Chronic pancreatitis
Chronic pancreatitis is a long-lasting condition and slowly destroys the functions of the pancreas. Chronic pancreatitis represents the end result of a continuous, prolonged, inflammatory, and scarring process that affects the pancreas. The pancreas does not heal or improve. Instead, it gets worse over time, which can lead to permanent damage to your pancreas endocrine and exocrine tissues and in some cases, development of pancreatic cancer 1, 2. For example, the pancreas may lose its ability to produce insulin and glucagon and digestive enzymes (pancreatic digestive juices). As a result, you can develop glucose intolerance or diabetes. Chronic pancreatitis can also cause weight loss because your body may be unable to digest fat and key elements of food. Chronic pancreatitis different from acute pancreatitis, where the pancreatic inflammation is only short term. Most people with chronic pancreatitis have had 1 or more attacks of acute pancreatitis. Chronic pancreatitis is less common than acute pancreatitis, with about 86,000 hospital stays per year 3. In the US, chronic pancreatitis affects African Americans more frequently than caucasians 4. In addition, chronic pancreatitis due to alcohol is more common in males, whereas that due to hyperlipidemia is more common in females 4. The median age at diagnosis of chronic pancreatitis is 45.
The most common cause of chronic pancreatitis in adults is excessive alcohol consumption in developed countries 5, 6, 7, 8, 9, 10. Chronic pancreatitis was formerly thought that malnutrition in developing countries was a cause of chronic pancreatitis, but this myth has since been dispelled 11. Cigarette smoking has been reported to be an independent risk factor for developing chronic pancreatitis 12 and increases its rate of progression 13, 14.
The major risk factors for the development of chronic pancreatitis may be categorized according to the TIGAR-O system 15:
- T: Toxic-metabolic (e.g. alcohol)
- I: Idiopathic, recent guidelines recommend that cystic fibrosis needs to be ruled out in these patients before calling it idiopathic 16
- G: Genetic more commonly seen in children 17
- A: Autoimmune pancreatitis
- R: Recurrent pancreatitis. Repeated episodes of acute pancreatitis can lead to chronic pancreatitis.
- O: Obstructive (e.g. choledocholithiasis, pancreatic head tumor)
Complications of chronic pancreatitis include:
- chronic pain in your abdomen
- maldigestion, when you can’t digest food properly
- malnutrition and malabsorption
- problems with how well your pancreas works
- scars in your pancreas
- diabetes
- pancreatic cancer, which is more likely in people with both diabetes and pancreatitis
- osteopenia, osteoporosis and bone fractures
Diagnosing chronic pancreatitis relies on changes in diagnostic imaging and blood work in addition to clinical symptoms. The histologic hallmarks of chronic pancreatitis include scar tissue (fibrosis), chronic inflammation, and loss of acinar cells 2. Because histologic diagnosis is rarely pursued given the potential for complications, there is a need for a noninvasive biomarker of pancreatic fibrosis. As defined by the International Study Group of Pediatric Pancreatitis: In Search for a Cure (INSPPIRE) consortium 18, chronic pancreatitis requires imaging findings characteristic of and consistent with chronic pancreatitis (specifically, radiographically evident calcifications, and pancreatic duct irregularities, such as strictures and dilations) along with 1 of the following 3: abdominal pain consistent with pancreatic origin, evidence of exocrine pancreatic insufficiency, or evidence of endocrine pancreatic insufficiency, or a pancreatic biopsy specimen demonstrating histological evidence of chronic pancreatitis.
In chronic pancreatitis, the pancreas is permanently damaged, but treatment can help control the condition and reduce any symptoms. People with chronic pancreatitis are usually advised to make lifestyle changes, such as stopping drinking alcohol and stopping smoking. They’re also given medicine to relieve pain. Surgery may also be an option for those experiencing severe pain.
People with severe pain or who are losing weight may need to stay in the hospital for:
- Pain medicines.
- Fluids given through a vein (IV).
- Stopping food or fluid by mouth to limit the activity of the pancreas, and then slowly starting an oral diet.
- Inserting a tube through the nose or mouth to remove the contents of the stomach (nasogastric suctioning) may sometimes be done. The tube may stay in for 1 to 2 days, or sometimes for 1 to 2 weeks.
The right diet is important for people with chronic pancreatitis to keep a healthy weight and get the correct nutrients. A nutritionist can help you create a diet that includes:
- Drinking plenty of liquids
- Limiting fats
- Eating small, frequent meals (this helps reduce digestive symptoms)
- Getting enough vitamins and calcium in the diet, or as extra supplements
- Limiting caffeine
Your doctor may prescribe pancreatic enzymes. You must take these medicines with every meal, and even with snacks. The enzymes will help you digest food better, gain weight and reduce diarrhea.
Avoid smoking and drinking alcoholic beverages, even if your pancreatitis is mild.
Other treatments may involve:
- Pain medicines or a surgical nerve block to relieve pain
- Taking insulin to control blood sugar (glucose) level
- Steroid medicine is recommended for people with chronic pancreatitis caused by problems with the immune system because it helps to relieve the inflammation of the pancreas.
Surgery may be performed if a blockage is found. In severe cases, a part of or the entire pancreas may be removed.
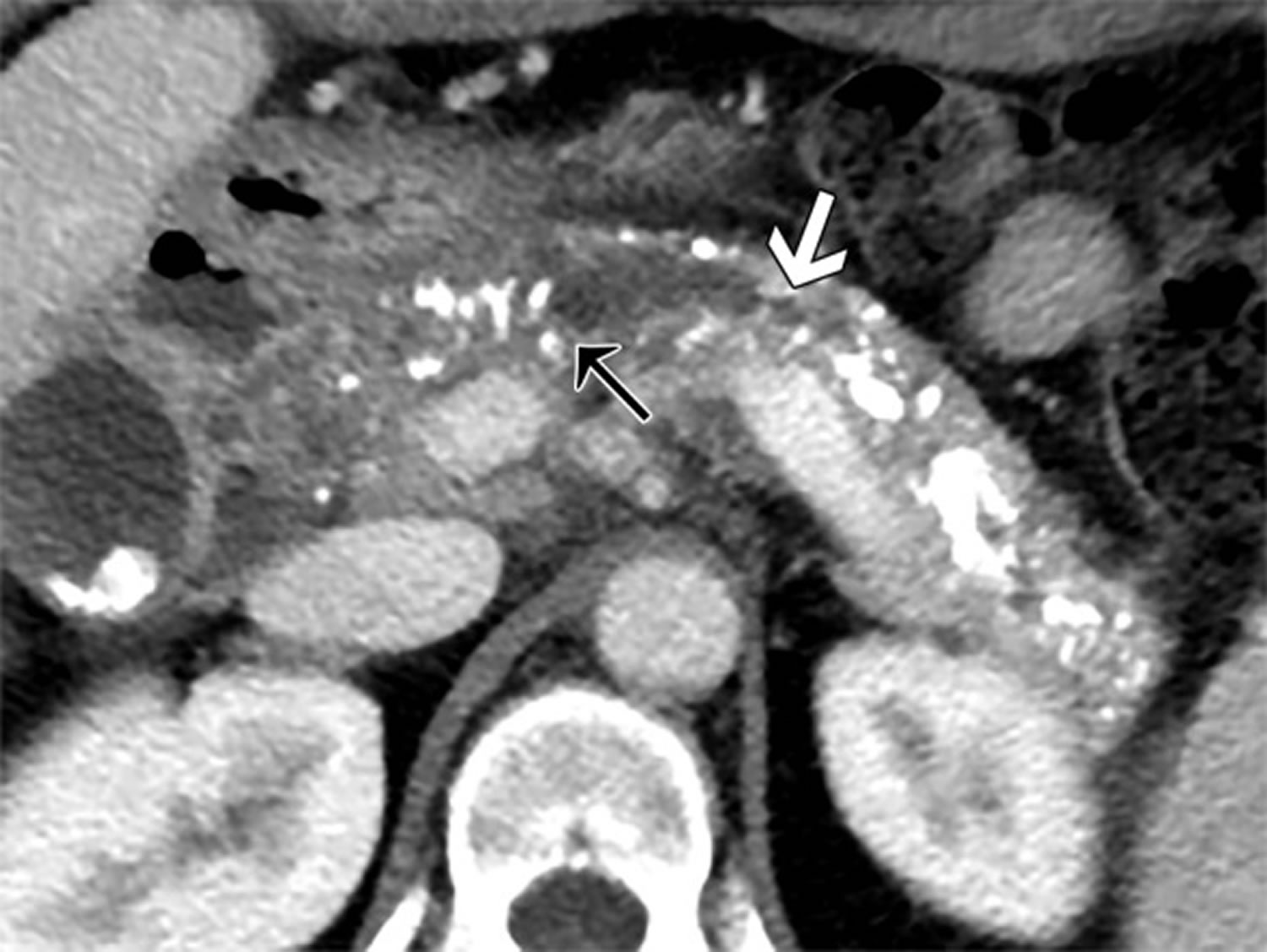
Figure 1. Chronic pancreatitis (contrast material–enhanced CT image of chronic pancreatitis)
Footnote: Images in a 49-year-old patient with history of alcohol abuse, chronic pancreatitis and cirrhosis. (a) Oblique axial contrast material–enhanced CT image shows moderately to markedly irregular pancreatic duct contour (white arrow) and stricture in pancreatic neck causing upstream ductal dilatation (black arrow). Distribution of findings is greater than 70% because there are features of chronic pancreatitis involving entire pancreas. Relatively less enhancement of parenchyma is shown in region of head compared with body and tail. Delayed enhancement pattern is nonspecific; however, it can be seen with fibrosis. (b) Oblique coronal contrast-enhanced CT image shows head, neck, and body of pancreas with more than seven coarse calcifications (arrow). Largest pancreatic duct caliber is measured as shown with white line. (c) Axial contrast-enhanced CT image shows measurement of pancreatic thickness in patient with pancreatic duct dilatation, measured perpendicular to longitudinal axis of parenchyma at level of lateral margin of adjacent vertebral body (VB) or upstream to pancreatic duct calculus or stricture. Splenic vein (SV) and artery in measurement should be avoided.
[Source 2 ]See your doctor right away for the following symptoms of severe pancreatitis:
- pain or tenderness in the abdomen that is severe or becomes worse
- nausea and vomiting
- fever or chills
- fast heartbeat
- shortness of breath
- yellowish color of the skin or whites of the eyes, called jaundice
These symptoms may be a sign of:
- serious infection
- inflammation
- blockage of the pancreas, gallbladder, or a bile and pancreatic duct
Left untreated, these problems can be fatal.
What is acute pancreatitis?
Acute pancreatitis occurs suddenly and is a short-term condition, meaning it appears suddenly and lasts for days 19, 20. Most people with acute pancreatitis get better, and it goes away in several days with treatment. But some people can have a more severe form of acute pancreatitis and go on to develop serious complications, which requires a lengthy hospital stay. Acute pancreatitis is different to chronic pancreatitis, where the pancreas has become permanently damaged from inflammation over many years. Acute pancreatitis affects men more often than women. Acute pancreatitis is becoming more common, for reasons that are not clear 21. Acute pancreatitis is the cause of up to 275,000 hospitalizations in the United States per year, incurring nearly $2.6 billion in hospitalization costs 22, 23, 24. The incidence of acute pancreatitis in children is at 10–15 cases per 100,000 children 25. While mild acute pancreatitis carries a mortality of <1%, mortality rates for severe pancreatitis can reach as high as 30% 26. Drugs are responsible for 0.1%-2% of acute pancreatitis incidents. The majority of drug-induced pancreatitis cases are mild to moderate in severity; however, severe and even fatal cases can occur. Although pancreatitis is rare in children, the number of children with acute pancreatitis has grown.
Repeat episodes of acute pancreatitis may lead to chronic pancreatitis. Other complications of acute pancreatitis include:
- dehydration
- bleeding
- infection
Most cases of pancreatitis are caused by overuse of alcohol 27, 28. Alcohol use is responsible for up to 70% of cases of acute pancreatitis in the United States. About 5 to 8 drinks per day for 5 or more years can damage the pancreas 29. Gallstones are the next most common cause of acute pancreatitis 30, 31. When the gallstones travel out of the gallbladder into the bile ducts, they block the opening that drains bile and enzymes. The bile and enzymes “back up” into the pancreas and cause swelling. Besides overuse of alcohol and gallstones, other causes of pancreatitis include:
- Heredity — Hereditary pancreatitis is a rare genetic disorder that predisposes a person to develop the disease, usually before age 20 32.
- Genetic causes — Mutations of the cystic fibrosis gene is the most widely recognized genetic cause.
- Blockage of the duct that drains digestive enzymes from the pancreas — If the enzymes don’t drain properly, they can back up and damage the pancreas. Blockage can be caused by gallstones, scarring from prior surgery, tumors, pancreatic cancer or abnormalities of the pancreas or of the shape or location of the pancreatic duct. If the blockage is found early, surgery or a procedure called endoscopic retrograde cholangiopancreatography (ERCP) to relieve the blockage may help to prevent damage to the pancreas. Endoscopic retrograde cholangiopancreatography (ERCP), a procedure used to treat gallstones, also can lead to pancreatitis.
- Autoimmune pancreatitis — For unexplained reasons, some people develop antibodies that attack their own pancreas.
- Very high blood triglyceride levels (hypertriglyceridemia) – most often above 1,000 mg/dL 33, 34, 35, 36.
- Damage to the ducts or pancreas during surgery
- After certain procedures used to diagnose gallbladder and pancreas problems (endoscopic retrograde cholangiopancreatography, ERCP) or ultrasound guided biopsy 37, 38
- Injury to the pancreas from an accident
- Overactive parathyroid gland (hyperparathyroidism)
- Reye syndrome
- Use of certain medicines (especially estrogens, corticosteroids, sulfonamides, thiazides, and azathioprine) 39, 40
- Certain infections, such as viral infections (e.g. mumps, cytomegalovirus, coxsackie B virus) that involve the pancreas
- Smoking. Although heavy smokers tended to be heavy drinkers, smoking itself was a significant risk factor for acute pancreatitis, recurrent acute pancreatitis and chronic pancreatitis 41, 42
Sometimes, a cause for pancreatitis is never found. This is known as idiopathic pancreatitis.
The most common symptoms of acute pancreatitis include 43:
- suddenly getting severe pain in the center of your abdomen (belly) that radiates to your back
- feeling or being sick (nausea and vomiting)
- a high temperature of 38°C (100.4°F) or more (fever)
Acute pancreatitis is usually diagnosed in hospital, where you’ll receive treatment and be monitored for any complications. A doctor will ask you about your symptoms, family history and may feel your abdomen – it will be very tender if you have acute pancreatitis. They’ll also do a blood test, and sometimes a CT scan, to help confirm the diagnosis.
At first, it can be difficult to tell whether your acute pancreatitis is mild or severe. You’ll be monitored closely for signs of serious problems, such as organ failure.
You may have further tests to help determine the severity of your condition and assess your risk of developing more serious complications. These may include:
- a CT scan of your abdomen – where a series of X-rays are taken to build up a more detailed image of your pancreas
- a MRI scan of your abdomen – where strong magnetic fields and radio waves are used to produce a detailed image of the inside of your body
- an ultrasound scan of your abdomen – where sound waves are used to create a picture of your gallbladder to detect gallstones, and a picture of your pancreas
A diagnosis of acute pancreatitis requires 2 out of 3 criteria 44, 45:
- Abdominal pain consistent with pancreatitis,
- A serum amylase or lipase three or more times the upper limit of normal, and
- Findings consistent with pancreatitis on cross-sectional abdominal imaging [in adults: computed tomography (CT) or magnetic resonance imaging (MRI); in children CT, MRI or in some cases transabdominal ultrasound (TUS)].
Acute pancreatitis is treated in hospital, where you’ll be closely monitored for signs of serious problems and given supportive treatment, such as fluids, painkillers (analgesics), oxygen and nutrition 46. Treatment for acute pancreatitis aims to help control the condition and manage any symptoms you may have. You may be given fluids directly into a vein (intravenous fluids), pain relief, liquid food through a tube in your abdomen and oxygen through tubes in your nose. A nil-by-mouth regimen to rest the gut, routine use of prophylactic antibiotics, and avoidance of early opiate analgesia have been invalidated in randomised trials, and do not feature in international guidelines 47, 48, 45.
Most people with mild acute pancreatitis get better within a week and are well enough to leave hospital after a few days.
Recovery can take longer in severe cases, as some people can develop complications.
Those with severe acute pancreatitis can develop complications that require further treatment and may need to be admitted to a high-dependency unit or intensive care unit (ICU). Recovery may take much longer from severe acute pancreatitis, and there’s a risk it could be fatal 49.
What is hereditary pancreatitis?
Hereditary pancreatitis is a rare genetic condition characterized by multiple recurrent episodes of inflammation of the pancreas (pancreatitis), which can progress to chronic pancreatitis. Hereditary pancreatitis is a rare cause of acute, recurrent acute, and chronic pancreatitis 50. Hereditary pancreatitis symptoms include recurring bouts of severe abdominal pain, nausea, and vomiting, that usually require opiate analgesia for pain relief 51. Onset of symptoms typically occurs before the age of ten years, but can begin at any age 52, 50, 53. In the United States, it is estimated that at least 1,000 individuals are affected with hereditary pancreatitis. People with hereditary pancreatitis develop chronic pancreatitis, a constantly inflamed pancreas. This leads to symptoms which may include fatty stools, weight loss, and poor absorption of nutrients from food.
Adults with hereditary pancreatitis are at an increased risk for type 1 diabetes, pancreatic exocrine failure and pancreatic cancer (adenocarcinoma of the pancreas) 54, 55, 56, 57. Pancreatic cancer is the 4th most leading cause of cancer deaths among Americans. Individuals with hereditary pancreatitis appear to have a 40% lifetime risk of developing pancreatic cancer 58. This increased risk is heavily dependent upon the duration of chronic pancreatitis and environmental exposures to alcohol and smoking. One recent study suggested that individuals with chronic pancreatitis for more than 25 years had a higher rate of pancreatic cancer when compared to individuals in the general population 58. This increased pancreatic cancer rate appears to be due to the prolonged chronic pancreatitis rather than having a gene mutation (all cationic trypsinogen mutations). It is important to note that these risk values may be higher than expected because these studies on pancreatic cancer use a highly selective population rather than a randomly selected population.
Hereditary pancreatitis was first recognized by Comfort and Steinberg as a clinical syndrome in 1952 59 and mutations in the cationic trypsinogen (serine protease 1) gene (PRSS1 gene) on chromosome 7q were identified as major etiological factors in 1996 60, 54, 61, 62. More than twenty pathogenic PRSS1 gene mutations have since been described, accounting for 80% of all cases of hereditary pancreatitis in people of European ancestry 60, 54, 61, 62. The two most commonly identified pathogenic PRSS1 mutations are p.R122H and p.N29I (90%), while less common mutations include A16V, R122C, N29T, D22G, and K23R 63, 64. Other implicated genes include those that encode serine protease inhibitor Kazal‐type 1 (SPINK1, on chromosome 5q32), cystic fibrosis transmembrane conductance regulator (CFTR), chymotrypsin C (CTRC), and A‐type carboxypeptidase (CPA1) 54, 61, 65. Modifier genes, such as the calcium‐sensing receptor gene (CASR), also influence the risk of hereditary pancreatitis 66.
In most cases, hereditary pancreatitis is due to a PRSS1 gene that is not working correctly and is inherited in an autosomal dominant pattern 62, 67, 68. Internationally, the majority of PRSS1 mutations have been identified in the USA 69 and Europe 63, though few have also been reported from Japan 70, South America 71 and Thailand 72.To date, hereditary pancreatitis has not been reported in patients of African ancestry 60.
In hereditary pancreatitis, inflammation is initially restricted to the acinar and ductal cells, leading to pancreatic exocrine insufficiency; the pancreatic islets are affected only late in the disease process 52 and patients require pancreatic enzyme replacement long before diabetes develops 73, 74. As many as 60% of people with symptomatic pancreatitis will ultimately require surgical interventions (including endoscopic procedures) because of severe abdominal pain, with unavoidable loss of pancreatic function 57, 74.
Hereditary pancreatitis diagnosis is based on the symptoms, a clinical history and exam, and the results of genetic testing. Criteria for genetic testing for hereditary pancreatitis, when patients meet one or more of the following criteria 75:
- A family history of idiopathic chronic pancreatitis, recurrent acute pancreatitis, or childhood pancreatitis
- Relatives with known mutations associated with hereditary pancreatitis
- Unexplained pancreatitis in a child
- Idiopathic chronic pancreatitis in patients <25 years old
- Recurrent acute pancreatitis of uncertain etiology
- Patients who are eligible for enrollment in approved research trials
At this time, there is no cure for hereditary pancreatitis. Patients with hereditary pancreatitis should be managed in the same way as patients who present with pancreatitis from other causes 50. Treatment is focused on managing the symptoms and may include medications, surgery, and surveillance for diabetes and cancer. Patients may be prescribed pancreatic enzyme supplements to treat maldigestion, insulin to treat diabetes, analgesics and narcotics to control pain, and lifestyle changes to reduce the risk of pancreatic cancer (for example, no smoking and no drinking alcohol).
Dietary recommendations to help control pain with digestion include the consumption of small meals throughout the day that are high in carbohydrates and low in protein and fat. Pancreatic enzymes such as Creon, Pancrease, and Violiase are helpful in providing improved digestion and a reduction in diarrhea and pain for some patients with more advanced disease.
Exposure to smoking and alcohol are known to dramatically increase the risk for pancreatic attacks among individuals with hereditary pancreatitis. Smoking is strongly discouraged as it doubles the risk for pancreatic cancer 76. Similarly, alcohol consumption is not recommended for these patients as no safe amount of alcohol use has been documented, because alcohol is a known risk factor for both acute and chronic pancreatitis. Therefore it is recommended that all hereditary pancreatitis patients avoid smoking and alcohol consumption. Though harder to control, dietary fat and emotional stress are considered triggers and should also be avoided 64. Finally, the risk and benefit of the use of medications that have been associated with drug-induced pancreatitis (eg, ACE inhibitors, HMG co-A reductase inhibitors, and selective serotonin reuptake inhibitors) should be individualized to the patient 23.
Overall survival in hereditary pancreatitis PRSS1 carriers appears to be similar to the general population 60, 77. However, if a patient with hereditary pancreatitis develops pancreatic cancer, it usually presents 20 years earlier than in the general population and thus results in an earlier and increased mortality 78. Cancer was the most frequent cause of death in PRSS1 carriers (32.4%), of which less than half (42%) were from pancreatic cancer 79, 80. Interestingly, non-malignant pancreatic disease was mentioned on death reports as the second most common cause (21.6%), followed by cardiovascular disease (13.5%), which was different than the only other report on hereditary pancreatitis causes of death 77.
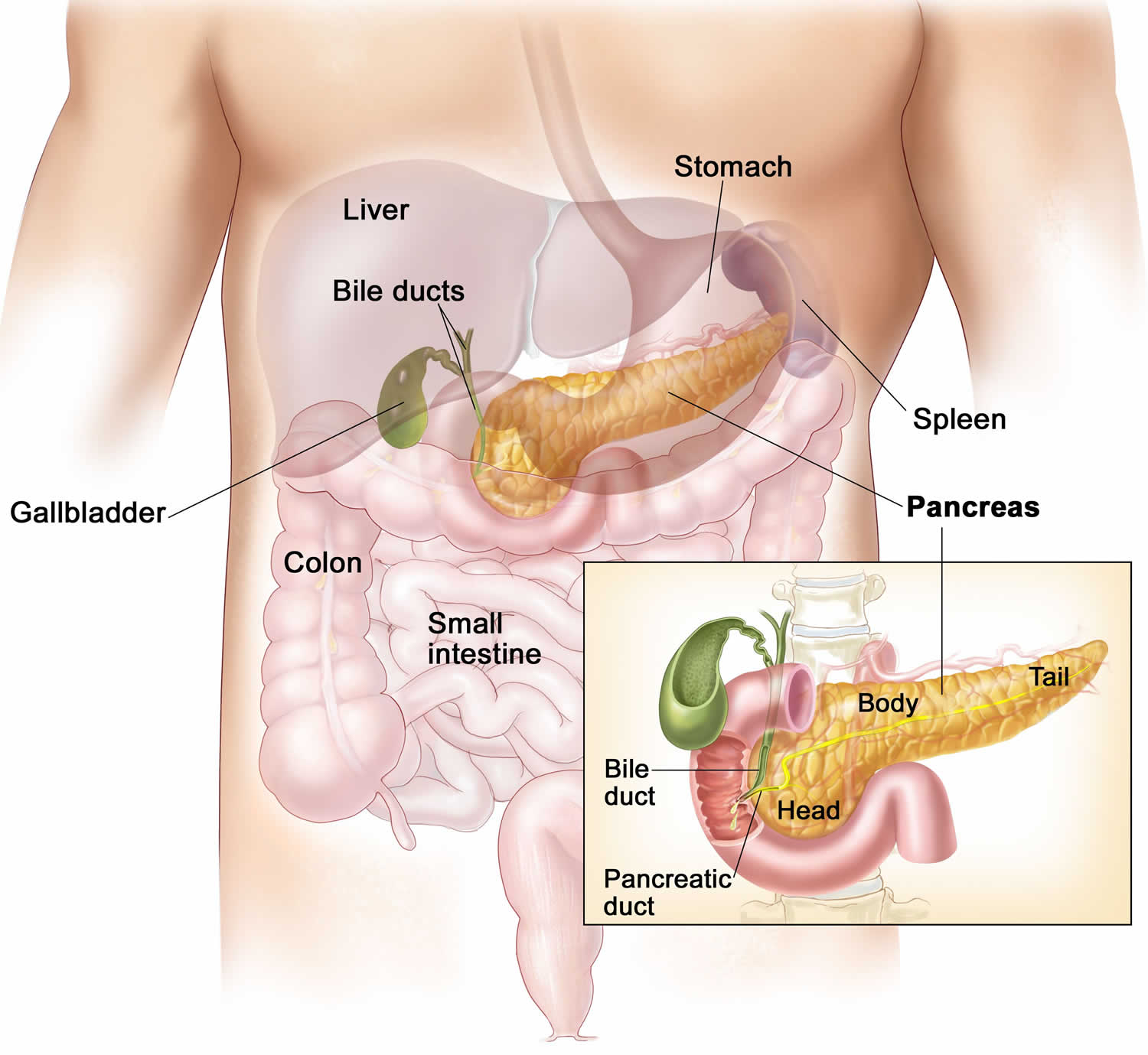
Pancreas anatomy
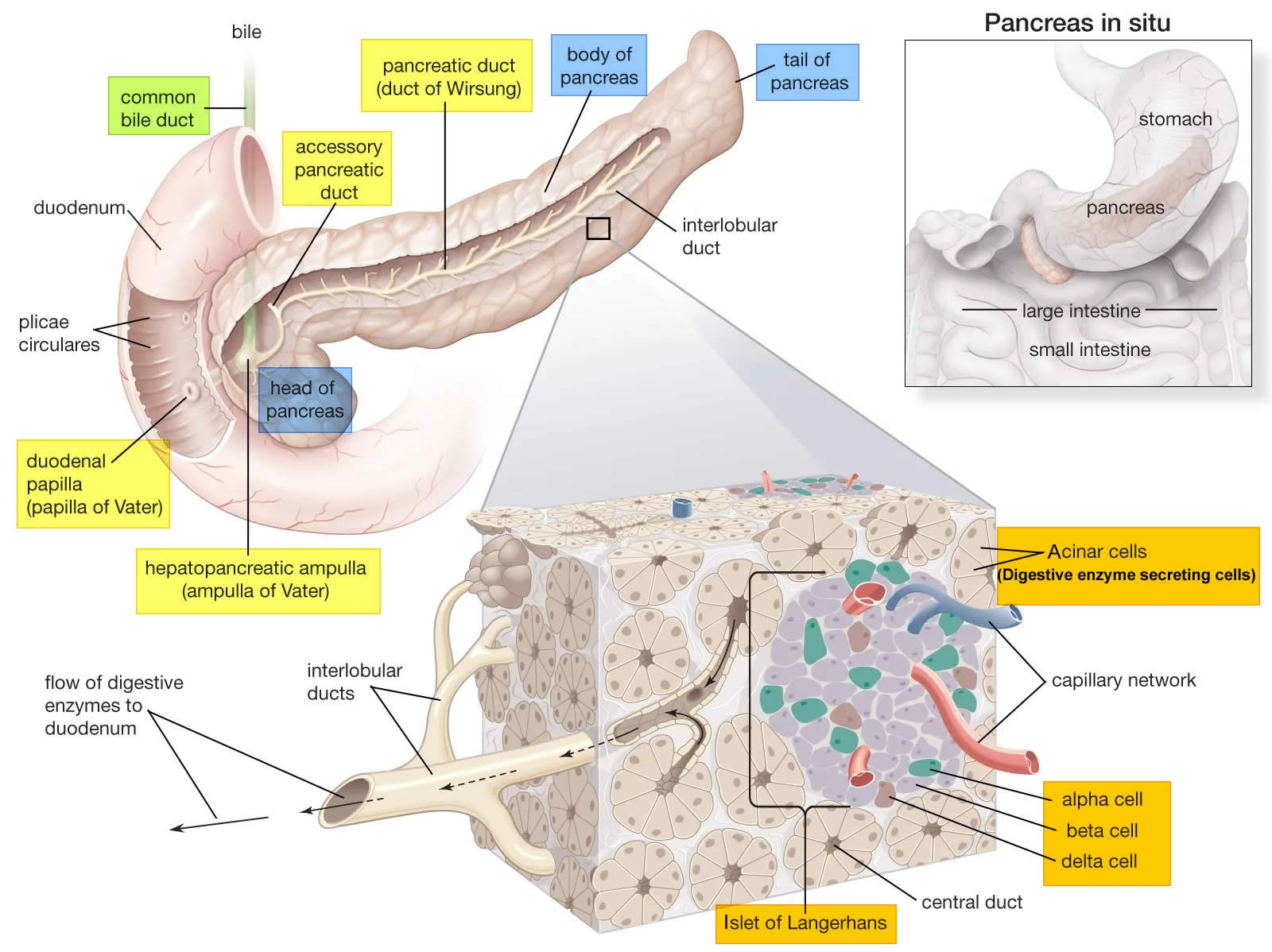
The pancreas is a large gland that sits behind the greater curvature of the stomach and close to the first part of the small intestine (the duodenum). The pancreas is shaped a bit like a fish with a wide head, a tapering body, and a narrow, pointed tail. In adults it’s about 12–15 cm (5–6 inches) long and 2.5 cm (1 in.) thick but less than 2 inches (5 centimeters) wide. The pancreas is both an endocrine and exocrine gland (see Figures 1 and 2).
The pancreas has 3 parts, the head, body, and tail.
- the wide end is called the head. The head of the pancreas is on the right side of the abdomen (belly), behind where the stomach meets the duodenum (the first part of the small intestine).
- the bit in the middle is called the body. The body of the pancreas is behind the stomach.
- the thin end is called the tail. The tail of the pancreas is on the left side of the abdomen next to the spleen.
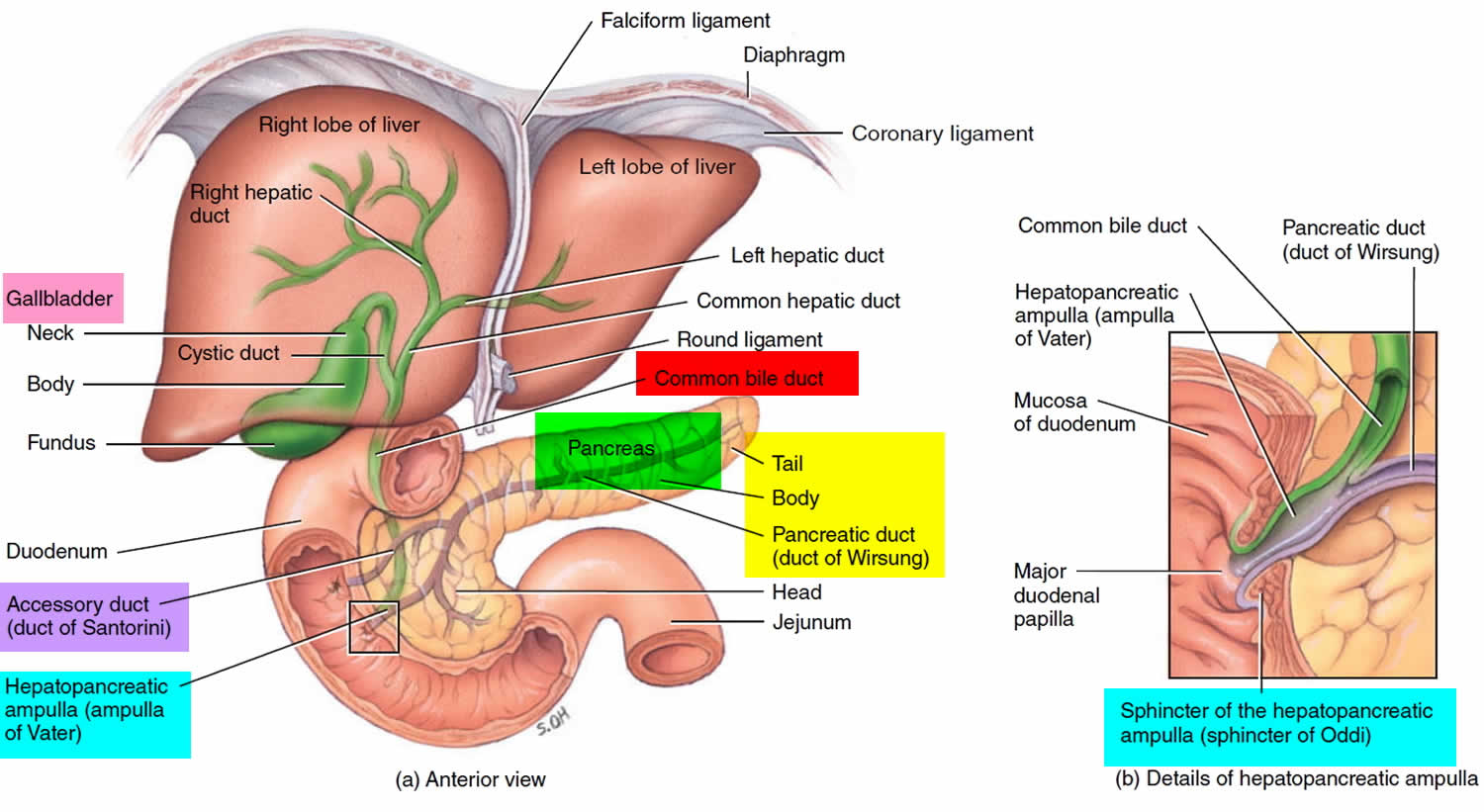
About 99% of the pancreas is exocrine tissue made up of small clusters of glandular epithelial cells called acinar cells (acini), which secretes 1,200 to 1,500 mL of pancreatic juice per day – that are released into the small intestines to help you digest foods (especially fats). The digestive enzymes are first released into tiny tubes called central ducts. These merge to form larger ducts, which empty into the pancreatic duct (duct of Wirsung). The pancreatic duct merges with the common bile duct (the duct that carries bile from the liver), and empties into the duodenum (the first part of the small intestine) at the ampulla of Vater (also known as the hepatopancreatic ampulla). The ampulla of Vater (hepatopancreatic ampulla) is where the pancreatic duct and bile duct join together to drain into the duodenum, which is the first part of the small intestine. The passage of pancreatic juice and bile through the hepatopancreatic ampulla (ampulla of Vater) into the duodenum of the small intestine is regulated by a mass of smooth muscle surrounding the ampulla known as the sphincter of the hepatopancreatic ampulla, or sphincter of Oddi. The other major duct of the pancreas, the accessory duct (duct of Santorini), that branches from the main pancreatic duct and opens independently into the duodenum about 2.5 cm (1 in.) superior to the hepatopancreatic ampulla (ampulla of Vater) at the minor duodenal papilla. The accessory duct (duct of Santorini) bypasses the sphincter and allows pancreatic juice to be released into the duodenum even when bile is held back.
The endocrine part of the pancreas consists of groups of cells that are closely associated with blood vessels. These remaining 1% of the cell clusters form “islands” of cells called pancreatic islets (Islets of Langerhans). The Islets of Langerhans cells secrete the hormones glucagon, insulin, somatostatin, and pancreatic polypeptide (PP). Islets of Langerhans main cell types are alpha cells (20%), beta cells (70%), and delta cells (5%). The pancreatic islets alpha cells secrete the hormone glucagon, and beta cells secrete the hormone insulin (Figure 2). Both insulin and glucagon are important hormones which help control blood sugar levels and are released directly into the bloodstream.
The Islets of Langerhans Delta (δ) cells, or D cells, secrete somatostatin (growth hormone–inhibiting hormone) concurrently with the release of insulin by the beta cells. Somatostatin is a peptide hormone that inhibits the secretion of glucagon and insulin by the nearby alpha and beta cells. Somatostatin also work with amylin to limit the secretion of stomach acid.
Other, minor types of pancreatic cells, about 5% of the total, are called pancreatic polypeptide (PP) and G cells. Pancreatic polypeptide (PP) cells secrete pancreatic polypeptide, a hormone that may inhibit the exocrine activity of the pancreas.
Pancreatic islets (Islets of Langerhans) are relatively concentrated in the tail of the pancreas, whereas the head is more exocrine. Over 90% of pancreatic cancers arise from the ducts of the exocrine portion (ductal carcinomas), so cancer is most common in the head of the pancreas.
Figure 2. The pancreas
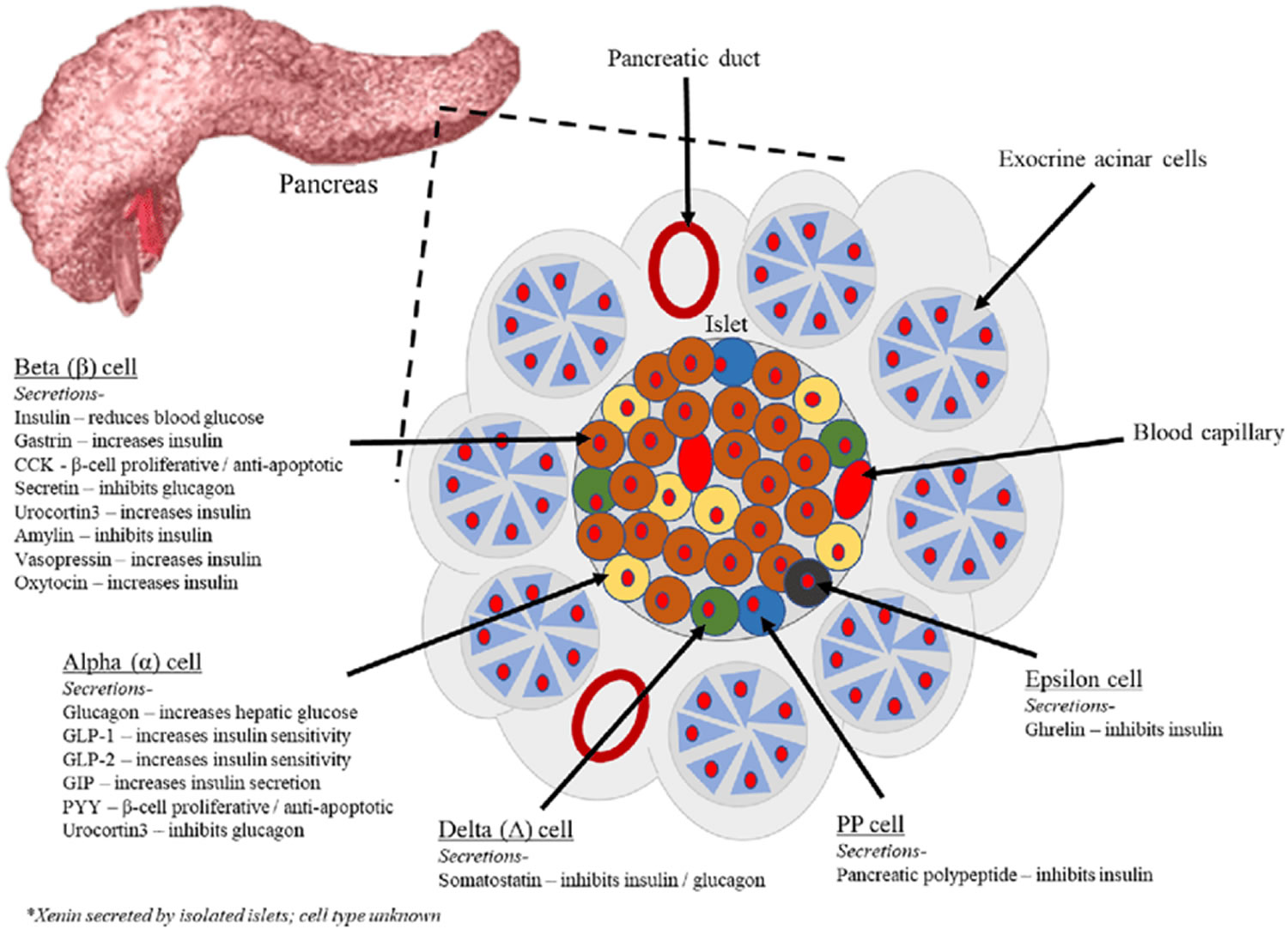
Figure 3. Pancreas cell types
Footnotes: Exocrine pancreatic acinar cells constitute most of the pancreatic tissue, these cells produce digestive enzymes which are transported via the pancreatic ducts. The endocrine pancreas is illustrated with all cell types; alpha, beta, delta, pancreatic polypeptide (PP) and epsilon. The endocrine pancreas cells are arranged in compact Islets of Langerhans and secrete a number of classical and ‘nonclassical’ peptides, as depicted.
Figure 4. Pancreas location
Figure 5. Relationship of the pancreas to the liver, gallbladder, and duodenum
Pancreas function
The pancreas has two main functions—to make insulin and to make digestive juices, or enzymes, to help you digest food in the intestine. About 99% of the pancreas is exocrine tissue made up of small clusters of glandular epithelial cells called acinar cells (acini), which secretes 1,200 to 1,500 mL of pancreatic juice per day – that are released into the small intestines to help you digest foods (especially fats). The cells of the secretory acini exhibit a high density of rough ER (endoplasmic reticulum) and secretory vesicles (zymogen granules). The acini open into a system of branched ducts that eventually converge on the main pancreatic duct. This duct runs lengthwise through the middle of the gland and joins the bile duct at the hepatopancreatic ampulla (ampulla of Vater). The hepatopancreatic sphincter (sphincter of Oddi) thus controls the release of both bile and pancreatic juice into the duodenum. Usually, however, there is a smaller accessory pancreatic duct (duct of Santorini) that branches from the main pancreatic duct and opens independently into the duodenum at the minor duodenal papilla, proximal to the major papilla. The accessory duct (duct of Santorini) bypasses the hepatopancreatic sphincter (sphincter of Oddi) and allows pancreatic juice to be released into the duodenum even when bile is held back.
Pancreatic juice is an alkaline mixture of water, enzymes, zymogens, sodium bicarbonate, and other electrolytes. The acini secrete the enzymes and zymogens, whereas the ducts secrete the sodium bicarbonate. The bicarbonate buffers HCl (hydrochloric acid) arriving from the stomach.
Sodium bicarbonate buffers the hydrochloric acid arriving from the stomach, with the reaction:
- HCl + NaHCO3 ⟶ NaCl + H2CO3 (carbonic acid).
The carbonic acid then breaks down to carbon dioxide (CO2) and water. CO2 is absorbed into the blood and ultimately exhaled. What is left in the small intestine, therefore, is salt water—sodium chloride (NaCl) and H2O. Sodium bicarbonate is therefore important in protecting the intestinal mucosa from hydrochloric acid (HCl) as well as raising the intestinal pH to the level needed for activity of the pancreatic and intestinal digestive enzymes.
The pancreatic zymogens are trypsinogen, chymotrypsinogen and procarboxypeptidase. When trypsinogen is secreted into the intestinal lumen, it is converted to trypsin by enteropeptidase, an enzyme on the brush border of the duodenum. Trypsin is autocatalytic—it converts trypsinogen into still more trypsin. Trypsin also converts the other two zymogens into chymotrypsin and carboxypeptidase, in addition to its primary role of digesting dietary protein.
Pancreatic acinar cells also secrete a protein called trypsin inhibitor that combines with any trypsin formed accidentally in the pancreas or in pancreatic juice and blocks its enzymatic activity.
Other pancreatic enzymes include pancreatic amylase, which digests starch; pancreatic lipase, which digests fat; and ribonuclease and deoxyribonuclease, which digest RNA and DNA, respectively. Unlike the zymogens, these enzymes are not altered after secretion. They become fully active, however, only upon exposure to bile or ions in the intestinal lumen.
Regulation of Pancreatic Secretion
Three stimuli are chiefly responsible for the release of pancreatic juice and bile.
- Acetylcholine (ACh), coming from the vagus nerves and enteric neurons. ACh stimulates the pancreatic acini to secrete their enzymes even during the cephalic phase of gastric control, before food is swallowed. The enzymes remain stored in the pancreatic acini and ducts, however, in preparation for release later when chyme enters the duodenum.
- Cholecystokinin (CCK), secreted by the mucosa of the duodenum and proximal jejunum (the next segment of the small intestine), primarily in response to fats in the small intestine. CCK also stimulates the pancreatic acini to secrete enzymes, but it is named for its strongly stimulatory effect on the gallbladder. It induces contractions of the gallbladder and relaxation of the hepatopancreatic sphincter, discharging bile into the duodenum.
- Secretin, produced by the same regions of the small intestine, mainly in response to the acidity of chyme from the stomach. Secretin stimulates the ducts of both the liver and pancreas to secrete an abundant sodium bicarbonate solution. In the pancreas, this flushes the enzymes into the duodenum.
Hormones of the Pancreatic Islets
The pancreas is primarily an exocrine digestive gland. Scattered throughout the exocrine tissue, are 1 to 2 million endocrine groups of cells that are closely associated with blood vessels called pancreatic islets (islets of Langerhans). Although they are less than 2% of the pancreatic tissue, the islets of Langerhans secrete the hormone glucagon and the hormone insulin of vital importance, especially in the regulation of glycemia, the blood glucose concentration. The pancreatic islets of Langerhans include two distinct types of cells—alpha cells, which secrete the hormone glucagon, and beta cells, which secrete insulin hormone. A typical islet measures about 75 × 175 μm and contains from a few to 3,000 cells. Islets of Langerhans main cell types are alpha cells (20%), beta cells (70%), and delta cells (5%). Islets of Langerhans respond directly to blood nutrient levels associated with the cycle of eating and fasting. Their functions are as follows:
- Alpha (α) cells, or A cells, secrete glucagon between meals when the blood glucose concentration falls below 100 mg/dL (5.6 mmol/L). Glucagon exerts two primary actions on the liver: (1) glycogenolysis, the breakdown of glycogen into glucose; and (2) gluconeogenesis, the synthesis of glucose from fats and proteins. These effects lead to the release of glucose into circulation, thus raising the blood glucose level. In adipose tissue, glucagon stimulates fat catabolism and the release of free fatty acids. Glucagon is also secreted in response to rising amino acid levels in the blood after a high-protein meal. It promotes amino acid absorption and thereby provides cells with the raw material for gluconeogenesis.
- Beta (β) cells, or B cells, secrete two hormones, insulin and amylin. Insulin, “the hormone of nutrient abundance,” is secreted during and immediately following a meal when blood nutrient levels are rising. Osteocalcin, a hormone from the osteoblasts of bone, also stimulates multiplication of beta cells, insulin secretion, and insulin sensitivity of other body tissues. The principal targets of insulin are the liver, skeletal muscles, and adipose tissue. In times of plenty, insulin stimulates cells to absorb glucose, fatty acids, and amino acids and to store or metabolize them; therefore, it lowers the level of blood glucose and other nutrients. It promotes the synthesis of glycogen, fat, and protein, thereby promoting the storage of excess nutrients for later use and enhancing cellular growth and differentiation. It also antagonizes glucagon, thus suppressing the use of already-stored fuels. The brain, liver, kidneys, and red blood cells absorb and use glucose without need of insulin, but insulin does promote glycogen synthesis in the liver. Insulin insufficiency or inaction is well known as the cause of diabetes. The beta cells also secrete another hormone, amylin, simultaneously with insulin. Amylin helps to reduce spikes in blood glucose by slowing the emptying of the stomach; modulating the secretion of gastric enzymes, acid, and bile; inhibiting glucagon secretion; and stimulating the sense of satiety (having had enough to eat).
- Delta (δ) cells, or D cells, secrete somatostatin (growth hormone–inhibiting hormone) concurrently with the release of insulin by the beta cells. Somatostatin is a peptide hormone that inhibits the secretion of glucagon and insulin by the nearby alpha and beta cells. Somatostatin also work with amylin to limit the secretion of stomach acid.
- Other, minor types of pancreatic cells, about 5% of the total, are called pancreatic polypeptide (PP) and G cells. Pancreatic polypeptide (PP) cells secrete pancreatic polypeptide, a hormone that may inhibit the exocrine activity of the pancreas.
Any hormone that raises blood glucose concentration is called a hyperglycemic hormone. You may have noticed that glucagon is not the only hormone that does so; so do growth hormone, epinephrine, norepinephrine, cortisol, and corticosterone. Insulin is called a hypoglycemic hormone because it lowers blood glucose levels.
Glucagon raises the blood sugar concentration by stimulating the liver to break down glycogen and convert certain noncarbohydrates, such as amino acids, into glucose. These actions raise the blood glucose concentration. Glucagon much more effectively elevates blood glucose than does epinephrine (adrenaline).
A negative feedback system regulates glucagon secretion. A low blood glucose concentration stimulates alpha cells to release glucagon. When the blood glucose concentration rises, glucagon secretion falls. This control prevents hypoglycemia when the blood glucose concentration is relatively low, such as between meals, or when glucose is used rapidly, such as during exercise.
The main effect of insulin is to lower the blood glucose level, exactly opposite that of glucagon. Insulin does this in part by promoting facilitated diffusion of glucose into cells that have insulin receptors, for use in cellular respiration. Such cells include those of adipose tissue, liver, and skeletal muscle. (Glucose uptake by active skeletal muscle does not require insulin.) Insulin also stimulates the liver to form glycogen from glucose and inhibits conversion of noncarbohydrates into glucose. In addition, insulin promotes transport of amino acids into cells, increases the rate of protein synthesis, and stimulates adipose cells to synthesize and store fat.
A negative feedback system sensitive to the blood glucose concentration regulates insulin secretion. When the blood glucose concentration is high, such as after a meal, beta cells release insulin. Insulin helps prevent too high a blood glucose concentration by promoting glycogen formation in the liver and entrance of glucose into adipose and muscle cells.
When glucose concentration falls, such as between meals or during the night, insulin secretion decreases. As insulin secretion decreases, less glucose enters adipose and resting muscle cells. Cells that lack insulin receptors and are therefore not dependent on insulin, such as nerve cells, can still take up glucose from the blood. At the same time that insulin is decreasing, glucagon secretion is increasing. Nerve cells, including those of the brain, obtain glucose by a facilitated diffusion mechanism that does not require insulin, but rather depends only on the blood glucose concentration. For this reason, nerve cells are particularly sensitive to changes in blood glucose concentration. Conditions that cause such changes—for example, oversecretion of insulin leading to decreased blood glucose—are likely to affect brain functions.
Insulin and glucagon are coordinated to maintain a relatively stable blood glucose concentration, despite great variation in the amount of carbohydrates a person eats. About 85% to 90% of people with diabetes mellitus have type 2 diabetes, in which the beta cells produce insulin but body cells lose the ability to recognize it. On the other hand, type 1 diabetes mellitus usually appears before age twenty and it is an autoimmune disease: the immune system destroys the beta cells of the pancreas.
Stages of chronic pancreatitis
Standardized reporting terminology has been suggested for chronic pancreatitis 2. The most used classification is the Cambridge classification, based on the status of the main pancreatic duct and the presence of side branches abnormalities.
Cambridge classification 2:
- Grade 0. Normal.
- Normal: main pancreatic duct;
- Abnormal side branches: none
- Grade 1. Equivocal.
- Normal: main pancreatic duct;
- Abnormal side branches: < 3
- Grade 2. Mild chronic pancreatitis.
- Normal: main pancreatic duct;
- Abnormal side branches: ≥ 3
- Grade 3. Moderate chronic pancreatitis.
- Abnormal: main pancreatic duct;
- Abnormal side branches: > 3
- Grade 4. Severe chronic pancreatitis.
- Abnormal: main pancreatic duct;
- Abnormal side branches: presence of filling defect, severe dilatation, irregularity, obstruction or one (or more) large cavity
Chronic pancreatitis causes
Repeated episodes of acute pancreatitis can lead to chronic pancreatitis. Instead of the inflammation getting better as in acute pancreatitis, the inflammation continues in some more susceptible people and causing permanent damage to the pancreas. In some cases, genetics may be a factor. However, sometimes, the cause is unknown.
The most common causes of chronic pancreatitis are:
- heavy alcohol use
- genetic disorders of your pancreas (cystic fibrosis, hereditary pancreatitis)
Other causes of chronic pancreatitis include:
- blockage in your pancreatic duct that drain enzymes from the pancreas (tumors, gallstones)
- high levels of blood fats, called lipids (hypertriglyceridemia)
- high level of calcium in your blood (hypercalcemia)
- autoimmune diseases such as systemic lupus erythematosus (SLE), celiac disease, inflammatory bowel disease (IBD) or autoimmune pancreatitis 81, 82, 83
- smoking or tobacco use. Cigarette smoking has been reported to be an independent risk factor for developing chronic pancreatitis 12 and increases its rate of progression 13, 14.
- overactive parathyroid gland (hyperparathyroidism)
- trauma
- use of certain medicines (especially sulfonamides, thiazides, and azathioprine)
- pancreas divisum
The results of past studies that have noted a significant association of very heavy alcohol consumption and cigarette smoking with chronic pancreatitis. This study is the first to demonstrate that a significant increase in the risk of chronic pancreatitis occurs only above a threshold of ≥5 alcohol drinks per day 84.
New studies are finding that deficiencies in certain vitamins and antioxidants may be linked to chronic pancreatitis 85, 86.
In many cases, doctors can’t find the cause of pancreatitis. This is called idiopathic pancreatitis.
Chronic pancreatitis prevention
Finding the cause of acute pancreatitis and treating it quickly may help prevent chronic pancreatitis. Limit the amount of alcohol you drink and quit smoking can reduce your risk of developing chronic pancreatitis.
Maintain a healthy weight or lose weight safely
Maintaining a healthy lifestyle and a healthy weight or losing weight if you’re overweight or obese can help to:
- make your pancreas work better
- lower your chance of getting gallstones, a leading cause of pancreatitis
- prevent obesity, a risk factor for pancreatitis
- prevent diabetes, a risk factor for pancreatitis
Avoid alcohol use
Alcohol use can cause acute and chronic pancreatitis. Talk with your doctor if you need help to stop drinking alcohol.
Avoid smoking
Smoking is a common risk factor for pancreatitis and the chances of getting pancreatitis are even higher in people who smoke and drink alcohol. Talk with your doctor if you need help to stop smoking.
Chronic pancreatitis signs and symptoms
The most common symptom of chronic pancreatitis is repeated episodes of severe pain in your upper abdomen, although some people have no pain at all. The pain usually develops in the middle or left side of your belly and can move along your back.
The upper abdominal pain may:
- spread to your back
- become constant and severe
- become worse after eating
- go away as your condition gets worse
The pain been described as a burning or shooting pain that comes and goes, but may last for several hours or days. Although the pain sometimes comes on after eating a meal, there’s often no trigger. Some people might feel sick and vomit. As the condition progresses, the painful episodes may become more frequent and severe. Eventually, a constant dull pain can develop in your abdomen, between episodes of severe pain. This is most common in people who continue to drink alcohol after being diagnosed with chronic pancreatitis.
Some people who stop drinking alcohol and stop smoking may find the pain is less severe.
Some people with chronic pancreatitis may not have symptoms until they have complications.
Other symptoms may include:
- diarrhea
- nausea
- oily, foul-smelling stools (steatorrhea)
- vomiting
- weight loss without trying
- jaundice (your skin or the whites of your eyes turn yellow)
Advanced chronic pancreatitis
Other symptoms develop as the damage to the pancreas progresses and it becomes unable to produce digestive juices, which help to break down food. The absence of digestive juices means it’s harder to break down fats and some proteins. This can cause your poop to become very smelly and greasy (steatorrhea), and make it difficult to flush down the toilet. The pancreas usually only loses these functions many years after the first symptoms started.
You may also experience:
- weight loss
- loss of appetite
- your skin or the whites of your eyes turn yellow (jaundice)
- symptoms of diabetes – such as feeling very thirsty, needing to pee more often than usual and feeling very tired
- ongoing nausea and sickness (vomiting).
Chronic pancreatitis complications
Chronic pancreatitis complications may include:
- Ascites
- Blockage (obstruction) of the small intestine or bile ducts
- Blood clot in the vein of the spleen
- Fluid collections in the pancreas (pancreatic pseudocysts) that may become infected
- Diabetes
- Poor absorption of fat, nutrients, and vitamins (most often the fat-soluble vitamins, A, D, E, or K)
- Iron deficiency anemia
- Vitamin B12 deficiency
Gastroparesis
The frequency of gastroparesis in adult patients with chronic pancreatitis is ~3.6% 87. Gastroparesis is frequently seen in patients with small duct chronic pancreatitis and can present with similar symptoms as seen in acute pancreatitis. Gastroparesis may also cause confusion in diagnosis, and affect the efficacy of pancreatic enzyme replacement therapy, complicating both evaluation and treatment of chronic pancreatitis 88. As per 1 publication, patients with abdominal pain who do not respond to pancreatic enzyme replacement therapy or express early satiety should be evaluated for gastroparesis 88. Treatment of gastroparesis is challenging in patients with chronic pancreatitis. Management for mild cases includes dietary modifications, such as small and frequent meals and prokinetic agents such as erythromycin and metoclopramide. Severe gastroparesis may require intrapyloric botulinum toxin injections, gastrostomy feeding tube placement, or implantation of gastric electrical stimulator 87.
Small Intestinal Bacterial Overgrowth (SIBO)
Small intestinal bacterial overgrowth (SIBO) is defined as excessive bacteria in the small intestine. Exocrine pancreatic insufficiency, impaired motility secondary to inflammation/narcotic use, and/or proton pump inhibitors (PPIs) can predispose patients with chronic pancreatitis to SIBO 89. Various adult studies have shown of the prevalence of SIBO in chronic pancreatitis patients to be between 22% and 67% 90. The presentation of SIBO may mimic exocrine pancreatic insufficiency or constipation and includes abdominal pain, bloating, diarrhea, steatorrhea, weight loss, weakness, neuropathy, and excessive flatulence 91. Small intestinal bacterial overgrowth (SIBO) should be considered in patients with chronic pancreatitis and suggestive symptoms not responsive to other therapy 89.
Pancreatic cancer
Meta-analysis reveals a relative risk of 13.3 for the development of pancreatic cancer among adults with chronic pancreatitis 92, 93, 94. The risk for the development of pancreatic cancer among children with chronic pancreatitis is not yet known. Genetic etiologies account for the most common cause of chronic pancreatitis among children. These are associated with a lifetime risk as high as 40% to 50% for the development of pancreatic cancer among adults, although genetic predisposition may be confounded by smoking, drinking, and other factors 87, 95. More recent analysis controlling for smoking exposure showed relative risk for the development of pancreatic cancer to be ~7% with those patients with hereditary pancreatitis with a PRSS1 mutation 60, whereas certain cystic fibrosis transmembrane conductance regulator (CFTR) mutations were associated with a more modest risk and SPINK1 mutations showing no increased association 96. Cigarette smoking, alcohol, and diabetes mellitus may further increase the risk for the development of pancreatic cancer in chronic pancreatitis 97, 87.
Whether chronic/recurrent inflammation or the genetic mutations alone account for the increased risk of pancreatic cancer is unknown. No reliable screening tests or screening recommendations have been developed to distinguish sub-groups of patients with chronic pancreatitis who are at risk for developing pancreatic cancer or whether age of diagnosis impacts future risk. Recently, the intestinal microbiota or more precisely, the mycobiota have been implicated in the pathogenesis of pancreatic cancer 98. Future studies are critical to determine the impact of hereditary pancreatitis among children on long-term pancreatic cancer risk especially as it pertains to earlier surgical intervention to potentially decrease this risk.
Pancreatic or peripancreatic fluid collections
Pancreatic fluid collections include acute fluid collections. Acute fluid collections are those <4 weeks old and divided into acute peri-pancreatic fluid collections and acute necrotic fluid collections. More mature fluid collections >4 weeks old are divided into walled off necrosis and pancreatic pseudocysts. Pancreatic pseudocysts are typically a consequence of acute pancreatitis, pancreatic trauma, and/or chronic pancreatitis 99. Among adults, the prevalence of pseudocysts in chronic pancreatitis have been reported to be between 10% and 40%, though this is likely overestimated because of previous inclusion of acute fluid collections and walled off necrosis within the definition of pancreatic pseudocysts 100. Although usually asymptomatic, mass effect from pancreatic pseudocysts can cause abdominal pain, early satiety, nausea, vomiting, jaundice, weight loss; secondary complications, such as gastric outlet obstruction, biliary obstruction, infection, or vascular complications, such as splenic vein thrombosis or intra-cystic hemorrhage can also present with pain 101. Asymptomatic pseudocysts are usually managed conservatively with observation, and pain control 102. Drainage, however, should be considered when fluid collection becomes clinically symptomatic (uncontrolled nausea/vomiting or pain), infected, causes obstructive complications (blood vessels, common bile duct, gastric outlet obstruction), bleeding, or fistula formation. Even when asymptomatic, endoscopic drainage has been proposed if the pseudocyst measures >5 cm in diameter without spontaneous regression 103. Previously walled off necrosis was felt to require definite intervention via surgery or advanced endoscopic techniques but recently care has evolved and many of these collections may also be managed conservatively if the patient remains clinically stable during the course 104.
Pancreatic duct disease
Pancreatic duct abnormalities are most often characterized by strictures and intraductal stone formation 105. For some patients, ductal obstruction may cause increased pressure resulting in ductal dilation, and is the assumed cause of pain. Endoscopic therapy is often offered for decompression of these strictures and removal of pancreatic duct stones when this is believed to be the cause of pain or for recurrent pancreatitis episodes 53, 106.
Vascular complications
Splenic vein thrombosis is a reported complication in about 10% to 20% of adult patients with chronic pancreatitis and is often related to the presence of a pancreatic pseudocyst or severe acute pancreatitis, in general 107. Most patients with splenic vein thrombosis are asymptomatic but they may develop varices, which are at high risk of gastrointestinal bleeding 108. Less commonly, deep vein thrombosis and pulmonary embolism have been reported, although are more typical in acute pancreatitis. Management is usually conservative. The use of anticoagulation is not well studied in chronic pancreatitis though no significant difference has been demonstrated in acute pancreatitis patients with regard to recanalization rate 109.
Chronic pancreatitis diagnosis
To diagnose pancreatitis and find its causes, doctors use:
- Your medical history. Your doctor will ask:
- about your symptoms
- if you have a history of health conditions or concerns that make you more likely to get pancreatitis—including medicines you are taking
- if you have a personal or family medical history of pancreatitis or gallstones
- A physical exam. During a physical exam, your doctor will:
- examine your body
- check your abdomen for pain, swelling, or tenderness
- Lab and imaging tests
Tests and procedures used to diagnose pancreatitis include:
- Blood tests to look for elevated levels of pancreatic enzymes, along with white blood cells, kidney function and liver enzymes
- Abdominal ultrasound to look for gallstones and pancreas inflammation
- Computerized tomography (CT) scan of your abdomen to look for gallstones and assess the extent of pancreas inflammation
- Magnetic resonance imaging (MRI) of your abdomen to look for abnormalities in the gallbladder, pancreas and ducts
- Endoscopic ultrasound to look for inflammation and blockages in the pancreatic duct or bile duct
- Stool tests in chronic pancreatitis to measure levels of fat that could suggest your digestive system isn’t absorbing nutrients adequately
- Biopsy.
Your doctor may recommend other tests, depending on your particular situation.
Lab tests
Lab tests to help diagnose pancreatitis include the following:
- Blood tests. Your physician may take a blood sample from you and send the sample to a lab to test for:
- high amylase and lipase levels—digestive enzymes made in your pancreas
- high blood glucose, also called blood sugar
- high levels of blood fats, called lipids
- signs of infection or inflammation of the bile ducts, pancreas, gallbladder, or liver
- pancreatic cancer
- Stool tests. Your doctor may test a stool sample to find out if a person has fat malabsorption. At present, measurement of fecal elastase is the most popular test to evaluate pancreatic exocrine insufficiency. Low levels of fecal elastase (<200 µg/g stool, although even lower levels are more specific) or serum trypsin (<20 ng/mL) are usually observed in patients with pancreatic exocrine insufficiency 110.
Biopsy
Sometimes the symptoms of chronic pancreatitis can be very similar to pancreatic cancer. You may need a biopsy, where a small sample of cells is taken from the pancreas and sent to a laboratory to be checked, to rule this out.
Imaging tests
Your doctor also use imaging tests to diagnose pancreatitis. A technician performs most tests in an outpatient center, a hospital, or a doctor’s office. You don’t need anesthesia, a medicine to keep you calm, for most of these tests.
- Ultrasound. Ultrasound uses a device called a transducer, which bounces safe, painless sound waves off your organs to create a picture of their structure. Ultrasound can find gallstones.
- Computed tomography (CT) scan. CT scans create pictures of your pancreas, gallbladder, and bile ducts. CT scans can show pancreatitis or pancreatic cancer. CT features of chronic pancreatitis include dilatation of the main pancreatic duct, pancreatic calcification, changes in pancreatic size (i.e. atrophy), shape, and contour and pancreatic pseudocysts.
- Magnetic resonance cholangiopancreatography (MRCP). MRCP uses a magnetic resonance imaging (MRI) machine, which creates pictures of your organs and soft tissues without x-rays. Your doctor or a specialist may use MRCP to look at your pancreas, gallbladder, and bile ducts for causes of pancreatitis.
- Endoscopic ultrasound (EUS). Your doctor inserts an endoscope—a thin, flexible tube—down your throat, through your stomach, and into your small intestine. The doctor turns on an ultrasound attachment to create pictures of your pancreas and bile ducts. Your doctor may send you to a gastroenterologist to perform this test.
- Pancreatic Function Test (PFT). Your doctor may use this test to measure how your pancreas responds to secretin, a hormone made by the small intestine. This test is done only at some centers in the United States.
Cross-sectional imaging is the most commonly used method to establish a diagnosis of acute pancreatitis and chronic pancreatitis. Among cross-sectional imaging studies, CT is readily available and performed often in the setting of acute pancreatitis. It is widely acknowledged that MRI with MR cholangiopancreatography (MRCP) is more sensitive than is CT for detection of ductal and subtle parenchymal changes, especially during early stages of chronic pancreatitis 111. However, the two modalities are complementary because CT is the modality of choice for evaluation of parenchymal and ductal calcifications.
Both the size and number of pancreatic calcifications should be graded because the degree of calcification in chronic pancreatitis may parallel the course of the disease 112. Coarse pancreatic calcifications are accepted as a definite sign of chronic pancreatitis 113, 114. Senescent pancreatic parenchymal calcifications are usually 1–3 mm and may not always imply chronic pancreatitis. Senescent calcifications are seen in peripheral ducts and lack association with clinical signs and symptoms of chronic pancreatitis, hypercalcemia, or alcoholism 115. Innumerable punctate calcifications, on the other hand, strongly suggest chronic pancreatitis. Calcification seen in chronic pancreatitis has rarely been scored and/or graded. Selection of seven as the number of coarse calcifications to discriminate more severe from less severe chronic pancreatitis is somewhat arbitrary but provides distinction. A cutoff of 50 punctate calcifications was chosen as a surrogate for innumerable calcifications 2.
Currently, no standardized reporting system for CT, MRI, or MR cholangiopancreatography (MRCP) is universally used. The Cambridge classification 116, which was developed for endoscopic retrograde cholangiopancreatography, has been suggested for translational use in CT and MRI or MR cholangiopancreatography interpretation by recent guidelines of the American Pancreatic Association 117. Although useful, the Cambridge classification only acknowledges ductal changes 118. Hence, the contribution of parenchymal observations is not captured.
Cambridge classification
- Grade 0. Normal.
- Normal: main pancreatic duct;
- Abnormal side branches: none
- Grade 1. Equivocal.
- Normal: main pancreatic duct;
- Abnormal side branches: < 3
- Grade 2. Mild chronic pancreatitis.
- Normal: main pancreatic duct;
- Abnormal side branches: ≥ 3
- Grade 3. Moderate chronic pancreatitis.
- Abnormal: main pancreatic duct;
- Abnormal side branches: > 3
- Grade 4. Severe chronic pancreatitis.
- Abnormal: main pancreatic duct;
- Abnormal side branches: presence of filling defect, severe dilatation, irregularity, obstruction or one (or more) large cavity
Chronic pancreatitis differential diagnosis
chronic pancreatitis differential diagnoses may include 119:
- Ampullary carcinoma
- Cholangitis
- Acute cholecystitis
- Chronic gastritis
- Community-Acquired Pneumonia (CAP)
- Crohn disease
- Intestinal perforation
- Mesenteric artery ischemia
- Myocardial infarction
- Pancreatic cancer
- Peptic ulcer disease
Chronic pancreatitis treatment
In chronic pancreatitis, the pancreas is permanently damaged, but treatment can help control the condition and manage any symptoms. Treatment for chronic pancreatitis may help relieve pain, improve how well the pancreas works, and manage complications.
Depending on your situation, your doctor may prescribe or provide the following additional treatments:
- Pain management. Chronic pancreatitis can cause persistent abdominal pain. Your doctor will evaluate you for causes of chronic pancreatitis and may recommend medications to control your pain. If necessary, you may be referred to a pain specialist. Severe pain may be relieved with options such as endoscopic ultrasound or injections to block nerves that send pain signals from the pancreas to the brain.
- Enzymes to improve digestion. In chronic pancreatitis leading to diarrhea or weight loss, pancreatic enzyme supplements can help your body break down and process the nutrients in the foods you eat. Pancreatic enzymes are taken with each meal.
- Changes to your diet. Your doctor may refer you to a dietitian who can help you plan low-fat meals that are high in nutrients.
- Medicines and vitamins. Long-term fat malabsorption may also lead to fat-soluble vitamin (A, D, E, and K) deficiencies 120 as well as deficiencies in calcium, magnesium, zinc, thiamine, and folic acid 121. Your doctor may give you vitamins A, D, E, and K if you have malabsorption. He or she may also give you vitamin B-12 shots if you need them.
- Treatment for diabetes. Chronic pancreatitis may cause diabetes. If you get diabetes, your doctor and health care team will work with you to create an eating plan and a routine of medicine, blood glucose monitoring, and regular checkups.
- Surgery. Your doctor may recommend surgery to relieve pressure or blockage in your pancreatic duct, or to remove a damaged or infected part of your pancreas. Surgery is done in a hospital, where you may have to stay a few days. In patients who do not get better with other treatments, surgeons may perform surgery to remove your whole pancreas, followed by islet auto-transplantation. Islets of Langerhans are groups of cells in your pancreas that make hormones, including insulin. After removing your pancreas, doctors will take islets from your pancreas and transplant them into your liver. The islets will begin to make hormones and release them into your bloodstream.
- Procedures. Your doctor may suggest a nerve block, which is a shot of numbing medicine through your skin and directly into nerves that carry the pain message from your pancreas. If you have stones blocking your pancreatic duct, your doctor may use a procedure to break up and remove the stones.
Living with chronic pain can cause mental as well as physical strain. See your doctor if you’re experiencing stress, anxiety or depression caused by chronic pancreatitis.
Some people with chronic pancreatitis will eventually develop a type of diabetes known as type 3c diabetes 122. This occurs when the pancreas can no longer produce insulin because it’s become so damaged. There are currently no universally accepted diagnostic criteria for type 3c diabetes 122. Conceptually, the diagnosis can be made in patients who meet the three following criteria: those who fulfil the diagnostic criteria for diabetes, those who have a disease of the exocrine pancreas, and those whose diabetes is reasonably certain to be secondary to their exocrine pancreatic disease 122.
People with chronic pancreatitis can sometimes develop sacs of fluid on the surface of their pancreas (pancreatic pseudocysts). These can cause bloating, indigestion and dull tummy pain. These cysts often disappear on their own. But sometimes they need to be drained using a technique called endoscopic ultrasound drainage, or endoscopic transpapillary drainage.
Chronic pancreatitis increases your risk of pancreatic cancer, although the chance is still small.
Pain relief
Pain relief is an important part of the treatment of chronic pancreatitis.
Mild painkillers
In most cases, the first painkillers used are paracetamol, or anti-inflammatories such as ibuprofen.
But taking anti-inflammatory painkillers on a long-term basis can increase your risk of developing stomach ulcers, so you may be prescribed a proton pump inhibitor (PPI) to protect against this.
Stronger painkillers
If paracetamol or anti-inflammatories don’t control the pain, you may need an opiate-based painkiller, such as codeine or tramadol. Side effects include constipation, nausea, vomiting and drowsiness.
Constipation can be particularly difficult to manage, so you may be prescribed a laxative to help relieve this. See the page on constipation for more information.
If you feel drowsy after taking an opiate-based painkiller, avoid driving and using heavy tools or machines.
Severe pain
If your pain is severe, you may be referred to a specialist (a gastroenterologist or pancreatico-biliary surgeon) or pain center for further assessment.
You may be offered surgery to help relieve the pain or treat any complications.
In some cases, additional medicine – called amitriptyline, gabapentin or pregabalin – may be recommended to help relieve the pain. Tricyclic antidepressants (TCA), serotonin-norepinephrine reuptake inhibitors (SNRI), and anticonvulsants have been long established through a multitude of studies as effective agents in treating chronic neuropathic pain 123. These medications provide pain relief by modulating central pain signaling, thus reducing central sensitization. Tricyclic antidepressants (TCAs) include amitriptyline and nortriptyline. Both are equally effective; however, nortriptyline is associated with fewer side effects 124. Serotonin-norepinephrine reuptake inhibitors (SNRIs) include duloxetine and anticonvulsants, such as gabapentin and pregabalin. Ketamine has been used to treat both acute and chronic pain of numerous types in patients of all ages, and there is modest evidence to support its use in inpatient pancreatitis care 125.
If this isn’t effective, severe pain can sometimes be relieved for a few weeks or months using an injection called a nerve block. This blocks the pain signals from the pancreas. Celiac plexus blockade has been used in adults for chronic pancreatitis for decades. Overall results have been mixed and largely short-term, based on moderate levels of data 126, 127.
Severe episodes
If the inflammation of your pancreas suddenly gets worse, you may need a short stay in hospital for treatment. This might involve having fluids delivered directly into a vein and oxygen through tubes into your nose.
Surgery
Surgery can be used to treat severe pain in people with chronic pancreatitis.
Endoscopic surgery
Patients with gallstones in the opening of their pancreas (the pancreatic duct) may benefit from endoscopic surgery and a treatment called lithotripsy. Lithotripsy involves using shock waves to break the stones into smaller pieces. An endoscope is then used to access the pancreatic duct so the pieces can be removed. This treatment may improve pain to some extent, but the benefit may not be permanent.
Pancreas resection
In cases where specific parts of the pancreas are inflamed and causing severe pain, they can be surgically removed. This is called a pancreas resection and may also be offered if endoscopic treatment doesn’t work.
The exact technique used for pancreas resection depends on which parts need to be removed. Speak with your surgical team about the benefits and risks of the procedure before deciding to go ahead with it.
Total pancreatectomy
In the most serious cases of chronic pancreatitis, where the pancreas has been extensively damaged, it may be necessary to remove the entire pancreas (total pancreatectomy). This can be very effective in treating pain, but you wont be able to produce the insulin that’s needed by your body any more. To overcome this problem, a technique called autologous pancreatic islet cell transplantation is sometimes used. During autologous pancreatic islet cell transplantation, the islet cells (Islets of Langerhans) responsible for producing hormones including insulin are removed from your pancreas before your pancreas is surgically removed. The islet cells are then mixed with a special solution and injected into your liver.
If autologous pancreatic islet cell transplantation is successful, the islet cells remain in your liver and begin to produce make hormones (e.g., insulin) and release them into your bloodstream.
In the short term, autologous pancreatic islet cell transplantation appears to be effective, but you may need additional insulin treatment in the long term.
On-going tests and checks
If you’ve been diagnosed with chronic pancreatitis, you should be offered:
- annual checks (every 6 months in under-16s) to make sure your diet is giving you the nutrients you need
- a bone density assessment every 2 years – problems with digesting foods may affect your bone health
- a blood test for diabetes every 6 months
- an annual check for pancreatic cancer if the cause of chronic pancreatitis is hereditary
Chronic pancreatitis prognosis
The prognostic factors associated with chronic pancreatitis are age at diagnosis, smoking, continued use of alcohol, and the presence of liver cirrhosis. Chronic pancreatitis is associated with poor prognosis in patients who continued to use alcohol, smoke and the presence of end-stage liver disease 4. At 10 years, there is a survival of 70%, which drops to 45% at 20 years 4. Pancreatic pseudocyst formation, mechanical obstruction of the bile duct and duodenum are major complications. Addition complications include diabetes (30% of patients), development of gastric varices and pseudoaneurysm formation.
- Kwon CI, Cho JH, Choi SH, Ko KH, Tirkes T, Gromski MA, Lehman GA. Recent advances in the diagnosis and management of chronic pancreatitis. Korean J Intern Med. 2019 Mar;34(2):242-260. doi: 10.3904/kjim.2019.051[↩]
- Tirkes T, Shah ZK, Takahashi N, Grajo JR, Chang ST, Venkatesh SK, Conwell DL, Fogel EL, Park W, Topazian M, Yadav D, Dasyam AK; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Reporting Standards for Chronic Pancreatitis by Using CT, MRI, and MR Cholangiopancreatography: The Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Radiology. 2019 Jan;290(1):207-215. doi: 10.1148/radiol.2018181353[↩][↩][↩][↩][↩][↩]
- Ketwaroo GA, Freedman SD, Sheth SG. Approach to patients with suspected chronic pancreatitis: a comprehensive review. Pancreas. 2015 Mar;44(2):173-80. doi: 10.1097/MPA.0000000000000239[↩]
- Benjamin O, Lappin SL. Chronic Pancreatitis. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482325[↩][↩][↩][↩]
- Shanbhogue AK, Fasih N, Surabhi VR, Doherty GP, Shanbhogue DK, Sethi SK. A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics. 2009 Jul-Aug;29(4):1003-26. doi: 10.1148/rg.294085748[↩]
- Lankisch PG, Assmus C, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic diseases in Lüneburg County. A study in a defined german population. Pancreatology. 2002;2(5):469-77. doi: 10.1159/000064713[↩]
- Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984 May;86(5 Pt 1):820-8.[↩]
- Andersen BN, Pedersen NT, Scheel J, Worning H. Incidence of alcoholic chronic pancreatitis in Copenhagen. Scand J Gastroenterol. 1982 Mar;17(2):247-52. doi: 10.3109/00365528209182047[↩]
- Talamini G, Bassi C, Falconi M, Sartori N, Salvia R, Rigo L, Castagnini A, Di Francesco V, Frulloni L, Bovo P, Vaona B, Angelini G, Vantini I, Cavallini G, Pederzoli P. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci. 1999 Jul;44(7):1303-11. doi: 10.1023/a:1026670911955[↩]
- Robles-Díaz G, Vargas F, Uscanga L, Fernández-del Castillo C. Chronic pancreatitis in Mexico City. Pancreas. 1990 Jul;5(4):479-83. doi: 10.1097/00006676-199007000-00017[↩]
- Apte, M.V., Pirola, R.C. and Wilson, J.S. (2008), Malnutrition as a cause of chronic pancreatitis: Myth dispelled?. Journal of Gastroenterology and Hepatology, 23: 1312-1314. https://doi.org/10.1111/j.1440-1746.2008.05542.x[↩]
- Lin Y, Tamakoshi A, Hayakawa T, Ogawa M, Ohno Y; Research Committee on Intractable Pancreatic Diseases. Associations of alcohol drinking and nutrient intake with chronic pancreatitis: findings from a case-control study in Japan. Am J Gastroenterol. 2001 Sep;96(9):2622-7. doi: 10.1111/j.1572-0241.2001.04121.x[↩][↩]
- Maisonneuve P, Lowenfels AB, Müllhaupt B, Cavallini G, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L, Frulloni L, Ammann RW. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005 Apr;54(4):510-4. doi: 10.1136/gut.2004.039263[↩][↩]
- Imoto M, DiMagno EP. Cigarette smoking increases the risk of pancreatic calcification in late-onset but not early-onset idiopathic chronic pancreatitis. Pancreas. 2000 Aug;21(2):115-9. doi: 10.1097/00006676-200008000-00002[↩][↩]
- Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001 Feb;120(3):682-707. doi: 10.1053/gast.2001.22586[↩]
- Löhr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, Haas S, Akisik F, Kartalis N, Iglesias-Garcia J, Keller J, Boermeester M, Werner J, Dumonceau JM, Fockens P, Drewes A, Ceyhan G, Lindkvist B, Drenth J, Ewald N, Hardt P, de Madaria E, Witt H, Schneider A, Manfredi R, Brøndum FJ, Rudolf S, Bollen T, Bruno M; HaPanEU/UEG Working Group. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017 Mar;5(2):153-199. doi: 10.1177/2050640616684695[↩]
- Schwarzenberg SJ, Bellin M, Husain SZ, Ahuja M, Barth B, Davis H, Durie PR, Fishman DS, Freedman SD, Gariepy CE, Giefer MJ, Gonska T, Heyman MB, Himes R, Kumar S, Morinville VD, Lowe ME, Nuehring NE, Ooi CY, Pohl JF, Troendle D, Werlin SL, Wilschanski M, Yen E, Uc A. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr. 2015 Apr;166(4):890-896.e1. doi: 10.1016/j.jpeds.2014.11.019[↩]
- Morinville VD, Husain SZ, Bai H, Barth B, Alhosh R, Durie PR, Freedman SD, Himes R, Lowe ME, Pohl J, Werlin S, Wilschanski M, Uc A; INSPPIRE Group. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr. 2012 Sep;55(3):261-5. doi: 10.1097/MPG.0b013e31824f1516. Erratum in: J Pediatr Gastroenterol Nutr. 2013 Apr;56(4):459. Abu-Al-Haija, Maisam [corrected to Abu-El-Haija, Maisam][↩]
- Szatmary P, Grammatikopoulos T, Cai W, Huang W, Mukherjee R, Halloran C, Beyer G, Sutton R. Acute Pancreatitis: Diagnosis and Treatment. Drugs. 2022 Aug;82(12):1251-1276. doi: 10.1007/s40265-022-01766-4[↩]
- Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013 Jun;144(6):1252-61. doi: 10.1053/j.gastro.2013.01.068[↩]
- Definition & Facts for Pancreatitis. https://www.niddk.nih.gov/health-information/digestive-diseases/pancreatitis/definition-facts[↩]
- Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, Jensen ET, Lund JL, Pasricha S, Runge T, Schmidt M, Shaheen NJ, Sandler RS. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology. 2015 Dec;149(7):1731-1741.e3. doi: 10.1053/j.gastro.2015.08.045[↩]
- Jones MR, Hall OM, Kaye AM, Kaye AD. Drug-induced acute pancreatitis: a review. Ochsner J. 2015 Spring;15(1):45-51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4365846[↩][↩]
- Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016 Nov 17;375(20):1972-1981. doi: 10.1056/NEJMra1505202[↩]
- Della Corte C, Faraci S, Majo F, Lucidi V, Fishman DS, Nobili V. Pancreatic disorders in children: new clues on the horizon. Dig Liver Dis. 2018;50(9):886–893. doi: 10.1016/j.dld.2018.06.016[↩]
- Vege SS, Yadav D, Chari ST. Pancreatitis. In: Talley NJ, GR Locke, Saito YA, editors. GI Epidemiology. Malden, MA: Blackwell Publishing;; 2007.[↩]
- Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–1261. doi: 10.1053/j.gastro.2013.01.068[↩]
- Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17(2):155–165. doi: 10.1016/j.pan.2017.01.005[↩]
- Nielsen JK, Olafsson S, Bergmann OM, Runarsdottir V, Hansdottir I, Sigurdardottir R, et al. Lifetime drinking history in patients with alcoholic liver disease and patients with alcohol use disorder without liver disease. Scand J Gastroenterol. 2017;52(6–7):762–767. doi: 10.1080/00365521.2017.1295466[↩]
- Lankisch PG, Assmus C, Lehnick D, Maisonneuve P, Lowenfels AB. Acute pancreatitis: does gender matter? Dig Dis Sci. 2001;46(11):2470–2474. doi: 10.1023/A:1012332121574[↩]
- Matta B, Gougol A, Gao X, Reddy N, Talukdar R, Kochhar R, Goenka MK, Gulla A, Gonzalez JA, Singh VK, Ferreira M, Stevens T, Barbu ST, Nawaz H, Gutierrez SC, Zarnescu NO, Capurso G, Easler J, Triantafyllou K, Pelaez-Luna M, Thakkar S, Ocampo C, de-Madaria E, Cote GA, Wu BU, Paragomi P, Pothoulakis I, Tang G, Papachristou GI. Worldwide Variations in Demographics, Management, and Outcomes of Acute Pancreatitis. Clin Gastroenterol Hepatol. 2020 Jun;18(7):1567-1575.e2. doi: 10.1016/j.cgh.2019.11.017[↩]
- Whitcomb DC, LaRusch J, Krasinskas AM, Klei L, Smith JP, Brand RE, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet. 2012;44(12):1349–1354. doi: 10.1038/ng.2466[↩]
- Pownall HJ, Ballantyne CM, Kimball KT, Simpson SL, Yeshurun D, Gotto AM., Jr Effect of moderate alcohol consumption on hypertriglyceridemia: a study in the fasting state. Arch Intern Med. 1999;159(9):981–987. doi: 10.1001/archinte.159.9.981[↩]
- Carr RA, Rejowski BJ, Cote GA, Pitt HA, Zyromski NJ. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology. 2016;16(4):469–476. doi: 10.1016/j.pan.2016.02.011[↩]
- Murphy MJ, Sheng X, MacDonald TM, Wei L. Hypertriglyceridemia and acute pancreatitis. JAMA Intern Med. 2013;173(2):162–164. doi: 10.1001/2013.jamainternmed.477[↩]
- Kiss L, Fur G, Matrai P, Hegyi P, Ivany E, Cazacu IM, et al. The effect of serum triglyceride concentration on the outcome of acute pancreatitis: systematic review and meta-analysis. Sci Rep. 2018;8(1):14096. doi: 10.1038/s41598-018-32337-x[↩]
- Kochar B, Akshintala VS, Afghani E, Elmunzer BJ, Kim KJ, Lennon AM, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81(1):143 e9–149 e9. doi: 10.1016/j.gie.2014.06.045[↩]
- Njei B, McCarty TR, Muniraj T, Sharma P, Jamidar PA, Aslanian HR, et al. Comparative effectiveness of pharmacologic and endoscopic interventions for prevention of post-ERCP pancreatitis: a network meta-analysis. Endosc Int Open. 2020;8(1):E29–e40. doi: 10.1055/a-1005-6366[↩]
- Vinklerová I, Procházka M, Procházka V, Urbánek K. Incidence, severity, and etiology of drug-induced acute pancreatitis. Dig Dis Sci. 2010;55(10):2977–2981. doi: 10.1007/s10620-010-1277-3[↩]
- Spanier BW, Tuynman HA, van der Hulst RW, Dijkgraaf MG, Bruno MJ. Acute pancreatitis and concomitant use of pancreatitis-associated drugs. Am J Gastroenterol. 2011;106(12):2183–2188. doi: 10.1038/ajg.2011.303[↩]
- Yadav D, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169(11):1035–1045. doi: 10.1001/archinternmed.2009.125[↩]
- Setiawan VW, Pandol SJ, Porcel J, Wilkens LR, Le Marchand L, Pike MC, et al. Prospective study of alcohol drinking, smoking, and pancreatitis: the multiethnic cohort. Pancreas. 2016;45(6):819–825. doi: 10.1097/MPA.0000000000000657[↩]
- Kiriyama S, Gabata T, Takada T, Hirata K, Yoshida M, Mayumi T, et al. New diagnostic criteria of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17(1):24–36. doi: 10.1007/s00534-009-0214-3[↩]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779[↩]
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15. doi: 10.1016/j.pan.2013.07.063[↩][↩]
- Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute technical review. Gastroenterology. 2018;154(4):1103–1139. doi: 10.1053/j.gastro.2018.01.031[↩]
- Pancreatitis. https://www.nice.org.uk/guidance/ng104/chapter/Recommendations[↩]
- Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N, Sartelli M, Wolbrink D, van Goor H, Baiocchi G, Ansaloni L, Biffl W, Coccolini F, Di Saverio S, Kluger Y, Moore E, Catena F. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019 Jun 13;14:27. doi: 10.1186/s13017-019-0247-0[↩]
- Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):175–184. doi: 10.1038/s41575-018-0087-5[↩]
- Raphael KL, Willingham FF. Hereditary pancreatitis: current perspectives. Clin Exp Gastroenterol. 2016 Jul 26;9:197-207. doi: 10.2147/CEG.S84358[↩][↩][↩]
- Wu D, Bampton TJ, Scott HS, Brown A, Kassahn K, Drogemuller C, De Sousa SM, Moore D, Ha T, Chen JW, Khurana S, Torpy DJ, Radford T, Couper R, Palmer L, Coates PT. The clinical and genetic features of hereditary pancreatitis in South Australia. Med J Aust. 2022 Jun 20;216(11):578-582. doi: 10.5694/mja2.51517[↩]
- Rebours V, Lévy P, Ruszniewski P. An overview of hereditary pancreatitis. Dig Liver Dis. 2012 Jan;44(1):8-15. doi: 10.1016/j.dld.2011.08.003[↩][↩]
- Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, Mayerle J, Drewes AM, Rebours V, Akisik F, Muñoz JED, Neoptolemos JP. Chronic pancreatitis. Nat Rev Dis Primers. 2017 Sep 7;3:17060. doi: 10.1038/nrdp.2017.60[↩][↩]
- Weiss FU. Pancreatic cancer risk in hereditary pancreatitis. Front Physiol. 2014 Feb 20;5:70. doi: 10.3389/fphys.2014.00070[↩][↩][↩][↩]
- Lévy P, Domínguez-Muñoz E, Imrie C, Löhr M, Maisonneuve P. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United European Gastroenterol J. 2014 Oct;2(5):345-54. doi: 10.1177/2050640614548208[↩]
- Eldredge, J., Couper, M.R., Moore, D.J., Khurana, S., Chen, J.W., Couper, J.J., Drogemuller, C.J., Radford, T., Kay, T.W., Loudovaris, T., Wilks, M., Coates, P.T. and Couper, R.T. (2021), South Australian experience with paediatric total pancreatectomy and islet autotransplantation for PRSS1-associated hereditary pancreatitis. Med J Aust, 215: 294-296.e1. https://doi.org/10.5694/mja2.51247[↩]
- Bellin MD, Freeman ML, Gelrud A, Slivka A, Clavel A, Humar A, Schwarzenberg SJ, Lowe ME, Rickels MR, Whitcomb DC, Matthews JB; PancreasFest Recommendation Conference Participants; Amann S, Andersen DK, Anderson MA, Baillie J, Block G, Brand R, Chari S, Cook M, Cote GA, Dunn T, Frulloni L, Greer JB, Hollingsworth MA, Kim KM, Larson A, Lerch MM, Lin T, Muniraj T, Robertson RP, Sclair S, Singh S, Stopczynski R, Toledo FG, Wilcox CM, Windsor J, Yadav D. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology. 2014 Jan-Feb;14(1):27-35. doi: 10.1016/j.pan.2013.10.009[↩][↩]
- Hereditary Pancreatitis. https://pancreasfoundation.org/hereditary-pancreatitis[↩][↩]
- Comfort MW, Steinberg AG. Pedigree of a family with hereditary chronic relapsing pancreatitis. Gastroenterology. 1952;21(1):54–63.[↩]
- Shelton CA, Umapathy C, Stello K, Yadav D, Whitcomb DC. Hereditary Pancreatitis in the United States: Survival and Rates of Pancreatic Cancer. Am J Gastroenterol. 2018 Sep;113(9):1376. doi: 10.1038/s41395-018-0194-5[↩][↩][↩][↩][↩]
- Rosendahl J, Bödeker H, Mössner J, Teich N. Hereditary chronic pancreatitis. Orphanet J Rare Dis. 2007 Jan 4;2:1. doi: 10.1186/1750-1172-2-1[↩][↩][↩]
- Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996 Oct;14(2):141-5. doi: 10.1038/ng1096-141[↩][↩][↩]
- Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, Truninger K, Ammann R, Cavallini G, Charnley RM, Uomo G, Delhaye M, Spicak J, Drumm B, Jansen J, Mountford R, Whitcomb DC, Neoptolemos JP; European Registry of Hereditary Pancreatitis and Pancreatic Cancer (EUROPAC). Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004 Mar;2(3):252-61. doi: 10.1016/s1542-3565(04)00013-8[↩][↩]
- Rebours V, Boutron-Ruault MC, Schnee M, Férec C, Le Maréchal C, Hentic O, Maire F, Hammel P, Ruszniewski P, Lévy P. The natural history of hereditary pancreatitis: a national series. Gut. 2009 Jan;58(1):97-103. doi: 10.1136/gut.2008.149179[↩][↩]
- Witt H, Beer S, Rosendahl J, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nat Genet. 2013 Oct;45(10):1216-20. doi: 10.1038/ng.2730[↩]
- Felderbauer P, Klein W, Bulut K, Ansorge N, Dekomien G, Werner I, Epplen JT, Schmitz F, Schmidt WE. Mutations in the calcium-sensing receptor: a new genetic risk factor for chronic pancreatitis? Scand J Gastroenterol. 2006 Mar;41(3):343-8. doi: 10.1080/00365520510024214[↩]
- Whitcomb DC, Preston RA, Aston CE, Sossenheimer MJ, Barua PS, Zhang Y, Wong-Chong A, White GJ, Wood PG, Gates LK Jr, Ulrich C, Martin SP, Post JC, Ehrlich GD. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996 Jun;110(6):1975-80. doi: 10.1053/gast.1996.v110.pm8964426[↩]
- Gorry MC, Gabbaizedeh D, Furey W, Gates LK Jr, Preston RA, Aston CE, Zhang Y, Ulrich C, Ehrlich GD, Whitcomb DC. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology. 1997 Oct;113(4):1063-8. doi: 10.1053/gast.1997.v113.pm9322498[↩]
- Applebaum-Shapiro SE, Finch R, Pfützer RH, Hepp LA, Gates L, Amann S, Martin S, Ulrich CD 2nd, Whitcomb DC. Hereditary pancreatitis in North America: the Pittsburgh-Midwest Multi-Center Pancreatic Study Group Study. Pancreatology. 2001;1(5):439-43. doi: 10.1159/000055844[↩]
- Otsuki M, Nishimori I, Hayakawa T, Hirota M, Ogawa M, Shimosegawa T; Research Committee on Intractable Disease of the Pancreas. Hereditary pancreatitis: clinical characteristics and diagnostic criteria in Japan. Pancreas. 2004 Mar;28(2):200-6. doi: 10.1097/00006676-200403000-00012[↩]
- Dytz MG, Mendes de Melo J, de Castro Santos O, da Silva Santos ID, Rodacki M, Conceição FL, Ortiga-Carvalho TM. Hereditary Pancreatitis Associated With the N29T Mutation of the PRSS1 Gene in a Brazilian Family: A Case-Control Study. Medicine (Baltimore). 2015 Sep;94(37):e1508. doi: 10.1097/MD.0000000000001508[↩]
- Pho-Iam T, Thongnoppakhun W, Yenchitsomanus PT, Limwongse C. A Thai family with hereditary pancreatitis and increased cancer risk due to a mutation in PRSS1 gene. World J Gastroenterol. 2005 Mar 21;11(11):1634-8. doi: 10.3748/wjg.v11.i11.1634[↩]
- Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010 Mar;7(3):131-45. doi: 10.1038/nrgastro.2010.6[↩]
- Bampton, T.J., Holmes-Walker, D.J., Drogemuller, C.J., Radford, T., Anderson, P., Etherton, C., Russell, C.H., Khurana, S., Torpy, D.J., Couper, J.J., Couper, R.L.T., Macintyre, P., Neo, E.L., Benitez-Aguirre, P., Thomas, G., Loudovaris, T., Thomas, H.E., Palmer, L.J., Wu, D., Rogers, N.M., Williams, L., Hawthorne, W.J., O’Connell, P.J., Kay, T.W., Pleass, H., Chen, J.W., Coates, P.T. and (2021), Australian experience with total pancreatectomy with islet autotransplantation to treat chronic pancreatitis. ANZ Journal of Surgery, 91: 2663-2668. https://doi.org/10.1111/ans.16853[↩][↩]
- Patel MR, Eppolito AL, Willingham FF. Hereditary pancreatitis for the endoscopist. Therap Adv Gastroenterol. 2013 Mar;6(2):169-79. doi: 10.1177/1756283X12467565[↩]
- Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, DiMagno EP. Cigarette Smoking as a Risk Factor for Pancreatic Cancer in Patients With Hereditary Pancreatitis. JAMA. 2001;286(2):169–170. doi:10.1001/jama.286.2.169[↩]
- Rebours V, Boutron-Ruault MC, Jooste V, Bouvier AM, Hammel P, Ruszniewski P, Lévy P. Mortality rate and risk factors in patients with hereditary pancreatitis: uni- and multidimensional analyses. Am J Gastroenterol. 2009 Sep;104(9):2312-7. doi: 10.1038/ajg.2009.363[↩][↩]
- Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, DiMagno EP. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001 Jul 11;286(2):169-70. doi: 10.1001/jama.286.2.169[↩]
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol. 2011 Dec;106(12):2192-9. doi: 10.1038/ajg.2011.328. Epub 2011 Sep 27. Erratum in: Am J Gastroenterol. 2011 Dec;106(12):2209.[↩]
- Bang UC, Benfield T, Hyldstrup L, Bendtsen F, Beck Jensen JE. Mortality, cancer, and comorbidities associated with chronic pancreatitis: a Danish nationwide matched-cohort study. Gastroenterology. 2014 Apr;146(4):989-94. doi: 10.1053/j.gastro.2013.12.033[↩]
- Sadr-Azodi O, Sanders DS, Murray JA, et al.: Patients with celiac disease have an increased risk for pancreatitis. 2012;10(10):1136–1142.e3. 10.1016/j.cgh.2012.06.023[↩]
- Pitchumoni CS, Chari S: Ulcerative colitis and autoimmune pancreatitis. 2013;47(6):469. 10.1097/MCG.0b013e31828a7099[↩]
- Gupte AR, Forsmark CE: Chronic pancreatitis. 2014;30(5):500–5. 10.1097/MOG.0000000000000094[↩]
- Yadav D, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, Bishop MD, Baillie J, Sherman S, DiSario J, Burton FR, Gardner TB, Amann ST, Gelrud A, Lawrence C, Elinoff B, Greer JB, O’Connell M, Barmada MM, Slivka A, Whitcomb DC; North American Pancreatic Study Group. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009 Jun 8;169(11):1035-45. doi: 10.1001/archinternmed.2009.125. Erratum in: Arch Intern Med. 2011 Apr 11;171(7):710.[↩]
- Singhvi A, Yadav D. Myths and realities about alcohol and smoking in chronic pancreatitis. Curr Opin Gastroenterol. 2018 Sep;34(5):355-361. doi: 10.1097/MOG.0000000000000466[↩]
- Pham A, Forsmark C. Chronic pancreatitis: review and update of etiology, risk factors, and management. F1000Res. 2018 May 17;7:F1000 Faculty Rev-607. doi: 10.12688/f1000research.12852.1[↩]
- Nassar Y, Richter S. Gastroparesis in Non-Diabetics: Associated Conditions and Possible Risk Factors. Gastroenterology Res. 2018 Oct;11(5):340-345. doi: 10.14740/gr1060w[↩][↩][↩][↩]
- Chowdhury RS, Forsmark CE, Davis RH, Toskes PP, Verne GN. Prevalence of gastroparesis in patients with small duct chronic pancreatitis. Pancreas. 2003 Apr;26(3):235-8. doi: 10.1097/00006676-200304000-00005[↩][↩]
- Ní Chonchubhair HM, Bashir Y, Dobson M, Ryan BM, Duggan SN, Conlon KC. The prevalence of small intestinal bacterial overgrowth in non-surgical patients with chronic pancreatitis and pancreatic exocrine insufficiency (PEI). Pancreatology. 2018 Jun;18(4):379-385. doi: 10.1016/j.pan.2018.02.010[↩][↩]
- Capurso G, Signoretti M, Archibugi L, Stigliano S, Delle Fave G. Systematic review and meta-analysis: Small intestinal bacterial overgrowth in chronic pancreatitis. United European Gastroenterol J. 2016 Oct;4(5):697-705. doi: 10.1177/2050640616630117[↩]
- Singh VV, Toskes PP. Small Bowel Bacterial Overgrowth: Presentation, Diagnosis, and Treatment. Curr Treat Options Gastroenterol. 2004 Feb;7(1):19-28. doi: 10.1007/s11938-004-0022-4[↩]
- Rösch T, Daniel S, Scholz M, Huibregtse K, Smits M, Schneider T, Ell C, Haber G, Riemann JF, Jakobs R, Hintze R, Adler A, Neuhaus H, Zavoral M, Zavada F, Schusdziarra V, Soehendra N; European Society of Gastrointestinal Endoscopy Research Group. Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy. 2002 Oct;34(10):765-71. doi: 10.1055/s-2002-34256[↩]
- Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010 Jun;24(3):349-58. doi: 10.1016/j.bpg.2010.02.007[↩]
- Duell EJ, Lucenteforte E, Olson SH, Bracci PM, Li D, Risch HA, Silverman DT, Ji BT, Gallinger S, Holly EA, Fontham EH, Maisonneuve P, Bueno-de-Mesquita HB, Ghadirian P, Kurtz RC, Ludwig E, Yu H, Lowenfels AB, Seminara D, Petersen GM, La Vecchia C, Boffetta P. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012 Nov;23(11):2964-2970. doi: 10.1093/annonc/mds140[↩]
- Uc A, Husain SZ. Pancreatitis in Children. Gastroenterology. 2019 May;156(7):1969-1978. doi: 10.1053/j.gastro.2018.12.043[↩]
- Cazacu IM, Farkas N, Garami A, Balaskó M, Mosdósi B, Alizadeh H, Gyöngyi Z, Rakonczay Z Jr, Vigh É, Habon T, Czopf L, Lazarescu MA, Erőss B, Sahin-Tóth M, Hegyi P. Pancreatitis-Associated Genes and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Pancreas. 2018 Oct;47(9):1078-1086. doi: 10.1097/MPA.0000000000001145[↩]
- Ramsey ML, Conwell DL, Hart PA. Complications of Chronic Pancreatitis. Dig Dis Sci. 2017 Jul;62(7):1745-1750. doi: 10.1007/s10620-017-4518-x[↩]
- Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, Shadaloey SA, Wu D, Preiss P, Verma N, Guo Y, Saxena A, Vardhan M, Diskin B, Wang W, Leinwand J, Kurz E, Kochen Rossi JA, Hundeyin M, Zambrinis C, Li X, Saxena D, Miller G. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019 Oct;574(7777):264-267. doi: 10.1038/s41586-019-1608-2[↩]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013 Jan;62(1):102-11. doi: 10.1136/gutjnl-2012-302779[↩]
- Dumonceau JM, Delhaye M, Tringali A, Dominguez-Munoz JE, Poley JW, Arvanitaki M, Costamagna G, Costea F, Devière J, Eisendrath P, Lakhtakia S, Reddy N, Fockens P, Ponchon T, Bruno M. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2012 Aug;44(8):784-800. doi: 10.1055/s-0032-1309840[↩]
- Hao, L., Pan, J., Wang, D., Bi, Y.-W., Ji, J.-T., Xin, L., Liao, Z., Du, T.-T., Lin, J.-H., Zhang, D., Zeng, X.-P., Ye, B., Zou, W.-B., Chen, H., Xie, T., Li, B.-R., Zheng, Z.-H., Hu, L.-H., and Li, Z.-S. (2017) Risk factors and nomogram for pancreatic pseudocysts in chronic pancreatitis: A cohort of 1998 patients. Journal of Gastroenterology and Hepatology, 32: 1403– 1411. doi: 10.1111/jgh.13748[↩]
- Pohl JF, Limbers CA, Kay M, Harman A, Rollins M, Varni JW. Health-related quality of life in pediatric patients with long-standing pancreatitis. J Pediatr Gastroenterol Nutr. 2012 May;54(5):657-63. doi: 10.1097/MPG.0b013e3182407c4f[↩]
- Lerch MM, Stier A, Wahnschaffe U, Mayerle J. Pancreatic pseudocysts: observation, endoscopic drainage, or resection? Dtsch Arztebl Int. 2009 Sep;106(38):614-21. doi: 10.3238/arztebl.2009.0614[↩]
- Dalsania R, Willingham FF. Treatment of walled-off pancreatic necrosis. Curr Opin Gastroenterol. 2019 Sep;35(5):478-482. doi: 10.1097/MOG.0000000000000564[↩]
- Adler JM, Gardner TB. Endoscopic Therapies for Chronic Pancreatitis. Dig Dis Sci. 2017 Jul;62(7):1729-1737. doi: 10.1007/s10620-017-4502-5[↩]
- Liu QY, Gugig R, Troendle DM, Bitton S, Patel N, Vitale DS, Abu-El-Haija M, Husain SZ, Morinville VD. The Roles of Endoscopic Ultrasound and Endoscopic Retrograde Cholangiopancreatography in the Evaluation and Treatment of Chronic Pancreatitis in Children: A Position Paper From the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Pancreas Committee. J Pediatr Gastroenterol Nutr. 2020 May;70(5):681-693. doi: 10.1097/MPG.0000000000002664[↩]
- Agarwal AK, Raj Kumar K, Agarwal S, Singh S. Significance of splenic vein thrombosis in chronic pancreatitis. Am J Surg. 2008 Aug;196(2):149-54. doi: 10.1016/j.amjsurg.2007.07.039[↩]
- Morinville VD, Husain SZ, Bai H, Barth B, Alhosh R, Durie PR, Freedman SD, Himes R, Lowe ME, Pohl J, Werlin S, Wilschanski M, Uc A; INSPPIRE Group. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr. 2012 Sep;55(3):261-5. doi: 10.1097/MPG.0b013e31824f1516. Erratum in: J Pediatr Gastroenterol Nutr. 2013 Apr;56(4):459. Abu-Al-Haija, Maisam [corrected to Abu-El-Haija, Maisam].[↩]
- Harris S, Nadkarni NA, Naina HV, Vege SS. Splanchnic vein thrombosis in acute pancreatitis: a single-center experience. Pancreas. 2013 Nov;42(8):1251-4. doi: 10.1097/MPA.0b013e3182968ff5[↩]
- Chowdhury, R.S. and Forsmark, C.E. (2003), Pancreatic function testing. Alimentary Pharmacology & Therapeutics, 17: 733-750. https://doi.org/10.1046/j.1365-2036.2003.01495.x[↩]
- Tirkes T, Lin C, Fogel EL, Sherman SS, Wang Q, Sandrasegaran K. T1 mapping for diagnosis of mild chronic pancreatitis. J Magn Reson Imaging. 2017 Apr;45(4):1171-1176. doi: 10.1002/jmri.25428[↩]
- Lesniak RJ, Hohenwalter MD, Taylor AJ. Spectrum of causes of pancreatic calcifications. AJR Am J Roentgenol. 2002 Jan;178(1):79-86. doi: 10.2214/ajr.178.1.1780079[↩]
- Klöppel G, Maillet B. Chronic pancreatitis: evolution of the disease. Hepatogastroenterology. 1991 Oct;38(5):408-12.[↩]
- Andersen PL, Madzak A, Olesen SS, Drewes AM, Frøkjaer JB. Quantification of parenchymal calcifications in chronic pancreatitis: relation to atrophy, ductal changes, fibrosis and clinical parameters. Scand J Gastroenterol. 2018 Feb;53(2):218-224. doi: 10.1080/00365521.2017.1415372[↩]
- Nagai H, Ohtsubo K. Pancreatic lithiasis in the aged. Its clinicopathology and pathogenesis. Gastroenterology. 1984 Feb;86(2):331-8.[↩]
- Axon AT, Classen M, Cotton PB, Cremer M, Freeny PC, Lees WR. Pancreatography in chronic pancreatitis: international definitions. Gut. 1984 Oct;25(10):1107-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1432537/pdf/gut00395-0097.pdf[↩]
- Conwell DL, Lee LS, Yadav D, Longnecker DS, Miller FH, Mortele KJ, Levy MJ, Kwon R, Lieb JG, Stevens T, Toskes PP, Gardner TB, Gelrud A, Wu BU, Forsmark CE, Vege SS. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: evidence-based report on diagnostic guidelines. Pancreas. 2014 Nov;43(8):1143-62. doi: 10.1097/MPA.0000000000000237[↩]
- Beyer G, Mahajan UM, Budde C, Bulla TJ, Kohlmann T, Kuhlmann L, Schütte K, Aghdassi AA, Weber E, Weiss FU, Drewes AM, Olesen SS, Lerch MM, Mayerle J. Development and Validation of a Chronic Pancreatitis Prognosis Score in 2 Independent Cohorts. Gastroenterology. 2017 Dec;153(6):1544-1554.e2. doi: 10.1053/j.gastro.2017.08.073[↩]
- Chronic Pancreatitis Differential Diagnoses. https://emedicine.medscape.com/article/181554-differential[↩]
- Witt H, Apte MV, Keim V, et al.: Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. 2007;132(4):1557–73. 10.1053/j.gastro.2007.03.001[↩]
- Duggan S, O’Sullivan M, Feehan S, et al.: Nutrition treatment of deficiency and malnutrition in chronic pancreatitis: a review. 2010;25(4):362–70. 10.1177/0884533610373772[↩]
- Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, Goodarzi MO, Habtezion A, Korc M, Kudva YC, Pandol SJ, Yadav D, Chari ST; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer(CPDPC). Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016 Nov;1(3):226-237. doi: 10.1016/S2468-1253(16)30106-6[↩][↩][↩]
- Freeman AJ, Maqbool A, Bellin MD, Goldschneider KR, Grover AS, Hartzell C, Piester TL, Szabo F, Kiernan BD, Khalaf R, Kumar R, Rios M, Husain SZ, Morinville VD, Abu-El-Haija M. Medical Management of Chronic Pancreatitis in Children: A Position Paper by the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Pancreas Committee. J Pediatr Gastroenterol Nutr. 2021 Feb 1;72(2):324-340. doi: 10.1097/MPG.0000000000003001[↩]
- Wilson PR. Multidisciplinary management of chronic pain. A practical guide for clinicians. Pain Med 2016;17:1376–8.[↩]
- Sheehy KA, Lippold C, Rice AL, Nobrega R, Finkel JC, Quezado ZM. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: a single-center cohort study. J Pain Res. 2017 Apr 5;10:787-795. doi: 10.2147/JPR.S131156[↩]
- Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009 Nov;54(11):2330-7. doi: 10.1007/s10620-008-0651-x[↩]
- Stevens T, Costanzo A, Lopez R, Kapural L, Parsi MA, Vargo JJ. Adding triamcinolone to endoscopic ultrasound-guided celiac plexus blockade does not reduce pain in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2012 Feb;10(2):186-91, 191.e1. doi: 10.1016/j.cgh.2011.09.006[↩]