 400 | 350 | |
| 19–30 years | 1000 | 2500 | 200 | 2000 | 700 | 3500 | 350 | 350 | |
| 31–50 years | 1000 | 2500 | 200 | 2000 | 700 | 3500 | 360 | 350 | |
| Lactation: | |||||||||
| <18 years | 1300 | 2500 | 200 | 2000 | 1250 | 4000 | 360 | 350 | |
| 19–3 years | 1000 | 2500 | 200 | 2000 | 700 | 4000 | 310 | 350 | |
| 31–50 years | 1000 | 2500 | 200 | 2000 | 700 | 4000 | 320 | 350 |
Footnote: Represents intake from pharmacological agents only, does not include intake from food and water.
Abbreviations: AI = Adequate Intakes; UL = Tolerable Upper Intake Levels (represents intake from pharmacological agents only, does not include intake from food and water.); RDA = Recommended Daily Allowances; ND = Not Determinable
[Source 129 ]Vitamin K and Osteoporosis
Vitamin K is the term used to name a family of fat-soluble compounds that is naturally present in some foods and is available as a dietary supplement that is important for blood clotting and healthy bones and other diverse physiological functions 130. The three main forms are vitamin K are phylloquinone (vitamin K1), menaquinones (vitamin K2) and menadione (vitamin K3) 131. Vitamin K has thus been clinically applied for the treatment and prevention of osteoporosis 132, 133, 134, 135.
Vitamin K1 (phylloquinone), which is the major dietary source, is concentrated in leafy vegetables (e.g., green vegetables) because it is directly involved in photosynthesis and is the vitamin K form best characterized in terms of food composition and dietary intakes. Vitamin K1 (phylloquinone) is active in animals and is responsible for the production of coagulation factors. Vitamin K1 (phylloquinone) is also can be converted into vitamin K2 (menaquinones) in animals 131. Vitamin K2 or menaquinones are the product of bacterial production or intestinal bacteria conversion from dietary vitamin K1 (phylloquinone) and are also found in fermented foods (e.g., cheeses and the Japanese soybean product natto) (Figure 1) 136, 137. Vitamin K2 (menaquinones) have unsaturated isoprenyl side chains and are designated as MK-4 through MK-13, based on the length of their side chain 131, 138. MK-4, MK-7, and MK-9 are the most well-studied menaquinones. Food composition databases are limited for vitamin K2 (menaquinones) and their presence in foods varies by region. Dietary intakes of all forms of vitamin K vary widely among age groups and population subgroups. Similarly, the utilization of vitamin K from different forms and food sources appear to vary, although our understanding of vitamin K is still rudimentary in light of new developments regarding the vitamin K2 (menaquinones) 139.
In the United States, vitamin K3 (menadione) is used in poultry feed and some swine feeds as a source of vitamin K 131. As such, menaquinone-4 (MK-4) formed from vitamin K3 (menadione) is present in poultry and pork products in the U.S. food supply and is the primary dietary source of MK-4 140. Menaquinone-4 (MK-4) is present at high concentrations in human, poultry and pork tissues 141. Although humans generally obtain vitamin K1 (phylloquinone) and menaquinone-7 (MK-7) from the diet, intake of menaquinone-4 (MK-4) in animal foods is extremely low. Vitamin K3 (menadione), a synthetic vitamin K analog (Figure 1), is the primary source of vitamin K in poultry feed and some swine feeds, along with small amounts of phylloquinone (vitamin K1) 131. As such, MK-4 formed from vitamin K3 (menadione) is present in poultry and pork products in the U.S. food supply and is the primary dietary source of MK-4 140. Although menaquinone-4 (MK-4) is also formed from tissue-specific conversion of phylloquinone (vitamin K1) 142, the impact on dietary intake from this conversion is likely negligible as animal organs containing high MK-4 concentrations including kidney, brain, and pancreas, are not commonly consumed in most regions of the world. Menaquinone-4 (MK-4) is also found in modest amounts in milk, butter, and cheeses, which may make a small contribution to total vitamin K intake. The high consumption of poultry, pork, and dairy products in the United States 143, however, suggests that MK-4 may make a relevant contribution to total vitamin K intake. In regions where food systems do not use vitamin K3 (menadione) in animal feed or consumption of dairy products is low, MK-4 is most likely not an important dietary source of vitamin K. For example, MK-4 has been estimated to account for ∼3% of total vitamin K intake in the Netherlands 144 and is found in animal products in relatively lower amounts compared with the United States and Japan 141.
Menaquinone-4 (MK-4) is unique among the vitamin K2 (menaquinones) in that it is produced by the body from vitamin K1 (phylloquinone) via a conversion process that does not involve bacterial action. Instead, menaquinone-4 (MK-4) is formed by a realkylation step from vitamin K3 (menadione) present in animal feeds or is the product of tissue-specific conversion directly from dietary vitamin K1 (phylloquinone) 145, 146, 147. In the United States, vitamin K3 (menadione) is the synthetic form of vitamin K used in poultry feed. As such, MK-4 formed from vitamin K3 (menadione) is present in poultry products in the US food supply 148. However, MK-4 formed from vitamin K1 (phylloquinone) is limited to organs not commonly consumed in the diet including kidney. The exceptions are dairy products with menaquinone-4 (MK-4) found in milk, butter, and cheese, albeit in modest amounts. Therefore it is unlikely that menaquinone-4 (MK-4) is an important dietary source of vitamin K in food supplies that do not use vitamin K3 (menadione) for poultry feed nor are rich in dairy products.
Matrix Gla-protein, a vitamin K-dependent protein present in vascular smooth muscle, bone, and cartilage, is the focus of considerable scientific research because it might help reduce abnormal calcification 149, 150. Low plasma concentrations of vitamin K are associated with a high risk of bone fractures in both northern Europeans and Asians populations of both sexes 151, 152, 153.
Vitamin K is a cofactor for the gamma-carboxylation of many proteins, including osteocalcin, one of the main proteins in bone 154. Some research indicates that high serum levels of undercarboxylated osteocalcin are associated with lower bone mineral density 130, 154. Some, but not all, studies also link higher vitamin K intakes with higher bone mineral density and/or lower hip fracture incidence 155, 156, 157, 158, 159, 160.
Although vitamin K is involved in the carboxylation of osteocalcin, it is unclear whether supplementation with any form of vitamin K reduces the risk of osteoporosis. Wu et al. 161 showed that both phylloquinone (vitamin K1) and vitamin K2 or menaquinones (MK-4 and MK-7) inhibit osteoclast-mediated effects on bone resorption in a dose dependent manner. Furthermore, Rangel et al. 162 demonstrated increased compact bone mass, increased bone formation markers and decreased bone resorption markers in ovariectomized mice supplemented with vitamin K. Also, the effect of coadministration of vitamin K2 (menaquinones) and other antiosteoporotic drugs, such as Teriparatide in ovariectomized mice 163 and bisphosphonates, has been investigated in uremic osteoporosis (chronic kidney disease–related osteoporosis) 164.
In 2006, Cockayne and colleagues conducted a systematic review and meta-analysis of randomized controlled trials that examined the effects of vitamin K supplementation on bone mineral density and bone fracture 165. Most of the trials were conducted in Japan and involved postmenopausal women; trial duration ranged from 6 to 36 months. Thirteen trials were included in the systematic review, and 12 showed that supplementation with either phytonadione or MK-4 improved bone mineral density. Seven of the 13 trials also had fracture data that were combined in a meta-analysis. All of these trials used MK-4 at either 15 mg/day (1 trial) or 45 mg/day (6 trials). MK-4 supplementation significantly reduced rates of hip fractures, vertebral fractures, and all nonvertebral fractures.
In their meta-analysis, Hao et al. 166 showed a statistically significant inverse association between dietary vitamin phylloquinone (vitamin K1) intake and risk of fractures (highest vs. the lowest intake). The authors did not find any significant association between low vitamin phylloquinone (vitamin K1) and bone mineral density (BMD) 166. Recently, 374 postmenopausal women with osteoporosis were studied showing a lower serum vitamin phylloquinone (vitamin K1) in the group with fractures (prevalent fractures: 0.53 (0.41), no fractures: 0.65 (0.66) μg/L) and independently associated with fracture risk 167. Dp-uc MGP was detectable in 97 (75%) participants with serum vitamin phylloquinone (vitamin K1) of 0.26 (0.15) μg/L, whilst vitamin K dependent protein PIVKA-II was above the clinical threshold in only 3.8% 167.
To date, a limited number of randomized controlled trials have evaluated the effects of phylloquinone (vitamin K1) and menaquinones (vitamin K2) supplementation on fracture risk showing a potential positive effect and few trials are ongoing 168, 169, 170, 171. In a double blind, randomized, controlled study, 244 postmenopausal women received vitamin K2 MK-7 (180 μg MK-7/day) capsules or placebo for 3 years to investigate its effect on vertebral fractures. MK-7 significantly decreased the loss in vertebral height of the lower thoracic region at the mid-site of the vertebrae after 2 and 3 years 171. An interventional study 241 osteoporotic patients were enrolled in a 24-month randomized open-label study: in the control group (without treatment; n = 121) and the vitamin K2–treated group (n = 120), which received 45 mg/day orally MK-4 (45 mg/day orally). They found a reduction in the vitamin K2-treated group of the incidence of bone fractures lower than the control group 172.
In Japan, vitamin K2 MK-4 was approved for a drug for osteoporosis treatment in 1995 based on domestic clinical trials showing the efficacy on bone mineral density. Now, MK-4 is in use for osteoporosis treatment in several Asian countries. For the last two decades, interventional clinical trials were conducted throughout the world. Many trials used MK-4 as vitamin K treatment, MK-7 or vitamin K1 was also used in some trials as well. According to the most recent meta-analysis 173, a favorable effect of vitamin K on clinical fractures was statistically significant, although the effect on vertebral or hip fractures was not statistically significant. Among the clinical trials, the largest one was conducted in Japan 174. This study involved more than 4000 Japanese women with three years of intervention and a one-year follow-up period 174. This study failed to demonstrate the fracture-preventive effect of vitamin K2 (MK-4) in the whole group of subjects 174. However, the significant effect on new vertebral fractures was observed in the subgroup analysis of high-risk patients with at least five pre-existing vertebral fractures 175.
Based on this information, long-term supplementation with vitamin K in postmenopausal women with osteoporosis might have some potential utility, in particular, given its negligible risk of serious side effects. This is also supported by the fact that vitamin K2 MK-4 in relatively high doses (45 mg) is registered in Japan and other parts of Asia for postmenopausal osteoporosis 176, 177, 130. The European Food Safety Authority has approved a health claim for vitamin K, noting that “a cause and effect relationship has been established between the dietary intake of vitamin K and the maintenance of normal bone” 178. The FDA has not authorized a health claim for vitamin K in the United States.
Is magnesium good for my bones?
Magnesium is an abundant mineral in your body, is naturally present in many foods, added to other food products, available as a dietary supplement, and present in some medicines (such as antacids and laxatives) 179. Approximately 30% to 40% of the dietary magnesium consumed is typically absorbed by the body 180. Magnesium is a cofactor in more than 300 enzyme systems that regulate diverse biochemical reactions in your body, including protein synthesis, muscle and nerve function, blood glucose control, and blood pressure regulation 181. Magnesium is required for energy production, oxidative phosphorylation, and glycolysis. Magnesium is involved in bone formation and influences the activities of osteoblasts and osteoclasts 182. Magnesium also affects the concentrations of both parathyroid hormone (PTH) and the active form of vitamin D, which are major regulators of bone homeostasis. Several population-based studies have found positive associations between magnesium intake and bone mineral density in both men and women 183. Other research has found that women with osteoporosis have lower serum magnesium levels than women with osteopenia and those who do not have osteoporosis or osteopenia 184. These and other findings indicate that magnesium deficiency might be a risk factor for osteoporosis 182.
Magnesium is also required for the synthesis of DNA, RNA, and the antioxidant glutathione. Magnesium also plays a role in the active transport of calcium and potassium ions across cell membranes, a process that is important to nerve impulse conduction, muscle contraction, and normal heart rhythm 181.
Although limited in number, studies suggest that increasing magnesium intakes from food or supplements might increase bone mineral density in postmenopausal and elderly women 185. For example, one short-term study found that 290 mg/day elemental magnesium (as magnesium citrate) for 30 days in 20 postmenopausal women with osteoporosis suppressed bone turnover compared with placebo, suggesting that bone loss decreased 186.
Diets that provide recommended levels of magnesium enhance bone health, but further research is needed to elucidate the role of magnesium in the prevention and management of osteoporosis.
An adult body contains approximately 25 g magnesium, with 50% to 60% present in the bones and most of the rest in soft tissues 187. Less than 1% of total magnesium is in blood serum, and these levels are kept under tight control. Normal serum magnesium concentrations range between 0.75 and 0.95 millimoles (mmol)/L 188. Hypomagnesemia is defined as a serum magnesium level less than 0.75 mmol/L 189. Magnesium homeostasis is largely controlled by the kidney, which typically excretes about 120 mg magnesium into the urine each day 190. Urinary excretion is reduced when magnesium status is low 185.
Assessing magnesium status is difficult because most magnesium is inside cells or in bone 181. The most commonly used and readily available method for assessing magnesium status is measurement of serum magnesium concentration, even though serum levels have little correlation with total body magnesium levels or concentrations in specific tissues 189. Other methods for assessing magnesium status include measuring magnesium concentrations in red blood cells, saliva, and urine; measuring ionized magnesium concentrations in blood, plasma, or serum; and conducting a magnesium-loading (or “tolerance”) test. No single method is considered satisfactory 191. Some experts 187 but not others 181 consider the tolerance test (in which urinary magnesium is measured after parenteral infusion of a dose of magnesium) to be the best method to assess magnesium status in adults. To comprehensively evaluate magnesium status, both laboratory tests and a clinical assessment might be required 189.
How much magnesium do I need?
The amount of magnesium you need depends on your age and sex. Average daily recommended amounts are listed below in milligrams (mg). Table 9 lists the current Recommended Dietary Allowances (RDAs) for magnesium 185. For infants from birth to 12 months, the Food and Nutrition Board at the Institute of Medicine of the National Academies established an Adequate Intake (AI) for magnesium that is equivalent to the mean intake of magnesium in healthy, breastfed infants, with added solid foods for ages 7–12 months.
- Recommended Dietary Allowance (RDA): average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy people.
- Adequate Intake (AI): established when evidence is insufficient to develop an RDA and is set at a level assumed to ensure nutritional adequacy.
- Tolerable Upper Intake Level (UL): maximum daily intake unlikely to cause adverse health effects 185.
Table 9. Recommended Dietary Allowances (RDAs) for Magnesium
| Age | Male | Female | Pregnancy | Lactation |
| Birth to 6 months | 30 mg* | 30 mg* | ||
| 7–12 months | 75 mg* | 75 mg* | ||
| 1–3 years | 80 mg | 80 mg | ||
| 4–8 years | 130 mg | 130 mg | ||
| 9–13 years | 240 mg | 240 mg | ||
| 14–18 years | 410 mg | 360 mg | 400 mg | 360 mg |
| 19–30 years | 400 mg | 310 mg | 350 mg | 310 mg |
| 31–50 years | 420 mg | 320 mg | 360 mg | 320 mg |
| 51+ years | 420 mg | 320 mg |
Footnotes:
Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA).
[Source 185 ]What foods provide magnesium?
Magnesium is found naturally in many foods and is added to some fortified foods. Green leafy vegetables, such as spinach, legumes, nuts, seeds, and whole grains, are good sources 181. You can get recommended amounts of magnesium by eating a variety of foods, including the following:
- Legumes, nuts, seeds, whole grains, and green leafy vegetables (such as spinach)
- Fortified breakfast cereals and other fortified foods
- Milk, yogurt, and some other milk products
In general, foods containing dietary fiber provide magnesium. Magnesium is also added to some breakfast cereals and other fortified foods. Some types of food processing, such as refining grains in ways that remove the nutrient-rich germ and bran, lower magnesium content substantially 185. Selected food sources of magnesium are listed in Table 10.
Tap, mineral, and bottled waters can also be sources of magnesium, but the amount of magnesium in water varies by source and brand (ranging from 1 mg/L to more than 120 mg/L) 181.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides comprehensive list of foods containing magnesium arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Magnesium-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/Magnesium-Food.pdf).
Table 10. Magnesium content of selected foods
| Food | Milligrams (mg) per serving | Percent DV* |
| Pumpkin seeds, roasted, 1 ounce | 156 | 37 |
| Chia seeds, 1 ounce | 111 | 26 |
| Almonds, dry roasted, 1 ounce | 80 | 19 |
| Spinach, boiled, ½ cup | 78 | 19 |
| Cashews, dry roasted, 1 ounce | 74 | 18 |
| Peanuts, oil roasted, ¼ cup | 63 | 15 |
| Cereal, shredded wheat, 2 large biscuits | 61 | 15 |
| Soymilk, plain or vanilla, 1 cup | 61 | 15 |
| Black beans, cooked, ½ cup | 60 | 14 |
| Edamame, shelled, cooked, ½ cup | 50 | 12 |
| Peanut butter, smooth, 2 tablespoons | 49 | 12 |
| Potato, baked with skin, 3.5 ounces | 43 | 10 |
| Rice, brown, cooked, ½ cup | 42 | 10 |
| Yogurt, plain, low fat, 8 ounces | 42 | 10 |
| Breakfast cereals, fortified with 10% of the DV for magnesium, 1 serving | 42 | 10 |
| Oatmeal, instant, 1 packet | 36 | 9 |
| Kidney beans, canned, ½ cup | 35 | 8 |
| Banana, 1 medium | 32 | 8 |
| Salmon, Atlantic, farmed, cooked, 3 ounces | 26 | 6 |
| Milk, 1 cup | 24–27 | 6 |
| Halibut, cooked, 3 ounces | 24 | 6 |
| Raisins, ½ cup | 23 | 5 |
| Bread, whole wheat, 1 slice | 23 | 5 |
| Avocado, cubed, ½ cup | 22 | 5 |
| Chicken breast, roasted, 3 ounces | 22 | 5 |
| Beef, ground, 90% lean, pan broiled, 3 ounces | 20 | 5 |
| Broccoli, chopped and cooked, ½ cup | 12 | 3 |
| Rice, white, cooked, ½ cup | 10 | 2 |
| Apple, 1 medium | 9 | 2 |
| Carrot, raw, 1 medium | 7 | 2 |
Footnote: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for magnesium is 420 mg for adults and children aged 4 years and older. FDA does not require food labels to list magnesium content unless magnesium has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 192 ]Magnesium supplements
Magnesium supplements are available in a variety of forms, including magnesium oxide, citrate, and chloride 181. The Supplement Facts panel on a dietary supplement label declares the amount of elemental magnesium in the product, not the weight of the entire magnesium-containing compound.
Absorption of magnesium from different kinds of magnesium supplements varies. Forms of magnesium that dissolve well in liquid are more completely absorbed in the gut than less soluble forms 193. Small studies have found that magnesium in the aspartate, citrate, lactate, and chloride forms is absorbed more completely and is more bioavailable than magnesium oxide and magnesium sulfate 194. One study found that very high doses of zinc from supplements (142 mg/day) can interfere with magnesium absorption and disrupt the magnesium balance in the body 195.
Several types of medications have the potential to interact with magnesium supplements or affect magnesium status. A few examples are provided below. People taking these and other medications on a regular basis should discuss their magnesium intakes with their healthcare providers.
- Bisphosphonates: Magnesium-rich supplements or medications can decrease the absorption of oral bisphosphonates, such as alendronate (Fosamax®), used to treat osteoporosis 196. Use of magnesium-rich supplements or medications and oral bisphosphonates should be separated by at least 2 hours.
- Antibiotics: Magnesium can form insoluble complexes with tetracyclines, such as demeclocycline (Declomycin®) and doxycycline (Vibramycin®), as well as quinolone antibiotics, such as ciprofloxacin (Cipro®) and levofloxacin (Levaquin®). These antibiotics should be taken at least 2 hours before or 4–6 hours after a magnesium-containing supplement 197.
- Diuretics: Chronic treatment with loop diuretics, such as furosemide (Lasix®) and bumetanide (Bumex®), and thiazide diuretics, such as hydrochlorothiazide (Aquazide H®) and ethacrynic acid (Edecrin®), can increase the loss of magnesium in urine and lead to magnesium depletion 198. In contrast, potassium-sparing diuretics, such as amiloride (Midamor®) and spironolactone (Aldactone®), reduce magnesium excretion 198.
- Proton pump inhibitors: Prescription proton pump inhibitor (PPI) drugs, such as esomeprazole magnesium (Nexium®) and lansoprazole (Prevacid®), when taken for prolonged periods (typically more than a year) can cause hypomagnesemia 199. In cases that FDA reviewed, magnesium supplements often raised the low serum magnesium levels caused by PPIs. However, in 25% of the cases, supplements did not raise magnesium levels and the patients had to discontinue the PPI. FDA advises healthcare professionals to consider measuring patients’ serum magnesium levels prior to initiating long-term PPI treatment and to check magnesium levels in these patients periodically 199.
Health risks from excessive magnesium
Too much magnesium from food does not pose a health risk in healthy individuals because the kidneys eliminate excess amounts in the urine 200. However, high doses of magnesium from dietary supplements or medications often result in diarrhea that can be accompanied by nausea and abdominal cramping 185. Forms of magnesium most commonly reported to cause diarrhea include magnesium carbonate, chloride, gluconate, and oxide 193. The diarrhea and laxative effects of magnesium salts are due to the osmotic activity of unabsorbed salts in the intestine and colon and the stimulation of gastric motility.
Very large doses of magnesium-containing laxatives and antacids (typically providing more than 5,000 mg/day magnesium) have been associated with magnesium toxicity 201, including fatal hypermagnesemia in a 28-month-old boy 202 and an elderly man 203. Symptoms of magnesium toxicity, which usually develop after serum concentrations exceed 1.74–2.61 mmol/L, can include hypotension, nausea, vomiting, facial flushing, retention of urine, ileus, depression, and lethargy before progressing to muscle weakness, difficulty breathing, extreme hypotension, irregular heartbeat, and cardiac arrest 200. The risk of magnesium toxicity increases with impaired renal function or kidney failure because the ability to remove excess magnesium is reduced or lost 200.
The Food and Nutrition Board at the Institute of Medicine of the National Academies has established Tolerable Upper Intake Levels (ULs) for magnesium that apply only to supplemental magnesium for healthy infants, children, and adults (see Table 11) 185.
Table 11. Tolerable Upper Intake Levels (ULs) for Supplemental Magnesium
| Age | Male | Female | Pregnant | Lactating |
| Birth to 12 months | None established | None established | ||
| 1–3 years | 65 mg | 65 mg | ||
| 4–8 years | 110 mg | 110 mg | ||
| 9–18 years | 350 mg | 350 mg | 350 mg | 350 mg |
| 19+ years | 350 mg | 350 mg | 350 mg | 350 mg |
Exercise
Weight-bearing, strength, and balance-training exercises are also an important part of any osteoporosis prevention and treatment program, regardless of age. They can help increase or preserve bone mass and may also help reduce the risk of falling. All types of physical activity can contribute to bone health. Activities that are weight bearing or involve impact are most useful for increasing or maintaining bone mass. Since continued physical activity provides a positive stimulus for bone, muscle, and other aspects of health, a lifelong commitment to physical activity and exercise is critical. Ending a physical activity regimen will result in bone mass returning to the level that existed before the activity began. Since repetitive programs of physical activity may be discontinued due to lack of motivation or interest, variety and creativity are important if physical activity is to be continued over the long term.
Physical activity will only affect bone at the skeletal sites that are stressed (or loaded) by the activity. In other words, physical activity programs do not necessarily benefit the whole skeleton, although any type of activity provides more benefit to bone than does no activity at all. For bone gain to occur, the stimulus must be greater than that which the bone usually experiences. Static loads applied continuously (such as standing) do not promote increased bone mass.
Complete lack of activity, such as periods of immobility, causes bone loss. When it is not possible to avoid immobility (e.g., bed rest during sickness), even brief daily weight-bearing movements can help to reduce bone loss. General physical activity every day and some weight-bearing, strength-building, and balance-enhancing activities 2 or more times a week are generally effective for promoting bone health for most persons.
Any activity that imparts impact (such as jumping or skipping) may increase bone mass more than will low- and moderate-intensity, endurance-type activities, such as brisk walking. However, endurance activities may still play an important role in skeletal health by increasing muscle mass and strength, balance, and coordination, and they may also help prevent falls in the elderly. Endurance activity is also very important for other aspects of health, such as helping to prevent obesity, diabetes, or cardiovascular disease.
Load-bearing physical activities such as jumping need not be engaged in for long periods of time to provide benefits to skeletal health. In fact, 5–10 minutes daily may suffice. Most adults should begin with weight-bearing exercise and gradually add some skipping and jumping activity. Longer periods (30–45 minutes) may be needed for weight training or walking/jogging. Those who have been inactive should work up to this amount of time gradually using a progressive program, e.g., start with shorter times and easier activities (light weights or walking) and then increase time or intensity slowly (by no more than 10 percent each week) in order to avoid injury.
Activities that are not weight bearing or are low impact may help improve balance and coordination and maintain muscle mass, which can help prevent falls. To encourage increased levels of physical activity among all age groups, “Physical Activity and Health: A Surgeon General’s Report” 204 recommends a “minimum of 30 minutes of physical activity of moderate intensity (such as brisk walking) on most, if not all, days of the week”. Since the skeleton responds preferentially to strength training and short bouts of high-load impact activity (such as skipping or jumping), the same report recommends that adults supplement their cardiorespiratory endurance activity with strength-developing exercise at least two times per week.
For those who cannot engage in regular physical activity due to disability, mechanical stimulation of the skeleton might prove beneficial. Recent, small studies found that use of vibrating platforms increased BMD (bone mineral density) and slowed bone loss 205, 206, 207. This may provide another way to reduce fracture risk both in the elderly and in younger individuals with disabling conditions that limit their ability to exercise. However, the long-term safety and efficacy of such approaches remain to be determined, and therefore specific rehabilitation and exercise programs aimed at increasing activity and function remain critically important in the frail elderly and in younger individuals with neuromuscular disabilities.
As noted earlier, the evidence does not lead to a specific set of exercises or practices but rather a set of principles that can be applied and varied according to the age and current physical condition of an individual. Many of these principles have been reviewed by expert panels of the American College of Sports Medicine 208 and they lead to the following suggestions for the frequency, intensity, length, and type of physical activity regimens to benefit bone health for individuals of all ages:
- Physical activities that include a variety of loading patterns (such as strength training or aerobic classes) may promote increased bone mass more than do activities that involve normal or regular loading patterns (such as running).
- General physical activity every day and some weight-bearing, strength-building, and balance-enhancing activities 2 or more times a week are generally effective for promoting bone health for most persons.
- Any activity that imparts impact (such as jumping or skipping) may increase bone mass more than will low- and moderate-intensity, endurance-type activities, such as brisk walking. However, endurance activities may still play an important role in skeletal health by increasing muscle mass and strength, balance, and coordination, and they may also help prevent falls in the elderly. Endurance activity is also very important for other aspects of health, such as helping to prevent obesity, diabetes, or cardiovascular disease.
- Load-bearing physical activities such as jumping need not be engaged in for long periods of time to provide benefits to skeletal health. In fact, 5–10 minutes daily may suffice. Most adults should begin with weight-bearing exercise and gradually add some skipping and jumping activity. Longer periods (30–45 minutes) may be needed for weight training or walking/jogging. Those who have been inactive should work up to this amount of time gradually using a progressive program, e.g., start with shorter times and easier activities (light weights or walking) and then increase time or intensity slowly (by no more than 10 percent each week) in order to avoid injury.
- Physical activities that include a variety of loading patterns (such as strength training or aerobic classes) may promote increased bone mass more than do activities that involve normal or regular loading patterns (such as running).
Physical Activity for Older Adults
Most elderly individuals should strongly consider engaging in regular physical activity. Physical activity is the only single therapy that can simultaneously improve muscle mass, muscle strength, balance, and bone strength. As a result, it may decrease the risk of fractures, in part by reducing the risk of falling. In fact, fall-risk reduction may be the biggest benefit of physical activity for the elderly.
This summary of a Cochrane review 209 presents what we know from research about the effect of exercise on bone mass in postmenopausal women. The review shows that for postmenopausal women the results suggest a relatively small statistically significant, but possibly important, effect of exercise on bone density compared with control groups. Exercise has the potential to be a safe and effective way to avert bone loss in postmenopausal women.
- Exercise will improve bone mineral density slightly.
- Exercise will reduce the chances of having a fracture slightly.
The following guidelines should be used to maximize the potential fall prevention benefits of physical activity in the elderly:
- Physical activity needs to be of sufficient intensity to improve muscle strength, since poor muscle strength is a known risk factor for falls. Strength or resistance training is best for building muscle, but even aerobic endurance activity can yield some improvements in muscle strength.
- Improving balance can be an important component of any physical activity program designed to decrease falls. This program may include balance training exercises or a movement activity such as Tai Chi. Any activity that requires weight bearing and challenges the postural system can improve balance and potentially help reduce falls.
- Physical activity must be performed on average 3 times per week for 30–45 minutes per session for at least three months for strength and balance benefits to be realized, and it must be continued if benefits are to be maintained.
- Those who suffer a fall that requires a visit to a health care provider or an emergency room should ask for a fall risk assessment that includes a program of physical activity.
Fall Prevention
Falls are not just the result of getting older and falls represent perhaps the biggest threat to the bone health and the functional independence of older individuals. Falls are common and frequently are the precipitating event that leads to a fracture or fractures in an individual. Thus, fall prevention offers another important opportunity to protect the bones throughout life, but particularly in those over age 60. Falls occur for a variety of reasons, with multiple factors often contributing to a single fall. These factors include problems with balance, mobility, vision, lower extremity weakness, and/or blood pressure or circulation. Often these problems are compounded by an acute illness (e.g., infection, fever, dehydration, arrhythmia), a new medication, or an environmental stress (e.g., standing or walking on an unsafe surface, poor lighting) that leads to the fall. To reduce the risk of falls, a variety of fall prevention measures should be encouraged for frail, elderly individuals. These include regular vision checks; elimination (where possible) of medications and/or dosages that may cause dizziness, low blood pressure, or confusion; and addressing environmental problems or obstacles that can lead to falls, including removing throw rugs, installing night lights, installing railings on stairs and grab bars in showers, encouraging use of rubber-soled shoes and slippers, and attaching phone cords and other wires to the baseboard of the wall. Hip protectors or hip pads might also be useful in reducing the impact of those falls that do occur.
Many falls can be prevented. Falls are usually caused by a number of things. By changing some of these things, you can lower your chances of falling:
- Begin a regular exercise program: Exercise is one of the most important ways to reduce your chances of falling. It makes you stronger and helps you feel better. Exercises that improve balance and coordination (like tai chi) are the most helpful. Lack of exercise leads to weakness and increases your chances of falling. Ask your doctor or health care worker about the best type of exercise program for you.
- Have your eyes checked by an eye doctor. You may be wearing the wrong glasses or have a condition such as glaucoma or cataracts that limits your vision. Poor vision can increase your chances of falling.
- Make your home safer: About half of all falls happen at home. To make your home safer:
- Remove things you can trip over (such as papers, books, clothes, and shoes) from stairs and places where you walk.
- Remove small throw rugs or use double-sided tape to keep the rugs from slipping.
- Keep items you use often in cabinets you can reach easily without using a stepstool.
- Have grab bars put in next to your toilet and in the tub or shower.
- Use nonslip mats in the bathtub and on shower floors.
- Improve the lighting in your home. As you get older, you need brighter lights to see well. Lamp shades or frosted bulbs can reduce glare.
- Have handrails and lights put in on all staircases.
- Wear shoes that give good support and have thin nonslip soles. Avoid wearing slippers and athletic shoes with deep treads.
What is osteoporosis?
Osteoporosis literally means “porous bone.” Osteoporosis is a disease of the bones that causes you to lose bone mass. Having osteoporosis raises your risk of experiencing fractures 210, 211. Osteoporosis is characterized by too little bone formation, excessive bone loss, or a combination of both, leading to bone fragility and an increased risk of fractures of the hip, spine and wrist 212.
Osteoporosis occurs most often in older adults. Osteoporosis affects men and women of all races. But white and Asian women, especially older women who are past menopause, are at highest risk. This is due to several factors. Women have less bone mass than men to begin with. Women also tend to live longer and absorb less calcium. In women, the rate of bone loss speeds up after menopause, when estrogen levels decrease. Since the ovaries make estrogen, faster bone loss may occur if both ovaries are removed by surgery.
Bone is living tissue that is constantly being broken down and replaced. Normally, bone formation and resorption are closely balanced. Osteoblasts (cells that make the organic matrix of bone and then mineralize bone) and osteoclasts (cells that resorb bone) are regulated by parathyroid hormone (PTH), calcitonin, estrogen, vitamin D, various cytokines, and other local factors such as prostaglandins 213. Osteoporosis occurs when the creation of new bone doesn’t keep up with the loss of old bone.
You may not know you have osteoporosis until your symptoms are severe. There typically are no symptoms in the early stages of bone loss. But once your bones have been weakened by osteoporosis, you might have signs and symptoms of osteoporosis that include:
- Back pain, caused by a fractured or collapsed vertebra
- Loss of height over time
- A stooped posture or a hunched back
- A bone that breaks much more easily than expected
Signs of osteoporosis include frequent broken bones or fractures, low back pain, or a hunched back. You may get shorter over time due to osteoporosis. Osteoporosis can cause your vertebrae (the bones in your spine) to collapse. These problems tend to occur after a lot of bone calcium has already been lost.
The good news is that medications, healthy diet and weight-bearing exercise can help prevent bone loss or strengthen already weak bones.
To diagnose osteoporosis, your doctor will do a bone density scan called a dual energy X-ray absorptiometry (DEXA or DXA) scan. This is a common test that measures your bone density. The DEXA bone scan often checks your hips, spine, and wrist. These are the most common places to have osteoporosis.
The American Academy of Family Physicians does not recommend that doctors use DEXA scans for women younger than 65 or men younger than 70 unless there are risk factors 214. The American Academy of Family Physicians recommends that women who are 65 years and older or have an equal or greater fracture risk be screened for osteoporosis 214.
What causes osteoporosis?
There are two ways osteoporosis can occur. You can lose too much bone or your body cannot produce enough bone. Some people have both issues. When you’re young, your bones are dense and strong. Osteoporosis makes your bones brittle and breakable.
It is natural to lose some bone mass as you age. For most adults, this begins in their mid 20s. Other factors can increase your risk of osteoporosis. Some of these risk factors are out of your control. For others, you can take steps to reduce your risk. Talk to your doctor about your risk factors.
- Uncontrollable risk factors:
- Sex: Osteoporosis is more common in women than men.
- Age: The older you are, the greater your chance of having osteoporosis.
- Race: Caucasians and Asians are more likely to have osteoporosis.
- Genetics: Your risk of osteoporosis is higher if it runs in your family.
- Menopause: This period in a woman’s life causes physical and hormonal effects. For example, it lowers your estrogen. These changes can increase your risk of osteoporosis. Your risk is even higher if you have early menopause (before age 45).
- Body frame: People who have small, thin frames are more likely to develop osteoporosis.
- Health: Certain conditions, such as cancer or stroke, can lead to osteoporosis.
- Controllable risk factors:
- Alcohol abuse
- Cancer (eg, multiple myeloma)
- Chronic obstructive pulmonary disease (COPD) (due to the disorder itself, as well as tobacco use and/or treatment with glucocorticoids). Chronic obstructive pulmonary disease is airflow limitation caused by an inflammatory response to inhaled toxins, often cigarette smoke. Alpha-1 antitrypsin deficiency and various occupational exposures are less common causes in nonsmokers.
- Chronic kidney disease
- Drugs (eg, glucocorticoids, anticonvulsants, medroxyprogesterone, aromatase inhibitors, rosiglitazone, pioglitazone, thyroid replacement therapy, heparin, ethanol, tobacco)
- Endocrine disease or hormonal imbalances (eg, glucocorticoid excess, hyperparathyroidism, hyperthyroidism, hypogonadism, hyperprolactinemia, diabetes mellitus)
- Eating disorders, such as anorexia nervosa
- Hypercalciuria
- Hypervitaminosis A
- Hypophosphatasia
- Hypovitaminosis D
- Inactive lifestyle or lack of exercise
- Lack of calcium and/or vitamins
- Liver disease
- Long-term use of certain medicines. Examples include corticosteroids and proton pump inhibitors (PPIs). Corticosteroids treat inflammation, pain, and chronic conditions, such as asthma and rheumatoid arthritis. Proton pump inhibitors (PPIs) help reduce stomach acid. These medicines can make it hard for your body to absorb calcium and cause osteoporosis.
- Malabsorption syndromes
- Prolonged weightlessness (as occurs in space flight)
- Rheumatoid arthritis
- Smoking or tobacco use.
How do I know if I have osteoporosis?
Osteoporosis does not become clinically apparent until a fracture occurs and so is sometimes referred to as the “silent disease.” Since osteoporosis does not have any symptoms until a bone breaks, it is important to talk to your doctor about your bone health. If your doctor feels that you are at risk for osteoporosis, he or she may order a bone density test. A bone density test measures how strong – or dense – your bones are and whether you have osteoporosis. It can also tell you what your chances are of breaking a bone. Bone density tests are quick, safe, and painless.
Bones affected by osteoporosis may become so fragile that fractures occur spontaneously or as the result of:
- Minor falls, such as a fall from standing height that would not normally cause a break in a healthy bone.
- Normal stresses such as bending, lifting, or even coughing.
Two-thirds of vertebral fractures are painless, although patients may complain of the resulting stooped or hunched posture (kyphosis) and height loss. Typical findings in patients with painful vertebral fractures may include the following:
- The episode of acute pain may follow a fall or minor trauma.
- Pain is localized to a specific, identifiable, vertebral level in the midthoracic to lower thoracic or upper lumbar spine.
- The pain is described variably as sharp, nagging, or dull; movement may exacerbate pain; in some cases, pain radiates to the abdomen.
- Pain is often accompanied by paravertebral muscle spasms exacerbated by activity and decreased by lying supine.
- Patients often remain motionless in bed because of fear of exacerbating the pain.
- Acute pain usually resolves after 4-6 weeks; in the setting of multiple fractures with severe kyphosis, the pain may become chronic.
Patients who have sustained a hip fracture may experience the following:
- Pain in the groin, posterior buttock, anterior thigh, medial thigh, and/or medial knee during weight-bearing or attempted weight-bearing of the involved extremity
- Diminished hip range of motion (ROM), particularly internal rotation and flexion
- External rotation of the involved hip while in the resting position
Figure 2. Common bone fracture areas in osteoporosis
[Source 215 ]Figure 3. Spine x-rays showing severe compression fractures due to osteoporosis
Footnotes: Spine x-ray imaging as obtained from on DEXA scanner. Normal spine (a); young woman with vertebral deformities (arrows) after post partum osteoporosis (b); severe osteoporosis in 68 year old woman with multiple moderate and severe compression fractures (c)
[Source 216 ]How is osteoporosis diagnosed?
See your doctor if you have signs of osteoporosis or if it runs in your family. To diagnose osteoporosis, your doctor will do a bone density scan called a dual energy X-ray absorptiometry (DEXA or DXA) scan. This is a common test that measures your bone density. The DEXA bone scan often checks your hips, spine, and wrist. These are the most common places to have osteoporosis.
The American Academy of Family Physicians does not recommend that doctors use DEXA scans for women younger than 65 or men younger than 70 unless there are risk factors 214. The American Academy of Family Physicians recommends that women who are 65 years and older or have an equal or greater fracture risk be screened for osteoporosis 214.
Bone mineral density (BMD) measurement is recommended in the following patients 2:
- Women age 65 years and older and men age 70 years and older, regardless of clinical risk factors
- Postmenopausal women and men above age 50–69, younger postmenopausal women and women in menopausal transition based on risk factor profile
- Postmenopausal women and men age 50 and older who have had an adult-age fracture, to diagnose and determine the degree of osteoporosis
- Adults with a condition (e.g., rheumatoid arthritis) or taking medication (e.g., glucocorticoids in a daily dose ≥5 mg prednisone or equivalent for ≥3 months) associated with low bone mass or bone loss
Baseline laboratory studies include the following:
- Complete blood count: May reveal anemia
- Serum chemistry levels: Usually normal in persons with primary osteoporosis
- Liver function tests
- Thyroid-stimulating hormone (TSH) level: Thyroid dysfunction has been associated with osteoporosis
- 25-Hydroxyvitamin D [25(OH)D] level: Vitamin D insufficiency can predispose to osteoporosis
- Serum protein electrophoresis: Multiple myeloma may be associated with osteoporosis
- 24-hour urine calcium/creatinine: Hypercalciuria may be associated with osteoporosis; further investigation with measurement of intact parathyroid hormone (PTH) and urine pH may be indicated; hypocalciuria may indicate malabsorption, which should be further evaluated with a serum vitamin D measurement and consideration of testing for malabsorption syndromes such as celiac sprue
- Testosterone (total and/or free) and luteinizing hormone (LH)/follicle-stimulating hormone (FSH): Male hypogonadism is associated with osteoporosis
Bone density scan
Bone density scan also called bone densitometry, dual-energy x-ray absorptiometry, DEXA or DXA, uses a very small dose of ionizing radiation to produce pictures of the inside of your body (usually the lower lumbar spine and hips) to measure bone mineral density (BMD) or bone loss.
DEXA scan is most often used to diagnose osteoporosis, to assess an individual’s risk for developing osteoporotic fractures. DEXA is also effective in tracking the effects of treatment for osteoporosis and other conditions that cause bone loss.
The DEXA bone scan can also assess an individual’s risk for developing fractures. The risk of fracture is affected by age, body weight, history of prior fracture, family history of osteoporotic fractures and life style issues such as cigarette smoking and excessive alcohol consumption. These factors are taken into consideration when deciding if a patient needs therapy.
DEXA scan is simple, quick and noninvasive. It’s also the most commonly used and the most standard method for diagnosing osteoporosis. The DEXA bone density test is usually completed within 10 to 30 minutes, depending on the equipment used and the parts of the body being examined.
Bone density testing is strongly recommended if you 217:
- are a post-menopausal woman and not taking estrogen.
- have a personal or maternal history of hip fracture or smoking.
- are a post-menopausal woman who is tall (over 5 feet 7 inches) or thin (less than 125 pounds).
- are a man with clinical conditions associated with bone loss, such as rheumatoid arthritis, chronic kidney disease or liver disease.
- use medications that are known to cause bone loss, including corticosteroids such as Prednisone, various anti-seizure medications such as Dilantin and certain barbiturates, or high-dose thyroid replacement drugs.
- have type 1 diabetes, liver disease, kidney disease or a family history of osteoporosis.
- have high bone turnover, which shows up in the form of excessive collagen in urine samples.
- have a thyroid condition, such as hyperthyroidism (overactive thyroid).
- have a parathyroid condition, such as hyperparathyroidism.
- have experienced a fracture after only mild trauma.
- have had x-ray evidence of vertebral fracture or other signs of osteoporosis.
An additional procedure called Vertebral Fracture Assessment (VFA) is now being done at many centers. The Vertebral Fracture Assessment (VFA) is a low-dose x-ray examination of the spine to screen for vertebral fractures that is performed on the DEXA machine, may be recommended for older patients, especially if:
- they have lost more than an inch of height.
- have unexplained back pain.
- if a DEXA scan gives borderline readings.
- the DEXA images of the spine suggest a vertebral deformity or fracture.
The VFA test adds only a few minutes to the DEXA procedure.
The dual-energy x-ray absorptiometry (DEXA) scan requires little to no special preparation. You will probably be asked to fill out a questionnaire that will help the doctor determine if you have medical conditions or take certain medications that either increase or decrease your risk of a fracture.
Tell your doctor and the technologist if there is a possibility you are pregnant or if you recently had a barium exam or received an injection of contrast material for a CT or radioisotope scan. Leave jewelry at home and wear loose, comfortable clothing. You may be asked to wear a gown. You should not take calcium supplements for at least 24 hours before your exam.
DEXA scan results
A radiologist, a doctor trained to supervise and interpret radiology examinations, will analyze your dual-energy x-ray absorptiometry (DEXA) scan images. The radiologist will send a signed report to your primary care or referring physician who will discuss the results with you.
Dual-energy x-ray absorptiometry (DEXA) scans are also interpreted by other physicians such as rheumatologists and endocrinologists.
Your DEXA test results will be in the form of two scores:
- T score: This number shows the amount of bone you have compared with a young adult of the same gender with peak bone mass. A T-score of -1 (minus 1) and above is considered normal. A T-score between -1.1 and -2.4 is classified as osteopenia (low bone mass). A T-score of -2.5 and below is defined as osteoporosis. The T-score is used to estimate your risk of developing a fracture and also to determine if treatment is required.
- Z score: This number reflects the amount of bone you have compared with other people in your age group and of the same size and gender. If your Z-score is unusually high or low, it may indicate a need for further medical tests.
Small changes may normally be observed between scans due to differences in positioning and usually are not significant.
What are the limitations of a bone density scan?
- A DEXA test cannot predict who will experience a fracture but can provide a relative risk and it is used to determine whether treatment is required.
- Despite its effectiveness as a method of measuring bone density, DEXA is of limited use in people with a spinal deformity or those who have had previous spinal surgery. The presence of vertebral compression fractures or osteoarthritis may interfere with the accuracy of the test; in such instances, CT scans may be more useful.
- Central DEXA devices are more sensitive and better standardized than peripheral DEXA devices but they are also somewhat more expensive.
- In the central DEXA examination, which measures bone density of the hip and spine, the patient lies on a padded table. An x-ray generator is located below the patient and an imaging device, or detector, is positioned above. To assess the spine, the patient’s legs are supported on a padded box to flatten the pelvis and lower (lumbar) spine. To assess the hip, the patient’s foot is placed in a brace that rotates the hip inward. In both cases, the detector is slowly passed over the area, generating images on a computer monitor. You must hold very still and may need to hold your breath for a few seconds while the technologist takes the x-ray. This helps reduce the possibility of a blurred image. The technologist will walk behind a wall or into the next room to activate the x-ray machine.
- In the peripheral DEXA scan, the finger, hand, forearm or foot is placed in a small device that obtains a bone density reading within a few minutes.
- A test done on a peripheral location, such as the heel or wrist, may help predict the risk of fracture in the spine or hip. These tests are not as helpful in following response to treatment, however, and if they indicate that drug therapy is needed, a baseline central DEXA scan should be obtained.
- Follow-up DEXA exams should be performed at the same institution and ideally with the same machine. Bone density measurements obtained with different DXA equipment cannot be directly compared.
Osteoporosis treatment
Treatment for osteoporosis starts with changes to your diet and lifestyle. You need to get enough calcium and vitamin D. Your doctor will want you to increase your physical activity. This helps to strengthen your bones and increase your bone mass. Examples of weight-bearing exercises include walking, jogging, and climbing steps. You also should stop smoking and limit alcohol.
If you’re at risk for falls, reduce your risk by getting rid of tripping hazards in your home. For example, remove rugs, avoid slick surfaces, and move electrical cords. You can install grab bars in certain places, such as your bathroom and shower. The bars can help you move around more easily and safely.
Maintaining a healthy weight can also help you manage osteoporosis. Stopping smoking and reducing your alcoholic consumption to just 2 standard drinks a day can improve your bone strength.
Treatment recommendations are often based on an estimate of your risk of breaking a bone in the next 10 years using information such as the bone density test. If your risk isn’t high, treatment might not include medication and might just focus instead on modifying risk factors for bone loss and falls.
Your doctor may prescribe medicine(s) to help treat osteoporosis. There are several types and forms.
- Biophosphonates. For both men and women at increased risk of fracture, the most widely prescribed osteoporosis medications are bisphosphonates. Biophosphonates help reduce your risk of breaks and fractures. It also increases bone density. It comes in oral (pill) form or intravenous (IV or injection) form. Side effects can include nausea, abdominal pain and heartburn-like symptoms. You may have irritation of the esophagus (the tube that connects your mouth and stomach). These are less likely to occur if the medicine is taken properly. Some people cannot take biophosphonates. This includes people who have kidney disease or low levels of calcium in their blood, and women who are pregnant or nursing. Examples of biophosphonates include:
- Alendronate (Binosto, Fosamax): This medicine is used to help prevent and treat osteoporosis. They help reduce your risk of fractures by decreasing the rate of bone loss. They are available in pill form. Their most common side effect is an upset stomach.
- Risedronate (Actonel, Atelvia): This medicine is used to help prevent and treat osteoporosis. They help reduce your risk of fractures by decreasing the rate of bone loss. They are available in pill form. Their most common side effect is an upset stomach.
- Ibandronate (Boniva): This medicine helps to slow bone loss and increase bone density. It is available as a pill or injection. You have 2 options for the pill. You can take it daily or monthly. For the injection, your doctor or nurse will give you a shot every 3 months. Side effects may include lower back or side pain, shortness of breath, tightness in your chest, and bloody or cloudy urine.
- Zoledronic acid (Reclast, Zometa). This medicine is given through IV once a year. Intravenous forms of bisphosphonates don’t cause stomach upset but can cause fever, headache and muscle aches for up to three days. It might be easier to schedule a quarterly or yearly injection than to remember to take a weekly or monthly pill, but it can be more costly to do so.
- Hormone-related therapy:
- Estrogen, especially when started soon after menopause, can help maintain bone density. However, estrogen therapy can increase the risk of blood clots, endometrial cancer, breast cancer and possibly heart disease. Therefore, estrogen is typically used for bone health in younger women or in women whose menopausal symptoms also require treatment.
- Raloxifene (Evista) is not a hormone, but it mimics estrogen’s beneficial effects on bone density in postmenopausal women, without some of the risks associated with estrogen. Taking this drug can reduce the risk of some types of breast cancer. Hot flashes are a common side effect. Raloxifene also may increase your risk of blood clots.
- In men, osteoporosis might be linked with a gradual age-related decline in testosterone levels. Testosterone replacement therapy can help improve symptoms of low testosterone, but osteoporosis medications have been better studied in men to treat osteoporosis and thus are recommended alone or in addition to testosterone.
- Bone-building medications: If you can’t tolerate the more common treatments for osteoporosis — or if they don’t work well enough — your doctor might suggest trying:
- Teriparatide (Forteo) is a synthetic form of parathyroid hormone (PTH) and it stimulates new bone growth. This medicine helps prevent and treat osteoporosis in women. It’s given by daily injection under the skin. You inject it in your thigh or stomach once a day. After two years of treatment with teriparatide, another osteoporosis drug is taken to maintain the new bone growth. Common side effects are nausea, stomach pain, headache, muscle weakness, fatigue, and loss of appetite.
- Abaloparatide (Tymlos) is another drug similar to parathyroid hormone. You can take it for only two years, which will be followed by another osteoporosis medication.
- Romosozumab (Evenity). This is the newest bone-building medication to treat osteoporosis. It is given as an injection every month at your doctor’s office. It is limited to one year of treatment, followed by other osteoporosis medications.
- Calcitonin. This is a hormone that helps slow down bone loss. It is available as an injection or nasal spray. Side effects of the injection include diarrhea, stomach pain, nausea, and vomiting. Side effects of the nose spray include headache and irritation of your nose lining.
- Monoclonal antibody medications: This drug is used when other drugs don’t work or if you can’t tolerate other treatment options. Denosumab (Prolia, Xgeva) increases your bone density. Compared with bisphosphonates, Denosumab produces similar or better bone density results and reduces the chance of all types of fractures. Denosumab is delivered via a shot under the skin every six months. It can be used by both women and men. Side effects can include lower calcium levels, skin rash, or pain in the arms and legs. If you take denosumab, you might have to continue to do so indefinitely. Recent research indicates there could be a high risk of spinal column fractures after stopping the drug.
- A very rare complication of bisphosphonates and denosumab is a break or crack in the middle of the thighbone.
- A second rare complication is delayed healing of the jawbone (osteonecrosis of the jaw). This can occur after an invasive dental procedure such as removing a tooth.
You should have a dental examination before starting these medications, and you should continue to take good care of your teeth and see your dentist regularly while on them. Make sure your dentist knows that you’re taking these medications.
Exercise
Exercise is an important part of an osteoporosis treatment program. Exercise can strengthen your bones and muscles and decrease your risk of falling. Research shows that the best physical activities for bone health include strength training or resistance training, like lifting weights and weight bearing exercises (exercise done while on your feet so you bear your own weight) like brisk walking, jogging, tennis or volleyball. Because bone is living tissue, during childhood and adulthood, exercise can make bones stronger. However, for older adults, exercise no longer increases bone mass. Instead, regular exercise can help older adults:
- Build muscle mass and strength and improve coordination and balance. This can help lower your chance of falling.
- Improve daily function and delay loss of independence.
An exercise program for osteoporosis should include four components:
- Weight-bearing exercises force your body to work against gravity, which helps to strengthen bones. Examples include walking, climbing stairs, playing tennis, and dancing. Higher-impact activities strengthen bone more than lower-impact exercises, but only do what your fitness level allows.
- Muscle-strengthening exercises use weights or your body’s own resistance to work against gravity. Examples include lifting free weights, using a weight machine, working with resistance bands, and lifting your own body weight. Do these types of exercises at least twice a week.
- Balance exercises improve your ability to hold yourself upright and help prevent falls. Examples include tai chi and yoga. Perform balance exercises at least twice a week.
- Flexibility exercises keep your muscles limber and joints mobile. They include yoga and stretching. Try to stretch for at least five to 10 minutes after every workout. Hold each stretch for 10 to 30 seconds.
Exercises that help improve your balance are useful to help avoid falling over in the future. Examples of some exercises that can help you improve your balance are:
- Tai Chi
- standing with your feet close together
- standing on one leg
- walking backwards
Reducing your likelihood of falling is also important. Consider how you might arrange your home and workplace so you are less likely to trip — for example by fixing down rugs and keeping the floor clear. Wearing sensible shoes and glasses if you need them can also help to keep you stable as your move around.
Although exercise is beneficial for people with osteoporosis, it should not put any sudden or excessive strain on your bones. If you have osteoporosis, you should avoid high-impact exercise. Your doctor or physiotherapist can help you build a safe exercise plan which suits your needs and reduces your risk of fracturing your bones. To help prevent injury and fractures, a physical therapist or rehabilitation medicine specialist can:
- Recommend specific exercises to strengthen and support your back.
- Teach you safe ways of moving and carrying out daily activities.
- Recommend an exercise program that is tailored to your circumstances.
Exercise specialists, such as exercise physiologists, may also help you develop a safe and effective exercise program.
References- Khosla S, Riggs BL. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am. 2005 Dec;34(4):1015-30, xi. doi: 10.1016/j.ecl.2005.07.009
- Cosman, F., de Beur, S. J., LeBoff, M. S., Lewiecki, E. M., Tanner, B., Randall, S., Lindsay, R., & National Osteoporosis Foundation (2014). Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 25(10), 2359–2381. https://doi.org/10.1007/s00198-014-2794-2
- Barton DW, Behrend CJ, Carmouche JJ. Rates of osteoporosis screening and treatment following vertebral fracture. Spine J. 2019 Mar;19(3):411-417. doi: 10.1016/j.spinee.2018.08.004
- Varacallo MA, Fox EJ. Osteoporosis and its complications. Med Clin North Am. 2014 Jul;98(4):817-31, xii-xiii. doi: 10.1016/j.mcna.2014.03.007
- Porter JL, Varacallo M. Osteoporosis. [Updated 2020 Nov 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441901
- Walzak LC, Loken Thornton W. The role of illness burden in theory of mind performance among older adults. Exp Aging Res. 2018 Oct-Dec;44(5):427-442. doi: 10.1080/0361073X.2018.1521494
- Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010.
- Harvard University, Harvard School of Public Health. Calcium: What’s Best for Your Bones and Health ? https://www.hsph.harvard.edu/nutritionsource/calcium-full-story/
- Optimal calcium intake. NIH Consens Statement. 1994 Jun 6-8;12(4):1-31.
- Heaney RP, Recker RR, Stegman MR, Moy AJ. Calcium absorption in women: relationships to calcium intake, estrogen status, and age. J Bone Miner Res. 1989 Aug;4(4):469-75. doi: 10.1002/jbmr.5650040404
- Weaver CM, Heaney RP. Isotopic exchange of ingested calcium between labeled sources. Evidence that ingested calcium does not form a common absorptive pool. Calcif Tissue Int. 1991 Oct;49(4):244-7. doi: 10.1007/BF02556212
- Weaver CM, Heaney RP, Martin BR, Fitzsimmons ML. Human calcium absorption from whole-wheat products. J Nutr. 1991 Nov;121(11):1769-75. doi: 10.1093/jn/121.11.1769
- Heaney RP. Bone mass, nutrition, and other lifestyle factors. Nutr Rev. 1996 Apr;54(4 Pt 2):S3-10. doi: 10.1111/j.1753-4887.1996.tb03891.x
- Kerstetter JE, O’Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab. 2005 Jan;90(1):26-31. doi: 10.1210/jc.2004-0179
- Barrett-Connor E, Chang JC, Edelstein SL. Coffee-associated osteoporosis offset by daily milk consumption. The Rancho Bernardo Study. JAMA. 1994 Jan 26;271(4):280-3. doi: 10.1001/jama.1994.03510280042030
- Massey LK, Whiting SJ. Caffeine, urinary calcium, calcium metabolism and bone. J Nutr. 1993 Sep;123(9):1611-4. doi: 10.1093/jn/123.9.1611
- Hirsch PE, Peng TC. Effects of alcohol on calcium homeostasis and bone. In: Anderson J, Garner S, eds. Calcium and Phosphorus in Health and Disease. Boca Raton, FL: CRC Press, 1996:289-300.
- U.S. Department of Agriculture. Results from the United States Department of Agriculture’s 1994-96 Continuing Survey of Food Intakes by Individuals/Diet and Health Knowledge Survey, 1994-96.
- Heaney RP, Rafferty K. Carbonated beverages and urinary calcium excretion. Am J Clin Nutr. 2001 Sep;74(3):343-7. doi: 10.1093/ajcn/74.3.343
- Fenton TR, Eliasziw M, Lyon AW, Tough SC, Hanley DA. Meta-analysis of the quantity of calcium excretion associated with the net acid excretion of the modern diet under the acid-ash diet hypothesis. Am J Clin Nutr. 2008 Oct;88(4):1159-66. doi: 10.1093/ajcn/88.4.1159
- National Center for Biotechnology Information, U.S. National Library of Medicine – Bone Health and Osteoporosis: A Report of the Surgeon General.- https://www.ncbi.nlm.nih.gov/books/NBK45523/
- National Center for Biotechnology Information, U.S. National Library of Medicine. – Prevention and Treatment for Those Who Have Bone Diseases – https://www.ncbi.nlm.nih.gov/books/NBK45501/
- Office on Women’s Health, the U.S. Department of Health and Human Services. Osteoporosis. https://www.womenshealth.gov/a-z-topics/osteoporosis
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011.
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, et al. Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006 Feb 16;354(7):669-83. doi: 10.1056/NEJMoa055218. Erratum in: N Engl J Med. 2006 Mar 9;354(10):1102.
- Wallace RB, Wactawski-Wende J, O’Sullivan MJ, Larson JC, Cochrane B, Gass M, Masaki K. Urinary tract stone occurrence in the Women’s Health Initiative (WHI) randomized clinical trial of calcium and vitamin D supplements. Am J Clin Nutr. 2011 Jul;94(1):270-7. doi: 10.3945/ajcn.110.003350
- Candelas G, Martinez-Lopez JA, Rosario MP, Carmona L, Loza E. Calcium supplementation and kidney stone risk in osteoporosis: a systematic literature review. Clin Exp Rheumatol. 2012 Nov-Dec;30(6):954-61. https://www.clinexprheumatol.org/abstract.asp?a=5491
- Kahwati LC, Weber RP, Pan H, Gourlay M, LeBlanc E, Coker-Schwimmer M, Viswanathan M. Vitamin D, Calcium, or Combined Supplementation for the Primary Prevention of Fractures in Community-Dwelling Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018 Apr 17;319(15):1600-1612. doi: 10.1001/jama.2017.21640
- Calcium. https://ods.od.nih.gov/factsheets/Calcium-Consumer
- U.S. Department of Agriculture, Center for Nutrition Policy and Promotion. ChooseMyPlate.gov 2011. https://www.choosemyplate.gov/
- Calcium. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional
- The National Institute of Health, Office of Dietary Supplements. Calcium. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional
- Straub DA. Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr Clin Pract. 2007;22:286-96. https://www.ncbi.nlm.nih.gov/pubmed/17507729
- Andon MB, Peacock M, Kanerva RL, De Castro JAS. Calcium absorption from apple and orange juice fortified with calcium citrate malate (CCM). J Am Coll Nutr 1996;15:313-6. https://www.ncbi.nlm.nih.gov/pubmed/8935449
- Protective effects of dietary lactulose and calcium phosphate against Salmonella infection. Bovee-Oudenhoven I, Van der Meer R. Scand J Gastroenterol Suppl. 1997; 222:112-4. https://www.ncbi.nlm.nih.gov/pubmed/9145462/
- Ditscheid B, Keller S, Jahreis G. Cholesterol metabolism is affected by calcium phosphate supplementation in humans. J Nutr. 2005;135:1678–1682. https://www.ncbi.nlm.nih.gov/pubmed/15987849
- Govers MJ, Van der Meer R. Effects of dietary calcium and phosphate on the intestinal interactions between calcium, phosphate, fatty acids, and bile acids. Gut. 1993;34:365–370. doi: 10.1136/gut.34.3.365. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1374143/
- Trautvetter U, Ditscheid B, Kiehntopf M, Jahreis G. A combination of calcium phosphate and probiotics beneficially influences intestinal lactobacilli and cholesterol metabolism in humans. Clin Nutr. 2012;31:230–237. doi: 10.1016/j.clnu.2011.09.013. https://www.ncbi.nlm.nih.gov/pubmed/22019281
- Trautvetter U, Kiehntopf M, Jahreis G. Postprandial effects of calcium phosphate supplementation on plasma concentration-double-blind, placebo-controlled cross-over human study. Nutr J. 2013;12:30. doi: 10.1186/1475-2891-12-30. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3599792/
- Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. https://www.ncbi.nlm.nih.gov/pubmed/1331788
- Nutr J. 2014; 13: 6. Published online 2014 Jan 17. doi: 10.1186/1475-2891-13-6. Effect of calcium phosphate and vitamin D3 supplementation on bone remodelling and metabolism of calcium, phosphorus, magnesium and iron. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3898568/
- National Cancer Institute. Calcium and Cancer Prevention. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/calcium-fact-sheet
- Michaëlsson K, Melhus H, Warensjö Lemming E, Wolk A, Byberg L. Long term calcium intake and rates of all cause and cardiovascular mortality: community based prospective longitudinal cohort study. BMJ. 2013 Feb 12;346:f228. doi: 10.1136/bmj.f228
- Lowe SA, Bowyer L, Lust K, McMahon LP, Morton M, North RA, Paech M, Said JM. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol. 2015 Oct;55(5):e1-29. doi: 10.1111/ajo.12399
- Caruso JB, Patel RM, Julka K, Parish DC. Health-behavior induced disease: Return of the milk-alkali syndrome. Journal of General Internal Medicine 2007; 22(7):1053–1055. https://www.ncbi.nlm.nih.gov/pubmed/17483976
- Cooke AM. Alkalosis occurring in the alkaline treatment of peptic ulcers. Quart J Med. 1932;25:527
- Life-threatening milk-alkali syndrome resulting from antacid ingestion during pregnancy. Gordon MV, McMahon LP, Hamblin PS. Med J Aust. 2005 Apr 4; 182(7):350-1. https://www.ncbi.nlm.nih.gov/pubmed/15804228/
- Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. New England Journal of Medicine 2006; 354(7):684–696. https://www.ncbi.nlm.nih.gov/pubmed/16481636
- Calcium. In: Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. National Academy Press, Washington, DC. https://www.nap.edu/read/5776/chapter/6
- Gordon MV, Hamblin PS, McMahon LP. Life-threatening milk-alkali syndrome resulting from antacid ingestion during pregnancy. Medical Journal of Australia 2005; 182(7):350–351. https://www.ncbi.nlm.nih.gov/pubmed/15804228
- Beall DP, Henslee HB, Webb HR, Scofield RH. Milk-alkali syndrome: A historical review and description of the modern version of the syndrome. American Journal of the Medical Sciences 2006; 331(5):233–242. https://www.ncbi.nlm.nih.gov/pubmed/16702792
- Xiao Q, Murphy RA, Houston DK, Harris TB, Chow WH, Park Y. Dietary and supplemental calcium intake and cardiovascular disease mortality: the National Institutes of Health-AARP diet and health study. JAMA Intern Med. 2013 Apr 22;173(8):639-46. doi: 10.1001/jamainternmed.2013.3283
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011 Apr 19;342:d2040. doi: 10.1136/bmj.d2040
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. Clarification of DRIs for calcium and vitamin D across age groups. J Am Diet Assoc. 2011 Oct;111(10):1467. doi: 10.1016/j.jada.2011.08.022
- Bronner F. Mechanisms of intestinal calcium absorption. Journal of Cellular Biochemistry 2003; 88(2):387–393. https://www.ncbi.nlm.nih.gov/pubmed/12520541
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357(3):266-81. doi: 10.1056/NEJMra070553
- Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: a systematic review. Nutr Rev. 2018 Jan 1;76(1):60-76. doi: 10.1093/nutrit/nux034
- Tripkovic L, Wilson LR, Hart K, Johnsen S, de Lusignan S, Smith CP, Bucca G, Penson S, Chope G, Elliott R, Hyppönen E, Berry JL, Lanham-New SA. Daily supplementation with 15 μg vitamin D2 compared with vitamin D3 to increase wintertime 25-hydroxyvitamin D status in healthy South Asian and white European women: a 12-wk randomized, placebo-controlled food-fortification trial. Am J Clin Nutr. 2017 Aug;106(2):481-490. doi: 10.3945/ajcn.116.138693
- Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington (DC): National Academy Press; 1997.
- Heaney RP, Weaver CM. Calcium and vitamin D. Endocrinol Metab Clin North Am. 2003 Mar;32(1):181–94. vii–viii. Review.
- Cranney A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, Fang M, Yazdi F, Garritty C, Sampson M, Barrowman N, Tsertsvadze A, Mamaladze V. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007 Aug;(158):1-235.
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010
- Jones G. Vitamin D. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease, 11th ed. Philadelphia: Lippincott Williams & Wilkins, 2014.
- Norman AW, Henry HH. Vitamin D. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition, 10th ed. Washington DC: Wiley-Blackwell, 2012.
- National Institute of Health. Vitamin D. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019,10, 1082–1093.
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010.
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of the extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014,144, 22–27.
- Haussler MR, Haussler CA, Jurutka PW, Thompson PD, Hsieh JC, Remus LS, Selznick SH, Whitfield GK. The vitamin D hormone and its nuclear receptor: molecular actions and disease states. J Endocrinol. 1997 Sep;154 Suppl:S57-73.
- Brunette MG, Chan M, Ferriere C, Roberts KD. Site of 1,25(OH)2 vitamin D3 synthesis in the kidney. Nature. 1978 Nov 16;276(5685):287-9. doi: 10.1038/276287a0
- Vitamin D Tests. https://www.testing.com/tests/vitamin-d-tests
- Gani, L. U., & How, C. H. (2015). PILL Series. Vitamin D deficiency. Singapore medical journal, 56(8), 433–437. https://doi.org/10.11622/smedj.2015119
- Taylor CL, Rosen CJ, Dwyer JT. Considerations in Dietetic Counseling for Vitamin D. J Acad Nutr Diet. 2019 Jun;119(6):901-909. doi: 10.1016/j.jand.2018.04.013
- Agency for Healthcare Research and Quality. Screening for vitamin D deficiency: Systematic review for the U.S. Preventive Services Task Force recommendation. Evidence Synthesis Number 118. AHRQ-Pub No. 13-05183-EF-1. June 2014.
- LeFevre ML; U.S. Preventive Services Task Force. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015 Jan 20;162(2):133-40. doi: 10.7326/M14-2450
- Sahay M, Sahay R. Rickets–vitamin D deficiency and dependency. Indian Journal of Endocrinology and Metabolism. 2012;16(2):164-176. doi:10.4103/2230-8210.93732. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3313732/
- Christakos S, Ajibade dV, dhawan P, Fechner AJ, Mady LJ. vitamin D: Metabolism. Endocrinol Metab Clin North Am. 2010;39:243. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879391/
- Portale AA, Halloran BP, Morris RC., Jr Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J Clin Invest. 1989;83:1494. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC303852/
- Iida K, Shinki T, Yamaguchi A, DeLuca HF, Kurokawa K, Suda T. A possible role of vitamin D receptors in regulating vitamin D activation in the kidney. Proc Natl Acad Sci U S A. 1995;92:6112. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC41652/
- Prié D, Friedlander G. Reciprocal control of 1,25-dihydroxyvitamin D and FGF23 formation involving the FGF23/Klotho system. Clin J Am Soc Nephrol. 2010;5:1717. http://cjasn.asnjournals.org/content/5/9/1717.long
- Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–15. http://jasn.asnjournals.org/content/17/5/1305.long
- Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2630868/
- Wharton B, Bishop N. Rickets. Lancet. 2003 Oct 25;362(9393):1389-400. doi: 10.1016/S0140-6736(03)14636-3
- Holick MF. Vitamin D. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease, 10th ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Wharton B, Bishop N. Rickets. Lancet 2003;362:1389-400. https://www.ncbi.nlm.nih.gov/pubmed/14585642
- American Academy of Dermatology. Position statement on vitamin D. November 1, 2008. https://www.aad.org/Forms/Policies/Uploads/PS/PS-Vitamin%20D.pdf
- Uday S, Högler W. Erratum to: Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised Concepts, Public Health, and Prevention Strategies. Curr Osteoporos Rep. 2017;15(5):507. doi:10.1007/s11914-017-0395-7
- Goldring SR, Krane S, Avioli LV. Disorders of calcification: osteomalacia and rickets. In: DeGroot LJ, Besser M, Burger HG, Jameson JL, Loriaux DL, Marshall JC, et al., eds. Endocrinology. 3rd ed. Philadelphia: WB Saunders, 1995:1204-27.
- Chesney RW. Rickets: an old form for a new century. Pediatr Int. 2003 Oct;45(5):509-11. doi: 10.1046/j.1442-200x.2003.01783.x
- Uday S, Högler W. Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised Concepts, Public Health, and Prevention Strategies. Curr Osteoporos Rep. 2017 Aug;15(4):293-302. doi: 10.1007/s11914-017-0383-y. Erratum in: Curr Osteoporos Rep. 2017 Aug 14
- Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O, Ramos-Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW, Bhatia V, Fu J, Goldberg G, Sävendahl L, Khadgawat R, Pludowski P, Maddock J, Hyppönen E, Oduwole A, Frew E, Aguiar M, Tulchinsky T, Butler G, Högler W. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab. 2016 Feb;101(2):394-415. doi: 10.1210/jc.2015-2175
- Norman AW, Henry HH. Vitamin D. In: Bowman BA, Russell RM, eds. Present Knowledge in Nutrition, 9th ed. Washington DC: ILSI Press, 2006.
- Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008;88:582S-6S. https://www.ncbi.nlm.nih.gov/pubmed/18689406?dopt=Abstract
- Cranney C, Horsely T, O’Donnell S, Weiler H, Ooi D, Atkinson S, et al. Effectiveness and safety of vitamin D. Evidence Report/Technology Assessment No. 158 prepared by the University of Ottawa Evidence-based Practice Center under Contract No. 290-02.0021. AHRQ Publication No. 07-E013. Rockville, MD: Agency for Healthcare Research and Quality, 2007. https://www.ncbi.nlm.nih.gov/pubmed/18088161?dopt=Abstract
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. https://www.ncbi.nlm.nih.gov/pubmed/17634462?dopt=Abstract
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911-30. doi: 10.1210/jc.2011-0385. Epub 2011 Jun 6. Erratum in: J Clin Endocrinol Metab. 2011 Dec;96(12):3908.
- Brown LL, Cohen B, Tabor D, Zappalà G, Maruvada P, Coates PM. The vitamin D paradox in Black Americans: a systems-based approach to investigating clinical practice, research, and public health – expert panel meeting report. BMC Proc. 2018 May 9;12(Suppl 6):6. doi: 10.1186/s12919-018-0102-4
- Carter GD. 25-hydroxyvitamin D assays: the quest for accuracy. Clin Chem 2009;55:1300-02.
- Hollis BW. Editorial: the determination of circulating 25-hydroxyvitamin D: no easy task. J. Clin Endocrinol Metab 2004;89:3149-3151.
- Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab 2004;89:3152-57. https://www.ncbi.nlm.nih.gov/pubmed/15240586?dopt=Abstract
- National Institute of Standards and Technology. NIST releases vitamin D standard reference material, 2009. https://www.nist.gov/news-events/news/2009/07/nist-releases-vitamin-d-standard-reference-material
- Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press; 1997. p. 432.
- Zaheer S, LeBoff MS. Osteoporosis: Prevention and Treatment. [Updated 2018 Nov 26]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279073
- Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared to 2000–2004. Am J Clin Nutr. 2008;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2745830/
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. https://www.ncbi.nlm.nih.gov/pubmed/10966885
- Pettifor JM. Nutritional and drug-induced rickets and osteomalacia. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 5. Washington, DC: American Society for Bone and Mineral Research; 2003. pp. 399–407.
- Haden ST, Fuleihan GE, Angell JE, Cotran NM, LeBoff MS. Calcidiol and PTH levels in women attending an osteoporosis program. Calcif Tissue Int. 1999 Apr;64(4):275-9. doi: 10.1007/s002239900618
- U.S. Department of Agriculture, Agricultural Research Service. 2011. USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory Home Page. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/
- Ovesen L, Brot C, Jakobsen J. Food contents and biological activity of 25-hydroxyvitamin D: a vitamin D metabolite to be reckoned with? Ann Nutr Metab. 2003;47(3-4):107-13. doi: 10.1159/000070031
- Roseland JM, Phillips KM, Patterson KY, Pehrsson PR, Taylor CL. Vitamin D in foods: An evolution of knowledge. Pages 41-78 in Feldman D, Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M, eds. Vitamin D, Volume 2: Health, Disease and Therapeutics, Fourth Edition. Elsevier, 2018.
- U.S. Food and Drug Administration. Food additives permitted for direct addition to food for human consumption; vitamin D2 mushroom powder. Federal Register 2020;85:41916-20.
- Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. 2015;55(9):1193-205. doi: 10.1080/10408398.2012.688897
- Taylor, C. L., Patterson, K. Y., Roseland, J. M., Wise, S. A., Merkel, J. M., Pehrsson, P. R., & Yetley, E. A. (2014). Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. The Journal of nutrition, 144(5), 654–659. https://doi.org/10.3945/jn.113.189811
- Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1710S-6S. doi: 10.1093/ajcn/80.6.1710S
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008 Aug;88(2):558S-564S. doi: 10.1093/ajcn/88.2.558S
- Vitamin D for Milk and Milk Alternatives. https://www.fda.gov/food/food-additives-petitions/vitamin-d-milk-and-milk-alternatives
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. https://fdc.nal.usda.gov/index.html
- Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122:1142-1152. https://www.ncbi.nlm.nih.gov/pubmed/18977996?dopt=Abstract
- Wharton B, Bishop N. Rickets. Lancet 2003;362:1389-400. https://www.ncbi.nlm.nih.gov/pubmed/14585642?dopt=Abstract
- Holick MF. Photobiology of vitamin D. In: Feldman D, Pike JW, Glorieux FH, eds. Vitamin D, Second Edition, Volume I. Burlington, MA: Elsevier, 2005.
- Wolpowitz D, Gilchrest BA. The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol 2006;54:301-17. https://www.ncbi.nlm.nih.gov/pubmed/16443061?dopt=Abstract
- Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes 2002;9:87-98.
- International Agency for Research on Cancer Working Group on ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer 2006;120:1116-22. https://www.ncbi.nlm.nih.gov/pubmed/17131335?dopt=Abstract
- Whiting SJ, Healey A, Psiuk S, Mirwald R, Kowalski K, Bailey DA. Relationship between carbonated and other low nutrient dense beverages and bone mineral content of adolescents. Nutr Res. 2001 Aug;21 (8):1107–15.
- Fitzpatrick L, Heaney RP. Got soda. J Bone Miner Res. 2003 Sep;18(9):1570–2. https://www.ncbi.nlm.nih.gov/pubmed/12968665
- Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press; 1997. p. 432.
- Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2000. p. 800.
- IOM 1997 Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press; 1997. p. 432.
- Suttie JW. Vitamin K. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:851-60.
- Booth S. L. (2012). Vitamin K: food composition and dietary intakes. Food & nutrition research, 56, 10.3402/fnr.v56i0.5505. https://doi.org/10.3402/fnr.v56i0.5505
- Hirota, Yoshihisa & Suhara, Yoshitomo. (2019). New Aspects of Vitamin K Research with Synthetic Ligands: Transcriptional Activity via SXR and Neural Differentiation Activity. International Journal of Molecular Sciences. 2019, 20(12), 3006; https://doi.org/10.3390/ijms20123006
- Fusaro M, Cianciolo G, Brandi ML, Ferrari S, Nickolas TL, Tripepi G, Plebani M, Zaninotto M, Iervasi G, La Manna G, Gallieni M, Vettor R, Aghi A, Gasperoni L, Giannini S, Sella S, M Cheung A. Vitamin K and Osteoporosis. Nutrients. 2020 Nov 25;12(12):3625. doi: 10.3390/nu12123625
- Fusaro M., Mereu M.C., Aghi A., Iervasi G., Gallieni M. Vitamin K and bone. Clin. Cases Miner. Bone Metab. 2017;14:200–206. doi: 10.11138/ccmbm/2017.14.1.200
- Bouckaert J.H., Said A.H. Fracture healing by vitamin K. Nature. 1960;185:849. doi: 10.1038/185849a0
- Booth SL, Tucker KL, Chen H, Hannan MT, Gagnon DR, Cupples LA, Wilson PW, Ordovas J, Schaefer EJ, Dawson-Hughes B, Kiel DP. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr. 2000 May;71(5):1201-8. doi: 10.1093/ajcn/71.5.1201
- Thane CW, Paul AA, Bates CJ, Bolton-Smith C, Prentice A, Shearer MJ. Intake and sources of phylloquinone (vitamin K1): variation with socio-demographic and lifestyle factors in a national sample of British elderly people. Br J Nutr. 2002 Jun;87(6):605-13. doi: 10.1079/BJNBJN2002583
- Ferland G. Vitamin K. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:230-47.
- National Institute of Health, Office of Dietary Supplements. Vitamin K. https://ods.od.nih.gov/factsheets/VitaminK-HealthProfessional/
- Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SL. Vitamin k contents of meat, dairy, and fast food in the u.s. Diet. J Agric Food Chem. 2006 Jan 25;54(2):463-7. doi: 10.1021/jf052400h
- Walther, B., Karl, J. P., Booth, S. L., & Boyaval, P. (2013). Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Advances in nutrition (Bethesda, Md.), 4(4), 463–473. https://doi.org/10.3945/an.113.003855
- Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008 Apr 25;283(17):11270-9. doi: 10.1074/jbc.M702971200
- Wells HF, Buzby JC. Dietary assessment of major trends in U.S. food consumption, 1970–2005. Economic Information Bulletin No (EIB-33) p 27, March 2008.
- Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YT. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. 2009 Sep;19(7):504-10. doi: 10.1016/j.numecd.2008.10.004
- Suttie JW. Vitamin K. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:305-16.
- Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. Davidson RT, Foley AL, Engelke JA, Suttie JW. J Nutr. 1998 Feb; 128(2):220-3. https://www.ncbi.nlm.nih.gov/pubmed/9446847/
- Menadione is a metabolite of oral vitamin K. Thijssen HH, Vervoort LM, Schurgers LJ, Shearer MJ. Br J Nutr. 2006 Feb; 95(2):260-6. https://www.ncbi.nlm.nih.gov/pubmed/16469140/
- Vitamin K contents of meat, dairy, and fast food in the U.S. Diet. Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SL. J Agric Food Chem. 2006 Jan 25; 54(2):463-7. https://www.ncbi.nlm.nih.gov/pubmed/16417305/
- Schurgers LJ. Vitamin K: key vitamin in controlling vascular calcification in chronic kidney disease. Kidney Int. 2013 May;83(5):782-4. doi: 10.1038/ki.2013.26
- Fusaro M., Gallieni M., Rizzo M.A., Stucchi A., Delanaye P., Cavalier E., Moysés R.M.A., Jorgetti V., Iervasi G., Giannini S., et al. Vitamin K plasma levels determination in human health. Clin. Chem. Lab. Med. 2017;55:789–799. doi: 10.1515/cclm-2016-0783
- Torbergsen A.C., Watne L.O., Wyller T.B., Frihagen F., Strømsøe K., Bøhmer T., Mowe M. Vitamin PK and 25(OH)D are independently and synergistically associated with a risk for hip fracture in an elderly population: A case control study. Clin. Nutr. 2015;34:101–106. doi: 10.1016/j.clnu.2014.01.016
- Finnes T.E., Lofthus C.M., Meyer H.E., Søgaard A.J., Tell G.S., Apalset E.M., Gjesdal C., Grimnes G., Schei B., Blomhoff R., et al. A combination of low serum concentrations of vitamins PK and D is associated with increased risk of hip fractures in elderly Norwegians: A NOREPOS study. Osteoporos. Int. 2016;27:1645–1652. doi: 10.1007/s00198-015-3435-0
- Yaegashi Y., Onoda T., Tanno K., Kuribayashi T., Sakata K., Orimo H. Association of hip fracture incidence and intake of calcium, magnesium, vitamin D, and vitamin K. Eur. J. Epidemiol. 2008;23:219–225. doi: 10.1007/s10654-008-9225-7
- Gundberg CM, Lian JB, Booth SL. Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv Nutr 2012;3:149-57. https://www.ncbi.nlm.nih.gov/pubmed/22516722?dopt=Abstract
- Yaegashi Y, Onoda T, Tanno K, Kuribayashi T, Sakata K, Orimo H. Association of hip fracture incidence and intake of calcium, magnesium, vitamin D, and vitamin K. Eur J Epidemiol 2008;23:219-25. https://www.ncbi.nlm.nih.gov/pubmed/18214692?dopt=Abstract
- Rejnmark L, Vestergaard P, Charles P, Hermann AP, Brot C, Eiken P, et al. No effect of vitamin K1 intake on bone mineral density and fracture risk in perimenopausal women. Osteoporos Int 2006;17:1122-32. https://www.ncbi.nlm.nih.gov/pubmed/16683180?dopt=Abstract
- Feskanich D, Weber P, Willett WC, Rockett H, Booth SL, Colditz GA. Vitamin K intake and hip fractures in women: a prospective study. Am J Clin Nutr 1999;69:74-9. https://www.ncbi.nlm.nih.gov/pubmed/9925126?dopt=Abstract
- Booth SL, Broe KE, Gagnon DR, Tucker KL, Hannan MT, McLean RR, et al. Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr 2003;77:512-6. https://www.ncbi.nlm.nih.gov/pubmed/12540415?dopt=Abstract
- Booth SL, Tucker KL, Chen H, Hannan MT, Gagnon DR, Cupples LA, et al. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr 2000;71:1201-8. https://www.ncbi.nlm.nih.gov/pubmed/10799384?dopt=Abstract
- Chan R, Leung J, Woo J. No association between dietary vitamin K intake and fracture risk in chinese community-dwelling older men and women: a prospective study. Calcif Tissue Int 2012;90:396-403. https://www.ncbi.nlm.nih.gov/pubmed/22451220?dopt=Abstract
- Wu W.J., Kim M.S., Ahn B.Y. The inhibitory effect of vitamin K on RANKL-induced osteoclast differentiation and bone resorption. Food Funct. 2015;6:3351–3358. doi: 10.1039/C5FO00544B
- Rangel L.B., de Siqueira D., do Rosário Soares O., Santana H.S., de Castro Miguel E., da Cunha M., de Abreu Oliveira A.L., Pedrosa D.F., Resgala L.C., Neto H.A., et al. Vitamin K supplementation modulates bone metabolism and ultra-structure of ovariectomized mice. Cell. Physiol. Biochem. 2018;51:356–374. doi: 10.1159/000495234
- Nagura N, Komatsu J, Iwase H, Hosoda H, Ohbayashi O, Nagaoka I, Kaneko K. Effects of the combination of vitamin K and teriparatide on the bone metabolism in ovariectomized rats. Biomed Rep. 2015 May;3(3):295-300. doi: 10.3892/br.2015.431
- Kazama JJ, Iwasaki Y, Fukagawa M. Uremic osteoporosis. Kidney Int Suppl (2011). 2013 Dec;3(5):446-450. doi: 10.1038/kisup.2013.93
- Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:1256-61. https://www.ncbi.nlm.nih.gov/pubmed/16801507?dopt=Abstract
- Hao G., Zhang B., Gu M., Chen C., Zhang Q., Zhang G., Cao X. Vitamin K intake and the risk of fractures: A meta-analysis. Med. (Baltim.) 2017;96:e6725. doi: 10.1097/MD.0000000000006725
- Moore A.E., Kim E., Dulnoan D., Dolan A.L., Voong K., Ahmad I., Gorska R., Harrington D.J., Hampson G. Serum vitamin K. Bone. 2020:115630. doi: 10.1016/j.bone.2020.115630
- Tanaka S., Miyazaki T., Uemura Y., Miyakawa N., Gorai I., Nakamura T., Fukunaga M., Ohashi Y., Ohta H., Mori S., et al. Comparison of concurrent treatment with vitamin K. J. Bone Miner. Metab. 2017;35:385–395. doi: 10.1007/s00774-016-0768-5
- Jiang Y., Zhang Z.L., Zhu H.M., Wu Y.Y., Cheng Q., Wu F.L., Xing X.P., Liu J.L., Yu W., Meng X.W. Menatetrenone versus alfacalcidol in the treatment of Chinese postmenopausal women with osteoporosis: A multicenter, randomized, double-blinded, double-dummy, positive drug-controlled clinical trial. Clin. Interv. Aging. 2014;9:121–127. doi: 10.2147/CIA.S54107
- Kasukawa Y., Miyakoshi N., Ebina T., Aizawa T., Hongo M., Nozaka K., Ishikawa Y., Saito H., Chida S., Shimada Y. Effects of risedronate alone or combined with vitamin K2 on serum undercarboxylated Osteocalcin and Osteocalcin levels in postmenopausal osteoporosis. J. Bone Miner. Metab. 2014;32:290–297. doi: 10.1007/s00774-013-0490-5
- Knapen M.H., Drummen N.E., Smit E., Vermeer C., Theuwissen E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos. Int. 2013;24:2499–2507. doi: 10.1007/s00198-013-2325-6
- Shiraki M., Shiraki Y., Aoki C., Miura M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J. Bone Miner. Res. 2000;15:515–521. doi: 10.1359/jbmr.2000.15.3.515
- Mott A., Bradley T., Wright K., Cockayne E.S., Shearer M.J., Adamson J., Lanham-New S.A., Torgerson D.J. Effect of vitamin K on bone mineral density and fractures in adults: An updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019 doi: 10.1007/s00198-019-04949-0
- Inoue T., Fujita T., Kishimoto H., Makino T., Nakamura T., Nakamura T., Sato T., Yamazaki K. Randomized controlled study on the prevention of osteoporotic fractures (OF study): A phase IV clinical study of 15-mg menatetrenone capsules. J. Bone Miner. Metab. 2009;27:66–75. doi: 10.1007/s00774-008-0008-8
- Azuma K, Inoue S. Multiple Modes of Vitamin K Actions in Aging-Related Musculoskeletal Disorders. Int J Mol Sci. 2019 Jun 11;20(11):2844. doi: 10.3390/ijms20112844
- Vermeer C, Schurgers LJ. A comprehensive review of vitamin K and vitamin K antagonists. Hematol Oncol Clin North Am. 2000 Apr;14(2):339-53. doi: 10.1016/s0889-8588(05)70137-4
- Rodríguez-Olleros Rodríguez C, Díaz Curiel M. Vitamin K and Bone Health: A Review on the Effects of Vitamin K Deficiency and Supplementation and the Effect of Non-Vitamin K Antagonist Oral Anticoagulants on Different Bone Parameters. J Osteoporos. 2019 Dec 31;2019:2069176. doi: 10.1155/2019/2069176
- European Food Safety Authority. Scientific opinion on the substantiation of health claims related to vitamin K and maintenance of bone pursuant to Article 13(1) of Regulation (EC) No 1924/2006. The EFSA Journal 2009;7:1228.
- Magnesium. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional
- Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Intestinal absorption of magnesium from food and supplements. J Clin Invest. 1991 Aug;88(2):396-402. doi: 10.1172/JCI115317
- Rude RK. Magnesium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, Mass: Lippincott Williams & Wilkins; 2012:159-75.
- Rude RK, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr. 2009 Apr;28(2):131-41. doi: 10.1080/07315724.2009.10719764
- Tucker KL. Osteoporosis prevention and nutrition. Curr Osteoporos Rep. 2009 Dec;7(4):111-7. doi: 10.1007/s11914-009-0020-5
- Mutlu M, Argun M, Kilic E, Saraymen R, Yazar S. Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J Int Med Res. 2007 Sep-Oct;35(5):692-5. doi: 10.1177/147323000703500514
- Institute of Medicine (IOM). Food and Nutrition Board. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press, 1997.
- Aydin H, Deyneli O, Yavuz D, Gözü H, Mutlu N, Kaygusuz I, Akalin S. Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporotic women. Biol Trace Elem Res. 2010 Feb;133(2):136-43. doi: 10.1007/s12011-009-8416-8
- Volpe SL. Magnesium. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Ames, Iowa; John Wiley & Sons, 2012:459-74.
- Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res. 2010 Dec;23(4):S194-8. doi: 10.1684/mrh.2010.0213
- Gibson, RS. Principles of Nutritional Assessment, 2nd ed. New York, NY: Oxford University Press, 2005.
- Rude RK. Magnesium. In: Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, eds. Encyclopedia of Dietary Supplements. 2nd ed. New York, NY: Informa Healthcare; 2010:527-37.
- Witkowski M, Hubert J, Mazur A. Methods of assessment of magnesium status in humans: a systematic review. Magnes Res. 2011 Dec;24(4):163-80. doi: 10.1684/mrh.2011.0292
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. https://fdc.nal.usda.gov
- Ranade VV, Somberg JC. Bioavailability and pharmacokinetics of magnesium after administration of magnesium salts to humans. Am J Ther. 2001 Sep-Oct;8(5):345-57. doi: 10.1097/00045391-200109000-00008
- Walker AF, Marakis G, Christie S, Byng M. Mg citrate found more bioavailable than other Mg preparations in a randomised, double-blind study. Magnes Res. 2003 Sep;16(3):183-91.
- Spencer H, Norris C, Williams D. Inhibitory effects of zinc on magnesium balance and magnesium absorption in man. J Am Coll Nutr. 1994 Oct;13(5):479-84. doi: 10.1080/07315724.1994.10718438
- Dunn CJ, Goa KL. Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease. Drugs. 2001;61(5):685-712. doi: 10.2165/00003495-200161050-00013
- Arayne MS, Sultana N, Hussain F. Interactions between ciprofloxacin and antacids–dissolution and adsorption studies. Drug Metabol Drug Interact. 2005;21(2):117-29. doi: 10.1515/dmdi.2005.21.2.117
- Sarafidis PA, Georgianos PI, Lasaridis AN. Diuretics in clinical practice. Part II: electrolyte and acid-base disorders complicating diuretic therapy. Expert Opin Drug Saf. 2010 Mar;9(2):259-73. doi: 10.1517/14740330903499257
- FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-low-magnesium-levels-can-be-associated-long-term-use-proton-pump
- Musso CG. Magnesium metabolism in health and disease. Int Urol Nephrol. 2009;41(2):357-62. doi: 10.1007/s11255-009-9548-7
- Kutsal E, Aydemir C, Eldes N, Demirel F, Polat R, Taspnar O, Kulah E. Severe hypermagnesemia as a result of excessive cathartic ingestion in a child without renal failure. Pediatr Emerg Care. 2007 Aug;23(8):570-2. doi: 10.1097/PEC.0b013e31812eef1c
- McGuire JK, Kulkarni MS, Baden HP. Fatal hypermagnesemia in a child treated with megavitamin/megamineral therapy. Pediatrics. 2000 Feb;105(2):E18. doi: 10.1542/peds.105.2.e18
- Onishi S, Yoshino S. Cathartic-induced fatal hypermagnesemia in the elderly. Intern Med. 2006;45(4):207-10. doi: 10.2169/internalmedicine.45.1482
- US Department of Health and Human Services. Physical activity and health: A report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, President’s Council on Physical Fitness and Sports; 1996. p. 300.
- Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, Mcleod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: A clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004 Mar;19(3):343–51. https://www.ncbi.nlm.nih.gov/pubmed/15040821
- Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: A randomized controlled pilot study. J Bone Miner Res. 2004 Mar;19(3):352–9. https://www.ncbi.nlm.nih.gov/pubmed/15040822
- Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004 Mar;19(3):360–9. https://www.ncbi.nlm.nih.gov/pubmed/15040823
- Kraemer et al. 2002, ACSM 1998a, ACSM 1998b
- Cochrane Review 6 July 2011. – Exercise for preventing and treating osteoporosis in postmenopausal women – http://www.cochrane.org/CD000333/MUSKEL_exercise-for-preventing-and-treating-osteoporosis-in-postmenopausal-women
- Kanis JA, McCloskey EV, Johansson H, et al. A reference standard for the description of osteoporosis. Bone 42(4) 67–75. 2008.
- National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation, Washington, DC. 1–36. 2008.
- National Center for Biotechnology Information, U.S. National Library of Medicine. – Osteoporosis – https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0024680/
- Merck Sharp & Dohme Corp., Merck Manual. Osteoporosis. https://www.merckmanuals.com/professional/musculoskeletal-and-connective-tissue-disorders/osteoporosis/osteoporosis
- DEXA for Osteoporosis. https://www.aafp.org/family-physician/patient-care/clinical-recommendations/all-clinical-recommendations/cw-osteoporosis.html
- National Osteoporosis Foundation. Osteoporosis: What is it?. Washington, DC: National Osteoporosis Foundation; [Cited 2004 Mar 1]. Available from: http://www.nof.org/osteoporosis/index.htm.
- Rev Endocr Metab Disord. 2012 Sep; 13(3): 209–223. Published online 2011 Jun 28. doi: 10.1007/s11154-011-9187-z. Treatment of osteopenia. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3411311/
- Bone Density Scan (DEXA or DXA). https://www.radiologyinfo.org/en/info/dexa