Pancreatitis
Pancreatitis is inflammation of the pancreas. Pancreatitis happens when the pancreas digestive enzymes (digestive juices) start digesting the pancreas itself. Pancreatitis can occur as acute pancreatitis — meaning it appears suddenly and lasts for days. Some people develop chronic pancreatitis, which is pancreatitis that occurs over many years. Either form is serious and can lead to complications. Signs and symptoms of pancreatitis may vary, depending on which type you experience.
Chronic pancreatitis does not heal or improve. It gets worse over time and leads to permanent damage. The most common cause is heavy alcohol use. Other causes include cystic fibrosis and other inherited disorders, high levels of calcium or fats in the blood, some medicines, and autoimmune conditions. Chronic pancreatitis symptoms include nausea, vomiting, weight loss, and oily stools. Treatment may also be a few days in the hospital for intravenous (IV) fluids, medicines to relieve pain, and nutritional support. After that, you may need to start taking enzymes and eat a special diet. It is also important to not smoke or drink alcohol.
Most cases of pancreatitis are caused by overuse of alcohol. Besides overuse of alcohol, other causes of pancreatitis include:
- Heredity — Hereditary pancreatitis is a rare genetic disorder that predisposes a person to develop the disease, usually before age 20.
- Genetic causes — Mutations of the cystic fibrosis gene is the most widely recognized genetic cause.
- Blockage of the duct that drains digestive enzymes from the pancreas — If the enzymes don’t drain properly, they can back up and damage the pancreas. Blockage can be caused by gallstones, scarring from prior surgery, tumors, pancreatic cancer or abnormalities of the pancreas or of the shape or location of the pancreatic duct. If the blockage is found early, surgery or a procedure called endoscopic retrograde cholangiopancreatography (ERCP) to relieve the blockage may help to prevent damage to the pancreas. Endoscopic retrograde cholangiopancreatography (ERCP), a procedure used to treat gallstones, also can lead to pancreatitis.
- Autoimmune pancreatitis — For unexplained reasons, some people develop antibodies that attack their own pancreas.
- Very high blood triglyceride levels (hypertriglyceridemia).
Sometimes, a cause for pancreatitis is never found. This is known as idiopathic pancreatitis.
See your doctor right away for the following symptoms of severe pancreatitis:
- pain or tenderness in the abdomen that is severe or becomes worse
- nausea and vomiting
- fever or chills
- fast heartbeat
- shortness of breath
- yellowish color of the skin or whites of the eyes, called jaundice
These symptoms may be a sign of:
- serious infection
- inflammation
- blockage of the pancreas, gallbladder, or a bile and pancreatic duct
Left untreated, these problems can be fatal.
Who is more likely to get pancreatitis?
Certain groups of people are more likely to get acute or chronic pancreatitis than others:
- Men are more likely to get pancreatitis than women 1
- African Americans have a higher risk of getting pancreatitis 2
- People with a family history of pancreatitis have a higher risk.
- People with a personal or family history of gallstones also have a higher risk.
- You are more likely to get pancreatitis if you have one of the following health conditions:
- diabetes
- gallstones
- high triglycerides (hypertriglyceridemia)
- genetic disorders of the pancreas
- certain autoimmune conditions
- cystic fibrosis
- You are also more likely to get pancreatitis if you:
- have obesity
- are a heavy alcohol user
- are a smoker
What are the most common causes of pancreatitis?
The top two causes of pancreatitis are:
These causes together represent about 80% of pancreatitis cases.
Can I die from pancreatitis?
You can die from complications of acute pancreatitis if it’s very severe. In a small percentage of people, severe acute pancreatitis causes a systemic reaction that affects the whole body. This can lead to shock and multiple organ failure, which can be fatal if it isn’t treated quickly. You may not be able to tell how severe your pancreatitis is, so you should go to the emergency room if you have symptoms.
What does the pain of pancreatitis feel like?
Abdominal pain from pancreatitis may be moderate to severe and may radiate to your back. Acute pancreatitis tends to be more severe, with a penetrating quality. Your abdomen may feel tender to the touch. With chronic pancreatitis, the pain may vary in intensity. It may come and go, but it typically doesn’t go away completely. You may notice it more after eating. For some people, the pain is constant.
It may feel worse when you:
- Lie flat.
- Cough.
- Exercise.
- Eat more.
It may feel better when you:
- Sit upright.
- Lean forward.
- Curl in a ball.
- Eat less.
What symptoms indicate that my pancreas isn’t working properly?
When long-term, chronic pancreatitis begins to affect your pancreatic function, you’ll notice it in your digestive system first. When your pancreas can no longer make and deliver its digestive enzymes, your body won’t be able to break down and absorb all the nutrients from your food. You may feel discomfort after eating and may begin passing undigested fats in your poop. Over time, you may notice weight loss.
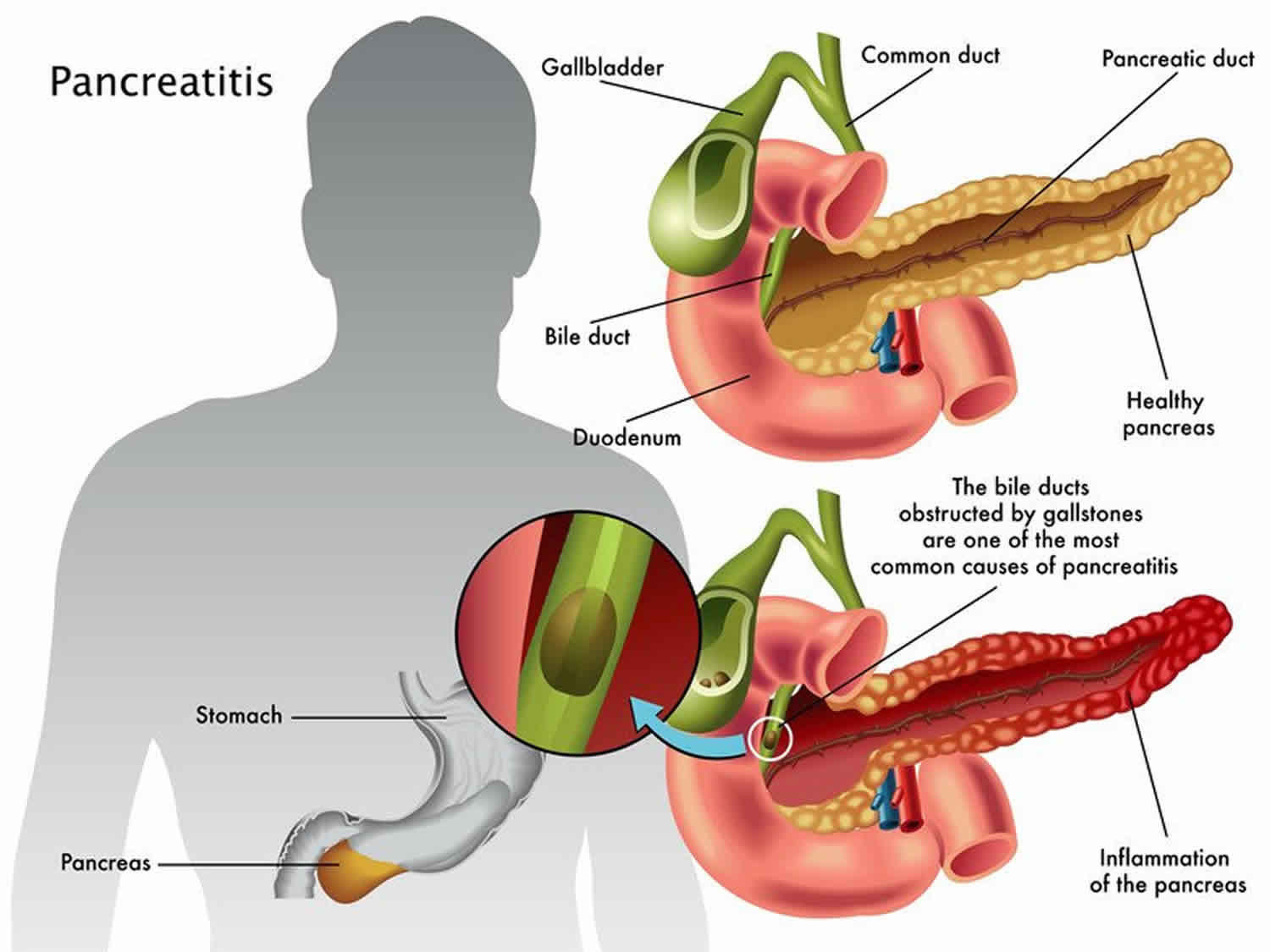
What is gallstone pancreatitis?
Your common bile duct empties bile from your gallbladder into your intestine through the same opening as your pancreatic duct. If a gallstone enters the common bile duct and gets stuck at that junction, it can temporarily block the drainage of pancreatic juice from the pancreatic duct. This traps the enzymes inside your pancreas. As pressure builds up behind the obstruction, it activates the enzymes inside your pancreas and they begin digesting the pancreas itself. This causes the inflammatory response of gallstone pancreatitis.
Do acute and chronic pancreatitis have the same causes?
Most of the time, pancreatitis is acute and temporary. But causes that are chronic and don’t go away, such as inherited disorders, can cause chronic pancreatitis. Repeat episodes of acute pancreatitis can also lead to chronic pancreatitis. If your pancreas becomes inflamed too many times from repeated stress and injury, your body may learn to keep it constantly inflamed, even after the injury has stopped.
Pancreas anatomy
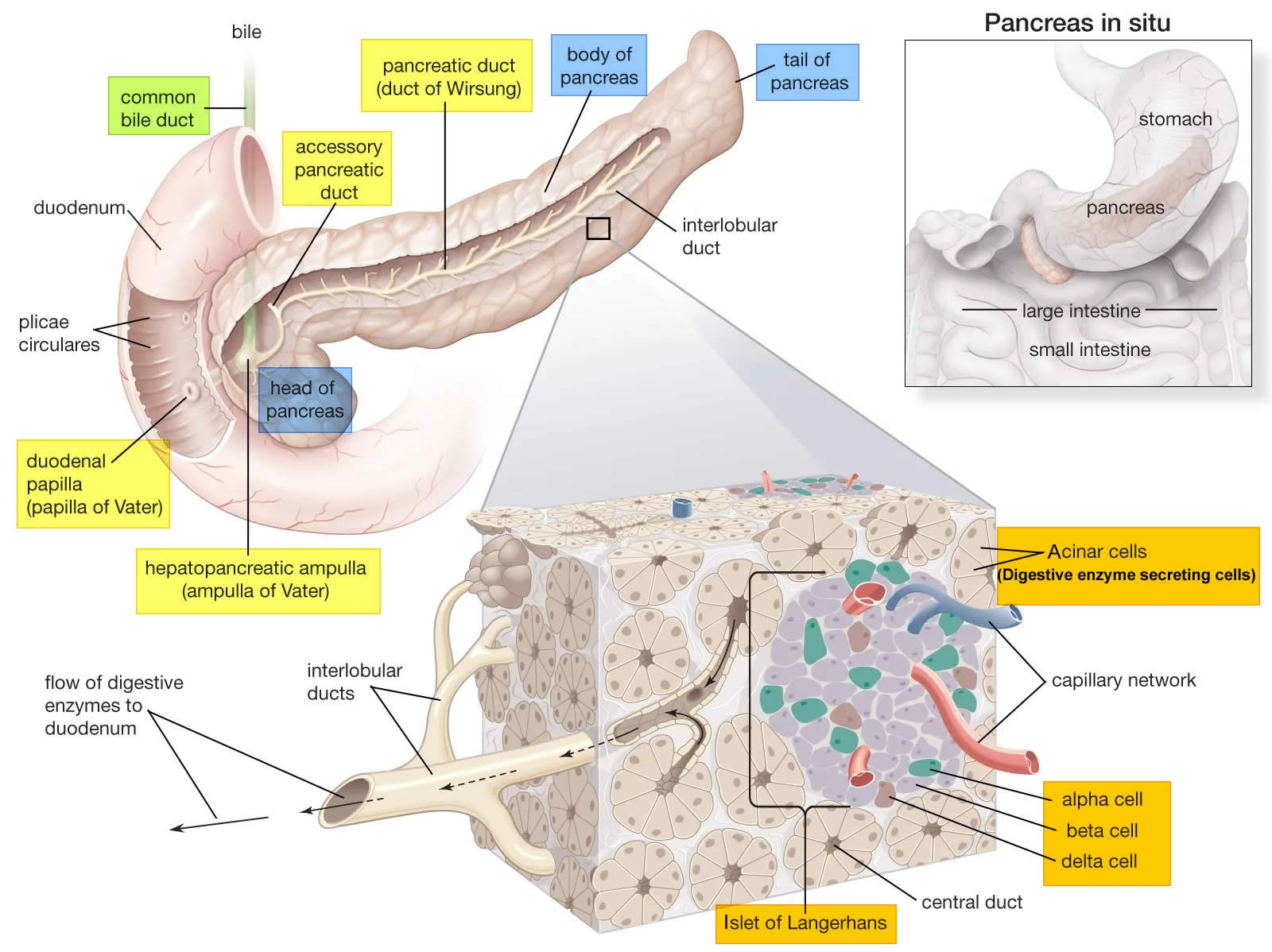
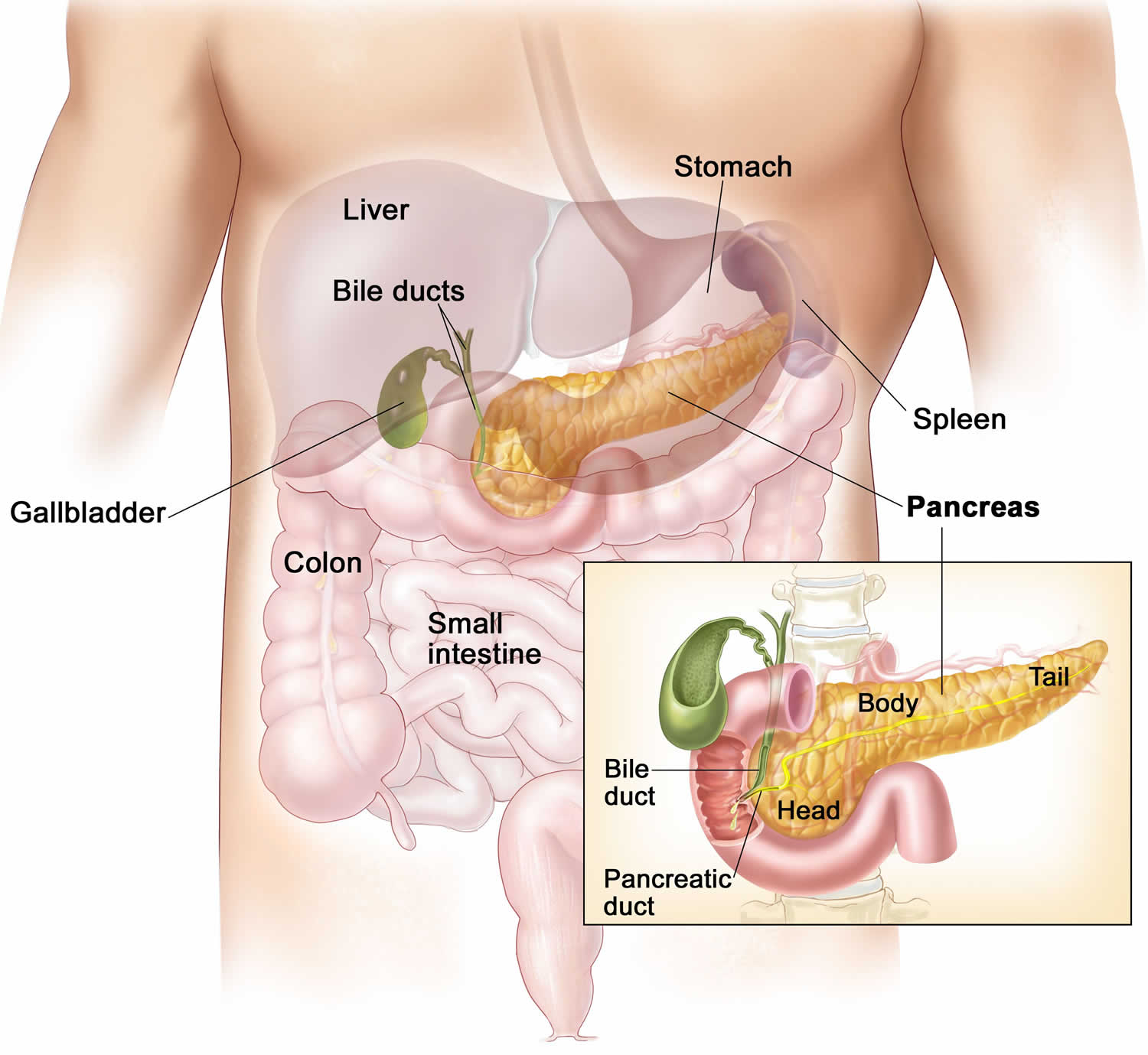
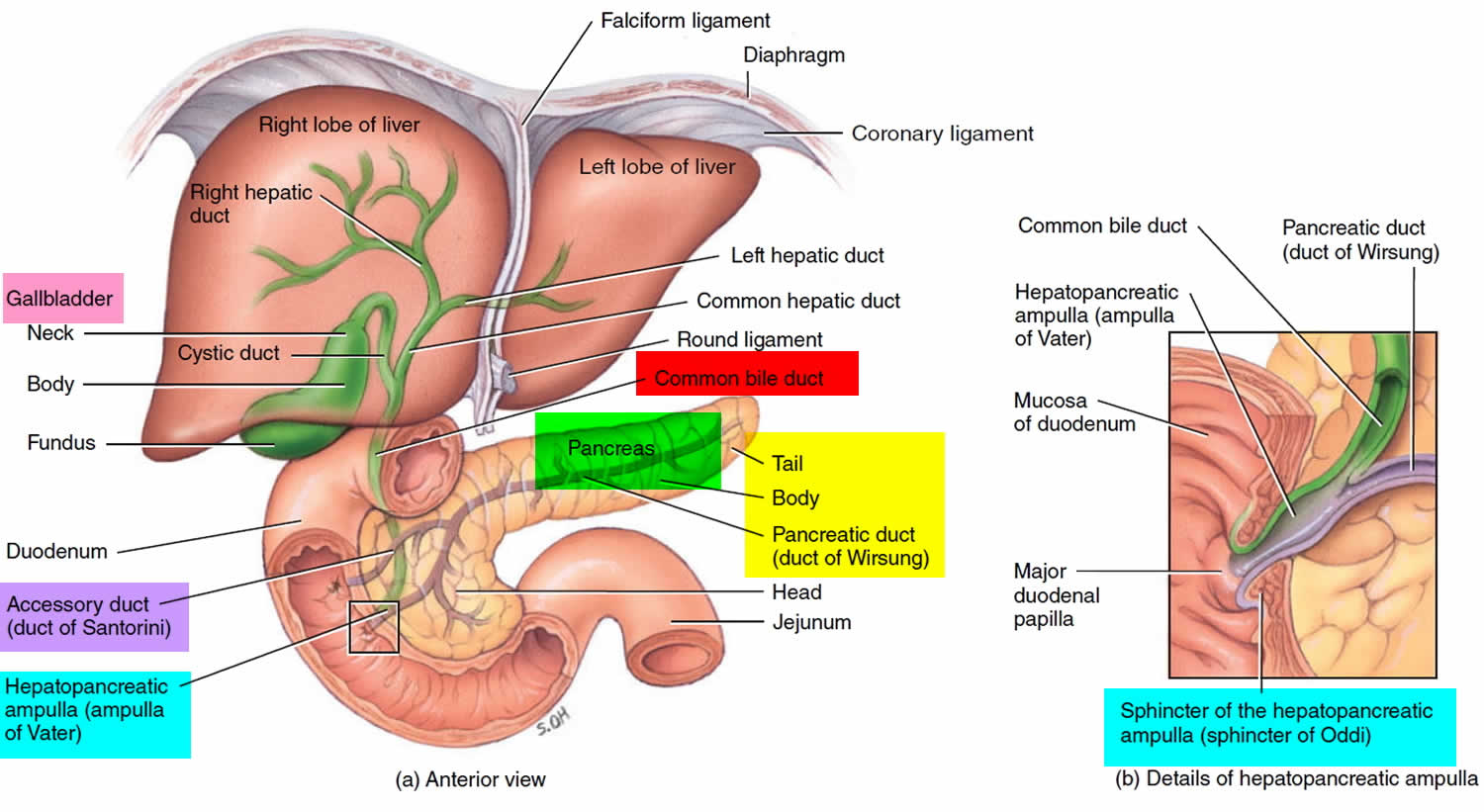
The pancreas is a large gland that sits behind the greater curvature of the stomach and close to the first part of the small intestine (the duodenum). The pancreas is shaped a bit like a fish with a wide head, a tapering body, and a narrow, pointed tail. In adults it’s about 12–15 cm (5–6 inches) long and 2.5 cm (1 in.) thick but less than 2 inches (5 centimeters) wide. The pancreas is both an endocrine and exocrine gland (see Figures 1 and 2).
The pancreas has 3 parts, the head, body, and tail.
- the wide end is called the head. The head of the pancreas is on the right side of the abdomen (belly), behind where the stomach meets the duodenum (the first part of the small intestine).
- the bit in the middle is called the body. The body of the pancreas is behind the stomach.
- the thin end is called the tail. The tail of the pancreas is on the left side of the abdomen next to the spleen.
About 99% of the pancreas is exocrine tissue made up of small clusters of glandular epithelial cells called acinar cells (acini), which secretes 1,200 to 1,500 mL of pancreatic juice per day – that are released into the small intestines to help you digest foods (especially fats). The digestive enzymes are first released into tiny tubes called central ducts. These merge to form larger ducts, which empty into the pancreatic duct (duct of Wirsung). The pancreatic duct merges with the common bile duct (the duct that carries bile from the liver), and empties into the duodenum (the first part of the small intestine) at the ampulla of Vater (also known as the hepatopancreatic ampulla). The ampulla of Vater (hepatopancreatic ampulla) is where the pancreatic duct and bile duct join together to drain into the duodenum, which is the first part of the small intestine. The passage of pancreatic juice and bile through the hepatopancreatic ampulla (ampulla of Vater) into the duodenum of the small intestine is regulated by a mass of smooth muscle surrounding the ampulla known as the sphincter of the hepatopancreatic ampulla, or sphincter of Oddi. The other major duct of the pancreas, the accessory duct (duct of Santorini), that branches from the main pancreatic duct and opens independently into the duodenum about 2.5 cm (1 in.) superior to the hepatopancreatic ampulla (ampulla of Vater) at the minor duodenal papilla. The accessory duct (duct of Santorini) bypasses the sphincter and allows pancreatic juice to be released into the duodenum even when bile is held back.
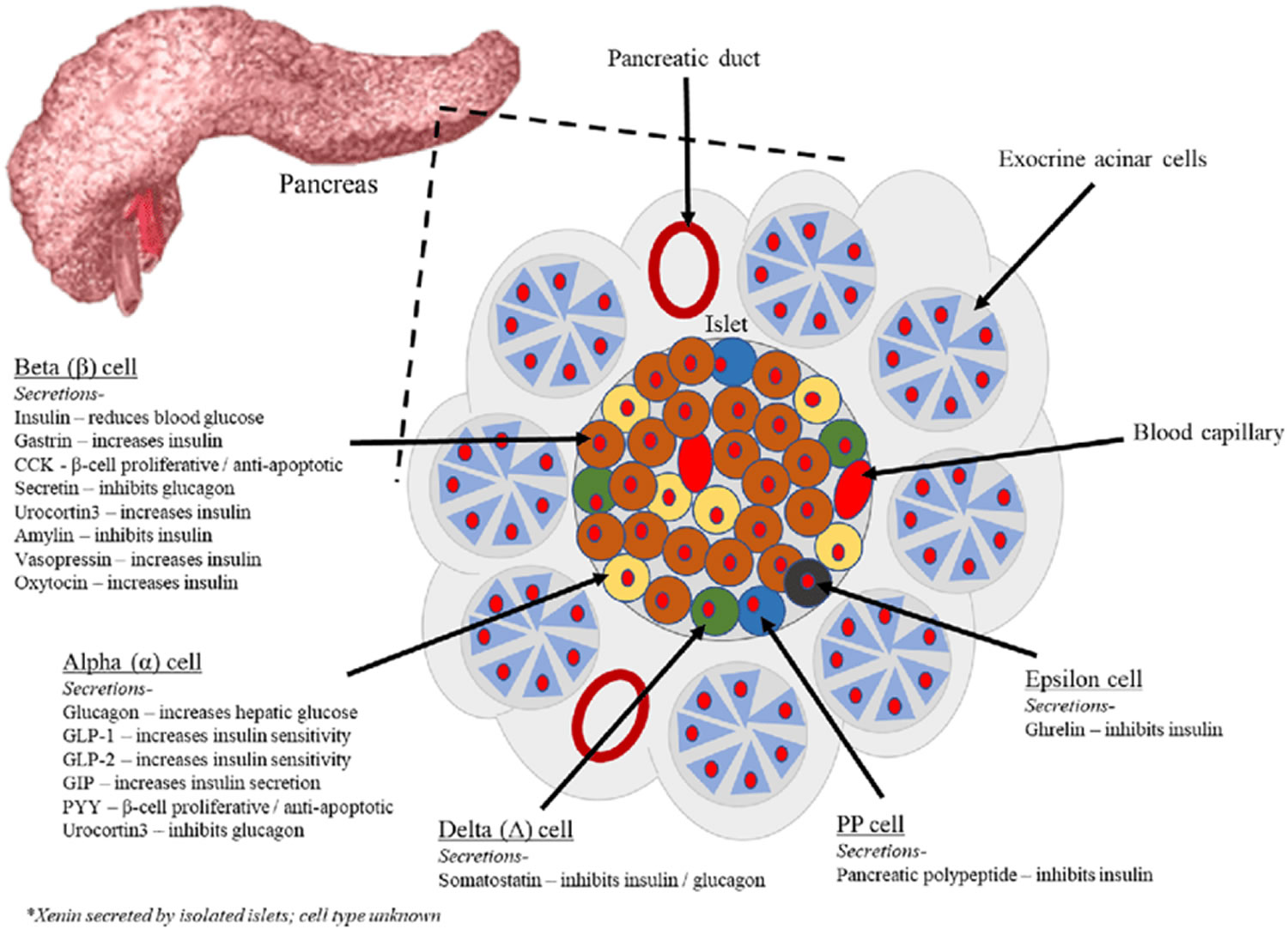
The endocrine part of the pancreas consists of groups of cells that are closely associated with blood vessels. These remaining 1% of the cell clusters form “islands” of cells called pancreatic islets (Islets of Langerhans). The Islets of Langerhans cells secrete the hormones glucagon, insulin, somatostatin, and pancreatic polypeptide (PP). Islets of Langerhans main cell types are alpha cells (20%), beta cells (70%), and delta cells (5%). The pancreatic islets alpha cells secrete the hormone glucagon, and beta cells secrete the hormone insulin (Figure 2). Both insulin and glucagon are important hormones which help control blood sugar levels and are released directly into the bloodstream.
The Islets of Langerhans Delta (δ) cells, or D cells, secrete somatostatin (growth hormone–inhibiting hormone) concurrently with the release of insulin by the beta cells. Somatostatin is a peptide hormone that inhibits the secretion of glucagon and insulin by the nearby alpha and beta cells. Somatostatin also work with amylin to limit the secretion of stomach acid.
Other, minor types of pancreatic cells, about 5% of the total, are called pancreatic polypeptide (PP) and G cells. Pancreatic polypeptide (PP) cells secrete pancreatic polypeptide, a hormone that may inhibit the exocrine activity of the pancreas.
Pancreatic islets (Islets of Langerhans) are relatively concentrated in the tail of the pancreas, whereas the head is more exocrine. Over 90% of pancreatic cancers arise from the ducts of the exocrine portion (ductal carcinomas), so cancer is most common in the head of the pancreas.
Figure 1. The pancreas
Figure 2. Pancreas cell types
Footnotes: Exocrine pancreatic acinar cells constitute most of the pancreatic tissue, these cells produce digestive enzymes which are transported via the pancreatic ducts. The endocrine pancreas is illustrated with all cell types; alpha, beta, delta, pancreatic polypeptide (PP) and epsilon. The endocrine pancreas cells are arranged in compact Islets of Langerhans and secrete a number of classical and ‘nonclassical’ peptides, as depicted.
Figure 3. Pancreas location
Figure 4. Relationship of the pancreas to the liver, gallbladder, and duodenum
Pancreas function
The pancreas has two main functions—to make insulin and to make digestive juices, or enzymes, to help you digest food in the intestine. About 99% of the pancreas is exocrine tissue made up of small clusters of glandular epithelial cells called acinar cells (acini), which secretes 1,200 to 1,500 mL of pancreatic juice per day – that are released into the small intestines to help you digest foods (especially fats). The cells of the secretory acini exhibit a high density of rough ER (endoplasmic reticulum) and secretory vesicles (zymogen granules). The acini open into a system of branched ducts that eventually converge on the main pancreatic duct. This duct runs lengthwise through the middle of the gland and joins the bile duct at the hepatopancreatic ampulla (ampulla of Vater). The hepatopancreatic sphincter (sphincter of Oddi) thus controls the release of both bile and pancreatic juice into the duodenum. Usually, however, there is a smaller accessory pancreatic duct (duct of Santorini) that branches from the main pancreatic duct and opens independently into the duodenum at the minor duodenal papilla, proximal to the major papilla. The accessory duct (duct of Santorini) bypasses the hepatopancreatic sphincter (sphincter of Oddi) and allows pancreatic juice to be released into the duodenum even when bile is held back.
Pancreatic juice is an alkaline mixture of water, enzymes, zymogens, sodium bicarbonate, and other electrolytes. The acini secrete the enzymes and zymogens, whereas the ducts secrete the sodium bicarbonate. The bicarbonate buffers HCl (hydrochloric acid) arriving from the stomach.
Sodium bicarbonate buffers the hydrochloric acid arriving from the stomach, with the reaction:
- HCl + NaHCO3 ⟶ NaCl + H2CO3 (carbonic acid).
The carbonic acid then breaks down to carbon dioxide (CO2) and water. CO2 is absorbed into the blood and ultimately exhaled. What is left in the small intestine, therefore, is salt water—sodium chloride (NaCl) and H2O. Sodium bicarbonate is therefore important in protecting the intestinal mucosa from hydrochloric acid (HCl) as well as raising the intestinal pH to the level needed for activity of the pancreatic and intestinal digestive enzymes.
The pancreatic zymogens are trypsinogen, chymotrypsinogen and procarboxypeptidase. When trypsinogen is secreted into the intestinal lumen, it is converted to trypsin by enteropeptidase, an enzyme on the brush border of the duodenum. Trypsin is autocatalytic—it converts trypsinogen into still more trypsin. Trypsin also converts the other two zymogens into chymotrypsin and carboxypeptidase, in addition to its primary role of digesting dietary protein.
Pancreatic acinar cells also secrete a protein called trypsin inhibitor that combines with any trypsin formed accidentally in the pancreas or in pancreatic juice and blocks its enzymatic activity.
Other pancreatic enzymes include pancreatic amylase, which digests starch; pancreatic lipase, which digests fat; and ribonuclease and deoxyribonuclease, which digest RNA and DNA, respectively. Unlike the zymogens, these enzymes are not altered after secretion. They become fully active, however, only upon exposure to bile or ions in the intestinal lumen.
Regulation of Pancreatic Secretion
Three stimuli are chiefly responsible for the release of pancreatic juice and bile.
- Acetylcholine (ACh), coming from the vagus nerves and enteric neurons. ACh stimulates the pancreatic acini to secrete their enzymes even during the cephalic phase of gastric control, before food is swallowed. The enzymes remain stored in the pancreatic acini and ducts, however, in preparation for release later when chyme enters the duodenum.
- Cholecystokinin (CCK), secreted by the mucosa of the duodenum and proximal jejunum (the next segment of the small intestine), primarily in response to fats in the small intestine. CCK also stimulates the pancreatic acini to secrete enzymes, but it is named for its strongly stimulatory effect on the gallbladder. It induces contractions of the gallbladder and relaxation of the hepatopancreatic sphincter, discharging bile into the duodenum.
- Secretin, produced by the same regions of the small intestine, mainly in response to the acidity of chyme from the stomach. Secretin stimulates the ducts of both the liver and pancreas to secrete an abundant sodium bicarbonate solution. In the pancreas, this flushes the enzymes into the duodenum.
Hormones of the Pancreatic Islets
The pancreas is primarily an exocrine digestive gland. Scattered throughout the exocrine tissue, are 1 to 2 million endocrine groups of cells that are closely associated with blood vessels called pancreatic islets (islets of Langerhans). Although they are less than 2% of the pancreatic tissue, the islets of Langerhans secrete the hormone glucagon and the hormone insulin of vital importance, especially in the regulation of glycemia, the blood glucose concentration. The pancreatic islets of Langerhans include two distinct types of cells—alpha cells, which secrete the hormone glucagon, and beta cells, which secrete insulin hormone. A typical islet measures about 75 × 175 μm and contains from a few to 3,000 cells. Islets of Langerhans main cell types are alpha cells (20%), beta cells (70%), and delta cells (5%). Islets of Langerhans respond directly to blood nutrient levels associated with the cycle of eating and fasting. Their functions are as follows:
- Alpha (α) cells, or A cells, secrete glucagon between meals when the blood glucose concentration falls below 100 mg/dL (5.6 mmol/L). Glucagon exerts two primary actions on the liver: (1) glycogenolysis, the breakdown of glycogen into glucose; and (2) gluconeogenesis, the synthesis of glucose from fats and proteins. These effects lead to the release of glucose into circulation, thus raising the blood glucose level. In adipose tissue, glucagon stimulates fat catabolism and the release of free fatty acids. Glucagon is also secreted in response to rising amino acid levels in the blood after a high-protein meal. It promotes amino acid absorption and thereby provides cells with the raw material for gluconeogenesis.
- Beta (β) cells, or B cells, secrete two hormones, insulin and amylin. Insulin, “the hormone of nutrient abundance,” is secreted during and immediately following a meal when blood nutrient levels are rising. Osteocalcin, a hormone from the osteoblasts of bone, also stimulates multiplication of beta cells, insulin secretion, and insulin sensitivity of other body tissues. The principal targets of insulin are the liver, skeletal muscles, and adipose tissue. In times of plenty, insulin stimulates cells to absorb glucose, fatty acids, and amino acids and to store or metabolize them; therefore, it lowers the level of blood glucose and other nutrients. It promotes the synthesis of glycogen, fat, and protein, thereby promoting the storage of excess nutrients for later use and enhancing cellular growth and differentiation. It also antagonizes glucagon, thus suppressing the use of already-stored fuels. The brain, liver, kidneys, and red blood cells absorb and use glucose without need of insulin, but insulin does promote glycogen synthesis in the liver. Insulin insufficiency or inaction is well known as the cause of diabetes. The beta cells also secrete another hormone, amylin, simultaneously with insulin. Amylin helps to reduce spikes in blood glucose by slowing the emptying of the stomach; modulating the secretion of gastric enzymes, acid, and bile; inhibiting glucagon secretion; and stimulating the sense of satiety (having had enough to eat).
- Delta (δ) cells, or D cells, secrete somatostatin (growth hormone–inhibiting hormone) concurrently with the release of insulin by the beta cells. Somatostatin is a peptide hormone that inhibits the secretion of glucagon and insulin by the nearby alpha and beta cells. Somatostatin also work with amylin to limit the secretion of stomach acid.
- Other, minor types of pancreatic cells, about 5% of the total, are called pancreatic polypeptide (PP) and G cells. Pancreatic polypeptide (PP) cells secrete pancreatic polypeptide, a hormone that may inhibit the exocrine activity of the pancreas.
Any hormone that raises blood glucose concentration is called a hyperglycemic hormone. You may have noticed that glucagon is not the only hormone that does so; so do growth hormone, epinephrine, norepinephrine, cortisol, and corticosterone. Insulin is called a hypoglycemic hormone because it lowers blood glucose levels.
Glucagon raises the blood sugar concentration by stimulating the liver to break down glycogen and convert certain noncarbohydrates, such as amino acids, into glucose. These actions raise the blood glucose concentration. Glucagon much more effectively elevates blood glucose than does epinephrine (adrenaline).
A negative feedback system regulates glucagon secretion. A low blood glucose concentration stimulates alpha cells to release glucagon. When the blood glucose concentration rises, glucagon secretion falls. This control prevents hypoglycemia when the blood glucose concentration is relatively low, such as between meals, or when glucose is used rapidly, such as during exercise.
The main effect of insulin is to lower the blood glucose level, exactly opposite that of glucagon. Insulin does this in part by promoting facilitated diffusion of glucose into cells that have insulin receptors, for use in cellular respiration. Such cells include those of adipose tissue, liver, and skeletal muscle. (Glucose uptake by active skeletal muscle does not require insulin.) Insulin also stimulates the liver to form glycogen from glucose and inhibits conversion of noncarbohydrates into glucose. In addition, insulin promotes transport of amino acids into cells, increases the rate of protein synthesis, and stimulates adipose cells to synthesize and store fat.
A negative feedback system sensitive to the blood glucose concentration regulates insulin secretion. When the blood glucose concentration is high, such as after a meal, beta cells release insulin. Insulin helps prevent too high a blood glucose concentration by promoting glycogen formation in the liver and entrance of glucose into adipose and muscle cells.
When glucose concentration falls, such as between meals or during the night, insulin secretion decreases. As insulin secretion decreases, less glucose enters adipose and resting muscle cells. Cells that lack insulin receptors and are therefore not dependent on insulin, such as nerve cells, can still take up glucose from the blood. At the same time that insulin is decreasing, glucagon secretion is increasing. Nerve cells, including those of the brain, obtain glucose by a facilitated diffusion mechanism that does not require insulin, but rather depends only on the blood glucose concentration. For this reason, nerve cells are particularly sensitive to changes in blood glucose concentration. Conditions that cause such changes—for example, oversecretion of insulin leading to decreased blood glucose—are likely to affect brain functions.
Insulin and glucagon are coordinated to maintain a relatively stable blood glucose concentration, despite great variation in the amount of carbohydrates a person eats. About 85% to 90% of people with diabetes mellitus have type 2 diabetes, in which the beta cells produce insulin but body cells lose the ability to recognize it. On the other hand, type 1 diabetes mellitus usually appears before age twenty and it is an autoimmune disease: the immune system destroys the beta cells of the pancreas.
Acute pancreatitis
Acute pancreatitis occurs suddenly and is a short-term condition, meaning it appears suddenly and lasts for days. Most people with acute pancreatitis get better, and it goes away in several days with treatment. But some people can have a more severe form of acute pancreatitis and go on to develop serious complications, which requires a lengthy hospital stay. Acute pancreatitis is different to chronic pancreatitis, where the pancreas has become permanently damaged from inflammation over many years. Acute pancreatitis is becoming more common, for reasons that are not clear 3. Acute pancreatitis is the cause of up to 275,000 hospitalizations in the United States per year 4, 1. While mild acute pancreatitis carries a mortality of <1%, mortality rates for severe pancreatitis can reach as high as 30% 5. Drugs are responsible for 0.1%-2% of acute pancreatitis incidents. The majority of drug-induced pancreatitis cases are mild to moderate in severity; however, severe and even fatal cases can occur. Although pancreatitis is rare in children, the number of children with acute pancreatitis has grown.
Repeat episodes of acute pancreatitis may lead to chronic pancreatitis. Other complications of acute pancreatitis include:
- dehydration
- bleeding
- infection
Acute pancreatitis causes
The most common cause of acute pancreatitis is having gallstones. Gallstones cause inflammation of your pancreas as stones pass through and get stuck in a bile or pancreatic duct. This condition is called gallstone pancreatitis. However, in approximately 30 percent of cases, a cause cannot be identified. Sometimes injury to the abdomen — such as a bicycle or playground accident or sports injury — can cause acute pancreatitis, or common medications and conditions, including:
- anti-seizure medications
- certain antibiotics
- specific types of chemotherapy
- infections
- problems when the immune system attacks the body
- blockage of the tubes (ducts) that drain enzymes from the pancreas
- high levels of fats, called triglycerides, in the blood
- overactive parathyroid gland
Other chronic conditions may cause pancreatitis such as inflammatory bowel disease, cystic fibrosis or celiac disease.
Table 1. Drugs associated with acute pancreatitis
| Angiotensin-converting enzyme (ACE) inhibitors | Estrogens | Pentamidine |
| Acetaminophen | Ethacrynic acids | Pergolide |
| Adrenocorticotrophic hormones | Exenatide | Phenolphthalein |
| Ezetimibe | Pilocarpine | |
| Alendronate | Fibrates | Prazosin |
| All-trans-retinoic acid | Finasteride | Procainamide |
| Alpha-methyldopa | Fluoroquinolones | Propofol |
| Aminosalicylates | 5-Fluorouracil | Propoxyphene |
| Amiodarone | Furosemide | Proton pump inhibitors |
| Amlodipine | Gabapentin | Quinupristin/dalfopristin |
| Ampicillin | Gold | Ranitidine |
| Antivirals | HAART agents | Repaglinide |
| Aspirin | HMG-CoA reductase inhibitors | Rifampin |
| Atypical antipsychotics | Rifapentine | |
| Azathioprine | Ifosfamide | Rivastigmine |
| Bupropion | Indomethacin | Ropinirole |
| Calcitriol | Interferon/ribavirin | Saw palmetto |
| Cannabis | Interleukin-2 | Selective serotonin receptor antagonists |
| Capecitabine | Irbesartan | |
| Carbamazepine | Isoniazid | Sirolimus |
| Ceftriaxone | Isotretinoin | Sodium stibogluconate |
| Cimetidine | Lamotrigine | Somatropin |
| Cisplatin | L-asparaginase | Sulfamethoxazole |
| Clomiphene | Macrolides | Sulfasalazine |
| Codeine | Mefenamic acid | Sumatriptan |
| Colchicine | 6-Mercaptopurine | Tacrolimus |
| Corticosteroids | Mesalamine | Tamoxifen |
| COX-2 inhibitors | Metformin | Tetracyclines |
| Cyclophosphamide | Methimazole | Thiazide diuretics |
| Cyclosporine | Methyldopa | Thrombolytic agents |
| Cyproheptadine | Metronidazole | TNF-alpha inhibitors |
| Cytosine | Mirtazapine | Topiramate |
| Danazol | Montelukast | Trimethoprim-sulfamethizole |
| Dapsone | Mycophenolate | |
| Diazoxide | Nitrofurantoin | Valproic acid |
| Diphenoxylate | NSAIDs | Venlafaxine |
| Dipyridamole | Octreotide | Vincristine |
| Doxercalciferol | Paclitaxel | Voriconazole |
| Doxorubicin | Pegaspargase | Zolmitriptan |
| Ertapenem | Penicillin |
Acute pancreatitis symptoms
People with acute pancreatitis may feel pain in their upper abdomen that may spread to their back.
Acute pancreatitis usually starts with pain that:
- begins slowly or suddenly in your upper abdomen
- sometimes spreads to your back
- can be mild or severe
- may last for several days
Other symptoms may include:
- fever
- nausea and vomiting
- fast heartbeat
- swollen or tender abdomen
People with acute pancreatitis usually look and feel seriously ill and need to see a doctor right away.
Acute pancreatitis diagnosis
To diagnose pancreatitis and find its causes, doctors use:
- Your medical history. Your doctor will ask:
- about your symptoms
- if you have a history of health conditions or concerns that make you more likely to get pancreatitis—including medicines you are taking
- if you have a personal or family medical history of pancreatitis or gallstones
- A physical exam. During a physical exam, your doctor will:
- examine your body
- check your abdomen for pain, swelling, or tenderness
- Lab and imaging tests
Tests and procedures used to diagnose pancreatitis include:
- Blood tests to look for elevated levels of pancreatic enzymes, along with white blood cells, kidney function and liver enzymes
- Abdominal ultrasound to look for gallstones and pancreas inflammation
- Computerized tomography (CT) scan of your abdomen to look for gallstones and assess the extent of pancreas inflammation
- Magnetic resonance imaging (MRI) of your abdomen to look for abnormalities in the gallbladder, pancreas and ducts
- Endoscopic ultrasound to look for inflammation and blockages in the pancreatic duct or bile duct
- Stool tests in chronic pancreatitis to measure levels of fat that could suggest your digestive system isn’t absorbing nutrients adequately
Your doctor may recommend other tests, depending on your particular situation.
Lab tests
Lab tests to help diagnose pancreatitis include the following:
- Blood tests. Your physician may take a blood sample from you and send the sample to a lab to test for:
- high amylase and lipase levels—digestive enzymes made in your pancreas
- high blood glucose, also called blood sugar
- high levels of blood fats, called lipids
- signs of infection or inflammation of the bile ducts, pancreas, gallbladder, or liver
- pancreatic cancer
- Stool tests. Your doctor may test a stool sample to find out if a person has fat malabsorption.
Imaging tests
Your doctor also use imaging tests to diagnose pancreatitis. A technician performs most tests in an outpatient center, a hospital, or a doctor’s office. You don’t need anesthesia, a medicine to keep you calm, for most of these tests.
- Ultrasound. Ultrasound uses a device called a transducer, which bounces safe, painless sound waves off your organs to create a picture of their structure. Ultrasound can find gallstones.
- Computed tomography (CT) scan. CT scans create pictures of your pancreas, gallbladder, and bile ducts. CT scans can show pancreatitis or pancreatic cancer.
- Magnetic resonance cholangiopancreatography (MRCP). MRCP uses a magnetic resonance imaging (MRI) machine, which creates pictures of your organs and soft tissues without x-rays. Your doctor or a specialist may use MRCP to look at your pancreas, gallbladder, and bile ducts for causes of pancreatitis.
- Endoscopic ultrasound (EUS). Your doctor inserts an endoscope—a thin, flexible tube—down your throat, through your stomach, and into your small intestine. The doctor turns on an ultrasound attachment to create pictures of your pancreas and bile ducts. Your doctor may send you to a gastroenterologist to perform this test.
- Pancreatic Function Test (PFT). Your doctor may use this test to measure how your pancreas responds to secretin, a hormone made by the small intestine. This test is done only at some centers in the United States.
Acute pancreatitis treatment
Mild acute pancreatitis usually goes away in a few days with rest and treatment.
If your pancreatitis is more severe, your treatments for acute pancreatitis in the hospital may include:
- Early eating. Old data suggested to stop eating for a couple of days in the hospital in order to give your pancreas a chance to recover. This is no longer practiced. Newer data have suggested that eating as soon as you tolerate food helps heal the pancreas. As the inflammation in your pancreas improves and pain symptoms improve, you should begin drinking clear liquids and eating bland foods. With time, you can go back to your normal diet. If your pancreatitis symptoms persist and you still experience pain when eating, your doctor may recommend a feeding tube to help you get nutrition.
- Pain medications. Pancreatitis can cause severe pain. Your health care team will give you medications to help control the pain.
- Intravenous (IV) fluids. As your body devotes energy and fluids to repairing your pancreas, you may become dehydrated. For this reason, you’ll receive extra fluids through a vein in your arm during your hospital stay.
Pancreatitis-related pain in some patients has been shown to respond to pancreatic enzyme replacement 7 and possibly with supplementation of antioxidants such as S-Adenosyl Methionine (SAMe) (800 mg per day), Vitamin C (180 mg per day), Vitamin E (30 mg per day), Vitamin A (2,400 microg per day), and selenium (75 microg per day) 8. The central nervous system and pain processing system have been implicated as a mechanism of pancreatitis-related pain, so the use of tricyclic antidepressants or gabapentin may be helpful 9. Additionally, while nonsteroidal anti-inflammatory drugs (NSAIDs) are preferred over opioid analgesics for pain relief, they may be contraindicated due to the patient’s comorbidities or not tolerated due to side effects. If opioid analgesics are to be prescribed, long-acting formulations are preferred over short or intermediate acting forms 10.
Once your pancreatitis is under control, your doctor will evaluate and treat the underlying cause of your pancreatitis.
Depending on the cause of your acute pancreatitis, treatment may include:
- Procedures to remove bile duct obstructions. Pancreatitis caused by a narrowed or blocked bile duct may require procedures to open or widen the bile duct. A procedure called endoscopic retrograde cholangiopancreatography (ERCP) uses a long tube with a camera on the end to examine your pancreas and bile ducts. The tube is passed down your throat, and the camera sends pictures of your digestive system to a monitor. Anesthesia is used for this procedure. Your gastroenterologist may use ERCP to diagnose problems in the bile duct and pancreatic duct and in removing obstructions, such as gallstones. In some people, however, ERCP can also lead to acute pancreatitis.
- Gallbladder surgery. If gallstones caused your pancreatitis, your doctor will recommend surgery to remove your gallbladder called cholecystectomy. Having surgery to remove the gallbladder within a few days after you are admitted to the hospital lowers the chance of complications. If you have severe pancreatitis, your doctor may advise delaying surgery to first treat complications.
- Pancreas procedures. Endoscopic procedures may be necessary to drain fluid in your abdomen if you have an abscess or infected pseudocyst, or a large pseudocyst causing pain or bleeding. Your doctor may remove damaged tissue from your pancreas.
- Treatment for alcohol dependence. Drinking several drinks a day over many years can cause pancreatitis. If this is the cause of your pancreatitis, your doctor may recommend you enter a treatment program for alcohol addiction. Continuing to drink alcohol would worsen your pancreatitis and lead to serious complications.
- Medication changes. If a medication is deemed to be a cause of acute pancreatitis, your doctor may stop the medication and work with you to find alternative options.
Acute pancreatitis prognosis
Acute pancreatitis is a severe disorder that still carries a mortality of 5 to 15%, depending on the cause, patient age, and comorbidity 11. Patients with gallstone pancreatitis generally have higher mortality than those with alcoholic pancreatitis 11. In addition, the presence of type 2 diabetes significantly increases the risk of complications and death. In patients with multiorgan involvement, the mortality can be as high as 20% 11. Most deaths are due to multiorgan failure and hypotensive shock. Various classifications have been developed to assess the prognosis of patients with acute pancreatitis, but most are cumbersome for practical use 12, 13, 14.
Chronic pancreatitis
Chronic pancreatitis is a long-lasting condition and slowly destroys the functions of the pancreas. The pancreas does not heal or improve. Instead, it gets worse over time, which can lead to lasting damage to your pancreas. For example, the pancreas may lose its ability to produce insulin and glucagon and digestive enzymes (pancreatic digestive juices). As a result, you can develop glucose intolerance or diabetes. Chronic pancreatitis can also cause weight loss because your body may be unable to digest fat and key elements of food. Chronic pancreatitis is less common than acute pancreatitis, with about 86,000 hospital stays per year 15. In the US, chronic pancreatitis affects African Americans more frequently than caucasians 16. In addition, chronic pancreatitis due to alcohol is more common in males, whereas that due to hyperlipidemia is more common in females 16. The median age at diagnosis is 45.
Complications of chronic pancreatitis include:
- chronic pain in your abdomen
- maldigestion, when you can’t digest food properly
- malnutrition and malabsorption
- problems with how well your pancreas works
- scars in your pancreas
- diabetes
- pancreatic cancer, which is more likely in people with both diabetes and pancreatitis
- osteopenia, osteoporosis and bone fractures
Diagnosing chronic pancreatitis relies on changes in diagnostic imaging and blood work in addition to clinical symptoms.
Chronic pancreatitis causes
Repeated episodes of acute pancreatitis can lead to chronic pancreatitis. Instead of the inflammation getting better as in acute pancreatitis, the inflammation continues in some more susceptible people and causing permanent damage to the pancreas. In some cases, genetics may be a factor. However, sometimes, the cause is unknown.
The most common causes of chronic pancreatitis are:
- heavy alcohol use
- genetic disorders of your pancreas (cystic fibrosis, hereditary pancreatitis)
Other causes of chronic pancreatitis include:
- blockage in your pancreatic duct
- high levels of blood fats, called lipids (hypertriglyceridemia)
- high level of calcium in your blood (hypercalcemia)
- autoimmune diseases such as systemic lupus erythematosus (SLE), celiac disease, inflammatory bowel disease (IBD) or autoimmune pancreatitis 17, 18, 19
New studies are finding that deficiencies in certain vitamins and antioxidants may be linked to chronic pancreatitis 20, 21.
In many cases, doctors can’t find the cause of pancreatitis. This is called idiopathic pancreatitis.
Chronic pancreatitis symptoms
Most people with chronic pancreatitis feel pain in the upper abdomen, although some people have no pain at all.
The upper abdominal pain may:
- spread to your back
- become constant and severe
- become worse after eating
- go away as your condition gets worse
People with chronic pancreatitis may not have symptoms until they have complications.
Other symptoms may include:
- diarrhea
- nausea
- oily, foul-smelling stools (steatorrhea)
- vomiting
- weight loss without trying
Chronic pancreatitis diagnosis
To diagnose pancreatitis and find its causes, doctors use:
- Your medical history. Your doctor will ask:
- about your symptoms
- if you have a history of health conditions or concerns that make you more likely to get pancreatitis—including medicines you are taking
- if you have a personal or family medical history of pancreatitis or gallstones
- A physical exam. During a physical exam, your doctor will:
- examine your body
- check your abdomen for pain, swelling, or tenderness
- Lab and imaging tests
Tests and procedures used to diagnose pancreatitis include:
- Blood tests to look for elevated levels of pancreatic enzymes, along with white blood cells, kidney function and liver enzymes
- Abdominal ultrasound to look for gallstones and pancreas inflammation
- Computerized tomography (CT) scan of your abdomen to look for gallstones and assess the extent of pancreas inflammation
- Magnetic resonance imaging (MRI) of your abdomen to look for abnormalities in the gallbladder, pancreas and ducts
- Endoscopic ultrasound to look for inflammation and blockages in the pancreatic duct or bile duct
- Stool tests in chronic pancreatitis to measure levels of fat that could suggest your digestive system isn’t absorbing nutrients adequately
Your doctor may recommend other tests, depending on your particular situation.
Lab tests
Lab tests to help diagnose pancreatitis include the following:
- Blood tests. Your physician may take a blood sample from you and send the sample to a lab to test for:
- high amylase and lipase levels—digestive enzymes made in your pancreas
- high blood glucose, also called blood sugar
- high levels of blood fats, called lipids
- signs of infection or inflammation of the bile ducts, pancreas, gallbladder, or liver
- pancreatic cancer
- Stool tests. Your doctor may test a stool sample to find out if a person has fat malabsorption. At present, measurement of fecal elastase is the most popular test to evaluate pancreatic exocrine insufficiency. Low levels of fecal elastase (<200 µg/g stool, although even lower levels are more specific) or serum trypsin (<20 ng/mL) are usually observed in patients with pancreatic exocrine insufficiency 22.
Imaging tests
Your doctor also use imaging tests to diagnose pancreatitis. A technician performs most tests in an outpatient center, a hospital, or a doctor’s office. You don’t need anesthesia, a medicine to keep you calm, for most of these tests.
- Ultrasound. Ultrasound uses a device called a transducer, which bounces safe, painless sound waves off your organs to create a picture of their structure. Ultrasound can find gallstones.
- Computed tomography (CT) scan. CT scans create pictures of your pancreas, gallbladder, and bile ducts. CT scans can show pancreatitis or pancreatic cancer.
- Magnetic resonance cholangiopancreatography (MRCP). MRCP uses a magnetic resonance imaging (MRI) machine, which creates pictures of your organs and soft tissues without x-rays. Your doctor or a specialist may use MRCP to look at your pancreas, gallbladder, and bile ducts for causes of pancreatitis.
- Endoscopic ultrasound (EUS). Your doctor inserts an endoscope—a thin, flexible tube—down your throat, through your stomach, and into your small intestine. The doctor turns on an ultrasound attachment to create pictures of your pancreas and bile ducts. Your doctor may send you to a gastroenterologist to perform this test.
- Pancreatic Function Test (PFT). Your doctor may use this test to measure how your pancreas responds to secretin, a hormone made by the small intestine. This test is done only at some centers in the United States.
Chronic pancreatitis treatment
Treatment for chronic pancreatitis may help relieve pain, improve how well the pancreas works, and manage complications.
Depending on your situation, your doctor may prescribe or provide the following additional treatments:
- Pain management. Chronic pancreatitis can cause persistent abdominal pain. Your doctor will evaluate you for causes of chronic pancreatitis and may recommend medications to control your pain. If necessary, you may be referred to a pain specialist. Severe pain may be relieved with options such as endoscopic ultrasound or injections to block nerves that send pain signals from the pancreas to the brain.
- Enzymes to improve digestion. In chronic pancreatitis leading to diarrhea or weight loss, pancreatic enzyme supplements can help your body break down and process the nutrients in the foods you eat. Pancreatic enzymes are taken with each meal.
- Changes to your diet. Your doctor may refer you to a dietitian who can help you plan low-fat meals that are high in nutrients.
- Medicines and vitamins. Long-term fat malabsorption may also lead to fat-soluble vitamin (A, D, E, and K) deficiencies 23 as well as deficiencies in calcium, magnesium, zinc, thiamine, and folic acid 24. Your doctor may give you vitamins A, D, E, and K if you have malabsorption. He or she may also give you vitamin B-12 shots if you need them.
- Treatment for diabetes. Chronic pancreatitis may cause diabetes. If you get diabetes, your doctor and health care team will work with you to create an eating plan and a routine of medicine, blood glucose monitoring, and regular checkups.
- Surgery. Your doctor may recommend surgery to relieve pressure or blockage in your pancreatic duct, or to remove a damaged or infected part of your pancreas. Surgery is done in a hospital, where you may have to stay a few days. In patients who do not get better with other treatments, surgeons may perform surgery to remove your whole pancreas, followed by islet auto-transplantation. Islets of Langerhans are groups of cells in your pancreas that make hormones, including insulin. After removing your pancreas, doctors will take islets from your pancreas and transplant them into your liver. The islets will begin to make hormones and release them into your bloodstream.
- Procedures. Your doctor may suggest a nerve block, which is a shot of numbing medicine through your skin and directly into nerves that carry the pain message from your pancreas. If you have stones blocking your pancreatic duct, your doctor may use a procedure to break up and remove the stones.
Chronic pancreatitis prognosis
Chronic pancreatitis is associated with poor prognosis in patients who continued to use alcohol, smoke and the presence of end-stage liver disease 16. At 10 years, there is a survival of 70%, which drops to 45% at 20 years 16. Pancreatic pseudocyst formation, mechanical obstruction of the bile duct and duodenum are major complications. Addition complications include diabetes (30% of patients), development of gastric varices and pseudoaneurysm formation.
Hereditary pancreatitis
Hereditary pancreatitis is a rare genetic condition characterized by multiple recurrent episodes of inflammation of the pancreas (pancreatitis), which can progress to chronic pancreatitis. Hereditary pancreatitis is a rare cause of acute, recurrent acute, and chronic pancreatitis 25. Hereditary pancreatitis symptoms include recurring bouts of severe abdominal pain, nausea, and vomiting, that usually require opiate analgesia for pain relief 26. Onset of symptoms typically occurs before the age of ten years, but can begin at any age 27, 25, 28. In the United States, it is estimated that at least 1,000 individuals are affected with hereditary pancreatitis. People with hereditary pancreatitis develop chronic pancreatitis, a constantly inflamed pancreas. This leads to symptoms which may include fatty stools, weight loss, and poor absorption of nutrients from food.
Adults with hereditary pancreatitis are at an increased risk for type 1 diabetes, pancreatic exocrine failure and pancreatic cancer (adenocarcinoma of the pancreas) 29, 30, 31, 32. Pancreatic cancer is the 4th most leading cause of cancer deaths among Americans. Individuals with hereditary pancreatitis appear to have a 40% lifetime risk of developing pancreatic cancer 33. This increased risk is heavily dependent upon the duration of chronic pancreatitis and environmental exposures to alcohol and smoking. One recent study suggested that individuals with chronic pancreatitis for more than 25 years had a higher rate of pancreatic cancer when compared to individuals in the general population 33. This increased pancreatic cancer rate appears to be due to the prolonged chronic pancreatitis rather than having a gene mutation (all cationic trypsinogen mutations). It is important to note that these risk values may be higher than expected because these studies on pancreatic cancer use a highly selective population rather than a randomly selected population.
Hereditary pancreatitis was first recognized by Comfort and Steinberg as a clinical syndrome in 1952 34 and mutations in the cationic trypsinogen (serine protease 1) gene (PRSS1 gene) on chromosome 7q were identified as major etiological factors in 1996 35, 29, 36, 37. More than twenty pathogenic PRSS1 gene mutations have since been described, accounting for 80% of all cases of hereditary pancreatitis in people of European ancestry 35, 29, 36, 37. The two most commonly identified pathogenic PRSS1 mutations are p.R122H and p.N29I (90%), while less common mutations include A16V, R122C, N29T, D22G, and K23R 38, 39. Other implicated genes include those that encode serine protease inhibitor Kazal‐type 1 (SPINK1, on chromosome 5q32), cystic fibrosis transmembrane conductance regulator (CFTR), chymotrypsin C (CTRC), and A‐type carboxypeptidase (CPA1) 29, 36, 40. Modifier genes, such as the calcium‐sensing receptor gene (CASR), also influence the risk of hereditary pancreatitis 41.
In most cases, hereditary pancreatitis is due to a PRSS1 gene that is not working correctly and is inherited in an autosomal dominant pattern 37, 42, 43. Internationally, the majority of PRSS1 mutations have been identified in the USA 44 and Europe 38, though few have also been reported from Japan 45, South America 46 and Thailand 47.To date, hereditary pancreatitis has not been reported in patients of African ancestry 35.
In hereditary pancreatitis, inflammation is initially restricted to the acinar and ductal cells, leading to pancreatic exocrine insufficiency; the pancreatic islets are affected only late in the disease process 27 and patients require pancreatic enzyme replacement long before diabetes develops 48, 49. As many as 60% of people with symptomatic pancreatitis will ultimately require surgical interventions (including endoscopic procedures) because of severe abdominal pain, with unavoidable loss of pancreatic function 32, 49.
Hereditary pancreatitis diagnosis is based on the symptoms, a clinical history and exam, and the results of genetic testing. Criteria for genetic testing for hereditary pancreatitis, when patients meet one or more of the following criteria 10:
- A family history of idiopathic chronic pancreatitis, recurrent acute pancreatitis, or childhood pancreatitis
- Relatives with known mutations associated with hereditary pancreatitis
- Unexplained pancreatitis in a child
- Idiopathic chronic pancreatitis in patients <25 years old
- Recurrent acute pancreatitis of uncertain etiology
- Patients who are eligible for enrollment in approved research trials
At this time, there is no cure for hereditary pancreatitis. Patients with hereditary pancreatitis should be managed in the same way as patients who present with pancreatitis from other causes 25. Treatment is focused on managing the symptoms and may include medications, surgery, and surveillance for diabetes and cancer. Patients may be prescribed pancreatic enzyme supplements to treat maldigestion, insulin to treat diabetes, analgesics and narcotics to control pain, and lifestyle changes to reduce the risk of pancreatic cancer (for example, no smoking and no drinking alcohol).
Dietary recommendations to help control pain with digestion include the consumption of small meals throughout the day that are high in carbohydrates and low in protein and fat. Pancreatic enzymes such as Creon, Pancrease, and Violiase are helpful in providing improved digestion and a reduction in diarrhea and pain for some patients with more advanced disease.
Exposure to smoking and alcohol are known to dramatically increase the risk for pancreatic attacks among individuals with hereditary pancreatitis. Smoking is strongly discouraged as it doubles the risk for pancreatic cancer 50. Similarly, alcohol consumption is not recommended for these patients as no safe amount of alcohol use has been documented, because alcohol is a known risk factor for both acute and chronic pancreatitis. Therefore it is recommended that all hereditary pancreatitis patients avoid smoking and alcohol consumption. Though harder to control, dietary fat and emotional stress are considered triggers and should also be avoided 39. Finally, the risk and benefit of the use of medications that have been associated with drug-induced pancreatitis (eg, ACE inhibitors, HMG co-A reductase inhibitors, and selective serotonin reuptake inhibitors) should be individualized to the patient 4.
Overall survival in hereditary pancreatitis PRSS1 carriers appears to be similar to the general population 35, 51. However, if a patient with hereditary pancreatitis develops pancreatic cancer, it usually presents 20 years earlier than in the general population and thus results in an earlier and increased mortality 52. Cancer was the most frequent cause of death in PRSS1 carriers (32.4%), of which less than half (42%) were from pancreatic cancer 53, 54. Interestingly, non-malignant pancreatic disease was mentioned on death reports as the second most common cause (21.6%), followed by cardiovascular disease (13.5%), which was different than the only other report on hereditary pancreatitis causes of death 51.
Pancreatitis causes
Pancreatitis occurs when the pancreas digestive enzymes become activated while still inside the pancreas, irritating the cells of your pancreas and causing inflammation. With repeated bouts of acute pancreatitis, damage to the pancreas can occur and lead to chronic pancreatitis. Scar tissue may form in the pancreas, causing loss of function. A poorly functioning pancreas can cause digestion problems and diabetes.
The most common causes of both acute and chronic pancreatitis are:
- Gallstones
- Heavy alcohol use
- Genetic disorders of your pancreas
- Some medicines
Other conditions that can lead to pancreatitis include:
- High triglyceride levels in the blood (hypertriglyceridemia)
- High calcium levels in the blood (hypercalcemia), which may be caused by an overactive parathyroid gland (hyperparathyroidism)
- Pancreatic cancer
- Abdominal surgery
- Cystic fibrosis
- Infections, such as viruses or parasites
- Injury to your abdomen
- Obesity
- Trauma
- Pancreas divisum a birth defect in which parts of the pancreas do not join together. The pancreas is a long, flat organ located between the stomach and spine. It helps in food digestion.
Sometimes, a cause for pancreatitis is never found. This is known as idiopathic pancreatitis.
Risk factors for getting pancreatitis
Factors that increase your risk of pancreatitis include:
- Excessive alcohol consumption. Research shows that heavy alcohol users (people who consume four to five drinks a day) are at increased risk of pancreatitis.
- Cigarette smoking. Smokers are on average three times more likely to develop chronic pancreatitis, compared with nonsmokers. The good news is quitting smoking decreases your risk by about half.
- Obesity. You’re more likely to get pancreatitis if you’re obese.
- Diabetes. Having diabetes increases your risk of pancreatitis.
- Family history of pancreatitis. The role of genetics is becoming increasingly recognized in chronic pancreatitis. If you have family members with the condition, your odds increase — especially when combined with other risk factors.
Pancreatitis pathophysiology
The pancreas normally synthesizes and secretes several pancreatic enzymes involved in digestion. These enzymes are produced in pancreatic acinar cells as inactive zymogens and secreted into the duodenum via the pancreatic duct and sphincter of Oddi where they are activated. The activation process begins as enterokinase, an enzyme produced in the duodenal crypts of Lieberkühn, encounters the pancreatic zymogen trypsinogen. Enterokinase binds to trypsinogen and cleaves an acidic propeptide, leaving active trypsin to initiate a cascade of proteolytic reactions. The reactions lead to the activation of other pancreatic zymogens such as chymotrypsinogen, proelastase, prophospholipase, and procarboxypeptidases that are necessary for digestion 55, 56.
If, however, the zymogens are activated prematurely before they exit the pancreas, autodigestion of the peripancreatic tissue and pancreatic parenchyma can occur. To guard against premature activation, the pancreas has several defense mechanisms. The first is an enzyme, the pancreatic secretory trypsin inhibitor, that can bind to and inactivate 20% of trypsin activity. A second defense mechanism is autolysis of prematurely activated trypsin, and a third defense mechanism involves the action of nonspecific proteases such as alpha-1 antitrypsin 57, 58. For pancreatitis to occur, an initial event must overwhelm these defense mechanisms. Several well-known causes exist, with gallstone obstruction and alcohol abuse the two most prevalent causes. Gallstone obstruction of the ampulla of Vater, which is responsible for 35%-40% of acute pancreatitis cases in the United States, is thought to induce pancreatitis via stasis and reflux of bile into the pancreatic duct 59. Prevailing theories posit that the blockage initiates the pancreatic zymogen cascade that then damages surrounding tissue. Cholecystectomy and bile duct clearance resolve the symptoms, confirming the cause-and-effect relationship 60.
Ethanol abuse is the second most common cause of pancreatitis in the United States, responsible for approximately 30% of cases 61. The pathogenic details of ethanol-induced pancreatitis are yet to be confirmed, but several mechanisms have been proposed. The first involves an oversensitization of pancreatic acinar cells to cholecystokinin and premature zymogen activation. The second suggests that ethanol induces acinar cells to overproduce enzymes that are activated prematurely because of buildup and stasis within the pancreas 62.
Other causes involve smoking, scorpion venom, hypertriglyceridemia, endoscopic retrograde cholangiopancreatography (ERCP), hypercalcemia, steroids, malignancy, infection, trauma, and drugs 63, 64, 65, 66, 67, 68.
Regardless of the mechanism underlying an episode of pancreatitis, once activated, the enzymes will begin to digest the cell membranes of the pancreas, thereby activating an inflammatory response. This response increases the vascular permeability of the pancreas 6. Bleeding, edema, ischemia, and necrosis can ensue 6. The severity of acute pancreatitis can vary as it progresses to systemic inflammatory response syndrome, sepsis, and multiple organ failure 69. Approximately 3%-13% of acute pancreatitis cases develop into chronic pancreatitis 70.
Pancreatitis prevention
You can’t prevent pancreatitis, but you can take steps to help you stay healthy.
Maintain a healthy weight or lose weight safely
Maintaining a healthy lifestyle and a healthy weight or losing weight if you’re overweight or obese can help to:
- make your pancreas work better
- lower your chance of getting gallstones, a leading cause of pancreatitis
- prevent obesity, a risk factor for pancreatitis
- prevent diabetes, a risk factor for pancreatitis
Avoid alcohol use
Alcohol use can cause acute and chronic pancreatitis. Talk with your doctor if you need help to stop drinking alcohol.
Avoid smoking
Smoking is a common risk factor for pancreatitis and the chances of getting pancreatitis are even higher in people who smoke and drink alcohol. Talk with your doctor if you need help to stop smoking.
Pancreatitis signs and symptoms
Signs and symptoms of pancreatitis may vary, depending on which type you experience. In general, patients with pancreatitis typically present with abdominal pain that may spread to your back, nausea, and vomiting. However, people with acute pancreatitis or chronic pancreatitis may feel the pain in different ways.
Acute pancreatitis signs and symptoms include:
- Upper abdominal pain
- Abdominal pain that radiates to your back
- Tenderness when touching the abdomen
- Fever
- Rapid pulse
- Nausea
- Vomiting
Chronic pancreatitis signs and symptoms include:
- Upper abdominal pain
- Abdominal pain that feels worse after eating
- Losing weight without trying
- Oily, smelly stools (steatorrhea)
A patient with mild acute pancreatitis may experience only minimal tenderness to abdominal palpation. Nevertheless, the pain is constant, usually located in the epigastrium, and generally described as knifelike and radiating to the midcentral back. Patients are restless and may bend forward, bringing their knees to their chest in an effort to alleviate the pain 71, 72.
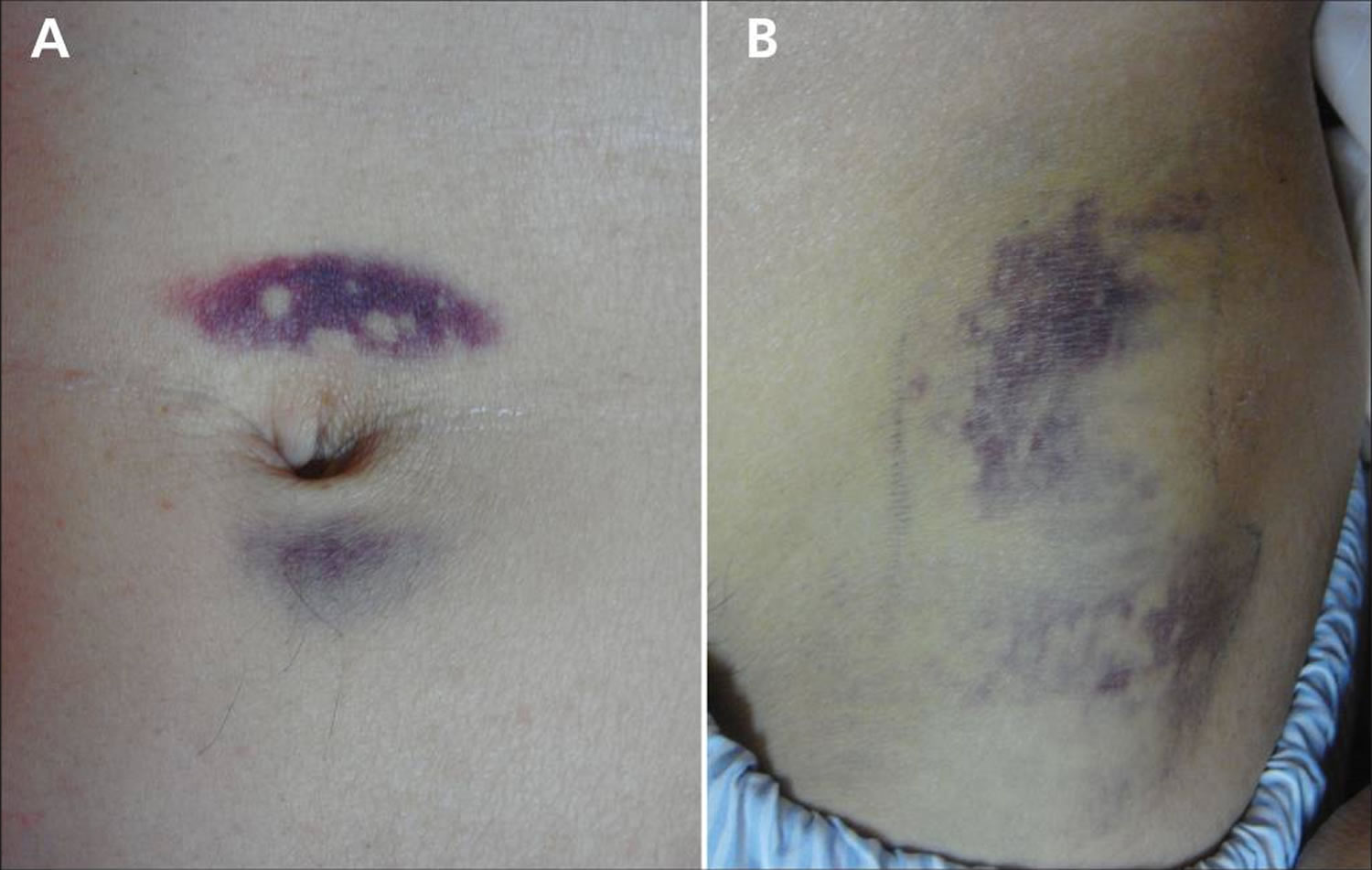
Jaundice (a yellow color of the skin, mucus membranes, or eyes) is a common finding. In 3% of patients with severe acute pancreatitis, flank ecchymosis (Grey Turner sign) or periumbilical ecchymosis (Cullen sign) develops and is suggestive of retroperitoneal hemorrhage. Patients with severe acute pancreatitis can also develop fever, rapid breathing, hypoxemia, and hypotension 73, 74.
Some patients display alterations in mental status. This symptom is more common in drug-induced acute pancreatitis and reflects exposure to drugs or to alcohol but may also result from hypotension, hypoxemia, or the massive release of toxic agents from the inflamed pancreas 66, 75.
Figure 5. Cullen sign
Figure 6. Cullen and Grey Turner sign
Footnote: (A) Periumbilical ecchymosis (Cullen sign) and (B) connecting patches of ecchymosis on the left flank (Grey Turner sign) in a 47-year-old man with sudden onset of left-flank pain.
[Source 77 ]Pancreatitis complications
Both acute and chronic pancreatitis can lead to serious complications that include:
- Kidney failure. Acute pancreatitis may cause kidney failure, which can be treated with dialysis if the kidney failure is severe and persistent.
- Breathing problems. Acute pancreatitis can cause chemical changes in your body that affect your lung function, causing the level of oxygen in your blood to fall to dangerously low levels (hypoxemia).
- Infection. Acute pancreatitis can make your pancreas vulnerable to bacteria and infection. Pancreatic infections are serious and require intensive treatment, such as surgery to remove the infected tissue.
- Narrowing or blockage in a bile or pancreatic duct
- Leakage from the pancreatic duct
- Pancreatic pseudocyst. Acute pancreatitis can cause fluid and debris to collect in cystlike pockets in your pancreas. A large pseudocyst that ruptures can cause complications such as internal bleeding and infection.
- Malnutrition. Both acute and chronic pancreatitis can cause your pancreas to produce fewer of the enzymes that are needed to break down and process nutrients from the food you eat. This can lead to malnutrition, diarrhea and weight loss, even though you may be eating the same foods or the same amount of food.
- Diabetes. Damage to insulin-producing cells in your pancreas from chronic pancreatitis can lead to diabetes, a disease that affects the way your body uses blood sugar.
- Pancreatic cancer. Long-standing inflammation in your pancreas caused by chronic pancreatitis is a risk factor for developing pancreatic cancer.
- Death.
Pancreatitis complications may arise that include local and systemic consequences. Local complications include fluid accumulation, pancreatic pseudocyst, necrotic collection, and walled-off necrosis. The fluids and necrotic tissue can become secondarily infected, leading to systemic inflammatory response syndrome and sepsis. Systemic complications include splanchnic vein thrombosis, abdominal compartment syndrome, pseudoaneurysm, acute respiratory distress syndrome, and exacerbation of underlying comorbidities such as coronary artery disease and chronic lung disease 78, 79, 80.
The majority of severe pancreatitis complications occur within 48 hours of onset. Ranson’s Criteria (Table 2) was published in 1974 as one of the first prognostic tools established to help clinicians identify severity and mortality of patients with acute pancreatitis 81. A Ranson score of 2 or less is associated with a mortality rate of 0-3% 1.
Table 2. Ranson criteria for acute pancreatitis based on initial lab values
| Ranson criteria at admission | |
|---|---|
| White blood cell count > 16,000 cells/mm³ | 1 point |
| Age > 55 years of age | 1 point |
| Blood glucose > 200 mg/dL | 1 point |
| Aspartate transaminase (AST) > 250 IU/L | 1 point |
| Lactate dehydrogease (LDH) > 350 IU/L | 1 point |
| Ranson criteria within 48 hours | |
| Serum calcium <8.0 mg/dL | 1 point |
| Hematocrit drop >10% | 1 point |
| PaO2 <60 mmHg | 1 point |
| Blood urea nitrogen (BUN) > 5 mg/dL | 1 point |
| Base deficit > 4.0 mEq/L | 1 point |
| Fluid sequestration > 6L | 1 point |
Footnote: Ranson Criteria for acute pancreatitis total point indicates a prognosis for the patient as follows:
- 0 to 2 points: Severe pancreatitis is unlikely; Mortality 0% to 3%
- 3 to 4 points: Severe pancreatitis is likely; Mortality 15%
- 5 to 6 points: Severe pancreatitis is likely; Mortality 40%
- 7 to 11 points: Severe pancreatitis is likely; Mortality nearly 100%
Alternatively, the Bedside Index for Severity in Acute Pancreatitis (BISAP) was developed in 2008 and has been shown to be a more accurate prognostic tool that predicts the mortality risk in acute pancreatitis based on the data within the first 24 hour (Table 3) 83. The Bedside Index for Severity in Acute Pancreatitis (BISAP) scoring index is to be used in the first 24 hour of presentation and the variables included in Bedside Index of Severity in Acute Pancreatitis (BISAP) score include 83:
- Blood urea nitrogen (BUN) > 25 mg/dL
- Abnormal mental status (Glasgow coma score <15)
- Evidence of systemic inflammatory response syndrome (SIRS)
- Greater than or equal to 60 years of age
- Presence of pleural effusion
To calculate the BISAP, sum the number of positive variables (0–5). Multiple studies have validated the performance of the BISAP and pooled estimates demonstrate its accuracy in predicting acute pancreatitis severity 84, 85.
Table 3. Bedside Index for Severity in Acute Pancreatitis (BISAP) score
| Bedside Index for Severity in Acute Pancreatitis (BISAP) score for pancreatitis mortality | |
|---|---|
| Blood urea nitrogen > 25 mg/dL | 1 point |
| Impaired mental status (disorientation, lethargy, somnolence, coma, stupor) | 1 point |
≥ 2 Systemic Inflammatory Response Syndrome (SIRS) Criteria
| 1 point |
| Age ≥ 60 years old | 1 point |
| Pleural effusion present on imaging | 1 point |
Footnotes: Bedside Index for Severity in Acute Pancreatitis (BISAP) score of 0 has < 1% and scores ≤ 2 have 1.9% mortality risk, respectively.
[Source 86 ]Pancreatitis diagnosis
To diagnose pancreatitis and find its causes, doctors use:
- Your medical history. Your doctor will ask:
- about your symptoms
- if you have a history of health conditions or concerns that make you more likely to get pancreatitis—including medicines you are taking
- if you have a personal or family medical history of pancreatitis or gallstones
- A physical exam. During a physical exam, your doctor will:
- examine your body
- check your abdomen for pain, swelling, or tenderness
- Lab and imaging tests
Tests and procedures used to diagnose pancreatitis include:
- Blood tests to look for elevated levels of pancreatic enzymes, along with white blood cells, kidney function and liver enzymes
- Abdominal ultrasound to look for gallstones and pancreas inflammation
- Computerized tomography (CT) scan of your abdomen to look for gallstones and assess the extent of pancreas inflammation
- Magnetic resonance imaging (MRI) of your abdomen to look for abnormalities in the gallbladder, pancreas and ducts
- Endoscopic ultrasound to look for inflammation and blockages in the pancreatic duct or bile duct
- Stool tests in chronic pancreatitis to measure levels of fat that could suggest your digestive system isn’t absorbing nutrients adequately
Your doctor may recommend other tests, depending on your particular situation.
Lab tests
Lab tests to help diagnose pancreatitis include the following:
- Blood tests. Your physician may take a blood sample from you and send the sample to a lab to test for:
- high amylase and lipase levels—digestive enzymes made in your pancreas
- high blood glucose, also called blood sugar
- high levels of blood fats, called lipids
- signs of infection or inflammation of the bile ducts, pancreas, gallbladder, or liver
- pancreatic cancer
- Stool tests. Your doctor may test a stool sample to find out if a person has fat malabsorption. At present, measurement of fecal elastase is the most popular test to evaluate pancreatic exocrine insufficiency. Low levels of fecal elastase (<200 µg/g stool, although even lower levels are more specific) or serum trypsin (<20 ng/mL) are usually observed in patients with pancreatic exocrine insufficiency 22.
Imaging tests
Your doctor also use imaging tests to diagnose pancreatitis. A technician performs most tests in an outpatient center, a hospital, or a doctor’s office. You don’t need anesthesia, a medicine to keep you calm, for most of these tests.
- Ultrasound. Ultrasound uses a device called a transducer, which bounces safe, painless sound waves off your organs to create a picture of their structure. Ultrasound can find gallstones.
- Computed tomography (CT) scan. CT scans create pictures of your pancreas, gallbladder, and bile ducts. CT scans can show pancreatitis or pancreatic cancer.
- Magnetic resonance cholangiopancreatography (MRCP). MRCP uses a magnetic resonance imaging (MRI) machine, which creates pictures of your organs and soft tissues without x-rays. Your doctor or a specialist may use MRCP to look at your pancreas, gallbladder, and bile ducts for causes of pancreatitis.
- Endoscopic ultrasound (EUS). Your doctor inserts an endoscope—a thin, flexible tube—down your throat, through your stomach, and into your small intestine. The doctor turns on an ultrasound attachment to create pictures of your pancreas and bile ducts. Your doctor may send you to a gastroenterologist to perform this test.
- Pancreatic Function Test (PFT). Your doctor may use this test to measure how your pancreas responds to secretin, a hormone made by the small intestine. This test is done only at some centers in the United States.
Pancreatitis treatment
Treatment for acute or chronic pancreatitis may include:
- Hospital stay to treat dehydration with intravenous (IV) fluids and, if you can swallow them, fluids by mouth
- Pain medicine, and antibiotics by mouth or through an IV if you have an infection in your pancreas
- A low-fat diet or nutrition by feeding tube or IV if you can’t eat
Your doctor may send you to a gastroenterologist or surgeon for one of the following treatments, depending on the type of pancreatitis that you have.
Acute pancreatitis treatment
Mild acute pancreatitis usually goes away in a few days with rest and treatment.
If your pancreatitis is more severe, your treatments for acute pancreatitis in the hospital may include:
- Early eating. Old data suggested to stop eating for a couple of days in the hospital in order to give your pancreas a chance to recover. This is no longer practiced. Newer data have suggested that eating as soon as you tolerate food helps heal the pancreas. As the inflammation in your pancreas improves and pain symptoms improve, you should begin drinking clear liquids and eating bland foods. With time, you can go back to your normal diet. If your pancreatitis symptoms persist and you still experience pain when eating, your doctor may recommend a feeding tube to help you get nutrition.
- Pain medications. Pancreatitis can cause severe pain. Your health care team will give you medications to help control the pain.
- Intravenous (IV) fluids. As your body devotes energy and fluids to repairing your pancreas, you may become dehydrated. For this reason, you’ll receive extra fluids through a vein in your arm during your hospital stay.
Pancreatitis-related pain in some patients has been shown to respond to pancreatic enzyme replacement 7 and possibly with supplementation of antioxidants such as S-Adenosyl Methionine (SAMe) (800 mg per day), Vitamin C (180 mg per day), Vitamin E (30 mg per day), Vitamin A (2,400 microg per day), and selenium (75 microg per day) 8. The central nervous system and pain processing system have been implicated as a mechanism of pancreatitis-related pain, so the use of tricyclic antidepressants or gabapentin may be helpful 9. Additionally, while nonsteroidal anti-inflammatory drugs (NSAIDs) are preferred over opioid analgesics for pain relief, they may be contraindicated due to the patient’s comorbidities or not tolerated due to side effects. If opioid analgesics are to be prescribed, long-acting formulations are preferred over short or intermediate acting forms 10.
Once your pancreatitis is under control, your doctor will evaluate and treat the underlying cause of your pancreatitis.
Depending on the cause of your acute pancreatitis, treatment may include:
- Procedures to remove bile duct obstructions. Pancreatitis caused by a narrowed or blocked bile duct may require procedures to open or widen the bile duct. A procedure called endoscopic retrograde cholangiopancreatography (ERCP) uses a long tube with a camera on the end to examine your pancreas and bile ducts. The tube is passed down your throat, and the camera sends pictures of your digestive system to a monitor. Anesthesia is used for this procedure. Your gastroenterologist may use ERCP to diagnose problems in the bile duct and pancreatic duct and in removing obstructions, such as gallstones. In some people, however, ERCP can also lead to acute pancreatitis.
- Gallbladder surgery. If gallstones caused your pancreatitis, your doctor will recommend surgery to remove your gallbladder called cholecystectomy. Having surgery to remove the gallbladder within a few days after you are admitted to the hospital lowers the chance of complications. If you have severe pancreatitis, your doctor may advise delaying surgery to first treat complications.
- Pancreas procedures. Endoscopic procedures may be necessary to drain fluid in your abdomen if you have an abscess or infected pseudocyst, or a large pseudocyst causing pain or bleeding. Your doctor may remove damaged tissue from your pancreas.
- Treatment for alcohol dependence. Drinking several drinks a day over many years can cause pancreatitis. If this is the cause of your pancreatitis, your doctor may recommend you enter a treatment program for alcohol addiction. Continuing to drink alcohol would worsen your pancreatitis and lead to serious complications.
- Medication changes. If a medication is deemed to be a cause of acute pancreatitis, your doctor may stop the medication and work with you to find alternative options.
Chronic pancreatitis treatment
Treatment for chronic pancreatitis may help relieve pain, improve how well the pancreas works, and manage complications.
Depending on your situation, your doctor may prescribe or provide the following additional treatments:
- Pain management. Chronic pancreatitis can cause persistent abdominal pain. Your doctor will evaluate you for causes of chronic pancreatitis and may recommend medications to control your pain. If necessary, you may be referred to a pain specialist. Severe pain may be relieved with options such as endoscopic ultrasound or injections to block nerves that send pain signals from the pancreas to the brain.
- Enzymes to improve digestion. In chronic pancreatitis leading to diarrhea or weight loss, pancreatic enzyme supplements can help your body break down and process the nutrients in the foods you eat. Pancreatic enzymes are taken with each meal.
- Changes to your diet. Your doctor may refer you to a dietitian who can help you plan low-fat meals that are high in nutrients.
- Medicines and vitamins. Long-term fat malabsorption may also lead to fat-soluble vitamin (A, D, E, and K) deficiencies 23 as well as deficiencies in calcium, magnesium, zinc, thiamine, and folic acid 24. Your doctor may give you vitamins A, D, E, and K if you have malabsorption. He or she may also give you vitamin B-12 shots if you need them.
- Treatment for diabetes. Chronic pancreatitis may cause diabetes. If you get diabetes, your doctor and health care team will work with you to create an eating plan and a routine of medicine, blood glucose monitoring, and regular checkups.
- Surgery. Your doctor may recommend surgery to relieve pressure or blockage in your pancreatic duct, or to remove a damaged or infected part of your pancreas. Surgery is done in a hospital, where you may have to stay a few days. In patients who do not get better with other treatments, surgeons may perform surgery to remove your whole pancreas, followed by islet auto-transplantation. Islets of Langerhans are groups of cells in your pancreas that make hormones, including insulin. After removing your pancreas, doctors will take islets from your pancreas and transplant them into your liver. The islets will begin to make hormones and release them into your bloodstream.
- Procedures. Your doctor may suggest a nerve block, which is a shot of numbing medicine through your skin and directly into nerves that carry the pain message from your pancreas. If you have stones blocking your pancreatic duct, your doctor may use a procedure to break up and remove the stones.
Pancreatitis treatment at home
Once you have recovered from your pancreatitis and left the hospital, you can take steps to continue your recovery from pancreatitis, such as:
- Stop drinking alcohol. Even if alcohol was not deemed to be the cause of acute pancreatitis, it is prudent to stop drinking alcohol while recovering. If you’re unable to stop drinking alcohol on your own, ask your doctor for help. Your doctor can refer you to local programs to help you stop drinking. Continuing to drink alcohol when you have acute pancreatitis can lead to:
- more episodes of acute pancreatitis
- chronic pancreatitis
- When people with chronic pancreatitis caused by alcohol use continue to drink alcohol, the condition is more likely to lead to severe complications and even death.
- Stop smoking. If you smoke, quit. If you don’t smoke, don’t start. If you can’t quit on your own, ask your doctor for help. Medications and counseling can help you quit smoking. Smoking with acute pancreatitis, especially if it’s caused by alcohol use, greatly raises the chances that your pancreatitis will become chronic. Smoking with pancreatitis also may raise your risk of pancreatic cancer.
- Choose a low-fat diet. Choose a diet that limits fat and emphasizes fresh fruits and vegetables, whole grains, and lean protein.
- Drink more fluids. Pancreatitis can cause dehydration, so drink more fluids throughout the day and limit caffeine. It may help to keep a water bottle or glass of water with you. Health care professionals strongly advise people with pancreatitis not to drink any alcohol, even if your pancreatitis is mild.
Alternative medicine
Alternative therapies can’t treat pancreatitis, but some alternative therapies may help you cope with the pain associated with pancreatitis.
People with chronic pancreatitis may experience constant pain that isn’t easily controlled with medications. Using complementary and alternative medicine therapies along with medications prescribed by your doctor may help you feel more in control of your pain.
Examples of alternative therapies that may help you cope with pain include:
- Meditation
- Relaxation exercises
- Yoga
- Acupuncture
Pancreatitis prognosis
The overall mortality in patients with acute pancreatitis is 10%-15% 87. Patients with biliary pancreatitis tend to have a higher mortality than patients with alcoholic pancreatitis 87. This rate has been falling over the last 2 decades as improvements in supportive care have been initiated. Type 2 diabetes mellitus has also been associated with higher severity and mortality in the setting of acute pancreatitis 88. In patients with severe disease (organ failure), who account for about 20% of presentations, mortality is approximately 30% 89. This figure has not decreased in the past 10 years. In patients with pancreatic necrosis without organ failure, the mortality approaches zero 87.
In the first week of illness, most deaths result from multiorgan system failure 87. In subsequent weeks, infection plays a more significant role, but organ failure still constitutes a major cause of mortality. Acute respiratory distress syndrome (ARDS), acute renal failure, cardiac depression, hemorrhage, and hypotensive shock all may be systemic manifestations of acute pancreatitis in its most severe form.
Identifying patients in the greatest need of aggressive medical treatment by differentiating their disease severity as mild or severe is recommended. In mild disease, the pancreas exhibits interstitial edema, an inflammatory infiltrate without hemorrhage or necrosis, and, usually, minimal or no organ dysfunction. In severe disease, the inflammatory infiltrate is severe, associated with necrosis of the parenchyma, often accompanied by evidence of severe gland dysfunction, and it may be associated with multiorgan system failure.
The International Association of Pancreatology and American Pancreatic Association guidelines states that developing a systemic inflammatory response syndrome (SIRS) at admission and persistent for 48 hours predict severe acute pancreatitis 90. A persistent SIRS is associated with a mortality of 25% when compared with 8% for a transient SIRS 91. However, the sensitivity and specificity for predicting mortality with persistent SIRS are 77-89% and 79-86%, respectively 92. SIRS on day 1 predicted severe disease with high sensitivity (85%-100%). The absence of SIRS on day 1 was associated with a high negative predictive value (98%-100%). Patients with a higher number of systemic inflammatory response (SIR) criteria on day 1 and persistent SIRS had an increased risk for severe disease 93.
Different strategies have been used to assess the severity of acute pancreatitis and predict outcome. Several clinical scoring systems (eg, Ranson criteria, BISAP, Glasgow, Imrie) are available. The APACHE II scoring system, though cumbersome, appears to be the best validated 87 (https://reference.medscape.com/calculator/12/apache-ii). Biological markers have also been used for this purpose. Genetic markers are being studied and have not yet come into clinical use.
Bedside Index for Severity in Acute Pancreatitis (BISAP) is a relatively recent addition (see Table 3 above). The Bedside Index for Severity in Acute Pancreatitis (BISAP) index has good predictive performance for both severe acute pancreatitis and mortality and has been validated prospectively, and it is simple and easy to calculate from initial presentation data. Compared with other scoring systems like Ranson criteria (see Table 2 above) and APACHE II score (https://reference.medscape.com/calculator/12/apache-ii), the BISAP score outperforms in specificity but has a suboptimal sensitivity for mortality 94.
Suppiah et al 95 examined the prognostic value of the neutrophil-lymphocyte ratio (NLR) in 146 consecutive patients with acute pancreatitis. They found that elevation of the neutrophil-lymphocyte ratio during the first 48 hours of hospital admission was significantly associated with severe acute pancreatitis and was an independent negative prognostic indicator 95. The neutrophil-lymphocyte ratio is calculated from the white cell differential and provides an indication of inflammation.
Peritoneal lavage has a high specificity (93%); however, it has a low sensitivity (54%). Dynamic CT scanning of the abdomen is widely available and useful in predicting the outcome of acute pancreatitis. When the Balthazar criteria are used, sensitivity is 87% and specificity is 88%. The modified CT Severity Index (see Table 4) is a sum of scores retrieved from the Balthazar score and those from the evaluation of pancreatic necrosis. It can aid in predicting mortality and detecting any necrosis on CT imaging as a predictor of high mortality 96. Total points are out of ten which helps determine the grade of pancreatitis and aid in treatment; 0 to 2 is mild, 4 to 6 is moderate, and 8 to 10 is severe.
Table 4. CT Severity Index for acute pancreatitis
| Prognostic indicator | Points |
|---|---|
| Pancreatic inflammation | |
| 1. Normal pancreas | 0 |
| 2. Intrinsic pancreatic abnormalities with or without inflammatory changes in peripancreatic fat | 2 |
| 3. Pancreatic or peripancreatic fluid collection and peripancreatic fat necrosis | 4 |
| Pancreatic necrosis | |
| 1. None | 0 |
| 2. Less than or equal to 30% | 2 |
| 3. Greater than 30% | 4 |
| Extrapancreatic complications ( one or more of the following complications, i.e., pleural effusions, abdominal ascites, vascular complications, parenchymal or gastrointestinal tract involvement) | 2 |
Khan et al 97 examined the prognostic value of Modified Early Warning Score (MEWS) in identifying severe acute pancreatitis in 200 patients admitted to a single institution (Table 5). The investigators tracked the highest and mean daily scores. They found that patients with a high Modified Early Warning Score (MEWS) value > 2 on day one or mean value > 1.2 on day two was most accurate in predicting severe acute pancreatitis 97. The investigators concluded that MEWS is a reliable, safe, and inexpensive score that can be used easily at all levels of health care for prognosticating patients with acute pancreatitis.
Table 5. Modified Early Warning Score for acute pancreatitis
[Source 97 ]In a retrospective study of data from 822 patients hospitalized with acute pancreatitis, Mikolasevic et al 98 found that patients who had nonalcoholic fatty liver at admission (n = 198; 24.1%) had a statistically higher incidence of moderately severe (35.4% vs 14.6%) and severe acute pancreatitis (20.7% vs 9.6%) than those without nonalcoholic fatty liver. Moreover, these patients had higher (1) C-reactive protein levels not only on the day of admission but also at day 3, (2) APACHE II scores at admission, (3) CT scan severity index, and (4) occurrence of organ failure and local complications. Although mortality was also higher in the nonalcoholic fatty liver group compared to the group without this disease, the difference was not statistically significant 98.
References- Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016 Nov 17;375(20):1972-1981. doi: 10.1056/NEJMra1505202
- Acute Pancreatitis. https://emedicine.medscape.com/article/181364-overview#a5
- Definition & Facts for Pancreatitis. https://www.niddk.nih.gov/health-information/digestive-diseases/pancreatitis/definition-facts
- Jones MR, Hall OM, Kaye AM, Kaye AD. Drug-induced acute pancreatitis: a review. Ochsner J. 2015 Spring;15(1):45-51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4365846
- Vege SS, Yadav D, Chari ST. Pancreatitis. In: Talley NJ, GR Locke, Saito YA, editors. GI Epidemiology. Malden, MA: Blackwell Publishing;; 2007.
- Kaurich T. Drug-induced acute pancreatitis. Proc (Bayl Univ Med Cent). 2008 Jan;21(1):77-81. doi: 10.1080/08998280.2008.11928366
- Halgreen H, Pedersen NT, Worning H. Symptomatic effect of pancreatic enzyme therapy in patients with chronic pancreatitis. Scand J Gastroenterol. 1986 Jan;21(1):104-8. doi: 10.3109/00365528609034631
- Uomo G, Talamini G, Rabitti PG. Antioxidant treatment in hereditary pancreatitis. A pilot study on three young patients. Dig Liver Dis. 2001 Jan-Feb;33(1):58-62. doi: 10.1016/s1590-8658(01)80136-5
- Frøkjær JB, Olesen SS, Gram M, Yavarian Y, Bouwense SA, Wilder-Smith OH, Drewes AM. Altered brain microstructure assessed by diffusion tensor imaging in patients with chronic pancreatitis. Gut. 2011 Nov;60(11):1554-62. doi: 10.1136/gut.2010.236620
- Patel MR, Eppolito AL, Willingham FF. Hereditary pancreatitis for the endoscopist. Therap Adv Gastroenterol. 2013 Mar;6(2):169-79. doi: 10.1177/1756283X12467565
- Gapp J, Tariq A, Chandra S. Acute Pancreatitis. [Updated 2022 Nov 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482468
- Jin T, Jiang K, Deng L, Guo J, Wu Y, Wang Z, Shi N, Zhang X, Lin Z, Asrani V, Jones P, Mittal A, Phillips A, Sutton R, Huang W, Yang X, Xia Q, Windsor JA. Response and outcome from fluid resuscitation in acute pancreatitis: a prospective cohort study. HPB (Oxford). 2018 Nov;20(11):1082-1091. doi: 10.1016/j.hpb.2018.05.018
- Amas Gómez L, Zubia Olaskoaga F. Results of the modification of an acute pancreatitis management protocol in Intensive Care medicine. Med Intensiva (Engl Ed). 2019 Dec;43(9):546-555. English, Spanish. doi: 10.1016/j.medin.2018.05.004
- Garret C, Péron M, Reignier J, Le Thuaut A, Lascarrou JB, Douane F, Lerhun M, Archambeaud I, Brulé N, Bretonnière C, Zambon O, Nicolet L, Regenet N, Guitton C, Coron E. Risk factors and outcomes of infected pancreatic necrosis: Retrospective cohort of 148 patients admitted to the ICU for acute pancreatitis. United European Gastroenterol J. 2018 Jul;6(6):910-918. doi: 10.1177/2050640618764049
- Ketwaroo GA, Freedman SD, Sheth SG. Approach to patients with suspected chronic pancreatitis: a comprehensive review. Pancreas. 2015 Mar;44(2):173-80. doi: 10.1097/MPA.0000000000000239
- Benjamin O, Lappin SL. Chronic Pancreatitis. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482325
- Sadr-Azodi O, Sanders DS, Murray JA, et al.: Patients with celiac disease have an increased risk for pancreatitis. 2012;10(10):1136–1142.e3. 10.1016/j.cgh.2012.06.023
- Pitchumoni CS, Chari S: Ulcerative colitis and autoimmune pancreatitis. 2013;47(6):469. 10.1097/MCG.0b013e31828a7099
- Gupte AR, Forsmark CE: Chronic pancreatitis. 2014;30(5):500–5. 10.1097/MOG.0000000000000094
- Singhvi A, Yadav D. Myths and realities about alcohol and smoking in chronic pancreatitis. Curr Opin Gastroenterol. 2018 Sep;34(5):355-361. doi: 10.1097/MOG.0000000000000466
- Pham A, Forsmark C. Chronic pancreatitis: review and update of etiology, risk factors, and management. F1000Res. 2018 May 17;7:F1000 Faculty Rev-607. doi: 10.12688/f1000research.12852.1
- Chowdhury, R.S. and Forsmark, C.E. (2003), Pancreatic function testing. Alimentary Pharmacology & Therapeutics, 17: 733-750. https://doi.org/10.1046/j.1365-2036.2003.01495.x
- Witt H, Apte MV, Keim V, et al.: Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. 2007;132(4):1557–73. 10.1053/j.gastro.2007.03.001
- Duggan S, O’Sullivan M, Feehan S, et al.: Nutrition treatment of deficiency and malnutrition in chronic pancreatitis: a review. 2010;25(4):362–70. 10.1177/0884533610373772
- Raphael KL, Willingham FF. Hereditary pancreatitis: current perspectives. Clin Exp Gastroenterol. 2016 Jul 26;9:197-207. doi: 10.2147/CEG.S84358
- Wu D, Bampton TJ, Scott HS, Brown A, Kassahn K, Drogemuller C, De Sousa SM, Moore D, Ha T, Chen JW, Khurana S, Torpy DJ, Radford T, Couper R, Palmer L, Coates PT. The clinical and genetic features of hereditary pancreatitis in South Australia. Med J Aust. 2022 Jun 20;216(11):578-582. doi: 10.5694/mja2.51517
- Rebours V, Lévy P, Ruszniewski P. An overview of hereditary pancreatitis. Dig Liver Dis. 2012 Jan;44(1):8-15. doi: 10.1016/j.dld.2011.08.003
- Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, Mayerle J, Drewes AM, Rebours V, Akisik F, Muñoz JED, Neoptolemos JP. Chronic pancreatitis. Nat Rev Dis Primers. 2017 Sep 7;3:17060. doi: 10.1038/nrdp.2017.60
- Weiss FU. Pancreatic cancer risk in hereditary pancreatitis. Front Physiol. 2014 Feb 20;5:70. doi: 10.3389/fphys.2014.00070
- Lévy P, Domínguez-Muñoz E, Imrie C, Löhr M, Maisonneuve P. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United European Gastroenterol J. 2014 Oct;2(5):345-54. doi: 10.1177/2050640614548208
- Eldredge, J., Couper, M.R., Moore, D.J., Khurana, S., Chen, J.W., Couper, J.J., Drogemuller, C.J., Radford, T., Kay, T.W., Loudovaris, T., Wilks, M., Coates, P.T. and Couper, R.T. (2021), South Australian experience with paediatric total pancreatectomy and islet autotransplantation for PRSS1-associated hereditary pancreatitis. Med J Aust, 215: 294-296.e1. https://doi.org/10.5694/mja2.51247
- Bellin MD, Freeman ML, Gelrud A, Slivka A, Clavel A, Humar A, Schwarzenberg SJ, Lowe ME, Rickels MR, Whitcomb DC, Matthews JB; PancreasFest Recommendation Conference Participants; Amann S, Andersen DK, Anderson MA, Baillie J, Block G, Brand R, Chari S, Cook M, Cote GA, Dunn T, Frulloni L, Greer JB, Hollingsworth MA, Kim KM, Larson A, Lerch MM, Lin T, Muniraj T, Robertson RP, Sclair S, Singh S, Stopczynski R, Toledo FG, Wilcox CM, Windsor J, Yadav D. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology. 2014 Jan-Feb;14(1):27-35. doi: 10.1016/j.pan.2013.10.009
- Hereditary Pancreatitis. https://pancreasfoundation.org/hereditary-pancreatitis
- Comfort MW, Steinberg AG. Pedigree of a family with hereditary chronic relapsing pancreatitis. Gastroenterology. 1952;21(1):54–63.
- Shelton CA, Umapathy C, Stello K, Yadav D, Whitcomb DC. Hereditary Pancreatitis in the United States: Survival and Rates of Pancreatic Cancer. Am J Gastroenterol. 2018 Sep;113(9):1376. doi: 10.1038/s41395-018-0194-5
- Rosendahl J, Bödeker H, Mössner J, Teich N. Hereditary chronic pancreatitis. Orphanet J Rare Dis. 2007 Jan 4;2:1. doi: 10.1186/1750-1172-2-1
- Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996 Oct;14(2):141-5. doi: 10.1038/ng1096-141
- Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, Truninger K, Ammann R, Cavallini G, Charnley RM, Uomo G, Delhaye M, Spicak J, Drumm B, Jansen J, Mountford R, Whitcomb DC, Neoptolemos JP; European Registry of Hereditary Pancreatitis and Pancreatic Cancer (EUROPAC). Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004 Mar;2(3):252-61. doi: 10.1016/s1542-3565(04)00013-8
- Rebours V, Boutron-Ruault MC, Schnee M, Férec C, Le Maréchal C, Hentic O, Maire F, Hammel P, Ruszniewski P, Lévy P. The natural history of hereditary pancreatitis: a national series. Gut. 2009 Jan;58(1):97-103. doi: 10.1136/gut.2008.149179
- Witt H, Beer S, Rosendahl J, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nat Genet. 2013 Oct;45(10):1216-20. doi: 10.1038/ng.2730
- Felderbauer P, Klein W, Bulut K, Ansorge N, Dekomien G, Werner I, Epplen JT, Schmitz F, Schmidt WE. Mutations in the calcium-sensing receptor: a new genetic risk factor for chronic pancreatitis? Scand J Gastroenterol. 2006 Mar;41(3):343-8. doi: 10.1080/00365520510024214
- Whitcomb DC, Preston RA, Aston CE, Sossenheimer MJ, Barua PS, Zhang Y, Wong-Chong A, White GJ, Wood PG, Gates LK Jr, Ulrich C, Martin SP, Post JC, Ehrlich GD. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996 Jun;110(6):1975-80. doi: 10.1053/gast.1996.v110.pm8964426
- Gorry MC, Gabbaizedeh D, Furey W, Gates LK Jr, Preston RA, Aston CE, Zhang Y, Ulrich C, Ehrlich GD, Whitcomb DC. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology. 1997 Oct;113(4):1063-8. doi: 10.1053/gast.1997.v113.pm9322498
- Applebaum-Shapiro SE, Finch R, Pfützer RH, Hepp LA, Gates L, Amann S, Martin S, Ulrich CD 2nd, Whitcomb DC. Hereditary pancreatitis in North America: the Pittsburgh-Midwest Multi-Center Pancreatic Study Group Study. Pancreatology. 2001;1(5):439-43. doi: 10.1159/000055844
- Otsuki M, Nishimori I, Hayakawa T, Hirota M, Ogawa M, Shimosegawa T; Research Committee on Intractable Disease of the Pancreas. Hereditary pancreatitis: clinical characteristics and diagnostic criteria in Japan. Pancreas. 2004 Mar;28(2):200-6. doi: 10.1097/00006676-200403000-00012
- Dytz MG, Mendes de Melo J, de Castro Santos O, da Silva Santos ID, Rodacki M, Conceição FL, Ortiga-Carvalho TM. Hereditary Pancreatitis Associated With the N29T Mutation of the PRSS1 Gene in a Brazilian Family: A Case-Control Study. Medicine (Baltimore). 2015 Sep;94(37):e1508. doi: 10.1097/MD.0000000000001508
- Pho-Iam T, Thongnoppakhun W, Yenchitsomanus PT, Limwongse C. A Thai family with hereditary pancreatitis and increased cancer risk due to a mutation in PRSS1 gene. World J Gastroenterol. 2005 Mar 21;11(11):1634-8. doi: 10.3748/wjg.v11.i11.1634
- Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010 Mar;7(3):131-45. doi: 10.1038/nrgastro.2010.6
- Bampton, T.J., Holmes-Walker, D.J., Drogemuller, C.J., Radford, T., Anderson, P., Etherton, C., Russell, C.H., Khurana, S., Torpy, D.J., Couper, J.J., Couper, R.L.T., Macintyre, P., Neo, E.L., Benitez-Aguirre, P., Thomas, G., Loudovaris, T., Thomas, H.E., Palmer, L.J., Wu, D., Rogers, N.M., Williams, L., Hawthorne, W.J., O’Connell, P.J., Kay, T.W., Pleass, H., Chen, J.W., Coates, P.T. and (2021), Australian experience with total pancreatectomy with islet autotransplantation to treat chronic pancreatitis. ANZ Journal of Surgery, 91: 2663-2668. https://doi.org/10.1111/ans.16853
- Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, DiMagno EP. Cigarette Smoking as a Risk Factor for Pancreatic Cancer in Patients With Hereditary Pancreatitis. JAMA. 2001;286(2):169–170. doi:10.1001/jama.286.2.169
- Rebours V, Boutron-Ruault MC, Jooste V, Bouvier AM, Hammel P, Ruszniewski P, Lévy P. Mortality rate and risk factors in patients with hereditary pancreatitis: uni- and multidimensional analyses. Am J Gastroenterol. 2009 Sep;104(9):2312-7. doi: 10.1038/ajg.2009.363
- Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, DiMagno EP. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001 Jul 11;286(2):169-70. doi: 10.1001/jama.286.2.169
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol. 2011 Dec;106(12):2192-9. doi: 10.1038/ajg.2011.328. Epub 2011 Sep 27. Erratum in: Am J Gastroenterol. 2011 Dec;106(12):2209.
- Bang UC, Benfield T, Hyldstrup L, Bendtsen F, Beck Jensen JE. Mortality, cancer, and comorbidities associated with chronic pancreatitis: a Danish nationwide matched-cohort study. Gastroenterology. 2014 Apr;146(4):989-94. doi: 10.1053/j.gastro.2013.12.033
- Kitamoto Y, Yuan X, Wu Q, McCourt DW, Sadler JE. Enterokinase, the initiator of intestinal digestion, is a mosaic protease composed of a distinctive assortment of domains. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7588-92. doi: 10.1073/pnas.91.16.7588
- Yamashina I. The action of enterokinase on trypsinogen. Biochim Biophys Acta. 1956 May;20(2):433–434. doi: 10.1016/0006-3002(56)90329-8
- Aoun E, Chang CC, Greer JB, Papachristou GI, Barmada MM, Whitcomb DC. Pathways to injury in chronic pancreatitis: decoding the role of the high-risk SPINK1 N34S haplotype using meta-analysis. PLoS One. 2008 Apr 16;3(4):e2003. doi: 10.1371/journal.pone.0002003
- Rowntree, R.K. and Harris, A. (2003), The Phenotypic Consequences of CFTR Mutations. Annals of Human Genetics, 67: 471-485. https://doi.org/10.1046/j.1469-1809.2003.00028.x
- Forsmark CE, Baillie J; AGA Institute Clinical Practice and Economics Committee; AGA Institute Governing Board. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007 May;132(5):2022-44. doi: 10.1053/j.gastro.2007.03.065
- Moreau JA, Zinsmeister AR, Melton LJ 3rd, DiMagno EP. Gallstone pancreatitis and the effect of cholecystectomy: a population-based cohort study. Mayo Clin Proc. 1988 May;63(5):466-73. doi: 10.1016/s0025-6196(12)65644-4
- Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008 Mar 24;168(6):649-56. doi: 10.1001/archinte.168.6.649
- Apte MV, Wilson JS, McCaughan GW, Korsten MA, Haber PS, Norton ID, Pirola RC. Ethanol-induced alterations in messenger RNA levels correlate with glandular content of pancreatic enzymes. J Lab Clin Med. 1995 May;125(5):634-40.
- Toskes PP. Hyperlipidemic pancreatitis. Gastroenterol Clin North Am. 1990 Dec;19(4):783-91.
- Mithöfer K, Fernández-del Castillo C, Frick TW, Lewandrowski KB, Rattner DW, Warshaw AL. Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology. 1995 Jul;109(1):239-46. doi: 10.1016/0016-5085(95)90290-2
- Wilmink T, Frick TW. Drug-induced pancreatitis. Drug Saf. 1996 Jun;14(6):406-23. doi: 10.2165/00002018-199614060-00006
- Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007 Jun;5(6):648-61; quiz 644. doi: 10.1016/j.cgh.2006.11.023
- Sadr-Azodi O, Mattsson F, Bexlius TS, Lindblad M, Lagergren J, Ljung R. Association of oral glucocorticoid use with an increased risk of acute pancreatitis: a population-based nested case-control study. JAMA Intern Med. 2013 Mar 25;173(6):444-9. doi: 10.1001/jamainternmed.2013.2737
- Aliperti G. Complications related to diagnostic and therapeutic endoscopic retrograde cholangiopancreatography. Gastrointest Endosc Clin N Am. 1996 Apr;6(2):379-407.
- Neoptolemos JP, Raraty M, Finch M, Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut. 1998 Jun;42(6):886-91. doi: 10.1136/gut.42.6.886
- Sekimoto M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Hirota M, Kimura Y, Takeda K, Isaji S, Koizumi M, Otsuki M, Matsuno S; JPN. JPN Guidelines for the management of acute pancreatitis: epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13(1):10-24. doi: 10.1007/s00534-005-1047-3
- Silen W. Acute pancreatitis. In: Silen W, Cope Z, editors. Cope’s Early Diagnosis of the Acute Abdomen. 18th ed. New York, NY: Oxford University Press; 1999. pp. 123–131.
- Mitchell RM, Byrne MF, Baillie J. Pancreatitis. Lancet. 2003 Apr 26;361(9367):1447-55. doi: 10.1016/s0140-6736(03)13139-x
- Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004 Jun 16;291(23):2865-8. doi: 10.1001/jama.291.23.2865
- Dickson AP, Imrie CW. The incidence and prognosis of body wall ecchymosis in acute pancreatitis. Surg Gynecol Obstet. 1984 Oct;159(4):343-7.
- McARTHUR, K.E. (1996), Review article: Drug-induced pancreatitis. Alimentary Pharmacology & Therapeutics, 10: 23-38. https://doi.org/10.1111/j.1365-2036.1996.tb00174.x
- Parikh RP, Upadhyay KJ. Cullen’s sign for acute haemorrhagic pancreatitis. Indian J Med Res. 2013;137(6):1210. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3734730
- Chung KM, Chuang SS. Cullen and Grey Turner signs in idiopathic perirenal hemorrhage. CMAJ. 2011;183(16):E1221. doi:10.1503/cmaj.101548 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3216450
- Nadkarni NA, Khanna S, Vege SS. Splanchnic venous thrombosis and pancreatitis. Pancreas. 2013 Aug;42(6):924-31. doi: 10.1097/MPA.0b013e318287cd3d
- Morgan DE, Baron TH, Smith JK, Robbin ML, Kenney PJ. Pancreatic fluid collections prior to intervention: evaluation with MR imaging compared with CT and US. Radiology. 1997 Jun;203(3):773-8. doi: 10.1148/radiology.203.3.9169703
- Ranson JH, Turner JW, Roses DF, Rifkind KM, Spencer FC. Respiratory complications in acute pancreatitis. Ann Surg. 1974 May;179(5):557-66. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1356020/pdf/annsurg00243-0045.pdf
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974 Jul;139(1):69-81.
- Ducarme G, Maire F, Chatel P, Luton D, Hammel P. Acute pancreatitis during pregnancy: a review. J Perinatol. 2014 Feb;34(2):87-94. doi: 10.1038/jp.2013.161
- Papachristou GI, Muddana V, Yadav D, O’Connell M, Sanders MK, Slivka A, Whitcomb DC. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010 Feb;105(2):435-41; quiz 442. doi: 10.1038/ajg.2009.622
- Chandra S, Murali A, Bansal R, Agarwal D, Holm A. The Bedside Index for Severity in Acute Pancreatitis: a systematic review of prospective studies to determine predictive performance. J Community Hosp Intern Med Perspect. 2017 Sep 19;7(4):208-213. doi: 10.1080/20009666.2017.1361292
- Pérez Campos A, Bravo Paredes E, Prochazka Zarate R, Bussalleu A, Pinto Valdivia J, Valenzuela Granados V. BISAP-O y APACHE-O: utilidad en la predicción de severidad en la pancreatitis aguda según la clasificación modificada de Atlanta [BISAP-O y APACHE-O: utility in predicting severity in acute pancreatitis in modified Atlanta classification]. Rev Gastroenterol Peru. 2015 Jan;35(1):15-24. Spanish.
- Kothari S, Kalinowski M, Kobeszko M, Almouradi T. Computed tomography scan imaging in diagnosing acute uncomplicated pancreatitis: Usefulness vs cost. World J Gastroenterol. 2019 Mar 7;25(9):1080-1087. doi: 10.3748/wjg.v25.i9.1080
- Acute Pancreatitis. https://emedicine.medscape.com/article/181364-overview#a6
- Huh JH, Jeon H, Park SM, Choi E, Lee GS, Kim JW, Lee KJ. Diabetes Mellitus is Associated With Mortality in Acute Pancreatitis. J Clin Gastroenterol. 2018 Feb;52(2):178-183. doi: 10.1097/MCG.0000000000000783
- Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006 May 18;354(20):2142-50. doi: 10.1056/NEJMcp054958
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15. doi: 10.1016/j.pan.2013.07.063
- R Mofidi, M D Duff, S J Wigmore, K K Madhavan, O J Garden, R W Parks, Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis, British Journal of Surgery, Volume 93, Issue 6, June 2006, Pages 738–744, https://doi.org/10.1002/bjs.5290
- A Buter, C W Imrie, C R Carter, S Evans, C J McKay, Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis, British Journal of Surgery, Volume 89, Issue 3, March 2002, Pages 298–302, https://doi.org/10.1046/j.0007-1323.2001.02025.x
- Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Mortele KJ, Banks PA. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol. 2009 Nov;7(11):1247-51. doi: 10.1016/j.cgh.2009.08.012
- Gao W, Yang HX, Ma CE. The Value of BISAP Score for Predicting Mortality and Severity in Acute Pancreatitis: A Systematic Review and Meta-Analysis. PLoS One. 2015 Jun 19;10(6):e0130412. doi: 10.1371/journal.pone.0130412. Erratum in: PLoS One. 2015;10(10):e0142025
- Suppiah A, Malde D, Arab T, Hamed M, Allgar V, Smith AM, Morris-Stiff G. The prognostic value of the neutrophil-lymphocyte ratio (NLR) in acute pancreatitis: identification of an optimal NLR. J Gastrointest Surg. 2013 Apr;17(4):675-81. doi: 10.1007/s11605-012-2121-1
- Mortele KJ, Wiesner W, Intriere L, Shankar S, Zou KH, Kalantari BN, Perez A, vanSonnenberg E, Ros PR, Banks PA, Silverman SG. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol. 2004 Nov;183(5):1261-5. doi: 10.2214/ajr.183.5.1831261
- Khan A, Sarma D, Gowda C, Rodrigues G. The Role of Modified Early Warning Score (MEWS) in the Prognosis of Acute Pancreatitis. Oman Med J. 2021 May 31;36(3):e272. doi: 10.5001/omj.2021.72
- Mikolasevic I, Orlic L, Poropat G, Jakopcic I, Stimac D, Klanac A, Carovic F, Milic S. Nonalcoholic fatty liver and the severity of acute pancreatitis. Eur J Intern Med. 2017 Mar;38:73-78. doi: 10.1016/j.ejim.2016.10.019