Methotrexate
Methotrexate (MTX) is a prescription medicine called a folic acid antagonist that is used as a disease‐modifying anti‐rheumatic drug (DMARD) to treat the symptoms of psoriatic arthritis and severe psoriasis (a skin disease in which red, scaly patches form on some areas of the body). Methotrexate is also used along with rest, physical therapy, and sometimes other medications to treat severe adult rheumatoid arthritis and juvenile rheumatoid arthritis (a condition in which the body attacks its own joints, causing pain, swelling, and loss of function) that cannot be controlled by certain other medications. Methotrexate is also used to treat certain types of cancer including cancers that begin in the tissues that form around a fertilized egg in the uterus, breast cancer, lung cancer, certain cancers of the head and neck, certain types of lymphoma, and leukemia (cancer that begins in the white blood cells). Methotrexate is also sometimes used to treat Crohn’s disease (condition in which the immune system attacks the lining of the digestive tract, causing pain, diarrhea, weight loss and fever), multiple sclerosis or MS (a condition in which the immune system attacks the nerves, causing weakness, numbness, loss of muscle coordination, and problems with vision, speech, and bladder control), and other autoimmune diseases (conditions that develop when the immune system attacks healthy cells in the body by mistake). Methotrexate injection is used “off-label” for treating ectopic pregnancies when surgery is not necessary, because it stops the embryo’s cells from growing and dividing, which eventually ends the pregnancy.
Methotrexate is in a class of medications called antimetabolites. Methotrexate treats cancer by slowing the growth of cancer cells. Methotrexate treats psoriasis by slowing the growth of skin cells to stop scales from forming. Methotrexate may treat rheumatoid arthritis by decreasing the activity of the immune system.
Methotrexate is available as an oral tablet, oral solution, and as an injection (intramuscular, intravenous, intrathecal, or subcutaneous) 1.. Your doctor will tell you how often you should take methotrexate. The schedule depends on the condition you have and on how your body responds to the medication. Your doctor may tell you to take methotrexate on a rotating schedule that alternates several days when you take methotrexate with several days or weeks when you do not take the medication. Follow these directions carefully and ask your doctor or pharmacist if you do not know when to take your medication.
- Methotrexate Orally: Usual dosing is as a weekly “pulse,” administered as a single dose or in three divided doses over 8 hourly in 24 hours every week. Folate supplementation with 1 mg per day or 5 to 7 mg once weekly should be considered for all patients to prevent bone marrow suppression. In adults, oral absorption is dependent upon the dose taken. Peak serum levels are achievable within one to two hours.
- Methotrexate Injection: Single-dose auto-injector can deliver methotrexate in certain doses such as: 7.5 mg, 10 mg, 12.5 mg, 15 mg, 17.5 mg, 20 mg, 22.5 mg, 25 mg, 27.5 mg, and 30 mg 2.
- After initiation of medical therapy with methotrexate, follow-up tests should include monitoring of complete blood count (CBC), renal function test, and liver function tests are recommended weekly for 4 weeks and then at least bi-monthly 3.
If you are taking methotrexate to treat psoriasis or rheumatoid arthritis, your doctor may tell you to take the medication once a week. Pay close attention to your doctor’s directions. Some people who mistakenly took methotrexate once daily instead of once weekly experienced very severe side effects or died.
Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take methotrexate exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
If you are taking methotrexate to treat psoriasis or rheumatoid arthritis, your doctor may start you on a low dose of the medication and gradually increase your dose. Follow these directions carefully.
If you are taking methotrexate to treat rheumatoid arthritis, it may take 3 to 6 weeks for your symptoms to begin to improve, and 12 weeks or longer for you to feel the full benefit of methotrexate. Continue to take methotrexate even if you feel well. Do not stop taking methotrexate without talking to your doctor.
Common side effects of methotrexate include headache, nausea, vomiting, abdominal pain, loss of appetite and mouth ulcers 4, 5. Rare but serious side effects include bone marrow suppression (myelosuppression), liver toxicity (hepatotoxicity), infection, and lung fibrosis 4. Daily supplementation with folic acid or folinic acid can alleviate liver toxicity and gastrointestinal adverse effects 6. A small elevation of aminotransferases level is common, but it is uncommon to have liver steatosis, liver fibrosis, and cirrhosis when taking a low dose of methotrexate. Over a long duration of treatment, ultrasound scanning and liver biopsy are required to ascertain the level of liver damage. Ask your doctor about the risks of using methotrexate for your condition.
With high methotrexate doses, patients may also experience mucosal ulceration. It may also be a sign of impending methotrexate toxicity. Alopecia, fatigue, fever, increased risk of infection, low white cell count, gastrointestinal bleeding, pancreatitis, bone marrow suppression (aplastic anemia), malignancy (lymphoproliferative disorders), infections, interstitial pneumonitis, and renal failure are other potentially life-threatening side effects 7, 8.
Methotrexate belongs to category X, which means it is absolutely contraindicated for use in pregnancy. Using methotrexate while you are pregnant can harm your unborn baby. Methotrexate may also cause birth defects if it is used by the father when his sexual partner becomes pregnant. If you are a woman who can bear children, your doctor may give you a pregnancy test before you start using methotrexate to make sure you are not pregnant. Female patients should use an effective form of birth control during treatment and for at least 3 months after the last dose. Male patients who have female partners should use an effective form of birth control during treatment and for at least 3 months after the last dose. Tell your doctor right away if pregnancy occurs while you are using methotrexate.
Methotrexate may cause very serious, life-threatening side effects. You should only take methotrexate to treat cancer or certain other conditions that are very severe and that cannot be treated with other medications. Talk to your doctor about the risks of taking methotrexate for your condition.
Tell your doctor if you have or have ever had excess fluid in your stomach area or in the space around your lungs and if you have or have ever had kidney disease. Also tell your doctor if you are taking nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin, choline magnesium trisalicylate (Tricosal, Trilisate), ibuprofen (Advil, Motrin), magnesium salicylate (Doan’s), naproxen (Aleve, Naprosyn), or salsalate. These conditions and medications may increase the risk that you will develop serious side effects of methotrexate. Your doctor will monitor you more carefully and may need to give you a lower dose of methotrexate or stop your treatment with methotrexate.
Methotrexate may cause a decrease in the number of blood cells made by your bone marrow. Tell your doctor if you have or have ever had a low number of any type of blood cells or any other problem with your blood cells. Call your doctor immediately if you experience any of the following symptoms: sore throat, chills, fever, or other signs of infection; unusual bruising or bleeding; excessive tiredness; pale skin; or shortness of breath.
Methotrexate may cause liver damage, especially when it is taken for a long period of time. If you drink or have ever drunk large amounts of alcohol or if you have or have ever had liver disease, your doctor may tell you not to take methotrexate unless you have a life-threatening form of cancer because there is a higher risk that you will develop liver damage. The risk that you will develop liver damage may also be higher if you are elderly, obese, or have diabetes. Tell your doctor if you are taking any of the following medications: acitretin (Soriatane), azathioprine (Imuran), isotretinoin (Accutane), sulfasalazine (Azulfidine), or tretinoin (Vesanoid). Ask your doctor about the safe use of alcoholic beverages while you are taking methotrexate. Call your doctor immediately if you experience any of the following symptoms: nausea, extreme tiredness, lack of energy, loss of appetite, pain in the upper right part of the stomach, yellowing of the skin or eyes, or flu-like symptoms. Your doctor may order liver biopsies (removal of a small piece of liver tissue to be examined in a laboratory) before and during your treatment with methotrexate.
Methotrexate may cause lung damage. Tell your doctor if you have or have ever had lung disease. Call your doctor immediately if you experience any of the following symptoms: dry cough, fever, or shortness of breath.
Methotrexate may cause damage to the lining of your mouth, stomach, or intestines. Tell your doctor if you have or have ever had stomach ulcers or ulcerative colitis (a condition which causes swelling and sores in the lining of the colon [large intestine] and rectum). If you experience any of the following symptoms, stop taking methotrexate and call your doctor right away: mouth sores, diarrhea, black, tarry, or bloody stools, or vomit that is bloody or looks like coffee grounds.
Taking methotrexate may increase the risk that you will develop lymphoma (cancer that begins in the cells of the immune system). If you do develop lymphoma, it might go away without treatment when you stop taking methotrexate, or it might need to be treated with chemotherapy.
If you are taking methotrexate to treat cancer, you may develop certain complications as methotrexate works to destroy the cancer cells. Your doctor will monitor you carefully and treat these complications if they occur.
Methotrexate may cause serious or life-threatening skin reactions. If you experience any of the following symptoms, call your doctor immediately: fever, rash, blisters, or peeling skin.
Methotrexate may decrease the activity of your immune system, and you may develop serious infections. Tell your doctor if you have any type of infection and if you have or have ever had any condition that affects your immune system. Your doctor may tell you that you should not take methotrexate unless you have life-threatening cancer. If you experience signs of infection such as a sore throat, cough, fever, or chills, call your doctor immediately.
If you take methotrexate while you are being treated with radiation therapy for cancer, methotrexate may increase the risk that the radiation therapy will cause damage to your skin, bones, or other parts of your body.
Keep all appointments with your doctor and the laboratory. Your doctor will order certain lab tests before, during, and after your treatment to check your body’s response to methotrexate and to treat side effects before they become severe.
Tell your doctor if you or your partner is pregnant or plan to become pregnant. If you are female, you will need to take a pregnancy test before you begin taking methotrexate. Use a reliable method of birth control so that you or your partner will not become pregnant during or shortly after your treatment. If you are male, you and your female partner should continue to use birth control for 3 months after you stop taking methotrexate. If you are female, you should continue to use birth control until you have had one menstrual period that began after you stopped taking methotrexate. If you or your partner become pregnant, call your doctor immediately. Methotrexate may cause harm or death to the fetus.
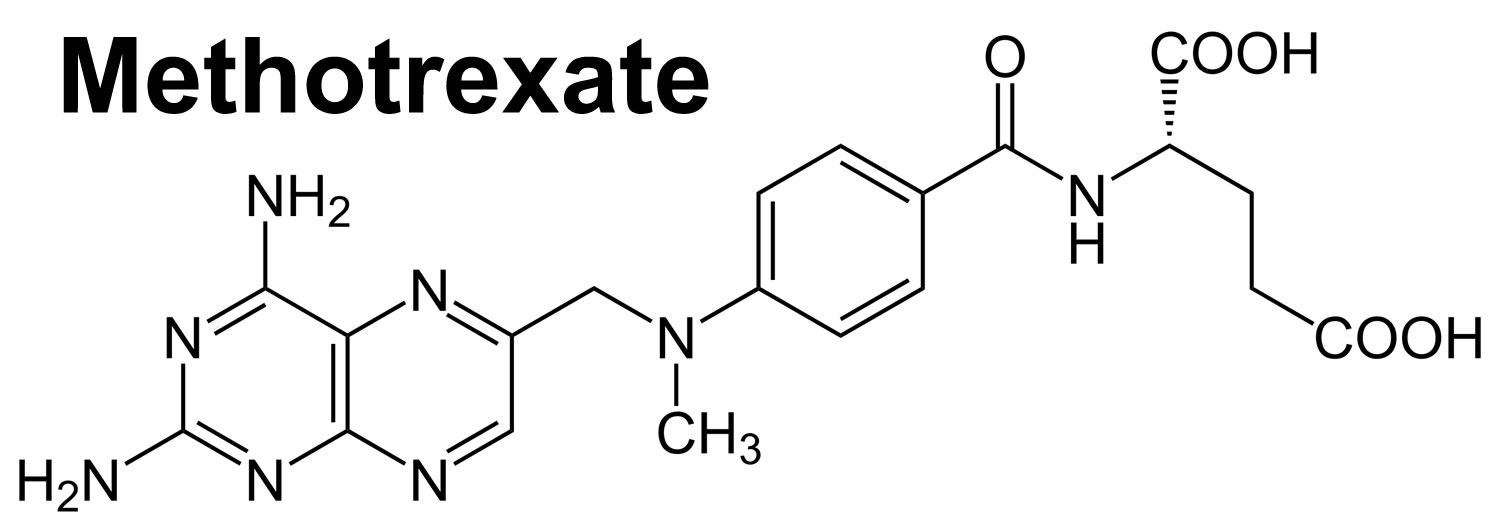
Figure 1. Methotrexate structure
How does methotrexate work?
Methotrexate is used to treat many conditions and has a distinct mechanism of action regarding its use in chemotherapy and immunosuppression in autoimmune diseases, depending on the disease and the dose 9. At high doses used for cancer, methotrexate acts as an antifolate antimetabolite and blocks the folic acid metabolic pathway 10. In cancer, methotrexate is taken up into the cell by carriers called the human reduced folate carriers (SLC19A1), and it forms methotrexate-polyglutamate. Both the methotrexate and the methotrexate-polyglutamate inhibit the enzyme dihydrofolate reductase, which catalyzes the conversion of dihydrofolate into tetrahydrofolate, the active form of folic acid 11. Tetrahydrofolate is necessary for the synthesis of the nucleotides of both DNA and RNA. Methotrexate-polyglutamate further inhibits the de novo purine synthesis of both purine and thymidylate synthase, thereby inhibiting DNA synthesis. This disrupts production of nucleotide bases, triggering several cellular processes that culminate in apoptosis 10. This mechanism is utilized in the treatment of cancer because of its cytotoxic effect 12.
At low doses used for autoimmune diseases or inflammatory diseases such as psoriatic arthritis, this pathway does not adequately explain its effects 10. Methotrexate inhibits enzyme AICAR transformylase, leading to hindrance in Adenosine and Guanine metabolism, Adenosine accumulation; and due to anti-inflammatory action of adenosine, leads to repression of T-cell activation, down-regulation of B-cells, increasing activated CD-95 T cells sensitivity; and repression of methyltransferase activity, inhibition of the binding of beta-1 interleukin to its cell surface receptor 13. The biochemical mechanisms that explain these effects remain incompletely understood.
Drug interactions- As methotrexate is highly plasma protein bound, any drug that displaces methotrexate from proteins can increase its blood levels.
Also, if any drug has effects on the renal clearance of methotrexate, its concentration may rise.
Nonsteroidal Anti-inflammatory Drugs (NSAIDs), salicylates, trimethoprim, penicillin, warfarin, valproate, proton pump inhibitors (PPIs), cyclosporin, cisplatin increases the risk of methotrexate toxicity in the blood; aminoglycosides, neomycin, probenecid reduces the absorption of methotrexate 14. The most significant and serious interactions are with NSAIDs and proton pump inhibitors (PPIs) since these are very common therapeutic choices 15.
How long does it take for methotrexate to work?
Methotrexate can start working for rheumatoid arthritis within 3 to 6 weeks and symptoms continue to improve over 3 months. For other people it might take a few months before they notice any improvement in their rheumatoid arthritis symptoms. It is important to keep taking the methotrexate as usually methotrexate is working at reducing inflammation and swelling, it just takes a while before you notice pain relief and have less joint stiffness.
How do I know if methotrexate is working for rheumatoid arthritis?
Methotrexate is an immunosuppressant drug that is used to treat rheumatoid arthritis, an autoimmune disease, as well as other inflammatory conditions. It works to reduce the inflammation, pain, swelling, redness, morning stiffness, fatigue and other symptoms associated with rheumatoid arthritis.
Methotrexate also slows down how quickly rheumatoid arthritis progresses and damages joints, which is why it belongs to a group of drugs known as disease-modifying anti-rheumatic drugs (DMARDs).
To tell if methotrexate treatment is working for your rheumatoid arthritis:
- Your doctor will conduct regular blood tests and check-ups
- You can keep a track of your symptoms and looks for signs of improvement
- Imaging tests, such as x-rays and musculoskeletal ultrasound, may be used
Blood tests and check-ups are used to tell you if methotrexate is working for rheumatoid arthritis
When you first start treatment with methotrexate your doctor will conduct blood tests and check-ups every 1-2 weeks. The results from these tests will help you to know whether methotrexate is working for you and also if it’s causing side effects.
Based on the results of your tests your doctor may adjust the dose of methotrexate you take until this medication is working well for you. Once you are on a dose that is working for you, blood tests and check-ups are usually conducted every 2 to 3 months.
Blood tests that help to determine how active your rheumatoid arthritis is and show whether methotrexate is working for you include:
- Erythrocyte sedimentation rate (ESR) test – measures the amount of inflammation in your body by measuring how fast red blood cells cling together and settle on the bottom of a test turn over the course of one hour
- C-reactive protein (CRP) test – also measures inflammation by measuring CRP, which is a protein produced by the liver when there is inflammation somewhere in your body
- Multibiomarker Disease Activity (MBDA, Vectra DA) test – measures 12 biomarkers )including proteins, hormone and growth factors) to provide a single measure of disease activity. It can help to determine the aggressiveness of your disease and likelihood of a symptom flare-up should your medications change.
You’ll also know if methotrexate is working for you because your rheumatoid arthritis symptoms will start to improve. You may notice a reduction in your rheumatoid arthritis symptoms in as little as 3 to 6 weeks after starting treatment with methotrexate, but it can take up to 12 weeks after starting on a dose that works well for you for the full effects of the drug to be seen.
If methotrexate is working for you, you’re likely to experience fewer swollen and painful joints, less morning stiffness and be able to move about and perform daily activities more easily.
It’s important to remember that during the first weeks and months of treatment you may not notice any benefit in terms of improvement in your symptoms, but this does not mean this medication won’t work for you in time.
If methotrexate does not work well enough on its own, it can also be combined with painkillers, other DMARDs, and biological drugs to provide symptom relief.
Imaging tests can tell you if methotrexate is working to prevent joint damage
Imaging tests, including x-rays, musculoskeletal ultrasound and magnetic resonance imaging (MRI), are used to diagnose rheumatoid arthritis and can also be used to check for worsening joint damage while you’re on methotrexate.
These radiographic tests are used to detect inflammation, bone erosions and joint space narrowing, which indicate that rheumatoid arthritis is damaging your joints. MUSU and MRI are better at picking up bone erosion and inflammation than x-rays.
When methotrexate works it helps to slow joint damage and the progression of rheumatoid arthritis. In some people taking methotrexate, no or limited damage may be picked up on these tests (radiographic progression). However, methotrexate isn’t always able to prevent disease progression, especially in people with severe rheumatoid arthritis.
How long does methotrexate work for?
A clinical trial studied rheumatoid arthritis patients who took methotrexate for 12 weeks and then methotrexate was stopped. The methotrexate continued to be effective for 3 to 6 weeks after methotrexate was stopped and after this time the rheumatoid arthritis symptoms began to return.
- Methotrexate starts to show improvements in rheumatoid arthritis symptoms in 3 to 6 weeks.
- Methotrexate is taken long term to reduce rheumatoid arthritis symptoms and reduce disease progression.
- If you stop taking methotrexate it will take 3 to 6 weeks for the methotrexate effects to diminish and your rheumatoid arthritis symptoms to reappear.
How long can I take methotrexate for?
Methotrexate can be taken long term to help treat rheumatoid arthritis symptoms and also to help slow disease progression and joint damage. Rheumatoid arthritis is a chronic illness that does not have a cure, so medicines like methotrexate are taken to reduce the symptoms and reduce that damage caused by the disease.
There have been clinical trials that have lasted 11 years which have studied rheumatoid arthritis patients taking long term methotrexate.
These studies showed:
- sustained positive response with a 50% improvement in joint pain
- greater than 65% reduction in joint swelling index
- 11% of the patients left the study due to methotrexate toxic effects.
Why should I take folic acid with methotrexate?
Folate occurs naturally as vitamin B-9 and is an essential nutrient your body needs for cell metabolism and growth. You need folate to help with skin, hair and nail growth. Folic acid is a man-made form of this B vitamin available as a supplement. Your doctor may prescribe folic acid to be taken with your methotrexate. You should take folic acid with methotrexate to help prevent a folate deficiency. Taking methotrexate can lower levels of folate in your body. A folate deficiency can lead to symptoms like upset stomach, low blood cell counts, tiredness, muscle weakness, mouth sores, liver toxicity and nervous system symptoms. Methotrexate is a medicine that is commonly prescribed for people with rheumatoid arthritis, cancer or psoriasis.
Common symptoms of folate deficiency can include:
- tiredness or fatigue, lack of energy
- muscle weakness
- headache
- dizziness
- shortness of breath
- neurological (nervous system) problems, like the feeling of pins and needles, tingling, or burning in your hands, arms or legs
- mental health problems such as depression, confusion, memory problems, and difficulty with judgement and understanding
- upset stomach (nausea, vomiting) or stomach pain
- diarrhea
- weight loss
Before taking methotrexate
- tell your doctor and pharmacist if you are allergic to methotrexate, any other medications, or any of the ingredients in methotrexate tablets. Ask your doctor or pharmacist for a list of the ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention the medications listed in the IMPORTANT WARNING section and any of the following: certain antibiotics such as chloramphenicol (chloromycetin), penicillins, and tetracyclines; folic acid (available alone or as an ingredient in some multivitamins); other medications for rheumatoid arthritis; phenytoin (Dilantin); probenecid (Benemid); sulfonamides such as co-trimoxazole (Bactrim, Septra), sulfadiazine, sulfamethizole (Urobiotic), and sulfisoxazole (Gantrisin); and theophylline (Theochron, Theolair). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if you have or have ever had any of the conditions mentioned in the IMPORTANT WARNING section or a low level of folate in your blood.
- tell your doctor right away if you have a change in how much or how often you urinate, rapid weight gain, swelling in the legs, ankles, or feet, or trouble breathing. These could be symptoms of a serious kidney problem.
- do not breast-feed while you are taking methotrexate and for at least 1 week after your last dose.
- methotrexate may affect fertility (ability to have children) in both men and women. However, it is important to use birth control to prevent pregnancy because methotrexate may harm the baby if a pregnancy does occur.
- limit alcohol use with methotrexate. Alcohol may increase the risk for liver problems.
- check with your doctor right away if you have pain or tenderness in the upper stomach, pale stools, dark urine, loss of appetite, nausea, vomiting, or yellow eyes or skin. These could be symptoms of a serious liver problem.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking methotrexate.
- check with your doctor right away if you have cough, fever, or trouble breathing. These could be symptoms of a serious lung or breathing problems (eg, acute or chronic interstitial pneumonitis).
- plan to avoid unnecessary or prolonged exposure to sunlight or ultraviolet light (tanning beds and sunlamps) and to wear protective clothing, sunglasses, and sunscreen. Methotrexate may make your skin sensitive to sunlight or ultraviolet light. If you have psoriasis, your sores may get worse if you expose your skin to sunlight while you are taking methotrexate.
- do not have any vaccinations during your treatment with methotrexate without talking to your doctor. Methotrexate may lower your body’s resistance and the vaccine may not work as well or you might get the infection the vaccine is meant to prevent. In addition, you should not be around other persons living in your household who receive live virus vaccines because there is a chance they could pass the virus on to you. Some examples of live vaccines include measles, mumps, influenza (nasal flu vaccine), poliovirus (oral form), rotavirus, and rubella. Do not get close to them and do not stay in the same room with them for very long. If you have questions about this, talk to your doctor.
- methotrexate can lower the number of white blood cells in your blood, which increases the chance of getting an infection. It can also lower the number of platelets, which are necessary for proper blood clotting. If this occurs, there are certain precautions you can take, especially when your blood count is low, to reduce the risk of infection or bleeding:
- If you can, avoid people with infections. Check with your doctor immediately if you think you are getting an infection or if you get a fever or chills, cough or hoarseness, lower back or side pain, or painful or difficult urination.
- Check with your doctor immediately if you notice any unusual bleeding or bruising, black, tarry stools, blood in the urine or stools, or pinpoint red spots on your skin.
- Be careful when using a regular toothbrush, dental floss, or toothpick. Your medical doctor, dentist, or nurse may recommend other ways to clean your teeth and gums. Check with your medical doctor before having any dental work done.
- Do not touch your eyes or the inside of your nose unless you have just washed your hands and have not touched anything else in the meantime.
- Be careful not to cut yourself when you are using sharp objects such as a safety razor or fingernail or toenail cutters.
- Avoid contact sports or other situations where bruising or injury could occur.
- serious skin reactions (eg, toxic epidermal necrolysis, Stevens-Johnson syndrome, exfoliative dermatitis, skin necrosis, or erythema multiforme) can occur with methotrexate. Check with your doctor right away if you have blistering, peeling, or loosening of the skin, blue-green to black skin discoloration, cough, cracks in the skin, diarrhea, itching, joint or muscle pain, loss of heat from the body, red irritated eyes, red skin lesions, often with a purple center, sore throat, sores, ulcers, or white spots in the mouth or on the lips, fever or chills, or unusual tiredness or weakness while you are using methotrexate.
- methotrexate may cause serious nerve problems. Check with your doctor right away if you have seizures, confusion, tingling or numbness in your hands, feet, or lips, trouble seeing, or headache.
- methotrexate may increase your risk for other cancers, including blood or skin cancer. The risk for skin cancer may be increased if you take cyclosporine after receiving treatment with methotrexate for psoriasis.
- methotrexate may cause a serious reaction called tumor lysis syndrome. Your doctor may give you a medicine to help prevent this. Tell your doctor right away if you have a change in urine amount, joint pain, stiffness, or swelling, lower back, side, or stomach pain, rapid weight gain, swelling of the feet or lower legs, or unusual tiredness or weakness.
Drug interactions
Although certain medicines should not be used together at all, in other cases two different medicines may be used together even if an interaction might occur. In these cases, your doctor may want to change the dose, or other precautions may be necessary. When you are taking methotrexate, it is especially important that your healthcare professional know if you are taking any of the medicines listed below. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using methotrexate with any of the following medicines is not recommended. Your doctor may decide not to treat you with this medication or change some of the other medicines you take.
- Measles Virus Vaccine, Live
- Mumps Virus Vaccine, Live
- Rotavirus Vaccine, Live
- Rubella Virus Vaccine, Live
- Varicella Virus Vaccine, Live
- Zoster Vaccine, Live
Using methotrexate with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Aceclofenac
- Acemetacin
- Acetazolamide
- Adenovirus Vaccine
- Amoxicillin
- Amtolmetin Guacil
- Asparaginase
- Aspirin
- Bacillus of Calmette and Guerin (BCG) Vaccine, Live
- Beet Root
- Bentiromide
- Bromfenac
- Bufexamac
- Capecitabine
- Capmatinib
- Celecoxib
- Chloral Hydrate
- Cholera Vaccine, Live
- Choline Salicylate
- Clonixin
- Dantrolene
- Darolutamide
- Dengue Tetravalent Vaccine, Live
- Dexibuprofen
- Dexketoprofen
- Dexlansoprazole
- Diclofenac
- Dicloxacillin
- Diflunisal
- Dipyrone
- Doxifluridine
- Doxycycline
- Droxicam
- Enasidenib
- Encorafenib
- Esomeprazole
- Etodolac
- Etofenamate
- Felbinac
- Fenbufen
- Fenoprofen
- Fepradinol
- Feprazone
- Floctafenine
- Floxacillin
- Flufenamic Acid
- Fluorouracil
- Flurbiprofen
- Foscarnet
- Fosphenytoin
- Furosemide
- Hydrochlorothiazide
- Ibuprofen
- Ibuprofen Lysine
- Indomethacin
- Influenza Virus Vaccine, Live
- Ketoprofen
- Ketorolac
- Leflunomide
- Levetiracetam
- Lornoxicam
- Loxoprofen
- Lumiracoxib
- Meclofenamate
- Mefenamic Acid
- Meloxicam
- Mezlocillin
- Midostaurin
- Morniflumate
- Nabumetone
- Naproxen
- Nepafenac
- Niflumic Acid
- Nimesulide
- Nimesulide Beta Cyclodextrin
- Nitrous Oxide
- Omeprazole
- Oxaprozin
- Oxyphenbutazone
- Pantoprazole
- Parecoxib
- Penicillin G
- Penicillin V
- Pexidartinib
- Phenylbutazone
- Phenytoin
- Piketoprofen
- Piperacillin
- Piroxicam
- Pirprofen
- Poliovirus Vaccine, Live
- Pristinamycin
- Probenecid
- Proglumetacin
- Proguanil
- Propionic Acid
- Propyphenazone
- Proquazone
- Pyrimethamine
- Rabeprazole
- Salicylic Acid
- Salsalate
- Sapropterin
- Silver Sulfadiazine
- Simeprevir
- Smallpox Vaccine
- Sodium Salicylate
- Sulfacetamide
- Sulfacytine
- Sulfadiazine
- Sulfamethizole
- Sulfamethoxazole
- Sulfapyridine
- Sulfisoxazole
- Sulindac
- Tafamidis
- Tamoxifen
- Tegafur
- Tenoxicam
- Teriflunomide
- Tiaprofenic Acid
- Ticarcillin
- Tolfenamic Acid
- Tolmetin
- Triamterene
- Trimethoprim
- Typhoid Vaccine, Live
- Valdecoxib
- Warfarin
- Yellow Fever Vaccine
- Zonisamide
Using methotrexate with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Amiodarone
- Cyclosporine
- Eltrombopag
- Etoricoxib
- Mercaptopurine
- Procarbazine
- Rofecoxib
- Theophylline
Other interactions
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using methotrexate with any of the following is usually not recommended, but may be unavoidable in some cases. If used together, your doctor may change the dose or how often you use methotrexate, or give you special instructions about the use of food, alcohol, or tobacco.
- Cola
Other medical problems
The presence of other medical problems may affect the use of methotrexate. Make sure you tell your doctor if you have any other medical problems, especially:
- Alcohol abuse, or history of or
- Anemia or
- Leukopenia (low white blood cells) or
- Liver disease, severe or
- Thrombocytopenia (low platelet blood level) or
- Weak immune system—methotrexate tablets should not be used in patients with these conditions.
- Ascites (extra fluid in the stomach area) or
- Kidney disease or
- Pleural effusion (extra fluid in the lung)—Use with caution. The effects may be increased because of slower removal of the medicine from the body.
- Diabetes or
- Obesity or
- Stomach or bowel problem (eg, peptic ulcers, ulcerative colitis)—Use with caution. May cause side effects to become worse.
- Infection (eg, bacteria, fungus, virus)—Use with caution. May decrease your ability to fight an infection.
Methotrexate uses
Methotrexate is an FDA-approved folic acid antagonist indicated for the treatment of rheumatoid arthritis because of its high potency and efficacy in such patients; it can also be useful in patients with juvenile idiopathic arthritis 16. Gubner first suggested methotrexate use in rheumatoid arthritis after performing a double blinded-placebo controlled clinical trial of methotrexate in patients with rheumatoid arthritis 17. Today methotrexate is one of the major chemotherapeutic choices for various types of cancers. Methotrexate is also safe and effective for patients with psoriasis, systemic lupus erythematosus (SLE), inflammatory bowel disease, vasculitis, and many other connective tissue diseases 18. Methotrexate is also effective in patients with organ transplantation because of its anti-inflammatory and immunomodulatory activity 19. Also, methotrexate can be combined with anti-TNF agents and has shown effective in managing patients with ulcerative colitis, lymphoma (non-Hodgkin’s type), carcinoma of the breast, small-cell carcinoma of the lung, epidermal tumors of the head and neck, and carcinoma of the ovary 5. Methotrexate has the same effects as cyclosporin for patients with graft-versus-host disease (GVHD). Methotrexate is used off-label in mycosis fungoides, dermatomyositis, pityriasis rubra pilaris, eczema, sarcoidosis, non-Hodgkin’s lymphoma (advanced stage), Burkitt’s lymphoma and non-metastatic osteosarcoma.
Methotrexate monitoring
Patients taking methotrexate should undergo monitoring of complete blood count (CBC), serum creatinine, transaminases is recommended weekly for the first 4 weeks and then at least bimonthly. A complete list of the current medications should be revised to avoid any possible drug interactions before prescribing methotrexate. Liver function tests (monitoring serum AST, ALT, serum albumin levels), liver biopsy can also be done in cases of hepatotoxicity 20. Creatinine clearance requires monitoring (50 ml/min is necessary before prescribing methotrexate) to avoid possible kidney toxicity 7. Monitoring for lung toxicity is also required as the patients may have a dry cough, fever, shortness of breath (dyspnea). Baseline chest X-rays are recommended to detect interstitial, and alveolar infiltrates, hilar adenopathy, pleural effusions, and pulmonary fibrosis 8. Methotrexate may also cause reactivation of tuberculosis in endemic countries, so tests to eliminate the presence of tuberculosis are required. Also, monitory for bone marrow toxicity as myelosuppression can occur due to folate deficiency. A sudden dip in blood counts must alert to that possibility.
Methotrexate contraindications
You should not use methotrexate if you are allergic to it. You may not be able to take methotrexate if you have:
- alcoholism, cirrhosis, or chronic liver disease;
- low blood cell counts;
- a weak immune system or bone marrow disorder; or
- if you are pregnant or breastfeeding.
Methotrexate can harm an unborn baby if the mother or the father is using methotrexate.
- If you are a woman, you may need to have a negative pregnancy test before starting methotrexate. Use effective birth control to prevent pregnancy while you are using methotrexate and for at least 6 months after your last dose.
- If you are a man, use effective birth control if your sex partner is able to get pregnant. Keep using birth control for at least 3 months after your last dose.
- Tell your doctor right away if a pregnancy occurs while either the mother or the father is using methotrexate.
Pregnant or breastfeeding women should avoid using methotrexate due to the elevated risk of teratogenicity and excretion into breast milk.
Methotrexate may cause injury or death to an unborn baby and should not be used during pregnancy to treat arthritis or psoriasis. However, methotrexate is sometimes used to treat cancer during pregnancy. Tell your doctor if you are pregnant or plan to become pregnant.
Do not use methotrexate to treat psoriasis or rheumatoid arthritis if you have low blood cell counts, a weak immune system, alcoholism or chronic liver disease, or if you are breastfeeding.
Caution is necessary for using methotrexate for patients who have pre-existing blood disorders, such as bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia 20. In cases of rheumatoid arthritis or psoriasis, it is contraindicated to use methotrexate in patients with chronic liver disease, liver cirrhosis, alcoholic hepatitis, or chronic alcoholism. It is also not recommended to use methotrexate in HIV/AIDS, blood dyscrasias, renal dysfunction and during radiotherapy 7.
Methotrexate can cause serious or fatal side effects. Tell your doctor if you have diarrhea, mouth sores, cough, shortness of breath, upper stomach pain, dark urine, numbness or tingling, muscle weakness, confusion, seizure, or skin rash that spreads and causes blistering and peeling.
To make sure methotrexate is safe for you, tell your doctor if you have ever had:
- liver problems, especially fluid in your stomach (ascites);
- kidney disease;
- lung problems, especially fluid in the lungs (pleural effusion);
- radiation treatments; or
- a stomach ulcer or ulcerative colitis.
Methotrexate dosage
Take methotrexate only as directed by your doctor. The dose of methotrexate will be different for different patients. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take methotrexate depend on the medical problem for which you are using methotrexate. Follow your doctor’s orders or the directions on the label. The following information includes only the average doses of methotrexate. If your dose is different, do not change it unless your doctor tells you to do so.
Do not use more of it, do not use it more often, and do not use it for a longer time than your doctor ordered. Do not take methotrexate everyday to treat conditions other than cancer. Read and follow the patient instructions that come with methotrexate. Talk to your doctor or pharmacist if you have any questions.
Measure the oral liquid medicine with a marked measuring spoon, oral syringe, or medicine cup.
Swallow the tablet whole. Do not crush, break, or chew it. Do not take the tablet if you cannot swallow it.
For patients with rheumatoid arthritis, psoriasis, or polyarticular juvenile idiopathic arthritis: Your doctor may give you folic acid or folinic acid supplement to help reduce the unwanted effects of methotrexate.
For patients with cancer: Do not take folic acid or folinic acid supplement unless directed by your doctor.
Do not take methotrexate with foods that are rich in milk.
For oral methotrexate dosage form (tablets)
- For acute lymphoblastic leukemia (ALL)
- Adults and children—Dose is based on body size and must be determined by your doctor. At first, 20 milligrams (mg) per meter squared (m²) of body size once a week. Your doctor may adjust your dose as needed and tolerated.
- For mycosis fungoides
- Adults—
- Used alone: 25 to 75 milligrams (mg) once a week.
- Used with other medicines: Dose is based on body size and must be determined by your doctor. The dose is usually 10 mg per meter squared (m²) of body size 2 times a week.
- Children—Use and dose must be determined by your doctor.
- Adults—
- For non-Hodgkin lymphoma
- Adults—2.5 milligrams (mg) 2 to 4 times a week. Your doctor may adjust your dose as needed and tolerated. However, the dose is usually not more than 10 mg per week.
- Children—Use and dose must be determined by your doctor.
- For polyarticular juvenile arthritis (pJIA)
- Children—Dose is based on body size and must be determined by your doctor. At first, 10 milligrams (mg) per meter squared (m²) once a week. Your doctor may adjust your dose as needed and tolerated.
- For psoriasis
- Adults—At first, 10 to 25 milligrams (mg) once a week. Your doctor may adjust your dose as needed and tolerated. However, the dose is usually not more than 30 mg per week.
- Children—Use and dose must be determined by your doctor.
- For rheumatoid arthritis
- Adults—At first, 7.5 milligrams (mg) once a week Your doctor may adjust your dose as needed and tolerated.
- Children—Use and dose must be determined by your doctor.
For oral methotrexate dosage form (solution)
- For acute lymphoblastic leukemia (ALL)
- Children—Dose is based on body size and must be determined by your doctor. At first, 20 milligrams (mg) per meter squared (m(2)) of body size once a week. Your doctor may adjust your dose as needed.
- For polyarticular juvenile idiopathic arthritis (pJIA)
- Children—Dose is based on body size and must be determined by your doctor. At first, 10 milligrams (mg) per meter squared (m(2)) of body size once per week. Your doctor may adjust your dose as needed.
Adult dose for Acute Lymphoblastic Leukemia (ALL)
Use: Acute lymphoblastic leukemia (ALL)
Note: A variety of combination chemotherapy regimens have been used for both induction and maintenance therapy in acute lymphoblastic leukemia. The physician should be familiar with the new advances in anti-leukemic therapy.
- Induction: 3.3 mg/m²/day orally or parenterally (in combination with prednisone 60 mg/m²) daily for 4 to 6 weeks
- Maintenance dose during remission: 30 mg/m² orally or IM 2 times a week
- Alternate maintenance dose during remission: 2.5 mg/kg IV every 14 days
Comments:
- When relapse occurs, reinduction of remission can usually be obtained by repeating the initial induction regimen.
- Acute lymphoblastic leukemia in children and young adolescents is the most responsive to present day chemotherapy. In young adults and older patients, clinical remission is more difficult to obtain and early relapse is more common.
Adult dose for Choriocarcinoma
Use: Gestational trophoblastic disease (GTD) including gestational choriocarcinoma, chorioadenoma destruens, and hydatidiform mole
- 15 to 30 mg orally or IM daily for a 5-day course; courses are usually repeated for 3 to 5 times, with rest periods of one or more weeks between courses, until any manifesting toxic symptoms subside
Comments:
- The effectiveness of therapy is evaluated by 24-hour quantitative analysis of urinary chorionic gonadotropin (hCG), which should return to normal or less than 50 IU/24 hr usually after the third or fourth course and usually be followed by a complete resolution of measurable lesions in 4 to 6 weeks.
- One to two courses of therapy after normalization of hCG is usually recommended.
- Since hydatidiform mole may precede choriocarcinoma, prophylactic chemotherapy with this drug has been recommended.
- Chorioadenoma destruens is an invasive form of hydatidiform mole. This drug is administered in these disease states in doses like those recommended for choriocarcinoma.
Adult dose for Trophoblastic Disease
Use: Gestational trophoblastic disease (GTD) including gestational choriocarcinoma, chorioadenoma destruens, and hydatidiform mole
- 15 to 30 mg orally or IM daily for a 5-day course; courses are usually repeated for 3 to 5 times, with rest periods of one or more weeks between courses, until any manifesting toxic symptoms subside
Comments:
- The effectiveness of therapy is evaluated by 24-hour quantitative analysis of urinary chorionic gonadotropin (hCG), which should return to normal or less than 50 IU/24 hr usually after the third or fourth course and usually be followed by a complete resolution of measurable lesions in 4 to 6 weeks.
- One to two courses of therapy after normalization of hCG is usually recommended.
- Since hydatidiform mole may precede choriocarcinoma, prophylactic chemotherapy with this drug has been recommended.
- Chorioadenoma destruens is an invasive form of hydatidiform mole. This drug is administered in these disease states in doses like those recommended for choriocarcinoma.
Adult dose for Burkitt’s Tumor and Lymphoma
Uses: Burkitt’s tumor and lymphoma
- Burkitt’s tumor Stages 1 to 2: 10 to 25 mg orally once a day for 4 to 8 days
- Burkitt’s tumor Stage 3: Methotrexate is commonly given concomitantly with other antitumor agents
- Duration of therapy: All stages usually require several courses of therapy interposed with 7 to 10 day rest periods
- Lymphosarcoma Stage 3: 0.625 to 2.5 mg/kg orally daily as a part of combination chemotherapy
Adult dose for Meningeal Leukemia
Use: Treatment and prophylaxis of meningeal leukemia
- 12 mg (maximum 15 mg) intrathecally every 2 to 5 days until the cell count of the CSF returns to normal; at this point, one additional dose is advisable
Comments:
- Administration at intervals of less than 1 week may result in increased subacute toxicity.
- The preserved formulations of this drug contain benzyl alcohol and must not be used for intrathecal or high dose therapy.
Adult dose for Mycosis Fungoides
Use: Mycosis fungoides (cutaneous T cell lymphoma)
Early stage dosing: 5 to 50 mg orally or parenterally once a week; alternatively, 15 to 37.5 mg 2 times a week may be used in patients who have responded poorly to weekly therapy
Comments:
- Therapy with this drug as a single agent appears to produce clinical responses in up to 50% of patients treated.
- Dose reduction or cessation is guided by patient response and hematologic monitoring.
Adult dose for Osteosarcoma
Use: Osteosarcoma
Initial dose: 12 g/m² IV as a 4-hour infusion (in combination with other chemotherapeutic agents); if this dose is not adequate to achieve a peak serum concentration of 1000 micromolar at the end of the infusion, the dose may be increased to 15 g/m²
Treatments may occur at 4, 5, 6, 7, 11, 12, 15, 16, 29, 30, 44, and 45 weeks after surgery.
Comments:
- If the patient is vomiting or unable to tolerate oral medication, leucovorin given IV or IM should be added to this regimen at the same dose and schedule as the methotrexate.
- Consult product labeling or local protocol for dosage of concomitant medications in the chemotherapy regimen.
Adult dose for Psoriasis
Use: For the symptomatic control of severe, recalcitrant, disabling psoriasis that is not adequately responsive to other forms of therapy, but only when the diagnosis has been established, as by biopsy and/or after dermatologic consultation. It is important to ensure that a psoriasis “flare” is not due to an undiagnosed concomitant disease affecting immune responses.
- Single dose: 10 to 25 mg/week orally, IM, IV, or subcutaneously until adequate response is achieved
- Divided dose: 2.5 mg orally every 12 hours for 3 doses once a week
- Maximum dose: 30 mg/week
Comments:
- Once optimal clinical response has been achieved, each dosage schedule should be reduced to the lowest possible amount of drug and to the longest possible rest period.
- The use of methotrexate may permit the return to conventional topical therapy, which should be encouraged.
Adult dose for Rheumatoid Arthritis
Use: For severe active rheumatoid arthritis in patients who have had an insufficient therapeutic response to, or are intolerant of, an adequate trial of first-line therapy including full dose nonsteroidal anti-inflammatory agents (NSAIDs)
- Single dose: 7.5 mg orally or subcutaneously once a week
- Divided dose: 2.5 mg orally every 12 hours for 3 doses once a week
- Maximum weekly dose: 20 mg
- Duration of therapy: Unknown
Comments:
- Dosages may be adjusted gradually to achieve optimal response.
- Limited experience shows a significant increase in the incidence and severity of serious toxic reactions, especially bone marrow suppression, at doses greater than 20 mg per week.
- Therapeutic response usually begins within 3 to 6 weeks and the patient may continue to improve for another 12 weeks or more.
Children dose for Acute Lymphoblastic Leukemia
Use: Childhood acute lymphoblastic leukemia (ALL)
Note: A variety of combination chemotherapy regimens have been used for both induction and maintenance therapy in acute lymphoblastic leukemia. The physician should be familiar with the new advances in anti-leukemic therapy.
- Induction: 3.3 mg/m²/day orally or parenterally (in combination with prednisone 60 mg/m²) daily for 4 to 6 weeks
- Alternate induction: 20 mg/m² orally once a week as a component of a multi-agent combination
- Maintenance dose during remission: 30 mg/m² orally or IM 2 times a week
- Alternate maintenance dose during remission: 2.5 mg/kg IV every 14 days
Comments:
- When relapse occurs, reinduction of remission can usually be obtained by repeating the initial induction regimen.
- Acute lymphoblastic leukemia in pediatric patients and young adolescents is the most responsive to present day chemotherapy. In young adults and older patients, clinical remission is more difficult to obtain and early relapse is more common.
Children dose for Meningeal Leukemia
Use: Treatment and prophylaxis of meningeal leukemia
- Less than 1 year old: 6 mg intrathecally every 2 to 5 days until the cell count of the CSF returns to normal; at this point, one additional dose is advisable
- One year old: 8 mg intrathecally every 2 to 5 days until the cell count of the CSF returns to normal; at this point, one additional dose is advisable
- Two years old: 10 mg intrathecally every 2 to 5 days until the cell count of the CSF returns to normal; at this point, one additional dose is advisable
- Three years and older: 12 mg intrathecally every 2 to 5 days until the cell count of the CSF returns to normal; at this point, one additional dose is advisable
Comments:
- Administration at intervals of less than 1 week may result in increased subacute toxicity.
- The preserved formulations of this drug contain benzyl alcohol and must not be used for intrathecal or high dose therapy.
Children dose for Juvenile Rheumatoid Arthritis
Use: For children with active polyarticular-course juvenile rheumatoid arthritis who have had an insufficient therapeutic response to, or are intolerant of, an adequate trial of first-line therapy including full dose nonsteroidal anti-inflammatory agents (NSAIDs)
- Initial dose: 10 mg/m² orally or subcutaneously once a week
- Maximum dose: 20 mg/m²/week (although there is experience with doses up to 30 mg/m²/week in children, there are too few published data to assess how doses over 20 mg/m²/week might affect the risk of serious toxicity in children; experience suggests that children receiving 20 to 30 mg/m²/week [0.65 to 1 mg/kg/week] may have better absorption and fewer GI side effects if this drug is administered either IM or subcutaneously)
Comments:
- Dosages may be adjusted gradually to achieve optimal response.
- Limited experience shows a significant increase in the incidence and severity of serious toxic reactions, especially bone marrow suppression, at doses greater than 20 mg per week.
- Therapeutic response usually begins within 3 to 6 weeks and the patient may continue to improve for another 12 weeks or more.
What should I do if I forget a dose?
Take the missed dose as soon as you remember it. However, if it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
Dose adjustments
Administration of methotrexate should be delayed until recovery if:
- The white blood cell (WBC) count is less than 1500/microliter
- The neutrophil count is less than 200/microliter
- The platelet count is less than 75,000/microliter
- The serum bilirubin level is greater than 1.2 mg/dL
- The SGPT level is greater than 450 Units
- Mucositis is present, until there is evidence of healing
- Persistent pleural effusion is present; this should be drained dry prior to infusion
Methotrexate side effects
Get emergency medical help if you have signs of an allergic reaction to methotrexate: hives, difficult breathing, swelling in your face or throat or a severe skin reaction (fever, sore throat, burning in your eyes, skin pain, red or purple skin rash that spreads and causes blistering and peeling).
Methotrexate may cause side effects. See your doctor if any of these symptoms are severe or do not go away:
- dizziness
- drowsiness
- headache
- swollen, tender gums
- decreased appetite
- reddened eyes
- hair loss
Some side effects can be serious. If you experience any of these symptoms or those listed in the IMPORTANT WARNING section, see your doctor immediately:
- blurred vision or sudden loss of vision
- seizures
- confusion
- weakness or difficulty moving one or both sides of the body
- sudden chest pain, wheezing, dry cough, cough with mucus, chest pain, feeling short of breath
- fever, chills, swollen lymph glands, night sweats, weight loss
- blisters or ulcers in your mouth, red or swollen gums, trouble swallowing
- vomiting, diarrhea, blood in your urine or stools
- skin changes such as redness, warmth, swelling, or oozing
- loss of consciousness
- low blood cell counts – fever, chills, tiredness, mouth sores, skin sores, easy bruising, unusual bleeding, pale skin, cold hands and feet, feeling light-headed or short of breath
- kidney problems – little or no urination, swelling in your feet or ankles
- liver problems – swelling around your midsection, right-sided upper stomach pain, nausea, loss of appetite, dark urine, jaundice (yellowing of the skin or eyes)
- nerve problems – confusion, weakness, drowsiness, coordination problems, feeling irritable, headache, neck stiffness, vision problems, loss of movement in any part of your body, seizure
- signs of tumor cell breakdown – tiredness, weakness, muscle cramps, nausea, vomiting, diarrhea, fast or slow heart rate, tingling in your hands and feet or around your mouth.
Methotrexate may cause other side effects. See your doctor if you have any unusual problems while taking methotrexate.
Methotrexate toxicity
High-dose methotrexate is the term for methotrexate doses higher than 500 mg/ml. Patients may experience nausea, mucosal ulceration, alopecia, fatigue, fever, increased risk of infection, leukopenia, gastrointestinal bleeding, pancreatitis, cirrhosis, aplastic anemia, cancer (lymphoproliferative disorders), infections, interstitial pneumonitis, renal impairment, and teratogenesis 20. To manage methotrexate toxicity: immediate leucovorin administration. In the case of renal failure, adequate hydration and urinary alkalinization with sodium bicarbonate are necessary.
The three antidotes used for methotrexate toxicity are leucovorin, thymidine, and glucarpidase 2. Leucovorin is the reduced active form of folic acid. It rescues normal cells from the toxic effects caused by methotrexate’s inhibition of reduced folates 21. Leucovorin is particularly effective in preventing myelosuppression, gastrointestinal toxicity, and neurotoxicity during methotrexate treatment. Thymidine rescues cells from the cytotoxic effects of methotrexate; however, its use is still under investigation and is always given together with the other drugs. Glucarpidase converts methotrexate into DAMPA and glutamate, two nontoxic metabolites, thus rapidly removing methotrexate in patients with renal dysfunction. Glucarpidase, in combination with leucovorin, is a common therapy for methotrexate toxicity. A single dose of glucarpidase reduces plasma methotrexate concentrations by 97% or more within 15 minutes. Hydration and urine alkalinization is also continued in patients requiring glucarpidase. Leucovorin therapy should continue for 48 hours after glucarpidase administration 21.
Hemodialysis and hemoperfusion can also lower methotrexate levels. Intrathecal overdoses require CSF drainage and exchange, steroids, antidotes, and suspension of the medications that interfere with methotrexate clearance (e.g., NSAIDs, salicylates, trimethoprim, penicillin, warfarin, valproate, proton pump inhibitors, cyclosporin, cisplatin).
What can I do to help prevent the potential side-effects from methotrexate?
The following steps are essential:
- Get a blood test every 4-6 weeks.
- Take 1-mg of folic acid (a vitamin) every morning. This will help reduce the chance of developing mouth ulcers.
- Keep in close touch with your doctor. It is very important that your doctor check your progress at regular visits to make sure methotrexate is working properly and to check for unwanted effects. Blood and urine tests may be needed to check for unwanted effects.
- L. Cheng, Precision dosage of methotrexate in psoriasis, British Journal of Dermatology, Volume 181, Issue 4, 1 October 2019, Pages 660–661, https://doi.org/10.1111/bjd.18273
- Lucas CJ, Dimmitt SB, Martin JH. Optimising low-dose methotrexate for rheumatoid arthritis-A review. Br J Clin Pharmacol. 2019 Oct;85(10):2228-2234. doi: 10.1111/bcp.14057
- Patel V, Wang Y, MacDonald JK, McDonald JW, Chande N. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014 Aug 26;2014(8):CD006884. doi: 10.1002/14651858.CD006884.pub3
- Alarcón GS. Methotrexate use in rheumatoid arthritis. A Clinician’s perspective. Immunopharmacology. 2000 May;47(2-3):259-71. doi: 10.1016/s0162-3109(00)00184-3
- Chande N, Wang Y, MacDonald JK, McDonald JW. Methotrexate for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2014 Aug 27;2014(8):CD006618. doi: 10.1002/14651858.CD006618.pub3
- Shea B, Swinden MV, Tanjong Ghogomu E, Ortiz Z, Katchamart W, Rader T, Bombardier C, Wells GA, Tugwell P. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013 May 31;2013(5):CD000951. doi: 10.1002/14651858.CD000951.pub2
- Kremer JM, Petrillo GF, Hamilton RA. Pharmacokinetics and renal function in patients with rheumatoid arthritis receiving a standard dose of oral weekly methotrexate: association with significant decreases in creatinine clearance and renal clearance of the drug after 6 months of therapy. J Rheumatol. 1995 Jan;22(1):38-40.
- A. Gohar, Response to ‘Reply to Gohar on “Lungs, methotrexate and psoriasis”, a comment on “Fatal, incidental, idiopathic pulmonary fibrosis in a patient receiving long‐term low‐dose methotrexate for psoriasis”’, Clinical and Experimental Dermatology, Volume 44, Issue 8, 1 December 2019, Page 948, https://doi.org/10.1111/ced.14002
- Wilsdon TD, Whittle SL, Thynne TR, Mangoni AA. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019 Jan 18;1(1):CD012722. doi: 10.1002/14651858.CD012722.pub2
- Cronstein BN, Chan ES. Treatment of inflammatory disease. In: Cronstein BN, Bertino JR editor(s). Methotrexate. Basel: Birkhauser, 2000:65‐82.
- Mikhaylov D, Hashim PW, Nektalova T, Goldenberg G. Systemic Psoriasis Therapies and Comorbid Disease in Patients with Psoriasis: A Review of Potential Risks and Benefits. J Clin Aesthet Dermatol. 2019 Jun;12(6):46-54. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6624011
- Singh RK, van Haandel L, Kiptoo P, Becker ML, Siahaan TJ, Funk RS. Methotrexate disposition, anti-folate activity and efficacy in the collagen-induced arthritis mouse model. Eur J Pharmacol. 2019 Jun 15;853:264-274. doi: 10.1016/j.ejphar.2019.03.052
- Cutolo M, Seriolo B, Pizzorni C, Craviotto C, Sulli A. Methotrexate in psoriatic arthritis. Clin Exp Rheumatol. 2002 Nov-Dec;20(6 Suppl 28):S76-80.
- Tukukino C, Wallerstedt SM. Drug information centre queries and responses about drug interactions over 10 years-A descriptive analysis. Basic Clin Pharmacol Toxicol. 2020 Jan;126(1):65-74. doi: 10.1111/bcpt.13294
- Hannoodee M, Mittal M. Methotrexate. [Updated 2022 Jan 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556114
- Braun J, Rau R. An update on methotrexate. Curr Opin Rheumatol. 2009 May;21(3):216-23. doi: 10.1097/BOR.0b013e328329c79d
- Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, Trentham DE. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985 Mar 28;312(13):818-22. doi: 10.1056/NEJM198503283121303
- Bedoui Y, Guillot X, Sélambarom J, Guiraud P, Giry C, Jaffar-Bandjee MC, Ralandison S, Gasque P. Methotrexate an Old Drug with New Tricks. Int J Mol Sci. 2019 Oct 10;20(20):5023. doi: 10.3390/ijms20205023
- Chan ES, Cronstein BN. Methotrexate–how does it really work? Nat Rev Rheumatol. 2010 Mar;6(3):175-8. doi: 10.1038/nrrheum.2010.5
- Shetty A, Cho W, Alazawi W, Syn WK. Methotrexate Hepatotoxicity and the Impact of Nonalcoholic Fatty Liver Disease. Am J Med Sci. 2017 Aug;354(2):172-181. doi: 10.1016/j.amjms.2017.03.014
- Van der Beek JN, Oosterom N, Pieters R, de Jonge R, van den Heuvel-Eibrink MM, Heil SG. The effect of leucovorin rescue therapy on methotrexate-induced oral mucositis in the treatment of paediatric ALL: A systematic review. Crit Rev Oncol Hematol. 2019 Oct;142:1-8. doi: 10.1016/j.critrevonc.2019.07.003