Kidney stones
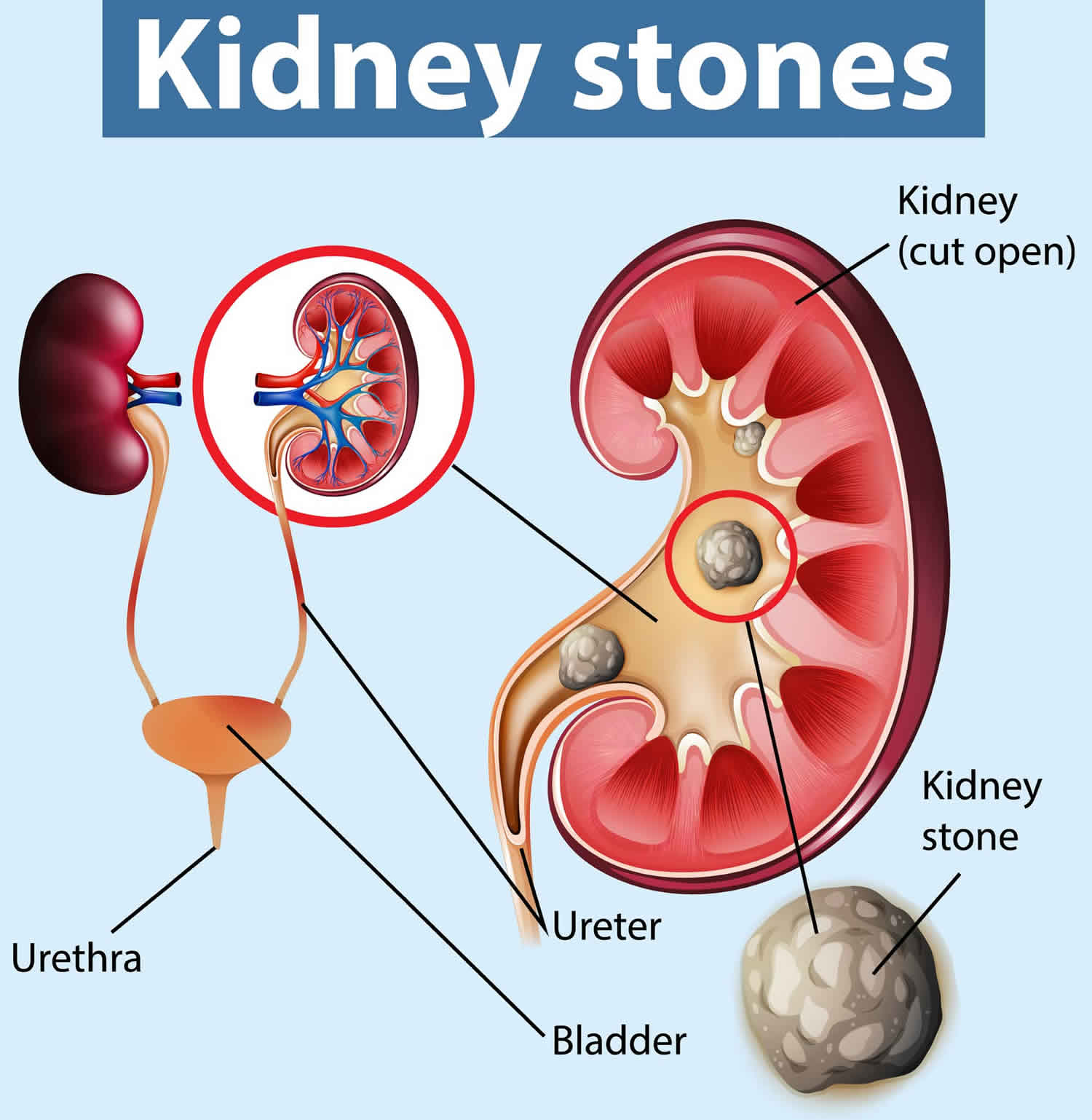
Kidney stones also called renal calculi or nephroliths, are hard, solid lumps that form in your kidney. You may hear doctors call kidney stones as nephrolithiasis, urolithiasis, or urinary stones. Kidney stones vary in size and shape. Some kidney stones are as small as a grain of sand. Others are as large as a tiny pebble to kidney stones several centimeters in diameter. Rarely, some kidney stones are as big as golf balls. A large kidney stone, called a staghorn calculus, can fill an entire renal calyceal system. As a general rule, the larger the kidney stone, the more noticeable are your symptoms. Kidney stones are made out of the waste products in your urine. A kidney stone may stay in your kidney. Kidney stone also may travel down the urinary tract. The urinary tract includes the ureters, bladder, and the urethra (Figure 1 and 2). If the stone is big enough, it can get stuck in your kidney or urinary tract. This can be very painful.
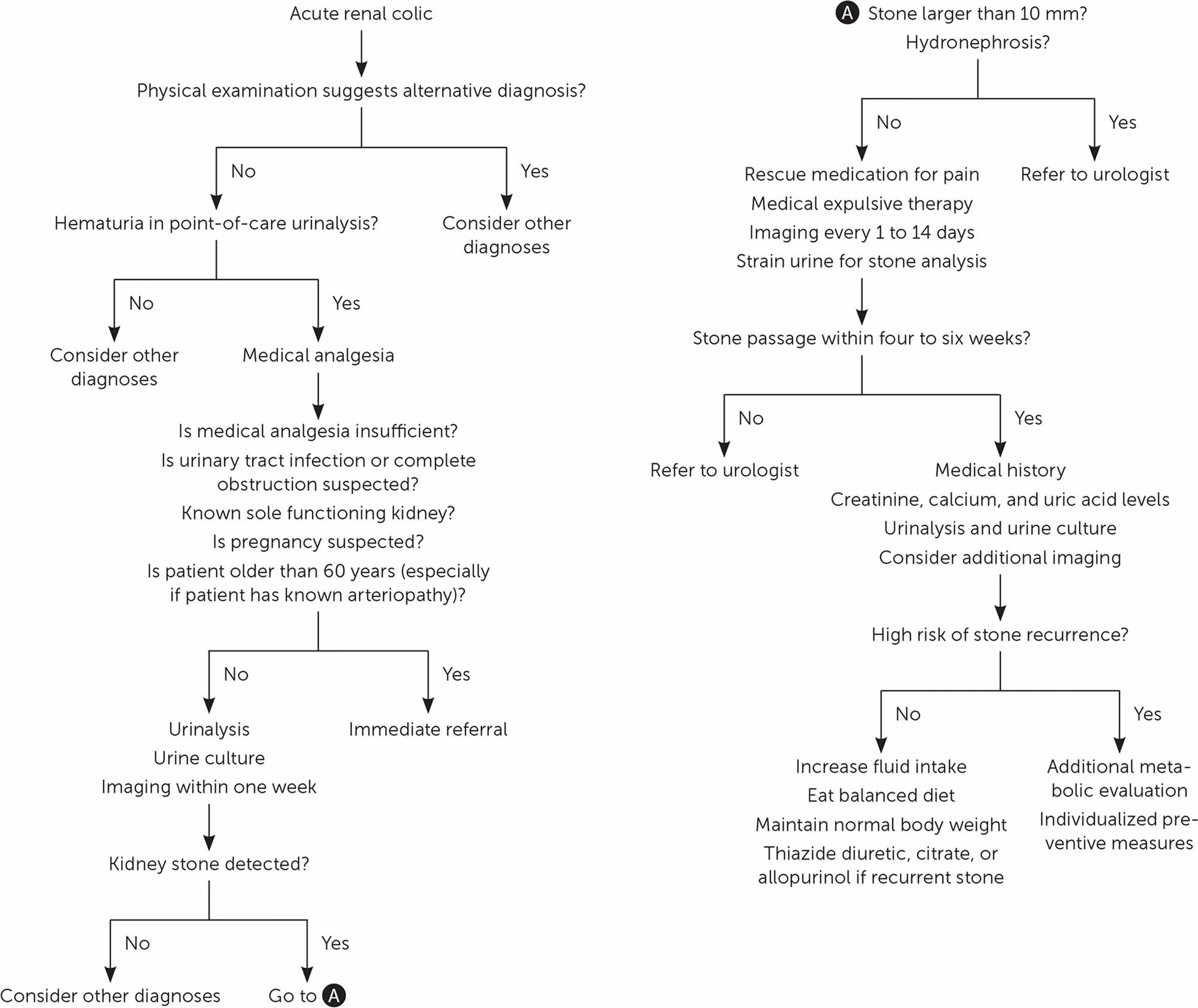
Kidney stones may be smooth or jagged and are usually yellow or brown. Most ureteral stones under 5 mm pass spontaneously 1. About 90% of ureteric stones smaller than 5 mm pass spontaneously, compared with about 50% of stones between 5 mm and 10 mm, so conservative management is preferred for ureteric stones 2. Depending on the size of the kidney stone, the average time to pass kidney stone ranges between one week and three weeks, and the passage of the stone is most accurately assessed by a plain film (kidney-ureter-bladder or KUB view) every one to two weeks to monitor progression. An observation period of three to four weeks is reasonable unless urgent intervention is indicated for intractable symptoms, infection, or obstruction 1.
Kidney stones affect up to 12% of the world population, with a lifetime risk of passing a kidney stone of about 8-10% 3, 4, 5. The prevalence of kidney stones was 10% during 2013–2014. People are most likely to develop kidney stones between ages 40 and 60, though the stones can appear at any age. In the United States, up to 19% of men and 9% of women will develop kidney stones in their lifetime 6, 7, 8, 9. Caucasians are more likely to develop kidney stones than African Americans. About 1/1000 adults in the US is hospitalized annually because of kidney stones, which are also found in about 1% of all autopsies 9. Research shows that 35 to 50 percent of people who have one kidney stone will develop additional stones, usually within 10 years of the first stone.
Increased incidence of kidney stones in the industrialized world is associated with improved standards of living and is strongly associated with race or ethnicity and region of residence 10. Other diseases such as high blood pressure, diabetes, and obesity may increase the risk for kidney stones. A seasonal variation is also seen, with high urinary calcium oxalate saturation in men during summer and in women during early winter 11. Men are more likely to develop kidney stones than women and kidney stones form twice as often in men as women 1. The peak age in men is 30 years; women have a bimodal age distribution, with peaks at 35 and 55 years 1. Once a kidney stone forms, the probability that a second stone will form within five to seven years is approximately 50% 4. In postmenopausal women, the occurrence of kidney stones is associated with a history of hypertension and a low dietary intake of magnesium and calcium 12.
The prevalence of kidney stones (nephrolithiasis) is increasing in women and with increasing age. Table 1 includes rates of different types of kidney stones in children and adults 13, 14, 15. Contributing risk factors for kidney stones are obesity, insulin resistance, gastrointestinal pathology, living in warmer climates, and certain dietary patterns and medications 16, 17.
Table 1. Incidence of kidney stones in children and adults
| Type | Children (%) | Adult (%) |
|---|---|---|
| Calcium oxalate | 45 to 65 | 56 to 61 |
| Calcium phosphate | 24 to 30 | 8 to 18* |
| Cystine | 5 to 8 | 1 |
| Struvite (magnesium ammonium phosphate) | 7 to 13 | 2 to 4 |
| Uric acid | 2 to 4 | 9 to 17 |
| Other | 4 | 2 |
Footnote: *Incidence is as high as 75 percent in pregnant women
[Source 18 ]Healthy kidneys remove waste products from your blood. These waste products leave your body in the urine your kidneys make. When the waste products don’t properly leave your kidneys, it can result in kidney stones.
Kidney stone starts to hurt when it causes irritation or blockage to the flow of urine out of the kidneys 19 . This builds rapidly to extreme pain. Kidney stones can cause a severe cramping pain in your lower back or side. The pain usually moves down toward your abdomen, groin, or genitals as the stone moves down the urinary tract. Other symptoms may include:
- Nausea and vomiting
- Cloudy or bloody urine
- Urine that smells bad or looks cloudy.
- Fever and chills
- Feeling like you need to go to the bathroom more often than usual
An episode of renal colic (kidney stone pain) has a sudden onset, with fluctuation and intensification over 15 to 45 minutes. Pain caused by a kidney stone may change — for instance, shifting to a different location or increasing in intensity — as the stone moves through your urinary tract.
Kidney stones may obstruct the urinary tract and impair kidney function. There is increased risk of infection with chronic obstruction. Bleeding may be chronic and accompany obstruction. The size, number, and metabolic composition of new stones strongly influence the natural history and complication rates.
In most cases, kidney stones pass without causing damage, but usually not without causing a lot of pain. Pain relievers may be the only treatment needed for small stones. Other treatment may be needed, especially for those stones that cause lasting symptoms or other complications. In severe cases, however, surgery may be required.
To find out if you have a kidney stone, your doctor will ask you about your symptoms. He or she will take a sample of your urine. Your doctor will order images of your kidneys and urinary tract.
If your kidney stone is small enough, you might be able to pass it in your urine. Most of the time, you’ll be able to pass your kidney stone without the help of a doctor.
If your kidney stone is too big to pass in your urine, your kidney stone will get stuck in the urinary tract and block the flow of your urine, causing severe pain or bleeding. If your kidney stone is causing an infection, you may need a doctor, such as a urologist’s help. Your doctor can give you medicine to help with your kidney stone pain.
If you have symptoms of kidney stones, including severe pain or bleeding, seek medical professional care right away. A doctor, such as a urologist, can treat any pain and prevent further problems, such as a urinary tract infection (UTI).
The following may be signs of kidney stones that need a doctor’s help:
- Extreme pain in your back or side that will not go away
- Blood in your urine
- Fever and chills
- Vomiting
- Urine that smells bad or looks cloudy
- A burning feeling when you urinate
Kidney stone pain can be very bad. Over-the-counter pain medicines (for example, ibuprofen and naproxen), either alone or along with narcotics, can be very effective. Some people with severe pain from kidney stones need to stay in the hospital. You may need to get fluids through an IV into your vein.
Treatment for kidney stones usually depends on their size, location, and what they are made of 20. Approximately 86% of kidney stones pass spontaneously; this proportion is lower for stones larger than 6 mm (59% vs. 90% for smaller stones) 21. Although stones larger than 6 mm in diameter are often removed by urologists (urinary tract surgical specialists) 22, these are the stones that have greatest benefit from medical expulsive therapy 23. Medical expulsive therapy with alpha blockers (e.g., tamsulosin [Flomax], 0.4 mg per day; doxazosin [Cardura], 4 mg per day) hastens and increases the likelihood of kidney stone passage, reduces pain, and prevents surgical interventions and hospital admissions 23, 22. These medications should be offered to patients with distal ureteral stones 5 to 10 mm in diameter 23. Tamsulosin is the most studied medication, but other alpha blockers seem equally effective 23. Calcium channel blockers (e.g., nifedipine) are less effective and may be no more effective than placebo 24, 25. Coadministration of oral corticosteroids or increasing fluid intake does not hasten stone passage or alleviate renal colic 26, 22.
Your doctor may also use a special machine that uses shock waves to break up the stone into smaller pieces. This is called extracorporeal shock wave lithotripsy, or ESWL. Shock-wave lithotripsy is a noninvasive procedure that uses high-energy sound waves to blast the stones into fragments that are then more easily passed out in the urine.
A urologist (urinary tract surgical specialist) can put a very thin instrument called an endoscope through your urethra and into your bladder and ureters to find the stone, the procedure is called uteroscopy. He or she can then pull the stone out or break it into smaller pieces. If a doctor does this, you will be given medicine to numb the area first.
Rarely, for very large or complicated stones, doctors will use percutaneous nephrolithomy or nephrolithotripsy.
Surgery is also an option and is sometimes the only way to get rid of a kidney stone.
Surgery is often needed if:
- The kidney stone is too large to pass on its own.
- The kidney stone is growing.
- The kidney stone is blocking urine flow and causing an infection or kidney damage.
- The pain cannot be controlled.
Make an appointment with your doctor if you have any signs and symptoms that worry you.
See your doctor if you have symptoms of a kidney stone:
- Severe pain in your back or side that will not go away
- Blood in your urine
- Fever and chills
- Nausea and vomiting
- Urine that smells bad or looks cloudy
- A burning feeling when you urinate
If you have been diagnosed with blockage from a kidney stone, passage of the kidney stone must be confirmed either by capture in a strainer during urination or by follow-up x-ray. Being pain free does not confirm that the kidney stone has passed. Kidney damage or kidney scarring can occur if treatment is delayed for too long.
Does the type of kidney stone I had affect food choices I should eat?
Yes. If you have already had kidney stones, ask your doctor which type of kidney stone you had. Based on the type of kidney stone you had, you may be able to prevent kidney stones by making changes in how much sodium, animal protein, calcium, or oxalate is in the food you eat.
You may need to change what you eat and drink for these types of kidney stones:
- Calcium oxalate stones
- Calcium phosphate stones
- Uric acid stones
- Cystine stones
A dietitian who specializes in kidney stone prevention can help you plan meals to prevent kidney stones.
Kidney anatomy
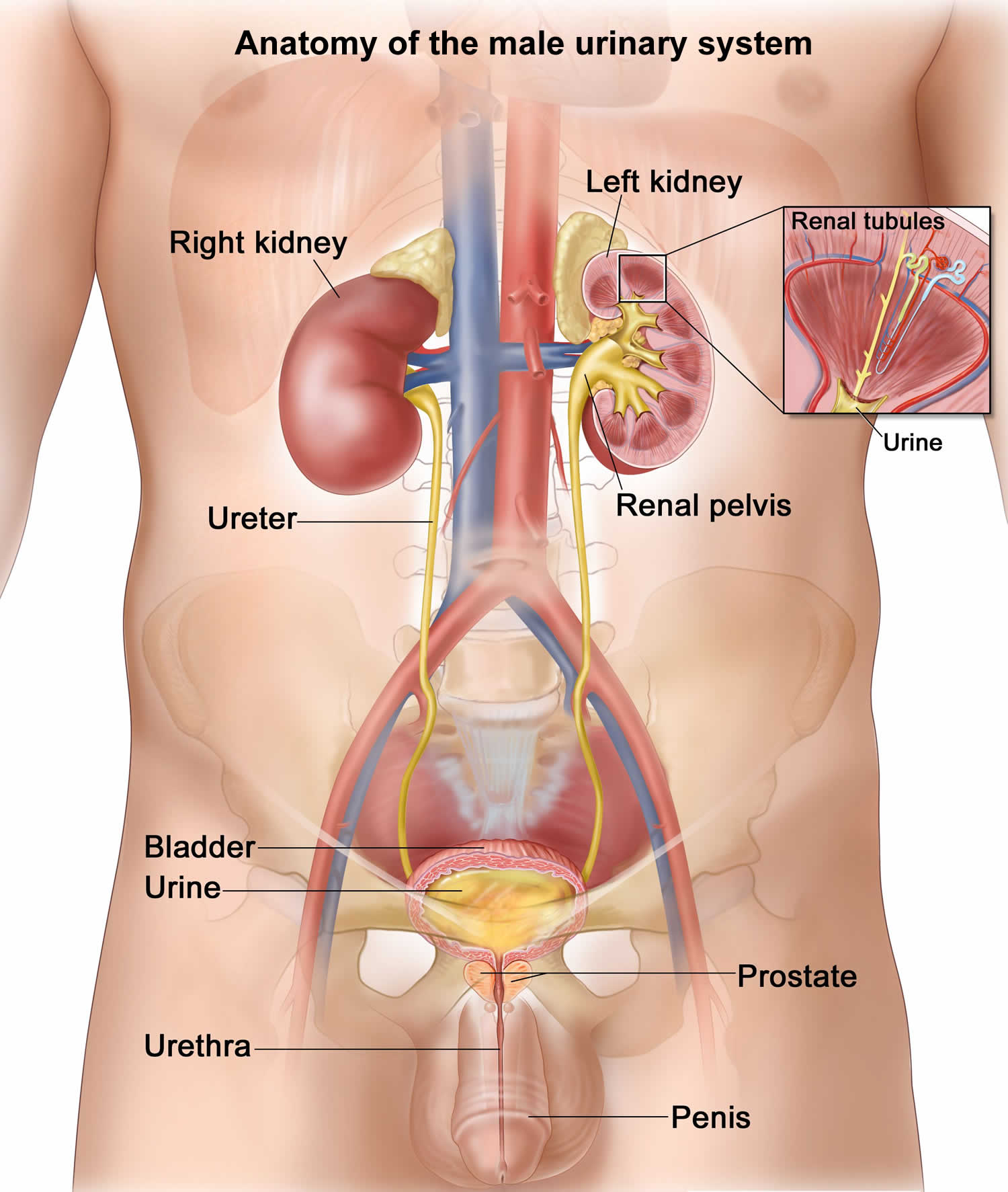
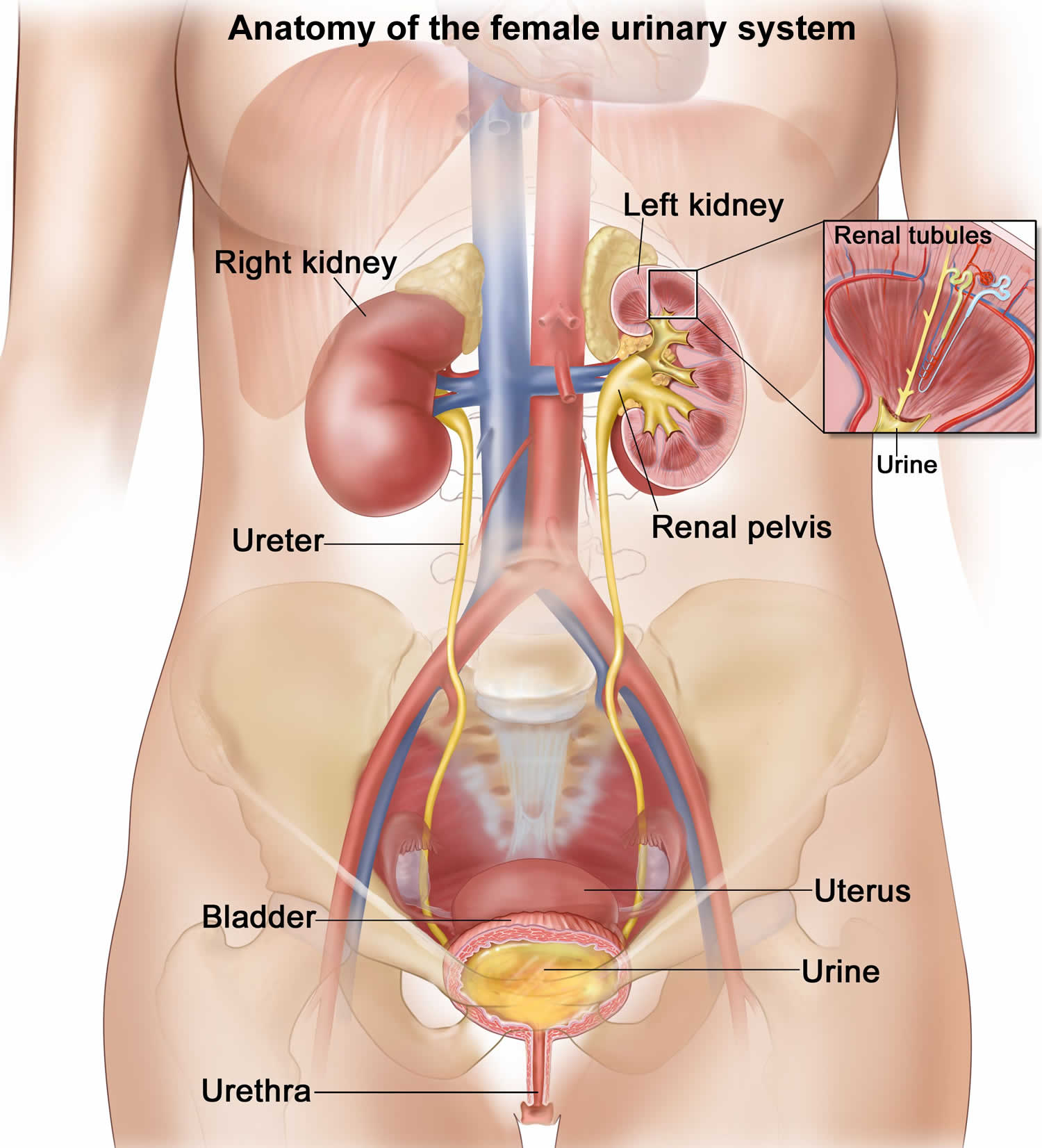
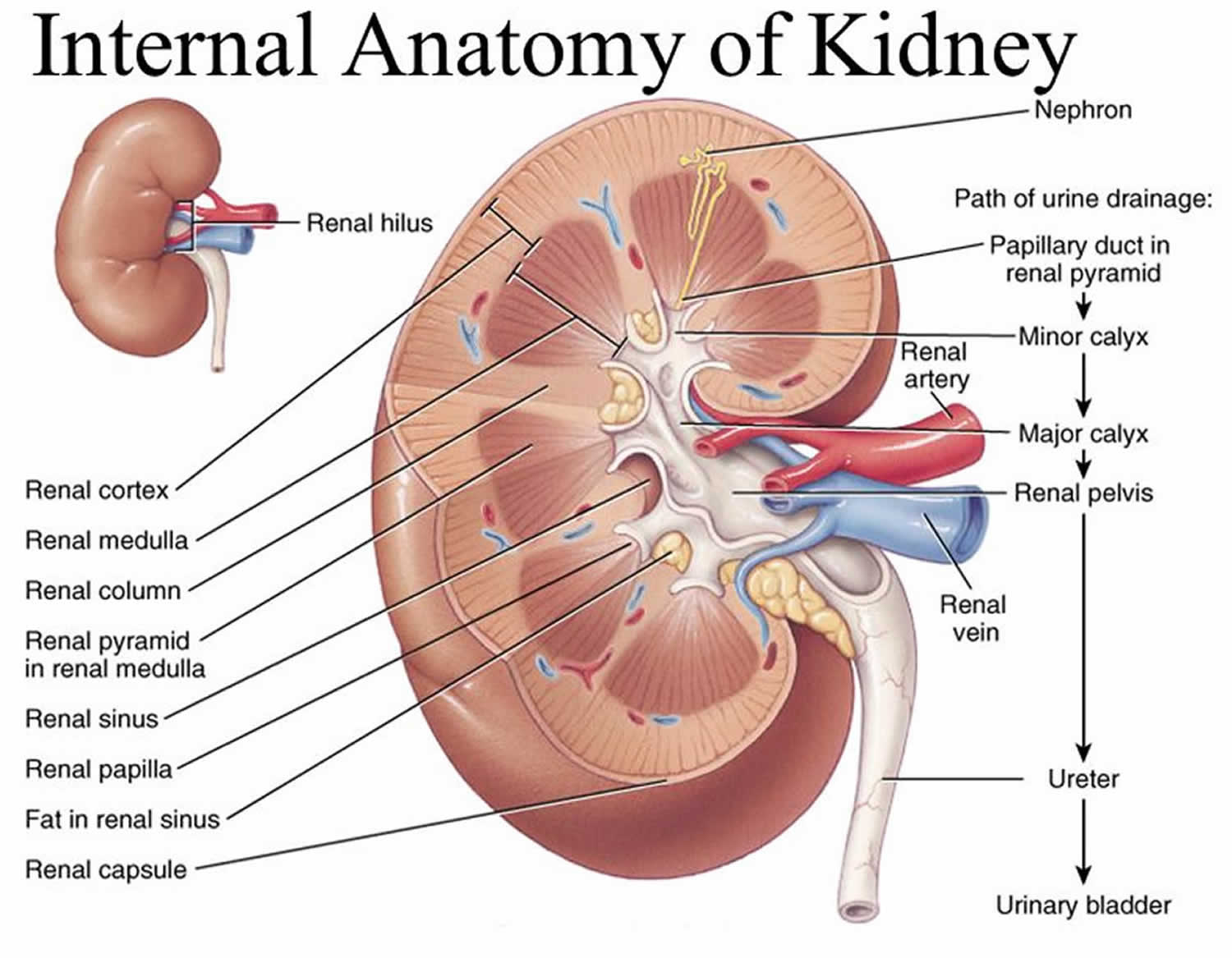
The kidneys are a pair of bean-shaped organs, each about the size of a fist. They are attached to the upper back wall of the abdomen and protected by the lower rib cage. One kidney is just to the left and the other just to the right of the backbone. The upper and lower portions of each kidney are sometimes called the superior pole and inferior pole.
The kidneys’ main job is to remove excess water, salt, and waste products from your blood coming in from the renal arteries. These substances become urine. Urine collects in the center of each kidney in an area called the renal pelvis and then leaves the kidneys through long slender tubes called ureters. The ureters lead to the bladder, where the urine is stored until you urinate.
Urine is generally considered sterile. The urinary system can be divided into the upper urinary tract and lower urinary tract:
- Upper urinary tract consists of the kidneys (renal parenchyma and collecting system) and the ureters
- Kidneys. Two bean-shaped organs, each about the size of a fist. They are located just below your rib cage, one on each side of your spine. Every day, your kidneys filter about 120 to 150 quarts of blood to remove wastes and balance fluids. This process produces about 1 to 2 quarts of urine per day.
- Ureters. Thin tubes of muscle that connect your kidneys to your bladder and carry urine to the bladder.
- Lower urinary tract includes the bladder (responsible for storage and elimination of urine), the urethra (tube through which urine exits the bladder to the outside world), and prostate in men. In the female, the urethra exits the bladder near the vaginal area, the vagina could contribute to contamination of urine specimens. In the male, the urethra exits the bladder, passes through the prostate, and then through the penile urethra. The foreskin when present may contribute to infection in select instances.
- Bladder. A hollow, muscular, balloon-shaped organ that expands as it fills with urine. The bladder sits in your pelvis between your hip bones. A normal bladder acts like a reservoir. It can hold 1.5 to 2 cups of urine. Although you do not control how your kidneys function, you can control when to empty your bladder. Bladder emptying is known as urination.
- Urethra. A tube located at the bottom of the bladder that allows urine to exit the body during urination.
All parts of the urinary tract—the kidneys, ureters, bladder, and urethra—must work together to urinate normally. The urinary tract is important because it filters wastes and extra fluid from the bloodstream and removes them from the body.
The kidneys also have other jobs:
- They help control blood pressure by making a hormone called renin.
- They help make sure the body has enough red blood cells by making a hormone called erythropoietin. This hormone tells the bone marrow to make more red blood cells.
Your kidneys are important, but you can function with only one kidney. Many people in the United States are living normal, healthy lives with just one kidney.
Some people do not have working kidneys at all, and survive with the help of a medical procedure called dialysis. The most common form of dialysis uses a specially designed machine that filters blood much like a real kidney would.
Figure 1. Anatomy of the male urinary system
Figure 2. Anatomy of the female urinary system
Figure 3. Normal Kidney Anatomy
Figure 4. Kidney stones
Kidney stones causes
Kidney stones often have no definite, single cause, although several factors such as your age, sex, genetics, and extrinsic factors such as geography, climate, dietary, mineral composition, and water intake may increase your risk 27, 28. Your urine has various wastes dissolved in it. When there is too much waste in too little liquid, crystals begin to form. The crystals attract other elements and join together to form a solid that will get larger unless it is passed out of the body with the urine. These crystals can develop into stones over weeks or months. Usually, these chemicals are eliminated in the urine by your kidneys. In most people, having enough liquid washes them out or other chemicals in urine stop a stone from forming. The cause of different types of kidney stones depends on the type of stone. The stone-forming chemicals are calcium, oxalate, urate, cystine, xanthine, and phosphate. Calcium stones are most common. Calcium stones are most likely to occur in men between ages 20 to 30. Calcium can combine with other substances to form the stone. Oxalate is the most common of these. Oxalate is a substance made daily by your liver or absorbed from your diet. Oxalate is present in certain foods such as spinach as well as nuts and chocolate. Oxalate is also found in vitamin C supplements. Dietary factors, high doses of vitamin D, intestinal bypass surgery, diseases of the small intestine and several metabolic disorders can increase the concentration of calcium or oxalate in urine.
Calcium stones can also form from combining with phosphate or carbonate. This type of stone is more common in metabolic conditions, such as renal tubular acidosis. It may also be associated with certain medications used to treat migraines or seizures, such as topiramate (Topamax, Trokendi XR, Qudexy XR).
Other types of kidney stones include:
- Struvite stone (magnesium ammonium phosphate): A struvite stone is more common in women. Struvite stone usually forms after a chronic urinary tract infection (UTI). These stones are usually made of ammonia. Struvite stones can grow very large and can block the kidney, ureter, or bladder.
- Uric acid stone: Uric acid stone is another common type of kidney stone. A uric acid stone forms when there is too much uric acid in the urine. You may be at risk for this type of stone if you eat a high-protein diet or if you’ve received chemotherapy. Foods such as organ meats and shellfish have high concentrations of a natural chemical compound known as purines. High purine intake leads to a higher production of monosodium urate, which, under the right conditions, may form stones in the kidneys. The formation of uric acid stones tends to run in families. Uric acid stones can form in people who lose too much fluid because of chronic diarrhea or malabsorption and those with diabetes or metabolic syndrome. Certain genetic factors also may increase your risk of uric acid stones.
- Cystine stone: A cystine stone is rare. The disease that causes cystine stones to form runs in families and is called cystinuria. It affects both men and women.
Possible causes of kidney stones include drinking too little water, obesity, weight loss surgery, or eating food with too much salt or sugar. Infections and family history might be important in some people. Eating too much fructose correlates with increasing risk of developing a kidney stone. Fructose can be found in table sugar and high fructose corn syrup.
Other substances, such as certain medicines, also can form stones (see Table 2).
After kidney stone is formed, the stone may stay in your kidney or travel down the urinary tract into the ureter. Sometimes, tiny kidney stones move out of your body in the urine without causing too much pain. But kidney stones that don’t move may cause a back-up of urine in the kidney, ureter, the bladder, or the urethra. This is what causes the pain.
You are more likely to develop kidney stones if you have these conditions, including:
- anatomical abnormalities that increase the risk of stone disease
- a blockage of the urinary tract
- chronic, or long-lasting, inflammation of the bowel
- cystic kidney diseases, which are disorders that cause fluid-filled sacs to form on the kidneys
- cystinuria
- digestive problems or a history of gastrointestinal tract surgery
- gout, a disorder that causes painful swelling of the joints
- hypercalciuria, a condition that runs in families in which urine contains unusually large amounts of calcium; this is the most common condition found in people who form calcium stones
- hyperoxaluria, a condition in which urine contains unusually large amounts of oxalate
- hyperparathyroidism, a condition in which the parathyroid glands release too much parathyroid hormone, causing extra calcium in the blood
- hyperuricosuria, a disorder in which too much uric acid is in the urine
- obesity
- repeated, or recurrent, urinary tract infections (UTIs)
- renal tubular acidosis, a disease that occurs when the kidneys fail to remove acids into the urine, which causes a person’s blood to remain too acidic.
Anatomical abnormalities that increase the risk of kidney stone disease
- Obstruction of the pelviureteral junction
- Hydronephrotic renal pelvis or calices
- Calyceal diverticulum
- Horseshoe kidney
- Ureterocele
- Vesicoureteral reflux (VUR)
- Ureteral stricture
- Tubular ectasia (medullary sponge kidney)
People who take certain medicines. You are more likely to develop kidney stones if you are taking one or more of the following medicines over a long period of time:
- diuretics, often called water pills, which help rid your body of water
- calcium-based antacids
- indinavir, a protease inhibitor used to treat HIV infection
- topiramate, an anti-seizure medication
Drug-induced kidney stones
Drug-induced kidney stones accounts for about 1% of all stone types 29. Drugs such as guaifenesin, triamterene, atazanavir, and sulfa drugs induce these kidney stones. For instance, people who take the protease inhibitor indinavir sulphate, a drug used to treat HIV infection, are at risk of developing kidney stones 30. Such lithogenic drugs or its metabolites may deposit to form a nidus or on renal calculi already present. On the other hand, these drugs may induce the formation of calculi through its metabolic action by interfering with calcium oxalate or purine metabolisms 31.
Table 2. Medications associated with kidney stone formation
| Type of medication | Examples |
|---|---|
| Agents that decrease uric acid production | Allopurinol (Zyloprim) |
| Laxatives (specific to ammonium urate stones), especially if abused | Overuse of any laxative resulting in electrolyte losses |
| Antibiotics | Sulfonamides, ampicillin, amoxicillin, ceftriaxone (Rocephin), quinolones, furans, pyridines |
| Carbonic anhydrase inhibitors | Acetazolamide, topiramate (Topamax) |
| Ephedra alkaloids (banned in the United States) | Herbal products used as stimulants and appetite suppressants |
| Potassium channel blockers | Amiodarone, sotalol (Betapace), dalfampridine (Ampyra; multiple sclerosis therapy) |
| Potassium-sparing diuretics | Triamterene (Dyrenium) |
| Reverse transcriptase inhibitors and protease inhibitors | HAART (highly active antiretroviral therapy) |
| Sulfonylureas | Various therapies for type 2 diabetes mellitus |
Hypercalciuria
Hypercalciuria is defined as excretion of urinary calcium exceeding 200 mg in a 24 hour collection or an excess of 4 mg calcium/kg/24 hour 1. Hypercalciuria has also been variously defined as 24 hour urinary calcium >300 mg/day in men, >250 mg/day in women or >200 mg/day in either sex while on a diet restricted in calcium, sodium, and animal protein 32, 33.
Hypercalciuria is the most common metabolic abnormality in patients with calcium oxalate stones and results from various mechanisms 1. Hypercalciuria may be a manifestation of systemic diseases such as primary hyperparathyroidism or sarcoidosis, but is considered idiopathic if no underlying cause can be identified 34. While idiopathic hypercalciuria has been shown to have a genetic predisposition in some cases, it can also be influenced by environmental factors such as diet 35. Although kidney stone formers have a higher risk of bone fracture than those in the general population36, it is not clear that hypercalciuria is a cause. In a retrospective study of 250 men and 182 post-menopausal women on or off estrogen therapy, no significant relationship was found between urine calcium levels and bone mineral density. Hypercalciuria may not cause low bone mineral density and increased fracture rate among stone formers 37.
A normal calcium intake (1 000–1 200 mg/day elemental calcium or approximately three servings of dairy daily) is recommended for patients with idiopathic hypercalciuria 38, 39. Both dairy and non-dairy dietary sources of calcium have been shown to have a protective effect against incident stone formation 40. On the other hand, severe calcium restriction should be avoided as it may accelerate bone loss and lead to hyperoxaluria due to the interaction between calcium and oxalate in the intestinal lumen by which calcium binds to oxalate and forms a calcium oxalate complex. In the setting of low calcium intake, excess uncomplexed oxalate is absorbed, ultimately leading to increased urinary oxalate excretion 41, 42, 43. Calcium in the form of food is preferred over calcium supplements as supplementation has been shown in epidemiologic studies to be associated with an increased risk of incident stone formation 44, 45, 46. If calcium supplements are indicated, they should be taken with meals, allowing the ingested calcium to complex with oxalate, thereby reducing intestinal oxalate absorption and counteracting the effect of increased urinary calcium.
- Absorptive hypercalciuria: Increased absorption of calcium from the gut results in increased circulating calcium, resulting in increased renal filtered load. The exact mechanism is unknown but seems to be inherited in an autosomal dominant fashion, and the jejunal mucosa is hyper-responsive to vitamin D. Absorptive hypercalciuria is very common, but most patients remain asymptomatic and do not experience stone formation.
- Renal hypercalciuria: Increased excretion of calcium in urine results from impaired renal tubular absorption of calcium. This occurs in about 2% of patients with recurrent stone formation.
- Resorptive hypercalciuria: Increased resorption of bone occurs as a result of primary hyperparathyroidism. This occurs in about 5% of patients with recurrent stone formation. The risk of renal stones is increased in primary hyperparathyroidism and returns to baseline about 10 years after parathyroidectomy. Patients who had stones before undergoing parathyroidectomy have a 27 times greater risk of stone formation after parathyroidectomy than do patients without hyperparathyroidism 47.
Hyperuricosuria
Uric acid is the end product of purine metabolism and is either derived from exogenous (dietary) sources (animal protein is a rich source of purines) or produced endogenously during cell turnover. Chronic metabolic acidosis can result in protein metabolism and thus increased excretion of urate and formation of kidney stones. Urinary uric acid has been shown to reduce the effectiveness of naturally occurring macromolecular inhibitors of calcium oxalate crystallization 48. In addition, protein derived from animal sources increases stone risk by increasing urinary calcium and oxalate and reducing pH and citrate. Animal protein provides an acid load through the high content of sulfur-containing amino acids, which leads to a state of mild chronic metabolic acidosis, low urine pH and hypocitraturia 49, 50. Since high protein diets are often additionally devoid of sufficient fruits and vegetables, hypocitraturia may also ensue from the lack of alkali 51. Interestingly, a study comparing idiopathic calcium stone formers with a control group found a higher mean renal acid load in the stone formers, even though animal protein intake was similar between the two groups 52. The authors attributed these findings to a lower intake of fruits and vegetables among stone formers 52. Furthermore, a small metabolic study simulating the three phases of the Atkins diet (a low carbohydrate, high protein diet) demonstrated increases in urinary uric acid and calcium and decreases in pH and citrate during both the stringent induction phase and the less stringent maintenance phases of the diet compared to baseline 53. While the acid load conferred by animal protein has been presumed to promote hypercalciuria by increasing bone resorption 54, Maalouf and colleagues 55 found that administration of potassium citrate failed to prevent protein-induced hypercalciuria, suggesting that the hypercalciuria may be attributable to a renal etiology.
Pure uric acid stones are rare but recur frequently. Low urinary pH (pH < 5.5) is the most common and important factor in uric acid nephrolithiasis; in normouricosuric stone disease the primary defect seems to be in the renal excretion of ammonia and is linked to an insulin resistant state. Hyperuricosuria occurs in 10% of patients with calcium stones, where uric acid crystals form the nidus for deposition of calcium and oxalate. A history of gout doubles the risk of kidney stones in men.
The recommended dietary allowance of protein is 0.8 g/kg/day 56, and animal protein restriction should include all forms of meat, including beef, poultry, and fish. A 3-phase randomized, crossover metabolic study in 25 normal subjects comparing three different animal protein sources revealed higher levels of urinary uric acid during the fish phase compared to the beef or poultry phase, although urinary saturation of calcium oxalate did not simply reflect urinary uric acid levels 57. Additionally, the question often arises among stone patients whether ingestion of whey protein, a dairy-derived protein supplement popular among athletes because it is thought to increase muscle mass and improve exercise performance, is also a risk factor for stone formation. A recent 2-phase metabolic study in which whey protein or albumin was given to 18 healthy volunteers on a controlled diet for 3 days showed no significant change from baseline in urinary stone risk factors with either supplement 58.
Hyperoxaluria
Hyperoxaluria occurs in approximately 10%–15% of calcium stone formers. Hyperoxaluria is defined as urinary excretion of oxalate in excess of 45 mg/day 1. Increased urinary oxalate can occur as a consequence of excessive dietary intake (oxalate gluttons), endogenous oxalate overproduction or intestinal oxalate overabsorption (enteric hyperoxaluria).
On the basis of the mechanism, hyperoxaluria is classified as follows:
- Enteric hyperoxaluria: This results from increased intestinal absorption due to ileal disease (Crohn’s disease, ileal bypass) or short bowel syndrome, low calcium intake, or gastrointestinal decolonisation of Oxalobacter formigenes. Oxalobacter is an intestinal bacterium that degrades dietary oxalate, and decolonisation of the gut results in increased absorption of oxalate. Oral administration of Oxalobacter has been shown to decrease urinary oxalate concentration in animals and humans 59, 60.
- Increased ingestion (oxalate gluttons): Dietary oxalate contributes to about half of the urinary oxalate and is inversely proportional to calcium intake in healthy people without gastrointestinal disease 61. Spinach, rhubarb, beets, chocolate, nuts, tea, wheat bran, strawberries, and soya foods are known to increase urinary oxalate concentrations 62. Vitamin C supplementation may increase urinary oxalate excretion and the risk of calcium oxalate crystallisation in patients who form calcium stones 63. Ingestion of grapefruit juice increases excretion of both oxalate and citrate in urine with no net change in its lithogenicity 64.
- Primary hyperoxaluria: This is an inborn error of metabolism (glycolic aciduria).
In normal individuals, approximately 10% of ingested oxalate is absorbed while the rest is eliminated through the stool 65, 66. For reasons not clearly understood, calcium oxalate stone formers absorb a slightly higher proportion of oxalate from the intestine than do normal subjects 65. Oxalate absorption depends not only on the amount of dietary oxalate, but also on dietary calcium intake. Higher calcium diets lead to reduced oxalate absorption, while calcium-restricted diets are associated with enhanced oxalate absorption and subsequently increased urinary oxalate excretion 66, 67. As such, a normal calcium diet in association with oxalate restriction is recommended in hyperoxaluric patients. High oxalate foods such as spinach, rhubarb, beets, nuts, chocolate, potatoes, bran, legumes and tea should be avoided 68. Some juices have been found to have a high oxalate content, including cranberry 69, grapefruit 70 and carambola juice (starfruit) 71. Spinach is frequently added to homemade fruit and vegetable juices, raising the oxalate content 72. Since the leaves are not cooked or processed to enhance removal or reduction of soluble oxalate, this practice may pose a threat to calcium oxalate stone formers. The addition of small amounts of calcium ions, especially in the form of calcium chloride, has been recommended by some to convert the oxalate into an insoluble form that is less likely to be absorbed in the digestive tract 72. However, any added calcium should be considered when calculating total dietary calcium intake.
Interestingly, no studies have directly shown a correlation between urinary oxalate and recurrent idiopathic calcium oxalate stone formation, and therefore recommendation of dietary oxalate restriction is empiric. Indeed, Noori and colleagues 73 randomized 57 patients with hyperoxaluria and recurrent calcium oxalate stones to a low oxalate diet or to the Dietary Approaches to Stop Hypertension (DASH) diet, which is a diet high in fruits, vegetables, nuts and legumes (high oxalate content) and low in sodium and red and processed meats. Although urinary oxalate increased in the group assigned to the DASH diet and decreased in those adhering to a low oxalate (4.8 mg/day vs. −4.2 mg/day, respectively), urinary saturation of calcium oxalate declined more on the DASH diet (−2.14) than on the low oxalate diet (−0.90), suggesting that other dietary measures had greater impact on reducing urinary stone risk than a low oxalate diet.
Enzymatic defects in the oxalate biosynthetic pathway lead to markedly high levels of urinary oxalate leading to aggressive calcium oxalate stone formation and oxalosis. Among the three forms of primary hyperoxaluria (I–III), renal failure is typically seen only with primary hyperoxaluria type 1. Strict dietary oxalate restriction is recommended in all forms of primary hyperoxaluria 74.
Patients with malabsorptive disorders from intestinal resection, roux-en-Y gastric bypass surgery, Crohn’s disease, celiac sprue, pancreatitis or use of fat-malabsorbing medications such as orlistat, are at risk of enteric hyperoxaluria because luminal calcium binds to poorly absorbed fatty acids, leading to higher levels of uncomplexed oxalate that is subsequently absorbed and excreted in the urine 75. In these patients, strict dietary oxalate restriction, along with a low fat diet and use of calcium supplements with meals to bind luminal oxalate is an effective strategy for stone prevention.
In experimental animals, testosterone promotes stone formation by suppressing osteopontin expression in the kidney and increasing urinary oxalate excretion. Estrogen seems to inhibit stone formation by increasing osteopontin expression in the kidney and decreasing urinary oxalate excretion 76.
Finally, O. formigenes is a Gram-negative anaerobic bacterium that resides in the intestine and uses oxalate as its sole source for energy and growth 77. Animal models have shown that absence of O. formigenes colonization can result in reduced degradation of oxalate in the intestinal lumen as well as reduced enteric oxalate secretion 78. Other animal models demonstrated that colonization with O. formigenes in addition to a low fat diet decreased urinary oxalate excretion 79. A case-control study in human subjects noted that despite a strong inverse correlation between colonization and risk of recurrent stone formation, no significant difference in median urinary oxalate levels was detected between patients who were or were not colonized with O. formigenes 80. Further work is needed to clarify the therapeutic role of this organism or its enzymes in preventing calcium oxalate stone formation.
Hypocitriuria
Hypocitraturia has been reported in 15%–63% of patients with kidney stones and is often seen in conjunction with other metabolic disorders 81. Hypocitriuria is defined as urinary citrate excretion of < 250 mg in 24 hours. Urinary citrate forms a soluble complex with calcium that inhibits the formation and propagation of crystals 1. It is a common correctable cause of recurrent pure calcium phosphate or brushite stones. Women excrete more citrate and have lower incidence of stone formation than men. Citrate is an important inhibitor of calcium stone formation because it directly inhibits nucleation, agglomeration and growth of calcium oxalate and/or calcium phosphate crystals and by complexing with calcium to reduce urinary saturation of calcium salts 82. Renal citrate excretion is modulated primarily by acid–base status; acidosis increases citrate reabsorption and alkalosis enhances citrate production and excretion in the renal proximal tubule.
Urinary citrate is mainly derived endogenously through the tricarboxylic acid cycle and is excreted by renal tubular cells. Intracellular acidosis, acidic diets (diets rich in animal proteins), and hypokalaemia decrease urinary citrate excretion. Fruits such as oranges and grapefruits are the main exogenous sources of urinary citrate. Hormonal replacement therapy in postmenopausal women results in higher urinary calcium excretion, but it also increases urinary excretion of citrate and leads to net inhibition of crystal precipitation, thereby decreasing the risk of calcium stones 83.
Fruits and vegetables increase urinary citrate because of their high alkali content, but not all fruits and juices have the same citraturic effect. Orange juice has shown the most consistent benefit because it has a high content of potassium citrate that confers an alkali load 84, 85, 86. Lemonade, which is high in citric acid, does not affect urine pH and has less citraturic effect. While fruit juices offer a more palatable and less costly therapy than potassium citrate medication, fruit juices can be high in calories and oxalate content and this may temper their use 70, 87. Fruits with a high malic acid (precursor to citrate) content, such as pears, may theoretically increase urinary citrate but few studies have examined this 88. Unfortunately, no citrus fruits or juices have been tested in a randomized trial to assess their benefit in reducing stone recurrence rates.
The Dietary Approaches to Stop Hypertension (DASH) diet, as an overall healthy diet, has been suggested to reduce the rate of stone formation 89. The high alkali content, among other factors, may contribute to improvement in urinary stone risk factors 90. Among three large cohorts of both men and women [Nurses’ Health Study I (NHS 1), Nurses’ Health Study II (NHS 2), and Health Professionals Follow-up Study (HPFS)], those subjects adhering most closely to the DASH diet had the lowest risk of incident stone formation on multivariate analysis 91. That is to say, when patients were given a score relating to how closely their diet resembled the DASH diet, those in the lowest quintile of DASH scores had the highest rate of incident stone formation in Health Professionals Follow-up Study (HPFS) (odds ratio (OR) = 1.53; Nurses’ Health Study 1 (OR = 1.47), and Nurses’ Health Study 2 (OR = 1.37).
Struvite (triple phosphate) and cystine stones
Various anatomical abnormalities promote urine stasis and increase the risk of stone formation by promoting precipitation of crystals. Urinary infection with urea splitting organisms (Proteus, Klebsiella, Serratia, and Mycoplasma) creates alkaline urine that promotes the formation of struvite stones. Urinary saturation with struvite occurs only when supranormal excretion of ammonia and alkaline urine occur together. Alkalaemia suppresses renal ammoniagenesis, but the hydrolysis of urea by bacteria liberates ammonia that alkalises urine.
Cystinuria (cystine stones) is an autosomal recessive trait, with an inborn error in the transport of dicarboxylic acids—cystine, ornithine, lysine, and arginine, commonly known as “COLA.” The low solubility of cystine results in its precipitation and stone formation.
Urinary glycoproteins
Various urinary glycoproteins (Tamm-Horsfall proteins, bikunin, nephrocalcin, urinary prothrombin fragment I) are inhibitors of stone formation. Their deficiency may promote stone formation.
Drugs that may increase the risk of stone disease
- Decongestants: ephedrine, guaifenesin
- Diuretics: triamterene
- Protease inhibitors: indinavir
- Anticonvulsants: felbamate, topiramate, and zonisamide
Risk factors for kidney stones
Factors that increase your risk of developing kidney stones include 92, 22, 93:
- Not drinking enough water. The biggest risk factor for kidney stones is not drinking enough fluids. Kidney stones are more likely to occur if you make less than 1 liter (32 ounces) of urine a day.
- Family history of kidney stones. If someone in your family has had kidney stones, you’re more likely to develop stones, too.
- Personal history of kidney stone. If you’ve already had one or more kidney stones, you’re at increased risk of developing another.
- Eating a diet high in protein and sodium but low in fiber may increase your risk of some types of kidney stones. This is especially true with a high-sodium diet. Too much salt in your diet increases the amount of calcium your kidneys must filter and significantly increases your risk of kidney stones.
- Being a man. Men are more likely to develop kidney stones than women. About 19 percent of men and 9 percent of women in the United States have kidney stones at least once during their lifetime 6, 7, 8.
- Being between 20 and 70 years of age
- Being bedridden or immobile for a long period of time
- Spinal cord injury
- Neurogenic bladder
- Certain supplements and medications, such as vitamin C, dietary supplements, laxatives (when used excessively), calcium-based antacids, and certain medications used to treat migraines or depression, can increase your risk of kidney stones.
- Being overweight. High body mass index (BMI), large waist size and weight gain have been linked to an increased risk of kidney stones.
- Digestive diseases and surgery. Bowel resection (e.g. colon resection, ileostomy, small bowel resection, Roux-en-Y gastric bypass surgery), inflammatory bowel disease, chronic pancreatitis, or chronic diarrhea can cause changes in the digestive process that affect your absorption of calcium and water, increasing the amounts of stone-forming substances in your urine.
- Other medical conditions such as renal tubular acidosis, cystinuria, hyperparathyroidism, sarcoidosis, cystic fibrosis, metabolic syndrome (e.g. obesity, type 2 diabetes mellitus, dyslipidemia, hypertension), gout and repeated urinary tract infections also can increase your risk of kidney stones.
- Renal tubular acidosis
- Polycystic kidney disease
- Cystinuria
- Primary hyperoxaluria
- Anatomical abnormalities that result in impaired urine flow (e.g. medullary sponge kidney, ureteral stricture, horseshoe kidney, etc.)
Kidney stone in children
Kidney stones are found in children as young as 5 years. More children are developing kidney stones, which is attributed to poor food choices such as not drinking enough fluids and eating foods that are high in salt. Sodas and other sweetened beverages can also increase the risk of kidney stones if they contain high fructose corn syrup. The increase in children with kidney stones the United States has been attributed to several factors, mostly related to the rise in diabetes, obesity, and high blood pressure (hypertension) in this population 16, 94. In fact, this problem is so common in children that some hospitals conduct ‘stone’ clinics for pediatric patients. Because increasing age is a risk factor for kidney stones, adolescents are more likely to form kidney stones than younger children. The underlying causes and resulting treatments differ in children and adults. Children with kidney stones are more likely to have anatomic and metabolic abnormalities 94, increased urinary calcium excretion, decreased urinary oxalate and citrate excretion, and much higher urinary calcium oxalate saturations than children with no history of kidney stones 16. Children with cystinuria and other hereditary forms of kidney stones are at increased risk of decline in renal function compared with age-matched controls, although progression to end-stage renal disease is uncommon 16.
Kidney stone in pregnant women
Pregnant women are twice as likely to have calcium phosphate stones compared with age-matched nonpregnant women, and are two to three times more likely to have calcium phosphate stones than oxalate stones 14. The incidence of kidney stones during pregnancy increases in the second and third trimesters. Women have an increased glomerular filtration rate and higher urinary calcium excretion throughout pregnancy, with higher urine pH in the second and third trimesters, which may predispose them to calcium phosphate stones. Ultrasonography is considered the imaging modality of choice in pregnant women. Kidney stones during pregnancy increase the risk of urinary tract infections, and pregnant women with renal colic have nearly double the risk of pre-term delivery compared with women who do not have kidney stones 95.
Types of kidney stones
A kidney stone is a solid piece of material that forms in your kidney from substances in your urine.
There are four types of kidney stones:
- Calcium oxalate stone: Calcium oxalate stone is the most common type of kidney stone, which is created when calcium combines with oxalate in your urine. Calcium that isn’t used by your bones and muscles goes into your kidneys. Usually, the kidneys will get rid of the extra calcium through the urine. Calcium stones occur when some of the calcium remains in the kidneys and collects over time. Inadequate calcium and fluid intake, as well other conditions, may contribute to calcium oxalate stone formation.
- Struvite stone (magnesium ammonium phosphate): A struvite stone is more common in women. Struvite stone usually forms after a chronic urinary tract infection (UTI). These stones are usually made of ammonia. Struvite stones can grow very large and can block the kidney, ureter, or bladder.
- Uric acid stone: Uric acid stone is another common type of kidney stone. A uric acid stone forms when there is too much uric acid in the urine. You may be at risk for this type of stone if you eat a high-protein diet or if you’ve received chemotherapy. Foods such as organ meats and shellfish have high concentrations of a natural chemical compound known as purines. High purine intake leads to a higher production of monosodium urate, which, under the right conditions, may form stones in the kidneys. The formation of uric acid stones tends to run in families. Uric acid stones can form in people who lose too much fluid because of chronic diarrhea or malabsorption and those with diabetes or metabolic syndrome. Certain genetic factors also may increase your risk of uric acid stones.
- Cystine stone: A cystine stone is rare. The disease that causes cystine stones to form runs in families and is called cystinuria.
About 85% of kidney stones in the US are composed of calcium, mainly calcium oxalate (see Table 3. Composition of Kidney Stones); 10% are uric acid; 2% are cystine; most of the remainder are magnesium ammonium phosphate (struvite).
Studying your kidney stone can help understand why you have it and how to reduce the risk of further stones. The most common type of stone contains calcium. Calcium is a normal part of a healthy diet. The kidney usually removes extra calcium that the body doesn’t need. Often people with calcium stones keep too much calcium. This calcium combines with waste products like oxalate to form a stone. The most common combination is called calcium oxalate.
Less common types of stones are: Infection-related stones, containing magnesium and ammonia called struvite stones and stones formed from monosodium urate crystals, called uric acid stones, which might be related to obesity and dietary factors. The rarest type of stone is a cvstine stone that tends to run in families.
Table 3. Composition of kidney stones
| Composition | Percentage of All Calculi | Common Causes |
|---|---|---|
| Calcium oxalate | 70% | Hypercalciuria Hyperparathyroidism Hypocitruria Renal tubular acidosis |
| Calcium phosphate | 15% | Hypercalciuria Hyperparathyroidism Hypocitruria Renal tubular acidosis |
| Cystine | 2% | Cystinuria |
| Magnesium ammonium phosphate (struvite) | 3% | Urinary Tract Infection (UTI) caused by urea-splitting bacteria |
| Uric acid | 10% | Urine pH < 5.5 Occasionally hyperuricosuria |
Calcium stones
Calcium stones, including calcium oxalate stones and calcium phosphate stones, are the most common types of kidney stones. Calcium oxalate stones are more common than calcium phosphate stones.
Most patients (up to 80%) with calcium stones have one or more of the metabolic risk factors and about 25% of stones are idiopathic (cause is unknown) in origin.
Metabolic risk factors for calcium stones:
- Hypercalciuria (40-60%)
- Hyperuricosuria (25%)
- Hyperoxaluria
- Hypocitriuria
- Other (vitamin A deficiency, hot climates, immobilization, urinary tract anomalies)
Calcium from food does not increase your chance of having calcium oxalate stones. Normally, extra calcium that isn’t used by your bones and muscles goes to your kidneys and is flushed out with urine. When this doesn’t happen, the calcium stays in the kidneys and joins with other waste products to form a kidney stone.
- For calcium stones, risk factors vary by population. The main risk factor in the US is hypercalciuria (condition of elevated calcium in the urine), a hereditary condition present in 50% of men and 75% of women with calcium calculi; thus, patients with a family history of calculi are at increased risk of recurrent calculi 9. These patients have normal serum calcium, but urinary calcium is elevated > 250 mg/day (> 6.2 mmol/day) in men and > 200 mg/day (> 5.0 mmol/day) in women 9.
- Hypocitruria (urinary citrate < 350 mg/day [1820 μmol/day]), present in about 40 to 50% of calcium calculi-formers, promotes calcium calculi formation because citrate normally binds urinary calcium and inhibits the crystallization of calcium salts 9. The activity of citrate is thought to be related to its concentration in urine, where it exhibits a dual action, opposing crystal formation by both thermodynamic and kinetic mechanisms. Citrate retards stone formation by inhibiting the calcium oxalate nucleation process and the growth of both calcium oxalate and calcium phosphate stones, largely by its ability to bind with urinary calcium and reduce the free calcium concentration, thereby reducing the supersaturation of urine. Citrate binds to the calcium oxalate crystal surface, inhibiting crystal growth and aggregation 96. There is also evidence that citrate blocks the adhesion of calcium oxalate monohydrate crystals to renal epithelial cells 97. Medical interventions to increase urinary citrate are a primary focus in the medical management of urolithiasis 98. The amount of diet-derived citrate that may escape in body conversion to bicarbonate is reportedly minor 99. Nonetheless, a prior study reported increased urinary citrate after 1 week on 4 ounces lemon juice per day, diluted in 2 L water, in stone formers with hypocitraturia 100. Two retrospective studies showed an effect in calcium stone formers of lemon juice and/or lemonade consumption on urinary citrate 101, but a recent clinical trial showed no influence of lemonade on urinary citrate 102.
- Hypocitraturia, if severe and/or persistent, usually requires pharmacologic therapy in the form of potassium citrate, which enhances urine pH and also citrate excretion. The identification and promotion of consumption of fluids that add to the crystal inhibitory potential of urine is appealing, not only to promote fluid intake but to enhance urinary citrate excretion. Citric acid is a naturally-occurring organic acid present in multiple fruits, such as lemon, lime, grapefruit, tangerine, and orange and their juices 103. Data on the citric acid content of fresh fruit juices and commercially-available fruit juice beverages may therefore prove useful in constructing nutrition therapy regimens for calcium stone formers.Lemon and lime juice, both from the fresh fruit and from juice concentrates, provide more citric acid per liter than ready-to-consume grapefruit juice, ready-to-consume orange juice, and orange juice squeezed from the fruit 103. Lemon and lime juices are rich sources of citric acid, containing 1.44 and 1.38 g/oz, respectively, comprising as much as 8% of the dry fruit weight 104. These data concur with those previously reported 105. As lemon and lime juice contain 38 and 35 mg potassium/oz, respectively, about the same as grapefruit juice and about 60% that of orange juice, ingestion of lemon or lime juice on a daily basis could provide dietary alkali that would decrease renal tubular reabsorption of citrate, resulting in enhanced urinary citrate excretion. The distribution of lemon or lime juice in ample water or other fluid, consumed throughout the day, would also add to the volume of fluids ingested, resulting in enhanced urine output 106 and reduced urine supersaturation.Further research should determine the bioavailability of dietary citric acid from various sources and characterize the response to dietary citric acid in kidney stone formers who are hypocitraturic, as well as those who are normocitraturic. The impact of diet-derived citrate on urinary concentrations among calcium stone formers consuming different diets (e.g., high fruit/vegetable intake versus low fruit/vegetable intake; high meat intake versus low meat intake) should be assessed, as dietary patterns are known to influence urinary citrate concentrations 107.
- About 5 to 8% of calculi are caused by renal tubular acidosis. About 1 to 2% of patients with calcium calculi have primary hyperparathyroidism 9. Rare causes of hypercalciuria are sarcoidosis, vitamin D intoxication, hyperthyroidism, multiple myeloma, metastatic cancer, and hyperoxaluria.
- Hyperoxaluria (urinary oxalate > 40 mg/day [> 440 μmol/day]) can be primary or caused by excess ingestion of oxalate-containing foods (eg, rhubarb, spinach, cocoa, nuts and nut products, peanuts [peanuts are legumes not nuts], wheat bran, pepper, tea) or by excess oxalate absorption due to various enteric diseases (eg, bacterial overgrowth syndromes, chronic pancreatic or biliary disease) or ileojejunal (eg, bariatric) surgery.
- Other risk factors include taking high doses of vitamin C (ie, > 2000 mg/day), a calcium-restricted diet (possibly because dietary calcium binds dietary oxalate), and mild hyperuricosuria. Mild hyperuricosuria, defined as urinary uric acid > 800 mg/day (> 5 mmol/day) in men or > 750 mg/day (> 4 mmol/day) in women, is almost always caused by excess intake of purine (in proteins, usually from meat, fish, and poultry); it may cause calcium oxalate calculus formation (hyperuricosuric calcium oxalate nephrolithiasis) 9.
Uric acid stones
Uric acid is the end product of purine metabolism and is either derived from exogenous (dietary) sources or produced endogenously during cell turnover. For example, eating a lot of fish, shellfish, and meat—especially organ meat—may increase uric acid in urine. Chronic metabolic acidosis can result in protein metabolism and thus increased excretion of urate and formation of kidney stones 108. Pure uric acid stones are rare but recur frequently. Low urinary pH (pH < 5.5) – urine acidity – is the most common and important factor in uric acid kidney stone formation or rarely with severe hyperuricosuria (urinary uric acid > 1500 mg/day [> 9 mmol/day]), which crystallizes undissociated uric acid; in normouricosuric stone disease the primary defect seems to be in the renal excretion of ammonia and is linked to an insulin resistant state 109. Hyperuricosuria occurs in 10% of patients with calcium stones, where uric acid crystals form the nidus for deposition of calcium and oxalate. A history of gout doubles the risk of kidney stones in men 110. Uric acid crystals may comprise the entire calculus or, more commonly, provide a nidus on which calcium or mixed calcium and uric acid calculi can form.
Struvite stones
Struvite stones also known as magnesium ammonium phosphate calculi or infection stones, may form after you have a urinary tract infection (UTI) caused by urea-splitting bacteria (eg, Proteus sp, Klebsiella sp) 30, 111, 9. Urease is necessary to split/cleave urea to ammonia and CO2, making urine more alkaline which elevates pH (typically > 7). Phosphate is less soluble at alkaline versus acidic pH, so phosphate precipitates on to the insoluble ammonium products, yielding to a large staghorn stone formation 112. Struvite stones can develop suddenly and become large quickly. The struvite stones must be treated as infected foreign bodies and removed in their entirety. Unlike other types of kidney stones, struvite stones (magnesium ammonium phosphate calculi) occur 3 times more frequently in women 9.
Cystine stones
Less than 3% of urinary tract stones are cystine stones 113. Cystine stones result from a inherited disorder called cystinuria that is passed down through families. Cystinuria is caused by a defect in the rBAT gene on chromosome 2, which causes the amino acid cystine to leak through your kidneys and into the urine 114. Cystine does not dissolve in urine and leads to cystine stone formation 19. People who are homozygous for cystinuria excrete more than 600 millimole insoluble cystine per day 30. The development of urinary cystine is the only clinical manifestation of this cystine stone disease 114.
Kidney stones prevention
Most people who have kidney stones have a 50% chance of developing another kidney stone within 10 years. But there are things you can do to lower your risk:
- Drink at least 2 liters of water per day. Your doctor may have you measure your urine output to be sure you’re drinking the right amount of fluids. Drinking enough water will help keep your urine less concentrated with waste products. Darker urine is more concentrated, so your urine should appear very light yellow to clear if you are well hydrated. Most of the fluid you drink should be water. Most people should drink more than 12 glasses of water a day. Speak with a healthcare professional about the right amount of water that’s best for you. Water is better than soda, sports drinks or coffee/tea. lf you exercise or if it is hot outside, you should drink more. Sugar and high-fructose corn syrup should be limited to small quantities.
- Don’t eat more than 1,500 mg of salt per day (about 1 teaspoon). This includes salt in pre-packaged food. What foods are high in salt? Everyone thinks of salty potato chips and French fries. Those should be rarely eaten. There are other products that are salty: sandwich meats, canned soups, packaged meals, and even sports drinks. Check nutrition labels to see how much salt (sodium) is in your food.
- Eat fewer oxalate-rich foods. If you tend to form calcium oxalate stones, your doctor may recommend restricting foods rich in oxalates. These include rhubarb, beets, okra, spinach, Swiss chard, sweet potatoes, nuts, tea, chocolate, black pepper and soy products.
- Try not to eat more than 2 servings of meat per day. Each serving should be no more than 6 to 8 ounces (170 to 227 grams). You need adequate protein, but it needs to be part of a balanced diet. Seek guidance from a registered dietitian when embarking on a weight loss diet or any dietary interventions to reduce the risk of kidney stones.
- Eat more fruits and vegetables, which make the urine less acid. When the urine is less acid, then stones may be less able to form. Animal protein produces urine that has more acid, which can then increase your risk for kidney stones.
- Get to a normal weight if you are overweight. But, high-protein weight loss diets that include high amounts of animal-based protein, as well as crash diets can add to the risk of kidney stone formation.
If you have had more than one kidney stone, your doctor might send you to a specialist to find the exact cause of your kidney stones. Some people need medicine to keep from getting another kidney stone.
Don’t be confused about having a “calcium stone”. Dairy products have calcium, but they actually help prevent kidney stones, because calcium binds with oxalate before it gets into the kidneys. People with the lowest dietary calcium intake have an increased risk of kidney stones. Continue eating calcium-rich foods unless your doctor advises otherwise.
A kidney stone can form from salt, the waste products of protein, and potassium. The most common type of kidney stone is a calcium oxalate stone. Most kidney stones are formed when oxalate, a by product of certain foods, binds to calcium as urine is being made by the kidneys. Both oxalate and calcium are increased when the body doesn’t have enough fluids and also has too much salt. Based on blood and urine tests, your doctor will determine which types of dietary changes are needed in your particular case.
Some herbal substances are promoted as helping prevent stones. You should know that there is insufficient published medical evidence to support the use of any herb or supplement in preventing stones.
See your doctor and/or a registered dietitian about making diet changes if you have had a stone or think you could be at increased risk for getting a kidney stone. A dietitian who can help you develop an eating plan that reduces your risk of kidney stones.
Drinking a lot of water
Drinking a lot of water is important for treating and preventing all types of kidney stones. Staying hydrated (having enough fluid in your body) will keep your urine diluted. This makes it harder for stones to form.
- You can also drink ginger ale, lemon-lime sodas, and fruit juices.
- Drink enough liquids throughout the day to make at least 2 quarts (2 liters) of urine every 24 hours.
- Drink enough to have light-colored urine. Dark yellow urine is a sign you are not drinking enough.
Limit your coffee, tea, and cola to 1 or 2 cups (250 or 500 milliliters) a day. Caffeine may cause you to lose fluid too quickly, which can make you dehydrated.
Diet and calcium stones
Follow these guidelines if you have calcium kidney stones:
- Drink plenty of fluids, particularly water.
- Eat less salt. Your chance of developing kidney stones increases when you eat more sodium. Sodium is a part of salt. Sodium is in many canned, packaged, and fast foods. It is also in many condiments, seasonings, and meats. Chinese and Mexican food, tomato juice, regular canned foods, and processed foods are often high in salt. Look for low-salt or unsalted products.
- Have only 2 or 3 servings a day of foods with a lot of calcium, such as milk, cheese, yogurt, oysters, and tofu.
- Eat lemons or oranges, or drink fresh lemonade. Citrate in these foods prevents stones from forming.
- Limit how much protein you eat. Eating animal protein may increase your chances of developing kidney stones. Choose lean meats. Your doctor may tell you to limit eating animal protein, including:
- beef, chicken, and pork, especially organ meats
- eggs
- fish and shellfish
- milk, cheese, and other dairy products
- Consider replacing some of the meat and animal protein you would typically eat with beans, dried peas, and lentils, which are plant-based foods that are high in protein and low in oxalate.
- Eat a low-fat diet.
Do not take extra calcium or vitamin D, unless your doctor who is treating your kidney stones recommends it.
- Watch out for antacids that contain extra calcium. Ask your doctor which antacids are safe for you to take.
- Even though calcium sounds like it would be the cause of calcium stones, it’s not. Your body still needs the normal amount of calcium you get from your daily diet. Limiting calcium may actually increase the chance that stones will form. In the right amounts, calcium can block other substances in the digestive tract that may cause kidney stones. Talk with a dietitian or your doctor about how much calcium you should eat to help prevent getting more calcium oxalate stones and to support strong bones. It may be best to get calcium from low-oxalate, plant-based foods such as calcium-fortified juices, cereals, breads, some kinds of vegetables, and some types of beans. Ask a dietitian or your doctor which foods are the best sources of calcium for you.
Ask your doctor before taking vitamin C or fish oil. They may be harmful to you.
If your doctor says you have calcium oxalate stones, you may also need to limit foods that are high in oxalate. If you’ve had calcium oxalate stones, you may want to avoid these foods to help reduce the amount of oxalate in your urine:
- Fruits: rhubarb, currants, canned fruit salad, strawberries, and Concord grapes
- Vegetables: beets, leeks, summer squash, sweet potatoes, spinach, and tomato soup
- Drinks: tea and instant coffee
- Other foods: grits, tofu, nuts, wheat bran and chocolate. Peanuts—which are legumes, not nuts, and are high in oxalate
Talk with a doctor or dietitian about other food sources of oxalate and how much oxalate should be in what you eat.
If your doctor says you have calcium phosphate stones, your chance of developing kidney stones increases when you eat more sodium (salt). Sodium is a part of salt. Sodium is in many canned, packaged, and fast foods. Sodium or salt is also in many condiments, seasonings, and meats. Ask your doctor about how much sodium should be in what you eat. See tips to reduce your sodium intake.
- Limit animal protein. Eating animal protein may increase your chances of developing calcium phosphate kidney stones. Your dietitian or doctor may tell you to limit eating animal protein, including:
- beef, chicken, and pork, especially organ meats
- eggs
- fish and shellfish
- milk, cheese, and other dairy products
- Although you may need to limit how much animal protein you have each day, you still need to make sure you get enough protein. Consider replacing some of the meat and animal protein you would typically eat with some of these plant-based foods that are high in protein:
- legumes such as beans, dried peas, lentils, and peanuts
- soy foods, such as soy milk, soy nut butter, and tofu
- nuts and nut products, such as almonds and almond butter, cashews and cashew butter, walnuts, and pistachios
- sunflower seeds
- Talk with a dietitian or doctor about how much total protein you should eat and how much should come from animal or plant-based foods.
- Get enough calcium from foods. Even though calcium sounds like it would be the cause of calcium stones, it’s not. In the right amounts, calcium can block other substances in the digestive tract that may lead to stones. Talk with a dietitian or doctor about how much calcium you should eat to help prevent getting more calcium phosphate stones and to support strong bones. It may be best to get calcium from plant-based foods such as calcium-fortified juices, cereals, breads, some kinds of vegetables, and some types of beans. Ask a dietitian or doctor which foods are the best sources of calcium for you.
Diet and uric acid stones
Avoid these foods if you have uric acid stones:
- Alcohol
- Anchovies
- Asparagus
- Baking or brewer’s yeast
- Cauliflower
- Consommé
- Gravy
- Herring
- Legumes (dried beans and peas)
- Mushrooms
- Oils
- Organ meats (liver, kidney, and sweetbreads)
- Sardines
- Spinach
Other suggestions for your diet include:
- Do not eat more than 3 ounces (85 grams) of meat at each meal. Your doctor or dietition may tell you to limit eating animal protein, including:
- beef, chicken, and pork, especially organ meats
- eggs
- fish and shellfish
- milk, cheese, and other dairy products
- Consider replacing some of the meat and animal protein you would typically eat with some of these plant-based foods that are high in protein:
- legumes such as beans, dried peas, lentils, and peanuts
- soy foods, such as soy milk, soy nut butter, and tofu
- nuts and nut products, such as almonds and almond butter, cashews and cashew butter, walnuts, and pistachios
- sunflower seeds
- Avoid fatty foods such as salad dressings, ice cream, and fried foods.
- Eat enough carbohydrates.
- Eat more lemons and oranges, and drink lemonade because the citrate in these foods stops stones from forming.
- Drink plenty of fluids, particularly water.
Losing weight if you are overweight is especially important for people who have had uric acid stones. If you are losing weight, lose it slowly. Quick weight loss may cause uric acid stones to form.
Diet and cystine stones
Drinking enough liquid, mainly water, is the most important lifestyle change you can make to prevent cystine stones. Talk with a doctor about how much liquid you should drink.
Medications
Medications can control the amount of minerals and salts in your urine and may be helpful in people who form certain kinds of stones. The type of medication your doctor prescribes will depend on the kind of kidney stones you have. Here are some examples:
- Calcium stones. To help prevent calcium stones from forming, your doctor may prescribe a thiazide diuretic or a phosphate-containing preparation.
- Uric acid stones. Your doctor may prescribe allopurinol (Zyloprim, Aloprim) to reduce uric acid levels in your blood and urine and a medicine to keep your urine alkaline. In some cases, allopurinol and an alkalizing agent may dissolve the uric acid stones.
- Struvite stones. To prevent struvite stones, your doctor may recommend strategies to keep your urine free of bacteria that cause infection, including drinking fluids to maintain good urine flow and frequent voiding. In rare cases long-term use of antibiotics in small or intermittent doses may help achieve this goal. For instance, your doctor may recommend an antibiotic before and for a while after surgery to treat your kidney stones.
- Cystine stones. Along with suggesting a diet lower in salt and protein, your doctor may recommend that you drink more fluids so that you produce a lot more urine,. If that alone doesn’t help, your doctor may also prescribe a medication that increases the solubility of cystine in your urine.
Kidney stones signs and symptoms
You may not have symptoms until your kidney stones move around within your kidney or pass into one of the ureters. The ureters are the tubes that connect your kidneys and bladder through which urine empties into your bladder. If a kidney stone becomes lodged in the ureters, it may block the flow of urine and cause the kidney to swell and the ureter to spasm, which can be very painful. At that point, you may experience symptoms. Kidney stones can cause a severe cramping pain on either side of your lower back or side. The pain usually moves down toward your abdomen, groin (groin pain) or genitals (testicle pain in men, and labia or vaginal pain in women) as the stone moves down the urinary tract. Other symptoms may include:
- Vague pain or stomach ache that doesn’t go away
- Nausea and vomiting
- Cloudy urine or blood in the urine
- Urine that smells bad or looks cloudy
- Fever and chills
- Feeling like you need to go to the bathroom more often than usual
Kidney stone starts to hurt when it causes irritation or blockage to the flow of urine out of the kidneys. This builds rapidly to extreme pain.
Pain caused by a kidney stone may change — for instance, shifting to a different location or increasing in intensity — as the stone moves through your urinary tract.
In most cases, kidney stones pass without causing damage, but usually not without causing a lot of pain. Pain relievers may be the only treatment needed for small stones. Other treatment may be needed, especially for those stones that cause lasting symptoms or other complications. In severe cases, however, surgery may be required.
Kidney stones complications
Complication of kidney stones may include the obstruction of the ureter (acute unilateral obstructive uropathy). Having kidney stones also increases your risk of developing chronic kidney disease. lf you have had one kidney stone, you are at increased risk of having another kidney stone. Those who have developed one kidney stone are at approximately 50% risk for developing another within 5 to 7 years.
Several complications can arise due to kidney stones, and subsequently, stones that cause obstruction. These include:
- Abscess formation
- Urosepsis
- Urinary fistula formation
- Ureteral scarring and stenosis
- Ureteral perforation
- Renal function loss due to long-standing obstruction
Kidney stones diagnosis
Diagnosis of a kidney stone starts with a medical history, physical examination, and imaging tests. Your doctors will want to know the exact size and shape of your kidney stones. This can be done with a high resolution CT scan from the kidneys down to the bladder or an x-ray called a “KUB x-ray” (kidney-ureter-bladder x-ray) which will show the size of the stone and its position. The KUB x-ray is often obtained by the surgeons to determine if the stone is suitable for shock wave treatment. The KUB test (kidney-ureter-bladder x-ray) may be used to monitor your stone before and after treatment, but the CT scan is usually preferred for diagnosis. In some people, doctors will also order an intravenous pyelogram or lVP, a special type of X- ray of the urinary system that is taken after injecting a dye.
Second, your doctors will decide how to treat your stone. The health of your kidneys will be evaluated by blood tests and urine tests. Your overall health, and the size and location of your stone will be considered.
Later, your doctor will want to find the cause of the stone. The stone will be analyzed after it comes out of your body, and your doctor will test your blood for calcium, phosphorus and uric acid. The doctor may also ask that you collect your urine for 24 hours to test for calcium and uric acid.
Kidney stone analysis
A kidney stone analysis is a test to find out what type of stone your kidney stones are made of. This information helps your doctor develop a plan to help you reduce your risk of forming more kidney stones in the future. Your treatment plan will depend on the type of stone you have, but most plans include drinking plenty of water and changing some of the foods you eat.
There are four types of kidney stones:
- Calcium oxalate stone: Calcium oxalate stone is the most common type of kidney stone, which is created when calcium combines with oxalate in your urine. Calcium that isn’t used by your bones and muscles goes into your kidneys. Usually, the kidneys will get rid of the extra calcium through the urine. Calcium stones occur when some of the calcium remains in the kidneys and collects over time. Inadequate calcium and fluid intake, as well other conditions, may contribute to calcium oxalate stone formation.
- Struvite stone:A struvite stone is more common in women. Struvite stone usually forms after a chronic urinary tract infection (UTI). These stones are usually made of ammonia.
- Uric acid stone: Uric acid stone is another common type of kidney stone. A uric acid stone forms when there is too much uric acid in the urine. You may be at risk for this type of stone if you eat a high-protein diet or if you’ve received chemotherapy. Foods such as organ meats and shellfish have high concentrations of a natural chemical compound known as purines. High purine intake leads to a higher production of monosodium urate, which, under the right conditions, may form stones in the kidneys. The formation of uric acid stones tends to run in families.
- Cystine stone: A cystine stone is rare. The disease that causes cystine stones to form runs in families and is called cystinuria.
About 85% of kidney stones in the US are composed of calcium, mainly calcium oxalate (see Table 3. Composition of Kidney Stones); 10% are uric acid; 2% are cystine; most of the remainder are magnesium ammonium phosphate (struvite).
To collect a kidney stone, you will need a kidney stone strainer to filter your urine and a clean container for your stone. A kidney stone strainer is a device made of fine mesh or gauze. Your doctor may give you a strainer, or you may get one from a drug store.
To collect your kidney stone, you will:
- Filter all your urine through the strainer.
- Check the strainer carefully after each time you urinate to look for a stone. It may look like a grain of sand or a tiny piece of gravel.
- If you find a stone, put it in a clean container with a lid.
- DO NOT add anything to the container. The stone must be kept dry.
- DO NOT put tape or tissue on the stone.
- Return the container to your doctor or lab as instructed.
A kidney stone may pass at any time of the day or night. So, it’s important to filter all your urine every time you urinate until you find a stone.
Your doctor may tell you to drink a lot of water to help pass the stone. If you have pain while the stone is passing, ask your doctor about pain medicine.
If your kidney stone is too large to pass, you may need a minor surgical procedure to remove the stone for testing. If you’ve already passed a kidney stone and you kept it, ask your doctot about testing it.
Blood testing
Blood tests may reveal too much calcium or uric acid in your blood. Blood test results help monitor the health of your kidneys and may lead your doctor to check for other medical conditions.
Urine testing
The 24-hour urine collection test may show that you’re excreting too many stone-forming minerals or too few stone-preventing substances. For this test, your doctor may request that you perform two urine collections over two consecutive days.
Your health care professional also may ask you to collect your urine for 24 hours after the kidney stone has passed or been removed. The health care professional can then measure how much urine you produce in a day, along with mineral levels in your urine. You are more likely to form stones if you don’t make enough urine each day or have a problem with high mineral levels.
Urinalysis
Urinalysis involves a health care professional testing your urine sample. You will collect a urine sample at a doctor’s office or at a lab, and a health care professional will test the sample. Urinalysis can show whether your urine has blood in it and minerals that can form kidney stones. White blood cells and bacteria in the urine mean you may have a urinary tract infection.
Imaging tests
Imaging tests may show kidney stones in your urinary tract. High-speed or dual energy computerized tomography (CT) may reveal even tiny stones. CT scans use a combination of x-rays and computer technology to create images of your urinary tract. Although a CT scan without contrast medium is most commonly used to view your urinary tract, a health care professional may give you an injection of contrast medium. Contrast medium is a dye or other substance that makes structures inside your body easier to see during imaging tests. You’ll lie on a table that slides into a tunnel-shaped device that takes the x-rays. CT scans can show the size and location of a kidney stone, if the stone is blocking the urinary tract, and conditions that may have caused the kidney stone to form.
Simple abdominal X-rays are used less frequently because this kind of imaging test can miss small kidney stones.
Ultrasound, a noninvasive test that is quick and easy to perform, is another imaging option to diagnose kidney stones.
Kidney stones treatment
The treatment for kidney stones is similar in children and adults and it depends on the type of stone, the cause and the severity of your symptoms.
Most kidney stones pass out of the body without help from a doctor. You may be asked to drink a lot of water. Drink at least 6 to 8 glasses of water per day to produce a large amount of urine. This will help the stone pass. But sometimes a kidney stone will not go away. Kidney stone may get stuck in the urinary tract, blocking the flow of urine and cause great pain. If you have a kidney stone that won’t pass on its own, you may need treatment.
Approximately 86% of kidney stones pass spontaneously; this proportion is lower for stones larger than 6 mm (59% vs. 90% for smaller stones) 21. Although stones larger than 6 mm in diameter are often removed by urologists (urinary tract surgical specialists) 22, these are the stones that have greatest benefit from medical expulsive therapy 23. Medical expulsive therapy with alpha blockers (e.g., tamsulosin [Flomax], 0.4 mg per day; doxazosin [Cardura], 4 mg per day) hastens and increases the likelihood of kidney stone passage, reduces pain, and prevents surgical interventions and hospital admissions 23, 22. These medications should be offered to patients with distal ureteral stones 5 to 10 mm in diameter 23. Tamsulosin is the most studied medication, but other alpha blockers seem equally effective 23. Calcium channel blockers (e.g., nifedipine) are less effective and may be no more effective than placebo 24, 25. Coadministration of oral corticosteroids or increasing fluid intake does not hasten stone passage or alleviate renal colic 26, 22.
Your doctor may also use a special machine that uses shock waves to break up the stone into smaller pieces. This is called extracorporeal shock wave lithotripsy, or ESWL. Shock-wave lithotripsy is a noninvasive procedure that uses high-energy sound waves to blast the stones into fragments that are then more easily passed out in the urine. Extracorporeal shock wave lithotripsy (ESWL) is used to remove stones slightly smaller than one half an inch (1.25 centimeters) that are located in the kidney or ureter.
A urologist (urinary tract surgical specialist) can put a very thin instrument called an endoscope through your urethra and into your bladder and ureters to find the stone, the procedure is called uteroscopy. In ureteroscopy, an endoscope is inserted through the ureter to retrieve or a laser is used to break up the stone. If a doctor does this, you will be given medicine to numb the area first. Rarely, for very large or complicated stones, doctors will use percutaneous nephrolithomy or nephrolithotripsy.
Your doctors will try to let the kidney stone pass without surgery. You may also get medication to help make your urine less acid. But if your kidney stone is too large, or if it blocks the flow of urine, or if there is a sign of infection, it is removed with open surgery or nephrolithotomy.
For some types of stones, your doctor may prescribe medicine to prevent stones from forming or help break down and remove the material that is causing the stone. These medicines can include:
- Allopurinol (for uric acid stones)
- Antibiotics (for struvite stones)
- Diuretics (water pills)
- Phosphate solutions
- Sodium bicarbonate or sodium citrate
- Water pills (thiazide diuretics)
- Tamsulosin to relax the ureter and help the stone pass
Surgery is also an option and is sometimes the only way to get rid of a kidney stone.
Surgery is often needed if:
- The kidney stone is too large to pass on its own.
- The kidney stone is growing.
- The kidney stone is blocking urine flow and causing an infection or kidney damage.
- The pain cannot be controlled.
Figure 5. Diagnosis and management of acute kidney stones
[Source 115 ]Kidney stone pain relief
Passing a small kidney stone can cause some discomfort. To relieve mild pain associated with kidney stone, your doctor may recommend nonsteroidal anti‐inflammatory drugs (NSAIDs) pain relievers such as ibuprofen (Advil, Motrin IB, others) or naproxen sodium (Aleve). For more severe pain, your doctor may prescribe nonsteroidal anti-inflammatory drugs (e.g., ketorolac, 30 to 60 mg intramuscularly) 22, 116. If an opioid is used, meperidine (Demerol) should be avoided because of the significant risk of nausea and vomiting 117. Neither scopolamine nor increased fluid intake alleviates renal colic 116, 26.
Table 4. Acute Management of Kidney Stones in Adults
| Management type | Therapy | Dosage | |
|---|---|---|---|
| Fluids | Oral intake of water, or intravenous normal saline if patient is unable to take oral fluids | At least 2 L of water per 24 hours | |
| 0.9% normal saline solution if blood pressure is low; consider decreasing sodium chloride in patients with calciuria (5% dextrose in water and 0.45% normal saline) | |||
| Antispasmodics to facilitate stone passage* | Alpha blockers | ||
| Doxazosin (Cardura) | 4 mg orally per day | ||
| Tamsulosin (Flomax) | 0.4 mg orally per day | ||
| Calcium channel blockers | |||
| Nifedipine (Procardia, sustained release) | 30 mg orally per day | ||
| Pain management† | Opioid narcotics | ||
| Codeine/acetaminophen | One or two tablets (5 to 10 mg codeine/325 to 500 mg acetaminophen) orally every four to six hours as needed | ||
| Hydrocodone/acetaminophen (Vicodin) | 5 to 10 mg orally every four to six hours as needed |
Footnote: People who are unable to take oral medications and people with low blood pressure or other signs of early hemodynamic instability should be treated intravenously.
* Often administered for up to four weeks before performing follow-up imaging studies to determine whether the stone has passed.
† Avoid using nonsteroidal anti-inflammatory drugs (NSAIDs) because they tend to lower kidney blood flow and glomerular filtration.
[Source 18 ]Small kidney stones with minimal symptoms
Most small kidney stones won’t require invasive treatment. You may be able to pass a small stone by 13:
- Drinking water. Drinking as much as 2.3 to 3.6 liters of water a day or achieving urine volume of at least 2-2.5 liters per day will keep your urine dilute and may prevent stones from forming. Unless your doctor tells you otherwise, drink enough fluid — ideally mostly water — to produce clear or nearly clear urine.
- Pain relievers. Passing a small stone can cause some discomfort. To relieve mild pain, your doctor may recommend pain relievers such as ibuprofen (Advil, Motrin IB, others) or naproxen sodium (Aleve).
- Medical therapy. Your doctor may give you a medication to help pass your kidney stone. This type of medication, known as an alpha blocker, relaxes the muscles in your ureter, helping you pass the kidney stone more quickly and with less pain. Examples of alpha blockers include tamsulosin (Flomax) and the drug combination dutasteride and tamsulosin (Jalyn).
Medicines to treat and prevent kidney stones
If you have had a kidney stone, your doctor may also prescribe medicines to prevent future kidney stones. Depending on the type of kidney stone you had and what type of medicine your doctor prescribes, you may have to take the medicine for a few weeks, several months, or longer. For example, if you had struvite stones, you may have to take an oral antibiotic for 1 to 6 weeks, or possibly longer. If you had another type of stone, you may have to take a potassium citrate tablet 1 to 3 times daily. You may have to take potassium citrate for months or even longer until your doctor says you are no longer at risk for kidney stones.
Thiazide diuretics, allopurinol, and citrate supplementation are effective in preventing calcium stones that recur despite lifestyle modification, even in the absence of hyperuricemia, urinary acidosis, hypocitraturia, or hyperuricosuria 92, 39, 82, 118. The effectiveness of thiazide diuretics has been documented only with high dosages (e.g., hydrochlorothiazide, 50 mg per day; chlorthalidone, 25 to 50 mg per day; indapamide, 2.5 mg per day); lower dosages have fewer adverse effects, but their effectiveness is unknown 118, 119.
Allopurinol should be started at 100 mg once per day and increased gradually to 100 mg three times per day 39. There is no evidence that combination therapy with thiazide diuretics or alkaline citrates is more effective than monotherapy 39,92, 118, 119. Allopurinol is one of the mainstays of therapy for patients with calcium stones, but most patients with uric acid stones have acidic urine that requires treatment with alkaline citrates 39, 92.
Citrate supplementation is used not only for calcium stones, but also for uric acid (urine pH target 6.0 to 7.5 or greater) and cystine stones (urine pH target of 7.0 to 7.5 or greater) 39,92. The preferred salt for supplementation is potassium citrate at a target dosage of 5 to 12 g per day 39,92, 82. The initial dosage should be 9 g per day, divided into three doses and taken within 30 minutes of meals or a bedtime snack. The most common adverse effects are gastrointestinal symptoms. Potassium citrate supplementation may correct low serum potassium levels caused by thiazide diuretics, but there is no evidence that combination therapy is more effective than monotherapy with either agent 39,92. Sodium citrate is an alternative for citrate supplementation, but the resulting excretion of sodium and calcium may partially counteract the intended effect 39,92. Unsweetened lemonade is a more palatable and less expensive alternative for citrate supplementation. Although there is no direct evidence of its effectiveness in preventing stone recurrence, the dilution of lemon juice in water should help patients meet the recommended fluid intake 120.
If medication or citrate supplementation is prescribed, serum potassium levels (for patients taking thiazide diuretics or potassium citrate) and liver enzymes (allopurinol) should be monitored to detect potentially serious adverse effects 92. Potassium levels should be monitored before prescription, within two weeks of prescription, and then every 12 months (earlier if illness occurs or another medication is added) 121. There are no recommendations on the frequency of monitoring for hepatotoxicity.
Always speak to your doctor about your health history prior to taking kidney stone medicines. Some kidney stone medicines have minor to serious side effects. Side effects are more likely to occur the longer you take the medicine and the higher the dose. Tell your doctor about any side effects that occur when you take kidney stone medicine.
Table 5. Medicines to treat kidney stones
| Type of kidney stone | Possible medicines prescribed by your doctor |
|---|---|
| Calcium stones |
|
| Uric acid stones |
|
| Struvite stones |
|
| Cystine stones |
|
Large kidney stones and those that cause symptoms
Kidney stones that are too large to pass on their own or cause bleeding, kidney damage or ongoing urinary tract infections may require more-extensive treatment. Procedures may include:
- Extracorporeal shock wave lithotripsy (ESWL) uses sound waves to create strong vibrations (shock waves) to break up stones into tiny pieces that can be passed in your urine. For certain kidney stones — depending on size and location — your doctor may recommend a procedure called extracorporeal shock wave lithotripsy (ESWL). The procedure lasts about 45 to 60 minutes and can cause moderate pain, so you may be under sedation or light anesthesia to make you comfortable. Extracorporeal shock wave lithotripsy (ESWL) can cause blood in the urine, bruising on the back or abdomen, bleeding around the kidney and other adjacent organs, and discomfort as the stone fragments pass through the urinary tract.
- Surgery to remove very large stones in the kidney. A procedure called percutaneous nephrolithotomy involves surgically removing a kidney stone using small telescopes called a nephroscope and instruments inserted through a small incision in your back. For larger kidney stones, the doctor also may use a laser to break the kidney stones into smaller pieces. You will receive general anesthesia during the surgery and be in the hospital for one to two days while you recover. After these procedures, sometimes the urologist may leave a thin flexible tube, called a ureteral stent, in your urinary tract to help urine flow or a stone to pass. Once the kidney stone is removed, your doctor sends the kidney stone or its pieces to a lab to find out what type it is. Your doctor may recommend percutaneous nephrolithotomy if extracorporeal shock wave lithotripsy (ESWL) is unsuccessful.
- Using a scope to remove stones. To remove a smaller stone in your ureter or kidney, your doctor may pass a thin lighted tube (ureteroscope) equipped with a camera through your urethra and bladder to your ureter. Once the stone is located, special tools can snare the stone or break it into pieces that will pass in your urine. Your doctor may then place a small tube (stent) in the ureter to relieve swelling and promote healing. You may need general or local anesthesia during this procedure.
- Parathyroid gland surgery. Some calcium phosphate stones are caused by overactive parathyroid glands, which are located on the four corners of your thyroid gland, just below your Adam’s apple. When these parathyroid glands produce too much parathyroid hormone (hyperparathyroidism), your calcium levels can become too high and kidney stones may form as a result. Hyperparathyroidism sometimes occurs when a small, benign tumor forms in one of your parathyroid glands or you develop another condition that leads these glands to produce more parathyroid hormone. Removing the growth from the parathyroid gland stops the formation of kidney stones. Or your doctor may recommend treatment of the condition that’s causing your parathyroid gland to overproduce the hormone.
Asymptomatic kidney stones
Many kidney stones are asymptomatic and found on imaging; each year, 10% to 25% become symptomatic or require intervention 22. Conservative management is an option for adults who are healthy, unfit for surgery, or pregnant, and who have access to health care and can adhere to active surveillance (imaging after six months, then annually) 22, 122. The patient should be referred for stone removal if symptoms, obstruction, or recurrent infection develops, or if the stone grows larger 22, 122. Stone removal should be considered if the patient prefers removal to conservative management; plans to conceive in the near future; has calyceal diverticular stones, stones larger than 10 mm (possibly larger than 4 mm), or renal pathology; or is unsuited for conservative management 122.
For prevention of calcium oxalate, cystine, and uric acid stones, urine should be alkalinized
For prevention of calcium oxalate, cystine, and uric acid stones, urine should be alkalinized [increase urine pH to 6.5 to 7] 123, 124. Western diets are characteristically high in acid-producing foods, such as grains, dairy products, legumes, and meat. Alkalinizing urine involves eating a diet high in fruits and vegetables, taking supplemental or prescription citrate, or drinking alkaline mineral waters 125.
How To Alkalinize Your Urine
Alkalinize urine (i.e., increase urine pH to 6.5 to 7) with dietary changes or oral supplementation, or until 24-hour urine citrate levels are in the normal range:
- Potassium citrate: 10 to 20 mEq orally with meals (prescription required)
- Calcium citrate: two 500-mg tablets per day with meals (each tablet contains 120 mg of calcium and 6 mEq of bicarbonate)
For prevention of calcium phosphate and struvite stones, urine should be acidified
For prevention of calcium phosphate and struvite stones, urine should be acidified [lower urine pH to 7 or less] 126. Cranberry juice or betaine can lower urine pH without the adverse effects associated with acid-producing foods. Although table salt (sodium chloride) also lowers urine pH, it can increase blood pressure, insulin excretion, and urine calcium excretion.
How to Acidify Your Urine
Acidify urine (i.e., lower urine pH to 7 or less) with dietary changes or oral supplementation:
- Cranberry juice: at least 16 oz per day
- Betaine: 650 mg orally three times per day with meals
Can Bacterial Infection Trigger Recurrence?
Bacteria exert both pathogenic and protective roles. Struvite stones are associated with recurrent infections because of high urinary pH levels from urease splitting bacteria and the body’s inability to rid the urinary tract of bacteria that become embedded in the stones 127.
Oxalobacter formigenes is an anaerobic bacterium that colonizes the intestinal tract, where it metabolizes oxalate to formate and carbon dioxide. Absence of O. formigenes colonization predisposes persons to oxalate stones 128. Preliminary studies of O. formigenes ingestion in healthy patients 128 and in patients with primary hyperoxaluria demonstrated up to a 90 percent decrease in urinary oxalate levels 129. Larger studies of this potential therapy are ongoing.
Specific treatments to prevent recurrent stones
Calcium stones
Normocalciuria
- Oral administration of potassium citrate—increases urine pH and citrate excretion in the urine.
Hypercalciuria
- Thiazide diuretics—decrease urinary calcium excretion by augmenting tubular reabsorption of calcium, but do not decrease intestinal absorption in absorptive hypercalciuria; the effect may be attenuated or lost after two or more years of treatment
- Addition of potassium citrate may help to control the diuretic induced hypokalaemia
- If magnesium loss is a concern because of chronic diuretic use, consider potassium magnesium citrate
- Potassium phosphate—may suppress calcitriol synthesis and thereby decrease calcium absorption
Hyperuricaemia or hyperuricosuria
- Allopurinol—to inhibit uric acid synthesis and decrease urinary uric acid excretion
- Potassium citrate should be given in addition to increase urine pH, as uric acid precipitates in acidic urine
Hyperoxaluria
- No specific drugs are available to reduce oxalate excretion in the urine
- Pyridoxine, a cofactor in the alanine-glycoxylate pathway, may reduce production of oxalate by inducing enzyme activity; in an observational study, high intake of vitamin B6 (> 40 mg/day) was inversely associated with risk of oxalate stone formation in women
- Calcium supplementation (250-1000 mg four times a day) to control enteric hyperoxaluria; urinary oxalate may decrease, but a concurrent rise in calcium may negate the beneficial effect
- Cholestyramine reduces intestinal absorption of oxalate, but no trials have shown its efficacy in preventing recurrent stones
- Probiotic treatment with Oxalobacter formigenes has recently been shown to significantly reduce oxalate excretion in both animals and humans; however, trials are pending to show its role in clinical practice 59, 130.
Hypocitriuria
- Potassium citrate—to increase citrate excretion
Struvite stones
- Treatment of infection is mandatory and may be needed in the long term
- Acetohydroxamic acid, a urease inhibitor, has been shown to reduce the urinary saturation of struvite but is associated with high frequency of side effects (deep vein thrombosis, haemolytic anaemia), which limits its use.
Cystine stones
- Treatment must include increasing urine output to about 3 l/day and adequate alkalinisation (urine pH > 7.0) with potassium citrate.
- In addition, specific agents such as α mercaptopropionylglycine or d-penicillamine that form soluble complexes with cystine are used.
Medications to prevent future kidney stones
If you have had a kidney stone, your doctor also may prescribe medicines to prevent future kidney stones. Medications can control the amount of minerals and salts in your urine and may be helpful in people who form certain kinds of stones. The type of medication your doctor prescribes will depend on the kind of kidney stones you have and you may have to take the medicine for a few weeks, several months, or longer. For example, if you had struvite stones, you may have to take an oral antibiotic for 1 to 6 weeks, or possibly longer. If you had another type of stone, you may have to take a potassium citrate tablet 1 to 3 times daily. You may have to take potassium citrate for months or even longer until a health care professional says you are no longer at risk for kidney stones.
Here are some examples:
- Calcium stones. To help prevent calcium stones from forming, your doctor may prescribe a thiazide diuretic often called water pills to help rid your body of water, a phosphate-containing preparation or potassium citrate, which is used to raise the citrate and pH levels in urine.
- Uric acid stones. Your doctor may prescribe allopurinol (Zyloprim, Aloprim) to reduce uric acid levels in your blood and urine and a medicine to keep your urine alkaline. In some cases, allopurinol and an alkalizing agent such as potassium citrate may dissolve the uric acid stones.
- Struvite stones. To prevent struvite stones, your doctor may recommend strategies to keep your urine free of bacteria that cause infection, including drinking fluids to maintain good urine flow and frequent voiding. In rare cases long-term use of antibiotics in small or intermittent doses may help achieve this goal. Acetohydroxamic acid, a strong antibiotic, used with another long-term antibiotic medication to prevent infection. For instance, your doctor may recommend an antibiotic before and for a while after surgery to treat your kidney stones.
- Cystine stones. Along with suggesting a diet lower in salt and protein, your doctor may recommend that you drink more fluids so that you produce a lot more urine,. If that alone doesn’t help, your doctor may also prescribe a medication called mercaptopropionyl glycine (tiopronin) that increases the solubility of cystine in your urine or potassium citrate to raise the pH levels of your urine.
Talk with your doctor about your health history prior to taking kidney stone medicines. Some kidney stone medicines have minor to serious side effects. Side effects are more likely to occur the longer you take the medicine and the higher the dose. Tell your doctor about any side effects that occur when you take kidney stone medicine.
Table 6. Medications to prevent future kidney stones
| Medication | Rationale | Dose | Specifics/side effects | Monitoring |
|---|---|---|---|---|
| Calcium oxalate stones | ||||
| Thiazide | Hypercalciuria | Hydrochlorothiazide 25–50 mg BID, chlorthalidone 25–50 mg/day, indapamide 1.25–5 mg/day | Hypokalemia, hyperlipidemia, hyperuricemia, hyperglycemia, hypocitraturia, hyperuricosuria, fatigue, erectile dysfunction | basic metabolic profile, uric acid, lipid profile |
| Potassium citrate (oral) | Hypocitraturia, low urine pH | 10–30 mEq BID | gastrointestinal side effects | Serum creatinine & potassium |
| Potassium citrate (liquid) | Enteric hyperoxaluria, chronic diarrhea | 15–30 mEq TID–QID (titrate to reduce oxalate) | gastrointestinal side effects, take with two largest meals | Serum creatinine & potassium |
| Allopurinol | Hyperuricosuria | 100–300 mg/day | Hypertransaminasemia, Stevens–Johnson syndrome | Liver enzymes |
| Uric acid stones | ||||
| Potassium citrate (oral) a | Alkalinization | 10–30 mEq BID (titrate dose to pH 6–6.5) | gastrointestinal side effects | Serum creatinine & potassium |
| Sodium bicarbonate | Alkalinization | 650 mg BID–QID | Increased sodium load may increase risk of calcium stones | basic metabolic profile |
| Allopurinol | Hyperuricosuria 2nd line therapy when alkalinization not successful | 100–300 mg/day | Hypertransaminasemia, Stevens–Johnson syndrome | Liver enzymes |
| Cystine stones | ||||
| Tiopronin (α-mercaptopropionyl glycine) | Increase cystine solubility | Initial 400 mg/day titrate to effect | Hematologic effects, tachyphylaxis, proteinuria, nausea, diarrhea, vitamin B6 deficiency (long-term use) | complete blood count, basic metabolic profile, urine protein |
| Potassium citrate (oral) | Alkalinization | 10–30 mEq BID (titrate dose to pH 7–7.5) | gastrointestinal side effects | Serum creatinine & potassium |
| Struvite stones | ||||
| Acetohydroxamic acid | Urease-inhibitor | 250 mg BID–TID | Headache, anemia, thrombophlebitis, rash, tremulousness | complete blood count |
Footnote: a First-line therapy.
[Source 34 ]Calcium stones
Thiazides
Thiazide diuretics are recommended by both the American Urological Association and European Association of Urology guidelines for patients with recurrent calcium-based stones and hypercalciuria 92, 39. Thiazides enhance calcium reabsorption directly in the distal renal tubule and indirectly in the proximal renal tubule. A meta-analysis of six randomized controlled trials (RCTs) found that thiazides were associated with a 47% relative risk reduction in stone recurrence rates compared with placebo or no treatment 119. The benefit of thiazides may additionally extend to normocalciuric patients since the proportion of patients with documented hypercalciuria in these randomized controlled trials was variable, and indeed in some trials the metabolic background of patients was unknown.
Thiazide doses and regimens tested in randomized controlled trials include hydrochlorothiazide 25 mg twice daily, chlorthalidone 25 mg daily or indapamide 2.5 mg daily. Interestingly, one study noted that only 35% of patients treated with thiazides were prescribed a dose shown in randomized controlled trials to be beneficial for stone prevention 131. The most common side effect of thiazides is hypokalemia, which may lead to hypocitraturia due to intracellular acidosis. As such, potassium supplementation is recommended to prevent hypokalemia, to avoid increased cardiovascular risk and to minimize glucose intolerance. The choice of potassium citrate versus potassium chloride to prevent thiazide-induced hypokalemia should be based on initial or follow-up urine pH and citrate; if either is low, potassium citrate may be the preferred potassium supplement.
Because hypercalciuria has been shown to be a risk factor for osteoporosis, a multidisciplinary panel of stone experts authored a consensus paper in which they recommended that stone formers at increased risk of bone loss undergo measurement of bone mineral density by dual emission X-ray absorptiometry (DXA) 132. The fracture risk associated with stone disease varies by skeletal site and demonstrates a higher risk of incident wrist fracture, but not hip fracture, in women and men 133. The use of thiazides in hypercalciuric men was shown to reduce urinary calcium and improve bone mineral density in a short-term study 134. Likewise, Pak and colleagues 135 observed significant increases in bone mineral density at the lumbar spine, femoral neck and radial shaft in 28 recurrent idiopathic hypercalciuric stone formers treated with thiazides and potassium citrate for a mean of 3.7 years.
Potassium citrate
Potassium citrate is used to correct hypocitraturia, which occurs alone or in combination with other abnormalities in about 10%–60% of calcium stone formers 136. Barcelo and coworkers 137 showed a benefit of potassium citrate therapy among 57 recurrent, hypocitraturic calcium stone formers randomized to receive either 30–60 mEq of potassium citrate daily or placebo in a 3-year trial assessing stone remission rates. Patients in the potassium citrate group had a significantly higher remission rate compared to the placebo group (72% vs. 20%, respectively). Potassium citrate has also been shown to improve stone remission rates after shock wave lithotripsy. A recent meta-analysis evaluated the efficacy of potassium citrate in reducing stone recurrence rates 12 months after shock wave lithotripsy in four trials comprising 374 participants 138. A significantly lower stone recurrence rate was seen 12 months after shock wave lithotripsy in the potassium citrate group compared to the control group. Finally, patients with hypocitraturia due to distal renal tubular acidosis have also been shown to benefit from potassium citrate therapy, as the alkali load corrects the metabolic acidosis, increases urinary citrate excretion and reduces stone recurrence rates 139.
Potassium citrate may also reduce urinary calcium excretion as an additional benefit of treatment. Song and associates 140 observed a mean decrease in urine calcium of 30% among 22 hypocitraturic calcium oxalate stone formers receiving 30–60 mEq of potassium citrate daily for at least 3 months. They speculated that the reduction in urinary calcium may be accounted for by a decrease in bone turnover due to systemic alkalinization, by binding of calcium by citrate in the intestine and reducing calcium absorption or as a result of a direct effect on the distal renal tubule affecting calcium reabsorption 140.
The optimal dose and formulation of potassium citrate remains to be determined 82. However, potassium citrate is usually administered as a sustained-release wax matrix tablet in doses ranging from 10–30 mEq twice daily. However, for patients with chronic diarrhea, use of a liquid formulation is advised because the rapid intestinal transit in these patients precludes sufficient absorption of sustained-release potassium citrate 141.
The most common side effects of potassium citrate are gastrointestinal upset, abdominal pain, and diarrhea. Taking the medication with meals may prevent these symptoms in some patients. Periodic monitoring with serum electrolytes and creatinine is recommended. For patients at risk of hyperkalemia or those who are unable to tolerate potassium citrate, sodium alkali such as sodium bicarbonate or a combination of sodium citrate and citric acid may be considered, although the sodium load conferred by these medications may induce hypercalciuria.
Allopurinol
Hyperuricosuria is seen in approximately 20% of patients with calcium stones 142. Although dietary restriction of animal protein is recommended as first line therapy in patients with hyperuricosuria, allopurinol, a xanthine oxidase inhibitor that lowers serum and urinary uric acid, is indicated in patients who are unable to normalize urinary uric acid with diet alone or in those with a genetic predisposition to hyperuricosuria. Allopurinol may additionally have antioxidant effects that contribute to the prevention of calcium stone formation, independent of xanthine oxidase inhibition 143. Among four randomized controlled trials evaluating the effect of allopurinol in recurrent calcium oxalate stone formers, the only trial to show a significant benefit in reducing recurrence rates is one in which only hyperuricosuric, normocalciuric patients were enrolled 144.
Allopurinol is generally well tolerated but may, in rare cases, be associated with Stevens–Johnson syndrome. Any patient reporting a rash with allopurinol should be instructed to discontinue the medication immediately. Hypertransaminasemia is another side effect that is generally reversible with discontinuation of the medication. Liver enzymes should be monitored soon after starting allopurinol and periodically thereafter 145.
Pyridoxine (vitamin B6)
Nondietary urinary oxalate is derived from the conversion of glyoxylate to oxalate by the enzyme lactate dehydrogenase 146, 147. Pyridoxine, a component of vitamin B6, is a necessary cofactor for alanine:glyoxyalate aminotransferase (AGT) which is involved in the alternate metabolic conversion of glyoxylate to glycine. Patients with primary hyperoxaluria type 1 have a deficiency in AGT by which they are unable to divert glyoxylate metabolism to the alternate pathway. While the only curative treatment for this disease is liver transplantation, pyridoxine can be used to decrease urinary oxalate. A prospective, uncontrolled open label trial in which 12 patients with primary hyperoxaluria type I were given pyridoxine incrementally up to a dosage of 20 mg/kg per day showed a 25.5% mean relative reduction in urinary oxalate, with benefit seen in 50% of patients 148.
Pyridoxine (vitamin B6) has been proposed as a preventative treatment in patients with idiopathic hyperoxaluria as well. One retrospective study evaluating the effect of pyridoxine on urinary oxalate in 95 idiopathic hyperoxaluric stone formers found that 75% of patients treated with pyridoxine and dietary counseling versus only 52% of patients treated with dietary counseling alone showed a reduction in urinary oxalate 149. On the other hand, three large cohort studies (HPFS, NHS I, NHS II) found no association between vitamin B6 intake and risk of incident kidney stones 150. The American Urological Association Medical Management of Kidney Stones Guideline found insufficient evidence to recommend pyridoxine for the management of patients with idiopathic hyperoxaluria 92. However if it is used, pyridoxine should be started at a low dose and titrated in a stepwise fashion to no more than 200 mg/day 151. Doses excessing 500 mg/day should be avoided because of the risk of developing severe sensory neuropathy.
Uric acid stones
The prevention of uric acid stone formation involves the administration of alkali to increase urine pH. A target pH of 6–6.5 can effectively prevent and dissolve uric acid stones 152. Potassium citrate, titrated to achieve the target pH, is first-line therapy for uric acid stone formers, with sodium alkali (sodium bicarbonate) reserved for those unable to tolerate potassium citrate or for whom renal dysfunction or hyperkalemia precludes its use. Of note, even large amounts of uric acid are soluble if the urine pH is sufficiently high; in contrast, even small amounts of uric acid will precipitate out in acidic urine. As such, allopurinol should not constitute first line therapy for idiopathic uric acid stone formers and is reserved only for patients who continue to form uric acid stones despite adequate urinary alkalinization 92. In addition to pharmacologic treatment to prevent uric acid stones, lifestyle changes such as weight loss and exercise should be recommended by physicians for patients with metabolic syndrome.
Dissolution therapy for uric acid stones is also achievable with alkali therapy using a target urine pH of 6–6.5. However, the success of dissolution therapy relies on accurate diagnosis of pure uric acid stones. One group of investigators determined that computed tomography (CT) Hounsfield units ≤500, pH ≤ 5.5 and stone size > 4 mm on non-enhanced CT had an 86% sensitivity, 98% specificity, and 90% positive predictive value for diagnosing uric acid stones 153. In an in vivo study of 30 patients, dual energy CT was shown to have a 100% positive predictive value for detecting uric acid stones 154. Patients with 100% pure uric acid stones tend to be older (60 vs. 55 years), heavier (34.3 vs. 29 kg/m²), have higher serum uric acids (6.53 vs. 5.89 mg/dL) and have lower pH (5.62 vs. 5.89) than those with 10%–20% uric acid stone composition 155. Combining these demographics with stone size and Hounsfield units on CT scan may contribute to better diagnostic accuracy in identifying patients with pure uric acid stones who may be successfully treated with dissolution therapy. Complete dissolution may occur in as little as 6 weeks to 6 months or more 156, 157, likely due to the varying percentages of uric acid stone composition 155.
Cystine stones
The therapeutic goal in treating patients with cystinuria is to reduce cystine concentration or raise cystine solubility above the solubility limit of 250 mg/L. Reducing cystine concentration can be accomplished with increased fluid intake, typically enough fluid intake to produce a urine volume of at least 3 L/day, or by reducing cystine excretion. Restriction of sodium and animal protein have been shown in randomized trials to decrease urinary cystine excretion 158, 159, 160. Like all stone formers, cystinuric patients are advised to limit their sodium intake to less than 2300 mg/day (100 mEq/day) 92.
Cystine solubility is influenced by urine pH and the presence of urinary macromolecules. Because cystine solubility increases significantly only above pH 7.5 and most cystinuric patients have high urine pH at baseline, urinary alkalinization has a limited therapeutic role, but constitutes second line therapy after diet and fluids, with the goal of treatment to achieve a urine pH of 7.0–7.5. At pH > 7, the risk of calcium phosphate stone formation becomes more pronounced, and repeat stone analysis, if one is available during treatment, should be pursued 161, 162. Potassium alkali (potassium citrate) is preferred over sodium alkali for urinary alkalinization because sodium enhances urinary cystine excretion.
For more severe cystinuria, cystine-binding thiol drugs (CBTD) constitute third line therapy 163, 164. Agents most commonly used include α-mercaptopropionyl glycine (tiopronin) and d-penicillamine. Thiol compounds contain sulfhydryl groups that undergo a disulfide exchange reaction with cystine to produce two molecules of cysteine bound to the cystine-binding thiol drugs (CBTD), a complex that is 50 times more soluble than cystine. The effect of the drugs is dose-dependent.
Tiopronin, as a second generation cystine-binding thiol drugs (CBTD), is considered the first line drug, with dosing starting at 200 mg two or three times daily and titrated to achieve a urine cystine concentration of less than 250 mg/L. Adverse effects include fever, gastrointestinal upset, asthenia, rash, joint aches, loss of taste, thrombocytopenia, aplastic anemia, proteinuria, and changes in mental status 165.
d-penicillamine has a more extensive side effect profile than tiopronin and therefore is used much less commonly 166. Dosing typically starts at 250 mg two or three times daily and is titrated to effect. One study reported adverse effects in 65% of patients taking tiopronin compared with 84% of those taking d-penicillamine 167. Adverse reactions necessitating cessation of treatment were also less common with tiopronin (31% vs. 69%, respectively). Long-term therapy with d-penicillamine may lead to pyridoxine (vitamin B6) deficiency, which may require oral supplementation (50 mg/day).
Captopril is a third generation angiotensin-converting-enzyme inhibitor that also contains a free sulfhydryl group and has been shown in vitro to increase cystine solubility 168. However, the recommended doses of captopril in vivo are not thought to be sufficient to induce a therapeutic effect on cystine stone formation and the drug has not been tested in rigorous clinical trials.
Recently, a widely available nutritional supplement, alpha-lipoic acid, has been investigated as a treatment for cystinuria 169. Using the SLC3A1−/− mouse model which grows urinary bladder stones at a rate of 1 mm³/day, treatment with alpha-lipoic acid was found to not only attenuate growth of existing cystine stones but also prevent initiation of new stone formation. The protective effect is thought to be derived from increased urinary cystine solubility due to excretion of downstream α-lipoic acid metabolites into the urine. Clinical trials are necessary to prove its efficacy in humans.
Although traditionally the goal of treatment has been to reduce urinary cystine concentration below the solubility level 163, most assays are unable to distinguish free cystine from soluble thiol drug-cysteine complexes. A proprietary test, cystine capacity (Litholink Corporation, Chicago, IL, USA), has been shown to reliably estimate stone forming propensity even in the presence of cystine-binding thiol drugs (CBTD) and offers an alternative means of monitoring therapeutic efficacy 170, 171.
Struvite stones
Struvite stones form as a consequence of repeated infection with urease-producing organisms, leading to alkaline urine and precipitation of magnesium ammonium phosphate crystals. Because these stones incorporate bacteria inside them, aggressive surgical stone removal is essential to eradicate the bacteria and prevent recurrent infections and stone. Culture-specific antibiotic therapy is recommended both pre-operatively and for some time post-operatively to sterilize the urine and prevent re-infection. A recent multi-institutional study on patterns of infection and colonization of struvite stones revealed that the bacteriology has shifted away from traditional urea-splitting organisms such as Proteus to Enterococcus and Escherichia coli 172. The high prevalence of infection with Enterococcus (18%) suggests that 1st and 2nd generation cephalopshorins may be ineffective and antibiotic coverage should include agents appropriate for Enterococcus. Nonetheless, there is a lack of evidence on the role of antibiotics in preventing stone growth/recurrence and the optimal duration of therapy.
Acetohydroxamic acid (AHA), a potent urease inhibitor, decreases the risk of struvite stone formation by preventing bacterial-induced urease from altering the urinary milieu. Acetohydroxamic acid has been shown in a number of randomized controlled trials to reduce stone growth and prolong the time interval to stone growth 173, 174, 175. However, adverse events are common, leading to high attrition rates. Side effects include tremor, palpitations, edema, proteinuria, headache, rash, alopecia, anemia, gastrointestinal discomfort, and thromboembolic phenomena. In light of these limitations, acetohydroxamic acid, along with suppressive antibiotics, are reserved for patients at high risk of recurrent struvite stone formation and/or those unable to undergo surgical stone removal. Using a lower dose of 250 mg twice daily has been proposed to reduce the overall adverse events, although deep vein thrombosis/pulmonary embolus was still prevalent 176.
Kidney Stone Diet
- All kidney stone formers should drinking enough water to achieve a urine volume of at least 2.5 liters daily is the most important thing you can do to prevent kidney stones. Unless you have kidney failure, many health care professionals recommend that you drink six to eight, 8-ounce (237.2 ml) glasses a day. Talk with a health care professional about how much liquid you should drink.
- Studies have shown that being overweight increases your risk of kidney stones. A dietitian can help you plan meals to help you lose weight.
- Studies have shown that the Dietary Approaches to Stop Hypertension (DASH) diet can reduce the risk of kidney stones 89.
- If you have already had kidney stones, ask your health care professional which type of kidney stone you had. Based on the type of kidney stone you had, you may be able to prevent kidney stones by making changes in how much sodium, animal protein, calcium, or oxalate is in the food you eat. You may need to change what you eat and drink for these types of kidney stones:
- Calcium Oxalate Stones
- Calcium Phosphate Stones
- Uric Acid Stones
- Cystine Stones
- A dietitian who specializes in kidney stone prevention can help you plan meals to prevent kidney stones. Search for credentialed nutrition and dietetics practitioners by location, specialty, language or insurance and payment options here (https://www.eatright.org/find-a-nutrition-expert).
Calcium Oxalate Stones
- Reduce oxalate. If you’ve had calcium oxalate stones, you may want to avoid these foods to help reduce the amount of oxalate in your urine:
- nuts and nut products
- peanuts—which are legumes, not nuts, and are high in oxalate
- rhubarb
- spinach
- wheat bran
- Talk with a dietitian or health care professional about other food sources of oxalate and how much oxalate should be in what you eat.
- Reduce sodium. Your chance of developing kidney stones increases when you eat more sodium. Sodium is a part of salt. Sodium is in many canned, packaged, and fast foods. It is also in many condiments, seasonings, and meats. Most Americans consume too much sodium. Talk with a health care professional about how much sodium should be in what you eat. Adults should aim to consume less than 2,300 mg a day 177. One teaspoon of table salt has 2,325 milligrams (mg) of sodium. If you have had calcium oxalate or calcium phosphate stones, you should follow this guideline, even if you take medicine to prevent kidney stones.
- Check labels for ingredients and hidden sodium, such as:
- sodium bicarbonate, the chemical name for baking soda
- baking powder, which contains sodium bicarbonate and other chemicals
- disodium phosphate
- monosodium glutamate, or MSG
- sodium alginate
- sodium nitrate or nitrite
- Here are some tips to help you reduce your sodium intake:
- Check the Percent Daily Value (%DV) for sodium on the Nutrition Facts label found on many foods. Low in sodium is 5% or less, and high in sodium is 20% or more.
- Consider writing down how much sodium you consume each day.
- When eating out, ask about the sodium content in the food.
- Cook from scratch. Avoid processed and fast foods, canned soups and vegetables, and lunch meats.
- Look for foods labeled: sodium free, salt free, very low sodium, low sodium, reduced or less sodium, light in sodium, no salt added, unsalted, and lightly salted.
- Check labels for ingredients and hidden sodium, such as:
- Limit animal protein. Eating animal protein may increase your chances of developing kidney stones. Although you may need to limit how much animal protein you eat each day, you still need to make sure you get enough protein. Consider replacing some of the meat and animal protein you would typically eat with beans, dried peas, and lentils, which are plant-based foods that are high in protein and low in oxalate. Talk with a health care professional about how much total protein you should eat and how much should come from animal or plant-based foods.
- A health care professional may tell you to limit eating animal protein, including:
- beef, chicken, and pork, especially organ meats
- eggs
- fish and shellfish
- milk, cheese, and other dairy products
- A health care professional may tell you to limit eating animal protein, including:
- Get enough calcium from foods. Even though calcium sounds like it would be the cause of calcium stones, it’s not. In the right amounts, calcium can block other substances in your digestive tract that may cause stones. Talk with a health care professional about how much calcium you should eat to help prevent getting more calcium oxalate stones and to support strong bones. It may be best to get calcium from low-oxalate, plant-based foods such as calcium-fortified juices, cereals, breads, some kinds of vegetables, and some types of beans. Ask a dietitian or other health care professional which foods are the best sources of calcium for you.
Calcium Phosphate Stones
- Reduce sodium. Your chance of developing kidney stones increases when you eat more sodium. Sodium is a part of salt. Sodium is in many canned, packaged, and fast foods. It is also in many condiments, seasonings, and meats. Most Americans consume too much sodium. Talk with a health care professional about how much sodium should be in what you eat. Adults should aim to consume less than 2,300 mg a day 177. One teaspoon of table salt has 2,325 milligrams (mg) of sodium. If you have had calcium oxalate or calcium phosphate stones, you should follow this guideline, even if you take medicine to prevent kidney stones.
- Check labels for ingredients and hidden sodium, such as:
- sodium bicarbonate, the chemical name for baking soda
- baking powder, which contains sodium bicarbonate and other chemicals
- disodium phosphate
- monosodium glutamate, or MSG
- sodium alginate
- sodium nitrate or nitrite
- Here are some tips to help you reduce your sodium intake:
- Check the Percent Daily Value (%DV) for sodium on the Nutrition Facts label found on many foods. Low in sodium is 5% or less, and high in sodium is 20% or more.
- Consider writing down how much sodium you consume each day.
- When eating out, ask about the sodium content in the food.
- Cook from scratch. Avoid processed and fast foods, canned soups and vegetables, and lunch meats.
- Look for foods labeled: sodium free, salt free, very low sodium, low sodium, reduced or less sodium, light in sodium, no salt added, unsalted, and lightly salted.
- Check labels for ingredients and hidden sodium, such as:
- Limit animal protein. Eating animal protein may increase your chances of developing kidney stones. Although you may need to limit how much animal protein you eat each day, you still need to make sure you get enough protein.
- Consider replacing some of the meat and animal protein you would typically eat with these plant-based foods that are high in protein:
- legumes such as beans, dried peas, lentils, and peanuts
- soy foods, such as soy milk, soy nut butter, and tofu
- nuts and nut products, such as almonds and almond butter, cashews and cashew butter, walnuts, and pistachios
- sunflower seeds
- A health care professional may tell you to limit eating animal protein, including:
- beef, chicken, and pork, especially organ meats
- eggs
- fish and shellfish
- milk, cheese, and other dairy products
- Consider replacing some of the meat and animal protein you would typically eat with these plant-based foods that are high in protein:
- Get enough calcium from foods. Even though calcium sounds like it would be the cause of calcium stones, it’s not. In the right amounts, calcium can block other substances in the digestive tract that may lead to stones. Talk with a health care professional about how much calcium you should eat to help prevent getting more calcium phosphate stones and to support strong bones. It may be best to get calcium from plant-based foods such as calcium-fortified juices, cereals, breads, some kinds of vegetables, and some types of beans. Ask a dietitian or other health care professional which foods are the best sources of calcium for you.
Uric Acid Stones
- Limit animal protein. Eating animal protein may increase your chances of developing kidney stones. Although you may need to limit how much animal protein you eat each day, you still need to make sure you get enough protein. Talk with a health care professional about how much total protein you should eat and how much should come from animal or plant-based foods.
- Consider replacing some of the meat and animal protein you would typically eat with some of these plant-based foods that are high in protein:
- legumes such as beans, dried peas, lentils, and peanuts
- soy foods, such as soy milk, soy nut butter, and tofu
- nuts and nut products, such as almonds and almond butter, cashews and cashew butter, walnuts, and pistachios
- sunflower seeds
- A health care professional may tell you to limit eating animal protein, including:
- beef, chicken, and pork, especially organ meats
- eggs
- fish and shellfish
- milk, cheese, and other dairy products
- Consider replacing some of the meat and animal protein you would typically eat with some of these plant-based foods that are high in protein:
- Losing weight. Losing weight if you are overweight is especially important for people who have had uric acid stones.
Cystine Stones
Drinking enough liquid, mainly water, is the most important lifestyle change you can make to prevent cystine stones. Talk with a health care professional about how much liquid you should drink.
Follow-up
The success of a medical prophylactic program hinges on improvement in urinary stone risk factors and ultimately on reduction in stone recurrence rate. By monitoring 24 hour urine parameters, a change in stone risk might be anticipated and corrected before stones recur. The frequency of follow-up depends on the metabolic activity of the individual. Periodic imaging can detect failure of medical therapy and prompt early surgical treatment and modulation of the medical regimen. In addition, follow-up should include blood and urine testing for adverse effects of treatment, such as hypokalemia with thiazides or proteinuria with α-mercaptopropionyl glycine (tiopronin).
- Clinicians should obtain a single 24-hour urine specimen for stone risk factors within six months of the initiation of treatment to assess response to dietary and/or medical therapy.
- After the initial follow-up, clinicians should obtain a single 24-hour urine specimen annually or with greater frequency, depending on stone activity, to assess patient adherence and metabolic response.
- Clinicians should obtain periodic blood testing to assess for adverse effects in patients on pharmacological therapy.
- Clinicians should obtain a repeat stone analysis, when available, especially in patients not responding to treatment.
- Clinicians should monitor patients with struvite stones for reinfection with urease-producing organisms and utilize strategies to prevent such occurrences.
- Clinicians should periodically obtain follow-up imaging studies to assess for stone growth or new stone formation based on stone activity (plain abdominal imaging, renal ultrasonography or low dose computed tomography [CT scan]).
Kidney stones prognosis
Kidney stones are painful, but most of the time can be removed from the body without causing lasting damage. Kidney stones that do not pass can become obstructive and can subsequently cause acute renal failure, or it can also become a nidus for infection, which can eventually be lethal.
Kidney stones often come back. This occurs more often if the cause is not found and treated. You are at risk for:
- Urinary tract infection
- Kidney damage or scarring if treatment is delayed for too long
Kidney stones have been associated with an increased risk of chronic kidney diseases 178, end-stage renal failure 179, cardiovascular diseases such as heart attack 180, 181, diabetes, and hypertension 182. It has been suggested that kidney stone may be a systemic disorder linked to the metabolic syndrome. Kidney stone is responsible for 2 to 3% of end-stage renal cases if it is associated with nephrocalcinosis 183.
If the patient undergoes nephrostomy tube placement, then there is a chance of bleeding, renal collecting system injury, injury of visceral organs, pulmonary complications, thromboembolic complications, and extrarenal stone migration 184.
References- Parmar MS. Kidney stones. BMJ : British Medical Journal. 2004;328(7453):1420-1424. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC421787/
- Segura JW, Preminger GM, Assimos DG, Dretler SP, Kahn RL, Lingeman JE, et al. Ureteral stones clinical guidelines panel summary report on the management of ureteral calculi. J Urol 1997;158: 1915-21. https://www.ncbi.nlm.nih.gov/pubmed/9334635
- Alelign T, Petros B. Kidney Stone Disease: An Update on Current Concepts. Adv Urol. 2018 Feb 4;2018:3068365. doi: 10.1155/2018/3068365
- Asplin JR, Favus MJ, Coe FL. Nephrolithiasis. In: Brenner BM, ed. Brenner and Rector’s the kidney. 5th ed. Philadelphia: Saunders, 1996: 1893-935.
- López M, Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol. 2010 Jan;25(1):49-59. doi: 10.1007/s00467-008-0960-5
- Taguchi K, Yasui T, Milliner DS, Hoppe B, Chi T. Genetic Risk Factors for Idiopathic Urolithiasis: A Systematic Review of the Literature and Causal Network Analysis. Eur Urol Focus. 2017 Feb;3(1):72-81. doi: 10.1016/j.euf.2017.04.010
- Coe FL, Worcester EM, Evan AP. Idiopathic hypercalciuria and formation of calcium renal stones. Nat Rev Nephrol. 2016 Sep;12(9):519-33. doi: 10.1038/nrneph.2016.101
- Gee HY, Jun I, Braun DA, Lawson JA, Halbritter J, Shril S, Nelson CP, Tan W, Stein D, Wassner AJ, Ferguson MA, Gucev Z, Sayer JA, Milosevic D, Baum M, Tasic V, Lee MG, Hildebrandt F. Mutations in SLC26A1 Cause Nephrolithiasis. Am J Hum Genet. 2016 Jun 2;98(6):1228-1234. doi: 10.1016/j.ajhg.2016.03.026
- Merck Sharp & Dohme Corp., Merck Manual. Urinary Calculi. https://www.merckmanuals.com/professional/genitourinary-disorders/urinary-calculi/urinary-calculi
- Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Kidney Int. 2003 May; 63(5):1817-23. https://www.ncbi.nlm.nih.gov/pubmed/12675858/
- Gender differences in seasonal variation of urine stone risk factors. Parks JH, Barsky R, Coe FL. J Urol. 2003 Aug; 170(2 Pt 1):384-8. https://www.ncbi.nlm.nih.gov/pubmed/12853781/
- Hall WD, Pettinger M, Oberman A, Watts NB, Johnson KC, Paskett ED, et al. Risk factors for kidney stones in older women in Southern United States. Am J Med Sci 2001;322: 12-8. https://www.ncbi.nlm.nih.gov/pubmed/11465241
- Song L, Maalouf NM. Nephrolithiasis. [Updated 2020 Mar 9]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279069
- Ross AE, Handa S, Lingeman JE, Matlaga BR. Kidney stones during pregnancy: an investigation into stone composition. Urol Res. 2008;36(2):99–102.
- Costa-Bauza A, Ramis M, Montesinos V, et al. Type of renal calculi: variation with age and sex. World J Urol. 2007;25(4):415–21.
- Acar B, Inci Arikan F, Emeksiz S, Dallar Y. Risk factors for nephrolithiasis in children. World J Urol. 2008;26(6):627–630.
- Pietrow PK, Karellas ME. Medical management of common urinary calculi. Am Fam Physician. 2006;74(1):86–94.
- Treatment and Prevention of Kidney Stones: An Update. Am Fam Physician. 2011 Dec 1;84(11):1234-1242. http://www.aafp.org/afp/2011/1201/p1234.html
- Kumar S. B. N., Kumar K. G., Srinivasa V., Bilal S. A review on urolithiasis. International Journal of Universal Pharmacy and Life Sciences. 2012;2(2):269–280.
- U.S. Department of Health and Human Services. The National Institute of Diabetes and Digestive and Kidney Diseases Health Information Center. Definition & Facts for Kidney Stones. https://www.niddk.nih.gov/health-information/urologic-diseases/kidney-stones/definition-facts
- Tchey DU, Ha YS, Kim WT, Yun SJ, Lee SC, Kim WJ. Expectant Management of Ureter Stones: Outcome and Clinical Factors of Spontaneous Passage in a Single Institution’s Experience. Korean J Urol. 2011 Dec;52(12):847-51. doi: 10.4111/kju.2011.52.12.847
- Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T. EAU Guidelines on Diagnosis and Conservative Management of Urolithiasis. Eur Urol. 2016 Mar;69(3):468-74. doi: 10.1016/j.eururo.2015.07.040
- Hollingsworth JM, Canales BK, Rogers MA, Sukumar S, Yan P, Kuntz GM, Dahm P. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016 Dec 1;355:i6112. doi: 10.1136/bmj.i6112
- Wang H, Man LB, Huang GL, Li GZ, Wang JW. Comparative efficacy of tamsulosin versus nifedipine for distal ureteral calculi: a meta-analysis. Drug Des Devel Ther. 2016 Mar 29;10:1257-65. doi: 10.2147/DDDT.S99330
- Pickard R, Starr K, MacLennan G, Lam T, Thomas R, Burr J, McPherson G, McDonald A, Anson K, N’Dow J, Burgess N, Clark T, Kilonzo M, Gillies K, Shearer K, Boachie C, Cameron S, Norrie J, McClinton S. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015 Jul 25;386(9991):341-9. doi: 10.1016/S0140-6736(15)60933-3
- Worster AS, Bhanich Supapol W. Fluids and diuretics for acute ureteric colic. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD004926. doi: 10.1002/14651858.CD004926.pub3
- Moe O. W. Kidney stones: pathophysiology and medical management. The Lancet. 2006;367(9507):333–344. doi: 10.1016/s0140-6736(06)68071-9
- Sayer J. A. The Genetics of nephrolithiasis. Nephron Experimental Nephrology. 2008;110(2):37–43. doi: 10.1159/000151730
- Giannossi L., Summa V. A review of pathological biomineral analysis techniques and classification schemes. In: Aydinalp C., editor. An Introduction to the Study of Mineralogy. InTech, IMAA-CNR, Italy: InTechOpen; 2012.
- Barbasa C., Garciaa A., Saavedraa L., Muros M. Urinary analysis of nephrolithiasis markers. Journal of Chromatography B. 2002;781(1-2):433–455. doi: 10.1016/s1570-0232(02)00557-3
- Dursun M., Otunctemur A., Ozbek E. Kidney stones and ceftriaxone. European Medical Journal of Urology. 2015;3(1):68–74.
- Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol. 2008 Mar;28(2):120-32. doi: 10.1016/j.semnephrol.2008.01.005
- Pak CY, Sakhaee K, Moe OW, Poindexter J, Adams-Huet B, Pearle MS, Zerwekh JE, Preminger GM, Wills MR, Breslau NA, Bartter FC, Brater DC, Heller HJ, Odvina CV, Wabner CL, Fordtran JS, Oh M, Garg A, Harvey JA, Alpern RJ, Snyder WH, Peters PC. Defining hypercalciuria in nephrolithiasis. Kidney Int. 2011 Oct;80(7):777-82. doi: 10.1038/ki.2011.227
- Sorokin I, Pearle MS. Medical therapy for nephrolithiasis: State of the art. Asian J Urol. 2018 Oct;5(4):243-255. doi: 10.1016/j.ajur.2018.08.005
- Coe FL, Parks JH, Moore ES. Familial idiopathic hypercalciuria. N Engl J Med. 1979 Feb 15;300(7):337-40. doi: 10.1056/NEJM197902153000703
- Melton LJ 3rd, Crowson CS, Khosla S, Wilson DM, O’Fallon WM. Fracture risk among patients with urolithiasis: a population-based cohort study. Kidney Int. 1998 Feb;53(2):459-64. doi: 10.1046/j.1523-1755.1998.00779.x
- Sakhaee K, Maalouf NM, Poindexter J, Adams-Huet B, Moe OW. Relationship between Urinary Calcium and Bone Mineral Density in Patients with Calcium Nephrolithiasis. J Urol. 2017 Jun;197(6):1472-1477. doi: 10.1016/j.juro.2017.01.002
- Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, Monga M, Penniston KL, Preminger GM, Turk TM, White JR; American Urological Assocation. Medical management of kidney stones: AUA guideline. J Urol. 2014 Aug;192(2):316-24. https://www.auajournals.org/doi/10.1016/j.juro.2014.05.006
- Skolarikos A, Straub M, Knoll T, Sarica K, Seitz C, Petřík A, Türk C. Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur Urol. 2015 Apr;67(4):750-63. doi: 10.1016/j.eururo.2014.10.029
- Taylor EN, Curhan GC. Dietary calcium from dairy and nondairy sources, and risk of symptomatic kidney stones. J Urol. 2013 Oct;190(4):1255-9. doi: 10.1016/j.juro.2013.03.074
- Sakhaee K, Maalouf NM, Sinnott B. Clinical review. Kidney stones 2012: pathogenesis, diagnosis, and management. J Clin Endocrinol Metab. 2012 Jun;97(6):1847-60. doi: 10.1210/jc.2011-3492
- Hess B, Jost C, Zipperle L, Takkinen R, Jaeger P. High-calcium intake abolishes hyperoxaluria and reduces urinary crystallization during a 20-fold normal oxalate load in humans. Nephrol Dial Transplant. 1998 Sep;13(9):2241-7. doi: 10.1093/ndt/13.9.2241
- Domrongkitchaiporn S, Sopassathit W, Stitchantrakul W, Prapaipanich S, Ingsathit A, Rajatanavin R. Schedule of taking calcium supplement and the risk of nephrolithiasis. Kidney Int. 2004 May;65(5):1835-41. doi: 10.1111/j.1523-1755.2004.00587.x
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997 Apr 1;126(7):497-504. doi: 10.7326/0003-4819-126-7-199704010-00001
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med. 2004 Apr 26;164(8):885-91. doi: 10.1001/archinte.164.8.885
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993 Mar 25;328(12):833-8. doi: 10.1056/NEJM199303253281203
- Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. Mollerup CL, Vestergaard P, Frøkjaer VG, Mosekilde L, Christiansen P, Blichert-Toft M. BMJ. 2002 Oct 12; 325(7368):807. https://www.ncbi.nlm.nih.gov/pubmed/12376441/
- Zerwekh JE, Holt K, Pak CY. Natural urinary macromolecular inhibitors: attenuation of inhibitory activity by urate salts. Kidney Int. 1983 Jun;23(6):838-41. doi: 10.1038/ki.1983.103
- Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995 Jul;95(7):791-7. doi: 10.1016/S0002-8223(95)00219-7
- Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988 Jan;66(1):140-6. doi: 10.1210/jcem-66-1-140
- Nouvenne A, Ticinesi A, Morelli I, Guida L, Borghi L, Meschi T. Fad diets and their effect on urinary stone formation. Transl Androl Urol. 2014 Sep;3(3):303-12. doi: 10.3978/j.issn.2223-4683.2014.06.01
- Vezzoli G, Dogliotti E, Terranegra A, Arcidiacono T, Macrina L, Tavecchia M, Pivari F, Mingione A, Brasacchio C, Nouvenne A, Meschi T, Cusi D, Spotti D, Montanari E, Soldati L. Dietary style and acid load in an Italian population of calcium kidney stone formers. Nutr Metab Cardiovasc Dis. 2015 Jun;25(6):588-93. doi: 10.1016/j.numecd.2015.03.005
- Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002 Aug;40(2):265-74. doi: 10.1053/ajkd.2002.34504
- Buclin T, Cosma M, Appenzeller M, Jacquet AF, Décosterd LA, Biollaz J, Burckhardt P. Diet acids and alkalis influence calcium retention in bone. Osteoporos Int. 2001;12(6):493-9. doi: 10.1007/s001980170095
- Maalouf NM, Moe OW, Adams-Huet B, Sakhaee K. Hypercalciuria associated with high dietary protein intake is not due to acid load. J Clin Endocrinol Metab. 2011 Dec;96(12):3733-40. doi: 10.1210/jc.2011-1531
- Eisenstein J, Roberts SB, Dallal G, Saltzman E. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev. 2002 Jul;60(7 Pt 1):189-200. doi: 10.1301/00296640260184264
- Tracy CR, Best S, Bagrodia A, Poindexter JR, Adams-Huet B, Sakhaee K, Maalouf N, Pak CY, Pearle MS. Animal protein and the risk of kidney stones: a comparative metabolic study of animal protein sources. J Urol. 2014 Jul;192(1):137-41. doi: 10.1016/j.juro.2014.01.093
- Hattori CM, Tiselius HG, Heilberg IP. Whey protein and albumin effects upon urinary risk factors for stone formation. Urolithiasis. 2017 Oct;45(5):421-428. doi: 10.1007/s00240-017-0975-0
- Rapid reversal of hyperoxaluria in a rat model after probiotic administration of Oxalobacter formigenes. Sidhu H, Allison MJ, Chow JM, Clark A, Peck AB. J Urol. 2001 Oct; 166(4):1487-91. https://www.ncbi.nlm.nih.gov/pubmed/11547118/
- Oxalobacter formigenes and its potential role in human health. Duncan SH, Richardson AJ, Kaul P, Holmes RP, Allison MJ, Stewart CS. Appl Environ Microbiol. 2002 Aug; 68(8):3841-7. https://www.ncbi.nlm.nih.gov/pubmed/12147479/
- Contribution of dietary oxalate to urinary oxalate excretion. Holmes RP, Goodman HO, Assimos DG. Kidney Int. 2001 Jan; 59(1):270-6. https://www.ncbi.nlm.nih.gov/pubmed/11135080/
- Grentz L, Massey LK. Contribution of dietary oxalate to urinary oxalate in health and disease. Topics in Clinical Nutrition 2002;17: 60-70.
- Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Baxmann AC, De O G Mendonça C, Heilberg IP. Kidney Int. 2003 Mar; 63(3):1066-71. https://www.ncbi.nlm.nih.gov/pubmed/12631089/
- Effect of grapefruit juice on urinary lithogenicity. Goldfarb DS, Asplin JR. J Urol. 2001 Jul; 166(1):263-7. https://www.ncbi.nlm.nih.gov/pubmed/11435883/
- Hesse A, Schneeberger W, Engfeld S, Von Unruh GE, Sauerbruch T. Intestinal hyperabsorption of oxalate in calcium oxalate stone formers: application of a new test with [13C2]oxalate. J Am Soc Nephrol. 1999 Nov;10 Suppl 14:S329-33.
- Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int. 2001 Jan;59(1):270-6. doi: 10.1046/j.1523-1755.2001.00488.x
- von Unruh GE, Voss S, Sauerbruch T, Hesse A. Dependence of oxalate absorption on the daily calcium intake. J Am Soc Nephrol. 2004 Jun;15(6):1567-73. doi: 10.1097/01.asn.0000127864.26968.7f
- Massey LK. Dietary influences on urinary oxalate and risk of kidney stones. Front Biosci. 2003 May 1;8:s584-94. doi: 10.2741/1082
- Gettman MT, Ogan K, Brinkley LJ, Adams-Huet B, Pak CY, Pearle MS. Effect of cranberry juice consumption on urinary stone risk factors. J Urol. 2005 Aug;174(2):590-4; quiz 801. doi: 10.1097/01.ju.0000165168.68054.f8
- Goldfarb DS, Asplin JR. Effect of grapefruit juice on urinary lithogenicity. J Urol. 2001 Jul;166(1):263-7.
- Huynh NK, Nguyen HVH. Effects of Juice Processing on Oxalate Contents in Carambola Juice Products. Plant Foods Hum Nutr. 2017 Sep;72(3):236-242. doi: 10.1007/s11130-017-0615-4
- Bong WC, Vanhanen LP, Savage GP. Addition of calcium compounds to reduce soluble oxalate in a high oxalate food system. Food Chem. 2017 Apr 15;221:54-57. doi: 10.1016/j.foodchem.2016.10.031
- Noori N, Honarkar E, Goldfarb DS, Kalantar-Zadeh K, Taheri M, Shakhssalim N, Parvin M, Basiri A. Urinary lithogenic risk profile in recurrent stone formers with hyperoxaluria: a randomized controlled trial comparing DASH (Dietary Approaches to Stop Hypertension)-style and low-oxalate diets. Am J Kidney Dis. 2014 Mar;63(3):456-63. doi: 10.1053/j.ajkd.2013.11.022
- Hoppe B. An update on primary hyperoxaluria. Nat Rev Nephrol. 2012 Jun 12;8(8):467-75. doi: 10.1038/nrneph.2012.113
- Solomon LR, Nixon AC, Ogden L, Nair B. Orlistat-induced oxalate nephropathy: an under-recognised cause of chronic kidney disease. BMJ Case Rep. 2017 Nov 12;2017:bcr2016218623. doi: 10.1136/bcr-2016-218623
- The influence of sex hormones on renal osteopontin expression and urinary constituents in experimental urolithiasis. Yagisawa T, Ito F, Osaka Y, Amano H, Kobayashi C, Toma H. J Urol. 2001 Sep; 166(3):1078-82. https://www.ncbi.nlm.nih.gov/pubmed/11490302/
- Allison MJ, Dawson KA, Mayberry WR, Foss JG. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol. 1985 Feb;141(1):1-7. doi: 10.1007/BF00446731
- Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 2006 Feb;69(4):691-8. doi: 10.1038/sj.ki.5000162
- Canales BK, Hatch M. Oxalobacter formigenes colonization normalizes oxalate excretion in a gastric bypass model of hyperoxaluria. Surg Obes Relat Dis. 2017 Jul;13(7):1152-1157. doi: 10.1016/j.soard.2017.03.014
- Kaufman DW, Kelly JP, Curhan GC, Anderson TE, Dretler SP, Preminger GM, Cave DR. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol. 2008 Jun;19(6):1197-203. doi: 10.1681/ASN.2007101058
- Zuckerman JM, Assimos DG. Hypocitraturia: pathophysiology and medical management. Rev Urol. 2009 Summer;11(3):134-44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2777061
- Phillips R, Hanchanale VS, Myatt A, Somani B, Nabi G, Biyani CS. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst Rev. 2015 Oct 6;2015(10):CD010057. doi: 10.1002/14651858.CD010057.pub2
- Dey J, Creighton A, Lindberg JS, Fuselier HA, Kok DJ, Cole FE, et al. Estrogen replacement increased the citrate and calcium excretion in rates in postmenopausal women with recurrent urolithiasis. J Urol 2002;167: 169-71. https://www.ncbi.nlm.nih.gov/pubmed/11743298
- Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol. 2006 Nov;1(6):1269-74. doi: 10.2215/CJN.00800306
- Hönow R, Laube N, Schneider A, Kessler T, Hesse A. Influence of grapefruit-, orange- and apple-juice consumption on urinary variables and risk of crystallization. Br J Nutr. 2003 Aug;90(2):295-300. doi: 10.1079/bjn2003897
- Wabner CL, Pak CY. Effect of orange juice consumption on urinary stone risk factors. J Urol. 1993 Jun;149(6):1405-8. doi: 10.1016/s0022-5347(17)36401-7
- Tracy CR, Pearle MS. Update on the medical management of stone disease. Curr Opin Urol. 2009 Mar;19(2):200-4. doi: 10.1097/MOU.0b013e328323a81d
- Manfredini R, De Giorgi A, Storari A, Fabbian F. Pears and renal stones: possible weapon for prevention? A comprehensive narrative review. Eur Rev Med Pharmacol Sci. 2016;20(3):414-25. https://www.europeanreview.org/wp/wp-content/uploads/414-425.pdf
- Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol. 2009 Oct;20(10):2253-9. doi: 10.1681/ASN.2009030276
- Taylor EN, Stampfer MJ, Mount DB, Curhan GC. DASH-style diet and 24-hour urine composition. Clin J Am Soc Nephrol. 2010 Dec;5(12):2315-22. doi: 10.2215/CJN.04420510
- Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Dietary and Lifestyle Risk Factors Associated with Incident Kidney Stones in Men and Women. J Urol. 2017 Oct;198(4):858-863. doi: 10.1016/j.juro.2017.03.124
- Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, Monga M, Penniston KL, Preminger GM, Turk TM, White JR; American Urological Assocation. Medical management of kidney stones: AUA guideline. J Urol. 2014 Aug;192(2):316-24. doi: 10.1016/j.juro.2014.05.006
- Dion M, Ankawi G, Chew B, Paterson R, Sultan N, Hoddinott P, Razvi H. CUA guideline on the evaluation and medical management of the kidney stone patient – 2016 update. Can Urol Assoc J. 2016 Nov-Dec;10(11-12):E347-E358. doi: 10.5489/cuaj.4218
- Sas DJ, Hulsey TC, Shatat IF, Orak JK. Increasing incidence of kidney stones in children evaluated in the emergency department. J Pediatr. 2010;157(1):132–137.
- Swartz MA, Lydon-Rochelle MT, Simon D, Wright JL, Porter MP. Admission for nephrolithiasis in pregnancy and risk of adverse birth outcomes. Obstet Gynecol. 2007;109(5):1099–1104.
- Ryall RL. Urinary inhibitors of calcium oxalate crystallization and their potential role in stone formation. World J Urol. 1997;15:155 https://www.ncbi.nlm.nih.gov/pubmed/9228722
- Sheng X, Jung T, Wesson JA, et al. Adhesion at calcium oxalate crystal surfaces and the effect of urinary constituents. Proc Natl Acad Sci USA. 2005;102:267. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC544292/
- Heilberg IP. Update on dietary recommendations and medical treatment of renal stone disease. Nephrol Dial Transplant. 2000;15:117 https://www.ncbi.nlm.nih.gov/pubmed/10607782
- Meschi T, Maggiore U, Fiaccadori E, et al. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004;66:2402. https://www.ncbi.nlm.nih.gov/pubmed/15569332
- Seltzer MA, Low RK, McDonald M, et al. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J Urol. 1996;156:907. https://www.ncbi.nlm.nih.gov/pubmed/8709360
- Kang D, Haleblian GE, Sur RL, et al. Long-term lemonade based dietary manipulation in patients with hypocitraturic nephrolithia-sis. J Urol. 2007;177:1358 https://www.ncbi.nlm.nih.gov/pubmed/17382731
- Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol. 2006;1:1269 http://cjasn.asnjournals.org/content/1/6/1269.long
- PENNISTON KL, NAKADA SY, HOLMES RP, ASSIMOS DG. Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-Available Fruit Juice Products. Journal of endourology / Endourological Society. 2008;22(3):567-570. doi:10.1089/end.2007.0304. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2637791/
- Penniston KL, Nakada SY, Holmes RP, et al. : Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J Endourol 2008;22:567–570 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2637791/
- Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. Seltzer MA, Low RK, McDonald M, Shami GS, Stoller ML. J Urol. 1996 Sep; 156(3):907-9. https://www.ncbi.nlm.nih.gov/pubmed/8709360/
- Lemonade therapy increases urinary citrate and urine volumes in patients with recurrent calcium oxalate stone formation. Penniston KL, Steele TH, Nakada SY. Urology. 2007 Nov; 70(5):856-60. https://www.ncbi.nlm.nih.gov/pubmed/17919696/
- Meschi T, Maggiore U, Fiaccadori E, et al. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004;66:2402 https://www.kidney-international.org/article/S0085-2538(15)50347-5/fulltext
- Carbohydrate intolerance and kidney stones in children in the Goldfields. Baldwin DN, Spencer JL, Jeffries-Stokes CA. J Paediatr Child Health. 2003 Jul; 39(5):381-5. https://www.ncbi.nlm.nih.gov/pubmed/12887672/
- Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Kidney Int. 2002 Sep; 62(3):971-9. https://www.ncbi.nlm.nih.gov/pubmed/12164880/
- The association between gout and nephrolithiasis in men: The Health Professionals’ Follow-Up Study. Kramer HJ, Choi HK, Atkinson K, Stampfer M, Curhan GC. Kidney Int. 2003 Sep; 64(3):1022-6. https://www.ncbi.nlm.nih.gov/pubmed/12911552/
- Coe F. L., Evan A., Worcester E. Kidney stone disease. Journal of Clinical Investigation. 2005;115(10):2598–2608. doi: 10.1172/jci26662
- Griffith D. P. Struvite stones. Kidney International. 1978;13(5):372–382. doi: 10.1038/ki.1978.55
- U.S. National Library of Medicine. Cystinuria. https://medlineplus.gov/ency/article/000346.htm
- Ahmed K., Dasgupta P., Khan M. S. Cystine calculi: challenging group of stones. Postgraduate Medical Journal. 2006;82(974):799–801. doi: 10.1136/pgmj.2005.044156
- Kidney Stones: Treatment and Prevention. Am Fam Physician. 2019;99(8):490-496. https://www.aafp.org/pubs/afp/issues/2019/0415/p490.html
- Afshar K, Jafari S, Marks AJ, Eftekhari A, MacNeily AE. Nonsteroidal anti-inflammatory drugs (NSAIDs) and non-opioids for acute renal colic. Cochrane Database Syst Rev. 2015 Jun 29;(6):CD006027. doi: 10.1002/14651858.CD006027.pub2
- Pathan SA, Mitra B, Cameron PA. A Systematic Review and Meta-analysis Comparing the Efficacy of Nonsteroidal Anti-inflammatory Drugs, Opioids, and Paracetamol in the Treatment of Acute Renal Colic. Eur Urol. 2018 Apr;73(4):583-595. doi: 10.1016/j.eururo.2017.11.001
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014 Nov 4;161(9):659-67. doi: 10.7326/M13-2908
- Fink HA, Wilt TJ, Eidman KE, Garimella PS, MacDonald R, Rutks IR, Brasure M, Kane RL, Ouellette J, Monga M. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med. 2013 Apr 2;158(7):535-43. doi: 10.7326/0003-4819-158-7-201304020-00005. Erratum in: Ann Intern Med. 2013 Aug 6;159(3):230-2.
- Prezioso D, Strazzullo P, Lotti T, Bianchi G, Borghi L, Caione P, Carini M, Caudarella R, Ferraro M, Gambaro G, Gelosa M, Guttilla A, Illiano E, Martino M, Meschi T, Messa P, Miano R, Napodano G, Nouvenne A, Rendina D, Rocco F, Rosa M, Sanseverino R, Salerno A, Spatafora S, Tasca A, Ticinesi A, Travaglini F, Trinchieri A, Vespasiani G, Zattoni F. ERRATUM: Dietary treatment of urinary risk factors for renal stone formation. A review of CLU Working Group. Arch Ital Urol Androl. 2016 Mar 31;88(1):76. doi: 10.4081/aiua.2016.1.76. Erratum for: Arch Ital Urol Androl. 2015 Jun;87(2):105-20.
- McDowell SE, Thomas SK, Coleman JJ, Aronson JK, Ferner RE. A practical guide to monitoring for adverse drug reactions during antihypertensive drug therapy. J R Soc Med. 2013 Mar;106(3):87-95. doi: 10.1258/jrsm.2012.120137. Erratum in: J R Soc Med. 2013 Apr;106(4):119.
- Streeper NM. Asymptomatic Renal Stones-to Treat or Not to Treat. Curr Urol Rep. 2018 Mar 17;19(5):29. doi: 10.1007/s11934-018-0782-3
- Trinchieri A, Esposito N, Castelnuovo C. Dissolution of radiolucent renal stones by oral alkalinization with potassium citrate/potassium bicarbonate. Arch Ital Urol Androl. 2009;81(3):188–191.
- Sakhaee K, Nicar M, Hill K, Pak CY. Contrasting effects of potassium citrate and sodium citrate therapies on urinary chemistries and crystallization of stone-forming salts. Kidney Int. 1983;24(3):348–352.
- Long LO, Park S. Update on nephrolithiasis management. Minerva Urol Nefrol. 2007;59(3):317–325.
- Pizzarelli F, Peacock M. Effect of chronic administration of ammonium sulfate on phosphatic stone recurrence. Nephron. 1987;46(3):247–252.
- Jarrar K, Boedeker RH, Weidner W. Struvite stones: long term follow up under metaphylaxis. Ann Urol (Paris). 1996;30(3):112–117.
- Siva S, Barrack ER, Reddy GP, et al. A critical analysis of the role of gut Oxalobacter formigenes in oxalate stone disease. BJU Int. 2009;103(1):18–21.
- Hoppe B, Beck B, Gatter N, et al. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int. 2006;70(7):1305–1311.
- Oxalobacter formigenes and its potential role in human health. Duncan SH, Richardson AJ, Kaul P, Holmes RP, Allison MJ, Stewart CS. Appl Environ Microbiol. 2002 Aug; 68(8):3841-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC124017/
- Vigen R, Weideman RA, Reilly RF. Thiazides diuretics in the treatment of nephrolithiasis: are we using them in an evidence-based fashion? Int Urol Nephrol. 2011 Sep;43(3):813-9. doi: 10.1007/s11255-010-9824-6
- Gambaro G, Croppi E, Coe F, Lingeman J, Moe O, Worcester E, Buchholz N, Bushinsky D, Curhan GC, Ferraro PM, Fuster D, Goldfarb DS, Heilberg IP, Hess B, Lieske J, Marangella M, Milliner D, Preminger GM, Reis Santos JM, Sakhaee K, Sarica K, Siener R, Strazzullo P, Williams JC; Consensus Conference Group. Metabolic diagnosis and medical prevention of calcium nephrolithiasis and its systemic manifestations: a consensus statement. J Nephrol. 2016 Dec;29(6):715-734. doi: 10.1007/s40620-016-0329-y
- Taylor EN, Feskanich D, Paik JM, Curhan GC. Nephrolithiasis and Risk of Incident Bone Fracture. J Urol. 2016 May;195(5):1482-1486. doi: 10.1016/j.juro.2015.12.069
- Adams JS, Song CF, Kantorovich V. Rapid recovery of bone mass in hypercalciuric, osteoporotic men treated with hydrochlorothiazide. Ann Intern Med. 1999 Apr 20;130(8):658-60. doi: 10.7326/0003-4819-130-8-199904200-00012
- Pak CY, Heller HJ, Pearle MS, Odvina CV, Poindexter JR, Peterson RD. Prevention of stone formation and bone loss in absorptive hypercalciuria by combined dietary and pharmacological interventions. J Urol. 2003 Feb;169(2):465-9. doi: 10.1097/01.ju.0000047341.55340.19
- Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med. 1995 Jan;98(1):50-9. doi: 10.1016/S0002-9343(99)80080-1
- Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol. 1993 Dec;150(6):1761-4. doi: 10.1016/s0022-5347(17)35888-3
- Carvalho M, Erbano BO, Kuwaki EY, Pontes HP, Liu JWTW, Boros LH, Asinelli MO, Baena CP. Effect of potassium citrate supplement on stone recurrence before or after lithotripsy: systematic review and meta-analysis. Urolithiasis. 2017 Oct;45(5):449-455. doi: 10.1007/s00240-016-0950-1
- Preminger GM, Sakhaee K, Skurla C, Pak CY. Prevention of recurrent calcium stone formation with potassium citrate therapy in patients with distal renal tubular acidosis. J Urol. 1985 Jul;134(1):20-3. doi: 10.1016/s0022-5347(17)46963-1
- Song Y, Hernandez N, Shoag J, Goldfarb DS, Eisner BH. Potassium citrate decreases urine calcium excretion in patients with hypocitraturic calcium oxalate nephrolithiasis. Urolithiasis. 2016 Apr;44(2):145-8. doi: 10.1007/s00240-015-0819-8
- Sakhaee K, Pak C. Superior calcium bioavailability of effervescent potassium calcium citrate over tablet formulation of calcium citrate after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013 Sep-Oct;9(5):743-8. doi: 10.1016/j.soard.2011.11.011
- Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med. 1977 Oct;87(4):404-10. doi: 10.7326/0003-4819-87-4-404
- Arowojolu O, Goldfarb DS. Treatment of calcium nephrolithiasis in the patient with hyperuricosuria. J Nephrol. 2014 Dec;27(6):601-5. doi: 10.1007/s40620-014-0084-x
- Pearle MS, Roehrborn CG, Pak CY. Meta-analysis of randomized trials for medical prevention of calcium oxalate nephrolithiasis. J Endourol. 1999 Nov;13(9):679-85. doi: 10.1089/end.1999.13.679
- Morgan MS, Pearle MS. Medical management of renal stones. BMJ. 2016 Mar 14;352:i52. doi: 10.1136/bmj.i52
- Holmes RP, Assimos DG. Glyoxylate synthesis, and its modulation and influence on oxalate synthesis. J Urol. 1998 Nov;160(5):1617-24.
- Williams HE, Wandzilak TR. Oxalate synthesis, transport and the hyperoxaluric syndromes. J Urol. 1989 Mar;141(3 Pt 2):742-9. doi: 10.1016/s0022-5347(17)40999-2
- Hoyer-Kuhn H, Kohbrok S, Volland R, Franklin J, Hero B, Beck BB, Hoppe B. Vitamin B6 in primary hyperoxaluria I: first prospective trial after 40 years of practice. Clin J Am Soc Nephrol. 2014 Mar;9(3):468-77. doi: 10.2215/CJN.06820613
- Ortiz-Alvarado O, Miyaoka R, Kriedberg C, Moeding A, Stessman M, Monga M. Pyridoxine and dietary counseling for the management of idiopathic hyperoxaluria in stone-forming patients. Urology. 2011 May;77(5):1054-8. doi: 10.1016/j.urology.2010.08.002
- Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Vitamin B6 intake and the risk of incident kidney stones. Urolithiasis. 2018 Jun;46(3):265-270. doi: 10.1007/s00240-017-0999-5
- Jaeger P, Portmann L, Jacquet AF, Burckhardt P. La pyridoxine peut normaliser l’oxalurie dans la lithiase rénale idiopathique [Pyridoxine can normalize oxaluria in idiopathic renal lithiasis]. Schweiz Med Wochenschr. 1986 Dec 13;116(50):1783-6. French.
- Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens. 2004 Mar;13(2):181-9. doi: 10.1097/00041552-200403000-00006
- Spettel S, Shah P, Sekhar K, Herr A, White MD. Using Hounsfield unit measurement and urine parameters to predict uric acid stones. Urology. 2013 Jul;82(1):22-6. doi: 10.1016/j.urology.2013.01.015
- Bonatti M, Lombardo F, Zamboni GA, Pernter P, Pycha A, Mucelli RP, Bonatti G. Renal stones composition in vivo determination: comparison between 100/Sn140 kV dual-energy CT and 120 kV single-energy CT. Urolithiasis. 2017 Jun;45(3):255-261. doi: 10.1007/s00240-016-0905-6
- Reichard C, Gill BC, Sarkissian C, De S, Monga M. 100% uric acid stone formers: what makes them different? Urology. 2015 Feb;85(2):296-8. doi: 10.1016/j.urology.2014.10.029
- Trinchieri A, Esposito N, Castelnuovo C. Dissolution of radiolucent renal stones by oral alkalinization with potassium citrate/potassium bicarbonate. Arch Ital Urol Androl. 2009 Sep;81(3):188-91.
- Sinha M, Prabhu K, Venkatesh P, Krishnamoorthy V. Results of urinary dissolution therapy for radiolucent calculi. Int Braz J Urol. 2013 Jan-Feb;39(1):103-7. doi: 10.1590/S1677-5538.IBJU.2013.01.13
- Jaeger P, Portmann L, Saunders A, Rosenberg LE, Thier SO. Anticystinuric effects of glutamine and of dietary sodium restriction. N Engl J Med. 1986 Oct 30;315(18):1120-3. doi: 10.1056/NEJM198610303151803
- Lindell A, Denneberg T, Edholm E, Jeppsson JO. The effect of sodium intake on cystinuria with and without tiopronin treatment. Nephron. 1995;71(4):407-15. doi: 10.1159/000188760
- Rodman JS, Blackburn P, Williams JJ, Brown A, Pospischil MA, Peterson CM. The effect of dietary protein on cystine excretion in patients with cystinuria. Clin Nephrol. 1984 Dec;22(6):273-8.
- Sakhaee K, Poindexter JR, Pak CY. The spectrum of metabolic abnormalities in patients with cystine nephrolithiasis. J Urol. 1989 Apr;141(4):819-21. doi: 10.1016/s0022-5347(17)41019-6
- Parks JH, Coe FL, Evan AP, Worcester EM. Urine pH in renal calcium stone formers who do and do not increase stone phosphate content with time. Nephrol Dial Transplant. 2009 Jan;24(1):130-6. doi: 10.1093/ndt/gfn420
- Barbey F, Joly D, Rieu P, Méjean A, Daudon M, Jungers P. Medical treatment of cystinuria: critical reappraisal of long-term results. J Urol. 2000 May;163(5):1419-23. doi: 10.1016/s0022-5347(05)67633-1
- Saravakos P, Kokkinou V, Giannatos E. Cystinuria: current diagnosis and management. Urology. 2014 Apr;83(4):693-9. doi: 10.1016/j.urology.2013.10.013
- Tiopronin. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=211843
- DeBerardinis RJ, Coughlin CR 2nd, Kaplan P. Penicillamine therapy for pediatric cystinuria: experience from a cohort of American children. J Urol. 2008 Dec;180(6):2620-3. doi: 10.1016/j.juro.2008.08.057
- Pak CY, Fuller C, Sakhaee K, Zerwekh JE, Adams BV. Management of cystine nephrolithiasis with alpha-mercaptopropionylglycine. J Urol. 1986 Nov;136(5):1003-8. doi: 10.1016/s0022-5347(17)45188-3
- Sloand JA, Izzo JL. Captopril Reduces Urinary Cystine Excretion in Cystinuria. Arch Intern Med. 1987;147(8):1409–1412. doi:10.1001/archinte.1987.00370080045011
- Zee T, Bose N, Zee J, Beck JN, Yang S, Parihar J, Yang M, Damodar S, Hall D, O’Leary MN, Ramanathan A, Gerona RR, Killilea DW, Chi T, Tischfield J, Sahota A, Kahn A, Stoller ML, Kapahi P. α-Lipoic acid treatment prevents cystine urolithiasis in a mouse model of cystinuria. Nat Med. 2017 Mar;23(3):288-290. doi: 10.1038/nm.4280
- Goldfarb DS, Coe FL, Asplin JR. Urinary cystine excretion and capacity in patients with cystinuria. Kidney Int. 2006 Mar;69(6):1041-7. doi: 10.1038/sj.ki.5000104
- Dolin DJ, Asplin JR, Flagel L, Grasso M, Goldfarb DS. Effect of cystine-binding thiol drugs on urinary cystine capacity in patients with cystinuria. J Endourol. 2005 Apr;19(3):429-32. doi: 10.1089/end.2005.19.429
- Parkhomenko E, De Fazio A, Tran T, Thai J, Blum K, Gupta M. A Multi-Institutional Study of Struvite Stones: Patterns of Infection and Colonization. J Endourol. 2017 May;31(5):533-537. doi: 10.1089/end.2016.0885
- Griffith DP, Gleeson MJ, Lee H, Longuet R, Deman E, Earle N. Randomized, double-blind trial of Lithostat (acetohydroxamic acid) in the palliative treatment of infection-induced urinary calculi. Eur Urol. 1991;20(3):243-7. doi: 10.1159/000471707
- Williams JJ, Rodman JS, Peterson CM. A randomized double-blind study of acetohydroxamic acid in struvite nephrolithiasis. N Engl J Med. 1984 Sep 20;311(12):760-4. doi: 10.1056/NEJM198409203111203
- Griffith DP, Khonsari F, Skurnick JH, James KE. A randomized trial of acetohydroxamic acid for the treatment and prevention of infection-induced urinary stones in spinal cord injury patients. J Urol. 1988 Aug;140(2):318-24. doi: 10.1016/s0022-5347(17)41592-8
- Iqbal MW, Youssef RF, Neisius A, Kuntz N, Hanna J, Ferrandino MN, Preminger GM, Lipkin ME. Contemporary Management of Struvite Stones Using Combined Endourologic and Medical Treatment: Predictors of Unfavorable Clinical Outcome. J Endourol. 2016 Jul;30(7):771-7. doi: 10.1089/end.2013.0257
- Dietary Guidelines for Americans. https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines
- Sigurjonsdottir V. K., L.Runolfsdottir H., Indridason O. S., et al. Impact of nephrolithiasis on kidney function. BMC Nephrology. 2015;16(1):p. 149. doi: 10.1186/s12882-015-0126-1
- El-Zoghby Z. M., Lieske J. C., Foley R. N., et al. Urolithiasis and the risk of ESRD. Clinical Journal of the American Society of Nephrology. 2012;7(9):1409–1415. doi: 10.2215/cjn.03210312
- Rule A. D., Roger V. L., Melton L. J., et al. Kidney stones associate with increased risk for myocardial infarction. Journal of the American Society of Nephrology. 2010;21(10):1641–1644. doi: 10.1681/asn.2010030253
- Worcester E. M., Coe F. L. Nephrolithiasis. Primary Care. 2008;35(2):369–391. doi: 10.1016/j.pop.2008.01.005
- Taylor E. N., Stampfer M. J., Curhan G. C. Obesity, weight gain and the risk of kidney stones. Journal of the American Medical Association. 2005;293(4):455–462. doi: 10.1001/jama.293.4.455
- Courbebaisse M., Prot-Bertoye C., Bertocchio J., et al. Nephrolithiasis of adult: from mechanisms to preventive medical treatment. Revue Medicale Internationale. 2017;38(1):44–52. doi: 10.1016/j.revmed.2016.05.013
- Expert Panel on Interventional Radiology; Scheidt MJ, Hohenwalter EJ, Pinchot JW, Ahmed O, Bjurlin MA, Braun AR, Kim CY, Knavel Koepsel EM, Schramm K, Sella DM, Weiss CR, Lorenz JM. ACR Appropriateness Criteria® Radiologic Management of Urinary Tract Obstruction. J Am Coll Radiol. 2020 May;17(5S):S281-S292. doi: 10.1016/j.jacr.2020.01.039